Abstract
Background: After the LACC trial publication in 2018, the minimally invasive approach (MIS) has severely decreased in favor of open surgery: MIS radical hysterectomy was associated with worse oncological outcomes than open surgery, but urological complications were never extensively explored in pre- versus post-LACC eras, even if they had a great impact on post-operative QoL. The purpose of this meta-analysis is to compare functional and organic urological complication rates before and after LACC trial. Methods: An independent search of the literature was conducted 4 years before and after the LACC trial and 50 studies were included. Results: The overall rate of urologic complications was higher in pre-LACC studies while no differences were found for organic urological complications. Conversely, the overall risk of dysfunctional urological complications showed a higher rate in the pre-LACC era. This is probably related to a sudden shift to open surgery, with potential lower thermal damage to the urinary tract autonomic nervous fibers. Conclusions: This meta-analysis showed that the incidence of urological complications in radical cervical cancer surgery was higher before the LACC trial, potentially due to the shift to open surgery. Nevertheless, further studies are needed to shed light on the connection between minimally invasive surgery and urological damage.
Keywords: urological complications, cervical cancer, radical surgery, LACC trial
1. Introduction
Background. Cervical cancer is the fourth most common cancer in women worldwide, however the number of cases has continuously declined in countries where screening and vaccination programs have been implemented [1]. The mean age of diagnosis is 45 years. Fertility sparing surgery is possible according to tumor and patient risk factors at early stages (FIGO Staging IA1–IB1) [2,3,4]. Nevertheless, radical hysterectomy with bilateral adnexectomy is the conventional treatment for women with no fertility desire or in a post-menopausal status [5]. Since the 2018 advent of the LACC clinical trial by P. T. Ramirez et al., the number of mini-invasive surgeries for cervical cancer has drastically decreased [6]. This trial compared oncological outcomes (disease-free survival, recurrence rates, and overall survival rates) between minimally invasive radical hysterectomy and open radical hysterectomy. After the randomized LACC trial publication, a sudden shift in the surgical approach for cervical cancer has occurred, with a significant increase in open radical hysterectomy versus radical minimally invasive surgery [7].
This meta-analysis aims to investigate the implications and rates of both functional and organic urological complications after radical surgery for cervical cancer treatment. We compared the 4 years before and the 4 years after the LACC trial publication in 2018.
Indeed, the minimally invasive radical hysterectomy surgical technique was associated with lower rates of disease-free survival and overall survival than open abdominal radical hysterectomy, among women with early-stage cervical cancer and in turn with worst oncological outcomes [6]. Moreover, two secondary analyses of the LACC trial, published in 2020, have shown that minimally invasive and open surgery correlated with similar morbidity rates and post-operative quality of life (QoL) [8,9]. In terms of urological complications, previous studies have not explored differences on this sensitive topic by comparing pre- and post-LACC eras. Nevertheless, these types of complications have a significant impact on post-operative QoL for patients undergoing a radical treatment for cervical cancer [10]. Accordingly, the European Society for Gynecologic Oncology (ESGO), in 2020, included urological complications in the list of the fifteen quality indicators (QIs) for cervical cancer surgical treatment [11], considering them of the same influence as other indicators, such as parametrial margins, upstaging surgical treatment, or relapse rates within two years after surgery.
LACC trial results have led to an important discussion in the field of gynecologic oncologic surgery, due to several biases shown in the subsequent literature. Therefore, a new trial, the RACC trial, has been set up and is ongoing to confirm these results, given the scientific skepticism surrounding the LACC trial. For these reasons, it is important to study the secondary and indirect effects of the shift in the LACC surgical approach, such as the impact on urological complications. These have not been taken into account until now, even though they have a great impact on patients’ QoL. Our meta-analysis could open a further discussion in the scientific literature to consider all potential factors to define the surgical approach that minimizes complications. This includes the urological aspect and ensures better radicality for cervical cancer treatment. This notion is supported by the fact that ESGO has mentioned urological complications in its QIs.
Objectives. Therefore, this meta-analysis was designed to assess urological complications in patients who underwent radical surgery for cervical cancer before and after LACC trial era to investigate if the shift to open surgery could influence the rate of urological complications.
2. Materials and Methods
This meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1. Literature Search
2.1.1. Search Strategy
Comprehensive systematic research was carried out by using MEDLINE, PubMed and Embase over the 4 years before and after the LACC trial (from January 2014 to October 2022). All studies following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were included.
Three authors (M.R., A.L., G.B.) independently read and evaluated the selected manuscripts to define the eligibility according to the main objective of the analysis, which was to include all relevant studies evaluating urological complications of radical hysterectomy for cervical cancer, performed by minimally invasive approach (either laparoscopy-assisted hysterectomy or robot-assisted hysterectomy, or for one study vaginal-assisted hysterectomy) or open laparotomy surgical technique (Table 1).
Table 1.
The main characteristics of the included studies.
| Author and Year | Type and Criteria of Clinical Study | Number of Patients | N of ARH Patients | N of LRH Patients | N of RRH Patients | N of Urological Complications | N of Functional Complications | N of Organic Complications |
|---|---|---|---|---|---|---|---|---|
| An Segaert 2015 [12] | Retrospective | 109 | 0 | 0 | 109 | 19 | 7 | 12 |
| Balaya 2018 [13] | Retrospective | 248 | 26 | 88 | 9 + 125 vaginal | 109 | 46 | 63 |
| Bogani 2014 ** [14] | Prospective | 90 | 45 | 45 | 0 | 6 | 1 | 5 |
| Bogani 2014 [15] | Prospective | 130 | 65 | 65 | 0 | 5 | 1 | 4 |
| Bogani 2014 [16] | Prospective | 40 | 40 | 0 | 1 | 0 | 1 | |
| Bogani 2014 [17] | Prospective | 96 | 0 | 96 | 0 | 13 | 13 | 0 |
| Boruta 2014 [18] | Retrospective | 22 | 22 | 0 | 0 | 0 | 0 | |
| Chai 2014 [19] | Retrospective | 148 | 148 | 0 | 0 | 46 | 42 | 4 |
| Chen 2014 * [20] | Retrospective | 100 | 44 | 32 | 24 | 3 | 0 | 3 |
| Chen 2015 [21] | Prospective | 65 | 0 | 65 | 0 | NR | NR | NR |
| Cheng Luo 2018 [22] | Retrospective | 60 | 0 | 30 | 30 | 4 | 0 | 4 |
| Corrado 2015 [23] | Retrospective | 60 | 30 | 30 | 5 | 1 | 4 | |
| Corrado 2016 [24] | Prospective | 125 | 43 | 41 | 41 | 49 | 42 | 7 |
| Corrado 2018 [25] | Retrospective | 341 | 101 | 152 | 88 | 14 | 6 | 8 |
| Ditto 2015 [26] | Prospective | 120 | 60 | 60 | 0 | 6 | 3 | 3 |
| Gabriel J. Rendón 2016 [27] | Retrospective | 76 | 0 | 76 | 0 | 5 | 0 | 5 |
| Gallotta 2014 [28] | Prospective | 3 | 0 | 3 | 0 | 0 | 0 | 0 |
| Gallotta 2015 [29] | Prospective | 58 | 0 | 58 | 0 | 6 | 3 | 3 |
| Gallotta 2017 [30] | Prospective | 40 | 0 | 0 | 40 | 3 | 2 | 1 |
| Hoogendam 2014 [31] | Prospective | 100 | 0 | 0 | 100 | 14 | 0 | 14 |
| Kanao 2014 [32] | Prospective | 53 | 53 | 0 | 0 | 0 | ||
| Katrin C Asciutto 2015 [33] | Prospective | 249 | 185 | 0 | 64 | 4 | 0 | 4 |
| Kim 2014 [34] | Prospective | 92 | 0 | 69 | 23 | 6 | 0 | 6 |
| Kim 2021 [35] | Prospective | 20.905 | 12.068 | 8.837 | 0 | 1.546 | 0 | 1.546 |
| Kong 2014 [36] | Retrospective | 88 | 48 | 40 | 0 | 31 | 20 | 11 |
| Kovachev 2021 [37] | Retrospective | 76 | 76 | 0 | 10 | 0 | 10 | |
| Laterza 2016 [38] | Retrospective | 150 | 68 | 82 | 0 | 22 | 15 | 7 |
| Lei 2017 [39] | Prospective | 243 | 243 | 0 | 17 | 14 | 3 | |
| Li 2021 [40] | Prospective | 1207 | 661 | 546 | 0 | 9 | 0 | 9 |
| Liu 2020 [41] | Retrospective | 21.026 | 13.452 | 7.574 | 0 | 324 | 0 | 324 |
| Lu 2022 [42] | Prospective | 148 | 0 | 148 | 0 | 17 | 17 | 0 |
| Makowski 2014 [43] | Prospective | 73 | 73 | 0 | 0 | 5 | 0 | 0 |
| Mendivil 2016 [44] | Retrospective | 146 | 39 | 49 | 58 | 4 | 0 | 4 |
| Nie 2017 [45] | Prospective | 933 | 833 | 100 | 85 | 0 | 85 | |
| Obermair 2020 [8] | Prospective | 536 | 257 | 279 * | 19 | NR | NR | |
| Park 2016 [46] | Retrospective | 293 | 107 | 186 | 0 | 87 | 77 | 10 |
| Pellegrino 2017 [47] | Prospective | 52 | 0 | 18 | 34 | 2 | 00 | 2 |
| Raspagliesi 2016 [48] | Prospective | 30 | 20 | 10 | 0 | 3 | 2 | 1 |
| Raspagliesi 2017 [49] | Prospective | 75 | 0 | 75 | 0 | 3 | 1 | 2 |
| Shah 2017 [50] | Prospective | 311 | 202 | 0 | 109 | 13 | 6 | 7 |
| Shi 2015 [51] | Retrospective | 106 | 0 | 106 | 0 | 8 | 4 | 4 |
| Vizza 2015 [52] | Prospective | 50 | 0 | 25 | 25 | 8 | 5 | 3 |
| Vizza 2018 [53] | Prospective | 20 | 0 | 0 | 20 | 0 | 0 | 0 |
| Wallin 2017 [54] | Retrospective | 304 | 155 | 0 | 149 | 8 | 0 | 8 |
| Yim 2014 [55] | Retrospective | 102 | 0 | 42 | 60 | 11 | 7 | 4 |
| Yim 2017 [56] | Prospective | 142 | 0 | 0 | 142 | 18 | 8 | 10 |
| Yin 2018 [57] | Prospective | 150 | 150 | 0 | 40 | 40 | 0 | |
| Zaccarini 2020 [58] | Retrospective | 93 | 32 | 61 * | 12 | 6 | 6 | |
| Zanagnolo 2016 [59] | Retrospective | 307 | 104 | 0 | 203 | 6 | 0 | 6 |
| Zhang 2017 [60] | Retrospective | 77 | 42 | 35 | 0 | 2 | 2 | 0 |
| Zhongyu Liu 2016 [61] | Prospective | 120 | 120 | 0 | 84 | 84 | 0 |
ARH: Abdominal radical hysterectomy; LRH: laparoscopic radical hysterectomy; RRH: robotic radical hysterectomy; NR: not retrievable. Studies in green: Pre-LACC trial. Studies in or-ange: Post-LACC trial. *, studies including minimally invasive approach, not specifying in LRH or RRH. **, it explains that even if they are called the same, different studies are involved.
The searched keywords and their MESH terms were: “cervical cancer” “hysterectomy” or “radical hysterectomy” + “urologic complications”; “cervical cancer surgery” + “urologic complications”.
Abstracts, full texts, and cross-referenced studies from the retrieved articles were screened to obtain all pertinent information. A revised reference list was also created to ensure no relevant manuscripts were excluded. Duplicate records were removed. Two other authors (BC, VB) verified the search for accuracy and pertinence.
2.1.2. Selection of Studies and Methodologic Quality Assessment
The key criteria for inclusion in our meta-analysis were: (1) original studies published in English, in peer-reviewed journals; (2) histopathological diagnosis of cervical cancer; (3) detailed reports of incidence of urological complication.
Exclusion criteria considered were: (1) editorials, review articles, and conference abstracts; (2) studies with incomplete or absent data on outcomes of interest; (3) no report regarding surgical approaches performed; (4) no report of urological complication; (5) studies in languages other than English.
Selected studies were comprehensively examined, and relevant data were extracted for each paper and entered into a spreadsheet. The information extracted included: journal, author, year of publication, main objective, study design (retrospective or prospective, randomized controlled trials, mono or multicentric), age of patients, histotype, type of surgery performed, report about urological complications incidence, typology of urological complications. Three of the authors (G.B., A.L., M.R.) carried out data extraction and quality assessment from all the retrieved studies based on full-text articles. Discrepancies between the investigators were resolved through discussions between all authors until a consensus was reached.
The meta-analysis included all identified controlled studies, which were qualitatively classified according to the Cochrane Handbook for Systematic reviews of Interventions guidelines. For bias risk assessment of included studies, we used the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) method.
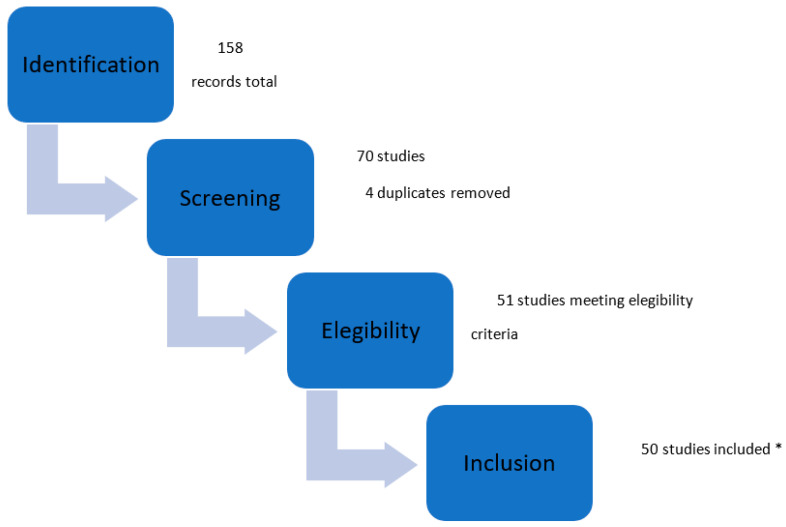
The selection of studies is shown in Figure 1. A search of the MEDLINE (PubMed) database from January 2014 to October 2022 resulted in 158 relevant articles. Further searches in Embase, Cochrane Library, and Google Scholar databases resulted in no additional articles. Finally, 51 articles met the inclusion criteria, including a total of 50,183 patients [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
Figure 1.
Selection study steps flow chart. Main characteristics of included studies. * Exclusion motivation: 1 study was excluded because it reported the numbers of combined complications and it was not possible to determine how many patients experienced combined or individual complications [21].
2.2. Selection and Inclusion Criteria
Our meta-analysis was designed for the following PICOS queries:
Population: Patients with histologically confirmed cervical cancer who underwent radical MIS or laparotomy surgery, 4 years before and after the LACC trial.
Intervention: radical MIS or laparotomy surgery for cervical cancer treatment.
Comparison: patients who underwent radical surgery for cervical cancer 4 years before and after the LACC trial.
Outcomes: dysfunctional and organic urological complications.
Study design: Observational studies (randomized control trials, retrospective and prospective studies, case-control, and cohort series) in which post-operative urological complications (both organic and dysfunctional) were recorded in cases of minimally invasive (laparoscopic and robotic surgery) and open surgery for radical cervical cancer treatment, during the 4 years before and the 4 years after the LACC trial were included. Reviews, letters to the editor, and congress abstracts were excluded. Only manuscripts written in English were included.
A flow chart summarizing the study selection process is available in Figure 1. The main characteristics of the included studies are shown in Table 1.
Dysfunctional urologic complications included: urinary retention and urinary incontinence, renal failure requiring dialysis, nycturia, and dysuria.
Organic urologic complications included: bladder and ureteral injury, ureterovaginal and vesicovaginal fistula, ureteral stenosis, ureteral fistula (resulting in uroperitoneum), urinary tract infection, and hematuria.
2.3. Main, Subgroup Analyses and Outcome Measures
Urologic complication rates linked to cervical cancer radical surgery were investigated, particularly in pre- versus post-LACC trial era. A subgroup analysis was also performed according to different types of urologic complications in the pre- and post-LACC trial: dysfunctional vs. organic lesions. Finally, we compared the incidence of urological complications between open surgery and MIS.
2.4. Statistical Method
Event rate (ER) and 95% confidence intervals (CIs) were assessed. Heterogeneity was evaluated by X2Q test and I2 statistic. For the Q test, p < 0.05 indicated significant heterogeneity; for the I2 statistics, an I2 value of >50% was considered significant. The pooled ER estimate was calculated using a random-effect model. Our results are displayed graphically as forest plots, with ER and CI 95% for each study. Publication bias was evaluated by a visual inspection of funnel plots. Calculations were performed using the Comprehensive Meta-Analysis Software, version v.2.0 (CMA; Biostat, Englewood, NJ, USA).
For the comparison of urological complications’ incidence in open surgery versus MIS, chi-square tests, or Fisher’s exact tests, as appropriate, were used since they are defined as categorical variables. All significance was defined at the p < 0.05 level. The SPSS (SPSS Inc., Chicago, IL, USA) and GraphPad Prism ver. 9.0.2 (GraphPad Software, San Diego, CA, USA) statistical programs were used for this analysis.
3. Results
3.1. Pre- and Post- LACC Urologic Complications Rate
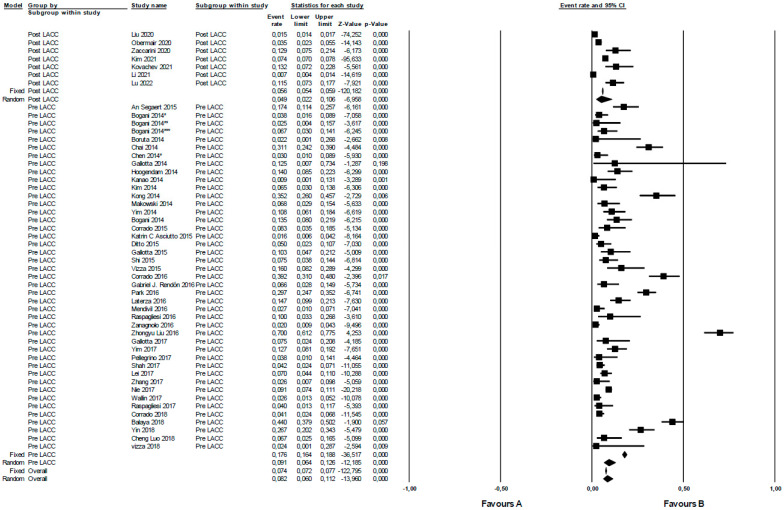
The rate of urologic complications was analyzed across 43 studies pre- and 7 studies post-LACC trials.
The urologic complications rate was higher in pre- than post-LACC studies, even though the difference is not statistically significant (p = 0.156). In a random effects meta-analysis, the overall risk of urological complications pre-LACC in terms of OR was 9.1 (95% CI 6.4–12.6), while that in the post-LACC period was 4.9 (95% CI 2.2–10.6). The examined studies pre- and post-LACC demonstrated significant heterogeneity, with I2 values of 94.1 (p = 0.000) and 99.2 (p = 0.000), respectively (Figure 2). Additionally, most examined cohorts were not very large with the exception of two of them (Kim 2021, with 20,905 patients and Liu 2022, with 21,026 patients).
Figure 2.
Urologic complications in radical surgery for cervical cancer pre- versus post-LACC trial. Event rate: urological complications rate.
3.2. Subgroup Meta-Analysis
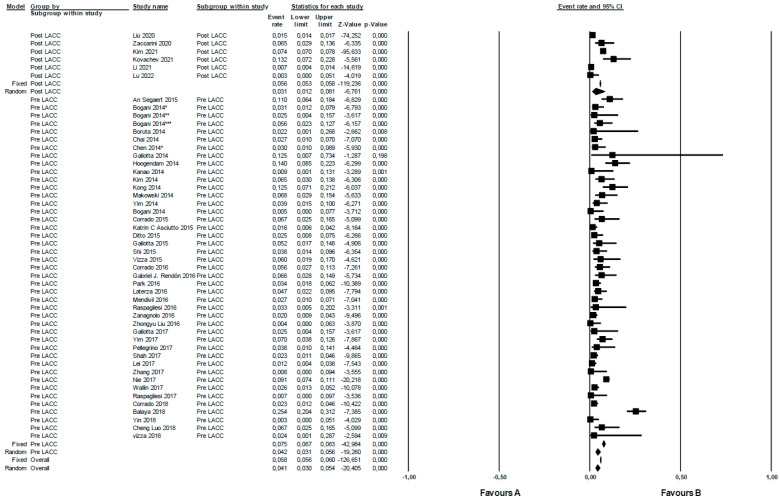
The rate of organic urologic complications was analyzed across the included studies pre- (n 43) versus post-LACC (n 7) trial. In a random effects meta-analysis, the overall risk of organic urological complications pre-LACC in terms of OR was 4.2 (95% CI 3.1–5.6). In contrast, for post-LACC, it was 3.1 (95% CI 1.2–8.1). When comparing pre- versus post-LACC, the rate of organic complications was higher in the pre-LACC era. However, this difference was not statistically significant (p = 0.59). The studies from pre- and post-LACC periods displayed significant heterogeneity, with I2 values of 82.2 (p = 0.000) and 99.3 (p = 0.000), respectively (Figure 3).
Figure 3.
Organic urologic complications in radical surgery for cervical cancer pre- versus post-LACC trial. Event rate: organic urological complications rate.
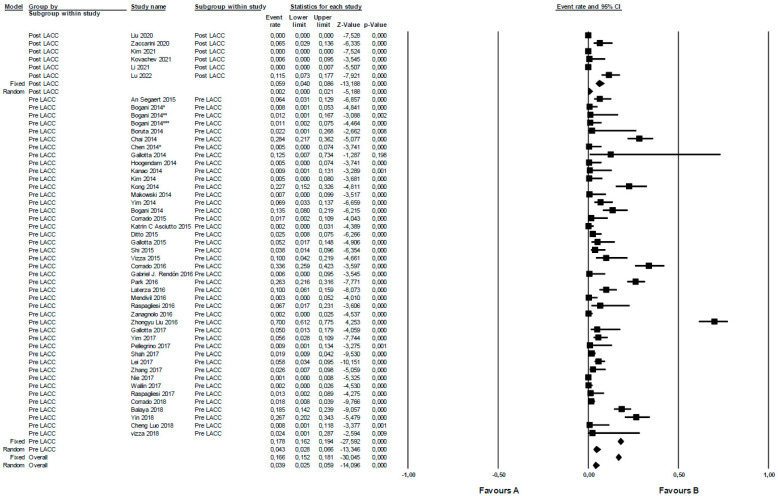
The rate of functional urologic complications was analyzed in the included studies pre- (n 43) and post-LACC (n 6) trial. In a random effects meta-analysis, the overall risk of functional urological complications pre-LACC in terms of OR was 4.3 (95% CI 2.8–6.6). For post-LACC, it was 0.2 (95% CI 0–2.1). When comparing the pre- versus post-LACC periods, a statistically significant difference (p = 0.012) was found, with a higher rate of functional urological complications in the pre-LACC period. Studies pre- and post-LACC demonstrated notable heterogeneity, I2 91.9 (p = 0.000) and 94.1 (p = 0.000), respectively (Figure 4).
Figure 4.
Functional urologic complications in radical surgery for cervical cancer pre- versus post-LACC trial. Event rate: functional urological complications rate.
In summary, even if the included studies have shown a great heterogeneity, we have found a non-significant difference regarding the total and the organic urological complications between pre- and post-LACC eras, while a statistically significant difference has been highlighted when considering only the functional urological complications.
Furthermore, it was observed that the incidence of urological complications in open surgery for all studies included was lower (3.9%) than for MIS (5.2%) (p value < 0.00001). In detail, the number of urological complications in open surgery was 1040 out of 26,159, while for MIS it was 943 out of 18,003.
4. Discussion
Cervical cancer surgery is associated with a high risk of complications, due to the disease’s local dissemination, leading to disruption of the normal anatomy of the female pelvis. In recent years, there has been extensive research into which type of surgical approach is associated with lower complication rates. Since the advent of the LACC trial, it has been shown that patients who underwent an open surgical approach had higher disease-free survival, overall survival and lower recurrence rates [6]. However, when considering urologic complications, clear differences between the open and minimally invasive approaches have not been identified [62]. The LACC trial brought about a radical change in cervical cancer surgery by leading to a dramatic shift to the surgical open technique [35].
To ensure more homogeneous cohorts for comparisons, all suitable studies 4 years before and 4 years after the LACC trial (since 4 years have elapsed from the publication of the LACC trial) were selected to investigate if there were any differences in terms of the urologic complication rate (Table 1). Among the 50 studies analyzed, none exhibited a statistically significant difference in terms of urological complications when comparing surgical techniques (LRH versus ARH versus RRH). Four studies [14,33,55,59] demonstrated that a minimally invasive approach could reduce overall postoperative complications, and ten studies indicated an association with shorter hospital stays compared to open surgery [14,15,20,26,33,36,38,44,48,59].
In radical surgery for cervical cancer, extensive dissection of peri-ureteral tissue is required and performed to isolate uterine arteries. The most dangerous surgical time in a radical hysterectomy for urological structure is during the dissection of the distal portion of the ureter, near its entry into the bladder, and also during bladder dissection to obtain the necessary vaginal resection margin. While a minimally invasive approach may require more training than open surgery, it was initially believed that it might be associated with greater complications. Upon analyzing all individual studies, there does not seem to be a clear correlation between the minimally invasive approach and an increased incidence of urological complications. However, on dividing the studies over time it seems that in the pre-LACC period there were greater rates of urological complications (Figure 2). This could be influenced by the discordant results of different studies, which show a great variability in the number of complications. In addition, in some studies, patients were evaluated both in the early post-operative period and in the long-term follow-up, so this could explain the varying incidences reported. Most of the studies, however, did not specify when the complication occurred, so it was not possible to further characterize this aspect.
In particular, the novelty of our study lies in the fact that functional urological outcomes between the two surgical eras were compared. A reduced rate in the post-LACC period (Figure 4) was found, suggesting that the open approach could be associated with a lower rate of urological complications. In contrast, no statistically significant differences were found in terms of organic complications (Figure 3).
A possible explanation for the higher rate of dysfunctional urological injuries could be related to the post-LACC radical and sudden shift to open surgery. This change might result in potentially lower thermal damage of autonomic fibers which innervate the lower urinary tracts when and where parametrium and uterosacral ligaments dissection is performed. In detail, sympathetic denervation enhances parasympathetic transmission to the low urinary tract which can explain dysfunctional urological complications after radical hysterectomy. This is further compounded by the loss of periureteral tone due to potential denervation of the pelvic plexus and pudendal nerves [63].
This hypothesis linked to use of the advanced sealing device is supported by our further analysis, where urological complication incidence in open surgery for all studies included was found to be lower than for MIS.
Advanced sealing devices can be dangerous for either visceral or vascular lesions. This is because inadvertent tissue contact may occur in the case of lateral thermal spread: the development of these technologies might have contributed to the rising incidence of urological complications in MIS, as previously reported [64].
Thermal injury due to the use energy devices can also occur when these coagulation tools are applied to tissues lying in the proximity of the ureter through an indirect mechanism. Extensive ureterolysis can lead to ureter devascularization and in turn to long-term dysfunctional complications. This is due to indirect lesion of the mesoureteral tissue, which not only hosts vascular but also nervous terminations [65].
Within the timeframe of 8 years, out of a total of 50,183 patients who underwent surgery, a non-significant difference in total and organic urological complications has emerged; meanwhile, a significant difference has been found between functional and organic urological complications, resulting in fewer functional complications during the post-LACC period. This finding could be due to the decreased number of minimally invasive surgeries that were performed after the LACC trial publication, together with the hypothesis that advanced sealing techniques used in minimally invasive surgery could produce collateral thermal damage affecting tissue response and healing process. It can also be speculated that the different surgical training needed for minimally invasive surgery versus laparotomy could affect the rate of urological complications, but further studies are needed.
Nevertheless, our meta-analysis has several limitations since:
-
-
Many of the studies we found were retrospective, which could reduce the level of evidence;
-
-
We excluded all non-English studies, potentially introducing a bias to our findings;
-
-
Stratification based on the cervical cancer stage was not possible, since few studies provided this information, even if cervical cancer stage is one of the most relevant risk factors for surgical urologic complications [35];
-
-
The group for urological organic complications could appear to be too heterogeneous, since it includes both severe and mild damages. Most case studies did not provide the specific grade of complication and subsequently our statistical method was not powered to outline any significant differences in the severity of complications.
The lack of evidence on urological complications after the LACC trial could compromise the quantitative analysis; therefore, there is a need for further studies to reach a convincing comparison.
In summary, despite the previously reported limitations, by comparing urological complication rates caused by radical surgery for cervical cancer in pre- versus post- LACC era in 50,183 patients, urological complications were found to be more frequent in the pre-LACC era, and in particular functional complications. This meta-analysis could be relevant for subsequent studies because it has shown that in the post-LACC era there is a reduction in functional complications that greatly impact patients’ quality of life [10]. However, our results must be confirmed by solid evidence based on randomized controlled trials. This will help in defining the optimal surgical approach, with fewer complications, including those in the urological field, and at the same time will ensure a better radicality for cervical cancer treatment, since the ESGO has also mentioned urological complications amongst its fifteen Quality Indicators.
Moreover, since the LACC trial introduced a controversial and extensive scientific discussion in the gynecologic oncologic surgery field, all factors contributing to the optimal global post-surgical outcome for oncological patients should be included in further trials evaluating radical cervical cancer treatment.
In this regard, more attention should be attributed to the QoL of oncological patients, to reach the proper balance between radicality and global clinical and psychological health, in order to ensure an optimal management of everyday life, not only restricted to the status of patient but also with a significant beneficial impact also on their caregivers.
By tailoring the proper surgical approach and minimizing surgical complications that greatly impact patients QoL, we could potentially achieve shorter hospital stays, a faster return to working or family activities, and a significant reduction in social and economic costs with the maximum benefit.
5. Conclusions
This meta-analysis revealed that the rate of urologic complications was higher in pre-LACC studies compared to post-LACC studies even if it is not statistically significant. This observation held true even for organic complications. However, when comparing pre- versus post-LACC, there was a statistically significant difference, with the pre-LACC era showing a higher rate of functional urological complications. Given the limitations described above, further prospective studies are necessary to confirm these findings.
Author Contributions
Conceptualization, V.B. and B.C.; methodology and statistical analysis, I.S.; investigation, A.L., G.B. and M.R.; data revision, D.S.; writing—original draft preparation, V.B, A.L. and G.B.; writing—review and editing, D.S., E.M., U.A. and L.F.; supervision, E.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Our study does not require ethical approval.
Informed Consent Statement
Patient consent was waived due to the anonymous design of the retrospective study.
Data Availability Statement
Raw data would be available on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro Cubal A.F., Ferreira Carvalho J.I., Costa M.F.M., Branco A.P.T. Fertility-Sparing Surgery for Early-Stage Cervical Cancer. Int. J. Surg. Oncol. 2012;2012:11. doi: 10.1155/2012/936534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatla N., Aoki D., Sharma D.N., Sankaranarayanan R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018;143((Suppl. S2)):22–36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- 4.Schlaerth J.B., Spirtos N.M., Schlaerth A.C. Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer. Am. J. Obstet. Gynecol. 2003;188:29–34. doi: 10.1067/mob.2003.124. [DOI] [PubMed] [Google Scholar]
- 5.Cibula D., Pötter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Meder C.H., Köhler C., Landoni F., Lax S., Lindegaard J.C., et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer. 2018;28:641–655. doi: 10.1097/IGC.0000000000001216. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez P.T., Frumovitz M., Pareja R., Lopez A., Vieira M., Ribeiro M., Buda A., Yan X., Shuzhong Y., Chetty N., et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 7.Lewicki P.J., Basourakos S.P., Qiu Y., Hu J.C., Sheyn D., Hijaz A., Shoag J.E. Effect of a Randomized, Controlled Trial on Surgery for Cervical Cancer. N. Engl. J. Med. 2021;384:1669–1671. doi: 10.1056/NEJMc2035819. [DOI] [PubMed] [Google Scholar]
- 8.Obermair A., Asher R., Pareja R., Frumovitz M., Lopez A., Moretti-Marques R., Rendon G., Ribeiro R., Tsunoda A., Behan V., et al. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: Results of a randomized controlled trial. Am. J. Obstet. Gynecol. 2020;222:249.e1–249.e10. doi: 10.1016/j.ajog.2019.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frumovitz M., Obermair A., Coleman R.L., Pareja R., Lopez A., Ribero R., Isla D., Rendon G., Bernardini M.Q., Ramirez P.T., et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): A secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020;21:851–860. doi: 10.1016/S1470-2045(20)30081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valente S. Urological problems and quality of life after treatment in early cervical cancer. Eur. J. Gynaecol. Oncol. 1988;9:424–427. [PubMed] [Google Scholar]
- 11.Cibula D., Planchamp F., Fischerova D., Fotopoulou C., Kohler C., Landoni F., Mathevet P., Naik R., Ponce J., Raspagliesi F., et al. European Society of Gynaecological Oncology quality indicators for surgical treatment of cervical cancer. Int. J. Gynecol. Cancer. 2020;30:3–14. doi: 10.1136/ijgc-2019-000878. [DOI] [PubMed] [Google Scholar]
- 12.Segaert A., Traen K., Van Trappen P., Peeters F., Leunen K., Goffin F., Vergote I. Robot-Assisted Radical Hysterectomy in Cervical Carcinoma: The Belgian Experience. Int. J. Gynecol. Cancer. 2015;25:1690–1696. doi: 10.1097/IGC.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 13.Balaya V., Mathevet P., Magaud L., Delomenie M., Bonsang-Kitzis H., Ngô C., Huchon C., Bats A., Lecuru F. Predictive factors of severe perioperative morbidity of radical hysterectomy with lymphadenectomy in early-stage cervical cancer: A French prospective multicentric cohort of 248 patients. Eur. J. Surg. Oncol. 2019;45:650–658. doi: 10.1016/j.ejso.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 14.Bogani G., Cromi A., Serati M., Di Naro E., Uccella S., Donadello N., Ghezzi F. Predictors of postoperative morbidity after laparoscopic versus open radical hysterectomy plus external beam radiotherapy: A propensity-matched comparison. J. Surg. Oncol. 2014;110:893–898. doi: 10.1002/jso.23747. [DOI] [PubMed] [Google Scholar]
- 15.Bogani G., Cromi A., Uccella S., Serati M., Casarin J., Pinelli C., Ghezzi F. Laparoscopic versus open abdominal management of cervical cancer: Long-term results from a propensity-matched analysis. J. Minim. Invasive Gynecol. 2014;21:857–862. doi: 10.1016/j.jmig.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Bogani G., Serati M., Nappi R., Cromi A., di Naro E., Ghezzi F. Nerve-sparing approach reduces sexual dysfunction in patients undergoing laparoscopic radical hysterectomy. J. Sex. Med. 2014;11:3012–3020. doi: 10.1111/jsm.12702. [DOI] [PubMed] [Google Scholar]
- 17.Bogani G., Cromi A., Uccella S., Serati M., Casarin J., Pinelli C., Nardelli F., Ghezzi F. Nerve-sparing versus conventional laparoscopic radical hysterectomy: A minimum 12 months’ follow-up study. Int. J. Gynecol. Cancer. 2014;24:787–793. doi: 10.1097/IGC.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 18.Boruta D.M., Fagotti A., Bradford L.S., Escobar P.F., Scambia G., Kushnir C.L., Michener C.M., Fader A.N. Laparoendoscopic single-site radical hysterectomy with pelvic lymphadenectomy: Initial multi-institutional experience for treatment of invasive cervical cancer. J. Minim. Invasive Gynecol. 2014;21:394–398. doi: 10.1016/j.jmig.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Chai Y., Wang T., Wang J., Yang Y., Gao Y., Gao J., Gao S., Wang Y., Zhou X., Liu Z. Radical hysterectomy with adjuvant radiotherapy versus radical radiotherapy for FIGO stage IIB cervical cancer. BMC Cancer. 2014;14:63. doi: 10.1186/1471-2407-14-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C.H., Chiu L.H., Chang C.W., Yen Y.K., Huang Y.H., Liu W.M. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int. J. Gynecol. Cancer. 2014;24:1105–1111. doi: 10.1097/IGC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 21.Chen L., Zhang W.-N., Zhang S.-M., Yang Z.-H., Zhang P. Effect of Laparoscopic Nerve-sparing Radical Hysterectomy on Bladder Function, Intestinal Function Recovery and Quality of Sexual Life in Patients with Cervical Carcinoma. Asian Pac. J. Cancer Prev. 2015;15:10971–10975. doi: 10.7314/APJCP.2014.15.24.10971. [DOI] [PubMed] [Google Scholar]
- 22.Luo C., Liu M., Li X. Efficacy and safety outcomes of robotic radical hysterectomy in Chinese older women with cervical cancer compared with laparoscopic radical hysterectomy. BMC Womens Health. 2018;18:61. doi: 10.1186/s12905-018-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corrado G., Fanfani F., Ghezzi F., Fagotti A., Uccella S., Mancini E., Sperduti I., Stevenazzi G., Scambia G., Vizza E. Mini-laparoscopic versus robotic radical hysterectomy plus systematic pelvic lymphadenectomy in early cervical cancer patients. A multi-institutional study. Eur. J. Surg. Oncol. 2015;41:136–141. doi: 10.1016/j.ejso.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Corrado G., Cutillo G., Saltari M., Mancini E., Sindico S., Vici P., Sergi D., Sperduti I., Patrizi L., Pomati G., et al. Surgical and Oncological Outcome of Robotic Surgery Compared With Laparoscopic and Abdominal Surgery in the Management of Locally Advanced Cervical Cancer After Neoadjuvant Chemotherapy. Int. J. Gynecol. Cancer. 2016;26:539–546. doi: 10.1097/IGC.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 25.Corrado G., Vizza E., Legge F., Anchora L.P., Sperduti I., Fagotti A., Mancini E., Gallotta V., Zampa A., Chiofalo B., et al. Comparison of Different Surgical Approaches for Stage IB1 Cervical Cancer Patients: A Multi-institution Study and a Review of the Literature. Int. J. Gynecol. Cancer. 2018;28:1020–1028. doi: 10.1097/IGC.0000000000001254. [DOI] [PubMed] [Google Scholar]
- 26.Ditto A., Martinelli F., Bogani G., Gasparri M.L., Di Donato V., Zanaboni F., Lorusso D., Raspagliesi F. Implementation of laparoscopic approach for type B radical hysterectomy: A comparison with open surgical operations. Eur. J. Surg. Oncol. EJSO. 2015;41:34–39. doi: 10.1016/j.ejso.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 27.Rendón G.J., Echeverri L., Echeverri F., Sanz-Lomana C.M., Ramirez P.T., Pareja R. Outpatient laparoscopic nerve-sparing radical hysterectomy: A feasibility study and analysis of perioperative outcomes. Gynecol. Oncol. 2016;143:352–356. doi: 10.1016/j.ygyno.2016.08.233. [DOI] [PubMed] [Google Scholar]
- 28.Gallotta V., Fanfani F., Scambia G. Minilaparoscopic nerve sparing radical hysterectomy in locally advanced cervical cancer after neoadjuvant radiochemotherapy. Gynecol. Oncol. 2014;132:758–759. doi: 10.1016/j.ygyno.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Gallotta V., Ferrandina G., Chiantera V., Fagotti A., Fanfani F., Ercoli A., Legge F., Costantini B., Alletti S.G., Bottoni C., et al. Laparoscopic Radical Hysterectomy After Concomitant Chemoradiation in Locally Advanced Cervical Cancer: A Prospective Phase II Study. J. Minim. Invasive Gynecol. 2015;22:877–883. doi: 10.1016/j.jmig.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Gallotta V., Chiantera V., Conte C., Vizzielli G., Fagotti A., Nero C., Costantini B., Lucidi A., Cicero C., Scambia G., et al. Robotic Radical Hysterectomy After Concomitant Chemoradiation in Locally Advanced Cervical Cancer: A Prospective Phase II Study. J. Minim. Invasive Gynecol. 2017;24:133–139. doi: 10.1016/j.jmig.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Hoogendam J.P., Verheijen R.H.M., Wegner I., Zweemer R.P. Oncological outcome and long-term complications in robot-assisted radical surgery for early stage cervical cancer: An observational cohort study. BJOG. 2014;121:1538–1545. doi: 10.1111/1471-0528.12822. [DOI] [PubMed] [Google Scholar]
- 32.Kanao H., Fujiwara K., Ebisawa K., Hada T., Ota Y., Andou M. Various types of total laparoscopic nerve-sparing radical hysterectomies and their effects on bladder function. J. Gynecol. Oncol. 2014;25:198. doi: 10.3802/jgo.2014.25.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asciutto K.C., Kalapotharakos G., Löfgren M., Högberg T., Borgfeldt C. Robot-assisted surgery in cervical cancer patients reduces the time to normal activities of daily living. Acta Obstet. Gynecol. Scand. 2015;94:260–265. doi: 10.1111/aogs.12561. [DOI] [PubMed] [Google Scholar]
- 34.Kim T.-H., Choi C.H., Choi J.-K., Yoon A., Lee Y.-Y., Lee J.-W., Bae D.-S., Kim B.-G. Robotic versus laparoscopic radical hysterectomy in cervical cancer patients: A matched-case comparative study. Int. J. Gynecol. Cancer. 2014;24:1466–1473. doi: 10.1097/IGC.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 35.Kim H., Jeong H.J., Kim B.W., Hwang J.H. The incidence of urologic complications requiring urologic procedure in radical hysterectomy and difference between abdominal radical hysterectomy and laparoscopic radical hysterectomy. J. Gynecol. Oncol. 2021;32:e84. doi: 10.3802/jgo.2021.32.e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong T.W., Chang S.J., Lee J., Paek J., Ryu H.S. Comparison of laparoscopic versus abdominal radical hysterectomy for FIGO stage IB and IIA cervical cancer with tumor diameter of 3 cm or greater. Int. J. Gynecol. Cancer. 2014;24:280–288. doi: 10.1097/IGC.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 37.Kovachev S.M., Kovachev M.S. The role of perioperative ureteral stenting for urologic complications in radical surgery of cervical cancer. Urol. J. 2021;88:348–354. doi: 10.1177/03915603211001178. [DOI] [PubMed] [Google Scholar]
- 38.Laterza R.M., Uccella S., Casarin J., Morosi C., Serati M., Koelbl H., Ghezzi F. Recurrence of Early Stage Cervical Cancer After Laparoscopic Versus Open Radical Surgery. Int. J. Gynecol. Cancer. 2016;26:547–552. doi: 10.1097/IGC.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 39.Lei H., Gui D., He Y. Short-and long-term outcomes of laparoscopic radical hys-terectomy for obese patients with cervical cancer. Chemotherapy. 2017;29:35. [PubMed] [Google Scholar]
- 40.Li Z., Chen C., Liu P., Duan H., Liu M., Xu Y., Li P., Zhang W., Jiang H., Bin X., et al. Comparison of oncological outcomes and major complications between laparoscopic radical hysterectomy and abdominal radical hysterectomy for stage IB1 cervical cancer with a tumour size less than 2 cm. Eur. J. Surg. Oncol. 2021;47:2125–2133. doi: 10.1016/j.ejso.2021.03.238. [DOI] [PubMed] [Google Scholar]
- 41.Liu P., Liang C., Lu A., Chen X., Liang W., Li D., Yin L., Li Z., Cao Y., Bin X., et al. Risk factors and long-term impact of urologic complications during radical hysterectomy for cervical cancer in China, 2004–2016. Gynecol. Oncol. 2020;158:294–302. doi: 10.1016/j.ygyno.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 42.Lu Q., Wu L.H., Qi L.Y., Tie P., Guan Z. Effect of Comprehensive Care Based on Appropriate Chinese Medicine Techniques on Urinary Retention and Bladder Function Recovery after Total Hysterectomy in Patients with Cervical Cancer. Comput. Math. Methods Med. 2022;2022:7495418. doi: 10.1155/2022/7495418. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Makowski M., Nowak M., Szpakowski M., Władziński J., Serwach-Nowińska A., Janas Ł., Wilczyński J.R. Classical radical hysterectomy and nerve-sparing radical hysterectomy in the treatment of cervical cancer. Przegląd Menopauzalny. 2014;13:180. doi: 10.5114/pm.2014.43822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendivil A.A., Rettenmaier M.A., Abaid L.N., Brown J.V., Micha J.P., Lopez K.L., Goldstein B.H. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: A five year experience. Surg. Oncol. 2016;25:66–71. doi: 10.1016/j.suronc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Nie J.C., Yan A.Q., Liu X.S. Robotic-Assisted Radical Hysterectomy Results in Better Surgical Outcomes Compared With the Traditional Laparoscopic Radical Hysterectomy for the Treatment of Cervical Cancer. Int. J. Gynecol. Cancer. 2017;27:1990. doi: 10.1097/IGC.0000000000001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J.-Y., Kim D., Suh D.-S., Kim J.-H., Kim Y.-M., Kim Y.-T., Nam J.-H. The Role of Laparoscopic Radical Hysterectomy in Early-Stage Adenocarcinoma of the Uterine Cervix. Ann. Surg. Oncol. 2016;23:825–833. doi: 10.1245/s10434-016-5489-4. [DOI] [PubMed] [Google Scholar]
- 47.Pellegrino A., Damiani G.R., Loverro M., Pirovano C., Fachechi G., Corso S., Trojano G. Comparison of Robotic and laparoscopic Radical type-B and C hysterectomy for cervical cancer: Long term-outcomes. Acta Biomed. 2017;88:289–296. doi: 10.23750/abm.v%vi%i.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raspagliesi F., Bogani G., Martinelli F., Signorelli M., Chiappa V., Scaffa C., Sabatucci I., Adorni M., Lorusso D., Ditto A. Incorporating 3D laparoscopy for the management of locally advanced cervical cancer: A comparison with open surgery. Tumori J. 2016;102:393–397. doi: 10.5301/tj.5000527. [DOI] [PubMed] [Google Scholar]
- 49.Raspagliesi F., Bogani G., Martinelli F., Signorelli M., Scaffa C., Sabatucci I., Lorusso D., Ditto A. 3D vision improves outcomes in early cervical cancer treated with laparoscopic type B radical hysterectomy and pelvic lymphadenectomy. Tumori J. 2017;103:76–80. doi: 10.5301/tj.5000572. [DOI] [PubMed] [Google Scholar]
- 50.Shah C.A., Beck T., Liao J.B., Giannakopoulos N.v., Veljovich D., Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J. Gynecol. Oncol. 2017;28:e82. doi: 10.3802/jgo.2017.28.e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi R., Wei W., Jiang P. Laparoscopic Nerve-Sparing Radical Hysterectomy for Cervical Carcinoma: Emphasis on Nerve Content in Removed Cardinal Ligaments. Int. J. Gynecol. Cancer. 2015;26:192–198. doi: 10.1097/IGC.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 52.Vizza E., Corrado G., Mancini E., Vici P., Sergi D., Baiocco E., Patrizi L., Saltari M., Pomati G., Cutillo G. Laparoscopic versus robotic radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical cancer: A case control study. Eur. J. Surg. Oncol. 2015;41:142–147. doi: 10.1016/j.ejso.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 53.Vizza E., Chiofalo B., Cutillo G., Mancini E., Baiocco E., Zampa A., Bufalo A., Corrado G. Robotic single site radical hysterectomy plus pelvic lymphadenectomy in gynecological cancers. J. Gynecol. Oncol. 2018;29:e2. doi: 10.3802/jgo.2018.29.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallin E., Flöter Rådestad A., Falconer H. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: Impact on complications, costs and oncologic outcome. Acta Obstet. Gynecol. Scand. 2017;96:536–542. doi: 10.1111/aogs.13112. [DOI] [PubMed] [Google Scholar]
- 55.Yim G.W., Kim S.W., Nam E.J., Kim S., Kim H.J., Kim Y.T. Surgical outcomes of robotic radical hysterectomy using three robotic arms versus conventional multiport laparoscopy in patients with cervical cancer. Yonsei Med. J. 2014;55:1222–1230. doi: 10.3349/ymj.2014.55.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yim G.W., Eoh K.J., Chung Y.S., Kim S.W., Kim S., Nam E.J., Lee J.Y., Kim Y.T. Perioperative Outcomes of 3-Arm Versus 4-Arm Robotic Radical Hysterectomy in Patients with Cervical Cancer. J. Minim. Invasive Gynecol. 2018;25:823–831. doi: 10.1016/j.jmig.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Yin S., Ma S.-N., Zhang Y.-Q., Shi T.-Y., Xiang L.-B., Ren Y.-L., Zang R.-Y. Surgical and oncological outcomes of an improved nerve-sparing radical hysterectomy technique: 6 years of experience at two centres. Surg. Oncol. 2018;27:380–386. doi: 10.1016/j.suronc.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Zaccarini F., Santy A., Dabi Y., Lavoue V., Carcopino X., Bendifallah S., Benbara A., Collinet P., Canlorbe G., Raimond E., et al. Comparison of survival outcomes between laparoscopic and abdominal radical hysterectomy for early-stage cervical cancer: A French multicentric study. J. Gynecol. Obstet. Human Reprod. 2021;50:102046. doi: 10.1016/j.jogoh.2020.102046. [DOI] [PubMed] [Google Scholar]
- 59.Zanagnolo V., Minig L., Rollo D., Tomaselli T., Aletti G., Bocciolone L., Landoni F., Rebollo J.M.C., Maggioni A. Clinical and Oncologic Outcomes of Robotic Versus Abdominal Radical Hysterectomy for Women With Cervical Cancer: Experience at a Referral Cancer Center. Int. J. Gynecol. Cancer. 2016;26:568–574. doi: 10.1097/IGC.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S., Ma L., Meng Q.W., Zhou D., Moyiding T. Comparison of laparoscopic-assisted radical vaginal hysterectomy and abdominal radical hysterectomy in patients with early stage cervical cancer: A retrospective study. Medicine. 2017;96:e8005. doi: 10.1097/MD.0000000000008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z., Li X., Tao Y., Li W., Yang Y., Yao Y., Zhu T. Clinical efficacy and safety of laparoscopic nerve-sparing radical hysterectomy for locally advanced cervical cancer. Int. J. Surg. 2016;25:54–58. doi: 10.1016/j.ijsu.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Li Y., Kong Q., Wei H., Wang Y. Comparison of the complications between minimally invasive surgery and open surgical treatments for early-stage cervical cancer: A systematic review and meta-analysis. PLoS ONE. 2021;16:e0253143. doi: 10.1371/journal.pone.0253143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chou M.-H., Meng E., Wu S.-T., Cha T.-L., Sun G.-H., Yu D.-S., Chung C.-H., Chien W.-C. Increased incidence of neurogenic bladder after radical hysterectomy for cervical cancer: A nationwide population-based cohort study. J. Chin. Med. Assoc. 2021;84:942–950. doi: 10.1097/JCMA.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 64.Adelman M.R., Bardsley T.R., Sharp H.T. Urinary tract injuries in laparoscopic hysterectomy: A systematic review. J. Minim. Invasive Gynecol. 2014;21:558–566. doi: 10.1016/j.jmig.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Sharp H.T., Adelman M.R. Prevention, Recognition, and Management of Urologic Injuries During Gynecologic Surgery. Obstet. Gynecol. 2016;127:1085–1096. doi: 10.1097/AOG.0000000000001425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data would be available on reasonable request.