Abstract
The detection of circulating tumor DNA (ctDNA) in liquid biopsy samples as an oncological marker is being used in clinical trials at every step of clinical management. As ctDNA-based liquid biopsy kits are developed and used in clinics, companies work towards increased convenience, accuracy, and cost over solid biopsies and other oncological markers. The technology used to differentiate ctDNA and cell-free DNA (cfDNA) continues to improve with new tests and methodologies being able to detect down to mutant allele frequencies of 0.001% or 1/100,000 copies. Recognizing this development in technology, the FDA has recently given pre-market approval and breakthrough device designations to multiple companies. The purpose of this review is to look at the utility of measuring total cfDNA, techniques used to differentiate ctDNA from cfDNA, and the utility of different ctDNA-based liquid biopsy kits using relevant articles from PubMed, clinicaltrials.gov, FDA approvals, and company newsletters. Measuring total cfDNA could be a cost-effective, viable prognostic marker, but various factors do not favor it as a monitoring tool during chemotherapy. While there may be a place in the clinic for measuring total cfDNA in the future, the lack of standardization means that it is difficult to move forward with large-scale clinical validation studies currently. While the detection of ctDNA has promising standardized liquid biopsy kits from various companies with large clinical trials ongoing, their applications in screening and minimal residual disease can suffer from lower sensitivity. However, researchers are working towards solutions to these issues with innovations in technology, multi-omics, and sampling. With great promise, further research is needed before liquid biopsies can be recommended for everyday clinical management.
Keywords: liquid biopsy, cfDNA, ctDNA
1. Introduction
Screening and early diagnosis are essential strategies to decrease mortality, treatment cost, and disease burden of cancer [1]. Diagnosis of various cancers at earlier stages increases five-year survival and cure rate, and diagnosis at later stages has dramatically higher medical costs [2,3]. After diagnosis, determining prognosis and molecular profiling plays a central role in patient management, especially as precision medicine becomes the status quo of treatment selection [4,5].
As described by the U.S. Preventive Services Task Force (USPSTF), active screening methods, such as mammograms for breast cancer, pap smears for cervical cancer, or colonoscopy for colorectal cancer, have been an effective means of early detection in older patients [6]. Mammograms prevent 21 deaths per 10,000 women screened over 10 years, pap smears prevent up to 8.34 deaths per 1000 women screened to the age of 65, and colonoscopies prevent up to 28 deaths per 1000 adults screened from the age of 45 to 65 [7,8,9]. Additionally, low-dose computed tomography is recommended as a screening method for lung cancer in patients aged 50–80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years which would prevent 503 deaths per 100,000 adults over a lifetime of screening. Even with these screening methods, limited sensitivity, specificity, and low incidence of cancer keeps them from being recommended to healthy individuals, and each method only screens for one cancer type [6].
For diagnosing, grading, and profiling tumors, solid tissue biopsy is the gold standard. However, solid biopsies are skill-intensive, invasive, risk missing tumor heterogeneity, and have low sensitivity [10]. Further, due to its invasive nature, a solid biopsy cannot be carried out for screening and/or monitoring during treatment. For cancers without screening, diagnosis with solid biopsy comes at later stages when patients are symptomatic. This contributes to the poor prognosis of lung, pancreatic, ovarian cancers, and other cancers [11,12,13].
Liquid biopsy (LB) is a technique that is being studied at every step of cancer management for screening, minimal residual disease (MRD), monitoring for recurrence in the adjuvant setting, and treatment selection for advanced cancer. LB mostly uses peripheral blood, but can also include other body fluids, such as urine, saliva, feces, pleural effusions, ascites, and cerebrospinal fluid (CSF) [14]. LB methods have been used to detect circulating tumor DNA (ctDNA), RNA (ctRNA), circulating tumor cells (CTCs), microRNA (miRNA), extracellular vesicles (EVs), and tumor-educated platelets (TEPs).
As a method of early detection, LB can be used to screen for multiple cancers at once, becoming more cost-effective than the array of screening methods used currently [15]. For use in diagnosis, LB has several advantages over solid tissue biopsy. LBs are non-invasive, samples are easier to obtain, and result in faster turnaround time [16]. In addition, LB can be performed repeatedly to monitor for response and progression and achieve better detail of the tumor’s spatial heterogeneity [10,17]. However, LB does not allow for histological evaluation, which would require a solid biopsy, and most panels have a limited number of genes. While there are some FDA-approved LB tests for prognosis and treatment selection, such as CellSearch, cobas EGFR Mutation Test v2, Guardant360 CDx, and FoundationOne Liquid CDx [18], LB tests for screening and diagnosis are still being clinically validated [4,17].
Here, we review methods of isolating ctDNA, as well as their recent applications for the purposes of screening, diagnosing, and management through ctDNA kinetics of various tumor types.
2. Circulating Free DNA
Circulating cell-free DNA (cfDNA) are extracellular fragments of dsDNA between 120–220 bp long, centered around 167 bp, which is associated with the nucleosome pattern of cfDNA in apoptosis [19]. cfDNA has a short half-life that varies from 4 min to 2 h, which lends itself to applications in monitoring. cfDNA can be found in various body fluids, such as blood, urine, or cerebrospinal fluid [20,21,22,23]. Under normal conditions, cfDNA can come from apoptosis, neutrophil extracellular traps (NETs), and erythroblast enucleation [24,25,26]. In plasma, cfDNA originates from granulocytes (32%), erythrocyte progenitors (30%), lymphocytes (12%), monocytes (11%), vascular endothelial cells (9%), and hepatocytes (1%) [27]. The cfDNA can be increased in normal physiological processes, such as physical exercise, or in pathological processes that increase cell death, such as inflammation, sepsis, or myocardial infarction [28,29,30,31].
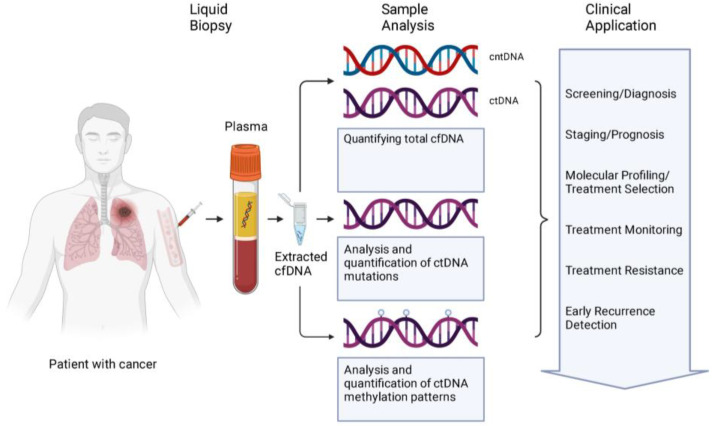
The ctDNA is the fraction of cfDNA that originates from tumor cells, which comes from three sources: apoptosis, necrosis, and active secretion. While ctDNA can come from apoptosis with fragment lengths similar to healthy patients, ctDNA is more fragmented or shorter than cfDNA [20,32,33]. In necrosis, chromatin does not fragment in a nucleosome pattern but is cleaved at random generating fragments of various sizes, which contributes to both shorter and longer fragments of >10,000 bp [34]. While apoptosis and necrosis are straightforward answers to the source of ctDNA, the concentration of cfDNA cannot be fully explained by the death of tumor cells [35]. However, the exact mechanisms of the actively secreted DNA are not fully understood with possible mechanisms being exosomes or amphisomes [36,37]. Overall, in patients with cancer, clinically relevant information from cfDNA could include a concentration of cfDNA/ctDNA, methylation patterns, and gene mutations (Figure 1).
Figure 1.
The cfDNA from liquid biopsy samples are analyzed in several ways: quantification of total cfDNA, qualitative and quantitative analysis of ctDNA mutations, and quantitative analysis of ctDNA methylation patterns. The analysis is then applied in the clinic at every step of patient management from screening to recurrence. Abbreviations: cntDNA: circulating non-tumor DNA; ctDNA: circulating tumor DNA; cfDNA: cell free DNA. Created with Biorender.com (accessed on 10 August 2023).
3. Total cfDNA Concentration
In healthy individuals, the concentration of cfDNA in plasma is between 0–10 ng/mL with the serum concentration of cfDNA being 10 times higher [38,39,40]. Most cfDNA in serum concentration comes from the process of clotting in the collection tube, which makes plasma better for clinical applications [41,42]. In patients with cancer, the concentration of cfDNA in plasma can be from 0 to over 1000 ng/mL [39,40,43,44]. This varies amongst cancer types. For instance, patients with gliomas had less ctDNA than other solid tumors, such as the pancreas, colon, breast, or ovary [45]. The concentration of cfDNA also varies with stage, where metastatic cancers have more cfDNA followed by locally advanced and then localized cancers [40]. A major challenge in the implementation of cfDNA in clinical use is the variance from the lack of standardization in methodology, such as collecting samples from serum versus plasma, extracting cfDNA with different kits, measuring samples using different techniques, time of collection, and timing for response [36,46,47].
Total cfDNA concentration has been studied extensively for its application as an early detection biomarker. For instance, a meta-analysis showed that using cfDNA concentration for diagnosis of lung cancer yielded a pooled sensitivity of 80% and specificity of 77% [36]. A study on differentiating prostate cancer from benign hyperplasia showed a sensitivity of 73.2% and specificity of 72.7% using cfDNA concentration, which aligns with a more recent study stating the low sensitivity and specificity result in poor diagnostic utility [37,48]. A study on breast cancer screening found that there was a significant difference in total cfDNA concentration between invasive breast cancer vs. disease-free subjects, but no significant difference between in situ cancer, benign tumors, and invasive cancer [49]. A study on gastrointestinal (GI) tract malignancies, including esophageal cancer, stomach cancer, and colorectal cancer, found an overall sensitivity of 75.8% and specificity of 95.8% [50]. The sensitivity and specificity of total cfDNA concentration in most studies is equal to that of serum proteins, which are generally not recommended for use in diagnosis or screening. Increased cfDNA in numerous processes and lower levels of cfDNA in earlier stages of cancer also decrease the utility of cfDNA concentration for these applications.
However, total cfDNA concentration could be an effective measure for prognosis determination and patient monitoring. Tumor markers (e.g., PSA, CEA, CA-125, etc.) are used in prognosis determination or monitoring during/after treatment for patients with cancer. In pancreatic cancer, the American Society of Clinical Oncologists (ASCO) guidelines recommend that CA19-9 in serum and CT are used for monitoring during treatment [51]. However, CA19-9 is not recommended for prognostic prediction of outcomes, and 5–10% of the population are Lewis antigen negative, with little to no secretion of CA19-9 [52,53]. In prostate cancer, PSA continues to be a mainstay in screening, prognosis, and monitoring after treatment in conjunction with other modalities [54,55]. However, the use of PSA may be associated with overdiagnosis and overtreatment for indolent cancers [56]. In breast cancer, ASCO guidelines state that CA15-3 and CA27-29 are serum proteins with prognostic value. CA15-3, CA27-29, and carcinoembryonic antigen (CEA) could be used for monitoring patients during active therapy in conjunction with other modalities. There were no serum proteins that were recommended for detecting recurrence after therapy [57]. In colorectal cancer (CRC), CEA is a recommended marker for determining prognosis, monitoring during treatment, and monitoring postoperatively for evaluation of metastatic disease [58].
Total cfDNA concentration has been shown in studies of pancreatic, prostate, breast, and colorectal cancer to be a cost-effective tool for prognosis, monitoring during, and monitoring for recurrence, which could fill the gaps in utility that some serum proteins have. There are also studies on lung cancer, which do not have any recognized biochemical markers (Table 1).
Table 1.
Studies using total cfDNA to predict patient prognosis.
| Cancer Type | # of Patients | cfDNA concentration |
PFS HR | OS HR | Additional Info | Citation |
|---|---|---|---|---|---|---|
| PDAC | 74 | >9.71 ng/mL | 6.85 | 4.16 | Average of cfDNA concentration before and after chemotherapy treatment. | [59] |
| PDAC | - | - | 1.96 | 3.39 | Subgroup analysis of cfDNA during treatment. |
[60] |
| PCa | - | >cut-off | log(HR) = 0.84 | log(HR) = 0.60 | Meta-analysis of studies using different cut-off points. | [61] |
| CRPC | - | >cut-off | log(HR) = 0.65 | log(HR) = 0.59 | Meta-analysis of studies using different cut-off points. | [61] |
| MBC | 194 | >0.306 ng/uL | 1.193 | 1.199 | Majority of samples were collected during treatment. |
[62] |
| MBC | 117 | high cfDNA | 1.64 | 2.73 | High cfDNA was determined by comparing to previous samples. HR based on comparing low and high cfDNA levels. |
[63] |
| mCRC | 1076 | >cut-off | - | 2.39 | Meta-analysis of studies using different cut-off points. | [64] |
| mCRC | 43 | >26 ng/mL | 1.51 | 2.02 | PFS and OS determined from samples before treatment. | [65] |
| NSCLC | - | >cut-off | 1.32 | 1.64 | meta-analysis of studies using different cut-off points. | [66] |
| NSCLC | 177 | >70 ng/mL | 2.6 | 2.63 | PFS and OS determined from samples before treatment. | [67] |
Abbreviations: PDAC: pancreatic ductal adenocarcinoma; PCa: prostate cancer; CRPC: castration resistant prostate cancer; MBC: metastatic breast cancer; mCRC: metastatic colorectal cancer; NSCLC: non-small cell lung cancer.
In a study of 74 patients with advanced/metastatic pancreatic cancer, high levels of total cfDNA were associated with new distant metastasis (NDM) with 91% sensitivity and 95% specificity. Researchers also found that total cfDNA concentration was associated with worse progression-free survival (PFS) and overall survival (OS). In two cases, they found that cfDNA was elevated in the first month after treatment of chemotherapy while CA19-9 levels were below detection prior to detection of NDM on CT scans [59]. In a meta-analysis subgroup of 3 studies, elevated levels of pre-operative total cfDNA had prognostic value associated with recurrence after surgery, and post-operative elevation of total cfDNA was also associated with recurrence and poor prognosis [60].
For prostate cancer, in a meta-analysis of 23 studies, total cfDNA concentration was found to be a poor diagnostic tool as previously discussed, but total cfDNA had similar prognostic value to PSA for PFS and OS [61]. Interestingly, PSA and cfDNA were independent of each other, and the combination of the two increased the discrimination between indolent vs. lethal prostate cancer [61].
A study of 194 patients with metastatic breast cancer (MBC) comparing total cfDNA, CTCs, and CA15-3 showed that cfDNA was a good predictor of OS and the best predictor of PFS [62]. For monitoring response to treatment, total cfDNA concentration had a discriminatory power that was 10% higher than CA15-3. However, the overall accuracy was low for all modalities studied [62]. A study of 117 patients with MBC, found that elevated cfDNA concentration was independently associated with unfavorable PFS and OS after adjusting for CTCs. They also found that decreased cfDNA compared to baseline levels was associated with increased treatment response [63]. In two studies of locally advanced breast cancer, there were differing conclusions regarding the concentration of cfDNA where one study found that increased cfDNA after chemotherapy was associated with improved treatment response [68]. Similarly, the other study found increased cfDNA after one cycle, but over the course of 6–8 cycles decreased cfDNA was associated with tumor response [69].
In a meta-analysis of seven studies on metastatic colorectal cancer (mCRC), high cfDNA levels were associated with poor overall survival [64]. In a study of 43 patients with mCRC, researchers also found that elevated cfDNA prior to treatment was correlated with poor overall survival and found that patients with worse response to treatment had significantly higher cfDNA concentrations at the end of treatment than those with stable disease [65]. In various gastrointestinal malignancies, elevated pre- and postoperative cfDNA concentrations were associated with tumor recurrence. In patients with tumor progression after treatment, cfDNA levels were more sensitive than CEA in monitoring for recurrence, with elevated cfDNA being detected earlier than elevated serum CEA levels [50].
In non-small cell lung cancer (NSCLC), a meta-analysis of 22 studies found that patients with elevated cfDNA concentration tend to have shorter PFS and OS [66]. A study within that meta-analysis of 218 patients found that individuals in the top third of cfDNA concentration before treatment showed a significantly shorter PFS and OS than patients in the lower two-thirds. However, cfDNA concentration during or after treatment did not have any association with the response to treatment [70]. Another study of 177 patients found that cfDNA had prognostic value predicting PFS and OS. Again, they found that there was no association of cfDNA concentration during or after treatment with the response to treatment [67]. In contrast, a number of other studies have found significant differences in cfDNA concentration in different response groups [38,71,72,73] (Table 1).
Overall, total cfDNA concentration has promise as a biomarker for prognosis in cancers discussed above, often being as accurate or better than serum protein biomarkers currently used for pancreatic, prostate, and breast cancer [59,61,62]. The meta-analysis for CRC noted that due to a lack of CEA measurements comparative conclusions cannot be made, but cfDNA could be a viable prognostic marker [64]. Total cfDNA also showed promise in NSCLC as a prognostic marker [66,67,70]. Total cfDNA concentration may also have utility in monitoring patients after treatment to detect recurrence or disease progression, with total cfDNA elevation being detected earlier than serum biomarkers [50,59,60,65].
The consensus for the application of total cfDNA concentration for monitoring is inconclusive with some studies showing decreased cfDNA [38,62,63,65,69,71,72,73] and others showing no correlation/increased cfDNA [67,68,70] while monitoring response to treatment. This discrepancy in cfDNA concentration could be explained by the increase of apoptosis and therefore cfDNA induced by chemotherapy, which would confound the results of these studies [67,68,70]. Another plausible reasoning could be related to different time points in sample collection and different chemotherapies utilized across trials [68].
While CTCs and ctDNA are often more accurate measures of tumor burden, cfDNA concentration may still have a place in the management of cancer. Total cfDNA concentration is cost-effective compared to ctDNA or CTCs, which require expensive assays. Due to the repetitive nature of monitoring during and after treatment, this cost is amplified over time [62,63,73]. Because most clinical labs lack CTC platforms, total cfDNA would be easier to implement in clinical settings with greater accessibility [62,73]. Monitoring ctDNA with specific targeted sequences presents a unique challenge as the vast genomic diversity of cancer and additional mutations driven by therapeutic selective pressure, make it difficult to identify effective target sequences [62]. However, measuring cfDNA concentration does not rely on known mutations, and could give a more holistic view of the tumor burden during treatment. In the future, total cfDNA concentration should continue to be studied in applications of prognosis and monitoring with plans to move forward with large-scale studies for clinical validation. However, large-scale clinical validation will require a standardization of the methodology to increase reproducibility in the clinical setting.
4. ctDNA
While cfDNA can be increased in healthy patients for various reasons, ctDNA detection is more specific to tumors. It has been established that ctDNA matches sequencing from tumor tissue, with the concordance of mutations in ctDNA and tumor tissue greater than 60–80% in various cancers [45,74,75,76,77,78,79]. Mutations are sequenced from ctDNA through targeted analysis or whole genome sequencing. The ctDNA can also be differentiated from cfDNA through analysis of aberrant methylation.
4.1. Techniques
Targeted sequencing looks for specific gene mutations or rearrangements that are common in a particular tumor type, which requires prior knowledge of the region of interest to design the proper assay. Generally, targeted sequencing uses PCR-based methods, such as droplet digital PCR (ddPCR), beads, emulsions, amplification, magnetics (BEAMing), and amplification refractory mutation system (ARMS) qPCR. In ddPCR, sample DNA molecules are separated into droplets, which are amplified by end-point PCR. Then, using fluorescent probes, positive and negative reactions are quantified where the copy number of target DNA is quantified by comparing the two [80]. The analytical sensitivity of ddPCR was 0.001%, detecting 1 mutant copy in 100,000 wild-type copies [80]. With BEAMing, sample DNA is separated onto beads with common primers attached, which are emulsified in oil. The strands attached to primers are then amplified, centrifuged, and collected with a magnet [81]. The beads can then be analyzed for mutations with fluorescent probes and flow cytometry, which can detect target DNA with a sensitivity of 0.01% (1/10,000 copies) [81,82,83]. In ARMS qPCR, target DNA is amplified with primers that are complementary to the mutant sequence. If the primer does not anneal properly then extension does not occur, with qPCR detecting the extension of target DNA [84]. ARMS qPCR can detect target DNA with a sensitivity of 0.1% (1/1000 copies) [85]. Another method using a specialized polymerase, SNPase-ARMS qPCR was able to achieve a sensitivity of 0.001% (1/100,000+ copies) [86].
There are also methods that apply next-generation sequencing (NGS), which has higher throughput than PCR, to a target region, such as Targeted Error Correction Sequencing (TEC-Seq), Tagged-Amplicon deep sequencing (TAm-Seq), Safe-Sequencing System (Safe-SeqS), CAncer Personalized Profiling by deep sequencing (CAPP-Seq), or Personalized Analysis of Rearranged Ends (PARE). TEC-Seq uses primers with predetermined barcodes for targeted capture of multiple regions and deep sequencing of captured DNA fragments. TEC-Seq was able to detect 100% and 89% of mutations present at 0.2% and 0.1% mutation allele frequency (MAF) respectively [40]. TAm-Seq uses specialized primers to amplify regions of interest, identifying mutations of 2% MAF with a sensitivity of over 97% [87]. Safe-SeqS adds a unique identifier (UID), which is able to detect target DNA with a sensitivity of 0.05% (1/2000) [88,89] CAPP-Seq combines optimized library preparation with bioinformatics to create a “selector”, which reflects recurrent mutations. The selector is applied to tumor DNA to identify the patient’s mutations, and then applied to ctDNA for quantification, detecting target DNA down to 0.02% (1/5000 copies) MAF [90]. PARE uses mate-paired tags and NGS to find rearranged sequences with a sensitivity of 0.001% (1/100,000+ copies) [91].
DNA from tumors also have aberrant DNA methylation, which occurs early during tumorigenesis, provides information on the tumor’s origin, and are homogenous across populations [27,92,93]. Almost every tumor type is characterized by progressive CpG-island-specific hypermethylation and global CpG hypomethylation [94]. The GRAIL test uses whole genome bisulfite sequencing (WGBS) targeted at over 100,000 methylation regions combined with machine learning to detect cancer and predict tissue of origin (TOO) localization detecting ctDNA down to 0.023% (~1/5000 copies) [95,96]. The OverC test uses an altered WGBS technique called enhanced linear splinter amplification sequencing (ELSA-seq), which mitigates the damage to DNA caused by bisulfite treatment and is able to detect ctDNA down to 0.02% (1/5000) [97,98]. Similarly, the PanSeer assay relies on whole genome bisulfite sequencing with 595 regions sequenced at higher depth, being able to detect cancer DNA with a sensitivity of 0.1% (1/1000 copies) [99]. The higher depth is achieved with newer methodologies, such as cell-free methylated DNA immunoprecipitation and high throughput sequencing (cfMeDIP-seq). cfMeDIP-seq uses beads that bind to methylated DNA and NGS to analyze CpG methylation patterns [100] (Table 2).
Table 2.
The analytical sensitivity of different technologies used to differentiate ctDNA from cfDNA.
| Technology Used | Analytical Sensitivity | Cancer Type | Test | Citation |
|---|---|---|---|---|
| RT-PCR | 0.1–1% MAF | NSCLC | cobas EGFR | [101] |
| ddPCR | 0.001% MAF | BRAF V600E | [80] | |
| BEAMing | 0.01% MAF | CRC, MBC | [82,83] | |
| ARMS-qPCR | 0.1% MAF | mCRC | [85] | |
| SNPase-ARMS qPCR | 0.001% MAF | Melanoma | [86] | |
| TEC-Seq | 0.1% MAF | BC, LC, OVC, CRC | [40] | |
| TAm-Seq | 0.002% MAF | Various | RaDaR | [102] |
| TAm-Seq | 0.01% MAF | BC | Signatera | [103] |
| SafeSEQ | 0.05% MAF | Various | CancerSEEK | [88,89,104] |
| CAPP-Seq | 0.02% MAF | NSCLC | [90] | |
| PARE | 0.001% MAF | BC, CRC | [91] | |
| WGBS | 0.023% MAF | Various | Galleri | [95,96] |
| ELSA-seq | 0.02% MAF | Various | OverC | [97,98] |
| cfMeDIP-seq | 0.1% MAF | Various | PanSeer | [99] |
NSCLC: non-small cell lung cancer; CRC: colorectal cancer; MBC: metastatic breast cancer; mCRC: metastatic colorectal cancer; BC: breast cancer; LC: lung cancer; OVC: ovarian cancer; MAF: mutant allele frequency; RT-PCR: real-time PCR; ddPCR: droplet digital PCR; BEAMing: beads, emulsions, amplification, magnetics; ARMS-qPCR: amplification refractory mutation system qPCR; TEC-Seq: targeted error correction sequencing; TAm-Seq: tagged-amplicon deep sequencing; Safe-SeqS: safe-sequencing system; CAPP-Seq: cancer personalized profiling by deep sequencing; PARE: personalized analysis of rearranged ends; WGBS: whole genome bisulfite sequencing; ELSA-seq: enhanced linear splinter amplification sequencing; cfMeDIP-seq: cell-free methylated DNA immunoprecipitation and high throughput sequencing.
The technology used to differentiate ctDNA from cfDNA has continued to develop at a breakneck pace with the “Agilent Resolution ctDx FIRST assay” being approved by the FDA for use as a companion diagnostic tool on 12 December 2022 [105]. Other assays that have pre-market approval by the FDA include Myriad’s BRACAnalysis CDx® (19 December 2014), Epi proColon® (12 April 2016), Roche’s cobas® EGFR Mutation Test v2 (1 June 2016), FoundationOne®Liquid CDx (26 August 2020), and Guardant360® CDx (7 August 2020) [106,107,108,109,110]. The FDA has also granted the Breakthrough Device designation to multiple ctDNA based assays for a variety of uses in the past few years: CancerSeek Assay (2019), Grail’s Galleri™ test (13 March 2019), Inivata’s RaDaR™ Assay (9 March 2021), Bluestar Genomics’ 5hmC Assay (21 March 2021), Natera’s Signatera Assay (24 March 2021), PredicineCARE cfDNA Assay (20 September 2022), Burning Rock’s OverC Multi-Cancer Detection (MCD) Blood Test (3 January 2023), [111,112,113,114,115,116,117]. While not all modes of utilization of ctDNA in cancer management have been clinically validated, there are hundreds of clinical trials on the use of ctDNA for early detection, diagnosis, prognosis, treatment selection, and monitoring.
4.2. Early Detection/Screening
Multiple tests have been developed for the purposes of screening or early detection of cancer in asymptomatic patients. Some tests, such as Epi proColon® and Bluestar Genomics’ 5hmC Assay, are geared towards detecting a single type of cancer, and others, such as CancerSeek, Galleri™, and OverC MCD Assay, are used as multi-cancer early detection tests. Overall, the goal of any screening test is to have high sensitivity in the early stages, specificity to limit false positives and lower costs for patient adherence. With multi-cancer detection, there is also an added requirement of determining the tissue of origin (Table 3).
Table 3.
Sensitivity and specificity of ctDNA-based screening tests in various cancer types.
| Test | # of Patients * | Cancer Type (Stage) | Sensitivity | Specificity | Citation |
|---|---|---|---|---|---|
| Epi proColon | 1544 | CRC | 68% | 80% | [118] |
| Epi proColon | 290 | CRC | 73.3% | 81.5% | [119] |
| Bluestar | 748 | PDAC | 51.9% | 100% | [120] |
| CancerSEEK ** | 9911 | Various | 27.1% | 98.9% | [121] |
| CancerSEEK | 1817 | Various (1–3) | 70% | >99% | [104] |
| Galleri | 944 | Various | 36–74% | 98% | [122] |
| Galleri | 1264 | Various | 54.9% | >99% | [95] |
| Galleri | 4077 | Various | 51.5% | >99% | [123] |
| OverC | 492 | Various | 72.4% | 99.2% | [124] |
| OverC | 639 | Various (1–3) | 80.6% | >99% | [125] |
| OverC | 360 | Various | 74.8% | 98.1% | [126] |
| OverC | 1010 | Various (1–3) | 68.5% | 96.3% | [127] |
* Total number of patients in the testing/validation set used to determine sensitivity and specificity. ** An earlier version of the CancerSEEK test was used in this clinical trial. Abbreviations: CRC: colorectal cancer; PDAC: pancreatic ductal adenocarcinoma.
Currently, the only FDA premarket-approved liquid biopsy test used for screening is the Epi proColon®, which can be offered to patients who are unwilling or unable to be screened by other recommended methods. It is performed by detecting methylated SEPT9 DNA using real-time PCR and a fluorescent probe [107]. Epi proColon® cites three clinical validation trials that led to the FDA’s premarket approval: a multi-center study of 1544 patients across Germany and the U.S. comparing Epi proColon® to colonoscopies for screening of CRC, which achieved a sensitivity of 68% and a specificity of 80% [118]; a study of 290 patients comparing Epi proColon® with a fecal immunochemical test (FIT) found the Epi proColon® test was statistically non-inferior to FIT. The sensitivity for CRC detection was 73.3% for Epi proColon versus 68.0% for FIT. Specificity was 81.5% for Epi proColon and 97.4% for FIT respectively [119]; and finally, a small two-site randomized controlled trial of 413 patients comparing the adherence to the Epi proColon® blood test, which had significantly higher uptake than FIT (99.5% vs. 88.1%) [128]. Other meta-analyses of 8643 and 2271 patients respectively, evaluating the diagnostic Epi proColon® test agree that it has high diagnostic value for CRC, especially with different algorithms, symptomatic patients, and patients with low compliance [129,130]. While the USPSTF acknowledges that there has been more evidence for the effectiveness of the Epi proColon® test, the USPSTF recommendation does not include serum tests “because of limited available evidence”, and more research is needed to continue evaluating the accuracy and effectiveness of these tests [131,132]. To that end, a search of clinicaltrials.gov for proColon shows 7 completed studies and 2 studies currently recruiting. Only the PERT study pertains to CRC, and it plans to recruit 4500 participants to evaluate the long-term performance of Epi proColon with respect to test accuracy and adherence compared to colonoscopies [133].
The Bluestar Genomics’ 5hmC Assay is another test used for single cancer type detection of FDA interest, receiving the Breakthrough Device designation in 2021 [114]. In 2020, Bluestar Genomics compared 64 patients with pancreatic ductal adenocarcinoma (PDAC) to 243 patients without cancer and demonstrated the ability to differentiate and classify the methylation patterns of PDAC [134]. Since then, a pre-print article from 2021 used 89 patients with PDAC and 596 control patients to generate a methylation library, and the predictive model was validated against 79 patients with PDAC, 506 patients with other types of cancer, and 163 patients without cancer. When used with the validation patients, the assay achieved an overall sensitivity of 51.9% and specificity of 100.0%, and for patients with new-onset diabetes, the assay achieved a sensitivity of 55.2% and specificity of 98.4% [120]. With Breakthrough Device designation in hand, Bluestar Genomics has announced two new clinical trials, NODMED and EpiDetect studies, that aim to enroll 6550 and 10,000 patients with newly diagnosed type 2 diabetes respectively. [135,136,137].
The CancerSEEK Assay developed by John Hopkins University is unique in that it uses SafeSeqS to detect 1933 distinct mutations in ctDNA and a protein biomarker assay to determine the cancer’s tissue of origin (TOO) [104]. In 2018, they studied the CancerSeek Assay, comparing 1005 patients with previously diagnosed stage 1–3 cancers and 812 healthy control patients. The overall sensitivity of CancerSEEK for the eight cancer types (ovary, liver, stomach, pancreas, esophagus, colorectum, lung, and breast) was 70%, ranging from 98% for ovarian cancer to 33% for breast cancer. The sensitivity varied across stages as well, with 78% for stage 3, 73% for stage 2, and 43% in stage 1. The overall specificity was greater than 99% [104]. By combining the detection of ctDNA and protein biomarkers, CancerSEEK increased sensitivity and specificity and localized the cancer to two TOO in 83% of patients and one TOO in 63% of patients [104]. In the DETECT-A study of 10,006 women with no history of cancer, an early version of CancerSEEK, without enhancements from machine learning, was used to screen for cancer. From 10,000 participants, 490 patients had positive baseline testing, 134 patients were confirmed positive by excluding clonal hematopoiesis of indeterminate potential (CHIP) and confirming the original mutation, 127 patients were evaluated by imaging (PET-CT and others), 64 patients had imaging concerning cancer, and 26 patients were diagnosed with cancer. Overall, the DETECT-A assay achieved a sensitivity of 27.1% and specificity of 98.9% [121]. However, the authors noted that newer generations of CancerSEEK have higher sensitivity and specificity, which would not require a two-step baseline and confirmatory test [104,121]. In January of 2023, the ASCEND study concluded, comparing the CancerSEEK assay against 1000 patients with known or suspected cancer and 2000 control patients. The data collected from the ASCEND study will be used to calibrate the CancerSEEK assay with future clinical trials yet to be announced [138].
GRAIL’s Galleri test was developed, tested, and continues clinical validation in the Circulating Cell-free Genome Atlas Study (CCGA) [139]. Initially, the CCGA compared three sequencing assays, targeted sequencing, whole genome sequencing, and methylation profiling (WGBS), in 2402 participants, which were divided into training and test sets. In the training set of 878 patients with cancer and 580 control patients, methylation sequencing using WGBS achieved the highest sensitivity, ranging from 54–92% in various cancers. In the testing set of 576 patients with cancer and 368 control patients, sensitivity ranged from 36% in breast cancer to 74% in pancreatic cancer, with overall specificity being 98% [122]. Following this, a larger study of 6689 participants was again divided into training and testing sets to build on the WGBS methylation-based assay. The training set included 1531 patients with cancer and 1521 control patients, and the validation set included 1264 patients with cancer and 610 control patients. The overall sensitivity for all stages and cancers in both sets matched closely, being 55.2% and 54.9% in training and validation sets respectively. Sensitivity was lower for cancers in stage 1 being 18% rising to 93% for cancers in stage 4. Specificity for both sets was greater than 99% [96]. Towards further clinical validation, a study of 4077 participants, with 2823 patients with cancer, and 1254 control patients, were tested with the refined Galleri WGBS assay and will continue to follow-up for 5 years. In this large, independent validation set, the Galleri test achieved an overall sensitivity of 51.5% increasing with the stage (stage 1 = 16.8%, stage 2 = 40.4%, stage 3 = 77.0%, and stage 4 = 90.1%). The specificity was greater than 99% [123]. The CCGA clinical trial is ongoing and is set to end in March 2024 [139]. More ongoing observational clinical trials using the Galleri test are the STRIVE study, which has enrolled nearly 100,000 women who are undergoing mammograms with the goal of independently validating Galler’s ability to detect and localize cancers in this population, the SUMMIT study, which is evaluating 13,000 participants with a high risk of cancer from smoking, and the REFLECTION study, which observes Galleri’s effect in clinical settings and patients [95,140,141,142]. There is also progress in interventional studies with the PATHFINDER study screening 4033 asymptomatic patients getting a positive predictive value (PPV) of 45% since the last update in 2021 [143]. This closely matches the estimated PPV of 49% [144]. A continuation of the PATHFINDER study (PATHFINDER-2) plans to enroll 20,000 participants to further evaluate the Galleri test in the general population [145,146].
The latest ctDNA-based screening test to be announced as receiving the FDA Breakthrough Device designation is Burning Rock’s OverC MCD blood test early in 2023 [117]. The OverC assay uses a technique called ELSA-seq, which is a type of WGBS, with increased yield, methylome coverage, and reproducibility [97]. Taking this technique, a prospective multicenter study (PROMISE, NCT04972201) compared and combined cfDNA methylation, cfDNA mutation, and microRNA expression assays in the early detection of 9 cancers. Participants were split into training and test sets with 981 and 492 patients respectively. The methylation model performed the best out of the three, with a sensitivity of 72.4% and a sensitivity of 99.2% overall. The combination of three tests correctly predicted the TOO 75.3% of the time, with 90.9% of cases being in the top two predictions [124,147]. Further development of the ELSA-seq technique occurred in the THUNDER study: a prospective, multi-center study including 1108 participants. A training set of 274 patients with cancer and 195 control patients and a validation set of 351 patients with cancer and 288 control patients were created. In the validation set, the sensitivity of ELSA-seq for patients with early-stage cancer (1–3) was 80.6%, the specificity was greater than 99% and correctly predicted the TOO in 81% of cases [125]. In the single-blind test phase of the THUNDER study, an independent validation set of 360 patients with 202 patients with cancer and 158 control patients used ELSA-seq achieving a sensitivity of 74.8%, specificity of 98.1%, and identified the TOO in 80.8% of cases [126]. An update to the THUNDER study used a training and validation set to create 2 tests with different predetermined cutoffs. Then, the two predictive models were used on an independent validation set of 505 patients with cancer and 505 control patients. Test 1 achieved a sensitivity of 76.2%, a specificity of 98.9%, and predicted the TOO in 79.1% of cases. Test 2 achieved a sensitivity of 70.2%, a specificity of 99.3%, and predicted the TOO in 83% of cases [127]. While the THUNDER study has been completed, Burning Rock plans to perform 2 larger multicenter studies with an estimated 14,000 and 11,879 participants to continue developing and expanding the capabilities of the OverC test to include more types of cancer [148,149]. They are also recruiting for a large prospective, multicenter, interventional study of approximately 12,500 participants, which will evaluate the performance of the OverC MCD blood test in asymptomatic individuals with an increased risk of cancer [150].
Through the FDA approval of Epi proColon®’s use in screening for CRC in patients who are unwilling to undergo other recommended screening methods, they confirm the niche that liquid biopsies have in the screening space. However, the reluctance of the FDA and USPSTF to approve and recommend other liquid biopsy screening tests shows there is still a long way to go before overcoming challenges in sensitivity, clinical validation, and cost. One challenge that was previously a concern was the lower specificity of detecting ctDNA due to CHIP, which are mutations associated with myeloid cancers and frequently mutated in healthy patients [151]. However, DETECT-A was able to exclude CHIP mutations and achieve a specificity of 98.9% [121]. Overall, the specificity of ctDNA-based liquid biopsy tests were ~99% with a few exceptions. Most notably, Epi proColon had a specificity of 80% [118] (Table 3).
Of greater concern for the utility of liquid biopsies is the lack of sensitivity, which could range from 27.1% to 80.6%, and gets lower with earlier-stage cancers (Table 3). Low ctDNA shedding tumors with low tumor burden or less metastatic spread are likely contributors to lowering the sensitivity of the test through sampling bias [152]. While various tests have been analytically validated to detect ctDNA in MAF down to 1/100,000, clinically, once the MAF drops below 0.01% (1/10,000 copies) the use of a 10 mL sample of blood will not contain a single ctDNA fragment to sequence or detect [153]. However, newer approaches are working on increasing the sensitivity of the liquid biopsy tests. Multi-omics combines the analysis of ctDNA with other biomarkers, such as fragmentation length or serum proteins. Based on a shorter fragment length ctDNA, Mouliere et al. were able to increase the sensitivity to 90% without a loss in specificity of 98%. However, there was sometimes a loss of sequencing data from ctDNA because it was not integral to detecting the tumor [32]. Similarly, a machine learning approach was able to achieve a sensitivity of 95.5% and specificity of 95% in 971 early-stage cancer patients [154]. CancerSeek used multi-omics as described above and reported an increase in sensitivity and specificity after applying serum proteins to the test [104]. Another study found similar results in pancreatic cancer increasing the sensitivity from 30% to 64% [154].
Another prospect to increase sensitivity might be to increase the sample volume. It is estimated a blood sample of 150 to 300 mL would be required to achieve a sensitivity of 95% for screening early breast cancer, which is not possible with a simple blood draw [155]. A method already being studied in the collection of CTCs is the application of an apheresis machine to collect a larger quantity of cells than would be possible through simple blood drawing. In advanced prostate cancer, Lambros et al found an increase in CTC yield from 167 per patient to 12,546 [156]. In a similar study on breast cancer, they found a 205× increase in the yield of CTCs [157]. For the application of apheresis in ctDNA, researchers from Johns Hopkins University have developed a system that can capture ctDNA from the flowing unaltered plasma running through an apheresis machine [158]. However, further development of the technology is needed [158].
Despite the continuous improvement in the analytical and clinical ability of liquid biopsies, none of the tests above have been recommended by the USPSTF. In part, this is due to the limitations of screening for some types of cancer, but the USPSTF cites a lack of clinical validation in its recommendation for epi proColon [131,159]. With that being said, there are over 200,000 patients being recruited across 10 clinical trials, including interventional trials used to determine the actual clinical benefits of the tests.
4.3. Treatment Selection/Companion Diagnostics
The FDA defines a companion diagnostic device as an in vitro test that provides information that is essential for the safe and effective use of a corresponding therapeutic product [160]. These are tested analytically and clinically to ensure that companion diagnostic devices do not lead to withholding appropriate therapy or administering inappropriate therapy [160]. In an era of increasing precision medicine, companion diagnostics are an integral part of identifying targetable mutations in the patient’s cancer. Recognizing this, the FDA has given 5 ctDNA-based tests pre-market approval. Some are PCR-based tests, such as Roche’s cobas® EGFR Mutation Test v2, and Qiagen’s therascreen test. Some are NGS-based tests, such as the FoundationOne®Liquid CDx, Guardant360® CDx, and Agilent’s Resolution ctDx FIRST assay. The PredicineCARE cfDNA Assay has received the FDA Breakthrough Device designation and also utilizes NGS (Table 4).
Table 4.
Companion diagnostic tests and the related concordance testing cited in the FDA Premarket Approval summaries.
| Diagnostic Test | Biomarker/ Details |
Cancer Type | Drug Name | Clinical Trial # | Compared Test | Concordance | Citations |
|---|---|---|---|---|---|---|---|
| BRACAnalysis | gBRCA1/2 | OC | Olaparib |
NCT00753545 NCT01874353 |
local testing | 99.2% (259/261) | [161] |
| BRACAnalysis | gBRCA1/2 | BC | Olaparib | NCT02000622 | BRACAnalysis | n/a | [162] |
| BRACAnalysis | gBRCA1/2 | BC | Talazoparib | NCT01945775 | BRACAnalysis | n/a | [163] |
| BRACAnalysis | gBRCA1/2 | PDAC | Olaparib | NCT02184195 | BRACAnalysis | n/a | [164] |
| BRACAnalysis | gBRCA1/2 | OC | Rucaparib | NCT01891344 | Foundation Medicine T5 |
n/a | [165,166] |
| BRACAnalysis | gBRCA1/2 | PC | Olaparib | NCT02987543 | FoundationOne CDx | n/a | [167,168] |
| FoundationOne Liquid CDx |
BRCA1/2 | OC | Rucaparib | NCT01891344 | Foundation Medicine T5 |
96.3% (209/217) | [169] |
| FoundationOne Liquid CDx |
BRCA1/2 | mCRPC | Rucaparib | NCT02952534 | FoundationOne LDT FoundationOne Liquid LDT local testing |
89.4% (144/161) | [170] |
| FoundationOne Liquid CDx | BRCA1/2 | mCRPC | Olaparib | NCT02987543 | FoundationOne CDx | n/a | [170] |
| cobas EGFR Plasma Test v2 | EGFR * | NSCLC | Erlotinib | NCT01310036 | cobas EGFR
Mutation Test v1 |
84.4% (757/897) | [171] |
| cobas EGFR Plasma Test v2 | EGFR ** | NSCLC | Osimertinib | NCT02094261 | cobas EGFR
Mutation Test v1 |
65.0% (206/317) | [172] |
| cobas EGFR Plasma Test v2 | EGFR * | NSCLC | Gefitinib | - | - | - | [173] |
| FoundationOne Liquid CDx |
EGFR * EGFR ** |
NSCLC | Erlotinib Osimertinib Gefitinib |
- | cobas EGFR
Mutation Test v2 |
94.4% (167/177) | [174] |
| Guardant360 CDx |
EGFR * EGFR ** |
NSCLC | Osimertinib | NCT02296125 | cobas EGFR
Mutation Test v2 |
79.5% (372/468) | [175] |
| Guardant360 CDx | KRAS G12C | NSCLC | Sotorasib | NCT03600883 | therascreen KRAS RGQ PCR Kit | 82.0% (155/189) | [176,177] |
| Resolution ctDx | KRAS G12C | NSCLC | Adagrasib | NCT03785249 | local testing | 95.1% (212/223) | [178] |
gBRCA1/2 = germline BRCA variants detected from DNA extracted from non-tumor cells. EGFR * = EGFR exon 19 deletions and exon 21 L858R; EGFR ** = EGFR T790M. Abbreviations: OC: ovarian cancer; BC: breast cancer; PDAC: pancreatic ductal adenocarcinoma; PC: prostate cancer; mCRPC: metastatic castration resistant prostate cancer; NSCLC: non-small cell lung cancer.
4.3.1. BRCA
While not detecting ctDNA, the first liquid biopsy-based companion diagnostic test was Myriad’s BRACAnalysis CDx®, which is approved for the identification of deleterious germline BRCA (gBRCA) variants from whole blood products in breast, ovarian, pancreatic, and metastatic castration-resistant prostate cancer (mCRPC), using Sanger sequencing and multiplex PCR. The results provide aid in identifying eligible patients for a PARP inhibitor, such as olaparib, talazoparib, or rucaparib [106]. The initial FDA priority review hinged on the completion of a randomized, double-blind, placebo-controlled, phase 2 study of olaparib, which showed a significantly prolonged progression-free survival with BRCA mutation-positive patients most likely to benefit from treatment [179]. In a pooled analysis of 300 patients from phase 1 and 2 trials, olaparib elicited durable responses in patients with relapsed gBRCA mutations [180]. The FDA analysis noted a limited representation of BRCA1/2 variants in the previous clinical trials and requested data from the ongoing clinical trials [161]. In those follow-ups, confirmatory, phase 3 trials (SOLO2, SOLO3), olaparib demonstrated a clinically significant survival benefit in the same patient population [181,182]. In the initial approval of BRACAnalysis, Myriad provided evidence of the analytical sensitivity and specificity being ~99% for both, and the clinical validity of BRACAnalysis was confirmed through a concordance rate between local test results and BRACAnalysis of 96.7% during clinical trials [161]. In the subsequent extension of BRACAnalysis’s indications to other PARP inhibitors and cancer types, some of the clinical trials used BRACAnalysis as part of the inclusion criteria, and that showed the efficacy of drugs being tested on the specific type of cancer [162,163,164]. In the other indications, the clinical trials cited use Foundation Medicine’s NGS-based assay. The study of Rucaparib in ovarian carcinoma (ARIEL2) used the Foundation Medicine T5 NGS assay, and the study of olaparib in mCRPC (PROfound) used the FoundationOne CDx NGS assay. Both of the aforementioned tests use solid tissue biopsy samples and included mutations other than BRCA1/2 [165,166,167,168]. While niraparib was not officially on the list of companion diagnostic indications, the final label update for BRACAnalysis notes that in a randomized, double-blind, phase 3 trial testing niraparib on 203 patients with gBRCA mutations, and 350 patients without gBRCA mutations the progression-free survival for the gBRCA+ cohort was significantly longer than the non-gBRCA cohort. This study used the myChoice HRD test on solid biopsy samples to identify the BRCA1/2 mutations [183,184].
NGS from Foundation Medicine was approved by the FDA on 19 December 2016 in the form of the FoundationFocus CDxBRCA Assay for use in detecting BRCA1/2 alterations following the Rucaparib ARIEL1/2 clinical trials [185]. The initial study enrolled patients with BRCA mutations detected by local testing. Later, the BRCA mutations were confirmed at a central Foundation Medicine lab using the Foundation Medicine T5a Panel [167,185]. Similar to BRACAnalaysis, the approval of FoundationOne Liquid CDx was based on a bridging study measuring the concordance compared to the clinical trial tissue assay, as well as the effectiveness of the FoundationOne liquid biopsy test to select patients for treatment with rucaparib [169,174]. When 217/491 patients from the ARIEL2 patient population were tested, the positive percent agreement was 93.8% (60/64) and the negative percent agreement was 97.4% (149/153) [169]. In the patients treated with rucaparib, there were 26 BRCA-positive patients as determined by FoundationOne® Liquid CDx and 61 BRCA-positive patients as determined by the clinical trial assay. The overall response rate was similar in the two groups being 53.8% (14/26) and 54.1% (33) respectively [169]. The bridging studies were conducted in accordance with the approval of the FoundationOne Liquid CDx assay for use in identifying BRCA-positive mCRPC for treatment with rucaparib [170,186]. The FoundationOne Liquid CDx assay is also approved for use in identifying BRCA-positive mCRPC, but no bridging study was cited by the FDA in the summary sheet [170].
4.3.2. EGFR
Similar to complementary diagnostic tests for detecting BRCA mutations, tests for detecting EGFR mutations required both analytical and clinical validation. Roche’s cobas® EGFR Mutation Test v1 was an RT-PCR test for the detection of exon 19 deletions or exon 21 L858R missense mutations in non-small cell lung cancer (NSCLC), for which erlotinib is indicated [187]. The EURTAC study was a phase 3 trial that screened 1044 patients using a clinical trial assay with a combination of methods for comparing erlotinib vs. cisplatin chemotherapy [188]. The FDA then approved the tissue-based cobas® EGFR Mutation Test v1 test based on retrospective testing of 487 samples, which had 432 results that could be compared to the clinical trial assay [187,189]. When compared, the cobas® EGFR Mutation Test v1 had an overall percent agreement of 96.3% (416/432) [189]. While retrospective analysis of the progression-free survival from the EURTAC study was not completed, the cobas® EGFR Mutation Test v1 was used to enroll 217 patients for treatment with erlotinib vs. gemcitabine/cisplatin showing a significant increase in PFS using erlotinib in EGFR mutation-positive patients [190]. The ASPIRATION and FAST-ACT2 studies also supported the effectiveness of erlotinib in patients with EGFR mutations detected by the cobas® EGFR Mutation Test v1 [191,192]. In another bridging study, Roche retrospectively tested samples from the ENSURE, ASPIRATION, and FAST-ACT2 studies with the cobas® EGFR Plasma Test v2 [101,171]. In a pooled analysis of the 897 paired samples available, an imperfect concordance with a positive predictive agreement (PPA) of 72.1% (339/470) and negative predictive agreement (NPA) of 97.9 (418/427) was recorded [171]. However, a negative plasma test would lead to patients using a solid tissue biopsy to determine the EGFR status, so a positive predictive value (PPV) of 97.6% was enough to approve the first liquid biopsy test for detecting exon 19 deletion and exon 21 L858R mutation in 2016 - the cobas® EGFR Plasma Test v2. [193]. With osimertinib, cobas EGFR Mutation Test v1 was used in the inclusion criteria for the AURA2 phase 2 clinical trial [194]. Then, in a bridging study, the cobas EGFR Plasma Test v2 test detected a T790M gatekeeper mutation with a PPA of 56.8% and a NPA of 80.2% [172]. Osimertinib was also established as a first-line treatment option for patients with exon 19 deletion and L858R mutation, where both plasma and tissue are now used [195,196,197]. Plasma or tissue was also approved as a companion diagnostic for gefitinib, but no trials are cited in the approval [173].
In a deviation from previous approvals that paired a single test result with a single drug based on clinical trials and bridging studies, the FDA approved companion diagnostic tests that detected the exon 19 del or L858R mutation in EGFR as suitable for treatment with a tyrosine kinase inhibitor (TKI) approved for that indication [198]. On 27 October 2020, the new group labeling for the cobas® EGFR Mutation Test v2 added two new tyrosine kinase inhibitors to its indication when detecting exon 19 deletion or L858R mutation with afatinib or dacomitinib. However, these would not be added to the plasma test [198]. In a similar progression, the FoundationOne CDx test was approved based on a bridging study comparing concordance with the cobas® v2 EGFR mutation test for the detection of EGFR exon 19 deletions, L858R, and T790M substitutions being eligible for treatment with afatinib, gefitinib, erlotinib, or osimertinib [199,200]. Another bridging study was later completed to approve the Foundation One Liquid CDx, with a PPA and NPA of above 95% across multiple tests, for the detection of EGFR exon 19 del, and L858R mutation with gefitinib, osimertinib, and erlotinib [169,185]. Again, the group approval in 2022 of FoundationOne CDx added tyrosine kinase inhibitors, dacomitinib, to its indication, but did not add new TKIs, afatinib or dacomitinib, to FoundationOne Liquid CDx’s [201,202]. Another liquid biopsy-based test, Guardant360 liquid biopsy CDx, was approved for the detection of EGFR alterations for use in only one TKI, osimertinib. The approval of Guardant360 CDx came from a bridging study measuring the concordance between Guardant360 and the cobas® EGFR mutation test using samples from the FLAURA clinical trial with a PPA of ~75% and NPA of ~99% [175].
4.3.3. Other
The Guardant360 CDx has also been approved for the detection of KRAS G12C mutations to indicate usage of sotorasib based on concordance studies comparing samples from the CodeBreaK100 study originally analyzed with the Therascreen KRAS RGQ PCR Kit [176,203,204]. With 189 patients compared, the PPA was 70.7% (82/116) and the NPA was 100% (73/73). Importantly, the Guardant360 CDx had no false positives and an overall response rate in the positive patient population of 38% [176,177]. The newest approval from the FDA of the Agilent Resolution ctDx FIRST assay was also for the detection of the KRAS G12C mutation for use with adagrasib, another KRAS inhibitor [178]. In a bridging study measuring the concordance between the Resolution FIRST assay and locally confirmed detections from the KRYSTAL-1 study, they compared 223 samples getting a PPA of 87% (47/54) and NPA of 97.6% (165/169) [178,205,206,207].
Predicine announced that the FDA granted the breakthrough device designation to the PredicineCARE companion diagnostic assay on 20 September 2022 [116]. This followed a clinical trial using their NGS-based assay to detect mutations in ctDNA in different types of breast cancer [208]. Among 141 patients with advanced breast cancer, 112 (79.4%) had plasma samples with mutations detected. Then, 21 patients had solid biopsies to compare to their plasma samples with the same single nucleotide variant detected in 6 plasma samples out of 10 tissue samples (6/10) in the PIK3CA gene, 5/9 in TP53, and 5/6 in ERBB2 [209]. While a study in 2020 showed strong concordance between the PredicinePLUS NGS assay and Guardant360, it only included 15 patients and should be carried out with a set PredicineCARE assay as they move forward [210].
With the ongoing introduction and approval of numerous companion diagnostic tests and the expansion of clinical utility through grouping therapeutic products to determine the change in labeling for companion diagnostic devices from specific products to a group of therapeutic products, the FDA added guidance for the industry in April 2020 [211]. At the heart of the guidance is still ensuring proper labeling to ensure the correct identification of patients for therapeutic treatment, which is now a specific group of products approved for the same indications based on molecular alterations. The goal of the FDA’s newer grouped approval comes with the following considerations: identifying the specific alterations that include or exclude the use of a group of therapeutics, identifying at least two approved therapeutics for those alterations, and demonstrating analytical and clinical validation [211]. Having the grouped labeling of both companion diagnostic devices and therapeutic treatments should make it easier for both products to gain traction and utility. Therapeutic treatments that are linked to a specific molecular alteration could be detected by multiple companion diagnostic devices. A new companion diagnostic device that can detect molecular alteration could be used for indicating multiple treatments. For instance, Guardant360 is already approved for use with one TKI and could make the argument to expand its coverage to all TKIs.
4.4. Minimal Residual Disease
In patients with curative disease, monitoring after treatment generally consists of serial measurements of various serum proteins and serial radiographic imaging [212,213]. However, there are some gaps in the utility of certain serum proteins, such as in pancreatic and breast cancer, as previously discussed [52,53,57]. Serum proteins also run into issues with limited sensitivity and specificity [212]. While imaging improves the detection of recurrence, it can only detect macroscopic disease [214]. Also, imaging is associated with a risk of radiation exposure and inconclusive readings due to normal reactions to treatment [212,215]. Analysis of ctDNA has shown utility for detecting relapse and resistance mutations before clinical progression occurs [216,217,218]. The FDA has given the breakthrough device designation to two ctDNA-based tests: Inivata’s RaDaR™ Assay and Natera’s Signatera Assay (Table 5).
Table 5.
Clinical trials using minimal residual disease tests to predict recurrence with median lead time.
| MRD Test | Cancer Type | # of Patients | Specificity | Sensitivity | Lead Time | Additional Info | Citation |
|---|---|---|---|---|---|---|---|
| RaDaR™ | NSCLC | 77 | 95.9% (47/49) | 64.8% (18/28) | 212.5 days | ctDNA detected >2 weeks after surgery | [219] |
| RaDaR™ | HNSCC | 17 | 100% (12/12) | 100% (5/5) | 122 days | ctDNA detected from samples taken during routine follow-up visits |
[220] |
| RaDaR™ | BC | 22 | 100% (5/5) | 100% (17/17) | 386.7 days | ctDNA detected from samples taken during routine follow-up visits |
[221] |
| RaDaR™ | BC | 38 | 94.1% (16/17) | 71.4% (15/21) | 92 days | ctDNA detected from samples taken at detection of recurrence or 3 years later |
[222] |
| Signatera | BC | 49 | 100% (31/31) | 89% (16/18) | 267 days | ctDNA detected from samples taken every 6 months | [103] |
| Signatera | CRC | 75 | 98.3% (58/59) | 87.5% (14/16) | 261 days * | ctDNA detected from samples taken at 1 month and every 3 months. | [223] |
| Signatera | UC | 68 | 98% (48/49) | 100% (13/13) | 96 days | ctDNA detected from samples taken during routine follow-up visits |
[224] |
| Signatera | mCRC | 112 | 93.3% (14/15) | 91.4% (32/35) | 95 days | ctDNA detection with samples from two-time points | [225] |
* Only average lead time was reported. Abbreviations: NSCLC: non-small cell lung cancer; HNSCC: head and neck squamous cell carcinoma; BC: breast cancer; CRC: colorectal cancer; UC: urothelial carcinoma; mCRC: metastatic colorectal cancer.
Inivata’s RaDaR™ Assay uses an enhanced version of the TAm-Seq technology, which originally covered 6 genes with a 97% sensitivity and specificity at 2% MAF [87]. The InVisionFirst assay used 36 genes targeted for NSCLC and was able to detect target DNA down to 0.25 MAF with a sensitivity of 92.46% [226]. Building on that, the RaDaR Assay uses personalized targets based on whole genome sequencing of tumor tissue, which amplifies up to 48 patient-specific tumor variants from cfDNA [102]. With 48 variants, the assay had a sensitivity of 100% at 0.002% MAF, and with only 16 variants, the assay had a sensitivity of 95% at 0.004% MAF [102]. This approach has since been studied for the detection of MRD in NSCLC, head and neck squamous cell carcinoma (HNSCC), breast cancer, and melanoma. In the “LUng cancer—Circulating tumor DNA” (LUCID) study, 88 patients had their ctDNA levels measured before and after treatment with surgery [227]. For the 77 patients undergoing observation >2 weeks after the end of treatment, ctDNA was detected with a clinical specificity of 98.7% (150/152) samples in 64.3% (18/28) patients who later experienced a clinical recurrence of their first tumor, with lead times of ~200 days from detection to disease recurrence [219]. For HNSCC, 17 patients with stage 3 or 4 diseases had samples taken before and after surgery with chemoradiotherapy as needed [220]. With later-stage diseases, the RaDaR assay was able to detect pre-operative ctDNA in 100% of patients, and ctDNA was detected 108 to 253 days before clinical recurrence occurred in 100% (5/5) of patients with no false positives [220]. In a larger study of 38 patients with recurrent or metastatic HNSCC, the RaDaR assay was used to monitor patients on platinum-based chemotherapy or immunotherapy [228]. Preliminary data from this study has shown that a decrease in ctDNA MAF from baseline to after first treatment was correlated with improved PFS [229]. Out of 22 early-stage breast cancer patients undergoing surgical treatment, RaDaR successfully identified MRD in 100% (17/17) of patients with clinical recurrence and no false positives [221]. In a follow-up study of 38 patients, the RaDaR assay detected 92% (12/13) of distant recurrence and 38% (3/8) of local recurrence. However, in the 6 cases missed by the RaDaR assay, 100% had indications of a possible alternative origin or second primary tumor. Only 1 patient of 17 who did not have disease recurrence had detectable ctDNA at 0.0085% MAF. [222]. There are also two clinical trials with ongoing monitoring. The TRACER trial, which is a sub-trial of the LIBERATE trial, has recruited 145 patients with breast cancer with the percentage of patients with detectable ctDNA dropping with treatment of various neoadjuvant therapy after surgery [230,231]. The other trial, cTRAK-TN, has developed RaDaR assays for 142 patients and has achieved a longer median lead time and higher sensitivity compared to dPCR [232]. In another ongoing trial of 47 patients with melanoma, 12 patients had ctDNA detected after surgical treatment with detection possibly preceding recurrence and being associated with lower PFS and OS [233]. While not monitoring for post-treatment MRD, RaDaR is being studied in advanced urothelial cancer for monitoring during treatment with immunotherapy with decreases in plasma ctDNA levels being associated with a pathological response [234].
Natera’s Signatera Assay is also based on a personalized targeted multiplex PCR-based NGS technology, which can track up to 16 clonal variants from cfDNA in plasma [103,223]. In the initial studies applying the technology to 49 patients with breast cancer, the Signatera Assay was able to detect ctDNA down to MAF of 0.01% and ctDNA was detected in 94% (16/17) patients 0.5–24 months prior to distant metastatic recurrence with detection in 89% (16/18) of overall recurrence. Again, there were no false positives from this study [103]. Similar findings were demonstrated in 122 early-stage CRC and 68 urothelial bladder carcinoma patients detecting 87.5% (14/16) of recurrence with an average lead time of 8.7 months and 100% (13/13) of recurrence with a median lead time of 96 days respectively [223,224]. In the urothelial bladder carcinoma study, the specificity was 98% (48/49) [224]. In another study of 112 patients with mCRC undergoing metastatic resection, 96.7% (59/61) of patients with detectable ctDNA had disease progression with a median lead time of 3.16 months. With one sample collected, the sensitivity was lower at 72% (59/82) and specificity of 93.3% (28/30). With two samples the sensitivity was up to 91.4% (32/35) and no change in specificity of 93.3% (14/15) [225]. In CIRCULATE-Japan, their biggest study of CRC, Signatera was used in 1039 patients with stage 2–4 resectable CRC to determine eligibility for adjuvant therapy after surgery based on the risk of recurrence [235,236]. In the cohort of 1039 patients, ctDNA positivity 4 weeks after surgery was associated with much higher rates of recurrence than ctDNA negative patients, 61.4% (115/187) versus 9.5% (81/852) respectively [237]. They also found that ctDNA positivity was better than CEA for relapse detection [237]. Monitoring for MRD is still ongoing for the CIRCULATE-Japan study, and another application of the Signatera test, the BESPOKE CRC study, is a multicentre study in California that plans to recruit 2000 patients to determine adjuvant therapy [238,239]. In a follow-up in urothelial carcinoma, 581 patients were monitored for MRD to determine adjuvant therapy that detected ctDNA prior to radiological relapse 59% of the time [240].
Similar to the use of ctDNA-based liquid biopsies in screening, the detection of ctDNA is highly specific for the presence of cancer, with the detection of MRD having a specificity >90% in all the completed studies [103,219,220,221,222,224,225]. (Table 5) The decrease in specificity is likely due to the repetition of the test over a shorter time frame, and possible detection of dormant disease [222]. With the detection of MRD, clinicians can obtain information on disease processes weeks and months ahead of current monitoring by serum proteins or imaging [225,231]. For example, the MRD tests have promising utility in monitoring for response to systemic therapy [224,234,237,240]. However, with the high specificity comes a lower sensitivity. The analytical sensitivity of both the RaDaR Assay and Signatera Assay are impressive being 0.002% MAF and 0.01% MAF respectively [102,222]. However, the clinical sensitivity could range from 64.3% (18/28) to 100% (13/13) [219,221,224]. (Table 5) The sensitivity of the tests increased with the disease burden being better at detecting distant over local recurrence [103,222]. Sensitivity also increased with more longitudinal samples with little decrease in specificity [225]. The increase and decrease in sensitivity of these tests fall in line with our understanding of sampling bias as discussed earlier [152,153]. Increasing the sample size by taking samples every month should increase the likelihood of detecting a rare ctDNA fragment when disease burden is low, such as in MRD. The future application of ctDNA in the detection of MRD is likely unhampered by the sensitivity, specificity, or lead times, especially as the technology continues to be refined. However, the most important part of getting the technology into the clinic will be the clinical trials that are ongoing, and future expansions for clinical validation [230,231,235,236,238,239].
5. Conclusions
The clinical applications of quantitative and qualitative analysis of cfDNA using liquid biopsy can be exciting solutions to weaknesses of solid biopsy, serum proteins, and imaging. While the clinical utility of measuring total cfDNA as a screening tool is low, there are promising studies using it to determine patients’ prognosis, treatment response, and recurrence. However, total cfDNA being increased in normal physiological processes, a lack of consensus for levels of cfDNA during treatment, and an overall lack of standardization in methodology holds it back from being studied in large-scale clinical trials. In contrast, a variety of ctDNA-based companion diagnostic devices have been clinically validated and are used in clinics under pre-market approval from the FDA for treatment selection. An increase in positive percent agreement might increase the clinical utility of these companion diagnostic devices, but the innovation has mostly been in the addition of group approvals, which will make it easier for the industry to create and push new companion diagnostic devices to market. Using ctDNA detection for other parts of clinical management, such as screening and MRD, is promising with clinical trials completed showing high specificity for detecting cancer. However, several factors, such as low tumor burden and sampling bias, contribute to low sensitivity, but there are new innovations in technology, multi-omics, and sampling to find solutions to these issues. With that said, larger clinical trials using ctDNA-based liquid biopsy tests are still ongoing to prove their clinical validity and propel them to mainstream use.
Author Contributions
Conceptualization, D.M. (Daruka Mahadevan); writing—original draft preparation, J.D.; writing—review and editing, P.J.C., B.S., D.M. (Devi Meyyappan), S.R. and D.M. (Daruka Mahadevan); visualization, J.D. and S.R.; supervision, D.M. (Daruka Mahadevan); project administration, D.M. (Daruka Mahadevan). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cancer World Health Organization. [(accessed on 15 March 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Incidence and Relative Survival by Stage at Diagnosis for Common Cancers Centers for Disease Control and Prevention. Published 10 November 2021. [(accessed on 15 March 2023)]; Available online: https://www.cdc.gov/cancer/uscs/about/data-briefs/no25-incidence-relative-survival-stage-diagnosis.htm.
- 3.Reddy S.R., Broder M.S., Chang E., Paydar C., Chung K.C., Kansal A.R. Cost of cancer management by stage at diagnosis among Medicare beneficiaries. Curr. Med. Res. Opin. 2022;38:1285–1294. doi: 10.1080/03007995.2022.2047536. [DOI] [PubMed] [Google Scholar]
- 4.Halabi S., Owzar K. The importance of identifying and validating prognostic factors in oncology. Semin. Oncol. 2010;37:e9–e18. doi: 10.1053/j.seminoncol.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambardella V., Tarazona N., Cejalvo J.M., Lombardi P., Huerta M., Roselló S., Fleitas T., Roda D., Cervantes A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers. 2020;12:1009. doi: 10.3390/cancers12041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A & B Recommendations A and B Recommendations|United States Preventive Services Taskforce. [(accessed on 15 March 2023)]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation-topics/uspstf-a-and-b-recommendations.
- 7.Recommendation: Breast Cancer: Screening|United States Preventive Services Taskforce. [(accessed on 15 March 2023)]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening#tab1.
- 8.Recommendation: Cervical Cancer Screening|United States Preventive Services Taskforce. Published 21 August 2018. [(accessed on 15 March 2023)]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/cervical-cancer-screening.
- 9.Recommendation: Colorectal Cancer Screening|United States Preventive Services Taskforce. Published 18 May 2021. [(accessed on 15 March 2023)]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening.
- 10.Gilson P., Merlin J.L., Harlé A. Deciphering Tumour Heterogeneity: From Tissue to Liquid Biopsy. Cancers. 2022;14:1384. doi: 10.3390/cancers14061384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. World Journal of Gastroenterology. Volume 24. Baishideng Publishing Group Co.; Pleasanton, CA, USA: 2018. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes; pp. 4846–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers T.K. Minimising diagnostic delay in lung cancer. Thorax. 2019;74:319. doi: 10.1136/thoraxjnl-2018-212927. [DOI] [PubMed] [Google Scholar]
- 13.Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician. 2016;93:937–944. [PubMed] [Google Scholar]
- 14.Pantel K., Alix-Panabières C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Killock D. CancerSEEK and destroy—A blood test for early cancer detection. Nat. Rev. Clin. Oncol. 2018;15:133. doi: 10.1038/nrclinonc.2018.21. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y., Taniguchi H., Ikeda M., Bando H., Kato K., Morizane C., Esaki T., Komatsu Y., Kawamoto Y., Takahashi N. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 2020;26:1859–1864. doi: 10.1038/s41591-020-1063-5. [DOI] [PubMed] [Google Scholar]
- 17.Sama S., Le T., Ullah A., Elhelf I.A., Kavuri S.K., Karim N.A. The Role of Serial Liquid Biopsy in the Management of Metastatic Non-Small Cell Lung Cancer (NSCLC) Clin. Pract. 2022;12:419–424. doi: 10.3390/clinpract12030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liquid Biopsy: What It Is & Procedure Details. Cleveland Clinic. [(accessed on 15 March 2023)]. Available online: https://my.clevelandclinic.org/health/diagnostics/23992-liquid-biopsy.
- 19.Giacona M.B., Ruben G.C., Iczkowski K.A., Roos T.B., Porter D.M., Sorenson G.D. Cell-Free DNA in Human Blood Plasma: Length Measurements in Patients with Pancreatic Cancer and Healthy Controls. Pancreas. 1998;17:89–97. doi: 10.1097/00006676-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Alcaide M., Cheung M., Hillman J., Rassekh S.R., Deyell R.J., Batist G., Karsan A., Wyatt A.W., Johnson N., Scott D.W., et al. Evaluating the quantity, quality and size distribution of cell-free DNA by multiplex droplet digital PCR. Sci. Rep. 2020;10:12564. doi: 10.1038/s41598-020-69432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorenson G.D., Pribish D.M., Valone F.H., Memoli V.A., Bzik D.J., Yao S.-L. Soluble Normal and Mutated DNA Sequences from Single-Copy Genes in Human Blood. Volume 3. [(accessed on 15 March 2023)]. Available online: http://aacrjournals.org/cebp/article-pdf/3/1/67/2287024/67.pdf. [PubMed]
- 22.Streleckiene G., Reid H.M., Arnold N., Bauerschlag D., Forster M. Quantifying cell free DNA in urine: Comparison between commercial kits, impact of gender and inter-individual variation. BioTechniques. 2018;64:225–230. doi: 10.2144/btn-2018-0003. [DOI] [PubMed] [Google Scholar]
- 23.Escudero L., Martínez-Ricarte F., Seoane J. ctDNA-Based Liquid Biopsy of Cerebrospinal Fluid in Brain Cancer. Cancers. 2021;13:1989. doi: 10.3390/cancers13091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki N., Kamataki A., Yamaki J., Homma Y. Characterization of circulating DNA in healthy human plasma. Clin. Chim. Acta. 2008;387:55–58. doi: 10.1016/j.cca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 26.Kawane K., Fukuyama H., Kondoh G., Takeda J., Ohsawa Y., Uchiyama Y., Nagata S. Requirement of DNase II for Definitive Erythropoiesis in the Mouse Fetal Liver. Science. 2001;292:1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 27.Moss J., Magenheim J., Neiman D., Zemmour H., Loyfer N., Korach A., Samet Y., Maoz M., Druid H., Arner P., et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018;9:5068. doi: 10.1038/s41467-018-07466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hummel E.M., Hessas E., Müller S., Beiter T., Fisch M., Eibl A., Wolf O.T., Giebel B., Platen P., Kumsta R., et al. Cell-free DNA release under psychosocial and physical stress conditions. Transl. Psychiatry. 2018;8:236. doi: 10.1038/s41398-018-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duvvuri B., Lood C. Frontiers in Immunology. Volume 10. Frontiers Media S.A.; Lausanne, Switzerland: 2019. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saukkonen K., Lakkisto P., Pettilä V., Varpula M., Karlsson S., Ruokonen E., Pulkki K., for the Finnsepsis Study Group Cell-Free Plasma DNA as a Predictor of Outcome in Severe Sepsis and Septic Shock. Clin. Chem. 2008;54:1000–1007. doi: 10.1373/clinchem.2007.101030. [DOI] [PubMed] [Google Scholar]
- 31.Chang C.P.-Y., Chia R.-H., Wu T.-L., Tsao K.-C., Sun C.-F., Wu J.T. Elevated cell-free serum DNA detected in patients with myocardial infarction. Clin. Chim. Acta. 2003;327:95–101. doi: 10.1016/S0009-8981(02)00337-6. [DOI] [PubMed] [Google Scholar]
- 32.Mouliere F., Chandrananda D., Piskorz A.M., Moore E.K., Morris J., Ahlborn L.B., Mair R., Goranova T., Marass F., Heider K., et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018;10:eaat4921. doi: 10.1126/scitranslmed.aat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang P., Chan C.W.M., Chan K.C.A., Cheng S.H., Wong J., Wong V.W.-S., Wong G.L.H., Chan S.L., Mok T.S.K., Chan H.L.Y., et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA. 2015;112:E1317–E1325. doi: 10.1073/pnas.1500076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.-D., Knippers R. DNA Fragments in the Blood Plasma of Cancer Patients: Quantitations and Evidence for Their Origin from Apoptotic and Necrotic Cells1. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 35.Stroun M., Lyautey J., Lederrey C., Olson-Sand A., Anker P. About the possible origin and mechanism of circulating DNA: Apoptosis and active DNA release. Clin. Chim. Acta. 2001;313:139–142. doi: 10.1016/S0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R., Shao F., Wu X., Ying K. Value of quantitative analysis of circulating cell free DNA as a screening tool for lung cancer: A meta-analysis. Lung Cancer. 2010;69:225–231. doi: 10.1016/j.lungcan.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Feng J., Gang F., Li X., Jin T., Houbao H., Yu C., Guorong L. Plasma cell-free DNA and its DNA integrity as biomarker to distinguish prostate cancer from benign prostatic hyperplasia in patients with increased serum prostate-specific antigen. Int. Urol. Nephrol. 2013;45:1023–1028. doi: 10.1007/s11255-013-0491-2. [DOI] [PubMed] [Google Scholar]
- 38.Gautschi O., Bigosch C., Huegli B., Jermann M., Marx A., Chassé E., Ratschiller D., Weder W., Joerger M., Betticher D.C., et al. Circulating Deoxyribonucleic Acid As Prognostic Marker in Non–Small-Cell Lung Cancer Patients Undergoing Chemotherapy. J. Clin. Oncol. 2004;22:4157–4164. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 39.Mouliere F., el Messaoudi S., Pang D., Dritschilo A., Thierry A.R. Multi-marker analysis of circulating cell-free DNA toward personalized medicine for colorectal cancer. Mol. Oncol. 2014;8:927–941. doi: 10.1016/j.molonc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phallen J., Sausen M., Adleff V., Leal A., Hruban C., White J., Anagnostou V., Fiksel J., Cristiano S., Papp E., et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017;9:eaan2415. doi: 10.1126/scitranslmed.aan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee T.-H., Montalvo L., Chrebtow V., Busch M.P. Quantitation of genomic DNA in plasma and serum samples: Higher concentrations of genomic DNA found in serum than in plasma. Transfusion. 2001;41:276–282. doi: 10.1046/j.1537-2995.2001.41020276.x. [DOI] [PubMed] [Google Scholar]
- 42.Pittella-Silva F., Chin Y.M., Chan H.T., Nagayama S., Miyauchi E., Low S.-K., Nakamura Y. Plasma or Serum: Which Is Preferable for Mutation Detection in Liquid Biopsy? Clin. Chem. 2020;66:946–957. doi: 10.1093/clinchem/hvaa103. [DOI] [PubMed] [Google Scholar]
- 43.Frattini M., Gallino G., Signoroni S., Balestra D., Battaglia L., Sozzi G., Leo E., Pilotti S., Pierotti M.A. Quantitative Analysis of Plasma DNA in Colorectal Cancer Patients. Ann. N. Y. Acad. Sci. 2006;1075:185–190. doi: 10.1196/annals.1368.025. [DOI] [PubMed] [Google Scholar]
- 44.Allen D., Butt A., Cahill D., Wheeler M., Popert R., Swaminathan R. Role of cell-free plasma DNA as a diagnostic marker for prostate cancer. Ann. N. Y. Acad. Sci. 2004;1022:76–80. doi: 10.1196/annals.1318.013. [DOI] [PubMed] [Google Scholar]
- 45.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M., et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit J., Carroll G., Gould T., Pockney P., Dun M., Scott R.J. Cell-Free DNA as a Diagnostic Blood-Based Biomarker for Colorectal Cancer: A Systematic Review. J. Surg. Res. 2019;236:184–197. doi: 10.1016/j.jss.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Bronkhorst A.J., Ungerer V., Holdenrieder S. Comparison of methods for the quantification of cell-free DNA isolated from cell culture supernatant. Tumor Biol. 2019;41:1010428319866369. doi: 10.1177/1010428319866369. [DOI] [PubMed] [Google Scholar]
- 48.Chen E., Cario C.L., Leong L., Lopez K., Márquez C.P., Chu C., Li P.S., Oropeza E., Tenggara I., Cowan J., et al. Cell-free DNA concentration and fragment size as a biomarker for prostate cancer. Sci. Rep. 2021;11:5040. doi: 10.1038/s41598-021-84507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stebbing J., Takis P.G., Sands C.J., Maslen L., Lewis M.R., Gleason K., Page K., Guttery D., Fernandez-Garcia D., Primrose L., et al. Comparison of phenomics and cfDNA in a large breast screening population: The Breast Screening and Monitoring Study (BSMS) Oncogene. 2023;42:825–832. doi: 10.1038/s41388-023-02591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lan Y.-T., Chen M.-H., Fang W.-L., Hsieh C.-C., Lin C.-H., Jhang F.-Y., Yang S.-H., Lin J.-K., Chen W.-S., Jiang J.-K., et al. Clinical relevance of cell-free DNA in gastrointestinal tract malignancy. Oncotarget. 2016;8:3009–3017. doi: 10.18632/oncotarget.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khorana A.A., Mangu P.B., Berlin J., Engebretson A., Hong T.S., Maitra A., Mohile S.G., Mumber M., Schulick R., Shapiro M., et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017;35:2324–2328. doi: 10.1200/JCO.2017.72.4948. [DOI] [PubMed] [Google Scholar]
- 52.Bergquist J.R., Puig C.A., Shubert C., Groeschl R.T., Habermann E., Kendrick M.L., Nagorney D.M., Smoot R.L., Farnell M.B., Truty M.J. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J. Am. Coll. Surg. 2016;223:52–65. doi: 10.1016/j.jamcollsurg.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Guo M., Luo G., Lu R., Shi W., Cheng H., Lu Y., Jin K., Yang C., Wang Z., Long J., et al. Distribution of Lewis and Secretor polymorphisms and corresponding CA19-9 antigen expression in a Chinese population. FEBS Open Bio. 2017;7:1660–1671. doi: 10.1002/2211-5463.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao Y.-H., Demissie K., Shih W., Mehta A.R., Stein M.N., Roberts C.B., DiPaola R.S., Lu-Yao G.L. Contemporary Risk Profile of Prostate Cancer in the United States. J. Natl. Cancer Inst. 2009;101:1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dess R.T., Sun Y., Jackson W.C., Jairath N.K., Kishan A.U., Wallington D.G., Mahal B.A., Stish B.J., Zumsteg Z.S., Den R.B., et al. Association of Presalvage Radiotherapy PSA Levels After Prostatectomy with Outcomes of Long-term Antiandrogen Therapy in Men With Prostate Cancer. JAMA Oncol. 2020;6:735–743. doi: 10.1001/jamaoncol.2020.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ilic D., Djulbegovic M., Jung J.H., Hwang E.C., Zhou Q., Cleves A., Agoritsas T., Dahm P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harris L., Fritsche H., Mennel R., Norton L., Ravdin P., Taube S., Somerfield M.R., Hayes D.F., Bast R.C. American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer. J. Clin. Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 58.Locker G.Y., Hamilton S., Harris J., Jessup J.M., Kemeny N., Macdonald J.S., Somerfield M.R., Hayes D.F., Bast R.C. ASCO 2006 Update of Recommendations for the Use of Tumor Markers in Gastrointestinal Cancer. J. Clin. Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 59.Huang C.-J., Huang W.-Y., Chen C.-Y., Chao Y.-J., Chiang N.-J., Shan Y.-S. Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: A prospective cohort study. Ther. Adv. Med. Oncol. 2022;14:17588359221106558. doi: 10.1177/17588359221106558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bunduc S., Gede N., Váncsa S., Lillik V., Kiss S., Dembrovszky F., Eróss B., Szakács Z., Gheorghe C., Mikó A., et al. Prognostic role of cell-free DNA biomarkers in pancreatic adenocarcinoma: A systematic review and meta–analysis. Crit. Rev. Oncol./Hematol. 2022;169:103548. doi: 10.1016/j.critrevonc.2021.103548. [DOI] [PubMed] [Google Scholar]
- 61.Liu H., Gao Y., Vafaei S., Gu X., Zhong X. The Prognostic Value of Plasma Cell-Free DNA Concentration in the Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021;11:599602. doi: 10.3389/fonc.2021.599602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandez-Garcia D., Hills A., Page K., Hastings R.K., Toghill B., Goddard K.S., Ion C., Ogle O., Boydell A.R., Gleason K., et al. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res. 2019;21:149. doi: 10.1186/s13058-019-1235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye Z., Wang C., Wan S., Mu Z., Zhang Z., Abu-Khalaf M.M., Fellin F.M., Silver D.P., Neupane M., Jaslow R.J., et al. Association of clinical outcomes in metastatic breast cancer patients with circulating tumour cell and circulating cell-free DNA. Eur. J. Cancer. 2018;106:133–143. doi: 10.1016/j.ejca.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spindler K.-L.G., Boysen A.K., Pallisgård N., Johansen J.S., Tabernero J., Sørensen M.M., Jensen B.V., Hansen T.F., Sefrioui D., Andersen R.F., et al. Cell-Free DNA in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Oncol. 2017;22:1049–1055. doi: 10.1634/theoncologist.2016-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pastor B., André T., Henriques J., Trouilloud I., Tournigand C., Jary M., Mazard T., Louvet C., Azan S., Bauer A., et al. Monitoring levels of circulating cell-free DNA in patients with metastatic colorectal cancer as a potential biomarker of responses to regorafenib treatment. Mol. Oncol. 2021;15:2401–2411. doi: 10.1002/1878-0261.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ai B., Liu H., Huang Y., Peng P. Circulating cell-free DNA as a prognostic and predictive biomarker in non-small cell lung cancer. Oncotarget. 2016;7:44583–44595. doi: 10.18632/oncotarget.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyun M.H., Sung J.S., Kang E.J., Choi Y.J., Park K.H., Shin S.W., Lee S.Y., Kim Y.H. Quantification of circulating cell-free DNA to predict patient survival in non-small-cell lung cancer. Oncotarget. 2017;8:94417–94430. doi: 10.18632/oncotarget.21769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma G., Wang J., Huang H., Han X., Xu J., Veeramootoo J.S., Xia T., Wang S. Identification of the plasma total cfDNA level before and after chemotherapy as an indicator of the neoadjuvant chemotherapy response in locally advanced breast cancer. Cancer Med. 2020;9:2271–2282. doi: 10.1002/cam4.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehner J., Stötzer O.J., Fersching D., Nagel D., Holdenrieder S. Circulating plasma DNA and DNA integrity in breast cancer patients undergoing neoadjuvant chemotherapy. Clin. Chim. Acta. 2013;425:206–211. doi: 10.1016/j.cca.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 70.Tissot C., Toffart A.-C., Villar S., Souquet P.-J., Merle P., Moro-Sibilot D., Pérol M., Zavadil J., Brambilla C., Olivier M., et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur. Respir. J. 2015;46:1773. doi: 10.1183/13993003.00676-2015. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S., Guleria R., Singh V., Bharti A.C., Mohan A., Das B.C. Plasma DNA level in predicting therapeutic efficacy in advanced nonsmall cell lung cancer. Eur. Respir. J. 2010;36:885. doi: 10.1183/09031936.00187909. [DOI] [PubMed] [Google Scholar]
- 72.Pan S., Xia W., Ding Q., Shu Y., Xu T., Geng Y., Lu Y., Chen D., Xu J., Wang F., et al. Can plasma DNA monitoring be employed in personalized chemotherapy for patients with advanced lung cancer? Biomed. Pharmacother. 2012;66:131–137. doi: 10.1016/j.biopha.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 73.Gristina V., Barraco N., La Mantia M., Castellana L., Insalaco L., Bono M., Perez A., Sardo D., Inguglia S., Iacono F., et al. Clinical Potential of Circulating Cell-Free DNA (cfDNA) for Longitudinally Monitoring Clinical Outcomes in the First-Line Setting of Non-Small-Cell Lung Cancer (NSCLC): A Real-World Prospective Study. Cancers. 2022;14:6013. doi: 10.3390/cancers14236013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuo Y.B., Chen J.S., Fan C.W., Li Y.S., Chan E.C. Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer. Clin. Chim. Acta. 2014;433:284–289. doi: 10.1016/j.cca.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Chi K.N., Barnicle A., Sibilla C., Lai Z., Corcoran C., Williams J.A., Barrett J.C., Adelman C.A., Qiu P., Easter A., et al. Concordance of BRCA1, BRCA2 (BRCA), and ATM mutations identified in matched tumor tissue and circulating tumor DNA (ctDNA) in men with metastatic castration-resistant prostate cancer (mCRPC) screened in the PROfound study. J. Clin. Oncol. 2021;39:26. doi: 10.1200/JCO.2021.39.6_suppl.26. [DOI] [Google Scholar]
- 76.Vandekerkhove G., Lavoie J.-M., Annala M., Sundahl N., Sano T., Struss W.J., Todenhöfer T., Ost P., Chi K.N., Black P.C., et al. Genomic concordance between profiling of circulating tumor DNA (ctDNA) and matched tissue in metastatic urothelial carcinoma. J. Clin. Oncol. 2019;37:457. doi: 10.1200/JCO.2019.37.7_suppl.457. [DOI] [Google Scholar]
- 77.Chae Y.K., Davis A.A., Jain S., Santa-Maria C., Flaum L., Beaubier N., Platanias L.C., Gradishar W., Giles F.J., Cristofanilli M. Concordance of Genomic Alterations by Next-Generation Sequencing in Tumor Tissue versus Circulating Tumor DNA in Breast Cancer. Mol. Cancer Ther. 2017;16:1412–1420. doi: 10.1158/1535-7163.MCT-17-0061. [DOI] [PubMed] [Google Scholar]
- 78.Xu B., Shan G., Wu Q., Li W., Wang H., Li H., Yang Y., Long Q., Zhao P. Concordance of Genomic Alterations between Circulating Tumor DNA and Matched Tumor Tissue in Chinese Patients with Breast Cancer. J. Oncol. 2020;2020:4259293. doi: 10.1155/2020/4259293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sacher A.G., Paweletz C., Dahlberg S.E., Alden R.S., O’connell A., Feeney N., Mach S.L., Jänne P.A., Oxnard G.R. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol. 2016;2:1014–1022. doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., Bright I.J., Lucero M.Y., Hid-dessen A.L., Legler T.C., et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diehl F., Li M., He Y., Kinzler K.W., Vogelstein B., Dressman D. BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods. 2006;3:551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 82.Diehl F., Li M., Dressman D., He Y., Shen D., Szabo S., Diaz L.A., Goodman S.N., David K.A., Juhl H., et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc. Natl. Acad. Sci. USA. 2005;102:16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heitzer E., Auer M., Hoffmann E.M., Pichler M., Gasch C., Ulz P., Lax S., Waldispuehl-Geigl J., Mauermann O., Mohan S., et al. Establishment of tumor-specific copy number alterations from plasma DNA of patients with cancer. Int. J. Cancer. 2013;133:346–356. doi: 10.1002/ijc.28030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carrera P., Righetti P.G., Gelfi C., Ferrari M. Amplification Refractory Mutation System Analysis of Point Mutations by Capillary Electrophoresis. In: Mitchelson K.R., Cheng J., editors. Capillary Electrophoresis of Nucleic Acids: Volume II: Practical Applications of Capillary Electrophoresis. Humana Press; Totowa, NJ, USA: 2001. pp. 95–108. [DOI] [PubMed] [Google Scholar]
- 85.Spindler K.-L.G., Pallisgaard N., Vogelius I., Jakobsen A. Quantitative Cell-Free DNA, KRAS, and BRAF Mutations in Plasma from Patients with Metastatic Colorectal Cancer during Treatment with Cetuximab and Irinotecan. Clin. Cancer Res. 2012;18:1177–1185. doi: 10.1158/1078-0432.CCR-11-0564. [DOI] [PubMed] [Google Scholar]
- 86.Stadler J., Eder J., Pratscher B., Brandt S., Schneller D., Müllegger R., Vogl C., Trautinger F., Brem G., Burgstaller J.P. SNPase-ARMS qPCR: Ultrasensitive Mutation-Based Detection of Cell-Free Tumor DNA in Melanoma Patients. PLoS ONE. 2015;10:e0142273. doi: 10.1371/journal.pone.0142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forshew T., Murtaza M., Parkinson C., Gale D., Tsui DW Y., Kaper F., Dawson S.-J., Piskorz A.M., Jimenez-Linan M., Bentley D., et al. Noninvasive Identification and Monitoring of Cancer Mutations by Targeted Deep Sequencing of Plasma DNA. Sci. Transl. Med. 2012;4:ra68–ra136. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 88.Ultra-Sensitive NGS-Based Liquid Biopsy Technology in Companion Diagnostics. 2021. [(accessed on 15 May 2023)]. Available online: https://www.amp.org/AMP/assets/File/education/ocw/QIAGEN_Sysmex_Inostics_Partnership_08252021.pdf?pass=99.
- 89.Kinde I., Wu J., Papadopoulos N., Kinzler K.W., Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc. Natl. Acad. Sci. USA. 2011;108:9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Newman A.M., Bratman S.V., To J., Wynne J.F., Eclov N.C.W., Modlin L.A., Liu C.L., Neal J.W., Wakelee H.A., Merritt R.E., et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leary R.J., Kinde I., Diehl F., Schmidt K., Clouser C., Duncan C., Antipova A., Lee C., McKernan K., De La Vega F.M., et al. Development of Personalized Tumor Biomarkers Using Massively Parallel Sequencing. Sci. Transl. Med. 2010;2:20ra14. doi: 10.1126/scitranslmed.3000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robertson K.D. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20:3139–3155. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 93.Roadmap Epigenomics Consortium. Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J., et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esteller M. Epigenetics in Cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 95.Liu M.C., Oxnard G.R., Klein E.A., Swanton C., Seiden M.V., CCGA Consortium Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020;31:745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desai M., Shchegrov S.R., Chai S., Zhou Y., Nguyen T., Cho Y., Melton C., Scott E., Roychowdhury-Saha M., Chang P.-Y., et al. Abstract LB297: Analytical validation of a tissue-free, multi-cancer, post-diagnosis cancer research test that uses cell-free DNA methylation profiling. Cancer Res. 2023;83:LB297. doi: 10.1158/1538-7445.AM2023-LB297. [DOI] [Google Scholar]
- 97.Liang N., Li B., Jia Z., Wang C., Wu P., Zheng T., Wang Y., Qiu F., Wu Y., Su J., et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat. Biomed. Eng. 2021;5:586–599. doi: 10.1038/s41551-021-00746-5. [DOI] [PubMed] [Google Scholar]
- 98.Li B., Su J., Zhang G., Xu J., Peng J., Zhou Y., Qiu F., Fang S., Wen X., Wang G., et al. Abstract 5116: Analytical performance of ELSA-seq, a blood-based test for early detection of multiple cancers. Cancer Res. 2022;82:5116. doi: 10.1158/1538-7445.AM2022-5116. [DOI] [Google Scholar]
- 99.Chen X., Gole J., Gore A., He Q., Lu M., Min J., Yuan Z., Yang X., Jiang Y., Zhang T., et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020;11:3475. doi: 10.1038/s41467-020-17316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen S.Y., Burgener J.M., Bratman S.V., De Carvalho D.D. Preparation of cfMeDIP-seq libraries for methylome profiling of plasma cell-free DNA. Nat. Protoc. 2019;14:2749–2780. doi: 10.1038/s41596-019-0202-2. [DOI] [PubMed] [Google Scholar]
- 101.Mok T., Wu Y.-L., Lee J.S., Yu C.-J., Sriuranpong V., Sandoval-Tan J., Ladrera G., Thongprasert S., Srimuninnimit V., Liao M., et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin. Cancer Res. 2015;21:3196–3203. doi: 10.1158/1078-0432.CCR-14-2594. [DOI] [PubMed] [Google Scholar]
- 102.Marsico G., Sharma G., Perry M., Hackinger S., Forshew T., Howarth K., Platt J., Rosenfeld N., Osborne R. Abstract 3097: Analytical development of the RaDaRTM assay, a highly sensitive and specific assay for the monitoring of minimal residual disease. Cancer Res. 2020;80:3097. doi: 10.1158/1538-7445.AM2020-3097. [DOI] [Google Scholar]
- 103.Coombes R.C., Page K., Salari R., Hastings R.K., Armstrong A.C., Ahmed S., Ali S., Cleator S.J., Kenny L.M., Stebbing J., et al. Personalized Detection of Circulating Tumor DNA Antedates Breast Cancer Metastatic Recurrence. Clin. Cancer Res. 2019;25:4255–4263. doi: 10.1158/1078-0432.CCR-18-3663. [DOI] [PubMed] [Google Scholar]
- 104.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L., Douville C., Javed A.A., Wong F., Mattox A., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Premarket Approval (PMA) [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P210040.
- 106.Premarket Approval (PMA) [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140020.
- 107.Devices@FDA. [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=320540.
- 108.Devices@FDA. [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=320651.
- 109.Devices@FDA. [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=452325.
- 110.Devices@FDA. [(accessed on 15 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=454228.
- 111.CancerSEEK Early Detection Research Network. [(accessed on 15 May 2023)]; Available online: https://edrn.nci.nih.gov/data-and-resources/biomarkers/cancerseek/
- 112.GRAIL Announces Significant Progress with Multi-Cancer Early Detection Test Including FDA Breakthrough Device Designation. GRAIL. [(accessed on 15 May 2023)]. Available online: https://grail.com/press-releases/grail-announces-significant-progress-with-multi-cancer-early-detection-test-including-fda-breakthrough-device-designation/
- 113.FDA Grants Breakthrough Device Designation for Inivatas Radar Assay. Inivata. [(accessed on 15 May 2023)]. Available online: https://www.inivata.com/fda-grants-breakthrough-device-designation-for-inivatas-radar-assay/
- 114.Nilson E. Bluestar Genomics Receives FDA Breakthrough Device Designation for First-of-Its-Kind Pancreatic Cancer Screening Test. ClearNote Health. [(accessed on 15 May 2023)]. Available online: https://www.clearnotehealth.com/press-release/bluestar-genomics-receives-fda-breakthrough-device-designation-for-first-of-its-kind-pancreatic-cancer-screening-test/
- 115.FDA Grants Two New Breakthrough Device Designations for Natera’s SignateraTM MRD Test. Natera. [(accessed on 15 May 2023)]. Available online: https://www.natera.com/company/news/fda-grants-two-new-breakthrough-device-designations-for-nateras-signatera-mrd-test-2/
- 116.Predicine’s Liquid Biopsy Next-Generation Sequencing (NGS) Assay is Granted Breakthrough Device Designation by U.S. Food and Drug Administration—Predicine|Advancing Precision Cancer Therapies. Precidine. [(accessed on 15 May 2023)]. Available online: https://www.predicine.com/2022/09/20/predicines-liquid-biopsy-next-generation-sequencing-ngs-assay-is-granted-breakthrough-device-designation-by-u-s-food-and-drug-administration/
- 117.Burning Rock Received FDA Breakthrough Device Designation for its OverC Multi-Cancer Detection Blood Test. Burning Rock. [(accessed on 15 May 2023)]. Available online: https://ir.brbiotech.com/news-releases/news-release-details/burning-rock-received-fda-breakthrough-device-designation-its.
- 118.Potter N.T., Hurban P., White M.N., Whitlock K.D., Lofton-Day C.E., Tetzner R., Koenig T., Quigley N.B., Weiss G. Validation of a Real-Time PCR–Based Qualitative Assay for the Detection of Methylated SEPT9 DNA in Human Plasma. Clin. Chem. 2014;60:1183–1191. doi: 10.1373/clinchem.2013.221044. [DOI] [PubMed] [Google Scholar]
- 119.Johnson D.A., Barclay R.L., Mergener K., Weiss G., König T., Beck J., Potter N.T. Plasma Septin9 versus fecal immunochemical testing for colorectal cancer screening: A prospective multicenter study. PLoS ONE. 2014;9:e98238. doi: 10.1371/journal.pone.0098238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Haan D., Bergamaschi A., Guler G.D., Friedl V., Ning Y., Reggiardo R., Kesling M., Collins M., Gibb B., Pitea A., et al. Validation of a Pancreatic Cancer Detection Test in New-Onset Diabetes Using Cell-Free DNA 5-Hydroxymethylation Signatures. MedRxiv. 2021 doi: 10.1101/2021.12.27.21268450. [DOI] [Google Scholar]
- 121.Lennon A.M., Buchanan A.H., Kinde I., Warren A., Honushefsky A., Cohain A.T., Ledbetter D.H., Sanfilippo F., Sheridan K., Rosica D., et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science. 2020;369:eabb9601. doi: 10.1126/science.abb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu M.C., Klein E., Hubbell E., Maddala T., Aravanis A.M., Beausang J.F., Filippova D., Gross S., Jamshidi A., Kurtzman K., et al. 50O—Plasma cell-free DNA (cfDNA) assays for early multi-cancer detection: The circulating cell-free genome atlas (CCGA) study. Ann. Oncol. 2018;29:viii14. doi: 10.1093/annonc/mdy269.048. [DOI] [Google Scholar]
- 123.Klein E.A., Richards D., Cohn A., Tummala M., Lapham R., Cosgrove D., Chung G., Clement J., Gao J., Hunkapiller N., et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021;32:1167–1177. doi: 10.1016/j.annonc.2021.05.806. [DOI] [PubMed] [Google Scholar]
- 124.Gao Q., Wang C., Yang X., Fang S., Zhang Y., Wang G., Liu F., Wen X., Zhao J., Zhou G., et al. 909P A multi-cancer early detection model based on liquid biopsy of multi-omics biomarkers: A proof of concept study (PROMISE study) Ann. Oncol. 2022;33:S963–S964. doi: 10.1016/j.annonc.2022.07.1035. [DOI] [Google Scholar]
- 125.Gao Q., Li B., Cai S., Xu J., Wang C., Su J., Fang S., Qiu F., Wen X., Zhang Y., et al. Early detection and localization of multiple cancers using a blood-based methylation assay (ELSA-seq) J. Clin. Oncol. 2021;39:459. doi: 10.1200/JCO.2021.39.3_suppl.459. [DOI] [Google Scholar]
- 126.Gao Q., Li B., Xu J., Fang S., Qiu F., Su J., Wang G., Cai S., Zhao H., Zhang W., et al. Analysis of epigenomic signatures in cell-free DNA (cfDNA) from cancer patients and high-risk controls: A blinded test cohort of THUNDER-II study. J. Clin. Oncol. 2021;39:e22518. doi: 10.1200/JCO.2021.39.15_suppl.e22518. [DOI] [Google Scholar]
- 127.Gao Q., Zhang Y., Xu J., Wang G., Zhao J., Wen X., Li B., Zhang Z., Cai S., Fan J., et al. Clinical validation of a multicancer detection blood test by circulating cell-free DNA (cfDNA) methylation sequencing: The THUNDER study. J. Clin. Oncol. 2022;40:10544. doi: 10.1200/JCO.2022.40.16_suppl.10544. [DOI] [Google Scholar]
- 128.Liles E.G., Coronado G.D., Perrin N., Harte A.H., Nungesser R., Quigley N., Potter N.T., Weiss G., Koenig T., deVos T. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treat. Res. Commun. 2017;10:27–31. doi: 10.1016/j.ctarc.2016.12.004. [DOI] [Google Scholar]
- 129.Song L., Jia J., Peng X., Xiao W., Li Y. The performance of the SEPT9 gene methylation assay and a comparison with other CRC screening tests: A meta-analysis. Sci. Rep. 2017;7:3032. doi: 10.1038/s41598-017-03321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu J., Hu B., Gui Y.C., Tan Z.B., Xu J.W. Diagnostic Value and Clinical Significance of Methylated SEPT9 for Colorectal Cancer: A Meta-Analysis. Med. Sci. Monit. 2019;25:5813–5822. doi: 10.12659/MSM.915472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin J.S., Perdue L.A., Henrikson N.B., Bean S.I., Blasi P.R. Screening for Colorectal Cancer: An Evidence Update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2021 May. (Evidence Synthesis, No. 202) [(accessed on 15 May 2023)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK570913/ [PubMed]
- 132.Recommendation: Colorectal Cancer: Screening|United States Preventive Services Taskforce. [(accessed on 15 May 2023)]; Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening#fullrecommendationstart.
- 133.Longitudinal Performance of Epi proColon—Full Text View—ClinicalTrials.gov. [(accessed on 15 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03218423.
- 134.Guler G.D., Ning Y., Ku C.-J., Phillips T., McCarthy E., Ellison C.K., Bergamaschi A., Collin F., Lloyd P., Scott A., et al. Detection of early stage pancreatic cancer using 5-hydroxymethylcytosine signatures in circulating cell free DNA. Nat. Commun. 2020;11:5270. doi: 10.1038/s41467-020-18965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.New Onset Diabetes Management for Earlier Detection of Pancreatic Cancer (NODMED)—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05188586.
- 136.EpiDetect Study: Clinical Validation of a Pancreatic Cancer Detection Test in New-Onset Diabetes Patients—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05188573.
- 137.ClearNote Health Clinical Trials for Pancreatic Cancer. ClearNote Health. [(accessed on 20 May 2023)]. Available online: https://www.clearnotehealth.com/clinical-trials/
- 138.Detecting Cancers Earlier Through Elective Plasma-based CancerSEEK Testing—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04213326.
- 139.The Circulating Cell-free Genome Atlas Study—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02889978.
- 140.The STRIVE Study: Development of a Blood Test for Early Detection of Multiple Cancer Types—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03085888.
- 141.The SUMMIT Study: A Cancer Screening Study—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT03934866.
- 142.REFLECTION: A Clinical Practice Learning Program for Galleri®—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05205967.
- 143.Beer T.M., McDonnell C.H., Nadauld L., Liu M.C., Klein E.A., Reid R.L., Marinac C., Chung K., Lopatin M., Fung E.T., et al. Interim results of PATHFINDER, a clinical use study using a methylation-based multi-cancer early detection test. J. Clin. Oncol. 2021;39:3010. doi: 10.1200/JCO.2021.39.15_suppl.3010. [DOI] [Google Scholar]
- 144.Nadauld L.D., McDonnell C.H.I.I.I., Beer T.M., Liu M.C., Klein E.A., Hudnut A., Whittington R.A., Taylor B., Oxnard G.R., Lipson J., et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers. 2021;13:3501. doi: 10.3390/cancers13143501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test Into Clinical Practice—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04241796.
- 146.GRAIL, LLC The PATHFINDER 2 Study: Evaluating the Safety and Performance of the GRAIL Multi-Cancer Early Detection Test in an Eligible Screening Population. [(accessed on 20 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05155605.
- 147.A Proof of Concept Study of Pan-cancer Early Detection by Liquid Biopsy—Full Text View—ClinicalTrials.gov. [(accessed on 20 May 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04972201.
- 148.Pan-canceR Early DetectIon project—Full Text View—ClinicalTrials.gov. [(accessed on 21 May 2023)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04383353.
- 149.Pan-canceR Early-Stage deteCtion by lIquid Biopsy tEchNique project—Full Text View—ClinicalTrials.gov. [(accessed on 21 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04822792.
- 150.Multi-canceR Early-detection Test in Asymptomatic Individuals (PREVENT)—Full Text View—ClinicalTrials.gov. [(accessed on 21 May 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05227534.
- 151.Genovese G., Kähler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M., et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rolfo C., Mack P., Scagliotti G.V., Aggarwal C., Arcila M.E., Barlesi F., Bivona T., Diehn M., Dive C., Dziadziuszko R., et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021;16:1647–1662. doi: 10.1016/j.jtho.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 153.Fiala C., Diamandis E.P. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 2018;16:166. doi: 10.1186/s12916-018-1157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cohen J.D., Javed A.A., Thoburn C., Wong F., Tie J., Gibbs P., Schmidt C.M., Yip-Schneider M.T., Allen P.J., Schattner M., et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl. Acad. Sci. USA. 2017;114:10202–10207. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tangvarasittichai O., Jaiwang W., Tangvarasittichai S. The Plasma DNA Concentration as a Potential Breast Cancer Screening Marker. Indian J. Clin. Biochem. 2013;30:55–58. doi: 10.1007/s12291-013-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lambros M.B., Seed G., Sumanasuriya S., Gil V., Crespo M., Fontes M., Chandler R., Mehra N., Fowler G., Ebbs B., et al. Single-Cell Analyses of Prostate Cancer Liquid Biopsies Acquired by Apheresis. Clin. Cancer Res. 2018;24:5635–5644. doi: 10.1158/1078-0432.CCR-18-0862. [DOI] [PubMed] [Google Scholar]
- 157.Fehm T.N., Meier-Stiegen F., Driemel C., Jäger B., Reinhardt F., Naskou J., Franken A., Neubauer H., Neves R.P., Dalum G., et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytom. Part A. 2018;93:1213–1219. doi: 10.1002/cyto.a.23669. [DOI] [PubMed] [Google Scholar]
- 158.Downs B.M., Sukumar S. Capturing ctDNA from Unaltered Stationary and Flowing Plasma with dCas9. ACS Appl. Mater. Interfaces. 2022;14:24113–24121. doi: 10.1021/acsami.2c03186. [DOI] [PubMed] [Google Scholar]
- 159.Lin J.S., Perdue L.A., Henrikson N.B., Bean S.I., Blasi P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325:1978–1998. doi: 10.1001/jama.2021.4417. [DOI] [PubMed] [Google Scholar]
- 160.In Vitro Companion Diagnostic Devices Guidance for Industry and Food and Drug Administration Staff. [(accessed on 25 May 2023)];2014 Available online: https://www.fda.gov/media/81309/download.
- 161.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140020B.pdf.
- 162.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 163.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Golan T., Hammel P., Reni M., van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swisher E.M., Lin K.K., Oza A.M., Scott C.L., Giordano H., Sun J., Konecny G.E., Coleman R.L., Tinker A.v., O’Malley D.M., et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 166.Premarket Approval (PMA) [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140020S016.
- 167.de Bono J., Mateo J., Fizazi K., Saad F., Shore N., Sandhu S., Chi K.N., Sartor O., Agarwal N., Olmos D., et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 168.Premarket Approval (PMA) [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140020S020.
- 169.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200006B.pdf.
- 170.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/P190032B.pdf.
- 171.Wu Y.-L., Lee V., Liam C.-K., Lu S., Park K., Srimuninnimit V., Wang J., Zhou C., Appius A., Button P., et al. Clinical utility of a blood-based EGFR mutation test in patients receiving first-line erlotinib therapy in the ENSURE, FASTACT-2, and ASPIRATION studies. Lung Cancer. 2018;126:1–8. doi: 10.1016/j.lungcan.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 172.Goss G., Tsai C.-M., Shepherd F.A., Bazhenova L., Lee J.S., Chang G.-C., Crino L., Satouchi M., Chu Q., Hida T., et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 173.Premarket Approval (PMA) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P120019S019.
- 174.Woodhouse R., Li M., Hughes J., Delfosse D., Skoletsky J., Ma P., Meng W., Dewal N., Milbury C., Clark T., et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS ONE. 2020;15:e0237802. doi: 10.1371/journal.pone.0237802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200010B.pdf.
- 176.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200010S002B.pdf.
- 177.Bauml J.M., Li B.T., Velcheti V., Govindan R., Curioni-Fontecedro A., Dooms C., Takahashi T., Duda A.W., Odegaard J.I., Cruz-Guilloty F., et al. Clinical validation of Guardant360 CDx as a blood-based companion diagnostic for sotorasib. Lung Cancer. 2022;166:270–278. doi: 10.1016/j.lungcan.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 15 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210040B.pdf.
- 179.Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., Scott C.L., Meier W., Shapira-Frommer R., Safra T., et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 180.Matulonis U.A., Penson R.T., Domchek S.M., Kaufman B., Shapira-Frommer R., Audeh M.W., Kaye S., Molife L.R., Gelmon K.A., Robertson J.D., et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: A multistudy analysis of response rates and safety. Ann. Oncol. 2016;27:1013–1019. doi: 10.1093/annonc/mdw133. [DOI] [PubMed] [Google Scholar]
- 181.Poveda A., Floquet A., Ledermann J.A., Asher R., Penson R.T., Oza A.M., Korach J., Huzarski T., Pignata S., Friedlander M., et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:620–631. doi: 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed] [Google Scholar]
- 182.Penson R.T., Valencia R.V., Cibula D., Colombo N., Leath C.A., III, Bidziński M., Kim J.-W., Nam J.H., Madry R., Hernández C., et al. Olaparib Versus Nonplatinum Chemotherapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020;38:1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.BRACAnalysis CDx® Technical Information. [(accessed on 25 May 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140020S020C.pdf.
- 184.Mirza M.R., Monk B.J., Herrstedt J., Oza A.M., Mahner S., Redondo A., Fabbro M., Ledermann J.A., Lorusso D., Vergote I., et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 185.Kristeleit R.S., Oaknin A., Ray-Coquard I., Leary A., Balmaña J., Drew Y., Oza A.M., Shapira-Frommer R., Domchek S.M., Cameron T., et al. Antitumor activity of the poly(ADP-ribose) polymerase inhibitor rucaparib as monotherapy in patients with platinum-sensitive, relapsed, BRCA-mutated, high-grade ovarian cancer, and an update on safety. Int. J. Gynecol. Cancer. 2019;29:1396–1404. doi: 10.1136/ijgc-2019-000623. [DOI] [PubMed] [Google Scholar]
- 186.Hussain M., Corcoran C., Sibilla C., Fizazi K., Saad F., Shore N., Sandhu S., Mateo J., Olmos D., Mehra N., et al. Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib) Clin. Cancer Res. 2022;28:1518–1530. doi: 10.1158/1078-0432.CCR-21-3940. [DOI] [PubMed] [Google Scholar]
- 187.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf12/P120019B.pdf.
- 188.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 189.Benlloch S., Botero M.L., Beltran-Alamillo J., Mayo C., Gimenez-Capitán A., de Aguirre I., Queralt C., Ramirez J.L., Cajal S.R.Y., Klughammer B., et al. Clinical Validation of a PCR Assay for the Detection of EGFR Mutations in Non–Small-Cell Lung Cancer: Retrospective Testing of Specimens from the EURTAC Trial. PLoS ONE. 2014;9:e89518. doi: 10.1371/journal.pone.0089518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wu Y.-L., Zhou C., Liam C.-K., Wu G., Liu X., Zhong Z., Lu S., Cheng Y., Han B., Chen L., et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 191.Park K., Yu C.-J., Kim S.-W., Lin M.-C., Sriuranpong V., Tsai C.-M., Lee J.-S., Kang J.-H., Chan K.C.A., Perez-Moreno P., et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer. JAMA Oncol. 2016;2:305–308. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 192.Wu Y.-L., Lee J.S., Thongprasert S., Yu C.-J., Zhang L., Ladrera G., Srimuninnimit V., Sriuranpong V., Sandoval-Tan J., Zhu Y., et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): A randomised, double-blind trial. Lancet Oncol. 2013;14:777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 193.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150047B.pdf.
- 194.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150044B.pdf.
- 195.Premarket Approval (PMA) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P120019S018.
- 196.Cheng Y., He Y., Li W., Zhang H., Zhou Q., Wang B., Liu C., Walding A., Saggese M., Huang X., et al. Osimertinib Versus Comparator EGFR TKI as First-Line Treatment for EGFR-Mutated Advanced NSCLC: FLAURA China, A Randomized Study. Target. Oncol. 2021;16:165–176. doi: 10.1007/s11523-021-00794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 198.Premarket Approval (PMA) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P120019S031.
- 199.SUMMARY of SAFETY and EFFECTIVENESS DATA (SSED) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019B.pdf.
- 200.Milbury C.A., Creeden J., Yip W.-K., Smith D.L., Pattani V., Maxwell K., Sawchyn B., Gjoerup O., Meng W., Skoletsky J., et al. Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLoS ONE. 2022;17:e0264138. doi: 10.1371/journal.pone.0264138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Premarket Approval (PMA) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P170019S033.
- 202.Premarket Approval (PMA) [(accessed on 1 June 2023)]; Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P190032S008.
- 203.A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of Sotorasib (AMG 510) in Subjects with Solid Tumors With a Specific KRAS Mutation (CodeBreaK 100)—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03600883.
- 204.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Phase 1/2 Study of MRTX849 in Patients With Cancer Having a KRAS G12C Mutation KRYSTAL-1—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03785249.
- 206.Jänne P.A., Riely G.J., Gadgeel S.M., Heist R.S., Ou S.-H.I., Pacheco J.M., Johnson M.L., Sabari J.K., Leventakos K., Yau E., et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 207.Pekker I., Pollak J., Potts K., Chan P., Tsai C.-H., Liao A., Garrison C., Brown T., Stull P., Bianchi-Frias D., et al. Resolution ctDx FIRST plasma assay as a companion diagnostic for adagrasib and its application to longitudinal monitoring. J. Clin. Oncol. 2022;40:3057. doi: 10.1200/JCO.2022.40.16_suppl.3057. [DOI] [Google Scholar]
- 208.Prognosis and Targeted Therapy Related Molecular Screening Program for Patients of Breast Cancer in China—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03792529.
- 209.Liao H., Zhang J., Zheng T., Liu X., Zhong J., Shao B., Dong X., Wang X., Du P., King B.L., et al. Identification of mutation patterns and circulating tumour DNA-derived prognostic markers in advanced breast cancer patients. J. Transl. Med. 2022;20:211. doi: 10.1186/s12967-022-03421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gerratana L., Zhang Q., Shah A.N., Davis A.A., Zhang Y., Wehbe F., Qiang W., Flaum L., Finkelman B.S., Gradishar W.J., et al. Performance of a novel Next Generation Sequencing circulating tumor DNA (ctDNA) platform for the evaluation of samples from patients with metastatic breast cancer (MBC) Crit. Rev. Oncol./Hematol. 2020;145:102856. doi: 10.1016/j.critrevonc.2019.102856. [DOI] [PubMed] [Google Scholar]
- 211.Developing and Labeling in Vitro Companion Diagnostic Devices for a Specific Group or Class of Oncology Therapeutic Products Guidance for Industry DRAFT GUIDANCE. [(accessed on 20 June 2023)];2018 Available online: https://www.fda.gov/media/120340/download.
- 212.Chao M., Gibbs P. Caution Is Required Before Recommending Routine Carcinoembryonic Antigen and Imaging Follow-Up for Patients With Early-Stage Colon Cancer. J. Clin. Oncol. 2009;27:e279–e280. doi: 10.1200/JCO.2009.25.6156. [DOI] [PubMed] [Google Scholar]
- 213.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R., D’Amico T.A., DeCamp M.M., Dilling T.J., Dobelbower M., et al. Non–Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 214.Erdi Y.E. Limits of Tumor Detectability in Nuclear Medicine and PET. Mol. Imaging Radionucl. Ther. 2012;21:23–28. doi: 10.4274/Mirt.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huang K., Dahele M., Senan S., Guckenberger M., Rodrigues G.B., Ward A., Boldt R.G., Palma D.A. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)—Can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother. Oncol. 2012;102:335–342. doi: 10.1016/j.radonc.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 216.Garcia-Murillas I., Schiavon G., Weigelt B., Ng C., Hrebien S., Cutts R.J., Cheang M., Osin P., Nerurkar A., Kozarewa I., et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 217.Tie J., Wang Y., Tomasetti C., Li L., Springer S., Kinde I., Silliman N., Tacey M., Wong H.-L., Christie M., et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016;8:346ra92. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chaudhuri A.A., Chabon J.J., Lovejoy A.F., Newman A.M., Stehr H., Azad T.D., Khodadoust M.S., Esfahani M.S., Liu C.L., Zhou L., et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gale D., Heider K., Ruiz-Valdepenas A., Hackinger S., Perry M., Marsico G., Rundell V., Wulff J., Sharma G., Knock H., et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann. Oncol. 2022;33:500–510. doi: 10.1016/j.annonc.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Flach S., Howarth K., Hackinger S., Pipinikas C., Ellis P., McLay K., Marsico G., Forshew T., Walz C., Reichel C.A., et al. Liquid BIOpsy for MiNimal RESidual DiSease Detection in Head and Neck Squamous Cell Carcinoma (LIONESS)—A personalised circulating tumour DNA analysis in head and neck squamous cell carcinoma. Br. J. Cancer. 2022;126:1186–1195. doi: 10.1038/s41416-022-01716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cutts R.J., Coakley M., Garcia-Murillas I., Ulrich L., Howarth K., Emmett W., Perry M., Ellis P., Knape C., Johnston S.R., et al. Abstract 536: Molecular residual disease detection in early stage breast cancer with a personalized sequencing approach. Cancer Res. 2021;81:536. doi: 10.1158/1538-7445.AM2021-536. [DOI] [Google Scholar]
- 222.Janni W., Huober J., Huesmann S., Pipinikas C., Braun T., Müller V., Marsico G., Fink A., Freire-Pritchett P., Koretz K., et al. Abstract P2-01-07: Detection of early-stage breast cancer recurrence using a personalised liquid biopsy-based sequencing approach. Cancer Res. 2022;82:P2-01-07. doi: 10.1158/1538-7445.SABCS21-P2-01-07. [DOI] [Google Scholar]
- 223.Reinert T., Henriksen T.V., Christensen E., Sharma S., Salari R., Sethi H., Knudsen M., Nordentoft I.K., Wu H.-T., Tin A.S., et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients with Stages I to III Colorectal Cancer. JAMA Oncol. 2019;5:1124–1131. doi: 10.1001/jamaoncol.2019.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Christensen E., Birkenkamp-Demtröder K., Sethi H., Shchegrova S., Salari R., Nordentoft I., Wu H.-T., Knudsen M., Lamy P., Lindskrog S.V., et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019;37:1547–1557. doi: 10.1200/JCO.18.02052. [DOI] [PubMed] [Google Scholar]
- 225.Loupakis F., Sharma S., Derouazi M., Murgioni S., Biason P., Rizzato M.D., Rasola C., Renner D., Shchegrova S., Malashevich A.K., et al. Detection of Molecular Residual Disease Using Personalized Circulating Tumor DNA Assay in Patients With Colorectal Cancer Undergoing Resection of Metastases. JCO Precis. Oncol. 2021;5:1166–1177. doi: 10.1200/PO.21.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Plagnol V., Woodhouse S., Howarth K., Lensing S., Smith M., Epstein M., Madi M., Smalley S., Leroy C., Hinton J., et al. Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS ONE. 2018;13:e0193802. doi: 10.1371/journal.pone.0193802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.LUCID—LUng Cancer CIrculating Tumour Dna Study—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04153526.
- 228.Multi-Omic Assessment of Squamous Cell Cancers Receiving Systemic Therapy—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03712566.
- 229.Taylor K., Zou J., Fonseca Magalhaes Filho M.A., Howarth K., Marsico G., Terrell S., Jones G., Knape C., Forshew T., Oliva M., et al. Personalized circulating tumor DNA (ctDNA) analysis in patients with recurrent/metastatic head and neck squamous cell cancer (R/M HNSCC) J. Clin. Oncol. 2022;40:6052. doi: 10.1200/JCO.2022.40.16_suppl.6052. [DOI] [Google Scholar]
- 230.Liquid Biopsy Evaluation and Repository Development at Princess Margaret—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03702309.
- 231.Elliott M.J., Veitch Z., Bedard P., Amir E., Dou A., Antras J.F., Nadler M., Meti N., Gregorio N., Shah E., et al. Abstract P6-01-16: Circulating Tumour DNA (ctDNA) Detection and Dynamics in Patients with Early Breast Cancer (EBC): Results of the Neoadjuvant TRACER cohort. Cancer Res. 2023;83:P6-01-16. doi: 10.1158/1538-7445.SABCS22-P6-01-16. [DOI] [Google Scholar]
- 232.Coakley M., Sritharan P., Villacampa G., Swift C., Dunne K., Kilburn L., Goddard K., Rojas P., Joad A., Emmett W., et al. Abstract PD5-03: PD5-03 Comparison of a personalized sequencing assay and digital PCR for circulating tumor DNA based Molecular Residual Disease detection in early-stage triple negative breast cancer in the cTRAK-TN trial. Cancer Res. 2023;83:PD5-03. doi: 10.1158/1538-7445.SABCS22-PD5-03. [DOI] [Google Scholar]
- 233.Genta S., Araujo D.V., Keshavarzi S., Pimentel Muniz T., Saeed Kamil Z., Howarth K., Terrell S., Joad A., Ventura R., Covelli A., et al. Leveraging personalized circulating tumor DNA (ctDNA) for detection and monitoring of molecular residual disease in high-risk melanoma. J. Clin. Oncol. 2022;40:9579. doi: 10.1200/JCO.2022.40.16_suppl.9579. [DOI] [Google Scholar]
- 234.Stockem C., Gil Jimenez A., van Dorp J., van Dijk N., Alkemade M., Seignette I., Pipinikas C., Jones G., Rosenfeld N., van Montfoort M., et al. 1770P Updated follow-up data and biomarker analysis of pre-operative ipilimumab and nivolumab in locoregional advanced urothelial cancer (NABUCCO) Ann. Oncol. 2022;33:S1347. doi: 10.1016/j.annonc.2022.07.1848. [DOI] [Google Scholar]
- 235.Taniguchi H., Nakamura Y., Kotani D., Yukami H., Mishima S., Sawada K., Shirasu H., Ebi H., Yamanaka T., Aleshin A., et al. CIRCULATE-Japan: Circulating tumor DNA–guided adaptive platform trials to refine adjuvant therapy for colorectal cancer. Cancer Sci. 2021;112:2915–2920. doi: 10.1111/cas.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.NIPH Clinical Trials Search. [(accessed on 20 June 2023)]. Available online: https://rctportal.niph.go.jp/en/detail?trial_id=jRCT1031200006.
- 237.Kotani D., Oki E., Nakamura Y., Yukami H., Mishima S., Bando H., Shirasu H., Yamazaki K., Watanabe J., Kotaka M., et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023;29:127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.BESPOKE Study of ctDNA Guided Therapy in Colorectal Cancer—Full Text View—ClinicalTrials.gov. [(accessed on 20 June 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04264702.
- 239.Kasi P.M., Sawyer S., Guilford J., Munro M., Ellers S., Wulff J., Hook N., Krinshpun S., Malashevich A.K., Malhotra M., et al. BESPOKE study protocol: A multicentre, prospective observational study to evaluate the impact of circulating tumour DNA guided therapy on patients with colorectal cancer. BMJ Open. 2021;11:e047831. doi: 10.1136/bmjopen-2020-047831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Powles T., Assaf Z.J., Davarpanah N., Banchereau R., Szabados B.E., Yuen K.C., Grivas P., Hussain M., Oudard S., Gschwend J.E., et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595:432–437. doi: 10.1038/s41586-021-03642-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.