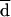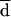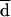Abstract
Three susceptibility testing procedures were compared to determine fluconazole, itraconazole, and ketoconazole MICs against 47 Candida albicans strains isolated sequentially from the oral cavities of five AIDS patients undergoing azole therapy. They included the broth microdilution method (BM), performed according to the National Committee for Clinical Laboratory Standards’ tentative standard, the agar dilution method (AD), and the Etest; the latter two tests were performed both in Casitone agar (AD-Cas and Etest-Cas) and in RPMI (AD-RPMI and Etest-RPMI). Twenty-four- and 48-h MICs obtained by AD and Etest were compared with 48-h MICs obtained by BM. The MICs of all the azoles determined by BM were usually lower than those obtained by the other methods, mainly due to different reading criteria. In order to assess the most appropriate way of evaluating the agreement of MICs obtained by different methods with those produced by the proposed reference method (BM), we used the mean differences calculated according to Bland and Altman’s method. Comparison of fluconazole MICs obtained by BM and AD-Cas yielded a mean difference of 3, and the percentages of agreement within ±2 dilutions were 98 and 100% at 24 and 48 h, respectively. For ketoconazole and itraconazole MICs, lower mean differences were noted, and agreement ranged from 96 to 100%. Agreement between the AD-RPMI and BM results was poor for all azoles, and an increase in MICs was always observed between the 1st- and 2nd-day readings. Similarly, Etest-Cas gave better agreement with BM than did Etest-RPMI for all the azoles. BM, AD-Cas, and Etest-Cas each demonstrated a progressive increase in fluconazole MICs against strains isolated sequentially from a given patient, in accordance with the decreased clinical response to fluconazole.
The rising incidence of fungal infections and the increasingly frequent use of antifungal agents have intensified the need for useful and reliable antifungal susceptibility test methods. Susceptibility testing of antifungal agents is greatly influenced by a variety of factors (5, 6, 10, 16, 18, 25, 26). After several collaborative studies, the Subcommittee on Antifungal Susceptibility Tests of the National Committee for Clinical Laboratory Standards (NCCLS) published a reference method for broth dilution antifungal susceptibility testing of yeasts which includes the less-expensive and less-cumbersome broth microdilution method (BM) (13). Another widely used antifungal susceptibility testing procedure is the agar dilution method (AD), which is easy to perform and allows simultaneous testing of a large number of organisms and the easy detection of microbial contaminants (6, 18, 28). Recently, several commercially available tests, such as Etest, have been introduced as alternative methods (4, 7, 14, 15, 22–24). Different media have been used with both AD and Etest (6, 9, 26).
In the last few years, the application of in vitro antifungal susceptibility tests for strains of Candida albicans causing thrush or esophagitis in AIDS patients showed higher MICs for isolates from patients refractory to azole treatment (8, 19). A progression from low to high MICs during the course of the infection was reported for C. albicans strains isolated sequentially from the oral cavities of patients with AIDS receiving azole therapy (2, 11, 12, 17, 20). Therefore, in vitro procedures could be helpful in monitoring antifungal therapy in this population of patients.
In the study described here, we compared three different methods and two media (RPMI 1640 and Casitone) for testing fluconazole, itraconazole, and ketoconazole against 47 strains of C. albicans isolated sequentially from the oral cavities of five AIDS patients undergoing azole treatment in order to verify the validity of AD and Etest.
(This work was presented as a poster at the Congress of the International Society for Human and Animal Mycology, Salsomaggiore Terme, Italy, 8 to 13 June 1997.)
MATERIALS AND METHODS
Sources of isolates.
Forty-seven strains of C. albicans isolated from the oral cavities of five AIDS patients who had recurrent episodes of oropharyngeal candidosis were used. All the patients were being treated with azoles. Yeasts were identified at species level by standard morphological and biochemical methods (27) and were stocked at −70°C in 10% glycerin until used. Candida krusei ATCC 6258 and Candida kefyr IP 706 were employed as quality control strains and tested in each run of the experiments for the three antifungal agents.
Susceptibility testing procedures.
The antifungal agents used in this study were an intravenous fluconazole preparation (Diflucan i.v.; Roerig Farmaceutici Italiani, Latina, Italy) and itraconazole and ketoconazole powders (kindly given by Janssen Pharmaceutica, Beerse, Belgium). Preliminary experiments showed no difference between the results obtained with the commercial preparation of fluconazole and those obtained with pure fluconazole (Pfizer Inc., New York, N.Y.). Azole susceptibility testing of all isolates of C. albicans were performed by three different methods: BM, AD, and Etest.
(i) BM.
BM was performed according to the NCCLS M27-T guidelines (13). Briefly, we used RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma Chemical Co.). Fluconazole was diluted to obtain final drug concentrations ranging from 0.125 to 64 μg/ml. Itraconazole and ketoconazole powders were dissolved in polyethylene glycol 400 (Janssen Chemicals, Beerse, Belgium) and diluted to obtain final drug concentrations ranging from 0.03 to 8.0 μg/ml for both drugs. Fifty microliters of the inoculum prepared spectrophotometrically as described elsewhere (13) was added to each well of the microtiter plates (final inoculum, 0.5 × 103 to 2.5 × 103 CFU/ml). Plates were incubated at 35°C for 48 h. The MIC was defined as the lowest concentration at which a prominent decrease in turbidity was observed (13).
(ii) AD.
AD was performed both in RPMI 1640 buffered to pH 7.0 with MOPS (AD-RPMI) and in phosphate-buffered Casitone agar, pH 7 (AD-Cas) (Casitone [Difco Laboratories, Detroit, Mich.], 9 g; yeast extract [Difco], 5 g; glucose, 20 g; sodium citrate, 10 g; Bacto Agar [Difco], 18 g; phosphate buffer [pH 7], 1 liter). Fluconazole was diluted in distilled water. Itraconazole and ketoconazole powders (10 mg of each) were dissolved in 1 ml of solvent (1 N HCl and ethyl alcohol in a 2:8 ratio) and then diluted with the same solvent mixture to 80 μg/ml. The drug solution was further diluted in distilled water. Twofold dilutions of each drug were added to RPMI and Casitone agar to obtain final concentrations ranging from 0.06 to 64 μg of fluconazole/ml and from 0.008 to 8 μg of itraconazole or ketoconazole/ml. Plates containing different antifungal dilutions and those containing diluents were inoculated by using a multipoint inoculator. Each pin delivered 1 μl of a yeast suspension containing 5 × 105 cells/ml from a 24-h culture. The inoculum size was determined by the count in Burker’s chamber. MICs were recorded as the lowest concentrations of drug that suppressed visible growth after 24 and 48 h of incubation at 35°C. All tests were performed in duplicate.
(iii) Etest.
Etest was performed in Casitone (Etest-Cas) and RPMI agar (Etest-RPMI). Petri plates (150 mm in diameter) containing 60 ml of medium were flooded with a yeast suspension of 5 × 105 cells/ml. Excess fluid was removed with a pipette, and after moisture was allowed to be absorbed, Etest strips were applied on each medium. The plates were incubated at 35°C and were read according to the manufacturer’s instructions at 24 and 48 h.
Analysis of the results.
All the AD and Etest MICs at 24 and 48 h were compared with the BM MICs at 48 h. Both on-scale and off-scale results were included in the analysis. MIC results obtained with the different procedures were compared according to the measuring agreement method reported by Bland and Altman (3). Every MIC was logarithmically transformed and deviated to avoid negative values (log-MIC) (e.g., 1, ≤0.12 μg/ml; 2, 0.25 μg/ml; 11, ≥128 μg/ml). The difference (d) between the log-MIC obtained by AD or Etest and that obtained by BM for each isolate was calculated, and the mean of these differences (mean difference, or 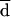 ) was determined. Results for MIC pairs obtained with different methodologies were considered in agreement when the difference between d and
) was determined. Results for MIC pairs obtained with different methodologies were considered in agreement when the difference between d and 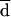 was within 1 (agreement ± 1 dilution) or 2 (agreement ± 2 dilutions) dilutions. The Mann-Whitney U test was applied to ascertain the distribution of itraconazole and ketoconazole MICs for fluconazole-susceptible and -resistant isolates. A P value of <0.05 was considered statistically significant.
was within 1 (agreement ± 1 dilution) or 2 (agreement ± 2 dilutions) dilutions. The Mann-Whitney U test was applied to ascertain the distribution of itraconazole and ketoconazole MICs for fluconazole-susceptible and -resistant isolates. A P value of <0.05 was considered statistically significant.
RESULTS
A total of 1,269 MIC data points were available for analysis. Table 1 summarizes azole MICs obtained by the three methods. Generally, MICs obtained by BM were lower than those obtained by the other methods for all three azole drugs.
TABLE 1.
Fluconazole, itraconazole, and ketoconazole MICs for 47 clinical isolates of C. albicans obtained by three methods
| Method | Time of reading (h) | MIC (μg/ml) ofa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fluconazole
|
Itraconazole
|
Ketoconazole
|
||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | ||
| BM | 48 | 0.125–32 | 4.0 | 16 | 0.03–0.5 | 0.125 | 0.25 | 0.03–0.5 | 0.06 | 0.25 |
| AD-Cas | 24 | 1.0–>64 | 32 | >64 | 0.03–2.0 | 0.125 | 0.25 | 0.03–0.5 | 0.125 | 0.25 |
| 48 | 1.0–>64 | 32 | >64 | 0.03–2.0 | 0.125 | 2.0 | 0.03–0.5 | 0.125 | 0.5 | |
| AD-RPMI | 24 | 0.25–>64 | 16 | >64 | 0.03–>8.0 | 0.125 | >8.0 | 0.03–8.0 | 0.125 | 0.25 |
| 48 | 8.0–>64 | >64 | >64 | 0.125–>8.0 | >8.0 | >8.0 | 0.03–>8.0 | 8.0 | >8.0 | |
| Etest-Cas | 24 | 0.5–>256 | 32 | 128 | 0.03–12 | 1.0 | 4.0 | 0.008–0.5 | 0.125 | 0.25 |
| 48 | 1.0–>256 | 64 | 128 | 0.06–12 | 2.0 | 8.0 | 0.008–0.75 | 0.25 | 0.5 | |
| Etest-RPMI | 24 | 0.125–>256 | 16 | >256 | 0.08–>16 | 1.0 | >16 | 0.008–>32 | 0.25 | 0.5 |
| 48 | 8.0–>256 | 128 | >256 | 0.08–>16 | 2.0 | >16 | 0.008–>32 | 0.5 | >32 | |
50% and 90%, MICs at which 50 and 90% of the isolates are inhibited, respectively.
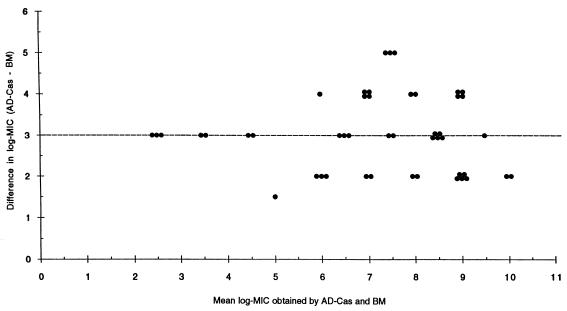
Fluconazole MICs generated in AD-CAs were always 3 dilutions higher than those obtained with BM. When 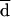 measured by Bland and Altman’s method was considered, the percentages of agreement within 2 dilutions were 98 and 100% at 24 and 48 h, respectively (Table 2; Fig. 1).
measured by Bland and Altman’s method was considered, the percentages of agreement within 2 dilutions were 98 and 100% at 24 and 48 h, respectively (Table 2; Fig. 1). 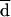 between BM and AD-Cas was lower (<1.0) for both itraconazole and ketoconazole MICs than for fluconazole MICs. The percentages of agreement within 2 dilutions were 98 and 96% for itraconazole at 24 and 48 h, respectively, and 100% for ketoconazole at both 24 and 48 h (Table 2). AD-RPMI yielded poorer agreement with BM than did AD-Cas for all azoles (Table 2). In this medium an increase of MICs was always observed between the 1st- and the 2nd-day readings for all azoles (Table 1).
between BM and AD-Cas was lower (<1.0) for both itraconazole and ketoconazole MICs than for fluconazole MICs. The percentages of agreement within 2 dilutions were 98 and 96% for itraconazole at 24 and 48 h, respectively, and 100% for ketoconazole at both 24 and 48 h (Table 2). AD-RPMI yielded poorer agreement with BM than did AD-Cas for all azoles (Table 2). In this medium an increase of MICs was always observed between the 1st- and the 2nd-day readings for all azoles (Table 1).
TABLE 2.
Agreement of MICs obtained by AD and by Etest with MICs obtained by NCCLS BM
| Method | Time of reading (h) | Fluconazole MICs
|
Itraconazole MICs
|
Ketoconazole MICs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
% Agreementa
|
|
% Agreementa
|
|
% Agreementa
|
||||||
| ±1 Dil | ±2 Dil | ±1 Dil | ±2 Dil | ±1 Dil | ±2 Dil | ||||||
| AD-Cas | 24 | 2.6 | 72 | 98 | 0.32 | 79 | 98 | 0.16 | 98 | 100 | |
| 48 | 3.0 | 91 | 100 | 0.79 | 74 | 96 | 0.57 | 83 | 100 | ||
| AD-RPMI | 24 | 2.5 | 49 | 83 | 2.1 | 23 | 70 | 0.07 | 75 | 84 | |
| 48 | 4.8 | 45 | 64 | 5.8 | 34 | 57 | 4.8 | 23 | 25 | ||
| Etest-Cas | 24 | 4.0 | 62 | 91 | 3.5 | 49 | 85 | 0.27 | 98 | 98 | |
| 48 | 3.5 | 64 | 94 | 3.6 | 51 | 87 | 0.5 | 96 | 100 | ||
| Etest-RPMI | 24 | 2.5 | 26 | 81 | 3.9 | 43 | 60 | 2.6 | 26 | 51 | |
| 48 | 5.0 | 45 | 64 | 4.5 | 23 | 43 | 4.3 | 6 | 20 | ||
% of agreement within 1 or 2 dilutions (Dil) referred to 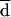 .
.
FIG. 1.
Difference from mean for fluconazole log-MIC (reading at 48 h). The horizontal line indicates 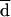 .
.
Similarly, Etest-Cas gave better agreement with BM than did Etest-RPMI for all azoles (Table 2). In general, interpretation of MICs by Etest was more difficult in RPMI than in Casitone due to the marked trailing observed in the former medium with most of the strains tested. The same phenomenon was also observed when selected isolates were tested on RPMI containing 2% glucose.
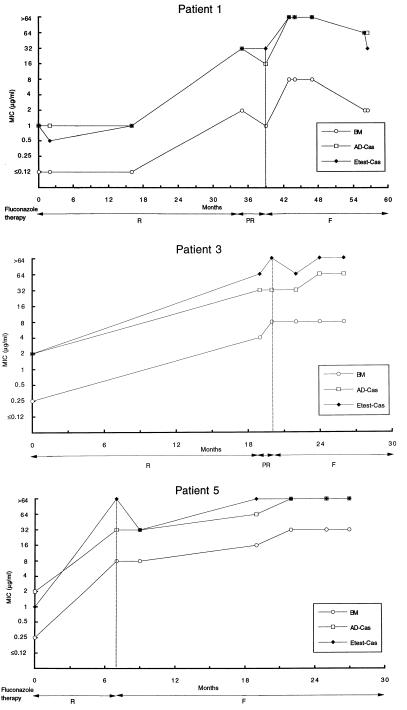
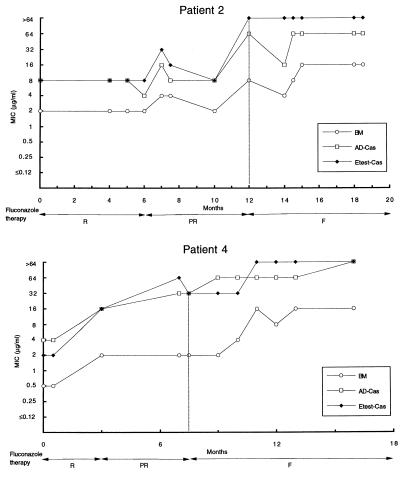
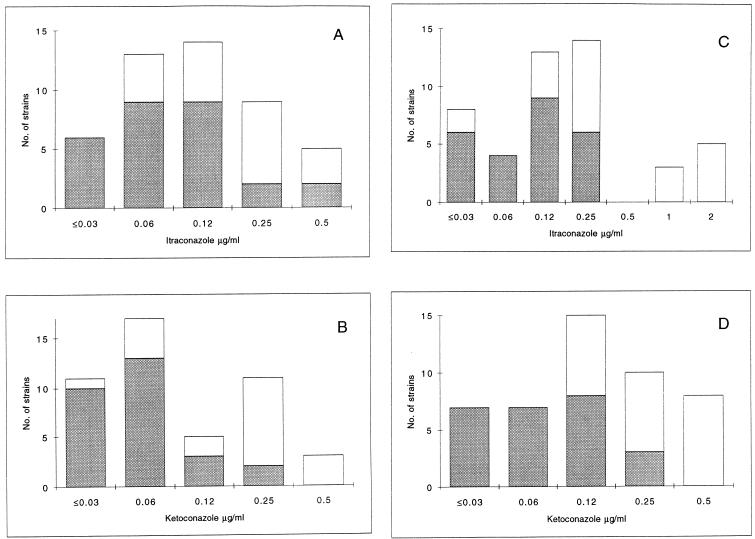
Overall, AD-Cas showed higher agreement with BM than did Etest-Cas for all azoles (Table 2). BM, AD-Cas, and Etest-Cas each demonstrated a progressive increase in fluconazole MICs against strains isolated sequentially from a given patient, in accordance with the decreased clinical response to fluconazole (Fig. 2). In order to detect possible cross-resistance among azoles, we further analyzed the distributions of itraconazole and ketoconazole MICs for fluconazole-susceptible and -resistant isolates of C. albicans. Because of the 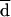 of 3 dilutions between fluconazole MICs obtained by BM and by AD-Cas, we arbitrarily chose fluconazole breakpoints of 4 and 32 μg/ml to define isolates susceptible to fluconazole by BM and by AD-Cas, respectively. When the Mann-Whitney U test was used to determine the distribution of itraconazole and ketoconazole MICs for the two groups of isolates, a statistically significant difference was found with both the methods employed (P = 0.0001) (Fig. 3). A decrease in susceptibility to itraconazole and ketoconazole was observed in strains that developed resistance to fluconazole.
of 3 dilutions between fluconazole MICs obtained by BM and by AD-Cas, we arbitrarily chose fluconazole breakpoints of 4 and 32 μg/ml to define isolates susceptible to fluconazole by BM and by AD-Cas, respectively. When the Mann-Whitney U test was used to determine the distribution of itraconazole and ketoconazole MICs for the two groups of isolates, a statistically significant difference was found with both the methods employed (P = 0.0001) (Fig. 3). A decrease in susceptibility to itraconazole and ketoconazole was observed in strains that developed resistance to fluconazole.
FIG. 2.
Trends of fluconazole MICs for C. albicans strains isolated sequentially from five AIDS patients undergoing azole therapy (readings at 48 h). Outcomes of fluconazole therapy are abbreviated as follows: R, response to 100 to 200 mg/day; PR, partial response to 200 mg/day; F, failure to respond to 200 to 400 mg/day. Vertical lines indicate the start of failure of fluconazole therapy.
FIG. 3.
Distribution of itraconazole and ketoconazole MICs for 47 strains of C. albicans obtained by BM (A and B) and AD-Cas (C and D). Shaded bars represent the distributions of itraconazole and ketoconazole MICs for fluconazole-susceptible isolates (fluconazole MICs, ≤4.0 and ≤32 μg/ml by BM and AD-Cas, respectively); open bars represent the distributions of itraconazole and ketoconazole MICs for fluconazole-resistant isolates (fluconazole MICs, ≥8.0 and ≥64 μg/ml by BM and AD-Cas, respectively). When the Mann-Whitney U test was applied to determine the distribution of itraconazole and ketoconazole MICs for the two groups of isolates, a statistically significant difference was found with both the methods employed (P = 0.0001).
DISCUSSION
Antifungal susceptibility tests, like those for antibacterial agents, may be an important aid in the decisions on starting antifungal treatment and in monitoring the outcome. So far, the use of these particular tests to monitor antifungal therapy has been limited by the lack of reproducibility and uncertain clinical relevance (5, 10). Following the increasing prevalence of fungal infections, new antifungal agents have been introduced and yeast isolates with reduced susceptibility to antifungal agents have been recognized (2, 8, 11, 12, 19, 20). Lately, the long-term use of azoles in the prophylaxis of systemic mycoses for bone marrow transplant patients and in suppressive therapy for AIDS patients has resulted in the selection of isolates that are more resistant to azole therapy (8, 11, 12, 19, 20).
Both BM and AD have been widely employed in clinical laboratories. Little information is available on the correlation between these two procedures. Since the NCCLS suggests performing the broth procedure in RPMI 1640 buffered with MOPS, we compared the azole MICs obtained by the proposed standard method with those obtained by agar dilution in the same medium and in Casitone medium buffered with phosphate.
Generally, AD MICs were higher than BM MICs. This could be explained by the different reading criteria considered in the definition of the endpoints. The different methods of reading will produce a consistent bias resulting in very poor agreement if the percentage of agreement is calculated, as usual, on the basis of discrepancies between MIC endpoints of no more than 2 dilutions. In order to assess the most appropriate way of evaluating agreement of MICs obtained by different methodologies, such as AD and BM, data analysis was performed according to Bland and Altman’s method (3). By this method of analysis, based on the notion that the mean of two measurements is the best estimate, since the “true” value is not known, it is possible to remove the consistent bias estimated by 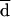 .
.
Interestingly, analysis of our data showed that the MICs of all three azoles determined by AD-Cas were in better agreement with the MICs obtained with BM than were those produced by AD-RPMI. The MICs of all three azoles obtained with AD-RPMI showed a marked increase between the 1st- and the 2nd-day readings. This was rarely observed for the MICs obtained by AD-Cas. A tendency to higher fluconazole MICs in assays with agar dilutions buffered with MOPS compared to those obtained in phosphate- or endomethylene-tetrahydrophthalic acid (EMTA)-buffered media has been reported (28).
Recently, the Etest has been introduced as a means of producing an accurate quantitative MIC result by using an agar diffusion format (4, 9, 24). So far, literature data comparing MICs obtained by Etest with those obtained by macro- or microdilution have shown variable results depending on the antifungal agent and the Candida species tested, as well as on the medium used for testing (4, 9, 21, 24). Sewell et al. (24) reported an agreement of 84% (±2 dilutions) between Etest-RPMI and broth macro- and microdilution methods for fluconazole susceptibility testing of Candida isolates when endpoint determinations were made after 24 h of incubation. Our data demonstrated that fluconazole, ketoconazole, and itraconazole MICs determined by Etest-Cas were in better agreement with the BM MICs than those produced in RPMI.
This study, which compared three different azole susceptibility testing procedures for strains of C. albicans isolated sequentially from the oral cavities of AIDS patients undergoing azole therapy, indicates that AD-Cas and Etest-Cas, as well as BM, can demonstrate progressive increases in fluconazole MICs against yeasts isolated from patients during the course of their oral infections, in accordance with the decrease in clinical response to fluconazole. In addition, all three methods were able to reveal a decrease in susceptibility to itraconazole and ketoconazole for some of the strains that developed resistance to fluconazole, as previously reported (1).
In conclusion, our study indicates that AD-Cas, unlike AD-RPMI, is a suitable method for testing azoles against isolates of C. albicans. AD-Cas produces results that agree with those obtained by BM, provided that an appropriate method is used to calculate the agreement. Etest-Cas can be used as an alternative method to determine azole susceptibility.
ACKNOWLEDGMENTS
This work was supported in part by grants from the IRCCS Ospedale Maggiore di Milano and the Istituto Superiore di Sanità, Roma, Italy (IX AIDS project).
REFERENCES
- 1.Barchiesi F, Colombo A L, McGough D A, Fothergill A W, Rinaldi M G. In vitro activity of itraconazole against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1530–1533. doi: 10.1128/aac.38.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi F, Hollis R J, McGough D A, Scalise G, Rinaldi M G, Pfaller M A. DNA subtypes and fluconazole susceptibilities of Candida albicans from the oral cavities of patients with AIDS. Clin Infect Dis. 1995;20:634–640. doi: 10.1093/clinids/20.3.634. [DOI] [PubMed] [Google Scholar]
- 3.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–311. [PubMed] [Google Scholar]
- 4.Colombo A L, Barchiesi F, McGough D A, Rinaldi M G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J Clin Microbiol. 1995;33:535–540. doi: 10.1128/jcm.33.3.535-540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook R A, McIntyre K A, Galgiani J N. Effects of incubation temperature, inoculum size, and medium on agreement of macro- and microdilution broth susceptibility test results for yeasts. Antimicrob Agents Chemother. 1990;34:1542–1545. doi: 10.1128/aac.34.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drouhet E, Dupont B, Improvisi L, Viviani M A, Tortorano A M. Disc agar diffusion and microplate automatized technics for in vitro evaluation of antifungal agents on yeasts and sporulated pathogenic fungi. In: Iwata K, Vanden Bossche H, editors. In vitro and in vivo evaluation of antifungal agents. Amsterdam, The Netherlands: Elsevier Science Publishers B.V; 1986. pp. 31–49. [Google Scholar]
- 7.Druetta A, Freydiere A, Guinet R, Gille Y. Evaluation of five commercial antifungal susceptibility testing systems. Eur J Clin Microbiol Infect Dis. 1993;12:336–342. doi: 10.1007/BF01964429. [DOI] [PubMed] [Google Scholar]
- 8.Dupont B, Improvisi L, Eliaszewicz M, Pialoux G the GEMO. Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. Resistance of Candida albicans to fluconazole in AIDS patients, abstr. 1203; p. 311. [Google Scholar]
- 9.Espinel-Ingroff A, Pfaller M, Erwin M E, Jones R N. An interlaboratory evaluation of Etest method for testing antifungal susceptibilities of pathogenic yeasts to five antifungal agents by using Casitone agar and solidified RPMI 1640 medium with 2% glucose. J Clin Microbiol. 1996;34:848–852. doi: 10.1128/jcm.34.4.848-852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galgiani J N. Antifungal susceptibility tests. Antimicrob Agents Chemother. 1987;31:1867–1870. doi: 10.1128/aac.31.12.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Tiballi R N, Zarins L T, Bradley S F, Sangeorzan J A, Kauffman C A. Azole resistance in oropharyngeal Candida albicans strains isolated from patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:2495–2497. doi: 10.1128/aac.38.10.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson E M, Warnock D W, Luker J, Porter S R, Scully C. Emergence of azole resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J Antimicrob Chemother. 1995;35:103–114. doi: 10.1093/jac/35.1.103. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Tentative standard M27-T. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 14.Pfaller M A, Buschelman B, Bale M J, Lancaster M, Espinel-Ingroff A, Rex J H, Rinaldi M G. Multicenter comparison of a colorimetric microdilution broth method with the reference macrodilution method for in vitro susceptibility testing of yeast isolates. Diagn Microbiol Infect Dis. 1994;19:9–13. doi: 10.1016/0732-8893(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller M A, Grant C, Mortland V, Rhine-Chalberg J. Comparative evaluation of alternative methods for broth dilution susceptibility testing of fluconazole against Candida albicans. J Clin Microbiol. 1994;32:506–509. doi: 10.1128/jcm.32.2.506-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller M A, Rinaldi M G. Antifungal susceptibility testing. Current state of technology, limitations, and standardization. Infect Dis Clin N Am. 1993;7:435–444. [PubMed] [Google Scholar]
- 17.Redding S, Smith J, Farinacci G, Rinaldi M G, Fothergill A, Rhine-Chalberg J, Pfaller M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation of in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis. 1994;18:240–242. doi: 10.1093/clinids/18.2.240. [DOI] [PubMed] [Google Scholar]
- 18.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhnke M, Eigler A, Engelmann E, Geiseler B, Trautmann M. Correlation between antifungal susceptibility testing of Candida isolates from patients with HIV infection and clinical results after treatment with fluconazole. Infection. 1994;22:132–136. doi: 10.1007/BF01739024. [DOI] [PubMed] [Google Scholar]
- 21.Ruhnke M, Schmidt-Westhausen A, Engelmann E, Trautmann M. Comparative evaluation of three antifungal susceptibility test methods for Candida albicans isolates and correlation with response to fluconazole therapy. J Clin Microbiol. 1996;34:3208–3211. doi: 10.1128/jcm.34.12.3208-3211.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez M L, Jones R N. Etest, an antimicrobial susceptibility testing method with broad clinical and epidemiologic application. Antimicrob Newsl. 1992;8:1–7. [Google Scholar]
- 23.Schmalreck, A. F., et al. 1996. Empfindlichkeitsprufung von Fluconazole: Auswertung einer Multizenter-Studie der Arbeitsgemeinschaft “Klinische Mykologie” der deutschsprachigen mykologischen Gesellschaft. Mycoses 39(Suppl. 2):1–11. [DOI] [PubMed]
- 24.Sewell D L, Pfaller M A, Barry A L. Comparison of broth macrodilution, broth microdilution, and E test antifungal susceptibility tests for fluconazole. J Clin Microbiol. 1994;32:2099–2102. doi: 10.1128/jcm.32.9.2099-2102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan, D. J., A. Espinel-Ingroff, L. S. Moore, and C. D. Webb. 1993. Antifungal susceptibility testing of yeasts: a brief overview. Clin. Infect. Dis. 17(Suppl. 2):S494–S500. [DOI] [PubMed]
- 26.Viviani M A, Tortorano A M, Cabrini E, Restelli A. Effetto del terreno di coltura sull’attività in vitro di tre derivati dell’imidazolo: miconazolo, econazolo, ketoconazolo. Chemioter Antimicrob. 1980;3:129–134. [Google Scholar]
- 27.Warren N G, Shadomy H J. Yeasts of medical importance. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 617–629. [Google Scholar]
- 28.Werner E, Seilbold M, Antweiler E. Susceptibility testing of Candida species for fluconazole: the role of buffering in the agar dilution assay. Mycoses. 1993;36:125–130. doi: 10.1111/j.1439-0507.1993.tb00699.x. [DOI] [PubMed] [Google Scholar]