Abstract
Gastrointestinal reflux disease (GERD) is a chronic, highly prevalent condition in the United States. GERD can significantly impact quality of life and lead to complications including aspiration pneumonia, esophageal stricture, Barrett’s esophagus (BE) and esophageal cancer. Obesity is a risk factor for GERD, which often improves with weight loss and bariatric surgery. Though the incidence of bariatric surgery, in particular, minimally invasive sleeve gastrectomy, has risen in recent years, emerging data has revealed that the severity or new onset of GERD may follow bariatric surgery. We performed a literature review to provide a detailed analysis of GERD with an emphasis on bariatric surgery as both the cure and the cause for GERD in the morbidly obese population. We also describe the pathophysiological mechanisms, management approach and treatment strategies of GERD following bariatric surgery.
Keywords: gastroesophageal, reflux, GERD, esophagitis, morbid, obesity, bariatric, Roux-en-Y, gastric, bypass, surgery, sleeve, gastrectomy
1. Introduction
It is reported that two out of five individuals have experienced reflux symptoms in the past and approximately one-third of individuals reported reflux symptoms within the past week, making GERD a common chronic condition in the United States [1]. The prevalence in the general population is estimated to be 18.1–27.8% [2]. GERD was the most frequent gastrointestinal diagnosis listed in outpatient clinic visits in the United States in 2009 [3].
The condition typically manifests as heartburn and regurgitation. Atypical or extraesophageal symptoms may be present in up to one-third of patients [4]. These include cough, laryngitis, asthma and chest pain [5]. GERD has been further classified as non-erosive reflux disease (NERD) and erosive reflux disease (ERD) based on endoscopic findings, and both can significantly impact quality of life and lead to complications, including Barrett’s esophagus and esophageal cancer [6,7].
Obesity is a risk factor for GERD and improves with weight loss [8,9]. Interestingly, as the incidence of bariatric surgery, particularly minimally invasive sleeve gastrectomy, has risen in recent years, emerging data has revealed that GERD may worsen after certain bariatric surgeries [10,11,12,13,14,15]. New-onset GERD can also develop post-bariatric surgery [12,13,16,17,18]. We conducted a literature review to provide a detailed analysis of GERD with an emphasis on bariatric surgery as both the cure and the cause in the morbidly obese population. We review the pathophysiological mechanisms, management approach and treatment strategies of GERD following bariatric surgery.
2. Methods
We performed a narrative review of the literature to provide a detailed analysis of GERD following bariatric surgery using PubMed computerized search and Google Scholar for articles with the title or keywords “GERD”, “gastroesophageal”, “reflux”, “obesity”, “bariatric surgery”, “Roux-en-Y gastric bypass”, “laparoscopic”, “sleeve gastrectomy” and “gastric sleeve”. Systematic reviews, literature reviews, meta-analyses, randomized controlled trials and clinical trials were prioritized. Articles that were not available in English or full text were excluded. Duplicate articles were removed. Articles regarding reflux in the context of pregnancy were excluded. The articles were independently reviewed by all authors.
3. Discussion
3.1. Obesity as the Cause of GERD
Obesity, defined as a body-mass index (BMI) of ≥30 kg/m2, has substantially increased over the past several decades in the United States with an estimated prevalence of 41.9% [19,20]. Obesity is a well-established risk factor for GERD independent of diet and a concomitant rise in the incidence of GERD has been noted [8,21,22,23]. A meta-analysis of nine studies by Hampel et al. revealed a pooled adjusted odds ratios of 1.43 for GERD symptoms for BMI of 25–30 kg/m2 and 1.94 for BMI > 30 kg/m2, highlighting the dose–response relationship between obesity and reflux [9]. The relationship between obesity and GERD has also been noted in other parts of the world, including Europe and Asia, where rates of obesity are overall lower than the Western hemisphere, but have been rising in recent years [2,24,25,26,27,28]. Additionally, there has been an increase in the complications of GERD, including Barrett’s esophagus and esophageal adenocarcinoma [29]. Central obesity, in particular, has been associated with an increase in the risk of Barrett’s esophagus (BE) which may be in part due to reflux [30]. Some studies have suggested a role for adiponectin and leptin from adipocytes in mediating obesity and BE [31,32].
There are multiple mechanisms that predispose obese patients to reflux (Table 1). Transient lower esophageal sphincter relaxations (TLESRs) consist of spontaneous relaxations of the lower esophageal sphincter and crural diaphragm, which are not preceded by swallowing. The most common stimulus for TLESRs has been suggested to be distension of the proximal stomach. Obese patients have a significantly increased number of total TLESRs, and thus reflux and acid exposure, especially in the postprandial phase [33,34]. In a prospective study of 84 patients, TLESRs were demonstrated to correlate with BMI and waist circumference [35]. Similarly, Corley et al. demonstrated a strong association between abdominal diameter and reflux symptoms, especially in Caucasians in a large, cross-sectional study [36]. The link between BMI and reflux symptoms seems to be partially mediated by abdominal diameter. A recent, prospective study of 771 patients proposed that the waist-to-hip ratio may be a better predictor of esophageal acid exposure compared to BMI [37].
Table 1.
Mechanical factors that have been suggested to predispose patients with obesity to gastroesophageal reflux disease.
| Pathophysiologic Mechanisms That Predispose Obese Patients to Reflux |
|---|
| Transient lower esophageal sphincter relaxations |
| Increased intra-abdominal pressure |
| Augmented gastroesophageal pressure gradient |
| Increased prevalence of hiatal hernia |
Obesity has been associated with an increased intra-abdominal pressure which may contribute to reflux of gastric contents into the esophagus [36]. Increased intra-abdominal pressure in obesity may result in the cephalad movement of the hiatal hernia and lead to reflux [38]. Pandolfino et al. explored the relationship between obesity and the esophagogastric junction pressure segment using high-resolution esophageal manometry in 285 patients [39]. The study concluded that obese patients were more likely to have an increased intragastric pressure, an augmented gastroesophageal pressure gradient, and disruption of esophagogastric junction, all of which can predispose to reflux. These findings were also correlated with waist circumference [39].
The gastroesophageal flap valve plays an important role in preventing the reflux of gastric contents into the esophagus. There is a high prevalence of hiatal hernias in obese patients, which leads to functional impairment of the esophagogastric junction [40]. Hiatal hernia involves herniation of the stomach through the esophageal hiatus into the mediastinum. Obesity has been linked to an increased separation between the lower esophageal sphincter and the crural diaphragm, which may predispose to the formation of a hiatal hernia [38]. In a study of 345 patients scheduled to undergo bariatric surgery, Suter et al. discovered a hiatal hernia on endoscopy in over 50% of patients [41]. More recently, Yen et al. found a hiatal hernia in 33% of patients with morbid obesity using high-resolution impedance manometry, and all patients with hiatal hernia had erosive esophagitis on endoscopy [42]. GERD is associated with a low esophageal sphincter pressure in patients with hiatal hernia, and hiatal hernias may also be larger in size in this population [43]. Of note, the baseline pressure of the lower esophageal sphincter, gastric volume, gastric acid production and gastric emptying are likely normal in obese patients, though the data are limited [44,45,46]. Gastric emptying may be delayed after a large, high-calorie meal which would increase risk of reflux [38]. The compromise in the esophagogastric junction and diaphragmatic hiatus coupled with a rise in intragastric pressure from abdominal obesity all provide the ideal conditions for the development of reflux.
3.2. Bariatric Surgery as the Treatment of GERD
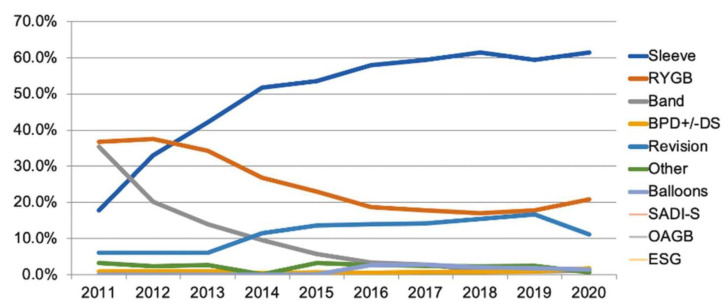
Bariatric surgery, specifically Roux-en-Y gastric bypass (RYGB), is highly effective for the treatment of GERD. The majority of bariatric surgeries are performed in BMIs of 35–55. GERD has been reported in as many as 62.4% to 73% of bariatric surgery candidates [47,48]. There has been an overall gradual increase in bariatric surgeries over the last two decades, with an increase from 43.5 per 100,000 to 70.6 per 100,000 from 2006 to 2009 and a subsequent plateau in bariatric surgeries from 2010 to 2015 [49]. Recent trends in bariatric surgeries performed are shown in Figure 1. There was a reduction in the overall volume of metabolic and bariatric surgeries that coincided with the COVID-19 pandemic due to the suspension of elective surgeries [10]. RYGB and sleeve gastrectomy (SG) are the most commonly performed bariatric surgeries [10,49]. There has been a significant increase in SG in recent years and it is the most widely performed bariatric surgery. In parallel, there was a steady decline in RYGB procedures at the end of 2012 and beginning of 2013, and a plateau in 2017, with an upward trend in 2019 and 2020. There has also been a large increase in minimally invasive bariatric surgeries compared to open bariatric surgeries [49].
Figure 1.
Trends in metabolic and bariatric surgery are shown from 2012 to 2020. BPD +/− DS = biliopancreatic diversion +/− duodenal switch; ESG = endoscopic sleeve gastroplasty; OAGB = one-anastomosis gastric bypass; RYGB = Roux-en-Y gastric bypass; SADI-S = single-anastomosis duodeno-ileostomy with sleeve [10].
High rates of GERD improvement have been observed following RYGB (Table 2). A randomized controlled trial of 217 patients with a 5-year follow-up period revealed that remission of reflux was observed in 60.4% of cases after RYGB compared to 25% of cases after SG [50]. There was no significant difference in weight loss between the two groups in the trial [50]. Frezza et al. revealed a significant reduction in reflux symptoms following RYGB in a study of 152 patients [51]. Similarly, Perry et al. demonstrated improvement or resolution of GERD in all 57 patients postoperatively following RYGB [52]. Laparoscopic RYGB (LRYGB) has been shown to be superior to laparoscopic SG (LSG) in improving reflux (p-value = 0.003, odd ratio, 3.16, 95% CI, 1.48–6.76) [53]. The superior improvement in GERD with RYGB may be related to higher weight loss achieved with RYGB. Additionally, pathophysiologic differences, including a major reduction in the mass of parietal cells responsible for gastric acid secretion with RYGB, and mechanical differences between the two procedures also contribute to decreased risk of reflux with RYGB compared to SG [54,55]. It is important to note that, due to the increased prevalence of reflux in patients with hiatal hernias, concomitant hiatal hernia should be repaired at the time of bariatric surgery, as it has been shown to be safe [56,57].
Table 2.
An overview of the literature pertaining to the remission of gastroesophageal reflux symptoms and the use of acid suppression medications pre- and post-Roux-en-Y gastric bypass. GERD = gastroesophageal reflux disease, PPI = proton pump inhibitors, RYGB = Roux-en-Y gastric bypass.
| Remission of Gastroesophageal Reflux Symptoms and Use of Acid Suppression Medication Pre- and Post-Roux-en-Y Gastric Bypass | ||||||
|---|---|---|---|---|---|---|
| Author | Year | Journal | Article Type | Number of Cases/Studies | GERD Symptom Remission | Pre- and Post-RYGB Usage of Acid Suppression Medications |
| Frezza et al. [51] | 2002 | Surgical Endoscopy | Prospective study | 152 | Heartburn 87% → 22%, p < 0.001 Water brash 18% → 7%, p < 0.05 Wheezing 40% → 5%, p < 0.001 Laryngitis 17% → 7%, p < 0.05 Aspiration 14% → 2%, p < 0.01 |
PPI: pre-RYGB 44% → post-RYGB 9%, p < 0.001 Histamine-2 receptor antagonists: pre-RYGB 60% → post-RYGB 10%, p < 0.01 |
| Perry et al. [52] | 2004 | Journal of Society of Laparoendoscopic surgeons | Prospective study | 57 | All patients reported improvement or no symptoms of GERD after RYGB | PPI: 31 patients pre-RYGB → 3 patients post-RYGB High-dose histamine-2 receptor antagonists: 17 patients pre-RYGB → all patients treated with ranitidine 150 mg per day post-RYGB |
3.3. Bariatric Surgery as the Cause of GERD: A Burning Issue
As the incidence of bariatric surgery rises, the persistent and de novo reflux following bariatric surgery has become a topic of concern. There has been mounting evidence that bariatric surgery, in particular SG, may lead to worsening of GERD (Table 3). According to a meta-analysis which compared the outcomes of SG and RYGB, the odds ratio for GERD after SG is five times higher (p < 0.001) [11]. In a retrospective review of 4832 patients, 84.1% of patients who underwent LSG continued to have GERD symptoms postoperatively, and 8.6% developed GERD postoperatively [16]. Preoperative GERD was associated with worse outcomes in the study [16]. Similarly, Sheppard et al. retrospectively reviewed 387 cases and noted increased PPI usage in patients after LSG in comparison to LRYGB [12]. Carter et al. concluded that LSG was associated with persistent reflux symptoms in patients who had preoperative GERD and increased risk of postoperative reflux symptoms in those who did not have these symptoms preoperatively [17]. Additional studies by Tai et al., Howard et al. and Matar et al. have also confirmed GERD following LSG [13,14,15].
Table 3.
Literature comparison of sleeve gastrectomy and Roux-en-Y gastric bypass in terms of remission of gastroesophageal reflux symptoms and the use of acid suppression medications. ACM = acid suppression medications, EE = erosive esophagitis, GERD = gastroesophageal reflux disease, OR = odds ratio, PPI = proton pump inhibitors, (L)RYGB = (laparoscopic) Roux-en-Y gastric bypass, (L)SG = (laparoscopic) sleeve gastrectomy.
| Comparison of Sleeve Gastrectomy and Roux-en-Y Gastric Bypass in Terms of Gastroesophageal Reflux Symptom Remission and the Use of Acid Suppression Medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Journal | Article Type | Number of Cases/Studies | GERD Symptom Remission—RYGB | Pre- and Post-Operative Usage of Acid Suppression Medications (ACM) | GERD Symptom Remission—SG | p-Value | Additional Comments |
| Peterli et al. [50] | 2018 | JAMA | Randomized controlled trial | 217 | 60.4% | N/R | 25% | 0.002 | De novo reflux in 31.6% after SG vs. 10.7% after RYGB (p = 0.01) |
| Alghamdi et al. [53] | 2022 | Frontiers in Surgery | Systematic review, meta-analysis | 16 | Odds ratio of GERD remission = 3.16 for LRYGB compared to LSG, p = 0.003, heterogeneity N/A Usage of ACM was not reported |
There was no significant statistical difference between LRYGB and LSG with regard to new-onset GERD; heterogeneity was noted | |||
| Gu et al. [11] | 2019 | Obesity Surgery | Systematic review, meta-analysis | 23 | OR for GERD after LSG compared to LRYGB = 5.10, p < 0.001 LRYGB had a better effect on GERD compared to LSG, OR = 0.19, p < 0.001 |
||||
| DuPree et al. [16] | 2014 | JAMA Surgery | Retrospective review | 4832 | 62.8% | N/A | 15.9% | p < 0.001 | New-onset GERD was noted in 8.6% in the LSG group |
| Sheppard et al. [12] | 2015 | Obesity Surgery | Retrospective review | 387 | Pre-operative PPI use in LSG: 28% → 2% were able to discontinue PPI after SG Pre-operative PPI use in LRGYB: 32% → 33% were able to discontinue PPI after RYGB |
||||
| Matar et al. [15] | 2020 | Obesity Surgery | Retrospective review | 517 | EE prevalence higher after SG than RYGB (37.9% vs. 17.6%, p = 0.0001) | ||||
Additionally, de novo GERD has also been documented following SG [12,13,16,17,18] (Table 4). A systematic review of 20 articles demonstrated that 8 out of 10 studies revealed rates of new-onset GERD following LSG [58,59]. The Swiss Multicenter Bypass or Sleeve Study (SM-BOSS) Trial of 217 patients reported de novo GERD in 31.6% of LSG patients vs. 10.7% in RYGB patients (p = 0.01) [50]. In a study of 110 patients who underwent LSG, the incidence of reflux symptoms and PPI intake were significantly increased compared to pre-operative values (68.1% vs. 33.6%, p < 0.0001; 57.2% vs. 19.1%, p < 0.0001) [60]. There was an increased incidence and severity of erosive esophagitis post-operatively. The study also newly diagnosed non-dysplastic Barrett’s esophagus postoperatively in 17.2% of patients [60]. A multi-center study of 90 patients, in which endoscopy prior to bariatric surgery was routinely performed and did not have evidence of Barrett’s esophagus preoperatively, noted a prevalence of Barrett’s esophagus of 18.8% in addition to increased prevalence of GERD symptoms, erosive esophagitis and PPI usage at a mean follow-up of 78 ± 15 months [61]. Barrett’s esophagus was significantly associated with weight loss failure in this study [61]. Similarly, Qumseya et al. performed a meta-analysis of 10 studies, including 680 patients who had undergone EGD from 6 months to 10 years after SG, and the pooled prevalence of BE was 11.6% (95% confidence interval [CI], 8.1–16.4%; p < 0.001; I2 = 28.7%) [62]. Most cases were noted after 3 years of follow-up and there was no significant association of BE with GERD symptoms in this study [62]. A meta-analysis of 46 studies with 10,718 patients revealed a prevalence of BE of 8% following SG [63]. There was a lack of correlation between symptoms and pathology as well [63]. This data suggests that there is a need for endoscopic surveillance following SG. The current studies regarding Barrett’s esophagus following SG have some limitations as outlined by a statement by American Society for Metabolic and Bariatric Surgery (ASMBS) [64]. The ASMBS has suggested to offer screening for BE in SG patients ≥3 years post-SG regardless of the presence of GERD symptoms [64].
Table 4.
An overview of the literature pertaining to the remission of gastroesophageal reflux symptoms and the use of acid suppression medications pre- and post-sleeve gastrectomy. ACM = acid suppression medication, GERD = gastroesophageal reflux disease, (L)SG = (laparoscopic) sleeve gastrecromy, N/R = not reported.
| Exacerbation or Remission of Gastroesophageal Reflux Symptoms and the Use of Acid Suppression Medications Pre- and Post-Sleeve Gastrectomy | |||||||
|---|---|---|---|---|---|---|---|
| Author | Year | Journal | Article Type | Number of Cases/Studies | GERD Symptom Remission | Pre- and Post-SG Usage of Acid Suppression Medications | Additional Comments |
| Carter et al. [17] | 2011 | Surgery for Obesity and Related Diseases | Retrospective review | 176 | Pre-SG, 34.6% had GERD symptoms Post-SG, 49% reported GERD symptoms within 30 days 47.2% had persistent GERD (>1 month after LSG) |
Pre-LSG: 22% → Post-LSG 33.8% of patients on GERD medication, p = 0.0428 | |
| Tai et al. [13] | 2013 | Surgical Endoscopy | Retrospective review | 66 | Prevalence of GERD symptoms pre-LSG 12.1% → 47% post-LSG, p < 0.001 | N/R | New-onset GERD after LSG: 44.8% Hiatal hernia: 6.1% → 27.3% |
| Howard et al. [14] | 2011 | Surgery for Obesity and Related Diseases | Retrospective review | 28 | 25% pre-LSG → 23% post-LSG, p < 0.05 | Pre-LSG: 25% → post-LSG 39% with GERD symptoms despite ACM use | New-onset GERD post-LSG: 22% |
Of note, reflux symptoms both pre- and post-operatively do not always correlate with objective evidence of GERD. A study involving obese patients under consideration for bariatric surgery reported 12.3% of patients with low GERD Questionnaire scores < 8 had erosive esophagitis [47].
There have been several proposed pathophysiological mechanisms for the development of de novo GERD or exacerbation of existing GERD following SG (Table 5).
Table 5.
Proposed mechanisms for de novo or increased gastroesophageal reflux following bariatric surgery.
| Proposed Mechanisms for De Novo or Increased Gastroesophageal Reflux following Bariatric Surgery | |
|---|---|
| Sleeve Gastrectomy | Roux-en-Y Gastric Bypass |
|
|
Gastric sleeve anatomy plays an important role in predisposing obese patients to GERD [65]. In general, the narrower the sleeve, the higher the intragastric pressure, which may increase reflux. Keidar et al. noted that a stenosis of the middle portion of the sleeve and the angular notch with upstream dilation was associated with higher rates of GERD following SG [66]. To reduce this risk, the use of a larger bougie size for sleeve calibration has been explored. When compared to a 32-French bougie, a 42-French bougie was associated with improvement in GERD symptoms in 80% of patients post-operatively [67]. However, data regarding the optimal bougie size for weight loss and decreased propensity of reflux has been mixed [68,69,70,71]. Regarding the ideal shape of the SG, it has been suggested that the sleeve should be a trapezoid, being widest at the antrum and narrowest at the cardia, to prevent sleeve stenosis and increased intragastric pressure [65]. The antrum should be preserved, as demonstrated by Garay et al., as it may accelerate gastric emptying, reduce intragastric pressure and subsequent reflux [72]. Thus, the placement of the first staple line has been proposed to be >5 cm from the pylorus [72].
Caution must be exercised to avoid disruption of the natural anatomy of the esophagogastric junction, namely the gastroesophageal flap valve, the angle of His and gastric sling fibers, which are protective against reflux. The last staple line should not be placed too close to the esophagus, to avoid mechanical injury of the sling fibers and angle of His, nor too far from the esophagus, resulting in accessory gastric tissue retention [65]. Concomitant hiatal hernia should be repaired to prevent functional impairment of the esophagogastric junction [40]. It has been suggested that impaired esophagogastric motility may contribute to postoperative GERD though alterations in motility are often induced as part of the mechanism of the surgery itself [73]. A dynamic contrast study may also reveal esophageal dysmotility as a possible etiology of reflux and, thus, some experts recommend these interventions in patients with post-SG GERD [74]. Cephalad migration of the Z-line has also been implicated in post-operative GERD, and can be detected on endoscopy or contrast studies [38,60].
3.4. Management and Treatment Approach to GERD Following Bariatric Surgery
Following bariatric surgery, lifestyle modifications are recommended to include gradual advancing of the diet from liquids to eventually smaller portions of healthy, protein-based food. Exercise, nutritional supplementation, management of underlying constipation and avoidance of factors that exacerbate reflux, i.e., alcohol, tobacco, caffeine, chocolate and postprandial supination are also emphasized. Overfilling of the stomach with large meal portions may lead to regurgitation or reflux-like symptoms. After exclusion of anatomic and functional etiologies, dietary education, counseling and training should be provided to these patients.
Patients often receive PPI prophylaxis for weeks to months to prevent marginal ulcers and reflux symptoms. A meta-analysis of seven studies revealed an OR of marginal ulcer formation in the PPI group versus the non-PPI group to be 0.50 with low heterogeneity, indicating the PPI group had two times less ulceration compared to the non-PPI group [75]. In patients with GERD following bariatric surgery, PPI and histamine-2 receptor antagonists (H2RA) regimens should be optimized following bariatric surgery. There has been emerging data regarding the efficacy of the novel potassium-competitive acid blocker, vonoprazan, in improving GERD symptoms refractory to PPI therapy [76,77,78,79,80]. However, its use in GERD following bariatric surgery has not been evaluated thus far.
Given the robust evidence linking SG to an increased risk of GERD, the authors recommend a discussion of the potential exacerbation of pre-existing GERD or an increased risk of developing new-onset GERD during the shared decision-making process for bariatric surgery selection. According to a multi-society consensus statement from the ASMBS, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), the American Society for Gastrointestinal Endoscopy (ASGE), the European Association of Endoscopic Surgery (EAES), the Society for Surgery of the Alimentary Tract (SSAT) and the Society of Thoracic Surgeons (STS), sleeve gastrectomy should not be performed as an anti-reflux procedure [81]. Patients with BMI > 35 and medically refractory GERD should be considered for either RYGB or fundoplication [81]. There are data to suggest an increased risk of hiatal hernia and GERD recurrence with fundoplication in patients with BMI > 35, and these patients may benefit from RYGB due to the additional weight loss benefit [81,82,83,84,85].
Post-surgical complications, including stenosis, kinking, angulation and marginal ulcers, must be recognized and treated appropriately. Stenosis, kinking and angulation may lead to postoperative GERD and obstructive symptoms due to increased intragastric pressure and reflux of gastric contents [86,87,88].
Endoscopic balloon dilation is a safe, effective and minimally invasive method for the management of gastric sleeve stenosis [89,90,91]. The optimal type and size of the balloon, including controlled radial expansion balloon and pneumatic achalasia balloons, and the number of dilations required are unclear [92].
Endoscopic balloon dilation has been demonstrated to be safe and effective for gastrojejunal strictures post-RYGB [93]. RYGB-associated strictures may also be safely treated with lumen-apposing metal stents (LAMS) [94,95,96]. In a prospective study of 412 patients who underwent RYGB, 14 patients developed a stricture, of which 12 had resolution of their stricture following LAMS insertion [94]. Two patients required surgery for refractory strictures that were associated with marginal ulceration of the gastrojejunostomy. A stent migration rate of 19% was noted, though stent migration did not seem to result in adverse patient outcomes in the study [94].
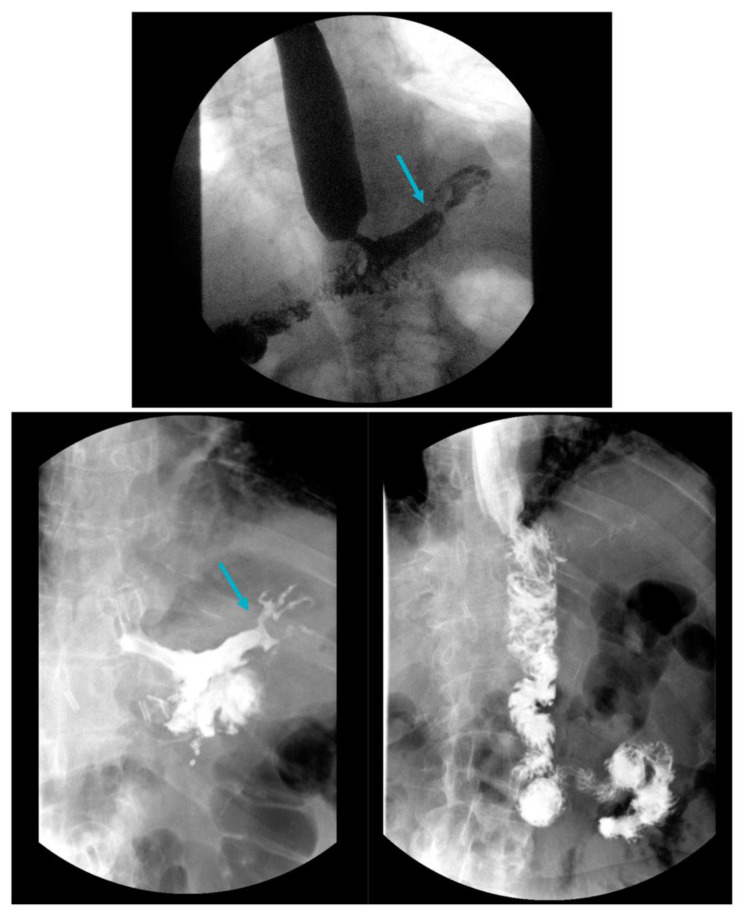
Gastrogastric (GG) fistulas are a serious complication that can occur in up to 6% of RYGB cases, and are often associated with abdominal pain, GERD and weight gain [97,98]. GERD in GG fistula has been attributed to the passage of gastric acid from the remnant stomach to the gastric pouch through the fistula (Figure 2 and Figure 3). Endoscopic repair offers a minimally invasive method to the treatment of GG fistulas (Figure 4 and Figure 5) [99]. Various endoscopic techniques have been utilized for endoscopic closure of GG fistulas, including over-the-scope clips, through-the-scope clips and endoscopic suturing [100,101,102,103,104]. The best outcomes for endoscopic repair of GG fistulas tend to occur with small fistulas that are <10 mm in size [105]. In a study of 95 patients, initial complete closure of GG fistula was successful in 95% of patients, though 65% had reopening [105]. There were no GG fistulas > 20 mm in size that remained closed during the follow-up period, compared to 32% of GG fistulas ≤ 10 mm in size that remained closed [105]. In another study of 99 RYGB patients with GGF, endoscopic GGF and surgical revision had similar rates of GERD resolution [106]. Endoscopic GGF was associated with a greater improvement in abdominal pain and less adverse events compared to surgical revision, which resulted in a greater weight loss [106]. Ultimately, surgical revision may be required to treat GG fistulas, especially with large defects [107].
Figure 2.
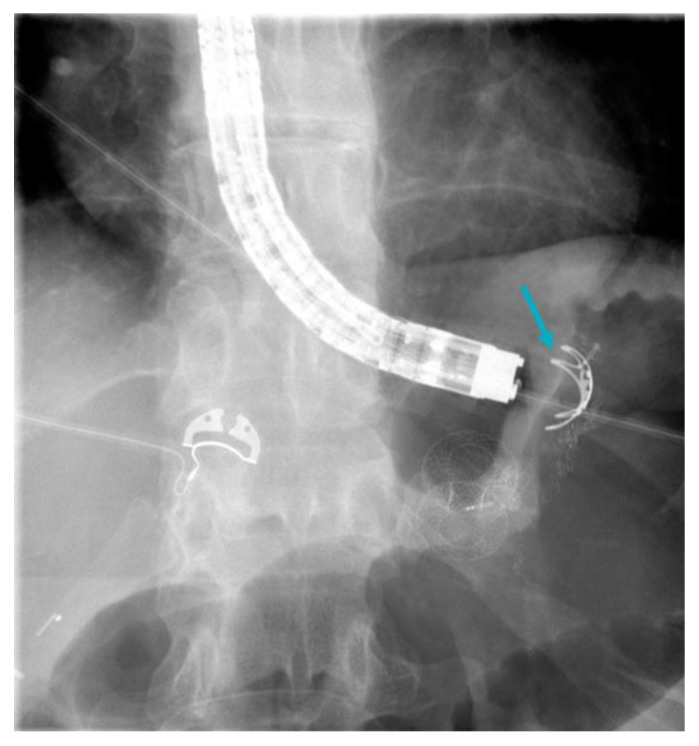
Upper gastrointestinal series demonstrating a Roux-en-Y gastric bypass with a gastrogastric fistula and leak under the left hemidiaphragm (arrows, top and bottom left) as evidenced by contrast filling of an irregularly shaped cavity lateral to the gastric pouch, which spontaneously empties back via the gastric pouch. This patient eventually required a distal esophagectomy, total gastrectomy and esophagojejunostomy (bottom right).
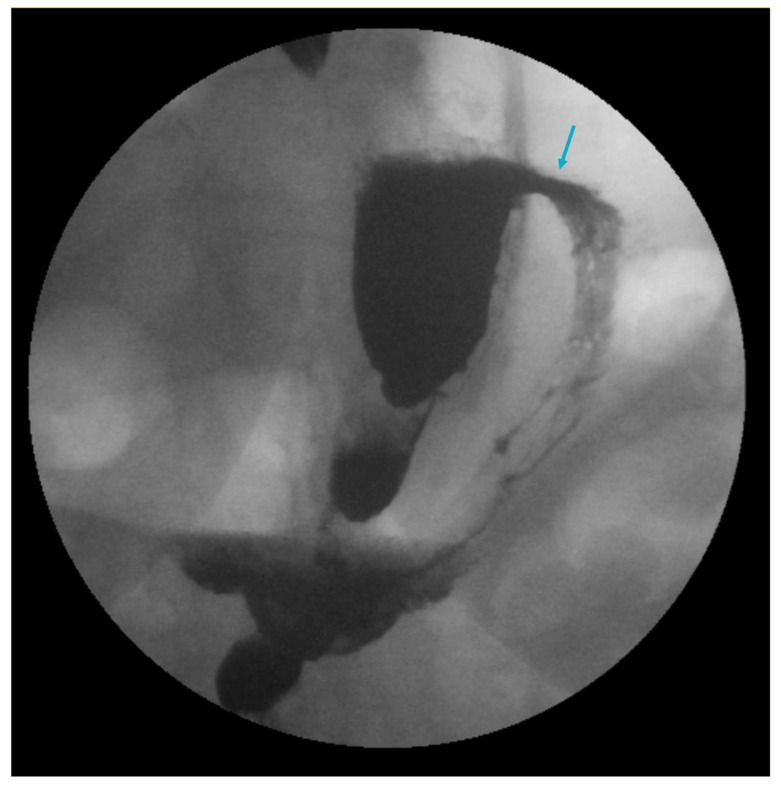
Figure 3.
A gastrogastric fistula (arrow) in a patient with a prior Roux-en-Y gastric bypass as demonstrated on upper gastrointestinal series.
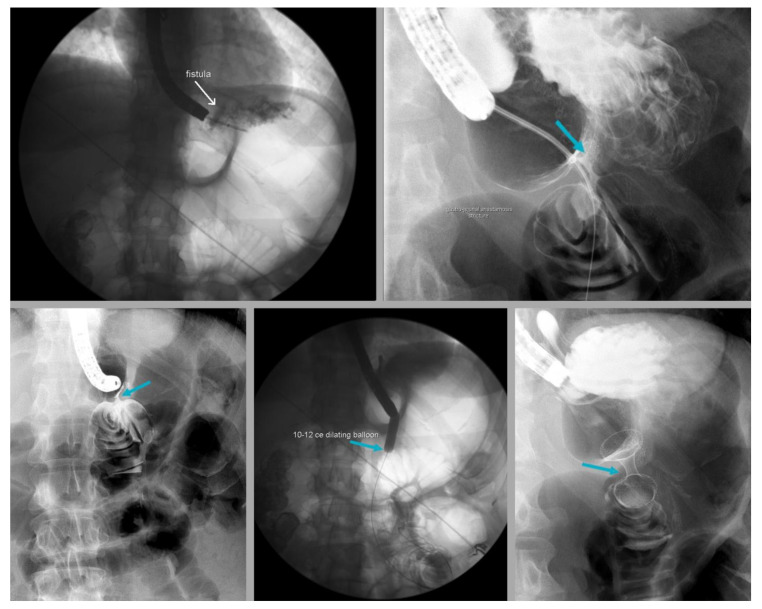
Figure 4.
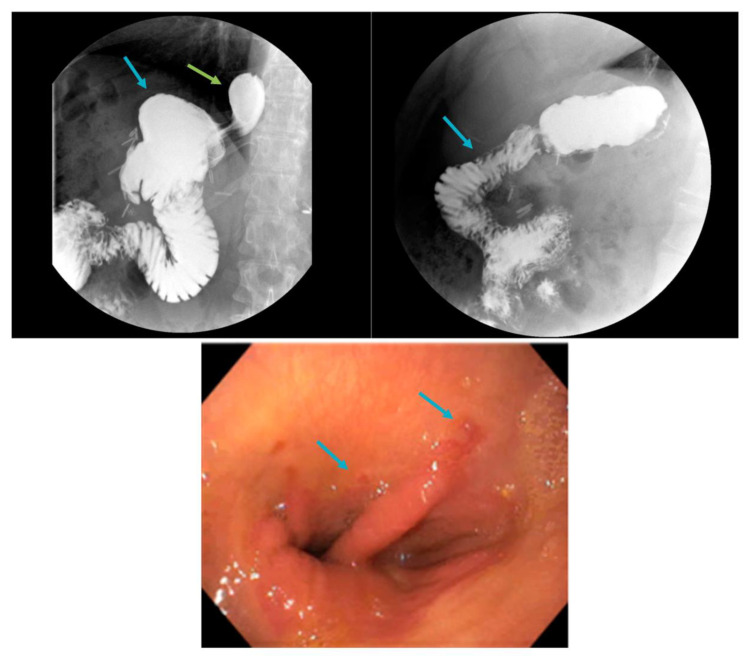
Upper gastrointestinal series in a patient with severe gastroesophageal reflux disease following Roux-en-Y gastric bypass, gastrogastric fistula (arrow, top left), gastrojejunal stricture (arrow, top right and arrow, bottom left), who underwent controlled radial expansion balloon dilation (arrow, bottom middle) and placement of lumen-apposing metal stent (arrow, bottom right).
Figure 5.
Persistent gastrogastric fistula despite suturing following Roux-en-Y gastric bypass which required endoscopic fistula closure utilizing an over-the-scope clip system “Bear Claw” Ovesco Endoscopy, Tübingen, Germany (arrow).
Post-operative leaks following bariatric surgery are a serious complication that can often result in significant morbidity and mortality. The use of endoscopic self-expandable metal stents has shown to be effective in the management of staple-line leaks following bariatric surgery [100,108]. Endoscopic internal drainage, in conjunction with perigastric abscess drainage, have also been shown to be promising [109].
It is important to note that the length of the Roux-en-Y limb, on average 120 cm, and the size of the gastric pouch are both critical to decrease the risk of complications such as GERD (Figure 6). It has been demonstrated that the optimal gastric pouch should be 20 to 30 cc in volume and primarily involve the lesser curvature of the stomach [110]. Revisional surgery may be needed to optimize the pouch size. A systematic review of 13 studies suggested that the length of the common limb >400 cm confers weight loss and metabolic benefits [111]. With regard to SG, surgical techniques and anatomy of the sleeve should be optimized as described in the section above to prevent post-SG GERD. If the sleeve gastrectomy has been performed correctly, an anti-reflux procedure should not be indicated in most cases.
Figure 6.
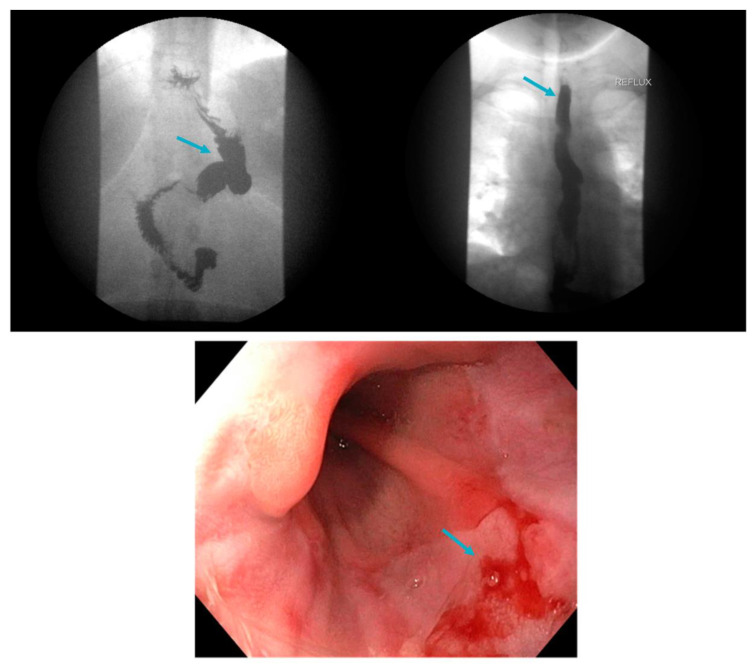
Large gastric pouch (blue arrow, top left) with sliding hiatal hernia (green arrow, top left) and a short, 20 cm Roux limb (top right) seen on upper gastrointestinal series, in addition to Los Angeles Grade A esophagitis on upper endoscopy (arrows, bottom).
Recently, endoscopic sleeve gastroplasty (ESG), also known as endoluminal vertical gastroplasty (EVG), has shown to be a safe and effective method for weight loss in the setting of weight recidivism post-LSG, but studies that have evaluated the effects of ESG on GERD are limited [112,113,114,115,116]. Fayad et al. revealed that new-onset GERD was notably lower in the ESG group than in the LSG group (1.9% vs. 14.5%, p < 0.05), due to sparing of the fundus of the stomach [117]. A study of 64 patients revealed no incidence of reflux symptoms at 1 year following EVG [118]. In the Transoral Gastric Volume Reduction as Intervention for Weight Management (TRIM) trial, which explored the safety and efficacy of transoral gastric volume reduction surgery using an endoscopic suturing system, 8 of 14 patients reported reflux symptoms prior to the procedure, and 5 out of 14 at 1-year follow-up [119].
Sleeve gastrectomy has also been combined with fundoplication in an attempt to minimize postoperative GERD. In a randomized clinical study of 278 patients comparing SG to SG with Rossetti fundoplication (RF) in terms of de novo GERD, there was a significant reduction in PPI use (4.3% vs. 17.1%) and esophagitis (2% vs. 23.4) in the SG with RF group compared to the SG group [120]. Olmi et al. described 220 obese patients who underwent LSG and modified RF with a 24-month follow-up. It was noted that 98.5% of cases did not report GERD or use PPIs postoperatively, and approximately 97% of cases had an improvement in esophagitis on endoscopy [121]. Uccelli et al. reported 127 patients who underwent sleeve gastrectomy with RF, of which 74.8% had GERD prior to the surgery and 95% did not have reflux symptoms at 5-year follow-up. Improvement in esophagitis on endoscopy was also noted, though the study had some limitations [122,123]. A significant increase in the lower esophageal sphincter tone was demonstrated on high-resolution esophageal manometry in 20 patients following LSG with RF, thereby potentially reducing the likelihood of developing long-term GERD [124]. Nocca et al. conducted a prospective study of 25 patients who underwent laparoscopic Nissen-sleeve gastrectomy, among which the majority had GERD symptoms, and 76% of patients remained asymptomatic without PPI use three months postoperatively [125]. Similar results in GERD improvement were noted in several other studies [126,127,128,129,130,131,132].
A systematic review and meta-analysis of 15 studies explored the efficacy of SG with hiatal hernia repair versus SG with fundoplication and concluded that, while both procedures were effective in GERD resolution and weight loss, SG with fundoplication was superior in terms of GERD resolution, though had a higher complication rate [133]. Carandina et al. reviewed the safety of combined sleeve gastrectomy with fundoplication in a review of seven studies with 487 patients, and revealed a postoperative complication rate of 9.4% [134]. Gastric perforation, bleeding and sleeve stenosis were the most common reported complications [134]. Further studies are warranted to determine the role of SG with fundoplication. Though SG with fundoplication seems to be a safe surgical procedure with an acceptable rate of early postoperative complications, the authors currently feel that there is a lack of high-quality, long-term data regarding the safety, efficacy and technical feasibility for SG with fundoplication to suggest this approach [133,134].
Ultimately, many patients require conversion from LSG to RYGB, which is highly effective in the treatment of post-bariatric surgery GERD (Table 6). The literature has revealed a 5–10% conversion rate from LSG to RYGB due to GERD [59,135,136,137]. Parmar et al. reviewed 22 conversions from LSG to LRYGB, among which 10 were for intractable GERD. Improvement in GERD was noted in all patients and 80% of patients discontinued acid suppression medications [138]. Similarly, Abdemur et al. reported complete resolution of GERD after LSG to LRYGB conversion in six out of nine patients [139]. Hendricks et al. reported four conversions from LSG to LRYGB, of which three had complete resolution of their symptoms and one patient had partial resolution [140]. Similar results were obtained by several other studies [141,142,143,144,145].
Table 6.
An overview of the literature on the effect of conversion from sleeve gastrectomy to Roux-en-Y gastric bypass on gastroesophageal reflux disease. ACM = acid suppression medication, GERD = gastroesophageal reflux disease, RYGB = Roux-en-Y gastric bypass, N/R = not reported.
| The Effect of Conversion from Sleeve Gastrectomy to Roux-en-Y Gastric Bypass on Gastroesophageal Reflux Disease | |||||
|---|---|---|---|---|---|
| Author | Year | Journal | Article Type | Conversion Rate to RYGB for GERD (%) | Effect on GERD Symptoms and Use of Acid Suppression Medications |
| Langer et al. [136] | 2010 | Obesity Surgery | Retrospective review | 11 | 100% of patients with severe reflux discontinued ACM |
| Salminen et al. [137] | 2018 | JAMA | Randomized clinical trial | 6 | N/R |
| Parmar et al. [138] | 2017 | Obesity Surgery | Prospective study | 45 | 100% of patients reported improvement in GERD symptoms 80% of patients were able to discontinue ACM |
| Abdemur et al. [139] |
2016 | Surgery for Obesity and Related Diseases | Retrospective review | 0.8 | 66% of patients had complete resolution of GERD symptoms |
| Hendricks et al. [140] | 2016 | Surgery for Obesity and Related Diseases | Retrospective review, comparative study | 10.5 | 75% of patients had complete resolution of GERD symptoms 25% of patients had partial resolution |
| Gautier et al. [141] | 2013 | Obesity Surgery | Retrospective review | 33.3 | 100% of patients discontinued ACM No recurrence of GERD was noted |
| Strauss et al. [142] | 2023 | Surgical Endoscopy | Retrospective review | 72.2 | 80.2% of patients had improvement in GERD symptoms 19.4% of patients were able to discontinue ACM |
| Felsenreich et al. [144] | 2022 | Obesity Surgery | Retrospective review | 34.2 | 29.9% of patients reported GERD symptoms following conversion |
| Peng et al. [145] | 2020 | Surgery for Obesity and Related Diseases | Systematic Review and Meta-analysis | N/R | 57.1–100% had remission or improvement in GERD symptoms |
In patients with medically refractory GERD following sleeve gastrectomy, the authors suggest obtaining an esophagram and selective esophagogastroduodenoscopy, manometry and pH testing to further assess the anatomy and physiology of the symptoms. It is important to determine the presence of a hiatal hernia, as this should be corrected at the time of conversion to RYGB (Figure 7). A small subset of surgeons have performed the Hill procedure using the phrenoesophageal bundle for persistent GERD following RYGB [146]. The widespread application of the Hill procedure for this purpose has been limited due to surgeon familiarity and expertise.
Figure 7.
Sliding hiatal hernia (arrow, top left) and reflux (arrow, top right) demonstrated on upper gastrointestinal series in addition to severe erosive esophagitis on upper endoscopy (arrow, below) in a patient following sleeve gastrectomy.
As discussed above, some studies have proposed endoscopic surveillance post-bariatric surgery to assess disease progression to Barrett’s esophagus and esophageal adenocarcinoma [147,148,149]. Endoscopy prior to bariatric surgery and obtaining a thorough history regarding gastroesophageal reflux symptoms may improve patient selection for bariatric surgery [135]. Carabotti et al. demonstrated that the incidence of endoscopic lesions was the same in patients who reported reflux symptoms and those who did not, and concluded that symptoms cannot be reliably considered as an indication for endoscopy in these patients [150]. The ASMBS has suggested to offer screening for BE in SG patients ≥3 years post-SG, regardless of the presence of GERD symptoms, in addition to the standard screening indications for GERD and Barrett’s esophagus [64].
Finally, the use of magnetic sphincter augmentation devices, such as the LINX© system following SG, has shown some promising results, though the data are limited to small, retrospective studies. Rausa et al. performed a meta-analysis of three retrospective studies with 33 patients who underwent magnetic sphincter augmentation (MSA) for GERD following bariatric surgery. The pooled mean difference in preoperative GERD-Health-Related Quality of Life Questionnaire (GERD-HRQL) score was 17.5, which was statistically significant [151]. In a multicenter, single-arm prospective study of 30 patients over 12 months, MSA following LSG revealed improved GERD symptoms, reduced PPI use and decreased distal acid exposure in a minimally invasive manner [152]. A retrospective review of 13 identified patients who underwent LINX placement after bariatric surgery also noted decreased PPI usage post-operatively, reduced GERD-HRQL scores and relative safety of the procedure [153]. Additional studies have corroborated these findings [136,137,138,154,155,156]. Further data are needed to establish the safety and efficacy of magnetic sphincter augmentation devices following bariatric surgery.
4. Conclusions
GERD is a common, chronic condition which can significantly impact quality of life and lead to serious complications. Obesity is a well-established risk factor for GERD, which often improves with weight loss and bariatric surgery. With the recent rise in bariatric surgery, especially SG, persistent and de novo reflux following bariatric surgery has become a topic of concern. Management of post-bariatric surgery GERD includes lifestyle modifications, optimization of PPI and H2RAs, treatment of postoperative complications and repair of hiatal hernia if present. Conversion to Roux-en-Y currently has the most robust evidence to support its safety and efficacy for the treatment of medically refractory GERD post-SG. Other options include magnetic sphincter augmentation, though data regarding safety and efficacy are limited. Currently and going forward, more precise, standardized methods are warranted to document GERD following bariatric surgery, due to the variability in the reported literature. As bariatric surgery can be both the cure and the cause for GERD in the morbidly obese population, careful patient selection and proper surgical technique are paramount for a favorable outcome.
Acknowledgments
The authors would like to thank Michael Larsen from the Department of Gastroenterology and Hepatology at Virginia Mason Franciscan Health for his assistance in providing a list of endoscopic images of interest. The authors would also like to thank Terri Davis Smith from the Center for Digestive Health at Virginia Mason Franciscan Health for her assistance in coordination and submission of the manuscript.
Author Contributions
Conceptualization, M.M., R.A.K., D.L. and S.B.D.; methodology, M.M., R.A.K., D.L. and S.B.D.; software, not applicable; validation, M.M., R.A.K., D.L. and S.B.D.; formal analysis, M.M., R.A.K., D.L. and S.B.D.; investigation, M.M., R.A.K., D.L. and S.B.D.; resources, M.M., R.A.K., D.L. and S.B.D.; data curation, M.M., R.A.K., D.L. and S.B.D.; writing—original draft preparation, M.M.; writing—review and editing, M.M., R.A.K., D.L. and S.B.D.; visualization, M.M., R.A.K., D.L. and S.B.D.; supervision, R.A.K., D.L. and S.B.D.; project administration, M.M. and R.A.K.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
An Institutional Review Board review or written Informed Consent was not applicable for this study.
Informed Consent Statement
This manuscript has not been previously published and is not currently under consideration elsewhere for publication.
Data Availability Statement
Not applicable.
Conflicts of Interest
No conflict of interest exist for all authors in this manuscript.
Funding Statement
All authors have no financial disclosure or support to report. There were no external sources of funding for this manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Delshad S.D., Almario C.V., Chey W.D., Spiegel B.M. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology. 2020;158:1250–1261.e2. doi: 10.1053/j.gastro.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag H.B., Sweet S., Winchester C.C., Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter J.E., Rubenstein J.H. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267–276. doi: 10.1053/j.gastro.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durazzo M., Lupi G., Cicerchia F., Ferro A., Barutta F., Beccuti G., Gruden G., Pellicano R. Extra-Esophageal Presentation of Gastroesophageal Reflux Disease: 2020 Update. J. Clin. Med. 2020;9:2559. doi: 10.3390/jcm9082559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaspersen D., Kulig M., Labenz J., Leodolter A., Lind T., Meyer-Sabellek W., Vieth M., Willich S.N., Lindner D., Stolte M., et al. Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: An analysis based on the ProGERD Study. Aliment. Pharmacol. Ther. 2003;17:1515–1520. doi: 10.1046/j.1365-2036.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz P.O., Gerson L.B., Vela M.F. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am. J. Gastroenterol. 2013;108:308–328. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 7.Katz P.O., Dunbar K.B., Schnoll-Sussman F.H., Greer K.B., Yadlapati R., Spechler S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022;117:27–56. doi: 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag H.B., Graham D.Y., Satia J.A., Rabeneck L. Obesity Is an Independent Risk Factor for GERD Symptoms and Erosive Esophagitis. Am. J. Gastroenterol. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 9.Hampel H., Abraham N.S., El-Serag H.B. Meta-Analysis: Obesity and the Risk for Gastroesophageal Reflux Disease and Its Complications. Ann. Intern. Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Clapp B., Ponce J., DeMaria E., Ghanem O., Hutter M., Kothari S., LaMasters T., Kurian M., English W. American Society for Metabolic and Bariatric Surgery 2020 estimate of metabolic and bariatric procedures performed in the United States. Surg. Obes. Relat. Dis. 2022;18:1134–1140. doi: 10.1016/j.soard.2022.06.284. [DOI] [PubMed] [Google Scholar]
- 11.Gu L., Chen B., Du N., Fu R., Huang X., Mao F., Khadaroo P.A., Zhao S. Relationship Between Bariatric Surgery and Gastroesophageal Reflux Disease: A Systematic Review and Meta-analysis. Obes. Surg. 2019;29:4105–4113. doi: 10.1007/s11695-019-04218-3. [DOI] [PubMed] [Google Scholar]
- 12.Sheppard C.E., Sadowski D.C., de Gara C.J., Karmali S., Birch D.W. Rates of Reflux Before and After Laparoscopic Sleeve Gastrectomy for Severe Obesity. Obes. Surg. 2015;25:763–768. doi: 10.1007/s11695-014-1480-y. [DOI] [PubMed] [Google Scholar]
- 13.Tai C.-M., Huang C.-K., Lee Y.-C., Chang C.-Y., Lee C.-T., Lin J.-T. Increase in gastroesophageal reflux disease symptoms and erosive esophagitis 1 year after laparoscopic sleeve gastrectomy among obese adults. Surg. Endosc. 2013;27:1260–1266. doi: 10.1007/s00464-012-2593-9. [DOI] [PubMed] [Google Scholar]
- 14.Howard D.D., Caban A.M., Cendan J.C., Ben-David K. Gastroesophageal reflux after sleeve gastrectomy in morbidly obese patients. Surg. Obes. Relat. Dis. 2011;7:709–713. doi: 10.1016/j.soard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Matar R., Maselli D., Vargas E., Veeravich J., Bazerbachi F., Beran A., Storm A.C., Kellogg T., Abu Dayyeh B.K. Esophagitis After Bariatric Surgery: Large Cross-sectional Assessment of an Endoscopic Database. Obes. Surg. 2020;30:161–168. doi: 10.1007/s11695-019-04164-0. [DOI] [PubMed] [Google Scholar]
- 16.DuPree C.E., Blair K., Steele S.R., Martin M.J. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease: A national analysis. JAMA Surg. 2014;149:328–334. doi: 10.1001/jamasurg.2013.4323. [DOI] [PubMed] [Google Scholar]
- 17.Carter P.R., LeBlanc K.A., Hausmann M.G., Kleinpeter K.P., Debarros S.N., Jones S.M. Association between gastroesophageal reflux disease and laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2011;7:569–572. doi: 10.1016/j.soard.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Borbély Y., Schaffner E., Zimmermann L., Huguenin M., Plitzko G., Nett P., Kröll D. De novo gastroesophageal reflux disease after sleeve gastrectomy: Role of preoperative silent reflux. Surg. Endosc. 2019;33:789–793. doi: 10.1007/s00464-018-6344-4. [DOI] [PubMed] [Google Scholar]
- 19.Stierman B., Afful J., Carroll M.D., Chen T.C., Davy O., Fink S., Fryar C.D., Gu Q., Hales C.M., Hughes J.P., et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Center for Health Statistics; Hyattsville, MD, USA: 2021. National Health Statistics Reports. No 158. [DOI] [Google Scholar]
- 20.Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. [(accessed on 22 April 2023)];2000 Volume 894:i–xii, 1–253. World Health Organ Technical Report Series. Available online: https://apps.who.int/iris/handle/10665/42330. [PubMed] [Google Scholar]
- 21.Fisher B.L., Pennathur A., Mutnick J.L.M., Little A.G. Obesity Correlates with Gastroesophageal Reflux. Dig. Dis. Sci. 1999;44:2290–2294. doi: 10.1023/A:1026617106755. [DOI] [PubMed] [Google Scholar]
- 22.Nandurkar S., Locke G.R., 3rd, Fett S., Zinsmeister A.R., Cameron A.J., Talley N.J. Relationship between body mass index, diet, exercise and gastro-oesophageal reflux symptoms in a community. Aliment. Pharmacol. Ther. 2004;20:497–505. doi: 10.1111/j.1365-2036.2004.02156.x. [DOI] [PubMed] [Google Scholar]
- 23.Shaheen N., Provenzale D. The Epidemiology of Gastroesophageal Reflux Disease. Am. J. Med. Sci. 2003;326:264–273. doi: 10.1097/00000441-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Rubio M., Moreno-Elola-Olaso C., Rey E., Locke G.R., III, Rodriguez-Artalejo F. Symptoms of gastro-oesophageal reflux: Prevalence, severity, duration and associated factors in a Spanish population. Aliment. Pharmacol. Ther. 2004;19:95–105. doi: 10.1046/j.1365-2036.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- 25.Nocon M., Labenz J., Willich S.N. Lifestyle factors and symptoms of gastro-oesophageal reflux—A population-based study. Aliment. Pharmacol. Ther. 2006;23:169–174. doi: 10.1111/j.1365-2036.2006.02727.x. [DOI] [PubMed] [Google Scholar]
- 26.Murray L., Johnston B., Lane A., Harvey I., Donovan J., Nair P., Harvey R. Relationship between body mass and gastro-oesophageal reflux symptoms: The Bristol Helicobacter Project. Leuk. Res. 2003;32:645–650. doi: 10.1093/ije/dyg108. [DOI] [PubMed] [Google Scholar]
- 27.Rey E., Moreno-Elola-Olaso C., Artalejo F.R., Locke G.R., 3rd, Diaz-Rubio M. Association between weight gain and symptoms of gastroesophageal reflux in the general population. Am. J. Gastroenterol. 2006;101:229–233. doi: 10.1111/j.1572-0241.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M., Johnsen R., Ye W., Hveem K., Lagergren J. Obesity and Estrogen as Risk Factors for Gastroesophageal Reflux Symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66. [DOI] [PubMed] [Google Scholar]
- 29.Cook M.B., Greenwood D.C., Hardie L.J., Wild C.P., Forman D. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am. J. Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 30.El-Serag H.B., Hashmi A., Garcia J., Richardson P., Alsarraj A., Fitzgerald S., Vela M., Shaib Y., Abraham N.S., Velez M., et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: A case-control study. Gut. 2014;63:220–229. doi: 10.1136/gutjnl-2012-304189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang P., Friedenberg F. Obesity and GERD. Gastroenterol. Clin. N. Am. 2014;43:161–173. doi: 10.1016/j.gtc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson O.M., Beresford S.A., Kirk E.A., Bronner M.P., Vaughan T.L. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity. 2010;18:2204–2211. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider J.H., Küper M., Königsrainer A., Brücher B. Transient Lower Esophageal Sphincter Relaxation in Morbid Obesity. Obes. Surg. 2009;19:595–600. doi: 10.1007/s11695-009-9809-7. [DOI] [PubMed] [Google Scholar]
- 34.Martín-Pérez J., Arteaga-González I., Martín-Malagón A., Díaz-Luis H., Casanova-Trujillo C., Carrillo-Pallarés A.A. Frequency of abnormal esophageal acid exposure in patients eligible for bariatric surgery. Surg. Obes. Relat. Dis. 2014;10:1176–1180. doi: 10.1016/j.soard.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Wu J.C., Mui L., Cheung C.M., Chan Y., Sung J.J. Obesity Is Associated With Increased Transient Lower Esophageal Sphincter Relaxation. Gastroenterology. 2007;132:883–889. doi: 10.1053/j.gastro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 36.A Corley D., Kubo A., Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56:756–762. doi: 10.1136/gut.2006.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringhofer C., Lenglinger J., Riegler M., Kristo I., Kainz A., Schoppmann S.F. Waist to hip ratio is a better predictor of esophageal acid exposure than body mass index. Neurogastroenterol. Motil. 2017;29:e13033. doi: 10.1111/nmo.13033. [DOI] [PubMed] [Google Scholar]
- 38.Anand G., Katz P.O. Gastroesophageal Reflux Disease and Obesity. Gastroenterol. Clin. N. Am. 2010;39:39–46. doi: 10.1016/j.gtc.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Pandolfino J.E., El–Serag H.B., Zhang Q., Shah N., Ghosh S.K., Kahrilas P.J. Obesity: A Challenge to Esophagogastric Junction Integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Che F., Nguyen B., Cohen A., Nguyen N.T. Prevalence of hiatal hernia in the morbidly obese. Surg. Obes. Relat. Dis. 2013;9:920–924. doi: 10.1016/j.soard.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Suter M., Dorta G., Giusti V., Calmes J. Gastro-esophageal Reflux and Esophageal Motility Disorders in Morbidly Obese Patients. Obes. Surg. 2004;14:959–966. doi: 10.1381/0960892041719581. [DOI] [PubMed] [Google Scholar]
- 42.Yen H.-H., Tseng P.-H., Shih M.-C., Yang P.-J., Lin M.-T., Lee P.-C. Derangement of esophageal anatomy and motility in morbidly obese patients: A prospective study based on high-resolution impedance manometry. Surg. Obes. Relat. Dis. 2020;16:2006–2015. doi: 10.1016/j.soard.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Van Herwaarden M.A., Samsom M., Smout A.J. Excess gastroesophageal reflux in patients with hiatus hernia is caused by mechanisms other than transient LES relaxations. Gastroenterology. 2000;119:1439–1446. doi: 10.1053/gast.2000.20191. [DOI] [PubMed] [Google Scholar]
- 44.Wisén O., Johansson C. Gastrointestinal function in obesity: Motility, secretion, and absorption following a liquid test meal. Metabolism. 1992;41:390–395. doi: 10.1016/0026-0495(92)90073-J. [DOI] [PubMed] [Google Scholar]
- 45.Harter R.L., Kelly W.B., Kramer M.G., Perez C.E., Dzwonczyk R.R. A comparison of the volume and pH of gastric contents of obese and lean surgical patients. Anesth. Analg. 1998;86:147–152. doi: 10.1213/00000539-199801000-00030. [DOI] [PubMed] [Google Scholar]
- 46.Mercer C.D., Wren S.F., Dacosta L.R., Beck I.T. Lower esophageal sphincter pressure and gastroesophageal pressure gradients in excessively obese patients. J. Med. 1987;18:135–146. doi: 10.1097/00132586-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Sharara A.I., Rustom L.B.O., Daher H.B., Rimmani H.H., Shayto R.H., Minhem M., Ichkhanian Y., Aridi H., Al-Abbas A., Shaib Y., et al. Prevalence of gastroesophageal reflux and risk factors for erosive esophagitis in obese patients considered for bariatric surgery. Dig. Liver Dis. 2019;51:1375–1379. doi: 10.1016/j.dld.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Merrouche M., Sabaté J.-M., Jouet P., Harnois F., Scaringi S., Coffin B., Msika S. Gastro-Esophageal Reflux and Esophageal Motility Disorders in Morbidly Obese Patients before and after Bariatric Surgery. Obes. Surg. 2007;17:894–900. doi: 10.1007/s11695-007-9166-3. [DOI] [PubMed] [Google Scholar]
- 49.Alalwan A.A., Friedman J., Park H., Segal R., Brumback B.A., Hartzema A.G. US national trends in bariatric surgery: A decade of study. Surgery. 2021;170:13–17. doi: 10.1016/j.surg.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Peterli R., Wölnerhanssen B.K., Peters T., Vetter D., Kröll D., Borbély Y., Schultes B., Beglinger C., Drewe J., Schiesser M., et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients with Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA. 2018;319:255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rrezza E.E., Ikramuddin S., Gourash W., Rakitt T., Kingston A., Luketich J., Schauer P.R. Symptomatic improvement in gastroesophageal reflux disease (GERD) following laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 2002;16:1027–1031. doi: 10.1007/s00464-001-8313-5. [DOI] [PubMed] [Google Scholar]
- 52.Perry Y., Courcoulas A.P., Fernando H.C., Buenaventura P.O., McCaughan J.S., Luketich J.D. Laparoscopic Roux-en-Y Gastric Bypass for Recalcitrant Gastroesophageal Reflux Disease in Morbidly Obese Patients. J. Soc. Laparoendosc. Surg. 2004;8:19–23. [PMC free article] [PubMed] [Google Scholar]
- 53.Alghamdi S., Mirghani H., Alhazmi K., Alatawi A.M., Brnawi H., Alrasheed T., Badoghaish W. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy effects on obesity comorbidities: A systematic review and meta-analysis. Front. Surg. 2022;9:953804. doi: 10.3389/fsurg.2022.953804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engevik A.C., Kaji I., Goldenring J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020;100:573–602. doi: 10.1152/physrev.00016.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siilin H., Wanders A., Gustavsson S., Sundbom M. The Proximal Gastric Pouch Invariably Contains Acid-Producing Parietal Cells in Roux-en-Y Gastric Bypass. Obes. Surg. 2005;15:771–777. doi: 10.1381/0960892054222849. [DOI] [PubMed] [Google Scholar]
- 56.Love M.W., Verna D.F., Kothari S.N., Scott J.D. Outcomes of Bariatric Surgery With Concomitant Hiatal Hernia Repair Using an Absorbable Tissue Matrix. Am. Surg. 2023;89:293–299. doi: 10.1177/00031348211023450. [DOI] [PubMed] [Google Scholar]
- 57.Kothari V., Shaligram A., Reynoso J., Schmidt E., McBride C.L., Oleynikov D. Impact on Perioperative Outcomes of Concomitant Hiatal Hernia Repair with Laparoscopic Gastric Bypass. Obes. Surg. 2012;22:1607–1610. doi: 10.1007/s11695-012-0714-0. [DOI] [PubMed] [Google Scholar]
- 58.Juodeikis Ž., Brimas G. Long-term results after sleeve gastrectomy: A systematic review. Surg. Obes. Relat. Dis. 2017;13:693–699. doi: 10.1016/j.soard.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Bou Daher H., Sharara A.I. Gastroesophageal reflux disease, obesity and laparoscopic sleeve gastrectomy: The burning questions. World J. Gastroenterol. 2019;25:4805–4813. doi: 10.3748/wjg.v25.i33.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genco A., Soricelli E., Casella G., Maselli R., Castagneto-Gissey L., Di Lorenzo N., Basso N. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: A possible, underestimated long-term complication. Surg. Obes. Relat. Dis. 2017;13:568–574. doi: 10.1016/j.soard.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 61.Sebastianelli L., Benois M., Vanbiervliet G., Bailly L., Robert M., Turrin N., Gizard E., Foletto M., Bisello M., Albanese A., et al. Systematic Endoscopy 5 Years After Sleeve Gastrectomy Results in a High Rate of Barrett’s Esophagus: Results of a Multicenter Study. Obes. Surg. 2019;29:1462–1469. doi: 10.1007/s11695-019-03704-y. [DOI] [PubMed] [Google Scholar]
- 62.Qumseya B.J., Qumsiyeh Y., Ponniah S.A., Estores D., Yang D., Johnson-Mann C.N., Friedman J., Ayzengart A., Draganov P.V. Barrett’s esophagus after sleeve gastrectomy: A systematic review and meta-analysis. Gastrointest. Endosc. 2021;93:343–352.e2. doi: 10.1016/j.gie.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 63.Yeung K.T.D., Penney N., Ashrafian L., Darzi A., Ashrafian H. Does Sleeve Gastrectomy Expose the Distal Esophagus to Severe Reflux? A Systematic Review and Meta-analysis. Ann. Surg. 2020;271:257–265. doi: 10.1097/SLA.0000000000003275. [DOI] [PubMed] [Google Scholar]
- 64.Campos G.M., Mazzini G.S., Altieri M.S., Docimo S., Jr., DeMaria E.J., Rogers A.M. ASMBS position statement on the rationale for performance of upper gastrointestinal endoscopy before and after metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2021;17:837–847. doi: 10.1016/j.soard.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Felinska E., Billeter A., Nickel F., Contin P., Berlth F., Chand B., Grimminger P., Mikami D., Schoppmann S.F., Müller-Stich B. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann. N. Y. Acad. Sci. 2020;1482:26–35. doi: 10.1111/nyas.14467. [DOI] [PubMed] [Google Scholar]
- 66.Keidar A., Appelbaum L., Schweiger C., Elazary R., Baltasar A. Dilated Upper Sleeve Can be Associated with Severe Postoperative Gastroesophageal Dysmotility and Reflux. Obes. Surg. 2010;20:140–147. doi: 10.1007/s11695-009-0032-3. [DOI] [PubMed] [Google Scholar]
- 67.Spivak H., Rubin M., Sadot E., Pollak E., Feygin A., Goitein D. Laparoscopic Sleeve Gastrectomy Using 42-French versus 32-French Bougie: The First-Year Outcome. Obes. Surg. 2014;24:1090–1093. doi: 10.1007/s11695-014-1199-9. [DOI] [PubMed] [Google Scholar]
- 68.Chang P.-C., Chen K.-H., Jhou H.-J., Chen P.-H., Huang C.-K., Lee C.-H., Chang T.-W. Promising effects of 33 to 36 Fr. bougie calibration for laparoscopic sleeve gastrectomy: A systematic review and network meta-analysis. Sci. Rep. 2021;11:15217. doi: 10.1038/s41598-021-94716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., Yi X.Y., Gong L.L., Li Q.F., Zhang J., Wang Z.H. The effectiveness and safety of laparoscopic sleeve gastrectomy with different sizes of bougie calibration: A systematic review and meta-analysis. Int. J. Surg. 2018;49:32–38. doi: 10.1016/j.ijsu.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Zarzycki P., Rymarowicz J., Małczak P., Pisarska-Adamczyk M., Mulek R., Binda A., Dowgiałło-Gornowicz N., Major P. PROSS Collaborative Study Group Differences in Technical Aspects of Primary Sleeve Gastrectomy Prior to Redo Bariatric Surgery—A Multicenter Cohort Study (PROSS Study) Medicina. 2023;59:799. doi: 10.3390/medicina59040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dhanabalsamy N., Rammohan R., Quirante F.P., Montorfano L., Menzo E.L., Szomstein S., Rosenthal R. Laparoscopic Sleeve Gastrectomy: Does Bougie Size Affect Weight Loss? Surg. Obes. Relat. Dis. 2016;12:S145. doi: 10.1016/j.soard.2016.08.263. [DOI] [Google Scholar]
- 72.Garay M., Balagué C., Rodríguez-Otero C., Gonzalo B., Domenech A., Pernas J.C., Gich I.J., Miñambres I., Fernández-Ananín S., Targarona E.M. Influence of antrum size on gastric emptying and weight-loss outcomes after laparoscopic sleeve gastrectomy (preliminary analysis of a randomized trial) Surg. Endosc. 2018;32:2739–2745. doi: 10.1007/s00464-017-5972-4. [DOI] [PubMed] [Google Scholar]
- 73.Kristo I., Paireder M., Jomrich G., Felsenreich D.M., Nikolic M., Langer F.B., Prager G., Schoppmann S.F. Modern Esophageal Function Testing and Gastroesophageal Reflux Disease in Morbidly Obese Patients. Obes. Surg. 2019;29:3536–3541. doi: 10.1007/s11695-019-04020-1. [DOI] [PubMed] [Google Scholar]
- 74.Küper M.A., Kramer K.M., Kischniak A., Zdichavsky M., Schneider J.H., Stüker D., Kratt T., Königsrainer A., Granderath F.A. Dysfunction of the lower esophageal sphincter and dysmotility of the tubular esophagus in morbidly obese patients. Obes. Surg. 2009;19:1143–1149. doi: 10.1007/s11695-009-9881-z. [DOI] [PubMed] [Google Scholar]
- 75.Ying V.W.C., Kim S.H.H., Khan K.J., Farrokhyar F., D’souza J., Gmora S., Anvari M., Hong D. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: A systematic review and meta-analysis of cohort studies. Surg. Endosc. 2015;29:1018–1023. doi: 10.1007/s00464-014-3794-1. [DOI] [PubMed] [Google Scholar]
- 76.Akiyama J., Hosaka H., Kuribayashi S., Moriyasu S., Hisada Y., Okubo H., Watanabe K., Imbe K., Nagata N., Kojima Y., et al. Efficacy of Vonoprazan, a Novel Potassium-Competitive Acid Blocker, in Patients with Proton Pump Inhibitor-Refractory Acid Reflux. Digestion. 2020;101:174–183. doi: 10.1159/000497775. [DOI] [PubMed] [Google Scholar]
- 77.Shirai Y., Kawami N., Iwakiri K., Kuwana M. Use of vonoprazan, a novel potassium-competitive acid blocker, for the treatment of proton pump inhibitor-refractory reflux esophagitis in patients with systemic sclerosis. J. Scleroderma Relat. Disord. 2022;7:57–61. doi: 10.1177/23971983211021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laine L., DeVault K., Katz P., Mitev S., Lowe J., Hunt B., Spechler S. Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology. 2023;164:61–71. doi: 10.1053/j.gastro.2022.09.041. [DOI] [PubMed] [Google Scholar]
- 79.Niikura R., Yamada A., Hirata Y., Hayakawa Y., Takahashi A., Shinozaki T., Takeuchi Y., Fujishiro M., Koike K. Efficacy of Vonoprazan for Gastroesophageal Reflux Symptoms in Patients with Proton Pump Inhibitor-resistant Non-erosive Reflux Disease. Intern. Med. 2018;57:2443–2450. doi: 10.2169/internalmedicine.0492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shinozaki S., Osawa H., Hayashi Y., Miura Y., Lefor A.K., Yamamoto H. Long-term vonoprazan therapy is effective for controlling symptomatic proton pump inhibitor-resistant gastroesophageal reflux disease. Biomed. Rep. 2021;14:32. doi: 10.3892/br.2021.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slater B.J., Collings A., Dirks R., Gould J.C., Qureshi A.P., Juza R., Rodríguez-Luna M.R., Wunker C., Kohn G.P., Kothari S., et al. Multi-society consensus conference and guideline on the treatment of gastroesophageal reflux disease (GERD) Surg. Endosc. 2023;37:781–806. doi: 10.1007/s00464-022-09817-3. [DOI] [PubMed] [Google Scholar]
- 82.Perez A.R., Moncure A.C., Rattner D.W. Obesity adversely affects the outcome of antireflux operations. Surg. Endosc. 2001;15:986–989. doi: 10.1007/s004640000392. [DOI] [PubMed] [Google Scholar]
- 83.Morgenthal C.B., Lin E., Shane M.D., Hunter J.G., Smith C.D. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg. Endosc. 2007;21:1978–1984. doi: 10.1007/s00464-007-9490-7. [DOI] [PubMed] [Google Scholar]
- 84.Kellogg T.A., Andrade R., Maddaus M., Slusarek B., Buchwald H., Ikramuddin S. Anatomic findings and outcomes after antireflux procedures in morbidly obese patients undergoing laparoscopic conversion to Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2007;3:52–57. doi: 10.1016/j.soard.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Prachand V.N., Alverdy J.C. Gastroesophageal reflux disease and severe obesity: Fundoplication or bariatric surgery? World J. Gastroenterol. 2010;16:3757–3761. doi: 10.3748/wjg.v16.i30.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levy J.L., Levine M.S., E Rubesin S., Williams N.N., Dumon K.R. Stenosis of gastric sleeve after laparoscopic sleeve gastrectomy: Clinical, radiographic and endoscopic findings. Br. J. Radiol. 2018;91:20170702. doi: 10.1259/bjr.20170702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Braghetto I., Korn O., Gutiérrez L., Torrealba A., Rojas J. Gastroesophageal Symptoms after Laparoscopic Gastric Bypass: Mistakes in Performing the Procedure? Arq. Bras. Cir. Dig. 2022;35:e1657. doi: 10.1590/0102-672020210002e1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim R., Beekley A., Johnson D.C., A Davis K. Early and late complications of bariatric operation. Trauma Surg. Acute Care Open. 2018;3:e000219. doi: 10.1136/tsaco-2018-000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang S.H., Popov V.B., Thompson C.C. Endoscopic balloon dilation for treatment of sleeve gastrectomy stenosis: A systematic review and meta-analysis. Gastrointest. Endosc. 2020;91:989–1002.e4. doi: 10.1016/j.gie.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 90.Mazer L., Yu J.X., Bhalla S., Platt K., Watts L., Volk S., Schulman A.R. Pneumatic Balloon Dilation of Gastric Sleeve Stenosis Is Not Associated with Weight Regain. Obes. Surg. 2022;32:1–6. doi: 10.1007/s11695-022-05957-6. [DOI] [PubMed] [Google Scholar]
- 91.Dhorepatil A.S., Cottam D., Surve A., Medlin W., Zaveri H., Richards C., Cottam A. Is pneumatic balloon dilation safe and effective primary modality of treatment for post-sleeve gastrectomy strictures? A retrospective study. BMC Surg. 2018;18:52. doi: 10.1186/s12893-018-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurien R., Menon S. Balloon dilation in sleeve gastrectomy stenosis: A simple solution to an occasionally tricky problem. Gastrointest. Endosc. 2020;91:1003–1004. doi: 10.1016/j.gie.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 93.Rossi T.R., Dynda D.I., Estes N.C., Marshall J.S. Stricture dilation after laparoscopic Roux-en-Y gastric bypass. Am. J. Surg. 2005;189:357–360. doi: 10.1016/j.amjsurg.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 94.Skidmore A.P. Use of lumen-apposing metal stents (LAMS) in the management of gastro jejunostomy stricture following Roux-en-Y Gastric Bypass for obesity: A prospective series. BMC Surg. 2021;21:314. doi: 10.1186/s12893-021-01310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansoor M.S., Tejada J., A Parsa N., Yoon E., Hida S. Off label use of lumen-apposing metal stent for persistent gastro-jejunal anastomotic stricture. World J. Gastrointest. Endosc. 2018;10:117–120. doi: 10.4253/wjge.v10.i6.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uchima H., Abu-Suboh M., Mata A., Cruz M., Espinos J. Lumen-apposing metal stent for the treatment of refractory gastrojejunal anastomotic stricture after laparoscopic gastric bypass. Gastrointest. Endosc. 2016;83:251. doi: 10.1016/j.gie.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Filho A.J.B., Kondo W., Nassif L.S., Garcia M.J., Tirapelle R.D.A., Dotti C.M. Gastrogastric Fistula: A Possible Complication of Roux-En-Y Gastric Bypass. J. Soc. Laparoendosc. Surg. 2006;10:326–331. [PMC free article] [PubMed] [Google Scholar]
- 98.Alyaqout K., Almazeedi S., Alhaddad M., Efthimiou E., Loureiro M.d.P. Gastrogastric Fistula after Roux-en-y Gastric Bypass: A Case Report and Review of Literature. Arq. Bras. Cir. Dig. 2020;33:e1509. doi: 10.1590/0102-672020190001e1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei M.T., Ahn J.Y., Friedland S. Over-the-Scope Clip in the Treatment of Gastrointestinal Leaks and Perforations. Clin. Endosc. 2021;54:798–804. doi: 10.5946/ce.2021.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rogalski P., Swidnicka-Siergiejko A., Wasielica-Berger J., Zienkiewicz D., Wieckowska B., Wroblewski E., Baniukiewicz A., Rogalska-Plonska M., Siergiejko G., Dabrowski A., et al. Endoscopic management of leaks and fistulas after bariatric surgery: A systematic review and meta-analysis. Surg. Endosc. 2021;35:1067–1087. doi: 10.1007/s00464-020-07471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niland B., Brock A. Over-the-scope clip for endoscopic closure of gastrogastric fistulae. Surg. Obes. Relat. Dis. 2016;13:15–20. doi: 10.1016/j.soard.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Kumar N., Larsen M.C., Thompson C.C. Endoscopic Management of Gastrointestinal Fistulae. Gastroenterol. Hepatol. 2014;10:495–502. [PMC free article] [PubMed] [Google Scholar]
- 103.Tsai C., Kessler U., Steffen R., Merki H., Zehetner J. Endoscopic Closure of Gastro-gastric Fistula After Gastric Bypass: A Technically Feasible Procedure but Associated with Low Success Rate. Obes. Surg. 2019;29:23–27. doi: 10.1007/s11695-018-3488-1. [DOI] [PubMed] [Google Scholar]
- 104.Sulz M.C., Bertolini R., Frei R., Semadeni G.-M., Borovicka J., Meyenberger C. Multipurpose use of the over-the-scope-clip system (“Bear claw”) in the gastrointestinal tract: Swiss experience in a tertiary center. World J. Gastroenterol. 2014;20:16287–16292. doi: 10.3748/wjg.v20.i43.16287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernandez-Esparrach G., Lautz D.B., Thompson C.C. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: A less-invasive approach. Surg. Obes. Relat. Dis. 2010;6:282–288. doi: 10.1016/j.soard.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 106.Dolan R.D., Jirapinyo P., Maahs E.D., Thompson C.C. Endoscopic closure versus surgical revision in the management of gastro-gastric fistula following Roux-en-Y gastric bypass. Endosc. Int. Open. 2023;11:E629–E634. doi: 10.1055/a-2037-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pina L., Wood G.C., Richardson S., Obradovic V., Petrick A., Parker D.M. Bariatric revisional surgery for gastrogastric fistula following Roux-en-Y gastric bypass positively impacts weight loss. Surg. Obes. Relat. Dis. 2023;19:626–631. doi: 10.1016/j.soard.2022.12.022. [DOI] [PubMed] [Google Scholar]
- 108.Tsai Y.-N., Wang H.-P., Huang C.-K., Chang P.-C., Lin I.-C., Tai C.-M. Endoluminal stenting for the management of leak following sleeve gastrectomy and loop duodenojejunal bypass with sleeve gastrectomy. Kaohsiung J. Med. Sci. 2018;34:43–48. doi: 10.1016/j.kjms.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chung Y., Park D.G., Kim Y.J. Endoscopic Management of Staple Line Leak after Bariatric Surgery: Surgeon’s Perspective. Clin. Endosc. 2021;54:805–809. doi: 10.5946/ce.2020.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christou N.V., Look D., MacLean L.D. Weight Gain After Short- and Long-Limb Gastric Bypass in Patients Followed for Longer Than 10 Years. Ann. Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hort A., Cheng Q., Morosin T., Yoon P., Talbot M. Optimal common limb length in Roux-en-Y gastric bypass surgery: Is it important for an ideal outcome?—A systematic review. ANZ J. Surg. 2023;93:851–858. doi: 10.1111/ans.18192. [DOI] [PubMed] [Google Scholar]
- 112.Novikov A.A., Afaneh C., Saumoy M., Parra V., Shukla A., Dakin G.F., Pomp A., Dawod E., Shah S., Aronne L.J., et al. Endoscopic Sleeve Gastroplasty, Laparoscopic Sleeve Gastrectomy, and Laparoscopic Band for Weight Loss: How Do They Compare? J. Gastrointest. Surg. 2018;22:267–273. doi: 10.1007/s11605-017-3615-7. [DOI] [PubMed] [Google Scholar]
- 113.Sharaiha R.Z., Kedia P., Kumta N., Aronne L.J., Kahaleh M. Endoscopic sleeve plication for revision of sleeve gastrectomy. Gastrointest. Endosc. 2015;81:1004. doi: 10.1016/j.gie.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 114.Maselli D.B., Alqahtani A.R., Abu Dayyeh B.K., Elahmedi M., Storm A.C., Matar R., Nieto J., Teixeira A., Al Khatry M., Neto M.G., et al. Revisional endoscopic sleeve gastroplasty of laparoscopic sleeve gastrectomy: An international, multicenter study. Gastrointest. Endosc. 2021;93:122–130. doi: 10.1016/j.gie.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 115.de Moura D.T.H., Barrichello S., Jr., de Moura E.G.H., de Souza T.F., Dos Passos Galvão Neto M., Grecco E., Sander B., Hoff A.C., Matz F., Ramos F., et al. Endoscopic sleeve gastroplasty in the management of weight regain after sleeve gastrectomy. Endoscopy. 2020;52:202–210. doi: 10.1055/a-1086-0627. [DOI] [PubMed] [Google Scholar]
- 116.Eid G. Sleeve gastrectomy revision by endoluminal sleeve plication gastroplasty: A small pilot case series. Surg. Endosc. 2017;31:4252–4255. doi: 10.1007/s00464-017-5469-1. [DOI] [PubMed] [Google Scholar]
- 117.Fayad L., Adam A., Schweitzer M., Cheskin L.J., Ajayi T., Dunlap M., Badurdeen D.S., Hill C., Paranji N., Lalezari S., et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: A case-matched study. Gastrointest. Endosc. 2019;89:782–788. doi: 10.1016/j.gie.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 118.Fogel R., De Fogel J., Bonilla Y., De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest. Endosc. 2008;68:51–58. doi: 10.1016/j.gie.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 119.Brethauer S.A., Chand B., Schauer P.R., Thompson C.C. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg. Obes. Relat. Dis. 2012;8:296–303. doi: 10.1016/j.soard.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 120.Olmi S., Cesana G., Gambioli A., Bonaldi M., Ferrari D., Uccelli M., Ciccarese F., Stefano D.C., Riccardo G., Lorenzo M. Effect of laparoscopic sleeve gastrectomy vs laparoscopic sleeve + Rossetti fundoplication on weight loss and de novo GERD in patients affected by morbid obesity: A randomized clinical study. Obes. Surg. 2022;32:1451–1458. doi: 10.1007/s11695-022-05955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olmi S., Uccelli M., Cesana G.C., Ciccarese F., Oldani A., Giorgi R., De Carli S.M., Villa R., Zanoni A.A.G., Bonaldi M. Modified laparoscopic sleeve gastrectomy with Rossetti antireflux fundoplication: Results after 220 procedures with 24-month follow-up. Surg. Obes. Relat. Dis. 2020;16:1202–1211. doi: 10.1016/j.soard.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 122.Uccelli M., Cesana G.C., Ciccarese F., Oldani A., Giorgi R., De Carli S.M., Villa R., Zanoni A.A.G., Ismail A., Di Capua F., et al. Laparoscopic sleeve gastrectomy with Rossetti fundoplication: Long-term (5-year) follow-up. Surg. Obes. Relat. Dis. 2022;18:1199–1205. doi: 10.1016/j.soard.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 123.Nocca D., Gautier T., Nedelcu M. Comment on: Laparoscopic sleeve gastrectomy with Rossetti fundoplication: Long-term (5-year) follow-up. Surg. Obes. Relat. Dis. 2022;18:1207–1208. doi: 10.1016/j.soard.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 124.Di Capua F., Cesana G.C., Uccelli M., De Carli S.M., Giorgi R., Ferrari D., Olmi S. Sleeve Gastrectomy with Rossetti Fundoplication Increases Lower Esophageal Sphincter Tone Preventing Gastroesophageal Reflux Disease: High-Resolution Manometry Assessment. J. Laparoendosc. Adv. Surg. Tech. 2023;33:44–51. doi: 10.1089/lap.2022.0123. [DOI] [PubMed] [Google Scholar]
- 125.Nocca D., Skalli E.M., Boulay E., Nedelcu M., Fabre J.M., Loureiro M. Nissen Sleeve (N-Sleeve) operation: Preliminary results of a pilot study. Surg. Obes. Relat. Dis. 2016;12:1832–1837. doi: 10.1016/j.soard.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 126.Ben Amor I., Casanova V., Vanbiervliet G., Bereder J.M., Habitan R., Kassir R., Gugenheim J. The Nissen-Sleeve (N-Sleeve): Results of a Cohort Study. Obes. Surg. 2020;30:3267–3272. doi: 10.1007/s11695-020-04469-5. [DOI] [PubMed] [Google Scholar]
- 127.Moon R.C., Teixeira A.F., Jawad M.A. Safety and effectiveness of anterior fundoplication sleeve gastrectomy in patients with severe reflux. Surg. Obes. Relat. Dis. 2017;13:547–552. doi: 10.1016/j.soard.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 128.Olmi S., Cesana G., D’angiolella L., Bonaldi M., Uccelli M., Mantovani L. Sleeve gastrectomy with tailored 360° fundoplication according to Rossetti in patients affected by obesity and gastroesophageal reflux: A prospective observational study. Surg. Obes. Relat. Dis. 2021;17:1057–1065. doi: 10.1016/j.soard.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 129.Lasnibat J.P., Braghetto I., Gutierrez L., Sanchez F. Sleeve Gastrectomy and Fundoplication as A Single Procedure in Patients with Obesity and Gastroesophageal Reflux. Arq. Bras. Cir. Dig. 2017;30:216–221. doi: 10.1590/0102-6720201700030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Le Page P.A., Martin D. Laparoscopic Partial Sleeve Gastrectomy with Fundoplication for Gastroesophageal Reflux and Delayed Gastric Emptying. World J. Surg. 2015;39:1460–1464. doi: 10.1007/s00268-015-2981-0. [DOI] [PubMed] [Google Scholar]
- 131.Da Silva L.E., Alves M.M., El-Ajouz T.K., Ribeiro P.C.P., Cruz R.J. Laparoscopic Sleeve-Collis-Nissen Gastroplasty: A Safe Alternative for Morbidly Obese Patients with Gastroesophageal Reflux Disease. Obes. Surg. 2015;25:1217–1222. doi: 10.1007/s11695-014-1523-4. [DOI] [PubMed] [Google Scholar]
- 132.del Genio G., Tolone S., Gambardella C., Brusciano L., Volpe M.L., Gualtieri G., del Genio F., Docimo L. Sleeve Gastrectomy and Anterior Fundoplication (D-SLEEVE) Prevents Gastroesophageal Reflux in Symptomatic GERD. Obes. Surg. 2020;30:1642–1652. doi: 10.1007/s11695-020-04427-1. [DOI] [PubMed] [Google Scholar]
- 133.Castagneto-Gissey L., Russo M.F., D’andrea V., Genco A., Casella G. Efficacy of Sleeve Gastrectomy with Concomitant Hiatal Hernia Repair versus Sleeve–Fundoplication on Gastroesophageal Reflux Disease Resolution: Systematic Review and Meta-Analysis. J. Clin. Med. 2023;12:3323. doi: 10.3390/jcm12093323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carandina S., Zulian V., Nedelcu A., Danan M., Vilallonga R., Nocca D., Nedelcu M. Is It Safe to Combine a Fundoplication to Sleeve Gastrectomy? Review of Literature. Medicina. 2021;57:392. doi: 10.3390/medicina57040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Madhok B.M., Carr W.R.J., McCormack C., Boyle M., Jennings N., Schroeder N., Balupuri S., Small P.K. Preoperative endoscopy may reduce the need for revisional surgery for gastro-oesophageal reflux disease following laparoscopic sleeve gastrectomy. Clin. Obes. 2016;6:268–272. doi: 10.1111/cob.12153. [DOI] [PubMed] [Google Scholar]
- 136.Langer F.B., Bohdjalian A., Shakeri-Leidenmühler S., Schoppmann S.F., Zacherl J., Prager G. Conversion from Sleeve Gastrectomy to Roux-en-Y Gastric Bypass—Indications and Outcome. Obes. Surg. 2010;20:835–840. doi: 10.1007/s11695-010-0125-z. [DOI] [PubMed] [Google Scholar]
- 137.Salminen P., Helmiö M., Ovaska J., Juuti A., Leivonen M., Peromaa-Haavisto P., Hurme S., Soinio M., Nuutila P., Victorzon M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA. 2018;319:241–354. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parmar C.D., Mahawar K.K., Boyle M., Schroeder N., Balupuri S., Small P.K. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass is Effective for Gastro-Oesophageal Reflux Disease but not for Further Weight Loss. Obes. Surg. 2017;27:1651–1658. doi: 10.1007/s11695-017-2542-8. [DOI] [PubMed] [Google Scholar]
- 139.Abdemur A., Han S.-M., Lo Menzo E., Szomstein S., Rosenthal R. Reasons and outcomes of conversion of laparoscopic sleeve gastrectomy to Roux-en-Y gastric bypass for nonresponders. Surg. Obes. Relat. Dis. 2016;12:113–118. doi: 10.1016/j.soard.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Hendricks L., Alvarenga E., Dhanabalsamy N., Menzo E.L., Szomstein S., Rosenthal R. Impact of sleeve gastrectomy on gastroesophageal reflux disease in a morbidly obese population undergoing bariatric surgery. Surg. Obes. Relat. Dis. 2016;12:511–517. doi: 10.1016/j.soard.2015.08.507. [DOI] [PubMed] [Google Scholar]
- 141.Gautier T., Sarcher T., Contival N., Le Roux Y., Alves A. Indications and Mid-Term Results of Conversion from Sleeve Gastrectomy to Roux-en-Y Gastric Bypass. Obes. Surg. 2013;23:212–215. doi: 10.1007/s11695-012-0782-1. [DOI] [PubMed] [Google Scholar]
- 142.Strauss A.L., Triggs J.R., Tewksbury C.M., Soriano I., Wernsing D.S., Dumon K.R., Williams N.N., Shao J.M. Conversion to Roux-En-Y Gastric Bypass: A successful means of mitigating reflux after laparoscopic sleeve gastrectomy. Surg. Endosc. 2023;37:5374–5379. doi: 10.1007/s00464-023-10024-x. [DOI] [PubMed] [Google Scholar]
- 143.El Chaar M., Stoltzfus J., Claros L., Miletics M. Indications for Revisions Following 630 Consecutive Laparoscopic Sleeve Gastrectomy Cases: Experience in a Single Accredited Center. J. Gastrointest. Surg. 2017;21:12–16. doi: 10.1007/s11605-016-3215-y. [DOI] [PubMed] [Google Scholar]
- 144.Felsenreich D.M., Steinlechner K., Langer F.B., Vock N., Eichelter J., Bichler C., Jedamzik J., Mairinger M., Kristo I., Prager G. Outcome of Sleeve Gastrectomy Converted to Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes. Surg. 2022;32:643–651. doi: 10.1007/s11695-021-05866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng B.-Q., Zhang G.-X., Chen G., Cheng Z., Hu J.-K., Du X. Gastroesophageal reflux disease complicating laparoscopic sleeve gastrectomy: Current knowledge and surgical therapies. Surg. Obes. Relat. Dis. 2020;16:1145–1155. doi: 10.1016/j.soard.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 146.Pescarus R., Sharata A.M., Dunst C.M., Shlomovitz E., Swanström L.L., Reavis K.M. Hill procedure for recurrent GERD post-Roux-en-Y gastric bypass. Surg. Endosc. 2016;30:2141–2412. doi: 10.1007/s00464-015-4442-0. [DOI] [PubMed] [Google Scholar]
- 147.Vilallonga R., Sanchez-Cordero S., Umpiérrez Mayor N., Molina A., Cirera de Tudela A., Ruiz-Úcar E., Carrasco M.A. GERD after Bariatric Surgery. Can We Expect Endoscopic Findings? Medicina. 2021;57:506. doi: 10.3390/medicina57050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kassir R., Kassir R., Deparseval B., Bekkar S., Serayssol C., Favre O., Garnier P.P. Routine surveillance endoscopy before and after sleeve gastrectomy? World J. Gastrointest. Endosc. 2019;11:1–4. doi: 10.4253/wjge.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jaruvongvanich V., Osman K., Matar R., Baroud S., Hanada Y., Chesta F., Maselli D.B., Mahmoud T., Wang K.K., Abu Dayyeh B.K. Impact of bariatric surgery on surveillance and treatment outcomes of Barrett’s esophagus: A stage-matched cohort study. Surg. Obes. Relat. Dis. 2021;17:1457–1464. doi: 10.1016/j.soard.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 150.Carabotti M., Avallone M., Cereatti F., Paganini A., Greco F., Scirocco A., Severi C., Silecchia G. Usefulness of Upper Gastrointestinal Symptoms as a Driver to Prescribe Gastroscopy in Obese Patients Candidate to Bariatric Surgery. A Prospective Study. Obes. Surg. 2016;26:1075–1080. doi: 10.1007/s11695-015-1861-x. [DOI] [PubMed] [Google Scholar]
- 151.Rausa E., Manfredi R., Kelly M.E., Bianco F., Aiolfi A., Bonitta G., Lucianetti A., Zappa M.A. Magnetic Sphincter Augmentation Placement for Recalcitrant Gastroesophageal Reflux Disease Following Bariatric Procedures: A Systematic Review and Bayesian Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. 2021;31:1034–1039. doi: 10.1089/lap.2020.0763. [DOI] [PubMed] [Google Scholar]
- 152.Khaitan L., Hill M., Michel M., Chiasson P., Woodworth P., Bell R., Sadek R., Hoffman A., Loing K., Veldhuis P., et al. Feasibility and Efficacy of Magnetic Sphincter Augmentation for the Management of Gastroesophageal Reflux Disease Post-Sleeve Gastrectomy for Obesity. Obes. Surg. 2023;33:387–396. doi: 10.1007/s11695-022-06381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Broderick R.C., Smith C.D., Cheverie J.N., Omelanczuk P., Lee A.M., Dominguez-Profeta R., Cubas R., Jacobsen G.R., Sandler B.J., Fuchs K.-H., et al. Magnetic sphincter augmentation: A viable rescue therapy for symptomatic reflux following bariatric surgery. Surg. Endosc. 2020;34:3211–3215. doi: 10.1007/s00464-019-07096-z. [DOI] [PubMed] [Google Scholar]
- 154.Riva C.G., Asti E., Lazzari V., Aquilino K., Siboni S., Bonavina L. Magnetic Sphincter Augmentation after Gastric Surgery. J. Soc. Laparosc. Robot. Surg. 2019;23:e2019.00035. doi: 10.4293/jsls.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hawasli A., Sadoun M., Meguid A., Dean M., Sahly M., Hawasli B. Laparoscopic placement of the LINX® system in management of severe reflux after sleeve gastrectomy. Am. J. Surg. 2019;217:496–499. doi: 10.1016/j.amjsurg.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 156.Desart K., Rossidis G., Michel M., Lux T., Ben-David K. Gastroesophageal Reflux Management with the LINX® System for Gastroesophageal Reflux Disease Following Laparoscopic Sleeve Gastrectomy. J. Gastrointest. Surg. 2015;19:1782–1786. doi: 10.1007/s11605-015-2887-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.