Abstract
This registry assessed the impact of conservative and invasive strategies on major adverse clinical events (MACE) in elderly patients with non-ST-elevation myocardial infarction (NSTEMI). Patients aged ≥75 years with NSTEMI were prospectively registered from European centers and followed up for one year. Outcomes were compared between conservative and invasive groups in the overall population and a propensity score-matched (PSM) cohort. MACE included cardiovascular death, acute coronary syndrome, and stroke. The study included 1190 patients (median age 80 years, 43% female). CAG was performed in 67% (N = 798), with two-thirds undergoing revascularization. Conservatively treated patients had higher baseline risk. After propensity score matching, 319 patient pairs were successfully matched. MACE occurred more frequently in the conservative group (total population 20% vs. 12%, adjHR 0.53, 95% CI 0.37–0.77, p = 0.001), remaining significant in the PSM cohort (18% vs. 12%, adjHR 0.50, 95% CI 0.31–0.81, p = 0.004). In conclusion, an early invasive strategy was associated with benefits over conservative management in elderly patients with NSTEMI. Risk factors associated with ischemia and bleeding should guide strategy selection rather than solely relying on age.
Keywords: coronary artery disease, non-ST-elevation myocardial infarction, elderly, conservative strategy, invasive strategy
1. Introduction
As life expectancy is advancing and the prevalence of coronary artery disease increases with age, the number of elderly patients with coronary artery disease (CAD) is rising [1,2]. Elderly patients with non-ST-elevation myocardial infarction (NSTEMI) are at higher risk of cardiovascular events, as well as treatment-related complications (especially bleeding) [3]. Moreover, as frailty is common, these patients are rarely included in clinical trials, which is why guidelines are often based on the extrapolation of data from a substantially younger and healthier population or studies with a small sample size. Due to this insufficient evidence, both the European Society of Cardiology (ESC) and American Heart Association/American College of Cardiology (AHA/ACC) can only provide valuable considerations but do not give specific recommendations for the treatment of elderly patients with an NSTEMI [4,5,6]. In practice, elderly patients less often receive guideline-recommended care, despite current guidelines recommending treating older patients with the same interventional strategies as younger patients [4,7].
The POPular AGE Registry was initiated in order to specifically capture the clinical treatment strategy and prognosis of this heterogeneous population. Further aims were to evaluate differences in cardiovascular risk between a conservative and invasive strategy and find predictors for major adverse cardiovascular events (MACE).
2. Materials and Methods
The POPular AGE registry is an investigator-initiated, prospective, observational, international, multicenter study of patients ≥75 years presenting with NSTEMI. There were no exclusion criteria. Patients were recruited between 1st August 2016 and December 2019 at 29 sites in the Netherlands, the United Kingdom and Austria. Decisions regarding medical therapy, the performance of invasive coronary angiography (CAG) and, if indicated, subsequent percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) were at the discretion of the attending physicians, except for a number of patients (N = 111) who were also enrolled in the POPular AGE trial and were randomized between ticagrelor and clopidogrel [8]. To assess differences in baseline characteristics and outcomes in different treatment strategies, the population was divided into two treatment groups: the invasive group, defined as patients who underwent CAG during the index hospitalization, and a conservative group receiving medical therapy alone. The invasive strategy was further stratified to CAG only, PCI or CABG. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Medical Research Ethics Committees United (ID: W17.021). All patients provided written informed consent.
Demographic, clinical and procedural characteristics and in-hospital and one-year follow-up data were collected. At one and twelve months, patients were sent a questionnaire inquiring about current medication use, events and quality of life by use of the Short Form Health Survey 12 (SF-12) and new hospital admissions. Frailty was assessed within one month after admission using the Groningen Frailty Indicator (GFI) (Appendix A). Cardiovascular events consisted of all-cause death, cardiovascular death, recurrent acute coronary syndrome (ACS), stroke, transient ischemic attack (TIA) and bleeding (Bleeding Academic Research Consortium [BARC] criteria) at one-year follow-up [9]. ACS included ST-segment elevation myocardial infarction (STEMI), NSTEMI, unstable angina pectoris, and type 2 myocardial infarction (MI), defined using the Fourth Definition of MI [10]. MACE was defined as a composite of cardiovascular death, ACS and stroke. Net adverse clinical events (NACE) were defined as a composite of all-cause death, ACS, definite stent thrombosis, stroke or Bleeding Academic Research Consortium (BARC 3 or 5) bleeding. Major bleeding was defined as a bleeding event of BARC type 3 or 5, and major or clinically relevant nonmajor bleeding was defined as a bleeding event of BARC type 2, 3 or 5. Antithrombotic therapy at discharge consisted of a P2Y12-inihibitor with aspirin or oral anticoagulation (OAC), or triple therapy consisting of aspirin, a P2Y12-inhibitor and OAC. Optimal medical treatment (OMT) was defined according to the guideline as the use of a beta-blocker, ACE-inhibitor or angiotensin II receptor blocker, and statin in combination with adequate antithrombotic therapy.
Continuous variables are presented as mean ± standard deviation (SD) or as median with interquartile range (IQR); categorical variables are presented as frequencies and percentages. Differences in baseline characteristics and events during follow-up between the invasive and conservative groups were tested with chi-square or Fisher exact tests for categorical variables and two-sample t-test or Mann-Whitney U test for continuous variables. Estimation of the incidence of the ischemic and bleeding endpoints was carried out using the Kaplan-Meier method. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated using Cox proportional-hazard models. Violation of the proportional hazards assumption was evaluated by calculating Schoenfeld residuals. To adjust for possible confounders, clinically relevant variables or characteristics that differed at baseline were selected for univariate regression analysis (Table S1). If there was a significant interaction in the univariate analysis, they were selected for multivariate regression analysis. Only those characteristics with a significant interaction in the multivariate analysis were included in the final model.
Propensity scores were estimated using a multivariable logistic regression model, with the invasive strategy as the dependent variable. Covariates were chosen based on clinical relevance and their relation with the treatment strategy or the clinical outcome, or both, as assessed using a regression model. The covariates included in the matching process were age, gender, diabetes mellitus, hypercholesterolemia, hypertension, smoking, previous PCI, previous MI, Killip class, left ventricular ejection fraction below 50%, ST-depression, peripheral artery disease, heart failure, chronic kidney disease, chronic obstructive pulmonary disease (COPD) and optimal medical treatment (OMT). Propensity score matching was performed using a one-to-one matching protocol without replacement (greedy-matching algorithm) within a caliper equal to 0.2 of the standard deviation of the logit of the propensity score. To assess the effect of frailty and bleeding risk on clinical outcomes, we performed sub-group analyses in frail patients (known frailty score of 4 or higher) and patients with high bleeding risk (HBR) (CRUSADE score of 40 or higher) [9]. Missing baseline covariates were imputed using the median before the propensity score model was finalized. Standardized mean differences of more than 0.10 were considered as evidence of imbalance. All tests were two-tailed and used a p-value < 0.05 to characterize statistical significance. All analyses were performed using R statistical software version 4.1.2.
3. Results
3.1. Baseline Characteristics
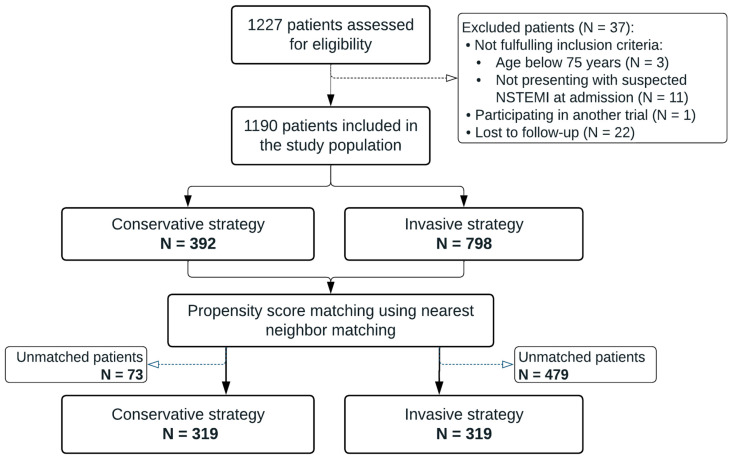
Between 1 August 2016 and 23 December 2019, a total of 1227 patients were enrolled in the registry. Fourteen patients did not meet the eligibility criteria, twenty-two patients were lost to follow-up, and one patient was enrolled in another clinical trial. The final population consisted of 1190 patients (Figure 1). The baseline characteristics of the study population are presented in Table 1. The median age was 80 (IQR 77–84) years and 43% of the population was female (N = 507). At discharge, 90% of patients were diagnosed with NSTEMI. The remaining patients had unstable angina pectoris (5%), non-specific chest pain (3%), ST-elevation MI (1%), Takotsubo cardiomyopathy (<1%), pericarditis (<1%) or exacerbation of COPD (<1%). Overall missing data at baseline was low, except for GRACE risk score (15%), Body Mass Index (26%), left ventricular ejection fraction (LVEF) at baseline (37%) and CRUSADE bleeding score (38%).
Figure 1.
Flowchart of the study. NSTEMI = non-ST-elevation myocardial infarction.
Table 1.
Baseline characteristics of the total study population before and after propensity score matching.
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Conservative (N = 392) |
Invasive (N = 798) |
p-Value | Conservative (N = 319) |
Invasive (N = 319) |
p-Value | |
| Age (years)—median, IQR | 83 (79–87) | 79 (77–83) | <0.001 | 82 (79–86) | 82 (79–85.5) | 0.757 |
| Female | 199 (51%) | 308 (39%) | <0.001 | 156 (49%) | 140 (44%) | 0.204 |
| BMI (mean ± SD) | 27 ± 5 | 27 ± 4 | 0.688 | 27 ± 5 | 27 ± 4 | 0.127 |
| Medical history | ||||||
| MI | 143 (36%) | 227 (29%) | 0.005 | 107 (34%) | 108 (34%) | 0.933 |
| Stroke | 69 (18%) | 111 (14%) | 0.095 | 58 (18%) | 57 (18%) | 0.918 |
| PAD | 30 (8%) | 94 (12%) | 0.029 | 26 (8%) | 29 (9%) | 0.672 |
| Heart failure | 48 (12%) | 31 (4%) | <0.001 | 21 (7%) | 26 (28%) | 0.449 |
| CKD | 70 (18%) | 66 (8%) | <0.001 | 38 (12%) | 41 (13%) | 0.718 |
| COPD | 65 (17%) | 87 (11%) | 0.006 | 43 (14%) | 48 (15%) | 0.571 |
| Atrial fibrillation | 94 (24%) | 112 (14%) | <0.001 | 70 (22%) | 60 (19%) | 0.326 |
| Dyslipidemia | 129 (33%) | 370 (46%) | <0.001 | 109 (34%) | 122 (38%) | 0.284 |
| Diabetes mellitus | 116 (30%) | 191 (24%) | 0.036 | 91 (29%) | 97 (30%) | 0.602 |
| Hypertension | 241 (62%) | 529 (66%) | 0.103 | 197 (62%) | 206 (65%) | 0.460 |
| Smoking | 149 (38%) | 367 (46%) | 0.009 | 126 (40%) | 135 (42%) | 0.469 |
| At admission | ||||||
| Killip class > II | 76 (19%) | 91 (11%) | <0.001 | 53 (17%) | 53 (17%) | 1.000 |
| LVEF < 50% | 73 (19%) | 142 (18%) | 0.727 | 55 (17%) | 59 (19%) | 0.679 |
| ST-depression | 117 (30%) | 240 (30%) | 0.936 | 94 (30%) | 100 (31%) | 0.606 |
| GRACE-score (mean ± SD) | 165 ± 41 | 161 ± 41 | 0.117 | 163 ± 40 | 165 ± 44 | 0.565 |
| CRUSADE score (mean ± SD) | 40 ± 12 | 34 ± 11 | <0.001 | 39 ± 12 | 37 ± 12 | 0.088 |
| Treatment | ||||||
| Optimal medical treatment | 110 (28%) | 364 (46%) | <0.001 | 101 (32%) | 110 (35%) | 0.449 |
| Aspirin | 220 (56%) | 638 (80%) | <0.001 | 181 (57%) | 237 (74%) | <0.001 |
| P2Y12-inhibitor | 265 (68%) | 662 (83%) | <0.001 | 222 (70%) | 247 (77%) | 0.025 |
| Beta-blocker | 254 (65%) | 607 (76%) | <0.001 | 215 (67%) | 223 (70%) | 0.495 |
| ACE-inhibitor | 151 (39%) | 418 (52%) | <0.001 | 132 (41%) | 146 (46%) | 0.264 |
| AT-II antagonist | 58 (15%) | 154 (19%) | 0.056 | 49 (15%) | 62 (19%) | 0.175 |
| Cholesterol inhibitor | 261 (67%) | 694 (87%) | <0.001 | 218 (68%) | 259 (81%) | <0.001 |
Data are n (%) unless stated otherwise. ACE-inhibitor = angiotensin-converting-enzyme inhibitor; AT-II = angiotensin-II; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PAD = peripheral arterial disease; PSM = propensity score matching.
3.1.1. Treatment
The median duration of hospital admission was 5 days (IQR 3–9). During the index hospital admission, CAG was performed in 67% of all patients (N = 798), mostly by radial artery access (72%) (N = 568). Significant coronary artery disease (>50% diameter reduction) was demonstrated in 85% of the cases (N = 676). Of patients undergoing CAG, 52% (N = 418) were subsequently treated with PCI, 14% (N = 111) with CABG and the remaining 34% (N = 270) only with medical treatment, of which one-third (N = 90) had no significant coronary artery disease (Table S2). On average, patients who underwent CAG were younger, more often male and had more risk factors for coronary artery disease (e.g., hypercholesterolemia, history of smoking) than patients in the conservative group (Table 1). After PSM, 319 pairs of patients were successfully matched. There was no significant difference in baseline characteristics between the two groups after PSM regarding the covariates used in the PSM (Table 1). Standardized differences were less than 0.10 for all covariates used in the PSM analysis, indicating that there was no evidence of imbalance between the groups (Figures S1 and S2).
3.1.2. Antithrombotic Therapy
At discharge, DAPT was the most commonly prescribed antithrombotic treatment (55%, N = 655) (Table S3). In patients receiving a P2Y12-inhibitor agent at discharge (N = 925, 480 with clopidogrel and 439 with ticagrelor), early discontinuation or switching of P2Y12-inhibitor occurred in 15% (N = 138). For both clopidogrel and ticagrelor, the most important reasons for early discontinuation or switching were undergoing CABG, bleeding and concomitant use of OAC. Of 74 patients who discontinued ticagrelor, 16 (22%) did so because of dyspnea. Early discontinuation was comparable within the invasive and conservative groups.
3.1.3. Frailty and Quality of Life
Frailty data at 1 month was available in 898 patients (Table 2). Of these patients, 60% (N = 541) had a GFI score of 4 or higher indicating frailty. Self-reported frailty was less common in the invasive group than in the conservative group (56% vs. 70%, p <0.001) (Table 2). The average quality of life within one month after admission was 37.1 ± 6.1 for the physical component summary (PCS) and 44.5 ± 6.0 for the mental component summary (MCS) of the SF-12 questionnaire. After PSM, there were no statically significant differences regarding frailty (68% vs. 62%, p = 0.224) and quality of life (PCS at 1 month: 36.4 vs. 37.0, p = 339, MCS at 1 month: 43.8 vs. 44.5, p = 0.256) between the conservative and invasive groups.
Table 2.
Frailty and quality of life outcomes.
| Questionnaire | Number of Patients (Conservative vs. Invasive) | Total Population | Conservative Group | Invasive Group | p-Value |
|---|---|---|---|---|---|
| GFI—median, IQR | 898 (357 vs. 541) | 4 (2–7) | 6 (3–8) | 4 (2–6) | <0.001 |
| Frailty (GFI ≥ 4) |
541 (60%) | 188 (70%) | 353 (56%) | <0.001 | |
| SF-12 at 1 month—mean ± SD PCS MCS |
728 (232 vs. 496) |
37.1 ±6.1 44.5 ±6.0 |
36.1 ± 6.6 43.4 ± 6.2 |
37.6 ± 5.7 44.9 ± 5.9 |
0.002 0.002 |
| SF-12 at 12 months—mean ± SD PCS MCS |
591 (150 vs. 441) |
37.8 ± 5.8 45.7 ± 5.5 |
36.4 ± 6.4 44.3 ± 6.2 |
38.3 ± 5.4 46.2 ± 5.1 |
0.001 0.001 |
Data are n (%) or mean ± SD. GFI = Groningen Frailty Indicator; IQR = interquartile range; PCS = physical component summary; MCS = mental component summary, SD = standard deviation. SF-12 = Short-Form 12.
3.2. Outcomes
3.2.1. Total Population
After one year, MACE occurred in 14% of patients (N = 171) in the total population. NACE occurred in one in four patients (25%, N = 295). The total bleeding rate was 13% (N = 153), of which clinically relevant minor and major bleeding occurred in 11% of patients (N = 135). In the multivariable analysis, age (HR 1.05, 95% CI 1.02–1.09, p = 0.001), diabetes mellitus (HR 1.59, 95% CI 1.15–2.22, p = 0.006), Killip class of 2 or higher (HR 1.53, 95% CI 1.04–2.27, p = 0.033), LVEF below 50% (HR 1.47, 95% CI 1.00–2.15, p = 0.049) and ST-depression (HR 1.66, 95% CI 1.19–2.33, p = 0.003) were independent predictors for MACE at baseline (Table 3).
Table 3.
Univariable and multivariable Cox Regression Analysis for Major Adverse Cardiovascular Events.
| Variables | Univariable Model | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI of HR | p-Value | HR | 95% CI of HR | p-Value | |
| Age | 1.06 | 1.03–1.09 | <0.001 | 1.05 | 1.02–1.09 | 0.001 |
| Diabetes Mellitus | 1.60 | 1.16–2.22 | 0.004 | 1.59 | 1.15–2.22 | 0.006 |
| Killip Class of 2 or higher | 1.94 | 1.32–2.84 | 0.001 | 1.53 | 1.04–2.27 | 0.033 |
| LVEF < 50% | 1.70 | 1.17–2.45 | 0.005 | 1.47 | 1.00–2.15 | 0.049 |
| ST-depression | 1.89 | 1.35–2.61 | <0.001 | 1.66 | 1.19–2.33 | 0.003 |
CI = confidence interval; HR = hazard ratio; LVEF = left ventricular ejection fraction.
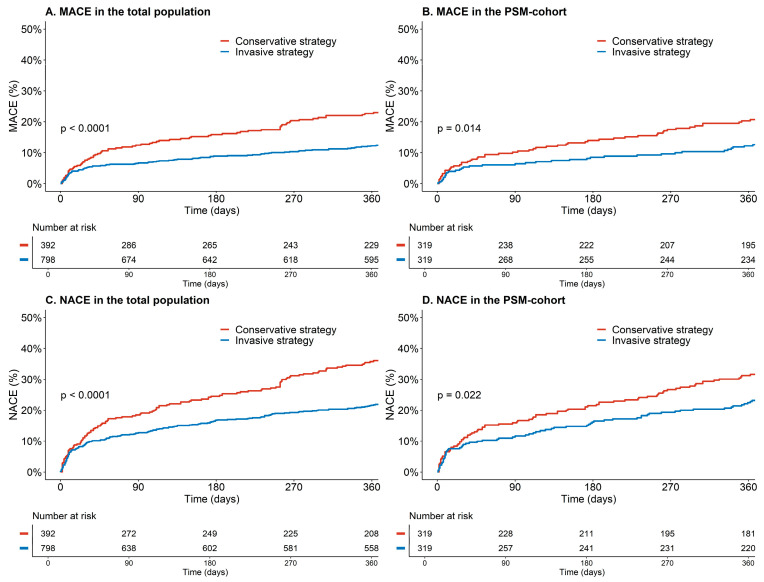
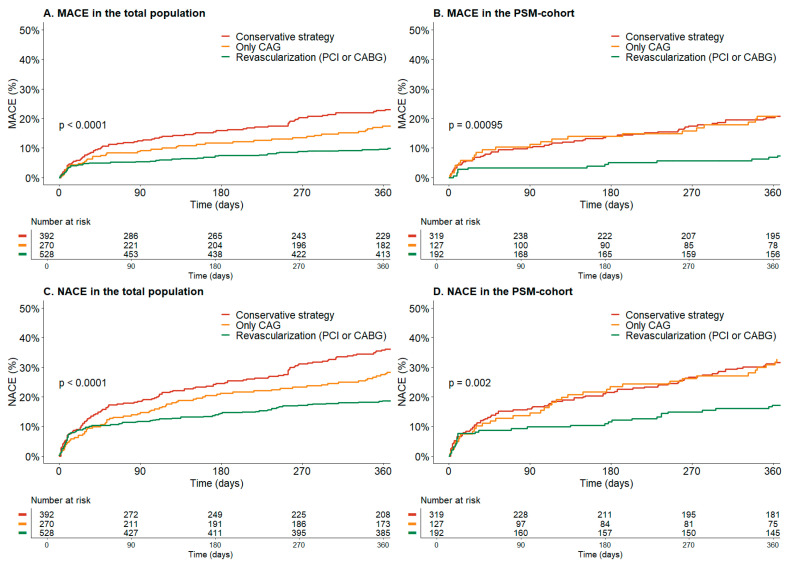
In the total population, the invasive strategy was associated with a lower risk for MACE after multivariable adjustment (12% vs. 20%, adjHR 0.53, 95% CI 0.37–0.77, p = 0.001) (Table 4 and Figure 2A). Undergoing revascularization with PCI or CABG was associated with an even lower risk of MACE after multivariable adjustment (adjHR0.39, 95% CI 0.26–0.61, p =< 0.001) (Figure 3A). There was a significant difference in clinically relevant major and minor bleeding (14% vs. 6%, adjHR 1.85, 95% CI 1.11–3.10, p = 0.012) between the invasive and conservative groups. Major bleeding was numerically higher, but not statistically significantly different (6% vs. 2%, adjHR 2.24, 95% CI 0.96–5.22, p = 0.062). In the invasive group, periprocedural bleeding occurred in 3% of patients (N = 27).
Table 4.
Cardiovascular outcomes before and after propensity score matching.
| Total Study Population | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Conservative (N = 392) |
Invasive (N = 798) |
HR * (95% CI) | p-Value | Conservative (N = 319) |
Invasive (N = 319) |
HR (95% CI) | p-Value | |
| MACE | 78 (20%) | 93 (12%) | 0.53 (0.37–0.77) | 0.001 | 57 (18%) | 38 (12%) | 0.50 (0.31–0.81) | 0.004 |
| NACE | 128 (33%) | 167 (21%) | 0.65 (0.49–0.86) | 0.003 | 91 (29%) | 70 (22%) | 0.69 (0.48–0.99) | 0.045 |
| All-cause death | 100 (26%) | 70 (9%) | 0.36 (0.25–0.52) | <0.001 | 70 (22%) | 37 (12%) | 0.46 (0.29–0.74) | 0.001 |
| CV death | 49 (13%) | 32 (4%) | 0.35 (0.21–0.60) | <0.001 | 36 (11%) | 19 (6%) | 0.47 (0.24–0.90) | 0.023 |
| Recurrent ACS | 32 (8%) | 49 (6%) | 0.47 (0.28–0.78) | 0.004 | 22 (7%) | 16 (5%) | 0.56 (0.27–1.17) | 0.123 |
| Stroke | 1 (0.3%) | 11 (1%) | 2.62 (0.31–22.3) | 0.378 | 1 (0.3%) | 0 | ||
| Major bleeding | 7 (2%) | 48 (6%) | 2.24 (0.96–5.22) | 0.062 | 4 (1%) | 24 (8%) | 4.37 (1.50–12.8) | 0.007 |
| Non-major and clinically relevant bleeding | 22 (6%) | 113 (14%) | 1.94 (1.16–3.23) | 0.012 | 17 (5%) | 53 (17%) | 2.52 (1.40–4.51) | 0.002 |
| Periprocedural bleeding | 0 | 27 (3%) | NA | NA | 0 | 13 (4%) | NA | NA |
* Hazard ratios in the total study population were adjusted for multiple baseline characteristics (Table S1). Data are n (%). ACS = acute coronary syndrome; CV = cardiovascular; MACE = Major Adverse Cardiac events; NACE = Net Adverse Clinical Events.
Figure 2.
Kaplan Meier Curve for major adverse cardiovascular events (MACE) and Net Adverse Clinical Events (NACE) in the invasive and conservative groups. (A) Kaplan Meier Curve for MACE and Net Adverse Clinical Events (NACE) in the total population before propensity score matching. (B) Kaplan Meier Curve for MACE after propensity score matching. (C) Kaplan Meier Curve for NACE in the total population before propensity score matching. (D) Kaplan Meier Curve for NACE after propensity score matching.
Figure 3.
Kaplan Meier Curve for major adverse cardiovascular events (MACE) and Net Adverse Clinical Events (NACE) between the different treatment strategies; conservative treatment, only coronary angiography (CAG) or revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). (A) Kaplan Meier Curve for MACE in the total population before propensity score matching. (B) Kaplan Meier Curve for MACE after propensity score matching. (C) Kaplan Meier Curve for NACE in the total population before propensity score matching. (D) Kaplan Meier Curve for NACE after propensity score matching.
3.2.2. PSM Cohort
After PSM, an invasive strategy remained associated with a lower risk for MACE compared to a conservative strategy (adjHR 0.50, 95% CI 0.31–0.81, p = 0.004) (Table 4 and Figure 2B). Similarly, revascularization with either PCI or CABG was associated with an even lower risk for MACE after PSM (adjHR 0.39, 95% CI 0.27–0.61, p < 0.001). An invasive strategy continued to demonstrate a significant decrease in MACE in the subgroup of patients aged 80 years or older (adjHR 0.54, 95% CI 0.31–0.92, p = 0.024). Bleeding rates for both major bleeding and non-major and clinically relevant bleeding remained higher in the invasive group (Table 4). However, NACE was still lower in the invasive group. This holds true for both the entire cohort (33% vs. 21%, HR 0.65, 95% CI 0.49–0.86, p = 0.003) and the PSM cohort (29% vs. 22%, HR 0.69, 95% CI 0.48–0.99, p = 0.045). Since differences in the use of aspirin, P2Y12 inhibitors and cholesterol inhibitors persisted after PSM, we conducted a sensitivity analysis with additional adjustments for these variables, which showed consistent results.
3.2.3. Frailty
Frail patients (N = 541) who underwent an invasive strategy had a lower incidence of MACE (9.1% vs. 18.1%, adjHR 0.49, 95% CI: 0.30–0.82, p = 0.006) and NACE (18.7% vs. 28.2%, adjHR 0.53, 95% CI 0.34–0.84, p = 0.007) than non-frail patients, but a higher rate of clinically relevant major and minor bleeding (13.9% vs. 5.9%, adjHR 2.29, 95% CI 1.19–4.42, p = 0.013). Results were consistent in the PSM cohort.
3.2.4. High Bleeding Risk
In HBR patients (N = 240), an invasive strategy reduced the risk for MACE (17.5% vs. 32.5%, adjHR 0.40, 95% CI 0.22–0.74, p = 0.003), while increasing the risk for clinically relevant major and minor bleeding (16.2% vs. 2.5%, adjHR 5.21, 95% CI 1.23–22.09, p = 0.025). However, in non-HBR patients (N = 496), the risk for both MACE (10.9% vs. 12.9%, adjHR 0.86, 95% CI 0.40–1.85, p = 0.70) and clinically relevant major and minor bleeding (14.8% vs. 12.9%, adjHR 0.85, 95% CI 0.44–1.64, p = 0.63) was comparable in both groups. The analysis yielded similar results in the PSM cohort.
4. Discussion
In this large, international, prospective, observational registry, we evaluated the treatment and survival of patients aged 75 years or older with NSTEMI. The main findings were (1) lower-risk patients were more likely to undergo CAG. (2) Age, diabetes mellitus, reduced LVEF below 50%, Killip class of 2 or higher and ST-depression at admission were independent predictors for MACE. (3) Patients who were conservatively treated had a higher risk of MACE than patients who underwent an invasive strategy, while undergoing revascularization was associated with an even lower risk of MACE, with robust results after propensity score analysis.
Treating elderly patients with NSTEMI is challenging. They more often present with atypical symptoms, and are a heterogeneous group with multiple comorbidities, variable frailty and functional status [11]. These patients are also at high risk for cardiovascular events, therefore, it is of utmost importance to perform adequate risk assessment when opting for invasive treatment. The identified predictors for MACE may assist in estimating this risk. Our data also suggest that although age is associated with a worse prognosis, older age alone should not be a reason to opt for a conservative strategy.
4.1. Invasive versus Conservative Strategy
The 2020 ESC Guidelines for the management of ACS in patients presenting without persistent ST-segment elevation recommends applying the same interventional strategies in older patients as for younger patients [4]. Unfortunately, these recommendations are largely based on studies in which older patients were underrepresented, studies with small sample sizes or highly selected populations [12,13,14,15]. Observational data has shown that an invasive strategy lowers the risk of all-cause death in elderly NSTEMI patients [16,17,18]. In these reports, conservatively treated patients were, as in our population, older and had more cardiovascular co-morbidities. It is highly plausible that these differences are due to indication bias, as physicians are less keen to invasively treat older and frail patients, probably reinforced by the fact that older patients also have a higher risk of procedure-related complications [19]. This has been described before as the risk-treatment paradox, meaning that the benefit of revascularization increases with cardiovascular risk, yet an increased cardiovascular risk results in those patients not being revascularized [20,21]. Randomization can overcome this paradox. The After Eighty study randomized a highly selected population of 457 patients with NSTEMI ≥80 years to an invasive or conservative strategy, as only 11% of eligible patients were finally included in the trial [13]. An invasive strategy was superior to the conservative strategy regarding the composite of myocardial infarction, need for urgent revascularization, stroke and death (HR 0.53, 95% CI 0.41–0.69). The findings in the PSM cohort are consistent with those of the After Eighty study, as evidenced by similar hazard ratios, particularly in the subgroup analysis comprising patients aged 80 years and above (adjHR 0.54, 95% CI 0.31–0.92). A meta-analysis, which incorporated the findings of the After Eighty study along with two smaller prematurely terminated RCTs, revealed similar results with a significant reduction in recurrent MI and urgent revascularization, but no survival benefit [11,22,23,24]. In contrast to the aforementioned studies, we observed a reduction in all-cause death and cardiovascular death. This difference may be due to unmeasured confounding and the selective indication for the invasive treatment of patients in healthier conditions. Ongoing randomized trials, such as the SENIOR-RITA trial (NCT03052036), will further refine existing evidence and contribute to solutions for this clinical dilemma.
Noteworthy is the notable difference in the Kaplan Meier curves between patients who underwent CAG without revascularization and those who were conservatively treated, before and after PSM (Figure 3C,D). This suggests that actual revascularization is necessary for the best clinical outcome. However, it should be noted that this difference may also be influenced by selection bias, as it is possible that more patients who only underwent CAG were diagnosed with type 2 MI, which has been associated with a higher mortality rate than type 1 MI in most studies [25].
As opposed to the After Eighty study, we did observe an increase in bleeding events in the invasive group, which can partly be explained by the difference in antithrombotic treatment and less use of the radial access for PCI leading to higher periprocedural bleeding event rates. Throughout the enrolment period, the prevailing standard of care in the invasive group was a 12-month duration of dual antiplatelet therapy (DAPT). This likely contributed to the elevated rates of bleeding observed, highlighting the importance of the recommendations in the current guidelines advocating for a shorter DAPT duration in patients at high risk of bleeding, for which age above 75 years is an important criterion [4]. Reducing the bleeding risk, especially in HBR patients, is an important issue that should be addressed, as bleeding events have a negative impact on prognosis and quality of life [26,27]. Therefore, it is important to carefully assess one’s risk of periprocedural and bleeding complications before opting for an invasive strategy. Our subgroup analysis revealed that effectively utilizing the CRUSADE score differentiated patients who were at high risk of bleeding in the invasive group. This highlights the role of clinical risk scores in facilitating risk stratification and treatment decisions.
4.2. Frailty and Quality of Life
Self-reported frailty and quality of life were assessed by the use of questionnaires at 1 and 12 months. The GFI score was missing in 25% of patients and SF-12 scores were missing in more than 40%. We deliberately chose to survey the questionnaires at one month, in order to prevent the hospitalization from influencing the score. Therefore, we did not use these data in our Cox regression models. The available data suggested that frailty was more common in patients in the conservative group. Quality of life also appeared to be worse in the conservative group. Clinical decision-making during hospitalization was probably partly based on functional status and frailty and, therefore, may explain these differences to some extent. This may have played a role in the difference in outcome between the conservative and invasive groups. However, when assessing frailty and quality of life after PSM, we saw no difference between the conservative and invasive groups. In frail patients, an invasive strategy remained the most beneficial treatment strategy based on the net clinical benefit, implying that an invasive strategy remains valuable in these patients. However, it should be noted that the assessment of frailty was based on questionnaires administered one month after hospitalization, which may limit the robustness of our results and warrants further investigation.
4.3. Strengths and Limitations
This registry is a large cohort study of elderly patients with NSTEMI in the Netherlands, the United Kingdom and Austria. Both academic and non-academic centers participated in this registry, making our data representative of NSTEMI patients in routine clinical practice. We made use of propensity score analyses to adjust for differences in patient characteristics, which is a well-accepted statistical methodology for the purpose of comparing non-randomized patient cohorts [28]. However, there were some limitations to our study. The first is its observational design. It is still probable that, despite using PSM, our results are subject to selection bias, which may account for the observed difference in risk and survival between the invasive and conservative groups. Similarly, unmeasured confounding may have influenced the treatment strategy and survival. Second, despite using questionnaires to assess frailty and quality of life, data regarding functional status and neuropsychiatric symptoms were limited. Third, the completeness of revascularization and reasons for clinical decision-making between the conservative and invasive groups were not registered. Finally, the presence of missing data might have biased the results.
5. Conclusions
In this prospective registry of real-world NSTEMI patients of 75 years or older, MACE and major bleeding were frequent. We found age, diabetes mellitus, reduced LVEF, Killip class and ST-depression at admission as independent predictors for MACE. In a population of elderly patients with NSTEMI, opting for an early invasive strategy was associated with benefits over conservative management. When deciding on the most suitable approach, it is essential to consider risk factors related to both ischemia and bleeding, rather than solely relying on age as the sole determining factor.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12175450/s1, Figure S1: Distribution of propensity scores; Figure S2: Covariate balance; Table S1: Cox regression models; Table S2: Coronary lesions; Table S3: Medical therapy at discharge.
Appendix A
Appendix A.1. The Groningen Frailty Questionnaire
The Groningen Frailty Questionnaire measures frailty by the use of self-assessment questionnaire. It uses 15 questions measuring the dimensions of physical and psychosocial vulnerability. Eight items have two response categories (yes/no), six items have three response categories (yes/sometimes/no), and one item has a Likert response category (1–10). All items were dichotomized to calculate GFI sum scores. A higher GFI sum score indicates a greater level of frailty, with a maximum score of 15. Higher scores indicate higher frailty levels. A person is considered to be frail when the GFI sum score is 4 points or higher.
Appendix A.2. SF-12
The SF-12 is a self-reported outcome measure assessing the impact of health on an individual’s everyday life, used to measure quality of life. It consists of 12 items, measuring eight concepts: physical functioning, role limitations due to physical health problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems and mental health. The SF-12 can be used to calculate the Physical Component Summary (PCS) and Mental Component Summary (MCS) scales. The average score for an average population is 50 points, with a standard deviation of 10 points. A score below 50 points will therefore indicate a lower quality of life compared to an average population.
Author Contributions
Conceptualization, M.E.G. and J.M.t.B.; methodology, M.E.G. and J.M.t.B.; validation, W.W.A.v.d.B., M.E.G. and J.M.t.B.; formal analysis, W.W.A.v.d.B., J.C.K.; investigation, W.W.A.v.d.B., M.E.G., R.S.H., C.R., R.F.S., D.A., R.O., J.C., G.G., R.J.W., D.A.A.M.S., S.L.B., H.K.T., M.G.S., A.A.C.M.H., D.N., M.E.E., A.W.J.v.’t.H., H.A., R.G., P.F.M.M.v.B., I.A., A.N., P.K., C.-J.B., A.L. and J.M.t.B.; resources, W.W.A.v.d.B. and M.E.G.; data curation, W.W.A.v.d.B., M.E.G. and J.C.K.; writing—original draft preparation, W.W.A.v.d.B. and J.M.t.B.; writing—review and editing, W.W.A.v.d.B., M.E.G., D.R.P.P.C.P.Y., J.A., R.S.H., C.R., R.F.S., D.A., R.O., J.C., G.G., R.J.W., D.A.A.M.S., S.L.B., H.K.T., M.G.S., A.A.C.M.H., D.N., M.E.E., A.W.J.v.’t.H., H.A., R.G., P.F.M.M.v.B., I.A., A.N., P.K., C.-J.B., A.L., J.C.K. and J.M.t.B.; visualization, W.W.A.v.d.B.; supervision, J.M.t.B.; project administration, M.E.G. and W.W.A.v.d.B.; funding acquisition, M.E.G. and J.M.t.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical research Ethics Committees United (MEC-U) (protocol code W17.021. and date of approval: 28 February 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
JtB report grants from the Netherlands Organization for Health Research and Development, a Dutch government institution called ZonMw, and AstraZeneca; and personal fees from AstraZeneca, Boehringer Ingelheim, Bayer, Ferrer, Pfizer, and Merck, outside the submitted work. RFS reports research grants and personal fees from AstraZeneca, Cytosorbents, GlyCardial Diagnostics and Thromboserin; and personal fees from Alnylam, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, Daiichi Sankyo, HengRui, Idorsia, Intas Pharmaceuticals, Medscape, Novartis, PhaseBio, and Sanofi Aventis. DA reports research grants from TA Sciences, and personal fees from Philips/Volcano, Pfizer, Astra Zeneca and Novartis. AvH reports unrestricted grants to institution from Medtronic, AZ, Boehringer Ingelheim, Abbott. All other authors have no conflict of interests to declare.
Funding Statement
This work was supported by AstraZeneca, grant number ESR-16-11872, and the St. Antonius Research fund, no grant number.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Eurostat Statistics Explained. Causes of Death Statistics. [(accessed on 11 January 2022)]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistics.
- 2.Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular Disease in Europe: Epidemiological Update 2016. Eur. Heart J. 2016;37:3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 3.Husted S., James S., Becker R.C., Horrow J., Katus H., Storey R.F., Cannon C.P., Heras M., Lopes R.D., Morais J., et al. Ticagrelor versus Clopidogrel in Elderly Patients with Acute Coronary Syndromes: A Substudy from the Prospective Randomized PLATelet Inhibition and Patient Outcomes (PLATO) Trial. Circ. Cardiovasc. Qual. Outcomes. 2012;5:680–688. doi: 10.1161/CIRCOUTCOMES.111.964395. [DOI] [PubMed] [Google Scholar]
- 4.Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2020;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Ganiats T.G., Holmes D.R., Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Damluji A.A., Forman D.E., Wang T.Y., Chikwe J., Kunadian V., Rich M.W., Young B.A., Page R.L., DeVon H.A., Alexander K.P., et al. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32–e62. doi: 10.1161/CIR.0000000000001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaman M.J., Stirling S., Shepstone L., Ryding A., Flather M., Bachmann M., Myint P.K. The Association between Older Age and Receipt of Care and Outcomes in Patients with Acute Coronary Syndromes: A Cohort Study of the Myocardial Ischaemia National Audit Project (MINAP) Eur. Heart J. 2014;35:1551–1558. doi: 10.1093/eurheartj/ehu039. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel M., Qaderdan K., Willemsen L., Hermanides R., Bergmeijer T., de Vrey E., Heestermans T., Tjon Joe Gin M., Waalewijn R., Hofma S., et al. Clopidogrel versus Ticagrelor or Prasugrel in Patients Aged 70 Years or Older with Non-ST-Elevation Acute Coronary Syndrome (POPular AGE): The Randomised, Open-Label, Non-Inferiority Trial. Lancet. 2020;395:1374–1381. doi: 10.1016/S0140-6736(20)30325-1. [DOI] [PubMed] [Google Scholar]
- 9.Mehran R., Rao S.V., Bhatt D.L., Gibson C.M., Caixeta A., Eikelboom J., Kaul S., Wiviott S.D., Menon V., Nikolsky E., et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth Universal Definition of Myocardial Infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 11.Brieger D., Eagle K.A., Goodman S.G., Steg P.G., Budaj A., White K., Montalescot G. Acute Coronary Syndromes Without Chest Pain, An Underdiagnosed and Undertreated High-Risk Group. Chest. 2004;126:461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 12.Sanchis J., Núñez E., Barrabés J.A., Marín F., Consuegra-Sánchez L., Ventura S., Valero E., Roqué M., Bayés-Genís A., del Blanco B.G., et al. Randomized Comparison between the Invasive and Conservative Strategies in Comorbid Elderly Patients with Non-ST Elevation Myocardial Infarction. Eur. J. Intern. Med. 2016;35:89–94. doi: 10.1016/j.ejim.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Tegn N., Abdelnoor M., Aaberge L., Endresen K., Smith P., Aakhus S., Gjertsen E., Dahl-Hofseth O., Ranhoff A.H., Gullestad L., et al. Invasive versus Conservative Strategy in Patients Aged 80 Years or Older with Non-ST-Elevation Myocardial Infarction or Unstable Angina Pectoris (After Eighty Study): An Open-Label Randomised Controlled Trial. Lancet. 2016;387:1057–1065. doi: 10.1016/S0140-6736(15)01166-6. [DOI] [PubMed] [Google Scholar]
- 14.Savonitto S., Cavallini C., Petronio A.S., Murena E., Antonicelli R., Sacco A., Steffenino G., Bonechi F., Mossuti E., Manari A., et al. Early Aggressive Versus Initially Conservative Treatment in Elderly Patients With Non–ST-Segment Elevation Acute Coronary Syndrome. JACC Cardiovasc. Interv. 2012;5:906–916. doi: 10.1016/j.jcin.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Bach R.G., Cannon C.P., Weintraub W.S., DiBattiste P.M., Demopoulos L.A., Anderson H.V., DeLucca P.T., Mahoney E.M., Murphy S.A., Braunwald E. The Effect of Routine, Early Invasive Management on Outcome for Elderly Patients with Non–ST-Segment Elevation Acute Coronary Syndromes. Ann. Intern. Med. 2004;141:186. doi: 10.7326/0003-4819-141-3-200408030-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kvakkestad K.M., Gran J.M., Eritsland J., Holst Hansen C., Fossum E., Andersen G.Ø., Halvorsen S. Long-Term Survival after Invasive or Conservative Strategy in Elderly Patients with Non-ST-Elevation Myocardial Infarction: A Prospective Cohort Study. Cardiology. 2019;144:79–89. doi: 10.1159/000503442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochar A., Chen A.Y., Sharma P.P., Pagidipati N.J., Fonarow G.C., Cowper P.A., Roe M.T., Peterson E.D., Wang T.Y. Long-Term Mortality of Older Patients with Acute Myocardial Infarction Treated in US Clinical Practice. J. Am. Heart Assoc. 2018;7:e007230. doi: 10.1161/JAHA.117.007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunniardy P., Koshy A.N., Meehan G., Murphy A.C., Ramchand J., Clark D.J., Farouque O., Yudi M.B. Invasive versus Conservative Management in Patients Aged ≥85 Years Presenting with non-ST-elevation Myocardial Infarction. Intern. Med. J. 2022;52:1167–1173. doi: 10.1111/imj.15258. [DOI] [PubMed] [Google Scholar]
- 19.Rosengren A. Better Treatment and Improved Prognosis in Elderly Patients with AMI: But Do Registers Tell the Whole Truth? Eur. Heart J. 2012;33:562–563. doi: 10.1093/eurheartj/ehr364. [DOI] [PubMed] [Google Scholar]
- 20.Saar A., Marandi T., Ainla T., Fischer K., Blöndal M., Eha J. The Risk-Treatment Paradox in Non-ST-Elevation Myocardial Infarction Patients According to Their Estimated GRACE Risk. Int. J. Cardiol. 2018;272:26–32. doi: 10.1016/j.ijcard.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 21.van der Sangen N.M.R., Azzahhafi J., Chan Pin Yin D.R.P.P., Peper J., Rayhi S., Walhout R.J., Tjon Joe Gin M., Nicastia D.M., Langerveld J., Vlachojannis G.J., et al. External Validation of the GRACE Risk Score and the Risk–Treatment Paradox in Patients with Acute Coronary Syndrome. Open Heart. 2022;9:e001984. doi: 10.1136/openhrt-2022-001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirlekar G., Libungan B., Karlsson T., Bäck M., Herlitz J., Albertsson P. Percutaneous Coronary Intervention in the Very Elderly with NSTE-ACS: The Randomized 80+ Study. Scand. Cardiovasc. J. 2020;54:315–321. doi: 10.1080/14017431.2020.1781243. [DOI] [PubMed] [Google Scholar]
- 23.de Belder A., Myat A., Blaxill J., Haworth P., O’Kane P.D., Hatrick R., Aggarwal R.K., Davie A., Smith W., Gerber R., et al. Revascularisation or Medical Therapy in Elderly Patients with Acute Anginal Syndromes: The RINCAL Randomised Trial. EuroIntervention. 2021;17:67–74. doi: 10.4244/EIJ-D-20-00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews C.J., Kirby J., Blaxill J.M., Greenwood J.P., Mozid A.M., Rossington J.A., Veerasamy M., Wassef N., Wheatcroft S.B., Bulluck H. Management of Non-ST-Segment Elevation Myocardial Infarction in Patients Aged ≥ 80 Years: A Meta-Analysis of Randomized Controlled Trials. J. Geriatr. Cardiol. 2022;19:789–790. doi: 10.11909/j.issn.1671-5411.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFilippis A.P., Chapman A.R., Mills N.L., de Lemos J.A., Arbab-Zadeh A., Newby L.K., Morrow D.A. Assessment and Treatment of Patients With Type 2 Myocardial Infarction and Acute Nonischemic Myocardial Injury. Circulation. 2019;140:1661–1678. doi: 10.1161/CIRCULATIONAHA.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin A.P., Wang T.Y., McCoy L., Bach R.G., Effron M.B., Peterson E.D., Cohen D.J. Impact of Bleeding on Quality of Life in Patients on DAPT. J. Am. Coll. Cardiol. 2016;67:59–65. doi: 10.1016/j.jacc.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valgimigli M., Costa F., Lokhnygina Y., Clare R.M., Wallentin L., Moliterno D.J., Armstrong P.W., White H.D., Held C., Aylward P.E., et al. Trade-off of Myocardial Infarction vs. Bleeding Types on Mortality after Acute Coronary Syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) Randomized Trial. Eur. Heart J. 2016;38:804–810. doi: 10.1093/eurheartj/ehw525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Agostino R.B. Propensity Scores in Cardiovascular Research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.