Abstract
Endoscopic management via retrograde ureteroscopic laser ablation of upper tract urothelial carcinoma (UTUC) has become the preferred treatment modality for low-risk tumors. The most popular ablative lasers over the past 15–20 years have been the holmium:yttrium-aluminum-garnet (Ho:YAG) and neodymium (Nd:YAG) lasers, but recently the thulium (Th:YAG) laser has emerged as a potential alternative. This review compares the mechanism of action, physiological properties and effects, and oncologic outcomes of Ho:YAG/Nd:YAG lasers versus the Th:YAG laser for UTUC treatment. Potential advantages of the Th:YAG laser over existing technologies are outlined, followed by a discussion of emerging laser technologies in UTUC management.
Keywords: Endoscopic management, Holmium laser, Laser ablation, Thulium laser, Upper tract urothelial carcinoma
1. Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare urological cancer that comprises 5%–10% of urothelial tumors, with an incidence of 1–2 per 100,000 person-years.[1–4] Traditionally, the criterion standard treatment for UTUC has been radical nephroureterectomy (RNU) of the affected kidney and ureter.[1] However, over the past 2 decades, kidney-sparing endoscopic management of UTUC has gained acceptance, as it offers preservation of ipsilateral renal function.
The European Association of Urology (EAU) guidelines on UTUC outline which patients may be recommended for endoscopic management. Only low-risk patients are eligible for elective kidney-sparing treatment, with low-risk being defined as the following: (1) unifocal tumor, (2) tumor size less than 2 cm, (3) low-grade cytology, (4) low-grade ureteroscopic biopsy, and (5) no invasive features on CT urography. Patients with imperative indications for kidney-sparing treatment (eg, patients with a solitary kidney or chronic kidney disease) may also be offered endoscopic management. All other patients are recommended to receive RNU.[1] Rigorous follow-up surveillance is also required after endoscopic management, due to high rates of tumor recurrence.[5–9] For patients treated endoscopically, cystoscopy, ureteroscopy, and CT urography are recommended at 3 and 6 months postoperatively and then yearly for 5 years.[1] Although the EAU guidelines define low-risk patients as those with tumors less than 2 cm, Scotland et al.[10] reported generally favorable oncologic outcomes in a long-term study of patients with tumors of 2 cm or greater treated endoscopically, suggesting that endoscopic treatment could be appropriate even for large tumors. However, recurrence was very high, with only a 10% recurrence-free survival (RFS) rate and a mean time to recurrence of 4.9 months.[10]
Options for endoscopic management include transurethral retrograde access with ureteroscopy, biopsy, and laser ablation or percutaneous antegrade access with resection and fulguration. The most widely adopted technique for endoscopic treatment is ureteroscopy with tumor laser ablation. This technique was first developed using electrocautery but has evolved to incorporate the much more efficient holmium:yttrium-aluminum-garnet (Ho:YAG) and neodymium:yttrium-aluminum-garnet (Nd:YAG) lasers. These lasers enable superior operative control over thermal cautery, hemostasis, incision, and ablation. Consequently, Ho:YAG and Nd:YAG lasers have become the standard for endoscopic treatment of UTUC over the past 20 years.[7–9] Recently, the thulium:yttrium-aluminum-garnet (Th:YAG) laser has risen in popularity as an alternative to Ho:YAG/Nd:YAG lasers.[11,12] Several retrospective single- and multi-institution studies have been published on the Th:YAG laser over the past few years, but there is a relative paucity of comparative studies. This review presents an overview of current laser therapy options for endoscopic treatment of UTUC. Furthermore, we explore the mechanisms and oncologic outcomes of standard Ho:YAG/Nd:YAG lasers and the new alternative Th:YAG laser, as well as future laser technologies that may be applied to endoscopic UTUC management.
2. Discussion of endoscopic lasers
2.1. Holmium and Neodymium lasers
2.1.1. Mechanism of action
Holmium and neodymium lasers, produced from different materials, offer distinctive laser characteristics and are often used in conjunction with one another. Both lasers continue to enjoy widespread clinical use, Ho:YAG having been developed more recently and Nd:YAG considered its predecessor. Nd:YAG is a solid-state laser that offers the following 4 wavelength options: 946, 1064, 1318, and 1444 nm. It operates in continuous and pulsatile modes and reaches a depth of penetration of 3–6 mm in soft tissue.[9,13] The holmium laser Ho:YAG, also a solid-state laser, emits a wavelength of 2120 nm. It only operates in pulstatile mode, with a much shallower depth of penetration of 0.5 mm. The different depths of tissue penetration allow for different advantageous uses of each laser. Nd:YAG may be used for deeper coagulation and debulking of larger or vascular tumors, whereas Ho:YAG may be preferred for ablation of surface papillary tumors when penetration into deeper tissues is not desirable.[9,14]
Because of Nd:YAG’s deeper depth of penetration, it confers an increased risk of ureteral stricture or perforation and therefore should be avoided for ureteral tumors.[14,15] Nd:YAG does not require direct tissue contact for tissue ablation. Recommended laser settings are 20–30 W of power for 2–3 seconds for a 200- to 600-μm diameter fiber.[9,14] Ho:YAG forms a vapor bubble at the tip of the laser that transmits a large amount of energy to the cells it contacts by quickly increasing the temperature of the surrounding tissue and causing vaporization of intracellular fluid. This mechanism of action requires the Ho:YAG laser to directly contact the tissue for ablation. Although its shallow depth of ablation can allow for better visualization of the target tissue and a more focused ablative area, direct contact can result in tissue adherence to the laser tip, which may impair visualization and require intraoperative interruptions for fiber cleaning. Recommended laser settings for Ho:YAG are 0.6–1.0 J per laser pulse at a repetition rate of 5–10 Hz.[9,14,15] While Ho:YAG has a much shallower depth of penetration and may be used in conjunction with Nd:YAG for most efficient tumor ablation, its more focused ablative target tissue area may be desired by some clinicians. Ho:YAG may be used alone to debulk and ablate larger tumors via a staged or sequential ablation pattern. This can be done with repetitive ablation during the same operation or subsequent operations to remove the entire tumor.
2.1.2. Oncologic outcomes with Ho:YAG/Nd:YAG
The Ho:YAG/Nd:YAG laser has been the standard laser used for endoscopic management of UTUC since the 1990s.[14,16,17] This comes with good reason, as the laser has shown itself to be easy to use and has yielded favorable oncologic and surgical outcomes. A meta-analysis comparing UTUC patients managed with endoscopic treatment versus RNU indicated no significant difference in cancer-specific survival (CSS) at 5 or 10 years of follow-up in appropriately selected patients. It is important to note, however, that all included studies were cohort studies, introducing potential selection bias, and there was in fact decreased CSS for patients receiving endoscopic treatment for high-grade UTUC. Rates of subsequent RNU therapy varied between 16.7% for low-grade tumors and 28.6% for high-grade tumors. [18] Systematic reviews of oncological outcomes for low-risk patients undergoing endoscopic treatment with the holmium laser have generally found consistently encouraging results. Outcomes reported in systematic reviews are summarized in Table 1. Overall survival (OS) ranged from 35% to 100% and CSS from 47% to 100%, although CSS was generally between 80% and 100% for the studies included.[7–9,14,19,20] Upper-tract recurrence rates were highly variable, with rates ranging between 14% and 77%, with 0%–36% requiring RNU.[8,9,14,20,21] Reported intraoperative or postoperative complications were typically mild. Ureteral perforation rates ranged between 0% and 10% and ureteral stricture rates between 5% and 14%, although strictures could also be caused by recurrent disease and not necessarily as a result of surgery.[8,9,14,19,21] Other than the previously mentioned reviews, a recent long-term retrospective outcomes study by Scotland et al.[22] followed 168 low- and high-grade patients treated endoscopically with a mean follow-up of 5.53 years. At 5 years, OS was 80.89%, CSS was 92.57%, and 74.79% of patients had at least one recurrence with a grade progression-free survival rate of 75.18%. The rate of complications was only 7.14%, with only 1 ureteral stricture.[22] Although recurrence rates for endoscopic management with Ho:YAG/Nd:YAG laser can be high, overall oncologic outcomes are excellent, and RNU can be avoided in most patients eligible for initial endoscopic management.
Table 1.
Oncologic outcomes reported in systematic reviews of endoscopic treatment of UTUC with Ho:YAG or Nd:YAG laser ablation.
| Authors | Year of publication | Follow-up time, mo | Upper tract recurrence rate, % | Bladder recurrence rate, % | Requiring RNU, % | CSS, % | OS, % | Renal salvage rate, % |
|---|---|---|---|---|---|---|---|---|
| Bader et al.[14] | 2009 | NR | 14–44 | 25–36 | NR | NR | NR | 78–81 |
| Adamis et al.[19] | 2011 | NR | 29–74 | 35–40 | NR | 86–100 | NR | 78–81 |
| Park and Jeon[8] | 2013 | 20–53 | 25–90 | 15–53 | 0–28 | 82–100 | 45–100 | NR |
| Verges et al.[7] | 2017 | 19–73 | 23–90 | 43 | 17–36 | 47–100 | NR | 64–83 |
| Petros et al.[9] | 2018 | 24–58 | 65 | 44 | 0–33 | 70–100 | 35–100 | NR |
2.2. Thulium laser
2.2.1. Mechanism of action
The thulium laser has several mechanical properties that make it a viable alternative to Ho:YAG and may provide potential advantages over its predecessor. Th:YAG operates at a wavelength of 1940–2013 nm, closer to the peak absorption point of water (Fig. 1).[12,23] Water is the primary chromophore, and as such, tissue ablation is mediated by vapor bubble contact, which allows for more efficient tissue ablation and coagulation at a very shallow depth. The depth of tissue penetration using Th:YAG is even shallower than that of Ho:YAG, with a depth of only 0.2–0.4 mm as compared with 0.5 mm.[12,24] This shallower depth of penetration decreases the thermal damage zone, which in turn may reduce postoperative complications such as ureteral stricture or perforation due to iatrogenic trauma to the adjacent urothelium.
Figure 1.
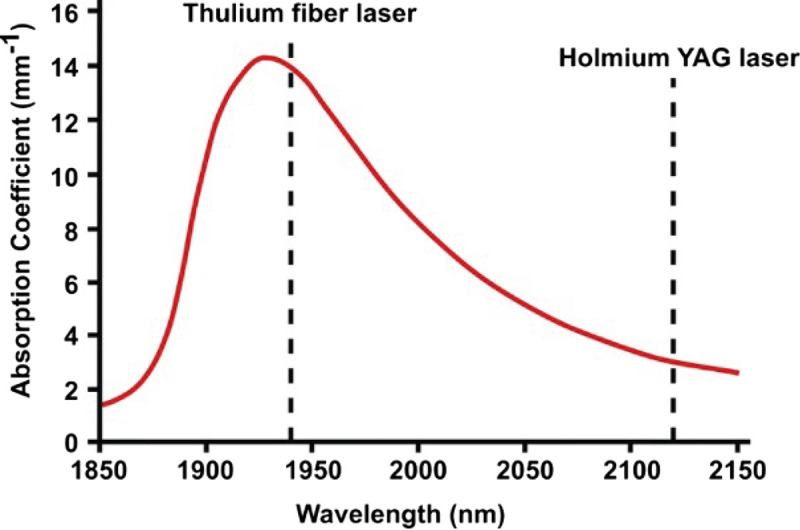
Absorptive capacity of liquid water at room temperature (22°C) for varying wavelengths. Figure reproduced with permission from Traxler and Keller. World J Urol, 2020, under the terms of the Creative Commons Attribution CC BY 4.0 International License (http://creativecommons.org/licenses/by/4.0/)[23]
Th:YAG is a diode laser, which allows for both pulsatile and continuous emission. While the pulsatile mode may be preferred for tissue ablation or resection, the continuous mode can provide stronger hemostatic potential and coagulation, as oxyhemoglobin requires a longer time to absorb laser wavelengths.[25] The continuous laser may also improve the precision of ablation over the pulsatile mode, as it forms smaller microbubbles at the tip of the laser. This in turn reduces fiber vibration, which can be a complicating factor during pulsed firing, and helps maintain a more predictable coagulation area during laser operation.[12,24,26] Because of the confined operating space in the upper urinary tract and the difficulty of visualization through ureteroscopy, any intraoperative complications that impair visualization can make the endoscopic surgery much more difficult. Improvements in visualization through reduced bleeding or fiber vibration would considerably enhance surgical technique. The improved hemostatic ability of the continuous firing mode may be one of the strongest potential advantages of Th:YAG over Ho:YAG. It is important to note, however, that hemostasis with Th:YAG has not been directly compared with Ho:YAG in vivo for UTUC. However, in an early study by Defidio et al.[12] investigating the use of Th:YAG for UTUC treatment, the participating surgeons, who were experienced with both Th:YAG and Ho:YAG, rated the Th:YAG higher on fiber tip stability, precision, and reduced bleeding. In an ex vivo study using porcine kidneys, Proietti et al.[24] found an increased coagulative area at the laser tip for Th:YAG over Ho:YAG. These findings do offer some evidence for improved hemostatic potential, which combined with its shallow depth of penetration make Th:YAG an ideal candidate for procedures involving the kidney and ureter. Further studies investigating the physiologic effects of its use in the genitourinary system are warranted. A comparison of the properties of the three major lasers used in UTUC laser ablation is displayed in Table 2.
Table 2.
Properties of lasers used for UTUC laser ablation.
| Continuous versus pulsed emission | Fiber diameter, μm | Wavelength, nm | Depth of penetration, mm | Recommended power settings | Preferred sites for use | |
|---|---|---|---|---|---|---|
| Neodymium laser | Continuous | 200–600 | 946, 1064, 1318, 1444 | 3–6 | 20–30 W | Renal pelvis |
| Holmium laser | Pulsed | 200, 272, 365, 600 | 2120 | 0.5 | 0.6–1.0 J/pulse at 5–10 Hz | Renal pelvis; Ureter |
| Thulium laser | Continuous or pulsed | 272, 365 | 1940–2013 | 0.2–0.4 | 10–20 W | Renal pelvis; Ureter |
UTUC = upper tract urothelial carcinoma;
2.2.2. Incision shape for holmium versus thulium lasers
The option of continuous laser emission with Th:YAG, as opposed to pulsed laser emission, may also offer an advantage over Ho:YAG in terms of incision precision. The Ho:YAG laser is limited to only pulsed laser emission. The laser emits a burst of energy, often at a power of 2–10 kW, for a period of less than 1 millisecond. This is followed by a pause between laser emissions that can be 20–100 times longer than that of the actual laser emission. The exact duration of the pause depends on the frequency settings for the laser. This setting, while effective for tissue or stone lithotripsy, may cause microfractures in soft tissue, which can result in irregular incision shapes. This is likely a consequence of the explosive nature of pulsed laser emissions and corresponding heat dissipation to surrounding tissue.[24,27]
In contrast, the Th:YAG laser offers both pulsed and continuous emission settings. Continuous emission allows for a more precise cut that generates a shallower and more regular incision shape. In addition, the power of the laser may be much lower than that of the Ho:YAG laser, which is typically set approximately 15 W for UTUC ablation.[12,28] These features contribute to Th:YAG being less efficient than Ho:YAG for stone lithotripsy but conversely may make it more effective for soft tissue ablation.[27]
Because of these mechanistic differences, Ho:YAG and Th:YAG produce incisions of different shapes and depths. An ex vivo study by Proietti et al.[24] of incisional characteristics and coagulative properties of Th:YAG versus Ho:YAG lasers using porcine kidneys and urothelium found that Ho:YAG resulted in deeper incisions (0.458 ± 0.194 vs. 0.346 ± 0.120 mm), but Th:YAG resulted in larger coagulative area (0.066 ± 0.035 vs. 0.125 ± 0.020 mm2) and total laser area (0.125 ± 0.055 vs. 0.264 ± 0.146 mm2; Table 3). The shallower incision depth of Th:YAG may allow for more precise removal of superficial tumors in the ureters, where vascular structures can begin at a depth of 0.4 mm.[24] The shape of incisions made using each laser also clearly differed when viewed under light microscopy. Incisions made using Ho:YAG were described as irregular, triangular, or saccular, whereas incisions made using Th:YAG were described as more regular (Fig. 2).[24]
Table 3.
Incision depth, vaporization area, coagulation area, and total laser area for Th:YAG versus Ho:YAG lasers upon porcine renal urothelium ex vivo.
| Th:YAG | Ho:YAG | p | |
|---|---|---|---|
| Power, mean ± SD, W | 15.21 ± 8.61 | 14.65 ± 7.79 | 0.930 |
| ID, mean ± SD, mm | 0.346 ± 0.120 | 0.458 ± 0.194 | 0.024* |
| VA, mean ± SD, mm2 | 0.070 ± 0.45 | 0.066 ± 0.46 | 0.572 |
| CA, mean ± SD, mm2 | 0.123 ± 0.020 | 0.066 ± 0.035 | 0.001* |
| TLA, mean ± SD, mm2 | 0.264 ± 0.146 | 0.125 ± 0.055 | 0.005* |
CA = coagulation area; ID = incision depth; TLA = total laser area; VA = vaporization area.
*p < 0.05.
Table reproduced with permission from Proietti et al.[24]
Figure 2.
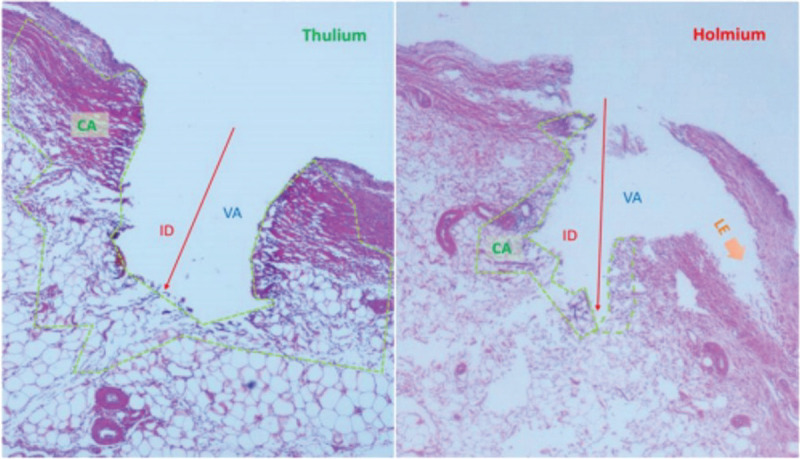
An example of the incisional shapes produced by of Th:YAG and Ho:YAG on soft tissue (porcine kidney). Laser settings were set at 10 W and 1 J at 10 Hz and long pulse, respectively. In the picture, ID, VA, and CA are shown. TLA = VA + CA. Orange arrows show LE dissection with the holmium laser. Figure reproduced with permission from Proietti et al.[24] CA = coagulation area; ID = incision depth; LE = lateral energy; TLA = total laser area; VA = vaporization area.
In a similar study, Huusmann et al.[29] compared incision depths and coagulation zones of pulsed Ho:YAG versus pulsed Th:YAG versus continuous Th:YAG at various power levels in porcine kidneys. Results were comparable with those of Proietti et al.,[24] with deeper incisions for pulsed Ho:YAG versus pulsed Th:YAG at all tested power levels (5, 40, 80 W) and deeper zones of necrotic tissue for Ho:YAG at lower power levels. Continuous Th:YAG did not demonstrate any significant depth of incision at 5 W and resulted in the shallowest incision depth and zone of necrotic tissue at 40 W. Moreover, subjective observations found that Th:YAG made a much smoother cut than Ho:YAG, and pulsed Th:YAG resulted in less scarring than continuous emission.[29] These findings indicate that pulsed Th:YAG may offer the benefit of minimal tissue trauma while maintaining sufficient depth for tumor ablation. Given the limited anatomic working space of the upper tract, a more regularly shaped shallower incision allows for greater procedural control and could reduce the risk of excess tissue loss and operative complications.
2.2.3. Oncologic outcomes with the thulium laser
As the Th:YAG laser was developed more recently than the Ho:YAG and Nd:YAG lasers, fewer studies have been published documenting clinical outcomes with the use of this laser. There has not yet been a prospective comparison study between Ho:YAG/Nd:YAG and Th:YAG lasers, due in part to the relatively low incidence of UTUC and guidelines advocating endoscopic management for only low-risk patients or patients for whom renal preservation is imperative. However, the few published studies available have demonstrated positive results with the use of the thulium laser.
Oncologic outcomes data from retrospective studies support the use of the thulium laser as a viable laser for the ablation of UTUC, as summarized in Table 4. In a retrospective cohort comparison of Th:YAG treatment versus RNU by Wen et al.,[30] laser treatment was associated with shorter hospitalization and lower creatinine levels on postoperative day one. However, relative recurrence rates were 21.9% versus 7.8%, and median follow-up was unspecified.[30] Defidio and colleagues[31] conducted a study of 101 patients treated with a dual thulium:holmium laser. At a median follow-up time of 18 months, 69.3% of patients were recurrence-free, 21.8% required subsequent endoscopic treatment, and 8.9% required eventual RNU, with an intention-to-treat kidney preservation rate of 91%. The kidney preservation rate among patients with imperative indications for kidney-sparing surgery (such as solitary kidney or poor global renal function among both kidneys) was also high at 87.5%. Recurrence-free survival was longer in patients without imperative indications for kidney-sparing surgery than those with imperative indications (33.17 vs. 26.87 months), which the authors attributed to a higher percentage of patients with high-grade tumors in the imperative versus the nonimperative group. No intraoperative or postoperative complications above Clavien-Dindo grade I were reported.[31] Musi et al.[26] conducted a prospective study with 42 patients that found similarly encouraging results. Median RFS was 44 months, 19% required subsequent ablative treatment, only 9.5% of patients were upgraded to RNU, and there were no Clavien-Dindo grade IV or V complications and only one grade III complication.[26] Bozzini and colleagues[28] performed a retrospective study with 47 low-risk patients treated solely with Th:YAG, resulting in a recurrence rate of 19.2% and no major complications at a mean follow-up time of 11.7 months. Among published studies of treatment with thulium laser for appropriately selected patients, the recurrence rate requiring further endoscopic treatment hovers approximately 20%, with approximately 9% requiring RNU, and ablation is generally free from major complications.
Table 4.
Oncologic outcomes with the thulium laser.
| Authors | Year of publication | Treatment modality | n | Low grade, n (%) | High grade, n (%) | Median follow-up time, mo | Recurrence rate, n (%) | Stage/grade progression rate, n (%) | Requiring upgrade to RNU, n (%) | RFS, mo | Patients with imperative indications, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Defidio et al.[12] | 2011 | Thulium laser | 59 | 36 (61.0) | 23 (39.0) | 26.4 | 22 (37.3) | NR | 8 (13.6) | NR | 9 (15.2) |
| Wen et al.[30] | 2018 | Thulium laser | 32 | 27 (84.4) | 5 (15.6) | NR | 7 (21.9) | NR | 3 (9.4) | NR | NR |
| RNU | 107 | 75 (70.1) | 32 (29.2) | NR | 8 (7.8) | NR | N/A | NR | N/A | ||
| Musi et al.[26] | 2018 | Thulium laser | 42 | 29 (69.1) | 5 (11.9)* | 26.3 | 8 (19.0) | 2 (4.8) | 4 (9.5) | 44 | 8 (19.0)† |
| Defidio et al.[31] | 2019 | Thulium:holmium laser | 101 | 82 (81.2) | 19 (18.8) | 18 | 31 (30.7) | 16 (15.8) | 9 (8.9) | 29.4 | 32 (31.7) |
| Bozzini et al.[28] | 2020 | Thulium laser | 78 | 49 (62.8) | 29 (37.2) | NR | 9 (19.2) | 0 (0.0) | 31 (39.8)‡ | NR | NR |
N/A = not applicable; NR = not recorded; RFS = recurrence-free survival; RNU = radical nephroureterectomy.
*Pathological grading on biopsy was inconclusive for 8 of patients (19.0%).
†A further 25 patients (59.3%) also had relatively imperative indications, such as advanced medical comorbidities.
‡All patients were biopsied before laser ablation, and all those who were found to have high grade disease received subsequent RNU. Twenty-nine of 31 RNU in the study were due to high-grade disease.
All patients in the thulium laser studies survived during the investigative periods. However, follow-up times were much shorter (11–26 months) than studies investigating the holmium laser, therefore limiting CSS and OS analysis. Direct comparison of oncologic outcomes between holmium and thulium lasers is difficult because of differences in study design. Whereas oncologic outcomes data from several large systematic reviews are available for holmium lasers, only smaller cohort or observational study data has been reported regarding thulium lasers. The recurrence rate seems to be lower with thulium than with the holmium laser, but this apparent difference could be due to shorter follow-up times reported in studies of the thulium laser. Further long-term outcome studies with the thulium laser are needed to fully evaluate recurrence and OS outcomes.
2.3. Emerging technologies
2.3.1. MOSES™ technology
The MOSES™ system (Lumenis, Clarion Medical Technologies) is a pulse-modulating technology for Ho:YAG laser that has shown revolutionary promise in lithotripsy of urinary calculi. It functions by “parting the water” between the target and the laser tip, which creates more efficient laser delivery to the target and decreased stone retropulsion.[32,33] It also demonstrated decreased marginal tissue damage and incision width when compared with normal Ho:YAG ablation in experiments with porcine ureters.[32] An abstract was published in the EAU 2020 Virtual Congress documenting the use of MOSES™ technology in UTUC ablation.[34] However, its potential superiority or inferiority as compared with existing technologies was not fully explored, and further research is required to establish its role in UTUC endoscopic treatment.
2.3.2. T-1470 LiteTouch™ laser
The T-1470 LiteTouch™ Laser (Convergent Laser Technologies, Alameda, California) is a novel diode laser that provides a 1470-nm wavelength. The 1470-nm diode lasers have documented usage in other surgical fields, such as colorectal surgery, vascular surgery, and otolaryngology.[35–37] The wavelength allows for soft tissue penetration as well as absorption by both water and oxyhemoglobin, allowing for excellent coagulation and hemostasis. Because of absorptive and coagulative capabilities of the laser, the T-1470 LiteTouch™ shows promise for its use in urologic procedures. The authors have published a recent report on the use of this laser for the enucleation and en bloc resection of UTUC, which is generally not possible with Ho:YAG/Nd:YAG or Th:YAG lasers.[38] This approach has potential for improved pathological staging and grading of excised tumor tissue, which would overcome some limitations currently encountered with conventional ureteroscopy biopsy and ablation tools.[39–41] This solid-state diode laser offers 3 modes, including continuous wave, pulse, and super pulse, along with adjustable frequencies of 10, 20, and 30 Hz, which allow for control over hemostasis by adjusting tissue absorptive time in between pulses. As the laser is still novel, further trials are warranted to fully assess both its enucleative and ablative capabilities for the treatment of UTUC.
3. Conclusions
Laser ablation of low-risk UTUC has become more accepted in the contemporary era because both endoscopic techniques and technologies have become optimized, offering intermediate-term RFS in 20%–40% of patients without increased cancer-specific mortality, and with the added advantage of nephron preservation. The Ho:YAG/Nd:YAG laser has been the standard of care since the 1990s, but a growing number of studies have highlighted the ablative and hemostatic advantages of the Th:YAG laser. Both lasers have demonstrated their respective value, and the opportunity remains for future direct comparison studies between the 2 lasers, as well as longer-term follow-up evaluations. Beyond the 2 currently established lasers, emerging technologies suggest the possibility that other lasers could be used for endoscopic UTUC treatment and operative techniques might be improved with better technology. The use of laser treatment for UTUC is rapidly evolving, and further research is well warranted to support the development of new technologies and refine this nephron-sparing technique.
Footnotes
How to cite this article: Zollinger BW, Shoen EJ, Gresham CF, Whalen MJ. Current laser therapy options for endoscopic treatment of upper tract urothelial carcinoma. Curr Urol 2023;17(1):62–67. doi: 10.1097/CU9.0000000000000158
Contributor Information
Ezra J. Shoen, Email: ezrashoen@gmail.com.
Charles F. Gresham, Email: trippgresham@gmail.com.
Michael J. Whalen, Email: mwhalen@mfa.gwu.edu.
Acknowledgments
None.
Statement of ethics
Not applicable.
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
Funding source
None.
Author contributions
All authors have contributed, take responsibility for, and agree with this manuscript;
BWZ, EJS, CFG, MJW: Conception and design of study;
BWZ, EJS: Data acquisition;
BWZ, EJS, MJW: Drafting the manuscript.
References
- 1.Rouprêt M Babjuk M Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol 2021;79(1):62–79. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Kallidonis P, Liatsikos E. Urothelial tumors of the upper urinary tract and ureter. In: Alan W Partin AW Peters CA, et al., eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021:2185–2198.e6. [Google Scholar]
- 4.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J Urol 2000;164(5):1523–1525. [PubMed] [Google Scholar]
- 5.Cornu JN Rouprêt M Carpentier X, et al. Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial cell carcinoma. World J Urol 2010;28(2):151–156. [DOI] [PubMed] [Google Scholar]
- 6.Bagley DH, Grasso M, 3rd. Ureteroscopic laser treatment of upper urinary tract neoplasms. World J Urol 2010;28(2):143–149. [DOI] [PubMed] [Google Scholar]
- 7.Verges DP, Lallas CD, Hubosky SG, Bagley DH, Jr. Endoscopic treatment of upper tract urothelial carcinoma. Curr Urol Rep 2017;18(4):31. [DOI] [PubMed] [Google Scholar]
- 8.Park BH, Jeon SS. Endoscopic management of upper urinary tract urothelial carcinoma. Korean J Urol 2013;54(7):426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petros FG, Li R, Matin SF. Endoscopic approaches to upper tract urothelial carcinoma. Urol Clin North Am 2018;45(2):267–286. [DOI] [PubMed] [Google Scholar]
- 10.Scotland KB Kleinmann N Cason D, et al. Ureteroscopic management of large ≥2 cm upper tract urothelial carcinoma: A comprehensive 23-year experience. Urology 2018;121:66–73. [DOI] [PubMed] [Google Scholar]
- 11.Emiliani E, Herrmann TR, Breda A. Thulium laser for the treatment of upper urinary tract carcinoma (UTUC)? Are we there, yet? World J Urol 2015;33(4):595–597. [DOI] [PubMed] [Google Scholar]
- 12.Defidio L, De Dominicis M, Di Gianfrancesco L, Fuchs G, Patel A. First collaborative experience with thulium laser ablation of localized upper urinary tract urothelial tumors using retrograde intra-renal surgery. Arch Ital Urol Androl 2011;83(3):147–153. [PubMed] [Google Scholar]
- 13.Bhatta N, Isaacson K, Bhatta KM, Anderson RR, Schiff I. Comparative study of different laser systems. Fertil Steril 1994;61(4):581–591. [DOI] [PubMed] [Google Scholar]
- 14.Bader MJ Sroka R Gratzke C, et al. Laser therapy for upper urinary tract transitional cell carcinoma: Indications and management. Eur Urol 2009;56(1):65–71. [DOI] [PubMed] [Google Scholar]
- 15.Moore HG, Thomas D, Chughtai B. Holmium enucleation of the prostate. In: Chughtai B, ed. A Comprehensive Guide to the Prostate. Cambridge, MA: Academic Press; 2018:73–79. [Google Scholar]
- 16.Lam JS, Gupta M. Ureteroscopic management of upper tract transitional cell carcinoma. Urol Clin North Am 2004;31(1):115–128. [DOI] [PubMed] [Google Scholar]
- 17.Schmeller NT, Hofstetter AG. Laser treatment of ureteral tumors. J Urol 1989;141(4):840–843. [DOI] [PubMed] [Google Scholar]
- 18.Seisen T Peyronnet B Dominguez-Escrig JL, et al. Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: A systematic review by the EAU non-muscle invasive bladder cancer guidelines panel. Eur Urol 2016;70(6):1052–1068. [DOI] [PubMed] [Google Scholar]
- 19.Adamis S, Varkarakis J. Minimally invasive approach in the management of upper- urinary-tract tumours. Scand J Urol Nephrol 2011;45(6):381–387. [DOI] [PubMed] [Google Scholar]
- 20.Grasso M, Fishman AI, Cohen J, Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: A 15-year comprehensive review of 160 consecutive patients. BJU Int 2012;110(11):1618–1626. [DOI] [PubMed] [Google Scholar]
- 21.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol 2007;4(8):432–443. [DOI] [PubMed] [Google Scholar]
- 22.Scotland KB Hubbard L Cason D, et al. Long term outcomes of ureteroscopic management of upper tract urothelial carcinoma. Urol Oncol 2020;38(11):850.e17–850.e26. [DOI] [PubMed] [Google Scholar]
- 23.Traxer O, Keller EX. Thulium fiber laser: The new player for kidney stone treatment? A comparison with holmium:YAG laser. World J Urol 2020;38(8):1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proietti S Rodríguez-Socarrás ME Eisner BH, et al. Thulium:YAG versus holmium:YAG laser effect on upper urinary tract soft tissue: Evidence from an ex vivo experimental study. J Endourol 2021;35(4):544–551. [DOI] [PubMed] [Google Scholar]
- 25.Tran H, Chung DE. Chapter 12—Thulium laser prostatectomy. In: Chughtai B, ed. A Comprehensive Guide to the Prostate. Cambridge, MA: Academic Press; 2018:81–97. [Google Scholar]
- 26.Musi G Mistretta FA Marenghi C, et al. Thulium laser treatment of upper urinary tract carcinoma: A multi-institutional analysis of surgical and oncological outcomes. J Endourol 2018;32(3):257–263. [DOI] [PubMed] [Google Scholar]
- 27.Blackmon RL, Irby PB, Fried NM. Comparison of holmium:YAG and thulium fiber laser lithotripsy: Ablation thresholds, ablation rates, and retropulsion effects. J Biomed Opt 2011;16(7):071403. [DOI] [PubMed] [Google Scholar]
- 28.Bozzini G Gastaldi C Besana U, et al. Thulium-laser retrograde intra renal ablation of upper urinary tract transitional cell carcinoma: An ESUT Study. Minerva Urol Nephrol 2021;73(1):114–121. [DOI] [PubMed] [Google Scholar]
- 29.Huusmann S, Lafos M, Meyenburg I, Muschter R, Teichmann HO, Herrmann T. Tissue effects of a newly developed diode pumped pulsed thulium:YAG laser compared to continuous wave thulium:YAG and pulsed holmium:YAG laser. World J Urol 2021;39(9):3503–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen J, Ji ZG, Li HZ. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: A retrospective single center study. BMC Cancer 2018;18(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Defidio L, Antonucci M, De Dominicis M, Fuchs G, Patel A. Thulium-holmium:YAG duo laser in conservative upper tract urothelial cancer treatment: 13 years experience from a tertiary national referral center. J Endourol 2019;33(11):902–908. [DOI] [PubMed] [Google Scholar]
- 32.Elhilali MM, Badaan S, Ibrahim A, Andonian S. Use of the Moses technology to improve holmium laser lithotripsy outcomes: A preclinical study. J Endourol 2017;31(6):598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim A, Badaan S, Elhilali MM, Andonian S. Moses technology in a stone simulator. Can Urol Assoc J 2018;12(4):127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmona O, Shvero A, Laufer M, Zilberman D, Winkler H, Kleinmann N. Treating UTUC with MOSES technology—Surgical and oncological outcomes. Euro Urol Open Sci 2020;19(Suppl 2):e330. [Google Scholar]
- 35.Weyand G, Theis CS, Fofana AN, Rüdiger F, Gehrke T. Laserhemorrhoidoplasty with 1470 nm diode laser in the treatment of second to fourth degree hemorrhoidal disease—A cohort study with 497 patients [in German]. Zentralbl Chir 2019;144(4):355–363. [DOI] [PubMed] [Google Scholar]
- 36.Vourliotakis G, Sahsamanis G, Evagelidis P, Aivatidi C. Endovascular laser treatment of incompetent saphenous veins using the 1470 nm diode laser and radial fiber. Ann Med Surg (Lond) 2018;25:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang F Xiao Z Chen R, et al. Transoral 980-nm/1470-nm dual-wavelength fiber laser microsurgery for early-stage glottic carcinoma. Oral Oncol 2019;96:66–70. [DOI] [PubMed] [Google Scholar]
- 38.Shoen E, Zollinger B, Gresham T, Rezaei KM, Whalen M. Use of the T-1470 LiteTouch™ laser in the en bloc resection of an upper tract urothelial cancer. Case Rep Urol 2021;2021:6623326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margolin EJ Matulay JT Li G, et al. Discordance between ureteroscopic biopsy and final pathology for upper tract urothelial carcinoma. J Urol 2018;199(6):1440–1445. [DOI] [PubMed] [Google Scholar]
- 40.Breda A Territo A Sanguedolce F, et al. Comparison of biopsy devices in upper tract urothelial carcinoma. World J Urol 2019;37(9):1899–1905. [DOI] [PubMed] [Google Scholar]
- 41.Kleinmann N, Healy KA, Hubosky SG, Margel D, Bibbo M, Bagley DH. Ureteroscopic biopsy of upper tract urothelial carcinoma: Comparison of basket and forceps. J Endourol 2013;27(12):1450–1454. [DOI] [PubMed] [Google Scholar]


