Abstract
Cellular therapy modalities, including autologous (Auto) hematopoietic cell transplantation (HCT), allogeneic (Allo) HCT, and now chimeric antigen receptor (CAR) T therapy, have demonstrated long term remissions in advanced hematologic malignancies. Auto and AlloHCT, through hematopoietic rescue, have permitted the use of higher doses of chemotherapy. AlloHCT also introduced nonspecific immune-mediated targeting of malignancy resulting in protection from relapse, although at the expense of similar targeting of normal host cells. In contrast, CAR T therapy, through genetically-engineered immunotherapeutic precision, allows for redirection of autologous immune effector cells against malignancy in an antigen-specific and MHC-independent fashion, with demonstrated efficacy in patients who are refractory to cytotoxic chemotherapy. It too has unique toxicities and challenges. Non-Hodgkin lymphoma (including large B-cell lymphoma, mantle cell lymphoma, and follicular lymphoma), B-cell acute lymphoblastic leukemia, and multiple myeloma are the three main diseases with fully developed CAR T products with widespread deployment. Recent and ongoing clinical trials are examining the interface between the three cellular therapy modalities (AutoHCT, AlloHCT, and CAR T), to determine whether they should be “complementary” or “competitive”. In this review, we examine the current state of this interface with respect to the most recent data and delve into the controversies and conclusions that may inform clinical decision-making.
Introduction
While previous paradigms of systemic therapy treated the immune system as expected collateral damage, immune cells, manipulated or engineered, now play a central role in current strategies for advanced hematologic malignancies. Nowhere is this more apparent than in the treatment modalities of hematopoietic cell transplantation (HCT) and chimeric antigen receptor T-cell (CAR T) therapy.
The current state of HCT can be subdivided into autologous HCT (AutoHCT) and allogeneic HCT (AlloHCT) with distinct aims targeting specific malignancies (Figure 1). AutoHCT allows for hematopoietic rescue following the administration of high-dose, myeloablative chemotherapy, predominantly in multiple myeloma, relapsed lymphomas, and germ cell tumors.1 In contrast, AlloHCT exploits not only cytotoxic chemotherapy but also the immunotherapeutic graft-versus-tumor effect from adoptive transfer of a donor immune system, albeit at the risk of non-specific immune-mediated toxicity in the form of graft-versus-host disease (GVHD).2 Successful management of acute lymphoblastic leukemia (ALL) and myeloid malignancies often hinges on the immunotherapeutic effect of AlloHCT, whereas many bone marrow failure syndromes, aplastic anemia, and even sickle cell disease respond to donor graft transplantation for hematopoietic purposes.
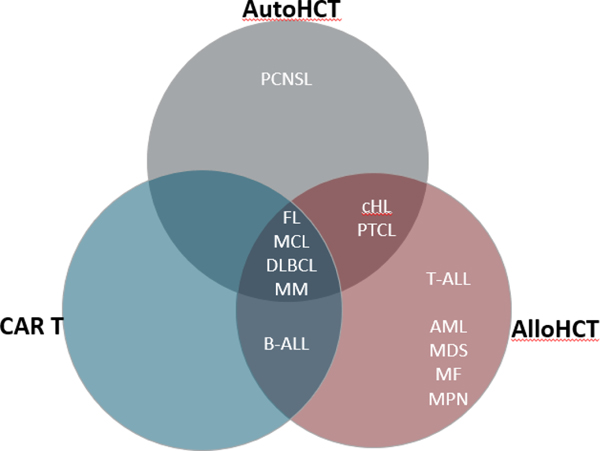
Figure 1. Current state of hematologic malignancy indications for CAR T, AlloHCT, and AutoHCT.
These diseases reflect potential indications for the three cellular therapy platforms. Importantly, however, these platforms are generally not interchangeable and the selection of the appropriate modality must be tailored to the disease biology and treatment history of each individual patient. MM, multiple myeloma; PCNSL, primary CNS lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; DLBCL, diffuse large B-cell lymphoma; B-ALL, B-cell acute lymphoblastic leukemia; cHL, classical Hodgkin lymphoma; PTCL, peripheral T-cell lymphoma; T-ALL, T-cell acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; MF, myelofibrosis; MPN, myeloproliferative neoplasm
In some contexts, such as multiple myeloma and myeloid malignancies, the roles of AutoHCT and AlloHCT, respectively, remain stable to date.3 In others such as B-cell non-Hodgkin’s lymphomas (NHL), CAR T therapy has challenged and successfully supplanted HCT in some situations.4,5 Questions remain in other disease states in which the roles of CAR T therapy and HCT are not competitive, rather complementary, as CAR T therapy may serve as a bridge to HCT.
The scenarios in which these strategies are employed require a nuanced approach and detailed knowledge of the evidence.
In this perspective review, we aim to highlight the current interface between AlloHCT, AutoHCT, and CAR T. While it is certainly worth acknowledging the explosion of cellular and immunotherapies (eg. T-cell redirecting therapies) in preclinical and early clinical development for a wide range of hematologic and solid malignancies (which may represent a future state of therapeutic options) they remain beyond the scope of the current review. Herein, we focus predominantly on B-cell ALL, B-cell NHL, and multiple myeloma as these diseases have the longest record of CAR T clinical development and the most volatility at the interface of these modalities.
Comparisons and Contrasts in the Management of HCT and CAR T Recipients
Several similarities and differences exist among the three modalities and the management of patients receiving them (Figure 2). In general, chemosensitivity and disease remission predict better outcomes for those receiving either AutoHCT or AlloHCT, whereas patients receiving CAR T treatment generally have active disease that has often proven refractory to cytotoxic chemotherapy.2,6 While CAR T therapy may be able to overcome chemorefractoriness, the pre-infusion period can be challenging as the manufacturing of autologous product can take a variable length of time and options for bridging therapy may be limited.7 In a “real world” study of axicabtagene ciloleucel (axi-cel) for LBCL, 16% of patients did not receive axi-cel due to disease progression, despite the majority receiving bridging therapy, and systemic bridging therapy was associated with inferior outcomes.8 Several early toxicities that occur following any of these cellular modalities are managed similarly including infections and hematologic toxicities.9 The modalities also have unique toxicities, such as acute and chronic GVHD in AlloHCT and cytokine release syndrome (CRS) and immune-effector cell associated neurotoxicity syndrome (ICANS) following CAR T.10,11 While ICANS had previously appeared to be acute or subacute, some recipients may experience delayed neurotoxicity and/or unconventional sequelae such as persistent neurocognitive and neuromuscular deficits.12 These toxicities require that not only the malignant hematologists have a specialized understanding of their recognition and management, but that all providers (eg. Infectious disease specialists, intensivists, neurologists, nursing, therapists, etc.) have specific training and experience in the management of such patients. Interestingly, seemingly different toxicities such as GVHD and CRS may have convergent therapeutic opportunities, such as targeting of the Janus kinase (JAK) and PI3 kinase pathways, therefore we are likely to see continued developments in the supportive management of cellular therapies concurrent with the development of the therapies themselves.13–18
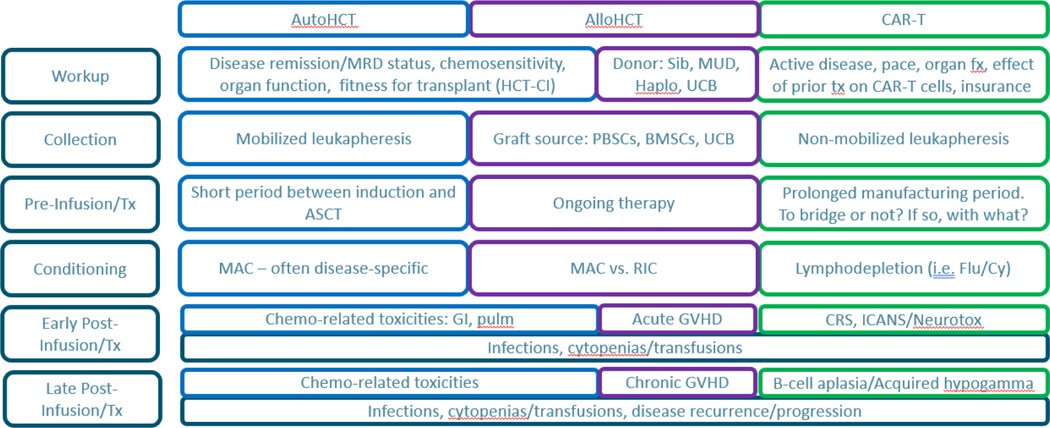
Figure 2. Similarities and differences in the workup and management of patients undergoing HCT or CAR T.
MRD, minimal residual disease. HCT-CI, HCT-comorbidity index. Sib, matched sibling donor; MUD, matched unrelated donor; Haplo, haploidentical; UCB, umbilical cord blood; PBSC, peripheral blood stem cell; BMSC, bone marrow stem cell; MAC, myeloablative conditioning; RIC, reduced intensity conditioning; GVHD, graft-versus-host disease; CRS, cytokine release syndrome; ICANS, immune effector cell associated neurotoxicity syndrome
CAR T Therapy Challenges Transplant-Based Standard of Care in Relapsed/Refractory Aggressive B-cell Non-Hodgkin’s Lymphomas
Until the introduction of CD19-targeting CAR T therapy, patients with large B-cell lymphoma (LBCL) who developed relapsed or refractory disease following salvage chemotherapy and AutoHCT had dismal outcomes with little chance of long-term survival.19 The studies which supported this salvage approach in the first place identified chemosensitivity as a prerequisite to success of AutoHCT, inherently identifying a subclass of patients that required a different approach to their disease.20,21 Some chemorefractory patients did respond to AlloHCT, suggesting the possibility of immunotherapeutic susceptibility, although the risks of high transplant-related morbidity and mortality limited its applicability.22
State of the art of CD19 CAR T for NHL and recent data on earlier usage
As no standard of care existed for LBCL patients who relapsed after AutoHCT or were ineligible principally due to chemorefractoriness, three CD19-targeting CAR T products received regulatory approval based on notable and durable responses as third-line treatment options for these patients.23–26 The preclinical, early phase, and pivotal studies that led to their designations have been reviewed in detail previously.2,25,27 Briefly, ZUMA-1 (axi-cel), JULIET (tisagenelecleucel), and TRANSCEND NHL-001 (lisocabtagene maraleucel, liso-cel) investigated their CAR T therapies in single-arm studies in LBCL relapsed after 2 lines and/or AutoHCT.23,25,26 Treatment-refractory patients comprised 79%, 55%, and 67%, respectively. Notably, bridging chemotherapy was not permitted in ZUMA-1 and need for bridging therapy was associated with worse response rate in JULIET. All had notable efficacy with complete response rates of 58%, 38%, and 53%, and 1-year PFS of 44%, 35%, and 44%, respectively.
With demonstrated efficacy in this highly-refractory population, questions regarding whether such benefits could be provided earlier in the treatment course led to the design and execution of randomized controlled trials pitting each of these products against standard-of-care (SoC) in the second-line setting. Data from these trials were recently presented and published (Table 1).28–30
Table 1.
Phase 3 clinical trials comparing CD19 CAR T therapy versus standard-of-care (SoC) in primary refractory or early relapsed large B-cell lymphomas.
| Trial | ZUMA-7 | BELINDA | TRANSFORM | |||
|---|---|---|---|---|---|---|
| Eligibility | Adults≥18 yo DLBCL including tFL, HGBCL, DLBCL NOS – PMBCL, FL3B, and CNS involvement excluded No impending organ compromise R/R ≤12 mo after 1L CIT ECOG PS ≤1, LVEF ≥ 50%; CrCl ≥ 60mL/min |
Adults≥18 yo Aggressive B-NHL: DLBCL NOS, FL3B, PMBCL, THRBCL, ALK+ LBCL, HGBCL (DH/TH and NOS), tFL, tMZL – active CNS disease excluded R/R ≤12 mo after 1L CIT ECOG PS ≤1, LVEF ≥ 45%; CrCl ≥ 60mL/min |
Adults (18–75 yo) DLBCL NOS, HGBCL (double/triple hit) with DLBCL histology, FL3B, PMBCL, THRBCL -- CNS involvement allowed R/R ≤12 mo after 1L CIT ECOG PS ≤1, LVEF ≥ 40%; CrCl > 45 mL/min |
|||
|
| ||||||
| Stratification | • Refractory vs Relapsed • sAAIPI 0/1 vs 2/3 |
• Refractory vs Relapsed <6mo vs. 6–12 mo • IPI 0/1 vs. 2+ • Geographic – US vs. non-US |
• Refractory vs Relapsed • sAAIPI 0/1 vs 2/3 |
|||
|
| ||||||
| Primary Endpoint | EFS (by BIRC) | EFS (by IRC) – at or after week 12 | EFS (by IRC) | |||
|
| ||||||
| Response Assessments | d50, 100, and 150 after randomization, the q3mo for 2y, then q6mo until 5y | Wk 6 and 12, q3mo for 1 yr, q6mo for 2y, annually to 5y | Wk 9 and 18, then Mo 6, 9, 12, 18, 24, 36 | |||
|
| ||||||
| Arm, N randomized | CAR T, 180 | SoC, 179 | CAR T, 162 | SoC, 160 | CAR T, 92 | SoC, 92 |
|
| ||||||
| Treatment | Axi-cel (2×106 CAR+ cells) • Infused, n= 170 • Bridging: glucocorticoids only, n= 65 • LDC = FluCy 30/500 × 3d |
2–3 cycles of CIT then HDT/AutoHCT for those with CR or PR • CIT, n=168 • AutoHCT, n=64 Crossover (off protocol), n= |
Tisagenlecleucel (0.6×108 CAR+ cells) • Infused, n= 162 • Bridging: CIT allowed multiple cycles/regimens, n= 135 • LDC = FluCy 25/250 × 3d or benda 90 x 2d |
2–3 cycles of CIT then HDT/AutoHCT for those with CR or PR, or second salvage CIT if not • CIT, n=155 • 2nd regimen, n=86 • AutoHCT, n=52 Crossover (on protocol), n=81 |
Liso-cel (100×106 CAR+ cells) • Infused, n=90 • Bridging: CIT allowed, n=58 • LDC = FluCy 30/300 × 3d |
3 cycles of CIT then HDT/AutoHCT for those with CR or PR • CIT, n=91 (CR, n=28) • AutoHCT, n=43 • Crossover (on protocol), n=50 |
|
| ||||||
| Refractory to 1°, n (%) | 133 (74) | 131 (73) | 107 (66) | 107 (67) | 67 (73) | 68 (74) |
|
| ||||||
| EFS | HR (95% CI) = 0.40 (0.31–0.51), P<0.001 Median EFS 8.3 vs. 2.0 mo |24-mo EFS 41% vs. 16% |
sHR (95% CI) = 1.07 (0.82–1.4), P=0.61 Median EFS 3.0 vs. 3.0 mo |
sHR (95% CI) = 0.35 (0.23–0.53), P<0.0001 6-mo EFS 63.3% vs. 33.4% | 12-mo EFS 44.5% vs. 23.7% |
|||
|
| ||||||
| PFS | HR (95% CI) = 0.73 (0.53–1.01), P=0.054 Median PFS 14.7 vs. 3.7 mo | 24-mo OS 46% vs. 27% |
Not reported | sHR (95% CI) = 0.41 (0.25–0.66), P=0.0001 6-mo PFS 69.4% vs. 47.8% | 12-mo PFS 52.3% vs. 33.9% |
|||
|
| ||||||
| OS | HR (95% CI) = 0.73 (0.53–1.01), P=0.054 Median OS NR vs. 35.1 mo |24-mo OS 61% vs. 52% |
sHR (95% CI) = 1.24 (0.83–1.85), P=NS | sHR (95% CI) = 0.51 (0.26–1.00), P=0.026 6-mo OS 91.8% vs. 89.4% |12-mo OS 79.1% vs. 64.2% |
|||
|
| ||||||
| Response, CR/ORR, % | 65/83 | 32/50 | 28.4/46.3 | 27.5/42.5 | 66/86 | 39/48 |
|
| ||||||
| TEAE, any/≥Gr 3,% | 100/91 | 100/83 | 99/84 | 99/90 | 100/92 | 99/87 |
|
| ||||||
| CRS, Any, % | 92 | N/A | 61 | N/A | 49 | N/A |
| Gr 3/4 | 6 | 5 | 1 | |||
| Gr 5 | 0 | 0 | 0 | |||
| Median onset, d | 3 | 4 | 5 | |||
|
| ||||||
| NE, Any, % | 69 | 20 | 10 | N/A | 12 | N/A |
| Gr 3/4 | 21 | 1 | 3 | 4 | ||
| Gr 5 | 0 | 0 | 0 | 0 | ||
| Median onset, d | 7 | 23 | 5 | 11 | ||
DLBCL, diffuse large B-cell lymphoma; tFL, transformed follicular lymphoma; HGBCL, high-grade B-cell lymphoma; NOS, not otherwise specified; PMBCL, primary mediastinal B-cell lymphoma; FL3B, follicular lymphoma grade 3B; CNS, central nervous system; R/R, relapsed/refractory; 1L CIT, first-line chemoimmunotherapy; sAAIPI, second-line age-adjusted international prognostic index; EFS, event-free survival; BIRC, blinded independent review committee; THRBCL, T-cell histiocyte rich large B-cell lymphoma; DH/TH, double-hit/triple-hit; tMZL, transformed marginal zone lymphoma;
ZUMA-7 (axi-cel), BELINDA (tisagenlecleucel), and TRANSFORM (liso-cel) all randomized patients 1:1 versus SoC, which consisted of several cycles of platinum-based salvage chemotherapy followed by AutoHCT for those who achieved a partial response (PR) or complete response (CR).28–30 In the primary analysis of ZUMA-7, axi-cel led to improved event-free survival (EFS) over SoC with a trend toward improved overall survival (OS) at a prespecified interim analysis. In a prespecified interim analysis of TRANSFORM, liso-cel led to improved EFS compared to the SoC arm. In BELINDA, there was not a statistically significant difference in EFS between the tisagenlecleucel and SoC arms, and therefore OS was not formally assessed. Response rates mirrored the EFS results and investigators concluded that the safety profiles were consistent with the prior pivotal studies for third-line therapy. Elsawy et al. reported health-related quality of life outcomes from ZUMA-7, highlighting significantly better Day 100 patient-reported outcomes among the axi-cel arm compared to SoC, which may help inform clinical decisions. 31
Distinctions between and limitations of second-line CD19 CAR T studies in LBCL
Despite the differences in outcomes, there are several important distinctions between the trials pertaining to the study design, populations, and interventions which limit the validity of comparisons between the cellular therapies themselves, although they did provide some clarity in select scenarios.5
All three trials, included adult patients with histologically-proven LBCL, although ZUMA-7, unlike the others, excluded primary mediastinal B-cell lymphoma and grade 3B follicular lymphoma. TRANSFORM was the only of the three to allow patients with active secondary central nervous system involvement, although very few were included (n = 4). The organ function requirements for ZUMA-7 were marginally more stringent, although it is unclear to what extent they had an impact.
The three trials have published data at different stages of maturity; the primary analysis of ZUMA-7 (N = 359) was published with a median follow-up of 24.9 months. BELINDA (N = 322) published the primary analysis of EFS and given the non-significant result of this primary endpoint is not formally testing OS. The prespecified interim analysis of TRANSFORM was presented, reporting a significantly different EFS, albeit with a smaller sample size of 184 patients and median follow-up of 6.2 months.
Event-free survival was chosen as the primary endpoint for these trials although was defined differently between trials. In ZUMA-7 and TRANSFORM EFS included, in addition to disease progression (PD) or death, failure to achieve response to therapy or change in therapy. EFS in BELINDA was defined as time from randomization to stable or progressive disease at or after week 12, or death, but need for third-line therapy was not considered an event. The investigators argued that stable or non-responsive disease in relapsed/refractory LBCL is associated with short time-to-progression and survival, justifying this endpoint. Importantly, participants in BELINDA had a response assessment at 6 weeks post-randomization that did not impact EFS but rather was used to evaluate disease burden prior to tisagenlecleucel infusion or to inform decisions to adjust salvage chemotherapy in the SoC arm.
Use and type of bridging therapy was a key difference in the studies’ designs and reflected the makeup of the populations that were enrolled. ZUMA-7 allowed only glucocorticoids as a bridging therapy in the axi-cel arm in order to avoid lymphoma progression during the manufacturing period. Therefore, patients who had impending organ compromise due to mass effect were excluded per protocol, potentially limiting the generalizability of the results. In contrast, both BELINDA and TRANSFORM permitted chemoimmunotherapy as bridging, therefore permitting the inclusion of patients with bulky or more rapidly-progressing disease. Notably in BELINDA, while the majority (83%) of patients received bridging therapy, 26% of patients had progressive disease before tisagenlecleucel infusion yet were included in the final analysis. A smaller majority (63%) of patients in TRANSFORM received bridging chemotherapy. ZUMA-7 and TRANSFORM had balanced baseline characteristics. Due to a stratification error by IPI score, BELINDA had an imbalance of high-grade B-cell lymphomas, activated B-cell (ABC) lymphomas, and IPI, with less aggressive disease biased toward the SoC arm. BELINDA and TRANSFORM had a higher proportion of ABC LBCL than ZUMA-7.
Axi-cel demonstrated superiority over SoC in patients with relapsed/refractory LBCL without impending organ compromise, whose disease can be controlled with glucocorticoid bridging therapy. The authors of ZUMA-7 do conclude that in the “real-world” bridging chemotherapy may be necessary, although it remains unclear if such patients would derive the same benefit either due to nature of their disease and/or the impact of bridging therapy itself. As TRANSFORM allowed for bridging chemotherapy, liso-cel appears more efficacious than SoC in such patients, although the follow-up for that trial is shorter and the population notably smaller than ZUMA-7 and BELINDA, therefore analysis of more mature data is awaited. Tisagenlecleucel did not result in a significant improvement in efficacy over SoC. Some argue that the eligibility criteria allowed for more advanced or more aggressive disease which was reflected in the majority receiving bridging chemotherapy, and it remains unclear if a trial with a more selected population would have yielded similar or different results.5 Importantly, salvage chemotherapy followed by AutoHCT remains standard practice for those who relapse >12 months from primary therapy as second-line CAR T has yet to be studied among such patients in randomized trials.
Outcomes of chemosensitive patients undergoing either AutoHCT or CD19 CAR T
As the aforementioned studies randomized patients at early relapse, we lack prospective data on the comparative efficacy between AutoHCT and CAR T in patients responding to second-line chemotherapy. Shadman and colleagues queried the Center for International Blood & Marrow Transplant Research (CIBMTR) registry database to examine outcomes in patients who achieved a PR and subsequently underwent either CAR T therapy or AutoHCT.32 Their analysis identified a significant difference in 2-year incidence of progression and OS favoring the AutoHCT cohort. These differences held true among patients with primary refractory disease or relapsed within 12 months. While this was a retrospective study and could not elucidate the reasons for choice of modality after achieving a PR to salvage chemotherapy, it potentially argues for reserving CAR T for those in this population who relapse after AutoHCT. Prospective studies addressing this question are warranted, especially since many patients intended for second-line CAR T will receive and potentially respond to bridging chemoimmunotherapy.
Potential earlier use of CAR T invites questions for subsequent use of both AutoHCT or AlloHCT in LBCL
Use of CAR T therapy in the second-line setting for LBCL will lead to more questions among those who progress after CAR T, such as whether there is a role for post-CAR T chemotherapy and AutoHCT, or should renewed consideration be given to AlloHCT. Dreger et al. recently published a perspective on CAR T and AlloHCT for LBCL.33 Their review of predominantly registry studies and one prospective trial of AlloHCT for aggressive B-cell lymphomas concluded that the 3-year PFS was approximately 35%, similar to that of CAR T therapy, albeit the non-relapse mortality was as high as 30%. A retrospective intention-to-treat analysis of patients with active LBCL intended for either AlloHCT (n=60) or CAR T therapy (n=41) of whom 65% and 73% received their respective cellular immunotherapy.34 CAR T therapy was associated with a numerically longer median OS (CAR T 475d vs. AlloHCT 285d, P=0.88), albeit without reaching statistical significance, and the non-relapse mortality was significantly lower than AlloHCT. Therefore, AlloHCT has been relegated to select indications in LBCL including those with suspicion for myelodysplasia or significant prolonged cytopenias.33 Its role after progression post-CAR T remains an area of interest, especially in patients ineligible for or unresponsive to subsequent clinical trials, although historically chemosensitivity has been predictive of AlloHCT success.
BCMA CAR T Therapy and HCT – Complements and Competitors
The current state of AutoHCT for multiple myeloma
High dose melphalan with AutoHCT remains a current standard in the treatment of multiple myeloma for those with acceptable performance status and organ function, even with the advent of novel therapies such as proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs).35 The Intergroupe Francophone du Myélome (IFM) 2009 demonstrated that bortezomib-lenalidomide-dexamethasone (RVd) induction followed by high dose melphalan and AutoHCT led to superior PFS relative to longer use of RVd induction without AutoHCT.36 However, several interesting observations relating to this trial have been made with extended follow-up and more granular analysis. Patients who achieved minimal residual disease (MRD) negativity in either arm had similar outcomes which were significantly better than those who never achieved MRD negativity.37 Additionally, analysis of long-term follow-up validated the concept that AutoHCT can be delayed until after first progression as there were not significant differences in PFS2 (defined as the time from randomization to progression on next line therapy or death) or OS between arms.38 The majority (76.7%) of those in the non-transplant cohort underwent AutoHCT at first relapse; a caveat to the delayed AutoHCT approach is that not all will be transplant-eligible at first relapse.39 In light of these data and potential mutagenic effects of high-dose melphalan, ongoing efforts aim to use aggressive quadruplet induction regimens and MRD-adaptive protocols to avoid or delay its use.40
Controversy and considerations pertaining to AlloHCT in multiple myeloma
While AutoHCT, whether early or delayed, yields a PFS and possibly OS benefit, patients almost invariably experience disease progression. Investigators have long sought to exploit the graft-versus-myeloma (GVM) effect of AlloHCT, although high non-relapse mortality and lack of early benefit have made it controversial.41 However, long-term data comparing modalities may yet validate the putative immunotherapeutic benefits in select populations. The BMT CTN 0102 trial compared tandem AutoHCT (auto-auto) to AutoHCT followed by AlloHCT (auto-allo) without specifying induction regimens and using biologic randomization.42 Early on, 3-year PFS and OS were comparable between cohorts and non-relapse mortality was significantly higher in the auto-allo cohort. Interestingly, those with chronic GVHD had lower incidence of relapse. With extended follow-up, those with standard risk disease had similar outcomes regardless of modality, but among those with high risk disease (defined as elevated beta-2-microglobulin or deletion chromosome 13) auto-allo had significantly better 6 and 10-year PFS compared to auto-auto, (31% vs. 13% [p=0.05] and 21% vs. 4% [p=0.03]), although higher NRM negated the relapse benefit as OS was comparable.43 A pooled analysis of extended follow-up of auto-auto versus auto-allo trials suggested that not only did auto-allo confer protection from initial relapse and longer PFS, but significantly longer post-relapse survival, implying that the GVM effect may be potentiated with novel therapies even at disease progression.44 Importantly, these studies were conducted prior to the widespread implementation of novel therapies and updated risk stratification, so their generalizability to modern myeloma patients is limited. However, they highlight that select subsets of patients could benefit from AlloHCT, especially with measures to reduce NRM, warranting continued study, and redirecting immune cells against myeloma will likely be an integral component of achieving functional cures.45
Pivotal and ongoing studies of BCMA CAR T therapy and their interface with AutoHCT
Targeted redirection of autologous T-cells against MM required a specific MM target for CAR generation. B-cell maturation antigen (BCMA) was identified as a highly- and specifically-expressed cell surface antigen on malignant plasma cell, ultimately leading to two commercially approved CAR T products, idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel).46–49 Both are approved for MM patients who have received at least four prior lines of therapy, including a proteasome inhibitor, immunomodulatory drug, and anti-CD38 monoclonal antibody. While not a prerequisite for these CAR T therapies, the majority of patients in the respective registration trials had undergone prior AutoHCT; in KarMMa-1 studying ide-cel, 94% had progressed following AutoHCT whereas 90% had undergone AutoHCT and progressed prior to receiving cilta-cel on the CARTITUDE-1 study. This is likely reflective of standard practice at this juncture. Interestingly, a small number of patients (n = 8) on CARTITUDE-1 had prior AlloHCT whereas such patients were excluded from KarMMa-1; the outcomes of this subgroup have not yet been described independently.
A principal focus of the ongoing KarMMa and CARTITUDE series of studies is the identification and treatment of high-risk populations, either by staging or clinical scenarios, in whom early CAR T therapy may augment AutoHCT or may be incorporated after first progression (Table 2). Two cohorts from CARTITUDE 2, cohort A (progressive disease after 1–3 prior lines of therapy) and cohort B (relapse within 12 months of AutoHCT or initiation of non-transplant-based MM therapy) have released early data on 20 and 18 patients, respectively.50,51 In cohort A, the majority had undergone prior AutoHCT, the ≥CR rate was 85% and at a median follow-up of 9.7 months the 6-month PFS was 90%. In cohort B, 14/18 (77.8%) had received prior AutoHCT, and at a median follow-up of 4.7 months, the ≥CR rate was 27.8%; among those with ≥3 month follow-up the ≥CR rate was 38%. These data are immature and primary endpoints yet to be analyzed, but of interest will be whether early relapse post-AutoHCT can be mitigated by early CAR T treatment, or whether this group of patients continues to be high risk and experience worse outcomes.
Table 2.
Ongoing clinical trials of BCMA CAR T therapy to augment response to or replace autologous HCT in multiple myeloma.
| Trial | KarMMa-2 NCT03601078 | KarMMa-4 NCT04196491 | BMT CTN 1902 NCT05032820 | CARTITUDE 2 NCT04133636 | CARTITUDE 5 NCT04923893 |
|---|---|---|---|---|---|
| Study Design | Phase 2, multicohort, open- label in clinically high-risk patients with RRMM | Phase 1, single-arm, dose-finding for treatment of high-risk NDMM | Phase 2, single-arm of ide-cel in patients with <VGPR after AutoHCT | Phase 2, multiple, small exploratory cohorts of patients with RRMM at different treatment stages | Randomized, Open Label Phase 3 of frontline therapy in patients in whom AutoHCT is not planned as initial therapy |
| Control | None | None | None | None | VRd x 8 → Rd |
| Experimental | Patients receive ide-cel after LDC, assigned to different cohorts | Induction x 3 cycles → Leukapheresis → Bridging x 1 (same as induction) → ide-cel |
AutoHCT → Len maintenance x ≥ 6 months → <VGPR → leukapheresis -→ ide-cel → Len maintenance |
Patients receive cilta-cel at different treatment stages depending on cohort enrolled | VRd x 6 → Leukapheresis → VRd x 2 bridging → cilta-cel |
| N | 181 (total) | 60 | 40 | 160 (total) | 650 |
| Primary Endpoint | Cohort 1: ORR Cohorts 2: CR rate |
DLT rates, AEs | CR rate | MRD- rate after 1 yr | PFS |
| Key Secondary Endpoints | TTR, DOR, PFS, TTP, OS, AEs, MRD- rate, HRQoL, PK | CR rate, ORR, DOR, TCR, feasibility of initiating maintenance, PFS, OS, PK |
Disease progression, best response, NRM, AEs, OS, maintenance feasibility, CAR T expansion and persistece |
ORR, ≥VGPR, CBR, DOR, TTR, MRD- CR rate after 1 yr, AEs |
Sustained MRD-, MRD- rate at 9 mo, MRD- CR rate, OS, ≥CR rate, PFS2, AEs, CAR T activation, expansion, persistence, HRQoL |
| Key Eligibility | Cohort dependent. • 1: RRMM subjects with ≥ 3 prior with rapid progression to most recent • 2a: R-ISS III and PD • <18 mo of induction + AutoHCT +Len • 2b: R-ISS III and PD • <18 mo since start of initial therapy, no AutoHCT • 2c: R-ISS III and <VGPR 70 to 110d post AutoHCT Measurable disease. ECOG ≤ 1. No CNS involvement, plasma cell leukemia, clinically significant chronic diseases, AutoHCT < 12 weeks from leukapheresis. |
NDMM, measurable disease, R-ISS III, ECOG ≤ 1. Must not receive Dara with cycles 2 or 3, and no dexamethasone with cycle 3. Adequate hematologic parameters and organ function, no CNS involvement, no clinically significant comorbidities |
AutoHCT (with melphalan > 140mg/m2) within 12 months, <VGPR after at least 6 months Len maintenance, adequate performance status, organ function, hematologic parameters; no prior AlloHCT, CNS, PCL, or amyloidosis; must have measurable disease, no clinically significant medical comordibities |
Cohort dependent. • A: PD after 1–3 lines • B: early relapse after 1st line • C: prior PI, IMID, CD38, and BCMA tx • D: <CR after 1st line AutoHCT • E: 1st line high risk with no AutoHCT planned • F: 1st line standard risk after initiation of therapy Measurable disease. ECOG ≤ 1. No major ongoing toxicity from prior therapy. No CNS involvement |
NDMM with measurable disease at screening, ECOG ≤ 1, no planned AutoHCT as initial treatment, adequate hematologic and organ function, no CNS involvement or hepatitis B or C |
RRMM, relapsed/refractory multiple myeloma; LDC, lymphodepleting chemotherapy; ORR, overall response rate; CR, complete response; TTR, time-to-response; DOR, duration of response; TTP, time-to-progression; HRQoL, health-related quality of life; R-iSS, revised International Staging System; Len, lenalidomide; NDMM, newly-diagnosed multiple myeloma; PCL, plasma cell leukemia; CBR, clinical benefit rate; PI, proteasome inhibitor; IMID, immunomodulatory drug; BCMA, B-cell maturation antigen; PFS2, progression-free survival after second-line therapy.
Ongoing and future studies of BCMA CAR T as a substitute or comparator for AutoHCT in MM
While these studies seek to improve the post-transplant response, other ongoing provocative studies seek to substitute AutoHCT altogether with CAR T (Table 2). Among these, the recently-announced phase 3 CARTITUDE-6 (NCT05257083) study will directly compare cilta-cel with AutoHCT in the upfront setting among patients receiving quadruplet (daratumumab-bortezomib-lenalidomide-dexamethasone) induction. The results of these studies could be practice-changing, and key questions beyond the efficacy endpoints will be addressed. The impact of lymphodepleting chemotherapy and BCMA CAR T treatment on hematopoietic progenitor cell mobilization and collection remains unanswered and is relevant as AutoHCT may be a therapeutic option at disease progression. Another salient question pertains to whether the less pretreated nature of these participants will impact the efficacy and safety of the CAR T products. Additionally, some of these studies include lenalidomide maintenance among those receiving CAR T therapy, which to date has an unknown impact on the efficacy and persistence of the CAR T cells.
As multiple myeloma is the most recent disease to obtain approved CAR T products and a disease in which HCT is quite entrenched in the treatment paradigm, it remains to be seen whether CAR T therapy will replace or be an adjunct to HCT. Additionally, as BCMA CAR T therapy has yet to demonstrate plateaus in relapse incidence and PFS, strategies to mitigate relapse could foreseeably include consolidative AlloHCT in select high-risk populations.
CD19 CAR T Therapy as a “Destination” or a Bridge to AlloHCT in B-cell Acute Lymphoblastic Leukemia
B-cell acute lymphoblastic leukemia (B-ALL) is a heterogenous disease with highly varied outcomes influenced by patient age, clinical factors, and molecular characteristics. While some may be cured with aggressive, prolonged chemotherapy regimens, others are directed toward early consolidative AlloHCT in light of their high risk of relapse.52–54 Relapsed B-ALL historically carries a dismal prognosis, even with the introduction of immunotherapies such as blinatumomab and inotuzumab-ozogamicin.55,56
CD19 CAR T therapy originated as a treatment for relapsed/refractory B-ALL, with initial approval of tisagenlecleucel for pediatric, adolescent and young adult (AYA) patients, and a recent indication for brexucabtagene autoleucel (brexu-cel) for adults. The preclinical and clinical development of CD19 CAR T products for B-ALL has been previously reviewed, extensively.2,57 Throughout the clinical trials and reviews, two interfaces exist between AlloHCT and CAR T – the efficacy and outcomes of CAR T among those who relapsed after prior AlloHCT and the role and impact of consolidative AlloHCT after CAR T.58
Among the larger trials of CD19 CAR T for B-ALL, around 40–60% of patients had undergone prior AlloHCT. In the pivotal ELIANA trial, complete response (CR) rates to tisagenlecleucel among children and AYAs were similar regardless of prior AlloHCT, although long-term PFS and OS subgroup data are unavailable.59,60 Similarly, prior AlloHCT did not significantly impact the CR/CRi rates of brexu-cel among adults in the pivotal ZUMA-3 study.61
In both of these studies a minority of patients proceeded to consolidative AlloHCT post-CAR T, and even among those who did not, a substantial proportion experienced durable remissions. The decision to proceed to consolidative AlloHCT is influenced by whether or not a patient has had a prior AlloHCT, and while these pivotal studies suggested that durable remissions are possible in its absence, others suggest that AlloHCT consolidation may be beneficial.62 Jiang et al. conducted a prospective study in which they compared 47 patients who received a 4–1BB CD19 CAR T product and achieved MRD negativity, who then either went on to AlloHCT or not.63 Twenty-one patients, all transplant naïve, proceeded to consolidative AlloHCT, and 26 did not for various reasons (5 prior AlloHCT, 5 contraindicated due to comorbidity, 3 lacking donor, 13 personal choice). While consolidative AlloHCT did not improve overall survival, there was a significant benefit relating to event-free and relapse-free survival, highlighting the potential added value of the graft-versus-leukemia effect.
Several disease and treatment-specific factors are posited to contribute to the risk of relapse post-CAR T and influence clinical decision-making regarding consolidative AlloHCT (Figure 3). For example, studies have demonstrated that CAR T persistence and/or persistent B-cell aplasia (a surrogate for persistence) predicts longer disease-free intervals in B-ALL, and some have suggested that CAR products with 4–1BB signaling domains demonstrate longer persistence. This concept is based mainly on the ELIANA trial in which 67 of 75 patients did not proceed to AlloHCT and the 12-month EFS was 50%, therefore may only be applicable to pediatric and young adult patients.59,60,64 Trials of tisagenlecleucel in older populations or other 4–1BB CAR T products, albeit smaller and heterogenous, tend to favor consolidative AlloHCT.63,65–67 Interestingly, available data on recipients of CD19 CAR T products with CD28 costimulatory domains suggests a reversal of the role of consolidative AlloHCT based on age group. Shah et al. suggested the importance of consolidative AlloHCT in children, adolescents, and young adults who received a CD19 CAR T product with CD28 costimulatory domain; 21 of 28 patients who achieved MRD-negative CR underwent AlloHCT (4 who had had prior AlloHCT) at a median of 54 days from CAR T infusion, with a 5-year EFS of 61.9% and cumulative incidence of relapse of 9.5% at 24 months.68 All seven who did not proceed to AlloHCT in MRD-negative CR after CAR T relapsed at a median of 152 days. Importantly, 6 of 7 had already relapsed after a first AlloHCT, therefore the disease risk, in addition to the treatment choice of CAR T only, likely contributed to the outcomes in this group. Interestingly, in the phase 2 study of brexu-cel (a product with a CD28 costimulatory domain) in adults with B-ALL, only a minority of patients (18%) underwent consolidative AlloHCT, and the duration of remission among responders was unchanged whether censoring for AlloHCT or not. The investigators in this study did not disclose the reasons for proceeding with AlloHCT in these ten patients. .61
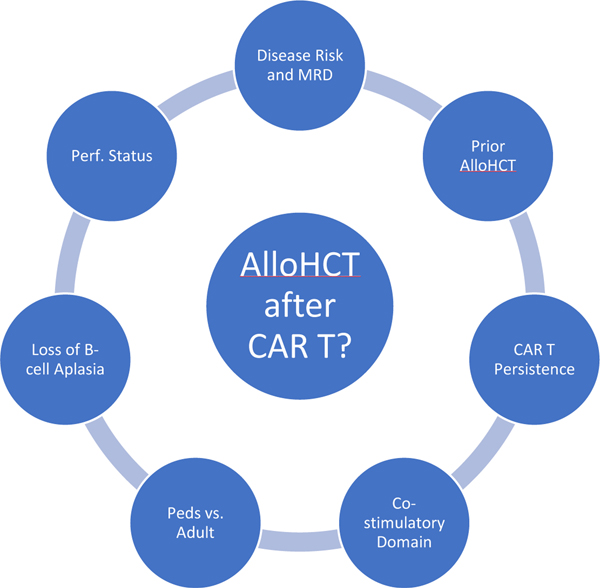
Figure 3.
Patient, disease, and treatment considerations in deciding on AlloHCT consolidation after CD19 CAR T therapy in B-cell acute lymphoblastic leukemia
While costimulatory domain and B-cell recovery have been informative regarding risk of relapse post-CAR T, their predictive values are limited. Recently, Pulsipher and colleagues published a translational study examining the predictive utility of serial MRD measurements among pediatric and AYA recipients of tisagenlecleucel in both the ENSIGN and ELIANA phase 2 trials. Patients who exhibited any bone marrow NGS-MRD positivity by 28d and 3 months (I.e. any detectable clonal sequence even if below the quoted limit of detection) had a dramatically higher risk of relapse than those with MRD-negativity, HR of 4.87 and 12, respectively. While B-cell recovery was predictive of early relapse if present by day 28, it lacked predictive value if occurring by 3 months, and those with CD19-negative relapses often displayed MRD-positivity despite persistent B-cell aplasia. A small number of patients who developed MRD-positivity underwent AlloHCT prior to overt relapse, usually because of B-cell recovery. While these patients were censored from the relapse analyses, most were alive by end of follow-up whereas most MRD-positive patients without subsequent AlloHCT died. Future studies are needed, however an evolving paradigm involves serial NGS-MRD measurements from both bone marrow and peripheral blood along with donor search for all patients, allowing for rapid intervention with AlloHCT at the time of MRD-positivity.
While ideally a prospective, randomized clinical trial would be able to answer the question of whether or not to consolidate with AlloHCT, such a trial may be pragmatically challenging due to the numerous confounding variables as well as the risk of ablating CAR T cells in a proportion of patients still benefiting from them. As larger numbers of patients are treated with CD19 CAR T for B-ALL, leveraging cellular therapy registries may provide insight and predictive modeling that could inform decisions on whether to proceed to consolidative AlloHCT or not after CD19 CAR T therapy.
Conclusion and Future Directions
The pioneering work of both AlloHCT and AutoHCT, while initially met with skepticism and frustrations, eventually demonstrated the curative potential of these modalities in advanced, otherwise fatal hematologic malignancies.21,69–73 By circumventing the dose-limiting toxicity of myeloablation, they greatly increased the therapeutic windows for cytotoxic chemotherapy regimens and, in the case of AlloHCT, unlocked the immunotherapeutic benefit of the donor immune system. Concurrent advances in supportive care have reduced the transplant-related morbidity and mortality and increased the accessibility of both HCT options.
The development of CAR T echoes that of HCT, following some decades behind. It required repeated trial and error with a “bench-to-bedside and back” approach in order to optimize signaling endodomains and create effective and persistent subsequent generations.74–79 Now well into clinical deployment of these refined models, CAR T therapy has once again demonstrated the ability of immune cells to treat and potentially cure advanced hematologic malignancies such as B-ALL, LBCL, and MM.
Both the cellular therapy technology and the manner in which they are used continue to evolve in tandem. The most recent CAR T versus SoC trials in LBCL signal potential updates to the second-line treatment algorithm in carefully-selected patients with early relapse or primary refractory disease. As such, the role, timing, and source of HCT will likely depend on factors such as disease burden and chemosensitivity, and its sequencing with CAR T will need to be tailored at the patient-level. Upcoming trials in multiple myeloma may signal similar change, although could just as easily demonstrate the superiority of HCT.
The regulatory approval and widespread usage of multiple CAR T products has demonstrated the potential and persistence of this therapeutic modality. Many other cellular therapy products are in clinical development, aiming for new targets in patients refractory to these approved products, employing techniques to allow for allogeneic “off-the-shelf” products, or targeting other hematologic and solid malignancies.80,81 Some will never interface with HCT. Others such as in acute myeloid leukemia, in which there is significant overlap between leukemic antigens and those found on normal hematopoietic progenitors, may require concurrent HCT with genetically-modified donor grafts in which the CAR target is edited from the normal hematopoietic cells.82,83
The advent of genetically-engineered cellular therapies has reinvigorated the conversation regarding the role of HCT in the treatment of hematologic malignancies. The question as to whether CAR T and HCT are competitive or complimentary is complicated and situationally dependent. As the technologies and data evolve, so too will the perspectives, and the challenge will continue to be understanding the nuances of the data so that they may be applied to individual patients in a tailored fashion.
Highlights.
The indications for and interface between AlloHCT, AutoHCT, and CAR T therapy are continuously evolving.
Management of the cellular therapy platforms overlaps in some respects and differs in others, necessitating a comprehensive training and continued innovation that can be translated between modalities.
Recent and ongoing studies comparing the platforms in hematologic malignancies have unique details that must be understood in order to appropriately apply the data.
Acknowledgements:
Grants: NIH/NCI R35 CA210084 NCI Outstanding Investigator Award (PI: DiPersio)
Declarations of Interest:
SRG: Consulting/advisory boards: Janssen, Sanofi-Genzyme, Wugen, Oncovalent; Travel: Adaptive Biosciences; Research Support: Adaptive Biosciences
AG: Research support from Kite Pharma-Gilead and Amgen. Advisory Board Member/Consultant for Kite Pharma-Gilead, Amgen, Celgene, EUSA, Atara, CRISPR Therapeutics, and Wugen.
JFD: Rivervest, consulting/advisory committee; Bioline, Incyte, NeoImmuneTech, Macrogenic, research collaborations; Washington University, employment/salary; Magenta, Wugen, ownership-equity.
BH: Research funding: Gilead, BMS; Consulting: Gilead, BMS, Novartis
MS: Consulting, Advisory Boards, steering committees or data safety monitoring committees: Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb,
Morphosys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate therapeutics and Atara Biotherapeutics; Research Funding: Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, Morphosys/Incyte
TJ: Institutional research support from CTI Biopharma, SyneosHealth, Incyte; Consultancy with Targeted Healthcare Communications; Advisory board participation with Care Dx, Bristol Myers Squibb, Incyte, Abbvie, and CTI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Majhail NS, Farnia SH, Carpenter PA, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21(11):1863–1869. doi: 10.1016/j.bbmt.2015.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldsmith SR, Ghobadi A, DiPersio JF. Hematopoeitic Cell Transplantation and CAR T-Cell Therapy: Complements or Competitors? Frontiers in Oncology. 2020;10. https://www.frontiersin.org/article/10.3389/fonc.2020.608916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanate AS, Majhail NS, Savani BN, et al. Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biology of Blood and Marrow Transplantation. 2020;26(7):1247–1256. doi: 10.1016/j.bbmt.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Jain T, Bar M, Kansagra AJ, et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biology of Blood and Marrow Transplantation. Published online 2019. doi: 10.1016/j.bbmt.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 5.Roschewski M, Longo DL, Wilson WH. CAR T-Cell Therapy for Large B-Cell Lymphoma — Who, When, and How? New England Journal of Medicine. 2021;386(7):692–696. doi: 10.1056/NEJMe2118899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CI, Roitman D, Tsang R, Stewart AK, Keating A, Crump M. ‘Relative’ chemotherapy sensitivity: the impact of number of salvage regimens prior to autologous stem cell transplant for relapsed and refractory aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplantation. 2002;30(12):885–891. doi: 10.1038/sj.bmt.1703772 [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Plastaras JP. Navigating the narrow bridge to CAR T-cell therapy. Blood Advances. 2020;4(13):2884–2885. doi: 10.1182/bloodadvances.2020002346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Advances. 2020;4(13):2871–2883. doi: 10.1182/bloodadvances.2020001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wudhikarn K, Palomba ML, Pennisi M, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer Journal. 2020;10(8):79. doi: 10.1038/s41408-020-00346-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. doi: 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PubMed] [Google Scholar]
- 12.Cohen AD, Parekh S, Santomasso BD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer Journal. 2022;12(2):32. doi: 10.1038/s41408-022-00629-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan J, Deng B, Ling Z, et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J Cell Mol Med. 2021;25(2):1089–1099. doi: 10.1111/jcmm.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenderian SS, Ruella M, Shestova O, et al. Ruxolitinib Prevents Cytokine Release Syndrome after Car T-Cell Therapy Without Impairing the Anti-Tumor Effect in a Xenograft Model. Biology of Blood and Marrow Transplantation. 2017;23(3):S19–S20. doi: 10.1016/j.bbmt.2016.12.003 [DOI] [Google Scholar]
- 15.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739–1749. doi: 10.1182/blood.2020004823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. New England Journal of Medicine. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635 [DOI] [PubMed] [Google Scholar]
- 17.Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. New England Journal of Medicine. 2021;385(3):228–238. doi: 10.1056/NEJMoa2033122 [DOI] [PubMed] [Google Scholar]
- 18.Amatya PN, Carter AJ, Ritchey JK, et al. The Dual PI3Kδγ Inhibitor Duvelisib Potently Inhibits IL-6 Production and Cytokine Release Syndrome (CRS) While Maintaining CAR-T Function in Vitro and In Vivo. Blood. 2020;136(Supplement 1):1–2. doi: 10.1182/blood-2020-13990432430499 [DOI] [Google Scholar]
- 19.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. New England Journal of Medicine. 1995;333(23):1540–1545. doi: 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- 21.Philip T, Armitage JO, Spitzer G, et al. High-Dose Therapy and Autologous Bone Marrow Transplantation after Failure of Conventional Chemotherapy in Adults with Intermediate-Grade or High-Grade Non-Hodgkin’s Lymphoma. New England Journal of Medicine. 1987;316(24):1493–1498. doi: 10.1056/NEJM198706113162401 [DOI] [PubMed] [Google Scholar]
- 22.Dreger P. Allogeneic stem cell transplant in non-Hodgkin lymphomas: Still an indication? Hematological Oncology. 2021;39(S1):100–103. doi: 10.1002/hon.2845 [DOI] [PubMed] [Google Scholar]
- 23.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2018;380(1):45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 26.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366–0 [DOI] [PubMed] [Google Scholar]
- 27.Jain T, Bar M, Kansagra AJ, et al. Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biology of Blood and Marrow Transplantation. 2019;25(12):2305–2321. doi: 10.1016/j.bbmt.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 28.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. New England Journal of Medicine. 2021;386(7):640–654. doi: 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 29.Bishop MR, Dickinson M, Purtill D, et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. New England Journal of Medicine. 2021;386(7):629–639. doi: 10.1056/NEJMoa2116596 [DOI] [PubMed] [Google Scholar]
- 30.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. The Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6 [DOI] [PubMed] [Google Scholar]
- 31.Elsawy M, Chavez JC, Avivi I, et al. Patient-Reported Outcomes in a Phase 3, Randomized, Open-Label Study Evaluating the Efficacy of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard of Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma (ZUMA-7). Blood. 2021;138(Supplement 1):430–430. doi: 10.1182/blood-2021-147598 [DOI] [Google Scholar]
- 32.Shadman M, Pasquini M, Ahn KW, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2022;139(9):1330–1339. doi: 10.1182/blood.2021013289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreger P, Fenske TS, Montoto S, et al. Cellular Immunotherapy for Refractory Diffuse Large B Cell Lymphoma in the Chimeric Antigen Receptor-Engineered T Cell Era: Still a Role for Allogeneic Transplantation? Biol Blood Marrow Transplant. 2020;26(4):e77–e85. doi: 10.1016/j.bbmt.2019.12.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreger P, Dietrich S, Schubert ML, et al. CAR T cells or allogeneic transplantation as standard of care for advanced large B-cell lymphoma: an intent-to-treat comparison. Blood Advances. 2020;4(24):6157–6168. doi: 10.1182/bloodadvances.2020003036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer Journal. 2019;9(4):44. doi: 10.1038/s41408-019-0205-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. New England Journal of Medicine. 2017;376(14):1311–1320. doi: 10.1056/NEJMoa1611750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrot A, Lauwers-Cances V, Corre J, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132(23):2456–2464. doi: 10.1182/blood-2018-06-858613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrot A, Lauwers-Cances V, Cazaubiel T, et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the IFM 2009 Trial. Blood. 2020;136(Supplement 1):39. doi: 10.1182/blood-2020-134538 [DOI] [Google Scholar]
- 39.Schiffer CA, Zonder JA. Transplantation for Myeloma — Now or Later? New England Journal of Medicine. 2017;376(14):1378–1379. doi: 10.1056/NEJMe1700453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landgren O, Hultcrantz M, Diamond B, et al. Safety and Effectiveness of Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab Combination Therapy for Patients With Newly Diagnosed Multiple Myeloma: The MANHATTAN Nonrandomized Clinical Trial. JAMA Oncology. 2021;7(6):862–868. doi: 10.1001/jamaoncol.2021.0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gahrton G, Iacobelli S, Garderet L, Yakoub-Agha I, Schönland S. Allogeneic Transplantation in Multiple Myeloma-Does It Still Have a Place? J Clin Med. 2020;9(7):2180. doi: 10.3390/jcm9072180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195–1203. doi: 10.1016/S1470-2045(11)70243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giralt S, Costa LJ, Maloney D, et al. Tandem Autologous-Autologous versus Autologous-Allogeneic Hematopoietic Stem Cell Transplant for Patients with Multiple Myeloma: Long-Term Follow-Up Results from the Blood and Marrow Transplant Clinical Trials Network 0102 Trial. Biol Blood Marrow Transplant. 2020;26(4):798–804. doi: 10.1016/j.bbmt.2019.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa LJ, Iacobelli S, Pasquini MC, et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant. 2020;55(9):1810–1816. doi: 10.1038/s41409-020-0887-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh N, Ye X, Tsai HL, et al. Allogeneic Blood or Marrow Transplantation with Post-Transplantation Cyclophosphamide as Graft-versus-Host Disease Prophylaxis in Multiple Myeloma. Biol Blood Marrow Transplant. 2017;23(11):1903–1909. doi: 10.1016/j.bbmt.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood. 2017;130(24):2594–2602. doi: 10.1182/blood-2017-06-793869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8 [DOI] [PubMed] [Google Scholar]
- 48.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. New England Journal of Medicine. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New England Journal of Medicine. 2021;384(8):705–716. doi: 10.1056/NEJMoa2024850 [DOI] [PubMed] [Google Scholar]
- 50.Agha ME, Cohen AD, Madduri D, et al. CARTITUDE-2: Efficacy and safety of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR T-cell therapy, in patients with progressive multiple myeloma (MM) after one to three prior lines of therapy. Journal of Clinical Oncology. 2021;39(15_suppl):8013. doi: 10.1200/JCO.2021.39.15_suppl.8013 [DOI] [Google Scholar]
- 51.van de Donk NWCJ, Delforge M, Agha M, et al. CARTITUDE-2: Efficacy and Safety of Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen (BCMA)-Directed Chimeric Antigen Receptor T-Cell Therapy, in Patients with Multiple Myeloma and Early Relapse after Initial Therapy. Blood. 2021;138(Supplement 1):2910. doi: 10.1182/blood-2021-146074 [DOI] [Google Scholar]
- 52.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–1559. doi: 10.1182/blood-2018-10-881961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta V, Richards S, Rowe J, Acute Leukemia Stem Cell Transplantation Trialists’ Collaborative Group. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121(2):339–350. doi: 10.1182/blood-2012-07-445098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(20):2380–2388. doi: 10.1200/JCO.2015.62.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. New England Journal of Medicine. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. New England Journal of Medicine. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connor MP, Frey N v. CAR T in adult ALL: When and for whom? Best Practice & Research Clinical Haematology. 2021;34(1):101256. doi: 10.1016/j.beha.2021.101256 [DOI] [PubMed] [Google Scholar]
- 58.Frey N v. Relapsed ALL: CAR T vs transplant vs novel therapies. Hematology. 2021;2021(1):1–6. doi: 10.1182/hematology.2021000225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grupp SA, Maude SL, Rives S, et al. Tisagenlecleucel for the Treatment of Pediatric and Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia: Updated Analysis of the ELIANA Clinical Trial. Biology of Blood and Marrow Transplantation. 2019;25(3):S126–S127. doi: 10.1016/j.bbmt.2018.12.410 [DOI] [Google Scholar]
- 60.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. The Lancet. 2021;398(10299):491–502. doi: 10.1016/S0140-6736(21)01222-8 [DOI] [PubMed] [Google Scholar]
- 62.Jacoby E. The role of allogeneic HSCT after CAR T cells for acute lymphoblastic leukemia. Bone Marrow Transplantation. 2019;54(2):810–814. doi: 10.1038/s41409-019-0604-3 [DOI] [PubMed] [Google Scholar]
- 63.Jiang H, Li C, Yin P, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial. Am J Hematol. 2019;94(10):1113–1122. doi: 10.1002/ajh.25582 [DOI] [PubMed] [Google Scholar]
- 64.Grupp SA, Maude SL, Rives S, et al. Updated Analysis of the Efficacy and Safety of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed/Refractory (r/r) Acute Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):895. doi: 10.1182/blood-2018-99-112599 [DOI] [Google Scholar]
- 65.Hay KA, Gauthier J, Hirayama A v, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652–1663. doi: 10.1182/blood-2018-11-883710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322–3331. doi: 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frey N v Shaw PA, Hexner EO, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020;38(5):415–422. doi: 10.1200/JCO.19.01892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah NN, Lee DW, Yates B, et al. Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. Journal of Clinical Oncology. 2021;39(15):1650–1659. doi: 10.1200/JCO.20.02262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas ED, Storb R, Clift RA, et al. Bone-Marrow Transplantation. New England Journal of Medicine. 1975;292(16):832–843. doi: 10.1056/NEJM197504172921605 [DOI] [PubMed] [Google Scholar]
- 70.Thomas ED, Storb R, Clift RA, et al. Bone-Marrow Transplantation. New England Journal of Medicine. 1975;292(17):895–902. doi: 10.1056/NEJM197504242921706 [DOI] [PubMed] [Google Scholar]
- 71.Perry AR, Linch DC. The history of bone-marrow transplantation. Blood Reviews. 1996;10(4):215–219. doi: 10.1016/S0268-960X(96)90004-1 [DOI] [PubMed] [Google Scholar]
- 72.Powles R, Morgenstern G, Kay H, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1(8325):612–615. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz E, Congdon C, Uphoff D. Modification of Acute Irradiation Injury in Mice and Guinea-Pigs by Bone Marrow Injections. Radiology. 1952;58(6):863–877. doi: 10.1148/58.6.863 [DOI] [PubMed] [Google Scholar]
- 74.Sadelain M. CAR therapy: the CD19 paradigm. J Clin Invest. 2015;125(9):3392–3400. doi: 10.1172/JCI80010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi: 10.1038/nrc971 [DOI] [PubMed] [Google Scholar]
- 77.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96(5):1999–2001. [PubMed] [Google Scholar]
- 78.Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188(4):619–626. doi: 10.1084/jem.188.4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nature Reviews Drug Discovery. 2020;19(3):185–199. doi: 10.1038/s41573-019-0051-2 [DOI] [PubMed] [Google Scholar]
- 81.MacLeod DT, Antony J, Martin AJ, et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Molecular Therapy. 2017;25(4):949–961. doi: 10.1016/j.ymthe.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim MY, Yu KR, Kenderian SS, et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173(6):1439–1453.e19. doi: 10.1016/j.cell.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu F, Zhang H, Sun L, et al. FIRST-IN-HUMAN CLL1-CD33 COMPOUND CAR (CCAR) T CELL THERAPY IN RELAPSED AND REFRACTORY ACUTE MYELOID LEUKEMIA. In: European</References> [Google Scholar]