Abstract
Background
Previous studies have demonstrated dexamethasone (DEX)'s efficacy for coronavirus disease 2019 (COVID-19). In contrast, patients with residual lung field shading and symptoms after DEX treatment have been observed, and the efficacy of additional corticosteroids (AC) is unknown.
Objectives
We aimed to investigate the efficacy of AC in patients with COVID-19 with residual respiratory symptoms or who required oxygen therapy or invasive mechanical ventilation after DEX treatment.
Methods
This was a single-center, retrospective observational study including 261 patients with community-onset COVID-19, aged ≥ 18 years, admitted to our hospital between March 1, 2020, and May 31, 2021. Finally, 34 patients were included in the study who met all four of the following criteria: (1) required oxygen therapy or invasive ventilation, (2) were treated with DEX, (3) had residual shading on chest imaging after DEX treatment, or (4) had unimproved respiratory symptoms or oxygen saturation < 90%. We reviewed the medical records and clinical courses of 14 patients who received AC therapy (AC group) and 20 patients who did not (non-additional corticosteroids or NC group).
Results
The 90-day mortality rate was 35.7% in the AC group and 25.0% in the NC group. There was no statistically significant difference between the two groups (p = 0.797). In addition, there was no difference between groups in the proportion of patients who required oxygen therapy at discharge (64% vs. 35%, p = 0.162). The time from the end of DEX therapy to discharge was significantly longer in the AC group (median 7.5 vs. 33 days, p = 0.019). Regarding serious adverse events, infection was statistically more common in the AC group than in the NC group (p = 0.005).
Conclusions
AC after DEX treatment does not improve clinical outcomes and may prolong hospital stay.
Keywords: corticosteroids in covid-19, sars-cov-2, acute hypoxemic respiratory failure, organizing pneumonia, covid-19
Introduction
By March 2023, coronavirus disease 2019 (COVID-19) caused more than 6.8 million deaths worldwide [1]. Among patients infected with COVID-19, 80% have mild disease, while approximately 5% develop shock and multiple organ failure, with an overall mortality rate of 2.3% [2]. The in-hospital mortality rate of COVID-19 was 14%, while those of the influenza virus, a typical virus that causes viral pneumonia, and severe acute respiratory syndrome coronavirus 2, were 6% [3]. This trend of higher mortality in COVID-19 compared to influenza was reportedly more pronounced in patients older than 50 years and younger than 18 years [4]. Thus, COVID-19 is as much or more of a threat to humanity than any current endemic or circulating virus.
There are a few established treatment options for critical patients with COVID-19, one of which is corticosteroids, which have potent anti-inflammatory effects. The RECOVERY trial conducted in the UK examined the efficacy of dexamethasone (DEX) treatment for COVID-19 [5]. The results showed a 28-day mortality rate of 22.9% in the DEX group compared to 25.7% in the standard-of-care group without DEX, especially in patients who received invasive mechanical ventilation [5]. In the CoDEX trial [6], a multicenter randomized control trial to evaluate the efficacy of DEX for patients with COVID-19 requiring ventilator management, ventilator-free days were found to be significantly higher in the DEX group than in the standard care group. The results of these studies support the usefulness of SC for the treatment of COVID-19.
However, the optimal duration and dosage of corticosteroids are still unknown. We often encountered patients with respiratory failure and clinical symptoms that persisted after the prescribed duration of corticosteroid therapy in the clinical setting. In addition, delayed viral excretion was reported in pneumonia caused by viruses that were prevalent in the past, such as Middle East respiratory syndrome coronavirus and influenza [7,8].
Therefore, we aimed to investigate the efficacy of additional corticosteroids (AC) in patients with COVID-19 who had residual respiratory symptoms or required oxygen therapy or invasive mechanical ventilation after DEX treatment.
Materials and methods
Study design and participants
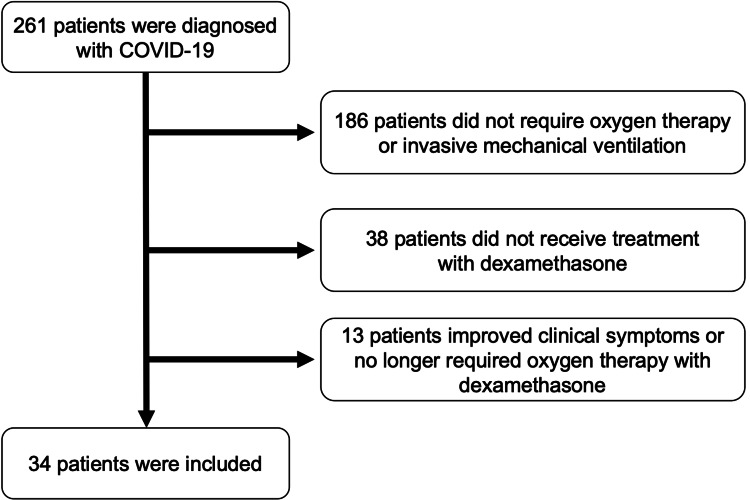
This study was an observational study conducted at a single center. We retrospectively reviewed the medical records of 261 patients aged 18 years and older with community-onset COVID-19 from March 1, 2020, to May 31, 2021. We confirmed the diagnosis of COVID-19 based on a positive polymerase chain reaction test by nasal swab. Among all patients, the following were excluded: 186 patients who did not require oxygen therapy or invasive mechanical ventilation, 38 patients who did not receive DEX treatment, and 13 patients whose symptoms improved or patients who no longer required oxygen therapy after a maximum of 10 days of treatment with a DEX dose of intravenous 6.6 mg/day. Finally, 34 patients were included (Figure 1). After the completion of DEX treatment, the patients were divided into two groups: 20 patients comprised the AC group, and 14 patients comprised the non-additional corticosteroids (NC) group. All patients in both groups were followed up for at least 28 days. Serious adverse effects were examined during the observation period, including infection, thrombosis, bleeding, acute renal failure, pneumothorax, and cardiac complications. The need for additional corticosteroid treatment was based on the consensus of multiple respiratory medicine and intensive care medicine specialists. All data were fully anonymized prior to access.
Figure 1. Study flowchart.
Of the 261 coronavirus disease 2019 (COVID-19) patients screened, 227 were excluded according to the criteria described in the methods section, and 34 were included.
Statistical analyses
Statistical analyses were performed using JMP software (Version 15; SAS Institute Japan Ltd., Minato-ku, Tokyo, Japan). Continuous and categorical variables were presented as medians (interquartile ranges) and numbers (percentages), respectively. The validity of the normal distribution was assessed using the Shapiro-Wilk test. Fisher's exact test and 95% confidence intervals (CIs) were used to test the differences between categorical variables; the Mann-Whitney U test was used for continuous variables. We excluded points that exceeded 1.5 times the upper and lower quantile range as outliers. Survival curves were estimated using the Kaplan-Meier method, followed by the log-rank test. P values less than 0.05 were considered statistically significant. All data were not fully anonymized before we accessed them.
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Showa University Ethics Committee (approval number: 21-093-B). After publishing a notice stating that this research would be based on the patient's clinical information on the website of the Showa University Ethics Committee, we obtained informed consent in the form of an opt-out. If the patient was under 20 years of age, consent was obtained from the parents or guardians.
Results
The median age of the AC group was 76.0 years, compared to 65.5 years in the NC group. There was no significant difference in the sex ratio or incidence of comorbidities between the two groups (Table 1). At the end of the DEX treatment, the mean platelet count was statistically significantly lower in the AC group than in the NC group. However, there were no significant differences in the other parameters (Table 2).
Table 1. Clinical characteristics of all patients.
AC, additional corticosteroids; BMI, body mass index; COPD, chronic obstructive pulmonary disease; NC, non-additional corticosteroids
| AC group (n = 14) | NC group (n = 20) | p value | |
| Age, year | 76.0 (70.5-83.2) | 65.5 (58.2-77.7) | 0.049 |
| Sex, male/female, n | 5/9 | 6/14 | 1.000 |
| BMI, kg/m2 | 23.1 (21.0-25.2) | 25.0 (21.6-27.0) | 0.420 |
| Smoking status, Former or Current, n | 6 (42.8) | 11 (55.0) | 0.728 |
| Respiratory support received at the end of dexamethasone, n (%) | |||
| Oxygen | 12 (85.7) | 17 (85.0) | 1.000 |
| Invasive mechanical ventilation | 7 (50.0) | 7 (35.0) | 0.486 |
| Other treatment during hospitalization, n (%) | |||
| Remdesivir | 10 (71.4) | 15 (75) | 1.000 |
| Favipiravir | 3 (21.4) | 4 (20.0) | 1.000 |
| Tocilizumab | 2 (14.2) | 4 (20.0) | 1.000 |
| Baricitinib | 1 (7.1) | 2 (10.0) | 1.000 |
| Extracorporeal membrane oxygenation | 2 (14.2) | 2 (10.0) | 1.000 |
| Comorbidities, n (%) | |||
| Hypertension | 10 (71.4) | 12 (60.0) | 0.717 |
| Diabetes mellitus | 5 (35.7) | 5 (25.0) | 0.704 |
| Dyslipidemia | 4 (28.5) | 6 (30.0) | 1.000 |
| Hyperuricemia | 1 (7.1) | 3 (15.0) | 0.627 |
| Asthma | 2 (14.2) | 1 (5.0) | 0.555 |
| COPD | 2 (14.2) | 2 (10.0) | 1.000 |
| Interstitial lung disease | 1 (7.1) | 0 (0) | 0.411 |
| Autoimmune disease | 3 (21.4) | 1 (5.0) | 0.283 |
| Chronic kidney disease | 1 (7.1) | 1 (5.0) | 1.000 |
| Malignancy | 3 (21.4) | 0 (0) | 0.060 |
| Chronic heart disease | 4 (28.5) | 6 (30) | 1.000 |
Table 2. Difference in blood examination between the two groups at the end of intravenous dexamethasone.
AC, additional systemic corticosteroids; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CRP, C-reactive protein; GTP, glutamyl transpeptidase; KL-6, Krebs von den Lungen-6, NC, non-additional systemic corticosteroids; PT, prothrombin time; SP, surfactant protein
| AC group (n = 14) | NC group (n = 20) | p value | |
| White blood cell, /μL | 11700 (7950-12750) | 9400 (6700-13850) | 0.624 |
| Neutrophil count, /μL | 10390 (6170-11960) | 7505 (5542-11175) | 0.391 |
| Lymphocyte count, /μL | 645 (417-1015) | 1085 (595-1750) | 0.089 |
| Hemoglobin, g/dL | 12.3 (10-13.5) | 12.7 (11-12.9) | 0.674 |
| Platelet count, ×104/μL | 20.0 (11.2-30.5) | 30.6 (18.7-37) | 0.030 |
| PT | 1.26 (1.17-1.36) | 1.13 (1.04-1.22) | 0.061 |
| APTT | 44.1 (31.6-65.5) | 36.1 (28.6-56.8) | 0.410 |
| Fibrinogen, mg/dL | 493 (319-758) | 476 (361-633) | 0.877 |
| D-dimer, ng/mL | 4.00 (1.39-5.92) | 1.87 (1.10-5.10) | 0.431 |
| Total protein, g/dL | 5.5 (5-5.9) | 5.5 (5.3-6.2) | 0.558 |
| Albumin, g/dL | 2.40 (2.35-2.65) | 2.65 (2.17-3.1) | 0.267 |
| Urea nitrogen, mg/dL | 22 (17.2-42) | 22.4 (17.7-39.6) | 0.888 |
| Creatinine, mg/dL | 0.67 (0.61-0.83) | 0.77 (0.66-1.12) | 0.151 |
| Uric acid, mg/dL | 3.8 (2.8-4.3) | 5 (3.3-6.8) | 0.149 |
| AST, U/L | 23.5 (19-33.7) | 29 (20.5-37.5) | 0.301 |
| ALT, U/L | 22 (14.5-49.2) | 27.5 (20-57.7) | 0.353 |
| Lactate dehydrogenase, U/L | 371 (255-451) | 300 (226-437) | 0.624 |
| ALP, U/L | 79 (64-104) | 71 (65-84.5) | 0.506 |
| γ-GTP, U/L | 42 (30-76) | 61 (36-117) | 0.258 |
| Creatine kinase, U/L | 40 (22-120.5) | 36 (22-108) | 0.841 |
| Sodium, mEq/L | 139.1 (136.1-143.5) | 139.2 (136.6-143.7) | 0.930 |
| Potassium, mEq/L | 4.3 (3.9-4.4) | 4.1 (3.9-4.6) | 0.712 |
| CRP, mg/dL | 2.87 (1.13-5.49) | 2.04 (0.42-6.74) | 0.611 |
| Ferritin, ng/mL | 839 (392-1180) | 768 (226-1608) | 0.779 |
| KL-6, U/mL | 1078 (788-1597) | 1013 (245-2143) | 0.748 |
| SP-D, ng/mL | 613 (149-1078) | 125 (107-459) | 0.182 |
| SP-A, ng/mL | 176 (176-176) | 55.1 (22.5-220) | 0.654 |
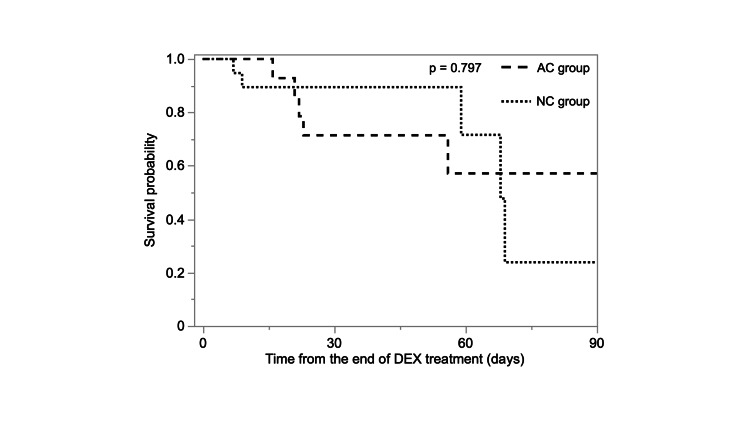
The 90-day mortality rate was 35.7% in the AC group and 25.0% in the NC group. There was no statistically significant difference between the two groups (p = 0.797, Figure 2). Regarding the other clinical outcomes, the proportion of patients discharged within 28 days of DEX treatment initiation was significantly higher in the NC group than in the AC group (p = 0.030, Table 3). Furthermore, the time from onset to discharge, time from COVID-19 diagnosis to discharge, time from admission to discharge, and time from the end of DEX treatment to discharge were all statistically significantly longer in the AC group (p = 0.014, p = 0.012, p = 0.011, and p = 0.018, respectively).
Table 3. Difference in clinical outcomes between the two groups.
AC, additional corticosteroids; DEX, dexamethasone; NC, non-additional corticosteroids
| Outcome | AC group (n = 14) | NC group (n = 20) | p value |
| Discharge in 28 days from the end of DEX, n (%) | 2 (14.2) | 11 (55.0) | 0.030 |
| Death during hospitalization, n (%) | 5 (35.7) | 5 (25.0) | 0.704 |
| Need oxygen at discharge, n (%) | 9 (64.2) | 7 (35.0) | 0.162 |
| Length, days | |||
| From onset to discharge | 49 (33.2-91) | 24.5 (21-68.2) | 0.014 |
| From diagnosis to discharge | 48 (30.7-89.7) | 20 (17.2-66) | 0.012 |
| From admission to discharge | 43 (29.2-85.5) | 17.5 (15-64) | 0.011 |
| From the end of DEX to discharge | 33 (21.7-73) | 7.5 (6-57.7) | 0.018 |
Figure 2. Kaplan-Meier survival curves for the 90-day survival with or without additional corticosteroids to DEX.
There was no statistically significant difference in the 90-day mortality between the two groups (p = 0.797).
AC, additional corticosteroids; DEX, dexamethasone; NC, non-additional corticosteroids
Regarding serious adverse events, infection was statistically more common in the AC group than in the NC group (p = 0.005, Table 4). While four patients were diagnosed with ventilator-associated pneumonia, two patients with pneumocystis pneumonia, two patients with invasive pulmonary aspergillosis, one patient with hospital-acquired pneumonia in the AC group, and three patients were diagnosed with ventilator-associated pneumonia in the NC group. Thrombosis, bleeding, acute renal failure, pneumothorax, and cardiac complications were not statistically significant between the two groups.
Table 4. Difference in serious adverse events between the two groups.
AC, additional corticosteroids; NC, non-additional corticosteroids
| Serious adverse events | AC group (n = 14) | NC group (n = 20) | p value |
| Infection | 9 | 3 | 0.005 |
| Thrombosis | 2 | 0 | 0.162 |
| Bleeding | 3 | 2 | 0.627 |
| Acute renal failure | 1 | 6 | 0.197 |
| Pneumothorax | 0 | 0 | 1.000 |
| Cardiac complications | 6 | 5 | 0.457 |
Discussion
This study is the first to demonstrate that corticosteroids, in addition to DEX, did not improve mortality and prolonged the length of hospital stay in patients with COVID-19. Furthermore, we found that infection was a significantly more common serious adverse effect in the AC group compared to the NC group.
In the RECOVERY trial, approximately 25% of the patients treated with DEX died at 28 days [5]. In a randomized controlled trial that compared the effects of 6 mg/day and 12 mg/day of DEX on patients with severe COVID-19, 12 mg/day of DEX did not improve mortality at 28 days [9]. This finding is consistent with the results of our study in that ACs did not improve mortality in patients with COVID-19. We would also expect mortality rates to vary depending on whether anti-interleukin-6 receptor antibodies [10,11], Janus kinase inhibitors [12,13], or antiviral drugs such as remdesivir [14,15] were used in combination. Although a meta-analysis is currently underway [16], we may also need to refer to evidence from other respiratory viral infections to determine the appropriate dose of corticosteroids for the treatment of COVID-19.
Our findings indicate that AC after DEX treatment prolonged the length of hospital stay. Recently, several studies comparing the efficacy of DEX and methylprednisolone (MP) in hospitalized patients with COVID-19 have been reported [17,18]. In a randomized controlled trial conducted in Iran, the group treated with 2 mg of MP per kg once daily, tapered to half the dose every five days, had a shorter length of hospital stay compared to the group treated with 6 mg of DEX daily for 10 days (7.43 ± 3.64 days vs. 10.52 ± 5.47 days, p = 0.015) [17]. Similarly, patients treated with 50 mg of oral prednisone for 14 days after three days of high-dose (250-500 mg/day) MP showed significant improvement in blood biomarkers such as C-reactive protein and lactate dehydrogenase compared to the DEX group [18]. Although it was unclear whether these studies reflect a difference in the pharmacological effects of DEX and MP, they indicate that a longer-term treatment strategy using different types of corticosteroids, other than DEX for COVID-19, might shorten the length of hospital stay.
In this study, there was a significantly higher incidence of infection in the AC group than in the NC group. Infection is a significant adverse event associated with corticosteroid use. COVID-19 is associated with bacterial and fungal infections in approximately 8% of patients [19] and is often critical. Previous reports have shown no statistical difference in the proportion of serious infections associated with different doses of steroids in patients with COVID-19 [9,12]. While we estimated that the median age of the AC group was 76 years in this study, the median age of the population was 55 [12] or 65 years [9] in previous studies. This fact may have potentially influenced their susceptibility to infection.
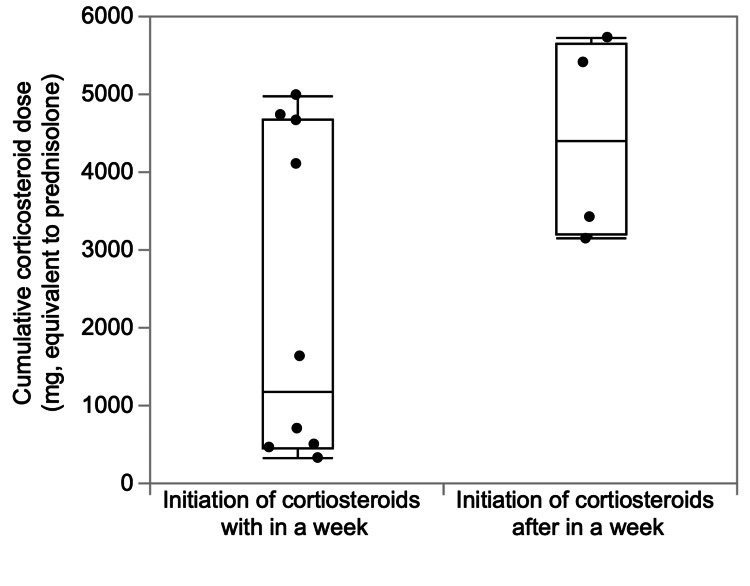
The strength of this study is that it is the first to prove that corticosteroids added to DEX did not benefit patients with moderate to severe COVID-19. It is not clear why the AC treatment did not improve clinical outcomes, but we hypothesized that this was partially due to the significant increase in infections with the ACs in our study. There are some limitations to this study. First, it was a small, single-center study; the sample size may have been insufficient to accurately examine the benefit of AC treatment after DEX. Second, the timing of the initiation of AC treatment and the total dosage was not uniform; there was no difference in the cumulative total corticosteroid doses between patients who started additional SC within one week after the end of DEX treatment and patients who started after one week (Figure 3). Third, it is unclear whether the conditions of the groups of included patients were uniform. It is often difficult to determine whether the patient’s condition after DEX treatment is the initial stage of sequelae [20], secondary organizing pneumonia [21], or acute respiratory distress syndrome [22], which is refractory to initial treatment. In a clinical trial of interstitial lung disease after COVID-19, it was reported that 4.8% of patients had interstitial lung disease with functional impairment four weeks after discharge [23]. Corticosteroids in these patients accelerated the recovery of respiratory function and resulted in radiological improvement [23]. A randomized controlled trial of corticosteroid treatment strategies for secondary organizing pneumonia after COVID-19 is underway at a university hospital in Spain [24].
Figure 3. Cumulative corticosteroid dose according to the timing of additional corticosteroid treatment initiation.
There was no significant difference in the timing of starting additional corticosteroids within or after a week (n = 14; p = 0.089).
Conclusions
In conclusion, this study found that AC after DEX treatment did not improve mortality or length of hospital stay in patients with moderate to severe COVID-19. Furthermore, the AC group induced significantly more infections than the control group. These results suggest that, while DEX is effective for COVID-19 with respiratory failure, if DEX does not completely recover the respiratory status caused by COVID-19, ACs may not be effective, regardless of the pathology. This is important in terms of the optimal dose and duration of corticosteroids for COVID-19. Consolidating findings in severe viral pneumonia other than SAR-CoV-2, such as influenza, in addition to findings in the sequelae of COVID-19 and secondary organizing pneumonia, we believe that further research will complement the current lack of understanding of the respiratory pathogenesis after DEX treatment for severe COVID-19.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Showa University Ethics Committee issued approval 21-093-B. This study was approved by the Showa University Ethics Committee (approval number: 21-093-B).
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.COVID-19 Map - Johns Hopkins Coronavirus Resource Center. [ Jul; 2023 ]. 2023. https://coronavirus.jhu.edu/map.html https://coronavirus.jhu.edu/map.html
- 2.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Wu Z, McGoogan JM. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany. Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Int J Infect Dis. 2021;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dexamethasone in hospitalized patients with Covid-19. Horby P, Lim WS, Emberson JR, et al. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID- 19: the CoDEX randomized clinical trial. Tomazini BM, Maia IS, Cavalcanti AB, et al. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viral loads and duration of viral shedding in adult patients hospitalized with influenza. Lee N, Chan PK, Hui DS, et al. J Infect Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Arabi YM, Mandourah Y, Al-Hameed F, et al. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 9.Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID steroid 2 randomized trial. Munch MW, Myatra SN, Vijayaraghavan BK, et al. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tocilizumab in patients hospitalized with Covid-19 pneumonia. Salama C, Han J, Yau L, et al. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. Rosas IO, Bräu N, Waters M, et al. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baricitinib plus remdesivir for hospitalized adults with Covid-19. Kalil AC, Patterson TF, Mehta AK, et al. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. Cao Y, Wei J, Zou L, et al. J Allergy Clin Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association of remdesivir treatment with survival and length of hospital stay among US veterans hospitalized with COVID-19. Ohl ME, Miller DR, Lund BC, et al. JAMA Netw Open. 2021;4:0. doi: 10.1001/jamanetworkopen.2021.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remdesivir for the treatment of Covid-19—final report. Beigel JH, Tomashek KM, Dodd LE, et al. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higher vs. standard doses of dexamethasone in patients with COVID-19 and hypoxia: a prospective meta-analysis. [ Dec; 2022 ];Granholm A. https://osf.io/fr5sv 2021 1:31–2021. [Google Scholar]
- 17.Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. BMC Infect Dis. 2021;21:337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. Pinzón MA, Ortiz S, Holguín H, et al. PLoS One. 2021;16:0. doi: 10.1371/journal.pone.0252057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Rawson TM, Moore LS, Zhu N, et al. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sequelae in adults at 6 months after COVID-19 infection. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. JAMA Netw Open. 2021;4:0. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Does COVID-19 pneumonia signify secondary organizing pneumonia?: a narrative review comparing the similarities between these two distinct entities. Chong WH, Saha BK, Chopra A. Heart Lung. 2021;50:667–674. doi: 10.1016/j.hrtlng.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Gibson PG, Qin L, Puah SH. Med J Aust. 2020;213:54–56. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Myall KJ, Mukherjee B, Castanheira AM, et al. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oral prednisone regimens to optimize the therapeutic strategy in patients with organizing pneumonia post-COVID-19 (NORCOVID) [ Dec; 2022 ]. 2022. https://classic.clinicaltrials.gov/ct2/show/NCT04534478 https://classic.clinicaltrials.gov/ct2/show/NCT04534478