Abstract
Three molecular typing methods, repetitive-sequence-based PCR (rep-PCR) fingerprinting, plasmid profiling, and arbitrarily primed PCR fingerprinting, were used to characterize isolates of Salmonella enterica serovar Saintpaul. Most of the isolates were obtained from epidemic human cases of food-borne salmonellosis, together with some from the food material suspected to be the source of infection, and a few were obtained from other cases apparently not related to the epidemic. All three methods adequately discriminated the epidemic strain from other strains of the serovar. In addition several isolates from human cases which are not identical to the epidemic strain were found. These isolates therefore must have been responsible for some sporadic infections, which were only temporally related to the epidemic. These strains showed a high degree of similarity to a strain isolated from a turkey. rep-PCR fingerprinting with REP-Dt primers and primer ERIC1R, applicable even to crude cell lysates, offers an attractive choice as a primary method for the discrimination of various Salmonella serotypes as well as isolates within serotype Saintpaul.
Identification of an epidemic strain is often critical to the success of epidemiological investigations aimed at preventing the spread of infection and eradicating its source (24, 25). Methods for epidemiological investigations of bacterial diseases which rely on phenotypical properties of the pathogens often lack the necessary resolution potential for strain discrimination. They are not able to detect minor primary changes in the bacterial genome that do not influence particular features (20, 34). Therefore, although it is true that phenotypic methods of microbial characterization are still useful, newer methods based on the molecular analysis of the genome have become important for the epidemiological characterization of pathogens. Plasmid profiling, arbitrarily primed PCR (AP-PCR) fingerprinting, and, lately, repetitive-sequence-based PCR (rep-PCR) fingerprinting have been extensively used to characterize strains of various bacterial species (1, 5–11, 13, 16–23, 27, 30–32). rep-PCR fingerprinting has been reported as being relatively simple, rapid, and sensitive for discriminating between closely related strains (4, 7, 21–23, 16, 19, 27–30, 33). However, this method has not been applied to date on field strains of Salmonella enterica serovar Saintpaul (referred to herein as S. saintpaul). This pathogen was recently implicated in a food-borne gastroenteritis epidemic in Germany; it, S. enterica serovar Rubislaw (S. rubislaw), and S. enterica serovar Javiana (S. javiana) are the most frequently isolated strains. They have been among 94 different serovars isolated from both patients and paprika-containing food items during the epidemic (12). The current study describes the usefulness of the three molecular typing methods to discriminate the epidemic strain of S. saintpaul from among several isolates of the serovar recovered from human stool samples during the course of the epidemic as well as from sources not related to the epidemic.
MATERIALS AND METHODS
Bacterial isolates.
Bacterial isolates of S. saintpaul used in this work were divided into three groups. Group 1 consisted of five strains obtained from suspected food material (potato chips) isolated between June and July 1993. Group 2 consisted of 39 strains from human cases of salmonellosis isolated between June and September 1993. Group 3 consisted of seven strains: five isolates from human cases not temporally related to the epidemic (one isolate from March 1993, one from November 1995, two from December 1995, and one from March 1996), one isolate obtained from poultry in November 1995, and one unrelated strain from the culture collection of our laboratory. All isolates were received as cultures on standard I agar (15 g of peptone, 3.3 g of beef extract, 6 g of sodium chloride, 1.01 g of glucose, 12 g of agar [pH 7.2]; Merck).
Preparation of crude chromosomal DNA.
The preparation of DNA for rep-PCR followed the boiling method described by Versalovic et al. (28), including the sonication step. Additionally, 1 volume of chloroform was added to the cell suspension after boiling, and the probes were centrifuged at 13,000 × g for 30 s. The clear supernatant was recovered and preserved as crude cell lysates containing DNA. Finally, the nucleic acid concentration was adjusted to 50 ng/μl and the suspension was stored at −20°C ready for use in rep-PCR. DNA from isolates that were used in AP-PCR was purified further by extraction with an equal volume of phenol-chloroform and subsequently with 1 volume of chloroform. DNA in the resulting supernatant was precipitated with 96% ethanol. Finally, the pellet was washed in 70% ethanol, dried under vacuum, and resuspended in 50 μl of T10E1 buffer. The purified DNA was then stored at −20°C for use in AP-PCR.
Conditions of PCR.
Each of the 50-μl PCR volumes was composed of autoclaved deionized water, magnesium-free reaction buffer (pH 9.0; Invitrogen GmbH), a deoxynucleotide triphosphate mixture (200 μM concentrations of dATP, dCTP, dTTP, and dGTP; Pharmacia Biotech), 2.5 U of Taq polymerase (Pharmacia), primer(s) (2 μM for single primers or 1 μM each for paired primers), template DNA representing 100 ng of DNA, and a wax bead containing 3.5 mM magnesium ions (Invitrogen GmbH). The primers used for rep-PCR were REP1R-Dt (5′-IIINCGNCGNCATCNGGC-3′) and REP2-Dt (5′-NCGNCTTATCNGGCCTAC-3′) as a pair (REP-Dt pair) and ERIC1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) as a single (28, 29). AP21 and AP22, used in AP-PCR, have been described by Ralph et al. (17). DNA amplification was done on either Biometra TRIO-Thermoblock (Göttingen, Germany) or on a Hybaid thermocycler (MWG Biotec, Ebersberg, Germany) under block control. The temperature profile for ERIC1R PCR was as described previously (28) with some modifications: 1 initial cycle at 94°C for 4 min, 35 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 65°C for 8 min, with a single final extension step at 65°C for 15 min. For the REP-Dt primer pair, the annealing temperature was 40°C for 1.30 min and the reaction was allowed to run for 30 cycles. Primer extension was at 72°C for 1.30 min, with a final extension temperature of 72°C for 10 min. For AP-PCR with AP21 and AP22, the annealing temperature was 30°C for 1 min and the primers were extended at 72°C for 2 min. The rest of the temperature profile was identical to that for the REP-Dt primer pair.
PCR amplicons were resolved on 1.2% (wt/vol) agarose (Biozym Diagnostik GmbH) by horizontal electrophoresis in TAE buffer (4.84 g of Tris base, 1.14 ml of glacial acetic acid, 0.1 mM EDTA; pH 8.0). Gels were stained with ethidium bromide (0.5 μg per ml of TAE buffer), visualized under UV transillumination, and photographed on a computer-controlled image analyzer (Vilber Lourmat Biotechnology, Torcy, France).
Plasmid profiling.
For plasmid profiling a small-scale procedure of plasmid preparation described by Beyer and Geue was used (3). Plasmid marker DNA from laboratory strains of Escherichia coli was extracted in the same way from agar plates containing appropriate antibiotics.
Plasmid DNA was analyzed by electrophoresis with 60 μl of lysate in 0.6% agarose (Biozym Diagnostik GmbH) in TAE electrophoresis buffer. Electrophoresis was performed at 22 V overnight for detection of high-molecular-weight plasmids and for 7 h for detection of smaller plasmids. E. coli marker DNA (25) for high-molecular-mass plasmids pIP40a (96 MDa), R27 (112 MDa), RP1 (38 MDa), R6K (26 MDa), and R1 (47 MDa) and for low-molecular-mass plasmid V517 (35.4, 4.75, 3.7, 3.4, 2.6, 1.98, 1.78, and 1.4 MDa) was included in the respective gel runs. The gels were stained with ethidium bromide after electrophoresis, and the separated plasmid DNA was visualized by UV transillumination.
Evaluation of fingerprint patterns.
Statistical comparisons of the PCR fingerprints were performed either as unweighted comparisons of peak positions or as comparisons of peak positions and the values of a densitometric scan, and the fingerprints were clustered according to the unweighted pair group method by arithmetic averaging with the computer-assisted analytical program DNA Fingerprint Analyzer (Wincam 2. × Modul; Cybertech, Berlin, Germany).
RESULTS
The aim of this study was to evaluate the usefulness of rep-PCR to discriminate an epidemic strain of S. saintpaul from several isolates of this pathogen obtained from cases of a documented outbreak of salmonellosis.
Table 1, in combination with Fig. 1 and 2, summarizes the results for the rep-PCR fingerprint patterns obtained from isolates of all three groups of S. saintpaul.
TABLE 1.
Fingerprint pattern categories and plasmid profile types obtained from isolates of S. saintpaul
| Isolate | Group | Date of isolation | Source | Pattern by rep-PCR with primer:
|
Plasmid profile type | |
|---|---|---|---|---|---|---|
| REP-Dt | ERIC1R | |||||
| 211054 | 3 | March 1993 | Human | AII | BII | 3 |
| 019171 | 2 | June 1993 | Human | AII | BII | 4 |
| XL4427 | 1 | June 1993 | Potato chips | AI | BI (reference) | 1 |
| 026625 | 2 | July 1993 | Human | AI | BI (reference) | 1 |
| 041120 | 2 | September 1993 | Human | AII | BII | 3 |
| GGD930D | 3 | November 1995 | Poultry | AIII | BII | 2 |
| 143095 | 3 | December 1995 | Human | AIII | BII | 1 |
| 206917 | 3 | March 1996 | Human | AI | BI | 5 |
| 41 | 3 | Unknown | Laboratory strain | AII | BIII | 1 |
FIG. 1.
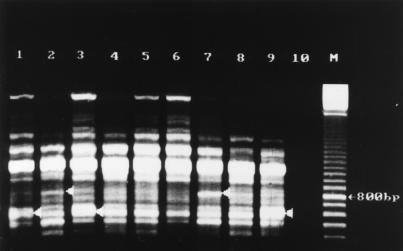
Rep-PCR fingerprint patterns of S. saintpaul obtained with the REP-Dt primer pair. Lanes (isolate, source, date of isolation): 1, XL4427, potato chips, July 1993; 2, GGD930D, poultry, November 1995; 3, 026625, human feces, July 1993; 4, 019171, human feces, June 1993; 5, 041120, human feces, September 1993; 6, 211054, human feces, March 1993; 7, 143095, human feces, December 1995; 8, laboratory strain (date unknown); 9, negative control; 10, 100-bp marker.
FIG. 2.
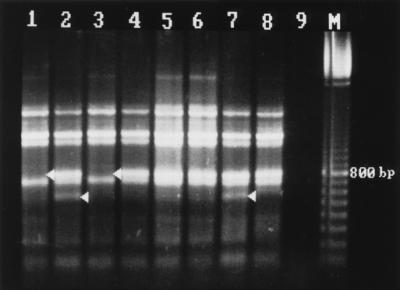
Rep-PCR fingerprint patterns of S. saintpaul with the ERIC1R primer. Lane assignments are the same as in Fig. 1.
Fingerprinting with primer pair REP-Dt (Fig. 1) generated patterns of seven major bands between 500 bp and 1.8 kbp, which were used for comparison analysis. Some minor bands of less than 400 bp and one minor band of more than 2.6 kbp which had poor reproducibility were not included in the analysis. Three different patterns, designated AI to AIII, were generated with this primer pair.
Fingerprinting with primer ERIC1R (Fig. 2) generated patterns of 10 major bands between 200 bp and 1.8 kbp, which were used for comparison analysis. Some additional minor bands were not included in the analysis. Again, three different banding patterns, designated BI to BIII, were generated. Isolates with any of these three patterns are not identical to isolates belonging to pattern groups AI to AIII.
Because all the patterns of group 1 isolates recovered from suspected contaminated human food material (potato chips) yielded identical fingerprints with each primer used (data not shown), only one, isolate XL4427 (Fig. 1 and 2, lanes 1), taken to be a reference strain, was fingerprinted together with each isolate from groups 2 and 3. It was noted that with either primers 37 of 39 isolates of group 2 yielded fingerprints identical to the one from isolate XL4427. For these isolates, isolate 026625 was taken as a reference in Fig. 1 and 2, lanes 3. This prevalent banding pattern has been designated AI/BI. Two of the group 2 isolates, 019171 isolated in June 1993 and 041120 isolated in September 1993, yielded fingerprints with notable variations (Fig. 1 and 2, lanes 4 and 5). These fingerprints belonged to pattern types AII and BII. For a given primer, pattern type II differed from pattern I by having either an additional band or by the lack of a major band. These and other variations in fingerprint patterns were also noted in four of seven of the group 3 isolates. Whereas the remaining three isolates yielded the same pattern as that of the epidemic strain, AI/BI, isolate 211054, obtained from a human in March 1993 (Fig. 1 and 2, lanes 6) had pattern type AII/BII. Isolates 143095 (from a human in November 1995) and GGD930D (from poultry in November 1995) yielded identical fingerprints with both primers, and these differed from the most prevalent pattern, AI/BI, by having an additional band of about 800 bp when REP-Dt was used (Fig. 1, lanes 7 and 2) and by having an additional band of about 1,000 bp as well as a missing band of about 300 bp when ERIC1R was used (Fig. 2, lanes 7 and 2). These differences made the banding patterns of the two isolates highly similar to banding pattern AII and identical to banding pattern BII. But with primer pair REP-Dt an additional band at about 550 bp generated a difference. Therefore, these two isolates had banding patterns belonging to pattern type AIII/BII. With strain 41, which had been isolated and stored in our laboratory about 10 years ago, a banding pattern of the type AII/BIII was generated (Fig. 1 and 2, lanes 8). It was the only strain generating a third pattern with ERIC1R, characterized by two major bands between 300 and 400 bp. Strain 206917, from a human in March 1996, as well as two strains from human feces isolated in November and December 1995 (data not shown) yielded fingerprints identical to those produced by the epidemic strain.
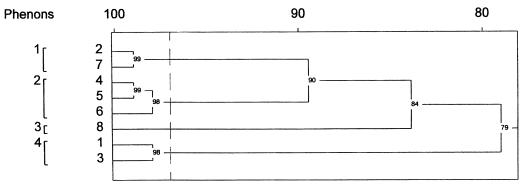
To demonstrate the relationships between the isolates with different fingerprint profiles, the summarized information gained by the banding patterns of both primers was analyzed statistically. As shown in Fig. 3 an analysis of the banding patterns by peak comparison as well as by a comparison of intensities grouped the isolates into two main clusters. One cluster, consisting of strains from group 1 and 2, represents the epidemic strain (XL4427 and 026625). The second cluster consists of two strains from group 2 and four strains from group 3, with a high degree of similarity between the strains from human feces (019171, 041120, 143095, and 211054) and the strain isolated from poultry (GGD930D) about 2 years after the end of the epidemic.
FIG. 3.
Dendrogram of the summarized fingerprint patterns obtained with primers REP-Dt and ERIC1R.
Plasmid profiling grouped the isolates into five types. Type 1, which did not contain any plasmid, consisted of all the strains from food as well as 37 of 39 strains from group 2. This is identical to the results obtained from rep-PCR. The laboratory strain (strain 41) and strain 143095, which have been clearly discriminated from the epidemic strain by rep-PCR, would not have been differentiated by this method. The second profile type contained two plasmids of 2.2 and 3 MDa and is represented by the strain from poultry, GGD930D. The third profile type containing only a 2.7-MDa plasmid was represented by strains 211054 and 041120, which are members of the second phenon produced by rep-PCR. The third strain of this phenon, 019171 from group 2, which had been isolated during the epidemic, showed a different plasmid profile (type 4) with three plasmids of 2.8, 3.0, and 3.4 MDa. Isolate 206917, which had been isolated more than 2 years after the end of the epidemic and which was grouped together with the epidemic strain by rep-PCR, showed a plasmid profile (type 5) with two plasmids of 3.7 and 11 MDa.
In the third molecular strain discrimination method, AP-PCR, all isolates that were discriminated from the epidemic strain by either of the other two techniques were investigated.
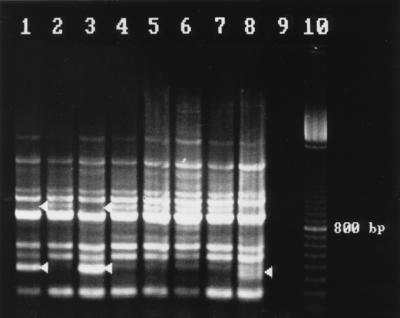
Fingerprinting with AP21 (Fig. 4) generated a pattern of nine bands between 500 bp and 1.7 kbp that was used for comparison analysis, as well as some additional shorter and longer fragments having a low degree of reproducibility. Discrimination between strains was mainly based on reproducible differences in the densities of some bands. Isolates XL4427, 026625, and 206917 (lanes 1, 3, and 9) were characterized by a prominent double band at about 600 bp. The rest of the strains differed from those by having a less prominent upper band of that double. Isolates GGD930D and 143095 could be discriminated by their more-prominent bands at 800 bp. These results are identical to what was obtained by rep-PCR with primer pair REP-Dt.
FIG. 4.
AP-PCR fingerprint patterns of S. saintpaul obtained with the AP21 primer. Lanes (isolate, source, date of isolation): 1, XL4427, potato chips, July 1993; 2, GGD930D, poultry, November 1995; 3, 026625, human feces, July 1993; 4, 019171, human feces, June 1993; 5, 041120, human feces, September 1993; 6, 211054, human feces, March 1993; 7, 143095, human feces, December 1995; 8, 41, laboratory strain (date unknown); 9, 206917, human feces, March 1996; 10, negative control; M, 100-bp marker.
Fingerprinting with AP22 (Fig. 5) generated easily readable patterns of up to six bands between 500 bp and 2.5 kbp, all of which were included in the comparison analysis. One band of about 350 bp had low reproducibility and has, therefore, not been considered further. AP-PCR with primer AP22 distinguished strains XL4427, 026625, and 206917 (lanes 1, 3, and 9, respectively) from the rest of the strains by the presence of an additional intense band at about 2,300 bp. All other strains were not further differentiated with the exception of strain 41, which showed an additional weak band at about 1,600 bp. This differentiation is identical to what was obtained by rep-PCR with primer ERIC1R.
FIG. 5.
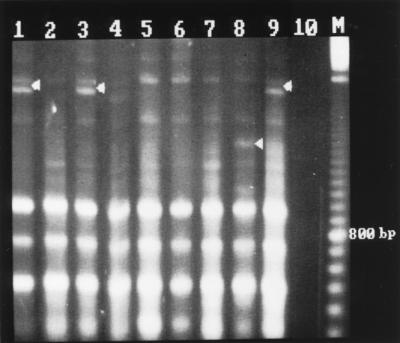
AP-PCR fingerprint patterns of S. saintpaul obtained with the AP22 primer. Lane assignments are the same as in Fig. 4.
Summarizing the information about both AP-PCRs for the nine isolates investigated, the differentiation of strains was the same as that achieved by rep-PCR, although the quality of AP-PCR fingerprints was not as high as that produced by rep-PCR.
DISCUSSION
In this study we investigated the suitability of rep-PCR fingerprinting with primers REP-Dt and ERIC1R for discriminating between strains of S. saintpaul and assessed the suitability of the method, together with plasmid profiling and AP-PCR, when applied to an epidemiological situation involving this pathogen. From among the published rep primers (28) those that showed efficient DNA amplification and good interserovar discrimination between eleven Salmonella serotypes as well as between isolates of the serotype S. saintpaul were selected. Nine single and three paired rep primers were used in a preceding experiment, and primer pair REP-Dt of the class I rep primers and primer ERIC1R of the class II rep primers were chosen because of their high levels of discriminatory power (15).
While it was expected that some strains of our group 3 isolates would be different from the epidemic strain, given their temporal and source diversity, it was noted that some human cases during the epidemic (group 2 isolates) may have been caused by different strains, which could not have been distinguished by conventional methods. These seemingly nonepidemic strains showed a high degree of similarity to some strains in group 3, raising the possibility of an alternative source of infection of humans by S. saintpaul during the epidemic.
From the two fingerprint patterns gained from rep-PCR the conclusion could be drawn that isolates XL4427 and 026625, representing group 1 and the majority of group 2 isolates, respectively, represented the epidemic strain. Both of the rep primers adequately discriminated this strain from other strains of the serovar as summarized in Fig. 3. Isolate XL4427 was different from isolates 019171 and 041120 from group 2 and from isolates 211054, 143095, 41, and GGD930D, all from group 3. All these were therefore considered nonepidemic strains. The fact that these nonepidemic strains were isolated at various times during the epidemic year and also much later (except strain 41) suggested that the strains were instead responsible for sporadic infections. The fingerprints from the nonepidemic strains were either identical (Fig. 2) or at least remarkably similar (Fig. 1) to each other.
Our data obtained from rep-PCR fingerprinting with REP-Dt and ERIC1R supported a conclusion from earlier epidemiological investigations of a nationwide outbreak of salmonellosis which occurred in Germany between April and September 1993. Lehmacher and his colleagues at the National Reference Center for Enteric Pathogens, Hamburg, Germany, used the methods of pulsed-field gel electrophoresis, ribotyping, random amplified polymorphic DNA typing, and plasmid profiling for the molecular typing of 13 strains each of S. saintpaul, S. javiana, and S. rubislaw, considered to be the prevailing strains of the epidemic. But only the data for S. javiana were published (12). Our investigations focused on the strains of S. saintpaul isolated by the State Food Inspection Office from patients and foodstuffs as well as on two strains of S. saintpaul isolated by the Veterinary Control Office for Animal Foodstuffs, both in Stuttgart, Germany. The rep-PCR fingerprinting of these strains yielded additional information. Two isolates, 019171 (recovered in June 1993) and 041120 (recovered in September 1993) from human epidemic cases were not clonally related to the epidemic strain. They rather represented a second strain of S. saintpaul present within the community at the time of the epidemic in south Germany. The identity of this strain with isolate 211054, collected in March 1993, and its high degree of similarity to strains GGD930D and 143095, collected in November 1995 from humans and turkeys, respectively, indicated a wider temporal dispersion, thus suggesting that the strain was responsible for rather sporadic infections independent from the incidence of epidemic cases.
Identification of this sporadic strain in turkeys suggested a possible role of poultry in the epidemiology of sporadic infections by S. saintpaul in south Germany.
The low percentage (only 4 of 42) of clinical isolates of S. saintpaul that contained plasmids limits the scope of plasmid profiling as a tool for an epidemiological investigation. Some differences between the results of rep-PCR and AP-PCR on the one hand and plasmid profiles on the other hand could be noted, raising the question of the value of plasmid profiling as an epidemiological tool. On the one hand all isolates except two that contained plasmids were ones that had been identified by rep-PCR fingerprinting as being different from the epidemic strain, which did not contain any plasmid. In contrast, strain 019171, which was isolated from human feces in June 1993 during the epidemic and which showed a pattern type, AII/BII, identical to that of two strains isolated in March and September 1993, had two additional plasmids. Furthermore, strain 143095, which was isolated from a human in December 1995 and which showed rep-PCR and AP-PCR fingerprints identical to those of strain GGD930D isolated from poultry in November 1995 and highly similar to those of strains 211054, 019171, and 041120, was plasmid free. Most important, strain 206917, isolated from a human in March 1996 and having fingerprint patterns identical to those of the epidemic strain, did contain two plasmids.
The absence or presence of plasmids in bacterial isolates cannot call into question their relatedness as determined by rep-PCR, because repetitive sequences are not known to be situated on plasmids. Bacterial strains of clinical relevance which persist in a host population over a long period of time or which are able to switch between animal and human hosts should be expected to differ in their plasmid content as a form of adaptation to changing environmental pressure. Therefore, the detection of plasmids in isolate 206917, obtained more than 2 years after the epidemic, did not signify a clonal difference between this strain and the one that caused the epidemic since the plasmids could well have been acquired within that span of time. Nevertheless, the absence or presence of plasmids could have a severe influence on the epidemiological interpretation of fingerprinting results, especially if the plasmid content is accompanied by resistance factors or even virulence factors. To test this feature, plasmid profiling should be followed by plasmid analysis (26). In this sense plasmid profiling is a valuable complementary method. Additionally, plasmid profiling should necessarily complement AP-PCR because plasmids can contribute to the banding pattern with arbitrary primers.
Considering the results yielded by AP-PCR fingerprinting, no additional or complementary information beyond what had been gathered by rep-PCR and plasmid profiling could be obtained. Banding patterns obtained with arbitrary primers AP21 and AP22 differed mainly in the densities of some bands and have therefore been rather difficult to interpret. Efforts to obtain reproducible differences between strains of serotype S. saintpaul have been greater than those needed for rep-PCR in terms of the purity of the DNA and the number of repeated experiments. At least for these two primers AP-PCR for the differentiation of isolates of serotype S. saintpaul is not recommended. Several other published random primers have also been used in accompanying experiments without greater success (data not shown).
From the results described in this work it was apparent that rep-PCR fingerprinting with the REP-Dt primer pair and primer ERIC1R, even with crude cell lysates, offers an attractive choice as a primary method for the discrimination of various Salmonella serotypes as well as isolates within serotype Saintpaul. Plasmid profiling can be a useful complementary method to rep-PCR fingerprinting to show if the persistence of a clinically relevant strain in a host population over a long time or the switch from an animal host to a human host (or vice versa) led to a change in plasmid content. This could be of importance for the epidemiological interpretation. The results obtained in this work and the well-documented problems of AP-PCR fingerprinting, especially the critical dependence of reproducibility on reaction conditions and quality of DNA (2, 14, 31), makes the technique comparatively less useful.
ACKNOWLEDGMENTS
This work was financially supported by DAAD.
We thank the technical team of the molecular laboratory of the Institute of Environmental and Animal Hygiene at the University of Hohenheim, Stuttgart, Germany, and in particular Sabine Hoche. We are also indebted to the staff of the Landesgesundheitsamt Baden-Württemberg, in particular R. Freerksen, and to the Staatliches Veterinäruntersuchungsamt Stuttgart, in particular H. Jaus, for supplying all the bacterial isolates used.
REFERENCES
- 1.Bamford K B, Bickley J, Collins J S A, Johnston B T, Potts S, Boston V, Owen R J, Sloan J M. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Syst Appl Microbiol. 1993;18:357–362. doi: 10.1136/gut.34.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassam B J, Caetano-Anolle G, Gresshoff P M. DNA amplification fingerprinting of bacteria. Appl Microbiol Biotechnol. 1992;38:70–76. doi: 10.1007/BF00169422. [DOI] [PubMed] [Google Scholar]
- 3.Beyer W, Geue L. Characterization of the virulence regions in the plasmids of three live salmonella vaccines. Zentbl Bakteriol. 1992;277:10–21. doi: 10.1016/s0934-8840(11)80865-0. [DOI] [PubMed] [Google Scholar]
- 4.De Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergenic consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorn C R, Silapanuntakul R, Angrick E J, Shipmam L D. Plasmid analysis and epidemiology of Salmonella enteritidis infection in three commercial layer flocks. Avian Dis. 1992;36:844–885. [PubMed] [Google Scholar]
- 6.Fitzgerald J R, Meaney W J, Hartigan P J, Smyth C J, Kapur V. Fine-structure molecular epidemiological analysis of Staphylococcus aureus recovered from cows. Epidemiol Infect. 1997;119:261–269. doi: 10.1017/s0950268897007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georghiou P R, Doggett A M, Kielhofner M A, Stout J E, Stout D A, Lupski J R, Hamill R J. Molecular fingerprinting of Legionella species by repetitive element PCR. J Clin Microbiol. 1994;32:2989–2994. doi: 10.1128/jcm.32.12.2989-2994.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graser Y, Klare I, Halle E, Gantenberg R, Buchholz P, Jacobi H D, Presber W, Schonian G. Epidemiological study of an Acinetobacter baumanni outbreak by using polymerase chain reaction fingerprinting. J Clin Microbiol. 1993;31:2417–2420. doi: 10.1128/jcm.31.9.2417-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grattard F, Berthelot P, Reyrolle M, Ros A, Etienne J, Pozzetto B. Molecular typing of nosocomial strains of Legionella pneumophila by arbitrarily primed PCR. J Clin Microbiol. 1996;34:1595–1598. doi: 10.1128/jcm.34.6.1595-1598.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmuth R, Shroeter A. Molecular typing methods for Salmonella enteritidis. Int J Food Microbiol. 1994;21:69–77. doi: 10.1016/0168-1605(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 11.Hermans P W M, Sluijter M, Hoogenboezem T, Heersma H, van Belkum A, de Groot R. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae strains. J Clin Microbiol. 1995;33:1606–1612. doi: 10.1128/jcm.33.6.1606-1612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmacher A, Bockemühl J, Aleksic S. Nationwide outbreak of human salmonellosis in Germany due to contaminated paprika and paprika-powdered potato chips. Epidemiol Infect. 1995;115:501–511. doi: 10.1017/s0950268800058660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu P Y-F, Lau Y-J, Hu B-S, Shyr J-M, Shi Z Y, Tsai W-S, Lin Y-H, Tseng C-Y. Analysis of clonal relationships among isolates of Shigella sonnei by different molecular typing methods. J Clin Microbiol. 1995;33:1779–1783. doi: 10.1128/jcm.33.7.1779-1783.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier J-R, Grimont P A D. Factors affecting reproducibility of random amplified polymorphic DNA fingerprinting. Res Microbiol. 1993;144:373–379. doi: 10.1016/0923-2508(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 15.Mukendi F. The use of repetitive DNA sequence-based polymerase chain reaction (rep-PCR) in genomic fingerprinting of Salmonella Saintpaul. M.S. Thesis. Berlin, Germany: Free University of Berlin; 1995. [Google Scholar]
- 16.Pooler M R, Ritchie D F, Hartung J S. Genetic relationships among strains of Xanthomonas fragariae based on random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus PCR data and generation of multiplexed PCR primers useful for the identification of this phytopathogen. Appl Environ Microbiol. 1996;62:3121–3127. doi: 10.1128/aem.62.9.3121-3127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph D, McClelland M, Welsh J, Baranton G, Perolat P. Leptospira species characterized by arbitrarily primed polymerase chain reaction (PCR) and by mapped restriction polymorphism in PCR-amplified rRNA genes. J Bacteriol. 1993;175:973–981. doi: 10.1128/jb.175.4.973-981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reboli A C, Houston E D, Monteforte J S, Wood C A, Hamill R J. Discrimination of epidemic and sporadic isolates of Acinetobacter baumannii. J Clin Microbiol. 1994;32:2635–2630. doi: 10.1128/jcm.32.11.2635-2640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Barradas M C, Hamill R J, Houston E D, Georghiou P R, Clarridge J E, Regnery R L, Koehler J E. Genomic fingerprinting of Bartonella species by repetitive element PCR for distinguishing species and isolates. J Clin Microbiol. 1995;33:1089–1093. doi: 10.1128/jcm.33.5.1089-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rychlik I, Sisak F, Lany P. Differentiation of Salmonella typhimurium by plasmid profile analysis and restriction endonuclease analysis of chromosomal DNA. Vet Med Czech. 1993;38:433–439. [PubMed] [Google Scholar]
- 21.Sadowsky M J, Kinkel L L, Bowers J H, Schottel J L. Use of repetitive intergenic DNA sequences to classify pathogenic and disease-suppressive Streptomyces strains. Appl Environ Microbiol. 1996;62:3489–3493. doi: 10.1128/aem.62.9.3489-3493.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selenska-Pobell S, Evguenieva-Hackenberg E, Schwickerath O. Random and repetitive primer amplified polymorphic DNA analysis of five soil and two clinical isolates of Rahnella aquatilis. System Appl Microbiol. 1995;18:425–438. [Google Scholar]
- 23.Selenska-Pobell S, Gigova L, Petrova N. Strain-specific fingerprints of Rhizobium galegae generated by PCR with arbitrary and repetitive primers. J Appl Bacteriol. 1995;79:425–431. doi: 10.1111/j.1365-2672.1995.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 24.Threlfall E J, Frost J A. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J Appl Bacteriol. 1990;68:5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 25.Tompkins L S, Troup N, Labigne-Roussel A, Cohen M L. Cloned, random chromosomal sequences as probes to identify Salmonella species. J Infect Dis. 1986;154:156–162. doi: 10.1093/infdis/154.1.156. [DOI] [PubMed] [Google Scholar]
- 26.Tschäpe H, Tietze E. Genetische und molekulare Grundlagen der Plasmidspezieshypothese. Biol Zentbl. 1981;102:385. [Google Scholar]
- 27.Vandamme P, Glupczynski Y, Lage A P, Lammens C, Quint W G V, Goossens H. Evaluation of random and repetitive motif primed polymerase chain reaction typing of Helicobacter pylori. System Appl Microbiol. 1995;18:357–362. [Google Scholar]
- 28.Versalovic J, Schneider M, de Bruijn F, Lupski J R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 29.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versalovic J, Kapur V, Koeuth T, Mazurek G H, Whittam T S, Musser J M, Lupski J R. DNA fingerprinting of pathogenic bacteria by fluorophore-enhanced repetitive sequence-based polymerase chain reaction. Arch Pathol Lab Med. 1995;119:23–29. [PubMed] [Google Scholar]
- 31.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods C R, Jr, Versalovic J, Koeuth T, Lupski J R. Analysis of relationships among isolates of Citrobacter diversus by using DNA fingerprints generated by repetitive sequence-based primers in the polymerase chain reaction. J Clin Microbiol. 1992;30:2921–2929. doi: 10.1128/jcm.30.11.2921-2929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wray C, Mclaren I, Parkinson N M, Beedell Y. Differentiation of Salmonella typhimurium DT204c by plasmid profile and biotyping. Vet Rec. 1987;28:514–516. doi: 10.1136/vr.121.22.514. [DOI] [PubMed] [Google Scholar]