Abstract
A PCR system that can quickly and accurately identify 14 species of human pathogenic yeasts was developed. The procedure distinguished between nine species of a closely related clade, Lodderomyces elongisporus, Candida parapsilosis, a new Candida sp., C. sojae, C. tropicalis, C. maltosa, C. viswanathii, C. albicans, and C. dubliniensis and between another five more divergent species, Pichia guilliermondii, C. glabrata, C. zeylanoides, C. haemulonii, and C. haemulonii type II. A rapid DNA extraction procedure that yields purified DNA in about 1 h is also described. The system uses uniform conditions with four primers for each reaction, two 40- to 50-mer universal primers that serve as a positive control and two 23- to 30-mer species-specific primers. Species-specific primers were derived from a 600-nucleotide variable region (D1/D2) at the 5′ end of the large-subunit (26S) ribosomal DNA gene and were generally designed to use mismatches at the 3′ end. Universal primers were developed from conserved nucleotide sequences in the small-subunit (18S) rRNA gene. In this system, a control 1,200- to 1,300-base DNA fragment was produced in all reactions and a smaller 114- to 336-base DNA fragment was produced if the chromosomal DNA from the target species was present. The PCR procedure is rapid and easy to interpret and may be used with mixed cultures.
Human pathogenic yeasts are ubiquitous in the environment, and some are normal inhabitants in the body. These yeasts are usually opportunistic organisms, causing acute-to-chronic infections when conditions in the host are favorable. Candida albicans and related species are the principal causes of human yeast infections. In the United States alone, this group is involved in millions of human infections a year and is responsible for over 90% of the systemic or deep infections, about 8,000 per year in the 1980s (1). At present, identification of these species, at both medical and research laboratories, is often based on standard laboratory techniques such as germ tube formation (19) and biochemical tests (17, 22, 27). These procedures require purification of the target organism, are time-consuming, and have an inherent weakness in that they may not be species specific. Additionally, strains of the same species can differ in key characteristics (14, 15). Further complicating traditional analyses is the increasing number of auxotrophs that do not grow on media required to perform the tests (1).
There is a need for new methods that can rapidly and accurately identify microorganisms, and molecular approaches using DNA probes based on chromosomal gene sequences have shown much promise (13, 28). Probes comprising short species-specific oligonucleotides complementary to ribosomal DNA (rDNA) have provided accurate identification of microorganisms (2, 10). Another approach uses specific oligonucleotides with PCR for the rapid identification of microorganisms. The advantage of the PCR method is that it is easy to use and is available to most laboratories. Recent reports have demonstrated that PCR methods can differentiate between related species of bacteria (4), filamentous fungi (21), and yeasts (5, 8, 11). Furthermore, large databases of partial rDNA sequences are becoming available for all types of microorganisms (7, 16), allowing the design of oligonucleotides that can distinguish target species from all other known microorganisms.
In this report we demonstrate the use of PCR for the rapid identification of human pathogenic yeasts. The system is based on 23- to 29-mer species-specific forward primers coupled with 26- to 30-mer partially specific reverse primers (primers specific for one to three species). A pair of universal primers is included so that four primers are present in each reaction mixture. In this PCR system, the universal primers produce a control DNA fragment in all reactions, and when chromosomal DNA of the target species is present, the species-specific primers produce a second smaller DNA fragment. We also describe a rapid DNA extraction method that yields purified DNA in about 1 h that is suitable for PCR. The PCR procedure reported here allows species to be identified in less than 1 day.
MATERIALS AND METHODS
Organisms.
The strains used in this study are listed in Table 1. All are maintained in the Agricultural Research Service Culture Collection (NRRL), National Center for Agricultural Utilization Research, Peoria, Ill.
TABLE 1.
Yeast species and strains used in this studya
| Speciesb | Strain designation
|
GenBank accession no. | |
|---|---|---|---|
| NRRL | CBSc | ||
| C. albicans* | Y-12983T | 562 | U45776 |
| Y-79 | 2730 | ||
| Y-81 | |||
| Y-82 | |||
| Y-107 | |||
| Y-116 | |||
| Y-302 | |||
| Y-477 | |||
| Y-6359 | |||
| Y-6943 | |||
| Y-17967 | |||
| Y-17968 | |||
| Y-17974 | |||
| Y-17976 | |||
| YB-3898 | 1912 | ||
| C. dubliniensis* | Y-17841T | 7987 | U57685 |
| Y-17512 | |||
| Y-17969 | |||
| Y-17971 | |||
| Y-17972 | |||
| Y-17973 | |||
| Y-17975 | |||
| C. glabrata* | Y-65T | 138 | U44808 |
| Y-1418 | |||
| Y-2242 | |||
| Y-17815 | |||
| YB-1333 | |||
| YB-3659 | |||
| YB-3660 | |||
| YB-4018 | |||
| YB-4319 | |||
| YB-4389 | |||
| C. haemulonii* | Y-6693T | 5149 | U44812 |
| Y-17799 | 5150 | ||
| Y-17800 | 7801 | ||
| C. haemulonii type II* | Y-17801T | 6915 | U44819 |
| Y-17802 | 7798 | ||
| C. lodderae | Y-17317T | 1924 | U45755 |
| C. maltosa* | Y-17677T | 5611 | U45745 |
| C. parapsilosis* | Y-12969T | 604 | U45754 |
| Y-543 | |||
| C. santamariae var. santamariae | Y-6656T | 4515 | U45794 |
| C. santamariae var. membranifaciens | Y-17647T | 5838 | U45785 |
| C. sojae* | Y-17909T | 7871 | U71070 |
| C. tropicalis* | Y-12968T | 94 | U45749 |
| Y-1552 | |||
| Y-5716 | 4913 | ||
| C. viswanathii* | Y-6660T | 4024 | U45752 |
| C. zeylanoides* | Y-1774T | 619 | U45832 |
| Candida sp.*d | Y-17456T | U45775 | |
| Lodderomyces elongisporus* | YB-4239T | 2605 | U45763 |
| Y-7681 | 5912 | ||
| Kluyveromyces delphensis | Y-2379T | U69576 | |
| K. yarrowii | Y-17763T | U68559 | |
| Pichia guilliermondii* | Y-2075T | 2030 | U45709 |
| Y-2076 | 2031 | ||
| Y-17466 | |||
| Y-17685 | |||
| Y-17818 | |||
| Y-17819 | |||
| Y-17820 | |||
| Y-17842 | |||
| Y-17857 | |||
| Y-17903 | |||
| Y-17904 | |||
| Y-17905 | |||
| Y-17970 | |||
| Zygosaccharomyces cidri | Y-12634T | 4575 | U84236 |
| Z. fermentati | Y-1559T | 707 | U84239 |
| Z. florentinus | Y-1560T | 746 | U72165 |
The nucleotide sequence of region D1/D2 of the large-subunit rDNA gene has been determined for each strain listed. In addition, a number of clinical isolates obtained from the CDC were tested with the primers developed in this study. The clinical isolates included strains of C. albicans (Y-27017, Y-27108, Y-27020, Y-27021, Y-27022, Y-27023, Y-27024, Y-27025, Y-27026, Y-27027, Y-27028, Y-27029, Y-27030, Y-27031, Y-27032, Y-27033, Y-27034, Y-27035, Y-27036, Y-27037, Y-27038, and Y-27039) and C. dubliniensis (Y-27014, Y-27015, Y-27016, and Y-27019). Of the nucleotide sequences of the D1/D2 regions for the CDC clinical isolates, only that for C. albicans Y-27022 has been determined.
Asterisks denote yeast species targeted for the design of specific primers.
CBS, Centraalbureau voor Schimelcultures, Delft, The Netherlands.
A new Candida species that will be described elsewhere.
Culture conditions and DNA isolation.
Culture and growth conditions have been previously described (16). For reference strains, DNA was isolated and purified by either a modification of the sodium dodecyl sulfate method of Raeder and Broda (23) as previously described (16) or a modification of the hexadecyltrimethyl-ammonium bromide (CTAB) buffer method (20a). In the CTAB method, lyophilized cells (50 to 100 mg) were placed in 1.5-ml microcentrifuge tubes and 700 μl of 2× CTAB buffer (100 mM Tris-HCl [pH 8], 1.4 M NaCl, 25 mM EDTA, 2% CTAB) was added. The cell mixture was vortexed to resuspend the cells, and 1 volume of phenol-chloroform was added. The mixture was vortexed again and centrifuged at 12,000 × g for 5 min. The upper aqueous phase was transferred to a new microcentrifuge tube, and the DNA was precipitated by adding an equal volume of isopropanol and centrifuging at 12,000 × g for 10 min. The DNA pellet was washed once in 70% ethanol and resuspended in 100 μl of TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). DNA concentrations of stock solutions were determined by ethidium bromide fluorescent quantitation (26) against known standards.
Rapid DNA isolation.
DNA from clinical isolates was obtained by a rapid-extraction method. Yeast cells were grown on YM agar slants (3 g of yeast extract, 3 g of malt extract, 5 g of peptone, 10 g of glucose, and 20 g of agar per liter of distilled water) at 25°C for about 48 h. One 4-mm loopful of yeast cells (about 2 × 108) was transferred to a 1.5-ml microcentrifuge tube containing 0.10 to 0.11 g (about a 50-μl volume) of 0.5-mm-diameter glass beads (Thomas Scientific, Swedesboro, N.J.) and 100 μl of 2× CTAB buffer. Microcentrifuge tubes were kept on ice between steps. The cell mixture was vortexed for 90 s with a VWR vortex mixer (Scientific Industries, Bohemia, N.Y.) at the maximum speed setting, and an equal volume of buffer-saturated phenol was added. The solution was mixed by vortexing and was centrifuged at 12,000 × g for 30 s. The supernatant was transferred to a new microcentrifuge tube, and DNA was purified with the Prep-A-Gene DNA purification kit (Bio-Rad Laboratories, Hercules, Calif.) as recommended by the manufacturer with the exception of the amount of DNA binding matrix used. In order to bind a controlled amount of DNA, a small amount of matrix (1.0 μl) was used per tube. DNA was redissolved in 50 μl of TE buffer, and 2.0 μl of this mixture was used per PCR assay. Results showed that a consistent and uniform concentration of DNA was obtained in each tube, about 10 ng/μl.
Oligonucleotide probes, phylogenetic relationships, and DNA sequences.
Oligonucleotides used as probes were synthesized in an ABI 292 DNA/RNA synthesizer (Applied Biosystems, Foster City, Calif.) as recommended by the manufacturer. The sequences of specific forward primers were checked against basidiomycetous and ascomycetous yeast databases to confirm that each primer represented a unique sequence. Duplex dissociation temperatures (Tds) were determined with the computer program of Rychlik and Rhoads (25). Species relationships were analyzed with the maximum-parsimony program of PAUP, version 3.1.1 by a simple heuristic search (29). Confidence limits were estimated from bootstrap analyses. DNA sequence reactions have been previously described (16), and sequence data are available as a computer file.
PCR methods.
PCRs were performed by “hot-start” or standard procedures. Standard reaction mixtures were prepared in various volumes containing 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.0 mM MgCl2, 0.4 mM concentrations of dATP, dCTP, dGTP, and dTTP, an 0.5 μM concentration of each oligonucleotide primer, and 5.0 U of Taq DNA polymerase or Tth DNA polymerase (Thermolase; Pharmacia Biotech) per 100 μl. The mixture was divided into 24-μl aliquots, and 1.0 μl of DNA was added (10 ng/μl). PCRs were performed in either a Perkin-Elmer GeneAmp PCR System 2400 or a System 9600. Tubes were incubated at 94°C for 4 min, and amplifications were performed for 35 cycles, with denaturation at 94°C for 20 s, annealing at 67°C for 1 min, and extension at 72°C for 20 s, followed by 72°C for 4 min. In the hot-start procedure, magnesium-free reaction mixtures were prepared and wax beads containing magnesium were added (HotWax Mg2+ beads; Invitrogen, San Diego, Calif.). Reactions were hot started when the wax beads melted at 68 to 72°C, releasing the magnesium and activating the polymerase. DNA fragments produced by PCR were visualized on 1.0 to 1.4% ethidium bromide-stained agarose gels in TAE buffer (0.04 M Tris-acetate [pH 8.0], 0.001 M EDTA).
RESULTS
Design of species-specific primers.
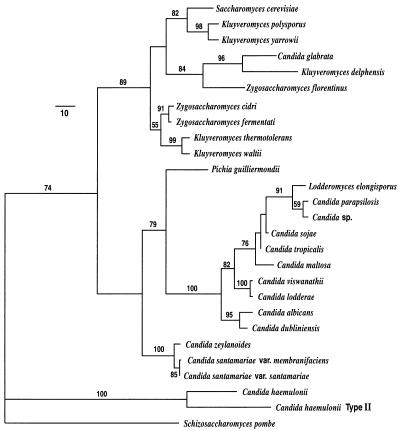
The four-primer PCR system distinguished between 14 species of pathogenic yeasts and closely related species (Table 1, Fig. 1). A phylogenetic analysis (Fig. 1) shows that nine species, Lodderomyces elongisporus, Candida parapsilosis, Candida sp., C. sojae, C. tropicalis, C. maltosa, C. viswanathii, C. albicans, and C. dubliniensis, form a closely related clade (C. albicans clade) with similar sequences. C. lodderae is considered a synonym of C. viswanathii and was not treated as a separate species (16). The other five species, Pichia guilliermondii, C. glabrata, C. zeylanoides, C. haemulonii, and C. haemulonii type II are more divergent from one another and from the other test species with the exception of C. zeylanoides, which is closely related to C. santamariae (16).
FIG. 1.
Phylogenetic tree of the Candida species studied and their nearest neighbors as represented by one of two most parsimonious trees derived from maximum-parsimony analysis. The phylogram was calculated from the divergence in large-subunit region D1/D2. Branch lengths are proportional to nucleotide differences as indicated on the marker bar. Numbers given on branches are the frequencies (expressed as percentages) with which a branch appeared in 100 bootstrap replicates. Frequencies under 50% are not shown. Tree length = 705; consistency index = 0.613; retention index = 0.749; rescaled consistency index = 0.459; homoplasy index = 0.387. Schizosaccharomyces pombe served as the outgroup species in the analysis.
Forward and reverse species-specific primers were designed from a database containing sequences comprising the 600-nucleotide variable region (D1/D2) at the 5′ end of the large-subunit (26S) rDNA gene (16). The database includes sequences for all known clinically important Candida species and selected reference species. Initial attempts to design species-specific forward primers coupled with a single universal reverse primer failed to distinguish all species. The universal reverse primers used were NL4 (5′-GGTCCGTGTTTCAAGACGG), NL4A (5′-GCGACTTAAGATCATTATGCC), and NL4A1 (5′-GCGACTTAAGATCATTATGCCAACATCC). The D1/D2 region did not show sufficient divergence to distinguish species of the C. albicans clade with a PCR system that used only specific forward primers. It became apparent that species-specific reverse primers would also be necessary. Reverse primers were designed on the basis of the sequence from an area as close as possible to the 3′ end of the D2 region since it was necessary for the PCR system to yield DNA fragments large enough to be easily visualized on standard agarose gels. An examination of the 3′ end indicated that there was insufficient diversity to design a specific primer for each species. Partially specific reverse primers could be designed for one to three species and are indicated in Table 2. For example, reverse primer NL4LEL1 was specific for L. elongisporus, C. parapsilosis, and Candida sp.
TABLE 2.
Primer pairs used to distinguish yeast species
| Species and primers useda | Sequenceb | Primer length (ntc) | Td (°C) | Fragment lengthd (bp) |
|---|---|---|---|---|
| L. elongisporus | ||||
| LEL4* | GGAGAATTGCGTAGGAATGTGGCT | 24 | 72.5 | 114 |
| NL4LEL1** | AGATCATTATGCCAACATCCTAGGCCG | 27 | 76.2 | |
| C. parapsilosis | ||||
| CPA4* | GCATCAGTTTGAGCGGTAGGATAAGC | 26 | 73.5 | 132 |
| NL4LEL1** | AGATCATTATGCCAACATCCTAGGCCG | 27 | 76.2 | |
| Candida sp.e | ||||
| CWO2* | GCATCAGTTTGGGCGGTAGGACG | 23 | 76.4 | 132 |
| NL4LEL1** | AGATCATTATGCCAACATCCTAGGCCG | 27 | 76.2 | |
| C. sojae | ||||
| CSO11* | AGCATCAGTTTGGGCGGTAGGAGG | 24 | 76.3 | 136 |
| NL4CMA1** | AAGATCATTATGCCAACATCCTAGGTAAT | 29 | 70.4 | |
| C. maltosa | ||||
| CMA3* | TTTGGACGGTAGGACAATTGCGGC | 24 | 74.7 | 128 |
| NL4CMA1** | AAGATCATTATGCCAACATCCTAGGTAAT | 29 | 70.4 | |
| C. tropicalis | ||||
| CTR22 | TGGGCGGTAGGAGAATTGCGTTA | 23 | 74.5 | 126 |
| NL4CTR1** | TAAGATCATTATGCCAACATCCTAGGTATA | 30 | 70.0 | |
| C. viswanathii | ||||
| CVI2* | TTGGGCGGCAGGACAATCGCGTG | 23 | 84.0 | 126 |
| NL4CTR1** | TAAGATCATTATGCCAACATCCTAGGTATA | 30 | 70.0 | |
| C. albicans | ||||
| CAL5* | TGTTGCTCTCTCGGGGGCGGCCG | 23 | 86.0 | 175 |
| NL4CAL** | AAGATCATTATGCCAACATCCTAGGTAAA | 29 | 71.2 | |
| NL5CAL*** | AGATCATTATGCCAACATCCTAGGTTAAA | 29 | 71.2 | |
| C. dubliniensis | ||||
| CDU2* | AGTTACTCTTTCGGGGGTGGCCT | 23 | 73.2 | 175 |
| NL4CAL** | AAGATCATTATGCCAACATCCTAGGTAAA | 29 | 71.2 | |
| P. guilliermondii | ||||
| PGU1* | GCCTTGCCTTCGTGGCGGGGTGA | 23 | 84.1 | 175 |
| NL4PGU** | TGGGATCATTATGCCAGCATCCTTGAT | 27 | 77.3 | |
| C. zeylanoides | ||||
| CZE2* | GTTGTAATTTGAAGAAGGTAACTTTGGTT | 29 | 68.7 | 336 |
| NL5CZE** | CGTGGAACCCAACCAAAAAGGAAGGG | 26 | 79.8 | |
| C. haemulonii | ||||
| CHA1* | TTGCACCCAGACACGGCCTTCTG | 23 | 77.9 | 163 |
| NL4CHA** | AACCATTGCGCCAGCATCCTTATTGCT | 27 | 80.0 | |
| C. haemulonii type II | ||||
| CHATII1* | GAAGGGCTTGCAGCTAGACAACT | 23 | 69.0 | 156 |
| NL4CHATII** | AGCCATTGCGCCAGCATCCTTCAAATC | 27 | 80.7 | |
| C. glabrata | ||||
| CGL1* | TGGGCTTGGGACTCTCGCAGCTC | 23 | 78.5 | 173 |
| NL4CGL1** | TAACCATTATGCCAGCATCCTAGATAAC | 28 | 70.5 |
*, species-specific forward primer; **, partially species-specific reverse primer; ***, partially species-specific reverse primer used in the five-primer system.
All sequences are listed 5′ to 3′.
nt, nucleotides.
Size of the DNA fragment produced by the forward primer and the corresponding reverse primer in a PCR with the target chromosomal DNA.
A new Candida species that will be described elsewhere.
Table 2 also shows species-specific forward primers which, when combined with the corresponding partially specific reverse primers, were able to distinguish individual yeast species. In a PCR, these primer pairs yielded small DNA fragments of between 114 and 336 bases when the corresponding species chromosomal DNA was present. The forward primers rely on single-base differences or the introduction of a base mismatch at the 3′ end of the primer. Table 3 compares the forward primers to the corresponding chromosomal DNAs for nine yeast species. The comparison indicates the types of terminal mismatches that yield PCR fragments only when the target species chromosomal DNA is present. Also included in Table 3, for comparison, are some primers that were not species specific (see Discussion section). With the exception of that of C. zeylanoides, the rDNA region chosen for design of the forward primers was an area between 105 and 182 bases upstream from the 3′ end of the D1/D2 region. There were sufficient single-base differences in this region among species to allow the design of specific primers. C. zeylanoides proved difficult to distinguish from C. santamariae, and a different area, which is 489 bases from the 3′ end, had to be chosen.
TABLE 3.
Influence of primer design on species specificity
| Species chromosomal DNA and primer(s) | Species specificitya | Sequenceb | No. of oligonucleotides 5′ to 3′c |
|---|---|---|---|
| L. elongisporus | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGA-GCGGTAGGAGAATTGCGTAGGAATGTGGCT | ||
| LEL4 | + | GGAGAATTGCGTAGGAATGTGGCT | 122 |
| C. parapsilosis | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGA-GCGGTAGGATAAGTGCAAAGAAATGTGGCA | ||
| CPA3 | − | AGCATCAGTTTGA-GCGGTAGGATAAG | 141 |
| CPA4 | + | GCATCAGTTTGA-GCGGTAGGATAAGc | |
| Candida sp.d | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGG-GCGGTAGGACAATTGCAAAGAAATGTGGCA | ||
| CWO1 | − | AGCATCAGTTTGG-GCGGTAGGAC | 144 |
| CWO2 | + | GCATCAGTTTGG-GCGGTAGGACg | |
| C. sojae | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGG-GCGGTAGGAGAATTGCGATGGAATGTGGCA | ||
| CSO11 | + | AGCATCAGTTTGG-GCGGTAGGAGg | 143 |
| C. maltosa | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGG-ACGGTAGGACAATTGCGGTGGAATGTGGCA | ||
| CMA3 | + | TTTGG-ACGGTAGGACAATTGCGGc | 135 |
| C. tropicalis | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGG-GCGGTAGGAGAATTGCGTTGGAATGTGGCA | ||
| CTR2 | − | TTG-GCGGTAGGAGAATTGCGTT | 132 |
| CTR21 | +e | TGG-GCGGTAGGAGAATTGCGTTt | |
| CTR22 | + | TGG-GCGGTAGGAGAATTGCGTTa | |
| C. viswanathii | |||
| Chromosomal DNA | GGGCCAGCATCAGTTTGG-GCGGCAGGACAATCGCGTGGGAATGTGGCA | ||
| CVI2 | + | TTGG-GCGGCAGGACAATCGCGTG | 132 |
| C. albicans | |||
| Chromosomal DNA | GGGCCAGCATCGGTTTGGAGCGGCAGGATAATGGCGGAGGAATGTGGCA | ||
| CAL4 | − | GGCCAGCATCGGTTTGGAGCGGC | 146 |
| C. dubliniensis | |||
| Chromosomal DNA | GGGCCAGCATCGGTTTGGAGCGGTAGGATAATGGCGGGGGAATGTGGCA | ||
| CDU1 | − | GGCCAGCATCGGTTTGGAGCGGT | 146 |
| C. albicans | |||
| Chromosomal DNA | TTGGTATTTTGCATGTTGCTCTCTCGGGGGCGGCCGCTGCGGTTTACCG | ||
| CAL5 | + | TGTTGCTCTCTCGGGGGCGGCCG | 182 |
| C. dubliniensis | |||
| Chromosomal DNA | TTGGTATTTTGCAAGTTACTCTTTCGGGGGTGGCCTCTGCGGTTTACCG | ||
| CDU2 | + | AGTTACTCTTTCGGGGGTGGCCT | 182 |
+, species-specific primer; −, primer that is not species-specific and that yielded PCR DNA fragments with chromosomal DNAs from two or more species.
Terminal nucleotides in lowercase represent deliberately introduced mismatches. C. albicans and C. dubliniensis have a base insertion (A at position 129 of C. albicans), and a gap has been inserted in the sequences of the first seven species in order to align the sequences (see reference 16 for aligned sequences of all species in this study).
Number of oligonucleotides from the 5′ end of the primer to the 3′ end of the D1/D2 sequence. Apparent discrepancies among starting positions and distances to the 3′ end as indicated in Table 2 (PCR fragment lengths) are due to insertions and deletions downstream from the starting position among the different species.
A new Candida species that will be described elsewhere.
CTR21 was able to distinguish C. tropicalis but consistently did not produce a DNA fragment as intense as that produced by CTR22.
Universal fungal primers used to yield control DNA fragments.
Universal fungal primers were based on conserved nucleotide sequences from the small-subunit (18S) rRNA gene. Previously described universal primers, NS1 to NS8 (33), did not yield PCR DNA fragments with a number of species at the reaction temperatures used in this study. New primers which were able to amplify rDNA from all strains listed in Table 1 at annealing temperatures of at least 67°C had to be developed. The new universal primers were developed from analyses of all available fungal rRNA sequences. A number of conserved regions were chosen, and a series of primers of various lengths was developed for each region. The most successful combination was the 40-mer forward primer NS395F (5′-AGAAACGGCTACCACATCCAAGGAAGGCAGCAGGCGCGCA) and the 42-mer reverse primer NS1654R (5′-CAATCGGTACTAGCGACGGGCGGTGTGTACAAAGGGCAGGGA). NS395F has a Td of 96.8°C, and NS1654R has a Td of 95.2°C. In developing these primers, many hundreds of combinations were tested. Combinations of shorter-length primers with lower Tds failed to yield PCR DNA fragments for some strains at temperatures above 65°C or failed to produce a control band when combined with species-specific primers at 67°C in a four-primer system. Regardless of the 18S rRNA gene region chosen, only combinations of primers with high Tds (around 95°C or above) yielded DNA fragments from all strains in a four-primer system at temperatures of 67 to 68°C. Primers NS395F and NS1654R yielded control DNA fragments of between 1,200 and 1,300 nucleotides, depending on the species of chromosomal DNA used. The control DNA fragments were easily distinguished from the smaller DNA fragments produced by the species-specific primers.
Identification of reference species and clinical isolates by PCR.
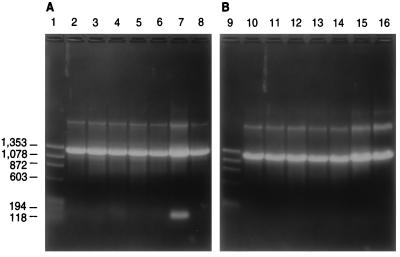
In this study, we found no noticeable differences between hot-start and standard PCR methods or between Taq DNA polymerase and Thermolase polymerase. All strain identifications were performed at an annealing temperature of 67°C with Taq DNA polymerase by standard PCR methods. Species-specific primers (forward and reverse pairs) produced distinct DNA fragments when chromosomal DNA from the target species was present and did not yield DNA fragments with chromosomal DNA from any other species. Figure 2 shows a series of PCRs; the PCR mixtures all contained a set of universal primers, NS395F and NS1654R, and a set of primers specific for C. albicans, CAL5 and NL4CAL, but differed in that each contained a different species chromosomal DNA. As shown, only the reaction mixture containing C. albicans DNA produced two bands, the large control fragment and the smaller species-specific fragment. The specific primer set was able to distinguish all other species including C. dubliniensis, which is closely related to C. albicans. Reactions with all other specific pairs gave similar results in that they yielded two distinct DNA bands only with the target species chromosomal DNA (data not shown). Chromosomal DNAs from several species could be mixed without affecting the specificities of the primers, suggesting that clinical isolates can be identified in mixed cultures. For instance, a DNA fragment was produced if the target species was part of a mixture containing chromosomal DNAs from two to four different species, but no fragment was produced if the target species DNA was not included in the mixture (data not shown).
FIG. 2.
Agarose gel electrophoresis of PCR products from 14 species of yeasts by using the four-primer system. All reaction mixtures for panels A and B contain universal primers NS395F and NS1654R and C. albicans species-specific primers CAL5 and NL4CAL as well as chromosomal DNAs from the following yeast strains: L. elongisporus YB-4239 (lane 2), C. parapsilosis Y-12969 (lane 3), Candida sp. Y-17456 (lane 4), C. sojae Y-17909 (lane 5), C. tropicalis Y-12968 (lane 6), C. albicans Y-12983 (lane 7), C. dubliniensis Y-17841 (lane 8), C. maltosa Y-17677 (lane 10), C. viswanathii Y-6660 (lane 11), P. guilliermondii Y-2075 (lane 12), C. zeylanoides Y-1774 (lane 13), C. haemulonii Y-6693 (lane 14), C. haemulonii type II Y-17801 (lane 15), C. glabrata Y-65 (lane 16). PCR products were visualized on 1.0% agarose gels. Lanes 1 and 9 contain ΦΧ174 replicative-form DNA cut with HaeIII. Molecular weight markers (in base pairs) are indicated.
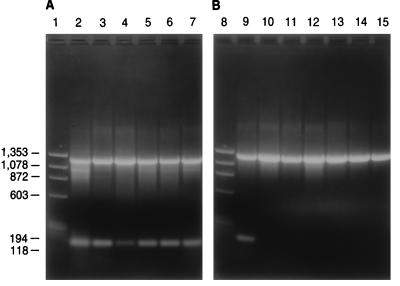
Figure 3 indicates that different strains of a species give the same pattern of DNA fragments. With the C. albicans probes, six strains of C. albicans yielded two bands, while all six strains of C. dubliniensis yielded only the control band. Modifying the PCR system to incorporate a rapid DNA extraction procedure (see Materials and Methods) yielded similar results. Identical DNA fragments were obtained regardless of the method of DNA extraction. With the use of the quick-extraction procedure, DNA can be isolated in about 1 h, and species identification can be performed in less than 1 day.
FIG. 3.
Agarose gel electrophoresis showing PCR products from six strains of C. albicans and six strains of C. dubliniensis. Reactions and conditions in panels A and B are similar to those described in the legend for Fig. 2 with the exception that chromosomal DNAs from the following strains were used: C. albicans Y-12983 (lane 2), C. albicans Y-79 (lane 3), C. albicans YB-3898 (lane 4), C. albicans Y-17967 (lane 5), C. albicans Y-17968 (lane 6), C. albicans Y-17974 (lane 7), C. albicans Y-12983 (lane 9), C. dubliniensis Y-17841 (lane 10), C. dubliniensis Y-17512 (lane 11), C. dubliniensis Y-17969 (lane 12), C. dubliniensis Y-17971 (lane 13), C. dubliniensis Y-17972 (lane 14), C. dubliniensis Y-17973 (lane 15). Lanes 1 and 8 contain ΦΧ174 replicative-form DNA cut with HaeIII. Molecular weight markers (in base pairs) are indicated.
Specific primer pairs were able to distinguish all strains of a species tested with the exception of two strains of C. albicans. NL4CAL and CAL5 identified 14 strains of C. albicans obtained from the Agricultural Research Service Culture Collection and 21 clinical isolates obtained from the Centers for Disease Control and Prevention (CDC) (Table 1). However, strains NRRL Y-17976 and NRRL Y-27022 (CDC strain) yielded a species-specific DNA fragment in only about 50% of the reactions. Sequence data revealed that both strains had a base insertion, an adenine, at position 33 from the 3′ end of the D1/D2 region. Position 33 is included in the DNA sequence used to design reverse primer NL4CAL. The insertion is four bases from the 3′ end of NL4CAL and apparently is enough of a modification to affect PCR fragment formation. To overcome the problem of strain variations in C. albicans, another reverse primer, NL5CAL, which incorporated the adenine base insertion, was added to the reaction mixture. In this case, a five-primer mixture containing equal molar amounts of NL4CAL and NL5CAL was used to distinguish all strains of C. albicans available to us. The five-primer system was used for all further testing of C. albicans strains.
DISCUSSION
The PCR system described is rapid and easy to interpret and may be used with mixed cultures; it was able to provide accurate identification of 14 medically important yeasts. To our knowledge, this is the first study that has shown that the PCR method can distinguish a large number of closely related species. In this study, we compared rDNA sequences and then designed primers to use naturally occurring mismatches at the 3′ end or we introduced a deliberate mismatch at the 3′ terminus. Internal mismatches were tested, but the results were often not predictable. The importance of primer termini has been discussed by Huang et al. (12) and Fell (8), who found that primer specificity is enhanced if a mismatched base is placed at the 3′ end. Fell (8) concluded that when comparing closely related species with sequences containing only two or three noncontiguous base differences, the oligonucleotide primers used must differ at the 3′ end.
Partially specific reverse primers were used to reduce the number of species to be distinguished by any single forward primer. With reverse primer NL4LEL1, for instance, the three-base sequence at the 3′ end differs from the corresponding sequences of the other reverse primers. In this case, only differences between L. elongisporus, C. parapsilosis, and Candida sp. were considered in designing the forward primers. Sequence position 99 in L. elongisporus had the pyrimidine base thymine versus the purine base adenine for the other two species. This single-base difference allowed forward primer LEL4 to differentiate L. elongisporus. A PCR DNA fragment was produced only with DNA from L. elongisporus, not with DNA from any other strain listed in Table 1. Primers CWO1 and CWO2 were designed to distinguish the new Candida sp. Sequence position 122 in this Candida sp. had a cytosine instead of the thymine in the corresponding position of C. parapsilosis and the guanine in L. elongisporus. Primer CWO1, using this mismatch, did not differentiate between Candida sp. and C. parapsilosis. These species had base differences involving a pyrimidine (cytosine) versus another pyrimidine (thymine). In general, terminal single-base mismatches which replaced a base with another base of the same class of bases were not enough to distinguish species. A specific PCR DNA fragment was synthesized if DNA from Candida sp. or C. parapsilosis was present. With CWO2, a deliberate mismatch was added to the 3′ end at position 121, and a guanine, which did not match the base of any of the three species, was added. CWO2 had a one-base mismatch compared with the sequence of Candida sp. and two mismatches compared with the sequences of the other two species. CWO2 yielded a PCR DNA fragment with Candida sp. DNA but not with DNA from the other two species.
The base differences, however, were not always so obvious as those discussed above, and the abilities of primer pairs to differentiate species often had to be determined experimentally. With reverse primers NL4CTR1 and NL4CAL, there was only one internal base difference (second base from the 3′ end), but when they were combined with forward primers they were able to identify the four species in the two groups. Other primers gave results similar to those for the L. elongisporus group. Primers CAL4 and CDU1, using position 124, which involved pyrimidine mismatches, failed to differentiate between C. albicans and C. dubliniensis, while CAL5 and CDU2, using position 160, which involved purine versus pyrimidine mismatches, distinguished between the two species. Primers CTR21 and CTR22 both distinguished C. tropicalis and demonstrated the use of different deliberately mismatched bases at the 3′ end. The purine guanine in sequence position 110 of C. tropicalis was replaced by another purine, adenine, in CTR22 and with the pyrimidine thymine in CTR21. Both primers yielded specific PCR DNA fragments with C. tropicalis DNA; however, CTR22 consistently yielded larger amounts of the fragment than CTR21. In designing deliberate mismatches, the terminal base was replaced with another base of the same class. A similar strategy was used with CPA4, CMA3, and CSO11.
The PCR system used uniform conditions for all reactions, thereby eliminating the need to make continual species-specific adjustments. The primers used were designed to distinguish among species when an annealing temperature of 67°C, which, in our study, was the lowest temperature that gave consistent results, was used. Based on earlier experiments, primers CPA3 and CTR2 were expected to differentiate C. parapsilosis and C. tropicalis, respectively. With the conditions used in this study, these primers were not species specific. For example, primer CTR2 yielded PCR DNA fragments with both C. tropicalis and C. viswanathii DNA. As the annealing temperature was raised to 68 to 69°C, however, both primers yielded DNA fragments only with the target species DNA, demonstrating that higher annealing temperatures can be used to increase primer specificity. Temperature also affected DNA fragment yield. Increasing temperature decreased the intensity of the DNA fragments on agarose gels to the point that they were not easily visualized. During the course of this research many of the original primers had to be redesigned and primer lengths and Tds had to be increased. Further work is needed to determine if higher annealing temperatures and longer-length primers are more advantageous.
Primer design relied mainly on a sequence of about 182 bases at the 3′ end of the D1/D2 region of the large-subunit rDNA gene. This short region alone contained sufficient divergence to design specific primer pairs for the nine closely related species of the C. albicans clade. With databases of rDNA gene sequences increasing rapidly, designing specific primer pairs should become easier. Developing primers to identify groups of microorganisms, i.e., genera, and then combining that system with species-specific primers would allow most laboratories to quickly and accurately identify microorganisms. However, the user of molecular probes must be aware of the potential impact of single-base changes in the target DNA. When identification is by sequencing, these substitutions are easy to evaluate, but for probes, a single change may markedly impact specificity, as we demonstrated for divergent strains of C. albicans.
Molecular identification techniques can be particularly useful to clinical laboratories since the number of opportunistic fungal pathogens is growing (6). At present, yeast infections are usually treated as a general fungal infection and agents such as the polyene amphotericin B or the newer azole drugs, which are intended to control a broad array of fungi, are used (31). The treatment is usually continued for an extended period of time. These agents are not always successful since the widespread use of these generalized drugs has resulted in the rapid development of antifungal drug resistance (32). An analysis of clinical isolates indicates that resistance is due not only to resistant strains of C. albicans but also to an increasing number of non-C. albicans strains. Various yeast species appear to develop resistance to the commonly used drugs at frequencies much higher than that for C. albicans. For instance, C. tropicalis and C. parapsilosis, which are both associated with endocarditis, are inherently resistant or can quickly develop resistance to polyenes and azole drugs (3, 9, 30). C. dubliniensis, which is associated with oral candidiasis, has been shown to develop stable fluconazole resistance at a high rate after exposure to azoles (18). The MICs of azole drugs for other yeast species such as C. glabrata, which is associated with cancer and bone marrow transplant patients, are significantly higher than those for C. albicans (20, 24). The present levels of drug doses used can suppress the growth of sensitive strains but allow the growth of the more resistant species. These organisms, which can quickly develop resistance or for which the MICs of the presently used drugs are higher, probably account for a large number of resistant yeast infections in certain populations.
Drug resistance is a major problem in treating yeast infections (32). Research in many laboratories is oriented to developing new drugs or drug delivery systems, but just as important an approach is the quick and accurate identification of disease-causing yeasts. This allows the delivery of the most effective drug and the use of the proper dose of drug for any particular infection. Molecular methods can give definitive identification with same-day results and can provide valuable information to physicians for patient management.
ACKNOWLEDGMENTS
We thank Christie J. Robnett for expert technical assistance and Stephen W. Peterson for expert advice.
REFERENCES
- 1.Ahearn D G. Yeasts pathogenic for humans. In: Kurtzman C P, Fell J W, editors. The yeasts, a taxonomic study. 4th ed. Amsterdam, The Netherlands: Elsevier Science B. V.; 1998. pp. 9–14. [Google Scholar]
- 2.Amann R I, Strombley J, Devereux R, Key R, Stahl D A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992;58:614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey G P. Azole antifungal agents. Clin Infect Dis. 1992;14:5161–5169. doi: 10.1093/clinids/14.supplement_1.s161. [DOI] [PubMed] [Google Scholar]
- 4.Candian U, Hofelein C, Luthy J. Polymerase chain reaction with additional primers allows identification of amplified DNA and recognition of specific alleles. Mol Cell Probes. 1992;6:13–19. doi: 10.1016/0890-8508(92)90066-7. [DOI] [PubMed] [Google Scholar]
- 5.Crampin A C, Matthews R C. Application of the polymerase chain reaction to the diagnosis of candidosis by amplification of an HSP-90 gene fragment. J Med Microbiol. 1993;39:233–238. doi: 10.1099/00222615-39-3-233. [DOI] [PubMed] [Google Scholar]
- 6.Dixon D M, Fromtling R A. Morphology, taxonomy, and classification of the fungi. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 699–708. [Google Scholar]
- 7.Fell J W, Statzell-Tallman A, Lutz M J, Kurtzman C P. Partial rRNA sequences in marine yeasts: a model for identification of marine eukaryotes. Mol Mar Biol Biotechnol. 1992;1:175–186. [PubMed] [Google Scholar]
- 8.Fell J W. Rapid identification of yeast species using three primers in a polymerase chain reaction. Mol Mar Biol Biotechnol. 1993;2:174–180. [PubMed] [Google Scholar]
- 9.Galgiani J N, Reiser J, Brass C, Espinel-Ingroff A, Gordon M A, Kerkering T M. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother. 1987;31:1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannoni S J, DeLong E F, Olsen G J, Pace N R. Phylogenetic group-specific oligonucleotide probes for identification of single microbial cells. J Bacteriol. 1988;170:720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes A R, Cannon R D, Shepherd M G, Jenkinson H F. Detection of Candida albicans and other yeasts in blood by PCR. J Clin Microbiol. 1994;32:228–231. doi: 10.1128/jcm.32.1.228-231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M, Arnheim N, Goodman M F. Extension of base mispairs by Taq polymerase: implications for single nucleotide discrimination in PCR. Nucleic Acids Res. 1992;20:4567–4573. doi: 10.1093/nar/20.17.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffnagle K E, Gander R M. Evaluation of Gen-Probe’s Histoplasma capsulatum and Cryptococcus neoformans AccuProbes. J Clin Microbiol. 1993;31:419–421. doi: 10.1128/jcm.31.2.419-421.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzman C P, Phaff H J. Molecular taxonomy of yeasts. In: Rose A H, Harrison J S, editors. The yeasts. 2nd ed. Vol. 1. New York, N.Y: Academic Press; 1987. pp. 63–94. [Google Scholar]
- 15.Kurtzman C P, Robnett C J. Molecular relationships among hyphal ascomycetous yeasts and yeastlike taxa. Can J Bot. 1995;73:S824–S830. [Google Scholar]
- 16.Kurtzman C P, Robnett C J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Land G A, Salkm I F, El-Zaatari M, McGinnis M R, Hashem G. Evaluation of the Baxter-MicroScan 4-hour enzyme-based yeast identification system. J Clin Microbiol. 1991;29:718–722. doi: 10.1128/jcm.29.4.718-722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and nonHIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 699–737. [Google Scholar]
- 20.Odds F C. Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother. 1993;31:463–471. doi: 10.1093/jac/31.4.463. [DOI] [PubMed] [Google Scholar]
- 20a.O’Donnell, K. Personal communication.
- 21.O’Donnell K, Gray L E. Phylogenetic relationships of the soybean sudden death syndrome pathogen Fusarium solani f. sp. phaseoli inferred from rDNA sequence data and PCR primers for its identification. Mol Plant-Microbe Interact. 1995;8:709–716. doi: 10.1094/mpmi-8-0709. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller M A, Preston T, Bale M, Koontz F P, Body B A. Comparison of the Quantum II, API Yeast Ident, and AutoMicrobic systems for identification of clinical yeast isolates. J Clin Microbiol. 1988;26:2054–2058. doi: 10.1128/jcm.26.10.2054-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raeder U, Broda P. Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol. 1985;1:17–20. [Google Scholar]
- 24.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rychlik W, Rhoads R E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing, and in vitro amplification of DNA. Nucleic Acids Res. 1989;17:8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.St. Germain G, Beauchesne D. Identification of the Microscan rapid yeast identification panel. J Clin Microbiol. 1991;29:2296–2299. doi: 10.1128/jcm.29.10.2296-2299.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockman L, Clark K A, Hunt J M, Roberts G D. Evaluation of commercially available acridinium ester-labeled chemiluminescent DNA probes for culture identification of Blastomyces dermatitidis, Coccidioides immitis, Cryptococcus neoformans, and Histoplasma capsulatum. J Clin Microbiol. 1993;31:845–850. doi: 10.1128/jcm.31.4.845-850.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swofford D L. PAUP: phylogenetic analysis using parsimony, version 3.1.1. Champaign, Ill: Illinois Natural History Survey; 1993. [Google Scholar]
- 30.Walsh T J, Pizzo A. Treatment of systemic fungal infections: recent progress and current problems. Eur J Clin Microbiol Infect Dis. 1988;7:460–475. doi: 10.1007/BF01962595. [DOI] [PubMed] [Google Scholar]
- 31.White T C. Antifungal drug resistance in Candida albicans. ASM News. 1997;63:427–433. [Google Scholar]
- 32.White T C, Bowden R A, Marr K A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press; 1990. pp. 315–322. [Google Scholar]