Abstract
The main aim of this systematic review and meta-analysis is to establish whether there is a correlation between the brain-derived neurotrophic factor (BDNF) level and electroconvulsive therapy (ECT) treatment and the reduction in psychotic symptoms in patients diagnosed with schizophrenia. A systematic search of PubMed/Medline, Cochrane Library, Web of Science, Scopus and Embase was conducted up to March 2023. Inclusion criteria: studies in which adult patients with schizophrenia treated with antipsychotic medication received ECT therapy and had the BDNF level measured before and after ECT treatment. Exclusion criteria: animal and in vitro studies or studies not involving complete information about the treatment and concentration of BDNF in plasma. The risk of bias was assessed using Egger’s regression-based test for meta-analysis with continuous outcomes. Six studies comprising 248 individuals with schizophrenia were included. A statistically significant increase in BDNF levels after ECT treatment was observed only in two studies (p < 0.001 and p < 0.027, respectively), whereas in four other studies, an upward trend without statistical significance was noticed. The estimated overall size effect revealed that ECT therapy caused a slight change in the BDNF level but without statistical significance (ES = −0.328). Different numbers of ECT procedures (4-10), final measurement of the BDNF level made at a different time point, using bilateral or unilateral electrode positioning during ECT and treatment with different combinations of typical or atypical antipsychotic medications may be potential reasons for the lack of statistical significance in the changes in BDNF levels after treatment. Data regarding the measurement of BDNF levels pre and post ECT therapy in patients with schizophrenia are very limited without an extended follow-up period and evaluation of mental health change. Our meta-analysis showed that treatment with ECT therapy and antipsychotic medication increases serum BDNF levels in patients with drug-resistant schizophrenia compared to patients treated with medication only; however, this effect is not statistically significant.
Keywords: schizophrenia, meta-analysis, electroconvulsive therapy (ECT), brain-derived neurotrophic factor (BDNF)
1. Introduction
Schizophrenia is a neuropsychiatric disorder that is characterized by deficits in thought processing, emotional responsiveness and cognition [1,2,3]. The cause of this disabling disorder remains unclear, but a growing body of evidence has pointed to neurodevelopmental processes in which neurotrophic factors are involved [4]. Brain-derived neurotrophic factor (BDNF) is an important neurotropin and can be found both in the human brain (cortex, hippocampus and forebrain) and peripheral blood [5]. BDNF plays a significant role in neurodevelopment and the survival, function and repair of neurons, including dopaminergic and serotoninergic neurons [5,6]. Also, BDNF may be an essential modulator of cognitive functions like memory, learning and thinking. Moreover, BDNF promotes neuronal plasticity in the nervous system by improving the survival, growth and differentiation of the nerve synapses [5,7,8].
Research conducted so far has revealed a reduction in BDNF concentrations both in peripheral blood [9,10,11,12] and in brain tissue (post-mortem studies) in patients with schizophrenia, [12,13] when compared to healthy control subjects. Additionally, the concentration of mRNA BDNF in the brain of schizophrenic patients is significantly decreased in comparison to healthy controls [12,14]. Based on these results, we may conclude that a low level of BDNF is likely to mediate in the neurodevelopmental pathway of schizophrenia by affecting new neuron development, synaptogenesis and neuronal communication. Decreased production of BDNF also correlates with cognitive dysfunctions (problems with memory and learning), neurodegeneration and the intensification of apoptosis observed in schizophrenia. Therefore, the effectiveness of antipsychotic drugs used in schizophrenia treatment or electroconvulsive therapy (ECT) used in drug-resistant schizophrenia should be associated with increased BDNF concentration and the resolution of disease symptoms. However, the results of scientific research are inconsistent in this matter. A number of studies demonstrated that serum BDNF concentrations were significantly increased in patients regularly medicated with antipsychotic drugs regardless of the dose [15,16] and in patients medicated for a long time, at least 10 years [17]. Moreover, Pedrini et al. [18] demonstrated that not only does chronic administration of clozapine increase BDNF concentration, but it is also correlated with clozapine dose. Also, in drug-naive first-episode schizophrenia, BDNF concentration is decreased as administration with antipsychotic drugs normalizes its level [19]. With regard to ECT, a twofold influence on the concentration of BDNF was observed. Li et al. [10] reported an increased concentration of BDNF after ECT and antipsychotic medication whereas Akbas et al. [20] and Fernandez et al. [21] obtained opposite results. Their studies demonstrated that BDNF concentration was increased when only antipsychotic drugs were used, not in combination with ECT therapy. It may suggest that an increased concentration of BDNF is a result of using antipsychotic medication, not ECT itself.
The aim of this systematic review and meta-analysis was to determine whether the use of ECT is associated with a change in the concentration of BDNF in patients diagnosed with treatment-resistant schizophrenia compared to the concentration of this factor in patients treated with antipsychotics only.
2. Material and Methods
2.1. Data Sources and Search Strategy
This meta-analysis was prepared according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines [22]. The protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO), registration number: CRD 42023417335.
Electronic literature search of PubMed, Medline, Medline Complete, Web of Science, Cochrane Library, Scopus and Embase from the earliest available date to March 2023 was conducted. The following terms/keywords were used: [‘schizophrenia’ or ‘psychotic disorder’], [‘electroconvulsive therapy’ or ‘ECT’] and [Brain-derived Neurotropic Factor or BDNF] were used in various combinations. Additional articles were searched by crosschecking the references and other available sources. Articles were selected by using a two-stage process: title and abstract screening and full-text assessment.
2.2. Data Extraction
Titles and abstracts were screened, and full-text articles were further assessed for eligibility in an independent manner (A.S.; B.K.). Data extraction of full-text articles was performed by 2 independent coders (A.S.; B.K.). The selection of the articles was conducted on the basis of the inclusion and exclusion criteria.
2.3. Study Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) prospective/retrospective case control studies/prospective/retrospective cohort studies; randomized control studies (RCT), (2) participants diagnosed with schizophrenia according to the International Classification of Diseases, tenth edition (ICD-10) or Diagnostic and Statistical Manual of Mental Disorders, third to fifth edition (DSM-3, DSM-4, DSM-4TR, DSM-5), (3) course of ECT with both bilateral and unilateral electrode placements included, (4) studies that reported BDNF concentration before and after treatment with ECT and (5) articles written in English and Russian. There was no restriction on antipsychotic medication type and dosage. Exclusion criteria: (1) in vitro studies, (2) animal studies, (3) literature reviews, (4) conference abstract without complete methodology and (5) articles with data overlapping with those articles that were already included in the meta-analysis.
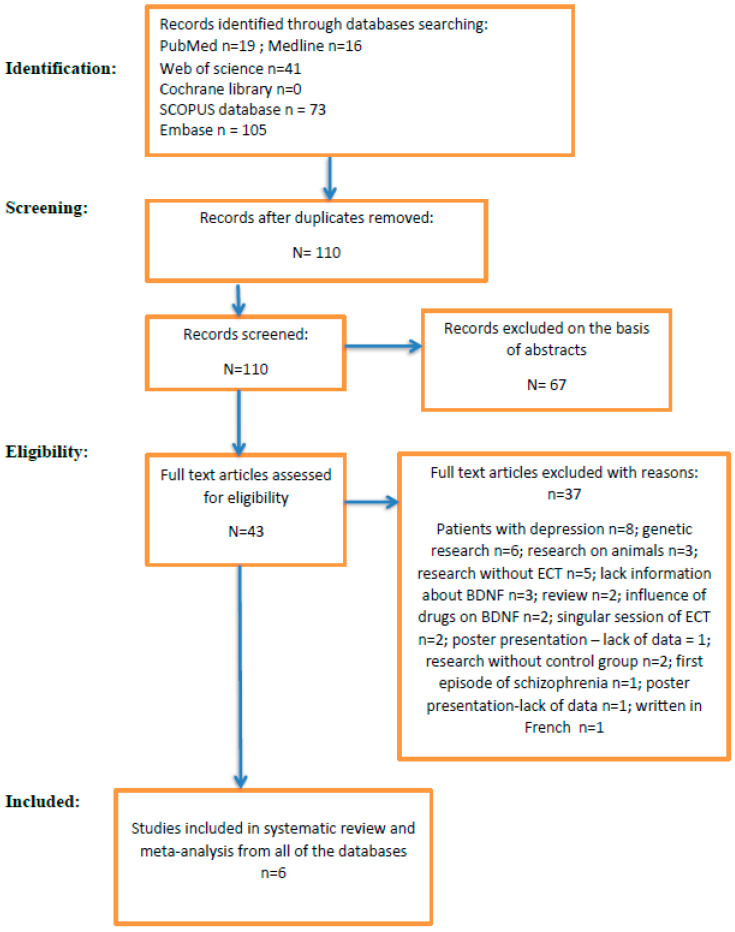
The PRISMA flowchart of the study selection process is presented in Figure 1.
Figure 1.
PRISMA 2020 flowchart for systematic reviews. Identification of new studies via databases and registers.
2.4. Data Collected
For the estimation of the overall effect size (changes in BDNF before and after ECT), meta-analysis was conducted with continuous outcomes on raw data that are provided on the prepared dataset after PRISMA analysis. Measures of central tendency (averages) and precision measures (as SD) were collected together with sample sizes. Sample averages of variables representing the measures of BDNF (at two time points: first pharmacology treatment and for the same patients with pharmacology treatment additionally after ECT—paired measures) were collected.
2.5. Data Synthesis and Statistical Analysis
The studies included in this meta-analysis were from different locations and performed on different populations, so the random-effect model was chosen. The overall effect summarizes well the individual studies if the variances between the different effects are natural, i.e., not large. The study of heterogeneity is proposed to determine to what extent differences between the results obtained by the individual studies affect the overall effect created in the meta-analysis. The heterogeneity was assessed by between-study variance τ2. Homogeneity was analyzed by testing whether the variability between studies τ2 is equal to zero. For this purpose, Q statistic with p-value based on chi-square distribution with k-1 degrees of freedom was applied (where k is the number of studies). Additionally, I2 and H2 were calculated to assess heterogeneity.
Effect size measures were adjusted by Hedges’ g [23,24]. Hedges’ g, called corrected effect size, is a measure of effect. It is the standardized effect size for the difference between means and uses the sample size weighted pooled standard deviation.
Random-effect weights were estimated by using inverse variance (weights including both within-study and between-study variance).
The estimation of the effect was performed by using the iterative method calculating the restricted maximum likelihood estimator (REML).
For adjustment of standard error, the truncated Knapp–Hartung [25] method was applied which truncates the value if it is less than 1 when estimating the variance–covariance matrix. Hartung–Knapp method for random-effect meta-analysis gives more adequate error rates than the Der Simonian and Laird method, especially for a few studies [26]. This method is also recommended when the contributed studies’ precisions vary [27].
Publication bias appears for the reason that studies with preferable outcomes are more likely to be published [28]. Consequently, the published results may be biased toward a certain direction. The publication bias analysis was performed by conducting Egger’s regression-based test for meta-analysis with continuous outcomes [29,30].
Egger test for asymmetry is obtained by regressing the standardized effect on the precision (1/SE):
| Effect/SE = α + β(1/SE) + ε, |
where Effect is the estimated effect, SE is the standard error of Effect, and ε is random noise. The size of α indicates the extent of asymmetry. Egger’s test estimates the statistics based on the t-distribution. Test of intercept α = 0 is based on t-distribution with k-2 degrees of freedom. Publication bias was additionally visualized using funnel plots. Funnel plot is visual method that relates the bias to the asymmetry of the funnel plot. On such plots, the studies’ effect sizes against their standard errors are presented.
The trim-and-fill analysis of publication bias [31,32] was also applied for the estimation of the effect size. It is a method of testing and adjusting for publication bias in meta-analysis. The side of imputation for trim-and-fill analysis based on Egger test was chosen.
Meta-analysis was performed with the usage of analytical tool reporting system PS IMAGO Pro 9.0 with the IBM SPSS Statistics 29 analytical engine (https://en.predictivesolutions.pl/ps-imago-pro,en, accessed on 1 August 2023).
3. Results
The search of Pub Med, Medline, Web of Science Cochrane Library, Scopus and Embase yielded 254 records. After removing 144 duplicate records and an additional 67 records on the basis of abstract content, 43 records were left for eligibility. After further analysis, only six of them fulfilled inclusion criteria and were included in this systematic review and meta-analysis.
The six included studies involved adult subjects with mean age varying from 32 [20] to 38 [10] years and a proportion of men from 70% [21] to 100% [20]. Each study was conducted in a different country, one in Brazil [21], one in China [10], one in Egipt [33], one in Lithuania [34], one in Russia [35] and one in Turkey [20]. The patients were diagnosed with schizophrenia [10,20], refractory schizophrenia [21] or treatment-resistant schizophrenia [33,34,35]. All patients were treated with different combinations of typical (haloperidol) and atypical (aripiprazole, clozapine, quetiapine, olanzapine, paliperidone, risperidone) drugs [10,20,21,33,34,35]. The ECT courses were conducted with bilateral frontotemporal electrode placement with one exception (unilateral frontotemporal electrode placement) [21]. The number of ECT procedures varied from 3 [35] to 20 [34]. Table 1 shows the characteristics of the included studies.
Table 1.
Study characteristics.
| Study | Study Design | Control Group | Patients Diagnosed with Schizophrenia That Underwent ECT | Patients Diagnosed with Schizophrenia on Antipsychotic Medication Only | ECT Characteristics | BDNF Mean Level | Symptom Rating Scales before and after Intervention | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | %F | Mean Age (years) +/− SD | N | %F | Mean Age (years) +/− SD | Duration of Illness (years) +/− SD |
N | %F | Mean Age (years) +/− SD | Duration of Illness (years) +/− SD |
Pre ECT |
Post ECT |
Med. Only Pretreatment | Med. Only Post Treatment | Control Group | ||||
| Fernandes et al. 2010 [21] | Pilot study | 21 | 30 | 35.27 ± 10.34 | 7 | 30 | 35.79 ± 10.85 | Data not available | n/a | Unilateral frontotemporal electrode placement. Charge delivered max 504 mC, current 0.9 A, frequency 30–70 Hz, pulse width 1 ms, duration max 8 s ECT session performed 3 times/week. |
0.14 mg/mL |
0.39 mg/mL |
n/a | n/a | 0.39 mg/mL |
BPRS, CGI-S | |||
| Li et al. 2016 [10] | Case control study | 77 | 44.2 | 40 ± 12.5 | 80 | 47.5 | 38.1 ± 11.1 | 11.3 ± 8.9 | 80 | 45 | 37.7 ± 12.1 | 11.4 ± 10.0 | Bilateral frontotemporal electrode placement. Maximum charge delivered 504 mC; output current = 0.9 A; frequency between 10–70 Hz; pulse width = 0.5 ms; maximum stimulus duration = 8 s. 8–10 ECT sessions every other day. |
9.7 ng/mL |
11.9 ng/mL |
9.8 ng/mL |
11.7 ng/mL | 12.4 ng/mL | PANSS |
| Ivanov et al. 2019 [35] | Case control study | n/a | 66 | 50 | 33.28 ± 8.7 |
6.6 ± 5.1 |
32 | 50 | 33.28 ± 8.7 |
6.6 ± 5.1 |
Bilateral frontotemporal electrode placement. Maximum charge delivered 550 mC; frequency between 27–40 Hz; pulse width = 1–1.5 ms; 3–12 ECT in session |
10.71 ng/mL |
12.30 ng/mL |
9.9 ng/mL |
9.53 ng/mL |
n/a | PANSS | ||
| Akbas et al. 2021 [20] | Case control study | 35 | 0 | 40.51 ± 7.16 | 19 | 0 | 32.47 ± 9.53 | 7.00 | 35 | 0 | 35.23 ± 11.64 | 9.00 | Bilateral temporal electrode placement. A maximum of three consecutive attempts were made to achieve adequate (25 s minimum) seizure per session. |
0.320 mg/mL |
0.315 mg/mL |
0.141 mg/mL |
0.468 mg/mL |
1.478 mg/mL |
PANSS |
| Valiuliene et al. 2021 [34] | Cohort study | 19 | 78.9 | 45.53 ± 15.02 | 31 | 19 | 34.48 ± 11.35 | Not provided | n/a | Bilateral temporal electrode placement. During the stimulation, 0.5 ms duration biphasic square impulses were applied. Impulse current strength was constant at 0.9 A. Stimulation duration ranged from 0.47 to 4.0 s at 70 Hz frequency. These parameters were adjusted according to each patient and increased gradually in succession. ECT was carried out every 2 days; the number of ECT procedures, depending on clinical progress, ranged from 10 to 20 sessions. |
28.98 ng/mL | 29.3 ng/mL | n/a | n/a | 30.12 ng/mL | PANSS | |||
| Shahin et al. 2022 [33] | Cohort study | n/a | 45 | 28 | 33.49 ± 10.14 | 7.43 ± 5.19 | 15 | 26.7 | 36.40 ± 7.18 | 7.60 ± 4.90 | Bilateral temporal electrode placement. The baseline parameters were pulse width (0.5 milliseconds), frequency (80 Hertz), duration (1 s) and current (800 milli ampere). These parameters were adjusted according to each patient and increased gradually in successive sessions. 4–10 sessions of ECT over 4 weeks. |
8.71 ng/mL |
9.26 ng/mL |
8.26 ng/mL |
8.90 ng/mL |
n/a | PANSS | ||
BDNF—brain-derived neurotrophic factor; BPRS—the Brief Psychiatry Rating Scale; CGI-S—the Clinical Global Impression-Severity Scale; ECT—electroconvulsive therapy; %F—percent of females; Med.—patients receiving only medication; N—number of patients; PANSS—the Positive and Negative Syndrome Scale.
As reports chosen for this systematic review and meta-analysis come from different locations, the random-effect model was chosen, and consequently, the heterogeneity of the data had to be evaluated. To study the heterogeneity of studies, Q, τ2, H2 and I2 values are given (Tables S1 and S2, attached as supplementary files). The higher the values, the greater the heterogeneity of the study. Analysis of homogeneity does not reject the hypothesis of homogeneity (Table S1 (p = 0.066), attached as a supplementary file). Heterogeneity measures given in Table S2, attached as a supplementary file, confirm the lack of considerable heterogeneity. For example, statistic I2 = (H2 − 1)/H2 = 52.29% indicates medium heterogeneity, where H2 = Q/(k − 1) = 10.342/5 = 2.096 (Table S2, attached as a supplementary file).
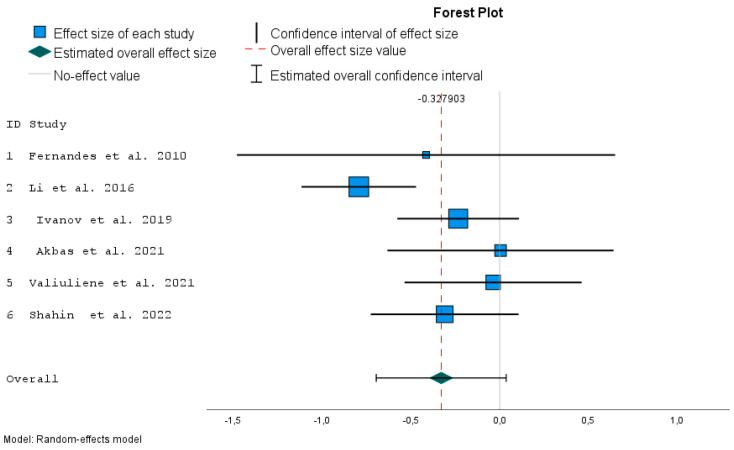
The I2 estimator indicates the percentage of the observed variance that comes from the true difference in the size of the individual studies’ effects. From a graphical point of view, the forest plot (Figure 2) reflects how much individual confidence intervals overlap.
Figure 2.
Forest plot for comparison of effect for schizophrenia patients before and after ECT with overall effect size [10,20,21,33,34,35].
τ2 (tau-squared) equal to 0.059 is the variance of observed effects. Because the variance between study populations is not too large, we have a basis for an overall summary of the effect. Additionally, we might expect a similar result for the common (fixed) effect model.
Lower and upper bounds of confidence intervals for individual papers and overall effect size measured by Hedges’ g are presented in the tables with p-values (Table 2 and Table 3). Forest plots give summary results of meta-analyses (Figure 2 and Figure 3).
Table 2.
Effect size estimates for individual studies.
| ID | Study | Effect Size | Std. Error a | t | Sig. (2-Tailed) |
95% Confidence Interval | Weight | Weight (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| 1 | Fernandes et al. 2010 [21] | −0.413 | 0.5424 | −0.762 | 0.446 | −1.477 | 0.650 | 2.832 | 5.7 |
| 2 | Li et al. 2016 [10] | −0.792 | 0.1643 | −4.817 | <0.001 | −1.114 | −0.470 | 11.649 | 23.5 |
| 3 | Ivanov et al. 2019 [35] | −0.233 | 0.1747 | −1.333 | 0.183 | −0.575 | 0.110 | 11.192 | 22.6 |
| 4 | Akbas et al. 2021 [20] | 0.005 | 0.3244 | 0.016 | 0.987 | −0.631 | 0.641 | 6.094 | 12.3 |
| 5 | Valiuliene et al. 2021 [34] | −0.036 | 0.2540 | −0.143 | 0.887 | −0.534 | 0.462 | 8.106 | 16.4 |
| 6 | Shahin et al. 2022 [33] | −0.309 | 0.2121 | −1.455 | 0.146 | −0.724 | 0.107 | 9.631 | 19.5 |
a Truncated Knapp–Hartung method is used for SE adjustment.
Table 3.
Overall effect size estimates.
| Effect Size | Std. Error a | t | Sig. (2-Tailed) | 95% Confidence Interval | 95% Prediction Interval b | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Overall | −0.328 | 0.1421 | −2.307 | 0.069 | −0.693 | 0.037 | −1.108 | 0.453 |
a Truncated Knapp–Hartung method is used for SE adjustment. b Based on t-distribution. Std. Error—standard error; Sig.—significance.
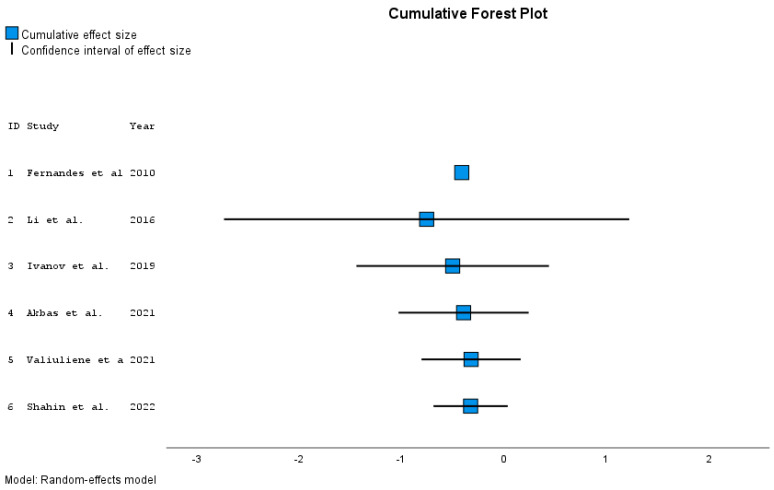
Figure 3.
Cumulative forest plot ordered by year of publication [10,20,21,33,34,35].
In the random-effect model, the weight wi of the study depends on the observed variability (column in Table 2) according to the formula
| wi = 1/(SE2 + τ2). |
The variability in the obtained effects for each study is due to sampling error (the error within each study SE) and the differences between the study populations (τ2: the variance between the studies). Values of wi or relative weights define the size of squares in the forest plot (Figure 2).
The confidence interval around the variable effect (whiskers represent 95% confidence intervals) depends on τ2 = 0.059 and individual SE. The random effect estimates a weighted mean of the true effects of each publication. The forest plot (Figure 2) is a graphical representation of the meta-analysis, where each row represents individual study results with the effect size measure. The gray solid vertical line (x = 0) dividing the graph into two parts is the line of no effect. The dashed vertical line shows the overall effect. The blue boxes represent the individual studies, with their size reflecting the weights (estimated by inverse variance). The green diamond represents the overall effect and so does the red vertical line (described by the value about 0.328).
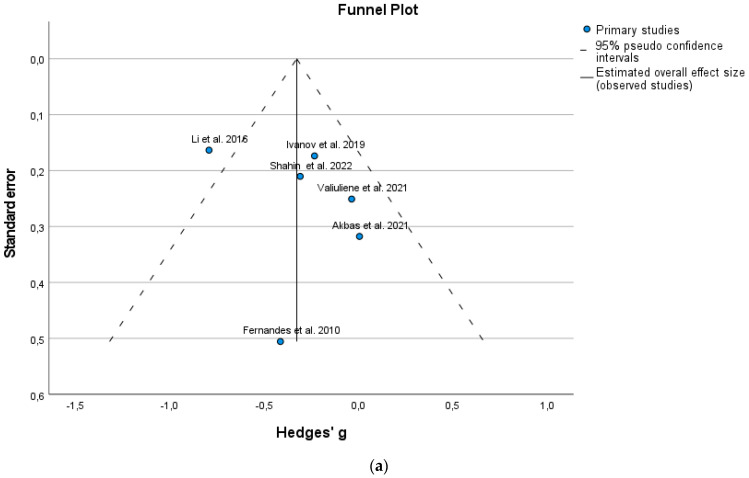
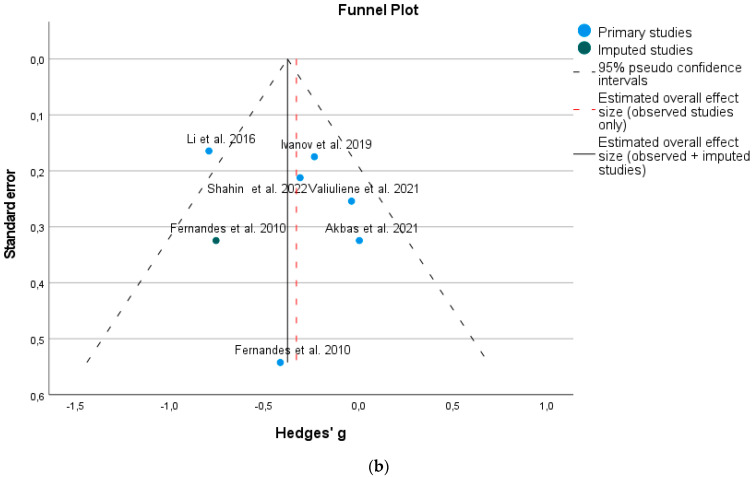
BDNF is a destimulant of psychotic diseases. The effect sizes (both individual and overall) have a negative sign, both for the individual and pooled effect. In the context of this meta-analysis, this represents a constructive outcome because it means that BDNF was lower after ECT. A confidence interval not reaching the gray line (zero value) suggests a significant effect, which is confirmed in Table 3. The effect measured by Hedges’ g is significant in Li et al. [10] (p < 0.001), while the overall effect measured by Hedges’ g is not significant (p = 0.069). On the other hand, only one publication imputed to the primary six studies according to the trim-and-fill procedure (Table 4) showed a significant result (p = 0.029). According to the Egger regression test for funnel plot asymmetry, the publication bias is not significant (Table 5; p = 0.185). However, from the funnel plot (Figure 4a), we can note visual asymmetry. This inconsistency can be explained by the fact that the Egger regression test has low power, especially when the number of studies is fewer than 10.
Table 4.
Effect size estimates for trim-and-fill analysis.
| Number | Effect Size | Std. Error a | t | Sig. (2-Tailed) |
95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Observed | 6 | −0.328 | 0.1421 | −2.307 | 0.069 | −0.693 | 0.037 |
| Observed + Imputed b | 7 | −0.375 | 0.1319 | −2.847 | 0.029 | −0.698 | −0.053 |
a Truncated Knapp–Hartung method is used for SE adjustment. b Number of imputed studies: 1; Std. Error—standard error; Sig.—significance.
Table 5.
Egger’s regression-based test a.
| Parameter | Coefficient | Std. Error | t | Sig. (2-Tailed) | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| (Intercept) | −0.596 | 0.3724 | −1.600 | 0.185 | −1.630 | 0.438 |
| SE b | 1.151 | 1.5053 | 0.764 | 0.487 | −3.029 | 5.330 |
a Random-effect meta-regression with the truncated Knapp–Hartung SE adjustment. b Standard error of effect size. Std. Error—standard error; Sig.—significance.
Figure 4.
(a) Funnel plot, original meta-analysis. (b) Funnel plot, bias-corrected meta-analysis [10,20,21,33,34,35].
To study how the effect has changed over the years, studies are added chronologically in the analysis, and the overall effect is calculated each time. Cumulative forest plot (Figure 3) presents changes in 95% confidence intervals for effects by year of publication (corresponding Table 6 gives the detailed values). Adding publications from subsequent years increases the precision of the joint effect (corresponding whiskers representing the reduction in 95% confidence intervals).
Table 6.
Effect size estimates for cumulative analysis.
| ID | Study | Effect Size | Std. Error a | t | Sig. (2-Tailed) |
95% Confidence Interval | Year b | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| 1 | Fernandes et al. 2010 [21] | −0.413 | 0.5424 | −0.762 | . | . | . | 2010 |
| 2 | Li et al. 2016 [10] | −0.760 | 0.1573 | −4.831 | 0.130 | −2.758 | 1,239 | 2016 |
| 3 | Ivanov et al. 2019 [35] | −0.503 | 0.2219 | −2.267 | 0.152 | −1.458 | 0.452 | 2019 |
| 4 | Akbas et al. 2021 [20] | −0.397 | 0.2019 | −1.965 | 0.144 | −1.039 | 0.246 | 2021 |
| 5 | Valiuliene et al. 2021 [34] | −0.323 | 0.1761 | −1.834 | 0.141 | −0.812 | 0.166 | 2021 |
| 6 | Shahin et al. 2022 [33] | −0.328 | 0.1421 | −2.307 | 0.069 | −0.693 | 0.037 | 2022 |
a Truncated Knapp–Hartung method is used for SE adjustment; cumulative analysis based upon the variables sorted in ascending order. b Std. Error—standard error; Sig.—significance.
In the funnel (triangle) plot, the studies with low precision are placed at the bottom and studies with greater precision at the top of the plot (Figure 4a,b). Funnel plots of the meta-analysis before (Figure 4a) and after applying the trim-and-fill method (Figure 4b) are given. Figure 4b shows the plot of the effect estimates from either the observed studies or both observed and imputed studies. The green circle represents the imputed result, whereas the blue circles represent the observed results. The side of the funnel plot, where the missing studies should be imputed, is chosen within the function depending on the results of Egger’s regression test. The side of imputation on the funnel plot is chosen automatically to the left (Figure 4a) on the basis of the negative Egger statistic (negative value −0.596).
The imputation of only one publication can change the result (Table 5) to obtain the effect measured by Hedges’ g −0.375 with a p-value smaller than the assumed significance of 0.05 (p = 0.029). According to Figure 4a, we can see that only one study (earliest publication by Li et al. [10]) is outside the boundary of the triangle (i.e., is not within 95% confidence intervals), but it is close to its edges.
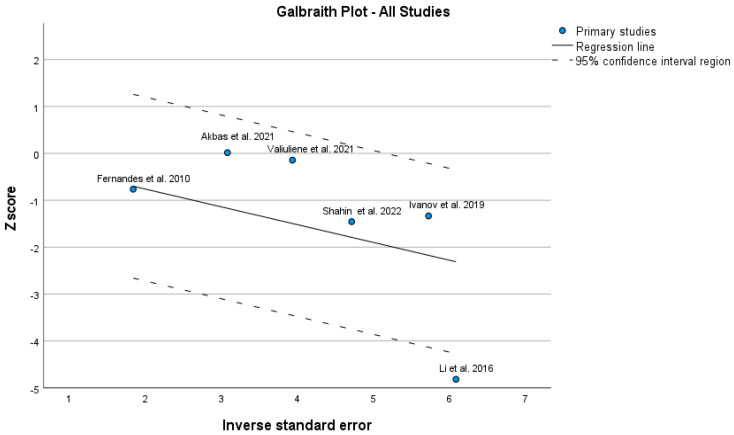
The Galbraith plot (Figure 5) shows an unweighted regression of z-scores (each estimate is divided by its standard error) on the inverse of the standard error. It gives information about the study-specific effect sizes, their precisions and the overall effect size which can help to detect potential outliers. In the Galbraith plot, horizontal line y = 0 represents no effect. One study (again Li et al. [10]) is outside 95% confidence intervals; however, this deviation is not very apparent.
Figure 5.
4. Discussion
The current meta-analysis was conducted to verify whether ECT therapy in drug-resistant schizophrenia is associated with a change in BDNF serum levels in comparison to treatment only with antipsychotic drugs. To our knowledge, to date, this is the first meta-analysis in this scope that selected exclusively patients with schizophrenia.
As is known, BDNF deficiencies play a role in the etiology of schizophrenia due to its fundamental involvement in brain function [36,37]. Several studies revealed that patients with schizophrenia have relatively lower BDNF levels compared to healthy controls [9,13,38,39,40]. Moreover, evidence showed that peripheral BDNF synthesis or release is reduced during acute episodes of schizophrenia [41,42], although it is not known whether it is a pathologic or compensatory effect. The articles included in this meta-analysis showed that, in the pre-ECT measurement, serum BDNF was decreased in drug-resistant patients with schizophrenia in comparison to healthy controls [10,20,21,33,34,35], and these differences were statistically significant.
Although there is a consensus regarding a lower BDNF level in patients with acute symptoms of schizophrenia compared to healthy persons, the changes in BDNF concentration after pharmaceutical treatment combined with ECT showed conflicting results. Recent research suggested the relative success in non-responders to pharmaceutical treatment in patients suffering from schizophrenia [43,44]. Therefore, in this meta-analysis, we expected the BDNF values to reach higher levels in the drug-resistant ECT group compared to the group that received medication only. Surprisingly, only in two studies [10,33], a statistically significant increase in the serum BDNF level after ECT treatment was observed (p < 0.001 and p < 0.027, respectively). In the case of four other studies included in this meta-analysis [20,21,34,35], ECT treatment did not cause a statistically significant change in serum BDNF levels; however, an upward trend in this matter was noticed. The estimated overall size effect revealed that ECT therapy caused a slight change in the BDNF level but without statistical significance (ES = −0.328). The obtained result may be attributed to the small sample size (overall 248 patients), various antipsychotic treatments used among patients and a short period (up to four weeks after ECT) when BDNF levels were measured. We may suspect that the BNDF levels of difficult-to-treat schizophrenia patients are more resistant to increase with treatment and may take some time after the last ECT course when an increase in the BDNF serum level will be gained. To verify this hypothesis, new studies in which BDNF levels are measured at a more extended follow-up period after completion of ECT are required. So far, Haghighi et al. [45] measured serum BDNF levels in patients with major depressive disorder (MDD), not only during ECT sessions but also at each follow-up visit after ECT up to 6 months after its completion. This study found a steady increase in BNDF up to one month after completion of ECT. However, a further increase in BDNF was less significant, and 6 months later, the BDNF level was similar to the pre-treatment levels [45,46]. Likewise, some other reports [47,48,49,50] have shown an increased level of BDNF after completion of ECT therapy in patients with MDD or in patients with bipolar disorder (BDNF levels were measured in the depressive state) [46,51,52,53].
Factors that could be responsible for the heterogeneity of studies on the BDNF level in schizophrenia have been recently identified in Ahmad et al.’s [54] review. At least some of them might influence the results of the studies on BDNF in ECT-treated individuals with schizophrenia:
Stage and/or clinical profile of disease, especially depressive symptoms [55,57,58,59,60,61,62];
Insomnia [71];
Physical activity [74];
Oxidative stress [65].
Moreover, other possible variables may be considered in this context, i.e., somewhat various ECT protocols applied in studies, diverse time-frames between ECT procedure and blood sampling or inconsistent criteria of clinical improvement. Also, different sets of anaesthesiology medications used in different centers, especially ketamine, may influence the BDNF level [75]. The above-specified factors should be controlled or standardized in future research to improve the accuracy of results.
With regard to the mechanism of action, ECT induces an increase in serum BDNF levels in schizophrenia. As our meta-analysis revealed, this increase was not statistically significant; however, it may be high enough to provide beneficial effects on schizophrenia symptoms. It was found that in chronic schizophrenia, not only is there increased striatal presynaptic dopamine synthesis [76,77,78] but also a dysregulation of neurotrophic factor levels, e.g., BDNF, during brain development, which could lead to the disorganization of neuronal networks. Inadequate neurotrophic support in the adult brain may decrease its capacity to adapt to changes and increase vulnerability to neurotoxic damage [10]. Therefore, deficits in BDNF production and utilization have been implicated in the pathology of schizophrenia [79,80]. Thus, ECT therapy induces an increase in the BDNF level, which itself enhances dopamine synthesis and turnover and is involved in the maintenance of midbrain dopaminergic neurons and in the regulation of synaptic plasticity [81,82].
From the authors’ point of view, further analysis of patients’ subgroups by using the meta-regression model seems to be unfeasible, as the heterogeneity of the studies persisted. Firstly, the patients received a different number of ECT procedures (4–10 [33], 8–10 [10], 3–12 [35], 12–18 [21] up to 10–20 [34]), so the final measurement of the BDNF level (blood collected on the last day of ECT) was performed at a different time point. What is more, in most of the cases [10,20,33,34], bilateral electrode positioning was used during ECT, but due to a lack of information [21,35], unilateral electrode positioning cannot be excluded. As is known, unilateral electrode positioning during ECT is putatively associated with a lower efficacy [83] and may have an impact on BDNF production [83,84]. Also, the patients were treated with different combinations of typical (haloperidol, zuclopenthixol) and atypical antipsychotic drugs (olanzapine, risperidone, clozapine, aripiprazole, quetiapine, paliperidone). Moreover, making definitive conclusions about whether there is a relation between an increased level of BDNF after ECT and schizophrenia improvement was impossible due to a lack of complete Positive and Negative Syndrome Scale (PANSS) results or because other scales, e.g., Brief Psychiatric Rating Scale (BPRS) and Clinical Global Impression Severity scale (CGIs), [21] were used. Therefore, it is clear that new studies to evaluate BDNF levels after ECT that will take into account antipsychotic treatment, the heterogeneity of schizophrenia itself and patients’ characteristics are required. The research should be extended to a bigger group of patients and have a longer follow-up period.
5. Limitations
This meta-analysis has some limitations too. First, there is limited clinical literature available in this field. Identified studies include small sample sizes of patients in both groups (treated with ECT and medication or only with medication), which may have an impact on the interpretation of the results and overall obtained results. Also, the use of medications with ECT may be a confounding factor that could not be avoided. What is more, different combinations of antipsychotic medication were used during patients’ therapy, which may have a direct impact on BDNF production. Therefore, a comparison between subgroups in which particular antipsychotics were used may point to whether there are any discrepancies in this matter or if the influence of different drugs on the BDNF level is similar. In the case of our meta-analysis, this confounding effect of medication was reduced as the BDNF levels were compared by using a paired-sample test that compares each patient to themselves before and after the intervention. Another limitation is the short (four-week) follow-up assessment of the BDNF level and a lack of evaluation of this parameter in an extended period, which should be complemented with psychiatric scales, e.g., PANSS or BPRS.
Lastly, it would be worth considering an assessment of the quality of BDNF measurement in each study. The methods and precalculations in the collection and storage of the samples could likely have an impact on the obtained results. To sum up, more comprehensive research including the issues mentioned above with a larger group of patients is recommended.
6. Summary
Our meta-analysis showed that treatment with ECT therapy and antipsychotic medication increases serum BDNF levels in patients with drug-resistant schizophrenia compared to patients treated with medication only. Though the overall effect size measured by Hedges’ g between “before” and “after ECT” is equal to –0.328, we can conclude on the basis of these six included publications that the obtained results are not statistically significant (p = 0.069).
Acknowledgments
The authors thank Karol Antczak, a native speaker, for proofreading this manuscript.
Abbreviations
Brain-derived neurotrophic factor (BDNF); Brief Psychiatric Rating Scale (BPRS); electroconvulsive therapy (ECT); Clinical Global Impression Severity scale (CGIs); major depressive disorder (MDD); Positive and Negative Syndrome Scale (PANSS).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12175728/s1, Table S1: Test of Homogeneity [24]; Table S2: Heterogeneity Measures [24].
Author Contributions
Author A.M.S. designed the study and wrote the protocol. The literature review and data extraction were conducted by A.M.S. and B.K. and supervised by W.D. M.Ć.-J. undertook the statistical analysis, prepared tables and figures and wrote the statistical part of the manuscript. A.M.S., B.K. and W.D. contributed to the interpretation of the results and wrote the manuscript. A.M.S. edited all drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Keshri N., Nandeesha H. Dysregulation of Synaptic Plasticity Markers in Schizophrenia. Indian J. Clin. Biochem. 2023;38:4–12. doi: 10.1007/s12291-022-01068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patlola S.R., Donohoe G., McKernan D.P. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2023;8:110668. doi: 10.1016/j.pnpbp.2022.110668. [DOI] [PubMed] [Google Scholar]
- 3.Zoupa E., Bogiatzidou O., Siokas V., Liampas I., Tzeferakos G., Mavreas V., Stylianidis S., Dardiotis E. Cognitive Rehabilitation in Schizophrenia-Associated Cognitive Impairment: A Review. Neurol. Int. 2022;15:12–23. doi: 10.3390/neurolint15010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashe P.C., Berry M.D., Boulton A.A. Schizophrenia, a neurodegenerative disorder with neurodevelopmental antecedents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25:691–707. doi: 10.1016/s0278-5846(01)00159-2. [DOI] [PubMed] [Google Scholar]
- 5.Bathina S., Das U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scalzo P., Kümmer A., Bretas T.L., Cardoso F., Teixeira A.L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010;257:540–5455. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- 7.Ventriglia M., Zanardini R., Bonomini C., Zanetti O., Volpe D., Pasqualetti P., Gennarelli M., Bocchio-Chiavetto L. Serum brain-derived neurotrophic factor levels in different neurological diseases. BioMed Res. Int. 2013;2013:901082. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng F., Zhou X., Moon C., Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. Int. J. Physiol. Pathophysiol. Pharmacol. 2012;4:188–200. [PMC free article] [PubMed] [Google Scholar]
- 9.Green M.J., Matheson S.L., Shepherd A., Weickert C.S., Carr V.J. Brain-derived neurotrophic factor levels in schizophrenia: A systematic review with meta-analysis. Mol. Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Ye F., Xiao W., Tang X., Sha W., Zhang X., Wang J. Increased serum brain-derived neurotrophic factor levels following electroconvulsive therapy or antipsychotic treatment in patients with schizophrenia. Eur. Psychiatry. 2016;36:23–28. doi: 10.1016/j.eurpsy.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Şimşek Ş., Gençoğlan S., Yüksel T., Kaplan İ., Aktaş H. Lower Brain-Derived Neurotropic Factor Levels in Untreated Adolescents with First-Episode Psychosis. J. Clin. Psychopharmacol. 2015;35:596–599. doi: 10.1097/JCP.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 12.Weickert C.S., Lee C.H., Lenroot R.K., Bruggemann J., Galletly C., Liu D., Balzan R., Pillai A., Buckley P., Weickert T.W. Increased plasma Brain-Derived Neurotrophic Factor (BDNF) levels in females with schizophrenia. Schizophr. Res. 2019;209:212–217. doi: 10.1016/j.schres.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Ray M.T., Shannon T., Weickert C., Webster M.J. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl. Psychiatry. 2014;4:e389. doi: 10.1038/tp.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong J., Hyde T.M., Cassano H.L., Deep-Soboslay A., Kleinman J.E., Weickert C.S. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169:1071–1084. doi: 10.1016/j.neuroscience.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes B.S., Steiner J., Berk M., Molendijk M.L., Gonzalez-Pinto A., Turck C.W., Nardin P., Gonçalves C.A. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: Meta-analysis and implications. Mol. Psychiatry. 2015;9:1108–1119. doi: 10.1038/mp.2014.117. [DOI] [PubMed] [Google Scholar]
- 16.Gama C.S., Andreazza A.C., Kunz M., Berk M., Belmonte-de-Abreu P.S., Kapczinski F. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neurosci. Lett. 2007;420:45–48. doi: 10.1016/j.neulet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Reis H.J., Nicolato R., Barbosa I.G., Teixeira do Prado P.H., Romano-Silva M.A., Teixeira A.L. Increased serum levels of brain-derived neurotrophic factor in chronic institutionalized patients with schizophrenia. Neurosci. Lett. 2008;439:157–199. doi: 10.1016/j.neulet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Pedrini M., Chendo I., Grande I., Lobato M.I., Belmonte-de-Abreu P.S., Lersch C., Walz J., Kauer-Sant’anna M., Kapczinski F., Gama C.S. Serum brain-derived neurotrophic factor and clozapine daily dose in patients with schizophrenia: A positive correlation. Neurosci. Lett. 2011;491:207–210. doi: 10.1016/j.neulet.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Leucht S. Measurements of response, remission, and recovery in schizophrenia and examples for their clinical application. J. Clin. Psychiatry. 2014;75:8–14. doi: 10.4088/JCP.13049su1c.02. [DOI] [PubMed] [Google Scholar]
- 20.Akbas I., Balaban O.D. Changes in serum levels of brain-derived neurotrophic factor with electroconvulsive therapy and pharmacotherapy and its clinical correlates in male schizophrenia patients. Acta Neuropsychiatr. 2022;34:99–105. doi: 10.1017/neu.2021.40. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes B.S., Massuda R., Torres M., Camargo D., Fries G.R., Gama C.S., Belmonte-de-Abreu P.S., Kapczinski F., Lobato M.I. Improvement of schizophrenia with electroconvulsive therapy and serum brain-derived neurotrophic factor levels: Lack of association in a pilot study. Psychiatry Clin. Neurosci. 2010;64:663–665. doi: 10.1111/j.1440-1819.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- 22.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedges L. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Edu. Stat. 1981;6:107–128. doi: 10.3102/10769986006002107. [DOI] [Google Scholar]
- 24.Hedges L.V., Olkin I. Statistical Methods for Meta-Analysis. Academic Press; San Diego, CA, USA: 1985. [Google Scholar]
- 25.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 26.IntHout J., Ioannidis J.P., Borm G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Röver C., Knapp G., Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med. Res. Meth. 2015;15:99. doi: 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simes R.J. Confronting publication bias: A cohort design for meta-analysis. Stat. Med. 1987;6:11–29. doi: 10.1002/sim.4780060104. [DOI] [PubMed] [Google Scholar]
- 29.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne J.A.C., Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein H.R., Sutton A.J., Borenstein M., editors. Publication Bias in Meta-Analysis: Prevention, Assessment, and Adjustments. Wiley; Chichester, UK: 2005. [Google Scholar]
- 31.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 32.Linyu S., Lifeng L. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine. 2019;98:e15987. doi: 10.1097/MD.0000000000015987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahin O., Gohar S.M., Ibrahim W., El-Makawi S.M., Fakher W., Taher D.B., Abdel Samie M., Khalil M.A., Saleh A.A. Brain-Derived neurotrophic factor (BDNF) plasma level increases in patients with resistant schizophrenia treated with electroconvulsive therapy (ECT) Int. J. Psychiatry Clin. Pract. 2022;26:370–375. doi: 10.1080/13651501.2022.2035770. [DOI] [PubMed] [Google Scholar]
- 34.Valiuliene G., Valiulis V., Dapsys K., Vitkeviciene A., Gerulskis G., Navakauskiene R., Germanavicius A. Brain stimulation effects on serum BDNF, VEGF, and TNFα in treatment-resistant psychiatric disorders. Eur. J. Neurosci. 2021;53:3791–3802. doi: 10.1111/ejn.15232. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov M.V., Zubov D.S. Electroconvulsive therapy in treatment of resistant schizophrenia: Biological markers of efficacy and safety. Zhurnal Nevrol. I Psikhiatrii Im. S.S. Korsakova. 2019;119:92–97. doi: 10.17116/jnevro201911903192. [DOI] [PubMed] [Google Scholar]
- 36.Lu B., Nagappan G., Lu Y. Handbook of Experimental Pharmacology. Volume 220. Springer; Berlin/Heidelberg, Germany: 2014. BDNF and synaptic plasticity, cognitive function, and dysfunction; pp. 223–250. [DOI] [PubMed] [Google Scholar]
- 37.Nagahara A.H., Tuszynski M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 38.Akyol E.S., Albayrak Y., Beyazyüz M., Aksoy N., Kuloglu M., Hashimoto K. Decreased serum levels of brain-derived neurotrophic factor in schizophrenic patients with deficit syndrome. Neuropsychiatr. Dis. Treat. 2015;11:865–872. doi: 10.2147/NDT.S79444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam F., Mulsant B.H., Voineskos A.N., Rajji T.K. Brain-Derived Neurotrophic Factor Expression in Individuals with Schizophrenia and Healthy Aging: Testing the Accelerated Aging Hypothesis of Schizophrenia. Curr. Psychiatry Rep. 2017;19:36. doi: 10.1007/s11920-017-0794-6. [DOI] [PubMed] [Google Scholar]
- 40.Koeva Y.A., Sivkov S.T., Akabaliev V.H. Brain-derived neurotrophic factor and its serum levels in schizophrenic patients. Folia Medica. 2014;56:20–23. doi: 10.2478/folmed-2014-0003. [DOI] [PubMed] [Google Scholar]
- 41.Peng S., Li W., Lv L., Zhang Z., Zhan X. BDNF as a biomarker in diagnosis and evaluation of treatment for schizophrenia and depression. Discov. Med. 2018;26:127–136. [PubMed] [Google Scholar]
- 42.Zakharyan R., Boyajyan A. Brain-derived neurotrophic factor blood levels are decreased in schizophrenia patients and associate with rs6265 genotypes. Clin. Biochem. 2014;47:1052–1055. doi: 10.1016/j.clinbiochem.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Chan C.Y.W., Abdin E., Seow E., Subramaniam M., Liu J., Peh C.X., Tor P.C. Clinical effectiveness and speed of response of electroconvulsive therapy in treatment-resistant schizophrenia. Psychiatry Clin. Neurosci. 2019;73:416–422. doi: 10.1111/pcn.12855. [DOI] [PubMed] [Google Scholar]
- 44.Petrides G., Malur C., Braga R.J., Bailine S.H., Schooler N.R., Malhotra A.K., Kane J.M., Sanghani S., Goldberg T.E., John M., et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: A prospective, randomized study. Am. J. Psychiatry. 2015;172:52–58. doi: 10.1176/appi.ajp.2014.13060787. [DOI] [PubMed] [Google Scholar]
- 45.Haghighi M., Salehi I., Erfani P., Jahangard L., Bajoghli H., Holsboer-Trachsler E., Brand S. Additional ECT increases BDNF-levels in patients suffering from major depressive disorders compared to patients treated with citalopram only. J. Psychiatr. Res. 2013;47:908–915. doi: 10.1016/j.jpsychires.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Luan S., Zhou B., Wu Q., Wan H., Li H. Brain-derived neurotrophic factor blood levels after electroconvulsive therapy in patients with major depressive disorder: A systematic review and meta-analysis. Asian J. Psychiatry. 2020;51:101983. doi: 10.1016/j.ajp.2020.101983. [DOI] [PubMed] [Google Scholar]
- 47.Brunoni A.R., Baeken C., Machado-Vieira R., Gattaz W.F., Vanderhasselt M.A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: A systematic review and meta-analysis. World J. Biol. Psychiatry. 2014;15:411–418. doi: 10.3109/15622975.2014.892633. [DOI] [PubMed] [Google Scholar]
- 48.Pelosof R., Santos L.A.D., Farhat L.C., Gattaz W.F., Talib L., Brunoni A.R. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: An updated systematic review and meta-analysis. World J. Biol. Psychiatry. 2023;24:24–33. doi: 10.1080/15622975.2022.2058083. [DOI] [PubMed] [Google Scholar]
- 49.Polyakova M., Schroeter M.L., Elzinga B.M., Holiga S., Schoenknecht P., de Kloet E.R., Molendijk M.L. Brain-Derived Neurotrophic Factor and Antidepressive Effect of Electroconvulsive Therapy: Systematic Review and Meta-Analyses of the Preclinical and Clinical Literature. PLoS ONE. 2015;10:e0141564. doi: 10.1371/journal.pone.0141564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salehi I., Hosseini S.M., Haghighi M., Jahangard L., Bajoghli H., Gerber M., Pühse U., Holsboer-Trachsler E., Brand S. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J. Psychiatr. Res. 2016;76:1–8. doi: 10.1016/j.jpsychires.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes B., Molendijk M.L., Köhler C.A., Soares J.C., Leite C.M., Machado-Vieira R., Ribeiro T.L., Silva J.C., Sales P.M., Quevedo J., et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: A meta-analysis of 52 studies. BMC Med. 2015;13:289. doi: 10.1186/s12916-015-0529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grønli O., Stensland G.Ø., Wynn R., Olstad R. Neurotrophic factors in serum following ECT: A pilot study. World J. Biol. Psychiatry. 2009;10:295–301. doi: 10.3109/15622970701586323. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto T., Yoshimura R., Ikenouchi-Sugita A., Hori H., Umene-Nakano W., Inoue Y., Ueda N., Nakamura J. Efficacy of electroconvulsive therapy is associated with changing blood levels of homovanillic acid and brain-derived neurotrophic factor (BDNF) in refractory depressed patients: A pilot study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1185–1190. doi: 10.1016/j.pnpbp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad R., Azman K.F., Yahaya R., Shafin N., Omar N., Ahmad A.H., Zakaria R., Wijaya A., Othman Z. Brain-derived neurotrophic factor (BDNF) in schizophrenia research: A quantitative review and future directions. AIMS Neurosci. 2023;10:5–32. doi: 10.3934/Neuroscience.2023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atake K., Nakamura T., Ueda N., Hori H., Katsuki A., Yoshimura R. The Impact of Aging, Psychotic Symptoms, Medication, and Brain-Derived Neurotrophic Factor on Cognitive Impairment in Japanese Chronic Schizophrenia Patients. Front. Psychiatry. 2018;9:232. doi: 10.3389/fpsyt.2018.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang F., Wang K., Du X., Deng H., Wu H.E., Yin G., Ning Y., Huang X., Teixeira A.L., de Quevedo J., et al. Sex difference in the association of body mass index and BDNF levels in Chinese patients with chronic schizophrenia. Psychopharmacology. 2019;236:753–762. doi: 10.1007/s00213-018-5107-1. [DOI] [PubMed] [Google Scholar]
- 57.Binford S.S., Hubbard E.M., Flowers E., Miller B.L., Leutwyler H. Serum BDNF Is Positively Associated with Negative Symptoms in Older Adults with Schizophrenia. Biol. Res. Nurs. 2018;20:63–69. doi: 10.1177/1099800417735634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heitz U., Papmeyer M., Studerus E., Egloff L., Ittig S., Andreou C., Vogel T., Borgwardt S., Graf M., Eckert A., et al. Plasma and serum brain-derived neurotrophic factor (BDNF) levels and their association with neurocognition in at-risk mental state, first episode psychosis and chronic schizophrenia patients. World J. Biol. Psychiatry. 2019;20:545–554. doi: 10.1080/15622975.2018.1462532. [DOI] [PubMed] [Google Scholar]
- 59.Tang X., Zhou C., Gao J., Duan W., Yu M., Xiao W., Zhang X., Dong H., Wang X., Zhang X. Serum BDNF and GDNF in Chinese male patients with deficit schizophrenia and their relationships with neurocognitive dysfunction. BMC Psychiatry. 2019;19:254. doi: 10.1186/s12888-019-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang X., Chen Y., Wang Y., Ren J., Zhang C. Depressive symptoms in schizophrenia patients: A possible relationship between SIRT1 and BDNF. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;20:109673. doi: 10.1016/j.pnpbp.2019.109673. [DOI] [PubMed] [Google Scholar]
- 61.Li S., Lu C., Kang L., Li Q., Chen H., Zhang H., Tang Z., Lin Y., Bai M., Xiong P. Study on correlations of BDNF, PI3K, AKT and CREB levels with depressive emotion and impulsive behaviors in drug-naïve patients with first-episode schizophrenia. BMC Psychiatry. 2023;23:225. doi: 10.1186/s12888-023-04718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manchia M., Isayeva U., Collu R., Primavera D., Deriu L., Caboni E., Iaselli M.N., Sundas D., Tusconi M., Pinna F., et al. Converging Evidence Points to BDNF as Biomarker of Depressive Symptoms in Schizophrenia-Spectrum Disorders. Brain Sci. 2022;4:666. doi: 10.3390/brainsci12121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang T.L. Effects of antipsychotics on the BDNF in schizophrenia. Curr. Med. Chem. 2013;20:345–350. doi: 10.2174/092986713804870729. [DOI] [PubMed] [Google Scholar]
- 64.Wu R.Q., Lin C.G., Zhang W., Lin X.D., Chen X.S., Chen C., Zhang L.J., Huang Z.Y., Chen G.D., Xu D.L., et al. Effects of Risperidone and Paliperidone on Brain-Derived Neurotrophic Factor and N400 in First-Episode Schizophrenia. Chin. Med. J. 2018;131:2297–2301. doi: 10.4103/0366-6999.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei C., Sun Y., Chen N., Chen S., Xiu M., Zhang X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. 2020;111:104473. doi: 10.1016/j.psyneuen.2019.104473. [DOI] [PubMed] [Google Scholar]
- 66.Xia H., Zhang G., Du X., Zhang Y., Yin G., Dai J., He M.X., Soares J.C., Li X., Zhang X. Suicide attempt, clinical correlates, and BDNF Val66Met polymorphism in chronic patients with schizophrenia. Neuropsychology. 2018;32:199–205. doi: 10.1037/neu0000383. [DOI] [PubMed] [Google Scholar]
- 67.Skibinska M., Groszewska A., Kapelski P., Rajewska-Rager A., Pawlak J., Dmitrzak-Weglarz M., Szczepankiewicz A., Twarowska-Hauser J. Val66Met functional polymorphism and serum protein level of brain-derived neurotrophic factor (BDNF) in acute episode of schizophrenia and depression. Pharmacol. Rep. 2018;70:55–59. doi: 10.1016/j.pharep.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Huang E., Hettige N.C., Zai G., Tomasi J., Huang J., Zai C.C., Pivac N., Nikolac Perkovic M., Tiwari A.K., Kennedy J.L. BDNF Val66Met polymorphism and clinical response to antipsychotic treatment in schizophrenia and schizoaffective disorder patients: A meta-analysis. Pharmacogenomics J. 2019;19:69–276. doi: 10.1038/s41397-018-0041-5. [DOI] [PubMed] [Google Scholar]
- 69.Kim E.J., Kim Y.K. 196G/A of the Brain-derived neurotrophic factor gene polymorphisms predicts suicidal behavior in schizophrenia patients. Psychiatry Investig. 2018;15:733–738. doi: 10.30773/pi.2018.02.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schweiger J.I., Bilek E., Schäfer A., Braun U., Moessnang C., Harneit A., Post P., Otto K., Romanczuk-Seiferth N., Erk S., et al. Effects of BDNF Val66Met genotype and schizophrenia familial risk on a neural functional network for cognitive control in humans. Neuropsychopharmacology. 2019;44:590–597. doi: 10.1038/s41386-018-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt K., Holsboer-Trachsler E., Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016;48:42–51. doi: 10.3109/07853890.2015.1131327. [DOI] [PubMed] [Google Scholar]
- 72.Wynn J.K., Green M.F., Hellemann G., Karunaratne K., Davis M.C., Marder S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018;195:572–573. doi: 10.1016/j.schres.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 73.Pawełczyk T., Grancow-Grabka M., Trafalska E., Szemraj J., Żurner N., Pawełczyk A. An increase in plasma brain derived neurotrophic factor levels is related to n-3 polyunsaturated fatty acid efficacy in first episode schizophrenia: Secondary outcome analysis of the OFFER randomized clinical trial. Psychopharmacology. 2019;236:2811–2822. doi: 10.1007/s00213-019-05258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gökçe E., Güneş E., Nalçaci E. Effect of Exercise on Major Depressive Disorder and Schizophrenia: A BDNF Focused Approach. Noro Psikiyatr. Ars. 2019;56:302–310. doi: 10.29399/npa.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meshkat S., Alnefeesi Y., Jawad M.Y.D., Di Vincenzo J.B., Rodrigues N., Ceban F., Mw Lui L., McIntyre R.S., Rosenblat J.D. Brain-Derived Neurotrophic Factor (BDNF) as a biomarker of treatment response in patients with Treatment Resistant Depression (TRD): A systematic review & meta-analysis. Psychiatry Res. 2022;317:114857. doi: 10.1016/j.psychres.2022.114857. [DOI] [PubMed] [Google Scholar]
- 76.Faden J., Citrome L. Schizophrenia: One Name, Many Different Manifestations. Med. Clin. N. Am. 2023;107:61–72. doi: 10.1016/j.mcna.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Howes O.D., Murray R.M. Schizophrenia: An integrated sociodevelopmental-cognitive model. Lancet. 2014;10:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu H., Yang F. The interplay of dopamine metabolism abnormalities and mitochondrial defects in the pathogenesis of schizophrenia. Transl. Psychiatry. 2022;12:464. doi: 10.1038/s41398-022-02233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieto R.R., Carrasco A., Corral S., Castillo R., Gaspar P.A., Bustamante M.L., Silva H. BDNF as a Biomarker of Cognition in Schizophrenia/Psychosis: An Updated Review. Front. Psychiatry. 2021;12:662407. doi: 10.3389/fpsyt.2021.662407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H.C., Du Y., Chen L., Yuan Z.Q., Cheng Y. MicroRNA schizophrenia: Etiology, biomarkers and therapeutic targets. Neurosci. Biobehav. Rev. 2023;146:105064. doi: 10.1016/j.neubiorev.2023.105064. [DOI] [PubMed] [Google Scholar]
- 81.Nucifora F.C., Jr., Woznica E., Lee B.J., Cascella N., Sawa A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2019;131:104257. doi: 10.1016/j.nbd.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenquist P.B., Miller B., Pillai A. The antipsychotic effects of ECT: A review of possible mechanisms. J. ECT. 2014;30:125–131. doi: 10.1097/YCT.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 83.Kellner C.H., Knapp R., Husain M.M., Rasmussen K., Sampson S., Cullum M., McClintock S.M., Tobias K.G., Martino C., Mueller M., et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: Randomised trial. Br. J. Psychiatry. 2010;196:226–234. doi: 10.1192/bjp.bp.109.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swartz C.M., Nelson A.I. Rational electroconvulsive therapy electrode placement. Psychiatry. 2005;2:37–43. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.