Abstract
The aim of this study was to investigate whether the use of nucleoside reverse transcriptase inhibitors (NRTIs) impacts the incidence of prediabetes or type 2 diabetes mellitus (T2DM) or the progression from prediabetes to T2DM in people living with HIV (PLWH). We conducted a retrospective cohort study using the U.S. Veterans Health Administration database among adult patients with an HIV diagnosis from the year 2000 until 2021 to determine the incidence of prediabetes and further progression to T2DM among NRTI exposed and unexposed patients. A multistate model was used to evaluate progression from normoglycemia to prediabetes and then to T2DM, and covariate adjustment with the Cox proportional hazards model was used to estimate the hazard ratios. Among 32,240 veterans diagnosed with HIV, prediabetes and T2DM were observed among 20.2% and 20.7% of patients, respectively. Among those diagnosed with prediabetes, 31.8% progressed to T2DM. Patients exposed to NRTIs at any time (86.6%), had a reduced risk of prediabetes [HR 0.50 (0.47–0.53 95% CI)] and among prediabetics, a lower risk of progression to T2DM [HR 0.73 (0.63–0.85 95% CI)] when compared to patients who never used NRTIs. In summary, NRTIs may reduce the risk of developing prediabetes and the progression from prediabetes to T2DM in PLWH.
Keywords: database, HIV, inflammasome, NRTI, prediabetes, T2DM, retrospective
Graphical Abstarct

In this 20-year retrospective analysis of a nationwide database, people living with HIV who were exposed to nucleoside reverse transcriptase inhibitors (NRTIs) had a decreased risk of developing prediabetes or progressing from prediabetes to type 2 diabetes mellitus.
1. INTRODUCTION
Prediabetes is a metabolic disease characterized by hyperglycemia due to a number of pathophysiologic factors including insulin hypersecretion, insulin resistance, and impaired incretin action (1). It is estimated to affect 88 million adults in the United States (2); however, only 15% are aware of this condition (2) and as many as 70% of these individuals will progress to type 2 diabetes mellitus (T2DM) (3). The prevalence of prediabetes and T2DM is rising in both developed and developing nations (4). Prediabetes and T2DM have historically been diagnosed by blood glucose tests, including impaired fasting glucose test and oral glucose tolerance test, and are currently diagnosed with hemoglobin A1c (HbA1c) testing, which measures glycated hemoglobin levels and indicates average blood glucose levels over a 2–3-month timespan prior to testing (5). Monitoring and controlling HbA1c level in patients with diabetes is crucial, as an elevated HbA1C predisposes patients to devastating microvascular complications, such as retinopathy, neuropathy, and nephropathy, and macrovascular complications, such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease (6).
Unfortunately, only a few modalities have been shown to alter the development of prediabetes or T2DM and subsequent complications. Improved glycemic control has been shown to slow, or even halt, the progression of microvascular and macrovascular complications. For patients with prediabetes, lifestyle changes aimed at weight loss, low-fat diet, and physical activity are the primary method of preventing progression to diabetes by combating hyperglycemia. Metformin, a biguanide, is the first-line agent in treating T2DM due to its efficacy and favorable side-effect profile, including a low risk of hypoglycemia following administration. Thiazolidinediones (glitazones), α-glucosidase inhibitors, orlistat, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide (GLP-1) analogues, and basal insulin are other options for medically managing diabetes (3). However, due to the limited efficacy of current disease-modifying diabetes medications, additional research is needed on this topic: specifically, the identification of new biological targets for the management of this endocrine disease.
Nucleoside reverse transcriptase inhibitors (NRTIs) are synthetic nucleoside analogues that function as competitive inhibitors of the viral reverse transcriptase (RT) enzyme. Due to their potent antiviral activity, NRTIs are approved for the treatment of human immunodeficiency virus (HIV) and Hepatitis B. In the treatment of HIV, NRTIs are a cornerstone of all aspects of HIV treatment: pre-exposure prophylaxis (PrEP)(7), post-exposure prophylaxis (PEP), and active infection. Anti-retroviral therapy (ARV) regimens typically consist of three drugs, to avoid resistance, including two NRTIs and a non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase inhibitor, or protease inhibitor. These drug classes have revolutionized HIV treatment and dramatically increased the life expectancy of people living with HIV (PWLH) (8). Previously, we have demonstrated that NRTIs can be repurposed to improve insulin sensitivity in animal models and are associated with reduced development of T2DM in humans (9). Specifically, we analyzed administrative claims data and identified a markedly reduced risk of developing T2DM in PLWH taking NRTIs that was replicated in multiple large national health insurance databases. We also found that NRTI treatment improved insulin-induced glucose uptake in both non-diabetic and diabetic human muscle and fat cells. Furthermore, NRTI treatment increased insulin sensitivity in high-fat diet-fed mice. These metabolic effects of NRTIs, as a class, can be attributed to their ability to block inflammasome activation (10), which is separate from their anti-viral activity (11,12).
Inflammasomes are an arm of innate immunity comprising multiprotein intracellular complexes that respond to exogenous and endogenous danger signals and toxic molecular patterns. In response to these signals, inflammasomes canonically activate caspase-1, an enzyme responsible for the proteolytic maturation of IL-1β and IL-18 to induce pyroptotic cell death. A number of inflammasomes have been documented in humans, most notably the NLR family (13).Known activators of inflammasomes include numerous molecular species presumed to promote T2DM, including: free fatty acids, glucose, islet amyloid polypeptide, and reactive oxygen species (14,15). As inflammasomes are key regulators of insulin resistance (16,17), a biochemical hallmark of prediabetes and T2DM, we sought to examine the association between NRTI use and the incidence of prediabetes and progression from prediabetes to overt T2DM among people living with HIV (PLWH) within the Veterans Affairs (VA) Administration System, the largest provider of HIV care in the United States (18). Our results suggest that NRTI use is indeed associated with a reduced risk of developing prediabetes and converting to T2DM.
2. RESULTS
2.1. Baseline Characteristics
A total of 32,240 HIV patients were included in this study. Patients were mostly male (97.2%) and, on average, 46 years old. Consistent with HIV care guidelines, NRTI exposure was identified among 27,906 (86.6%) individuals. Mean (SD) Charlson Comorbidity Index calculated before HIV diagnosis, was 0.23 (0.66) and 0.53 (1.17) in patients exposed and unexposed to NRTIs, respectively (Table 1). Black patients comprised approximately 45% of both groups. White patients comprised 45% of the NRTI exposed group and 39% of the unexposed group (Table 1).
Table 1:
Baseline sample characteristics
| NRTI exposure | ||||
|---|---|---|---|---|
| Unexposed N=4334 | Exposed N=27906 | P.value | ||
| Metformin use | 93(2.15%) | 539(1.93%) | 0.374 | |
| Age, years mean(SD) | 48.25(11.9) | 45.64(11.25) | <0.001 | |
| Sex | Female | 243(5.61%) | 635(2.28%) | <0.001 |
| Male | 4091(94.39%) | 27271(97.72%) | ||
| Race | Black | 1936(44.67%) | 12678(45.43%) | <0.001 |
| Other/Unknown | 676(15.6%) | 2705(9.69%) | ||
| White | 1722(39.73%) | 12523(44.88%) | ||
| Index year | 2007(5.64) | 2006.01(6.28) | <0.001 | |
| BMI 30+ | 832(19.2%) | 3783(13.56%) | <0.001 | |
| Charlson comorbidity mean(SD) | 0.53(1.17) | 0.23(0.66) | <0.001 | |
| Systemic hypertension | 1122(25.89%) | 4213(15.1%) | <0.001 | |
| Depression | 898(20.72%) | 4006(14.36%) | <0.001 | |
| Pure hypercholesteremia | 94(2.17%) | 352(1.26%) | <0.001 | |
| Hyperglycideremia | 434(10.01%) | 1838(6.59%) | <0.001 | |
| Ischemic heart disease | 180(4.15%) | 592(2.12%) | <0.001 | |
| Other heart diseases | 244(5.63%) | 836(3%) | <0.001 | |
| ALT (U/L) | <40 | 2984(68.85%) | 20729(74.28%) | <0.001 |
| >120 | 122(2.81%) | 633(2.27%) | ||
| 40–79 | 728(16.8%) | 4412(15.81%) | ||
| 80–120 | 145(3.35%) | 765(2.74%) | ||
| Missing | 355(8.19%) | 1367(4.9%) | ||
| AST (U/L) | <40 | 3118(71.94%) | 21943(78.63%) | <0.001 |
| >120 | 213(4.91%) | 1157(4.15%) | ||
| 40–79 | 693(15.99%) | 3699(13.26%) | ||
| 80–120 | 195(4.5%) | 872(3.12%) | ||
| Missing | 115(2.65%) | 235(0.84%) | ||
| Viral load(copies/ml) | >1000 | 1313(30.3%) | 14652(52.5%) | <0.001 |
| 0–500 | 2029(46.82%) | 11434(40.97%) | ||
| 501–1000 | 94(2.17%) | 895(3.21%) | ||
| Missing | 898(20.72%) | 925(3.31%) | ||
2.2. Pre-diabetes and progression to Type 2 Diabetes in PLWH
Over the entire study period, prediabetes and T2DM were observed among 6,513 (20.2%) and 6,666 (20.7%) individuals, respectively (Table 2, panel A). We detected 2072 patients (31.8%) with prediabetes who further developed T2DM.
Table 2:
Transition frequencies and hazard ratios according to NRTI exposure
| Panel A | To state | ||
|---|---|---|---|
| Overall† | Prediabetes (%) | T2DM (%) | |
| Entry | 6,513 (20.2) | 4,594 (14.3) | |
| From state | Prediabetes | 0 (0) | 2,072 (31.8) |
| T2DM | 0 (0) | 0 (0) | |
| Panel B‡ | |||
| Entry to Prediabetes | N (%) | Total N | |
| NRTI exposed | 5,251 (19.2) | 27,301 | |
| NRTI unexposed | 1,262 (25.6) | 4,939 | |
| Entry to T2DM | N (%) | Total N | |
| NRTI exposed | 3,704 (13.6) | 27,301 | |
| NRTI unexposed | 890 (18.0) | 4939 | |
| Prediabetes to T2DM | N (%) | Total N | |
| NRTI exposed | 1,812 (30.9) | 5,856 | |
| NRTI unexposed | 250 (39.6) | 657 | |
Abbreviations: type 2 diabetes mellitus (T2DM); nucleoside reverse transcriptase inhibitor (NRTI); hazard ratio (HR); confidence interval (CI).
Overall counts in the study by NRTI: NRTI exposed=27,906; NRTI unexposed=4,334.
NRTI use is coded in a time dependent manner and the total N represent those exposed prior to the transition of interest. Note, in Panel B, 605 NRTI unexposed patients became exposed later and switch to NRTI exposed group when assessing the transition from prediabetes to T2DM.
2.3. Influence of NRTIs in Hyperglycemic States
Prediabetes was detected among 5,251 (19.2%) and 1,262 (25.6%) NRTI exposed and unexposed patients, respectively. T2DM was detected among 3,705 (13.6%) and 890 (18.0%) NRTI exposed and unexposed patients, respectively. Figure 1 displays the Kaplan-Meier survival estimates for incident Prediabetes. Those exposed to NRTIs have consistently lower risk of prediabetes throughout the study period (Log-rank p value<0.001). Additionally, the progression from prediabetes to T2DM was identified in 1,812 (30.9%) of NRTI exposed and 250 (39.6%) of NRTI unexposed patients (Table 2, panel B). Figure 2 displays the Kaplan-Meier survival estimates for progressing to T2DM among those with prediabetes. Those exposed to NRTIs have a lower risk of T2DM progression (Log-rank p value<0001). In addition, NRTI exposed patients have a lower cumulative hazard of both prediabetes and T2DM compared to those unexposed (Figure 3). The risk of developing prediabetes, or the transition to T2DM, was analyzed among the study cohort accounting for diabetes risk factors such as demographics, comorbidities, and the use of metformin. The adjusted Cox proportional hazards model indicates that patients who had an NRTI prescription were at a significantly lower risk of developing prediabetes [HR 0.50 (0.47–0.53 95% CI), P<0.001] and T2DM [HR 0.52 (0.48–0.56 95% CI), P<0.001] from study entry when compared with those never exposed to NRTIs (Table 3).
Figure 1.
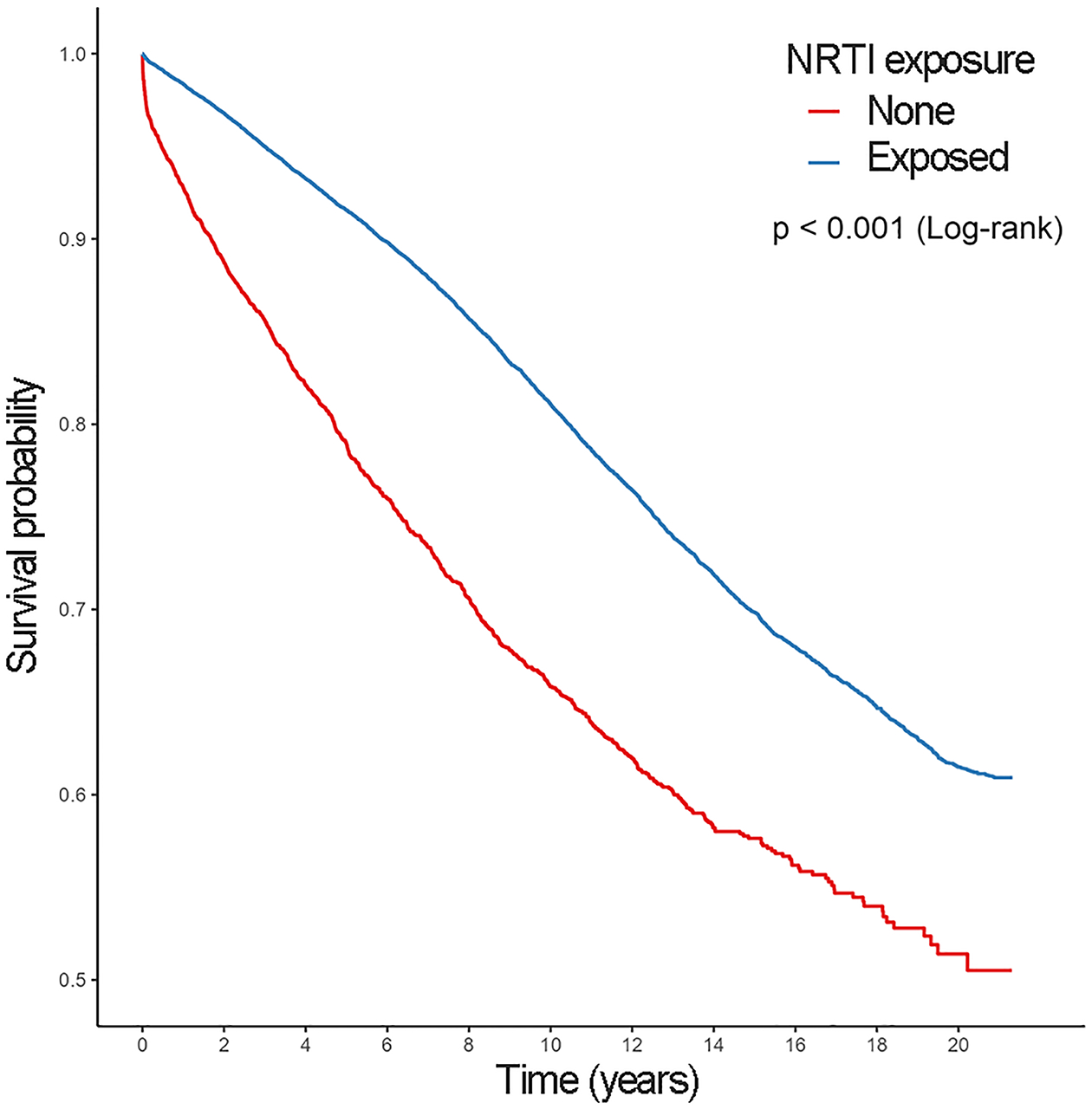
Kaplan-Meier surival estimates for incident prediabetes.
Figure 2.
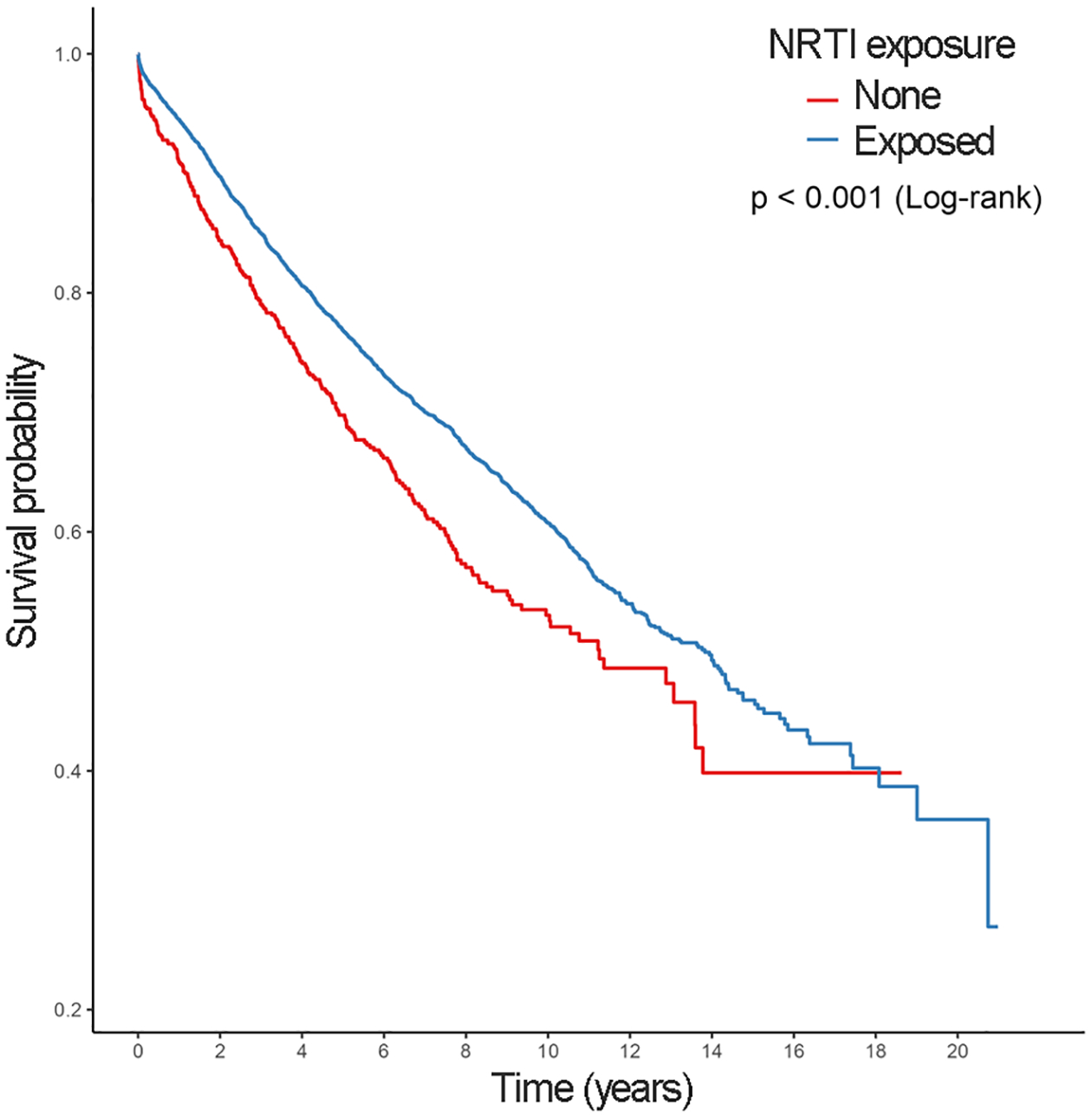
Kaplan-Meier surival estimates for progression to T2DM from prediabetes
Figure 3.
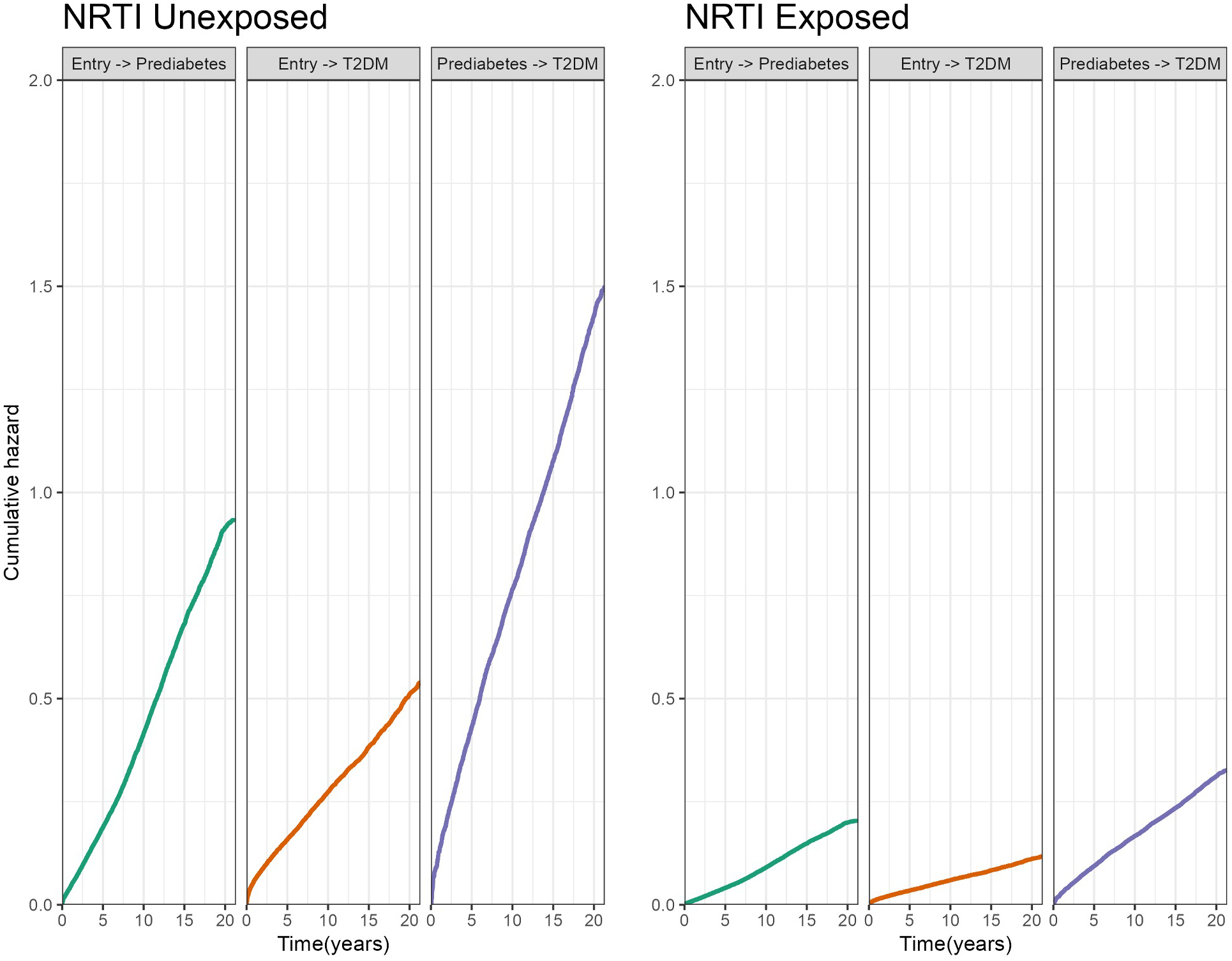
Baseline cumulative hazards (unadjusted for covariates).
Table 3:
Multi-state model showing Hazard Ratios (HRs) adjusted for covariates
| Entry to prediabetes | Entry to T2DM | Prediabetes to T2DM | ||
|---|---|---|---|---|
| Variable | HR(95% CI) | HR(95% CI) | HR(95% CI) | |
| NRTI use§ | 0.5(0.47–0.53) | 0.52(0.48–0.56) | 0.73(0.63–0.85) | |
| Metformin use | NA | NA | 2.79(2.37–3.28) | |
| Age | 1.02(1.02–1.03) | 1.04(1.04–1.04) | 1.01(1.01–1.02) | |
| Sex (Male vs Female) | 0.85(0.74–0.97) | 1.24(1.02–1.52) | 1.06(0.83–1.35) | |
| Race (reference=Black) | ||||
| Other/Unknown | 0.53(0.47–0.59) | 0.82(0.74–0.92) | 1.21(0.99–1.49) | |
| White | 0.65(0.62–0.69) | 0.75(0.7–0.8) | 1.14(1.04–1.25) | |
| Index year | 1.03(1.02–1.03) | 0.94(0.93–0.95) | 0.95(0.94–0.96) | |
| BMI 30+ | 1.38(1.29–1.48) | 2.07(1.93–2.22) | 1.39(1.25–1.55) | |
| Charlson comorbidity | 0.93(0.9–0.97) | 1.05(1.01–1.1) | 0.97(0.91–1.05) | |
| Systemic hypertension | 1.13(1.05–1.21) | 1.18(1.08–1.27) | 1.2(1.07–1.35) | |
| Depression | 1.05(0.98–1.13) | 0.99(0.9–1.08) | 0.97(0.85–1.1) | |
| Pure hypercholesteremia | 1.14(0.95–1.35) | 0.94(0.75–1.18) | 0.95(0.71–1.26) | |
| Hyperglycideremia | 1.39(1.27–1.52) | 1.01(0.9–1.14) | 1.18(1.02–1.37) | |
| Ischemic heart disease | 1.13(0.96–1.32) | 1.2(1.01–1.43) | 1.09(0.84–1.42) | |
| Other heart diseases | 1.13(0.98–1.3) | 0.93(0.79–1.11) | 0.82(0.63–1.07) | |
| ALT (reference=<40) | ||||
| >120 | 1.32(1.03–1.7) | 1.1(0.88–1.39) | 1.89(1.26–2.81) | |
| 40–79 | 1.18(1.09–1.27) | 1.14(1.05–1.25) | 1.34(1.18–1.52) | |
| 80–120 | 1.15(0.94–1.4) | 1.11(0.92–1.34) | 1.46(1.06–2) | |
| Missing | 0.85(0.74–0.97) | 1.02(0.88–1.17) | 0.86(0.66–1.11) | |
| AST (reference=<40) | ||||
| >120 | 0.54(0.43–0.67) | 1.09(0.91–1.3) | 1.14(0.8–1.62) | |
| 40–79 | 0.75(0.69–0.82) | 0.99(0.91–1.09) | 0.92(0.79–1.08) | |
| 80–120 | 0.49(0.39–0.6) | 1.24(1.05–1.46) | 1.29(0.9–1.83) | |
| Missing | 0.71(0.53–0.95) | 0.91(0.68–1.23) | 1.18(0.71–1.94) | |
| Viral load(copies/ml) (reference=>1000) | ||||
| 0–500 | 0.99(0.94–1.04) | 1.05(0.98–1.11) | 1(0.91–1.1) | |
| 501–1000 | 0.96(0.83–1.1) | 1.1(0.94–1.28) | 1.1(0.86–1.4) | |
| Missing | 0.71(0.62–0.8) | 0.79(0.68–0.91) | 0.82(0.65–1.04) | |
NRTI exposure compared to non-exposure.
Furthermore, NRTI users with prediabetes had a lower progression rate to T2DM [HR 0.73 (0.63–0.85 95% CI), P<0.001] (Table 3). Other factors statistically significantly associated with a higher risk of hyperglycemic states include older age, systemic hypertension, and having a BMI of greater than 30 (Table 3).
3. DISCUSSION
In this retrospective analysis of a nationwide database, we observed that PLWH exposed to NRTIs had a significant reduction in the risk of developing prediabetes and T2DM, as well as progressing from prediabetes to T2DM, compared to those who were unexposed to NRTIs.
PLWH are considered to be at higher risk for metabolic disorders than non-infected individuals because of their constitutively elevated chronic immune activation, driven by many mechanisms including inflammasome activity (19, 20), and exposure-related toxicity of certain anti-HIV drugs. Most notably, protease inhibitors, such as darunavir, ritonavir, and indinavir, are associated with a number of metabolic disorders including lipodystrophy, insulin resistance, T2DM, and central adiposity (21). Additionally, lipodystrophy is associated with several NRTIs including stavudine and zidovudine (22). However, our findings contrast with the studies from the beginning of the antiretroviral therapy (ART) era, when the use of earlier generation NRTIs and protease inhibitors were associated with increased risk of the aforementioned metabolic disorders (23–25). Earlier-generation NRTIs such as didanosine and stavudine have been shown to exhibit greater toxicity than later-generation NRTIs such as emtricitabine, lamivudine, and tenofovir, including lipodystrophy, myelosuppression, GI insufficiency, peripheral neuropathy, and mitochondrial toxicity. These earlier-introduced drugs are also associated with development of insulin resistance and, when combined with protease inhibitors as a part of a ARV regimen, with a higher risk of type 2 diabetes in HIV-affected people (23,26–28). Hence, our results are in line with the latest evidence that modern NRTIs can reduce, as opposed to promote, insulin resistance and decrease the risk of T2DM (9). Similarly, a Canadian database study reported that ART prescription, particularly after 2005, did not increase the risk of diabetes and, indeed, suggested a protective effect (29).
The greatest strength of our study is the large sample size observed over a 21-year period. We also employed a Markovian multistate model, which has several advantages over traditional survival analysis. MSMs allow for multiple transitions and possible competing risks, providing a better ability to evaluate the progression of disease. Allowing transitions from study entry to prediabetes and T2DM, and from prediabetes to T2DM, enables the model to more accurately fit the different states that patients may pass through during the course of disease. Moreover, the effects of covariates are allowed to vary over the different transitions allowing better insight into their effects over the course of disease progression. Multiple covariates, including patient demographics, comorbidities, and use of metformin, were included in the Cox model to mitigate confounding bias. Our study also has several limitations: 1) the results might not be generalizable to a population outside the VA Health Care System; 2) residual or unobserved confounding bias, stemming from unrecognized factors not controlled for, cannot be ruled out; 3) health insurance claim database analyses are subject to improper documentation and miscoding; 4) ICD codes and HbA1c values are less sensitive than impaired fasting glucose test, which may underestimate the number of prediabetic individuals; 5) genetic variants that could be associated with increased risk of T2DM were not evaluated in this study (21).
In addition, a limitation of our study, as with all retrospective administrative claims studies, is the potential that patients may not be adherent to the medications under study due to financial constraints, forgetfulness, social stigma, substance use, toxicity, or other factors. Although medication compliance is an issue in the management of many chronic diseases, adherence to a ARV regimen is especially important in the treatment of HIV in order to suppress viral load, slow disease progression, and prevent resistance (30). Because most patients on an ARV regimen take antiviral medications for an extended period of time, usually many years, the cumulative burden of cost and toxicity presents a major challenge to ensuring optimal patient compliance. As a result of imperfect adherence, we are likely underestimating the potential association between NRTI use and prevention of development of prediabetes or progression to T2DM. If patients were fully treatment-adherent, the treatment effect would likely be even greater than what our analysis revealed. Furthermore, and importantly, a claims database medication study captures real world patterns that represent actual patient adherence as a recorded NRTI exposure in the VA Administration System does not equate adherence to a ARV regimen. Therefore, although our investigation on NRTI effects is subject to the fact that real/actual medication use may not be completely identical to prescription/claims data, we think this is an advantage and further strengthens the real-world applicability of our findings of the association between NRTI use in practice and incident prediabetes or progression to T2DM. Additional limitations include challenges in controlling for NRTI exposure duration and dose, as NRTI-mediated reduction of hyperglycemia may be spectral and/or dose-dependent. While prediabetes and T2DM have established guidelines for diagnosis (>5.7% HbA1c for prediabetes and >6.5% HbA1c for diabetes), these somewhat arbitrary diagnostic cutoffs may exacerbate or reduce the apparent effect of NRTIs on glycemic state compared to its true effect. Future prospective cohort studies and clinical trials can provide additional causal insights into the beneficial effect of NRTI exposure on glycemic state in metabolic diseases, such as prediabetes and T2DM, in PLWH and the overall population.
4. MATERIALS AND METHODS
4.1. Data Source
This retrospective cohort study examining drug-disease association was conducted using data from the Department of Veterans Affairs. The Veterans Affairs Informatics and Computing Infrastructure (VINCI) was utilized to obtain individual-level information on demographics, administrative claims, and pharmacy dispensation. The study was conducted in compliance with the Department of Veterans Affairs requirements and received Institutional Review Board and Research and Development approval.
We extracted information from VINCI, including inpatient and outpatient claims coded with International Classification of Diseases (ICD) revision 9-CM and revision 10-CM, laboratory, and pharmacy claims. The study used data from January 1, 2000 to January 27, 2021.
4.2. Study Population
Study inclusion required at least one ICD-9 or ICD-10 code for HIV and one HIV laboratory result. The study index was based on the first HIV code ranging from January 1, 2000 to January 2021. Patients were followed until death or end of study follow-up on January 27, 2021. Inclusion criterion was HIV diagnosis in a patient 18 years old or older. Patients were excluded if they 1) had a diagnosis of pre-diabetes or diabetes before the index date, 2) had a prescription of metformin prior to pre-diabetes diagnosis, or prior to T2DM diagnosis without a pre-diabetes diagnosis.
4.3. Exposure Definition
Patients were classified as NRTI users if at least one outpatient pharmacy prescription was filled for one or more NRTI drugs in an isolated or combined formula. NRTI use was coded in a time dependent manner. Patients were only counted as NRTI exposed if they had an NRTI prescription prior to the transition of interest, or study end point for those with no prediabetes or T2DM. Once exposed they were considered exposed for the remainder of the study. As such the number of total patients exposed to an NRTI over the study is greater than those exposed prior to each transition outcome.
4.4. Outcomes
Prediabetes was defined as 790.29, R73.03, or two hemoglobin A1c (HbA1c) values between 5.7–6.4%. T2DM was defined as 250.x0, 250.x2, E11.xx, or two HbA1c values greater than 6.4%. Patients with prediabetes and T2DM diagnosis on the same day were considered as T2DM. Time to event during the follow-up period was the outcome variable. Data was right-censored at the end of the follow-up or if death occurred.
4.5. Statistical Analysis
Kaplan-Meier survival estimates were generated along with p values from Log-rank tests to examine the relationship between NRTI exposure and incident prediabetes and subsequent progression to T2DM from prediabetes (Figures 1,2). A multi-state model (MSM) was then used analyze the risk of developing prediabetes, or the transition to T2DM. MSMs allow multiple transition states to be modeled including the possibility of competing risks. We constructed a MSM with 3 possible transitions where patients could move from study entry to 1) pre-diabetes, 2) T2DM or from pre-diabetes to 3) T2DM. Using a Markov multistate model, effects of covariates are allowed to vary for the different transitions. The mstate package in R was used to set up the data to estimate the MSM via a Cox proportional hazards model (31,32). An unadjusted MSM is fit to plot the baseline cumulative hazards for each outcome transition. Figure 3 displays the baseline hazards for each transition stratified by NRTI exposure. An adjusted MSM included demographics and comorbidities to estimate hazard ratios and 95% confidence intervals using the Cox models with transition-specific effects. Missing data was considered as a separate category. All data management was performed using SAS (SAS Institute Inc., SAS 9.4, Cary, NC: SAS Institute Inc.) and R (R Foundation for Statistical Computing, Vienna, Austria), http://www.R-project.org/.
ACKNOWLEDGMENTS
This paper represents original research conducted using data from the Department of Veterans Affairs. This material is the result of work supported with resources and the use of facilities at the Dorn Research Institute, Columbia VA Health Care System, Columbia, South Carolina. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Funding.
J.A. has received support from NIH grants (R01EY028027, R01EY29799, R01EY031039, R01AG082108), DuPont Guerry, III, Professorship, the University of Virginia Strategic Investment Fund, and a gift from Mr. and Mrs. Eli W. Tullis.
CONFLICT OF INTEREST
J.A. has received support from the UVA Strategic Investment Fund and National Institutes of Health (NIH) grants (R01EY028027, R01EY029799, R01EY031039, R01AG082108). S.S.S., J.M., T.H.C. are supported by NIH grant R01DA054992 and the South Carolina Center for Rural and Primary Healthcare for projects unrelated to this study. J.A. is a co-founder of DiceRx, iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and, unrelated to this work, he has been a consultant for Allergan, Boehringer- Ingelheim, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences unrelated to this work. J.W.H. has received consulting fees from Celgene Corporation unrelated to this work. S.S.S has received research grants from Boehringer Ingelheim, Coherus BioSciences, Alexion Pharmaceuticals, and EMD Serono, all for projects unrelated to study. J.A., S.N., and F.P. are named as inventors on patent applications filed by the University of Virginia or the University of Kentucky.
DATA AVAILABILITY
Analyses of the Veterans Health Administration Database were performed using data within the US Department of Veterans Affairs secure research environment, the VA Informatics and Computing Infrastructure (VINCI). The completeness, utility, accuracy, validity, and access methods are described on the VA website, https://www.virec.research.va.gov.
REFERENCES
- 1.Khetan AK, Rajagopalan S. Prediabetes. Canadian Journal of Cardiology. 2018;34(5):615–623. [DOI] [PubMed] [Google Scholar]
- 2.CDC’S NATIONAL CENTER for CHRONIC DISEASE PREVENTION and HEALTH PROMOTION Diabetes and Prediabetes CDC Works to Prevent Type 2 Diabetes and Improve the Health of All People with Diabetes. 2022. https://www.cdc.gov/chronicdisease/pdf/factsheets/diabetes-H.pdf. Accessed November 28, 2022.
- 3.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. The Lancet. 2022; 400(10365):1803–1820. [DOI] [PubMed] [Google Scholar]
- 4.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. The Lancet. 2011;378(9785):31–40. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;36(Supplement_1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovascular Diabetology. 2021;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Özdener AE, Park TE, Kalabalik J, Gupta R. The future of pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) infection. Expert Review of Anti-infective Therapy. 2017;15(5):467–481. [DOI] [PubMed] [Google Scholar]
- 8.Cruciani M, Mengoli C, Serpelloni G, Parisi SG, Malena M, Bosco O. Abacavir-based triple nucleoside regimens for maintenance therapy in patients with HIV. The Cochrane Database of Systematic Reviews. 2013;(6):CD008270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambati J, Magagnoli J, Leung H, et al. Repurposing anti-inflammasome NRTIs for improving insulin sensitivity and reducing type 2 diabetes development. Nature Communications. 2020;11(1):4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler BJ, Gelfand BD, Kim Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346(6212):1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda S, Varshney A, Fowler BJ, et al. Cytoplasmic synthesis of endogenous Alu complementary DNA via reverse transcription and implications in age-related macular degeneration. Proceedings of the National Academy of Sciences. 2021;118(6):e2022751118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda S, Narendran S, Varshney A, et al. Alu complementary DNA is enriched in atrophic macular degeneration and triggers retinal pigmented epithelium toxicity via cytosolic innate immunity. Science Advances. 2021;7(40):eabj3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathinam VAK, Fitzgerald KA. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165(4):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nature Immunology. 2010;11(10):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology. 2011;12(5):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masters SL, Latz E, O’Neill LAJ. The inflammasome in atherosclerosis and type 2 diabetes. Science Translational Medicine. 2011;3(81):81ps17. [DOI] [PubMed] [Google Scholar]
- 18.VA.gov | Veterans Affairs. www.hiv.va.gov. Accessed November 28, 2022. https://www.hiv.va.gov/about-index.asp#:~:text=Contact%20This%20Site-
- 19.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annual Review of Medicine. 2011;62(1):141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association Between Systemic Inflammation and Incident Diabetes in HIV-Infected Patients After Initiation of Antiretroviral Therapy. Diabetes Care. 2010;33(10):2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holstein A, Plaschke A, Egberts EH. Lipodystrophy and metabolic disorders as complication of antiretroviral therapy of HIV infection. Experimental and Clinical Endocrinology & Diabetes. 2001;109(08):389–392. [DOI] [PubMed] [Google Scholar]
- 22.Margolis AM, Heverling H, Pham PA, Stolbach A. A Review of the Toxicity of HIV Medications. Journal of Medical Toxicology. 2013;10(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brambilla AM, Novati R, Calori G, et al. Stavudine or indinavir-containing regimens are associated with an increased risk of diabetes mellitus in HIV-infected individuals. AIDS. 2003;17(13):1993–1995. [DOI] [PubMed] [Google Scholar]
- 24.García-Benayas T, Rendón AL, Rodríguez-Novóa S, et al. Higher risk of hyperglycemia in HIV-infected patients treated with didanosine plus tenofovir. AIDS research and human retroviruses. 2006;22(4):333–337. [DOI] [PubMed] [Google Scholar]
- 25.Justman JE, Benning L, Danoff A, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV-infected women. Journal of Acquired Immune Deficiency Syndromes (1999). 2003;32(3):298–302. [DOI] [PubMed] [Google Scholar]
- 26.Wit SD, Sabin CA, Weber R, et al. Incidence and Risk Factors for New-Onset Diabetes in HIV-Infected Patients: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. Diabetes Care. 2008;31(6):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paula AA, Falcão MC, Pacheco AG. Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Research and Therapy. 2013;10(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledergerber B, Furrer H, Rickenbach M, et al. Factors Associated with the Incidence of Type 2 Diabetes Mellitus in HIV-Infected Participants in the Swiss HIV Cohort Study. Clinical Infectious Diseases. 2007;45(1):111–119. [DOI] [PubMed] [Google Scholar]
- 29.Samad F, Harris M, Puskas CM, et al. Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Research and Care. 2017;5(1):e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to Antiretroviral Therapy Among People Living with HIV. North American Journal of Medical Sciences. 2013;5(3):220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Wreede LC de, Fiocco M, Putter H. mstate: An R Package for the Analysis of Competing Risks and Multi-State Models. Journal of Statistical Software. 2011;38(7):1–30. [Google Scholar]
- 32.Therneau T (2022). A Package for Survival Analysis in R. R package version 3.4.0, https://CRAN.R-project.org/package=survival. Accessed November 28, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analyses of the Veterans Health Administration Database were performed using data within the US Department of Veterans Affairs secure research environment, the VA Informatics and Computing Infrastructure (VINCI). The completeness, utility, accuracy, validity, and access methods are described on the VA website, https://www.virec.research.va.gov.


