Abstract
Malignant tumors are one of the leading causes of death which impose an increasingly heavy burden on all countries. Therefore, the establishment of research models that closely resemble original tumor characteristics is crucial to further understanding the mechanisms of malignant tumor development, developing safer and more effective drugs, and formulating personalized treatment plans. Recently, organoids have been widely used in tumor research owing to their advantages including preserving the structure, heterogeneity, and cellular functions of the original tumor, together with the ease of manipulation. This review describes the history and characteristics of tumor organoids and the synergistic combination of three‐dimensional (3D) culture approaches for tumor organoids with emerging technologies, including tissue‐engineered cell scaffolds, microfluidic devices, 3D bioprinting, rotating wall vessels, and clustered regularly interspaced short palindromic repeats‐CRISPR‐associated protein 9 (CRISPR‐Cas9). Additionally, the progress in research and the applications in basic and clinical research of tumor organoid models are summarized. This includes studies of the mechanism of tumor development, drug development and screening, precision medicine, immunotherapy, and simulation of the tumor microenvironment. Finally, the existing shortcomings of tumor organoids and possible future directions are discussed.
Keywords: CRISPR‐Cas9, drug screening, precision medicine, three‐dimensional culture, tumor organoid
1. INTRODUCTION
Malignant tumors are one of the leading causes of death which impose a significant burden on individual countries worldwide. This burden will gradually increase over time, with the global cancer burden predicted to reach 28.4 million cases by 2040. 1 Despite some achievements in the diagnosis and treatment of malignant tumors, several challenges remain. 2 Little is known about the mechanisms involved in tumor development, progression, and the development of drug resistance. Different individuals with the same tumor may respond differently to treatment because of the complexity of tumors. Therefore, establishing models that closely resemble in vivo tumors will improve our understanding of malignant tumors and help formulate more effective treatment plans.
Currently, preclinical models applied in tumor research mainly include two‐dimensional (2D) cultured cell lines, patient‐derived tumor xenografts (PDXs), and patient‐derived tumor organoids (PDOs). 3 2D cultured cell lines have been used for the longest period of time compared to the other two models, and the methods for establishing this model simpler and easier to use for tumor research and drug screening. However, the composition of tumors is more complex, and cell line cultures are usually derived from a single cell that does not mimic the real state of tumor cells in vivo and their interaction with the in vivo environment, which may lead to a misestimation of the model's response to therapy in vivo. 4 , 5 The patient‐derived tumor xenograft is a preclinical model wherein patient‐derived tissue is transplanted into immunodeficient mice; this preserves the heterogeneity of the tumor and tumor–stroma interactions 6 , 7 where orthotopic transplantation is closer to the real environment than subcutaneous transplantation. 8 However, the tumor microenvironment (TME) transplanted into mice is gradually being replaced by murine‐derived cells. 9 Tumor cells exhibit different evolutionary trajectories in patients and mouse models 10 and the lack of a normal immune microenvironment makes PDXs‐based results potentially deviant from the real situation. 11 , 12 Moreover, drawbacks such as the complexity of the operation, low success rate, and unsuitability for gene editing limit the application of PDXs. Patient‐derived tumor organoids are three‐dimensional (3D) cultures of tumor tissue from patients that can preserve the histological and genetic characteristics and heterogeneity of the original tumor under in vitro culture conditions. 13 This method overcomes some of the drawbacks of 2D cultured cell lines and PDXs and has great potential for high‐throughput drug screening and personalized medicine. 14
In this review, we introduce the history and characteristics of tumor organoids, and analyze the synergistic combination of 3D culture methods for tumor organoids with emerging technologies, including tissue engineering cell scaffolds, microfluidic devices, 3D bioprinting, rotating wall vessels (RWVs), and clustered regularly interspaced short palindromic repeats‐CRISPR‐associated protein 9 (CRISPR‐Cas9). In addition, the research progress of tumor organoid models and their applications in basic and clinical research are discussed, including studies involving the mechanisms of tumorigenesis and tumor development, drug development and screening, precision medicine, immunotherapy, and simulation of the TME. Finally, the existing shortcomings of tumor organoids and scope for the future are discussed.
2. HISTORY OF TUMOR ORGANOIDS
Currently, the establishment of organoids is based on the ability of cells to self‐organize and differentiate, and form in vitro 3D culture. In 1907, Henry Van Peters Wilson demonstrated that isolated sponge cells can form regenerative tissues through self‐organization. 15 Subsequently, studies on the in vitro culture of animal organs slowly developed in the laboratory. 16 , 17 In 1981, pluripotent stem cells (PSC) were isolated from early mouse embryos cultured in a medium conditioned by teratocarcinoma stem cells. 18 Eighteen years later, ESCs were first extracted from human blastocysts. 19 The development of organoid research is increasingly flourishing as scientists continue to understand and study stem cells and reduce the use of animal models. In 1987, Li et al. 20 found that mouse mammary epithelial cells cultured on recombinant basement membranes from Engelbreth–Holm–Swarm tumors (EHS) form ducts and lumens. They exhibit a structure different from previous in vitro cell cultures and provide an idea for the development of 3D cell culture. In 2008, Eiraku et al. induced the differentiation of ESCs into self‐organized, functionally polarized cortical tissues using 3D aggregation culture. 21 This led to a shift in the study of organoids from 2D to 3D formats. Subsequently, Sato et al. successfully established 3D mouse crypt fossa structures in a new matrix gel culture system and maintained them for 8 months. 22 This was a landmark discovery in the history of organoid development in 2009, and almost all other tissue‐derived organoids are established based on this study. Since then, research on organoids has rapidly developed. To date, successfully established human organoids include the colon, 23 , 24 stomach, 25 , 26 liver, 27 , 28 , 29 pancreas, 29 , 30 lung, 31 , 32 , 33 and kidney. 34 , 35 The establishment of organoids can reduce the number of experimental animals to some extent. Also, they can reduce some of the experimental errors caused by species differences as they are based on human genetic backgrounds. Meanwhile, the establishment of PDOs has been increasingly improving owing to the demand for tumor research, including colon, 23 , 36 , 37 gastric, 38 prostate, 39 , 40 pancreatic, 41 , 42 breast, 43 , 44 bladder, 45 and esophageal cancers 46 (Figure 1). Currently, PDOs are also widely used because of their similar histological, genetic, and cellular behavioral characteristics as those of parental tumors.
FIGURE 1.

The timeline of tumor organoids development. Different organoid protocols for human tumors have been reported for the first time.
3. METHODS FOR CULTURING TUMOR ORGANOIDS
3.1. Traditional culture methods
The culture protocol for PDOs usually includes an ECM substitute (Matrigel or basement membrane extract), growth factors, molecule inhibitors, and a culture medium. 3 In general, the culture of tumor organoids follows the following procedure. First, the obtained tumor tissue is processed into small pieces or cells by mechanical methods and enzymatic digestion, then inoculated into the ECM substitute and cultured in a tissue‐specific medium. 14 Growth factors and ECM substitutes can be involved in the regulation of cell signaling which affects cell behavior. 47 , 48 For example, primary breast cancer epithelial cells of the same origin do not behave similarly when cultured in normal and abnormal ECM‐mimicking environments. In the normal ECM‐simulated environment, the tumor cells showed inert growth or aggregated growth. However, in the abnormal ECM‐simulated environment, the tumor cells showed protrusive migration and dissemination. In addition, removing the Wingless‐related integration site (Wnt) during organoid culture of colon cancer also achieved selective culture of cancer cells. 36 A mixture of different tumor tissue‐specific growth factors can mimic paracrine signaling. 14 Therefore, there is a slight variation in the composition of the medium and growth factors used for the organoid depending on the cell source and purpose of the study. Sometimes, a certain class of the component is necessary for one PDOs but not for another. For example, testosterone is required in prostate tumor organoids, but not in other tumor organoids. 49 The culture route is also important for the generation of tumor organoids. Commonly used methods include submerged culture for organoids of gastrointestinal (GI) origin and air–liquid interface for organoids of respiratory and renal origin. However, larger organoids cannot be cultured using these two methods owing to the limitation of nutrient accessibility. 50
Owing to a lack of vasculature, general organoids need to control their size during culturing. When organoids are large, cells near the center have difficulty exchanging oxygen and nutrients with the outer environment. Thus, the larger the structure size, the more number of cells die. Therefore, for organoids of normal cell or tissue origin, the maximum size of their culture does not exceed 4mm. 51 For PDO, the size of organoids usually needs to be controlled below 100 μm in order to reduce central necrosis and meet the requirements of high throughput. 52
The traditional culture protocol plays an extremely important role in the establishment and application of tumor organoids; however, this protocol has some drawbacks. For example, the commonly used ECM substitute (Matrigel) is derived from animals, but its composition is unclear, and its effect on the organoid grown in it is unknown. 53 Second, samples are mainly derived from a limited sample source: surgically resected/biopsied tissues of patients. Furthermore, most tumor organoids only contain tumor cells and lack TME components such as stromal cells. 54 Therefore, researchers have explored ways to improve existing ECM alternatives and to find new and more suitable ECM alternatives.
3.2. Emerging technologies
3.2.1. Tissue‐engineered cell scaffolds
Matrigel is an indispensable component of many PDOs culture protocols. It is derived from Engelbreth–Holm–Swarm mouse sarcoma and its composition is essentially identical to that of the tumor ECM, 55 including laminin (major component), collagen IV, heparin sulfate proteoglycan perlecan, entactin, and various growth factors. 56 Considering its origin, Matrigel may increase the risk of animal‐derived pathogenic microbial infections and inter‐ and intra‐lot variability in organoids. This can lead to uncertain and unreproducible experimental results. 57 , 58 , 59 These limitations have prompted researchers to search for and develop suitable ECM alternatives (Table 1).
TABLE 1.
Different types of materials used in organoid culture.
| Biomaterial | Material type | Features | Limitations | Reference |
|---|---|---|---|---|
| Matrigel | Natural |
Cheap Easy access Wide application |
Risk of animal‐derived pathogenic microbial infections Inter‐ and intra‐lot variability Poor control of mechanical properties |
59 |
| Natural biological macromolecules (e.g., collagen) | Natural |
Good histocompatibility Easy access Biodegradability |
Poor mechanical performance Biological and physical properties cannot be controlled independently Inter‐ and intra‐lot variability |
70, 71 |
| Synthetic polymer | Synthetic |
Controllable mechanical properties Repeatability Adjustable degradation rate |
Cell adherence only after modification Potential cytotoxicity Low similarity to natural tissue ECM |
71, 75 |
| Acellular ECM | Natural | Retains natural chemical and mechanical properties |
Acquisition difficulty Unclear composition |
80 |
| Hybrid hydrogels (combination of two or more materials) | Synthetic |
Suitable mechanical properties Low immunogenicity in vivo ECM‐like biology |
Research still in infancy | 236 |
Abbreviation: ECM, extracellular matrix.
In recent years, tissue‐engineered cell scaffolds provide support for cell growth and attachment and mimic the function of ECM in vivo with similar or superior results to Matrigel. 60 Hydrogels are highly promising 3D scaffolds for tissue engineering applications because of their high‐water content, porous structure, and uniform cell‐loading capacity. 61 Hydrogel scaffolds are composed of natural materials, synthetic hydrophilic polymers, or a mixture of both, with a water content of up to 95%, 62 and good histocompatibility and permeability. 63 , 64 , 65 Scaffolds with different compositions, molecular weights, and fabrication methods behave differently in terms of biochemical and biophysical aspects such as porosity, solubility, and compliance. 60 , 66 , 67 This can have an impact on cell phenotype and behavior; therefore, it is essential to select a suitable ECM substitute according to the experimental specifications. Scaffolds of natural origin can be composed of one or more components including proteins (such as collagen) and polysaccharides (alginate or alginate–chitosan mixtures, hyaluronic acid, and mixtures of hyaluronic acid and chitosan). 68 , 69 Enzymatically crosslinked hydrogels are comparable to Matrigel for colorectal tumor organoid culture; this preserves the sensitivity of colorectal tumors to various therapeutic agents. 70 Collagen or hyaluronic acid are natural components of ECM so they have good histocompatibility; however, their application is limited since they have poor mechanical properties, and their biological and physical properties cannot be independently controlled. 71 In addition, the impact of collagen on tumor cells varies depending on its cellular origin, resulting in distinct biological manifestations of the corresponding PDO. For instance, pancreatic cancer cells specifically produce type I collagen (Col1) homotrimer (α1/α1/α1). This homotrimer promotes pancreatic cancer cell proliferation and tumor progression, while exhibiting tumor immunity and microbiome oncogenicity in mice. Compared to pancreatic cancer PDO without Col1 knockout, pancreatic cancer PDO with Col1 knockout had more retarded growth and development. In contrast, the normal Col1 heterotrimer (α1/α2/α1) produced by fibroblasts in pancreatic ductal adenocarcinoma (PDAC) exhibited immunosuppression and promotion of tumor progression after knockdown. 72
Materials for synthetic scaffolds mainly include metals, ceramics, and organic or inorganic polymers such as polycaprolactone (PCL), polyacrylamide (PAM), and polyethylene glycol (PEG). 73 The cell adhesion of the resulting scaffold made from polymer alone is poor; therefore, additional modifications are required to enable cell adhesion. 74 Culturing of cancer‐associated fibroblasts (CAFs) on a PCL scaffold allows the deposition of CAF‐derived ECM on the scaffold; this promotes cell adhesion and growth through integrin‐mediated cell attachment. 75 The mechanical environment and ECM components required at different stages of organoid generation vary. The ability to control the physicochemical properties of synthetic scaffolds extends their applicability to basic and clinical research. 76
Recently, Below et al. 77 described a well‐defined, fully synthetic hydrogel scaffold based on 8‐arm PEG and successfully established pancreatic cancer organoids to achieve organoid growth and polarization. Furthermore, pancreatic cancer organoid growth was modulated by altering the hydrogel properties. Furthermore, when co‐cultured with the stroma, organoid can bind to the stromal cells in the hydrogel and exhibit signaling, phenotypic, and tumor behavior consistent with the in vivo model. Synthetic scaffolds have a well‐defined and controlled composition with insignificant batch‐to‐batch variation, and their use in combination with well‐defined media can largely avoid the possibility of unknown proteins and genes affecting cell culture. However, the biocompatibility and cytotoxicity of synthetic scaffolds must also be considered. 78 , 79 Preparation of a GI tissue‐derived ECM hydrogel using an optimized decellularization scheme is rich in GI tissue‐specific core matrisomes and nonmatricellular proteins; this is an effective alternative to Matrigel. 80 This allows for better support of the growth and development of gastrointestinal tract organoids and allows long‐term subculture and passaging of gastrointestinal tract organoids to generate more organoids. In addition to gastrointestinal sources, ECM hydrogel has now expanded to animal sources such as heart, bladder, kidney, lung, fat, tendon, and skeletal muscle, with the most prominent animal donor being the pig. 81 However, the ECM in this hydrogel is derived from animals; therefore, it has some similar limitations to that of the Matrigel. Unlike animal‐derived ECM hydrogel, human‐derived ECM hydrogel is well protected from differences in ECM composition, xenogeneic reactions, and transmission of animal‐derived pathogens. 82 Human‐derived ECM hydrogel can be derived from a variety of tissues/organ, such as skin, cartilage, tendon, adipose tissue, heart, lung, gastrointestinal tract, liver, pancreas‐kidney, gonads, uterus, umbilical cord, cornea, and peripheral nerves. 83 Both animal‐derived and human‐derived ECM hydrogel have been commercialized for clinical applications, such as In Matrico® (porcine or human‐derived), GraftJacket® (human dermis), and Prima™ Plus (porcine heart valves).
3.2.2. Microfluidic cell culture platforms and organ‐on‐a‐chip
Microfluidic devices are mainly used to simulate physiological and pathological parameters in a controlled 2D cell culture environment for drug screening. 84 In recent years, progress has been made in the application of microfluidics in 3D cell culture. Schuster et al. 85 described a microfluidic organoid culture system that is compatible with gel systems and allows automation and high throughput (Figure 2a). This culture system allows real‐time and reproducible analysis of organoids, and permits different combinations and temporal sequences to test the effects of drug treatments. Currently, microfluidic‐based organoid culture platforms (also referred to as organ‐on‐a‐chip or organoid‐on‐a‐chip) are reported for lung cancer, 86 gastrointestinal tumors, 87 and pancreatic cancer. 85 , 88 Microfluidic techniques can mimic the TME for tumor organoids, including blood supply, 89 interaction with the immune system, 90 and stromal cells. 91 Shirure et al. 89 established a microfluidic platform that mimics biological mass transport near the end of capillary arteries in a TME. This platform allows dynamic observation of the hallmark features of tumor progression (including cell proliferation, angiogenesis, cell migration, and intra‐tumor cell invasion) and induces sprouting angiogenesis. Microfluidics are more representative of the real in vivo tumor environment compared with tumor organoids alone, compensating for the lack of TME in tumor organoids, and are promising models for preclinical studies. 89 , 92 , 93 However, organ‐on‐a‐chip models are currently unsuitable for widespread use because most are disposable devices and consume considerable time, money, and labor. 94
FIGURE 2.

Tumor organoid culture based on microfluidic devices and tumor organoids based on 3D bioprinting. (a) Automated microfluidic 3D cellular and organoid culture platform for dynamical drug perturbations. (i) A programmable membrane valve‐based microfluidic chip. (ii) 3D culture chamber platform(scale bar 100 μm). (iii) Cross‐section of a pdms‐based bilayer multi‐chamber 3D culture chamber device(scale bar 100 μm). (iv) Automated and dynamic drug screens. (v) Continuous observation of organoid or 3D cellular structures by time‐lapse imaging. (vi) Quantification of organoid or 3D cellular structures. (Reproduced with permission from Reference 85. Copyright 2020, Springer Nature). (b) Schematic diagram of 3D bioprinting approaches. (Reproduced with permission from Reference 100. Copyright 2016, IOP Publishing). (c) Inkjet printing to quantify tumor heterogeneity. (Reproduced with permission from Reference 120. Copyright 2020, IOP Publishing).
Microfluidics can be used to study ECM surrogates. The dependence of breast cancer organoids on Matrigels has limited their application as preclinical models to some extent. Meanwhile, breast cancer organoids cultured in alginate microbeads using microfluidic droplet technology have similar structural and phenotypic properties to parental tumors while maintaining drug sensitivity. 95 This suggests that alginate microbeads are potentially suitable for breast cancer organoid culture.
In conclusion, the combination of microfluidics and organoid culture helps to achieve a high‐throughput generation of organoids and improves the applicability of organoids in drug screening and personalized therapeutic applications. However, many of the combined applications of microfluidics and organoids reported in the literature have complicated processes, high costs, and the inability to accommodate large‐sized organoids. In addition, the following limitations exist in achieving high‐throughput and automated screening of PDO: (1) as the size of the assay is scaled up, the volume and number of cells of the required organoids change, for example, during the conversion from screening using 384‐well plates to screening using 1536‐well plates, the size of the organoids is not simply reduced, but further testing and optimization have to be performed before screening can be performed 96 ; (2) high‐throughput screening saves material to some extent for organoid culture, but with it comes an increase in dead volume, which requires the purchase of additional equipment to address 97 ; (3) as the number of wells per plate increases, the smaller the volume available, the impact that edge effects can have during culture can be significant. 98
3.2.3. 3D bioprinting
3D bioprinting has rapidly developed in the last decade, with notable applications in regenerative medicine and tissue engineering. 99 Bioprinting is the use of cells and/or other biocompatible materials such as bioinks for printing entities with 3D structures, including organs or tissues. The structure is formed layer by layer with precise control of the spatial and temporal distribution of cells and ECM at micron‐level resolution (Figure 2b). 100 , 101 There are three main bioprinting techniques available: droplet bioprinting, 102 extrusion bioprinting, 103 and laser bioprinting. 104 Moreover, new bioprinting techniques include freeform reversible embedding of suspended hydrogels (FRESH), 105 , 106 acoustic, 107 stereolithography, 108 and magnetic. 109 Droplet bioprinting was one of the first bioprinting methods used 110 and involves a layer‐by‐layer printing of cells and inactive materials on a substrate via droplet encapsulation. 111 Droplet bioprinting has the advantages of low cost, high speed (10 kHz), and high cell survival after printing; however, the inability to use high‐viscosity materials leads to poor mechanical properties of the printed structures. 100 , 112 Unlike droplet bioprinting, extrusion bioprinting can print structures with high cell density using high‐viscosity materials and maintains a faster speed and fair cell viability; however, it has low spatial resolution. 113 Laser bioprinting is based on laser‐induced forward transfer and its post‐printing cell viability is higher than that of droplet bioprinting. Laser bioprinting allows for single‐cell control and does not suffer from clogging and high temperatures which are disadvantages of droplet and extrusion bioprinting; however, the high cost prevents the printing of large entities. 100 , 111 3D bioprinting of skin, 114 heart, 115 bone, 116 liver, 117 and brain‐like tissue 118 is reported, but complete organs that can be used for transplantation are not possible so far.
The combination of 3D bioprinting and tumor organoids is primarily used to develop high‐throughput PDOs models suitable for drug screening (Figure 2c). 119 , 120 Reid et al. 121 described a method to place stem cells directly into a hydrogel using a low‐cost 3D printer that retains function and viability of stem cells for 7 days. The authors used the method to print breast cancer cells into decellularized ECM hydrogels derived from rat and human breast tissues and build larger volumes of tumor organoids. 122 Maloney et al. 123 used an immersion printing technique to print glioblastoma (GBM) in 96‐well plates. Hou et al. 124 used magnetic bioprinting to consistently produce pancreatic cancer organoids in standard flat‐bottomed 384‐well and 1536‐well plates which may be very useful for scaling up PDOs production and improving the efficiency of drug screening. In addition, their bioprinter significantly increases the formation of breast cancer organoids in 3D collagen gels and precisely generates tumoroid arrays. They further demonstrated the ability of 3D bioprinting to study breast cancer tumorigenesis and microenvironmental control by co‐printing breast cancer cells with normal breast epithelial cells. 125 In addition, 3D printing can precisely control the shape and size of tumor organoids by controlling the number of cells and their coordinates. Reid et al. 126 demonstrated that only 10 cells could be used to form 3D structures in a single print. Over time, these structures can fuse with each other to form larger individual organoids, even though the spacing between prints can be as much as 500 μm. Both linear and nonlinear structures can generate dimensions up to 3 mm in length/diameter. These findings suggest that the synergistic application of bioprinting with organoids has great potential in preclinical model development and optimization.
3.2.4. RWV vessel bioreactors
RWV bioreactors are suspension culture systems that were originally developed by NASA for microgravity and Earth‐based cell science experiments. 127 It simulates microgravity by rotating to create a microgravity, low‐turbulence, low‐shear culture environment for cells, in which cells can grow, self‐aggregate, or aggregate into 3D structures around microcarriers or scaffolds. 128 RWV bioreactors may improve cell distribution and differentiation, together with nutrient and metabolite transport in cell‐inoculated scaffolds. 129 , 130 Moreover, the adverse effects of shear on cells are reduced, including induction of caspase‐mediated apoptosis, 131 damage to fragile organoid substructures, 132 and perturbed cellular metabolism. 133 , 134 RWV is commonly used in bone tissue engineering with good effects on in vivo bone repair in animal studies where the derived tissue constructs resemble natural bone. 135
In recent years, successful organoids based on RWV bioreactors were developed for the bladder, 136 vaginal epithelial tissue, 137 glioblastoma, 138 , 139 hepatocellular carcinoma, 140 and prostate cancer. 141 Cells cultured in RWV bioreactors resemble their in vivo parental tissues in many respects, including 3D spatial organization and polarity, cell differentiation, function, and response to external stimuli. 142 , 143 Skardal et al. 144 inoculated colon cancer cells (HCT116) in RWV bioreactors cultured with the hepatocyte cell line (Hep‐G2)‐derived liver organoid, wherein tumor cells express mesenchymal and metastatic markers in vivo and exhibit a phenotype similar to colon cancer metastasis in vivo. This tumor organoid is a more suitable model for metastatic tumors than 2D cell lines. Shortly thereafter, the same research team cultured host‐liver colorectal tumor organoids composed of primary human hepatocytes, mesenchymal stem cells (MSC), and colon cancer HCT116 cells in RWV bioreactors to make the tumor organoid model more consistent with in vivo characteristics. The RWV‐based tumor organoids have formed a good physiological correlation with tumor cells in vivo, which well generalizes the responses of tumor and stromal cells to chemotherapeutic agents. Furthermore, the generation of a large number of organoids in a single batch is achievable (Figure 3a). 140 Overall, the RWV bioreactor operating procedure is relatively simple, flexible, and suitable for different complex models of cell co‐culture and high‐throughput models for drug screening (Figure 3b). 142 , 145 However, bubbles sometimes form because of the gas exchange system in RWV bioreactors which leads to increased turbulence and fluid shear stress; this may adversely affect cell aggregation, viability, and differentiation which impairs organoid formation. 146
FIGURE 3.

Pattern diagram and structure of cultured tumor organoids in RWV. (a) Operational principles of rotating wall vessel (RWV) technology. (Reproduced with permission from Reference 140. Copyright 2017, IOP Publishing). (b) Colon carcinoma cell growth inside liver tumor organoids. (i) Schematic drawing of liver tumor organoid formation in the RWV bioreactor. (ii) Comparison of organoid cultures with and without MSCs. (iii) Bright light microscopy of liver tumor organoids. (iv) Fluorescence and bright light superimposed image of progressive growth of HCT116 cells within liver tumor organoids. (Reproduced with permission from Reference 142. Copyright 2010, Springer Nature).
3.2.5. Clustered regularly interspaced short palindromic repeats‐Cas9
Clustered regularly interspaced short palindromic repeats (CRISPR)‐Cas9 is a genome‐editing tool that targets and modifies specific DNA sequences in the genome. 147 , 148 The mechanism by which CRISPR‐Cas9‐mediated genome editing achieves activation/silencing of target sequences of target genes consists of the following three steps: (1) guide RNA targeting to recognize sequences of target genes, (2) Cas9 nuclease upstream of target sequences to break the double strand of the target gene, and (3) ligation or repair of the double‐strand break. 149 CRISPR‐Cas9 has been rapidly developing since its first application in RNA‐guided DNA cleavage in mammalian cells in 2013. Currently, CRISPR‐Cas9 is widely used to discover new targets for cancer therapy 150 , 151 and to identify genes that synergize with‐ or confer drug resistance. 152 , 153 Advances and developments in CRISPR‐Cas9 have helped reduce the difficulty of gene editing 154 which has benefited organoid models (Figure 4c). 155 It has enabled scientists to obtain tumor organoids from normal tissues, which distinguishes them from previous tumor organoids derived from the patient's tumor tissues. For example, the introduction of multiple mutations from human colorectal tumors into normal human intestinal epithelial organoids by CRISPR‐Cas9 showed that the isogenic organoids carrying these mutations exhibit tumorigenicity in mice. 156 Similarly, Dekkers et al. 157 obtained specific subtypes of breast cancer organoids after the targeted knockdown of four breast cancer‐related suppressor genes (P53, PTEN, RB1, and NF1) in normal breast cancer cells using CRISPR‐Cas9 (Figure 4a,b). Meanwhile, tumor organoids established by CRISPR‐Cas9 help determine whether genes are involved in tumorigenesis and their role in tumor development which advances the exploration and study of the mechanisms of carcinogenesis. For example, RBMS3 silencing in benign lung tumor organoids using CRISPR/Cas9 gene editing promotes the growth and progression of malignant lung cancer. Vaishnavi et al. 158 further analysis shows the role of RBMS3 as a suppressor of lung cancer and the possibility that RBMS3 silencing could lead to malignant progression. Notably, the newly developed CRISPR‐Cas9‐mediated homology‐independent organoid transgenesis (CRISPR‐HOT) system exhibits rapid and efficient generation of genetically engineered human liver duct organoids and human fetal hepatocyte organoids which have promising applications in cancer modeling and gene function studies. 28 However, the current CRISPR‐Cas9 technology is imperfect. Guide RNAs show poor function in organoids compared to cell lines, possibly because the existing methods do not accurately predict active gRNAs. 159 In addition, CRISPR‐Cas9‐mediated genome editing has the following limitations that need to be addressed: off‐target effects, original spacer adjacent motif requirement, DNA‐damage toxicity, and immunotoxicity.
FIGURE 4.

The application of CRISPR‐Cas9 in tumor organoid culture and organoids in the study of cancer invasion and progression. (a) Process of cancer modeling of human breast organoids. (b) Images of gene‐edited human breast organoids. (i) Whole‐mount three‐dimensional confocal image (left) and optical sections (right) of organoids(scale bar 200 μm; scale bar 30 μm). (ii) Representative brightfield images of control organoids or CRISPR‐Cas9‐edited organoids(scale bar 200 μm). (Reproduced with permission from Reference 157. Copyright 2020, Oxford University Press). (c) The mechanism of CRISPR/Cas9. (Reproduced with permission from Reference 155. Copyright 2018, Elsevier Ltd). (d) Invasive behavior of engineered colon cancer organoids after orthotopic transplantation. (i) Flow chart of orthotopic transplantation model and in vivo imaging. (ii) In vivo imaging shows high migratory behavior of tumor cells in colon cancer organoids(scale bar 100 μm). (Reproduced with permission from Reference 176. Copyright 2017, PNAS).
3.2.6. Air–liquid interphase
Traditional organoid culture methods cannot meet the requirements of in vitro simulation of TME, so researchers developed air–liquid interphase (ALI), a co‐culture of tumor tissue/cells with TME component cells. ALI is primarily achieved by placing tumor cells or chopped tumor tissue, endogenous homologous tumor‐infiltrating lymphocytes (TILs), and pre‐cured collagen gels containing Transwell into culture wells spiked with medium, exposing the organoid to air on one side and liquid medium on the other, a structure that facilitates increased oxygen concentration in the culture system. 160 ALI PDO encapsulates the T‐cell receptor spectrum of primordial origin and can functionally mimic PD‐1‐dependent immune checkpoint blockade, causing TILs activation and expansion leading to further cytotoxic responses, and in which immune cells can be retained for 30 days. 161 Compared to the traditional culture approach of PDO, ALI PDO can achieve a more accurate overview of the stem cell niche and mimic tumor cell–stromal cell interactions by co‐culturing tumor cells with stromal components, allowing studies to focus more on the intra‐tumor immune response. 50
3.3. Analysis and characterization of PDO
The established PDO model needs to be identified and characterized before proceeding to the next step of the experiment. PDO is most widely used as a model for drug evaluation and screening. Cell viability assays, on the other hand, are the main method to measure the response of the organoid to the tested drug, such as the CellTiter‐Glo® luminescent cell viability assay (Promega). 97 In addition to functional analysis, genome‐wide, and targeted CRISPR screens, single‐cell transcriptomics, image‐/imaging‐based assessment methods. 162 The combined use of multiple analytical methods (e.g., cell viability assays, single‐cell sequencing, histopathology, and real‐time imaging combined) can improve the predictive accuracy of organoids as drug screening models to some extent. 163 High content screening (HCS) uses imaging, image analysis, and data extraction to achieve multiple phenotypic data in organoids HCS uses imaging, image analysis, and data extraction to obtain multiple phenotypic data from a single cell in the organoid. Compared to single‐endpoint measurements of high‐throughput screening, the multiparametric readouts of HCS enable it to reveal multiple phenotypic changes and intercellular differences in cells in response to PDO drugs. 164
4. APPLICATION OF TUMOR ORGANOIDS
4.1. Study of tumorigenesis and development mechanisms
Tumorigenesis and progression are based on the accumulation of genetic mutations. Determining the mutational process is important for a deeper understanding of tumors. The introduction of pathological mutations into normal organoids by genetic modification to mimic the tumorigenic process is feasible. 156 , 157 , 165 , 166 , 167 , 168 , 169 , 170 Matano et al. 156 introduced driver pathway mutations (APC, SMAD4, TP53, KRAS, and PIK3CA) using CRISPR‐Cas9 genome‐editing technology, and established selective culture conditions in normal human colonic organoids to allow multiple mutation combinations in isogenic organoids. This class of organs exhibits tumorigenic capacity proportional to the number of mutations it carries after implantation in mice under the kidney subcapsule. This mouse model exhibits tumorigenicity compared to CRC organoids after the insertion of oncogenic mutations in human adenoma organoids with the chromosomal instability (CIN) phenotype. 156 Similarly, mutant organoids with invasive carcinoma features grow after transplantation into mice as tumors with the combined loss of APC and TP53 leading to the appearance of extensive aneuploidy (a hallmark of cancer progression). 165 In contrast, tumor cells show significantly higher mutation rates, and most of the mutations were acquired during the final dominant clonal expansion of cancer when comparing genetic differences between normal and tumor organoids using whole‐genome sequencing. 166 , 167 In addition, the mesenchymal niche of mutant stem cells is associated with tumorigenesis, and a paracrine coordinating mechanism is identified in organoid models. 168
Metastatic progression is a major cause of death in cancer patients and the migration and invasion of cancer cells are key drivers of cancer metastasis. 171 Co‐culture of near‐physiological microvessels with breast cancer organoids shows that tumor cells rapidly remodel, destroy, or integrate into existing vessels and form mosaic vessels which allow tumor cells to disseminate. 172 Meanwhile, Homophilic CD44 interactions between tumor cells and subsequent CD44–PAK2 interactions mediate migration and aggregation of circulating tumor clusters in breast cancer organoids, with aggregated tumor cells promoting destructive metastasis of tumors. 173 Furthermore, basal epithelial gene expression triggers collective invasion in major human breast cancer subtype organoids. 174 Similarly, inhibition of ROCK2 activity increases the invasiveness of colorectal adenocarcinoma organoids. 175 Clonal selection and metastatic capacity exhibit ecotype dependence (Figure 4d), 176 , 177 but this stem cell niche factor dependence is progressively lost with cancer progression. 178
4.2. Drug development and screening
Statistics from 2010 to 2017 show that the success rate of drug candidates from phase I clinical trials to the initiation of late‐stage development is below 10%, and the main reason for their failure is inadequate safety or efficacy. 179 Therefore, the establishment of effective preclinical models is beneficial to improve the success rate of drug development. 2D cell lines, PDXs, and PDOs can all be used as preclinical models for drug screening of tumors. Numerous studies show that PDOs pathologically characterizes and maintains genetic stability and can more accurately assess drug efficacy and drug sensitivity than 2D cell lines and PDXs (Figure 5b). 180 , 181 Moreover, uniform‐sized organoids can be obtained by using simple microfluidic devices for repeatable drug screening. 182 Grossman et al. 183 found that drug sensitivity test results for pancreatic ductal adenocarcinoma (PDAC) PDOs correlate with the clinical response of individual patients. The use of drug sensitivity data excludes ineffective drugs for patients and reduces the toxic reactions produced by these drugs. A study of 27 primary liver cancer organoid lines from different regions of the tumor (from 129 patients) shows that some anti‐cancer drugs are broadly effective against multiple lines, while others were effective against only one organoid class. 184 Furthermore, PDOs can provide preclinical models for therapeutic studies of rare tumors. Puca et al. 49 established a tumor organoid for neuroendocrine prostate cancer, and a fraction of single drugs and drug combinations in high‐throughput drug screens show new possibilities regarding the treatment of this disease. Moreover, the application of automated microfluidics to drug screening of tumor carcinoids allows the real‐time analysis of carcinoids. Chronological administration of drug treatments might be more effective than constant doses of individual drugs or combinations (Figure 5c). 85 Kong et al. 185 used pharmacogenomic data of colorectal and bladder carcinoids for network‐based machine learning, and the identified biomarkers accurately predict drug responses in 191 clinical patients (Figure 5a).
FIGURE 5.

Application of tumor organoids in drug screening. (a) Process for organic identification of drug response biomarkers by network‐based machine learning (ML). (Reproduced with permission from Reference 185. Copyright 2020, Springer Nature). (b) Flow of a week‐long drug sensitivity test on the InSMAR‐chip. (Reproduced with permission from Reference 181. Copyright 2021, Springer Nature). (c) Combinatorial drug treatment of human tumor organoids on microfluidic platform. (i) continuous fluorescence and phase imaging of drug treatment and stimulation during treatment. (ii) Different drug and concentration combinations for treatment of organoid and normal growth media(scale bar 100 μm). (Reproduced with permission from Reference 85. Copyright 2020, Springer Nature).
The inadequate safety of drug candidates is another major reason for drug development failure, and matching tumor organoids to normal organoids has potential applications in estimating drug safety and appropriate dosing. Herpers et al. 186 produced more than 500 dual‐targeting bispecific antibodies (bAbs) targeting the Wingless‐related integration site (WNT) and receptor tyrosine kinase (RTK). A large‐scale screening of bAbs was performed using a heterogeneous colorectal cancer PDOs biobank matched to normal colonic mucosal samples. The results identified that MCLA‐158 shows therapeutic benefits to colorectal cancer cells and is minimally toxic to normal LGR5+ colon stem cells. Photodynamic therapy (PDT) is an anti‐tumor therapy that relies on photosensitizers (PS), light activation at specific wavelengths, and oxygen levels to achieve tumor targeting through rapid accumulation of PS at the tumor site and light excitation of PS to produce reactive oxygen species. The 3D structure of the PDO model allows the cells in it to be in different oxygen concentration environments, which facilitates the testing of PDT efficacy. 187 Nanobody‐targeted PDT remains therapeutic in tumor head and neck squamous cell carcinoma (HNSCC) organoids with low EGFR expression levels, whereas the corresponding normal organoids did not respond to EGFR‐targeted PDT. 188 Valančiūtė et al. 189 tested the killing effect of methylene blue photodynamic therapy (MB‐PDT) in combination with light emitting diodes (LEDs) using a lung cancer carcinoid model. The results showed that MB‐PDT destroyed the structure of lung carcinoids and caused tumor cell death by inducing immunogenic cancer cell death (ICD). This suggests that the organoid could be an ideal platform for testing and evaluating PDT, providing more valuable data for preclinical studies of PDT.
Tumor organoids usually retain the genetic characteristics and heterogeneity of parental tumors, which offers the possibility to investigate the mechanisms of drug resistance and find new therapeutic targets. 45 CD44+ hepatocellular carcinoma (HCC) PDOs is significantly resistant to sorafenib, and sorafenib increases Hedgehog signaling protein and CD44 levels. 190 Meanwhile, sorafenib resistance is reversed in CD44+ HCC PDOs following the introduction of a Hedgehog signaling inhibitor (GANT61). 190 Stromal antigen (STAG) 3 promotes colorectal cancer cell migration and chemoresistance suggesting that STAG3 may be a potential therapeutic target and prognostic biomarker for colorectal cancer. 191 Similarly, microRNA 21 (miRNA21) may mediate resistance to HSP90 inhibitors in cholangiocarcinoma (CCA) organoids by reducing DNAJB5 levels. In other words, HSP90 inhibitors potentially treat CCA, while the presence of miRNA21 may reduce its effectiveness in treatment; therefore, its levels could be used as a diagnostic marker for suitability of the treatment method. 192
Although organoids have made significant contributions to the field of drug screening, the influence of their structure, spatial distribution, and size on drug efficacy should not be ignored. Studies have shown that compared with individual organoid cells, 3D organoids are less sensitive to drugs, and their efficacy is significantly lower. The presence of stents can delay the effective action time of drugs, and Matrigel can cause more obvious delays than synthetic hydrogels. In addition, among larger organoids (with a cross‐sectional area > 8000 μm2), the closer the cells were to the core, the later the drug response occurred. No similar phenomenon was observed in small organoids. 193 Hence, organoid‐based drug sensitivity data warrant further research and investigation by taking various factors into account.
4.3. Precision medicine
Precision medicine (also known as personalized medicine) involves the analysis of genetic traits and differences between individuals through genomics and uses the results to guide the development of effective interventions. Clinical, environmental, behavioral, and lifestyle aspects also need to be considered in this process. 194 , 195 Precision medicine requires preclinical models to closely resemble the in vivo environment to develop treatment plans and predict the clinical response to therapy. Organoids have potential applications in precision medicine research because of their good generalization of the in vivo environment. 196 , 197
Tumor organoids are known as precision oncology when applied to precision medicine. 198 Precision oncology provides an overview of the genetic and histological characteristics of a patient's tumor, inter‐, and intra‐tumor heterogeneity, and drug response and resistance. It also allows drug screening and safety testing to determine which patients are most likely to benefit from it and to develop an appropriate treatment plan (Figure 6a–d). 199 , 200 , 201 , 202 , 203 A proven treatment regimen will help increase patient survival and improve quality of life. 204 Vlachogiannis et al. 200 established a living biobank for PDOs derived from patients with metastatic gastrointestinal cancer. The phenotype and genotype of PDOs are similar to those of the parental tumor and molecular profiling is consistent with the drug screening results. The PDOs have 88% positive predictive value and 100% negative predictive value in forecasting patient response to targeted therapy or chemotherapy. Pauli et al. 199 combined genomic sequencing data with high‐throughput drug screening of tumor organoid models to develop a series of optimal treatment regimens. A combination of the PIK3 inhibitor (buparlisib) with the hypoxia signaling inhibitor (vorinostat) was identified as the optimal treatment option for patients with uterine carcinosarcoma. Meanwhile, the combination of buparlisib with PARP and HDAC inhibitor Olaparib was identified as the optimal treatment regimen for patients with endometrial adenocarcinoma, and there was good concordance between in vitro and in vivo tumor responses. Seppälä et al. 201 found that next‐generation sequencing (NGS) after biomass expansion of pancreatic ductal adenocarcinoma organoids improves detection of somatic mutations and quantifies copy number variants which improves clinical sequencing quality. Moreover, Song et al. 203 established 15 monoclonal tumor organoid lines from four different lesions in one colon cancer patient and each PDOs line showed heterogeneity in genotype and phenotype. This revealed time‐point heterogeneity in anticancer drug responses that can be used to pinpoint the optimal time frame for therapeutic drugs combined with high‐throughput drug screening and image‐based drug responsiveness tracing. The application of organoids significantly shortens the time to derive the analytical results used to guide treatment and facilitate real‐time patient assessment compared with traditional precision medicine research methods. 205 Notably, the association between predicted and true patient outcomes requires further validation in large‐sample studies.
FIGURE 6.
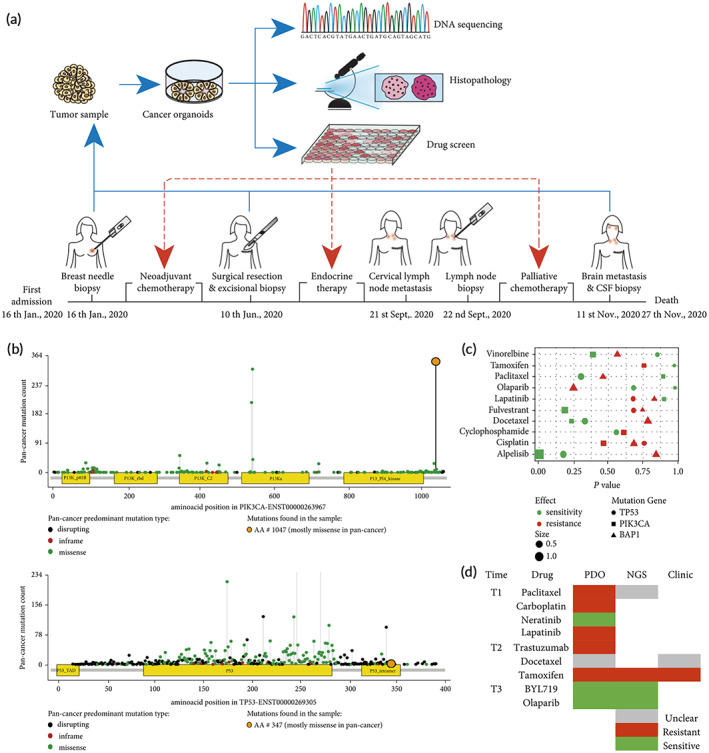
The application of breast cancer organoids in precision medicine. (a) Personalized treatment of metastatic ER+ breast cancer in a 41‐year‐old woman using tumor organoids. (b) Patients' mutations in PIK3CA and TP53 genes. (c) Scatter plot of the impact of drug treatment associated with gene mutations in the GDSC database. (d) Drug response prediction for PDOs and NGS and clinical response. (Reproduced with permission from Reference 202. Copyright 2022, Y. Liu et al.).
4.4. Immunotherapy
Immunotherapy is a new anti‐tumor strategy that uses the patient's immune system to kill tumor cells. Tumor‐specific mutations generate neoantigens and the immune system triggers a sufficient immune response to specifically kill them when the tumor cell exhibits sufficient immunogenicity. 206 , 207 Therefore, we can choose to actively target specific antigens on the tumor or augment the host immune system, including monoclonal antibodies, adoptive transfer of TILs, allogeneic cell‐based vaccines, chimeric antigen receptors T (CAR‐T) cells, and immune checkpoint inhibition when the strength of the immune response induced by tumor cell neoantigens is insufficient. 208 , 209 Currently, organoid models are used in tumor immunology studies including exploring potential immune mechanisms, 210 , 211 immunotherapy modeling, 161 , 212 , 213 and personalized immunotherapy testing. 214 , 215 , 216 T cells are key players in immunotherapy, and the co‐culture of autologous tumor organoids and peripheral blood lymphocytes could be used from mismatch repair‐deficient colorectal cancer and peripheral blood from patients with nonsmall cell lung cancer to enrich tumor‐reactive T cells and assess the efficiency of T‐cell‐mediated killing of matched tumor organoids. 217 This co‐culture model can also be used to assess the sensitivity of patients to immunotherapy and explain the possible mechanisms of drug resistance (Figure 7b). 212
FIGURE 7.

Tumor organoids are used as in vitro models for immunotherapy and simulation of tumor microenvironment in tumor organoids. (a) Flow of TEG co‐culture with PDOs. (i) Schematic diagram of TEG co‐culture with PDOs. (ii) BEHAV3D platform. (Reproduced with permission from Reference 210. Copyright 2023, J. F. Dekkers et al.). (b) Schematic diagram of PBMC co‐culture with patient‐derived tumor organoids. (Reproduced with permission from Reference 212. Copyright 2019, Springer Nature). (c) Schematic diagram of tumor organoids and immune cells co‐cultured to mimic the microenvironment. (Reproduced with permission from Reference 225. Copyright 2022, Elsevier Inc). (d) Morphological features of cancer‐associated fibroblast‐integrated pancreatic cancer carcinoid organs (CIPCO). (i) Immunofluorescence images of CK19‐ and vimentin‐labeled PCO and CIPCO models (scale bar 100 μm). (ii) Immunofluorescence images of type I collagen‐labeled PCOs and CIPCOs (scale bar 100 μm). (iii) Comparison of Masson's trichrome‐stained images of PCOs, CIPCOs, and human pancreatic cancer cells (scale bar 500 μm). (iv) Immunofluorescence detection of the sensitivity of cancer cells to different concentrations of gemcitabine in PCO and CIPCO models (scale bar 200 μm). (Reproduced with permission from Reference 226. Copyright 2022, Y.‐H. Go).
The TME can affect the immune system and immunotherapy, and understanding the interactions between the two can help optimize immunotherapy. Neal et al. 161 established primary tumor epithelial and endogenous immune matrix organoids in humans and mice using ALI approach and showed that tumor organoids in TILs encapsulate the primitive tumor T‐cell receptor (TCR) profile. Also, tumor organoids can mimic immune checkpoint blockade (ICB), anti‐PD‐1‐ and/or anti‐PD‐L1 amplification, activation of tumor antigen‐specific TILs, and trigger tumor cytotoxicity. In addition, microfluidic platforms allow tumor organoids to be co‐cultured with TME components which provides models for studying immune mechanisms in the TME, screening immunotherapeutic agents, and predicting clinical responses to therapy. 218
CAR‐T cells therapy is to recognize and attack tumor cells by modifying autologous or allogeneic T cells to target specific antigens of tumor cells. Currently, CAR‐T cells are mainly used in hematologic cancers and not widely used in solid tumors, but related research is rapidly developing. There is a possibility of adverse reactions during CAR‐T cells therapy, such as cytokine storm, and neurological toxicity, which may even threaten life safety. 219 Therefore, preclinical evaluation of the efficacy and toxicity of CAR‐T cells is necessary. As a preclinical model of CAR‐T cells, the advantages of PDO in retaining parental tumor cell heterogeneity and specific antibody expression give it a unique advantage. A live confocal imaging protocol was established that allows monitoring of effector cell recruitment and cytolytic activity at the level of individual colorectal carcinoids to assess CAR efficacy and cytotoxicity. 214 Jacob et al. 220 co‐cultured EGFRvIII‐specific CAR‐T cells with EGFRvIII+ and EGFRvIII‐ GBO, respectively, and proliferation of CAR‐T cells and a decrease in EGFRvIII/EGFR signaling intensity were clearly observed in EGFRvIII+ GBO. Further experiments revealed that the T‐cell killing effector granzyme B in the co‐culture model of CAR‐T cells with EGFRvIII+ GBO was clustered on the side close to the tumor cells expressing EGFRvIII, and the levels of cytokines suggestive of antigen recognition and T‐cell activation were also significantly increased.
Moreover, Teijeira et al. 215 co‐cultured tumor organoids, autologous fibroblasts, and T cells to test the therapeutic response of CEA‐CD3 T‐cell engagers against colon cancer and test drug combinations. Recently, Dekkers et al. 210 described a system that characterizes the behavioral phenotypic heterogeneity of cellular immunotherapies and investigates the dynamic interactions between immune cells and tumor organoids through imaging and transcriptomics (Figure 7a). These findings suggest that organoids have promising applications in tumor immunotherapy.
4.5. Simulation of the TME
The TME is a hypoxic and acidic environment produced by the tumor and includes cellular components such as tumor‐associated fibroblasts CAFs, endothelial cells, bone marrow‐derived suppressor cells (MDSC), immune cells, and noncellular components such as the ECM, cytokines, and growth factors. 221 The TME is shaped and governed by the tumor and plays an important role in tumor growth, progression, invasion, and acquisition of drug resistance. Its dynamic regulation leads to suppression of the body's immune cell function or apoptosis of anti‐tumor effector cells; thus, the tumor acquires an immune escape capability which is the main reason for immunotherapy failure. 222 Fortunately, the stromal cell types within the TME are genetically stable which represents the possibility of reducing tumor recurrence and drug resistance by targeting the TME. 223
The application of tumor organoids in the simulation of TME is reported (Figure 7c,d). 224 , 225 , 226 Tsai et al. co‐cultured human pancreatic cancer organoids, CAF, and T cells to establish an organic co‐culture model and observed ECM infiltration around tumor tissue which may be valuable for the evaluation of immunotherapeutic agents in the context of T‐cell infiltration. 227 Neal et al. 161 combined tumor organoids, CAFs, and TILs using the ALI method in co‐culture to preserve endogenous immune and nonimmune matrix elements and the in vivo association and function between native TILs and tumor cells. This allows for immuno‐oncology studies and personalized immunotherapy testing. However, fibroblasts and immune cells in these PDOs are progressively reduced and can only be maintained for 1–2 months, suggesting that they can only be used for short‐term disease modeling. 161 CAFs play an important role in cancer because they secrete various ECM components. They promote the growth of tumor organoids through paracrine signaling and make tumor organoids resistant to anticancer drugs to create a favorable environment for tumor development. 228 Öhlund et al. 229 co‐cultured mouse pancreatic stellate cells (PSCs) and pancreatic ductal adenocarcinoma (PDA) organoids. PSCs co‐cultured with tumor organoids acquired a CAF phenotype and promoted organoid proliferation compared to naive PSCs embedded in Matrigel alone. Furthermore, they described two PSC‐derived coexisting CAF isoforms with conflicting functions showing myofibroblastic and inflammatory phenotypes. Further research suggests that IL1 induces LIF expression and downstream JAK/STAT activation to generate inflammatory CAFs, whereas TGFβ antagonizes this process by downregulating IL1R1 expression and promoting differentiation into myofibroblasts. 230 The heterogeneity of CAFs within tumors suggests that selective targeting of CAFs may be beneficial for anti‐tumor therapy.
5. CONCLUSIONS AND PROSPECTS
Malignant tumors are one of the leading causes of death and impose an increasingly heavy burden on every country. Therefore, it is important to further understand the mechanisms of tumorigenesis and tumor development, develop safer and more effective drugs, and formulate more personalized treatment plans. The establishment of research models that closely resemble the original tumor characteristics will help solve these challenges. Previous tumor studies have relied on 2D cell line models and PDXs models; however, both have non‐negligible shortcomings. Tumor organoids provide a better simulation of the histological, phenotypic, and genotypic characteristics of parental tumors and retain inter‐ and intra‐tumor heterogeneity compared with 2D cell lines and PDXs models while avoiding animal‐related ethical issues. Meanwhile, the synergistic combination of tumor organoids with emerging technologies such as tissue engineering scaffolds, microfluidics, 3D bioprinting, bioreactors, and gene editing overcome some of the drawbacks of traditional culture methods and broaden the application of tumor organoids. In addition, the establishment of several types of tumor organoids, such as colorectal cancer, liver cancer, and breast cancer organoids, provide valuable in vitro models for the study of the mechanisms of the corresponding tumors, drug development and screening, and optimization of precision medicine and immunotherapy. In recent years, the development of iPSC‐ and circulating tumor cells(CTC)‐derived organoids has enriched the sources of tumor organoids and contributed to personalized medical treatment of non‐surgical tumor patients. 231 Moreover, iPSC‐derived organoids can retain tumor and nontumor cell components which helps restore cell–cell interactions within the tumor. 232
Tumor organoids provide models for tumors at various stages of development by combining genetic modifications and various omics analyses from the establishment of organoids to the creation of living biobanks through cryopreservation. However, they only partially simulate the characteristics and development of tumors. The following limitations persist: (1) the lack of standardized culture protocols and evaluation criteria and the low success rate of some tumor organoids prevents the reproducibility and repeatability of organoids and is not conducive to high‐throughput assays. Therefore, the development of standard culture and evaluation protocols for tumor organoids and the use of well‐defined materials are needed to improve the generation efficiency and provide more reliable results for tumor organoids as in vitro models. (2) Tumor organoids are mainly derived from epithelial cells and the number of studies on tumor organoids derived from nonepithelial cells is limited. Further focus on the establishment of nonepithelial‐derived tumor organoids will be beneficial to mechanistic studies and therapeutic optimization of related tumors. (3) Normal cells have an advantage over tumor cells during the long‐term culture of tumor organoids due to their genetic stability 40 and epigenetic drift may occur in long‐term cultured and passaged tumor organoids. 159 Therefore, it is necessary to explore the mechanism by which genetic drift occurs and to avoid normal cell contamination to make tumor organoids more mature and stable; some studies have been conducted to overcome this limitation. 233 (4) Tumor organoids must be enhanced to simulate interactions between cells, tissues, and organs. Although it is possible to co‐culture stromal cells and the ECM to mimic the TME, the role of the peripheral immune system in tumors cannot be assessed 234 and these models cannot be cultured for long periods of time. 161 Applying techniques from other models and tissue engineering to tumor organoids may allow for long‐term stable co‐culture of multiple tissues/organs which further improves the accuracy of predicting clinical responses. 235 Despite the deficiencies of tumor organoids, the combined multi‐organ and multi‐technology approach has great potential to help bridge the gap between tumor organoids and the original tumor to simulate a more complex and realistic state in vivo.
AUTHOR CONTRIBUTIONS
Qian Yang: Visualization (equal); writing – original draft (lead); writing – review and editing (equal). Mengmeng Li: Investigation (equal); supervision (equal); visualization (equal). Xinming Yang: Methodology (equal); project administration (equal); resources (equal); supervision (equal). Zian Xiao: Methodology (equal); project administration (equal); resources (equal). Xinying Tong: Data curation (equal); investigation (equal); validation (equal). Ayinuer Tuerdi: Data curation (equal); investigation (equal); validation (equal). Shisheng Li: Conceptualization (equal); funding acquisition (lead); writing – review and editing (equal). Lanjie Lei: Conceptualization (equal); methodology (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81870711) and the Natural Science Foundation of Hunan Province (No. 2020JJ4791, No. 2019JJ60078).
Yang Q, Li M, Yang X, et al. Flourishing tumor organoids: History, emerging technology, and application. Bioeng Transl Med. 2023;8(5):e10559. doi: 10.1002/btm2.10559
Contributor Information
Shisheng Li, Email: lissdoctor@csu.edu.cn.
Lanjie Lei, Email: leilanjie1988@163.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Zeng S, Tang Q, Xiao M, et al. Cell membrane‐coated nanomaterials for cancer therapy. Mater Today Bio. 2023;20:100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K. Organoid technology and applications in cancer research. J Hematol Oncol. 2018;11(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—a growing case for three‐dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405‐412. [DOI] [PubMed] [Google Scholar]
- 5. Nagle PW, Plukker JTM, Muijs CT, van Luijk P, Coppes RP. Patient‐derived tumor organoids for prediction of cancer treatment response. Semin Cancer Biol. 2018;53:258‐264. [DOI] [PubMed] [Google Scholar]
- 6. Izumchenko E, Paz K, Ciznadija D, et al. Patient‐derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28(10):2595‐2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown KM, Xue A, Mittal A, Samra JS, Smith R, Hugh TJ. Patient‐derived xenograft models of colorectal cancer in pre‐clinical research: a systematic review. Oncotarget. 2016;7(40):66212‐66225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffman RM. Patient‐derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451‐452. [DOI] [PubMed] [Google Scholar]
- 9. Cho SY, Sung CO, Chae J, et al. Alterations in the rho pathway contribute to Epstein‐Barr virus‐induced lymphomagenesis in immunosuppressed environments. Blood. 2018;131(17):1931‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben‐David U, Ha G, Tseng Y‐Y, et al. Patient‐derived xenografts undergo mouse‐specific tumor evolution. Nat Genet. 2017;49(11):1567‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zitvogel L, Pitt JM, Daillère R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16(12):759‐773. [DOI] [PubMed] [Google Scholar]
- 12. Saito R, Kobayashi T, Kashima S, Matsumoto K, Ogawa O. Faithful preclinical mouse models for better translation to bedside in the field of immuno‐oncology. Int J Clin Oncol. 2020;25(5):831‐841. [DOI] [PubMed] [Google Scholar]
- 13. Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23(6):882‐897.e11. [DOI] [PubMed] [Google Scholar]
- 14. Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 2018;36(4):358‐371. [DOI] [PubMed] [Google Scholar]
- 15. Wilson HV. A new method by which sponges may be artificially reared. Science. 1907;25(649):912‐915. [DOI] [PubMed] [Google Scholar]
- 16. Weiss P, Taylor AC. Reconstitution of complete organs from single‐cell suspensions of chick embryos IN advanced stages of differentiation. Proc Natl Acad Sci USA. 1960;46(9):1177‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86(3):287‐301. [PMC free article] [PubMed] [Google Scholar]
- 18. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634‐7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145‐1147. [DOI] [PubMed] [Google Scholar]
- 20. Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA. 1987;84(1):136‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eiraku M, Watanabe K, Matsuo‐Takasaki M, et al. Self‐organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3(5):519‐532. [DOI] [PubMed] [Google Scholar]
- 22. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262‐265. [DOI] [PubMed] [Google Scholar]
- 23. Sato T, Stange DE, Ferrante M, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762‐1772. [DOI] [PubMed] [Google Scholar]
- 24. Joosten SPJ, Zeilstra J, van Andel H, et al. MET signaling mediates intestinal crypt‐villus development, regeneration, and adenoma formation and is promoted by stem cell CD44 isoforms. Gastroenterology. 2017;153(4):1040‐1053.e4. [DOI] [PubMed] [Google Scholar]
- 25. Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148(1):126‐136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schlaermann P, Toelle B, Berger H, et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut. 2016;65(2):202‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huch M, Gehart H, van Boxtel R, et al. Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell. 2015;160(1‐2):299‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendriks D, Artegiani B, Hu H, de Sousa C, Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR‐Cas9‐based gene knockin and knockout in organoid cultures from human liver. Nat Protoc. 2021;16(1):182‐217. [DOI] [PubMed] [Google Scholar]
- 29. Broutier L, Andersson‐Rolf A, Hindley CJ, et al. Culture and establishment of self‐renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc. 2016;11(9):1724‐1743. [DOI] [PubMed] [Google Scholar]
- 30. Hohwieler M, Illing A, Hermann PC, et al. Human pluripotent stem cell‐derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 2017;66(3):473‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dye BR, Hill DR, Ferguson MA, et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife. 2015;4:e05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller AJ, Dye BR, Ferrer‐Torres D, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14(2):518‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann K, Obermayer B, Hönzke K, et al. Human alveolar progenitors generate dual lineage bronchioalveolar organoids. Commun Biol. 2022;5(1):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morizane R, Bonventre JV. Generation of nephron progenitor cells and kidney organoids from human pluripotent stem cells. Nat Protoc. 2017;12(1):195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan HHN, Siu HC, Ho SL, et al. Organoid cultures of early‐onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020;69(12):2165‐2179. [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Yuen ST, Xu J, et al. Whole‐genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46(6):573‐582. [DOI] [PubMed] [Google Scholar]
- 39. Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Servant R, Garioni M, Vlajnic T, et al. Prostate cancer patient‐derived organoids: detailed outcome from a prospective cohort of 81 clinical specimens. J Pathol. 2021;254(5):543‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160(1–2):324‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell‐ and patient‐derived tumor organoids. Nat Med. 2015;21(11):1364‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1‐2):373‐386.e10. [DOI] [PubMed] [Google Scholar]
- 44. Dekkers JF, van Vliet EJ, Sachs N, et al. Long‐term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protoc. 2021;16(4):1936‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee SH, Hu W, Matulay JT, et al. Tumor evolution and drug response in patient‐derived organoid models of bladder cancer. Cell. 2018;173(2):515‐528.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li X, Francies HE, Secrier M, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun. 2018;9(1):2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nguyen‐Ngoc KV, Cheung KJ, Brenot A, et al. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci USA. 2012;109(39):E2595‐E2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Subramaniam D, Angulo P, Ponnurangam S, et al. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020;11(2):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Puca L, Bareja R, Prandi D, et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun. 2018;9(1):2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rauth S, Karmakar S, Batra SK, Ponnusamy MP. Recent advances in organoid development and applications in disease modeling. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Driehuis E, Kretzschmar K, Clevers H. Establishment of patient‐derived cancer organoids for drug‐screening applications. Nat Protoc. 2020;15(10):3380‐3409. [DOI] [PubMed] [Google Scholar]
- 53. Seliktar D. Designing cell‐compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124‐1128. [DOI] [PubMed] [Google Scholar]
- 54. Corrò C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol. 2020;319(1):C151‐C165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79‐80:3‐18. [DOI] [PubMed] [Google Scholar]
- 56. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378‐386. [DOI] [PubMed] [Google Scholar]
- 57. Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 58. Liu H, Bockhorn J, Dalton R, et al. Removal of lactate dehydrogenase‐elevating virus from human‐in‐mouse breast tumor xenografts by cell‐sorting. J Virol Methods. 2011;173(2):266‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Talbot NC, Caperna TJ. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: relevance to stem cell responses. Cytotechnology. 2015;67(5):873‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aisenbrey EA, Murphy WL. Synthetic alternatives to matrigel. Nat Rev Mater. 2020;5(7):539‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Y, Chen H, Li J. Recent advances on gelatin methacrylate hydrogels with controlled microstructures for tissue engineering. Int J Biol Macromol. 2022;221:91‐107. [DOI] [PubMed] [Google Scholar]
- 62. Sathaye S, Mbi A, Sonmez C, et al. Rheology of peptide‐ and protein‐based physical hydrogels: are everyday measurements just scratching the surface? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(1):34‐68. [DOI] [PubMed] [Google Scholar]
- 63. Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307‐4314. [DOI] [PubMed] [Google Scholar]
- 64. van Vlierberghe S, Dubruel P, Schacht E. Biopolymer‐based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12(5):1387‐1408. [DOI] [PubMed] [Google Scholar]
- 65. Chavda H, Patel C. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int J Pharm Investig. 2011;1(1):17‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rao SS, Dejesus J, Short AR, Otero JJ, Sarkar A, Winter JO. Glioblastoma behaviors in three‐dimensional collagen‐hyaluronan composite hydrogels. ACS Appl Mater Interfaces. 2013;5(19):9276‐9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H, Zhang H, Chen Z, Zhao Y, Gu Z, Shang L. Polymer‐based responsive structural color materials. Prog Mater Sci. 2023;135:101091. [Google Scholar]
- 68. Kaur S, Kaur I, Rawal P, Tripathi DM, Vasudevan A. Non‐matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58‐66. [DOI] [PubMed] [Google Scholar]
- 69. Wang H, Zhang H, Xie Z, et al. Injectable hydrogels for spinal cord injury repair. Eng Regen. 2022;3(4):407‐419. [Google Scholar]
- 70. Ng S, Tan WJ, Pek MMX, Tan MH, Kurisawa M. Mechanically and chemically defined hydrogel matrices for patient‐derived colorectal tumor organoid culture. Biomaterials. 2019;219:119400. [DOI] [PubMed] [Google Scholar]
- 71. Cassereau L, Miroshnikova YA, Ou G, Lakins J, Weaver VM. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J Biotechnol. 2015;193:66‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen Y, Yang S, Tavormina J, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40(8):818‐834.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869‐1879. [DOI] [PubMed] [Google Scholar]
- 75. Nayak B, Balachander GM, Manjunath S, Rangarajan A, Chatterjee K. Tissue mimetic 3D scaffold for breast tumor‐derived organoid culture toward personalized chemotherapy. Colloids Surf B Biointerfaces. 2019;180:334‐343. [DOI] [PubMed] [Google Scholar]
- 76. Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560‐564. [DOI] [PubMed] [Google Scholar]
- 77. Below CR, Kelly J, Brown A, et al. A microenvironment‐inspired synthetic three‐dimensional model for pancreatic ductal adenocarcinoma organoids. Nat Mater. 2022;21(1):110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N‐isopropylacrylamide), poly(N‐vinylcaprolactam) and amphiphilically modified poly(N‐vinylcaprolactam). Biomaterials. 2005;26(16):3055‐3064. [DOI] [PubMed] [Google Scholar]
- 80. Kim S, Min S, Choi YS, et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat Commun. 2022;13(1):1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang W, Du A, Liu S, Lv M, Chen S. Research progress in decellularized extracellular matrix‐derived hydrogels. Regen Ther. 2021;18:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R. Tissue‐engineered grafts from human Decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci. 2018;19(12):4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brown M, Li J, Moraes C, Tabrizian M, Li‐Jessen NYK. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials. 2022;289:121786. [DOI] [PubMed] [Google Scholar]
- 84. Lee PJ, Ghorashian N, Gaige TA, Hung PJ. Microfluidic system for automated cell‐based assays. JALA (Charlottesville, VA). 2007;12(6):363‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schuster B, Junkin M, Kashaf SS, et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat Commun. 2020;11(1):5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jung DJ, Shin TH, Kim M, Sung CO, Jang SJ, Jeong GS. A one‐stop microfluidic‐based lung cancer organoid culture platform for testing drug sensitivity. Lab Chip. 2019;19(17):2854‐2865. [DOI] [PubMed] [Google Scholar]
- 87. Song SL, Li B, Carvalho MR, et al. Complex in vitro 3D models of digestive system tumors to advance precision medicine and drug testing: Progress, challenges, and trends. Pharmacol Ther. 2022;239:108276. [DOI] [PubMed] [Google Scholar]
- 88. Geyer M, Queiroz K. Microfluidic platforms for high‐throughput pancreatic ductal adenocarcinoma organoid culture and drug screening. Front Cell Dev Biol. 2021;9:761807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shirure VS, Bi Y, Curtis MB, et al. Tumor‐on‐a‐chip platform to investigate progression and drug sensitivity in cell lines and patient‐derived organoids. Lab Chip. 2018;18(23):3687‐3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boussommier‐Calleja A, Li R, Chen MB, Wong SC, Kamm RD. Microfluidics: a new tool for modeling cancer‐immune interactions. Trends Cancer. 2016;2(1):6‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu R, Zhou X, Wang S, Trinkle C. Tumor organoid models in precision medicine and investigating cancer‐stromal interactions. Pharmacol Ther. 2021;218:107668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gonçalves IM, Carvalho V, Rodrigues RO, et al. Organ‐on‐a‐Chip platforms for drug screening and delivery in tumor cells: a systematic review. Cancers (Basel). 2022;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park SE, Georgescu A, Huh D. Organoids‐on‐a‐chip. Science. 2019;364(6444):960‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lei L, Ma B, Xu C, Liu H. Emerging tumor‐on‐chips with electrochemical biosensors. TrAC Trends Anal Chem. 2022;153:116640. [Google Scholar]
- 95. Fang G, Lu H, Rodriguez de la Fuente L, et al. Mammary tumor organoid culture in non‐adhesive alginate for luminal mechanics and high‐throughput drug screening. Adv Sci. 2021;8(21):e2102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Du Y, Li X, Niu Q, et al. Development of a miniaturized 3D organoid culture platform for ultra‐high‐throughput screening. J Mol Cell Biol. 2020;12(8):630‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Booij TH, Cattaneo CM, Hirt CK. Tumor organoids as a research tool: how to exploit them. Cell. 2022;11(21):3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lundholt BK, Scudder KM, Pagliaro L. A simple technique for reducing edge effect in cell‐based assays. J Biomol Screen. 2003;8(5):566‐570. [DOI] [PubMed] [Google Scholar]
- 99. Vijayavenkataraman S, Yan WC, Lu WF, Wang CH, Fuh JYH. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296‐332. [DOI] [PubMed] [Google Scholar]
- 100. Arslan‐Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication. 2016;8(1):014103. [DOI] [PubMed] [Google Scholar]
- 101. Salaris F, Rosa A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: challenges and opportunities. Brain Res. 2019;1723:146393. [DOI] [PubMed] [Google Scholar]
- 102. Daly R, Harrington TS, Martin GD, Hutchings IM. Inkjet printing for pharmaceutics—a review of research and manufacturing. Int J Pharm. 2015;494(2):554‐567. [DOI] [PubMed] [Google Scholar]
- 103. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion‐based bioprinting. Biomaterials. 2016;76:321‐343. [DOI] [PubMed] [Google Scholar]
- 104. Antoshin AA, Churbanov SN, Minaev NV, et al. LIFT‐bioprinting, is it worth it? Bioprinting. 2019;15:e00052. [Google Scholar]
- 105. Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482‐487. [DOI] [PubMed] [Google Scholar]
- 106. Hinton TJ, Jallerat Q, Palchesko RN, et al. Three‐dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Demirci U. Acoustic Picoliter droplets for emerging applications in semiconductor industry and biotechnology. J Microelectromech Syst. 2006;15(4):957‐966. [Google Scholar]
- 108. Wang Z, Abdulla R, Parker B, Samanipour R, Ghosh S, Kim K. A simple and high‐resolution stereolithography‐based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2015;7(4):045009. [DOI] [PubMed] [Google Scholar]
- 109. Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three‐dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8(10):1940‐1949. [DOI] [PubMed] [Google Scholar]
- 110. Tasoglu S, Kaynak G, Szeri AJ, Demirci U, Muradoglu M. Impact of a compound droplet on a flat surface: a model for single cell epitaxy. Phys Fluids. 2010;22(8):082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hoch E, Tovar GE, Borchers K. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur J Cardiothorac Surg. 2014;46(5):767‐778. [DOI] [PubMed] [Google Scholar]
- 112. Gurkan UA, El Assal R, Yildiz SE, et al. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Mol Pharm. 2014;11(7):2151‐2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ferris CJ, Gilmore KG, Wallace GG, In het Panhuis M. Biofabrication: an overview of the approaches used for printing of living cells. Appl Microbiol Biotechnol. 2013;97(10):4243‐4258. [DOI] [PubMed] [Google Scholar]
- 114. Kim BS, Lee JS, Gao G, Cho DW. Direct 3D cell‐printing of human skin with functional transwell system. Biofabrication. 2017;9(2):025034. [DOI] [PubMed] [Google Scholar]
- 115. Jang J, Park HJ, Kim SW, et al. 3D printed complex tissue construct using stem cell‐laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264‐274. [DOI] [PubMed] [Google Scholar]
- 116. Byambaa B, Annabi N, Yue K, et al. Bioprinted osteogenic and Vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater. 2017;6(16):1700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee JW, Choi YJ, Yong WJ, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1):015007. [DOI] [PubMed] [Google Scholar]
- 118. Lozano R, Stevens L, Thompson BC, et al. 3D printing of layered brain‐like structures using peptide modified gellan gum substrates. Biomaterials. 2015;67:264‐273. [DOI] [PubMed] [Google Scholar]
- 119. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. [DOI] [PubMed] [Google Scholar]
- 120. Yoon WH, Lee HR, Kim S, et al. Use of inkjet‐printed single cells to quantify intratumoral heterogeneity. Biofabrication. 2020;12(3):035030. [DOI] [PubMed] [Google Scholar]
- 121. Reid JA, Mollica PA, Johnson GD, Ogle RC, Bruno RD, Sachs PC. Accessible bioprinting: adaptation of a low‐cost 3D‐printer for precise cell placement and stem cell differentiation. Biofabrication. 2016;8(2):025017. [DOI] [PubMed] [Google Scholar]
- 122. Mollica PA, Booth‐Creech EN, Reid JA, et al. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019;95:201‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Maloney E, Clark C, Sivakumar H, et al. Immersion bioprinting of tumor organoids in multi‐well plates for increasing chemotherapy screening throughput. Micromachines (Basel). 2020;11(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hou S, Tiriac H, Sridharan BP, et al. Advanced development of primary pancreatic organoid tumor models for high‐throughput phenotypic drug screening. SLAS Discov. 2018;23(6):574‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Reid JA, Palmer XL, Mollica PA, Northam N, Sachs PC, Bruno RD. A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci Rep. 2019;9(1):7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Reid JA, Mollica PA, Bruno RD, Sachs PC. Consistent and reproducible cultures of large‐scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res. 2018;20(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Begley CM, Kleis SJ. The fluid dynamic and shear environment in the NASA/JSC rotating‐wall perfused‐vessel bioreactor. Biotechnol Bioeng. 2000;70(1):32‐40. [DOI] [PubMed] [Google Scholar]
- 128. Schwarz RP, Goodwin TJ, Wolf DA. Cell culture for three‐dimensional modeling in rotating‐wall vessels: an application of simulated microgravity. J Tissue Cult Methods. 1992;14(2):51‐57. [DOI] [PubMed] [Google Scholar]
- 129. Gaspar DA, Gomide V, Monteiro FJ. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2012;2(4):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schröder M, Reseland JE, Haugen HJ. Osteoblasts in a perfusion flow bioreactor‐tissue engineered constructs of TiO(2) scaffolds and cells for improved clinical performance. Cell. 2022;11(13):1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Regmi S, Fu A, Luo KQ. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep. 2017;7:39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ovando‐Roche P, West EL, Branch MJ, et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther. 2018;9(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32(8):1053‐1060. [DOI] [PubMed] [Google Scholar]
- 134. Fuh KF, Withell J, Shepherd RD, Rinker KD. Fluid flow stimulation modulates expression of S100 genes in Normal breast epithelium and breast cancer. Cell Mol Bioeng. 2022;15(1):115‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jin F, Zhang Y, Xuan K, et al. Establishment of three‐dimensional tissue‐engineered bone constructs under microgravity‐simulated conditions. Artif Organs. 2010;34(2):118‐125. [DOI] [PubMed] [Google Scholar]
- 136. Smith YC, Grande KK, Rasmussen SB, O'Brien AD. Novel three‐dimensional organoid model for evaluation of the interaction of uropathogenic Escherichia coli with terminally differentiated human urothelial cells. Infect Immun. 2006;74(1):750‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hjelm BE, Berta AN, Nickerson CA, Arntzen CJ, Herbst‐Kralovetz MM. Development and characterization of a three‐dimensional organotypic human vaginal epithelial cell model. Biol Reprod. 2010;82(3):617‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Redden RA, Iyer R, Brodeur GM, Doolin EJ. Rotary bioreactor culture can discern specific behavior phenotypes in Trk‐null and Trk‐expressing neuroblastoma cell lines. In Vitro Cell Dev Biol Anim. 2014;50(3):188‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Park S, Avera AD, Kim Y. Biomanufacturing of glioblastoma organoids exhibiting hierarchical and spatially organized tumor microenvironment via transdifferentiation. Biotechnol Bioeng. 2022;119(11):3252‐3274. [DOI] [PubMed] [Google Scholar]
- 140. Devarasetty M, Wang E, Soker S, Skardal A. Mesenchymal stem cells support growth and organization of host‐liver colorectal‐tumor organoids and possibly resistance to chemotherapy. Biofabrication. 2017;9(2):021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhau HE, Goodwin TJ, Chang SM, Baker TL, Chung LW. Establishment of a three‐dimensional human prostate organoid coculture under microgravity‐simulated conditions: evaluation of androgen‐induced growth and PSA expression. In Vitro Cell Dev Biol Anim. 1997;33(5):375‐380. [DOI] [PubMed] [Google Scholar]
- 142. Barrila J, Radtke AL, Crabbé A, et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host‐pathogen interactions. Nat Rev Microbiol. 2010;8(11):791‐801. [DOI] [PubMed] [Google Scholar]
- 143. Radtke AL, Herbst‐Kralovetz MM. Culturing and applications of rotating wall vessel bioreactor derived 3D epithelial cell models. J Vis Exp. 2012;(62):3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Skardal A, Devarasetty M, Rodman C, Atala A, Soker S. Liver‐tumor hybrid organoids for modeling tumor growth and drug response In vitro. Ann Biomed Eng. 2015;43(10):2361‐2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kim W, Gwon Y, Park S, Kim H, Kim J. Therapeutic strategies of three‐dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2023;19:50‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Phelan MA, Gianforcaro AL, Gerstenhaber JA, Lelkes PI. An air bubble‐isolating Rotating Wall vessel bioreactor for improved spheroid/organoid formation. Tissue Eng Part C Methods. 2019;25(8):479‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mali P, Yang L, Esvelt KM, et al. RNA‐guided human genome engineering via Cas9. Science. 2013;339(6121):823‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Asmamaw M, Zawdie B. Mechanism and applications of CRISPR/Cas‐9‐mediated genome editing. Biol Theory. 2021;15:353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Hart T, Chandrashekhar M, Aregger M, et al. High‐resolution CRISPR screens reveal fitness genes and genotype‐specific cancer liabilities. Cell. 2015;163(6):1515‐1526. [DOI] [PubMed] [Google Scholar]
- 151. Aguirre AJ, Meyers RM, Weir BA, et al. Genomic copy number dictates a gene‐independent cell response to CRISPR/Cas9 targeting. Cancer Discov. 2016;6(8):914‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ruiz S, Mayor‐Ruiz C, Lafarga V, et al. A genome‐wide CRISPR screen identifies CDC25A as a determinant of sensitivity to ATR inhibitors. Mol Cell. 2016;62(2):307‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gayle S, Landrette S, Beeharry N, et al. Identification of apilimod as a first‐in‐class PIKfyve kinase inhibitor for treatment of B‐cell non‐Hodgkin lymphoma. Blood. 2017;129(13):1768‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fujii M, Clevers H, Sato T. Modeling human digestive diseases with CRISPR‐Cas9‐modified organoids. Gastroenterology. 2019;156(3):562‐576. [DOI] [PubMed] [Google Scholar]
- 155. Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106‐119. [DOI] [PubMed] [Google Scholar]
- 156. Matano M, Date S, Shimokawa M, et al. Modeling colorectal cancer using CRISPR‐Cas9‐mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256‐262. [DOI] [PubMed] [Google Scholar]
- 157. Dekkers JF, Whittle JR, Vaillant F, et al. Modeling breast cancer using CRISPR‐Cas9‐mediated engineering of human breast organoids. J Natl Cancer Inst. 2020;112(5):540‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vaishnavi A, Juan J, Jacob M, et al. Transposon mutagenesis reveals RBMS3 silencing as a promoter of malignant progression of BRAFV600E‐driven lung tumorigenesis. Cancer Res. 2022;82(22):4261‐4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Michels BE, Mosa MH, Streibl BI, et al. Pooled In vitro and In vivo CRISPR‐Cas9 screening identifies tumor suppressors in human colon organoids. Cell Stem Cell. 2020;26(5):782‐792.e7. [DOI] [PubMed] [Google Scholar]
- 160. DiMarco RL, Su J, Yan KS, Dewi R, Kuo CJ, Heilshorn SC. Engineering of three‐dimensional microenvironments to promote contractile behavior in primary intestinal organoids. Integr Biol. 2014;6(2):127‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972‐1988.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Corsini NS, Knoblich JA. Human organoids: new strategies and methods for analyzing human development and disease. Cell. 2022;185(15):2756‐2769. [DOI] [PubMed] [Google Scholar]
- 163. Shinozawa T, Kimura M, Cai Y, et al. High‐fidelity drug‐induced liver injury screen using human pluripotent stem cell‐derived organoids. Gastroenterology. 2021;160(3):831‐846.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lukonin I, Zinner M, Liberali P. Organoids in image‐based phenotypic chemical screens. Exp Mol Med. 2021;53(10):1495‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Drost J, van Jaarsveld RH, Ponsioen B, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521(7550):43‐47. [DOI] [PubMed] [Google Scholar]
- 166. Jager M, Blokzijl F, Sasselli V, et al. Measuring mutation accumulation in single human adult stem cells by whole‐genome sequencing of organoid cultures. Nat Protoc. 2018;13(1):59‐78. [DOI] [PubMed] [Google Scholar]
- 167. Roerink SF, Sasaki N, Lee‐Six H, et al. Intra‐tumour diversification in colorectal cancer at the single‐cell level. Nature. 2018;556(7702):457‐462. [DOI] [PubMed] [Google Scholar]
- 168. Roulis M, Kaklamanos A, Schernthanner M, et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature. 2020;580(7804):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lo YH, Kolahi KS, Du Y, et al. A CRISPR/Cas9‐engineered ARID1A‐deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 2021;11(6):1562‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Schutgens F, Rookmaaker MB, Margaritis T, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37(3):303‐313. [DOI] [PubMed] [Google Scholar]
- 171. Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895‐904. [DOI] [PubMed] [Google Scholar]
- 172. Silvestri VL, Henriet E, Linville RM, Wong AD, Searson PC, Ewald AJ. A tissue‐engineered 3D microvessel model reveals the dynamics of mosaic vessel formation in breast cancer. Cancer Res. 2020;80(19):4288‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Liu X, Taftaf R, Kawaguchi M, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient‐derived breast cancer models. Cancer Discov. 2019;9(1):96‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155(7):1639‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Libanje F, Raingeaud J, Luan R, et al. ROCK2 inhibition triggers the collective invasion of colorectal adenocarcinomas. EMBO J. 2019;38(14):e99299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Fumagalli A, Drost J, Suijkerbuijk SJ, et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci USA. 2017;114(12):E2357‐E2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Vaquero‐Siguero N, Schleussner N, Volk J, Mastel M, Meier J, Jackstadt R. Modeling colorectal cancer progression reveals niche‐dependent clonal selection. Cancers (Basel). 2022;14(17):4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Seino T, Kawasaki S, Shimokawa M, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. 2018;22(3):454‐467.e6. [DOI] [PubMed] [Google Scholar]
- 179. Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18(7):495‐496. [DOI] [PubMed] [Google Scholar]
- 180. Yang L, Yang S, Li X, et al. Tumor organoids: from inception to future in cancer research. Cancer Lett. 2019;454:120‐133. [DOI] [PubMed] [Google Scholar]
- 181. Hu Y, Sui X, Song F, et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun. 2021;12(1):2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ozcelik A, Abas BI, Erdogan O, Cevik E, Cevik O. On‐Chip organoid formation to study CXCR4/CXCL‐12 chemokine microenvironment responses for renal cancer drug testing. Biosensors. 2022;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Grossman JE, Muthuswamy L, Huang L, et al. Organoid sensitivity correlates with therapeutic response in patients with pancreatic cancer. Clin Cancer Res. 2022;28(4):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Li L, Knutsdottir H, Hui K, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4(2):e121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kong J, Lee H, Kim D, et al. Network‐based machine learning in colorectal and bladder organoid models predicts anti‐cancer drug efficacy in patients. Nat Commun. 2020;11(1):5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Herpers B, Eppink B, James MI, et al. Functional patient‐derived organoid screenings identify MCLA‐158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat Cancer. 2022;3(4):418‐436. [DOI] [PubMed] [Google Scholar]
- 187. Yu R, Maswikiti EP, Yu Y, et al. Advances in the application of preclinical models in photodynamic therapy for tumor: a narrative review. Pharmaceutics. 2023;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Driehuis E, Spelier S, Beltrán Hernández I, et al. Patient‐derived head and neck cancer organoids recapitulate EGFR expression levels of respective tissues and are responsive to EGFR‐targeted photodynamic therapy. J Clin Med. 2019;8(11):1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Valančiūtė A, Mathieson L, O'Connor RA, et al. Phototherapeutic induction of immunogenic cell death and CD8+ T cell‐Granzyme B mediated cytolysis in human lung cancer cells and organoids. Cancers (Basel). 2022;14(17):4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Wang S, Wang Y, Xun X, et al. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient‐derived organoids. J Exp Clin Cancer Res. 2020;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sasaki M, Miyoshi N, Fujino S, et al. The meiosis‐specific cohesin component stromal antigen 3 promotes cell migration and chemotherapeutic resistance in colorectal cancer. Cancer Lett. 2021;497:112‐122. [DOI] [PubMed] [Google Scholar]
- 192. Lampis A, Carotenuto P, Vlachogiannis G, et al. MIR21 drives resistance to heat shock protein 90 inhibition in cholangiocarcinoma. Gastroenterology. 2018;154(4):1066‐1079.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Van Hemelryk A, Mout L, Erkens‐Schulze S, French PJ, van Weerden WM, van Royen ME. Modeling prostate cancer treatment responses in the organoid era: 3D environment impacts drug testing. Biomolecules. 2021;11(11):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. König IR, Fuchs O, Hansen G, von Mutius E, Kopp MV. What is precision medicine? Eur Respir J. 2017;50(4):1700391. [DOI] [PubMed] [Google Scholar]
- 195. Carlsten C, Brauer M, Brinkman F, et al. Genes, the environment and personalized medicine: we need to harness both environmental and genetic data to maximize personal and population health. EMBO Rep. 2014;15(7):736‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jin MZ, Han RR, Qiu GZ, Ju XC, Lou G, Jin WL. Organoids: An intermediate modeling platform in precision oncology. Cancer Lett. 2018;414:174‐180. [DOI] [PubMed] [Google Scholar]
- 197. Grassi L, Alfonsi R, Francescangeli F, et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019;10(3):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol. 2013;31(15):1803‐1805. [DOI] [PubMed] [Google Scholar]
- 199. Pauli C, Hopkins BD, Prandi D, et al. Personalized In vitro and In vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient‐derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Seppälä TT, Zimmerman JW, Suri R, et al. Precision medicine in pancreatic cancer: patient‐derived organoid pharmacotyping is a predictive biomarker of clinical treatment response. Clin Cancer Res. 2022;28(15):3296‐3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Liu Y, Gan Y, AiErken N, et al. Combining organoid models with next‐generation sequencing to reveal tumor heterogeneity and predict therapeutic response in breast cancer. J Oncol. 2022;2022:9390912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Song MH, Park JW, Kim MJ, et al. Colon cancer organoids using monoclonal organoids established in four different lesions of one cancer patient reveal tumor heterogeneity and different real‐time responsiveness to anti‐cancer drugs. Biomed Pharmacother. 2022;152:113260. [DOI] [PubMed] [Google Scholar]
- 204. Veninga V, Voest EE. Tumor organoids: opportunities and challenges to guide precision medicine. Cancer Cell. 2021;39(9):1190‐1201. [DOI] [PubMed] [Google Scholar]
- 205. Ye W, Luo C, Li C, Huang J, Liu F. Organoids to study immune functions, immunological diseases and immunotherapy. Cancer Lett. 2020;477:31‐40. [DOI] [PubMed] [Google Scholar]
- 206. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69‐74. [DOI] [PubMed] [Google Scholar]
- 207. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science. 2015;348(6230):124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wang Y, Wang M, Wu HX, Xu RH. Advancing to the era of cancer immunotherapy. Cancer Commun. 2021;41(9):803‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Dekkers JF, Alieva M, Cleven A, et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat Biotechnol. 2023;41(1):60‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hai J, Zhang H, Zhou J, et al. Generation of genetically engineered mouse lung organoid models for squamous cell lung cancers allows for the study of combinatorial immunotherapy. Clin Cancer Res. 2020;26(13):3431‐3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cattaneo CM, Dijkstra KK, Fanchi LF, et al. Tumor organoid‐T‐cell coculture systems. Nat Protoc. 2020;15(1):15‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Holokai L, Chakrabarti J, Lundy J, et al. Murine‐ and human‐derived autologous organoid/immune cell Co‐cultures as pre‐clinical models of pancreatic ductal adenocarcinoma. Cancers (Basel). 2020;12(12):3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Schnalzger TE, de Groot MH, Zhang C, et al. 3D model for CAR‐mediated cytotoxicity using patient‐derived colorectal cancer organoids. EMBO J. 2019;38:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Teijeira A, Migueliz I, Garasa S, et al. Three‐dimensional colon cancer organoids model the response to CEA‐CD3 T‐cell engagers. Theranostics. 2022;12(3):1373‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Scognamiglio G, De Chiara A, Parafioriti A, et al. Patient‐derived organoids as a potential model to predict response to PD‐1/PD‐L1 checkpoint inhibitors. Br J Cancer. 2019;121(11):979‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor‐reactive T cells by Co‐culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586‐1598.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zhang J, Tavakoli H, Ma L, Li X, Han L, Li X. Immunotherapy discovery on tumor organoid‐on‐a‐chip platforms that recapitulate the tumor microenvironment. Adv Drug Deliv Rev. 2022;187:114365. [DOI] [PubMed] [Google Scholar]
- 219. Kumari R, Ouyang X, Wang J, et al. Preclinical pharmacology modeling of chimeric antigen receptor T therapies. Curr Opin Pharmacol. 2021;61:49‐61. [DOI] [PubMed] [Google Scholar]
- 220. Jacob F, Salinas RD, Zhang DY, et al. A patient‐derived glioblastoma organoid model and biobank recapitulates inter‐ and intra‐tumoral heterogeneity. Cell. 2020;180(1):188‐204.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zhong S, Jeong JH, Chen Z, Chen Z, Luo JL. Targeting tumor microenvironment by small‐molecule inhibitors. Transl Oncol. 2020;13(1):57‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904‐5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Liu PF, Cao YW, Zhang SD, et al. A bladder cancer microenvironment simulation system based on a microfluidic co‐culture model. Oncotarget. 2015;6(35):37695‐37705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Xu H, Jiao D, Liu A, Wu K. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Go YH, Choi WH, Bae WJ, et al. Modeling pancreatic cancer with patient‐derived organoids integrating cancer‐associated fibroblasts. Cancers (Basel). 2022;14(9):2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Tsai S, McOlash L, Palen K, et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018;18(1):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Liu J, Li P, Wang L, et al. Cancer‐associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell Mol Gastroenterol Hepatol. 2021;11(2):407‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Öhlund D, Handly‐Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Biffi G, Oni TE, Spielman B, et al. IL1‐induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9(2):282‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Praharaj PP, Bhutia SK, Nagrath S, Bitting RL, Deep G. Circulating tumor cell‐derived organoids: current challenges and promises in medical research and precision medicine. Biochim Biophys Acta Rev Cancer. 2018;1869(2):117‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sharma A, Sances S, Workman MJ, Svendsen CN. Multi‐lineage human iPSC‐derived platforms for disease modeling and drug discovery. Cell Stem Cell. 2020;26(3):309‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Shi R, Radulovich N, Ng C, et al. Organoid cultures as preclinical models of non‐small cell lung cancer. Clin Cancer Res. 2020;26(5):1162‐1174. [DOI] [PubMed] [Google Scholar]
- 234. Spitzer MH, Carmi Y, Reticker‐Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487‐502.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Bersini S, Jeon JS, Dubini G, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35(8):2454‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Cai MH, Chen XY, Fu LQ, et al. Design and development of hybrid hydrogels for biomedical applications: recent trends in anticancer drug delivery and tissue engineering. Front Bioeng Biotechnol. 2021;9:630943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


