Abstract
Background
Adverse pregnancy outcomes (APOs), hypertensive disorders of pregnancy, gestational diabetes mellitus, and preterm birth are associated with ischemic heart disease in later life.
Objectives
The authors aimed to study the features of premature myocardial infarction (MI) among women with and without prior APOs.
Methods
We performed a retrospective analysis of women with premature MI (<65 years of age) referred for left heart catheterization matched with a database of abstracted pregnancy data. We compared MI characteristics and epicardial coronary anatomy between women with and without APOs during their index pregnancy and evaluated time from delivery to MI.
Results
Of 391 women with premature MI and associated coronary angiography (age: 49 ± 8 years), 154 (39%) had a prior APO (hypertensive disorders of pregnancy n = 78, preeclampsia n = 35, gestational diabetes mellitus n = 28, and preterm birth n = 48). Women with APO history had a higher prevalence of diabetes (33% vs 16% without APO; P = 0.001) and presented earlier with MI following delivery (19.6 [IQR: 14.3-23.5] years vs those without APO 21.5 [IQR: 17.0-25.4] years; P = 0.012), driven by preeclampsia (17.1 [IQR: 12.7-22.4] years, P = 0.010). Women with and without APOs had similar MI features including rates of ST-segment elevation MI, obstructive and multi-vessel coronary artery disease, percutaneous coronary intervention, and shock.
Conclusions
Among women with premature MIs, 39% had a history of an APO. Women with APO history presented sooner after pregnancy but had similar MI characteristics vs those without APOs. Pregnancy history may identify women who warrant early, aggressive cardiovascular disease prevention.
Key words: gestational diabetes mellitus, hypertensive disorders of pregnancy, myocardial infarction, preeclampsia, pregnancy
Central Illustration
Adverse pregnancy outcomes (APOs), such as hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), and preterm birth (PTB) are associated with ischemic heart disease (IHD) in later life.1,2 Prior studies have shown that just 14 years after delivery, women with preeclampsia (PEC) history are at over double the risk of developing IHD compared with women without PEC history.1,2 GDM also carries a 2-fold higher risk of cardiovascular (CV) events that is independent of development of type 2 diabetes.3 Similarly, PTB is associated with an increased risk of future coronary heart disease and may be a prognostic marker of worse outcomes following coronary artery stenting.4,5
Atherosclerotic disease likely contributes to increased IHD risk, as women with PEC history have higher coronary artery calcium scores than women without PEC even after adjusting for hypertension and other traditional CV risk factors.6 APOs have also been associated with lower coronary flow reserve among women with signs and symptoms of ischemia without obstructive coronary artery disease, suggestive of a predominant microvascular pathology responsible for their ischemic physiology.7
The evaluation of premature myocardial infarction (MI before age of 65 years) characteristics, etiology, and severity among women with APOs as compared to those without APOs has been limited.7,8 Of prior studies investigating APOs and MI characteristics in later life,8,9 neither comprehensively assessed APOs known to be associated with future IHD nor evaluate PTB. Prior studies also lack detail on coronary disease patterns and timing of disease presentation with premature MI after pregnancy complicated by an APO.
Understanding timing of MI presentation after pregnancy could help guide risk assessments and inform management decisions. Furthermore, understanding the association of prior APOs with etiology of MI could influence MI management. Women with MIs are less likely to have obstructive coronary artery disease and more likely to have MIs with no obstructive coronary arteries10 including spontaneous coronary artery dissection (SCAD),11 particularly at younger ages, when compared with men. In women with premature MI, it is unknown whether APO history influences the development and severity of obstructive and nonobstructive atherosclerotic disease, or SCAD.
In this study, we aimed to determine features of premature MI among women with prior APOs including HDP (PEC or gestational hypertension [GH]), GDM, and PTB, compared with those without any APO history. We aimed to evaluate markers of clinical severity during MI presentation and coronary angiographic findings. Among women with premature MI, we hypothesized that women with APO history would present earlier with MI after pregnancy, have more severe disease presentation, higher burden of atherosclerotic disease, and would be less likely to present with obstructive coronary artery disease than women without APO history.
Methods
Retrospective cohort development
We developed a retrospective cohort of women with a history of premature MI between ages 18 and 64 years presenting within the University of Pittsburgh Medical Center system from the years 2010 to 2019. The University of Pittsburgh Medical Center system is comprised of over 40 hospitals throughout Pennsylvania and surrounding states (West Virginia, Ohio) that share a common electronic health record (EHR). We identified women with diagnosis of MI using hospital administrative claims data and collected all clinical data including baseline demographics and medical histories from the system’s EHR. For initial data extraction, we defined MI by 1 of 2 criteria: 1) an International Classification of Diseases-9th and 10th Revision (ICD-9 or ICD-10) diagnosis code of non-ST-segment elevation MI (NSTEMI) or ST-segment elevation MI (STEMI); or 2) an elevated troponin (≥0.1 ng/mL) and subsequent left heart catheterization or cardiac surgery. ICD diagnoses codes used in these analyses are listed in Supplemental Tables 1 and 2. The year 2010 was chosen as a starting point for MI admission due to the limitations of the EHR data prior to 2010. For those with recurrent MI, we analyzed data from the index presentation for MI.
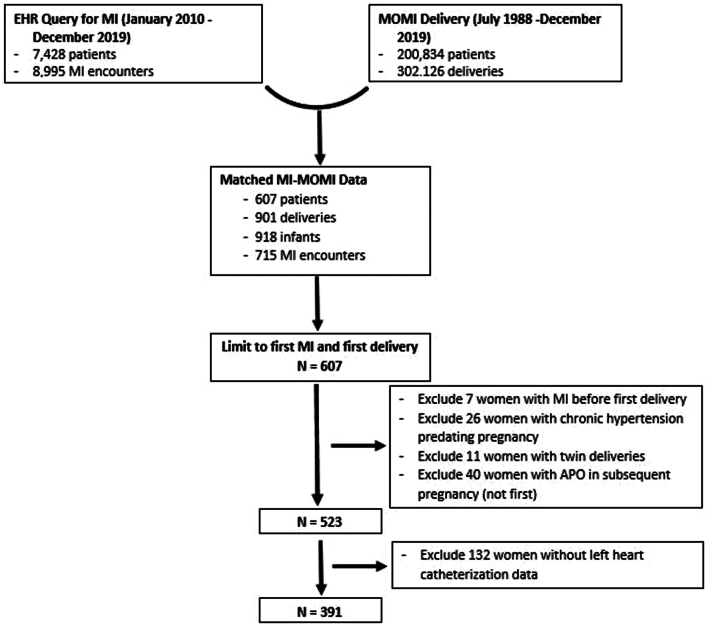
We then matched women identified as having an MI with the Magee Obstetric Maternal and Infant (MOMI) database that includes records of those who delivered within our system’s tertiary obstetric hospital, the Magee-Womens Hospital of University of Pittsburgh Medical Center, which is the region’s largest tertiary delivery hospital (Figure 1). The MOMI database collects information on maternal, fetal, and neonatal characteristics from admitting services, ICD-9 and -10 codes, electronic medical record abstraction, and electronic birth records of all women who have delivered at Magee-Womens Hospital since 1988. For multiparous women, we analyzed data from the first pregnancy between July 1988 and December 2019.
Figure 1.
Cohort Development of Women Aged <65 Years With MI and Prior Live Birth
APO = adverse pregnancy outcome; EHR = electronic health record; MI = myocardial infarction; MOMI = Magee Obstetric Maternal and Infant database.
We excluded those with twin deliveries (n = 11), prepregnancy hypertension (n = 26), prepregnancy MI (n = 7), APO in subsequent (not first) pregnancy (n = 40), and those without a left heart catheterization report available for more detailed data abstraction on coronary anatomy (n = 132) as shown in Figure 1.
APO definition
APOs were chosen for inclusion based on established association with IHD in prior studies and were obtained using the MOMI database. PEC and GH were defined by American College of Obstetricians and Gynecologists definitions at time of delivery based on ICD-9 and -10 codes from discharge diagnoses for the delivery encounter. These diagnoses have been validated in a subset by an honest broker by comparison to patient charts and examination of frequencies for outliers.12 PEC was evaluated alone and in combination with GH to form a composite HDP variable given the evidence of CV disease (CVD) in both groups that were analyzed collectively in multiple prior studies.1,13 Similarly, GDM was defined by ICD-9 and ICD-10 codes on discharge diagnoses. PTB was defined as delivery before 37 weeks gestation. In considering women may have multiple exposure variables during index pregnancy, we categorized patients by APO category in the following order, if present: 1) PEC; 2) HDP (PEC group plus those with GH without diagnosis of PEC; 3) GDM without HDP (only if no HDP diagnosis); and 4) normotensive PTB (only if no HDP nor GDM diagnosis).
MI data abstraction
Once all MI-related clinical data were abstracted from the EHR, 3 independent physician reviewers screened left heart catheterization reports to ensure that either acute coronary syndrome or concern for obstructive epicardial coronary disease were the reason for study. In addition, chart reviews of 132 patients, 57 (43%) with an APO history, who met the inclusion criteria for MI, but did not receive left heart catheterization, were reviewed to establish appropriate exclusion from the study. For the majority of patients without left heart catheterization, the treating team felt the event was consistent with supply-demand mismatch, (type II, MI) and elected not to refer for left heart catheterization (Supplemental Table 3). For women who had a left heart catheterization, we manually abstracted details from catheterization reports including type and severity of coronary lesions, type of intervention or plan for future coronary artery bypass grafting, invasive arterial blood pressure measurements, and left ventricular end diastolic pressure. Obstructive atherosclerotic disease was defined as a stenosis ≥50% and absence of evidence of alternative causes of stenosis such as SCAD or coronary vasospasm. Acute plaque rupture or thrombus were defined based on left catheterization report and included all those with STEMI. For those with no reported obstructive coronary artery disease, a subset (20%, n = 16) of the left heart catheterization films was reviewed by an interventional cardiologist who was blinded to the reports and clinical scenario. There was agreement with major report findings on 15/16 (94%). One was reported as normal coronaries, but upon blinded review, was felt to have possible subtle distal left anterior descending artery SCAD and was re-coded as such. There was no intracoronary imaging during catheterization or other systemic vascular imaging suggestive of fibromuscular dysplasia to otherwise verify SCAD diagnosis. In addition, there was excellent agreement between interventional cardiologist (A.L.) and general cardiologist (M.C.) on independent reviews of the initial 16 catheterization reviews (15/16, 94%). The remainder of the coronary angiograms was manually reviewed by 2 blinded cardiologists (M.C., A.K.) to examine for atherosclerotic disease or pathology including SCAD. There was no additional atherosclerotic disease or SCAD noted.
Other diagnostic and clinical variables
Comorbid conditions (hypertension, DM, and dyslipidemia) were obtained from the EHR based on diagnosis codes reported 6 months before and 6 months after MI hospitalization. Body mass index (BMI), blood pressure, and medication use were also abstracted from the EHR. The EHR search process for antiplatelet medication use included querying the discharge medication reconciliation for antiplatelets as well as the discharge summary text for antiplatelet use. Left ventricular ejection fraction was abstracted from echocardiogram reports. Mortality was assessed using the EHR and the U.S. Social Security Death Index. Inpatient readmissions and recurrent MI were recorded if they occurred within the system to allow capture by our EHR.
Statistical analyses
Demographics, medical history, and MI presentation variables were compared between women with and without an APO history overall and for the APO subgroups defined above. Continuous normally distributed variables were compared using the unpaired t-test. Wilcoxon rank sum test was used for non-normally distributed continuous variables. Categorical variables were compared using chi-square test or Fisher exact test for variables with expected counts <5. Cumulative incidence curves summarized occurrence of MI events over time by APO. Gray’s K sample test statistics were used to compare differences by APO status vs individuals with no APOs. Cox proportional-hazard models adjusted for age at delivery, self-reported race (as a social construct), diabetes, hypertension, and BMI. We truncated follow-up at 20 years (mean time from MI) for this model to create a “no MI” group for comparison, though it should be noted that all patients in this study were selected based on having a premature MI. Four missing BMI values were imputed by the median. The Cox Proportional Hazard assumption was tested by Schoenfeld residuals. P values <0.05 were considered statistically significant.
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Pittsburgh.14,15 This study was approved by the University of Pittsburgh Institutional Review Board (STUDY18120048 and STUDY21040178). Statistical analyses were performed using Stata version 16.0 (StataCorp LLC) and SAS version 9 (SAS Institute Inc).
Results
We identified 7,428 women with an MI from January 2010 to December 2019, of whom 607 had a delivery recorded in the MOMI database (Figure 1). After exclusions, our study cohort included 391 women who presented with an MI before 65 years and had at least one delivery before their MI (median age: 50 IQR: 44-55] years at the time of MI). From this group, 74 (32%) self-identified as Black and 154 (39%) had an APO in their index delivery. Women with HDP history were younger at presentation for first MI (median age: 48 [IQR: 40-54] years) compared with women without an APO (51 [IQR: 44-55] years; P = 0.040), which appeared to be driven by those with PEC (47 [IQR: 41-53] years; P = 0.033) (Table 1).
Table 1.
Demographic and Clinical Characteristics Among Women With an Myocardial Infarction Before 65 Years by Adverse Pregnancy Outcomes History (N = 391)
| No Pregnancy Complication (n = 237) | Any APO (n = 154) | HDP (GH or PEC) (n = 78) | PEC (n = 35) | Gestational DM (n = 28) | Normotensive PTB (n = 48) | |
|---|---|---|---|---|---|---|
| Age at MI (y) | 51 (44-55) | 50.5 (42-55) | 48 (40-54)a | 47 (41-53)a | 53 (48-58) | 52 (43-56) |
| Survival-free-of MI Time since delivery (y) | 21.5 (17.0-25.4) | 19.6 (14.3-23.5)a | 19.4 (13.5-22.7)a | 17.1 (12.7-22.4)a | 18.7 (14.9-22.4) | 20.8 (15.3-25.4) |
| Race | ||||||
| White | 160 (67.5) | 108 (70.1) | 56 (71.8) | 31 (88.6) | 21 (75.0) | 31 (64.6) |
| Black | 74 (31.6) | 46 (29.9) | 22 (28.2) | 4 (11.4) | 7 (25.0) | 17 (35.4) |
| Other | 3 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Preterm birthc | 0 (0) | 81 (52.6) | 22 (28.2) | 17 (48.6) | 4 (14.3) | - |
| Gestational DMc | 0 (0) | 40 (26.0) | 12 (15.4) | 4 (11.4) | - | 0 (0) |
| Number of deliveries | 1 (1-1) | 1 (1-2)a | 1 (1-2)a | 1 (1-2) | 1 (1-1) | 1 (1-2) |
| Recurrent APO after indexd | 0/54 (0) | 36/50 (72.0) | 22/30 (73.3) | 9/12 (75.0) | 5/5 (100) | 9/15 (60.0) |
| HDP after indexd | 0/54 (0) | 14/50 (28.0) | 10/30 (33.3) | 4/12 (33.3) | 2/5 (40.0) | 2/15 (13.3) |
| GDM after indexd | 0/54 (0) | 14/50 (28.0) | 9/30 (30.0) | 4/12 (33.3) | 4/5 (80.0) | 1/15 (6.7) |
| PTB after indexd | 0/54 (0) | 19/50 (38.0) | 10/30 (33.3) | 4/12 (33.3) | 1/5 (20.0) | 8/15 (53.3) |
| BMI (kg/m2) | 32.5 ± 9.0 | 31.5 ± 9.2 | 32.1 ± 8.2 | 33.8 ± 8.6 | 32.3 ± 7.7 | 29.9 ± 11.4 |
| Hypertension | 85/186 (45.7) | 60/126 (47.6) | 31/65 (47.7) | 15/33 (45.5) | 12/23 (52.2) | 17/38 (44.7) |
| Diabetes | 30/186 (16.1) | 41/126 (32.5)b | 24/65 (36.9)b | 16/33 (48.5)b | 11/23 (47.8)b | 6/38 (15.8) |
| Dyslipidemia | 70/186 (37.6) | 59/126 (46.8) | 27/65 (41.5) | 15/33 (45.5) | 14/23 (60.9)a | 18/38 (47.4) |
| Systolic BP on admission (mm Hg) | 142 ± 31 | 142 ± 34 | 142 ± 32 | 142 ± 36 | 144 ± 29 | 142 ± 41 |
| Diastolic BP on admission (mm Hg) | 85 ± 19 | 84 ± 19 | 85 ± 17 | 86 ± 17 | 80 ± 14 | 84 ± 24 |
| Statin before admission | 58 (24.5) | 40 (26.0) | 19 (24.4) | 7 (20.0) | 11 (39.3) | 10 (20.8) |
Values are median (IQR), n (%), n/N (%), or mean ± SD.
APO = adverse pregnancy outcome; BP = blood pressure; DM = diabetes mellitus; GH = gestational hypertension; HDP = hypertensive disorder of pregnancy; MI = myocardial infarction; PEC = preeclampsia; PTB = preterm birth.
P < 0.05 compared with no APO group.
P < 0.001 compared with no APO group.
Index pregnancy.
Denominator denotes number with delivery after index pregnancy.
Women with a prior APO also had higher prevalence of diabetes at the time of their MI (33% for any APO, 37% for HDP, and 48% for GDM) compared with those without an APO (16%), all with P ≤ 0.001. Women with GDM history had a higher prevalence of dyslipidemia compared with those without an APO. There were no other differences in traditional CV risk factors (Table 1).
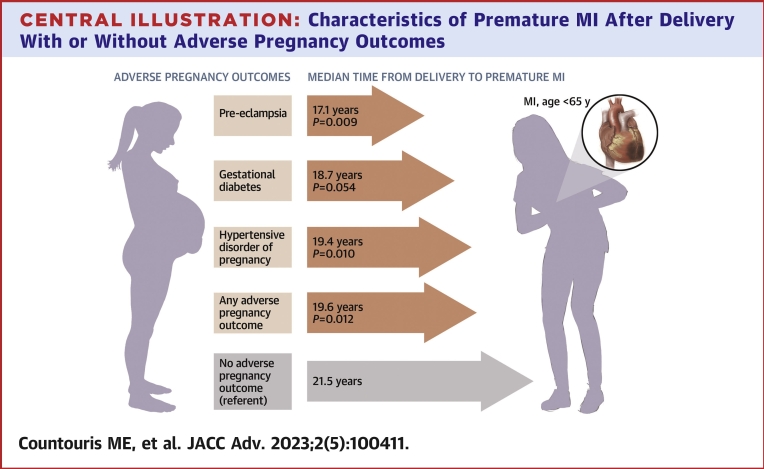
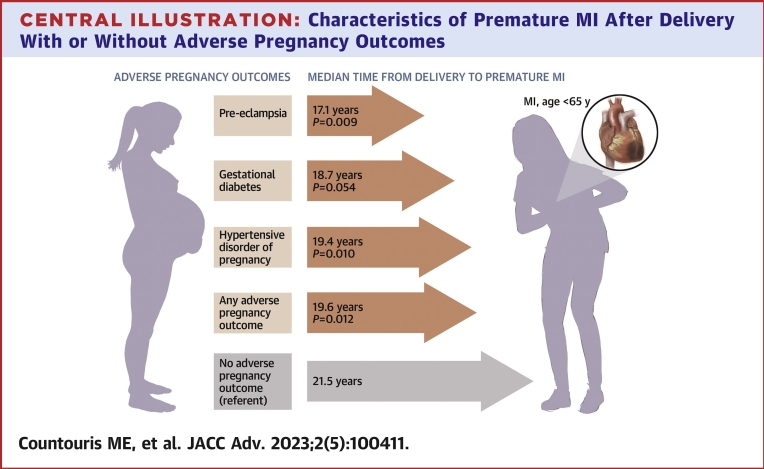
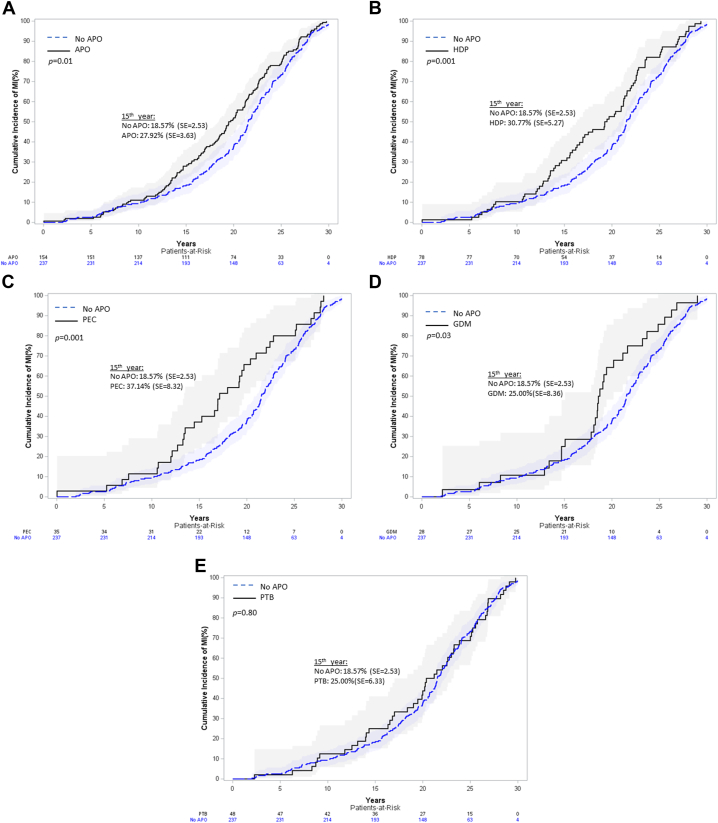
Women with an APO presented sooner after delivery with their MI compared with women without an APO history (19.6 [IQR: 14.3-23.5] years vs 21.5 [IQR: 17.0-25.4] years; P = 0.012) (Table 1). When dividing by APO group, women with HDP history (GH or PEC) presented with their MI sooner after delivery (19.4 [IQR: 13.5-22.7] years; P = 0.010) as did those with only PEC history (17.1 [IQR: 12.7-22.4] years; P = 0.009) (Central Illustration). Cumulative incidence curves (Figure 2) and adjusted HRs (Supplemental Table 4) demonstrate shorter time from delivery to MI for women with any APO, HDP, PEC, and GDM history compared with women without an APO in any pregnancy. Only one individual had an MI within 1 year of delivery. Sensitivity analyses looking at subgroups with single pregnancy with an APO, those with subsequent pregnancy without APO, and those with subsequent pregnancy with recurrent APO showed that those with APO recurrence did not present sooner with MI after delivery but did present at younger ages (Supplemental Table 5).
Central Illustration.
Characteristics of Premature MI After Delivery With or Without Adverse Pregnancy Outcomes
Women with preeclampsia, hypertensive disorder of pregnancy, or any adverse pregnancy outcome history presented sooner after delivery with their myocardial infarction (MI) compared with those without an adverse pregnancy outcome history. APO = adverse pregnancy outcome; CAD = coronary artery disease; MI = myocardial infarction; PCI = percutaneous intervention; PEC = preeclampsia; STEMI = ST-segment elevation MI.
Figure 2.
Time From Delivery to MI Cumulative Incidence Curves by Adverse Pregnancy Outcome
(A) Any APO vs no APO history. (B) HDP vs no APO history. (C) PEC vs no APO history. (D) GDM vs no APO history. (E) PTB vs no APO history. APO = adverse pregnancy outcome; GDM = gestational diabetes; HDP = hypertensive disorder of pregnancy; MI = myocardial infarction; PEC = preeclampsia; PTB = preterm birth.
Women with a left heart catheterization were older, further from the time of delivery, and more likely to have dyslipidemia compared with women who did not have a left heart catheterization (Supplemental Table 6).Women with APO history had similar rates of STEMI, obstructive and multivessel coronary artery disease, acute plaque rupture, percutaneous coronary intervention, cardiogenic shock, and 1-year mortality compared with those without APO history (Tables 2 and 3, Central Illustration). Peak troponin level, left ventricular end diastolic pressure at time of left heart catheterization, and left ventricular ejection fraction within 30 days of their MI were also similar between groups.
Table 2.
MI Outcomes Obtained From the Electronic Health Record Among Women With Adverse Pregnancy Outcomes Compared With Women Without Pregnancy Complications Who Had Left Heart Catheterizations (N = 391)
| No Pregnancy Complication (n = 237) | Any APO (n = 154) | HDP (GH or PEC) (n = 78) | PEC (n = 35) | Gestational DM (n = 28) | Normotensive PTB (n = 48) | |
|---|---|---|---|---|---|---|
| LVEF (%) (n = 346) | 54 ± 12 | 53 ± 13 | 52 ± 14 | 50 ± 14 | 55 ± 12 | 53 ± 14 |
| LVEF ≤35% | 27 (13.0) | 17 (12.2) | 10 (14.3) | 5 (15.6) | 1 (4.0) | 6 (14.0) |
| Highest troponin (ng/mL) | 2.1 (0.35-13.9) | 3.1 [0.64-18.1] | 3.5 (0.47-23.4) | 3.4 (0.45-15.8) | 1.7 (0.39-16.5) | 3.1 (0.54-18.7) |
| Discharge antiplatelet | 200 (85.8) | 126 (83.4) | 65 (85.5) | 27 (81.8) | 23 (82.1) | 38 (80.9) |
| Discharge statin | 136 (59.1) | 87 (58.8) | 44 (58.7) | 19 (59.4) | 19 (70.4) | 24 (52.2) |
| CABG | 22 (9.3) | 8 (5.2) | 5 (6.4) | 2 (5.7) | 2 (7.1) | 1 (2.1) |
| Median time to death from MI admission (y) | 1.5 (0.3-3.3) | 1.1 (0.3-1.9) | 0.7 (0.4-1.9) | 1.4 (0.6-3.1) | 1.6 (1.5-1.6) | 0.9 (0.1-2.0) |
| 1-y mortality | 9 (3.8) | 9 (5.8) | 4 (5.1) | 1 (2.9) | 0 (0.0) | 5 (10.4) |
| MI readmission within 1 y | 10 (4.2) | 5 (3.3) | 3 (3.9) | 1 (2.9) | 0 (0.0) | 2 (4.2) |
| Any cardiac readmission within 1 y | 48 (20.3) | 28 (18.2) | 11 (14.1) | 3 (8.6) | 1 (3.6)a | 16 (13.3) |
Values are mean ± SD, n (%), or median (IQR).
APO = adverse pregnancy outcome; CABG = coronary artery bypass graft; DM = diabetes mellitus; GH = gestational hypertension; HDP = hypertensive disorder of pregnancy; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PEC = preeclampsia; PTB = preterm birth.
P < 0.05 compared with no APO group.
Table 3.
MI Outcomes Obtained From Left Heart Catheterization Report Among Women With Adverse Pregnancy Outcomes Compared With Women Without Pregnancy Complications (N = 391)
| No Pregnancy Complication (n = 237) | Any APO (n = 154) | HDP (GH or PEC) (n = 78) | PEC (n = 35) | Gestational DM (n = 28) | Normotensive PTB (n = 48) | |
|---|---|---|---|---|---|---|
| ACS typea | ||||||
| Unstable angina | 15/198 (7.6) | 12/122 (9.8) | 9/61 (14.8) | 5/26 (19.2) | 2/22 (9.1) | 1/39 (2.6) |
| NSTEMI | 129 (65.2) | 77 (63.1) | 34 (55.7) | 12 (46.2) | 15 (68.2) | 28 (71.8) |
| STEMI | 54 (27.3) | 33 (27.1) | 18 (29.5) | 9 (34.6) | 5 (22.7) | 10 (25.6) |
| SCAD | 6 (2.6) | 4 (2.7) | 3 (4.0) | 2 (6.1) | 0 (0) | 1 (2.1) |
| Vasospasm | 7 (3.0) | 6 (4.0) | 4 (5.3) | 0 (0.0) | 2 (8.0) | 0 (0.0) |
| Atherosclerotic disease | ||||||
| No disease | 38 (16.2) | 27 (17.8) | 16 (20.5) | 6 (17.1) | 2 (7.7) | 9 (18.8) |
| Nonobstructive | 45 (19.2) | 29 (19.1) | 10 (12.8) | 5 (14.3) | 7 (26.9) | 12 (25.0) |
| Obstructive | 152 (64.7) | 96 (63.2) | 52 (66.7) | 24 (68.6) | 17 (65.4) | 27 (56.3) |
| Acute plaque rupture/thrombus | 66 (28.0) | 39 (25.3) | 21 (26.9) | 8 (22.9) | 7 (25.0) | 11 (22.9) |
| Multi-vessel disease (>1 vessel PCI, 3VD, or CABG) | 59 (38.8) | 34 (37.0) | 21 (42.0) | 11 (47.8) | 6 (37.5) | 7 (26.9) |
| PCI | 123 (51.9) | 79 (51.3) | 38 (48.7) | 19 (54.3) | 17 (60.7) | 24 (50.0) |
| Shock (systolic BP <90, MAP <65, or mechanical support) | 10/158 (6.3) | 14/115 (12.2) | 7/56 (12.5) | 3/24 (12.5) | 2/21 (9.5) | 5/38 (13.2) |
| Ao systolic BP (mm Hg) (n = 287) | 130 ± 22 | 133 ± 32 | 132 ± 31 | 127 ± 33 | 141 ± 34 | 131 ± 33 |
| Ao diastolic BP (mm Hg) (n = 275) | 74 ± 14 | 76 ± 16 | 76 ± 14 | 73 ± 15 | 77 ± 14 | 76 ± 19 |
| LVEDP (mm Hg) (n = 259) | 18 ± 7 | 19 ± 8 | 19 ± 8 | 19 ± 9 | 21 ± 9 | 19 ± 8 |
| LVEDP ≥15 mm Hg | 111/165 (67.3) | 66/94 (70.2) | 32/47 (68.1) | 14/22 (63.6) | 13/16 (81.3) | 21/31 (67.7) |
Values are n/N (%) or mean ± SD.
3VD = triple vessel disease; ACS = acute coronary syndrome; Ao = opening aortic; APO = adverse pregnancy outcome; CABG = coronary artery bypass graft; DBP = diastolic blood pressure; DM = diabetes mellitus; gHTN = gestational hypertension; HDP = hypertensive disorder of pregnancy; LVEDP = left ventricular end diastolic pressure; MAP = mean arterial pressure; NSTEMI = non-ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention; PEC = preeclampsia; PTB = preterm birth; SBP = systolic blood pressure; SCAD = spontaneous coronary artery dissection; STEMI = ST-segment elevation myocardial infarction; VF = ventricular fibrillation; VT = ventricular tachycardia.
STEMI/NSTEMI could only be assessed when documented in left heart catheterization report.
Discussion
Among women with history of early presentation of MI before 65 years, an impressive 39% had an APO history. Women with a history of APOs also presented in their late 40s as compared to their early 50s with MIs, a difference driven primarily by the group with HDP history. Among APOs, PEC was associated with the shortest time from pregnancy to premature MI in our cohort. Contrary to our hypothesis, women with APO history had similar MI characteristics, regarding likelihood of obstructive epicardial coronary artery disease and MI severity (STEMI, percutaneous intervention, and cardiogenic shock), compared with women without APO history.
We found that among a younger cohort of women presenting with premature MI, those with APO history presented at a median of 19.6 years after their affected pregnancy, which was closer to time of pregnancy than women without APO history. For women with HDP history, this corresponds to presentation in their late 40s vs early 50s of age. In light of increasing rates of HDP over the past 30 years,16 our findings might help explain the increase in MI presentations among young adult women, despite declines in other age and sex strata.17 Notably, 36% of women with an MI in our study had no obstructive coronary artery disease by invasive angiography, likely higher than previous reports due to our younger, female population.18 Our finding that among women with premature MI, those with APO experienced their MI even earlier than women without APO is consistent with prior studies based in Canada.8,9
Our findings suggest that APOs may accelerate pathologic processes leading to MI as soon as 15 to 20 years after delivery, when women are in their late 40s. One potential mechanism may be due to increased exposure to inflammation, as evidenced by elevated C-reactive protein, cystatin C, or inflammatory chemokines. These inflammatory biomarker abnormalities have been found at the time of delivery and persist in the years postpartum for women with HDP19, 20, 21 and GDM.22 The role of inflammation in development of atherosclerotic disease is well known23 and trials which target specific pathways of inflammation to reduce CV outcomes have shown promising results.24,25 Alternatively, the antiangiogenic state associated with PEC, which is also noted to persist several years following pregnancy, has been associated with adverse postpartum CV profiles; this may also play a role in development of premature coronary disease.26, 27, 28 Maternal vascular malperfusion lesions, including vasculopathy and infarct, of the placenta have been hypothesized to give insight onto mechanisms of future vascular disease development. Vascular lesions of the placenta have been linked to an adverse CV profile years after delivery, including peripheral microvascular changes, higher blood pressure, adverse lipid profile, and higher carotid intima media thickness.29, 30, 31 Future studies are needed to further investigate specific pro-inflammatory pathways or vascular mechanisms responsible for earlier presentation with MI.
Women with APOs are at increased risk of developing traditional CV risk factors which may impact accelerated MI presentation. Diabetes prevalence was significantly higher among women with HDP and GDM in our study compared with women without APO history. Prior work has shown that women with both HDP history and hypertension have a higher likelihood of LV remodeling in the decade after delivery.32 By studying only early MI, our referent group was, by design, a high-risk group with a high proportion of CV risk factors. Rates of hypertension were close to 50% in both APO and non-APO groups, findings that are consistent with other studies investigating early MI.17
The routine evaluation of women in the postpartum period in current practice provides a critical opportunity to screen early for CV risk factors and apply aggressive risk factor management to change a woman’s potential future CVD trajectory. It is of utmost importance that discussion of pregnancy outcomes become a routine part of our CV history taking. The American Heart Association (AHA), American College of Cardiology (ACC), and American College of Obstetricians and Gynecologists (ACOG) recommend early and frequent CV risk factor screening and modification for women with a history of APOs including HDP, GDM, and PTB, beginning after 3 months postpartum.33, 34, 35 Considering that close to 40% of women with an MI before 65 years in our cohort had an APO as compared to 13% to 20% in epidemiologic studies, pregnancy outcomes may provide a way to identify women at-risk for premature MI.36 Similarly, a recent study found that among women with a reported stroke before 60 years of age, 39% reported a history of at least one APO.37 PEC is also considered a CVD risk enhancer in the 2018 AHA/ACC Cholesterol Management Guidelines, and thus may influence prescription of statins in women aged over 40 years.38 Early postpartum follow-up with primary care physicians or cardiologists provides an opportunity for risk factor screening and counseling on lifestyle changes including diet improvement, weight loss, and exercise to improve CV health.39 Incorporation of APOs into traditional CVD risk prediction models has not consistently better re-classified women,40 although may better risk stratify women aged younger than 40 years. Thus, future research is needed to identify which women warrant the most aggressive prevention strategies.
This study has several strengths. First, we used a validated diagnosis of APO history through chart abstraction, not a self-report which is often the standard. Also, we present a diverse cohort with 32% of participants being of self-reported Black race. In addition, after initial extraction of EHR data for MI, all left heart catheterization reports were reviewed to ensure accurate inclusion for indication of acute coronary syndrome.
There are also limitations worth noting. First, this was a single medical system study, although there are multiple hospitals included. These hospitals are situated throughout diverse socioeconomic areas of Pennsylvania, West Virginia, and Ohio, and add to the generalizability of this study. Second, some women identified as having an MI did not have a left heart catheterization. This limits our analyses of angiographic findings to patients in whom the treating team considered the likelihood of underlying coronary artery disease to be high. Given that young women with MI are less likely to be referred for left heart catheterization, some type I MI may have been missed.17,41 Based on our chart review, of those who met inclusion criteria for MI, but did not have a left heart catheterization, a small minority (5%) were suspected to have had a type I MI (Supplemental Table 3). Third, for the purposes of this study, we excluded women with APO in a pregnancy other than the first in our system. Fourth, recurrent HDP has been associated with increased CVD risk; however, due to the small number of women with recurrent HDP in this study (n = 10), sensitivity analyses by APO were unable to be performed. Future larger studies are needed to investigate recurrent APO or APO in any pregnancy and impact on MI characteristics. Fifth, since this was a retrospective study, we were limited to using data available in the EHR. Thus, we were unable to include intrauterine growth restriction or miscarriages as APOs due to lack of available data. Similarly, some covariates were unavailable including parity, smoking status, alcohol use history, family history, and lipid levels. It is possible that antiplatelets were prescribed, but not documented. There may also be under prescribing of antiplatelet agents among women with acute MI, which has been reported in prior studies.17,42
Conclusions
Among women with premature MI, close to 40% had a history of an APO in their index pregnancy. Those with an APO presented with MI sooner after delivery and those with HDP history presented at younger ages, in their late 40s vs early 50s of age. Women with APOs had similar MI characteristics to those without an APO, with no observed increase in the likelihood of obstructive coronary artery disease, and MI with nonobstructive coronary artery disease, including SCAD. Considering a woman’s pregnancy history may help identify women at greater risk for premature MI who warranted more aggressive CV risk factor screening and modification.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Women with APOs comprised almost 40% of women with aged <65 years. Women with APOs presented at younger ages and sooner among those with first MI before 65 years.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Providers should recognize that pregnancy history can help identify women at risk for premature MI. Women with APOs require early and aggressive risk factor screening and modification to prevent future ischemic heart disease.
TRANSLATIONAL OUTLOOK: Women with and without APO history had similar MI characteristics. Future studies are needed to further investigate specific mechanisms responsible for earlier presentation with MI among women with APOs. Interventions tailored to prevent ischemic heart disease in this population will be essential.
Funding support and author disclosures
Dr Countouris is funded through the American Heart Association (CDA941351). Drs Catov and Countouris are also funded through the American Heart Association 16SFRN28930000. Drs Reynolds and Hausvater are funded through the American Heart Association 16SFRN28730004, 16SFRN2781006, and 812162. Dr Hausvater is supported by National Institutes of Health T32HL098129. Dr Koczo is supported by NIH T32HL129964. Dr Reynolds reports in-kind donation for research on MI in women from Abbott Vascular, Siemens and Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Dr Ashley Lee, an interventional cardiologist at the University of Pittsburgh Medical Center, for reviewing left heart catheterization films for this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Bellamy L., Casas J.P., Hingorani A.D., Williams D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P., Haththotuwa R., Kwok C.S., et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2) doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 3.Kramer C.K., Campbell S., Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 4.Wu P., Gulati M., Kwok C.S., et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(2) doi: 10.1161/JAHA.117.007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pehrson M., Edsfeldt A., Sarno G., et al. Long-term outcome following coronary artery stenting by history of preterm delivery. JACC: Adv. 2022;1(5) [Google Scholar]
- 6.White W.M., Mielke M.M., Araoz P.A., et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol. 2016;214:519.e1–519.e8. doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park K., Quesada O., Cook-Wiens G., et al. Adverse pregnancy outcomes are associated with reduced coronary flow reserve in women with signs and symptoms of ischemia without obstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. J Womens Health (Larchmt) 2020;29:487–492. doi: 10.1089/jwh.2019.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grand'Maison S., Pilote L., Schlosser K., Stewart D.J., Okano M., Dayan N. Clinical features and outcomes of acute coronary syndrome in women with previous pregnancy complications. Can J Cardiol. 2017;33:1683–1692. doi: 10.1016/j.cjca.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 9.McDonald E.G., Dayan N., Pelletier R., Eisenberg M.J., Pilote L. Premature cardiovascular disease following a history of hypertensive disorder of pregnancy. Int J Cardiol. 2016;219:9–13. doi: 10.1016/j.ijcard.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Smilowitz N.R., Mahajan A.M., Roe M.T., et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-get with the guidelines) Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003443. [DOI] [PubMed] [Google Scholar]
- 11.Hayes S.N., Kim E.S.H., Saw J., et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton E.F., Rogan S.C., Lopa S., Sharbaugh D., Muldoon M.F., Catov J.M. Early pregnancy blood pressure elevations and risk for maternal and neonatal morbidity. Obstet Gynecol. 2020;136:129–139. doi: 10.1097/AOG.0000000000003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo C.C.W., Lo A.C.Q., Leow S.H., et al. Future cardiovascular disease risk for women with gestational hypertension: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Xie X., Yuan T., et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021;21:364. doi: 10.1186/s12884-021-03809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora S., Stouffer G.A., Kucharska-Newton A.M., et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 19.Hubel C.A., Powers R.W., Snaedal S., et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51:1499–1505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Countouris M.E., Demirci J.R., Jeyabalan A., Catov J.M., Schwarz E.B. Relationship of postpartum levels of cystatin and high-sensitivity C-reactive protein and duration of lactation in mothers with previous gestational hypertension or preeclampsia. Breastfeed Med. 2019;14:408–415. doi: 10.1089/bfm.2018.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman D.J., McManus F., Brown E.A., et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 22.Lekva T., Michelsen A.E., Aukrust P., et al. CXC chemokine ligand 16 is increased in gestational diabetes mellitus and preeclampsia and associated with lipoproteins in gestational diabetes mellitus at 5 years follow-up. Diab Vasc Dis Res. 2017;14:525–533. doi: 10.1177/1479164117728011. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 24.Ridker P.M., Everett B.M., Thuren T., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 25.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 26.Benschop L., Schalekamp-Timmermans S., Broere-Brown Z.A., et al. Placental growth factor as an indicator of maternal cardiovascular risk after pregnancy. Circulation. 2019;139:1698–1709. doi: 10.1161/CIRCULATIONAHA.118.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noori M., Donald A.E., Angelakopoulou A., Hingorani A.D., Williams D.J. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 28.Garrido-Gimenez C., Mendoza M., Cruz-Lemini M., et al. Angiogenic factors and long-term cardiovascular risk in women that developed preeclampsia during pregnancy. Hypertension. 2020;76:1808–1816. doi: 10.1161/HYPERTENSIONAHA.120.15830. [DOI] [PubMed] [Google Scholar]
- 29.Catov J.M., Muldoon M.F., Gandley R.E., et al. Maternal vascular lesions in the placenta predict vascular impairments a decade after delivery. Hypertension. 2022;79:424–434. doi: 10.1161/HYPERTENSIONAHA.121.18394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaaban C.E., Rosano C., Cohen A.D., et al. Cognition and cerebrovascular reactivity in midlife women with history of preeclampsia and placental evidence of maternal vascular malperfusion. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.637574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catov J.M., Muldoon M.F., Reis S.E., et al. Preterm birth with placental evidence of malperfusion is associated with cardiovascular risk factors after pregnancy: a prospective cohort study. BJOG. 2018;125:1009–1017. doi: 10.1111/1471-0528.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Countouris M.E., Villanueva F.S., Berlacher K.L., Cavalcante J.L., Parks W.T., Catov J.M. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77:1057–1068. doi: 10.1016/j.jacc.2020.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosca L., Benjamin E.J., Berra K., et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho L., Davis M., Elgendy I., et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women. J Am Coll Cardiol. 2020;75:2602–2618. doi: 10.1016/j.jacc.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstet Gynecol. 2019;133:e320–e356. doi: 10.1097/AOG.0000000000003243. [DOI] [PubMed] [Google Scholar]
- 36.Wang M.C., Freaney P.M., Perak A.M., et al. Trends in prepregnancy obesity and association with adverse pregnancy outcomes in the United States, 2013 to 2018. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller E.C., Bello N.A., Davis R., et al. Women with adverse pregnancy outcomes have higher odds of midlife stroke: the population assessment of tobacco and health study. J Womens Health (Larchmt) 2021;31(4):503–512. doi: 10.1089/jwh.2021.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Hauspurg A., Countouris M.E., Catov J.M. Hypertensive disorders of pregnancy and future maternal health: how can the evidence guide postpartum management? Curr Hypertens Rep. 2019;21:96. doi: 10.1007/s11906-019-0999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuart J.J., Tanz L.J., Cook N.R., et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72:1252–1263. doi: 10.1016/j.jacc.2018.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ya'qoub L., Lemor A., Dabbagh M., et al. Racial, ethnic, and sex disparities in patients with STEMI and cardiogenic shock. J Am Coll Cardiol Intv. 2021;14:653–660. doi: 10.1016/j.jcin.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Peters S.A.E., Colantonio L.D., Zhao H., et al. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol. 2018;71:1729–1737. doi: 10.1016/j.jacc.2018.02.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.