Abstract
Purpose
Systemic inflammation plays an important role in the pathophysiology and progression of aneurysmal subarachnoid hemorrhage (aSAH). In this study, we aimed to investigate the association between a new biomarker, the inflammatory burden index (IBI) and the prognosis as well as in-hospital complications of aSAH patients.
Patients and Methods
We analyzed data from patients with aSAH between January 2019 and September 2022 who were included in the LongTEAM (Long-term Prognosis of Emergency Aneurysmal Subarachnoid Hemorrhage) registry study. The IBI was formulated as C-reactive protein × neutrophils/lymphocytes. The unfavorable functional prognosis was assessed by the modified Rankin Scale (mRS). Receiver operating characteristic (ROC) curve analysis was conducted to determine the optimal cut-off values for IBI to distinguish the unfavorable functional prognosis. Multivariate logistic regression was applied to investigate the association between IBI and in-hospital complications. Propensity score matching was adjusted for imbalances in baseline characteristics to assess the effect of IBI on prognosis.
Results
A total of 408 consecutive patients with aSAH enrolled in the study, of which 235 (57.6%) were female patients and the mean age was 55.28 years old. An IBI equal to 138.03 was identified as the best cut-off threshold to distinguish the unfavorable prognosis at 3 months (area under the curve [AUC] [95% CI] 0.637 [0.568–0.706]). ln IBI was independently associated with 3-month functional prognosis (OR [95% CI] 1.362 [1.148–1.615]; P<0.001), pneumonia (OR [95% CI] 1.427 [1.227–1.659]; P<0.001) and deep venous thrombosis (DVT). (OR [95% CI] 1.326 [1.124–1.564]; P=0.001). After propensity score matching (57:57), an increased proportion of patients with IBI ≥138.03 had a poor functional prognosis at 3 months and in-hospital complications including developed pneumonia and DVT.
Conclusion
In patients with aSAH, high IBI level at admission was associated with unfavorable functional prognosis as well as pneumonia and deep vein thrombosis.
Keywords: aneurysmal subarachnoid hemorrhage, inflammatory burden index, functional prognosis, complications
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a life-threatening neurological event. Most patients with aSAH continue to face complications after the initial rupture of the aneurysm, including delayed cerebral ischemia (DCI), intracranial infection, hypoproteinemia, pneumonia, and deep vein thrombosis (DVT). These complications can have a significant impact on the patient’s overall prognosis. Systemic inflammation may exacerbate the symptoms of aSAH, leading to poor prognosis, and plays a crucial role in mediating peripheral infection, meningitis, DCI and other complications.1–3
Studies suggest that aSAH leads to systemic inflammatory changes and immune dysregulation, including alterations in inflammatory cell subsets and levels of inflammatory cytokines.4–6 Peripheral inflammatory cell like neutrophils and lymphocytes are pivotal players in the progress of inflammation, leading to various pathological processes such as vascular endothelial damage, blood–brain barrier breakdown and vasospasm.7–9 Dynamic changes in peripheral inflammatory cells also result in immunosuppression, which may increase the risk of infection.10,11 To address this, systemic inflammatory biomarkers, such as neutrophils-to-lymphocytes ratio (NLR), monocytes-to-lymphocytes ratio (MLR), have gained significant attention.11–13 These biomarkers are easy to measure and can effectively reflect changes in inflammatory levels. Moreover, they have been found to be associated with the prognosis of patients with aSAH.14 The inflammatory burden index (IBI) is a novel inflammatory marker that is defined as C-reactive protein (CRP) × neutrophils/lymphocytes. Previous studies have demonstrated that IBI is associated with the prognosis of different tumors.15 And IBI has been shown to have the best predictive accuracy among systemic inflammatory biomarkers for the prognosis of patients with colorectal cancer.16
In this study, we aimed to explore the association between IBI and the prognosis as well as in-hospital complications of aSAH patients. We also systematically and comprehensively compared the IBI with previously proposed inflammatory biomarkers to assess the prognostic value.
Materials and Methods
Patient Selection
We analyzed the data of patients who participated in the LongTEAM trial (Registration No. NCT 04785976) between January 2019 and September 2022. The methods of this trial have been described previously.17
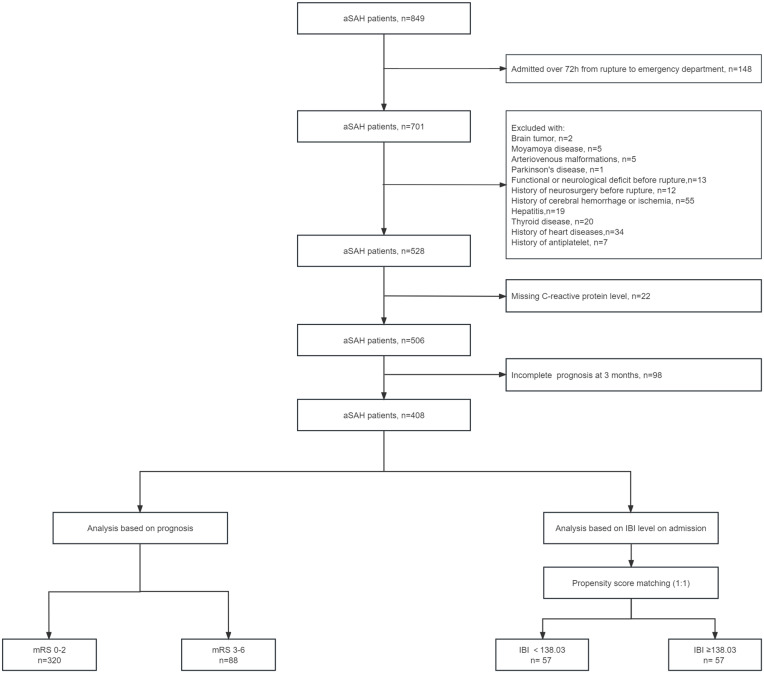
In the present study, all patients had angiographically documented aneurysms with subarachnoid hemorrhage (SAH), confirmed by either computed tomography (CT) or lumbar puncture. We set the inclusion criteria as follows: (1) age ≥18 years old; (2) emergency admission; (3) single aneurysm; (4) treated with surgical clipping or endovascular coiling; (5) less than 72 hours from the rupture to the admission and less than 72 hours from the admission to treatment. The main exclusion criteria were as follows: (1) previous SAH; (2) history of neurosurgery due to any reasons; (3) physical disability due to any previous disease; (4) treatment including external ventricular drainage, lumbar puncture, angiography, intubation, and/or mechanical ventilation at other hospitals before presentation to our hospital; (5) continued use of anti-inflammatory drugs within the past 6 months (6) missing data, including medical records, radiological, and laboratory information. Figure 1 shows the participant selection process.
Figure 1.
Flowchart of study patients.
Abbreviations: aSAH, aneurysmal subarachnoid hemorrhage, mRS, modified Rankin Scale; IBI, inflammatory burden index.
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital (KY 2021–008-01), and informed consent was obtained from all participants or their authorized representatives. All patients received standard management in accordance with the guidelines during their hospitalization.18
Baseline Characteristics
We collected data on various parameters including demographics (age, sex), medical history (hypertension, hyperlipidemia, diabetes mellitus and heart disease), aneurysm characteristics, clinical and radiological status upon admission (early seizures, early loss of consciousness, acute hydrocephalus, World Federation of Neurosurgical Societies [WFNS] grade, modified Fisher Scale [mFS] grade, Graeb score, Subarachnoid hemorrhage Early Brain Edema Score [SEBES] grade), treatment modality, and laboratory data (CRP, neutrophils, lymphocytes, glucose, hemoglobin, leukocytes, platelets and monocytes).
Calculation of Inflammatory Biomarkers
We used the following equations to calculate inflammatory biomarkers: IBI = CRP× neutrophils/lymphocytes, NLR = neutrophils/lymphocytes, MLR = monocytes/lymphocytes, Platelets-to-Lymphocytes Ratio (PLR) = platelets/lymphocytes, Systemic Inflammation Response Index (SIRI) = neutrophils × monocytes/lymphocytes, and Systemic Immune-inflammation Index (SII) = neutrophils × platelets/lymphocytes.
Outcome Measures
The primary outcome was prognosis at 3 months after discharge assessed by the modified Rankin Scale (mRS) (follow-up conducted by a neurosurgeon through telephone or outpatient appointment). The mRS is a stroke outcome scale ranging from 0 (asymptomatic) to 6 (death), with an unfavorable prognosis defined as an mRS score of ≥3. The secondary outcome was postoperative clinical complications during hospitalization including DCI, intracranial infection, hypoproteinemia, pneumonia, and DVT. Supplementary Table 1 shows the detailed diagnostic criteria for the associated complications.
Statistical Analysis
The statistical analyses were performed using R software version R 4.2.6 (Mice and VIM packages) and SPSS Statistics version 26.0 (IBM Corp.) All tests of significance were two-sided, and a p value of less than 0.05 was considered statistically significant.
Descriptive statistics are summarized as the mean ± SD or median (IQR) for continuous variables, and as frequencies (percentages) for categorical variables. Continuous variables were analyzed using independent Student’s t-test (for normal distribution) or Mann–Whitney U-test (for non-normal distribution). Categorical independent variables were analyzed using Pearson’s chi-squared test, continuity correction test, or Fisher’s exact test.
A receiver operating characteristic (ROC) curve and subsequent analysis of the area under the curve (AUC) was applied to evaluate the association between IBI and the prognosis at 3 months. To assess the clinical importance of IBI, other biomarkers of systemic inflammation, such as CRP, NLR, MLR, SIRI, and SII, were also included in the ROC analysis. Patients were then dichotomized according to the identified cut-off IBI value (The cut-off value was calculated by Youden’s index through the ROC analysis) and propensity score matching (PSM) (1:1 match, caliper 0.02) was performed to adjust for any confounding variables.
We conducted logistic regression analyses separately for the sequence. Binary outcomes (in-hospital complications and mRS score) were evaluated using univariate regression analysis. Variables with a p value less than 0.05 in univariate analysis were entered into a multivariate logistic regression analysis using a forward stepwise model to determine the independent parameter associated with adverse outcomes. Odds ratios and 95% confidence intervals (CI) were calculated for each variable.
Sensitivity analysis was conducted to address the issue of missing CRP data and prognosis data at 3 months in some patients. Multiple imputation method was used to impute the missing CRP data, while missing prognosis data at 3 months was not imputed. The completed data set was compared with the final patients included in the analysis to ensure the reliability of the subsequent analysis.
Results
Study Population and Baseline Characteristics
A total of 408 consecutive patients with aSAH enrolled in the LongTEAM study. Of the patients included, 235 (57.6%) were female patients, and the mean age was 55.28 years old.
Table 1 reports the participants’ baseline characteristics according to prognosis at 3 months. Patients with unfavorable outcome at 3 months (mRS score 3–6: 88/408 [21.6%]) were older and were more likely to have hypertension and heart disease and had worse clinical status, including higher WFNS grades, higher mFS grades and Graeb score. A higher proportion of patients with unfavorable outcome at 3 months experience early loss of consciousness on admission and had a higher incidence of postoperative complications including DCI, hypoproteinemia, pneumonia and DVT. Further, a significantly higher IBI on admission was observed in patients with unfavorable prognosis at 3 months (IBI median [IQR] 46.88 [13.26–220.80] vs 21.61 [9.11–58.66]; p<0.001).
Table 1.
Comparison of aSAH Patients According to Prognosis at 3 Months (mRS Score 0–2 Vs 3–6)
| Patient Characteristics | mRS Score at 3 Month | p value | |
|---|---|---|---|
| 0–2 (n=320) | 3–6 (n=88) | ||
| Age, yrs, mean±SD | 53.61±11.90 | 61.32±11.72 | <0.001 |
| Female, n (%) | 207 (64.7) | 52 (59.1) | 0.334 |
| Prior medical history | |||
| Hypertension, n (%) | 162 (50.6) | 60 (68.2) | 0.003 |
| Hyperlipidemia, n (%) | 23 (7.2) | 5 (5.7) | 0.621 |
| Diabetes mellitus, n (%) | 19 (5.9) | 6 (6.8) | 0.760 |
| Heart Disease, n (%) | 18 (5.6) | 13 (14.8) | 0.004 |
| Aneurysm characteristics | |||
| Posterior circulation, n (%) | 32 (10.0) | 10 (11.4) | 0.709 |
| Neurological data/score | |||
| Early seizures, n (%) | 13 (4.1) | 9 (10.2) | 0.023 |
| Early loss of consciousness, n (%) | 66 (20.6) | 44 (50.0) | <0.001 |
| WFNS grade 4–5, n (%) | 38 (11.9) | 45 (51.1) | <0.001 |
| mFS grade 3–4, n (%) | 206 (64.4) | 75 (85.2) | <0.001 |
| Graeb score 5–12, n (%) | 17 (5.3) | 15 (17.0) | <0.001 |
| SEBES score 3–4, n (%) | 138 (43.1) | 30 (34.1) | 0.127 |
| Acute hydrocephalus, n (%) | 126 (39.4) | 42 (47.7) | 0.159 |
| Laboratory values on admission | |||
| IBIa, median (IQR) | 21.61 (9.11–58.66) | 46.88 (13.26–220.80) | <0.001 |
| N/L, median (IQR) | 10.91 (6.97–15.79) | 14.37 (9.01–21.89) | 0.001 |
| C-reactive protein b, median (IQR) | 2.07 (0.92–5.46) | 3.93 (1.47–11.91) | 0.001 |
| Neutrophils c, median (IQR) | 10.66 (8.11–12.98) | 13.18 (9.67–17.10) | <0.001 |
| Lymphocytes d, median (IQR) | 0.99 (0.71–1.35) | 0.89 (0.70–1.37) | 0.279 |
| Glucose e, median (IQR) | 7.40 (6.48–8.90) | 8.63 (7.30–10.18) | <0.001 |
| Hemoglobin f, median (IQR) | 141 (129–152) | 143 (134–151) | 0.391 |
| Leukocytes g, median (IQR) | 12.16 (9.68–14.25) | 15.06 (11.63–18.50) | <0.001 |
| Platelet h, median (IQR) | 226 (192–269) | 223 (179–281) | 0.946 |
| Monocytes i, median (IQR) | 0.38 (0.27–0.52) | 0.54 (0.39–0.72) | <0.001 |
| Treatment modality | 0.092 | ||
| Surgical clipping, n (%) | 153 (47.8) | 51 (58.0) | |
| Endovascular coiling, n (%) | 167 (52.2) | 37 (42.0) | |
| In-hospital complications | |||
| Delayed cerebral ischemia, n (%) | 66 (20.6) | 50 (56.8) | <0.001 |
| Intracranial infection, n (%) | 31 (9.7) | 13 (14.8) | 0.173 |
| Hypoproteinemia, n (%) | 98 (30.6) | 49 (55.7) | <0.001 |
| Pneumonia, n (%) | 98 (30.6) | 69 (78.4) | <0.001 |
| Deep Vein Thrombosis, n (%) | 38 (11.9) | 33 (37.5) | <0.001 |
Notes: aUnit of measurement: mg/L. bUnit of measurement: mg/L. cUnit of measurement: 109/L. dUnit of measurement: 109/L. eUnit of measurement: mg/L. fUnit of measurement: g/L. gUnit of measurement: 109/L. hUnit of measurement. iUnit of measurement: 109/L. As indicated by the bold font, the p value reached statistical significance.
Abbreviations: aSAH, aneurysmal subarachnoid hemorrhage; mRS, modified Rankin Scale; ASMD, Absolute Standardized Mean Difference; WFNS, world federation of neurological societies; mFS, modified Fisher; SEBES, subarachnoid hemorrhage early brain edema score; IBI, inflammatory burden index; N/L, neutrophils to lymphocytes ratio.
ROC Curve Analysis
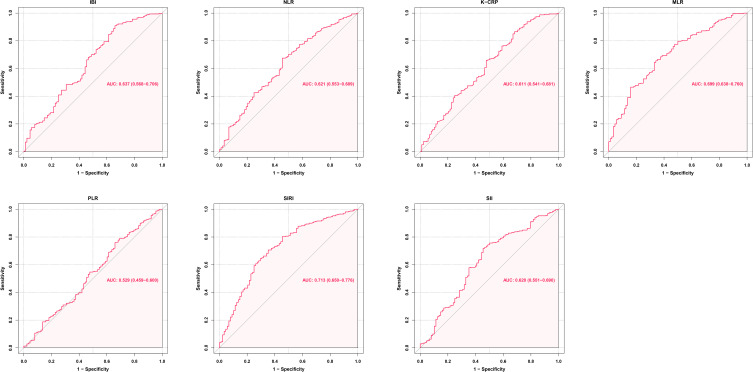
In ROC analysis (Figure 2), an IBI of 138.03 was identified as the optimal cut-off value to distinguish between favorable and unfavorable prognosis at 3 months (AUC [95% CI] 0.637 [0.568–0.706]; p<0.001). Using the IBI cut-off value of 138.03, the cohort can be divided into two groups: the lower IBI group (IBI ≤138.03 mg/L, n=349) and the higher IBI group (IBI >138.03 mg/L, n=59).
Figure 2.
ROC analysis of the association between multiple inflammatory biomarkers and prognosis at 3 months.
Abbreviations: AUC, area under the curve; CI, confidence interval; IBI, inflammatory burden index; NLR, neutrophils-to-lymphocytes ratio; CRP, C-reactive protein; PLR, platelets-to-lymphocytes ratio; MLR, monocytes-to-lymphocytes ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index.
In our cohort, IBI demonstrated a stronger association with the primary outcome compared to previously reported inflammatory biomarkers such as CRP (AUC [95% CI]: 0.611 [0.542–0.680]; p=0.035), NLR (AUC [95% CI]: 0.621 [0.554–0.688]; p=0.034), PLR (AUC [95% CI]: 0.529 [0.459–0.600]; p<0.001), and SII (AUC [95% CI]: 0.620 [0.551–0.690]; p=0.035). However, the AUC of IBI was lower than that of MLR and SIRI (MLR AUC [95% CI]: 0.699 [0.639–0.760]; p=0.031; SIRI AUC [95% CI]: 0.713 [0.650–0.776]; p=0.035) (Table 2).
Table 2.
Comparison of AUC Values of Inflammatory Markers Associated with Prognosis at 3 Months
| IBI | NLR | CRP | PLR | MLR | SIRI | SII | |
|---|---|---|---|---|---|---|---|
| AUC | 0.637 | 0.621 | 0.611 | 0.529 | 0.699 | 0.713 | 0.620 |
| 95% CI | 0.568–0.706 | 0.554–0.688 | 0.542–0.680 | 0.459–0.600 | 0.639–0.760 | 0.650–0.776 | 0.551–0.690 |
| p value | <0.001 | 0.001 | 0.001 | 0.398 | <0.001 | <0.001 | 0.001 |
| SEN | 0.341 | 0.545 | 0.318 | 0.341 | 0.659 | 0.648 | 0.545 |
| SPE | 0.909 | 0.675 | 0.866 | 0.759 | 0.656 | 0.706 | 0.719 |
| PPV | 0.508 | 0.316 | 0.394 | 0.280 | 0.345 | 0.499 | 0.348 |
| NPV | 0.834 | 0.844 | 0.822 | 0.807 | 0.875 | 0.377 | 0.852 |
Abbreviations: AUC, area under the curve; CI, confidence intervals; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; IBI, inflammatory burden index; NLR, neutrophils to lymphocytes ratio; CRP, C-reactive protein; PLR, platelets to lymphocytes ratio; MLR, monocytes to lymphocytes ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index.
Logistic Regression Analyses
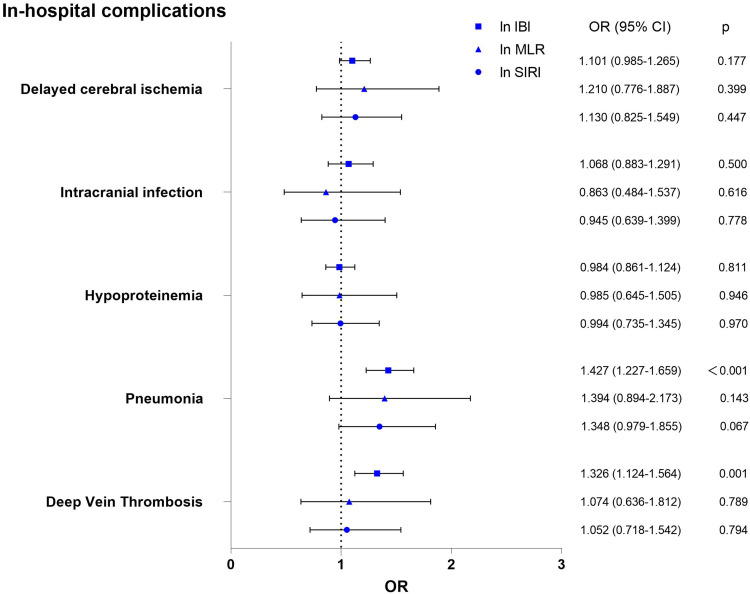
For ease of comparison, we logarithmically transformed the IBI, MLR, and SIRI indices using the natural logarithm as the base. After adjusting for factors such as female, WFNS grade, mFS grade, GRAEB score, SEBES score, acute hydrocephalus and surgical clipping. The multivariate regression analysis showed ln IBI was independently associated with higher mRS scores at 3 months (OR=1.362, 95% CI 1.148–1.615; p<0.001) and two kinds of in-hospital complications including pneumonia (OR=1.427, 95% CI 1.227–1.659; p<0.001) and DVT (OR=1.326, 95% CI 1.124–1.564; p=0.001). In contrast, there was no significant association between ln MLR as well as ln SIRI and in-hospital complications (Figure 3). Specific information about comparison of unadjusted and risk-adjusted outcomes by ln IBI, ln MLR and ln SIRI are presented respectively in Supplementary Tables 2–4.
Figure 3.
The multivariate regression analysis of the association between ln IBI, ln MLR, ln SIRI and in-hospital complications.
Notes: Multivariable regression: adjusted for Female, WFNS grade, mFS grade, GRAEB score, SEBES score, acute hydrocephalus and surgical clipping.
Abbreviations: ln IBI, the natural logarithm of “inflammatory burden index”; ln MLR, the natural logarithm of “monocytes to lymphocytes ratio”; ln SIRI, the natural logarithm of “Systemic Inflammation Response Index”.
Primary and Secondary Outcomes in the PSM Cohort
PSM (caliper 0.02, ratio 1:1, nearest neighbor approach) was performed according to the following parameters: female, early loss of consciousness, WFNS grade, mFS grade, GRAEB score, SEBES score, acute hydrocephalus and surgical clipping. After PSM, 2 evenly balanced cohorts consisting of 57 patients each were available for further analyses. After adjusting for confounding factors, there were no statistically significant differences in baseline characteristics between the higher IBI group and the lower IBI group after PSM (Table 3). And comparison of confounding factors before and after PSM is presented in Supplementary Figure 1. However, patients in the high IBI group still had a poorer prognosis, as reflected by higher mRS scores at 3 months (28/57 [49.1%] vs 11/57 [19.3%]; p=0.001) as well as a higher incidence of including complications including pneumonia (41/57 [71.9%] vs 24/57 [42.1%]; p=0.001) and DVT (17/57 [29.8%] vs 5/57 [8.8%]; p=0.004) (Table 4).
Table 3.
Patient Characteristics Before and After Propensity Score Matching by Identified IBI Threshold (138.03)
| Patient Characteristics | IBI on Admission Pre-PSM (n=408) | ASMD | IBI on Admission Post-PSM (n=114) | ASMD | ||
|---|---|---|---|---|---|---|
| <138.03 | ≥138.03 | <138.03 | ≥138.03 | |||
| 349 | 59 | 57 | 57 | |||
| Age, yrs, mean±SD | 55.32±12.16 | 54.88±13.01 | 0.022 | 53.86±11.88 | 54.61±13.11 | 0.077 |
| Female sex, n (%) | 227 (65.0) | 32 (54.2) | 0.217 | 32 (56.1) | 32 (56.1) | 0.000 |
| Prior medical history | ||||||
| Hypertension, n (%) | 186 (53.3) | 36 (61.0) | 0.158 | 32 (56.1) | 36 (63.2) | 0.141 |
| Hyperlipidemia, n(%) | 25 (7.2) | 3 (5.1) | 0.095 | 5 (8.8) | 3 (5.3) | 0.124 |
| Diabetes mellitus, n (%) | 23 (6.6) | 2 (3.4) | 0.177 | 4 (7.0) | 2 (3.5) | 0.137 |
| Heart Disease, n (%) | 27 (7.7) | 4 (6.8) | 0.038 | 7 (12.3) | 4 (7.0) | 0.160 |
| Aneurysm characteristics | ||||||
| Posterior circulation, n(%) | 35 (10.0) | 7 (11.9) | 0.057 | 5 (8.8) | 7 (12.3) | 0.124 |
| Neurological data/score | ||||||
| Early seizures, n (%) | 16 (4.6) | 6 (10.2) | 0.185 | 5 (8.8) | 6 (10.5) | 0.062 |
| Early loss of consciousness, n (%) | 81 (23.2) | 29 (49.2) | 0.519 | 27 (47.4) | 27 (47.4) | 0.000 |
| WFNS grade 4–5, n (%) | 60 (17.2) | 23 (39.0) | 0.447 | 20 (35.1) | 21 (36.8) | 0.037 |
| mFS grade 3–4, n (%) | 230 (65.9) | 51 (86.4) | 0.600 | 49 (86.0) | 49 (86.0) | 0.000 |
| Graeb score 5–12, n (%) | 23 (6.6) | 9 (15.3) | 0.241 | 8 (14.0) | 9 (15.8) | 0.051 |
| SEBES score 3–4, n (%) | 138 (39.5) | 30 (50.8) | 0.226 | 31 (54.4) | 28 (49.1) | 0.106 |
| Acute hydrocephalus, n (%) | 135 (38.7) | 33 (55.9) | 0.347 | 31 (54.4) | 31 (54.4) | 0.000 |
| Laboratory values on admission | ||||||
| IBI a, median (IQR) | 19.03 (8.49–44.40) | 295.74 (204.37–636.86) | 1.044 | 3.66 (1.50–8.76) | 288.28 (202.54–557.71) | 1.072 |
| N/L, median (IQR) | 10.63 (6.94–15.53) | 16.79 (12.19–26.27) | 0.811 | 8.90 (5.43–15.93) | 16.74 (12.05–25.96) | 0.971 |
| C-reactive protein b, median (IQR) | 1.76 (0.88–4.16) | 18.68 (10.95–35.38) | 0.919 | 0.45 (0.28–0.99) | 18.68 (10.63–35.26) | 0.983 |
| Neutrophils c, median (IQR) | 0.72 (8.07–13.12) | 13.93 (10.53–19.11) | 0.773 | 11.41 (7.37–35.26) | 13.72 (10.39–19.09) | 0.688 |
| Lymphocytes d, median (IQR) | 1.00 (0.73–1.41) | 0.81 (0.60–1.10) | 0.753 | 1.23 (0.84–1.92) | 0.81 (0.60–1.09) | 1.548 |
| Glucose e, median (IQR) | 7.50 (6.52–9.28) | 8.12 (7.06–9.11) | 0.111 | 7.83 (6.64–10.15) | 8.12 (7.07–9.19) | 0.067 |
| Hemoglobin f, median (IQR) | 140 (129–151) | 147 (136–155) | 0.273 | 139 (130–149) | 147 (135–155) | 0.204 |
| Leukocytes g, median (IQR) | 12.25 (9.65–14.71) | 15.09 (11.79–20.32) | 0.716 | 13.08 (9.50–15.98) | 15.06 (11.71–20.32) | 0.553 |
| Platelet h, median (IQR) | 224 (189–269) | 230 (189–293) | 0.170 | 223 (189–265) | 230 (194–294) | 0.300 |
| Monocytes i, median (IQR) | 0.39 (0.27–0.54) | 0.55 (0.37–0.76) | 0.452 | 0.46 (0.30–0.77) | 0.55 (0.36–0.73) | 0.010 |
| Treatment modality | 0.445 | 0.000 | ||||
| Surgical clipping, n (%) | 164 (47.0) | 40 (67.8) | 38 (66.7) | 38 (66.7) | ||
| Endovascular coiling, n (%) | 185 (53.0) | 19 (32.2) | 19 (33.3) | 19 (33.3) | ||
Notes: Propensity score matching (caliper 0.02, ratio 1:1, nearest neighbor approach) was performed according to the following parameters: Female, Early loss of consciousness, WFNS grade, mFS grade, GRAEB score, SEBES score, Acute hydrocephalus and surgical clipping. After PS matching, 2 evenly balanced cohorts consisting of 57 patients each and without significant differences in outcome-relevant parameters were available for further analyses. aUnit of measurement: mg/L. bUnit of measurement: mg/L. cUnit of measurement: 109/L. dUnit of measurement: 109/L. eUnit of measurement: mg/L. fUnit of measurement: g/L. gUnit of measurement: 109/L. hUnit of measurement: 109/L. iUnit of measurement: 109/L.
Abbreviations: IBI, inflammatory burden index; PSM, propensity score matching; ASMD, absolute standardized mean difference; WFNS, world federation of neurological societies; mFS, modified Fisher; SEBES, subarachnoid hemorrhage early brain edema score; N/L, neutrophils to lymphocytes ratio.
Table 4.
Outcome Parameters Before and After Propensity Score Matching by Identified IBI Threshold (138.03)
| Outcome Parameters | IBI on Admission Pre-PSM (n=408) | p value | IBI on Admission Post-PSM (n=114) | p value | ||
|---|---|---|---|---|---|---|
| <138.03 | ≥138.03 | <138.03 | ≥138.03 | |||
| 349 | 59 | 57 | 57 | |||
| mRS 3–6 at 3 months, n (%) | 58 (16.6) | 30 (50.8) | <0.001 | 11 (19.3) | 28 (49.1) | 0.001 |
| Delayed cerebral ischemia, n (%) | 91 (26.1) | 25 (42.4) | 0.010 | 14 (24.6) | 23 (40.4) | 0.072 |
| Intracranial infection, n (%) | 34 (9.7) | 10 (16.9) | 0.099 | 10 (17.5) | 10 (17.5) | 1.000 |
| Hypoproteinemia, n (%) | 122 (35.0) | 25 (42.4) | 0.272 | 25 (43.9) | 24 (42.1) | 0.850 |
| Pneumonia, n (%) | 124 (35.5) | 43 (72.9) | <0.001 | 24 (42.1) | 41 (71.9) | 0.001 |
| Deep Vein Thrombosis, n (%) | 53 (15.2) | 18 (30.5) | 0.004 | 5 (8.8) | 17 (29.8) | 0.004 |
Notes: Propensity score matching (caliper 0.02, ratio 1:1, nearest neighbor approach) was performed according to the following parameters: Female, Early loss of consciousness, WFNS grade, mFS grade, GRAEB score, SEBES score, Acute hydrocephalus and surgical clipping. After PS matching, 2 evenly balanced cohorts consisting of 57 patients each and without significant differences in outcome-relevant parameters were available for further analyses. As indicated by the bold font, the p value reached statistical significance.
Abbreviations: PSM, propensity score matching IBI, inflammatory burden index; mRS, modified Rankin Scale.
Sensitivity Analysis
The results of the sensitivity analyses using multiple imputation method are presented in Supplementary Table 5. Patient characteristics before and after multiple imputation method differed with respect to heart disease, surgical clipping and deep vein thrombosis. No significant differences were found in age, sex, other prior medical history, aneurysm characteristics, neurological data/score, laboratory values on admission, treatment modality and outcome parameters.
Discussion
This study aims to assess the inflammatory burden of aSAH patients using a novel biomarker called IBI and demonstrates its powerful prognostic value in these patients. These are several key findings from our study: First, IBI exhibits a stronger association with 3-month prognosis of aSAH patients compared to other biomarkers including NLR, SII, CRP, PLR. Secondly, we utilized IBI to grade the inflammatory burden in aSAH patients and obtained a good prognostic stratification. Our study showed that high IBI level was independently associated with an increased incidence of complications, such as pneumonia and DVT. Although the AUC of SIRI and MLR is greater than that of IBI, there was no significant association between MLR as well as SIRI and in-hospital complications in multivariate regression analysis.
Both aneurysmal and spontaneous non-aneurysmal subarachnoid hemorrhage can lead to excessive systemic inflammation and a thrombotic response, which may be associated with the severity of secondary brain injury.19,20 Blood samples collected upon admission offer a convenient source for searching for inflammatory markers that may associate with the prognosis and complications of aSAH patients. A single-center retrospective analysis revealed that several inflammatory biomarkers are associated with a poor prognosis at 90 days.21 The systemic inflammatory levels assessed through the SII are associated with the ability to predict the occurrence of cerebral vasospasm and DCI following aSAH.22,23 Similar to the findings of us, Giede-Jeppe A observed that NLR was an independent parameter associated with poor functional outcomes in aSAH patients. However, the limitations of their study are that they used propensity matching with a relatively low caliper of 0.2, which could affect the matching accuracy.24 Zhang investigated the associations between NLR with aneurysm size, prognosis, and DCI incidence, and reported an AUC of 0.698 for correlation with DCI. However, their study did not adjust for potential baseline confounders.25 Therefore, there is still a need to identify a simple, effective and practical inflammatory markers that have robust association with the prognosis and complications of aSAH patients.
IBI was initially proposed by Xie as a tool for evaluating the inflammatory burden of different cancers and predicting patients’ prognosis.15 Their study found that IBI not only differentiates the prognosis of patients with different levels of inflammation but also provides significant prognostic stratification for most cancer patients. Subsequent studies have systematically and comprehensively compared the predictive abilities of various combinations of serum inflammatory markers for predicting the prognosis of colorectal cancer. Among these studies, IBI was found to have the highest predictive accuracy.16
Consistent with previous research, our study demonstrates that IBI has a relatively more robust association with the prognosis at 3 months and complications of aSAH patients. One possible reason for this advantage is that IBI can integrate the benefits of various parameters. CRP is commonly used to detect inflammation levels in the body and plays a critical role in defending against infection and inflammation.25,26 Neutrophils and lymphocytes are essential components of the immune system and play significant roles in inflammatory responses, infection resistance, and immune regulation.27,28 IBI combines the strengths of these parameters and comprehensively reflects the body’s inflammation and immune status. This may help supervising physicians to assess patients’ clinical status preliminarily through IBI and give more attention to potential complications and prognosis during hospitalization in the early stage.
The mechanism underlying the alteration of IBI following intracranial aneurysm rupture remains unclear. Some studies suggest that changes in peripheral blood cells may be mediated through activation of the hypothalamic–pituitary–adrenal axis (HPA) and sympathetic nervous system. After central nervous system injury, the activation of the hypothalamic–pituitary–adrenal (HPA) axis mediates an increase in serum cortisol levels, which independently leads to changes in the numbers of neutrophils and lymphocytes in peripheral blood.29,30 Studies by Naredi have demonstrated that patients with subarachnoid hemorrhage had nearly three times more systemic norepinephrine spillover into the plasma within 48 hours post-injury, confirming the presence of significant sympathetic nervous system activation after subarachnoid hemorrhage.31 Catecholamines including norepinephrine could increase the number of circulating white blood cells by mobilizing cells from the marginal pool and peripheral storage sites such as the spleen and lungs.32 In addition, levels of inflammatory substances, including serum CRP, increase after aSAH, which may be associated with elevated levels of D-dimer.25,33 Furthermore, the combination of organ dysfunction caused by central nervous system injury and immune suppression mediated by HPA axis and sympathetic nervous system activation can contribute to the occurrence of infections and various complications.27
Our study has several limitations. Firstly, this study is a retrospective, single-center study. A multicenter study with a large sample size is needed to verify our results. Secondly, the data on the evaluation of systemic inflammatory response is collected from blood samples taken on admission. Studying the impact of the trajectory of the inflammatory markers during hospitalization on the prognosis of aSAH is valuable. Additionally, adding more detection markers in blood samples such as interleukins and tumor necrosis factor would be advantageous. Thirdly, 120 of the 528 patients (22.7%) were not included in the analysis because of lack of data on CRP at admission. This may have led to confirmatory bias. Although sensitivity analysis was performed to compare the excluded data with the included data and no significant differences were found, it is a fact that the sample size has been reduced.
Conclusions
This single-center retrospective cohort study suggests that IBI value of ≥138.03 on admission is independently associated with complications in aSAH patients, such as pneumonia and DVT. As a new inflammatory burden index, IBI is a feasible and promising biomarker for assessing the prognosis of patients with aSAH.
Acknowledgments
We acknowledge the contribution of all staff who participated in the present study.
Funding Statement
This study was supported by the National Key Research and Development Program of China (Grant Nos. 2021YFC2501101 and 2020YFC2004701), Beijing Municipal Administration of Hospitals Incubating Program, Beijing, China (Grant No. pX2020023), and the Natural Science Foundation of Beijing, China (Grant No. 7204253).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
This study has obtained approval from the Institutional Review Board of Beijing Tiantan Hospital (KY 2021-008-01). Informed consent for clinical analysis was obtained from all individual participants or their authorized representatives. All the analysis was performed according to the Declaration of Helsinki and the local ethics policies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no personal, financial, or institutional interest in any of the materials or methods used in this study or the findings specified in this paper.
References
- 1.Muhammad S, Hänggi D. Inflammation and anti-inflammatory targets after aneurysmal subarachnoid hemorrhage. Int J Mol Sci. 2021;22(14):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin J, Duan J, Du L, Xing W, Peng X, Zhao Q. Inflammation and immune cell abnormalities in intracranial aneurysm subarachnoid hemorrhage (SAH): relevant signaling pathways and therapeutic strategies. Front Immunol. 2022;13:1027756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn SH, Savarraj JPJ, Parsha K, et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J Neuroinflammation. 2019;16(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Jiang Y, Peng Y, Zhang M. The Quantitative and Functional Changes of Postoperative Peripheral Blood Immune Cell Subsets Relate to Prognosis of Patients with Subarachnoid Hemorrhage: a Preliminary Study. World Neurosurg. 2017;108:206–215. doi: 10.1016/j.wneu.2017.08.091 [DOI] [PubMed] [Google Scholar]
- 5.Zeyu Z, Yuanjian F, Cameron L, Sheng C. The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Exp Neurol. 2021;336:113535. [DOI] [PubMed] [Google Scholar]
- 6.Ray B, Ross SR, Danala G, et al. Systemic response of coated-platelet and peripheral blood inflammatory cell indices after aneurysmal subarachnoid hemorrhage and long-term clinical outcome. J Crit Care. 2019;52:1–9. doi: 10.1016/j.jcrc.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich V, Flores R, Muller A, Bi W, Peerschke EI, Sehba FA. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation. 2011;8:103. doi: 10.1186/1742-2094-8-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Xie G, Wang C, et al. Decreased progranulin levels in patients and rats with subarachnoid hemorrhage: a potential role in inhibiting inflammation by suppressing neutrophil recruitment. J Neuroinflammation. 2015;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Tamimi YZ, Bhargava D, Orsi NM, et al. Compartmentalisation of the inflammatory response following aneurysmal subarachnoid haemorrhage. Cytokine. 2019;123:154778. doi: 10.1016/j.cyto.2019.154778 [DOI] [PubMed] [Google Scholar]
- 10.Sarrafzadeh A, Schlenk F, Meisel A, Dreier J, Vajkoczy P, Meisel C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42(1):53–58. doi: 10.1161/STROKEAHA.110.594705 [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Zeng H, Tan X, Wu X, Qian C, Chen G. The role of the blood neutrophil-to-lymphocyte ratio in aneurysmal subarachnoid hemorrhage. Front Neurol. 2021;12:671098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feghali J, Kim J, Gami A, et al. Monocyte-based inflammatory indices predict outcomes following aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2021;44(6):3499–3507. doi: 10.1007/s10143-021-01525-1 [DOI] [PubMed] [Google Scholar]
- 13.Dowlati E, Mualem W, Carpenter A, et al. Early fevers and elevated neutrophil-to-lymphocyte ratio are associated with repeat endovascular interventions for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2022;36(3):916–926. doi: 10.1007/s12028-021-01399-7 [DOI] [PubMed] [Google Scholar]
- 14.Tao C, Wang J, Hu X, Ma J, Li H, You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26(3):393–401. doi: 10.1007/s12028-016-0332-0 [DOI] [PubMed] [Google Scholar]
- 15.Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–1243. [DOI] [PubMed] [Google Scholar]
- 16.Xie H, Ruan G, Wei L, et al. Comprehensive comparative analysis of prognostic value of serum systemic inflammation biomarkers for colorectal cancer: results from a large multicenter collaboration. Front Immunol. 2022;13:1092498. doi: 10.3389/fimmu.2022.1092498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin F, Chen Y, He Q, et al. Prognostic value of elevated cardiac troponin I after aneurysmal subarachnoid hemorrhage. Front Neurol. 2021;12:677961. doi: 10.3389/fneur.2021.677961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–1737. [DOI] [PubMed] [Google Scholar]
- 19.Cuoco JA, Guilliams EL, Adhikari S, et al. Systemic immune-inflammation index predicts acute symptomatic hydrocephalus after spontaneous nonaneurysmal subarachnoid hemorrhage. World Neurosurg. 2023;173:e378–e390. doi: 10.1016/j.wneu.2023.02.060 [DOI] [PubMed] [Google Scholar]
- 20.Bacigaluppi S, Bragazzi NL, Ivaldi F, Benvenuto F, Uccelli A, Zona G. Systemic inflammatory response in spontaneous subarachnoid hemorrhage from aneurysmal rupture versus subarachnoid hemorrhage of unknown origin. J Inflamm Res. 2022;15:6329–6342. doi: 10.2147/JIR.S380101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie Z, Lin F, Li R, Chen X, Zhao Y. A Pooled Analysis of Preoperative Inflammatory Biomarkers to Predict 90-Day Outcomes in Patients with an Aneurysmal Subarachnoid Hemorrhage: a Single-Center Retrospective Study. Brain Sci. 2023;13(2):257. doi: 10.3390/brainsci13020257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraghty JR, Lung TJ, Hirsch Y, et al. Systemic immune-inflammation index predicts delayed cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2021;89(6):1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Pandey S, Shen R, Xu Y, Zhang Q. Increased systemic immune-inflammation index is associated with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage patients. Front Neurol. 2021;12:745175. doi: 10.3389/fneur.2021.745175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giede-Jeppe A, Reichl J, Sprügel MI, et al. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;132(2):400–407. doi: 10.3171/2018.9.JNS181975 [DOI] [PubMed] [Google Scholar]
- 25.Zhang B, Lin L, Yuan F, et al. Clinical application values of neutrophil-to-lymphocyte ratio in intracranial aneurysms. Aging. 2021;13(4):5250–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurth H, Birkenhauer U, Steiner J, Schlak D, Hennersdorf F, Ebner FH. Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage - Serum D-dimer and C-reactive Protein as Early Markers. J Stroke Cerebrovasc Dis. 2020;29(3):104558. [DOI] [PubMed] [Google Scholar]
- 27.Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–786. [DOI] [PubMed] [Google Scholar]
- 29.Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–38. doi: 10.1016/j.coi.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Zetterling M, Engström BE, Hallberg L, et al. Cortisol and adrenocorticotropic hormone dynamics in the acute phase of subarachnoid haemorrhage. Br J Neurosurg. 2011;25(6):684–692. doi: 10.3109/02688697.2011.584638 [DOI] [PubMed] [Google Scholar]
- 31.Zierath D, Tanzi P, Shibata D, Becker KJ. Cortisol is More Important than Metanephrines in Driving Changes in Leukocyte Counts after Stroke. J Stroke Cerebrovasc Dis. 2018;27(3):555–562. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naredi S, Lambert G, Edén E, et al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901–906. doi: 10.1161/01.STR.31.4.901 [DOI] [PubMed] [Google Scholar]
- 33.Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77–91. [DOI] [PubMed] [Google Scholar]