Abstract
Susceptibility testing of Mycobacterium tuberculosis is seriously limited by the time required to obtain results. We show that susceptibility testing of clinical isolates of M. tuberculosis can be accomplished rapidly with acceptable accuracy by using flow cytometry. The susceptibilities of 35 clinical isolates of M. tuberculosis to various concentrations of isoniazid, rifampin, and ethambutol were tested by the agar proportion method and by flow cytometry. Agreement between the results from the two methods was 95, 92, and 83% for isoniazid, ethambutol, and rifampin, respectively. Only 11 discrepancies were detected among 155 total tests. The results of flow cytometric susceptibility tests were available within 24 h of inoculation of drug-containing medium, while the proportion method required 3 weeks to complete. The flow cytometric method is also simple to perform.
In response to the resurgence of tuberculosis and increases in resistance to antituberculosis drugs (3, 6–8, 28) the Centers for Disease Control and Prevention (CDC) have stated that rapid and accurate susceptibility testing of Mycobacterium tuberculosis is essential and should be performed for the control of the disease (6). Classically, susceptibility testing for M. tuberculosis has been performed by growing the tubercle bacillus on medium in the presence or absence of antimycobacterial agents for 2 or 3 weeks of incubation before obtaining results (5, 21, 22). A number of methods are practiced or have been proposed that greatly decrease the time required to obtain susceptibility test results. The most frequently used method, BACTEC-460, requires 4 to 12 days of incubation before results are available (13, 17, 22, 29, 32). Recently other methods, including a bioluminescence assay for detection of mycobacterial ATP (1, 23), the Gen-Probe DNA hybridization system (15, 18), the luciferase reporter gene assay (14), high-performance liquid chromatography mycolic acid analysis (12), the E-test MIC method (35), and a colorimetric method (36), have been proposed as high-throughput assays for performing rapid susceptibility testing. The results are available 3 to 14 days after initiation of testing procedures.
We showed previously that susceptibility testing of M. tuberculosis (24) and other mycobacteria (4) could be accomplished within 24 h after the mycobacteria were incubated with antimycobacterial agents. The method is based on the ability of mycobacteria to hydrolyze fluorescein diacetate (FDA) to free fluorescein via nonspecific cellular esterases. Accumulation of fluorescein in metabolically active mycobacterial cells can then be easily detected by using a flow cytometer. By contrast, mycobacteria that are killed or inhibited by antimycobacterial agents hydrolyze significantly less FDA and therefore have less fluorescence.
Although the feasibility of using flow cytometry and FDA staining for susceptibility testing of M. tuberculosis was demonstrated (24), the results were obtained with an attenuated strain (H37Ra) of M. tuberculosis. In this report, we demonstrate that flow cytometry and FDA staining can be used to detect the susceptibility or resistance of clinical isolates of M. tuberculosis to ethambutol (EMB), isoniazid (INH), and rifampin (RIF) 24 h after initiation of the testing procedure.
MATERIALS AND METHODS
Antimycobacterial agents.
EMB, INH, and RIF were obtained from Sigma Chemical Co., St. Louis, Mo. Stock solutions of EMB and INH were prepared at 10,000 μg/ml in distilled water and sterilized by filtration with a 0.2-μm-pore-size filter before being dispensed in 1.0-ml aliquots and stored at −70°C until used. RIF (10,000 μg/ml) was prepared similarly, except that it was dissolved in dimethyl sulfoxide (Sigma).
Mycobacteria and preparation.
Thirty-five clinical isolates of M. tuberculosis with varied resistances to antimycobacterial agents were obtained from the CDC. Each isolate was grown from frozen stocks in 10.0 ml of 7H9 broth (Difco, Detroit, Mich.) in a sterile 50.0-ml polypropylene screw-cap tube (Sarstedt, Newton, N.C.) at 37°C in the presence of 5% CO2 until the turbidity of the suspension was equivalent to a McFarland 1.0 standard. Approximately 5 to 14 days of incubation were required.
Agar proportion susceptibility testing.
The agar proportion method, similar to that recommended by the National Committee for Clinical Laboratory Standards (22), was used to determine the percentage of M. tuberculosis organisms resistant to each of the concentrations of antimycobacterial agents tested. Briefly, appropriate concentrations of EMB, INH, and RIF were added to 7H10 medium tempered at 50 to 52°C to yield 5.0 μg of EMB/ml; 0.2, 1.0, and 5.0 μg of INH/ml; and 1.0 μg of RIF/ml. Subsequently, 5.0 ml of medium containing each antimycobacterial agent was dispensed into labeled quadrants of sterile petri plates. One quadrant was reserved for 7H10 medium without any antituberculosis agent. After solidification of the agar, the plates were inoculated with 0.1 ml of 10−2 and 10−4 dilutions of a McFarland 1.0 concentration of a suspension of each isolate of M. tuberculosis. The inoculated plates were then incubated at 37°C in an atmosphere of 5% CO2 for 3 weeks. An isolate was considered susceptible to an antimycobacterial agent if the number of colonies that grew on the drug-containing plate was <1% of the number of colonies that grew on the drug-free control. An isolate was considered resistant if 1% or more grew on the drug-containing plate.
Flow cytometric susceptibility testing.
An aliquot (0.9 ml) of each actively growing M. tuberculosis isolate was transferred to a 2.0-ml screw-cap microtube (Sarstedt). The tubes were then inoculated with 0.1 ml of a working dilution of INH at 50.0, 10.0, 2.0, or 0.2 μg/ml. Similarly, tubes containing suspensions of M. tuberculosis isolates were inoculated with 0.1 ml of EMB at 50.0 μg/ml or RIF at 10.0 μg/ml. Drug-free suspensions of M. tuberculosis were also included as controls. The suspensions were then incubated for 24 h at 37°C in the presence of 5% CO2. After incubation, 0.2 ml of each assay suspension was removed and placed in a sterile 2.0-ml screw-cap microtube containing 0.2 ml of FDA (Sigma) prepared fresh daily at 500 ng/ml in phosphate-buffered saline at pH 7.4. The samples were then incubated at 37°C for 30 min before being analyzed with a Bryte HS flow cytometer with WinBryte software (Bio-Rad Laboratories, Hercules, Calif.).
Initially, unstained viable M. tuberculosis cells were detected and differentiated from non-M. tuberculosis particles in 7H9 medium by forward and side angle light scatter. Background events (particles) in the 7H9 medium and electronic noise were eliminated by thresholding. Subsequently, viable M. tuberculosis cells incubated in the presence or absence of antimycobacterial agents for 24 h were stained with FDA. For each isolate at each concentration of antimycobacterial agent the flow cytometer provided a histogram profile relating the number of M. tuberculosis organisms in each of 2,048 logarithmic channels of increasing fluorescence intensity, a mean channel fluorescence value, and a contour plot relating forward angle light scatter and intensity of fluorescence. Two thousand events were acquired for each sample. In addition, 1.5-μm-diameter fluorescent polystyrene beads (Bio-Rad Laboratories) were used daily for calibration of the instrument.
Flow cytometric susceptibility index.
The susceptibility index was determined by using the mean channel fluorescence value obtained from histogram profiles (channels 0 through 2048) of the population of FDA-stained M. tuberculosis cells in the presence or absence of antimycobacterial agents. Subsequently, these values were divided by 512, the number of channels per log decade. The antilog was then determined for these values to obtain the relative linear fluorescence value for each sample analyzed. Finally, for each isolate the relative fluorescence value of each drug-containing sample was divided by the relative fluorescence value of the drug-free control to obtain the susceptibility index. An isolate of M. tuberculosis was considered susceptible to an antimycobacterial agent if the susceptibility index was 0.75 or less. The calculation eliminates the variability among isolates of M. tuberculosis in their abilities to hydrolyze FDA in the absence of antimycobacterial agents.
RESULTS
Detection of M. tuberculosis by flow cytometry.
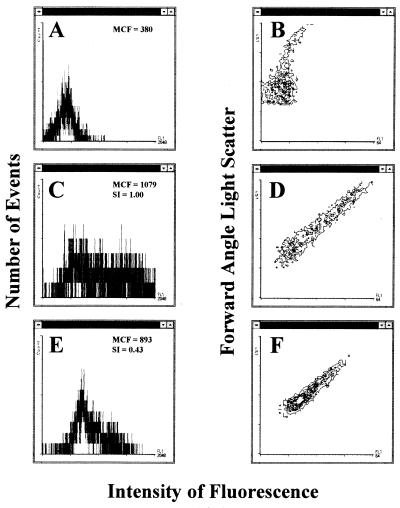
Unstained viable M. tuberculosis organisms were readily detected in 7H9 medium by flow cytometric analysis with forward angle light scatter and intensity of fluorescence displayed in a contour plot profile of the acquired data (Fig. 1B). A histogram profile of the unstained M. tuberculosis organisms (events) showing the low intensity of fluorescence for each event acquired was also obtained (Fig. 1A). When viable M. tuberculosis organisms were exposed to FDA (Fig. 1C and D), the intensity of fluorescence increased in the population. The contour plot profile (Fig. 1D) shows the intensity of fluorescence emitted by the viable M. tuberculosis population after hydrolysis of FDA relative to the degree of forward angle light scatter measured. The histogram profile was used to determine that the mean channel fluorescence of the population was 1,079 (Fig. 1C). By contrast, viable M. tuberculosis organisms incubated for 24 h with 5.0 μg of INH/ml (Fig. 1E and F), showed a significant decrease in the intensity of fluorescence displayed in the histogram after exposure to FDA. The mean channel fluorescence of the population had decreased to 893 (Fig. 1E). In addition, the contour plot (Fig. 1F) obtained for the M. tuberculosis organisms exposed to 5.0 μg of INH/ml was considerably different from the contour plot of the drug-free control (Fig. 1D). Furthermore, the susceptibility index value of 0.43 (Fig. 1E) demonstrated that the M. tuberculosis isolate was affected by 5.0 μg of INH/ml.
FIG. 1.
Intensity of fluorescence versus number of events (A, C, and E) and forward angle light scatter (B, D, and F) displayed as histogram or contour plot profiles, respectively, for M. tuberculosis organisms incubated for 24 h with (E and F) or without (C and D) 5.0 μg of INH/ml and then exposed to FDA. Other controls included M. tuberculosis organisms not incubated with INH or exposed to FDA (A and B). The mean channel fluorescence (MCF) values (the mean of the logarithmic intensity of fluorescence) of M. tuberculosis organisms with or without incubation with INH and exposed to FDA were used to calculate the susceptibility indices (SI). An index value of less than 0.75 suggested that M. tuberculosis organisms hydrolyzed less FDA than the drug-free control.
Susceptibility of clinical isolates of M. tuberculosis to antimycobacterial agents.
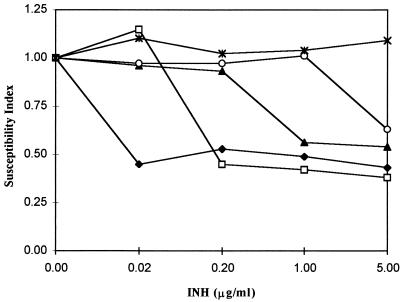
Thirty-five clinical isolates of M. tuberculosis with different susceptibilities to INH were obtained from the CDC. Subsequently, the abilities of the isolates to hydrolyze FDA after incubation for 24 h in various concentrations of INH were determined by flow cytometry. Figure 2 shows the flow cytometric susceptibility index values obtained for 5 of the 35 isolates with different susceptibilities to various concentrations of INH. The flow cytometric susceptibility index values decreased rapidly for four of the five isolates. Isolates 497, 941, and 810 were susceptible to 0.02, 0.20, and 1.0 μg or more of INH/ml, respectively. Isolate 065 was susceptible only to 5.0 μg of INH/ml, while isolate 024 was completely resistant to all concentrations of INH tested. The concentration of INH obtained by the susceptibility index for each of the isolates correlated with the inhibitory concentration obtained by the proportion method. Each of the isolates, except resistant isolate 024, had a susceptibility index value of 0.75 or less.
FIG. 2.
Susceptibility index values of 5 of the 35 clinical isolates of M. tuberculosis tested after incubation for 24 h with or without various concentrations of INH. Isolates 497 (⧫), 941 (□), 810 (▴), and 065 (○) were susceptible to 0.02, 0.20, 1.0, or 5.0 μg or more of INH/ml, respectively. Isolate 024 (∗) was resistant to all concentrations of INH tested.
We next determined the ability of the remaining 30 of the 35 clinical isolates of M. tuberculosis to hydrolyze FDA after incubation with various concentrations of INH for 24 h. The susceptibility results for each of the 35 isolates are listed in Table 1. The inhibitory concentration of INH obtained by the proportion method was also obtained by flow cytometry for all but four of the isolates. By flow cytometry, isolate 322 was resistant to 5.0 μg of INH/ml, but it was susceptible by the proportion method. Isolate 531 was resistant to 0.2 and 1.0 μg of INH/ml, but it was susceptible by the proportion method. Similarly, isolate 843 was resistant to 1.0 μg of INH/ml, but it was susceptible by the proportion method. By contrast, isolate 863 was resistant to 0.2 μg of INH/ml by the proportion method, but it was susceptible by the flow cytometric test. Agreement between the methods was 94, 94, and 97% at 0.2, 1.0, and 5.0 μg of INH/ml, respectively. Overall, the agreement was 95%.
TABLE 1.
Results of susceptibility tests for 35 clinical isolates of M. tuberculosis exposed to INHa
| Isolate | Susceptibility to indicated concentration of INH (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Proportion method
|
Flow cytometry
|
|||||
| 0.2 | 1.0 | 5.0 | 0.2 | 1.0 | 5.0 | |
| 024 | R | R | R | R | R | R |
| 026 | R | R | R | R | R | R |
| 065 | R | R | S | R | R | S |
| 072 | R | R | R | R | R | R |
| 126 | R | S | S | R | S | S |
| 127 | R | R | R | R | R | R |
| 152 | S | S | S | S | S | S |
| 204 | S | S | S | S | S | S |
| 212 | S | S | S | S | S | S |
| 231 | S | S | S | S | S | S |
| 232 | R | R | S | R | R | S |
| 243 | R | S | S | R | S | S |
| 246 | R | R | R | R | R | R |
| 253 | R | S | S | R | S | S |
| 289 | S | S | S | S | S | S |
| 292 | S | S | S | S | S | S |
| 299 | S | S | S | S | S | S |
| 322 | R | R | S | R | R | R |
| 495 | S | S | S | S | S | S |
| 497 | S | S | S | S | S | S |
| 498 | S | S | S | S | S | S |
| 508 | S | S | S | S | S | S |
| 509 | S | S | S | S | S | S |
| 514 | S | S | S | S | S | S |
| 531 | S | S | S | R | R | S |
| 567 | S | S | S | S | S | S |
| 705 | R | S | S | R | S | S |
| 710 | R | S | S | R | S | S |
| 798 | R | R | S | R | R | S |
| 810 | R | S | S | R | S | S |
| 813 | S | S | S | S | S | S |
| 843 | R | S | S | R | R | S |
| 863 | R | S | S | S | S | S |
| 941 | S | S | S | S | S | S |
| 982 | R | S | S | R | S | S |
Isolates of M. tuberculosis were exposed to 0.2, 1.0, or 5.0 μg of INH/ml. The isolates marked S for the proportion method showed less than 1% of the growth seen on drug-free control plates. The isolates marked S for flow cytometry had susceptibility indexes of 0.75 or less after exposure to INH for 24 h. Isolates marked R for both methods were considered resistant.
In other studies, 26 isolates of M. tuberculosis were tested for susceptibility to EMB by the flow cytometric and proportion methods (Table 2). Agreement between the methods was reached for 24 of the 26 isolates. Isolates 204 and 813 were resistant to 5.0 μg of EMB/ml by flow cytometry, but they were susceptible by the proportion method. Agreement was 92%. When 24 isolates of M. tuberculosis were tested for susceptibility to RIF by the flow cytometric and proportion methods, four discrepancies were detected (Table 3). Isolates 322, 509, and 843 were resistant to 1.0 μg of RIF/ml by the flow cytometric method but susceptible by the proportion method. By contrast, isolate 231 was resistant by the proportion method but susceptible by flow cytometry. The overall agreement was 83%.
TABLE 2.
Results of susceptibility tests for 26 clinical isolates of M. tuberculosis exposed to EMBa
| Isolate | Susceptibility to EMB (5.0 μg/ml)
|
|
|---|---|---|
| Proportion method | Flow cytometry | |
| 072 | R | R |
| 152 | S | S |
| 204 | S | R |
| 212 | S | S |
| 231 | S | S |
| 243 | S | S |
| 246 | R | R |
| 253 | S | S |
| 289 | S | S |
| 292 | S | S |
| 299 | S | S |
| 495 | S | S |
| 497 | S | S |
| 498 | S | S |
| 508 | S | S |
| 509 | S | S |
| 514 | S | S |
| 531 | S | S |
| 567 | S | S |
| 705 | S | S |
| 710 | S | S |
| 798 | R | R |
| 813 | S | R |
| 843 | S | S |
| 863 | S | S |
| 982 | S | S |
Isolates of M. tuberculosis were exposed to 5.0 μg of EMB/ml. The isolates marked S for the proportion method showed less than 1% of the growth seen on drug-free control plates. The isolates marked S for flow cytometry had susceptibility indexes of 0.75 or less after exposure to EMB for 24 h. Isolates marked R for both methods were considered resistant.
TABLE 3.
Results of susceptibility tests for 24 clinical isolates of M. tuberculosis exposed to RIFa
| Isolate | Susceptibility to RIF (1.0 μg/ml)
|
|
|---|---|---|
| Proportion method | Flow cytometry | |
| 072 | S | S |
| 126 | S | S |
| 204 | R | R |
| 212 | R | R |
| 231 | R | S |
| 243 | S | S |
| 246 | S | S |
| 253 | S | S |
| 289 | R | R |
| 292 | R | R |
| 299 | R | R |
| 322 | S | R |
| 497 | S | S |
| 498 | S | S |
| 508 | S | S |
| 509 | S | R |
| 514 | S | S |
| 531 | R | R |
| 705 | S | S |
| 710 | S | S |
| 813 | R | R |
| 843 | S | R |
| 863 | S | S |
| 982 | S | S |
Isolates of M. tuberculosis were exposed to 1.0 μg of RIF/ml. The isolates marked S for the proportion method showed less than 1% of the growth seen on drug-free control plates. The isolates marked S for flow cytometry had a susceptibility index of 0.75 or less after exposure to RIF for 24 h. Isolates marked R for both methods were considered resistant.
Reproducibility.
Table 4 shows the reproducibility of the flow cytometric susceptibility test. Three isolates (531, 126, and 072) with varied susceptibilities or resistances to INH, EMB, and RIF were tested three times. Isolates susceptible to INH, EMB, or RIF had susceptibility indexes ranging from 0.68 to 0.72, with an average standard deviation of 0.03. Similarly, isolates resistant to concentrations of INH, EMB, or RIF had susceptibility indexes ranging from 0.91 to 1.17. Their average standard deviation was 0.04.
TABLE 4.
Reproducibility of the flow cytometric susceptibility test for three isolates of M. tuberculosis with varied susceptibilities and resistances to INH, EMB, or RIF
| Isolate | Results of proportion methoda | Values for flow cytometric method
|
||
|---|---|---|---|---|
| Antimycobacterial agent (concn) | Mean of susceptibility index | Standard deviation | ||
| 531 | Control | 1.00 | 0.00 | |
| R | RIF (1.0 μg/ml) | 1.02 | 0.08 | |
| S | EMB (5.0 μg/ml) | 0.68 | 0.03 | |
| 126 | Control | 1.00 | 0.00 | |
| R | INH (0.2 μg/ml) | 0.91 | 0.02 | |
| S | INH (1.0 μg/ml) | 0.72 | 0.04 | |
| S | INH (5.0 μg/ml) | 0.71 | 0.02 | |
| S | RIF (1.0 μg/ml) | 0.70 | 0.03 | |
| 072 | Control | 1.00 | 0.00 | |
| R | INH (0.2 μg/ml) | 0.99 | 0.02 | |
| R | INH (1.0 μg/ml) | 1.00 | 0.01 | |
| R | INH (5.0 μg/ml) | 1.17 | 0.08 | |
| R | EMB (5.0 μg/ml) | 0.99 | 0.05 | |
DISCUSSION
The in vitro susceptibility testing of M. tuberculosis is seriously limited by the time required to obtain results (5, 13, 15, 17, 22, 29, 32). We demonstrated previously that susceptibility testing of M. tuberculosis, specifically an attenuated laboratory isolate (H37Ra), could be accomplished rapidly by using a flow cytometer (24). Results of tests were available within 24 h after M. tuberculosis organisms were incubated with antimycobacterial agents. Furthermore, multiplication of mycobacteria was not required to obtain susceptibility results. The flow cytometric susceptibility test method is based on the ability of M. tuberculosis organisms exposed to FDA to rapidly hydrolyze the compound to fluorescein by intrinsic esterases. In nonviable mycobacteria or mycobacteria susceptible to antimycobacterial agents, hydrolysis of FDA is reduced due to the decreased metabolic activity of the organisms. The use of flow cytometry allows rapid measurement (1 min or less per sample) of differences in the amounts of accumulated fluorescein among susceptible organisms and those resistant to, or untreated with, antimycobacterial agents. Consequently, determination of the susceptibility or resistance of mycobacteria can be accomplished rapidly. In this investigation, the feasibility of using flow cytometry to obtain susceptibility results for clinical isolates of M. tuberculosis 24 h after initiation of testing procedures was demonstrated.
We tested 35 clinical isolates of M. tuberculosis obtained from the CDC for susceptibility or resistance to INH by the flow cytometric and proportion methods. Overall, there was agreement between the two methods for 100 of the 105 total tests (95%). For two of the isolates, 531 and 843, discrepancies in INH inhibitory concentrations obtained by flow cytometry were corrected at the next higher concentration of INH. Isolate 863 was resistant to 0.2 μg of INH/ml by the proportion method but susceptible to this concentration of INH by flow cytometry. When higher concentrations (1.0 and 5.0 μg/ml) were tested, the same susceptibility results were obtained by the proportion method and by flow cytometry. Isolate 322 was resistant to 5.0 μg of INH/ml by flow cytometry, but it was susceptible by the proportion method. This isolate, however, was resistant to the lower concentrations of INH tested by the proportion method. Discrepancies were also noted for the susceptibilities of two and three isolates to inhibitory concentration of EMB and RIF, respectively. We classified these isolates as resistant by flow cytometry because their susceptibility indices did not match our chosen susceptibility cutoff value of 0.75. Another isolate, 231, was resistant to 1.0 μg of RIF/ml by the proportion method but susceptible to this concentration by flow cytometry.
A possible explanation for the discrepancies is that the majority of the population of M. tuberculosis cells was not in the exponential growth phase when tested by flow cytometry. Hydrolysis of FDA is affected by the metabolic activity of the mycobacterial cells. Similar levels of hydrolysis would occur in the drug-treated suspensions of M. tuberculosis cells and the drug-free controls if neither were metabolically active. Discrepancies have been detected if non-log-phase mycobacteria were used for testing by flow cytometry. Another explanation is the selection of a susceptibility index cutoff value. We conservatively set the cutoff value at 0.75. The value could have been 0.90, 0.85, 0.80, 0.76, or any numerical value within these numbers. By increasing the cutoff value to 0.90, only seven discrepancies among all of the tests performed would have been reported. Further experiments with the flow cytometric susceptibility test may reveal that the cutoff value for detection of susceptibility should be higher. Finally, it is assumed that the proportion method is correct. However, selection of a subpopulation of resistant or susceptible organisms within the population of M. tuberculosis organisms being tested has yielded conflicting results for the proportion method.
The use of flow cytometry for antimicrobial susceptibility testing is increasing (9–11, 16, 19, 20, 25–27, 30, 31, 33). A major advantage, besides rapidity and objectivity, is the ability to analyze bacterial cells individually or in small groups or clusters (9). Classically, susceptibility testing of M. tuberculosis depends on detection of growth (5, 13, 21, 22) or formation of colonies (5, 21, 22) to assess the effectiveness of an antimycobacterial agent. This may require weeks of incubation (5, 21, 22). By contrast, individual mycobacteria are examined by flow cytometry within hours of testing. Changes in individual mycobacterial cells can be assessed by forward or side angle light scatter or through the utilization of fluorescent dyes. The changes usually occur within 24 h after mycobacteria have been exposed to antimycobacterial agents (4, 9, 24, 34). In support of this observation, Bardou et al. (2) and Takayama et al. (34) showed by electron microscopy that there were dramatic changes in the cellular morphology of the tubercle bacillus after exposure to INH for 24 h or less. In this study, contour plots of forward angle light scatter obtained 24 h after M. tuberculosis cells were incubated in the presence or absence of INH also showed dramatic differences. This is consistent with the alterations in cellular morphology reported by Barbou et al. (2) and Takayama et al. (34). Furthermore, the ability to analyze individual mycobacteria accounts for the detection of lower concentrations of antimycobacterial agents that affect the population of mycobacteria than those detected by the standard methods (22). Although not shown here, several isolates of M. tuberculosis were determined to be susceptible to 0.2 μg of INH/ml by the proportion method, but they were shown to be susceptible to 0.02 μg/ml by the flow cytometric susceptibility test. Norden et al. (24) and Bownds et al. (4) also reported similar findings.
There are several concerns regarding the use of flow cytometry for susceptibility testing of M. tuberculosis. Biosafety is frequently considered the most important. Viable mycobacteria with or without incubation with antimycobacterial agents are presently processed by the instrument. Although the test is rapid, accurate, and reproducible, many clinical laboratories do not have the facilities to safely perform the procedure. However, the present format of the test could be utilized safely by public health laboratories or large reference laboratories that have a biosafety level-three tuberculosis laboratory. Safety is a primary concern, and it is being improved by developing procedures that kill the mycobacteria prior to testing without compromising their staining characteristics. Another concern is the cost of the flow cytometer. However, when the high costs of supplies for performing susceptibility testing with the radiometric instrument and increasing difficulties in disposing of radioactive materials are considered, the flow cytometer is less expensive. The reagents used for flow cytometry are also relatively inexpensive. Costs are restricted to the purchase of 7H9 broth, microtubes, FDA, and the antimycobacterial agents. Technician times for performing the radiometric and flow cytometric methods, however, are similar.
In conclusion, flow cytometry and FDA staining can be used to perform susceptibility testing of clinical isolates of M. tuberculosis. The assay is extremely simple to perform and, most importantly, can be completed in 24 h after initiation of testing.
ACKNOWLEDGMENTS
We thank Bio-Rad Laboratories in cooperation with the Gundersen Medical Foundation, Inc., LaCrosse, Wis., for support.
We greatly appreciate the support of Adolf L. Gundersen and Mark Connelly along with Herbert M. Heili. We also thank Louise Kubista, David Fett, Michelle Myrdal, and Daniel Muller for excellent advice and assistance.
REFERENCES
- 1.Arain T M, Resconi A E, Hickey M J, Stover C K. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob Agents Chemother. 1996;40:1536–1541. doi: 10.1128/aac.40.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardou F, Quémard A, Dupont M-A, Horn C, Marchal G, Daffé M. Effects of isoniazid on ultrastructure of Mycobacterium aurum and Mycobacterium tuberculosis and on production of secreted proteins. Antimicrob Agents Chemother. 1996;40:2459–2467. doi: 10.1128/aac.40.11.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch A B, Cauthen G M, Onorato I M, Dansbury K G, Kelly G D, Driver C R, Snider D E., Jr Nationwide survey of drug-resistant tuberculosis in the United States. JAMA. 1994;271:665–671. [PubMed] [Google Scholar]
- 4.Bownds S E, Kurzynski T A, Norden M A, Dufek J L, Schell R F. Rapid susceptibility testing for nontuberculosis mycobacteria using flow cytometry. J Clin Microbiol. 1996;34:1386–1390. doi: 10.1128/jcm.34.6.1386-1390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canetti G, Frosman S, Grosset J H, Hauduroy P, Langerova M, Mahler H T, Meissner G, Mitchison D A, Sula L. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull W H O. 1963;29:565–578. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Tuberculosis morbidity—United States, 1995. Morbid Mortal Weekly Rep. 1996;45:365–370. [PubMed] [Google Scholar]
- 7.Cohn, D. L., F. Bustreo, and M. C. Raviglione. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD global surveillance project. Clin. Infect. Dis. 24(Suppl. 1):S121–S130. [DOI] [PubMed]
- 8.Daniel T M, Debanne S M. Estimation of the annual risk of tuberculosis infection for white men in the United States. J Infect Dis. 1997;175:1535–1537. doi: 10.1086/516495. [DOI] [PubMed] [Google Scholar]
- 9.Davey H M, Kell D B. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durodie J, Coleman K, Simpson I N, Loughborough S H, Winstanley D W. Rapid detection of antimicrobial activity using flow cytometry. Cytometry. 1995;21:374–377. doi: 10.1002/cyto.990210409. [DOI] [PubMed] [Google Scholar]
- 11.Gant V A, Warnes G, Phillips I, Savidge G F. The application of flow cytometry to the study of bacterial responses to antibiotics. J Med Microbiol. 1993;39:147–154. doi: 10.1099/00222615-39-2-147. [DOI] [PubMed] [Google Scholar]
- 12.Garza-Gonzales E, Guerrero-Olazaran M, Tijerina-Menchaca R, Viader-Salvado J M. Determination of drug susceptibility of Mycobacterium tuberculosis through mycolic acid analysis. J Clin Microbiol. 1997;35:1287–1289. doi: 10.1128/jcm.35.5.1287-1289.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heifets L B, Iseman M D, Cook J L, Lindholm-Levy P J, Drupa I. Determination of in vitro susceptibility of Mycobacterium tuberculosis to cephalosporins by radiometric and conventional methods. Antimicrob Agents Chemother. 1985;27:11–15. doi: 10.1128/aac.27.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs W R, Jr, Barletta R G, Udani R, Chan J, Kalkut G, Sosne G, Kieser T, Sarkis G J, Hatfull G F, Bloom B R. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science. 1993;260:819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- 15.Kawa D E, Pennell D R, Kubista L N, Schell R F. Development of a rapid method for determining the susceptibility of Mycobacterium tuberculosis to isoniazid by using the Gen-Probe DNA hybridization system. Antimicrob Agents Chemother. 1989;33:1000–1005. doi: 10.1128/aac.33.7.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirk S M, Callister S M, Lim L C L, Schell R F. Rapid susceptibility testing of Candida albicans by flow cytometry. J Clin Microbiol. 1997;35:358–363. doi: 10.1128/jcm.35.2.358-363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C, Heifets L B. Determination of minimal concentrations of antituberculosis drugs by radiometric and conventional methods. Am Rev Respir Dis. 1987;136:349–352. doi: 10.1164/ajrccm/136.2.349. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Casabona N, Xairó Mimó D, González T, Rosselló J, Arcalis L. Rapid method for testing susceptibility of Mycobacterium tuberculosis by using DNA probes. J Clin Microbiol. 1997;35:2521–2525. doi: 10.1128/jcm.35.10.2521-2525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason D J, Allman R, Stark J M, Lloyd D. Rapid estimation of bacterial antibiotic susceptibility with flow cytometry. J Microsc. 1994;176:8–16. doi: 10.1111/j.1365-2818.1994.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 20.Mason D J, Gant V A. The application of flow cytometry to the estimation of bacterial antibiotic susceptibility. J Antimicrob Chemother. 1995;36:441–448. doi: 10.1093/jac/36.2.441. [DOI] [PubMed] [Google Scholar]
- 21.McClatchy J K. Susceptibility testing of mycobacteria. Lab Med. 1978;9:47–52. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing for Mycobacterium tuberculosis. Proposed standard M24-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 23.Nilsson L E, Hoffner S E, Ansehen S. Rapid susceptibility testing for Mycobacterium tuberculosis by bioluminescence assay of mycobacterial ATP. Antimicrob Agents Chemother. 1988;32:1208–1212. doi: 10.1128/aac.32.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norden M A, Kurzynski T A, Bownds S E, Callister S M, Schell R F. Rapid susceptibility testing of Mycobacterium tuberculosis (H37Ra) by flow cytometry. J Clin Microbiol. 1995;33:1231–1237. doi: 10.1128/jcm.33.5.1231-1237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Gorman M R G, Hopfer R L. Amphotericin B susceptibility testing of Candida species by flow cytometry. Cytometry. 1991;12:743–747. doi: 10.1002/cyto.990120808. [DOI] [PubMed] [Google Scholar]
- 26.Ordonez J V, Wehman N M. Rapid flow cytometric antibiotic susceptibility assay for Staphylococcus aureus. Cytometry. 1993;14:811–818. doi: 10.1002/cyto.990140714. [DOI] [PubMed] [Google Scholar]
- 27.Pore R S. Antibiotic susceptibility testing by flow cytometry. J Antimicrob Chemother. 1994;34:613–627. doi: 10.1093/jac/34.5.613. [DOI] [PubMed] [Google Scholar]
- 28.Raviglione M C, Snider D E, Jr, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 29.Roberts G D, Goodman N L, Heifets L, Larsh H W, Lindner T H, McClatchy J K, McGinnis M R, Siddiqi S H, Wright P. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983;18:689–696. doi: 10.1128/jcm.18.3.689-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro H M, editor. Practical flow cytometry. 3rd ed. New York, N.Y: Alan R. Liss, Inc.; 1995. pp. 412–425. [Google Scholar]
- 31.Shapiro H M. Flow cytometry in laboratory microbiology: new directions. ASM News. 1990;56:584–588. [Google Scholar]
- 32.Siddiqi S H, Hawkins J E, Laszio A. Interlaboratory drug susceptibility testing of Mycobacterium tuberculosis by a radiometric procedure and two conventional methods. J Clin Microbiol. 1985;22:919–923. doi: 10.1128/jcm.22.6.919-923.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steen H B. Flow cytometric studies of microorganisms. In: Melamed M R, Lindmo T, Mendelsohn M L, editors. Flow cytometry and sorting. 2nd ed. New York, N.Y: Wiley-Liss; 1990. pp. 605–622. [Google Scholar]
- 34.Takayama K, Wang L, Merkal R S. Scanning electron microscopy of the H37Ra strain of Mycobacterium tuberculosis exposed to isoniazid. Antimicrob Agents Chemother. 1973;4:62–65. doi: 10.1128/aac.4.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanger A, Mills K. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using Etest. J Clin Microbiol. 1996;34:1672–1676. doi: 10.1128/jcm.34.7.1672-1676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley W K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]