Abstract
Objective:
Chronic substance use and its effects on brain function and structure has long been of interest to clinicians and researchers. Prior cross-sectional comparisons of diffusion tensor imaging (DTI) metrics have suggested deleterious effects of chronic substance use (i.e., cocaine use) on white matter coherence. However, it is unclear how these effects may replicate across geographic regions when examined with similar technologies. In this study, we sought to conduct a replication of previous work in this area and determine whether there are any patterns of persistent differences in white matter microstructure between individuals with a history of cocaine use disorder (CocUD, according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) and healthy controls.
Method:
A total of 46 participants (21 healthy controls, 25 chronic cocaine users) were recruited from the Richmond, Virginia metropolitan area. Information regarding past and current substance use was collected from all participants. Participants also completed structural and DTI scans.
Results:
Consistent with previous DTI studies, significant differences were found between fractional anisotropy (FA) and axial diffusivity (AD) CocUD and controls, with CocUD showing lower FA and AD in the right inferior and superior longitudinal fasciculus, the genu, body, and splenium of the corpus callosum, and the anterior, posterior, and superior corona radiata, among several other regions. These differences were not significant for other diffusivity metrics. Lifetime alcohol consumption was greater in the CocUD group, but lifetime alcohol consumption did not show a significant linear relationship with any of the DTI metrics in within-group regression analyses.
Conclusions:
These data align with previously reported declines in white matter coherence in chronic cocaine users. However, it is less clear whether comorbid alcohol consumption results in an additive deleterious effect on white matter microstructure.
Substance Use Remains a public health concern in the United States, where roughly 20.3 million people age 12 years or older had a substance use disorder (SUD) in the past year (Substance Abuse and Mental Health Services Administration, 2019). Cocaine use is of particular concern for mortality and morbidity, given that it is increasing (Kerridge et al., 2019); it therefore is increasingly involved in deaths resulting from overdose in the United States and is the most common cause of drug-related visits to emergency departments (Han et al., 2019), with increased risk for cardiac disorders (Stankowski et al., 2015).
Increased stimulant use and stimulant use disorder are also problematic cognitively. Compared with cannabis use disorder, for example (Lee et al., 2019), stimulant use disorders are particularly associated with impairments in attention, memory, and executive function (EF) (e.g., Potvin et al., 2014; Vonmoos et al., 2013). This association could stem from either poor neurocognition conferring risk for drug use (Crews & Boettiger, 2009), chronic effects of cocaine degrading EF, or both. Causality of cocaine-induced toxicological damage is suggested by animal models, wherein controlled chronic cocaine administration results in impaired learning and memory (Mendez et al., 2008) and increased impulsivity (Mendez et al., 2010; Perry et al., 2007). These deficits are potentially attributable to decreases in white matter (WM) coherence in the corpus callosum, as well as to increases in neurofilament expression after continuous cocaine infusion in rodents (Narayana et al., 2009).
The pronounced association between stimulant use disorder and EF abnormalities adds urgency to the understanding of potential neurotoxic mechanisms of this association in humans. WM microstructural abnormalities in cocaine use disorder (CocUD) have been detected with diffusion tensor imaging (DTI), which quantifies the diffusion of water molecules through biological tissue (reviewed in Lindsey et al., 2023) and measures the degree to which water diffusion is anisotropic (or constrained) to a particular direction (Basser, 1995). Traditionally, tracts with less anisotropic diffusion are thought to be damaged in some way (Alexander et al., 2007). Of note, the cocaine exposure–related alterations in the corpus callosum found in the rodent studies (Narayana et al., 2009, 2014) were reflected in humans in a preliminary longitudinal study (Ma et al., 2017) that used DTI to examine people with CocUD. Other DTI studies have reported significant decrements in fractional anisotropy (FA) in the genu and body of the corpus callosum (Beard et al., 2019; Moeller et al., 2005), as well as the splenium (Beard et al., 2019; Ma et al., 2017) in persons with CocUD. Lane and colleagues (2010) used Tract Based Spatial Statistics (TBSS) to detect significantly lower FA and higher radial diffusivity in cocaine-dependent individuals in frontal and parietal regions and in the corpus callosum. Other studies have found decrements in diffusivity in CocUD outside of the corpus callosum (Suchting et al., 2021), such as in the anterior cingulate, uncinate fasciculus, and internal capsule (van Son et al., 2016).
Sample size in neuroimaging studies of stimulant use disorder has been limited in the past because of two challenges: (a) difficulties in recruitment and study attendance attrition in a population characterized by unreliability and poor EF, and (b) increased incidence of head-motion artifact and magnetic resonance imaging (MRI) data exclusion in persons with externalizing behaviors (Ekhtiari et al., 2019) or low EF and/or socioeconomic marginalization generally (Cosgrove et al., 2022). This is of concern in light of the increasing focus on rigor and reproducibility of scientific findings (Ioannidis, 2008), especially in neuroimaging (Button et al., 2013). Uncovering similar findings in a new sample of CocUD would provide confirmatory data on this population.
We therefore sought to replicate in a different community sample (Richmond, VA) previous cross-sectional findings reported in persons with CocUD in a different region of the United States (Houston, TX) (Lane et al., 2010; Ma et al., 2015, 2017) using nearly identical processing methodology. Replication cannot simply be assumed. Although unlikely, regional differences in typical intensity of cocaine use, cocaine availability, comorbid substance use, or even differences in cocaine product from nuances of smuggling or transit may have resulted in different patterns of WM abnormalities. This effort of replicating previous, similar findings would shed light on whether significant geographic variability exists in WM microstructure in subjects with CocUD. We investigated this using the TBSS approach in the FMRIB's Software Library (FSL; Smith et al., 2006) as in a previous publication (Lane et al 2010).
Finally, we sought an exploratory investigation of substance comorbidity effects in this population. One common theme within past work has been the acquisition of data on polysubstance use as the most common context for cocaine use. For example, previous findings have found that comorbid cocaine and alcohol use may exacerbate the effects of cocaine, as studies in rodent models have found that concurrent cocaine and alcohol use produces a number of toxic byproducts (Zheng et al., 2019). Cocaethylene, a byproduct of comorbid cocaine and alcohol use (Jatlow et al., 1996), has been shown to be toxic in humans (Shimomura et al., 2019). Most individuals with CocUD also meet lifetime criteria for alcohol use disorder (AUD; Bierut et al., 2008). More recent works have demonstrated that WM differences often reported in CocUD and other substance abuse literature may indicate a pre-existing vulnerability to polysubstance use because of WM abnormalities (Kaag et al., 2017). Although this is a possible explanation for previous positive findings of WM decrements in polysubstance use, many of the previous studies examining CocUD using DTI have included alcohol consumption and polysubstance use measures in their analyses. We believe it is relevant and important to consider how polysubstance use, specifically lifetime alcohol consumption, may interact with CocUD and WM microstructure.
We hypothesized that we would replicate previous findings of significant differences in diffusivity metrics (e.g., FA, mean diffusivity [MD], radial diffusivity [RD], and axial diffusivity [AD]) between individuals diagnosed with CocUD and healthy controls, including reduced FA and AD in CocUD participants in the superior corona radiata, the corticospinal tract, the corpus callosum, and the anterior corona radiata.
Method
This work was approved by the institutional review board at Virginia Commonwealth University and was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Informed consent was obtained from all participants.
Participants
We recruited regular cocaine users and neurotypical controls through advertisements from the Richmond, VA, metropolitan area. Most participants (n = 39) were recruited between 2014 and 2017, with the remaining eight participants having been recruited between 2018 and 2019. Prospective participants underwent screening for psychiatric disorders and SUD using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID-IV; First et al., 1995). SUD were further characterized using the Addiction Severity Index (McLellan et al., 1992). All psychiatric and substance use assessments were administered by staff who were trained by clinical psychologists and specialized didactic programs (SCID-IV). Each assessment was also presented to a research psychiatrist for concurrence. Participants were urine screened for amphetamine, barbiturates, buprenorphine, benzodiazepines, cocaine, methamphetamine, methylenedioxymethamphetamine (MDMA or Ecstasy), methadone, opiates, oxycodone, phencyclidine, propoxyphene, tricyclic antidepressants, and marijuana. Participants were also screened for recent alcohol use using an Alco-Sensor FST (Intoximeters, St. Louis, MO) to measure breath alcohol immediately before MRI scans. Participants also underwent a physical examination.
All eligible participants analyzed herein were between 27 and 60 years of age and free of alcohol (per breath screening) at time of MRI scanning (see Table 1 for demographic details). All CocUD participants specifically met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), criteria for current cocaine dependence. Healthy controls had no current or lifetime history of substance use or psychiatric disorder. Exclusion criteria included (a) being left-handed; (b) current or past DSM-IV Axis I disorder other than substance abuse or substance dependence (CocUD participants); (c) history of medical disorders or medications that target the central nervous system; (d) claustrophobia experienced during MRI or MRI simulator sessions; (e) any definite or suspected clinically significant abnormalities of the brain per board certified neuroradiologist; (f) positive urine drug screen (control participants); (g) positive pregnancy test result. CocUD subjects also underwent urine drug screening; those who had a positive urine drug screen were then assessed for intoxication by a clinician (MD or nurse practitioner) immediately before MRI scanning. No CocUD subjects were found to be intoxicated at the time of study participation. The final sample in this study consisted of 47 participants (20 healthy controls, 27 CocUD).
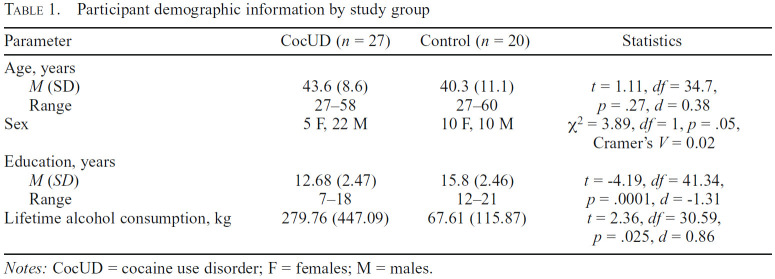
Table 1.
Participant demographic information by study group
| Parameter | CocUD (n = 27) | Control (n = 20) | Statistics |
|---|---|---|---|
| Age, years | |||
| M (SD) | 43.6 (8.6) | 40.3 (11.1) | t = 1.11, df= 34.7, |
| Range | 27-58 | 27-60 | p = .27, d =0.38 |
| Sex | 5 F, 22 M | 10 F, 10 M | χ2 = 3.89, df= 1, p =.05, Cramer's V = 0.02 |
| Education, years | |||
| M (SD) | 12.68 (2.47) | 15.8 (2.46) | t =-4.19, df =41.34, |
| Range | 7-18 | 12-21 | p = .0001, d = -1.31 |
| Lifetime alcohol consumption, kg | 279.76 (447.09) | 67.61 (115.87) | t =2.36, df = 30.59, |
Notes: CocUD = cocaine use disorder; F = females; M = males.
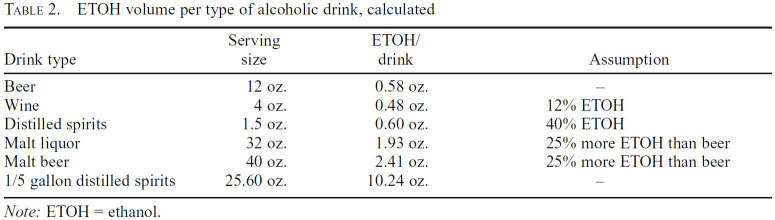
Lifetime alcohol consumption (in kg) was estimated using responses from the Addiction Severity Index (McLellan et al., 1992; see Table 2) per the following formula:
Table 2.
ETOH volume per type of alcoholic drink, calculated
| Drink type | Serving size | ETOH/drink | Assumption |
|---|---|---|---|
| Beer | 12 oz. | 0.58 oz. | - |
| Wine | 4 oz. | 0.48 oz. | 12% ETOH |
| Distilled spirits | 1.5 oz. | 0.60 oz. | 40% ETOH |
| Malt liquor | 32 oz. | 1.93 oz. | 25% more ETOH than beer |
| Malt beer | 40 oz. | 2.41 oz. | 25% more ETOH than beer |
| 1/5 gallon distilled spirits | 25.60 oz. | 10.24 oz. | .- |
Note: ETOH = ethanol.
Alcohol consumption (kg) = [(years of use)(past year of use)(number of drinks/occasion)(ETOH density)(standard ETOH oz per drink)] / 1,000
Ethanol (ETOH) density and standard ETOH volume per drink were calculated using the following formula:
ETOH density = 0.789g/ml = 23.33g/fl. oz
Participants also completed the Kreek–McHugh–Schluger–Kellogg (KMSK) scale, which quantifies self-exposure to opiates, cocaine, alcohol, and tobacco (Kellogg et al., 2003). Responses to the KMSK cocaine subscale were used to identify patterns, volume, and length of use. Higher scores on the cocaine subscale indicate more frequent use.
Neuroimaging acquisition
Whole-brain DTI data were acquired using a Philips 3T Ingenia wide-bore MRI scanner with a 32-channel receive head coil (Philips Medical Systems, Best, Netherlands). Whole-brain diffusion weighted images were acquired in the transverse plane using a single shot diffusion sensitized spin echo planar imaging sequence (Hasan & Narayana, 2003) with the following parameters: b-factors 1,000 s/mm2 and 0 s/mm2, SENSE in-plane acceleration factor 2.75, repetition time (TR) = 11.16 s, echo time (TE) = 75 ms, halfscan factor = 0.678, 42 diffusion directions, 6 repetitions of b-factor 0, FOV = 256 mm × 256 mm, acquisition matrix = 128 × 128, slice thickness 2 mm, no interslice gap, 69 transverse slices, flip angle = 90°, and voxel resolution 2 mm × 2 mm × 2 mm. The DTI acquisition time was approximately 10 minutes.
Neuroimaging preprocessing
DTI images were pre-processed using the FMIRB Software Library (FSL, Version 6.0; Jenkinson et al., 2012). Images were corrected for eddy current-induced distortions and head motion (Andersson & Sotiropoulos, 2016). The process for eddy current correction and head motion correction also identified slices in which head motion causes partial or complete signal dropout and corrected it using Gaussian Process prediction (Andersson et al., 2016). To calculate the number of outlier slices (defined by the eddy software to be four standard deviations lower than the expected intensity), we calculated the percent of outlier slices (across all volumes) for each individual subject (total number of outlying slices / total number of acquired slices). The average percent of outlying slices per subject was 0.08 (SD = 0.11). Within the CocUD group, the average percent of outlier slices was 0.11 (SD = 0.13), whereas in the healthy control group the average was 0.04 (SD = 0.06). Two sample t test revealed that this difference in outlier slices was statistically significant, t(45) = 2.22, p = .03. We also used the FSL functions eddy_quad and eddy_squad to examine subject-level and group-level and quality control issues (Bastiani et al., 2019). Results from eddy_squad also indicated that there are more outlier slices in the CocUD group than the healthy control group.
Brain tissue was extracted and an exclusion mask generated using FSL's Brain Extraction Tool (Smith, 2002). Fitting of diffusion tensors was accomplished using FMRIB's Diffusion Toolbox (Behrens et al., 2003), which generated FA, MD, and the three eigenvalues (L1, L2, and L3) for each voxel. Instead of directly using the three eigenvalues, two commonly used DTI measures were used in this study: axial/ longitudinal diffusivity (hereafter “axial diffusivity,” or AD), which is operationally defined as L1, and radial/transverse diffusivity (hereafter “radial diffusivity,” or RD), which is operationally defined as the average of L2 and L3 (Ma et al., 2015).
Tract-based spatial statistics statistical analysis
As in Lane et al. (2010), whole-brain voxelwise statistical analysis was conducted using the TBSS suite (Smith et al., 2006) in FSL (Smith et al., 2004). First, the tbss_1_preproc script was run, which slightly eroded the FA images from above. This script also zeroed the top-most slice and the bottom-most slice of each subject's FA image to remove likely outliers in FA values, because of the expected presence of non-brain tissue in these two extreme end slices.
This procedure was conducted for all subjects, and thus all subjects had the exact same number of slices (the two end slices) zeroed in their FA images. No subjects were excluded from this procedure. Second, images were subjected to a nonlinear registration to a 1 mm × 1 mm × 1 mm standard space of the FMRIB58_FA template (Andersson et al., 2007a, 2007b). Finally, the mean FA image was created, thinned, and thresholded (FA = 0.20) to create a mean FA WM skeleton representing the center of the WM tracts common to all participants. Each subject's aligned FA data were then projected onto this skeleton and the resulting data fed into voxelwise cross-subject statistics. This projection onto the WM skeleton was completed for all remaining DTI diffusivity metrics (AD, RD, and MD).
Group differences in FA and each of the other DTI diffusivity metrics were tested voxelwise across the entire whole-brain WM skeleton using the FSL program Randomise, which is a nonparametric statistical program that automatically conducts family-wise error (FWE) correction for multiple comparisons and allows for Threshold-Free Cluster Enhancement (TFCE). TFCE is a cluster-based thresholding method that does not require an arbitrary initial cluster-forming threshold (Salimi-Khorshidi et al., 2011; Smith & Nichols, 2009). All analyses here used the TFCE method with 10,000 random permutations. In the initial analyses, the two comparisons were CocUD greater than healthy controls and healthy controls greater than CocUD. Significant clusters were reported if they met the defined FWE-corrected significance threshold of p < .025 (corresponding to FWE p < .05, two tails, after Bonferroni correction). FWE significant clusters were not reported if they consisted of fewer than 10 voxels. Significant clusters were identified and labeled using the script autoaq (Winkler & Brumbaugh, 2011), which uses the FSL cluster command to threshold results based on researcher specification and identifies the location of the cluster using the FSL atlasquery command with a researcher-specified WM atlas. To this end, we specified the Johns Hopkins University DTI-based ICBM-DTI-81 WM labeled atlas in autoaq (Hua et al., 2008; Mori et al., 2005; Wakana et al., 2007).
Addressing confounding group differences in alcohol use and demographics
As shown in Table 1, there were statistically significant between-group differences in sex representation, lifetime alcohol consumption, and education. When baseline variables differ between groups by chance, an analysis of covariance (ANCOVA) can be used in primary comparisons between the groups to account for the potential confounding effects from the variables showing group differences. However, when baseline differences between groups differ because they are a component or characteristic of the diagnosis or syndrome that defines a group, this may not be appropriate (Miller & Chapman, 2001). If the baseline difference between groups in a variable (e.g., sex distribution) is related to a meaningful difference in group characteristics and not because of chance, then according to Miller and Chapman (2001), the removal of the variance in DTI metrics associated with that variable (e.g., sex distribution) could remove most variance in DTI metrics associated with CocUD.
In the present data set, substance group membership would be confounded with sex representation (as a covariate) in that men are far more likely to use cocaine than women (Kasperski et al., 2011). Instead, we conducted an exploratory analysis to determine the effect size from a small subset of subjects matched by sex (CocUD men n = 10, CocUD women n = 4; control men n = 9, control women n = 9) and age (CocUD Mage = 40.93, CocUD SD = 8.06; control Mage = 35.61, control SD = 11.46).
A similar argument applies to including alcohol as a covariate in ANCOVA herein, because binge alcohol use is endemic in (and frequently precedes) CocUD. For example, in a large urban sample, more than 73% of persons with CocUD also had a lifetime diagnosis of alcohol dependence (Bierut et al., 2008). Finally, there are similar trends in previous literature regarding low education in CocUD, with the 2015 National Survey on Drug Use and Health indicating that illicit drug use and crack cocaine use are lowest among adults who completed college (Center for Behavioral Health Statistics and Quality, 2016; Kerridge et al., 2019). This may be attributable, in part, to individuals at high risk having increased access to drugs, and subsequently experiencing increased adverse consequences from drug use (Braggio et al., 1993; Crum, 2014; Eggert & Herting, 1995). Therefore, we did not covary out the greater incidence of male sex, greater alcohol use, or lower education of the CocUD group in the primary cross-sectional comparisons because these were deemed fundamental features of CocUD itself. Rather, to gain insights into how cumulative alcohol exposure and education may have affected group-difference DTI findings, we instead performed regression analyses relating DTI metrics with these confounding variables directly within each group separately.
Results
Demographics
As seen in Table 1, the two groups were not different in age. Whereas the control group had equal numbers of men and women (10 men, 10 women), the CocUD group reflected the strong preponderance of men with CocUD in our community (22 men, 5 women). The healthy control group reported more years of education on average than the CocUD group, and most CocUD participants (and also some controls) reported high levels of alcohol consumption, with the CocUD group reporting significantly higher mean lifetime consumption than the healthy control group. The CocUD group on average reported first using cocaine at 22 years of age but with great variability (SD = 8.12). Most CocUD subjects reported smoking cocaine as their preferred route of administration (n = 18), although some reported intranasal use (n = 9). The CocUD group also reported an average of 15.79 years of regular use, also with large variability (SD = 8.10). CocUD subjects reported using cocaine twice in the past week before study participation on average (M = 2.48, SD = 2.24). Fifteen participants met criteria for past or current alcohol abuse, 7 met criteria for past or current abuse of another substance (e.g., marijuana), and 7 met criteria for both past/current alcohol abuse and abuse of another substance other than cocaine. In addition, two participants in the CocUD group met DSM-IV criteria for past substance-induced mood disorder, one met criteria for history of anxiety disorder, and one met criteria for history of adjustment disorder.
Group differences in white matter DTI metrics
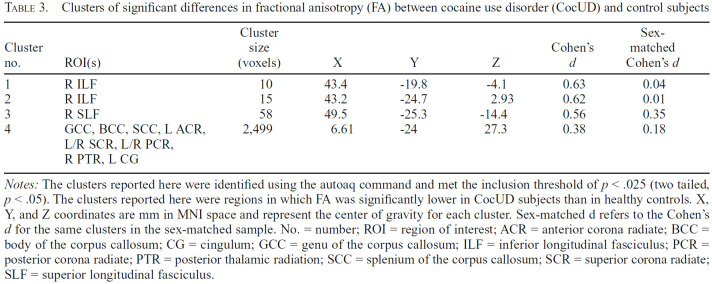
We found several significant group differences in regional FA and AD (Table 3), but not in MD or RD (Figures 1 and 2). For FA, we found one small cluster (58 voxels) of reduced FA in CocUD in the right superior longitudinal fasciculus, and two small clusters (of 15 and 10 voxels, respectively) in the right inferior longitudinal fasciculus. We found one large cluster (2,499 voxels) of significantly reduced FA in CocUD, which spanned the genu, body, and splenium of the corpus callosum, the left anterior corona radiata, the bilateral superior corona radiata, the bilateral posterior corona radiata, the right posterior thalamic radiation, and the left cingulate gyrus. For AD, we found three larger clusters (612, 598, and 119 voxels, respectively) of significantly lower AD in CocUD. The largest cluster spanned the right anterior limb of the internal capsule, the right posterior limb of the internal capsule, the right retrolenticular part of the internal capsule, and the right superior corona radiata (Table 4). The next largest clusters were located in the genu and body of the corpus callosum and the right anterior limb of the internal capsule and the right external capsule, respectively. Two small clusters (20 and 10 voxels, respectively) were found in the genu of the corpus callosum and the right external capsule. We used fslmaths to determine whether there were any overlapping significant clusters of voxels between the significant results for FA and AD but found no voxels in common between the two.
Table 3.
Clusters of significant differences in fractional anisotropy (FA) between cocaine use disorder (CocUD) and control subjects
| Cluster no. | ROI(s) | Cluster size (voxels) | X | Y | Z | Cohen's d | Sex-matched Cohen's d |
|---|---|---|---|---|---|---|---|
| 1 | RILF | 10 | 43.4 | -19.8 | -4.1 | 0.63 | 0.04 |
| 2 | RILF | 15 | 43.2 | -24.7 | 2.93 | 0.62 | 0.01 |
| 3 | R SLF | 58 | 49.5 | -25.3 | -14.4 | 0.56 | 0.35 |
| 4 | GCC, BCC, SCC, L ACR, L/R SCR, L/R PCR, R PTR, L CG | 2,499 | 6.61 | -24 | 27.3 | 0.38 | 0.18 |
Notes: The clusters reported here were identified using the autoaq command and met the inclusion threshold of p < .025 (two tailed, p < .05). The clusters reported here were regions in which FA was significantly lower in CocUD subjects than in healthy controls. X, Y, and Z coordinates are mm in MNI space and represent the center of gravity for each cluster. Sex-matched d refers to the Cohen's d for the same clusters in the sex-matched sample. No. = number; ROI = region of interest; ACR = anterior corona radiate; BCC = body of the corpus callosum; CG = cingulum; GCC = genu of the corpus callosum; ILF = inferior longitudinal fasciculus; PCR = posterior corona radiate; PTR = posterior thalamic radiation; SCC = splenium of the corpus callosum; SCR = superior corona radiate; SLF = superior longitudinal fasciculus.
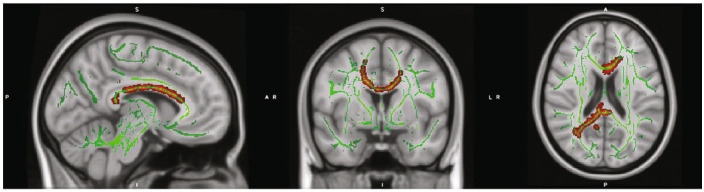
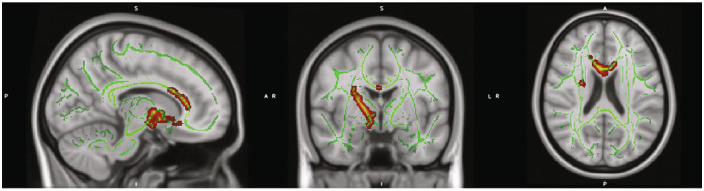
Figure 1.
Regions with significantly reduced fractional anisotropy (FA) in cocaine use disorder (CocUD) as compared with healthy controls. Notes: Results are displayed on the MNI_152 1 mm head template provided by FMRIB's Software Library. All results met an inclusion threshold of p < .025 (two tailed p < .05) and were significant for a contrast assuming that CocUD had lower FA than controls. All red voxels represent significant t values that are significant at least at p < .025 (two tailed p < .05). Green voxels represent the underlying FA skeleton, averaged across all subjects.
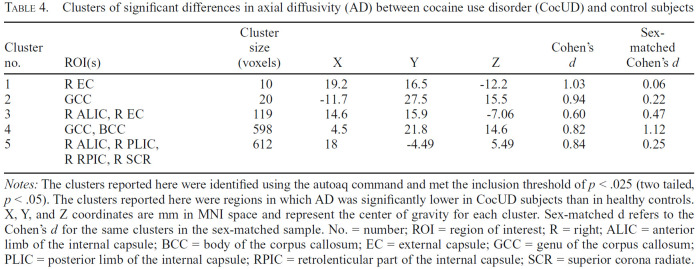
Figure 2.
Regions with significantly aberrant axial diffusivity (AD) in cocaine use disorder (CocUD) as compared with healthy controls. Notes: Results are displayed on the MNI_152 1 mm head template provided by FMRIB's Software Library. All results met an inclusion threshold of p < .025 (two tailed p < .05) and were significant for a contrast assuming that CocUD had lower AD than controls. All red voxels represent significant t values that are significant at least at p < .025 (two tailed p < .05). Green voxels represent the underlying fractional anisotropy (FA) skeleton, averaged across all subjects.
Table 4.
Clusters of significant differences in axial diffusivity (AD) between cocaine use disorder (CocUD) and control subjects
| Cluster no. | ROI(s) | Cluster size (voxels) | X | Y | Z | Cohen's d | Sex-matched Cohen's d |
|---|---|---|---|---|---|---|---|
| 1 | R EC | 10 | 19.2 | 16.5 | -12.2 | 1.03 | 0.06 |
| 2 | GCC | 20 | -11.7 | 27.5 | 15.5 | 0.94 | 0.22 |
| 3 | R ALIC, R EC | 119 | 14.6 | 15.9 | -7.06 | 0.60 | 0.47 |
| 4 | GCC, BCC | 598 | 4.5 | 21.8 | 14.6 | 0.82 | 1.12 |
| 5 | R ALIC, R PLIC, R RPIC, R SCR | 612 | 18 | -4.49 | 5.49 | 0.84 | 0.25 |
Notes: The clusters reported here were identified using the autoaq command and met the inclusion threshold of p < .025 (two tailed, p < .05). The clusters reported here were regions in which AD was significantly lower in CocUD subjects than in healthy controls. X, Y, and Z coordinates are mm in MNI space and represent the center of gravity for each cluster. Sex-matched d refers to the Cohen's d for the same clusters in the sex-matched sample. No. = number; ROI = region of interest; R = right; ALIC = anterior limb of the internal capsule; BCC = body of the corpus callosum; EC = external capsule; GCC = genu of the corpus callosum; PLIC = posterior limb of the internal capsule; RPIC = retrolenticular part of the internal capsule; SCR = superior corona radiate.
We also investigated whether participant age was related to DTI metrics because previous studies have noted signifi-cant relationships between the two (Burzynska et al., 2010; Molloy et al., 2021). For FA, MD, RD, and AD, there were no significant interactions of Group × Age, and no significant regression of any of these DTI metrics with age. We also conducted analyses investigating whether differences between men and women were present in DTI metrics, but t tests revealed no significant differences.
Relationships between DTI metrics and lifetime alcohol consumption and other demographic variables within groups
In light of the appreciable rates of lifetime alcohol use, especially in the CocUD group, we conducted post hoc exploratory brainwide analysis of whether DTI metrics varied as a function of alcohol or other demographic variables within each of the CocUD and healthy control groups singly. Each of these analyses used FA, MD, RD, and AD as outcome variables.
CocUD group. There were no significant clusters wherein FA, MD, AD, and RD correlated with lifetime alcohol consumption in the CocUD group. To ensure that alcohol correlations were not obscured by the skewed distribution of the alcohol consumption variable, analyses were re-performed after lifetime alcohol consumption was log transformed and mean corrected. This also failed to uncover any significant alcohol use–associated clusters for any diffusion metric in either the CocUD group or the control group. For education within the CocUD group, we found a significant cluster (145 voxels) in the superior corona radiata in which FA correlated positively with years of education. Likewise, we found three small clusters (450, 46, and 1 voxel, respectively) located in the body of the corpus callosum and the superior corona radiata in which RD correlated with education.
Given how education had a significant relationship with FA in the superior corona radiata in CocUD and how we had significant primary group-difference findings in the superior corona radiata, we calculated the degree of spatial overlap between the significant cluster found in the within-group education and primary analysis. Significant spatial overlap would suggest a possibility that reduced education in CocUD stemmed from abnormalities in this critical frontocortical WM tract. However, we found only 2 voxels from the significant education cluster that overlapped the primary analysis results, of the 2,499 voxels that showed group difference in the primary analysis (0.08% overlap).
We also performed a regression between FA, MD, RD, and AD and total scores on the KMSK cocaine subscale to determine whether frequency of cocaine use was significantly related to DTI metrics. The cocaine subscale of the KMSK has a maximum score of 16, with higher scores indicating more frequent cocaine use. Regressions between DTI measures of diffusivity and KMSK total scores indicated no significant relation for FA, MD, or RD. We did find a significant positive association between KMSK scores and AD (Table 5). The largest of these clusters consisted of 9,483 voxels and spanned multiple regions, including the genu, body, and splenium of the corpus callosum, the anterior/ posterior limbs of the internal capsule, the anterior/superior corona radiata, the external capsule, the superior longitudinal fasciculus, the superior fronto-occipital fasciculus, and the tapetum.
Table 5.
Significant clusters from regression between Kreek–McHugh–Schluger–Kellogg (KMSK) scale scores and axial diffusivity (AD) within the cocaine use disorder (CocUD) group
| Cluster no. | ROI(s) | Cluster size (voxels) | X | Y | Z | Effect size Cohen's d |
|---|---|---|---|---|---|---|
| 1 | FM | 31 | -15.2 | 46.3 | -10.2 | 0.69 |
| 2 | FM | 33 | 17.1 | 46.6 | 23.6 | 1.74 |
| 3 | L ACR | 37 | -21 | 32.2 | 13.9 | 0.89 |
| 4 | L PTR | 42 | -28.2 | -66.3 | 21.7 | 1.73 |
| 5 | R SLF | 62 | 31.7 | 3.45 | 45.5 | 0.86 |
| 6 | L ACR | 80 | -18 | 45.5 | -1.54 | 0.76 |
| 7 | L ACR | 94 | -24.2 | 32 | 5.16 | 0.94 |
| 8 | BCC, SCC | 101 | 13.7 | -27 | 28.9 | 0.69 |
| 9 | L ATR, L IFOF, L SLF, L UF | 210 | -32.1 | 35.9 | 3.26 | 0.94 |
| 10 | L ATR, L CG, FM, L IFOF, L SLF, L UF | 447 | -18.3 | 39 | 22.1 | 0.86 |
| 11 | L ACR, L SCR, L PCR, L EC, L SLF | 2,670 | -25 | -9.13 | 38.7 | 0.50 |
| 12 | GCC, BCC, SCC, | 9,483 | 24.5 | 1.76 | 24.6 | 1.18 |
Notes: The clusters reported here were identified using the autoaq command and met the inclusion threshold of p < .025 (two tailed p < .05). The clusters reported here were regions in which AD showed a positive, significant relationship with KMSK scores in CocUD subjects. X, Y, and Z coordinates are mm in MNI space and represent the center of gravity for each cluster. No. = number; ROI = region of interest; L = left; R = right; ACR = anterior corona radiate; ALIC = anterior limb of the internal capsule; ATR = anterior thalamic radiation; BCC = body of the corpus callosum; CG = cingulum; EC = external capsule; FM = forceps minor; GCC = genu of the corpus callosum; IFOF = inferior fronto-occipital fasciculus; PCR = posterior corona radiate; PLIC = posterior limb of the internal capsule; PTR = posterior thalamic radiation; SCR = superior corona radiate; SFOF = superior fronto-occipital fasciculus; SLF = superior longitudinal fasciculus; TP = tapetum; UF = uncinate fasciculus.
Control group. We repeated the same analyses of the CocUD group in the control group. Likewise, we found no significant clusters in controls that correlated with lifetime alcohol consumption for FA, MD, RD, or AD; we found no significant relation between years of education and DTI metrics; and we found no significant difference between men and women in FA, MD, RD, or AD.
Discussion
We probed whether participants with CocUD would show reduced WM coherence than healthy controls in a pattern found previously by our group (Lane et al., 2010) and others (Kaag et al., 2017; Tondo et al., 2021), and whether DTI metrics would be influenced by participant sex, education, or lifetime alcohol consumption in both groups with (CocUD) and without (controls) histories of chronic cocaine use. We were especially interested in how closely these data, collected from a different geographic region than previous studies (although similar in sample size, demographics, preprocessing, and analytical approach), would reflect previously reported findings. More specifically, we hypothesized that based on Lane et al. (2010) and on similar works (Kaag et al., 2017; Ma et al., 2015, 2017; Tondo et al., 2021) we would find decrements in DTI metrics (FA, MD, RD, and AD) in individuals with a history of chronic cocaine use compared with healthy controls in regions such as the superior corona radiata, the corticospinal tract, the corpus callosum, and the anterior corona radiata. This specific hypothesis was mostly supported, as we identified significant differences between groups in the superior and anterior corona radiata and in the corpus callosum, in replication of previous work. We did not identify any significant differences in the corticospinal tract.
In our primary group analysis, we found significantly lower FA in CocUD subjects in the right inferior longitudinal fasciculus; the right superior longitudinal fasciculus; the genu, splenium, and body of the corpus callosum; the bilateral superior corona radiate; the left anterior corona radiate; the bilateral posterior corona radiate; the right posterior thalamic radiation; and the left cingulate gyrus (Table 3). Second, we similarly found significantly lower AD in CocUD subjects than controls in the right external capsule, the genu and body of the corpus callosum, the right anterior limb of the internal capsule, the right posterior limb of the internal capsule, and the right superior corona radiata (Table 4).
It is promising that our primary group analysis found clusters of significant difference in FA and AD between CocUD and healthy controls in similar regions to those found in Lane et al. (2010), which is arguably our closest methodological comparator. Lane (2010) also noted differences in FA in the superior corona radiata and the corpus callosum, with similar cluster sizes to the findings reported here. This finding suggests that chronic cocaine use (or cocaine + alcohol) may be driving a significant difference in WM microstructure (or vice versa, since this is a correlational finding), and replicates previous findings of CocUD-related decrements in WM coherence (Lane et al., 2010; Ma et al., 2015, 2017). A recent report by the Enhancing NeuroImaging Genetics through Meta-Analysis (ENIGMA) consortium's Addiction working group revealed that CocUD patients displayed deficits in similar WM tracts to those reported here; these deficits were distinct enough that a machine learning algorithm was able to correctly identify individuals with CocUD and methamphetamine dependence relative to controls (Ottino-González et al., 2022).
Similarly, it is interesting that these results indicated significant differences in AD (but not RD) between CocUD and controls, whereas Lane (2010) reported differences in RD (but not AD). Previous work has examined how DTI parameters are influenced by underlying neural structure and has found that FA can be affected by several factors, including neural apoptosis, demyelination, and hypertrophy (Hutchinson et al., 2018; O’Donnell & Westin, 2011). Conversely, AD is thought to be associated with axonal morphology and degradation, with alterations in AD being indicative of disruption in tissue organization and axonal coherence, whereas RD is associated with myelin changes (Caeyenberghs et al., 2010). The findings in Lane (2010) for RD are reminiscent of our findings in AD, with Lane (2010) reporting significantly greater RD in CocUD compared with controls in the right corticospinal tract, the right superior corona radiata, the left posterior corona radiata, and the left superior longitudinal fasciculus, among other regions. It is possible that chronic cocaine use in our sample specifically affected axonal coherence and tissue organization, which drove our significant findings in this area. However, these differences in WM coherence between chronic cocaine users and non–drug-using controls could reflect preexisting traits; thus, further work is needed to clarify this relationship.
It is important to caution that however replicated, these findings are correlational, and provide no information on causality. Although rodent model studies indicate that chronic cocaine use alters WM (Narayana et al., 2014), a possibility remains that the association between CocUD and reduced FA stems in part or in whole from neurodevelopmentally degraded coherence of WM tracts that connect frontocortical control regions and subcortical incentive-motivational structures (e.g., anterior internal capsule or frontal aspects of corona radiata) reducing capacity for frontal cortex to regulate motivational neurocircuitry by late adolescence (Jacobus et al., 2013). This reduced cognitive control would in turn facilitate drug experimentation and other risky behavior in youth (Nigg et al., 2006).
In lieu of conducting questionable ANCOVAs that would have partialed out key components of the CocUD phenotype, we instead addressed the greater alcohol use, lower education, and greater male representation in the CocUD group by conducting potentially explanatory regression analyses within the CocUD and healthy control groups. Surprisingly, we found no evidence of correlation of alcohol use and DTI metrics. This was unexpected because of the numerous previous studies that have found individuals withAUD show significantly lower FA in WM tracts than healthy controls (De Santis et al., 2019; Hampton et al., 2019; McQueeny et al., 2009; Thayer et al., 2013). Further, work has demonstrated similar widespread deficits in hemispheric resting state functional connectivity and in both gray and white matter volume in chronic cocaine users, further bolstering the argument that chronic cocaine use is associated with neural disruption (Kelly et al., 2011; Lim et al., 2007). Although previous work has found that FA varies over the lifetime as WM density changes (Curran et al., 2016), it is unlikely that age influenced this finding, as we found no significant relationship between age and any DTI metrics, or any evidence of a significant Group × Age interaction. Some previous work, however, has also found significant associations between diffusivity metrics and years of cocaine use, but not years of alcohol use (Tannous et al., 2019; Tondo et al., 2021). It may be that compared with participants selected for AUD in these other reports, in drinkers whose primary substance of abuse is not alcohol, the cumulative alcohol exposure was not sufficient for neurotoxic effects detectable by DTI.
Within the CocUD group, we found that both education and KMSK cocaine subscale scores were associated with DTI metrics. We found that education was positively associated with FA in a cluster in the superior corona radiata. However, after calculating the percent spatial overlap (0.08%) between our primary group-difference and within-group correlation findings, it is unlikely that lower education in CocUD was driving most of our results. Recent work has found that lower education is associated with higher risk of SUD; conversely, in CocUD specifically, there has been a marked increase in cocaine use among individuals with higher education when compared to individuals with less education (Kerridge et al., 2019). Although it is unlikely that educational differences were driving our primary findings, future work may find use in more closely investigating how level of education is associated with CocUD and WM microstructure.
For the KMSK cocaine subscale, we found that AD was positively associated with KMSK total scores in a linear regression, with results finding several large clusters spanning multiple WM tracts. Although it is seemingly contradictory to have found elevated AD in a population that one would reasonably expect to display neurodegeneration, past work has suggested that decreases in AD in the acute phase of injury followed by normalization or increases in AD are common in several neurodegenerative disorders (e.g., multiple sclerosis; Schmierer et al., 2007; Winklewski et al., 2018). Compensatory responses are not uncommon in CocUD—recent work has shown that, when compared with current cocaine users, those remaining abstinent from cocaine show similar WM properties to control subjects (He et al., 2020). It is thought that this pattern of initial decrease in AD followed by an increase is attributable to clearing of axon fragments, but more research is needed to definitively understand this relationship (Della Nave et al., 2011).
It is also promising that our results were not markedly different from those conducted in a different geographic region. Given previous research suggesting geographic differences in types and administration method of substance consumption (Hand et al., 2017; Zoorob, 2019), we saw a potential for some differences from WM abnormalities reported in previous literature in this area. However, as we essentially replicated previous findings (Lane et al., 2010; Ma et al., 2017), it may be that among CocUD participants, patterns of use (and related toxicity) do not differ appreciably across different regions of the United States. Future work should investigate this phenomenon more in depth using more granular metrics of cocaine use to determine whether signifi-cant differences in DTI and CocUD between two geographic samples would emerge with larger sample sizes.
Although speculative, we posit that aberrant WM in fronto-limbic and frontotemporal pathways, such as the superior/ inferior longitudinal fasciculus, the anterior thalamic radiation, and the forceps major, may account for EF decrements characteristic of CocUD such as self-regulatory dysfunction, as has been noted in previous literature (Clark et al., 2012). Similar decrements have been found in individuals with a history of eating disorders or borderline personality disorder (Estella et al., 2020; Vandekerckhove et al., 2020). One study found that reductions in FA were significantly correlated with ratings of impulsivity and motor control in chronic marijuana users (Gruber et al., 2011). Although few studies have examined WM specifically in this context, functional imaging studies have demonstrated that CocUD patients (when presented with gambling tasks) demonstrate abnormal incentive processing, with elevated salience for rewards and punishment, but less prefrontal regulation after adverse outcomes (Vaquero et al., 2017). This dysregulation may be attributable to an inherent vulnerability to addiction; one study comparing CocUD patients to individuals with gambling disorder demonstrated similar patterns of WM coherence between the two groups that could not be explained solely by exposure to drugs or alcohol (Yip et al., 2017). Given these findings, we believe future work should examine this phenomenon closely, in both CocUD and other similar disorders that involve self-regulation dysfunction.
Limitations
There are several limitations to the present work. First, prospective study applicants were accepted as they presented if they met criteria, resulting in a heavy skew toward lower-educated, heavy-drinking men in the CocUD group compared with controls. Similarly, for lifetime alcohol consumption, the calculated lifetime consumption (in kg) for the CocUD group still falls far below what is commonly reported in AUD literature (e.g., Zhao et al., 2020). It may simply be that our sample, although meeting criteria for CocUD, did not concurrently consume enough alcohol throughout their lifetime to elicit similar WM decrements as seen in previous work. Best statistical practices, however, indicate that such covariates should not be included when they are a characteristic of a disorder, as this would almost completely remove all variance from the independent variable and render results from an ANCOVA meaningless (Miller & Chapman, 2001). Prevalence estimates have noted that during the period of recruitment for this study, men were more likely than women to use cocaine and seek treatment for CocUD (Kasperski et al., 2011; Kerridge et al., 2019; Kosten et al., 1993), perhaps because of more opportunity to engage in cocaine use (Kasperski et al., 2011), although the gender gap is narrowing (Kerridge et al., 2019). Similarly, meta-analytic evidence suggests that prevalence of concurrent and simultaneous alcohol use in cocaine users is between 74% and 77% (Liu et al., 2018). There are similar trends reporting findings of lower education among crack cocaine users (Kerridge et al., 2019). In our sample, we noted a statistically significant difference in years of education between the CocUD group and the control group. We were unable to eliminate this difference because of difficulty in recruitment of CocUD subjects with more varied years of education.
Thus, considering this evidence, coupled with the lack of correlation between alcohol use or years of education with DTI metrics in this sample, we decided against conducting ANCOVAs to avoid this violation of statistical assumptions. Conversely, truncating the CocUD participant sample to artificially attain an age- and sex-match with controls would compromise power to potentially result in uninterpretable negative findings. Going forward, we believe it is important to understand sex differences in the neuropathology of CocUD; thus, more studies are needed to include sufficient sample sizes. Although our post hoc investigations of how sex, education, and lifetime alcohol relate to DTI metrics directly do not suggest that group differences were driven by these factors, we cannot rule out that DTI differences between groups defined by CocUD were really driven by group differences. Future work should allow for such sex representation, cumulative alcohol use, or education by group analyses. This is especially true for sex representation, as the CocUD group only contained 5 women of 27 total participants. Future projects with extended time frames could selectively admit qualified applicants to balance for demographic factors.
Another potential limitation is that there were significantly more outlier diffusion MRI slices with lower intensity acquired in the CocUD group than the control group. This was mitigated by the fsl eddy software, which corrected outlier slices using Gaussian Process prediction (Andersson et al., 2016). Nevertheless, future studies should try to match this factor between groups.
The final limitation pertains to our MRI acquisition sequences. Although we used more diffusion directions (42) in our DTI acquisition sequences than previous studies (such as Lane, 2010), this is still far below what is currently possible in DTI research. Other recent studies have increased the number of diffusion directions acquired to between 60 and more than 100, which may be more suited to work with substance abuse data, such as those described here. We recommend that future work, with the ability to gather more diffusion directions, replicate these findings and those of other previous studies and determine whether increasing the number of diffusion directions even more may provide more sensitivity to detect WM change in chronic cocaine users.
Conclusions and future directions
The difficulty of recruiting “boutique” disorder samples for neuroimaging and resultant modest sample sizes poses challenges for rigor and reproducibility in these studies (Button et al., 2013). Here, we essentially replicated the findings from Lane (2010) and other reports, which can increase confidence in findings of cocaine-related WM alterations. Moreover, because our data capture enabled an approximate quantitation of lifetime alcohol consumption, this enabled follow-on analyses of direct alcohol exposure associations with WM abnormalities atop a background of chronic cocaine exposure as well as atop an otherwise neurotypical background (controls). These analyses did not reveal any alcohol–WM associations in either group. Nevertheless, despite the recruitment challenge, it may be preferred that future studies compare a CocUD sample to an AUD sample matched on lifetime alcohol quantity, to definitively isolate cocaine exposure-related WM abnormalities. Second, with advances in DTI acquisition technologies and data processing methods, newer acquisition parameters and sequences may be more sensitive to better reveal relationships between chronic substance use and WM coherence. Further, in light of increasing use of cocaine by women (Kerridge, 2019) and funder emphasis on uncovering sex differences (National Institutes of Health NOT-OD-15-102), future studies powered to detect Sex × Group interactions on WM abnormalities with cocaine use are of great interest. Finally, future DTI experiments can incorporate self-control and other EF tasks to determine the mechanistic or functional relevance of frontocortical WM abnormalities in CocUD.
Footnotes
This project was funded through National Institute on DrugAbuse Project numbers P50DA009262, U54DA038999, and R01 DA034131, and National Center forAdvancingTranslational Sciences Project number UL1TR002649.
References
- Alexander A. L., Lee J. E., Lazar M., Field A. S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. doi:10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (4th ed.). Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Andersson J. L. R., Graham M. S., Zsoldos E., Sotiropoulos S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 2016;141:556–572. doi: 10.1016/j.neuroimage.2016.06.058. doi:10.1016/j.neuroimage.2016.06.058. [DOI] [PubMed] [Google Scholar]
- Andersson J. L. R., Jenkinson M., Smith S. FMRIB Technical Report TR07JA1. Oxford, England: FMRIB Centre; 2007a. Non-linear optimization. Retrieved from https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja1/tr07ja1.pdf. [Google Scholar]
- Andersson J. L. R., Jenkinson M., Smith S. Oxford, England: FMRIB Centre; 2007b. Non-linear registration aka Spatial normalization: FMRIB Technical Report TR07JA2. Retrieved from https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf. [Google Scholar]
- Andersson J. L. R., Sotiropoulos S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. doi:10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P. J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8:333–344. doi: 10.1002/nbm.1940080707. doi:10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Bastiani M., Cottaar M., Fitzgibbon S. P., Suri S., Alfaro-Almagro F., Sotiropoulos S. N., Andersson J. L. R. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. NeuroImage. 2019;184:801–812. doi: 10.1016/j.neuroimage.2018.09.073. doi:10.1016/j.neuroimage.2018.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C. L., Schmitz J. M., Soder H. E., Suchting R., Yoon J. H., Hasan K. M., Lane S. D. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug and Alcohol Dependence. 2019;201:29–37. doi: 10.1016/j.drugalcdep.2019.03.023. doi:10.1016/j.drugalcdep.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T. E. J., Woolrich M. W., Smith S. M., Boulby P. A., Barker G. J., Sillery E. L., Matthews P. M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bierut L. J., Strickland J. R., Thompson J. R., Afful S. E., Cottler L. B. Drug use and dependence in cocaine dependent subjects, community-based individuals, and their siblings. Drug and Alcohol Dependence. 2008;95:14–22. doi: 10.1016/j.drugalcdep.2007.11.023. doi:10.1016/j.drugalcdep.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braggio J. T., Pishkin V., Gameros T. A., Brooks D. L. Academic achievement in substance-abusing and conduct-disordered adolescents. Journal of Clinical Psychology. 1993;49:282–291. doi: 10.1002/1097-4679(199303)49:2<282::aid-jclp2270490223>3.0.co;2-n. doi:10.1002/1097-4679(199303)49:2<282::AIDJCLP2270490223>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Burzynska A. Z., Preuschhof C., Bäckman L., Nyberg L., Li S. C., Lindenberger U., Heekeren H. R. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. NeuroImage. 2010;49:2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. doi:10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Button K. S., Ioannidis J. P. A., Mokrysz C., Nosek B. A., Flint J., Robinson E. S. J., Munafò M. R. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. doi:10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Leemans A., Geurts M., Taymans T., Linden C. V., Smits-Engelsman B. C. M., Swinnen S. P. Brain-behavior relationships in young traumatic brain injury patients: DTI metrics are highly correlated with postural control. Human Brain Mapping. 2010;31:992–1002. doi: 10.1002/hbm.20911. doi:10.1002/hbm.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. B., Chung T., Thatcher D. L., Pajtek S., Long E. C. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction. 2012;107:206–214. doi: 10.1111/j.1360-0443.2011.03566.x. doi:10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K. T., McDermott T. J., White E. J., Mosconi M. W., Thompson W. K., Paulus M. P., Aupperle R. L. Limits to the generalizability of resting-state functional magnetic resonance imaging studies of youth: An examination of ABCD Study® baseline data. Brain Imaging and Behavior. 2022;16:1919–1925. doi: 10.1007/s11682-022-00665-2. doi:10.1007/s11682-022-00665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F. T., Boettiger C. A. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry, and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. doi:10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum R. M. In The ASAM Principles of Addiction Medicine: Fifth Edition. Alphen aan den Rijn. The Netherlands: Wolters Kluwer Health; 2014. The epidemiology of substance use disorders. [Google Scholar]
- Curran K. M., Emsell L., Leemans A. Quantitative DTI measures. In: Van Hecke W., Emsell L., Sunaert S., editors. Diffusion Tensor Imaging: A Practical Handbook. New York, NY: Springer; 2016. pp. 65–87. Retrieved from . [DOI] [Google Scholar]
- Della Nave R., Ginestroni A., Diciotti S., Salvatore E., Soricelli A., Mascalchi M. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology. 2011;53:367–372. doi: 10.1007/s00234-010-0807-1. doi:10.1007/s00234-010-0807-1. [DOI] [PubMed] [Google Scholar]
- De Santis S., Bach P., Pérez-Cervera L., Cosa-Linan A., Weil G., Vollstädt-Klein S., Canals S. Microstructural white matter alterations in men with alcohol use disorder and rats with excessive alcohol consumption during early abstinence. JAMA Psychiatry. 2019;76:749–758. doi: 10.1001/jamapsychiatry.2019.0318. doi:10.1001/jamapsychiatry.2019.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert L. L., Herting J. R. Drug involvement among potential dropouts and “typical” youth. Journal of Drug Education. 1995;23:31–55. doi: 10.2190/9RCJ-DTYE-KL5L-HDRA. doi:10.2190/9RCJ-DTYE-KL5L-HDRA. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H., Kuplicki R., Yeh H. W., Paulus M. P. Physical characteristics not psychological state or trait characteristics predict motion during resting state fMRI. Scientific Reports. 2019;9:419. doi: 10.1038/s41598-018-36699-0. doi:10.1038/s41598-018-36699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-IP, Version 2.0) [Google Scholar]
- Gruber S. A., Silveri M. M., Dahlgren M. K., Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19:231–242. doi: 10.1037/a0023034. doi:10.1037/a0023034\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. G., Alhassoon O. M., Stern M. J., Wollman S. C., Kimmel C. L., Perez-Figueroa A., Radua J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: A neuroimaging meta-analysis. American Journal of Drug and Alcohol Abuse. 2015;41:290–299. doi: 10.3109/00952990.2015.1044607. doi:10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- Hampton W. H., Hanik I. M., Olson I. R. Substance abuse and white matter: Findings, limitations, and future of diffusion tensor imaging research. Drug and Alcohol Dependence. 2019;197:288–298. doi: 10.1016/j.drugalcdep.2019.02.005. doi:10.1016/j.drugalcdep.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B. H., Tuazon E., Kunins H. V., Mantha S., Paone D. Unintentional drug overdose deaths involving cocaine among middle-aged and older adults in New York City. Drug and Alcohol Dependence. 2019;198:121–125. doi: 10.1016/j.drugalcdep.2019.01.042. doi:10.1016/j.drugalcdep.2019.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand D. J., Short V. L., Abatemarco D. J. Substance use, treatment, and demographic characteristics of pregnant women entering treatment for opioid use disorder differ by United States census region. Journal of Substance Abuse Treatment. 2017;76:58–63. doi: 10.1016/j.jsat.2017.01.011. doi:10.1016/j.jsat.2017.01.011. [DOI] [PubMed] [Google Scholar]
- Hasan K. M., Narayana P. A. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: Theoretical analysis and validation. Magnetic Resonance in Medicine. 2003;50:589–598. doi: 10.1002/mrm.10552. doi:10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- He Q., Li D., Turel O., Bechara A., Hser Y. I. White matter integrity alternations associated with cocaine dependence and long-term abstinence: Preliminary findings. Behavioural Brain Research. 2020;379:112388. doi: 10.1016/j.bbr.2019.112388. doi:10.1016/j.bbr.2019.112388. [DOI] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D. S., Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. doi:10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E. B., Schwerin S. C., Avram A. V., Juliano S. L., Pierpaoli C. Diffusion MRI and the detection of alterations following traumatic brain injury. Journal of Neuroscience Research. 2018;96:612–625. doi: 10.1002/jnr.24065. doi:10.1002/jnr.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J. P. A. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–648. doi: 10.1097/EDE.0b013e31818131e7. doi:10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Thayer R. E., Trim R. S., Bava S., Frank L. R., Tapert S. F. White matter integrity, substance use, and risk taking in adolescence. Psychology of Addictive Behaviors. 2013;27:431–442. doi: 10.1037/a0028235. doi:10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow P., McCance E. F., Bradberry C. W., Elsworth J. D., Taylor J. R., Roth R. H. Alcohol plus cocaine: The whole is more than the sum of its parts. Therapeutic Drug Monitoring. 1996;18:460–464. doi: 10.1097/00007691-199608000-00026. doi:10.1097/00007691-199608000-00026. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C. F., Behrens T. E. J., Woolrich M. W., Smith S. M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. doi:10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kaag A. M., van Wingen G. A., Caan M. W. A., Homberg J. R., van den Brink W., Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addiction Biology. 2017;22:1048–1056. doi: 10.1111/adb.12375. doi:10.1111/adb.12375. [DOI] [PubMed] [Google Scholar]
- Kasperski S. J., Vincent K. B., Caldeira K. M., Garnier-Dykstra L. M., O’Grady K. E., Arria A. M. College students’ use of cocaine: Results from a longitudinal study. Addictive Behaviors. 2011;36:408–411. doi: 10.1016/j.addbeh.2010.12.002. doi:10.1016/j.addbeh.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg S. H., McHugh P. F., Bell K., Schluger J. H., Schluger R. P., LaForge K. S., Kreek M. J. The Kreek–McHugh–Schluger–Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. doi:10.1016/S0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kelly C., Zuo X.-N., Gotimer K., Cox C. L., Lynch L., Brock D., Milham M. P. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biological Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. doi:10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge B. T., Chou S. P., Pickering R. P., Ruan W. J., Huang B., Jung J., Hasin D. S. Changes in the prevalence and correlates of cocaine use and cocaine use disorder in the United States, 2001–2002 and 2012–2013. Addictive Behaviors. 2019;90:250–257. doi: 10.1016/j.addbeh.2018.11.005. doi:10.1016/j.addbeh.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Kosten T. A., Gawin F. H., Kosten T. R., Rounsaville B. J. Gender differences in cocaine use and treatment response. Journal of Substance Abuse Treatment. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. doi:10.1016/0740-5472(93)90100-G. [DOI] [PubMed] [Google Scholar]
- Lane S. D., Steinberg J. L., Ma L., Hasan K. M., Kramer L. A., Zuniga E. A., Moeller F. G. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. doi:10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. S. C., Hoppenbrouwers S., Franken I. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychology Review. 2019;29:14–26. doi: 10.1007/s11065-019-09402-x. doi:10.1007/s11065-019-09402-x. [DOI] [PubMed] [Google Scholar]
- Lim K. O., Wozniak J. R., Mueller B. A., Franc D. T., Specker S. M., Rodriguez C. P., Rotrosen J. P. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and Alcohol Dependence. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. doi:10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey H. M., Hodges C. B., Greer K. M., Wilde E. A., Merkley T. L. Diffusion-weighted imaging in mild traumatic brain injury: A systematic review of the literature. In Neuropsychology Review. 2023;33:42–121. doi: 10.1007/s11065-021-09485-5. doi:10.1007/s11065-021-09485-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Williamson V., Setlow B., Cottler L. B., Knackstedt L. A. The importance of considering polysubstance use: Lessons from cocaine research. Drug and Alcohol Dependence. 2018;192:16–28. doi: 10.1016/j.drugalcdep.2018.07.025. doi:10.1016/j.drugalcdep.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Steinberg J. L., Keyser-Marcus L., Ramesh D., Narayana P. A., Merchant R. E., Cifu D. X. Altered white matter in cocaine-dependent subjects with traumatic brain injury: A diffusion tensor imaging study. Drug and Alcohol Dependence. 2015;151:128–134. doi: 10.1016/j.drugalcdep.2015.03.015. doi:10.1016/j.drugalcdep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Steinberg J. L., Wang Q., Schmitz J. M., Boone E. L., Narayana P. A., Moeller F. G. A preliminary longitudinal study of white matter alteration in cocaine use disorder subjects. Drug and Alcohol Dependence. 2017;173:39–46. doi: 10.1016/j.drugalcdep.2016.12.016. doi:10.1016/j.drugalcdep.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan A. T., Kushner H., Metzger D., Peters R., Smith I., Gris-som G., Argeriou M. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. doi:10.1016/0740-5472(92)90062-S. [DOI] [PubMed] [Google Scholar]
- McQueeny T., Schweinsburg B. C., Schweinsburg A. D., Jacobus J., Bava S., Frank L. R., Tapert S. F. Altered white matter integrity in adolescent binge drinkers. Alcohol: Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. doi:10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I. A., Montgomery K. S., LaSarge C. L., Simon N. W., Bizon J. L., Setlow B. Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiology of Learning and Memory. 2008;89:185–191. doi: 10.1016/j.nlm.2007.08.005. doi:10.1016/j.nlm.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I. A., Simon N. W., Hart N., Mitchell M. R., Nation J. R., Wellman P. J., Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behavioral Neuroscience. 2010;124:470–477. doi: 10.1037/a0020458. doi:10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. A., Chapman J. P. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. doi:10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Moeller F. G., Hasan K. M., Steinberg J. L., Kramer L. A., Dougherty D. M., Santos R. M., Narayana P. A. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. doi:10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Nugent S., Bokde A. L. W. Alterations in diffusion measures of white matter integrity associated with healthy aging. The Journals of Gerontology: Series A, Biological Sciences and Medical Sciences. 2021;76:945–954. doi: 10.1093/gerona/glz289. doi:10.1093/gerona/glz289. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P. C., Nagae-Poetscher L. M. Oxford, England: Elsevier; 2005. MRI atlas of human white matter. [DOI] [PubMed] [Google Scholar]
- Narayana P. A., Ahobila-Vajjula P., Ramu J., Herrera J., Steinberg J. L., Moeller F. G. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Research: Neuroimaging. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. doi:10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana P. A., Herrera J. J., Bockhorst K. H., Esparza-Coss E., Xia Y., Steinberg J. L., Moeller F. G. Chronic cocaine administration causes extensive white matter damage in brain: Diffusion tensor imaging and immunohistochemistry studies. Psychiatry Research: Neuroimaging. 2014;221:220–230. doi: 10.1016/j.pscychresns.2014.01.005. doi:10.1016/j.pscychresns.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J. T., Wong M. M., Martel M. M., Jester J. M., Puttler L. I., Glass J. M., Zucker R. A. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. doi:10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- O’Donnell L. J., Westin C.-F. An introduction to diffusion tensor image analysis. Neurosurgery Clinics of North America. 2011;22:185–196. doi: 10.1016/j.nec.2010.12.004. doi:10.1016/j.nec.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino-González J., Uhlmann A., Hahn S., Cao Z., Cupertino R. B., Schwab N., Garavan H. White matter microstructure differences in individuals with dependence on cocaine, methamphetamine, and nicotine: Findings from the ENIGMA-Addiction working group. Drug and Alcohol Dependence. 2022;230:109185. doi: 10.1016/j.drugalcdep.2021.109185. doi:10.1016/j.drugalcdep.2021.109185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. L., Nelson S. E., Anderson M. M., Morgan A. D., Carroll M. E. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacology, Biochemistry, and Behavior. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. doi:10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S., Stavro K., Rizkallah E., Pelletier J. Cocaine and cognition: A systematic quantitative review. Journal of Addiction Medicine. 2014;8:368–376. doi: 10.1097/ADM.0000000000000066. doi:10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Smith S. M., Nichols T. E. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. NeuroImage. 2011;54:2006–2019. doi: 10.1016/j.neuroimage.2010.09.088. doi:10.1016/j.neuroimage.2010.09.088. [DOI] [PubMed] [Google Scholar]
- Schmierer K., Wheeler-Kingshott C. A., Boulby P. A., Scaravilli F., Altmann D. R., Barker G. J., Miller D. H. Diffusion tensor imaging of post mortem multiple sclerosis brain. NeuroImage. 2007;35:467–477. doi: 10.1016/j.neuroimage.2006.12.010. doi:10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura E. T., Jackson G. F., Paul B. D.2019Cocaine, crack cocaine, and ethanol: A deadly mix In Critical Issues in Alcohol and Drugs of Abuse Testing (2nd ed.,pp. 215–224.Cambridge, MA: Academic Press; doi:10.1016/B978-0-12-815607-0.00017-4 [Google Scholar]
- Smith S. M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. doi:10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T. E., Mackay C. E., Behrens T. E. J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi:10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Nichols T. E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi:10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Stankowski R. V., Kloner R. A., Rezkalla S. H. Cardiovascular consequences of cocaine use. Trends in Cardiovascular Medicine. 2015;25:517–526. doi: 10.1016/j.tcm.2014.12.013. doi:10.1016/j.tcm.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. 2019 HHS Publication No. PEP19-5068, NSDUH Series H-54 (Vol. 170). Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Suchting R., Beard C. L., Schmitz J. M., Soder H. E., Yoon J. H., Hasan K. M., Lane S. D. A meta-analysis of tract-based spatial statistics studies examining white matter integrity in cocaine use disorder. Addiction Biology. 2021;26:e12902. doi: 10.1111/adb.12902. doi:10.1111/adb.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous J., Mwangi B., Hasan K. M., Narayana P. A., Steinberg J. L., Walss-Bass C., Lane S. D. Measures of possible allostatic load in comorbid cocaine and alcohol use disorder: Brain white matter integrity, telomere length, and anti-saccade performance. PLoS One. 2019;14:e0199729. doi: 10.1371/journal.pone.0199729. doi:10.1371/journal.pone.0199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E., Callahan T. J., Weiland B. J., Hutchison K. E., Bryan A. D. Associations between fractional anisotropy and problematic alcohol use in juvenile justice-involved adolescents. American Journal of Drug and Alcohol Abuse. 2013;39:365–371. doi: 10.3109/00952990.2013.834909. doi:10.3109/00952990.2013.834909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondo L. P., Viola T. W., Fries G. R., Kluwe-Schiavon B., Rothmann L. M., Cupertino R., Grassi-Oliveira R. White matter deficits in cocaine use disorder: Convergent evidence from in vivo diffusion tensor imaging and ex vivo proteomic analysis. Translational Psychiatry. 2021;11:1–13. doi: 10.1038/s41398-021-01367-x. doi:10.1038/s41398-021-01367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Son D., Wiers R. W., Catena A., Perez-Garcia M., Verdejo-García A. White matter disruptions in male cocaine polysubstance users: Associations with severity of drug use and duration of abstinence. Drug and Alcohol Dependence. 2016;168:247–254. doi: 10.1016/j.drugalcdep.2016.09.023. doi:10.1016/j.drugalcdep.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M. M., Fallon J. H., Perry M., Gollub R. L., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. doi:10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A., Brumbaugh M. Automatic atlas queries in FSL. 2011 Retrieved from https://brainder.org/2012/07/30/automatic-atlas-queries-in-fsl/ [Google Scholar]
- Winklewski P. J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Frontiers in Neurology. 2018;9:92. doi: 10.3389/fneur.2018.00092. doi:10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S. W., Morie K. P., Xu J., Constable R. T., Malison R. T., Carroll K. M., Potenza M. N. Shared microstructural features of behavioral and substance addictions revealed in areas of crossing fibers. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2:188–195. doi: 10.1016/j.bpsc.2016.03.001. doi:10.1016/j.bpsc.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Pfefferbaum A., Podhajsky S., Pohl K. M., Sullivan E. V. Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder. Addiction Biology. 2020;25:e12746. doi: 10.1111/adb.12746. doi:10.1111/adb.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Shang L., Zhan C.-G., Zheng F. In vivo characterization of toxicity of norcocaethylene and norcocaine identified as the most toxic cocaine metabolites in male mice. Drug and Alcohol Dependence. 2019;204:107462. doi: 10.1016/j.drugalcdep.2019.04.033. doi:10.1016/j.drugalcdep.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoorob M. Fentanyl shock: The changing geography of overdose in the United States. International Journal on Drug Policy. 2019;70:40–46. doi: 10.1016/j.drugpo.2019.04.010. doi:10.1016/j.drugpo.2019.04.010. [DOI] [PubMed] [Google Scholar]