Abstract
Background
The management of suspected kidney stone disease in pregnancy is challenging. In cases of persistent flank pain and where investigations may have rendered equivocal results, ureteroscopy (URS) is a recognized diagnostic and therapeutic intervention. This study aimed to investigate the safety and outcomes associated with performing URS during pregnancy, as the technique has evolved over the past 4 decades at our center.
Materials and Methods
We performed a retrospective analysis of pregnant patients who underwent URS at our tertiary center between 1984 and 2022. Outcomes of interest included anesthetic approach, operative time, hospital stay, and complications.
Results
Eighty-seven pregnant patients underwent 96 URS procedures, and 60% (n = 57) of these procedures were performed during the third trimester. Overall, 58% (n = 56) of the procedures were achieved with local anesthesia and light sedation. During the most recent decade, the latter was successfully carried out in 97% of the procedures, with the remainder occurring under spinal anesthesia as per patient choice. Overall, 57% (n = 50) of the whole study group had ureteral calculi found at the time of surgery and in 88% (n = 44) of these cases, fragmentation/extraction was performed. The remainder had insertion of ureteral stent with definitive clearance deferred until postpartum. Mean operative time and postprocedure hospital stay was 33 minutes (range, 7–100 minutes) and 2.2 days (range, 0–16 days), respectively. The overall intraoperative and postoperative complication rates were 2% and 11%, respectively. During the final decade, the latter improved to 6% and all adverse events were minor (Clavien I/II), with the exception of a single case. Regarding exit strategy, ureteral stent was placed in 42% (n = 40) of the procedures, 23% (n = 22) had ureteral catheter inserted, and the remainder (35%, n = 34) had none.
Conclusions
Ureteroscopy can be safely performed during pregnancy using anesthetic approach with local anesthesia and light sedation. Development of a local protocol and multidisciplinary management algorithm are instrumental in enabling the delivery of such a service.
Keywords: Endourology, Lithotripsy, Pregnancy, Ureteroscopy, Urolithiasis
1. Introduction
Unilateral flank pain caused by suspected stone disease during pregnancy is a difficult clinical entity to manage in terms of both diagnosis and treatment.[1] However, initial misdiagnosis and delayed final diagnosis are not infrequent.[2] This is largely due to the restrictions that must be applied to the standard stone pathway to uphold important safety considerations for mother and fetus, such as avoidance of exposure to ionizing radiation.[3] While international guidelines have addressed this clinical problem, it remains a challenge for clinicians to adapt and apply these recommendations in the context of their local hospital setting.[4] To this end, practice patterns vary widely for this condition.[3] While a conservative management strategy is adopted wherever possible, intervention in the form of surgery can be required. Ureteroscopy (URS) is increasingly recognized to be a safe procedure for this special patient group, as long as it is delivered by a team with an appropriate level of expertise.[5–7] However, reports are lacking regarding how this intervention can be established within a formal management pathway where noncontrast computed tomography (NCCT) is not available as a standard diagnostic test.[8,9] Ureteroscopy during pregnancy was first performed at our center in the early 1980s. Since that time, our service has incorporated it as a diagnostic and therapeutic intervention where specific criteria are met, as well as a transition to performing it under local anesthesia (LA) and light sedation. This study aimed to share our experiences gained over 4 decades and to evaluate how URS can be implemented as part of a tailored and multidisciplinary management algorithm.
2. Materials and methods
Retrospective analysis was performed on data collected periodically since 1995 at our tertiary center in Western Norway. These have been compared with previously reported findings relating to the period between 1984 and 1994.[10] This allowed for evaluation of a continuous data set between January 1984 and June 2022 for pregnant patients who underwent URS at our center. Requirement for ethical approval was cleared by the regional ethics committee (REC-354396). All women undergoing URS for suspected kidney stone disease (KSD) during pregnancy were eligible for inclusion. Patients with complications, such as sepsis, which required emergency decompression were excluded (Fig. 1). Our hospital implements a multidisciplinary protocol, whereby pregnant patients admitted with flank pain due to suspected stone disease undergo 2 days of close clinical observation. The analgesic regimen during this period of assessment and initial management consists of regular paracetamol (1 g every 6 hours) and ketobemidone (2.5 mg every 4–6 hours) as required. Transabdominal ultrasound (US) is performed to identify upper tract dilatation, to determine the presence or absence of ureteric jet according to Doppler signals, and, if possible, to identify a stone. If a conservative strategy is appropriate, US is repeated every 24 hours to assess for change. In such cases where pain persists without signs of active infection and where US reveals KSD or equivocal results, URS is performed. However, it should be emphasized that it is not an absolute requirement for a stone to have been confirmed on US before performing URS. Therefore, the URS procedure serves as both diagnostic and therapeutic intervention, as laser lithotripsy can be subsequently performed to achieve stone clearance as required. Given the additional time and complication risk associated with performing laser lithotripsy for renal stones, patients found to have the latter at time of surgery undergo stent placement, and formal stone clearance is deferred until the postpartum period.
Figure 1.
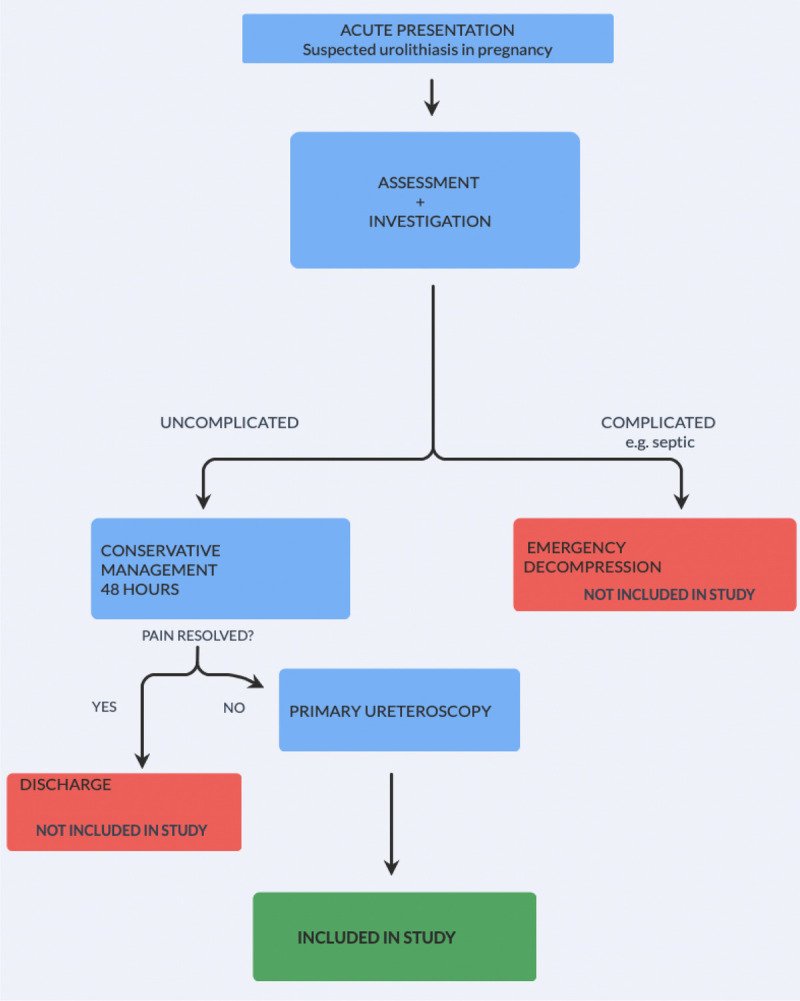
Flowchart for study inclusion.
2.1. Outcomes of interest
Primary outcome of interest was operative findings. Secondary outcomes of interest included anesthetic type and complications. Additional information was also collected on patient demographics, operative time, hospital stay, and exit strategy.
The results were divided into 3 periods based on equipment used during these periods for the cases: (1) 1984–1994: semirigid URS + grasping forceps, (2) 1995–2009: semirigid URS + grasping forceps/pneumatic lithoclast, and (3) 2010–2022: semirigid/flexible URS + grasping forceps/laser lithotripsy (Holmium/Thulium fiber laser).
2.2. Surgical technique
When performed under general anesthesia or regional anesthesia, URS is currently performed using a semirigid ureteroscope (8/9.8Ch; Richard Wolf Medical Instruments, Vernon Hills, IL) or a flexible ureteroscope (URF-V3 or P7; Olympus, Tokyo, Japan). This is carried out using a standardized technique described previously with the insertion of a JJ stent or ureteric catheter at the surgeon’s discretion.[4,10,11] The key difference in technique for this special patient group is the complete lack of fluoroscopy and avoidance of ureteral access sheath. Intraoperative transabdominal US is available to confirm correct placement of ureteral stent as required.
2.3. Modifications for URS under LA and light sedation
Local anesthesia includes 2% lidocaine gel (10 mL) inserted per urethra by the surgeon before insertion of the scope (no specific time interval is applied). The agent of choice at our center for light sedation during the operation is remifentanil. The latter is administered under careful control of the anesthetist. When URS is performed under LA and light sedation, preference is given to use of a smaller semirigid ureteroscope (4.5/6Ch; Richard Wolf Medical Instruments, Vernon Hills, IL). Irrigation devices, such as pressurized bags and handheld pumps, are avoided to minimize patient discomfort. The strategy for stone fragmentation is otherwise similar except that preference is given for dusting the stone to fine particles, which can pass spontaneously given that in our experience, additional instrumentation with basketing can trigger more patient discomfort. Patients are counselled regarding anesthetic choice and their choice is respected accordingly.
2.4. Statistics
Independent-sample t tests were performed to compare continuous data. Categorical variables were compared using χ2 tests. Statistical analysis was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY). A p value less than 0.05 determined statistical significance.
3. Results
Between 1984 and 2022, 87 pregnant patients underwent a total of 96 URS procedures (Table 1). A majority of the procedures (60%) were carried out during the third trimester, while 7% and 33% took place during the first and second trimesters, respectively. Overall, 58% (n = 56) procedures were performed under LA and sedation. In the first period, only one case was performed using LA/sedation, with the remainder undergoing regional anesthesia. There was a significant rise in the number of patients undergoing URS under LA from the first (4%) to the second period (64%) (p < 0.001), and again from the second (64%) to the third period (97%) (p < 0.05). Moreover, in this final period, no cases were performed under general anesthesia. Overall, 57% (n = 50) of the whole study group had calculi found at the time of surgery, and in 88% (n = 44) of these cases, fragmentation/extraction was performed, while the remainder had ureteral stent insertion with definitive clearance deferred until postpartum. Other endoscopic findings included distal ureteral edema with no calculi (15%), pelvic-ureteric junction obstruction (4%), blood clots in the renal pelvis (1%), and 21% had normal findings (Table 2). Mean operative time and postprocedure hospital stay were 33 minutes (range, 7–100 minutes) and 2.2 days (range, 0–16 days), respectively. Throughout the study period, 9 patients underwent 2 × URS procedures during their pregnancy. This was either due to bilateral stone disease (n = 3), repeat episode during a later pregnancy (n = 4), or repeat URS during the same pregnancy (n = 2). The latter were all associated with a nonendourologist having performed the surgery in the first sitting. Regarding exit strategy, ureteral stent was placed in 42% (n = 40) of the patients, while 23% (n = 22) had ureteral catheter inserted. The remainder (35%, n = 34) received neither. All ureteral catheters were removed within 48 hours after the surgery. The patients underwent postpartum imaging to confirm stone-free status. This is currently performed using NCCT, but before 2010, US was the standard imaging method. When using NCCT, the definition of zero residual fragments was applied and stone-free rate in a single session for ureteric stones was 100%.
Table 1.
Patient and operative information.
| Time period, yr | ||||
|---|---|---|---|---|
| Parameters | 1 (1984–1994) | 2 (1995–2009) | 3 (2010–2021) | Total |
| No. pregnant patients | 24 | 35 | 28 | 87 |
| No. URS procedures | 25 | 39 | 32 | 96 |
| Highest level of ureteroscope insertion | ||||
| Renal pelvis | 18 (72%) | 19 (49%) | 26 (81%) | 63 (66%) |
| Upper ureter | 6 (24%) | 14 (36%) | ‐ | 20 (21%) |
| Middle ureter | ‐ | 5 (13%) | ‐ | 5 (5%) |
| Lower ureter | 1 (4%) | 1 (2%) | 6 (19%) | 8 (8%) |
| Trimester (at time of URS) | ||||
| First | 3 (12%) | 2 (5%) | 2 (6%) | 7 (7%) |
| Second | 9 (36%) | 17 (43%) | 5 (16%) | 31 (33%) |
| Third | 13 (52%) | 20 (52%) | 25 (78%) | 58 (60%) |
| Anesthesia | ||||
| General | 0 | 4 (10%) | 0 | 4 (4%) |
| Regional | 24 (96%) | 10 (26%) | 1 (3%) | 36 (38%) |
| LA/sedation | 1 (4%) | 25 (64%) | 31 (97%) | 56 (58%) |
LA = local anesthesia; No. = number; URS = ureteroscopy.
Table 2.
Operative results.
| Time period, yr | ||||
|---|---|---|---|---|
| Parameters | 1 (1984–1994) | 2 (1995–2009) | 3 (2010–2021) | Total |
| Operating time, mean (range), min | 28 (10–65) | 33 (7–100) | 37 (13–80) | 33 |
| Post URS hospital stay, mean (range), d | 2.7 (0–16) | 2.3 (0–16) | 1.7 (0–6) | 2.2 |
| Main endoscopic findings* | ||||
| Ureteric/renal calculi | 12 (50%) | 24 (68%) | 14 (48%) | 50 (57%) |
| Distal ureteral edema, no calculi | 5 (21%) | ‐ | 8 (30%) | 13 (15%) |
| Pyonephrosis | ‐ | 1 (3%) | ‐ | 1 (1%) |
| PUJ obstruction | 2 (6%) | 1 (4%) | 3 (4%) | |
| Blood clots in renal pelvis | 1 (4%) | ‐ | ‐ | 1 (1%) |
| Megaloureter | ‐ | 1 (3%) | ‐ | 1 (1%) |
| Normal findings | 6 (25%) | 7 (20%) | 5 (18%) | 18 (21%) |
| Endoscopic stone treatment | ||||
| Stone disintegration and/or extraction | 12 (100%) | 21 (88%) | 10 (84%) | 44 (88%) |
| JJ stent inserted with stone in situ | ‐ | 2 (8%) | 2 (8%) | 4 (8%) |
| JJ stent inserted after stone distracted to renal pelvis | ‐ | 1 (4%) | 1 (7%) | 2 (4%) |
| Exit strategy | ||||
| JJ stent | 3 (12%) | 13 (33%) | 24 (75%) | 40 (42%) |
| Ureteral catheter | 4 (16%) | 15 (39%) | 3 (9%) | 22 (23%) |
| Nothing | 18 (72%) | 11 (28%) | 5 (16%) | 34 (35%) |
*At initial URS.
PUJ = pelvic ureteric junction; URS = ureteroscopy.
3.1. Intraoperative outcomes
While all cases performed with LA/sedation experienced no intraoperative complications, one case performed under regional anesthesia suffered total spinal anesthesia in the recovery room after the procedure had been completed (Table 3). This is a recognized, but extremely rare complication, which required emergency intubation until the block wore off and spontaneous ventilation resumed. Thereafter, the patient made a full recovery and had an uncomplicated pregnancy. Iatrogenic ureteral perforation (Grade 2) was the other intraoperative complication. This was identified on exiting the upper urinary system and was successfully managed with insertion of ureteral stent, which was kept until the postpartum period. The remaining period of this patient’s pregnancy was uneventful. The delivery was normal, and the child was healthy. Few cases that required a second URS procedure were associated with a nonendourologist performing the initial surgery, (p < 0.001).
Table 3.
Summary of complications.
| Time period, yr | ||||
|---|---|---|---|---|
| Parameters | 1 (1984–1994) | 2 (1995–2009) | 3 (2010–2021) | Total |
| Intraoperative complications | ||||
| Total spinal anesthesia requiring intubation | ‐ | ‐ | 1 (3%) | 1 (1%) |
| Ureteral perforation | 1 (4%) | ‐ | ‐ | 1 (1%) |
| Overall intraoperative complication rate | 4% | ‐ | 3% | 2 (2%) |
| Postoperative complications | ||||
| Post URS temperature >38.5°C | 3 (12%) | 3 (8%) | 1 (3%) | 7 (7%) |
| Pain (requiring stent insertion) | ‐ | ‐ | 1 (3%) | 1 (1%) |
| Pneumonia | ‐ | 1 (3%) | ‐ | 1 (1%) |
| Spinal headache | ‐ | 1 (3%) | ‐ | 1 (1%) |
| Overall postoperative complication rate | 12% | 13% | 6% | 11% |
URS = ureteroscopy.
3.2. Postoperative outcomes
The overall postoperative complication rate was 11%, and the most frequent adverse event was postoperative fever. Only one major complication was recorded. This was a patient who required stent insertion (under LA) 2 days postoperatively because of persistent flank pain. No maternal deaths were recorded. Over the past 4 decades, the postoperative complication rate improved from 12% in first period to 6% in the final period. No obstetric complications occurred at the time of surgery or during the early postoperative period over the whole study period.
3.3. Delivery outcomes
One case was lost to follow-up because the patient moved out of the area. Another patient elected for termination of pregnancy. Review of hospital care notes revealed that the decision for the latter appeared unrelated to their stone episode or URS procedure. Of the remaining patients, 85% and 15% underwent vaginal and caesarean delivery, respectively. In the former group, one patient had premature contractions and delivered 7 weeks prematurely. Another patient had vaginal delivery induced 1 week before the scheduled due date because of persistent stent related pain. None of the caesarean deliveries were performed because of reasons related to their previous URS procedure. Therefore, the rate of obstetric complications determined to be associated with KSD and intervention was less than 3%. Healthy baby status was confirmed in all but 2 of the cases. One case of cleft lip was recorded, but this occurred in a case during the third trimester and, therefore, long after the period of cleft formation during the first trimester. One case of cerebral palsy was documented as well. The URS procedures had been uncomplicated in this case, and postnatal assessment records had not determined URS to be a causative factor.
4. Discussion
Our results support the feasibility and safety of URS during pregnancy and its implementation in a tailored management algorithm, as both diagnostic and therapeutic intervention. Furthermore, anesthetic choice for URS in this special patient group can be LA and light sedation, if the treating center has appropriate experience and expertise. However, nearly all cases (97%) over the past decade at our institution have been performed this way. This was supported by the advent of next-generation ureteroscopes and newer laser technologies and accessories.[12,13] To this end, it has become our standard approach for URS during pregnancy. It is important to note that URS is only performed by designated endourologists, and their involvement is now sought for all cases. Our results showed an improvement in postoperative stay from 2.2 days in the first period to 1.7 days in the final period. However, when comparing changes in operative time for similar periods, the operative time did gradually increase. A likely explanation is the added time required for regular patient communication when performing URS with the LA and light sedation approach, as well as the heightened care required during instrumentation.
International guidelines now acknowledge low-dose computed tomography (CT) as a possible option for diagnostic imaging during pregnancy.[4] However, while CT does hold an obvious advantage of decreasing the rate of negative URS, it is still associated with an unknown and therefore unwanted radiation risk.[14] For this reason, it remains a policy in our hospital to not perform CT at all during pregnancy. Interestingly, despite the increased attention surrounding the role of CT, particularly in the United States, a recent study from a national database study in that same country revealed that of more than 14,000 hospital cases of pregnant women investigated for suspected symptomatic stone disease, no CT imaging was performed[15]; therefore, its current role in clinical practice remains unknown. Magnetic resonance imaging (MRI) is given as a second-line imaging option in guidelines, and it can serve to differentiate pathological from physiological dilatation of the ureter.[16] However, availability can be a challenge for clinicians, because expediting this modality in the acute setting can be difficult, especially out of normal working hours and even at a tertiary center.[4] Furthermore, as highlighted in a study by White et al.[14] (2013), the positive predictive value of MRI for detecting KSD in pregnancy (80%) is only marginally greater than for US (77%). As well as additional costs and prolonged examination time, MRI’s absolute safety during the first trimester when active organogenesis takes place is unknown.[17]
Ishii et al.[14] performed a systematic review of studies on pregnancy URS. From data of 271 procedures, intraoperative fluoroscopy was used in 24.5% of cases in contrast to none in our study. Only 5.1% had URS performed under LA or sedation. Diagnostic MRI was only used in 3.2% of cases. Mean stone-free rate was 84.65% (range, 72.7%–92.6%) and the overall complication rate was 16.1%. A multicenter study in United States recorded an obstetric complication rate of 4.3%. These were all cases of preterm labor, but no deaths were recorded.[5]
The rate of normal findings in this study was approximately 20%. In the other patients, a cause for the symptom profile could be identified, as well as a means for symptom resolution, such as stenting in the presence of pelvic-ureteric junction obstruction. In those with persistent pain who meet the threshold for URS, but are found to have normal findings, pathologies, such as stone disease, can be excluded. There was a rise in stent use for exit strategy in the final period. This reflects a shift toward inserting a stent and using, for example, a stent on string approach, which allows for removal after 24 hours and before discharge.
The results here represent how our center, a tertiary unit in Western Norway, has evolved in the management of this difficult condition. It is intended to add to the limited body of literature available and offer a safe and feasible way to manage this special patient group. Performing randomized trials is not feasible in this setting. These results and experiences can serve to complement other reports.
It is a limitation that this was a single-center study, as well as retrospective in nature. However, it offers another perspective on the role of URS in this special patient group and a relatively unique observation of practice over time. It is a limitation that there is the lack of quality-of-life assessment to compare between anesthetic type.[18] Linguistic validation of a Norwegian version of a patient-reported outcome measure for patients with KSD would be of great value in future evaluations.[19]
5. Conclusions
Ureteroscopy can be safely performed during pregnancy, including under LA and light sedation. It can serve as a therapeutic procedure but also a diagnostic intervention for pregnant patients with hydronephrosis and persistent flank pain, which is refractory to a conservative approach. Development of a multidisciplinary protocol in the hospital to triage these patients, as well as continuity of care with a named urologist, is a key component to delivery of this service.
Footnotes
How to cite this article: Juliebø-Jones P, Beisland C, Gjengstø P, Baug S, Ulvik Ø. Ureteroscopy during pregnancy: Outcomes and lessons learned over 4 decades at a tertiary center in norway. Curr Urol 2023;17(1):7–12. doi: 10.1097/CU9.0000000000000157
Contributor Information
Christian Beisland, Email: dr.beisland@gmail.com.
Peder Gjengstø, Email: peder.gjengsto@gmail.com.
Stephen Baug, Email: stephen.baug@gmail.com.
Øyvind Ulvik, Email: doc.ulvik@online.no.
Acknowledgment
The authors thank Dr Atle Ulvik for expertise regarding anesthesia and Dr Robert Geraghty for statistical advice.
Statement of ethics
Requirement for ethical approval was cleared by the regional ethics committee (REC-354396). The participants’ consent was exempted given it was registered as a clinical audit. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest statement
ØU has previously received honoraria for lectures on behalf of Olympus and Boston Scientific. The other authors declare that they have no conflicts of interest.
Funding source
None.
Author contributions
PJJ: Conceptualization, data curation, investigation, methodology, project administration, formal analysis, and writing;
CB: Project administration conceptualization, methodology, writing, supervision;
PG: Investigation, data curation, writing;
SB: Methodology, data curation, and writing;
ØU: Conceptualization, methodology, writing, supervision.
References
- 1.Pais VM, Jr., Payton AL, LaGrange CA. Urolithiasis in pregnancy. Urol Clin North Am 2007;34(1):43–52. [DOI] [PubMed] [Google Scholar]
- 2.Dai JC Nicholson TM Chang HC, et al. Nephrolithiasis in pregnancy: Treating for two. Urology 2021;151:44–53. [DOI] [PubMed] [Google Scholar]
- 3.Somani BK, Dellis A, Liatsikos E, Skolarikos A. Review on diagnosis and management of urolithiasis in pregnancy: An ESUT practical guide for urologists. World J Urol 2017;35(11):1637–1649. [DOI] [PubMed] [Google Scholar]
- 4.Türk C NeIsius A Petrik A, et al. EAU guidelines on urolithiasis. EAU Guidelines. Edn. presented at the EAU annual congress, Amsterdam, 2020. Available at: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urolithiasis-2020.pdf. Accessed January 6, 2022. [Google Scholar]
- 5.Keenan RA, Hegarty NJ, Davis NF. Symptomatic hydronephrosis and ureteral calculi in pregnancy: A narrative review with a proposed management protocol. J Endourol 2022;36(8):1099–1112. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EB, Pais VM, Jr. Obstetric complications of ureteroscopy during pregnancy. J Urol 2012;2012(12):11–12. [DOI] [PubMed] [Google Scholar]
- 7.Ishii H, Aboumarzouk OM, Somani BK. Current status of ureteroscopy for stone disease in pregnancy. Urolithiasis 2014;42(1):1–7. [DOI] [PubMed] [Google Scholar]
- 8.Rashid AO, Abdala RY. Safety and efficacy of flexible and semi-rigid ureteroscopy with laser lithotripsy for the management of ureteral calculi in pregnancy. Afr J Urol 2021;27:46. [Google Scholar]
- 9.Morgan K Rees CD Shahait M, et al. Urolithiasis in pregnancy: Advances in imaging modalities and evaluation of current trends in endourological approaches. Actas Urol Esp (Engl Ed) 2022;46(5):259–267. [DOI] [PubMed] [Google Scholar]
- 10.Ulvik NM, Bakke A, Høisaeter PA. Ureteroscopy in pregnancy. J Urol 1995;154(5):1660–1663. [PubMed] [Google Scholar]
- 11.Ulvik Ø, Harneshaug JR, Gjengstø P. Ureteral strictures following ureteroscopic stone treatment. J Endourol 2021;35(7):985–990. [DOI] [PubMed] [Google Scholar]
- 12.Keller EX, De Coninck V, Traxer O. Next-generation fiberoptic and digital ureteroscopes. Urol Clin North Am 2019;46(2):147–163. [DOI] [PubMed] [Google Scholar]
- 13.Keller EX Kronenberg P Tailly T, et al. Laser accessories: Surgical fibers, strippers, cleavers, and protective glasses. Curr Opin Urol 2022;32(3):330–338. [DOI] [PubMed] [Google Scholar]
- 14.White WM Johnson EB Zite NB, et al. Predictive value of current imaging modalities for the detection of urolithiasis during pregnancy: A multicenter, longitudinal study. J Urol 2013;189(3):931–934. [DOI] [PubMed] [Google Scholar]
- 15.Spradling K Sohlberg EM Li S, et al. Urinary stone disease in pregnancy: Current management practices in a large national cohort. Urology 2020;142:60–64. [DOI] [PubMed] [Google Scholar]
- 16.Kanal E Barkovich AJ, et al. Expert Panel on MR Safety . ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging 2013;37(3):501–530. [DOI] [PubMed] [Google Scholar]
- 17.Masselli G Derme M Laghi F, et al. Imaging of stone disease in pregnancy. Abdom Imaging 2013;38(6):1409–1414. [DOI] [PubMed] [Google Scholar]
- 18.Mehmi A, Jones P, Somani BK. Current status and role of patient-reported outcome measures (PROMs) in endourology. Urology 2021;148:26–31. [DOI] [PubMed] [Google Scholar]
- 19.Bhojani N Moussaoui G Nguyen DD, et al. Validation of the French version of the Wisconsin Quality of Life (WISQOL) questionnaire for patients with nephrolithiasis. Can Urol Assoc J 2021;15(4):E227–E231. [DOI] [PMC free article] [PubMed] [Google Scholar]


