Abstract
As a novel form of regulated cell death, ferroptosis is characterized by intracellular iron and lipid peroxide accumulation, which is different from other regulated cell death forms morphologically, biochemically, and immunologically. Ferroptosis is regulated by iron metabolism, lipid metabolism, and antioxidant defense systems as well as various transcription factors and related signal pathways. Emerging evidence has highlighted that ferroptosis is associated with many physiological and pathological processes, including cancer, neurodegeneration diseases, cardiovascular diseases, and ischemia/reperfusion injury. Noncoding RNAs are a group of functional RNA molecules that are not translated into proteins, which can regulate gene expression in various manners. An increasing number of studies have shown that noncoding RNAs, especially miRNAs, lncRNAs, and circRNAs, can interfere with the progression of ferroptosis by modulating ferroptosis-related genes or proteins directly or indirectly. In this review, we summarize the basic mechanisms and regulations of ferroptosis and focus on the recent studies on the mechanism for different types of ncRNAs to regulate ferroptosis in different physiological and pathological conditions, which will deepen our understanding of ferroptosis regulation by noncoding RNAs and provide new insights into employing noncoding RNAs in ferroptosis-associated therapeutic strategies.
Keywords: ferroptosis, ncRNAs, iron metabolism, lipid peroxidation, miRNAs, lncRNAs, circRNAs
1. Introduction
Iron is an essential trace element for virtually all living organisms. It plays important roles in many physiological and cellular processes, including oxygen transport, energy production, and cellular proliferation [1,2]. Because of its importance, iron levels are finely tuned in living organisms, and iron overload can damage an organism through a variety of mechanisms, including the induction of a kind of regulated cell death, ferroptosis. The term “ferroptosis” was first introduced in 2012, which refers to a type of regulated cell death resulting from iron overload and lipid peroxidation [3,4,5]. Therefore, it cannot be blocked by the specific inhibitors for other regulated cell death types, such as apoptosis, necrosis, necroptosis, and autophagy, but can be blocked by iron chelators (e.g., deferoxamine mesylate) and lipid peroxidation inhibitors (e.g., ferrostatin-1 and liproxstatin-1) [3,6,7]. As a novel form of regulated cell death, ferroptosis is regulated by its unique metabolism and regulatory mechanism, leading to different morphological, biochemical, and immunological features from apoptosis, autophagy, necroptosis, and pyroptosis [4,8]. Normally, ferroptotic cells exhibit morphological changes mainly in their mitochondria, including obviously smaller mitochondria, rupture of the mitochondrial outer membrane, and reduced or absent mitochondrial crista [3,5,6]. They also show necrosis-like features, such as plasma membrane integrity loss, cytoplasmic and cytoplasmic organelles swelling, and moderate chromatin condensation. However, there are no apoptotic bodies or autophagosomes in ferroptotic cells, which are signature morphological features in apoptosis and autophagy, respectively. Iron accumulation and lipid peroxidation are the main biochemical features of ferroptosis, both of which are related to the mechanism of ferroptosis [4,5,6]. Additionally, ferroptosis is considered a form of inflammatory cell death immunologically, which is associated with the release of damage-associated molecular pattern (DAMP) or lipid oxidation products [4]. Since it was discovered, increasing evidence has shown that ferroptosis plays special roles in many physiological and pathological processes, such as cancer, neurodegeneration diseases, cardiovascular diseases, and ischemia/reperfusion injury (IRI) [9,10,11,12,13,14].
Noncoding RNAs (ncRNAs) refer to the RNA molecules transcribed from genomes that do not encode proteins. With the advent of powerful sequencing technologies and in silico tools, it was found that up to 90% of genes in eukaryotic genomes have the capacity to be transcribed into RNAs [15,16,17]. Only 1–2% of the genes encode proteins, whereas the majority of the genes are transcribed as noncoding RNAs (ncRNAs). According to their length and shape, ncRNAs are divided into various types, including transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), circular RNAs (circRNAs), PIWI-interacting RNAs (piRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), long noncoding RNAs (lncRNAs), etc. Different from the message RNAs (mRNAs) that encode proteins, ncRNAs play important roles in transcriptional and posttranscriptional levels as well as in the epigenetic regulation of gene expression [18,19]. With in-depth research, ncRNAs have been found to participate in multiple biological processes, including both physiological and pathological processes. In the past several years, more and more ncRNAs have been reported to be involved in ferroptosis, which adds a complex network for the regulation of ferroptosis in different physiological and pathological conditions. In this review, we summarize the recent advances in the mechanism of ferroptosis, especially the role of ncRNAs, including the function of different types of ncRNAs in ferroptosis and the mechanism for ncRNAs regulating ferroptosis.
2. The Core Mechanism and Regulators of Ferroptosis
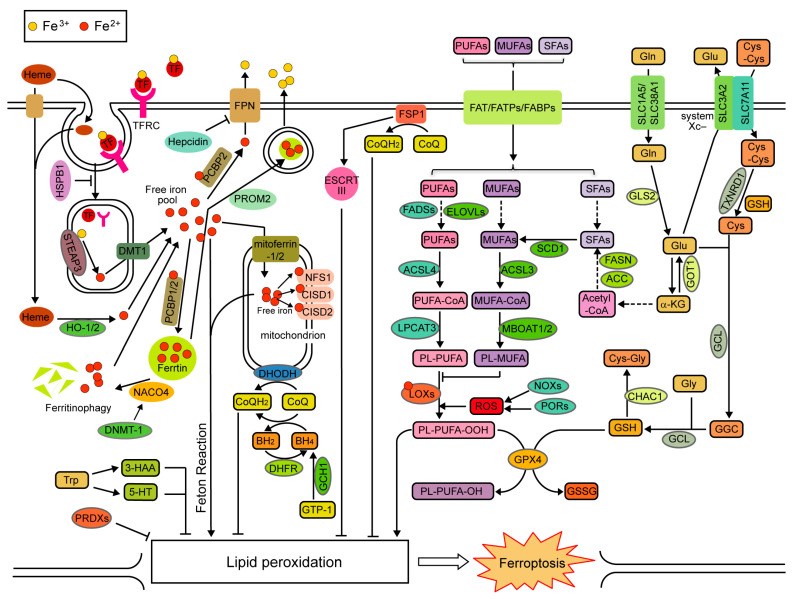
Ferroptosis is an iron-dependent, oxidative-damage-related type of regulated cell death [4,5]. Although ferroptosis has been studied for more than ten years, our knowledge of its mechanism is still limited. An increased iron accumulation and lipid peroxidation can induce ferroptosis, whereas the antioxidant defense systems inhibit lipid peroxidation and ferroptosis [4,5,6]. Generally, it is universally accepted that ferroptosis is regulated by iron metabolism (leading to abnormal iron accumulation), lipid metabolism (leading to increased lipid peroxidation), and the antioxidant defense systems (leading to the dysregulation of antioxidant defense) (Figure 1). Additionally, many proteins, especially transcription factors, regulate ferroptosis by directly or indirectly modulating iron metabolism, lipid metabolism, and the antioxidant defense systems.
Figure 1.
Schematic illustration of the core mechanisms of ferroptosis. Ferroptosis is regulated by iron metabolism, lipid metabolism, and antioxidant defense systems. α-KG: α-ketoglutarate; BH2: dihydrobiopterin; Cys: cysteine; Cys-Cys: cystine; Cys-Gly: cysteinylglycine; Gln: glutamine; GGC: γ-glutamyl cysteine; GSSG: glutathione oxidized; Glu: glutamate; Gly: glycine; and Trp: tryptophan.
2.1. Iron Metabolism
Ferroptosis is a form of regulated cell death dependent on iron, which exchanges between two states, ferrous (Fe2+) and ferric (Fe3+), in cells. Indeed, only intracellular free Fe2+ (but not Fe3+) induces ferroptosis. Fe2+ induces lipid peroxidation and ferroptosis in two ways, by directly generating ROS by the Fenton reaction or by activating iron-containing enzymes that catalyze lipid peroxidation (e.g., lipoxygenase) [5,11]. The absorption, storage, efflux, and utilization of iron, which regulate intracellular free Fe2+ levels, may affect ferroptosis [5,11].
Dietary non-heme iron is absorbed by proximal small intestinal mucosal absorptive cells and is then transported to the blood, where it is oxidized to Fe3+ and binds to transferrin (TF) [20]. TF with Fe3+ is recognized by the transferrin receptor (TFRC) in the cell membrane, and the Fe3+-TF-TFRC complex comes into the cytoplasm through endocytosing [20,21]. In the endosome, Fe3+ is reduced to Fe2+ by the ferrireductase activity of six-transmembrane epithelial antigens of the prostate 3 (STEAP3) [22]. The reduced Fe2+ is further released into a free iron pool in the cytoplasm by divalent metal transporter 1 (DMT1, also known as solute carrier family 11 member 2, SLC11A2) [20,21]. Elevation of TF, TFRC, STEAP3, or DMT1 was found to promote ferroptosis by enhancing iron uptake [5,21]. It was also found that heat shock protein family B (small) member 1 (HSPB1), when phosphorylated at serine 15 by protein kinase C (PKC), inhibits the iron uptake process by regulating the cytoskeleton organization, resulting in the suppression of ferroptosis [23]. Heme iron is taken up as heme by receptor-mediated endocytosis or heme transporters [24]. Internalized heme is then degraded by heme oxygenase 1 (HO-1) or HO-2, releasing the Fe2+ to the cytoplasm. Both HO-1 and HO-2 were reported to be ferroptosis regulators [25,26]. Two mitochondrial iron importers, mitoferrin-1 (also known as solute carrier family 25 member 37, SLC25A37) and mitoferrin-2 (also known as solute carrier family 25 member 28, SLC25A28), were found to facilitate ferroptosis by increasing mitochondrial free iron accumulation [27,28].
To maintain the free iron hemostasis in cells, excess intracellular Fe2+ is stored to avoid iron overload. Ferritin is a cytosolic iron-storage protein complex comprising 24 subunits of two types, ferritin-light chains (FTL) and ferritin-heavy chains 1 (FTH1) [21]. Increasing the expression of FTL and FTH1 will reduce intracellular free Fe2+ and inhibit ferroptosis [21]. When ferritin binds to nuclear receptor coactivator 4 (NCOA4), it is transported to autophagosome for lysosomal degradation (known as ferritinophagy), releasing ferritin-bound iron in order to increase intracellular free Fe2+ levels and result in ferroptosis [29]. DNA (cytosine-5)-methyltransferase 1 (DNMT-1) can enhance NCOA4 expression via DNA methylation in the NCOA4 promoter, leading to NCOA4-mediated ferritinophagy and ferroptosis [30]. Poly(RC)-binding proteins (PCBPs) act as iron chaperones to deliver Fe2+ to different proteins [21]. It was found that PCBP1 and PCBP2 deliver Fe2+ to ferritin, resulting in the suppression of ferroptosis [31,32]. PCBP2 was also reported to inhibit ferroptosis by transporting Fe2+ to ferroportin [33].
Iron-efflux protein ferroportin (FPN1, also known as solute carrier family 40 member 1, SLC40A1) is responsible for the exportation of intracellular free Fe2+ and the re-oxidation of Fe2+ to Fe3+ [34]. Hepcidin (encoded by the Hamp1 gene) can induce the internalization and degradation of FPN1 [35]. A decrease in FPN1 or an increase in hepcidin levels can promote ferroptosis by increasing intracellular free Fe2+ levels [21]. Prominin 2 (PROM2), a pentaspanin protein involved in the organization of plasma membrane microdomains, was also found to export iron by promoting a form of ferritin-containing multivesicular bodies, resulting in the resistance of ferroptosis [36,37]. Iron is also used for iron-sulfur cluster biogenesis. The expression of some mitochondrial proteins with an iron-sulfur cluster, such as NFS1, CISD1, and CISD2, may reduce the available iron levels and thus inhibit ferroptosis [38,39,40].
Moreover, iron-regulatory proteins (IRPs), including IRP1 (also known as aconitase 1, ACO1) and IRP2 (also known as iron-responsive element binding protein 2, IREB2), register cytosolic iron concentrations and post-transcriptionally regulate the expression of iron metabolism genes to optimize cellular iron availability [41,42]. IRPs can bind to iron-responsive elements (IREs), the specific RNA stem–loop structures located in 5′- or 3′- untranslated regions (UTR) of mRNA, leading to the opposite effect: the inhibition of translation at 5′- UTR or the promotion of translation at 3′- UTR. IRPs can bind to the mRNA of TFRC, DMT1, FTH1, FTL, and FPN, affecting ferroptosis sensitivity [3,21,43].
2.2. Lipid Metabolism
Ferroptosis directly results from lipid peroxidation, which occurs in polyunsaturated fatty acids (PUFAs) through both non-enzymatically spontaneous autoxidation and enzyme-mediated processes [44,45]. Lipoxygenases (LOXs) are the major enzymes responsible for enzymatical lipid peroxidation by catalyzing the oxygenation of PUFAs to generate various hydroperoxy PUFA derivatives, including the initial lipid hydroperoxides and the subsequent reactive toxic aldehydes (e.g., malondialdehyde and 4-hydroxynonenal). The inhibition of LOX activities or reducing their levels inhibit ferroptosis [46]. The arachidonate lipoxygenase (ALOX) family, a class of non-heme-iron-containing LOXs, play a tissue- or cell-dependent role in mediating ferroptosis [44,45]. Additionally, cyclooxygenase-2 (COX-2, encoded by the PTGS2 gene) oxidizes lysophospholipids but not phospholipids; therefore, it was considered as a biomarker but not a driver for ferroptosis in early studies [5,47]. However, recent studies have found that COX-2 can mediate lipid peroxidation and induce ferroptosis in certain conditions [48,49]. Alternatively, non-enzymatic lipid peroxidation requires free radicals, including ROS. Some membrane electron transfer proteins, including nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and cytochrome P450 oxidoreductases (PORs), contribute to ROS production and thus induce ferroptosis [50,51,52].
Lipid peroxidation occurs in PUFAs, which are supplied by the lipid synthesis and metabolism pathways. Ferroptosis results from the peroxidation of PUFA-phospholipids in the membrane [5,45]. Two PUFAs, arachidonic acid (AA) and adrenic acid (AdA), are the main substrates in PUFA-phospholipids during ferroptosis [44,45]. AdA/AA is ligated with CoA by Acyl-CoA synthetase long-chain family member 4 (ACSL4) to form CoA-AdA/AA, which then undergoes esterification with membrane phosphatidylethanolamine by lysophosphatidylcholine acyltransferase 3 (LPCAT3) to form PE-AdA/AA. A deficiency of either ACSL4 or LPCAT3 leads to the suppression of ferroptosis [53]. On the contrary, because of the lack of the bis-allylic positions readily available for peroxidation, monounsaturated fatty acids (MUFAs) in membrane phospholipids may inhibit ferroptosis by competing with PUFAs. Similarly, MUFAs are converted to MUFA-CoAs by Acyl-CoA synthetase long-chain family member 3 (ACSL3) and then form PE-MUFAs by membrane-bound O-acyltransferase domain containing 1/2 (MBOAT1/2). Therefore, promoting ACSL3 or MBOAT1/2 expression inhibits ferroptosis by elevating PE-MUFAs [54,55,56].
Long-chain PUFAs, including AA and AdA, are synthesized from dietary essential fatty acids (e.g., linoleic acid) by a series of enzymatic reactions in cells. These reactions are catalyzed by the elongation of very-long-chain fatty acid proteins (ELOVLs) and fatty acid desaturases (FADSs). It was reported that the inhibition or silencing of these enzymes, such as ELOVL5, FADS1, and FADS2, can repress ferroptosis [57,58]. Different from PUFAs, MUFAs come from saturated fatty acids (SFAs) produced by stearoyl-CoA desaturase 1 (SCD1), whereas SFAs can be de novo synthesized from acetyl-CoA by acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN). Increasing the expression of SCD1, FASN, and ACC protects cells from ferroptosis [55,59,60,61]. Alternatively, dietary fatty acids are another important source of PUFAs, MUFAs, and SFAs, especially for cells incapable of synthesizing fatty acids. Fatty acid translocase (FAT/CD36), fatty acid transport proteins (FATPs), and fatty-acid-binding proteins (FABPs) mediate the uptake of fatty acids [62]. It was reported that CD36 and FATP2, which mediate the absorption of AA and AdA, promote ferroptosis in certain cells [63,64]. FABP3, FABP4, and FABP7 were also reported to inhibit ferroptosis by regulating fatty acid uptake [55,65].
2.3. Antioxidant Defense Systems
Different from iron metabolism and lipid metabolism, the antioxidant defense system in cells suppresses lipid peroxidation, leading to the inhibition of ferroptosis. A selenocysteine-containing enzyme of the antioxidant defense system, glutathione peroxidase 4 (GPX4), plays a central role in ferroptosis inhibition. GPX4 is the only known enzyme that directly inhibits lipid peroxidation by using glutathione (GSH) to reduce toxic phospholipid hydroperoxides (PL-OOH) to non-toxic phospholipid alcohols (PL-OH) [66,67]. Therefore, its dysfunction always leads to the accumulation of lipid peroxides and ferroptosis. Many small-molecule ferroptosis inducers, such as RSL3, ML162, ML210, FIN56, and FINO2, induce ferroptosis by inhibiting GPX4 activity or promoting its degradation [66,67,68,69]. Interestingly, GPX4 can be regulated by lipid metabolism. GPX4 is a selenocysteine-containing protein, which needs selenocysteine tRNA for its synthesis. Selenocysteine tRNA comes from isopentenyl pyrophosphate (IPP), a product of the mevalonate pathway for lipid synthesis [70]. Thus, GPX4 levels are regulated by the mevalonate pathway via selenocysteine tRNA. Moreover, it was also reported that GPX4 is regulated by SCD1/FADS2, two important enzymes involved in lipid metabolism [71].
GPX4 uses GSH to reduce PL-OOH to PL-OH; therefore, the availability of GSH is also important for the inhibition of lipid peroxidation and ferroptosis by GPX4. GSH is synthesized from glutamate, cysteine, and glycine by glutamate cysteine ligase (GCL) and glutathione synthetase (GSS), whereas ChaC glutathione-specific γ-glutamyl-acyltransferase 1 (CHAC1) cleaves GSH into 5-oxo-L-proline and a Cys-Gly dipeptide. The inhibition of GCL or GSS, or activating CHAC1, may reduce GSH levels and induce ferroptosis [3,68,72,73,74]. Moreover, amino acid metabolism, which regulates the supply of glutamate, cysteine, or glycine, also plays crucial roles in ferroptosis. Among the metabolism of amino acids, the uptake of cystine, which can be further converted into cysteine by GSH and/or thioredoxin reductase 1 (TXNRD1), is considered to be a key mechanism for inducing ferroptosis [75]. Many small-molecule compounds, including erastin, sulfasalazine, and sorafenib, trigger ferroptosis by inhibiting system Xc−, a glutamic acid/cystine antiporter in the plasma membrane responsible for the uptake of cystine [68,75,76]. The suppression of the expression levels of its two subunits, solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2), also exhausts GSH and induces ferroptosis [77,78]. Cysteine is also synthesized through the trans-sulfuration pathway by cysteinyl-tRNA synthetase (CARS) and cystathionine-β-synthase (CBS), leading to resistance to ferroptosis [79,80,81,82]. Additionally, glutaminolysis, the catabolism of glutamate and glutamine, is another target for the regulation of ferroptosis. Glutamine is absorbed mainly by glutamine transport, solute carrier family 1 member 5 (SLC1A5), and solute carrier family 38 member 1 (SLC38A1) [83,84]. It is converted into glutamate by glutaminases, and glutamate can be further converted into α-ketoglutarate by glutamic oxaloacetic transaminase (GOT). Glutamate can help the system-Xc−-mediated absorption of cystine and can supply the GSH synthesis directly. Some studies showed that SLC1A5 and SLC38A1 suppress ferroptosis [85,86,87], whereas GOT1 promotes ferroptosis through elevating GSH levels [88]. However, more studies found that SLC1A5, SLC38A1, and glutaminase 2 (GLS2) promote glutaminolysis and ferroptosis [83,89,90,91,92,93,94,95,96]. These were attributed to the enhancement of α-ketoglutarate, which promotes ferroptosis probably by increasing lipid synthesis, the local iron level, and mitochondrial ROS [83,97,98]. It seems that glutaminolysis has a context-dependent dual function in ferroptosis.
In addition to the GPX4-GSH system, many other members of the antioxidant defense system were also found to regulate ferroptosis. Ferroptosis-suppressor protein 1 (FSP1, previously known as apoptosis-inducing factor mitochondria-associated 2, AIFM2) can trap lipid peroxides using NADPH to reduce non-mitochondrial coenzyme Q10 (CoQ), thereby repressing ferroptosis in a GPX4-independent manner [99,100]. Moreover, FSP1 was also reported to activate the ESCRT-III-dependent membrane repair system to inhibit ferroptosis, which is independent of its oxidoreductase function [101]. Tetrahydrobiopterin (BH4) is another radical-trapping antioxidant that protects lipid membranes from ferroptosis, which prevents two PUFA acyl tails from consuming phospholipids [102]. Both GTP cyclohydrolase-1 (GCH1) and dihydrofolate reductase (DHFR) can inhibit ferroptosis by producing BH4 [102,103]. In mitochondria, dihydroorotate dehydrogenase (DHODH) reduces CoQ to radical-trapping antioxidant ubiquinol (CoQH2), leading to the restoration of peroxide-damaged mitochondrial lipids and the inhibition of ferroptosis [104]. Additionally, several peroxiredoxins (PRDXs), members of a selenium-independent glutathione peroxidase family, were reported to inhibit ferroptosis [105,106,107]. It was also found recently that tryptophan can produce two radical-trapping antioxidants, serotonin (5-HT) and 3-hydroxyanthranilic acid (3-HAA), to eliminate lipid peroxidation, thereby inhibiting ferroptosis [108].
2.4. Transcription Factors Regulating Ferroptosis
Many transcription factors, as well as their related signaling pathways, are also involved in the regulation of ferroptosis by directly or indirectly affecting the above-mentioned mechanisms. Nuclear factor erythroid 2-related factor 2 (NRF2, also known as nuclear factor erythroid-derived 2-like 2, NFE2L2) is a master regulator of oxidative stress signaling and redox homeostasis [109,110]. NRF2 transcriptionally regulates a group of genes involved in antioxidant defense, such as SLC7A11, TXNRD1, GSS, GCLC, GCLM, CHAC1, GPX4, FSP1, ARK1C1, ALDH1A1, and NQO1, to inhibit ferroptosis. It was also reported that NRF2 can suppress ferroptosis by transcriptionally regulating the expression of FPN1, HO-1, FTL, FTH1, ABCB6, FECH, and HRG1 (SLC48A1) involved in iron metabolism and PPARG and NROB2 involved in lipid metabolism [109,110]. Sterol regulatory-element binding proteins (SREBPs) are transcription factors that regulate the expression of genes involved in lipid synthesis [111,112]. SREBP1 is the master regulator of lipogenesis through transcriptionally inducing the expression of ACC, FASN, and SCD1. The knockdown of SREBP1 or SCD1 will sensitize cancer cells to ferroptosis [55,61]. Signal transducer and activator of transcription 3 (STAT3), a transcription-factor-regulating gene associated with cell survival, the cell cycle, and immune reaction, was found to inhibit ferroptosis by increasing the expression of SLC7A11 and GPX4 for antioxidant defense, reducing ACSL4 expression for lipid metabolism, and increasing hepcidin expression for iron metabolism [113,114,115,116].
Some transcription factors have a dual function in ferroptosis. Tumor suppressor p53 is a transcription factor that mediates a variety of anti-proliferative processes via transcriptionally regulating many stress-response genes [117]. Normally, the activation of p53 induces ferroptosis by transcriptionally regulating SLC7A11, GLS2, CBS, and lncRNA-LINC00336/miR-6852/CBS involved in the anti-oxidant defense, ALOX12, SAT1/ALOX15, PTGS2, and iPLA2β involved in lipid metabolism, and FDXR, mitoferrin-2, and lncRNA-PVT1/miR-214/TFRC involved in iron metabolism [117,118]. p53 was also reported to be a ferroptosis suppressor by enhancing GSH levels via its target, p21, inducing mitophagy via Parkin and inhibiting NOX-mediated lipid peroxidation via directly binding the dipeptidyl peptidase DPP4 [117,118]. Thus, p53 regulates ferroptosis in a bidirectional and context-dependent way. Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen (hypoxia). HIF-1 and HIF-2, two HIFs mediating most of the cellular response to hypoxia, transcriptionally regulate TFRC, FTMT, and CA9 for iron metabolism, FABP3, FABP7, and lncRNA-CBSLR/CBS/ACSL4 for lipid metabolism, and PDK1, BNIP3, METTL14/SLC7A11, and lncRNA-PMAN/ELAVL1/SLC7A11 for antioxidant defense, eventually inhibiting ferroptosis in certain conditions, whereas under some different conditions, they can also transcriptionally regulate TF, TFRC, DMT1, ZIP8, and ZIP14 for iron metabolism, ACSL4, PTGS2, and HILPDA for lipid metabolism, and CHAC1 and SOD for antioxidant defense, leading to the promotion of ferroptosis [119]. Some activated transcription factors (ATFs), such as ATF3 and ATF4, which are activated in response to ER stress, also play dual roles in ferroptosis. Both ATF3 and ATF4 can transcriptionally induce SLC7A11 to inhibit ferroptosis [120,121,122,123,124]. It was also reported that ATF3 represses brucine-induced glioma cell ferroptosis by upregulating NOX4 and SOD1 to reduce ROS [125] and inhibits IRI-induced cardiomyocyte ferroptosis by transcriptionally inducing Fanconi anemia complementation group D2 (FANCD2) to affect the expression of GPX4, SLC7A11, FTH1, and PTGS2 [126]. ATF4 was also found to transcriptionally induce HSPA5, which in turn binds GPX4 and protects against GPX4 protein degradation, resulting in the suppression of ferroptosis [127,128]. On the other hand, both ATF3 and ATF4 can transcriptionally induce CHAC1 to reduce GSH and promote ferroptosis [72,129,130]. ATF3 is transcriptionally regulated by ATF4 to upregulate TFRC expression to promote ferroptosis [131]. ATF3 was also found to promote IFN-γ-driven ferroptosis by increasing the transcription of an miRNA miR-21-3p to repress TXNRD1 [132], whereas ATF4 induces B-cell translocation gene 1 (BTG1) to enhance the ferroptosis of hepatocytes [133].
Taken together, the mechanisms of ferroptosis are very complex. The core mechanisms of ferroptosis are iron accumulation, lipid peroxidation, and antioxidant defense, which are further regulated by many transcription factors and other proteins.
3. The Classification and Function of ncRNAs in Ferroptosis
ncRNAs can be divided into two subtypes, basic structural ncRNAs and regulatory ncRNAs, according to their function [134]. The former are also known as “housekeeping” ncRNAs, including tRNAs, rRNAs, snRNAs, and snoRNAs, which are constitutively expressed and function during the translation and splicing process. The regulatory ncRNAs contain miRNAs, siRNAs, circRNAs, piRNAs, and lncRNAs, which play important roles in the epigenetic regulation of gene expression. At present, the majority of ncRNAs involved in ferroptosis regulation are the regulatory ncRNAs, especially miRNAs, circRNAs, and lncRNAs.
3.1. MiRNAs in Ferroptosis
MiRNAs are small single-stranded ncRNAs of from 21 to 23 nucleotides derived from endogenous short-hairpin transcripts [135,136]. MiRNAs normally bind to complementary sequences within the 3′-UTRs of target mRNAs, leading to the cleavage, degradation, or translation inhibition of target mRNAs and eventually suppressing their expression [136,137].
In recent years, many studies have reported that miRNAs can target the ferroptosis-related genes to regulate ferroptosis. For instance, miRNA-214 (miR-214) targets and inhibits TFRC to reduce ferroptosis [138]; miR-424-5p and miR-4291 target and inhibit ACSL4 to suppress ferroptosis [139,140]; miR-30b-5p and miR-124 target and inhibit FPN1 to promote ferroptosis [141,142]; miR-541-3p and miR-324-3p target and inhibit GPX4 to enhance ferroptosis [143,144,145]; and miR-128-3p, miR-375, and miR-27a-3p target and inhibit SLC7A11 to induce ferroptosis [146,147,148].
In addition to mRNA, miRNA-binding sequences also exist in some other ncRNAs. These ncRNAs, including RNAs of pseudogenes, lncRNAs, and circRNAs, can compete with mRNA for the same miRNA pool, thereby regulating miRNA activity, which adds a level of regulation to the miRNA network [149,150].
3.2. LncRNAs in Ferroptosis
LncRNAs are a large group of RNAs, generally defined as transcripts of more than 200 nucleotides that are not translated into proteins [151,152]. LncRNAs regulate gene expression in a variety of ways at the epigenetic, including chromatin remodeling, transcriptional, translational, and post-translational regulations [153].
Some lncRNAs bind to miRNAs in a competitive manner as a miRNA sponge, leading to the inhibition of miRNAs. Therefore, some of these lncRNAs can regulate ferroptosis by inhibiting ferroptosis-related miRNAs. LncRNA PVT1 (lncPVT1) was found to inhibit miR-214 and enhance TFRC and p53 to induce ferroptosis in brain ischemia/reperfusion (I/R) [138]. LncOIP5-AS1 inhibits miR-128-3p to elevate SLC7A11 expression, eventually inhibiting ferroptosis in prostate cancer [146].
In addition, lncRNAs were also found to regulate ferroptosis by interacting with proteins to modulate mRNA stability and protein ubiquitination. For example, lncPMAN can bind to ELAVL1, leading to the stabilization of SLC7A11 mRNA to inhibit ferroptosis [154], whereas lncHEPFAL can promote SLC7A11 protein ubiquitination and degradation to promote ferroptosis [155]. Moreover, lncP53RRA can interact with Ras GTPase-activating protein-binding protein 1 (G3BP1) to sequester p53 in the nucleus, thus promoting ferroptosis [156].
3.3. CircRNAs in Ferroptosis
CircRNAs are a class of single-stranded RNAs that form a closed continuous loop from the 3′ to 5′ end, which are highly expressed in the eukaryotic transcriptome [157,158,159]. Unlike linear RNAs, circRNAs have covalently closed circular structures without a 5′ cap structure and a 3′ polyA tail and are derived from exons via alternative mRNA splicing. CircRNAs are much more stable than linear RNAs [158,160,161,162], which have a high degree of stability and a potential effect on gene regulation [163,164,165].
CircRNAs usually also act as competitive RNA or RNA sponges to bind miRNAs, thus regulating the target proteins of those miRNAs. In this way, many circRNAs regulate ferroptosis by modulating ferroptosis-related genes by inhibiting the corresponding miRNAs. For example, circRNA IL4R (circIL4R) binds to and inhibits miR-541-3p to enhance GPX4, leading to the inhibition of ferroptosis in hepatocellular carcinoma [143]. CircLMO1 inhibits miR-4291 and elevates ACSL4 levels to induce ferroptosis in cervical cancer [140].
In addition, circRNAs were also found to regulate ferroptosis by directly binding to proteins. Circ-cIARS (hsa_circ_0008367) was found to bind to and block the RNA-binding protein ALKBH5, which is a negative regulator of ferritinophagy and ferroptosis [166]. CircEXOC5 can directly bind to RNA-binding protein polypyrimidine tract binding protein 1 (PTBP1) to enhance ACSL4 mRNA stability, leading to ferroptosis in sepsis-induced acute lung injury [167]. It was reported that circRAPGEF5 interacts with and inhibits the splicing regulator RNA-binding protein fox-1 homolog 2 (RBFOX2) to confer ferroptosis resistance by modulating the alternative splicing of TFRC in endometrial cancer cells [168]. A recent study showed that circST6GALNAC6 interacts with small heat shock protein 1 (HSPB1) to inhibit its phosphorylation at the Ser-15 site, a phosphorylation site in the protective response to ferroptosis stress [169]. It was also found that circLRFN5 binds to PRRX2 protein and promotes its degradation, leading to the downregulation of GCH1 and inducing ferroptosis [170]. Moreover, circRNA circ101093 (cir93) was reported to interact with and increase fatty-acid-binding protein 3 (FABP3), which enhances the absorption and usage of AA to inhibit lipid peroxidation and ferroptosis [171].
Currently, circRNAs are reported to regulate ferroptosis in the above-mentioned manners, although they also show protein-coding and transcriptional regulation abilities.
3.4. PiRNAs in Ferroptosis
PiRNAs are also a type of regulatory ncRNAs. They are small ncRNAs, which are different from miRNAs in that they are larger, lack sequence conservation, and are more complex [172]. They can combine with piwi proteins to make up a piRNA/piwi complex, which can cause gene silencing via interacting with a target transcript [173].
Few studies on piRNAs and ferroptosis have been reported. It was found in breast cancer cells that piR-36712 inhibits SEPW1 expression by binding to SEPW1P (a retroprocessed pseudogene of SEPW1) RNA, blocking its competition with SEPW1 mRNA for miR-7 and miR-324, and it subsequently suppresses the ubiquitination of p53, enhancing the levels of p53 and its target p21 [174]. Since both p53 and p21 are regulators of ferroptosis [117], it is not surprising that piR-36712 may also be involved in the regulation of ferroptosis. Another study in prostate cancer showed that piR-31470 forms a complex with piwi-like RNA-mediated gene silencing 4 (PIWIL4) and then recruits DNMT1, DNA methyltransferase 3α, and methyl-CpG binding domain protein 2 to initiate and maintain the hypermethylation and inactivation of glutathione S-transferase P1 (GSTP1) [175]. Related studies have indicated that GSTP1 inactivation inhibits tumor cells from evading ferroptosis, leading to tumor growth [176], suggesting that piR-31470 may suppress ferroptosis through the inactivation of GSTP1.
3.5. Structural ncRNAs in Ferroptosis
In addition to regulatory ncRNAs, structural ncRNAs, such as tRNAs and rRNAs, are also involved in the regulation of ferroptosis.
TRNAs are typically from 76 to 90 nucleotides in length (in eukaryotes) and contribute to protein synthesis and serve as the physical link between mRNAs and the amino acid sequence of proteins [177]. TRNAs are required in the synthesis of ferroptosis-associated proteins; thus, changes in tRNAs may alter the expression of these proteins and then influence ferroptosis. Interestingly, studies have also found that the mutation of tRNA results in a decrease in selenoprotein expression, except for GPX4 and GPX1, and weak ferroptosis alteration [178,179,180]. Moreover, it was found that a loss of cysteinyl-tRNA synthetase reduces GSH synthesis by inhibiting trans-sulfuration and decreasing cysteine levels, leading to the suppression of erastin-induced ferroptosis [79]. It seems that tRNAs decrease GSH synthesis and increase ferroptosis without modulating GPX4. On the contrary, it was also reported that Queuine-modified tRNAs promote antioxidant defenses by activating catalase, SOD, GPX, and GSH reductase [181], and the deletion of the selenocysteine-tRNA gene leads to the accumulation of ROS [182], suggesting that tRNAs may inhibit ferroptosis by enhancing the antioxidant defense system’s abilities.
RRNAs are the primary component of ribosomes. They are also a kind of ribozyme that carries out protein synthesis in ribosomes [183]. Interestingly, a highly conserved 18S rRNA binding site was identified within the 5′-UTR of human NRF2 mRNA, which is required for internal translation initiation, suggesting that the 18S rRNA regulates NRF2 expression [184]. This is supported by another finding that mouse hepatoma cells with a 70% decrease in the 16S/18S rRNA ratio caused by long-term ethidium bromide treatment showed increased NRF2 expression [185]. Considering the important roles of NRF2 in ferroptosis, 18S rRNA may regulate ferroptosis by regulating NRF2 expression. On the other hand, nuclear mitotic apparatus protein (NuMA), which is downregulated in response to ROS, can bind to 18S and 28S rRNAs and localize to rDNA-promoter regions to promote nascent pre-rRNA synthesis [186]. It was also reported that treatment with iron chelator deferoxamine inhibited rRNA synthesis in leukemia HL-60 cells [187]. These findings suggest that rRNA synthesis may be regulated during ferroptosis.
SnRNAs mediate post-transcriptional splicing in gene expression, whereas snoRNAs mediate modifications to rRNAs, tRNAs, and snRNAs. Both of them are also structural ncRNAs. However, there are currently no reports on the relationship between ferroptosis and snRNAs or snoRNAs.
Taken together, the current research mainly focuses on the regulation of ferroptosis by regulatory ncRNAs, especially miRNAs, lncRNAs, and circRNAs. Further studies are needed to explore the functions of piRNAs, tRNA, rRNAs, snoRNAs, and snRNAs in ferroptosis.
4. ncRNAs Regulating Ferroptosis
MiRNAs, lncRNAs, and circRNAs are the major ncRNAs that have been reported to regulate ferroptosis. They were found to regulate ferroptosis by directly modulating enzymes and other proteins involved in iron metabolism, lipid metabolism, and antioxidant defense, or by modulating other ferroptosis-related genes and proteins (e.g., transcription factors) indirectly.
4.1. NcRNAs Regulating Ferroptosis through Iron Metabolism
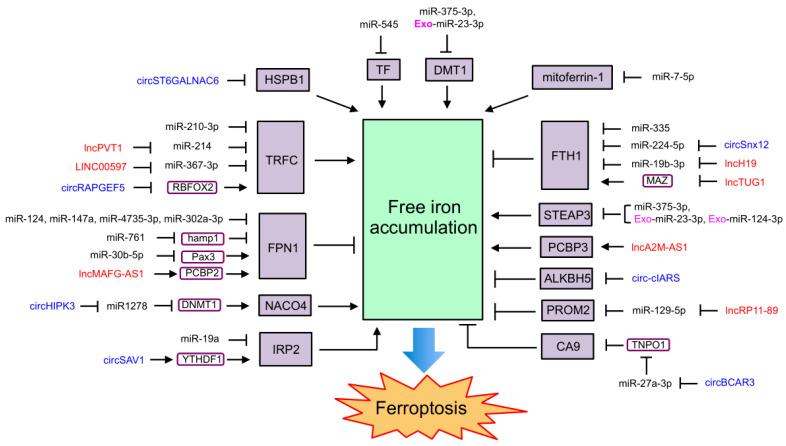
As one of the important mechanisms of ferroptosis, iron metabolism is regulated by many ncRNAs, which also affect ferroptosis (Figure 2). MiR-545 was reported to bind to the mRNA of TF and repress its expression to inhibit iron uptake and ferroptosis [188]. MiR-214 and miR-367-3p were found to target and repress TFRC to inhibit iron uptake and ferroptosis [138,189]. MiR-210-3p, which is enriched in hypoxia-conditioned cardiac microvascular endothelial cell-derived exosomes, also inhibits TFRC expression and attenuates erastin-induced myocardial cell ferroptosis [190]. LncPVT1, which competes with miR-214 in mouse brain I/R models, and lncRNA LINC00597, which competes with miR-367-3p in lung cancer cells, can increase TFRC expression to promote iron uptake and ferroptosis [138,189]. Moreover, circRAPGEF5 was reported to change the splicing of TFRC by binding to and inhibiting RBFOX2 and then inhibiting iron uptake to repress the ferroptosis of endometrial cancer cells [168].
Figure 2.
ncRNAs regulating ferroptosis through iron metabolism. MiRNAs are unboxed text in black. LncRNAs are unboxed text in red. CircRNAs are unboxed text in blue. Exosomal ncRNAs are marked with an “Exo-” in pink. Genes and proteins that directly involved in ferroptosis are black text boxed in black and shaded in purple. Other genes and proteins mediated the function of ncRNAs on ferroptosis are black text boxed in deep purple.
Furthermore, miR-124-3p enriched in HO-1-modified bone marrow mesenchymal-stem cell-derived exosomes inhibits STEAP3 by directly interacting with its mRNA, suppressing the hypoxia/reoxygenation (H/R)-induced ferroptosis of IAR20 (normal rat hepatocyte cell line) and LO2 (human fetal hepatocyte cell line) by blocking the reduction of Fe3+ to Fe2+ and decreasing free Fe2+ levels [191]. MiR-375-3p targets DMT1 and downregulates its expression to inhibit ferroptosis by blocking the release of Fe2+ to the free iron pool [192]. MiR-23a-3p, which is carried by the exosome from human umbilical cord blood-derived mesenchymal stem cells, targets and represses DMT1 expression to inhibit the ferroptosis of myocardial cells isolated from mouse models for acute myocardial infarction (AMI) and cardiomyocyte hypoxia injury [193]. CircST6GALNAC6 interacts with HSPB1 to inhibit its phosphorylation at Serine 15, leading to the promotion of iron uptake and ferroptosis [169]. MiR-7-5p attenuates mitoferrin-1 to block mitochondrial iron accumulation, leading to ferroptosis suppression and radiation resistance in cancer cell lines [194].
MiR-124, miR-147a, miR-4735-3p, and miR-302a-3p target FPN1 and block iron export to facilitate ferroptosis in neuronal cells [141], lung cancer cells [195], clear cell renal cell carcinoma cells [196], and glioblastoma cells [197]; miR-761 reduces hepcidin levels to suppress FPN1 degradation in the liver, leading to a decrease in iron deposition and ferroptosis [198]; and miR-30b-5p represses Pax3 (a transcription factor) to downregulate FPN1 transcription, thus inducing the ferroptosis of trophoblasts, leading to preeclampsia [142]. LncMAFG-AS1 binds to and stabilizes PCBP2 by the recruitment of deubiquitinase ubiquitin carboxyl-terminal hydrolase isozyme L5 (UCHL5) and then transports iron to FPN1 and suppresses ferroptosis [199]. MiR-129-5p targets and represses PROM2 to inhibit iron export, eventually promoting ferroptosis in non-small-cell lung cancer [200], whereas lncRP11-89 inhibits miR-129-5p to increase PROM2 expression, leading to the suppression of ferroptosis in bladder cancer [200].
MiR-335, miR-224-5p, and miR-19b-3p directly reduce FTH1 expression by binding to its mRNA, leading to an increase in free iron and the promotion of ferroptosis in Parkinson’s disease [201], heart failure [202], and lung cancer [203]. The inhibition of miR-224-5p by circSnx12 and the inhibition of miR-19b-3p by lncH19 elevate FTH1 levels to repress curcumenol-induced ferroptosis in heart failure [202] and lung cancer [203]. LncTUG1 targets MYC-associated zinc finger protein (MAZ) to reduce FTH1 expression and then enhances DHA-induced ferroptosis in glioma cells [204]. In gestational diabetes mellitus (GDM), circHIPK3 blocks miR-1278 to enhance DNMT1 expression and facilitates ferroptosis by inducing NCOA4-mediated ferritinophagy [30,205]. LncA2M-AS1 directly interacts with PCBP3, an iron chaperone, to promote ferroptosis in pancreatic cancer [206]. It was also reported that circ-cIARS binds to and blocks RNA-binding protein ALKBH5 to promote ferritinophagy and ferroptosis [166].
Additionally, miR-19a negatively regulates IRP2 to inhibit ferroptosis by modulating iron metabolism in colorectal cancer [207]. N6-methyladenosine-modified circSAV1 forms a complex with YTHDF1 and IRP2, which in turn facilitates IRP2 translation and accelerates cigarette-smoke-extract-induced ferroptosis in chronic obstructive pulmonary disease [208]. CircBCAR3 inhibits miR-27a-3p by the competitive RNA mechanism to upregulate transportin-1 (TNPO1) and then interacts with and inhibits carbonic anhydrase 9 to increase free iron accumulation by the upregulation of TFRC and the downregulation FTL and FTH1, eventually promoting ferroptosis [209,210].
4.2. NcRNAs Regulating Ferroptosis through Lipid Metabolism
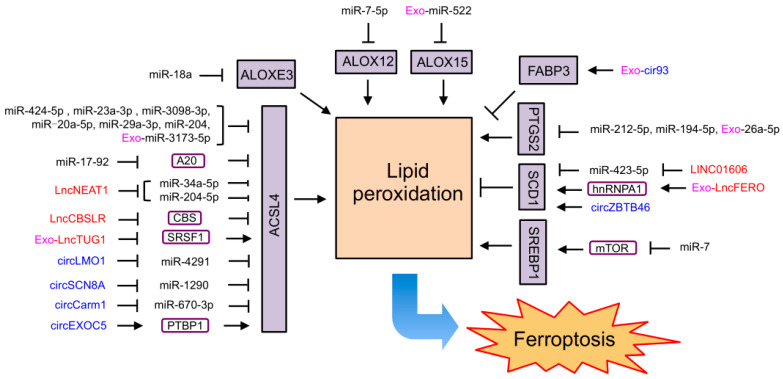
Many ncRNAs were found to affect ferroptosis by regulating lipid metabolism (Figure 3). As an important marker and driver of ferroptosis, ACSL4 was reported to be targeted by a group of ncRNAs to regulate ferroptosis. MiR-34a-5p and miR-204-5p in prostate cancer [211,212], miR-424-5p in ovarian cancer [139], miR-670-3p in glioblastoma [213], miR-23a-3p in hepatocellular carcinoma [214], miR-4291 in cervical cancer [140], miR-1290 in non-small-cell lung cancer [215], miR-3098-3p in neuronal cells [216], miR-20a-5p in an acute kidney injury mice model and patients with delayed graft function [217], miR-29a-3p in hippocampal neurons after intracerebral hemorrhage [218], and miR-204 in HIV-1 Tat protein-exposed mouse primary microglial cells [219] directly target and limit ACSL4 expression to inhibit ferroptosis. It was also reported that miR-3173-5p carried by exosomes derived from cancer-associated fibroblasts represses ACSL4 to inhibit ferroptosis in GEM-resistant pancreatic cancer [220]. Moreover, lncNEAT1 competing with miR-34a-5p and miR-204-5p, circLMO1 competing with miR-4291, circSCN8A competing with miR-1290, and circCarm1 competing with miR-670-3p elevate ASCL4 levels and promote ferroptosis in prostate cancer [211,212], cervical cancer [140], non-small-cell lung cancer [215], and neuronal cells [216]. MiR-17-92 increases ACSL4 expression by directly targeting the zinc lipoprotein A20 to protect endothelial HUVEC cells from erastin-induced ferroptosis [221]. LncTUG1 carried by urine-derived stem-cell-derived exosomes reduces ACSL4 levels by blocking serine/arginine splicing factor 1 (SRSF1) and inhibiting the H/R-induced ferroptosis of human proximal tubular epithelial cells in IRI-induced acute kidney injury [222]. CircEXOC5 binds to PTBP1 to promote ACSL4 mRNA stability, leading to ferroptosis in sepsis-induced acute lung injury [167]. LncCBSLR decreased CBS levels to promote ACSL4 ubiquitination and degradation, thus protecting gastric cancer cells from ferroptosis [223].
Figure 3.
ncRNAs regulating ferroptosis through lipid metabolism. MiRNAs are unboxed text in black. LncRNAs are unboxed text in red. CircRNAs are unboxed text in blue. Exosomal ncRNAs are marked with an “Exo-” in pink. Genes and proteins that directly involved in ferroptosis are black text boxed in black and shaded in purple. Other genes and proteins mediated the function of ncRNAs on ferroptosis are black text boxed in deep purple.
In addition to targeting ACSL4, miR-7-5p targets and blocks ALOX12 to inhibit lipid peroxidation and ferroptosis, leading to radio-resistance of cancer cells [224]. MiR-522 in exosomes secreted by cancer-associated fibroblasts reduces ALOX15 expression by directly interacting with its mRNA, leading to the inhibition of lipid peroxidation and ferroptosis in gastric cancer [225]. MiR-18a directly binds to ALOXE3 mRNA and represses its expression to inhibit ferroptosis in glioblastoma cells [226]. MiR-212-5p and miR-194-5p bind to the mRNA of PTGS2 to repress its expression, thus inhibiting ferroptosis in neuronal death after traumatic brain injury [48] and protecting against temporal lobe epilepsy in young rats [227]. MiR-26a-5p carried by endothelial progenitor cell-derived exosomes also inhibits ferroptosis by targeting PTGS2 mRNA to improve airway remodeling in chronic obstructive pulmonary disease [228].
Moreover, miR-423-5p targets and reduces SCD1 expression to promote the ferroptosis of colon cancer cells, which is inhibited by lncRNA LINC01606 [229]. CircZBTB46 also acts as an miRNA sponge to upregulate SCD1 and enhance RSL3-induced ferroptosis in acute myeloid leukemia (AML) cells [230]. In addition, exosomal lncFERO derived from gastric cancer cells was found to promote SCD1 expression by directly interacting with SCD1 mRNA and recruiting heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1), which resulted in the suppression of ferroptosis in gastric cancer stem cells [231]. Exosomal cir93 from lung adenocarcinoma patients increases FABP3 to inhibit lipid peroxidation and ferroptosis [171]. In prostate cancer cells, miR-7 targets mTOR to suppress SREBP1, leading to the promotion of icariin- and curcumol-induced ferroptosis [232].
4.3. NcRNAs Regulating Ferroptosis through Antioxidant Defense
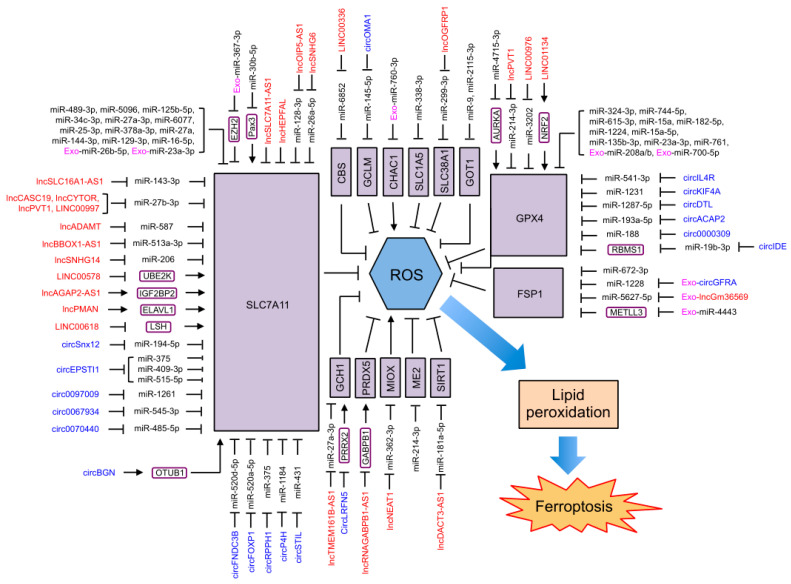
Approximately half of the current reported ferroptosis-regulating ncRNAs influence ferroptosis through antioxidant defense (Figure 4). SLC7A11 and GPX4, two important ferroptosis suppressors and markers, are targets not only for small molecules as ferroptosis inducers but also for a large group of ncRNAs regulating ferroptosis. MiR-409-3p, miR-515-5p, miR-375, miR-1261, miR-489-3p, miR-128-3p, miR-545-3p, miR-5096, miR-520d-5p, miR-125b-5p, miR-34c-3p, miR-143-3p, miR-26a-5p, miR-27b-3p, miR-587, miR-194-5p, miR-27a-3p, miR-520a-5p, miR-1184, miR-6077, miR-485-5p, miR-25-3p, miR-513a-3p, miR-206, and miR-431 have been reported to target and repress SLC7A11 to promote ferroptosis in cervical cancer [233], gastric cancer [148,234,235], liver cancer [236], prostate cancer [146], thyroid cancer [237], breast cancer [238], oral squamous cell carcinoma [239,240,241], kidney cancer [242,243], ovarian cancer [244,245], lung cancer [147,246,247,248,249], prostate cancer [250], esophageal squamous cell carcinoma [251], osteosarcoma [252], and colorectal cancer [253]. MiR-378a-3p, miR-27a, miR-144-3p, and exosomal miR-26b-5p from patients with acute myocardial infarction target and reduce SLC7A11 expression to induce ferroptosis in the IRI of the kidney [254], brain [255], and heart [256,257]. MiR-30b-5p directly binds to SLC7A11 mRNA and represses its levels to induce the ferroptosis of trophoblasts under hypoxic conditions, leading to preeclampsia [142]. MiR-129-3p reduces the expression of SLC7A11 to induce ferroptosis under Se deficiency conditions, leading to liver damage [258]. MiR-16-5p inhibits SLC7A11 to promote ferroptosis in adriamycin-induced cardiomyocyte injury [259]. Exosomal miR-23a-3p derived from cardiac fibroblasts inhibits SLC7A11 expression to promote ferroptosis in atrial fibrillation [260]. It was also reported that miR-30b-5p reduces Pax3 levels to transcriptionally repress the expression of SLC7A11 to promote the hypoxia-induced ferroptosis of trophoblasts during preeclampsia [142]. MiR-367-3p carried by bone marrow mesenchymal stem cells (BMSCs)-derived exosomes targets an enhancer of zeste homolog 2 (EZH2) and restrains EZH2 expression, thus elevating SLC7A11 levels indirectly, and thus inhibiting the erastin-induced ferroptosis of microglia [261]. Functioning as competitive RNA, lncOIP5-AS1 inhibits miR-128-3p, lncSLC16A1-AS1 inhibits miR-143-3p, lncSNHG6 inhibits miR-26a-5p, lncCASC19/lncCYTOR/lncPVT1/lncRNA-LINC00997 inhibits miR-27b-3p, lncADAMT inhibits miRNA-587, lncBBOX1-AS1 inhibits miR-513a-3p, lncSNHG14 inhibits miR-206, circSnx12 inhibits miR-194-5p, circEPSTI1 inhibits miR-375/miR-409-3p/miR-515-5p, circRNA circ0097009 inhibits miR-1261, circRNA circ0067934 inhibits miR-545-3p, circFNDC3B inhibits miR-520d-5p, circFOXP1 inhibits miR-520a-5p, circP4H inhibits miR-1184, circRNA circ0070440 inhibits miR-485-5p, circRPPH1 inhibits miR-375, and circSTIL inhibits miR-431 to increase SCL7A11 levels, resulting in the inhibition of ferroptosis and the progression of prostate cancer [146], kidney cancer [242,243], ovarian cancer [244,245], esophageal squamous cell carcinoma [251], osteosarcoma [252], cervical cancer [233], liver cancer [236], thyroid cancer [237], oral squamous cell carcinoma [239], lung cancer [246,248,249], gastric cancer [234], and colorectal cancer [253]. Moreover, lncHEPFAL reduces SLC7A11 by promoting its ubiquitination and degradation, eventually enhancing erastin-induced ferroptosis in hepatocellular carcinoma [155]. On the other hand, lncRNA LINC00578 also inhibits SLC7A11 ubiquitination by binding to UBE2K to inhibit ferroptosis in pancreatic cancer [262]. CircBGN directly binds to OTUB1 and SLC7A11, enhancing OTUB1-mediated SLC7A11 deubiquitination to inhibit ferroptosis in breast cancer [263]. Furthermore, lncAGAP2-AS1 stabilizes SLC7A11 mRNA via the IGF2BP2 pathway to suppress ferroptosis in melanoma cells [264]. LncPMAN stabilizes SLC7A11 mRNA by recruiting ELAVL1 to the cytoplasm to inhibit the ferroptosis of gastric cancer cells [154]. LncSLC7A11-AS1 inhibits SLC7A11 expression to induce ferroptosis in ovarian cancer [265]. Additionally, lncRNA LINC00618 attenuates the expression of lymphoid-specific helicase (LSH), which can bind to the promoter regions of SLC7A11 to promote its transcription. Therefore, lncRNA LINC00618 represses SLC7A11 expression to accelerate ferroptosis in human leukemia [266].
Figure 4.
ncRNAs regulating ferroptosis through antioxidant defense. MiRNAs are unboxed text in black. LncRNAs are unboxed text in red. CircRNAs are unboxed text in blue. Exosomal ncRNAs are marked with an “Exo-” in pink. Genes and proteins that directly involved in ferroptosis are black text boxed in black and shaded in purple. Other genes and proteins mediated the function of ncRNAs on ferroptosis are black text boxed in deep purple.
Similarly, GPX4 was reported to be targeted to induce ferroptosis by miR-541-3p and miR-214-3p in liver cancer [143,267], miR-324-3p in breast cancer [144] and kidney cancer [145], miR-1231 in thyroid cancer [268], miR-1287-5p/miR-744-5p/miR-615-3p in lung cancer [269,270], miR-1287-5p in osteosarcoma [271], miR-15a in prostate cancer [272], miR-193a-5p in cervical cancer [273], miR-3202 in cholangiocarcinoma [274], as well as by miR-182-5p, miR-1224, miR-15a-5p, miR-135b-3p in the IRI of kidney injury [254] and heart [275,276,277], miR-23a-3p in intracerebral hemorrhage [278], miR-188 in diabetic nephropathy [279], miR-761 in liver dysfunction of patients with polycystic ovary syndrome [198], exosomal miR-208a/b secreted from hypoxia-induced cardiomyocytes in cardiomyocytes and cardiac fibroblasts [280], and exosomal miR-700-5p from hypoxia-pretreated adipose-derived stem cells in UV-light-induced skin injury [281]. The inhibition of miR-214-3p by lncPVT1 [267] and the inhibition of miR-541-3p by circIL4R [143] in liver cancer, the inhibition of miR-3202 by lncRNA LINC00976 in cholangiocarcinoma [274], the inhibition of miR-1231 by circKIF4A in thyroid cancer [268], the inhibition of miR-1287-5p by circDTL in lung cancer [270], the inhibition of miR-193a-5p by circACAP2 in cervical cancer [273], and the inhibition of miR-188 by circRNA circ0000309 in diabetic nephropathy [279] elevate GPX4 levels to suppress ferroptosis. Moreover, circIDE inhibits miR-19b-3p as a sponge to elevate the expression of RBMS1, which in turn reduces the stability of GPX4 mRNA to facilitate ferroptosis in hepatocellular carcinoma [282]. LncRNA LINC01134 recruits NRF2 to the promoter region of GPX4 to enhance GPX4 transcription, leading to the suppression of ferroptosis in hepatocellular carcinoma [283]. MiR-4715-3p targets and represses Aurora kinase A (AURKA) to reduce GPX4 protein levels in an unknown mechanism and then induces ferroptosis in upper gastrointestinal adenocarcinoma [284].
In addition to SLC7A11 and GPX4, ncRNAs regulate other members of the GPX4-GSH system to affect ferroptosis. MiR-6852 targets and represses CBS to reduce cysteine and GSH synthesis, leading to the promotion of ferroptosis, whereas lncRNA LINC00336 acts as a sponge to block miR-6852 and inhibit ferroptosis in lung cancer [285]. MiR-145-5p directly binds to the mRNA of GCLM (a subunit of GCL) and represses its expression, resulting in a decrease in GSH levels and the induction of ferroptosis in prolactinomas, whereas circOMA1 inhibits miR-145-5p in a competitive manner to suppress ferroptosis [286]. It was also found that exosomal miR-760-3p from adipose-derived mesenchymal stem cells targets and represses CHAC1 to reduce GSH and attenuate ferroptosis in neurons [287].
NcRNAs also modulate ferroptosis by regulating glutaminolysis in two ways. MiR-338-3p targeting SLC1A5 in retinal pigment epithelium cells [87] and miR-299-3p targeting SLC38A1 in lung cancer cells [85] reduce glutamine absorption and GSH levels to promote ferroptosis, whereas lncOGFRP1 attenuates ferroptosis by blocking miR-299-3p in lung cancer cells [85]. MiR-9 and miR-2115-3p bind to the mRNA of GOT1 to repress its expression, leading to suppression of ferroptosis probably by the elevation of glutamate and GSH levels in melanoma cells and a preeclampsia model, respectively [288,289]. On the contrary, miR-137 targeting SLC1A5 in melanoma cells [89], miR-150-5p targeting SLC38A1 during pulmonary fibrosis [92], and miR-15b-5p and miR-190a-5p targeting GLS2 in pancreatic β-cells and rat cardiomyocyte cells [93,95] inhibit ferroptosis by blocking glutaminolysis and lipid synthesis, whereas lncZFAS1 acts as a competitive endogenous RNA to block miR-150-5p to facilitate ferroptosis during pulmonary fibrosis [92]. Moreover, lncATXN8OS stabilizes GLS2 mRNA to facilitate ferroptosis in glioma [94]. LncSnhg7 interacts with T-box transcription factor 5 (Tbx5) to transcriptionally induce GLS2 expression, resulting in the promotion of cardiomyocyte ferroptosis in cardiac hypertrophy [96].
Additionally, ncRNAs regulate ferroptosis via antioxidant systems other than the GPX4-GSH system. MiR-1228, miR-5627-5p, and miR-672-3p target and suppress FSP1 to promote ferroptosis in breast cancer cells [290], in neuronal cells [291], and in rats with contusive spinal cord injury [292], whereas circGFRA and lncGm36569 carried by mesenchymal-stem-cell-derived exosomes promote ferroptosis by directly targeting miR-1228 and miR-5627-5p, respectively [290,291]. It was also reported that exosomal miR-4443 from cisplatin-resistant lung cancer tissue inhibits ferroptosis by directly targeting METLL3, which regulates the m6A modification of FSP1 mRNA [293]. Moreover, LncTMEM161B-AS1 directly blocks miR-27a-3p to increase the expression of its target GCH1, leading to the suppression of ferroptosis in esophageal cancer [293]. CircLRFN5 interacts with PRRX2 and promotes its ubiquitination and proteasomal degradation, transcriptionally reducing PRRX2-mediated GCH1 expression, resulting in the induction of ferroptosis in glioma [170]. LncGABPB1-AS1 downregulates PRDX5 by blocking GABPB1 translation to promote the erastin-induced ferroptosis of hepatocellular carcinoma cells [106]. LncNEAT1 competitively binds to miR-362-3p to increase the expression of myo-inositol oxygenase (MIOX), a non-heme-iron enzyme, to promote ROS production and the ferroptosis of hepatocellular carcinoma cells [294]. MiR-214-3p targets malic enzyme 2 (ME2) to suppress the cellular antioxidant capacity and promote ferroptosis in neonatal rat cardiomyocytes [295]. Cancer-associated fibroblast-derived exosomal lncDACT3-AS1 inhibits miR-181a-5p to elevate the levels of its target sirtuin 1 (SIRT1), leading to an increase in antioxidant capacity to inhibit ferroptosis in gastric cancer [296].
4.4. NcRNAs Regulating Ferroptosis through Other Ferroptosis Regulators
There are also some ncRNAs that regulate ferroptosis through other ferroptosis regulators, e.g., transcription factors. MiR-365a-3p directly targets and suppresses NRF2 to promote ferroptosis in non-small-cell lung cancer [297]. LncMT1DP facilitates erastin-induced ferroptosis by stabilizing miR-365a-3p [297]. MiR-6077 and exosomal miR-125b-5p from adipose-derived stem cells directly repress KEAP1, a negative regulator of NRF that regulates its ubiquitination and degradation, to increase the expression and nucleus translocation of NRF2, leading to the alleviation of ferroptosis in lung adenocarcinoma cells [247] and pulmonary microvascular endothelial cells [298]. LncGMDS-AS1 and lncRNA LINC01128 promote ferroptosis by competitively blocking miR-6077 [247]. Moreover, lncRNA LINC00239 interacts with the NRF2 binding site of KEAP1 to increase NRF2 levels by ubiquitination inhibition and then inhibits ferroptosis in colorectal cancer [299]. Additionally, miR-130b-3p targets and represses Dickkopf1 to activate NRF2, leading to ferroptosis suppression in melanoma [300].
In addition to NRF2, miR-214 and let-7b-5p target and repress p53 to inhibit ferroptosis in brain I/R and acute myeloid leukemia, which are directly blocked by lncPVT1 and circKDM4C, respectively [138,301]. LncMeg3 directly interacts with p53 and enhances its stability and transcriptional activity, mediating ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells [302,303,304]. LncP53RRA can interact with Ras GTPase-activating protein-binding protein 1 (G3BP1) to sequester p53 in the nucleus, thus promoting ferroptosis [156]. LncPELATON forms a complex with p53 and the RNA-binding protein EIF4A3 to inhibit ferroptosis in glioblastoma [305].
Moreover, miR-221-3p was reported to target ATF3 to transcriptionally induce the expression of GPX4 and HRD1 and then promote the ubiquitination and degradation of ACSL4 by HRD1, resulting in ferroptosis suppression in gastric cancer cells [306]. LncDLEU1 binds to ZFP36 and facilitates the degradation of ATF3 mRNA by ZFP36, thus upregulating the expression of SLC7A11 to attenuate erastin-induced ferroptosis in glioblastoma [307]. MiR-214 and miR-3200-5p directly repress ATF4 expression to enhance ferroptosis in hepatocellular carcinoma cells [308,309], whereas LncHULC increases ATF4 levels by competing with miR-3200-5p and inhibits ferroptosis [308]. CircRHOT1 directly inhibits miR-106a-5p to increase its target STAT3, resulting in ferroptosis inhibition in breast cancer cells [310]. It was also reported that miR-125b-5p targets and represses STAT3 to induce ferroptosis in gastric cancer [311]. CircRNA circ0008035 increases the expression of E2F transcription factor 7 (E2F7) by blocking miR-302a, inhibiting dexmedetomidine-induced ferroptosis in gastric cancer cells [312]. CircASAP2 blocks miR-770-5p to enhance the expression of SRY-Box transcription factor 2 (SOX2) and then induces SLC7A11 expression to suppress ferroptosis in diabetic nephropathy [313]. Moreover, miR-7-5p upregulates HIF-1α expression by an unknown mechanism to inhibit ferroptosis in radioresistant HeLa (cervical carcinoma) and SAS (human tongue carcinoma) cell lines [224].
NcRNAs also regulate ferroptosis by other signaling pathways. CircTTBK2 inhibits miR-761 to modulate its target ITGB8, a subunit of integrin beta, to reduce ferroptosis in glioma [314]. CircRNA circ0007142 was found to block the effect of miR-874-3p on its target GDPD5, leading to ferroptosis suppression in colorectal cancer [315]. LncMEG8 inhibits miR-497-5p to increase NOTCH2, leading to the inhibition of ferroptosis by elevating SLC7A11 and GPX4 levels [316]. CircRNA circ0000745 competitively inhibits miR-494-3p to upregulate neuroepithelial cell transforming 1 (NET1), a guanine nucleotide exchange factor of RhoA, to suppress ferroptosis in acute lymphoblastic leukemia cells [317]. CircPVT1 competes with miR-30a-5p to elevate the levels of its target Frizzled3, activating Wnt/β-Catenin signaling to inhibit ferroptosis [318]. LncA2M-AS1 forms a complex with PCBP3 to facilitate p38 activation and inhibit the AKT-mTOR signaling pathway, contributing to the promotion of ferroptosis [206]. In addition, circABCB10 acts as a sponge for miR-326 to elevate CCL5 levels and attenuate the ferroptosis of rectal cancer cells [319]. LncRNA LINC00460 blocks miR-320a to enhance the expression of myelin and lymphocyte protein 2 (MAL2), inhibiting ferroptosis in breast cancer [320].
5. Conclusions and Perspectives
Ferroptosis is a novel type of regulated cell death that was first proposed in 2012 [3]. Ferroptosis has been found in diverse species, including humans, other mammals and vertebrates, invertebrates, plants, yeast, and bacteria [321,322,323,324]. Substantial studies have focused on exploring the mechanisms of ferroptosis and understanding how it is regulated in different cells, especially in humans and other mammals. Collectively, these studies have demonstrated that ferroptosis results from lipid peroxidation caused by the dysregulation of iron metabolism, lipid metabolism, and antioxidant defense, which is controlled by regulating enzymes and other proteins involved in these processes at the transcriptional, post-transcriptional, translational, and post-translational levels. NcRNAs have been proven to regulate gene expression in various manners. In recent years, numerous ncRNAs, including miRNAs, lncRNAs, and circRNAs, have been reported to regulate ferroptosis, which have been summarized in this review. These ncRNAs can directly target ferroptosis-related enzymes or proteins involved in iron metabolism, lipid metabolism, and antioxidant defense, or they can indirectly target other regulators of ferroptosis, such as transcription factors. Some ncRNAs also act as molecular sponges or form complexes with other ncRNAs to exert their functions, which adds another level of regulation on ferroptosis. Moreover, because of the differential expression of ncRNAs in different cells, these ncRNAs may affect the regulation of ferroptosis in a cell-type-dependent or tissue-type-dependent manner. Therefore, although great progress has been made in studying the molecular mechanism of ncRNA regulation of ferroptosis, a deeper understanding of the mechanisms of how ncRNAs regulate ferroptosis in cell- or tissue-specific manners is still required. Moreover, ferroptosis is also reported in fish, invertebrates, plants, yeast, and bacteria [321,322,323,324], which often contain different ncRNAs in their genomes. Few studies have reported the regulation of ferroptosis by ncRNAs in these species, which may be different from our current knowledge in humans and other mammals.
Ferroptosis functions are intricately involved in numerous physiological processes and various diseases, such as cancer, neurodegeneration disease, cardiovascular diseases, and the IRI of different organs such as the heart, brain, kidney, and liver [9,10,11,12,13,14]. Therefore, some ncRNAs regulating ferroptosis, including miRNAs, lncRNAs, and circRNAs, have the potential to be novel therapeutic methods and diagnostic biomarkers for these diseases, especially for cancer. On the one hand, more and more studies suggest that ncRNAs can be used as a biomarker to predict tumor progression and clinical prognosis. However, the most important ncRNAs associated with specific types of tumors are still not identified, which limits the application of ncRNAs as diagnostic biomarkers. On the other hand, according to studies on ncRNA-regulated ferroptosis, the ferroptosis process can be altered via targeting ncRNAs, thus affecting the ferroptosis-related pathological process, e.g., cell proliferation and chemoresistance for cancer cells. Current inducers and inhibitors of ferroptosis mainly target the proteins involved in the core mechanism of ferroptosis, which may induce metabolic dysregulation in normal cells and cause unexpected side effects in addition to regulating ferroptosis. Due to their cell- or tissue-specific manners of regulating ferroptosis, ncRNAs may be better targets for therapeutic methods. Furthermore, because of the heterogeneity of gene expression on a per individual basis, ncRNA-associated therapy and biomarkers can be applied to support the personalized treatment of ferroptosis-related disease. Additionally, ncRNAs are also promising therapeutic agents for ferroptosis-related diseases. Indeed, ncRNAs have already shown their effect of regulating ferroptosis in vitro. Their low in vivo bioavailability has limited their clinical application. In general, three strategies have been proposed for ncRNA-based therapies, including nanoparticles, ncRNA modification, and oncolytic adenovirus strategy [325]. Although no project has entered the clinical trial stage, progress has been achieved in the clinical application of ncRNA to regulate ferroptosis. Moreover, since the regulation of ferroptosis by ncRNAs derived from exosomes has been reported in many studies, exosomes can be used as excellent ncRNA transporters to induce or inhibit ferroptosis in cells. With more extensive research, loading selected ncRNAs into exosomes to induce ferroptosis may create novel opportunities for ncRNA-based therapies.
Taken together, ncRNAs form a complex network to regulate ferroptosis via proteins or genes involved in the core mechanism of ferroptosis or its regulators. This review has summarized the regulatory roles of several types of ncRNAs in ferroptosis, which are beneficial for understanding the pathogenesis of ferroptosis-related diseases. And these ferroptosis-related ncRNAs have great potential to act as therapeutic targets or agents for novel therapeutic methods, as well as diagnostic biomarkers, for these diseases, especially for cancers.
Author Contributions
Conceptualization, C.Z.; writing—original draft, X.Z. and C.Z.; writing—review and editing, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 82073065.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Estevao D., da Cruz-Ribeiro M., Cardoso A.P., Costa A.M., Oliveira M.J., Duarte T.L., da Cruz T.B. Iron metabolism in colorectal cancer: A balancing act. Cell Oncol. 2023 doi: 10.1007/s13402-023-00828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Huang X., Wang J., Huang R., Wan D. Regulation of Iron Homeostasis and Related Diseases. Mediat. Inflamm. 2020;2020:6062094. doi: 10.1155/2020/6062094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Cao F., Yin H.L., Huang Z.J., Lin Z.T., Mao N., Sun B., Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaghaghi Z., Motieian S., Alvandi M., Yazdi A., Asadzadeh B., Farzipour S., Abbasi S. Ferroptosis Inhibitors as Potential New Therapeutic Targets for Cardiovascular Disease. Mini Rev. Med. Chem. 2022;22:2271–2286. doi: 10.2174/1389557522666220218123404. [DOI] [PubMed] [Google Scholar]
- 8.Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschhorn T., Stockwell B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu W., Liang K., Zhu H., Zhao C., Hu H., Yin S. Ferroptosis and Its Role in Chronic Diseases. Cells. 2022;11:2040. doi: 10.3390/cells11132040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 2022;22:381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y., Xia X., Basnet D., Zheng J.C., Huang J., Liu J. Mechanisms of Ferroptosis and Emerging Links to the Pathology of Neurodegenerative Diseases. Front. Aging Neurosci. 2022;14:904152. doi: 10.3389/fnagi.2022.904152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Coillie S., Van San E., Goetschalckx I., Wiernicki B., Mukhopadhyay B., Tonnus W., Choi S.M., Roelandt R., Dumitrascu C., Lamberts L., et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 2022;13:1046. doi: 10.1038/s41467-022-28718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L., Han S., Guo J., Qiu T., Zhou J., Shen L. Ferroptosis-A New Dawn in the Treatment of Organ Ischemia-Reperfusion Injury. Cells. 2022;11:3653. doi: 10.3390/cells11223653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium E.P. The ENCODE (ENCyclopedia of DNA Elements) Project. Science. 2004;306:636–640. doi: 10.1126/science.1105136. [DOI] [PubMed] [Google Scholar]
- 16.Consortium E.P. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seal R.L., Chen L.L., Griffiths-Jones S., Lowe T.M., Mathews M.B., O’Reilly D., Pierce A.J., Stadler P.F., Ulitsky I., Wolin S.L., et al. A guide to naming human non-coding RNA genes. EMBO J. 2020;39:e103777. doi: 10.15252/embj.2019103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P., Wu W., Chen Q., Chen M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019;16:20190027. doi: 10.1515/jib-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frazer D.M., Anderson G.J. The regulation of iron transport. BioFactors. 2014;40:206–214. doi: 10.1002/biof.1148. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Yu C., Kang R., Tang D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020;8:590226. doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson M.D. Steap proteins: Implications for iron and copper metabolism. Nutr. Rev. 2007;65:335–340. doi: 10.1111/j.1753-4887.2007.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun X., Ou Z., Xie M., Kang R., Fan Y., Niu X., Wang H., Cao L., Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West A.R., Oates P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008;14:4101–4110. doi: 10.3748/wjg.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang S.K., Chen S.E., Chang L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2019;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consoli V., Sorrenti V., Pittala V., Greish K., D’Amico A.G., Romeo G., Intagliata S., Salerno L., Vanella L. Heme Oxygenase Modulation Drives Ferroptosis in TNBC Cells. Int. J. Mol. Sci. 2022;23:5709. doi: 10.3390/ijms23105709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C., Zhang Y., Cheng X., Yuan H., Zhu S., Liu J., Wen Q., Xie Y., Liu J., Kroemer G., et al. PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev. Cell. 2018;46:441–455.E8. doi: 10.1016/j.devcel.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., Guo M., Shen M., Kong D., Zhang F., Shao J., Tan S., Wang S., Chen A., Cao P., et al. The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol. 2020;36:101619. doi: 10.1016/j.redox.2020.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou W., Xie Y., Song X., Sun X., Lotze M.T., Zeh H.J., 3rd, Kang R., Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W., Li W., Wang Y., Leng Y., Xia Z. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 2021;7:267. doi: 10.1038/s41420-021-00656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frey A.G., Nandal A., Park J.H., Smith P.M., Yabe T., Ryu M.S., Ghosh M.C., Lee J., Rouault T.A., Park M.H., et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc. Natl. Acad. Sci. USA. 2014;111:8031–8036. doi: 10.1073/pnas.1402732111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Protchenko O., Baratz E., Jadhav S., Li F., Shakoury-Elizeh M., Gavrilova O., Ghosh M.C., Cox J.E., Maschek J.A., Tyurin V.A., et al. Iron Chaperone Poly rC Binding Protein 1 Protects Mouse Liver from Lipid Peroxidation and Steatosis. Hepatology. 2021;73:1176–1193. doi: 10.1002/hep.31328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanatori I., Richardson D.R., Imada K., Kishi F. Iron Export through the Transporter Ferroportin 1 Is Modulated by the Iron Chaperone PCBP2. J. Biol. Chem. 2016;291:17303–17318. doi: 10.1074/jbc.M116.721936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward D.M., Kaplan J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta. 2012;1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemeth E., Tuttle M.S., Powelson J., Vaughn M.B., Donovan A., Ward D.M., Ganz T., Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 36.Brown C.W., Amante J.J., Chhoy P., Elaimy A.L., Liu H., Zhu L.J., Baer C.E., Dixon S.J., Mercurio A.M. Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev. Cell. 2019;51:575–586.E4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown C.W., Chhoy P., Mukhopadhyay D., Karner E.R., Mercurio A.M. Targeting prominin2 transcription to overcome ferroptosis resistance in cancer. EMBO Mol. Med. 2021;13:e13792. doi: 10.15252/emmm.202013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan H., Li X., Zhang X., Kang R., Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem. Biophys. Res. Commun. 2016;478:838–844. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez S.W., Sviderskiy V.O., Terzi E.M., Papagiannakopoulos T., Moreira A.L., Adams S., Sabatini D.M., Birsoy K., Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim E.H., Shin D., Lee J., Jung A.R., Roh J.L. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180–190. doi: 10.1016/j.canlet.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Rouault T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D.L., Ghosh M.C., Rouault T.A. The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front. Pharmacol. 2014;5:124. doi: 10.3389/fphar.2014.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson G.J., Frazer D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017;106:1559S–1566S. doi: 10.3945/ajcn.117.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.Y., Kim W.K., Bae K.H., Lee S.C., Lee E.W. Lipid Metabolism and Ferroptosis. Biology. 2021;10:184. doi: 10.3390/biology10030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang D., Minikes A.M., Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell. 2022;82:2215–2227. doi: 10.1016/j.molcel.2022.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah R., Shchepinov M.S., Pratt D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lei P., Bai T., Sun Y. Mechanisms of Ferroptosis and Relations with Regulated Cell Death: A Review. Front. Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao X., Jiang Y., Liang W., Wang Y., Cao S., Yan H., Gao L., Zhang L. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain. 2019;12:78. doi: 10.1186/s13041-019-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y., Liu Y., Li K., Yuan D., Yang S., Zhou L., Zhao Y., Miao S., Lv C., Zhao J. COX-2/PGE2 Pathway Inhibits the Ferroptosis Induced by Cerebral Ischemia Reperfusion. Mol. Neurobiol. 2022;59:1619–1631. doi: 10.1007/s12035-021-02706-1. [DOI] [PubMed] [Google Scholar]
- 50.Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J., Zhong M., Yuan H., Zhang L., Billiar T.R., et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 51.Yang W.H., Ding C.C., Sun T., Rupprecht G., Lin C.C., Hsu D., Chi J.T. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 2019;28:2501–2508.e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou Y., Palte M.J., Deik A.A., Li H., Eaton J.K., Wang W., Tseng Y.Y., Deasy R., Kost-Alimova M., Dančík V., et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magtanong L., Ko P.J., To M., Cao J.Y., Forcina G.C., Tarangelo A., Ward C.C., Cho K., Patti G.J., Nomura D.K., et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019;26:420–432.e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tesfay L., Paul B.T., Konstorum A., Deng Z., Cox A.O., Lee J., Furdui C.M., Hegde P., Torti F.M., Torti S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019;79:5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang D., Feng Y., Zandkarimi F., Wang H., Zhang Z., Kim J., Cai Y., Gu W., Stockwell B.R., Jiang X. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell. 2023;186:2748–2764.E22. doi: 10.1016/j.cell.2023.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J.Y., Nam M., Son H.Y., Hyun K., Jang S.Y., Kim J.W., Kim M.W., Jung Y., Jang E., Yoon S.J., et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA. 2020;117:32433–32442. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vriens K., Christen S., Parik S., Broekaert D., Yoshinaga K., Talebi A., Dehairs J., Escalona-Noguero C., Schmieder R., Cornfield T., et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566:403–406. doi: 10.1038/s41586-019-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ates G., Goldberg J., Currais A., Maher P. CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biol. 2020;36:101648. doi: 10.1016/j.redox.2020.101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartolacci C., Andreani C., Vale G., Berto S., Melegari M., Crouch A.C., Baluya D.L., Kemble G., Hodges K., Starrett J., et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun. 2022;13:4327. doi: 10.1038/s41467-022-31963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yi J., Zhu J., Wu J., Thompson C.B., Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA. 2020;117:31189–31197. doi: 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagarajan S.R., Butler L.M., Hoy A.J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 2021;9:2. doi: 10.1186/s40170-020-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veglia F., Tyurin V.A., Blasi M., De Leo A., Kossenkov A.V., Donthireddy L., To T.K.J., Schug Z., Basu S., Wang F., et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–78. doi: 10.1038/s41586-019-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M., Chen P., Liu J., Zhu S., Kroemer G., Klionsky D.J., Lotze M.T., Zeh H.J., Kang R., Tang D. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019;5:eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seibt T.M., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Yang W.S., SriRamaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conrad M., Pratt D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019;15:1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 69.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., Brown L.M., Valenzuela C.A., Wolpaw A.J., Stockwell B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ingold I., Berndt C., Schmitt S., Doll S., Poschmann G., Buday K., Roveri A., Peng X., Porto Freitas F., Seibt T., et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. 2018;172:409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 71.Xuan Y., Wang H., Yung M.M., Chen F., Chan W.S., Chan Y.S., Tsui S.K., Ngan H.Y., Chan K.K., Chan D.W. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics. 2022;12:3534–3552. doi: 10.7150/thno.70194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen M.S., Wang S.F., Hsu C.Y., Yin P.H., Yeh T.S., Lee H.C., Tseng L.M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8:114588–114602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y., Wu D., Fu Q., Hao S., Gu Y., Zhao W., Chen S., Sheng F., Xu Y., Chen Z., et al. CHAC1 as a Novel Contributor of Ferroptosis in Retinal Pigment Epithelial Cells with Oxidative Damage. Int. J. Mol. Sci. 2023;24:1582. doi: 10.3390/ijms24021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koppula P., Zhuang L., Gan B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L., Liu R., Liu Y., Li G., Chen Q., Liu X., Ma S. Cystine-glutamate antiporter xCT as a therapeutic target for cancer. Cell Biochem. Funct. 2021;39:174–179. doi: 10.1002/cbf.3581. [DOI] [PubMed] [Google Scholar]
- 77.Tu H., Tang L.J., Luo X.J., Ai K.L., Peng J. Insights into the novel function of system Xc- in regulated cell death. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1650–1662. doi: 10.26355/eurrev_202102_24876. [DOI] [PubMed] [Google Scholar]
- 78.Liu M.R., Zhu W.T., Pei D.S. System Xc(-): A key regulatory target of ferroptosis in cancer. Investig. New Drugs. 2021;39:1123–1131. doi: 10.1007/s10637-021-01070-0. [DOI] [PubMed] [Google Scholar]
- 79.Hayano M., Yang W.S., Corn C.K., Pagano N.C., Stockwell B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimada K., Stockwell B.R. tRNA synthase suppression activates de novo cysteine synthesis to compensate for cystine and glutathione deprivation during ferroptosis. Mol. Cell Oncol. 2016;3:e1091059. doi: 10.1080/23723556.2015.1091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu N., Lin X., Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br. J. Cancer. 2020;122:279–292. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L., Cai H., Hu Y., Liu F., Huang S., Zhou Y., Yu J., Xu J., Wu F. A pharmacological probe identifies cystathionine beta-synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9:1005. doi: 10.1038/s41419-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGivan J.D., Bungard C.I. The transport of glutamine into mammalian cells. Front. Biosci. 2007;12:874–882. doi: 10.2741/2109. [DOI] [PubMed] [Google Scholar]
- 85.Liu L., Su S., Ye D., Yu Z., Lu W., Li X. Long non-coding RNA OGFRP1 regulates cell proliferation and ferroptosis by miR-299-3p/SLC38A1 axis in lung cancer. Anticancer Drugs. 2022;33:826–839. doi: 10.1097/CAD.0000000000001328. [DOI] [PubMed] [Google Scholar]
- 86.Venkateswaran G., McDonald P.C., Chafe S.C., Brown W.S., Gerbec Z.J., Awrey S.J., Parker S.J., Dedhar S. A Carbonic Anhydrase IX/SLC1A5 axis regulates glutamine metabolism dependent ferroptosis in hypoxic tumor cells. Mol. Cancer Ther. 2023:MCT-23-0041. doi: 10.1158/1535-7163.MCT-23-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J., Sun C., Dong X., Wang H. A novel miR-338-3p/SLC1A5 axis reprograms retinal pigment epithelium to increases its resistance to high glucose-induced cell ferroptosis. J. Mol. Histol. 2022;53:561–571. doi: 10.1007/s10735-022-10070-0. [DOI] [PubMed] [Google Scholar]
- 88.Kremer D.M., Nelson B.S., Lin L., Yarosz E.L., Halbrook C.J., Kerk S.A., Sajjakulnukit P., Myers A., Thurston G., Hou S.W., et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat. Commun. 2021;12:4860. doi: 10.1038/s41467-021-24859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo M., Wu L., Zhang K., Wang H., Zhang T., Gutierrez L., O’Connell D., Zhang P., Li Y., Gao T., et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25:1457–1472. doi: 10.1038/s41418-017-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu F., Wang H., Pei H., Zhang Z., Liu L., Tang L., Wang S., Ren B.C. SLC1A5 Prefers to Play as an Accomplice Rather Than an Opponent in Pancreatic Adenocarcinoma. Front. Cell Dev. Biol. 2022;10:800925. doi: 10.3389/fcell.2022.800925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y., Dong P., Liu N., Yang J.Y., Wang H.M., Geng Q. TRIM6 Reduces Ferroptosis and Chemosensitivity by Targeting SLC1A5 in Lung Cancer. Oxid. Med. Cell Longev. 2023;2023:9808100. doi: 10.1155/2023/9808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y., Tai W., Lu N., Li T., Liu Y., Wu W., Li Z., Pu L., Zhao X., Zhang T., et al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging. 2020;12:9085–9102. doi: 10.18632/aging.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou X., Zhuo M., Zhang Y., Shi E., Ma X., Li H. miR-190a-5p regulates cardiomyocytes response to ferroptosis via directly targeting GLS2. Biochem. Biophys. Res. Commun. 2021;566:9–15. doi: 10.1016/j.bbrc.2021.05.100. [DOI] [PubMed] [Google Scholar]
- 94.Luo J., Bai R., Liu Y., Bi H., Shi X., Qu C. Long non-coding RNA ATXN8OS promotes ferroptosis and inhibits the temozolomide-resistance of gliomas through the ADAR/GLS2 pathway. Brain Res. Bull. 2022;186:27–37. doi: 10.1016/j.brainresbull.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Wu F., Shang C., Jin T., Shi L. Hispidin Inhibits Ferroptosis Induced by High Glucose via the miR-15b-5p/GLS2 Axis in Pancreatic Beta Cells. Evid. Based Complement. Altern. Med. 2023;2023:9428241. doi: 10.1155/2023/9428241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q., Song C., Zhang M., Liu Y., Wang L., Xie Y., Qi H., Ba L., Shi P., Cao Y., et al. Super-enhancer-driven lncRNA Snhg7 aggravates cardiac hypertrophy via Tbx5/GLS2/ferroptosis axis. Eur. J. Pharmacol. 2023;953:175822. doi: 10.1016/j.ejphar.2023.175822. [DOI] [PubMed] [Google Scholar]
- 97.Shin D., Lee J., You J.H., Kim D., Roh J.L. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020;30:101418. doi: 10.1016/j.redox.2019.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suzuki S., Venkatesh D., Kanda H., Nakayama A., Hosokawa H., Lee E., Miki T., Stockwell B.R., Yokote K., Tanaka T., et al. GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma. Cancer Res. 2022;82:3209–3222. doi: 10.1158/0008-5472.CAN-21-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Goya Grocin A., Xavier da Silva T.N., Panzilius E., Scheel C.H., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 101.Dai E., Meng L., Kang R., Wang X., Tang D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 2020;522:415–421. doi: 10.1016/j.bbrc.2019.11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Muller C., Zandkarimi F., Merl-Pham J., Bao X., Anastasov N., Kossl J., et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soula M., Weber R.A., Zilka O., Alwaseem H., La K., Yen F., Molina H., Garcia-Bermudez J., Pratt D.A., Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020;16:1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mao C., Liu X., Zhang Y., Lei G., Yan Y., Lee H., Koppula P., Wu S., Zhuang L., Fang B., et al. Author Correction: DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;596:E13. doi: 10.1038/s41586-021-03820-9. [DOI] [PubMed] [Google Scholar]
- 105.Lovatt M., Adnan K., Kocaba V., Dirisamer M., Peh G.S.L., Mehta J.S. Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol. 2020;30:101417. doi: 10.1016/j.redox.2019.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qi W., Li Z., Xia L., Dai J., Zhang Q., Wu C., Xu S. LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci. Rep. 2019;9:16185. doi: 10.1038/s41598-019-52837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu B., Chen X.B., Hong Y.C., Zhu H., He Q.J., Yang B., Ying M.D., Cao J. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol. Sin. 2019;40:1334–1342. doi: 10.1038/s41401-019-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu D., Liang C.H., Huang B., Zhuang X., Cui W., Yang L., Yang Y., Zhang Y., Fu X., Zhang X., et al. Tryptophan Metabolism Acts as a New Anti-Ferroptotic Pathway to Mediate Tumor Growth. Adv. Sci. 2023;10:e2204006. doi: 10.1002/advs.202204006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anandhan A., Dodson M., Schmidlin C.J., Liu P., Zhang D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shakya A., McKee N.W., Dodson M., Chapman E., Zhang D.D. Anti-Ferroptotic Effects of Nrf2: Beyond the Antioxidant Response. Mol. Cells. 2023;46:165–175. doi: 10.14348/molcells.2023.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimano H., Sato R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017;13:710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 112.Snaebjornsson M.T., Janaki-Raman S., Schulze A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020;31:62–76. doi: 10.1016/j.cmet.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 113.Brown C.W., Amante J.J., Goel H.L., Mercurio A.M. The alpha6beta4 integrin promotes resistance to ferroptosis. J. Cell Biol. 2017;216:4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Q., Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol. Int. 2019;43:1245–1256. doi: 10.1002/cbin.11121. [DOI] [PubMed] [Google Scholar]
- 115.Qiang Z., Dong H., Xia Y., Chai D., Hu R., Jiang H. Nrf2 and STAT3 Alleviates Ferroptosis-Mediated IIR-ALI by Regulating SLC7A11. Oxid. Med. Cell Longev. 2020;2020:5146982. doi: 10.1155/2020/5146982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang L., Wang H., Yang X., Wu Q., An P., Jin X., Liu W., Huang X., Li Y., Yan S., et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct. Target. Ther. 2020;5:138. doi: 10.1038/s41392-020-00253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J., Zhang C., Wang J., Hu W., Feng Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int. J. Mol. Sci. 2020;21:8387. doi: 10.3390/ijms21218387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu R., Wang W., Zhang W. Ferroptosis and the bidirectional regulatory factor p53. Cell Death Discov. 2023;9:197. doi: 10.1038/s41420-023-01517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng X., Liang Y., Zhang C. Ferroptosis Regulated by Hypoxia in Cells. Cells. 2023;12:1050. doi: 10.3390/cells12071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y., Yan J., Zhao Q., Zhang Y., Zhang Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front. Pharmacol. 2022;13:904314. doi: 10.3389/fphar.2022.904314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L., Liu Y., Du T., Yang H., Lei L., Guo M., Ding H.F., Zhang J., Wang H., Chen X., et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ. 2020;27:662–675. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen D., Fan Z., Rauh M., Buchfelder M., Eyupoglu I.Y., Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen D., Rauh M., Buchfelder M., Eyupoglu I.Y., Savaskan N. The oxido-metabolic driver ATF4 enhances temozolamide chemo-resistance in human gliomas. Oncotarget. 2017;8:51164–51176. doi: 10.18632/oncotarget.17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He F., Zhang P., Liu J., Wang R., Kaufman R.J., Yaden B.C., Karin M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 2023;79:362–377. doi: 10.1016/j.jhep.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu S., Wang X.Z., He C., Wang L., Liang S.P., Wang C.C., Li C., Luo T.F., Feng C.S., Wang Z.C., et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 2021;42:1690–1702. doi: 10.1038/s41401-021-00700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu H., Mo H., Yang C., Mei X., Song X., Lu W., Xiao H., Yan J., Wang X., Yan J., et al. A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radic. Biol. Med. 2022;189:122–135. doi: 10.1016/j.freeradbiomed.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 127.Cui J., Zhou Q., Yu M., Liu Y., Teng X., Gu X. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf. 2022;242:113944. doi: 10.1016/j.ecoenv.2022.113944. [DOI] [PubMed] [Google Scholar]
- 128.Zhu S., Zhang Q., Sun X., Zeh H.J., 3rd, Lotze M.T., Kang R., Tang D. HSPA5 Regulates Ferroptotic Cell Death in Cancer Cells. Cancer Res. 2017;77:2064–2077. doi: 10.1158/0008-5472.CAN-16-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen J., Zaal E.A., Berkers C.R., Ruijtenbeek R., Garssen J., Redegeld F.A. Omega-3 Fatty Acids DHA and EPA Reduce Bortezomib Resistance in Multiple Myeloma Cells by Promoting Glutathione Degradation. Cells. 2021;10:2287. doi: 10.3390/cells10092287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang N., Zeng G.Z., Yin J.L., Bian Z.X. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt’s Lymphoma. Biochem. Biophys. Res. Commun. 2019;519:533–539. doi: 10.1016/j.bbrc.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 131.Liang Y., Chen S., Han S., Luo L., Shen F., Huang Z. Toosendanin induced hepatotoxicity via triggering PERK-eIF2alpha-ATF4 mediated ferroptosis. Toxicol. Lett. 2023;377:51–61. doi: 10.1016/j.toxlet.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 132.Guo W., Wu Z., Chen J., Guo S., You W., Wang S., Ma J., Wang H., Wang X., Wang H., et al. Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis. J. Immunother. Cancer. 2022;10:e004381. doi: 10.1136/jitc-2021-004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cho I.J., Kim D., Kim E.O., Jegal K.H., Kim J.K., Park S.M., Zhao R., Ki S.H., Kim S.C., Ku S.K. Cystine and Methionine Deficiency Promotes Ferroptosis by Inducing B-Cell Translocation Gene 1. Antioxid. 2021;10:1543. doi: 10.3390/antiox10101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaikkonen M.U., Lam M.T., Glass C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011;90:430–440. doi: 10.1093/cvr/cvr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Green D., Dalmay T., Chapman T. Microguards and micromessengers of the genome. Heredity. 2016;116:125–134. doi: 10.1038/hdy.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 138.Lu J., Xu F., Lu H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 2020;260:118305. doi: 10.1016/j.lfs.2020.118305. [DOI] [PubMed] [Google Scholar]
- 139.Ma L.L., Liang L., Zhou D., Wang S.W. Tumor suppressor miR-424-5p abrogates ferroptosis in ovarian cancer through targeting ACSL4. Neoplasma. 2021;68:165–173. doi: 10.4149/neo_2020_200707N705. [DOI] [PubMed] [Google Scholar]
- 140.Ou R., Lu S., Wang L., Wang Y., Lv M., Li T., Xu Y., Lu J., Ge R.S. Circular RNA circLMO1 Suppresses Cervical Cancer Growth and Metastasis by Triggering miR-4291/ACSL4-Mediated Ferroptosis. Front. Oncol. 2022;12:858598. doi: 10.3389/fonc.2022.858598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bao W.D., Zhou X.T., Zhou L.T., Wang F., Yin X., Lu Y., Zhu L.Q., Liu D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19:e13235. doi: 10.1111/acel.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang H., He Y., Wang J.X., Chen M.H., Xu J.J., Jiang M.H., Feng Y.L., Gu Y.F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020;29:101402. doi: 10.1016/j.redox.2019.101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu Q., Zhou L., Yang G., Meng F., Wan Y., Wang L., Zhang L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol. Int. 2020;44:2344–2356. doi: 10.1002/cbin.11444. [DOI] [PubMed] [Google Scholar]
- 144.Hou Y., Cai S., Yu S., Lin H. Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim. Biophys. Sin. 2021;53:333–341. doi: 10.1093/abbs/gmaa180. [DOI] [PubMed] [Google Scholar]
- 145.Yu R., Zhou Y., Shi S., Wang X., Huang S., Ren Y. Icariside II induces ferroptosis in renal cell carcinoma cells by regulating the miR-324-3p/GPX4 axis. Phytomedicine. 2022;102:154182. doi: 10.1016/j.phymed.2022.154182. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y., Guo S., Wang S., Li X., Hou D., Li H., Wang L., Xu Y., Ma B., Wang H., et al. LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol. Environ. Saf. 2021;220:112376. doi: 10.1016/j.ecoenv.2021.112376. [DOI] [PubMed] [Google Scholar]
- 147.Lu X., Kang N., Ling X., Pan M., Du W., Gao S. MiR-27a-3p Promotes Non-Small Cell Lung Cancer Through SLC7A11-Mediated-Ferroptosis. Front. Oncol. 2021;11:759346. doi: 10.3389/fonc.2021.759346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ni H., Qin H., Sun C., Liu Y., Ruan G., Guo Q., Xi T., Xing Y., Zheng L. MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis. Stem Cell Res. Ther. 2021;12:325. doi: 10.1186/s13287-021-02394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smillie C.L., Sirey T., Ponting C.P. Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit. Rev. Biochem. Mol. Biol. 2018;53:231–245. doi: 10.1080/10409238.2018.1447542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mattick J.S., Amaral P.P., Carninci P., Carpenter S., Chang H.Y., Chen L.L., Chen R., Dean C., Dinger M.E., Fitzgerald K.A., et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023;24:430–447. doi: 10.1038/s41580-022-00566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Perkel J.M. Visiting “noncodarnia”. Biotechniques. 2013;54:301, 303–304. doi: 10.2144/000114037. [DOI] [PubMed] [Google Scholar]
- 153.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lin Z., Song J., Gao Y., Huang S., Dou R., Zhong P., Huang G., Han L., Zheng J., Zhang X., et al. Hypoxia-induced HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the cytoplasmic translocation of ELAVL1 in peritoneal dissemination from gastric cancer. Redox Biol. 2022;52:102312. doi: 10.1016/j.redox.2022.102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang B., Bao W., Zhang S., Chen B., Zhou X., Zhao J., Shi Z., Zhang T., Chen Z., Wang L., et al. LncRNA HEPFAL accelerates ferroptosis in hepatocellular carcinoma by regulating SLC7A11 ubiquitination. Cell Death Dis. 2022;13:734. doi: 10.1038/s41419-022-05173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mao C., Wang X., Liu Y., Wang M., Yan B., Jiang Y., Shi Y., Shen Y., Liu X., Lai W., et al. A G3BP1-Interacting lncRNA Promotes Ferroptosis and Apoptosis in Cancer via Nuclear Sequestration of p53. Cancer Res. 2018;78:3484–3496. doi: 10.1158/0008-5472.CAN-17-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dawoud A., Ihab Zakaria Z., Hisham Rashwan H., Braoudaki M., Youness R.A. Circular RNAs: New layer of complexity evading breast cancer heterogeneity. Noncoding RNA Res. 2023;8:60–74. doi: 10.1016/j.ncrna.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kristensen L.S., Andersen M.S., Stagsted L.V.W., Ebbesen K.K., Hansen T.B., Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 159.Nisar S., Bhat A.A., Singh M., Karedath T., Rizwan A., Hashem S., Bagga P., Reddy R., Jamal F., Uddin S., et al. Insights into the Role of CircRNAs: Biogenesis, Characterization, Functional, and Clinical Impact in Human Malignancies. Front. Cell Dev. Biol. 2021;9:617281. doi: 10.3389/fcell.2021.617281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Enuka Y., Lauriola M., Feldman M.E., Sas-Chen A., Ulitsky I., Yarden Y. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–1383. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren S., Lin P., Wang J., Yu H., Lv T., Sun L., Du G. Circular RNAs: Promising Molecular Biomarkers of Human Aging-Related Diseases via Functioning as an miRNA Sponge. Mol. Ther. Methods Clin. Dev. 2020;18:215–229. doi: 10.1016/j.omtm.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wei G., Zhu J., Hu H.B., Liu J.Q. Circular RNAs: Promising biomarkers for cancer diagnosis and prognosis. Gene. 2021;771:145365. doi: 10.1016/j.gene.2020.145365. [DOI] [PubMed] [Google Scholar]
- 163.Anderson A.J., Culver H.R., Prieto T.R., Martinez P.J., Sinha J., Bryant S.J., Bowman C.N. Messenger RNA enrichment using synthetic oligo(T) click nucleic acids. Chem. Commun. 2020;56:13987–13990. doi: 10.1039/D0CC05815G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jie M., Wu Y., Gao M., Li X., Liu C., Ouyang Q., Tang Q., Shan C., Lv Y., Zhang K., et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol. Cancer. 2020;19:56. doi: 10.1186/s12943-020-01160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Prats A.C., David F., Diallo L.H., Roussel E., Tatin F., Garmy-Susini B., Lacazette E. Circular RNA, the Key for Translation. Int. J. Mol. Sci. 2020;21:8591. doi: 10.3390/ijms21228591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Z., Wang Q., Wang X., Xu Z., Wei X., Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6:72. doi: 10.1038/s41420-020-00306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang W., Xu R., Zhao H., Xiong Y., He P. CircEXOC5 promotes ferroptosis by enhancing ACSL4 mRNA stability via binding to PTBP1 in sepsis-induced acute lung injury. Immunobiology. 2022;227:152219. doi: 10.1016/j.imbio.2022.152219. [DOI] [PubMed] [Google Scholar]
- 168.Zhang J., Chen S., Wei S., Cheng S., Shi R., Zhao R., Zhang W., Zhang Q., Hua T., Feng D., et al. CircRAPGEF5 interacts with RBFOX2 to confer ferroptosis resistance by modulating alternative splicing of TFRC in endometrial cancer. Redox Biol. 2022;57:102493. doi: 10.1016/j.redox.2022.102493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang L., Wu S., He H., Ai K., Xu R., Zhang L., Zhu X. CircRNA-ST6GALNAC6 increases the sensitivity of bladder cancer cells to erastin-induced ferroptosis by regulating the HSPB1/P38 axis. Lab. Investig. 2022;102:1323–1334. doi: 10.1038/s41374-022-00826-3. [DOI] [PubMed] [Google Scholar]
- 170.Jiang Y., Zhao J., Li R., Liu Y., Zhou L., Wang C., Lv C., Gao L., Cui D. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J. Exp. Clin. Cancer Res. 2022;41:307. doi: 10.1186/s13046-022-02518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang X., Xu Y., Ma L., Yu K., Niu Y., Xu X., Shi Y., Guo S., Xue X., Wang Y., et al. Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun. 2022;42:287–313. doi: 10.1002/cac2.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Halbach R., Miesen P., Joosten J., Taskopru E., Rondeel I., Pennings B., Vogels C.B.F., Merkling S.H., Koenraadt C.J., Lambrechts L., et al. A satellite repeat-derived piRNA controls embryonic development of Aedes. Nature. 2020;580:274–277. doi: 10.1038/s41586-020-2159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y., Dou M., Song X., Dong Y., Liu S., Liu H., Tao J., Li W., Yin X., Xu W. The emerging role of the piRNA/piwi complex in cancer. Mol. Cancer. 2019;18:123. doi: 10.1186/s12943-019-1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tan L., Mai D., Zhang B., Jiang X., Zhang J., Bai R., Ye Y., Li M., Pan L., Su J., et al. PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA. Mol. Cancer. 2019;18:9. doi: 10.1186/s12943-019-0940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang L., Meng X., Pan C., Qu F., Gan W., Xiang Z., Han X., Li D. piR-31470 epigenetically suppresses the expression of glutathione S-transferase pi 1 in prostate cancer via DNA methylation. Cell Signal. 2020;67:109501. doi: 10.1016/j.cellsig.2019.109501. [DOI] [PubMed] [Google Scholar]
- 176.Tan X., Huang X., Niu B., Guo X., Lei X., Qu B. Targeting GSTP1-dependent ferroptosis in lung cancer radiotherapy: Existing evidence and future directions. Front. Mol. Biosci. 2022;9:1102158. doi: 10.3389/fmolb.2022.1102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sharp S.J., Schaack J., Cooley L., Burke D.J., Soll D. Structure and transcription of eukaryotic tRNA genes. CRC Crit. Rev. Biochem. 1985;19:107–144. doi: 10.3109/10409238509082541. [DOI] [PubMed] [Google Scholar]
- 178.Becker N.P., Martitz J., Renko K., Stoedter M., Hybsier S., Cramer T., Schomburg L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics. 2014;6:1079–1086. doi: 10.1039/C4MT00004H. [DOI] [PubMed] [Google Scholar]
- 179.De Spirt S., Eckers A., Wehrend C., Micoogullari M., Sies H., Stahl W., Steinbrenner H. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic. Biol. Med. 2016;91:164–171. doi: 10.1016/j.freeradbiomed.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 180.Kipp A.P., Frombach J., Deubel S., Brigelius-Flohe R. Selenoprotein W as biomarker for the efficacy of selenium compounds to act as source for selenoprotein biosynthesis. Methods Enzym. 2013;527:87–112. doi: 10.1016/B978-0-12-405882-8.00005-2. [DOI] [PubMed] [Google Scholar]
- 181.Pathak C., Jaiswal Y.K., Vinayak M. Queuine promotes antioxidant defence system by activating cellular antioxidant enzyme activities in cancer. Biosci. Rep. 2008;28:73–81. doi: 10.1042/BSR20070011. [DOI] [PubMed] [Google Scholar]
- 182.Hiramoto K., Satoh H., Suzuki T., Moriguchi T., Pi J., Shimosegawa T., Yamamoto M. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev. Res. 2014;7:835–844. doi: 10.1158/1940-6207.CAPR-14-0094. [DOI] [PubMed] [Google Scholar]
- 183.Aubert M., O’Donohue M.F., Lebaron S., Gleizes P.E. Pre-Ribosomal RNA Processing in Human Cells: From Mechanisms to Congenital Diseases. Biomolecules. 2018;8:123. doi: 10.3390/biom8040123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li W., Thakor N., Xu E.Y., Huang Y., Chen C., Yu R., Holcik M., Kong A.N. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010;38:778–788. doi: 10.1093/nar/gkp1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perez M.J., Gonzalez-Sanchez E., Gonzalez-Loyola A., Gonzalez-Buitrago J.M., Marin J.J. Mitochondrial genome depletion dysregulates bile acid- and paracetamol-induced expression of the transporters Mdr1, Mrp1 and Mrp4 in liver cells. Br. J. Pharmacol. 2011;162:1686–1699. doi: 10.1111/j.1476-5381.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jayaraman S., Chittiboyina S., Bai Y., Abad P.C., Vidi P.A., Stauffacher C.V., Lelievre S.A. The nuclear mitotic apparatus protein NuMA controls rDNA transcription and mediates the nucleolar stress response in a p53-independent manner. Nucleic Acids Res. 2017;45:11725–11742. doi: 10.1093/nar/gkx782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yung B.Y., Yang Y.H., Bor A.M. Nucleolar protein B23 translocation after deferoxamine treatment in a human leukemia cell line. Int. J. Cancer. 1991;48:779–784. doi: 10.1002/ijc.2910480524. [DOI] [PubMed] [Google Scholar]
- 188.Zheng S., Hu L., Song Q., Shan Y., Yin G., Zhu H., Kong W., Zhou C. miR-545 promotes colorectal cancer by inhibiting transferring in the non-normal ferroptosis signaling. Aging. 2021;13:26137–26147. doi: 10.18632/aging.203801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huang J., Deng C., Guo T., Chen X., Chen P., Du S., Lu M. Cinobufotalin Induces Ferroptosis to Suppress Lung Cancer Cell Growth by lncRNA LINC00597/hsa-miR-367-3p/TFRC Pathway via Resibufogenin. Anticancer. Agents Med. Chem. 2023;23:717–725. doi: 10.2174/1871520622666221010092922. [DOI] [PubMed] [Google Scholar]
- 190.Lei D., Li B., Isa Z., Ma X., Zhang B. Hypoxia-elicited cardiac microvascular endothelial cell-derived exosomal miR-210-3p alleviate hypoxia/reoxygenation-induced myocardial cell injury through inhibiting transferrin receptor 1-mediated ferroptosis. Tissue Cell. 2022;79:101956. doi: 10.1016/j.tice.2022.101956. [DOI] [PubMed] [Google Scholar]
- 191.Wu L., Tian X., Zuo H., Zheng W., Li X., Yuan M., Tian X., Song H. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J. Nanobiotechnol. 2022;20:196. doi: 10.1186/s12951-022-01407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Z., Gu Q., Chen R. Promotive role of IRF7 in ferroptosis of colonic epithelial cells in ulcerative colitis by the miR-375-3p/SLC11A2 axis. Biomol. Biomed. 2023;23:437–449. doi: 10.17305/bjbms.2022.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Song Y., Wang B., Zhu X., Hu J., Sun J., Xuan J., Ge Z. Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol. Toxicol. 2021;37:51–64. doi: 10.1007/s10565-020-09530-8. [DOI] [PubMed] [Google Scholar]
- 194.Tomita K., Fukumoto M., Itoh K., Kuwahara Y., Igarashi K., Nagasawa T., Suzuki M., Kurimasa A., Sato T. MiR-7-5p is a key factor that controls radioresistance via intracellular Fe(2+) content in clinically relevant radioresistant cells. Biochem. Biophys. Res. Commun. 2019;518:712–718. doi: 10.1016/j.bbrc.2019.08.117. [DOI] [PubMed] [Google Scholar]
- 195.Wei D., Ke Y.Q., Duan P., Zhou L., Wang C.Y., Cao P. MicroRNA-302a-3p induces ferroptosis of non-small cell lung cancer cells via targeting ferroportin. Free Radic. Res. 2021;55:821–830. doi: 10.1080/10715762.2021.1947503. [DOI] [PubMed] [Google Scholar]
- 196.Zhu C., Song Z., Chen Z., Lin T., Lin H., Xu Z., Ai F., Zheng S. MicroRNA-4735-3p Facilitates Ferroptosis in Clear Cell Renal Cell Carcinoma by Targeting SLC40A1. Anal. Cell. Pathol. 2022;2022:4213401. doi: 10.1155/2022/4213401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu P., Ge F.H., Li W.X., Xu Z., Wang X.L., Shen J.L., Xu A.B., Hao R.R. MicroRNA-147a Targets SLC40A1 to Induce Ferroptosis in Human Glioblastoma. Anal. Cell. Pathol. 2022;2022:2843990. doi: 10.1155/2022/2843990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zheng R., Lin C., Mao Y., Jin F. miR-761-hepcidin/Gpx4 pathway contribute to unexplained liver dysfunction in polycystic ovary syndrome by regulating liver iron overload and ferroptosis. Gynecol. Endocrinol. 2023;39:2166483. doi: 10.1080/09513590.2023.2166483. [DOI] [PubMed] [Google Scholar]
- 199.Xiang L., Zeng Q., Liu J., Xiao M., He D., Zhang Q., Xie D., Deng M., Zhu Y., Liu Y., et al. MAFG-AS1/MAFG positive feedback loop contributes to cisplatin resistance in bladder urothelial carcinoma through antagonistic ferroptosis. Sci. Bull. 2021;66:1773–1788. doi: 10.1016/j.scib.2021.01.027. [DOI] [PubMed] [Google Scholar]
- 200.Luo W., Wang J., Xu W., Ma C., Wan F., Huang Y., Yao M., Zhang H., Qu Y., Ye D., et al. LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis. 2021;12:1043. doi: 10.1038/s41419-021-04296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li X., Si W., Li Z., Tian Y., Liu X., Ye S., Huang Z., Ji Y., Zhao C., Hao X., et al. miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int. J. Mol. Med. 2021;47:61. doi: 10.3892/ijmm.2021.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zheng H., Shi L., Tong C., Liu Y., Hou M. circSnx12 Is Involved in Ferroptosis During Heart Failure by Targeting miR-224-5p. Front. Cardiovasc. Med. 2021;8:656093. doi: 10.3389/fcvm.2021.656093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang R., Pan T., Xiang Y., Zhang M., Xie H., Liang Z., Chen B., Xu C., Wang J., Huang X., et al. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater. 2022;13:23–36. doi: 10.1016/j.bioactmat.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gong H., Gao M., Lin Y., Liu J., Hu Z., Liu J. TUG1/MAZ/FTH1 Axis Attenuates the Antiglioma Effect of Dihydroartemisinin by Inhibiting Ferroptosis. Oxid. Med. Cell Longev. 2022;2022:7843863. doi: 10.1155/2022/7843863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jiang J., Gao H., Zhou W., Cai H., Liao L., Wang C. Circular RNA HIPK3 facilitates ferroptosis in gestational diabetes mellitus by regulating glutathione peroxidase 4 DNA methylation. J. Gene Med. 2023:e3526. doi: 10.1002/jgm.3526. [DOI] [PubMed] [Google Scholar]
- 206.Qiu X., Shi Q., Zhang X., Shi X., Jiang H., Qin S. LncRNA A2M-AS1 Promotes Ferroptosis in Pancreatic Cancer via Interacting with PCBP3. Mol. Cancer Res. 2022;20:1636–1645. doi: 10.1158/1541-7786.MCR-22-0024. [DOI] [PubMed] [Google Scholar]
- 207.Fan H., Ai R., Mu S., Niu X., Guo Z., Liu L. MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2. Bioengineered. 2022;13:12021–12029. doi: 10.1080/21655979.2022.2054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xia H., Wu Y., Zhao J., Cheng C., Lin J., Yang Y., Lu L., Xiang Q., Bian T., Liu Q. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023;30:1293–1304. doi: 10.1038/s41418-023-01138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xi Y., Shen Y., Wu D., Zhang J., Lin C., Wang L., Yu C., Yu B., Shen W. CircBCAR3 accelerates esophageal cancer tumorigenesis and metastasis via sponging miR-27a-3p. Mol. Cancer. 2022;21:145. doi: 10.1186/s12943-022-01615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li Z., Jiang L., Chew S.H., Hirayama T., Sekido Y., Toyokuni S. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol. 2019;26:101297. doi: 10.1016/j.redox.2019.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiang X., Guo S., Zhang Y., Zhao Y., Li X., Jia Y., Xu Y., Ma B. LncRNA NEAT1 promotes docetaxel resistance in prostate cancer by regulating ACSL4 via sponging miR-34a-5p and miR-204-5p. Cell Signal. 2020;65:109422. doi: 10.1016/j.cellsig.2019.109422. [DOI] [PubMed] [Google Scholar]
- 212.Wu H., Liu A. Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J. Int. Med. Res. 2021;49:300060521996183. doi: 10.1177/0300060521996183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bao C., Zhang J., Xian S.Y., Chen F. MicroRNA-670-3p suppresses ferroptosis of human glioblastoma cells through targeting ACSL4. Free Radic. Res. 2021;55:853–864. doi: 10.1080/10715762.2021.1962009. [DOI] [PubMed] [Google Scholar]
- 214.Lu Y., Chan Y.T., Tan H.Y., Zhang C., Guo W., Xu Y., Sharma R., Chen Z.S., Zheng Y.C., Wang N., et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022;41:3. doi: 10.1186/s13046-021-02208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu B., Ma H., Liu X., Xing W. CircSCN8A suppresses malignant progression and induces ferroptosis in non-small cell lung cancer by regulating miR-1290/ACSL4 axis. Cell Cycle. 2023;22:758–776. doi: 10.1080/15384101.2022.2154543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mao R., Liu H. Depletion of mmu_circ_0001751 (circular RNA Carm1) protects against acute cerebral infarction injuries by binding with microRNA-3098-3p to regulate acyl-CoA synthetase long-chain family member 4. Bioengineered. 2022;13:4063–4075. doi: 10.1080/21655979.2022.2032971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shi L., Song Z., Li Y., Huang J., Zhao F., Luo Y., Wang J., Deng F., Shadekejiang H., Zhang M., et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am. J. Transpl. 2023;23:11–25. doi: 10.1016/j.ajt.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 218.Chen H., Ren L., Ma W. Mechanism of SOX10 in ferroptosis of hippocampal neurons after intracerebral hemorrhage via the miR-29a-3p/ACSL4 axis. J. Neurophysiol. 2023;129:862–871. doi: 10.1152/jn.00374.2022. [DOI] [PubMed] [Google Scholar]
- 219.Kannan M., Sil S., Oladapo A., Thangaraj A., Periyasamy P., Buch S. HIV-1 Tat-mediated microglial ferroptosis involves the miR-204-ACSL4 signaling axis. Redox Biol. 2023;62:102689. doi: 10.1016/j.redox.2023.102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Qi R., Bai Y., Li K., Liu N., Xu Y., Dal E., Wang Y., Lin R., Wang H., Liu Z., et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist. Updat. 2023;68:100960. doi: 10.1016/j.drup.2023.100960. [DOI] [PubMed] [Google Scholar]
- 221.Xiao F.J., Zhang D., Wu Y., Jia Q.H., Zhang L., Li Y.X., Yang Y.F., Wang H., Wu C.T., Wang L.S. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem. Biophys. Res. Commun. 2019;515:448–454. doi: 10.1016/j.bbrc.2019.05.147. [DOI] [PubMed] [Google Scholar]
- 222.Sun Z., Wu J., Bi Q., Wang W. Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res. Ther. 2022;13:297. doi: 10.1186/s13287-022-02986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang H., Hu Y., Weng M., Liu X., Wan P., Hu Y., Ma M., Zhang Y., Xia H., Lv K. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res. 2022;37:91–106. doi: 10.1016/j.jare.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tomita K., Nagasawa T., Kuwahara Y., Torii S., Igarashi K., Roudkenar M.H., Roushandeh A.M., Kurimasa A., Sato T. MiR-7-5p Is Involved in Ferroptosis Signaling and Radioresistance Thru the Generation of ROS in Radioresistant HeLa and SAS Cell Lines. Int. J. Mol. Sci. 2021;22:8300. doi: 10.3390/ijms22158300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang H., Deng T., Liu R., Ning T., Yang H., Liu D., Zhang Q., Lin D., Ge S., Bai M., et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer. 2020;19:43. doi: 10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yang X., Liu J., Wang C., Cheng K.K., Xu H., Li Q., Hua T., Jiang X., Sheng L., Mao J., et al. miR-18a promotes glioblastoma development by down-regulating ALOXE3-mediated ferroptotic and anti-migration activities. Oncogenesis. 2021;10:15. doi: 10.1038/s41389-021-00304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yi T.T., Zhang L.M., Huang X.N. Glycyrrhizic acid protects against temporal lobe epilepsy in young rats by regulating neuronal ferroptosis through the miR-194-5p/PTGS2 axis. Kaohsiung J. Med. Sci. 2023;39:154–165. doi: 10.1002/kjm2.12642. [DOI] [PubMed] [Google Scholar]
- 228.Liu C., Lu J., Yuan T., Xie L., Zhang L. EPC-exosomal miR-26a-5p improves airway remodeling in COPD by inhibiting ferroptosis of bronchial epithelial cells via PTGS2/PGE2 signaling pathway. Sci. Rep. 2023;13:6126. doi: 10.1038/s41598-023-33151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luo Y., Huang S., Wei J., Zhou H., Wang W., Yang J., Deng Q., Wang H., Fu Z. Long noncoding RNA LINC01606 protects colon cancer cells from ferroptotic cell death and promotes stemness by SCD1-Wnt/beta-catenin-TFE3 feedback loop signalling. Clin. Transl. Med. 2022;12:e752. doi: 10.1002/ctm2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Long F., Lin Z., Long Q., Lu Z., Zhu K., Zhao M., Yang M. CircZBTB46 Protects Acute Myeloid Leukemia Cells from Ferroptotic Cell Death by Upregulating SCD. Cancers. 2023;15:459. doi: 10.3390/cancers15020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang H., Wang M., He Y., Deng T., Liu R., Wang W., Zhu K., Bai M., Ning T., Yang H., et al. Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis. 2021;12:1116. doi: 10.1038/s41419-021-04406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xu W., Ding J., Li B., Sun T., You X., He Q., Sheng W. Effects of icariin and curcumol on autophagy, ferroptosis, and lipid metabolism based on miR-7/m-TOR/SREBP1 pathway on prostate cancer. BioFactors. 2023;49:438–456. doi: 10.1002/biof.1927. [DOI] [PubMed] [Google Scholar]
- 233.Wu P., Li C., Ye D.M., Yu K., Li Y., Tang H., Xu G., Yi S., Zhang Z. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging. 2021;13:4663–4673. doi: 10.18632/aging.202518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liu J., Yang H., Deng J., Jiang R., Meng E., Wu H. CircRPPH1 promotes the stemness of gastric cancer cells by targeting miR-375/SLC7A11 axis. Environ. Toxicol. 2023;38:115–125. doi: 10.1002/tox.23668. [DOI] [PubMed] [Google Scholar]
- 235.Mao S.H., Zhu C.H., Nie Y., Yu J., Wang L. Levobupivacaine Induces Ferroptosis by miR-489-3p/SLC7A11 Signaling in Gastric Cancer. Front. Pharmacol. 2021;12:681338. doi: 10.3389/fphar.2021.681338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lyu N., Zeng Y., Kong Y., Chen Q., Deng H., Ou S., Bai Y., Tang H., Wang X., Zhao M. Ferroptosis is involved in the progression of hepatocellular carcinoma through the circ0097009/miR-1261/SLC7A11 axis. Ann. Transl. Med. 2021;9:675. doi: 10.21037/atm-21-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang H.H., Ma J.N., Zhan X.R. Circular RNA Circ_0067934 Attenuates Ferroptosis of Thyroid Cancer Cells by miR-545-3p/SLC7A11 Signaling. Front Endocrinol. 2021;12:670031. doi: 10.3389/fendo.2021.670031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yadav P., Sharma P., Sundaram S., Venkatraman G., Bera A.K., Karunagaran D. SLC7A11/ xCT is a target of miR-5096 and its restoration partially rescues miR-5096-mediated ferroptosis and anti-tumor effects in human breast cancer cells. Cancer Lett. 2021;522:211–224. doi: 10.1016/j.canlet.2021.09.033. [DOI] [PubMed] [Google Scholar]
- 239.Yang J., Cao X.H., Luan K.F., Huang Y.D. Circular RNA FNDC3B Protects Oral Squamous Cell Carcinoma Cells from Ferroptosis and Contributes to the Malignant Progression by Regulating miR-520d-5p/SLC7A11 Axis. Front. Oncol. 2021;11:672724. doi: 10.3389/fonc.2021.672724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sun K., Ren W., Li S., Zheng J., Huang Y., Zhi K., Gao L. MiR-34c-3p upregulates erastin-induced ferroptosis to inhibit proliferation in oral squamous cell carcinomas by targeting SLC7A11. Pathol. Res. Pr. 2022;231:153778. doi: 10.1016/j.prp.2022.153778. [DOI] [PubMed] [Google Scholar]
- 241.Yu Y., MohamedAl-Sharani H., Zhang B. EZH2-mediated SLC7A11 upregulation via miR-125b-5p represses ferroptosis of TSCC. Oral. Dis. 2023;29:880–891. doi: 10.1111/odi.14040. [DOI] [PubMed] [Google Scholar]
- 242.Xu F., Ji S., Yang L., Li Y., Shen P. Potential upstream lncRNA-miRNA-mRNA regulatory network of the ferroptosis-related gene SLC7A11 in renal cell carcinoma. Transl. Androl. Urol. 2023;12:33–57. doi: 10.21037/tau-22-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li Y.Z., Zhu H.C., Du Y., Zhao H.C., Wang L. Silencing lncRNA SLC16A1-AS1 Induced Ferroptosis in Renal Cell Carcinoma Through miR-143-3p/SLC7A11 Signaling. Technol. Cancer Res. Treat. 2022;21:15330338221077803. doi: 10.1177/15330338221077803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Qin K., Zhang F., Wang H., Wang N., Qiu H., Jia X., Gong S., Zhang Z. circRNA circSnx12 confers Cisplatin chemoresistance to ovarian cancer by inhibiting ferroptosis through a miR-194-5p/SLC7A11 axis. BMB Rep. 2023;56:184–189. doi: 10.5483/BMBRep.2022-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Cai L., Hu X., Ye L., Bai P., Jie Y., Shu K. Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by Targeting microRNA-587/solute carrier family 7 member 11 axis in epithelial ovarian cancer. Bioengineered. 2022;13:8226–8239. doi: 10.1080/21655979.2022.2049470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang W., Xie Y., Malhotra A. Potential of Curcumin and Quercetin in Modulation of Premature Mitochondrial Senescence and Related Changes during Lung Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2021;40:53–60. doi: 10.1615/JEnvironPatholToxicolOncol.2021039371. [DOI] [PubMed] [Google Scholar]
- 247.Bi G., Liang J., Zhao M., Zhang H., Jin X., Lu T., Zheng Y., Bian Y., Chen Z., Huang Y., et al. miR-6077 promotes cisplatin/pemetrexed resistance in lung adenocarcinoma via CDKN1A/cell cycle arrest and KEAP1/ferroptosis pathways. Mol. Ther. Nucleic Acids. 2022;28:366–386. doi: 10.1016/j.omtn.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pan C.F., Wei K., Ma Z.J., He Y.Z., Huang J.J., Guo Z.Z., Chen Z.P., Barr M.P., Shackelford R.E., Xia Y., et al. CircP4HB regulates ferroptosis via SLC7A11-mediated glutathione synthesis in lung adenocarcinoma. Transl. Lung Cancer Res. 2022;11:366–380. doi: 10.21037/tlcr-22-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao Y., Cui Q., Shen J., Shen W., Weng Y. Hsa_circ_0070440 promotes lung adenocarcinoma progression by SLC7A11-mediated-ferroptosis. Histol. Histopathol. 2023:18597. doi: 10.14670/hh-18-597. [DOI] [PubMed] [Google Scholar]
- 250.Jiang X., Guo S., Xu M., Ma B., Liu R., Xu Y., Zhang Y. TFAP2C-Mediated lncRNA PCAT1 Inhibits Ferroptosis in Docetaxel-Resistant Prostate Cancer Through c-Myc/miR-25-3p/SLC7A11 Signaling. Front. Oncol. 2022;12:862015. doi: 10.3389/fonc.2022.862015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pan C., Chen G., Zhao X., Xu X., Liu J. lncRNA BBOX1-AS1 silencing inhibits esophageal squamous cell cancer progression by promoting ferroptosis via miR-513a-3p/SLC7A11 axis. Eur. J. Pharmacol. 2022;934:175317. doi: 10.1016/j.ejphar.2022.175317. [DOI] [PubMed] [Google Scholar]
- 252.Li L., Zhang Y., Gao Y., Hu Y., Wang R., Wang S., Li Y., He Y., Yuan C. LncSNHG14 promotes nutlin3a resistance by inhibiting ferroptosis via the miR-206 /SLC7A11 axis in osteosarcoma cells. Cancer Gene Ther. 2023;30:704–715. doi: 10.1038/s41417-022-00581-z. [DOI] [PubMed] [Google Scholar]
- 253.Li Q., Li K., Guo Q., Yang T. CircRNA circSTIL inhibits ferroptosis in colorectal cancer via miR-431/SLC7A11 axis. Environ. Toxicol. 2023;38:981–989. doi: 10.1002/tox.23670. [DOI] [PubMed] [Google Scholar]
- 254.Ding C., Ding X., Zheng J., Wang B., Li Y., Xiang H., Dou M., Qiao Y., Tian P., Xue W. miR-182-5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell Death Dis. 2020;11:929. doi: 10.1038/s41419-020-03135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhu L., Feng Z., Zhang J., Du L., Meng A. MicroRNA-27a Regulates Ferroptosis Through SLC7A11 to Aggravate Cerebral ischemia-reperfusion Injury. Neurochem. Res. 2023;48:1370–1381. doi: 10.1007/s11064-022-03826-3. [DOI] [PubMed] [Google Scholar]
- 256.Ye J., Lyu T.J., Li L.Y., Liu Y., Zhang H., Wang X., Xi X., Liu Z.J., Gao J.Q. Ginsenoside Re attenuates myocardial ischemia/reperfusion induced ferroptosis via miR-144-3p/SLC7A11. Phytomedicine. 2023;113:154681. doi: 10.1016/j.phymed.2023.154681. [DOI] [PubMed] [Google Scholar]
- 257.Li H., Ding J., Liu W., Wang X., Feng Y., Guan H., Chen Z. Plasma exosomes from patients with acute myocardial infarction alleviate myocardial injury by inhibiting ferroptosis through miR-26b-5p/SLC7A11 axis. Life Sci. 2023;322:121649. doi: 10.1016/j.lfs.2023.121649. [DOI] [PubMed] [Google Scholar]
- 258.Zhang K., Gu X., Xia Y., Zhao X., Khoso Pervez A., Li S. MiR-129-3p regulates ferroptosis in the liver of Selenium-deficient broilers by targeting SLC7A11. Poult. Sci. 2023;102:102271. doi: 10.1016/j.psj.2022.102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chen Y., Deng Y., Chen L., Huang Z., Yan Y., Huang Z. miR-16-5p Regulates Ferroptosis by Targeting SLC7A11 in Adriamycin-Induced Ferroptosis in Cardiomyocytes. J. Inflamm. Res. 2023;16:1077–1089. doi: 10.2147/JIR.S393646. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 260.Liu D., Yang M., Yao Y., He S., Wang Y., Cao Z., Chen H., Fu Y., Liu H., Zhao Q. Cardiac Fibroblasts Promote Ferroptosis in Atrial Fibrillation by Secreting Exo-miR-23a-3p Targeting SLC7A11. Oxid. Med. Cell Longev. 2022;2022:3961495. doi: 10.1155/2022/3961495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fan J., Han Y., Sun H., Sun S., Wang Y., Guo R., Guo J., Tian X., Wang J., Wang J. Mesenchymal stem cell-derived exosomal microRNA-367-3p alleviates experimental autoimmune encephalomyelitis via inhibition of microglial ferroptosis by targeting EZH2. Biomed. Pharmacother. 2023;162:114593. doi: 10.1016/j.biopha.2023.114593. [DOI] [PubMed] [Google Scholar]
- 262.Li H., Wei Y., Wang J., Yao J., Zhang C., Yu C., Tang Y., Zhu D., Yang J., Zhou J. Long Noncoding RNA LINC00578 Inhibits Ferroptosis in Pancreatic Cancer via Regulating SLC7A11 Ubiquitination. Oxid. Med. Cell Longev. 2023;2023:1744102. doi: 10.1155/2023/1744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wang S., Wang Y., Li Q., Li X., Feng X. A novel circular RNA confers trastuzumab resistance in human epidermal growth factor receptor 2-positive breast cancer through regulating ferroptosis. Environ. Toxicol. 2022;37:1597–1607. doi: 10.1002/tox.23509. [DOI] [PubMed] [Google Scholar]
- 264.An L., Huang J., Ge S., Zhang X., Wang J. lncRNA AGAP2-AS1 Facilitates Tumorigenesis and Ferroptosis Resistance through SLC7A11 by IGF2BP2 Pathway in Melanoma. Comput. Math. Methods Med. 2022;2022:1972516. doi: 10.1155/2022/1972516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 265.Yuan J., Liu Z., Song R. Antisense lncRNA As-SLC7A11 suppresses epithelial ovarian cancer progression mainly by targeting SLC7A11. Pharmazie. 2017;72:402–407. doi: 10.1691/ph.2017.7449. [DOI] [PubMed] [Google Scholar]
- 266.Wang Z., Chen X., Liu N., Shi Y., Liu Y., Ouyang L., Tam S., Xiao D., Liu S., Wen F., et al. A Nuclear Long Non-Coding RNA LINC00618 Accelerates Ferroptosis in a Manner Dependent upon Apoptosis. Mol. Ther. 2021;29:263–274. doi: 10.1016/j.ymthe.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.He G.N., Bao N.R., Wang S., Xi M., Zhang T.H., Chen F.S. Ketamine Induces Ferroptosis of Liver Cancer Cells by Targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Devel Ther. 2021;15:3965–3978. doi: 10.2147/DDDT.S332847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chen W., Fu J., Chen Y., Li Y., Ning L., Huang D., Yan S., Zhang Q. Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging. 2021;13:16500–16512. doi: 10.18632/aging.203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Han B., Liu Y., Zhang Q., Liang L. Propofol decreases cisplatin resistance of non-small cell lung cancer by inducing GPX4-mediated ferroptosis through the miR-744-5p/miR-615-3p axis. J. Proteom. 2023;274:104777. doi: 10.1016/j.jprot.2022.104777. [DOI] [PubMed] [Google Scholar]
- 270.Wang S., Ma H., Fang J., Yu Y., Ren Y., Yu R. CircDTL Functions as an Oncogene and Regulates Both Apoptosis and Ferroptosis in Non-small Cell Lung Cancer Cells. Front. Genet. 2021;12:743505. doi: 10.3389/fgene.2021.743505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Xu Z., Chen L., Wang C., Zhang L., Xu W. MicroRNA-1287-5p promotes ferroptosis of osteosarcoma cells through inhibiting GPX4. Free Radic. Res. 2021;55:1119–1129. doi: 10.1080/10715762.2021.2024816. [DOI] [PubMed] [Google Scholar]
- 272.Xu P., Wang Y., Deng Z., Tan Z., Pei X. MicroRNA-15a promotes prostate cancer cell ferroptosis by inhibiting GPX4 expression. Oncol. Lett. 2022;23:67. doi: 10.3892/ol.2022.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Liu Y., Li L., Yang Z., Wen D., Hu Z. Circular RNA circACAP2 Suppresses Ferroptosis of Cervical Cancer during Malignant Progression by miR-193a-5p/GPX4. J. Oncol. 2022;2022:5228874. doi: 10.1155/2022/5228874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lei S., Cao W., Zeng Z., Zhang Z., Jin B., Tian Q., Wu Y., Zhang T., Li D., Hu C., et al. JUND/linc00976 promotes cholangiocarcinoma progression and metastasis, inhibits ferroptosis by regulating the miR-3202/GPX4 axis. Cell Death Dis. 2022;13:967. doi: 10.1038/s41419-022-05412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sun W., Shi R., Guo J., Wang H., Shen L., Shi H., Yu P., Chen X. miR-135b-3p Promotes Cardiomyocyte Ferroptosis by Targeting GPX4 and Aggravates Myocardial Ischemia/Reperfusion Injury. Front. Cardiovasc. Med. 2021;8:663832. doi: 10.3389/fcvm.2021.663832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li G., Jin J., Liu S., Ding K., Qian C. Inhibition of miR-1224 suppresses hypoxia/reoxygenation-induced oxidative stress and apoptosis in cardiomyocytes through targeting GPX4. Exp. Mol. Pathol. 2021;121:104645. doi: 10.1016/j.yexmp.2021.104645. [DOI] [PubMed] [Google Scholar]
- 277.Fan K., Huang W., Qi H., Song C., He C., Liu Y., Zhang Q., Wang L., Sun H. The Egr-1/miR-15a-5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur. J. Pharmacol. 2021;909:174403. doi: 10.1016/j.ejphar.2021.174403. [DOI] [PubMed] [Google Scholar]
- 278.Kong Y., Li S., Zhang M., Xu W., Chen Q., Zheng L., Liu P., Zou W. Acupuncture Ameliorates Neuronal Cell Death, Inflammation, and Ferroptosis and Downregulated miR-23a-3p After Intracerebral Hemorrhage in Rats. J. Mol. Neurosci. 2021;71:1863–1875. doi: 10.1007/s12031-020-01770-x. [DOI] [PubMed] [Google Scholar]
- 279.Jin J., Wang Y., Zheng D., Liang M., He Q. A Novel Identified Circular RNA, mmu_mmu_circRNA_0000309, Involves in Germacrone-Mediated Improvement of Diabetic Nephropathy Through Regulating Ferroptosis by Targeting miR-188-3p/GPX4 Signaling Axis. Antioxid. Redox Signal. 2022;36:740–759. doi: 10.1089/ars.2021.0063. [DOI] [PubMed] [Google Scholar]
- 280.Guo Y., Bie Z.D., Li X. Hypoxic cardiomyocyte-derived exosomes regulate cardiac fibroblast activation, apoptosis, migration and ferroptosis through miR-208a/b. Gen. Physiol. Biophys. 2023;42:149–158. doi: 10.4149/gpb_2022061. [DOI] [PubMed] [Google Scholar]
- 281.Zha J., Pan Y., Liu X., Zhu H., Liu Y., Zeng W. Exosomes from hypoxia-pretreated adipose-derived stem cells attenuate ultraviolet light-induced skin injury via delivery of circ-Ash1l. Photodermatol. Photoimmunol. Photomed. 2023;39:107–115. doi: 10.1111/phpp.12857. [DOI] [PubMed] [Google Scholar]
- 282.Zhai H., Zhong S., Wu R., Mo Z., Zheng S., Xue J., Meng H., Liu M., Chen X., Zhang G., et al. Suppressing circIDE/miR-19b-3p/RBMS1 axis exhibits promoting-tumour activity through upregulating GPX4 to diminish ferroptosis in hepatocellular carcinoma. Epigenetics. 2023;18:2192438. doi: 10.1080/15592294.2023.2192438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kang X., Huo Y., Jia S., He F., Li H., Zhou Q., Chang N., Liu D., Li R., Hu Y., et al. Silenced LINC01134 Enhances Oxaliplatin Sensitivity by Facilitating Ferroptosis Through GPX4 in Hepatocarcinoma. Front. Oncol. 2022;12:939605. doi: 10.3389/fonc.2022.939605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gomaa A., Peng D., Chen Z., Soutto M., Abouelezz K., Corvalan A., El-Rifai W. Epigenetic regulation of AURKA by miR-4715-3p in upper gastrointestinal cancers. Sci. Rep. 2019;9:16970. doi: 10.1038/s41598-019-53174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang M., Mao C., Ouyang L., Liu Y., Lai W., Liu N., Shi Y., Chen L., Xiao D., Yu F., et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wu N., Zhu D., Li J., Li X., Zhu Z., Rao Q., Hu B., Wang H., Zhu Y. CircOMA1 modulates cabergoline resistance by downregulating ferroptosis in prolactinoma. J. Endocrinol. Investig. 2023;46:1573–1587. doi: 10.1007/s40618-023-02010-w. [DOI] [PubMed] [Google Scholar]
- 287.Wang Y., Niu H., Li L., Han J., Liu Z., Chu M., Sha X., Zhao J. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnology. 2023;21:109. doi: 10.1186/s12951-023-01862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Deng Y., Lai W., Yu L., Zhang W., Ding Y. miR-2115-3p inhibits ferroptosis by downregulating the expression of glutamic-oxaloacetic transaminase in preeclampsia. Placenta. 2022;129:94–103. doi: 10.1016/j.placenta.2022.09.014. [DOI] [PubMed] [Google Scholar]
- 289.Zhang K., Wu L., Zhang P., Luo M., Du J., Gao T., O’Connell D., Wang G., Wang H., Yang Y. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol. Carcinog. 2018;57:1566–1576. doi: 10.1002/mc.22878. [DOI] [PubMed] [Google Scholar]
- 290.Bazhabayi M., Qiu X., Li X., Yang A., Wen W., Zhang X., Xiao X., He R., Liu P. CircGFRA1 facilitates the malignant progression of HER-2-positive breast cancer via acting as a sponge of miR-1228 and enhancing AIFM2 expression. J. Cell Mol. Med. 2021;25:10248–10256. doi: 10.1111/jcmm.16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shao C., Chen Y., Yang T., Zhao H., Li D. Mesenchymal Stem Cell Derived Exosomes Suppress Neuronal Cell Ferroptosis Via lncGm36569/miR-5627-5p/FSP1 Axis in Acute Spinal Cord Injury. Stem Cell Rev. Rep. 2022;18:1127–1142. doi: 10.1007/s12015-022-10327-x. [DOI] [PubMed] [Google Scholar]
- 292.Wang F., Li J., Zhao Y., Guo D., Liu D., Chang S., Qiao H., Li J., Yang Y., Zhang C., et al. miR-672-3p Promotes Functional Recovery in Rats with Contusive Spinal Cord Injury by Inhibiting Ferroptosis Suppressor Protein 1. Oxid. Med. Cell Longev. 2022;2022:6041612. doi: 10.1155/2022/6041612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Song Z., Jia G., Ma P., Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276:119399. doi: 10.1016/j.lfs.2021.119399. [DOI] [PubMed] [Google Scholar]
- 294.Zhang Y., Luo M., Cui X., O’Connell D., Yang Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022;29:1850–1863. doi: 10.1038/s41418-022-00970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Liu F., Jiang L.J., Zhang Y.X., Xu S.T., Liu S.L., Ye J.T., Liu P.Q. Inhibition of miR-214-3p attenuates ferroptosis in myocardial infarction via regulating ME2. Biochem. Biophys. Res. Commun. 2023;661:64–74. doi: 10.1016/j.bbrc.2023.04.031. [DOI] [PubMed] [Google Scholar]
- 296.Qu X., Liu B., Wang L., Liu L., Zhao W., Liu C., Ding J., Zhao S., Xu B., Yu H., et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist. Updat. 2023;68:100936. doi: 10.1016/j.drup.2023.100936. [DOI] [PubMed] [Google Scholar]
- 297.Gai C., Liu C., Wu X., Yu M., Zheng J., Zhang W., Lv S., Li W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020;11:751. doi: 10.1038/s41419-020-02939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shen K., Wang X., Wang Y., Jia Y., Zhang Y., Wang K., Luo L., Cai W., Li J., Li S., et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023;62:102655. doi: 10.1016/j.redox.2023.102655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Han Y., Gao X., Wu N., Jin Y., Zhou H., Wang W., Liu H., Chu Y., Cao J., Jiang M., et al. Long noncoding RNA LINC00239 inhibits ferroptosis in colorectal cancer by binding to Keap1 to stabilize Nrf2. Cell Death Dis. 2022;13:742. doi: 10.1038/s41419-022-05192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liao Y., Jia X., Ren Y., Deji Z., Gesang Y., Ning N., Feng H., Yu H., Wei A. Suppressive role of microRNA-130b-3p in ferroptosis in melanoma cells correlates with DKK1 inhibition and Nrf2-HO-1 pathway activation. Hum. Cell. 2021;34:1532–1544. doi: 10.1007/s13577-021-00557-5. [DOI] [PubMed] [Google Scholar]
- 301.Dong L.H., Huang J.J., Zu P., Liu J., Gao X., Du J.W., Li Y.F. CircKDM4C upregulates P53 by sponging hsa-let-7b-5p to induce ferroptosis in acute myeloid leukemia. Environ. Toxicol. 2021;36:1288–1302. doi: 10.1002/tox.23126. [DOI] [PubMed] [Google Scholar]
- 302.Zhu J., Liu S., Ye F., Shen Y., Tie Y., Zhu J., Wei L., Jin Y., Fu H., Wu Y., et al. Long Noncoding RNA MEG3 Interacts with p53 Protein and Regulates Partial p53 Target Genes in Hepatoma Cells. PLoS ONE. 2015;10:e0139790. doi: 10.1371/journal.pone.0139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhou Y., Zhong Y., Wang Y., Zhang X., Batista D.L., Gejman R., Ansell P.J., Zhao J., Weng C., Klibanski A. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 304.Chen C., Huang Y., Xia P., Zhang F., Li L., Wang E., Guo Q., Ye Z. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur. J. Histochem. 2021;65:3224. doi: 10.4081/ejh.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fu H., Zhang Z., Li D., Lv Q., Chen S., Zhang Z., Wu M. LncRNA PELATON, a Ferroptosis Suppressor and Prognositic Signature for GBM. Front. Oncol. 2022;12:817737. doi: 10.3389/fonc.2022.817737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shao C.J., Zhou H.L., Gao X.Z., Xu C.F. Downregulation of miR-221-3p promotes the ferroptosis in gastric cancer cells via upregulation of ATF3 to mediate the transcription inhibition of GPX4 and HRD1. Transl. Oncol. 2023;32:101649. doi: 10.1016/j.tranon.2023.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhao J., Yang S., Lv C., Liu Y. Cancer-associated fibroblasts suppressed ferroptosis in glioblastoma via upregulating lncRNA DLEU1. Am. J. Physiol. Cell Physiol. 2023;324:C1039–C1052. doi: 10.1152/ajpcell.00454.2022. [DOI] [PubMed] [Google Scholar]
- 308.Guan L., Wang F., Wang M., Han S., Cui Z., Xi S., Xu H., Li S. Downregulation of HULC Induces Ferroptosis in Hepatocellular Carcinoma via Targeting of the miR-3200-5p/ATF4 Axis. Oxid. Med. Cell Longev. 2022;2022:9613095. doi: 10.1155/2022/9613095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bai T., Liang R., Zhu R., Wang W., Zhou L., Sun Y. MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J. Cell Physiol. 2020;235:5637–5648. doi: 10.1002/jcp.29496. [DOI] [PubMed] [Google Scholar]
- 310.Zhang H., Ge Z., Wang Z., Gao Y., Wang Y., Qu X. Circular RNA RHOT1 promotes progression and inhibits ferroptosis via mir-106a-5p/STAT3 axis in breast cancer. Aging. 2021;13:8115–8126. doi: 10.18632/aging.202608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liu Y.P., Qiu Z.Z., Li X.H., Li E.Y. Propofol induces ferroptosis and inhibits malignant phenotypes of gastric cancer cells by regulating miR-125b-5p/STAT3 axis. World J. Gastrointest. Oncol. 2021;13:2114–2128. doi: 10.4251/wjgo.v13.i12.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Gao X., Wang X.L. Dexmedetomidine promotes ferroptotic cell death in gastric cancer via hsa_circ_0008035/miR-302a/E2F7 axis. Kaohsiung J. Med. Sci. 2023;39:390–403. doi: 10.1002/kjm2.12650. [DOI] [PubMed] [Google Scholar]
- 313.Li Q., Meng X., Hua Q. Circ ASAP2 decreased inflammation and ferroptosis in diabetic nephropathy through SOX2/SLC7A11 by miR-770-5p. Acta Diabetol. 2023;60:29–42. doi: 10.1007/s00592-022-01961-5. [DOI] [PubMed] [Google Scholar]
- 314.Zhang H.Y., Zhang B.W., Zhang Z.B., Deng Q.J. Circular RNA TTBK2 regulates cell proliferation, invasion and ferroptosis via miR-761/ITGB8 axis in glioma. Eur. Rev. Med. Pharmacol. Sci. 2020;24:2585–2600. doi: 10.26355/eurrev_202003_20528. [DOI] [PubMed] [Google Scholar]
- 315.Wang Y., Chen H., Wei X. Circ_0007142 downregulates miR-874-3p-mediated GDPD5 on colorectal cancer cells. Eur. J. Clin. Investig. 2021;51:e13541. doi: 10.1111/eci.13541. [DOI] [PubMed] [Google Scholar]
- 316.Ma Q., Dai X., Lu W., Qu X., Liu N., Zhu C. Silencing long non-coding RNA MEG8 inhibits the proliferation and induces the ferroptosis of hemangioma endothelial cells by regulating miR-497-5p/NOTCH2 axis. Biochem. Biophys. Res. Commun. 2021;556:72–78. doi: 10.1016/j.bbrc.2021.03.132. [DOI] [PubMed] [Google Scholar]
- 317.Yang X., Li Y., Zhang Y., Liu J. Circ_0000745 promotes acute lymphoblastic leukemia progression through mediating miR-494-3p/NET1 axis. Hematology. 2022;27:11–22. doi: 10.1080/16078454.2021.2008590. [DOI] [PubMed] [Google Scholar]
- 318.Yao W., Wang J., Meng F., Zhu Z., Jia X., Xu L., Zhang Q., Wei L. Circular RNA CircPVT1 Inhibits 5-Fluorouracil Chemosensitivity by Regulating Ferroptosis Through MiR-30a-5p/FZD3 Axis in Esophageal Cancer Cells. Front. Oncol. 2021;11:780938. doi: 10.3389/fonc.2021.780938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Xian Z.Y., Hu B., Wang T., Cai J.L., Zeng J.Y., Zou Q., Zhu P.X. CircABCB10 silencing inhibits the cell ferroptosis and apoptosis by regulating the miR-326/CCL5 axis in rectal cancer. Neoplasma. 2020;67:1063–1073. doi: 10.4149/neo_2020_191024N1084. [DOI] [PubMed] [Google Scholar]
- 320.Zhang C., Xu L., Li X., Chen Y., Shi T., Wang Q. LINC00460 Facilitates Cell Proliferation and Inhibits Ferroptosis in Breast Cancer Through the miR-320a/MAL2 Axis. Technol. Cancer Res. Treat. 2023;22:15330338231164359. doi: 10.1177/15330338231164359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Conrad M., Kagan V.E., Bayir H., Pagnussat G.C., Head B., Traber M.G., Stockwell B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes. Dev. 2018;32:602–619. doi: 10.1101/gad.314674.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Distefano A.M., Marchetti F., Zabaleta E., Pagnussat G.C. Measuring and Perturbing Ferroptosis in Plants. Methods Mol. Biol. 2022;2447:185–192. doi: 10.1007/978-1-0716-2079-3_15. [DOI] [PubMed] [Google Scholar]
- 323.Costa I., Barbosa D.J., Silva V., Benfeito S., Borges F., Remiao F., Silva R. Research Models to Study Ferroptosis’s Impact in Neurodegenerative Diseases. Pharmaceutics. 2023;15:1369. doi: 10.3390/pharmaceutics15051369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Guo Z., Sun J., Lv X., Zhang T., Yao H., Wu W., Xing Z., Kong N., Wang L., Song L. The ferroptosis in haemocytes of Pacific oyster Crassostrea gigas upon erastin treatment. Fish. Shellfish. Immunol. 2023;133:108556. doi: 10.1016/j.fsi.2023.108556. [DOI] [PubMed] [Google Scholar]
- 325.Wang W.T., Han C., Sun Y.M., Chen T.Q., Chen Y.Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019;12:55. doi: 10.1186/s13045-019-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.