Abstract
Background:
Iron deficiency, with or without anemia, is an adverse prognostic factor in heart failure (HF). In AFFIRM-AHF (a randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure), intravenous ferric carboxymaltose (FCM), although having no significant effect on the primary end point, reduced the risk of HF hospitalization (hHF) and improved quality of life versus placebo in iron-deficient patients stabilized after an acute HF (AHF) episode. These prespecified AFFIRM-AHF subanalyses explored the association between hemoglobin levels and FCM treatment effects.
Methods:
AFFIRM-AHF was a multicenter, double-blind, randomized, placebo-controlled trial of FCM in hospitalized AHF patients with iron deficiency. Patients were stratified by baseline hemoglobin level (<12 versus ≥12 g/dL). In each subgroup, the primary composite (total hHF and cardiovascular death) and secondary (total hHF; total cardiovascular hospitalizations and cardiovascular death; time to cardiovascular death, and time to first/days lost due to hHF or cardiovascular death) outcomes were assessed with FCM versus placebo at week 52. Sensitivity analyses using the World Health Organization anemia definition (hemoglobin level <12 g/dL [women] or <13 g/dL [men]) were performed, among others.
Results:
Of 1108 AFFIRM-AHF patients, 1107 were included in these subanalyses: 464 (FCM group, 228; placebo group, 236) had a hemoglobin level <12 g/dL, and 643 (FCM, 329; placebo, 314) had a hemoglobin level ≥12 g/dL. Patients with a hemoglobin level <12 g/dL were older (mean, 73.7 versus 69.1 years), with more frequent previous HF (75.0% versus 68.7%), serum ferritin <100 μg/L (75.4% versus 68.1%), and transferrin saturation <20% (87.9% versus 81.4%). For the primary outcome, annualized event rates per 100 patient-years with FCM versus placebo were 71.1 and 73.6 (rate ratio, 0.97 [95% CI, 0.66–1.41]), respectively, and 48.5 versus 72.9 (RR, 0.67 [95% CI, 0.48–0.93]) in the hemoglobin levels <12 and ≥12 g/dL subgroups, respectively. No significant interactions between hemoglobin subgroup and treatment effect were observed for primary (Pinteraction=0.15) or secondary outcomes. Changes from baseline in hemoglobin, serum ferritin and transferrin saturation were significantly greater with FCM versus placebo in both subgroups between weeks 6 and 52. Findings were similar using the World Health Organization definition for anemia.
Conclusions:
The effects of intravenous FCM on outcomes in iron-deficient patients stabilized after an AHF episode, including improvements in iron parameters over time, did not differ between patients with hemoglobin levels <12 and ≥12 g/dL.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02937454.
Keywords: anemia, heart failure, hemoglobins, iron deficiencies
Clinical Perspective.
What Is New?
In patients with iron deficiency and who have stabilized after an acute heart failure episode, the effect of intravenous ferric carboxymaltose versus placebo on clinical outcomes, including heart failure hospitalization and cardiovascular death, was irrespective of baseline hemoglobin levels.
What Are the Clinical Implications?
Results from the present prespecified subgroup analyses suggest that the effects of ferric carboxymaltose versus placebo observed in AFFIRM-AHF (randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure) were independent of hemoglobin level, with improved iron parameters and quality of life observed in both hemoglobin levels <12 g/dL and ≥12 g/dL subgroups.
Iron is involved in several biological processes that are essential for maintaining homeostasis and, as such, it appears to have a role that extends far beyond erythropoiesis.1 Iron deficiency is common in patients with heart failure (HF), affecting up to 50% of those with stable disease.1–3 In this context, iron deficiency has been associated with reduced physical well-being and a considerable impairment of quality of life (QoL),4,5 and has been shown to be an independent predictor of worse functional capacity and survival.3,6 Studies have shown that iron deficiency is an adverse prognostic factor in both chronic HF and acute HF (AHF), conferring an increased risk of hospitalization and death.1,2,7–10
Anemia (defined as a lower-than-normal hemoglobin level) is also a common comorbidity in patients with HF and has been associated with an increased risk of hospitalization and death.11 Although iron deficiency is a primary cause of low hemoglobin in HF,12 both the prognostic role of iron deficiency and the beneficial effects of treating it with ferric carboxymaltose (FCM) have been shown to be independent of baseline hemoglobin level in patients with chronic HF.1,8,13,14 Correction of hemoglobin levels alone (ie, using erythropoiesis-stimulating agents) has not been shown to improve cardiovascular outcomes in patients with HF.15,16 Therefore, it is important to understand the role of iron deficiency and its treatment in relation to hemoglobin levels in patients with recent AHF, a population with higher morbidity and mortality compared with a chronic HF population.
AFFIRM-AHF (a randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure) demonstrated that treatment with intravenous FCM, although having no significant effect on the composite primary end point of total HF hospitalizations and cardiovascular death, reduced the risk of HF hospitalizations and improved QoL in patients with iron deficiency who had stabilized after an episode of AHF.17 These prespecified, exploratory subgroup analyses of the AFFIRM-AHF trial aimed to investigate the effects of FCM versus placebo on clinical and QoL outcomes in iron-deficient patients with hemoglobin values <12 g/dL versus ≥12 g/dL after stabilization from an AHF episode.
Methods
AFFIRM-AHF Trial Design
The AFFIRM-AHF trial design has been previously published.17,18 Briefly, AFFIRM-AHF was an international, multicenter, double-blind, placebo-controlled, phase-IV, randomized trial comparing outcomes with FCM versus placebo in patients stabilized after an AHF episode and who experienced concomitant iron deficiency. The trial was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and local and national regulations. The study was approved by an institutional review board, and all patients provided their written informed consent to participate. The data that support the findings of this study are available from the corresponding author upon reasonable request.
AFFIRM-AHF Patient Population and Treatment
AFFIRM-AHF included patients ≥18 years of age who had been hospitalized with signs and symptoms typical of AHF (eg, elevated natriuretic peptides), treated with a minimum of 40 mg of IV furosemide (or equivalent intravenous diuretic), and had concomitant iron deficiency (defined as serum ferritin <100 μg/L or serum ferritin 100–299 μg/L with transferrin saturation [TSAT] <20%) and a left ventricular ejection fraction (LVEF) <50%. Patients with symptoms related to other medical conditions (eg, hemoglobin level <8 g/dL [<10 g/dL for sites in the Netherlands, Spain, and Singapore] or hemoglobin level >15 g/dL) were excluded. Patients were randomly assigned (1:1) to receive intravenous FCM or placebo before discharge from hospital, with the first dose administered shortly before discharge. Patients were assessed over 52 weeks.
Study End Points and Clinical Assessments
The primary end point was a composite of total HF hospitalizations and cardiovascular death during up to 52 weeks of follow-up. Secondary end points included a composite of total cardiovascular hospitalizations and cardiovascular death; cardiovascular death; total HF hospitalizations; time to first HF hospitalization or cardiovascular death; and days lost due to HF hospitalizations or cardiovascular death, which were all evaluated up to 52 weeks after randomization. Other end points included changes in disease-specific QoL (assessed using the self-administered 12-item Kansas City Cardiomyopathy Questionnaire [KCCQ-12] overall summary score [OSS] and clinical summary score [CSS]) from baseline to weeks 2, 4, 6, 12, 24, 36, and 52, and laboratory values (hemoglobin, serum ferritin, and TSAT) from baseline to weeks 6, 12, 24, and 52. The proportion of patients who had iron deficiency defined as serum ferritin <100 μg/L versus serum ferritin 100–299 μg/L with TSAT <20% at baseline was assessed, as was the number of study treatment doses administered during the study. Safety end points included a summary of adverse events (AEs).
Stratification Into Subgroups by Baseline Hemoglobin Level
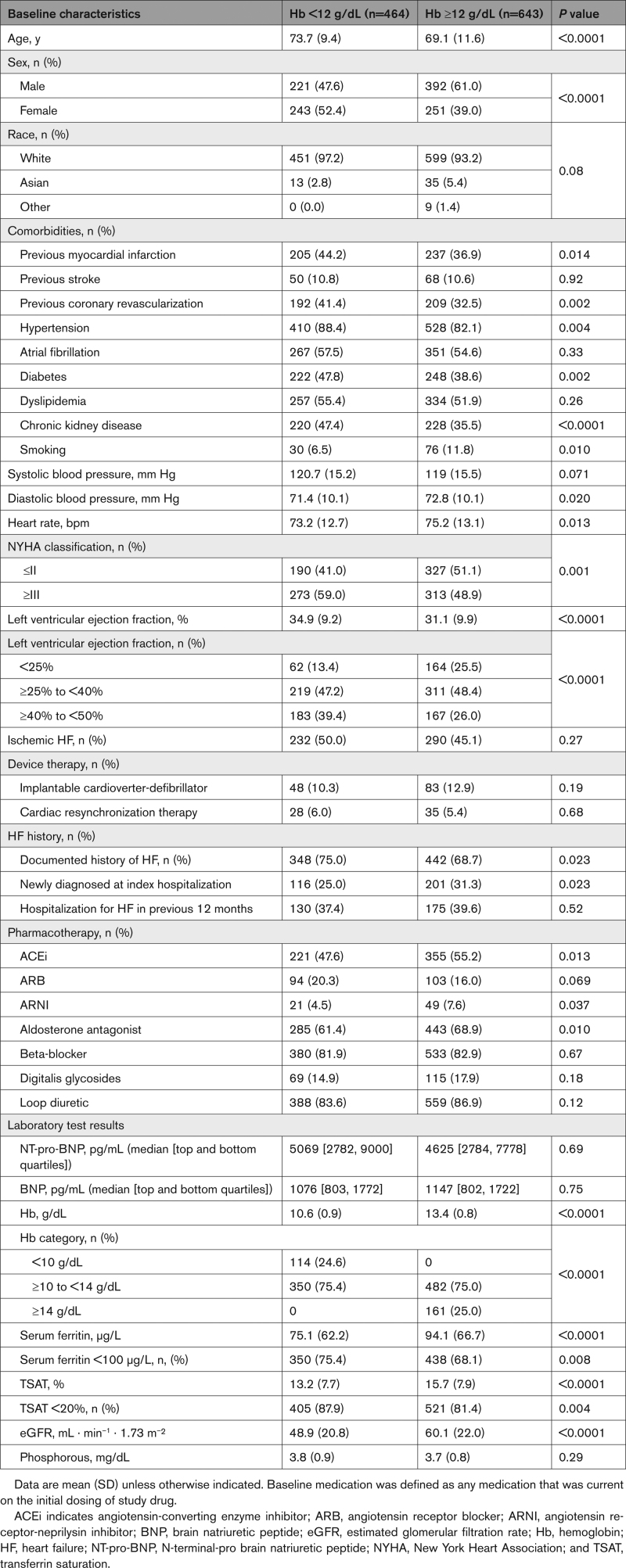
In the prespecified hemoglobin subgroup analyses, patients were stratified into 2 subgroups according to hemoglobin level at baseline: hemoglobin level <12 g/dL (low hemoglobin level subgroup) and hemoglobin level level ≥12 g/dL (normal hemoglobin level subgroup; patients with a hemoglobin level >15 g/dL were excluded from the AFFIRM-AHF trial population). For completeness, post hoc sensitivity analyses were performed with patients stratified into 2 subgroups according to the World Health Organization (WHO) definition of anemia: hemoglobin level <12 g/dL in women and hemoglobin level <13 g/dL in men (low hemoglobin subgroup) and hemoglobin level ≥12 g/dL in women and hemoglobin level ≥13 g/dL in men (normal hemoglobin subgroup).19 Post hoc sensitivity analyses with patients stratified by median hemoglobin value and hemoglobin quartiles at baseline were also performed.
Statistical Methods for Subgroup Analyses
All analyses were conducted in patients from the AFFIRM-AHF modified intention-to-treat (mITT) population who had an available hemoglobin value at baseline, except for safety and laboratory end points, which were assessed in patients in the safety analysis set who had an available hemoglobin value at baseline.
The following analyses were performed for the prespecified hemoglobin subgroup stratification (hemoglobin level <12 g/dL and hemoglobin level ≥12 g/dL), as well as for the subgroups stratified by the WHO definition of anemia. Baseline characteristics were descriptively summarized as mean (SD) for continuous variables and n (%) for discrete variables. Primary and secondary outcomes with FCM versus placebo within each subgroup were analyzed using a negative binomial model for recurrent end points (presented as event rate ratios [RRs] with 95% CI) and time to first event end points were analyzed using a Cox regression model (presented as hazard ratios [HRs] with 95% CI); both models were adjusted for baseline sex, age, HF etiology, HF duration, country, hemoglobin level, and interaction between treatment arm and hemoglobin level (covariates chosen a priori). Interaction P values (Pinteraction) for the association between hemoglobin subgroup and treatment outcomes were also generated. To assess the association between hemoglobin level and primary and secondary outcomes in patients who did not receive iron treatment, results in the placebo arms of the hemoglobin subgroups were also compared. The proportional hazards assumption was not tested for subgroup analyses.
In each hemoglobin subgroup, mean (SE) changes from baseline in KCCQ-12 OSS and CSS and in laboratory values (hemoglobin, serum ferritin, and TSAT) with FCM versus placebo were compared at each time point using repeated-measures analysis of variance. In the low-hemoglobin subgroups (defined according to prespecified cutoff and WHO anemia cutoff), the proportions of patients who had low hemoglobin levels according to the respective definition at weeks 24 and 52 were assessed in the FCM and placebo arms, as were the proportions of these patients who had iron deficiency at the respective time points. AEs were descriptively summarized in each hemoglobin subgroup and treatment arm as number of patients with events (%) and number of events.
An a priori–defined pre–COVID-19 pandemic sensitivity analysis, which censored patients in each country at the date when its first COVID-19 patient was reported, was performed for each hemoglobin subgroup to account for the impact of COVID-19 on primary and secondary outcomes; the methodology for this analysis has been previously described.17
Post hoc sensitivity analyses of the primary and secondary outcomes with patients stratified by baseline median hemoglobin and baseline hemoglobin quartiles were performed using the methodology described. Given that the kidneys play a key role in the generation of erythropoietin and erythropoietin deficiency is a major driver of low hemoglobin level in chronic kidney disease,20,21 a further post hoc sensitivity analysis of the primary outcome by estimated glomerular filtration rate (eGFR) tertile plus hemoglobin level (<12 versus ≥12 g/dL) was performed to explore the relationship between kidney function, hemoglobin level, and effect of FCM versus placebo on clinical end points.
All hemoglobin measurements from site visits throughout the study were examined to explore the association between time-updated hemoglobin and the effect of FCM versus placebo on the time-to–first HF hospitalization and cardiovascular death outcome. For this post hoc, time-updated, Cox regression analysis, time of event was aligned with time of hemoglobin measurement: the updated hemoglobin value was set to the most recent hemoglobin measurement preceding the event of interest for patients who experienced an event and to the most recent hemoglobin measurement before the censor time for patients who did not experience an event of interest. Only the value before the event or censor day was entered into the model. Time-updated HRs with 95% CIs and P values were generated for the hemoglobin level <12 g/dL and ≥12 g/dL subgroups, and the global effect of each of the following variables was assessed: treatment; sex, age, HF etiology, history of HF, and country at randomization; hemoglobin subgroup (<12 versus ≥12 g/dL) at baseline; time-updated hemoglobin value; interaction between treatment arm and hemoglobin subgroup (<12 g/dL and ≥12 g/dL); and interaction between treatment arm and time-updated hemoglobin value. The frequency of each time-updated hemoglobin level (stratified into 11 discrete levels ranging from 7–17 g/dL) and the HR (95% Wald CI) for the composite outcome of HF hospitalizations or cardiovascular death with FCM versus placebo associated with each time-updated hemoglobin level were also calculated post hoc; time-updated hemoglobin levels with <20 patients in at least one treatment arm were excluded.
To explore the influence of intrapatient variation in hemoglobin, serum ferritin, and TSAT levels over time on the time to first HF hospitalization or cardiovascular death outcome with FCM versus placebo, a Cox model adjusting for time-dependent hemoglobin, time-dependent serum ferritin, or time-dependent TSAT (in addition to baseline sex, age, HF etiology, history of HF, country, treatment arm, hemoglobin subgroup [<12 g/dL and ≥12 g/dL], and interaction between treatment arm and hemoglobin subgroup [<12 g/dL and ≥12 g/dL]) was used. For these 3 post hoc analyses, all hemoglobin, serum ferritin, or TSAT values recorded for each patient between baseline and the event or censor day were entered into the model.
For all analyses, SAS version 9.4 (SAS Institute, Inc, Cary, NC; 2000–2004) was used, with P values <0.05 considered statistically significant. Authors had full access to all the data in the study and take responsibility for its integrity and analyses.
Results
Prevalence of Low and Normal Hemoglobin at Baseline
Of the 1108 patients in the AFFIRM-AHF mITT analysis set, 1107 (FCM, 557; placebo, 550) patients had baseline hemoglobin data available and were included in these subgroup analyses. Overall, 464 (FCM, 228; placebo, 236) patients (41.9%) had hemoglobin levels <12 g/dL (low) and 643 (FCM, 329; placebo, 314) patients (58.1%) had hemoglobin levels ≥12 g/dL (normal) at baseline (Table). After the WHO anemia definition of a hemoglobin level <13 g/dL for men was applied to define low hemoglobin levels (sensitivity analysis), 140 of the 613 men included in the analyses were reclassified from having normal to low hemoglobin levels (Figure S1). Accordingly, the proportion of men classified as having low hemoglobin levels increased from 36.1% (n=221 of 613) when defined as hemoglobin level <12 g/dL to 58.9% (n=361 of 613) using the hemoglobin level <13 g/dL WHO definition; overall, 604 (FCM, 292; placebo, 312) patients (54.6%) and 503 (FCM, 265; placebo, 238) patients (45.4%) were classified as having low and normal hemoglobin levels at baseline according to the WHO anemia definition (Table S1).
Table.
Baseline Demographics and Clinical Characteristics by Baseline Hemoglobin Level
Baseline Characteristics by Hemoglobin Level
Patients with a hemoglobin level <12 g/dL were, on average, older (mean 73.7 versus 69.1 years of age; P<0.0001), had a higher prevalence of previously documented HF (75.0% versus 68.7%; P=0.023) and comorbidities, a lower eGFR (mean, 48.9 versus 60.1 mL · min–1 · 1.73 m–²; P<0.0001), LVEF <25% (13.4% versus 25.5%; P<0.0001), and New York Heart Association class ≤II (41.0% versus 51.1%; P=0.001) compared with patients with a hemoglobin level ≥12 g/dL (Table). Use of angiotensin-converting enzyme inhibitors (47.6% versus 55.2%; P=0.013) and asldosterone antagonists (61.4% versus 68.9%; P=0.010) was less frequent in patients with a hemoglobin level <12 g/dL compared with patients with a hemoglobin level ≥12 g/dL. Baseline characteristics for patients with low and normal hemoglobin levels, as defined by the WHO definition of anemia, are shown in Table S1.
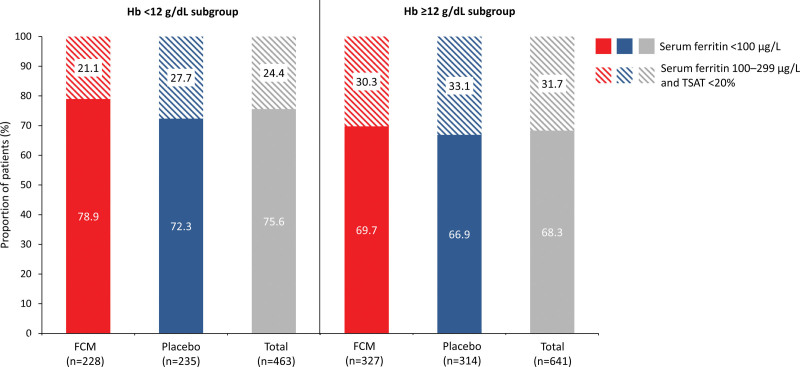
Regarding iron parameters, serum ferritin values <100 μg/L (75.4% versus 68.1%; P=0.008) and TSAT values <20% (87.9% versus 81.4%; P=0.004) were more common in patients with a hemoglobin level <12 g/dL compared with ≥12 g/dL (Table); 75.6% and 68.3% of patients with hemoglobin levels <12 g/dL and ≥12 g/dL, respectively, met the inclusion criteria for iron deficiency defined as serum ferritin <100 μg/L, whereas 24.4% and 31.7%, respectively, met the inclusion criteria for iron deficiency defined as serum ferritin 100 to 299 μg/L with TSAT <20% (Figure 1). Similar values were observed when stratifying patients into low and normal hemoglobin subgroups according to the WHO definition of anemia (Table S1; Figure S2).
Figure 1.
Proportion of patients in each baseline hemoglobin level subgroup who had iron deficiency (defined as serum ferritin <100 µg/L or serum ferritin 100–299 μg/L with TSAT <20%). Solid colors indicate serum ferritin <100 µg/L, and diagonally striped colors indicate serum ferritin 100–299 µg/L and TSAT <20%. Data were missing for one patient on placebo in the hemoglobin level <12 g/dL subgroup and 2 patients on FCM in the hemoglobin level ≥12 g/dL subgroup. Percentages are based on the number of patients with data available. FCM indicates ferric carboxymaltose; Hb, hemoglobin; and TSAT, transferrin saturation.
Treatment Exposure by Hemoglobin Level
In the hemoglobin level <12 g/dL subgroup, 27.5% and 60.7% of patients in the FCM and placebo arms, respectively, received ≥3 treatment doses, with corresponding values of 14.9% and 46.5% in the hemoglobin level ≥12 g/dL subgroup (Figure S3). The respective mean (SD) cumulative doses of FCM and placebo administered were 1.6 (0.6) g and 1.9 (0.6) g for patients with a hemoglobin level <12 g/dL, and 1.2 (0.5) g and 1.6 (0.7) g for patients with a hemoglobin level ≥12 g/dL. Mean (SD) time on study drug (calculated from the first day of study drug administration to the day after the last study drug administration) was also greater in patients with hemoglobin levels <12 g/dL (FCM, 67.4 [69.6] days; placebo, 120.3 [72.0] days) than those with hemoglobin ≥12 g/dL (FCM, 48.6 [63.7] days; placebo, 95.9 [77.3] days). Similar observations were reported in patients stratified into low and normal hemoglobin subgroups according to the WHO definition of anemia (Figure S4).
Primary and Secondary End Points by Hemoglobin Level
The annualized event rate for the primary outcome in the placebo arm was 62.4 per 100 patient-years in patients with a hemoglobin level <12 g/dL versus 58.0 per 100 patient-years in patients with a hemoglobin level ≥12 g/dL (RR, 1.08 [95% CI 0.76–1.53]; Figure S5), with similar results using the WHO anemia definition to delineate low and normal hemoglobin levels (Figure S6). Irrespective of definition, nominally higher rates of secondary outcomes were observed in the low hemoglobin subgroup versus the normal hemoglobin subgroup across most secondary end points (Figure S5 and Figure S6).
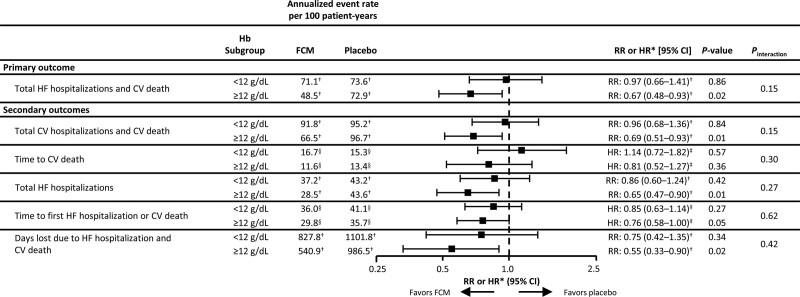
The annualized event rate for the primary outcome with FCM versus placebo was 71.1 versus 73.6 per 100 patient-years in those with a hemoglobin level <12 g/dL (RR, 0.97 [95% CI, 0.66–1.41]) and 48.5 versus 72.9 per 100 patient-years in those with a hemoglobin level ≥12 g/dL (RR, 0.67 [95% CI, 0.48–0.93]; Figure 2). Using the WHO anemia definition to delineate low and normal hemoglobin levels, the annualized event rate for the primary outcome with FCM versus placebo was 68.9 versus 81.3 per 100 patient-years in patients with low hemoglobin levels (RR, 0.85, 95% CI, 0.61–1.18) and 46.6 versus 65.4 per 100 patient-years in patients normal hemoglobin levels (RR, 0.71 [95% CI, 0.49–1.04]; Figure S7). There was no significant interaction between hemoglobin level and treatment effect for any of the primary or secondary end points (Pinteraction for primary end point based on a hemoglobin level <12 g/dL versus ≥12 g/dL definition: 0.15 [Figure 2]; Pinteraction for primary end point based on the WHO anemia definition: 0.50 [Figure S7]).
Figure 2.
Primary and secondary outcomes with FCM vs placebo at week 52, by baseline hemoglobin level. *RR or HR for FCM vs placebo in each subgroup. †Annualized event rate per 100 patient-years and RR analyzed using a negative binomial model. ‡HR for treatment difference analyzed using Cox regression model. §Percentage of patients with ≥1 event. Negative binomial model and Cox regression models both adjusted for baseline sex, age, HF etiology, HF duration, country, hemoglobin subgroup, and interaction between treatment arm and hemoglobin subgroup. Respective n-numbers for patients with hemoglobin levels <12 g/dL and ≥12 g/dL at baseline were 228 and 329 for FCM and 236 and 314 for placebo, respectively. CV indicates cardiovascular; FCM, ferric carboxymaltose; Hb, hemoglobin; HF, heart failure; HR, hazard ratio; and RR, rate ratio.
Pre–COVID-19 Sensitivity Analyses
For both prespecified and WHO anemia definitions of low versus normal hemoglobin levels, results of the COVID-19 sensitivity analyses for primary and secondary end points were similar to those observed with the mITT analysis set (Figure S8A and S8B).
Other Sensitivity Analyses
Sensitivity analyses with patients stratified by baseline median hemoglobin level (12.4 g/dL; Figure S9) and baseline hemoglobin quartiles (Figure S10) showed no significant interaction between hemoglobin level and treatment effect for any of the primary or secondary outcomes. Analyses of patients stratified by baseline eGFR tertile plus a hemoglobin level <12 or ≥12 g/dL (Figure S11) showed no significant interaction between baseline eGFR and hemoglobin level and treatment effect.
The time-updated hemoglobin analysis model yielded an HR for the effect of FCM versus placebo on time to first HF hospitalization or cardiovascular death of 1.48 (95% CI, 0.28–7.85; P=0.64) in the hemoglobin level <12 g/dL subgroup and 1.38 (95% CI, 0.20–9.27; P=0.74) in the hemoglobin level ≥12 g/dL subgroup (Table S2). Time-updated hemoglobin value had a significant effect on the statistical model (P<0.001), as did history of HF (P<0.001) and country (P=0.017). The global effect of the interaction between treatment arm and time-updated hemoglobin value was nonsignificant (P=0.60). The association between each discrete time-updated hemoglobin level and the risk of HF hospitalizations or cardiovascular death with FCM versus placebo is explored in Figure S12.
The 3 analyses exploring the effect of FCM versus placebo on time to first HF hospitalization or cardiovascular death when accounting for intrapatient variation in hemoglobin, serum ferritin, or TSAT over time yielded similar results to the main analysis, with no significant interaction detected between treatment effect and hemoglobin subgroup (Table S3). The global effects of time-dependent hemoglobin (P=0.0018) and time-dependent TSAT (P<0.0001) on the statistical model were significant, with an increase in hemoglobin level of 1 g/dL associated with a 12% lower likelihood of an event (HR, 0.88 [95% CI 0.81–0.95]) and an increase in TSAT of 1% associated with a 3% lower likelihood of an event (HR, 0.97 [95% CI, 0.96–0.98]). The global effect of serum ferritin (P=0.11) on the statistical model was not significant.
Disease-Specific QoL
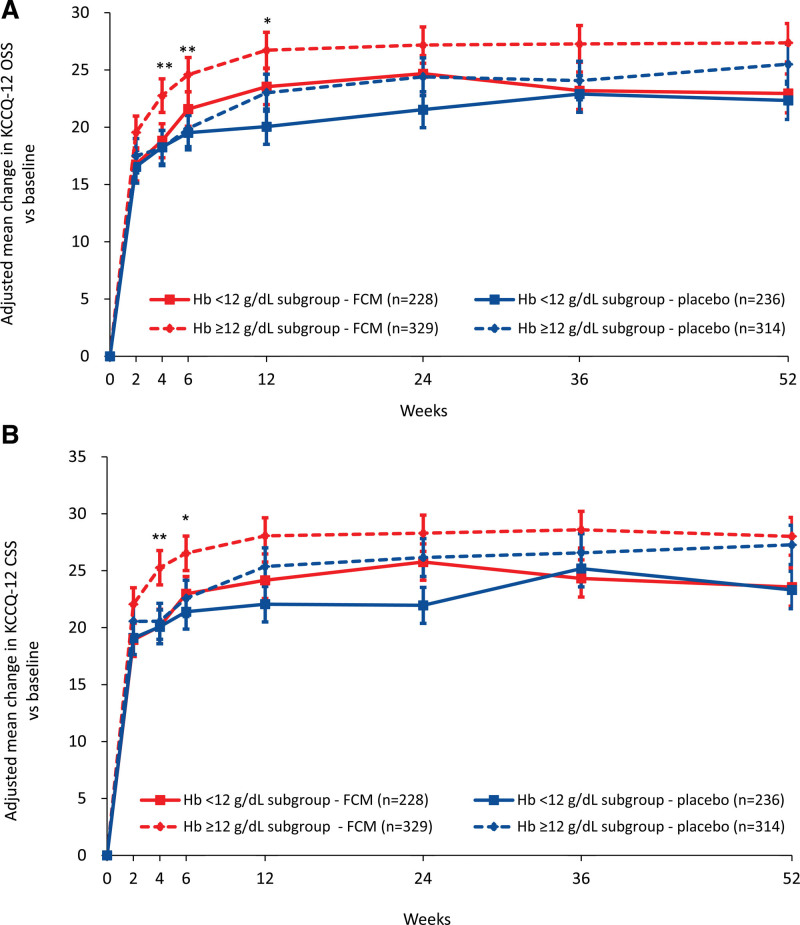
The adjusted mean changes from baseline in KCCQ-12 OSS (Figure 3A) and CSS (Figure 3B) were numerically greater with FCM versus placebo between weeks 2 and 52 in patients with a hemoglobin level ≥12 g/dL (reaching statistical significance at weeks 4 and 6 [OSS and CSS] and 12 [OSS only]) and between weeks 6 and 24 in patients with a hemoglobin level <12 g/dL. Using the WHO anemia definition to delineate low and normal hemoglobin levels, the adjusted mean changes from baseline in KCCQ-12 OSS were numerically greater with FCM versus placebo between weeks 2 and 52 in patients with a low hemoglobin level (reaching statistical significance at weeks 6, 12, and 24) and between weeks 4 and 52 in patients with normal hemoglobin levels (reaching statistical significance at week 4; Figure S13A); the adjusted mean changes from baseline in KCCQ-12 CSS were numerically greater with FCM versus placebo between weeks 2 and 24 in patients with low hemoglobin levels (reaching statistical significance at week 24) and weeks 4 and 12 in patients with normal hemoglobin levels (reaching statistical significance at week 4; Figure S13B).
Figure 3.
Adjusted mean change in KCCQ-12 with FCM versus placebo, by baseline hemoglobin level. A, KCCQ-12 OSS; B, KCCQ-12 CSS. *P<0.05, **P<0.01 for FCM vs placebo in the low hemoglobin level ≥12 g/dL subgroup only. Error bars are standard error of the mean. Estimates are based on a mixed-effect model of repeated measures using an unstructured covariance matrix: change score = baseline score + treatment + visit + treatment × visit + subgroup + subgroup × visit + subgroup × treatment + subgroup × treatment × visit + baseline covariates. CSS indicates clinical summary score; FCM, ferric carboxymaltose; Hb, hemoglobin; KCCQ-12, 12-item Kansas City Cardiomyopathy Questionnaire; and OSS, overall summary score.
The odds of an individual patient in the hemoglobin levels <12 g/dL and ≥12 g/dL subgroup experiencing a ≥5-point improvement or deterioration versus baseline in KCCQ-12 OSS and CSS at week 24 are shown in Figure S14. FCM was associated with numerically larger odds of a ≥5-point improvement in KCCQ-12 OSS versus placebo in both hemoglobin subgroups and numerically smaller odds of a ≥5-point deterioration in patients with hemoglobin levels ≥12 g/dL only. Numerically larger odds of a ≥5-point improvement and numerically smaller odds of a ≥5-point deterioration in the KCCQ-12 CSS were observed with FCM versus placebo in both hemoglobin levels <12 g/dL and ≥12 g/dL subgroups. None of these effects were significant.
Iron Parameters Over Time by Hemoglobin Level
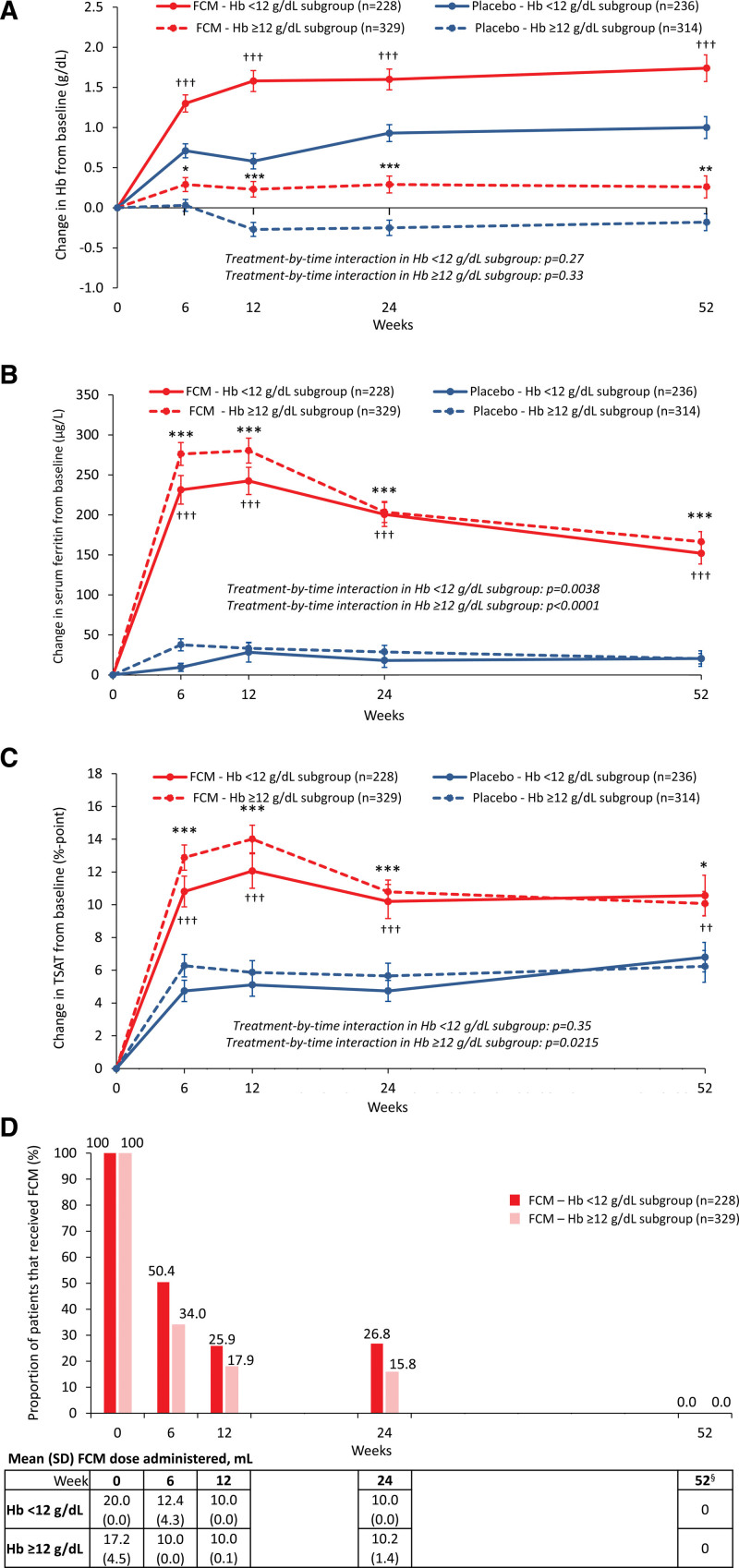
The mean changes from baseline in hemoglobin level (Figure 4A), serum ferritin (Figure 4B), and TSAT (Figure 4C) were significantly greater with FCM versus placebo between weeks 6 and 52 in both the hemoglobin levels <12 g/dL and ≥12 g/dL subgroups. Peak serum ferritin and TSAT values with FCM were observed around weeks 6 and 12 (Figure 4B and 4C), with the highest proportions of patients receiving FCM treatment at baseline and week 6 (<12 g/dL subgroup: 100% and 50%, respectively; hemoglobin level ≥12 g/dL subgroup: 100% and 34%, respectively; Figure 4D).
Figure 4.
Change vs baseline in hemoglobin, serum ferritin, and TSAT over time with FCM and placebo, and details of FCM administration at each time point by baseline hemoglobin level. A, Hemoglobin level; B, serum ferritin concentration; C, TSAT; and D, proportion of patients having received FCM at each time point. *P<0.05, **P<0.01 and ***P<0.001 for FCM vs placebo in the hemoglobin level ≥12 g/dL subgroup and †P<0.05, ††P<0.01 and †††P<0.001 for FCM vs placebo in the hemoglobin level <12 g/dL subgroup. §No study drug was administered after week 24, as per protocol. Error bars are standard error of the mean. FCM indicates ferric carboxymaltose; Hb, hemoglobin; and TSAT, transferrin saturation.
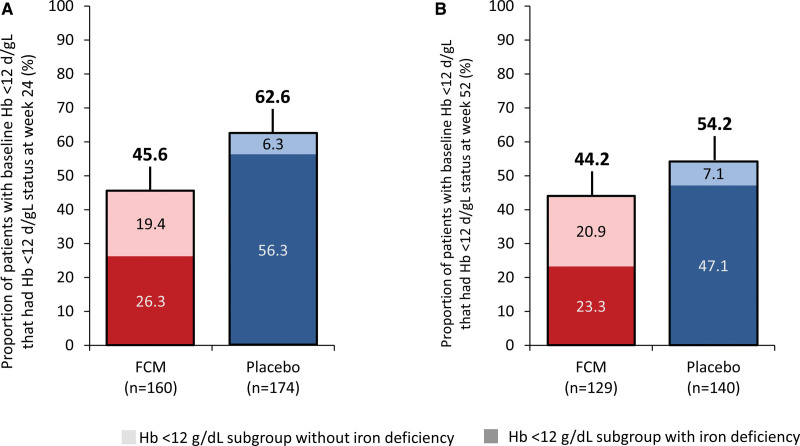
Of the patients with a hemoglobin level <12 g/dL at baseline, 45.6% (n=73 of 160) and 62.6% (n=109 of 174) of those in the FCM and placebo arms, respectively, had a persisting hemoglobin level <12 g/dL at week 24, whereas 44.2% (n=57 of 129) and 54.2% (n=76 of 140), respectively, had a persisting hemoglobin level <12 g/dL at week 52 (Figure 5A and 5B). Of those with a persisting hemoglobin level <12 g/dL at week 24, 57.5% (n=42 of 73) in the FCM arm and 89.9% (n=98 of 109) in the placebo arm also had persisting iron deficiency (defined according to the study protocol). Of those with persisting a hemoglobin level <12 g/dL at week 52, 52.6% (n=30 of 57) in the FCM arm and 86.8% (n=66 of 76) in the placebo arm also had persisting iron deficiency. Similar observations were reported when using the WHO definition of anemia to delineate low and normal hemoglobin levels (Figures S15A to S15D, S16A, and S16B).
Figure 5.
Proportion of patients with a hemoglobin level <12 g/dL at baseline who also had a hemoglobin level <12 g/dL at weeks 24 and 52, and the proportion of these patients who had iron deficiency at the corresponding time point. The darker block colors indicate patients with a hemoglobin level <12 g/dL without iron deficiency, and the lighter block colors indicate patients with a hemoglobin level <12 g/dL with iron deficiency. In A and B, 100% represents all patients who had a hemoglobin level <12 g/dL level at baseline who had nonmissing data for hemoglobin level and iron deficiency status at the respective time point. FCM indicates ferric carboxymaltose; and Hb, hemoglobin.
HF and Anemia Medication Use During the Study
HF medication use during the study is shown in Figure S17. At all time points, mineralocorticoid receptor agonist use was least frequent in patients in the placebo arm of the hemoglobin level <12 g/dL subgroup. Angiotensin-converting enzyme inhibitor and beta-blocker use was generally more frequent in patients with a hemoglobin level ≥12 g/dL compared with a hemoglobin level <12 g/dL across time points, irrespective of treatment arm. Only 2 patients (both in the FCM arm) received erythropoiesis-stimulating agents at any time during the course of the study.
Summary of AEs
A summary of AEs in hemoglobin levels <12 g/dL and ≥12 g/dL subgroups is shown in Table S4. In patients with a hemoglobin level <12 g/dL, treatment-emergent AEs occurred in 151 (65.9%) of 229 patients in the FCM arm and 161 (67.9%) of 237 patients in the placebo arm, leading to premature study treatment discontinuation in 48 (21.0%) and 45 (19.0%) of patients assigned to FCM and placebo, respectively. In patients with a hemoglobin level ≥12 g/dL, treatment-emergent AEs occurred in 205 (62.3%) of 329 patients in the FCM arm and 199 (63.4%) of 314 patients in the placebo arm, leading to premature study discontinuation in 49 (14.9%) and 51 (16.2%) of patients assigned to FCM and placebo, respectively. No hypophosphatemia was observed in either hemoglobin subgroup. A summary of AEs using the WHO definition of anemia to delineate low and normal hemoglobin levels is shown in Table S5.
Discussion
These prespecified, exploratory subgroup analyses of the AFFIRM-AHF study showed that treatment of iron deficiency with FCM in patients who had stabilized after an AHF episode reduced the risk of HF hospitalizations irrespective of baseline hemoglobin level. Nominally greater improvements in QoL with FCM versus placebo were also observed in both low and normal hemoglobin subgroups. These findings are in line with previous studies such as the FAIR-HF trial (Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure) on patients with chronic HF and iron deficiency, which demonstrated that treatment with intravenous FCM improved symptoms, functional capacity, and QoL in subgroups of patients with hemoglobin levels <12 g/dL and ≥12 g/dL.22 However, the patients enrolled in AFFIRM-AHF represent a population at increased risk of morbidity and mortality due to their recent AHF episode, and there are no randomized controlled trial data on the interaction between hemoglobin level and iron deficiency and the outcomes in this population. Therefore, the current study provides data to fill this evidence gap.
Anemia (defined as a lower-than-normal hemoglobin level) has been reported in 30% to 50% of patients with AHF,23 and has been associated with markers of more advanced and active heart disease,24 as well as an increased risk of hospitalization.11 Because iron deficiency is a common contributor to low hemoglobin levels in patients with HF,12 it is important to assess the impact of treating iron deficiency in this population. In addition, iron deficiency (with or without anemia) is independently associated with increased morbidity and mortality in HF;1,7,9,14 treatment of iron deficiency may be expected to benefit patients with HF, regardless of hemoglobin level.
No statistically significant interactions between hemoglobin level and outcomes with FCM versus placebo were observed in these exploratory analyses; however, a nominally smaller effect size was observed in patients with a hemoglobin level <12 g/dL than in patients with a hemoglobin level ≥12 g/dL, with modest sample sizes potentially masking interactions. The role of hemodilution in AHF, which may make low hemoglobin levels less clinically relevant when considering intravenous iron therapy, may merit further investigation, as might the role of a low hemoglobin level as a potential marker of more advanced disease that is less responsive to FCM. Nevertheless, a lack of significant interaction between hemoglobin level and the effect of FCM versus placebo on primary and secondary outcomes was observed in sensitivity analyses of patients stratified by the WHO definition of anemia, by hemoglobin quartile and by hemoglobin median value, with the treatment effect favoring FCM versus placebo across these stratifications. These consistent results suggest that the effect of FCM versus placebo in patients with iron deficiency who had stabilized after an AHF episode is irrespective of hemoglobin level.
Two different definitions of “low hemoglobin level” (<12 g/dL as prespecified in the AFFIRM-AHF protocol and <12 g/dL for women and <13 g/dL for men as per the WHO definition of anemia) were evaluated in this study, and these definitions altered the characteristics of the patients included within the subgroups. Despite these differences in definition, the same conclusion that the effect of FCM versus placebo in terms of primary and secondary outcomes was irrespective of hemoglobin level was reached. Furthermore, with both definitions, patients with low hemoglobin levels were, on average, older, had lower eGFR, and had a higher prevalence of comorbidities, previous documented HF, serum ferritin <100 μg/L, and TSAT <20% than patients with normal hemoglobin levels. These data suggest that patients with iron deficiency and low hemoglobin levels are more likely to have a higher baseline risk of cardiovascular events than those with iron deficiency and normal hemoglobin levels. Indeed, the annualized event rates in the placebo arm of patients with low hemoglobin levels were nominally higher for primary and secondary outcomes than those in the placebo arm of patients with normal hemoglobin levels. The proportion of patients with iron deficiency defined as serum ferritin <100 μg/L (compared with 100–299 μg/L and TSAT <20%) was also higher in those with low versus normal hemoglobin levels, with a higher exposure of patients with low hemoglobin levels to the study drug. The connection between type of iron deficiency (low ferritin concentration versus higher ferritin concentration but low TSAT) and response to FCM merits further investigation.
A prespecified QoL analysis showed that AFFIRM-AHF patients had severely impaired health-related QoL at baseline, and that those treated with intravenous FCM versus placebo experienced significantly greater improvements in health status starting at week 4, and continuing up to week 24.25 A mean KCCQ-12 change of ≥2 to 3 points was considered clinically meaningful for this patient population as a whole, with a change of ≥5 points considered clinically meaningful for an individual within the population.25 In these AFFIRM-AHF subgroup analyses, a significantly greater improvement in disease-specific QoL was observed with FCM versus placebo as early as week 4 in patients with a hemoglobin level ≥12 g/dL, persisting to week 12, with nominally greater improvements thereafter; in those with a hemoglobin level <12 g/dL, although no significant differences were observed, nominally greater improvements with FCM versus placebo were observed between weeks 6 and 24, and a trend toward a treatment effect was observed. Moreover, the odds of an individual patient experiencing a ≥5-point increase versus baseline in KCCQ-12 OSS or CSS scores at week 24 numerically favored FCM versus placebo in both the hemoglobin levels <12 g/dL and ≥12 g/dL subgroups, although the effect size was greater in the ≥12 g/dL subgroup. Although these observations should be considered in the context of an exploratory analysis, they suggest that FCM is beneficial for improving QoL irrespective of hemoglobin level, with patients who have normal hemoglobin levels perhaps standing to benefit most.
By week 52, the difference in adjusted mean change in KCCQ-12 between FCM and placebo had attenuated in both low and normal hemoglobin subgroups. This was likely due in part to the study drug dosing regimen, with FCM only administered up to week 24, with less than one-third of patients in the FCM arm receiving infusions after week 6.25 However, the attenuation effect appeared to be more pronounced in those with low hemoglobin levels, indicating that causes of anemia other than iron deficiency may also have impacted KCCQ-12 scores. Indeed, although 46% of FCM-treated patients with a hemoglobin level <12 g/dL at baseline had a hemoglobin level <12 g/dL at week 52, only about half were iron deficient, suggesting that causes other than iron deficiency may be responsible for the persisting low hemoglobin levels. Based on previous studies, these causes may have included ongoing inflammation, hemodilution, and deficient erythropoietin signaling.26 Because the kidneys play a key role in the generation of erythropoietin, and erythropoietin deficiency is a major driver of low hemoglobin levels in chronic kidney disease,20,21 the interaction between eGFR and hemoglobin level and treatment effect was assessed for the primary outcome; however, no significant interaction was found. Only 2 patients received erythropoiesis-stimulating agents during the study, suggesting limited impact. The frequency of beta-blocker use, which has previously been shown to increase hemoglobin levels in patients with HF,27 was lower in the low versus normal hemoglobin subgroup throughout the trial.
The hemoglobin curves mirrored the KCCQ-12 OSS and CSS curves, with greater improvements in the first 4 to 12 weeks with FCM versus placebo, followed by a subsequent plateau. Again, this is likely to be partly related to the study drug-dosing regimen and suggests that increasing hemoglobin may correlate with improvements in QoL. Although increases in hemoglobin, serum ferritin, and TSAT were greater with FCM versus placebo irrespective of hemoglobin level, limited “spontaneous improvements” in iron parameters (including hemoglobin) were also observed in the placebo arm of those who had low hemoglobin levels at baseline. Combined with the intensification of all relevant treatments after a hospital admission, this may partly explain the return to normal hemoglobin levels in more than one-third of patients on placebo at week 24.
Strengths and Limitations
Some strengths and limitations warrant consideration. First, these exploratory analyses were performed on data from a trial that did not meet its primary composite end point, potentially due, in part, to the effect of the COVID-19 pandemic (with an a priori–defined pre–COVID-19 sensitivity analysis showing a significant benefit of FCM versus placebo on the primary composite of total HF hospitalizations and cardiovascular death).17 Second, because these were subgroup analyses, patients were not randomized according to hemoglobin level at baseline, and there was limited statistical power after stratification of patients into subgroups; however, the AFFIRM-AHF trial was a robust clinical trial with well-defined populations of patients at high risk of HF. The agreement of main and sensitivity analyses (both of which suggest that the benefit of FCM is irrespective of hemoglobin level) adds to this robustness. The data presented herein should be considered exploratory and hypothesis generating only.
Conclusions
In AFFIRM-AHF, treating iron deficiency with intravenous FCM after stabilization from an AHF episode resulted in reductions in the risk of HF hospitalizations and improvements in disease-specific QoL, although the primary composite end point of HF hospitalization and cardiovascular death was not met.17,25 This prespecified subgroup analysis showed that the effects of FCM versus placebo observed in the AFFIRM-AHF trial were similar irrespective of baseline hemoglobin level, with improvements in iron parameters and QoL observed through time in patients with both low and normal hemoglobin levels.
Article Information
Acknowledgments
Editorial support was provided by Erin Knox-Macaulay of AXON Communications (London, UK) and funded by Vifor Pharma Ltd. Biostatistical analysis services were provided by Bernard Roubert of Vifor Pharma Ltd (Glattbrugg, Switzerland).
Sources of Funding
AFFIRM-AHF (a randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure) was funded by Vifor Pharma.
Disclosures
Dr Filippatos has received personal fees from Servier (lecture and registry committee member), Novartis (lecture fees and trial/registry committee member), and Boehringer Ingelheim (lecture and trial committee member). Dr Ponikowski has received research grants and personal fees from Vifor Pharma (principal investigator of AFFIRM-AHF [a randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure]; participation in clinical trials); personal fees from Amgen, Bayer, Novartis, Abbott Vascular, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Berlin Chemie, Cibiem, BMS, and Impulse Dynamics (participation in clinical trials). Dr Farmakis has received speaker honoraria and/or consultation fees from Abbott Laboratories, Bayer, Boehringer Ingelheim, Leo, Novartis, and Orion, outside the submitted work. Dr Anker has received grants from Abbott Vascular and Vifor International; and personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bioventrix, Brahms, Cardiac Dimensions, Cardior, Cordio, CVRx, Edwards, Impulse Dynamics, Janssen, Novartis, Occlutech, Respicardia, Servier, Vectorious and V-Wave, all outside the submitted work. Dr Butler has received personal fees from Vifor Pharma, Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and V-Wave Limited (consultant). Drs Fabien and Waechter have received personal fees from Vifor Pharma (Vifor Pharma employee). Dr Macdougall has received personal fees for consulting from Vifor Pharma and GlaxoSmithKline and played a leadership/fiduciary role in the ASCEND trial steering committee for GlaxoSmithKline. Dr Metra has received personal fees from Vifor Pharma (executive committee member), Amgen (executive committee member and national principal investigator), AstraZeneca, Abbott Vascular, Bayer (participation in advisory boards), Servier (participation in advisory boards and speeches at sponsored symposia), Edwards Therapeutics (speeches at sponsored symposia), Actelion (DMC member), LivaNova (executive committee member), and Windtree Therapeutics (executive committee member and advisory board). Dr Ruschitzka reports personal fees from Vifor for his role in the clinical event adjudication committee for Vifor in 2016 during the conduct of the study; he has not received personal payments by pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent in activities, such as participation in, and a steering committee member of, clinical trials, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research, educational, and/or travel grants from Abbott, Amgen, AstraZeneca, Bayer, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, V-Wave, Vascular Medical, Vifor, Wissens Plus, and ZOLL. Research and educational grants do not impact Dr Ruschitzka’s personal remuneration. The University Medical Center Groningen, which employs Dr van der Meer, has received research grants and fees from Vifor Pharma (executive committee, speaker); research grants from AstraZeneca, Ionis, Pfizer, Novo Nordisk, and Pharma Nord; and speaker fees from Novartis, Novo Nordisk, Pharmacosmos, BridgeBio and Abbott. Dr Jankowska has received research grants and personal fees from Vifor Pharma (co-principal investigator of the AFFIRM trial); personal fees from Bayer, Novartis, Abbott, Boehringer Ingelheim, Pfizer, Servier, AstraZeneca, Berlin Chemie, Cardiac Dimensions, Fresenius, and Gedeon Richter. Drs Kirwan and Rosano declare no competing interests.
Supplemental Material
Tables S1–S5
Figures S1–S17
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AFFIRM-AHF
- randomized, double-blind placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalizations and mortality in iron-deficient subjects admitted for acute heart failure
- AHF
- acute heart failure
- CSS
- clinical summary score
- FCM
- ferric carboxymaltose
- HF
- heart failure
- KCCQ-12
- 12-item Kansas City Cardiomyopathy Questionnaire
- mITT
- modified intention-to-treat
- OSS
- overall summary score
- QoL
- quality of life
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.060757.
For Sources of Funding and Disclosures, see page 1652.
Circulation is available at www.ahajournals.org/journal/circ.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Gerasimos Filippatos, Email: gfilippatos@gmail.com.
Piotr Ponikowski, Email: ppponikowski@gmail.com.
Dimitrios Farmakis, Email: dimitrios_farmakis@yahoo.com.
Stefan D. Anker, Email: s.anker@cachexia.de.
Javed Butler, Email: javed.butler@bswhealth.org.
Vincent Fabien, Email: Vincent.Fabien@viforpharma.com.
Bridget-Anne Kirwan, Email: bridget.kirwan@socar.ch.
Iain C. Macdougall, Email: iain.macdougall11@gmail.com.
Marco Metra, Email: metramarco@libero.it.
Giuseppe Rosano, Email: giuseppe.rosano@gmail.com.
Frank Ruschitzka, Email: frank.ruschitzka@usz.ch.
Peter van der Meer, Email: p.van.der.meer@umcg.nl.
Sandra Wächter, Email: Sandra.Waechter@viforpharma.com.
Ewa A. Jankowska, Email: ewa.jankowska@umw.edu.pl.
References
- 1.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, Banasiak W, Polonski L, Filippatos G, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–1880. doi: 10.1093/eurheartj/ehq158 [DOI] [PubMed] [Google Scholar]
- 2.Klip IT, Comín-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582.e3. doi: 10.1016/j.ahj.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 3.Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71:782–793. doi: 10.1016/j.jacc.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 4.Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, Borodulin-Nadzieja L, von Haehling S, Doehner W, Banasiak W, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. doi: 10.1016/j.cardfail.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 5.Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comín-Colet J. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174:268–275. doi: 10.1016/j.ijcard.2014.03.169 [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, Dahm JB, Angermann CE. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP Registry. Clin Res Cardiol. 2017;106:436–443. doi: 10.1007/s00392-016-1073-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleśkowska-Florek W, Zymliński R, Biegus J, Siwołowski P, Banasiak W, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J. 2014;35:2468–2476. doi: 10.1093/eurheartj/ehu235 [DOI] [PubMed] [Google Scholar]
- 8.Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2018;73:115–123. doi: 10.1080/00015385.2017.1351239 [DOI] [PubMed] [Google Scholar]
- 9.Nakano H, Nagai T, Sundaram V, Nakai M, Nishimura K, Honda Y, Honda S, Iwakami N, Sugano Y, Asaumi Y, et al. ; NaDEF investigators. Impact of iron deficiency on long-term clinical outcomes of hospitalized patients with heart failure. Int J Cardiol. 2018;261:114–118. doi: 10.1016/j.ijcard.2018.03.039 [DOI] [PubMed] [Google Scholar]
- 10.van der Wal HH, Grote Beverborg N, Dickstein K, Anker SD, Lang CC, Ng LL, van Veldhuisen DJ, Voors AA, van der Meer P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J. 2019;40:3616–3625. doi: 10.1093/eurheartj/ehz680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia H, Shen H, Cha W, Lu Q. The prognostic significance of anemia in patients with heart failure: a meta-analysis of studies from the last decade. Front Cardiovasc Med. 2021;8:632318. doi: 10.3389/fcvm.2021.632318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, et al. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034 [DOI] [PubMed] [Google Scholar]
- 13.Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart Rothe B, et al. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail. 2013;15:1267–1276. doi: 10.1093/eurjhf/hft099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangel I, Gonçalves A, de Sousa C, Leite S, Campelo M, Martins E, Amorim S, Moura B, Silva Cardoso J, Maciel MJ. Iron deficiency status irrespective of anemia: a predictor of unfavorable outcome in chronic heart failure patients. Cardiology. 2014;128:320–326. doi: 10.1159/000358377 [DOI] [PubMed] [Google Scholar]
- 15.Young JB, Anand IS, Diaz R, Maggioni AP, McMurray JJV, O’Connor C, Pfeffer MA, Solomon S, Tendera M, van Veldhuisen DJ, et al. Reduction of Events with Darbepoetin Alfa in Heart Failure (RED-HF) Trial (abstract). J Card Fail. 2006;12:S77. [Google Scholar]
- 16.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJV, O’Connor C, Pfeffer MA, et al. ; RED-HF Committees. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865 [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Kirwan B-A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, et al. ; AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396:1895–1904. doi: 10.1016/S0140-6736(20)32339-4 [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, et al. Rationale and design of the AFFIRM-AHF trial: a randomised, double-blind, placebo-controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron-deficient patients admitted for acute heart failure. Eur J Heart Fail. 2019;21:1651–1658. doi: 10.1002/ejhf.1710 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. 2011. Available at https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1. Accessed September 2022.
- 20.Batchelor E, Kapitsinou P, Pergole P, Kovesdy C, Jalal D. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis and treatment. J Am Soc Nephrol. 2020;31:456–468. doi: 10.1681/ASN.2019020213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kidney Dis. 2001;38:415–425. doi: 10.1053/ajkd.2001.26111 [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Comín-Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355 [DOI] [PubMed] [Google Scholar]
- 23.Chopra VK, Anker SD. Anaemia, iron deficiency and heart failure in 2020: facts and numbers. ESC Heart Fail. 2020;7:2007–2011. doi: 10.1002/ehf2.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Meara E, Rouleau JL, White M, Roy K, Blondeau L, Ducharme A, Neagoe PE, Sirois MG, Lavoie J, Racine N, et al. ; ANCHOR Investigators. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail. 2014;7:773–781. doi: 10.1161/CIRCHEARTFAILURE.114.001100 [DOI] [PubMed] [Google Scholar]
- 25.Jankowska EA, Kirwan BA, Kosiborod M, Butler J, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Filippatos G, Keren A, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: the results of the AFFIRM-AHF study. Eur Heart J. 2021;42:3011–3020. doi: 10.1093/eurheartj/ehab234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. doi: 10.1161/CIRCULATIONAHA.118.030099 [DOI] [PubMed] [Google Scholar]
- 27.Khan W, Deepak SM, Coppinger T, Waywell C, Borg A, Harper L, Williams SG, Brooks NH. Beta blocker treatment is associated with improvement in renal function and anaemia in patients with heart failure. Heart. 2006;92:1856–1857. doi: 10.1136/hrt.2005.083998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.