Abstract
Purpose of review
In 2010, the WHO-Informal Working Group on Echinococcosis (IWGE) published an Expert Consensus on the diagnosis and treatment of echinococcal infections. We provide an update on the diagnosis of cystic echinococcosis through a scoping review of the literature published after the release of the WHO-IWGE document.
Recent findings
Ultrasound accurately and reliably depicts the pathognomonic signs of cystic echinococcosis (CE) stages compared with other imaging techniques. Among these, T2-wighted MRI is to be preferred to computed tomography, which has poor performance for the etiological diagnosis of CE. A negative serology cannot exclude the diagnosis of CE, while a positive serology, applied after the visualization of a CE-compatible lesion, may confirm a CE diagnosis. Serology alone must not be used to define ‘CE’ nor as ‘screening’ tool for infection. Other imaging and laboratory techniques did not show clinically applicable performances.
Summary
In the absence of a focal lesion compatible with a CE cyst, no diagnosis of CE should be attempted. There is urgent need to achieve univocal CE case definitions and consensus diagnostic algorithm, as well as standardization of diagnostic methods and issue of a Target Product Profile of CE diagnostics, as advocated by the WHO in the 2021–2030 roadmap for neglected tropical diseases (NTDs).
Keywords: cystic echinococcosis, imaging, laboratory diagnosis, serology
INTRODUCTION
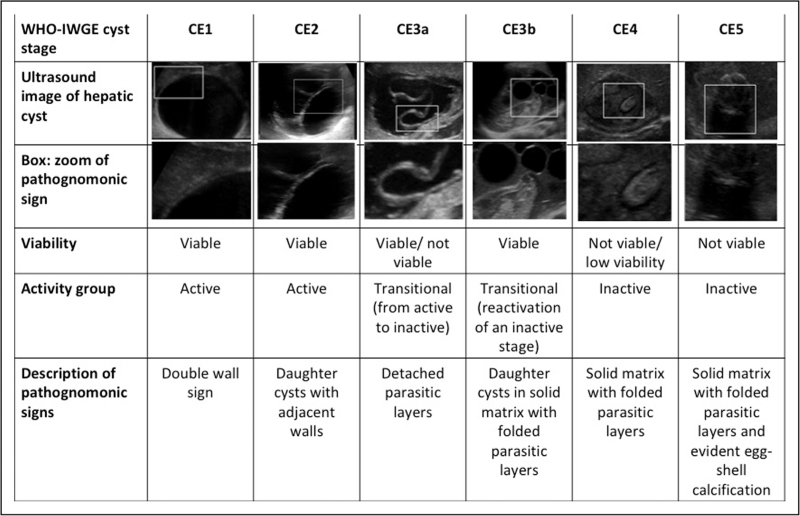
In the intermediate host, including humans, the larval stage (metacestode) of Echinococcus granulosus sensu lato, causing cystic echinococcosis (CE), develops as a well delimited cystic lesion (with the exception of the osseous localization, where infiltrative growth occurs [1]). CE cysts may present with variable morphology, classified in stages as schematized in Fig. 1[2]. Notably, there is a fairly good correspondence between these stages and the biological viability for what concerns CE1-CE2-CE3b cysts (biologically viable) and CE4-CE5 cysts (inactive/not viable) [3]. Exceptions are CE3a cysts, which can be viable or not viable [3], and a proportion of CE4 cysts, which can be still viable in a variable percentage of cases [4–6]. Currently, no assay can identify what individual CE3a or CE4 cyst is actually viable or not viable, implying the need for years-long follow-up with imaging to visualize morphological changes reflecting viability outcomes. The correct etiological identification of a focal lesion is of paramount importance to implement the appropriate clinical management and avoid misdiagnoses and mistreatment, which can have devastating consequences for the patient. However, despite the worldwide distribution of cystic echinococcosis, its diagnosis is still difficult, and a consensus diagnostic algorithm is unavailable. The main difficulties in the diagnosis of cystic echinococcosis are reaching a conclusive etiological diagnosis by noninvasive methods and evaluating CE cyst viability of CE3a and CE4 stages, which are pivotal for clinical decision-making.
FIGURE 1.
CE cyst stages according to the WHO-IWGE classification [2], viability, activity status, and description of pathognomonic signs on ultrasound.
Box 1.
no caption available
In 2010, the WHO-Informal Working Group on Echinococcosis (IWGE) published an Expert Consensus on the diagnosis and treatment of echinococcal infections [2], which included the cyst classification and case definitions. This article aims to provide an update on the diagnosis of cystic echinococcosis by means of a scoping review of the literature published after the release of this document [2]. We focus on tools for the diagnosis of patients with uncomplicated cystic echinococcosis, which is both the most common and probably the most diagnostically problematic clinical presentation. Studies not including diagnostic tools applied on humans/human samples and methods applied to cyst material obtained invasively, will not be reviewed here. So far, seroassays have proven poorly reliable for defining clinical outcome during the follow-up, which remains based on imaging, and this aspect would not be specifically reviewed here either.
LITERATURE SEARCH
On 6 February 2023, we performed a PubMed (MEDLINE) literature search using the strategy reported in Supplementary file 1. We restricted the search to human studies published after April 2010, when the WHO-IWGE Expert Consensus [2] was published. No language restriction was applied.
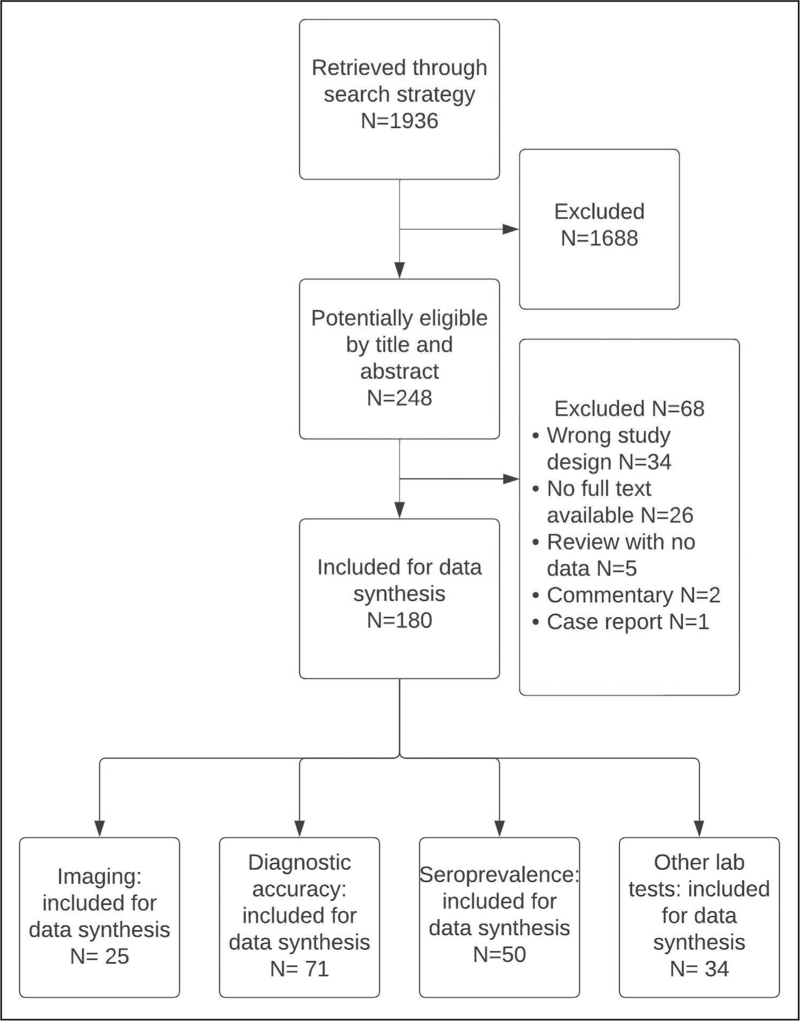
Original prospective and retrospective cohorts, case--control, cross-sectional and diagnostic accuracy studies, as well as systematic reviews of the topics of interest were reviewed. When the full text was not available, data were extracted from the abstract, when possible. The flow diagram of the electronic searches and selection of publications is shown in Fig. 2. The full list of papers finally included in this review is available in Supplementary file 2.
FIGURE 2.
Literature search and selection.
UPDATES ON IMAGING TECHNIQUES FOR THE ETIOLOGICAL DIAGNOSIS AND STAGING OF ECHINOCOCCAL CYSTS
In uncomplicated cystic echinococcosis, especially in the liver, diagnosis and cyst staging are two inseparable aspects, as cystic echinococcosis stages are characterized by pathognomonic features, the recognition of which allow both etiological diagnosis and staging (Fig. 1). On the basis of these features, differential diagnosis and clinical decision-making are based.
Ultrasonography, by which CE cyst stages are defined, accurately and reliably depicts the pathognomonic signs of CE cyst stages, both when compared with histopathology of surgically removed cysts [7], and to other imaging techniques [8▪▪]. The seminal study by Stojkovic et al.[8▪▪] rigorously evaluated the agreement on cyst staging between three imaging modalities, demonstrating that MRI had almost perfect agreement (Cohen's Kappa 0.8–1) with ultrasonography, while agreement of CT was only moderate (Cohen's Kappa 0.6–0.7). In particular, heavily T2-weighted sequences have excellent performance for cyst staging [8▪▪]. On the contrary, diffusion-weighted MRI (DW-MRI) performs poorly [9,10]. T2-weighted pulmonary MRI was also found having good performance compared with CT for the etiological diagnosis of lung cystic echinococcosis [11–13]. As known, CT better detects cyst calcifications [8▪▪,11,14]. However, the presence of peripheral ‘egg-shell’ calcification is not pathognomonic of CE cysts, therefore cannot, alone, lead to the diagnosis of cystic echinococcosis; on the contrary, the presence of isolated intra-lesion calcifications can exclude the diagnosis of cystic echinococcosis. Similarly, contrast enhancement of cyst components can exclude cystic echinococcosis, as the metacestode is not vascularized, but, clearly, absence of contrast-enhancement cannot confirm the diagnosis of cystic echinococcosis. The unreliable performance of CT in depicting signs diagnostic for cystic echinococcosis and in differentiating cyst stages, which need different management approaches, clearly make this technique less adequate than ultrasonography and MRI for the work-up of suspect cystic echinococcosis.
Differential diagnosis of CE cysts is wide, ranging from simple cysts to neoplasms. Of particular importance is the differential diagnosis with alveolar echinococcosis caused by Echinococcus multilocularis, especially in co-endemic areas, as cystic echinococcosis and alveolar echinococcosis are two very different diseases and dramatic consequences may derive from their inaccurate differentiation [15]. Several studies published in the last decade have explored the usefulness of advanced imaging techniques, including ultrasound elastography, CT, and DW-MRI, for the differential diagnosis of cystic echinococcosis and other lesions; however, results were variable and overall disappointing when coming to clinical usefulness [16–23]. FDG-PET/CT, which has a defined role in alveolar echinococcosis, has been also applied to cystic echinococcosis, with potential usefulness to evaluate the presence of inflammatory complications [24–26], but not for evaluation of CE cysts in terms of viability [27].
The interpretation (and execution, in the case of ultrasonography) of imaging techniques towards the recognition of pathognomonic signs of cystic echinococcosis (or which exclude cystic echinococcosis) is operator-dependent. Recently, several studies have tried to apply automatic classification algorithms to ultrasonography and CT images, to improve the diagnosis of cystic echinococcosis also by nonexpert personnel. When applied to the differential diagnosis of cystic echinococcosis vs. alveolar echinococcosis using CT, performance was clinically unsatisfactory [28▪▪]. Studies based on ultrasonography images aiming to automatically classify cyst stages had variable accuracy [29▪,30▪], but did not address the main interest of automatic imaging interpretation, that is differential diagnosis. On the contrary, the reliability of operators in recognizing CE cysts pathognomonic signs and stages by ultrasonography has been evaluated with positive results. When experts on ultrasonography diagnosis of cystic echinococcosis were involved in a study aiming to evaluate the reliability of WHO-IWGE US classification of CE cysts [31], it was found a substantial to almost perfect inter-observer and intra-observer agreement, confirming that experts can reliably identify and stage CE cysts based on ultrasonography. In the context of a four decades-long control programme for cystic echinococcosis in Argentina, using ultrasonography since 1997 as the only diagnostic tool for cystic echinococcosis population screening [32], a yearly FASE (Focused Assessment with Sonography for Echinococcosis) short course is used since year 2000 to train rural physicians [33▪▪]. The authors reported that on the first screening performed by trainees immediately after the course, all suspected cases, re-evaluated by an experienced operator, were not confirmed as cystic echinococcosis. This stresses that both technical ultrasonography skills and specific knowledge and experience in recognizing cystic echinococcosis pathognomonic imaging features are required for an accurate diagnosis. However, the authors also discuss that the cost of this counter-evaluation was lower than that which would be caused by complications of undiagnosed cystic echinococcosis.
UPDATES ON SEROLOGY AND OTHER LABORATORY TOOLS FOR THE DIAGNOSIS OF ECHINOCOCCAL CYSTS
In the WHO-IWGE Expert Consensus document [2], the application of ‘high-sensitivity serological tests, confirmed by a separate high specificity serological test’ is listed among diagnostic criteria for cystic echinococcosis. More recent studies allowed better framing several aspects of the serodiagnosis of cystic echinococcosis, such as the variables associated with seroassays’ results, the comparative performance of different seroassays and the rationale application of seroassays within the diagnostic work-up.
Seroassays for cystic echinococcosis are extremely heterogeneous in terms of format, antigen(s) used and robustness, as highlighted by recent studies comparing different assays or the same assay in different laboratories [34▪,35▪,36▪,37▪]. This stresses the recommendation that centres performing serology for cystic echinococcosis should evaluate the performance of tests they use, calculating accuracy and posttest probabilities in their own epidemiological context, by analysis of sera from clinically well characterized cohorts. Well known causes of cross-reactivity in seroassays for cystic echinococcosis include mainly E. multilocularis and Taenia solium/cysticercosis [38,39], among others. Furthermore, in endemic regions, it is well known that individuals seropositive to E. granulosus markedly outnumber actual cases of cystic echinococcosis. This is confirmed by recent population studies and hospital-based cohorts retrieved by this review [40–48], with seropositivity being found in a large proportion of imaging-negative people (two to eight times higher seroprevalence than prevalence of actual infection with the metacestode), including in areas where no cystic echinococcosis cases were detected. This seropositivity-only condition has been suggested to be in part due to ‘exposure’ to the parasite, but it appears not predictive of the future development of a CE cyst [49]. The parasite-specific antibody profiling of these seropositive imaging-negative individuals showed a profile compatible with a poorly specific immune response [50]. The misleading outcome of false positivity can be at least partially overcome by the application of serology only after a lesion compatible with cystic echinococcosis is visualized. This strategy increases the pretest probability of cystic echinococcosis infection and therefore improves the posttest probability result of the seroassay, especially if more than one test based on different antigens is applied [35▪,37▪,51]. False-negative results can be more difficult to cope with. False-negative results in confirmed cystic echinococcosis cases have been robustly associated with CE cyst stage (CE1 and CE4-CE5 stages vs. CE2-CE3a-CE3b), independently of the test format and antigen(s) used [52▪▪]. Other factors that are associated with higher proportion of false-negative serology results are CE cyst localization (extra-hepatic vs. hepatic) [53–65], size (small vs. large), number (single vs. multiple) and integrity (untreated/complicated vs. treated/complicated) [39,57,60,65,66]. In diagnostic accuracy and hospital cohort studies retrieved in this review [53–65], seropositivity rates in individuals with lung cystic echinococcosis ranged from 80% at best to as low as 17%, or 12% in individuals with cysts in uncommon localizations. These figures are always lower that those obtained with hepatic cystic echinococcosis. Despite their importance in seroassays’ results, these variables are infrequently reported in published studies, hampering a precise evaluation of their performances. Of 71 diagnostic accuracy studies retrieved by this review and available for data extraction (Supplementary file 3), only 23 (32.4%) described the samples cohort in terms of cyst stage, 40 (56.3%) described the localization of the cysts and 28 (40%) mentioned whether the serum was collected before or after treatment. Of note, only 22 out of 66 publications (33.8%) aiming to assess also test specificity included a clinically relevant control group (i.e. patients with other focal lesions, alveolar echinococcosis and so on). In synthesis, therefore, a negative serology cannot exclude the diagnosis of cystic echinococcosis while a positive serology, if carried out with validated assays and applied only after the visualization of a CE-compatible lesion, may confirm a diagnosis of cystic echinococcosis. Confirmation can also be achieved through observation of seroconversion and/or change in cyst morphology after treatment of a CE-compatible cyst with liquid content (ex-juvantibus).
Taken together, these results confirm and reinforce the indication that serology alone must not be used to define ‘CE’ nor as a ‘screening’ tool for infection, as the higher rate of seropositivity in population studies does not reflect high sensitivity but rather low specificity of serology for cystic echinococcosis, an infection with low-prevalence and therefore low pretest probability. Furthermore, its theoretical use for ‘early diagnosis’ or for ‘capturing active cysts’ is thwarted by its low sensitivity especially for ‘young’ CE1 cysts and by its positivity also in a proportion of cases with inactive CE cysts. Finally, its theoretical use for ‘screening for CE in localizations not explorable by US’ is contradicted by the low sensitivity for extra-hepatic cystic echinococcosis. Unfortunately, studies using only serology are still carried out and published [18/61 (29.5%) of population/surveillance-based surveys published in the target period of this review] (Supplementary file 3), which provide uninformative data on prevalence of infection in a population.
Other diagnostic assays different than those detecting antibodies have been applied for the diagnosis of cystic echinococcosis, or the definition of cyst's viability or potential usefulness for follow-up (Supplementary file 3). Of the 34 studies published in the target timeframe of this review, n = 9 investigated genomic/miRNA targets, n = 8 serum host-derived markers, n = 6 antigen detection, n = 5 proteomics/metabolomics, n = 4 in-vitro cytokine release and n = 2 spectrometric analyses. With the caveat that we did not evaluate here the study design in terms of cystic echinococcosis and control cohort characteristics, in general, these markers seem still quite far from possible use in practice. Of the 32 studies from which data were available, nine (28.1%) did not report practically applicable cut-off/accuracy data, and of the 23 studies wherein such practical interpretation was provided, 15 (65.2%) reported low (<80%) sensitivity. The other eight (34.8%) studies reporting higher sensitivities [67–74] encompassed very different targets, with antigen detection and PCR as the diagnostic techniques most easily implementable, should any of these targets be further validated and reach clinical practice.
THE WAY FORWARD TOWARDS THE FORMALIZATION OF CONSENSUS CYSTIC ECHINOCOCCOSIS CASE DEFINITIONS AND DIAGNOSTIC ALGORITHM
The WHO-IWGE Expert Consensus [2] included clinical and diagnostic criteria on which possible, probable and confirmed cystic echinococcosis cases were defined. However, in the light of current data available, these criteria need revision. For example, pathognomonic ultrasonography features were not taken into account and only intervention-related analyses (microscopy or molecular biology on invasively collected material; response to treatment) were envisaged in the definition of ‘confirmed CE’. Furthermore, ‘possible CE’ could be defined as seropositivity-only. In the opinion of who writes, a more practically useful definition of ‘confirmed’ vs. ‘suspect’ cystic echinococcosis is needed, which takes, in the first place, from the identification of an actual lesion compatible with cystic echinococcosis.
To complicate things further, other sources of data inaccuracy hamper the estimate of disease burden from official records. For example, in Europe, heterogeneous requirements apply for reporting cystic echinococcosis vs. alveolar echinococcosis as compared to ‘echinococcosis’ in general [75] and the cystic echinococcosis case definition included in current legislation is not even in line with that currently provided by the WHO, as it envisages only the ‘confirmed case’ category and this is also defined by seropositivity-only [76].
DISCUSSION/CONCLUSION
The diagnosis of cystic echinococcosis should be based on imaging (i.e. in the absence of a focal lesion compatible with a CE cyst, no diagnosis of cystic echinococcosis case should be attempted), while serology has a complementary role for cystic echinococcosis confirmation (but not for ruling cystic echinococcosis out). Clinical management of uncomplicated cystic echinococcosis depends on correct etiological/differential diagnosis and staging [2], and devastating consequences may derive from the misdiagnosis and mistreatment of cystic echinococcosis and other lesions entering in differential diagnosis [15]. There is therefore an urgent need to achieve univocal case definitions, to be then received by national and international stakeholders, and to achieve consensus on a diagnostic algorithm. The WHO-IWGE is carrying out, at the time of writing, a Delphi consensus study to this aim. Standardization of diagnostic methods is also terribly needed. Ultrasonography has been demonstrated to be reliable in expert hands [31], but is an operator-dependent exam and standardized cystic echinococcosis-focused training schemes would be advisable. Seroassays are not standardized, have variable performance and too often are not validated using an appropriate panel of ‘local’ sera. The actual issue of a Target Product Profile (TPP) of cystic echinococcosis diagnostics, as now clearly advocated by the WHO in the 2021–2030 roadmap on NTDs [77] will forcibly provide direction in this field, but so far TPP definition is not being worked on.
Acknowledgements
The authors thank Dr Andrea Fittipaldo at IRCCS Sacro Cuore Don Calabria Hospital for the support with the literature search.
Financial support and sponsorship
This work was supported by the Italian Ministry of Health ‘Fondi Ricerca Corrente’ to IRCCS Sacro Cuore Don Calabria Hospital – Linea 2.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Cattaneo L, Manciulli T, Cretu CM, et al. Cystic echinococcosis of the bone: a European multicenter study. Am J Trop Med Hyg 2019; 100:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16. [DOI] [PubMed] [Google Scholar]
- 3.Hosch W, Junghanss T, Stojkovic M, et al. Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed 2008; 21:734–754. [DOI] [PubMed] [Google Scholar]
- 4.Stojkovic M, Rosenberger KD, Steudle F, Junghanss T. Watch and wait management of inactive cystic echinococcosis: does the path to inactivity matter: analysis of a prospective patient cohort. PLoS Negl Trop Dis 2016; 10:e0005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldi F, De Silvestri A, Tamarozzi F, et al. Medical treatment versus «Watch and Wait» in the clinical management of CE3b echinococcal cysts of the liver. BMC Infect Dis 2014; 14:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lissandrin R, Tamarozzi F, Mariconti M, et al. Watch and wait approach for inactive echinococcal cyst of the liver: an update. Am J Trop Med Hyg 2018; 99:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Li ZY, Liu L, et al. Application of ultrasound in the diagnosis and preoperative evaluation of cystic hepatic echinococcosis. Zhonghua Yi Xue Za Zhi 2020; 100:3453–3456. [DOI] [PubMed] [Google Scholar]
- 8▪▪.Stojkovic M, Rosenberger K, Kauczor HU, et al. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis 2012; 6:e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compares the performance of ultrasonography, MRI and CT in depicting the pathognomonic signs which define cystic echinococcosis and its stages.
- 9.Çeçe H, Gündoğan M, Karakaş Ö, et al. The role of diffusion-weighted magnetic resonance imaging in the classification of hepatic hydatid cysts. Eur J Radiol 2013; 82:90–94. [DOI] [PubMed] [Google Scholar]
- 10.Koken D, Cagli B, Tuncel SA, et al. Efficacy of diffusion-weighted MRI in the differentiation of all liver hydatid cyst types: diffusion-weighted MRI in the liver hydatid cysts. J Med Imaging Radiat Oncol 2016; 60:59–65. [DOI] [PubMed] [Google Scholar]
- 11.Tandur R, Irodi A, Chacko BR, et al. Magnetic resonance imaging as an adjunct to computed tomography in the diagnosis of pulmonary hydatid cysts. Indian J Radiol Imaging 2018; 28:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodhi KS, Bhatia A, Samujh R, et al. Prospective comparison of MRI and contrast-enhanced MDCT for evaluation of pediatric pulmonary hydatid disease: added diagnostic value of MRI. Am J Roentgenol 2019; 212:982–987. [DOI] [PubMed] [Google Scholar]
- 13.Choh NA, Parry AH, Wani AH, et al. The spectrum of imaging findings in pulmonary hydatid disease and the additive value of T2-weighted magnetic resonance imaging in its diagnosis. Pol J Radiol 2021; 86:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller J, Stojkovic M, Kauczor HU, et al. Performance of magnetic resonance susceptibility-weighted imaging for detection of calcifications in patients with hepatic echinococcosis. J Comput Assist Tomogr 2018; 42:211–215. [DOI] [PubMed] [Google Scholar]
- 15.Stojkovic M, Mickan C, Weber T, Junghanss T. Pitfalls in diagnosis and treatment of alveolar echinococcosis: a sentinel case series. BMJ Open Gastroenterol 2015; 2:e000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oruc E, Yildirim N, Bolca Topal N, et al. Role of diffusion weighted magnetic resonance imaging (DW MRI) in classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol 2010; 16:279–287. [DOI] [PubMed] [Google Scholar]
- 17.Sonmez G, Sivrioglu AK, Mutlu H, et al. Is it possible to differentiate between hydatid and simple cysts in the liver by means of diffusion-weighted magnetic resonance imaging? Clin Imaging 2012; 36:41–45. [DOI] [PubMed] [Google Scholar]
- 18.Shanshan W, Hui L, Yan L, et al. The study of biochemical profile of cyst fluid and diffusion-weighted magnetic resonance imaging in differentiating hepatic hydatid cysts from liver simple cysts. J Clin Lab Anal 2018; 32:e22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksoy S, Erdil I, Hocaoglu E, et al. The role of diffusion-weighted magnetic resonance imaging in the differential diagnosis of simple and hydatid cysts of the liver. Niger J Clin Pr 2018; 21:212–216. [DOI] [PubMed] [Google Scholar]
- 20.Ran B, Aji T, Jiang T, et al. Differentiation between hepatic cystic echinococcosis types 1 and simple hepatic cysts: a retrospective analysis. Medicine (Baltimore) 2019; 98:e13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalcinoz K, Ikizceli T, Kahveci S, Karahan OI. Diffusion-weighted MRI and FLAIR sequence for differentiation of hydatid cysts and simple cysts in the liver. Eur J Radiol Open 2021; 8:100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durmaz F, Ozgokce M, Turkoglu S, et al. Ultrasound elastography in the differentiation of simple cyst and type I hydatid cyst of the liver. Ultrasound Q 2021; 37:129–132. [DOI] [PubMed] [Google Scholar]
- 23.Karakas E, Uzunköy A, Karakas EY, et al. Efficacy of diffusion-weighted magnetic resonance imaging in follow-up patients treated with open partial cystectomy of liver hydatid cysts. Int J Clin Exp Med 2014; 7:5090–5096. [PMC free article] [PubMed] [Google Scholar]
- 24.Ozmen O, Tatci E, Uslu Biner I, et al. Could SUVmax be an auxiliary parameter in the clinical management of pulmonary hydatid disease? Clin Respir J 2019; 13:58–65. [DOI] [PubMed] [Google Scholar]
- 25.Salvador F, Escolà-Vergé L, Barios M, et al. Usefulness of the FDG PET/CT in the management of cystic echinococcosis: a pilot study. Acta Trop 2022; 227:106295. [DOI] [PubMed] [Google Scholar]
- 26.Yoldaş B, Gürsoy S, Budak E, et al. FDG PET/CT signs of proven pulmonary hydatid cyst: is there any clue? Jpn J Radiol 2022; 40:1194–1200. [DOI] [PubMed] [Google Scholar]
- 27.Manciulli T, Tamarozzi F, D’Alessandro GL, et al. Comment on ‘Usefulness of the FDG PET/CT in the management of cystic echinococcosis: a pilot study’. Acta Trop 2023; 238:106775. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Xin S, Shi H, Jide A. Automatic lesion segmentation and classification of hepatic echinococcosis using a multiscale-feature convolutional neural network. Med Biol Eng Comput 2020; 58:659–668. [DOI] [PubMed] [Google Scholar]; This study explored artificial intelligence for the differentiation of cystic from alveolar echinococcosis using CT scan.
- 29▪.Wu M, Yan C, Wang X, et al. Automatic classification of hepatic cystic echinococcosis using ultrasound images and deep learning. J Ultrasound Med 2022; 41:163–174. [DOI] [PubMed] [Google Scholar]; This study explored artificial intelligence applied to ultrasound imaging for staging of cystic echinococcosis cysts.
- 30▪.Cheng J, Wang H, Li R, et al. A two-stage multiresolution neural network for automatic diagnosis of hepatic echinococcosis from ultrasound images: a multicenter study. Med Phys 2022; 49:3199–3212. [DOI] [PubMed] [Google Scholar]; This study explored artificial intelligence applied to ultrasound imaging for staging of cystic echinococcosis cysts.
- 31.Solomon N, Fields PJ, Tamarozzi F, et al. Expert reliability for the World Health Organization standardized ultrasound classification of cystic echinococcosis. Am J Trop Med Hyg 2017; 96:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lightowlers MW, Gasser RB, Hemphill A, et al. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int J Parasitol 2021; 51:1167–1192. [DOI] [PubMed] [Google Scholar]
- 33▪▪.Del Carpio M, Hugo Mercapide C, Salvitti JC, et al. Early diagnosis, treatment and follow-up of cystic echinococcosis in remote rural areas in Patagonia: impact of ultrasound training of nonspecialists. PLoS Negl Trop Dis 2012; 6:e1444. [DOI] [PMC free article] [PubMed] [Google Scholar]; Application of FASE (Focused Assessment with Sonography for Echinococcosis) short yearly training course for rural physicians in the context of a control programme for cystic echinococcosis.
- 34▪.Jercic MI, Santillan G, Elola S, et al. First inter-laboratory comparison of Echinococcus granulosus sensu lato diagnosis in Latin America. Rev Panam Salud Pública 2019; 43:1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Inter-laboratory evaluation of seroassays for cystic echinococcosis.
- 35▪.Tamarozzi F, Longoni SS, Vola A, et al. Evaluation of nine commercial serological tests for the diagnosis of human hepatic cyst echinococcosis and the differential diagnosis with other focal liver lesions: a diagnostic accuracy study. Diagnostics 2021; 11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparison of the performance of seroassays for cystic echinococcosis, evaluating pre and posttest probability applying one or a combination of assays.
- 36▪.Kronenberg PA, Deibel A, Gottstein B, et al. Serological assays for alveolar and cystic echinococcosis: a comparative multitest study in Switzerland and Kyrgyzstan. Pathogens 2022; 11:518. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparative evaluation of seroassays for cystic echinococcosis in two distinct countries.
- 37▪.Manciulli T, Enríquez-Laurente R, Tamarozzi F, et al. Field performance of a rapid diagnostic test for the serodiagnosis of abdominal cystic echinococcosis in the Peruvian highlands. Am J Trop Med Hyg 2021; 105:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparison of the performance of seroassays for cystic echinococcosis, evaluating pre and posttest probability applying one or a combination of assays.
- 38.Hernández-González A, Muro A, Barrera I, et al. Usefulness of four different Echinococcus granulosus recombinant antigens for serodiagnosis of unilocular hydatid disease (UHD) and postsurgical follow-up of patients treated for UHD. Clin Vaccine Immunol 2008; 15:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernández-González A, Santivañez S, García HH, et al. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis 2012; 6:e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang WB, Xing Y, Xu XC, et al. Community survey, treatment and long-term follow-up for human cystic echinococcosis in northwest China. Chin Med J Engl 2011; 124:3176–3179. [PubMed] [Google Scholar]
- 41.Xu XZ, Jin XL, Jiang WC, et al. Epidemiological survey of echinococcosis in some areas of Jiangsu Province. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2012; 24:697–699. [PubMed] [Google Scholar]
- 42.Huang XM, Lv LF, Liu JP, et al. Surveillance of echinococcosis in Liyang City from 2008 to 2012. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2013; 25:521–523. [PubMed] [Google Scholar]
- 43.Kilimcioğlu AA, Girginkardeşler N, Korkmaz M, et al. A mass screening survey of cystic echinococcosis by ultrasonography, Western blotting, and ELISA among university students in Manisa, Turkey. Acta Trop 2013; 128:578–583. [DOI] [PubMed] [Google Scholar]
- 44.Fortunato S, Castagna B, Monteleone MR, et al. Parasite prevalence in a village in Burkina Faso: the contribution of new techniques. J Infect Dev Ctries 2014; 8:670–675. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Wang H, Han XM, et al. Survey on echinococcosis in Maqing county of Qinghai province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2015; 33:269–272. [PubMed] [Google Scholar]
- 46.Gao C-H, Wang J-Y, Shi F, et al. Field evaluation of an immunochromatographic test for diagnosis of cystic and alveolar echinococcosis. Parasit Vectors 2018; 11:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moshfe A, Sarkari B, Arefkhah N, et al. Seroepidemiological study of cystic echinococcosis in nomadic communities in the southwest of Iran: A population-based study. J Immunoassay Immunochem 2019; 40:183–192. [DOI] [PubMed] [Google Scholar]
- 48.Harandi MF, Moazezi SS, Saba M, et al. Sonographical and serological survey of human cystic echinococcosis and analysis of risk factors associated with seroconversion in rural communities of Kerman, Iran: sonographical and serological survey of human cystic echinococcosis. Zoonoses Public Health 2011; 58:582–588. [DOI] [PubMed] [Google Scholar]
- 49.Hernández A, Cardozo G, Dematteis S, et al. Cystic echinococcosis: analysis of the serological profile related to the risk factors in individuals without ultrasound liver changes living in an endemic area of Tacuarembó, Uruguay. Parasitology 2005; 130:455–460. [DOI] [PubMed] [Google Scholar]
- 50.Mourglia-Ettlin G, Miles S, Hernández A, Dematteis S. Antibody profiling in ultrasound normal individuals with positive serology for cystic echinococcosis. Parasite Immunol 2016; 38:93–100. [DOI] [PubMed] [Google Scholar]
- 51.Vola A, Manciulli T, De Silvestri A, et al. Diagnostic performances of commercial elisa, indirect hemagglutination, and western blot in differentiation of hepatic echinococcal and nonechinococcal lesions: a retrospective analysis of data from a single referral centre. Am J Trop Med Hyg 2019; 101:1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52▪▪.Tamarozzi F, Silva R, Fittipaldo VA, et al. Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: a systematic review of the literature with meta-analysis. PLoS Negl Trop Dis 2021; 15:e0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a systematic review and meta-analysis showing the robust association between positivity rate of seroassays (sensitivity) and stage of hepatic echinococcal cysts.
- 53.Rahimi H, Sadjjadi S, Sarkari B. Performance of antigen B isolated from different hosts and cyst locations in diagnosis of cystic echinococcosis. Iran J Parasitol 2011; 6:12–19. [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J-Y, Gao C-H, Steverding D, et al. Differential diagnosis of cystic and alveolar echinococcosis using an immunochromatographic test based on the detection of specific antibodies. Parasitol Res 2013; 112:3627–3633. [DOI] [PubMed] [Google Scholar]
- 55.Jiao W, Fu C, Liu WL, et al. Diagnostic potential of five natural antigens from Echinococcus granulosus in the patients of cystic echinococcosis with different clinical status. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2014; 32:116–122. [PubMed] [Google Scholar]
- 56.Santivañez SJ, Rodriguez ML, Rodriguez S, et al. Evaluation of a new immunochromatographic test using recombinant antigen B8/1 for diagnosis of cystic echinococcosis. J Clin Microbiol 2015; 53:3859–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sánchez-Ovejero C, Akdur E, Manzano-Román R, et al. Evaluation of the sensitivity and specificity of GST-tagged recombinant antigens 2B2t, Ag5t and DIPOL in ELISA for the diagnosis and follow up of patients with cystic echinococcosis. PLoS Negl Trop Dis 2020; 14:e0008892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esenkaya Taşbent F, Yağci B, Kadiyoran C, İyisoy MS. Comparative evaluation of the efficacy of indirect hemagglutination test and radiological methods in the prediagnosis of cystic echinococcosis. Turk J Parasitol 2021; 45:22–27. [DOI] [PubMed] [Google Scholar]
- 59.Akcam AT, Ulku A, Koltas IS, et al. Clinical characterization of unusual cystic echinococcosis in southern part of Turkey. Ann Saudi Med 2014; 34:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aydin Y, Altuntas B, Kaya A, et al. The availability of Echinococcus IgG ELISA for diagnosing pulmonary hydatid cysts. Eurasian J Med 2018; 50:144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Khattabi W, Aichane A, Riah A, et al. Analysis of radioclinical semiology of pulmonary hydatid cyst. Rev Pneumol Clin 2012; 68:329–337. [DOI] [PubMed] [Google Scholar]
- 62.Ghoshal AG, Sarkar S, Saha K, et al. Hydatid lung disease: an analysis of five years cumulative data from Kolkata. J Assoc Physicians India 2012; 60:12–16. [PubMed] [Google Scholar]
- 63.Malik AA, ul Bari S, Younis M, et al. Primary splenic hydatidosis. Indian J Gastroenterol 2011; 30:175–177. [DOI] [PubMed] [Google Scholar]
- 64.Okus A, Sevinc B, Ay S, et al. Relation between serology and grow-up time in atypically localized hydatic cysts. Turk J Parasitol 2014; 37:257–261. [DOI] [PubMed] [Google Scholar]
- 65.Santivañez SJ, Arias P, Portocarrero M, et al. Serological diagnosis of lung cystic hydatid disease using the synthetic p176 peptide. Clin Vaccine Immunol 2012; 19:944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lissandrin R, Brunetti E, Tinelli C, et al. Factors Influencing the serological response in hepatic echinococcus granulosus infection. Am J Trop Med Hyg 2016; 94:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parija S, Chaya D. Evaluation of a newly designed sandwich enzyme linked immunosorbent assay for the detection of hydatid antigen in serum, urine and cyst fluid for diagnosis of cystic echinococcosis. Trop Parasitol 2013; 3:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue X, Li H, Tang J, et al. Rapid and label-free screening of echinococcosis serum profiles through surface-enhanced Raman spectroscopy. Anal Bioanal Chem 2020; 412:279–288. [DOI] [PubMed] [Google Scholar]
- 69.Alizadeh Z, Mahami-Oskouei M, Spotin A, et al. Parasite-derived microRNAs in plasma as novel promising biomarkers for the early detection of hydatid cyst infection and postsurgery follow-up. Acta Trop 2020; 202:105255. [DOI] [PubMed] [Google Scholar]
- 70.Toribio L, Santivanez S, Scott AL, et al. Diagnostic urinary cfDNA detected in human cystic echinococcosis. Mol Biochem Parasitol 2020; 239:111314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ciftci TT, Yabanoglu-Ciftci S, Unal E, et al. Metabolomic profiling of active and inactive liver cystic echinococcosis. Acta Trop 2021; 221:105985. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Gongsang Q, Ji J, et al. Characterizing dynamic changes of plasma cell-free Echinococcus granulosus DNA before and after cystic echinococcosis treatment initiation. Genomics 2021; 113:576–582. [DOI] [PubMed] [Google Scholar]
- 73.Ben Salah E, Sakly W, Barrera C, et al. Soluble programmed death-1 (sPD-1) as predictor of early surgical outcomes of paediatric cystic echinococcosis. Parasite Immunol 2021; 43:e12809. [DOI] [PubMed] [Google Scholar]
- 74.Shakra MY, Abou-Sheishaa GA, Hafez AO, Shalash IR. Conjugated silver nanoparticles as a diagnostic tool for circulating hydatid antigens. Egypt J Immunol 2022; 29:84–93. [PubMed] [Google Scholar]
- 75.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J 2022; 20:e07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. http://data.europa.eu/eli/dec_impl/2018/945/oj [Accessed 1 April 2023] [Google Scholar]
- 77. World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030.Geneva: WHO; 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.