Abstract
(1) Background: Sarcopenia has gained much interest in recent years due to an increase in morbidity. Sarcopenia is associated with type 2 diabetes mellitus (T2DM) and vice versa. There is a paucity of information regarding the prevalence and predictors of sarcopenia among T2DM individuals. The aim of the present study was to determine the prevalence and predictors of sarcopenia among T2DM individuals. (2) Methods: This study included 159 diabetics (cases) and 79 non-diabetics (controls) aged >50 years. The subjects were assessed for demographic and anthropometric parameters. Sarcopenia (according to the Asian Working Group for Sarcopenia 2019 criteria) was assessed using Jammer’s hydraulic dynamometer for handgrip strength, dual-energy X-ray absorptiometry for muscle mass, and 6m gait speed. The biochemical investigations included glycated hemoglobin; fasting and prandial glucose; fasting insulin; lipid, renal, liver, and thyroid profiles; serum calcium; phosphorous; vitamin D; and parathyroid hormone (PTH). Appropriate statistical methods were used to determine the significance of each parameter, and a multivariate regression analysis was applied to determine the predictors. (3) Results: The prevalence of sarcopenia was significantly higher among the cases than the controls (22.5% vs. 8.86%, p—0.012). Body mass index (BMI) (OR—0.019, CI—0.001–0.248), physical activity (OR—0.45, CI—0.004–0.475), serum calcium levels (OR—0.155, CI—0.035–0.687), hypertension (OR—8.739, CI—1.913–39.922), and neuropathy (OR—5.57, CI—1.258–24.661) were significantly associated with sarcopenia following multivariate regression analysis. (4) Conclusions: T2DM individuals are prone to sarcopenia, especially those with a low BMI, low physical activity, hypertension, neuropathy, and low serum calcium levels. Hence, by modifying these risk factors among the elderly T2DM, sarcopenia can be prevented.
Keywords: sarcopenia, diabetes mellitus, neuropathy, hypertension, calcium
1. Introduction
Sarcopenia is defined as a loss of muscle mass, muscle strength, and performance [1]. Of these, strength is the most important component and is the major predictor of mortality [2]. The term sarcopenia is derived from the Greek words “sarx”, which means flesh, and “penia”, which means loss [3]. Sarcopenia is mainly a disease of the elderly due to age-related muscle loss, which is called primary sarcopenia. Secondary sarcopenia is seen with a background of systemic illness that affects muscle health [4]. Sarcopenia has gained special interest among researchers and clinicians in recent decades and has also been given a separate code in the International Classification of Disease as an independent disease (i.e., M 62.84) in the tenth revision [5].
The prevalence of sarcopenia varies widely across the world due to the different age groups studied, different ethnicities, associated comorbidities, and different diagnostic criteria employed. The prevalence among community-dwelling elderly individuals ranges between 9.9 to 40% [6,7], whereas among the elderly with type 2 diabetes mellitus (T2DM), it ranges between 15 to 44% [8,9,10] using various diagnostic criteria. Due to the increase in the life expectancy, approximately 2 billion elderly individuals are estimated to be diagnosed with sarcopenia by the year 2050 [11].
Sarcopenia is associated with increased morbidity and mortality due to physical disability, falls, fractures, poor quality of life, depression, and hospitalization [4]. The causes of sarcopenia are multiple, including chronic disorders like neurological disorders, inflammatory and autoimmune diseases, malnourishment, hypogonadism, any chronic systemic illness, and multiple drugs, especially glucocorticoids [12]. Amongst its various causes, diabetes is one of the most commonly encountered entity and is a common cause of sarcopenia. Impaired muscle function contributes to a sedentary lifestyle in T2DM patients, which in turn contributes to metabolic alterations, and vice versa.
Apart from age, multiple factors are involved in the causation of sarcopenia among individuals with diabetes. These include HbA1c levels, degree of insulin resistance; microvascular complications, particularly neuropathy; and macrovascular complications [13]. Other factors that are responsible for causing sarcopenia are body mass index (BMI) and physical activity [14,15].
The diagnostic criteria for sarcopenia have been evolving over the past few years. Various working groups (European, Asian, South Asian, and International) on sarcopenia have distinct cutoffs for the parameters. The three components defining sarcopenia include muscle mass, muscle strength, and functioning, while a few other criteria use only two components. A comparison of the various diagnostic criteria is summarized in Table 1.
Table 1.
Comparison of various diagnostic criteria for sarcopenia and their cutoffs.
| Hand Grip Strength | Muscle Mass | Gait Speed | |
|---|---|---|---|
| AWGS 2019 [1] | <28 for men <18 for women |
ALM/Ht2: <7 kg/m2—men <5.4 kg/m2—women |
<1 m/s |
| AGWS 2014 [16] | <26 for men <18 for women |
ALM/Ht2: <7 kg/m2—men <5.4 kg/m2—women |
≤0.8 m/s |
| EWGSOP [11] | <30 for men <20 for women |
ALM/Ht2: <7.26 kg/m2—men <5.5 kg/m2—women |
≤0.8 m/s |
| EWGSOP2 [17] | <27 for men <16 for women |
ALM/Ht2: <7 kg/m2—men <6 kg/m2—women |
≤0.8 m/s |
| FNIH [18] | <26 for men <16 for women |
ALM/BMI: <0.789 kg/BMI—men <0.512 kg/BMI—women |
- |
| IWGS [19] | - | ALM/Ht2: <7.23 kg/m2—men <5.67 kg/m2—women |
<1 m/s |
| SWAG SARCO [20] | <27.5 for men <18 for women |
ALM/Ht2: <6.11 kg/m2—men <4.61 kg/m2—women |
≤0.8 m/s |
Various studies from different parts of the world have used different criteria to define sarcopenia, and it had many cutoffs based on race and gender. Only a few Indian studies have been conducted on sarcopenia, taking subjects of variable ages with or without T2DM. Most Indian studies have used the European criteria to define sarcopenia.
These European cutoffs are higher than the Asian criteria cutoffs, thus leading to an overestimation of the prevalence of sarcopenia among Indians. In addition, the methods used to assess the components of sarcopenia were different amongst various studies. There is a paucity of studies from the Indian subcontinent on elderly T2DM investigating sarcopenia using the Asian criteria and standard methods for the assessment of the components of sarcopenia. Hence, the present cross-sectional observational study was conducted among elderly T2 DM patients using standard methods to assess sarcopenia and covering all the three aspects of sarcopenia. We assessed the prevalence using the AWGS 2019 criteria and the predictors of sarcopenia among elderly T2DM.
2. Materials and Methods
2.1. Subjects
The present cross-sectional study included individuals with T2DM aged ≥50 years as cases and age, sex, body mass index (BMI) matched healthy non-diabetic individuals as controls. The source of the samples was the outpatient Department of Endocrinology and Metabolism of our institution. Individuals with (i) secondary and type 1 diabetes, (ii) chronic kidney disease with an estimated glomerular filtration rate (eGFR) ≤ 30 mLm2/min (stages 4 and 5), (iii) chronic liver disease, (iv) heart failure, (v) malabsorption, (vi) malignancies, (vii) autoimmune disorders, (viii) inflammatory diseases, (ix) stroke, (x) severe hip or knee osteoarthritis (xi) cognitive impairment (xii) amputations (xiii) spinal cord injuries, (xiv) acute and chronic ongoing infections, (xv) myopathies, (xvi) BMI ≥ 40 or ≤18 kg/m2, (xvii) those on hormonal or nutritional supplements with high protein content, (xviii) illicit drug users, (xix) athletes, and (xx) non-consenting individuals, were excluded from the study. Both the case and control groups were subjected to clinical evaluation and detailed history taking. The sample size was calculated based on the prevalence from the previous Indian study and using the formula n = 4pq/d2. Permission from the ethics committee of our institution was obtained prior to the beginning of the recruitment, and informed consent was obtained from all the participants.
Each patient was asked in a detailed interview about their age, sex, duration of diabetes, past medical illness, physical activity [using the International Physical Activity Questionnaire—Short Form (IPAQ-SF) score [21]], smoking, alcohol consumption, drug history including insulin, antidiabetics, anti-hypertensives, lipid-lowering agents, and others if any. A detailed clinical examination including height, weight, BMI, and waist circumference (measured at the maximal diameter, midway between the lowest rib margin and the iliac crest during mid-expiration), were taken. Blood pressure recording and detailed systemic examinations was performed. Peripheral neuropathy was assessed through a detailed neurological examination. The Revised Neuropathy Disability Score was used to label peripheral neuropathy [22]. Pinprick sensation, vibration sensation (using a 128 Hz tuning fork), monofilament test, temperature sensation (with the cold handle of a tuning fork), and ankle reflex were assessed. A total score of six or more was labeled as diabetic peripheral neuropathy [22]. Retinopathy was assessed and classified into NPDR (non-proliferative diabetic retinopathy) and PDR (proliferative diabetic retinopathy). Nephropathy was assessed biochemically using the estimated glomerular filtration rate (eGFR) and spot urinary albumin creatinine ratio (UACR).
Sarcopenia was evaluated using the Asian Working Group for Sarcopenia Criteria 2019 [1]. Lean body mass was measured using a dual X-ray absorptiometry (DXA) scan (GE Lunar Prodigy advance). The appendicular lean mass (ALM) was calculated as the lean body mass of all four limbs. The appendicular mass index (AMI) was calculated as ALM/Ht2 (in kg/m2). The cutoff values of <7.0 kg/m2 in men and <5.4 kg/m2 in women were taken as low muscle mass. Handgrip strength (HGS) was measured using JAMAR’s hydraulic handheld dynamometer. The dominant hand was tested. The subjects were asked to relax for 5 min and tested in a sitting position with their shoulders adducted and neutrally rotated, elbow flexed at 90°, forearm in a neutral position, and wrist slightly dorsiflexed and deviated in the ulnar direction. The subjects were asked to squeeze as hard as possible in one go and then relax. The scores of three successive trials were recorded, and an average was taken. Cutoff values of <28 kg for men and <18 kg for women were taken to describe decreased muscle strength. For gait speed, the participants were asked to stand still behind the start line and asked to walk at their normal pace until they were beyond the finish line. The walkway distance was 6 m. Timing started when the participant stepped on the start line and stopped as soon as they crossed the stop line. Their speed was calculated as distance/time in seconds. A cutoff value of <1 m/s was taken to define a decreased gait speed.
Various biochemical parameters were assessed. Fasting plasma glucose (FPG), postprandial plasma glucose (PPG), fasting lipid profile, serum calcium, serum phosphorous, serum albumin, serum alkaline phosphatase and serum creatinine, thyroid stimulating hormone (TSH) were performed by an automated analyzer. Glycated haemoglobin (HbA1c) was measured using high performance liquid chromatography as per the norms of National Glycohemoglobin Standardization Program. Sample for the serum intact parathyroid hormone (iPTH) was collected in fasting and transported in a cold chain, centrifuged and stored at −80 °C till processed and measured using electrochemiluminescence immunoassay (ECLIA). Serum 25-hydroxy vitamin D (25-OH-Vit D) and fasting insulin were measured using enzyme linked immunosorbent assay (ELISA). Insulin resistance was calculated using homeostatic model assessment for insulin resistance (HOMA-IR).
2.2. Statistical Analysis
All the data analyses were carried out using the statistical product services solution IBM SPSS version 29.0. Tests of the normality assumption of continuous data were performed using an appropriate statistical test. For normally distributed continuous variables, descriptive statistics such as mean, standard deviation, and range values were calculated. Comparisons between two group means were tested using a Student’s independent t-test. For non-normal data, the median values were calculated. The median values were compared using the nonparametric Mann–Whitney U-test. Regarding categorical variables, the data were presented as frequency and percent values. Frequency data across categories were compared using a Chi-square/Fisher’s exact test as appropriate. A two-sided probability of p < 0.05 was considered statistically significant for all statistical tests. To study the association between various factors and sarcopenia, univariate logistic regression analysis was used first, then a multivariate logistic regression analysis was performed.
3. Results
The present study included 159 individuals with T2DM as cases and 79 non-diabetic individuals as controls. The controls were age, sex, and BMImatched to the cases. The mean age of our study population was 57.4 ± 6.04 for the cases and 56 ± 5.82 for the control group. The sex distribution, male to female ratio, was 1.06 for the cases and 1.024 for the controls and mean BMI of the cases and controls were 27.4 and 26.9, respectively. The rest of the demographic parameters of the cases and controls are shown in Table 2.
Table 2.
Comparison of parameters between the cases and controls.
| Parameter | Cases (T2DM) | Controls (without T2DM) | p-Value |
|---|---|---|---|
| Total number (n) | 159 | 79 | - |
| Age (years) | 57.4 (±6.04) | 56 (±5.82) | 0.093 |
| Sex (male:female) | 1.066 | 1.024 | 0.891 |
| BMI (kg/m2) | 27.4 (±3.42) | 26.9 (±3.46) | 0.286 |
| Waist circumference (cms) | 98.2 (±11.64) | 95.8 (±10.01) | 0.115 |
| Alcohol (%) | 34 (21.4%) | 15 (19%) | 0.703 |
| Smoking (%) | 30 (18.8%) | 6 (7.6%) | 0.069 |
| Physical activity | |||
| Minimal | 86 (54.1%) | 36 (45.6%) | |
| Moderate | 73 (45.9%) | 43 (54.4%) | |
| Vigorous | 0 | 0 | 0.15 |
| Dyslipidemia (%) | 97 (61%) | 51 (64.55%) | 0.594 |
| Statin use (%) | 78 (49.05%) | 19 (11.94%) | <0.001 |
| Hypertension (%) | 81 (50.9%) | 27 (34.17%) | 0.014 |
| FPG | 140.39 ± 47.78 | 91.23 ± 8.86 | <0.001 |
| PPG | 214.92 ± 66.36 | 129.97 ± 14.26 | <0.001 |
| HbA1c | 8.4 ± 1.90 | 5.29 ± 0.34 | <0.001 |
| Total cholesterol | 176.62 ± 48.22 | 187.59 ± 40.13 | 0.082 |
| Triglycerides | 165.79 ± 109.68 | 150.70 ± 65.71 | 0.261 |
| HDL | 45.64 ± 13.69 | 47.08 ± 13.27 | 0.443 |
| LDL | 98.43 ± 37.97 | 115.09 ± 36.57 | 0.002 |
| VLDL | 30.94 ± 16.79 | 29.74 ± 12.93 | 0.581 |
| Serum albumin | 4.22 ± 0.51 | 4.24 ± 0.35 | 0.672 |
| ALP | 89.94 ± 30.00 | 86.36 ± 25.56 | 0.364 |
| eGFR | 83.09 ± 18.505 | 92.04 ± 14.502 | <0.001 |
| Serum calcium | 9.55 ± 0.529 | 9.55 ± 0.451 | 0.951 |
| Serum phosphorous | 3.66 ± 0.649 | 3.61 ± 0.540 | 0.557 |
| Serum 25-OH-vit D | 24.93 ± 10.65 | 23.99 ± 9.48 | 0.506 |
| Serum iPTH | 27.28 ± 12.612 | 24.55 ± 9.856 | 0.093 |
| TSH | 2.55 ± 1.056 | 2.68 ± 1.054 | 0.399 |
| AMI | 7.03 (±1.19) | 7.45 (±1.53) | 0.044 |
| HGS | 28.25 (±8.42) | 32.34 (±10.33) | 0.002 |
| GS | 1.011 (±0.158) | 1.113 (±0.16) | <0.001 |
| Total sarcopenia | 35 (22.01%) | 7 (8.86%) | 0.012 |
| Severe sarcopenia | 19 (11.9%) | 3 (3.7%) | 0.040 |
The prevalence of sarcopenia among the individuals with T2DM was 22%, which was significantly higher compared to 8.8% among the individuals without T2DM. According to the 2014 AWGS criteria, the prevalence of sarcopenia was 17.6%, which was less compared to that of 2019 criteria, which was 22%. However, this was not statistically significant. The comparison of cutoffs of the parameters of sarcopenia is shown in Table 3.
Table 3.
Comparison of the 2014 and 2019 AGWS criteria cutoffs among the cases (with T2DM).
| Parameter | 2014 AWGS Criteria | 2019 AWGS Criteria | p-Value |
|---|---|---|---|
| Low AMI | 37 (23.2%) | 37 (23.2%) | - |
| Low HGS | 26 (16.3%) | 31 (19.4%) | 0.55 |
| Low GS | 21 (13.2%) | 65 (40.8%) | <0.001 |
| Sarcopenia | 28 (17.6%) | 35 (22%) | 0.324 |
All the characteristics of the diabetic individuals, including the medications used and microvascular complications, are summarized in Table 4. The mean duration of diabetes among our diabetic group was 8.2 (±5.87) years, with 99 individuals (62.2%) having durations less than 10 years and 64 (40.2%) having 5 or fewer years of duration. Half of the study population were hypertensive, and the prevalence of dyslipidaemia was approximately 61%; however, statin intake was seen in only 49%. Approximately half (49%) of our study population had microvascular complications, of which neuropathy was the most common (36.4%), followed by nephropathy (25.7%) and retinopathy (20.7%). The mean HbA1c of our study population was 8.4%, and the mean HOMA-IR was 4.87. Approximately 82.3% had insulin resistance (HOMA-IR ≥ 2), and 61% had severe insulin resistance (HOMA-IR ≥ 3). Various antidiabetic drugs were used. A total of 73% (116) were on oral antidiabetic agents, of which metformin was the most frequently used, accounting for 90%. The majority of the cases (93.7%), were on a combination of ≥2 antidiabetic agents and insulin use in approximately 27%.
Table 4.
Glycemic parameters, antidiabetics used, and microvascular complications of diabetes in the cases group.
| Parameter | Values |
|---|---|
| Mean duration of diabetes | 8.2 (±5.87) years |
| Antidiabetic agents used | |
| Insulin | 43 (27%) |
| Metformin | 143 (89.9%) |
| Sulphonylureas | 81 (50.9%) |
| DPP4 inhibitors | 104 (65.4%) |
| SGLT 2 inhibitors | 37 (23.2%) |
| Alpha glucosidase inhibitors | 41 (25.7%) |
| Thiazolidinediones | 0 |
| Combination (>1 agent) | 149 (93.7%) |
| Statins use | 78 (49.05%) |
| Hypertension | 81 (50.9%) |
| Dyslipidemia | 97 (61%) |
| Nephropathy | 41 (25.7%) |
| Neuropathy | 58 (36.4%) |
| Retinopathy | 33 (20.7%) |
| Fasting insulin | 13.86 (±11.25) |
| HOMA-IR | 4.87 (±4.80) |
| Urine ACR | 0.227 (±0.327) |
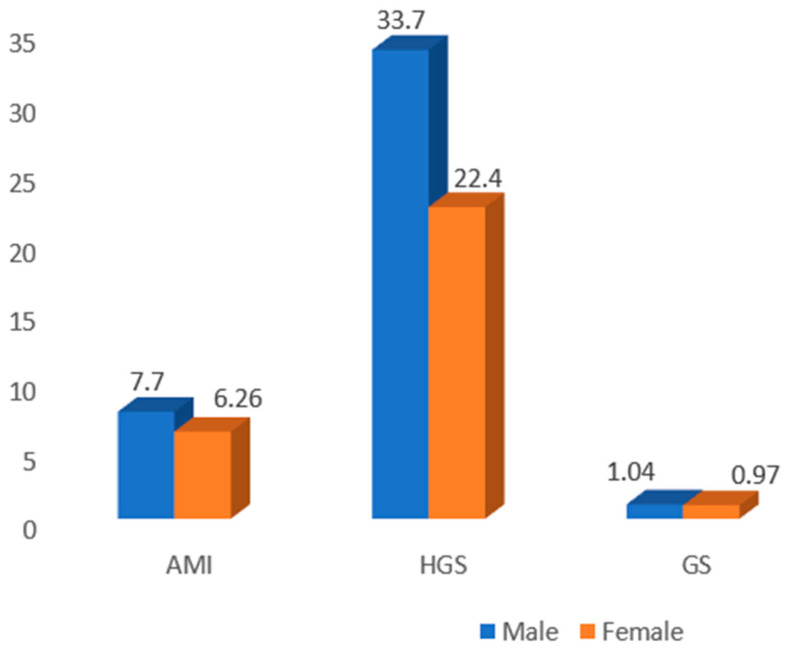
The mean appendicular mass index (AMI), handgrip strength (HGS), and gait speed (GS) were less in females than the males, which was expected and is shown in Figure 1. The means of all the parameters were significantly low in the sarcopenic group compared to the non-sarcopenic group (Table 5). Comparisons of all the baseline parameters between the sarcopenic and non-sarcopenic diabetics are shown in Table 6.
Figure 1.
Comparison of sarcopenic parameters between the male and female diabetics.
Table 5.
Comparisons of parameters of sarcopenia between the sarcopenia and no sarcopenia groups.
| Muscle Parameters | Sarcopenia (n = 35) | No Sarcopenia (n = 124) | p-Value |
|---|---|---|---|
| AMI | 5.81 (±0.80) | 7.37 (±1.04) | <0.001 |
| HS | 21.84 (±6.19) | 30.06 (±8.09) | <0.001 |
| GS | 0.84 (±0.12) | 1.05 (±0.13) | <0.001 |
Table 6.
Table showing comparisons of parameters between the sarcopenic and non-sarcopenic diabetics.
| Parameter | Sarcopenia | No Sarcopenia | p-Value |
|---|---|---|---|
| Total number | 35 (22%) | 124 (78%) | |
| Male:female | 18:17 | 64:60 | 0.985 |
| Mean age | 61 (±6.27) | 56.38 (±5.59) | <0.001 |
| Duration of diabetes | 10 (±7.49) | 7.69 (5.26) | 0.037 |
| Alcohol | 2 (5.7%) | 32 (25.8%) | 0.010 |
| Smoking | 3 (8.6%) | 27 (21.8%) | 0.018 |
| BMI | 24.95 (±3.02) | 28.09 (±3.21) | <0.001 |
| Waist circumference | 91.54 (±10.05) | 100.05 (±11.40) | <0.001 |
| Physical activity | 2 (5.7%) | 71 (57.3%) | <0.001 |
| Dyslipidemia | 19 (54.28%) | 78 (62.9%) | 0.356 |
| Statin | 20 (57.14%) | 58 (46.77%) | 0.279 |
| Hypertension | 26 (74.3%) | 55 (44.4%) | 0.002 |
| Insulin | 16 (45.7%) | 27 (21.8%) | 0.005 |
| Metformin | 29 (82.9%) | 114 (91.9%) | 0.115 |
| Sulphonylureas | 11 (31.4%) | 70 (56.5%) | 0.009 |
| DPP4 inhibitors | 22 (62.9%) | 82 (66.1%) | 0.719 |
| SGLT-2 inhibitors | 12 (34.3%) | 25 (20.2%) | 0.081 |
| Alpha glucosidase inhibitors | 6 (17.1%) | 35 (28.2%) | 0.186 |
| Combination of antidiabetics | 32 (91.4%) | 117 (94.35%) | 0.873 |
| AMI | 5.81 (±0.80) | 7.37 (±1.04) | <0.001 |
| HGS | 21.84 (±6.19) | 30.06 (±8.09) | <0.001 |
| GS | 0.84 (±0.12) | 1.05 (±0.13) | <0.001 |
| Neuropathy | 22 (62.9%) | 36 (29.0%) | <0.001 |
| Nephropathy | 17 (48.6%) | 24 (19.4%) | <0.001 |
| Retinopathy | 14 (40.0%) | 19 (15.3%) | 0.001 |
| FPG | 148.68 (±53.70) | 138.04 (±45.94) | 0.246 |
| PPG | 215.31(±77.82) | 214.81 (±63.108) | 0.969 |
| HbA1c | 8.169 (±1.87) | 8.467 (±1.92) | 0.423 |
| Fasting insulin | 13.41 (±10.53) | 13.98 (±11.48) | 0.793 |
| HOMA IR | 4.90 (±4.57) | 4.86 (±4.88) | 0.287 |
| Total cholesterol | 166.22 (±47.98) | 179.54 (±48.07) | 0.150 |
| Triglyceride | 130.85 (±48.18) | 175.64 (±119.86) | 0.032 |
| HDL | 49.45 (±15.29) | 44.56 (±13.07) | 0.062 |
| LDL | 91.82 (±39.4) | 100.35 (±37.503) | 0.244 |
| VLDL | 25.00 (±8.82) | 32.70 (±18.16) | 0.017 |
| Serum albumin | 4.15 (±0.628) | 4.23 (±0.48) | 0.392 |
| ALP | 89.11 (±30.00) | 90.17 (±30.12) | 0.854 |
| eGFR | 71.51 (±14.84) | 86.35 (±18.16) | <0.001 |
| Urine ACR | 0.40 (±0.43) | 0.17 (±0.27) | <0.001 |
| Serum calcium | 9.35 (±0.55) | 9.60 (±0.51) | 0.011 |
| Serum phosphorous | 3.76 (±0.67) | 3.62 (±0.64) | 0.276 |
| Serum 25-OH-vit D | 24.45 (±9.48) | 25.06 (±10.99) | 0.766 |
| Serum iPTH | 32.24 (±17.45) | 25.87 (±10.54) | 0.008 |
| TSH | 2.43 (±0.86) | 2.58 (±1.105) | 0.454 |
A univariate regression analysis revealed a significant association between sarcopenia and age, alcohol intake, physical activity, BMI, waist circumference, hypertension, insulin usage, sulphonylureas, neuropathy, nephropathy, retinopathy, triglyceride levels, VLDL (very low density lipoprotein) levels, eGFR, UACR, serum calcium, and parathyroid hormone (iPTH), as shown in Table 7.
Table 7.
Univariate regression analysis of various demographic and anthropometric parameters with sarcopenia as the dependent variable.
| Factors | Subgroup | β Estimate | Odds Ratio (Confidence Interval) |
p-Value |
|---|---|---|---|---|
| Age | 50–59 | Ref | ||
| ≥60 | 1.26 | 3.527 (1.62–7.67) | 0.001 | |
| Sex | F | Ref | ||
| M | −0.007 | 0.993 (0.469–2.103) | 0.985 | |
| Duration of diabetes | <10 | Ref | ||
| ≥10 | 0.541 | 1.71 (0.80–3.66) | 0.162 | |
| Alcohol intake | −1.75 | 0.174 (0.039–0.768) | 0.021 | |
| Smoking | −1.09 | 0.337 (0.09–1.18) | 0.337 | |
| Physical activity | Minimal | Ref | ||
| Moderate | −3.096 | 0.045 (0.01–0.197) | <0.001 | |
| BMI | 18–22.9 | Ref | ||
| 23–27.4 | −1.868 | 0.154 (0.047–0.505) | 0.002 | |
| ≥27.5 | −3.186 | 0.0413 (0.01–0.159) | <0.001 | |
| Waist circumference | High | Ref | ||
| Normal | −1.92 | 0.147 (0.048–0.449) | <0.001 | |
| Hypertension | No | Ref | ||
| Yes | 1.29 | 3.624 (1.569–8.367) | 0.003 | |
| Dyslipidemia | No | Ref | ||
| Yes | −0.391 | 0.676 (0.317–1.445) | 0.313 | |
| Statin use | No | Ref | ||
| Yes | 0.417 | 1.517 (0.712–3.234) | 0.280 | |
| Insulin | No | Ref | ||
| Yes | 1.11 | 3.025 (1.373–6.666) | 0.006 | |
| Metformin | No | Ref | ||
| Yes | −0.858 | 0.424 (0.142–1.26) | 0.123 | |
| Sulphonylureas | No | Ref | ||
| Yes | −1.04 | 0.354 (0.159–0.785) | 0.011 | |
| DPP-4 inhibitors | No | Ref | ||
| Yes | −0.143 | 0.867 (0.397–1.891) | 0.719 | |
| SGLT-2 inhibitors | No | Ref | ||
| Yes | 0.726 | 2.066 (0.906–4.712) | 0.084 | |
| Alpha glucosidase inhibitor | No | Ref | ||
| Yes | −0.642 | 0.526 (0.201–1.377) | 0.191 | |
| Combination of drugs | <3 | Ref | ||
| ≥3 | −0.204 | 0.816 (0.373–1.784) | 0.610 | |
| Neuropathy | No | Ref | ||
| Yes | 1.42 | 4.137 (1.881–9.094) | <0.001 | |
| Nephropathy | No | Ref | ||
| Yes | 1.37 | 3.935 (1.771–8.746) | <0.001 | |
| Retinopathy | No | Ref | ||
| Yes | 1.30 | 3.684 (1.60–8.485) | 0.002 | |
| FPG | 0.004 | 1.004 | ||
| (0.997–1.011) | 0.251 | |||
| PPG | 1.14 | 1.000 (0.994–1.006) | 0.969 | |
| HbA1c | <7 | Ref | ||
| ≥7 | −0.238 | 0.788 (0.317–1.956) | 0.607 | |
| Fasting insulin | −0.004 | 0.995 | ||
| (0.960–1.032) | 0.792 | |||
| HOMA-IR | <2 | Ref | ||
| ≥2 | −0.432 | 0.649 (0.258–1.633) | 0.359 | |
| Total Cholesterol | <200 | Ref | ||
| ≥200 | −0.123 | 0.884 (0.395–1.977) | 0.764 | |
| Triglycerides | <150 | Ref | ||
| ≥150 | −0.981 | 0.375 (0.166–0.846) | 0.018 | |
| LDL | <100 | Ref | ||
| ≥100 | −0.188 | 0.829 (0.388–1.771) | 0.628 | |
| HDL | 0.0233 | 1.023 (0.998–1.050) | 0.071 | |
| VLDL | −0.0481 | 0.953 (0.917–0.991) | 0.015 | |
| Albumin | −0.316 | 0.728 (0.353–1.50) | 0.390 | |
| ALP | −0.0012 | 0.999 (0.986–1.01) | 0.853 | |
| eGFR | −0.0532 | 0.948 (0.923–0.974) | <0.001 | |
| Urine ACR | 1.78 | 5.918 (2.036–17.20) | 0.001 | |
| TSH | −0.148 | 0.863 (0.588–1.27) | 0.451 | |
| Serum calcium | −0.980 | 0.375 (0.173–0.816) | 0.013 | |
| Serum phosphorous | 0.327 | 1.386 (0.771–2.491) | 0.275 | |
| Serum iPTH | 0.036 | 1.037 (1.007–1.067) | 0.013 | |
| 25-OH-Vit D | −0.005 | 0.994 (0.958–1.032) | 0.764 |
After applying the multivariate regression analysis (shown in Table 8), we found that physical activity was associated with a significant decrease in the risk of sarcopenia, with an odds ratio of 0.45 (CI of 0.004–0.475). We observed that the risk of sarcopenia was significantly lower in patients with a BMI > 27.5 kg/m2 (Table 8). Hypertension was significantly associated with sarcopenia, with an odds ratio of 8.739 (CI of 1.913–39.922, p—0.005). Of the microvascular complications, only neuropathy showed a significant positive association, with an odds ratio of 5.57 (CI of 1.258–24.66). Amongst all the biochemical parameters, only serum calcium was significantly negatively associated with sarcopenia, with an odds ratio of 0.155 (CI of 0.035–0.687), implying that the lower the serum calcium levels, the higher the risk of sarcopenia.
Table 8.
Results of multivariate logistic regression analysis with sarcopenia as the dependent and other factors as independent variables.
| Parameter | Comparison Values | β-Estimate | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|---|---|
| Age | <60 | Ref | ||
| ≥60 | −1.138 | 0.320 (0.063–1.630) | 0.170 | |
| Physical activity | Minimal | Ref | ||
| Moderate | −3.099 | 0.45 (0.004–0.475) | 0.010 | |
| Alcohol intake | No | Ref | ||
| Yes | −1.773 | 0.170 (0.014–1.993) | 0.158 | |
| Insulin use | No | Ref | ||
| Yes | 0.183 | 1.201 (0.257–5.607) | 0.816 | |
| Sulphonyl urea use | No | Ref | ||
| Yes | −1.269 | 0.281 (0.049–1.625) | 0.156 | |
| Hypertension | No | Ref | ||
| Yes | 1.500 | 8.739 (1.913–39.922) | 0.005 | |
| BMI | <23 | Ref | ||
| 23–27.4 | −1.865 | 0.155 (0.021–1.136) | 0.067 | |
| ≥27.5 | −3.973 | 0.019 (0.001–0.248) | 0.003 | |
| Waist circumference | Normal | Ref | ||
| High | 0.267 | 1.306 (0.164–10.425) | 0.801 | |
| Triglycerides | <150 | Ref | 0.273 | |
| ≥150 | −0.791 | 0.453 (0.110–1.863) | ||
| eGFR | - | −0.034 | 0.966 (0.924–1.010) | 0.129 |
| PTH | - | 0.008 | 1.008 (0.952–1.066) | 0.794 |
| Serum calcium | - | −1.865 | 0.155 (0.035–0.687) | 0.014 |
| Urine ACR | - | 1.272 | 3.566 (0.089–143.657) | 0.500 |
| Neuropathy | No | Ref | ||
| Yes | 1.717 | 5.570 (1.258–24.661) | 0.024 | |
| Nephropathy | No | Ref | ||
| Yes | −0.221 | 1.318 (0.083–20.843) | 0.845 | |
| Retinopathy | No | Ref | ||
| Yes | 0.266 | 1.305 (0.141–12.034) | 0.814 |
Other parameters like age, alcohol intake, insulin and sulphonylurea use, waist circumference, serum triglycerides, eGFR, urine ACR, serum iPTH, nephropathy, and retinopathy showed a significant association based on the univariate analysis; however, they were insignificant after the multivariate regression analysis. Lower physical activity, lower BMI, the presence of hypertension, presence of neuropathy, and low serum calcium levels were significant predictors of sarcopenia among the diabetics.
4. Discussion
To the best of our knowledge, this was the first study using AWGS 2019 criteria among elderly T2DM individuals in India. The prevalence of sarcopenia according to this criterion was 22% among elderly T2DM individuals compared to 8.8% among the age-, sex-, and BMI-matched healthy controls. This indicates that diabetes itself is an independent risk factor for sarcopenia. Earlier studies that followed the AWGS 2014 criteria reported a prevalence of 28.5% and 27.4%, which were slightly higher than in our study [23,24]. This difference could be due to the higher mean age of the study population. A recent Indian study reported a prevalence of sarcopenia in older diabetics (>45 years) of 31% among males and 20% among females using the AWGS 2014 criteria [25].
We also compared the 2014 and 2019 AWGS criteria cutoffs and found the prevalence of sarcopenia to be 17.6% and 22% using the 2014 and 2019 criteria, respectively. The absolute prevalence of low AMI was equal due to the same cutoffs in both criteria, whereas the absolute prevalence of low HGS and low GS was higher with the 2019 criteria, of which the prevalence of low GS was statistically significant. This was due to a change in the cutoff of HGS from 26 to 28 kgs for males and GS from 0.8m/s to 1m/s for both the sexes.
The mean AMI in diabetic males and females was 7.75 and 6.26, respectively, which was similar (7.0 and 5.8, respectively) to an earlier report by Zengin et.al [25] among older diabetics, but less than the values reported by Kaur et al. [10] who used the bio-impedance method to assess the skeletal muscle mass index. The study population included younger diabetics as well. The mean HGS in diabetic males and females was 33.72 and 22.43, respectively, which was similar to that reported by Kaur et al [10] (32.4 and 20.6, respectively), using JAMMAR’s hydraulic hand dynamometer, where a slightly lower HGS (26.6 and 17.6, respectively) was recorded in both the sexes using a different hand dynamometer, as described by Zengin et al. [25]. The mean gait speed was 1.04 m/s and 0.97 m/s among male and female diabetics, respectively.
Demographic characteristics like age, gender, and duration of diabetes did not show a significant association with sarcopenia. These findings were similar to reports by other researchers [26]. Neither alcohol nor smoking showed a significant association with sarcopenia, which is similar to that reported by two other studies [23,26]. However, a population-based study from China showed that chronic heavy alcohol intake is associated with an increased risk of sarcopenia [27], which was not seen in our study population due to their occasional and minimal intake of alcohol. This shows that heavy alcohol intake has an effect on muscle health, but minimal amounts may not have a significant effect.
The duration of diabetes did not show any significant association with sarcopenia. This was contrary to earlier findings reported by Sazlina et al., who showed a significant association, with an odds ratio of 1.85 with a duration >10 years [23]. This difference could be due to the higher mean duration of diabetes in their study population compared to our study population (10 ± 6.6 vs. 8.2 ± 5.8 years).
In the present study, physical activity had a significant association with sarcopenia, showing that moderate physical activity decreases the risk of sarcopenia (odds ratio of 0.045), which was persistently seen after multivariate logistic regression, also with an odds ratio of 0.45. This means that with regular physical activity, the risk of sarcopenia was decreased by 45%. This finding was similar to earlier published studies [10,23]. As physical activity increases, particularly moderate or vigorous physical activity, muscle mass and strength also increase [28]. A recent Korean population-based study conducted on the elderly population (>60 years) showed that muscle mass and handgrip strength were significantly higher among men and women engaged in moderate or vigorous physical activity compared to those who had minimal physical activity, with an odds ratio of 0.77 [28]. Thus, regular physical activity is a safe strategy for preventing sarcopenia (for both loss of muscle mass and muscle strength). Physical activity also indirectly benefits muscle health by improving glycemic control [29]. Physical activity increases muscle mass and improves insulin-independent glucose uptake by the skeletal muscle, which leads to decreased glycemia and improvements in diabetes and various other complications that lead to sarcopenia [29].
In the present study, BMI and waist circumference had a significant negative association with sarcopenia. Having a BMI within the overweight or obese ranges resulted in an odds ratio of 0.154 or 0.041, respectively. Waist circumference showed an odds ratio of 0.147. This is in agreement with the study by Sazlina et al., who reported odds ratios of 0.16 and 0.03 for the overweight and obese ranges, respectively [23]. Following the multivariate analysis, only the BMI in the obese range showed a significant association with sarcopenia, with an odds ratio of 0.019, which was also in line with that reported by Sazlina et al. [23] and Rahman et al. [30], with obese BMIs having odds ratios of 0.09 and 0.081, respectively [23,30]. Two other studies analyzing the risk factors of sarcopenia also showed that those with lower BMIs had higher risks of sarcopenia [15,31]. The probable explanation for this could be that having a low BMI is an indicator of poor nourishment, which affects protein synthesis, resulting in lesser muscle mass and strength [32].
The presence of hypertension was significantly associated with sarcopenia, with an odds ratio of 8.7; that is, individuals with T2DM and hypertension had an 8–9 times higher risk of sarcopenia. This could be due to the synergistic effect of both diabetes and hypertension on muscle health. This finding was different from previous published studies [23,26]. Several studies showed hypertension to be an independent risk factor of sarcopenia [33]. Hypertension can damage myocytes [33]. Hypertension showed a significant positive association after a multivariate analysis [33]. Dyslipidemia and statin use showed no significant association with sarcopenia, which was similar to two earlier reports [23,26].
Among the various antidiabetic agents, we found a positive association between insulin use and sarcopenia and a negative association between sulphonylurea use and sarcopenia. On the contrary, based on multivariate logistic regression analyses, there was no significant association. None of the other antidiabetic agents, including metformin, voglibose, DPP-4 inhibitors, and SGLT-2 inhibitors, had any significant association with sarcopenia. These findings are similar to earlier findings by Sazlina et al. [23]. However, a Japanese study reported that individuals with T2DM taking insulin showed an attenuation in the progression of sarcopenia [34]. This difference could be due to the cross-sectional design of our study, which could not assess the causal relation compared to the longitudinal design of the previous study. The present study showed no significant association between the combination of antidiabetics and sarcopenia. Though not analyzed in our study, Glucagon-like peptide 1 (GLP 1) receptor analogues were shown to be detrimental to sarcopenia [35].
All three microvascular complications showed a significant positive association with sarcopenia, with odds ratios of 4.13, 3.93, and 3.68 for neuropathy, nephropathy, and retinopathy, respectively. After a multivariate analysis, only neuropathy showed a significant association with sarcopenia, with an odds of 5.57. A recent study among T2DM individuals >50 years showed that neuropathy was associated with decreased muscle strength in the lower extremities [36]. This was in contrary to an earlier report by Sazlina et al., in which they found no association between any microvascular complications and sarcopenia [23]. Neuropathy causes sarcopenia through various mechanisms, including denervation atrophy [37]. A recent meta-analysis reported a significant positive association between microangiopathies and sarcopenia, with odds of 1.7, 2.8, and 4.8, for neuropathy, nephropathy, and retinopathy, respectively [38].
Glycemic parameters such as fasting, postprandial blood glucose, and glycated hemoglobin showed no significant association with sarcopenia. These findings are similar to earlier studies that assessed HbA1c [26,39]. Sugimoto et al. reported a significant association between HbA1c and sarcopenia (particularly with low muscle mass), even after adjusting for the other co-variates [40]. This discrepancy could be due to the age and size of the study population. Sugimoto et al. [40] studied 2067 elderly diabetics, with a mean age of 68 years, contrasting with our study, which included 159 diabetics, and the mean age was 57.4. The variability in HbA1c could be small, and our study sample size, which was small compared to the others, could be inadequate to assess this association. Other studies have also reported a significant association between higher HbA1c and sarcopenia, especially with those >8.5% [41,42].
Fasting insulin and HOMA-IR (insulin resistance index) were not associated with sarcopenia. Insulin sensitivity as a predictor of sarcopenia is a less well-studied entity. The available literature shows variable results. Lee CG et al. studied non-diabetic individuals and reported that the highest quartile of insulin resistance increased the odds of losing lean mass [43]. On the other hand, Abbatecola et al. reported a negative association between insulin resistance and sarcopenia [44].
Among the serum lipids, triglycerides and VLDL showed a significant negative association based on a univariate analysis, which was not evident based on a multivariate regression analysis. Thus, in our study, none of the serum lipid levels showed a significant association with sarcopenia. This is similar to earlier research findings [23], whereas a recent study reported that VLDL had association with a low skeletal muscle mass index [45].
Serum albumin levels did not have a significant association with sarcopenia. This parameter has not been well-studied in earlier research studies. Sugimoto et al. reported significantly low serum albumin levels in their sarcopenic group compared to their non-sarcopenic group [40]. Renal parameters such as eGFR and albuminuria (spot urine albumin to creatinine ratio) were significantly associated with sarcopenia. eGFR showed a negative association, and the urine albumin creatinine ratio had a positive association, with an odds ratio of 0.948 and 5.91, respectively. However, after a multivariate logistic regression analysis, these parameters had no significant association, like that of nephropathy. The findings of our study are similar to previously published results by Sazlina et al. [23]. Pechmann et al. reported a significant association between albuminuria and sarcopenia, with odds ratio of 2.84; however, they found no significant association with eGFR. This difference could be due to their higher mean age of 65.6 and a higher mean duration of diabetes of 15.4 years compared to our study population (57.4 and 8.2 years, respectively). As the duration of diabetes and age increases, the risk of diabetic nephropathy increases, and thus the proportion of patients with albuminuria also increases.
Among the bone mineral metabolic parameters, serum calcium showed a significant negative correlation, and serum intact PTH showed a significant positive correlation with sarcopenia. Serum phosphorous and 25(OH)vitamin D had no significant correlation with sarcopenia, which was similar to an earlier study that showed no association with serum vitamin D levels [30]. However, after a multivariate regression analysis, only serum calcium showed a significant negative correlation with sarcopenia. The higher the serum calcium level, the lesser is the risk, with odds ratio of 0.15. Admittedly, serum calcium as a predictor of sarcopenia did not receive significant attention earlier. However, a recent systematic review reported the role of various minerals in muscle health, and calcium intake showed a significant association with muscle health [46].
Calcium plays a vital role in muscle contraction, as it facilitates the effective interaction between actin and myosin muscle fibers. In striated muscle, calcium binds to troponin c on actin filaments, causing a shift in the position of troponin–tropomyosin complexes, resulting in exposure of the myosin binding sites. This allows myosin bound by ADP and inorganic phosphate (Pi) to form cross bridges with actin. The subsequent release of ADP and Pi generates the power stroke that drives muscle contraction [47]. Changes in calcium signaling can impact the regulation of contractile forces in differentiated muscle fibers. Research has shown that alterations in calcium homeostasis are associated with skeletal muscle weakness during the aging process. The sarcoplasmic reticulum has reduced calcium availability for contractions, resulting in diminished contractile force. This, coupled with an aging-related shift from faster to slower myosin/myosin light chain forms, leads to a decline in muscle power [48]. Furthermore, a study by Seo et al. demonstrated a negative correlation between calcium intake and total body fat mass, as well as a positive correlation with appendicular skeletal mass [49]. Other studies have shown a significant role of calcium intake in muscle mass [50,51]. A role of calcium in sarcopenia has been suggested via its modulation of calpains, which are cysteine proteases responsible for the regulation of key process in myogenesis. Therefore, a deficiency could potentially lead to sarcopenic outcomes [52].
In the present study, neither the duration of diabetes, the glycemic control parameters, nor the use of diabetes medications influenced the risk of sarcopenia. However, these parameters should be studied in a longitudinal design to assess their causal role in sarcopenia.
The present study provides a few recommendations by which sarcopenia can be prevented to some extent by simple, feasible lifestyle modifications. A particular emphasis should be given by both clinicians and national policy makers on the importance of physical activity, calcium intake, adequate control of hypertension, and maintaining a healthy BMI to prevent sarcopenia.
The strengths of the present study were that it included healthy age-, sex-, and BMI-matched controls, the use of the dual X-ray absorptiometry for appendicular mass index, Jammar’s hand-held dynamometer for handgrip strength, an assessment of the effects of antidiabetic medication, and assessment of all possible biochemical parameters that are responsible for muscle health. The present study, despite being done very meticulously using standard criteria and methods to assess sarcopenia, is not completely devoid of limitations. First, this was a cross-sectional study, which does not allow for the evaluation of causal relationships. Second, the prevalence in the study could be underestimated because we excluded all the patients with other chronic systemic illnesses who are usually more prone to sarcopenia. Third, protein intake was not included because the precise amount could not be elucidated; however, the nutritional status was also reflected by the serum albumin levels, which were assessed in our study. Fourth, the results of our study may not be applicable to the general population because the study population was taken form a single tertiary care center. Hence, large population-based and long-term follow-up studies are warranted to precisely determine the risk factors. In the future, interventional trials aimed at treating sarcopenia should be conducted.
5. Conclusions
The modifiable risk factors for T2DM may help for achieving better outcomes. It should not be forgotten that individuals with T2DM are more prone to sarcopenia. These include individuals with hypertension, a low BMI, less physical activity, neuropathy, and low serum calcium levels, who are at high risk and should be screened for sarcopenia. Every individual with T2DM should be educated about these modifiable risk factors and should be encouraged to maintain a healthy BMI; regular physical activity, especially muscle strengthening exercises; control of hypertension; and adequate calcium intake to prevent sarcopenia. Further longitudinal studies are warranted to assess the causal relationships between various factors and sarcopenia.
Acknowledgments
The authors express their heartful gratitude to the colleagues, Ankit Manglunia, Jaspreet Singh, Brij Teli, Kasukurthi Lavanya, Vejendla Soma Srinivas, and Siddharth Shankar Panigrahi for their help in completing this study.
Abbreviations
| ADP | adenosine diphosphate |
| ALM | appendicular lean mass |
| ALP | alkaline phosphatase |
| AMI | appendicular mass index |
| AWGS | Asian Working Group for Sarcopenia |
| BMI | body mass index |
| CI | confidence interval |
| DPP4 | Dipeptidyl peptidase |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| FNIH | Foundation for the National Institutes of Health biomarkers consortium |
| FPG | fasting plasma glucose |
| GFR | glomerular filtration rate |
| GS | gait speed |
| HbA1C | hemoglobin A1C |
| HDL | high-density lipoprotein |
| HGS | handgrip strength |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| HTN | hypertension |
| IWGS | International Working Group on Sarcopenia |
| LDL | low-density lipoprotein |
| OR | odds ratio |
| PPG | postprandial plasma glucose |
| PTH | parathyroid hormone |
| SGLT | Sodium-glucose co-transporters |
| SWAG SARCO | South Asian Working Action Group on SARCOpenia |
| T2DM | type 2 diabetes mellitus |
| TSH | thyroid stimulating hormone |
| Urine ACR | urine albumin creatinine ratio |
| VLDL | very low-density lipoprotein |
Author Contributions
Planning, conceptualizing, and designing the research—J.S., A.K.S., J.K. and S.M.; performing the study—S.L.S. and J.S.; analyzing the data—S.L.S., S.M., A.K.S. and P.J.; writing the manuscript—S.L.S. and S.D.; edited the manuscript—S.D., P.J. and J.K.; Final editing of the manuscript—S.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was performed with ethical approval (Ref No./DRI/IMS.SH/SOA/2021/122, dated 28 July 2021).
Informed Consent Statement
Signed informed consent was directly obtained from the patients.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a research grant obtained from the Endocrine Society of India (ESI). ESI trainee grant 2021/20 September 2021.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Newman A.B., Kupelian V., Visser M., Simonsick E.M., Goodpaster B.H., Kritchevsky S.B., Tylavsky F.A., Rubin S.M., Harris T.B. Strength, But Not Muscle Mass, Is Associated with Mortality in the Health, Aging and Body Composition Study Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg I.H. Sarcopenia: Origins and Clinical Relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 4.Haase C.B., Brodersen J.B., Bülow J. Sarcopenia: Early prevention or overdiagnosis? BMJ. 2022;376:e052592. doi: 10.1136/bmj-2019-052592. [DOI] [PubMed] [Google Scholar]
- 5.Cao L., Morley J.E. Sarcopenia Is Recognized as an Independent Condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J. Am. Med. Dir. Assoc. 2016;17:675–677. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft A.J., Landi F., Schneider S.M., Zuniga C., Arai H., Boirie Y., Chen L.K., Fielding R.A., Martin F.C., Michel J.P., et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayhew A.J., Amog K., Phillips S., Parise G., McNicholas P.D., De Souza R.J., Thabane L., Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: A systematic review and meta-analyses. Age Ageing. 2019;48:48–56. doi: 10.1093/ageing/afy106. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Feng X., Zhou J., Gong H., Xia S., Wei Q., Hu X., Tao R., Li L., Qian F., et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016;6:38937. doi: 10.1038/srep38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh N., Harshitha R., Bhargava M. Prevalence of sarcopenia in an elderly population in rural South India: A cross-sectional study. F1000Research. 2020;10:175. doi: 10.12688/f1000research.22580.1. [DOI] [Google Scholar]
- 10.Kaur P., Bansal R., Bhargava B., Mishra S., Gill H., Mithal A. Decreased handgrip strength in patients with type 2 diabetes: A cross-sectional study in a tertiary care hospital in north India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:325–329. doi: 10.1016/j.dsx.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borba V.Z.C., Costa T.L., Moreira C.A., Boguszewski C.L. Mechanisms of Endocrine Disease: Sarcopenia in endocrine and non-endocrine disorders. Eur. J. Endocrinol. 2019;180:R185–R199. doi: 10.1530/EJE-18-0937. [DOI] [PubMed] [Google Scholar]
- 13.Mesinovic J., Zengin A., De Courten B., Ebeling P.R., Scott D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019;12:1057–1072. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xin C., Sun X., Lu L., Shan L. Prevalence of sarcopenia in older Chinese adults: A systematic review and meta-analysis. BMJ Open. 2021;11:e041879. doi: 10.1136/bmjopen-2020-041879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L.K., Lee W.J., Chen L.Y., Hwang A.C., Lin M.H., Peng L.N., Chen L.K. Sarcopenia, and its association with cardiometabolic and functional characteristics in Taiwan: Results from I-Lan Longitudinal Aging Study: Sarcopenia in Taiwan. Geriatr. Gerontol. Int. 2014;14:36–45. doi: 10.1111/ggi.12208. [DOI] [PubMed] [Google Scholar]
- 16.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., Chou M.Y., Chen L.Y., Hsu P.S., Krairit O., et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., Ferrucci L., Guralnik J.M., Fragala M.S., Kenny A.M., et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., van Kan G.A., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhar M., Kapoor N., Suastika K., Khamseh M.E., Selim S., Kumar V., Raza S.A., Azmat U., Pathania M., Rai Mahadeb Y.P., et al. South Asian Working Action Group on SARCOpenia (SWAG-SARCO)—A consensus document. Osteoporos. Sarcopenia. 2022;8:35–57. doi: 10.1016/j.afos.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee P.H., Macfarlane D.J., Lam T., Stewart S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weintrob N., Amitay I., Lilos P., Shalitin S., Lazar L., Josefsberg Z. Bedside neuropathy disability score compared to quantitative sensory testing for measurement of diabetic neuropathy in children, adolescents, and young adults with type 1 diabetes. J. Diabetes Complicat. 2007;21:13–19. doi: 10.1016/j.jdiacomp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Sazlina S.G., Lee P.Y., Chan Y.M.A., Hamid M.S., Tan N.C. The prevalence and factors associated with sarcopenia among community living elderly with type 2 diabetes mellitus in primary care clinics in Malaysia. PLoS ONE. 2020;15:e0233299. doi: 10.1371/journal.pone.0233299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung F.Y., Koh Y.L.E., Malhotra R., Ostbye T., Lee P.Y., Shariff Ghazali S., Tan N.C. Prevalence of and factors associated with sarcopenia among multi-ethnic ambulatory older Asians with type 2 diabetes mellitus in a primary care setting. BMC Geriatr. 2019;19:122. doi: 10.1186/s12877-019-1137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zengin A., Kulkarni B., Khadilkar A.V., Kajale N., Ekbote V., Tandon N., Bhargava S.K., Sachdev H.S., Sinha S., Scott D., et al. Prevalence of Sarcopenia and Relationships Between Muscle and Bone in Indian Men and Women. Calcif. Tissue Int. 2021;109:423–433. doi: 10.1007/s00223-021-00860-1. [DOI] [PubMed] [Google Scholar]
- 26.Pechmann L.M., Jonasson T.H., Canossa V.S., Trierweiler H., Kisielewicz G., Petterle R.R., Moreira C.A., Borba V.Z.C. Sarcopenia in Type 2 Diabetes Mellitus: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2020;2020:7841390. doi: 10.1155/2020/7841390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai J., Ma B., Qin J., Lyu Q., Khatun P., Liang R., Cong M., Guo L., Kong Y. Alcohol consumption patterns and the risk of sarcopenia: A population-based cross-sectional study among chinese women and men from Henan province. BMC Public Health. 2022;22:1894. doi: 10.1186/s12889-022-14275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H.Y., Jung W.S., Kim S.W., Lim K. Relationship between Sarcopenia, Obesity, Osteoporosis, and Cardiometabolic Health Conditions and Physical Activity Levels in Korean Older Adults. Front. Physiol. 2021;12:706259. doi: 10.3389/fphys.2021.706259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sylow L., Kleinert M., Richter E.A., Jensen T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017;13:133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 30.Rahman R., Wilson B.P., Paul T.V., Yadav B., Kango Gopal G., Viggeswarpu S. Prevalence and factors contributing to primary sarcopenia in relatively healthy older Indians attending the outpatient department in a tertiary care hospital: A cross-sectional study. Aging Med. 2021;4:257–265. doi: 10.1002/agm2.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu R., Wong M., Leung J., Lee J., Auyeung T.W., Woo J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults: Sarcopenia incidence and its risk factors. Geriatr. Gerontol. Int. 2014;14:15–28. doi: 10.1111/ggi.12220. [DOI] [PubMed] [Google Scholar]
- 32.Vandewoude M.F.J., Alish C.J., Sauer A.C., Hegazi R.A. Malnutrition-Sarcopenia Syndrome: Is This the Future of Nutrition Screening and Assessment for Older Adults? J. Aging Res. 2012;2012:651570. doi: 10.1155/2012/651570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kara M., Kara Ö., Ceran Y., Kaymak B., Kaya T.C., Çıtır B.N., Durmuş M.E., Durmuşoğlu E., Razaq S., Doğan Y., et al. SARcopenia Assessment in Hypertension: The SARAH Study. SARcopenia Assessment in Hypertension: The SARAH Study. Am. J. Phys. Med. Rehabil. 2023;102:130–136. doi: 10.1097/PHM.0000000000002045. [DOI] [PubMed] [Google Scholar]
- 34.Bouchi R., Fukuda T., Takeuchi T., Nakano Y., Murakami M., Minami I., Izumiyama H., Hashimoto K., Yoshimoto T., Ogawa Y. Insulin Treatment Attenuates Decline of Muscle Mass in Japanese Patients with Type 2 Diabetes. Calcif. Tissue Int. 2017;101:1–8. doi: 10.1007/s00223-017-0251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikejima S., Kondo S., Sakai T., Taniai H., Takahashi T., Umezu J., Iseka M., Inoue M., Nishihara H., Murata K., et al. Novel Approach to Sarcopenia in Diabetic Patients Treated with GLP-1 Receptor Agonists (GLP-1RA) Diabetes. 2018;67((Suppl. 1)):673-P. doi: 10.2337/db18-673-P. [DOI] [Google Scholar]
- 36.Nomura T., Ishiguro T., Ohira M., Ikeda Y. Diabetic polyneuropathy is a risk factor for decline of lower extremity strength in patients with type 2 diabetes. J. Diabetes Investig. 2018;9:186–192. doi: 10.1111/jdi.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen H., Gjerstad M.D., Jakobsen J. Atrophy of Foot Muscles. Diabetes Care. 2004;27:2382–2385. doi: 10.2337/diacare.27.10.2382. [DOI] [PubMed] [Google Scholar]
- 38.Qiao Y.S., Chai Y.H., Gong H.J., Zhuldyz Z., Stehouwer C.D.A., Zhou J.B., Simó R. The Association between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences from Observational Studies. Front. Endocrinol. 2021;12:782391. doi: 10.3389/fendo.2021.782391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trierweiler H., Kisielewicz G., Hoffmann Jonasson T., Rasmussen Petterle R., Aguiar Moreira C., Zeghbi Cochenski Borba V. Sarcopenia: A chronic complication of type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2018;10:25. doi: 10.1186/s13098-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto K., Tabara Y., Ikegami H., Takata Y., Kamide K., Ikezoe T., Kiyoshige E., Makutani Y., Onuma H., Gondo Y., et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: The Multicenter Study for Clarifying Evidence for Sarcopenia in Patients with Diabetes Mellitus. J. Diabetes Investig. 2019;10:1471–1479. doi: 10.1111/jdi.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalyani R.R., Metter E.J., Egan J., Golden S.H., Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38:82–90. doi: 10.2337/dc14-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon J.W., Ha Y.C., Kim K.M., Moon J.H., Choi S.H., Lim S., Park Y.J., Lim J.Y., Kim K.W., Park K.S., et al. Hyperglycemia Is Associated with Impaired Muscle Quality in Older Men with Diabetes: The Korean Longitudinal Study on Health and Aging. Diabetes Metab. J. 2016;40:140. doi: 10.4093/dmj.2016.40.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee C.G., Boyko E.J., Barrett-Connor E., Miljkovic I., Hoffman A.R., Everson-Rose S.A., Lewis C.E., Cawthon P.M., Strotmeyer E.S., Orwoll E.S., et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbatecola A.M., Paolisso G., Lamponi M., Bandinelli S., Lauretani F., Launer L., Ferrucci L. Insulin resistance and executive dysfunction in older persons. J. Am. Geriatr. Soc. 2004;52:1713–1718. doi: 10.1111/j.1532-5415.2004.52466.x. [DOI] [PubMed] [Google Scholar]
- 45.Gong H., Liu Y., Lyu X., Dong L., Zhang X. Lipoprotein subfractions in patients with sarcopenia and their relevance to skeletal muscle mass and function. Exp. Gerontol. 2022;159:111668. doi: 10.1016/j.exger.2021.111668. [DOI] [PubMed] [Google Scholar]
- 46.Van Dronkelaar C., Van Velzen A., Abdelrazek M., Van Der Steen A., Weijs P.J.M., Tieland M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018;19:6–11.e3. doi: 10.1016/j.jamda.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Kuo I.Y., Ehrlich B.E. Signaling in Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2015;7:a006023. doi: 10.1101/cshperspect.a006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brotto M. Aging, sarcopenia and store-operated calcium entry: A common link? Cell Cycle. 2011;10:4201–4202. doi: 10.4161/cc.10.24.18645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seo M.H., Kim M.K., Park S.E., Rhee E.J., Park C.Y., Lee W.Y., Baek K.H., Song K.H., Kang M.I., Oh K.W. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: The fourth Korea national health and nutrition examination survey (KNHANES IV) 2009. Endocr. J. 2013;60:679–686. doi: 10.1507/endocrj.EJ12-0395. [DOI] [PubMed] [Google Scholar]
- 50.Kim M.K., Baek K.H., Song K.H., IL Kang M., Park C.Y., Lee W.Y., Oh K.W. Vitamin D Deficiency Is Associated with Sarcopenia in Older Koreans, Regardless of Obesity: The Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J. Clin. Endocrinol. Metab. 2011;96:3250–3256. doi: 10.1210/jc.2011-1602. [DOI] [PubMed] [Google Scholar]
- 51.Soares M.J., Chan She Ping-Delfos W., Ghanbari M.H. Calcium and vitamin D for obesity: A review of randomized controlled trials. Eur. J. Clin. Nutr. 2011;65:994–1004. doi: 10.1038/ejcn.2011.106. [DOI] [PubMed] [Google Scholar]
- 52.Dargelos E., Poussard S., Brulé C., Daury L., Cottin P. Calcium-dependent proteolytic system and muscle dysfunctions: A possible role of calpains in sarcopenia. Biochimie. 2008;90:359–368. doi: 10.1016/j.biochi.2007.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.