Abstract
PURPOSE
Chemoimmunotherapy for patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) is largely unchanged for decades. Both preclinical models and clinical data suggest the combination of lenalidomide and ibrutinib may have synergy in DLBCL, particularly in the non–germinal center B-cell-like subset.
METHODS
We enrolled 60 patients with newly diagnosed non–germinal center B-cell-like DLBCL in this investigator-initiated, single-arm phase II trial of rituximab, lenalidomide, and ibrutinib (RLI) with the sequential addition of chemotherapy (ClinicalTrials.gov identifier: NCT02636322). Patients were treated with rituximab 375 mg/m2 intravenous once on day 1, lenalidomide 25 mg once per day on days 1-10, and ibrutinib 560 mg once daily continuously of each 21-day cycle (RLI). After two cycles, standard chemotherapy was added to RLI for six additional cycles. The primary end points were overall response rate (ORR) after two cycles of RLI alone and complete response rate after completion of RLI with chemotherapy. In evaluable samples, circulating tumor DNA and DLBCL90 assays were performed.
RESULTS
The median age was 63.5 years (range, 29-83 years) with 28% age 70 years or older. The revised international prognostic index identified 42% as high risk, and 62% were double expressor of MYC and BCL2 protein. The ORR after two cycles of RLI was 86.2%, and the complete response rate at the end of RLI-chemotherapy was 94.5%. With a median follow-up of 31 months, the progression-free survival and overall survival were at 91.3% and 96.6% at 2 years, respectively.
CONCLUSION
Smart Start is the first study, to our knowledge, to treat newly diagnosed DLBCL with a targeted therapy combination before chemotherapy. RLI produced a high ORR, and RLI with chemotherapy resulted in durable responses. This establishes the potential for developing biologically driven and noncytotoxic first-line therapies for DLBCL.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL), the most common lymphoid cancer, represents two of every five lymphoma diagnoses worldwide.1 Therapy for newly diagnosed DLBCL is commonly rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), which cures approximately 60% of patients, despite originating before modern classification systems.2,3 Because a high rate (40%) of patients are either refractory to initial treatment or relapse afterward, there is an unmet need to identify novel approaches to incorporate new drugs in first-line treatment.4 Numerous randomized phase III trials have evaluated combining R-CHOP with a single agent (X) known to be active in relapsed DLBCL, but results have been disappointing,5-7 with the exception of the recent POLARIX trial, which replaced vincristine with polatuzumab vedotin.8 The R-CHOP + X approach is limited by factors including increased toxicity, lack of drug synergy, and biopsy-requiring or complicated eligibility criteria, which may prevent enrollment of patients with rapidly proliferating disease.9,10 The biology of DLBCL is heterogeneous, and is most frequently classified via the putative cell of origin into germinal center B-cell-like (GCB), activated B-cell-like (ABC), or unclassified subtypes. The ABC subtype, included in the non-GCB subtype if defined by immunohistochemistry (IHC),11 has inferior clinical outcomes with R-CHOP, achieving 2-year progression-free survival (PFS) rates of 40%-64%,6,7,12 yet has multiple targetable vulnerabilities including chronic activated B-cell receptor signaling and nuclear factor κB activation, associated with expression of IRF4, a key transcription factor for B-cell differentiation.13,14 Lenalidomide, an immunomodulatory drug targeting cereblon, has an overall response rate (ORR) of 28%-53% in patients with relapsed non-GCB DLBCL (9% in GCB DLBCL); however, median PFS is 2.6 months alone and 2.8 months combined with rituximab.15,16 Lenalidomide decreases expression of IRF4 in ABC DLBCL, resulting in increased interferon β signaling.17 Ibrutinib, a Bruton's tyrosine kinase (BTK) inhibitor, has an ORR of 37% in patients with relapsed ABC DLBCL (5% in GCB DLBCL); however, median PFS is 2 months.18 In preclinical ABC DLBCL models, lenalidomide combined with ibrutinib results in a complete block of IRF4 expression, synergistic increase in interferon β, resulting in a synthetic lethal response.17 In addition to direct antilymphoma activity, both lenalidomide and ibrutinib have significant immunomodulatory effects, which promote a shift from a tumor-mediated immune suppression to antitumor activity.19-21 In patients with relapsed non-GCB DLBCL, the combination of rituximab, lenalidomide, and ibrutinib (RLI) has an ORR of 65% and median PFS of 5.5 months, and when combined with infusional etoposide, doxorubicin, vincristine, with prednisone and cyclophosphamide (EPOCH) has 71% ORR and a median PFS of 6.5 months.22,23
CONTEXT
Key Objective
The Smart Start study was designed to evaluate if the targeted therapy combination of rituximab, lenalidomide, and ibrutinib would be effective and safe before and in combination with chemotherapy in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL) based upon preclinically predicted synthetic lethality.
Knowledge Generated
Smart Start demonstrated the significant clinical activity, manageable safety profile, and promising survival outcomes of targeted therapy alone and with chemotherapy as a frontline treatment for patients with newly diagnosed DLBCL, establishing the precedent that window trials are feasible.
Relevance (J.W. Friedberg)
-
The Smart Start trial demonstrates feasibility of using targeted therapy combinations alone before chemoimmunotherapy for patients with newly diagnosed DLBCL. These results indicate the potential for a new screening strategy paradigm to more rapidly identify efficacy of rational targeted therapies in biologically defined subsets of this disease.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Based upon preclinical synergy, promising clinical results in relapsed DLBCL, and the historical limitations of the R-CHOP + X approach, we designed Smart Start, an investigator-initiated, open-label, single-center, phase II trial of RLI in patients with newly diagnosed non-GCB DLBCL. We hypothesized that using a window of RLI alone in this subtype, followed by a combination of RLI with standard chemotherapy, would be effective and safe, allow partial evaluation of RLI alone, and when followed by a combination of RLI with standard chemotherapy would preserve or improve the chance for a curative outcome. Here, we present the primary analysis of Smart Start, the first trial, to our knowledge, to evaluate a targeted therapy combination without chemotherapy in patients with newly diagnosed DLBCL, and set the stage for the development of future trials to evaluate additional targeted therapy combinations in DLBCL.
METHODS
Study Design and Patient Population
The Smart Start study was an investigator-initiated, open-label, single-center, phase II clinical trial. Adult patients were eligible if they had previously untreated non-GCB DLBCL defined by IHC,11 Eastern Cooperative Oncology Group performance status of ≤ 3, with additional eligibility criteria listed in the Data Supplement (online only). The initial trial design was modified based upon new data from concurrent studies,5,24 including to allow eligibility regardless of revised International Prognostic Index (R-IPI) or Ki-67 expression, with modifications defined in the Data Supplement.
Treatment
Patients received RLI (rituximab 375 mg/m2 intravenous once on day 1, lenalidomide 25 mg orally once per day on days 1-10, and ibrutinib 560 mg orally once daily continuously of each 21-day cycle) alone for cycles 1 and 2, and with chemotherapy for cycles 3 through 8 (Data Supplement). Steroid prephase therapy was originally allowed if needed for symptom control; however, after a patient experienced a fungal infection, steroid prephase therapy was subsequently prohibited with no further fungal infections noted. The initial design of the trial defined chemotherapy as EPOCH with fixed dosing; however, when an unrelated first-line trial in DLBCL patients found R-CHOP equivalent to R-EPOCH,24 the study protocol was amended to allow the treating physician to choose EPOCH or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP; Data Supplement). When an unrelated randomized trial found ibrutinib 560 mg with R-CHOP was associated with increased toxicity in patients age > 65 years,5 the study protocol was amended to reduce ibrutinib dosing in subsequent patients age > 65 years to 420 mg daily. Prophylaxis for tumor lysis syndrome, venous thrombosis, infection, and neutropenia were mandatory (Data Supplement).
Response Assessments
Disease assessment was performed at baseline, end of cycle 2 and 4, therapy completion, every 3 months during year 1, and every 4 months in year 2. Imaging with 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) was performed during and at the end of therapy to determine response via Lugano criteria, with a Deauville score of 1-3 indicating complete metabolic response.25,26 At subsequent time points, response was defined by CT or PET-CT. In addition, screening and first restaging PET-CT were analyzed for total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG) using standard techniques (Data Supplement).27-29 Testing for circulating tumor DNA (ctDNA) was performed at baseline, on day 1 of cycles 1-3, and at the end of therapy using hybrid-capture techniques for exploratory analyses.30 Tumors were classified based upon cell of origin and double-hit signature by the DLBCL90 NanoString assay.31 Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.32
Study Oversight
The study concept and protocol were designed by the first author; MD Anderson Cancer Center had overall responsibility for the study and its conduct, and the Institutional Review Board approved the trial. All patients provided informed consent. Janssen and Celgene provided study drugs and funding, and were allowed to review the manuscript, but had no role in the conduct of the study, collection and/or analysis of data, and had no influence on the content or submission of the manuscript for publication. The study was conducted in accordance with ethical principles defined by the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Statistical Analysis
The primary end points were to estimate ORR at the end of cycle 2 of RLI and complete response (CR) rate at the end of all therapy. Because of the unprecedented design in patients with newly diagnosed DLBCL, we used independent Bayesian futility and toxicity monitoring rules in a rolling evaluation to ensure adequate clinical activity and acceptable toxicity (Data Supplement).33 Descriptive statistics including mean, standard deviation, median, and range for continuous variables such as age and marker scores, and frequency counts and percentages for categorical variables such as stage and response status are provided. Chi-square test or Fisher's exact test was used to evaluate the association between response and other variables. Wilcoxon rank sum test or Kruskal-Wallis test were used to evaluate the difference in a continuous variable between or among patient groups. Kaplan-Meier method was used to estimate the time-to-event end points including PFS and overall survival (OS, Data Supplement). Statistical software programs SAS 9.4 (SAS, Cary, NC) and S-Plus 8.2 (TIBCO Software Inc, Palo Alto, CA) were used for all the analyses.
RESULTS
Characteristics and Disposition of the Patients
From May 2016 through February 2019, 60 patients with non-GCB DLBCL were enrolled (Table 1). The median age was 63.5 years, and 28% were age 70 years or older. According to the R-IPI, 42% (25/60) of patients were considered poor risk.34 Of patients with testing for Ki-67, 45% (23/51) had ≥ 90% expression. Only 13% (n = 8) of patients had both R-IPI < 3 and Ki-67 < 80%. Of patients with testing for both MYC and BCL2 overexpression via IHC, 62% (24/39) were double expressor.35 Median time from diagnosis to treatment was 28 days (range, 9-138 days).36
TABLE 1.
Characteristics of All 60 Patients at Study Entry
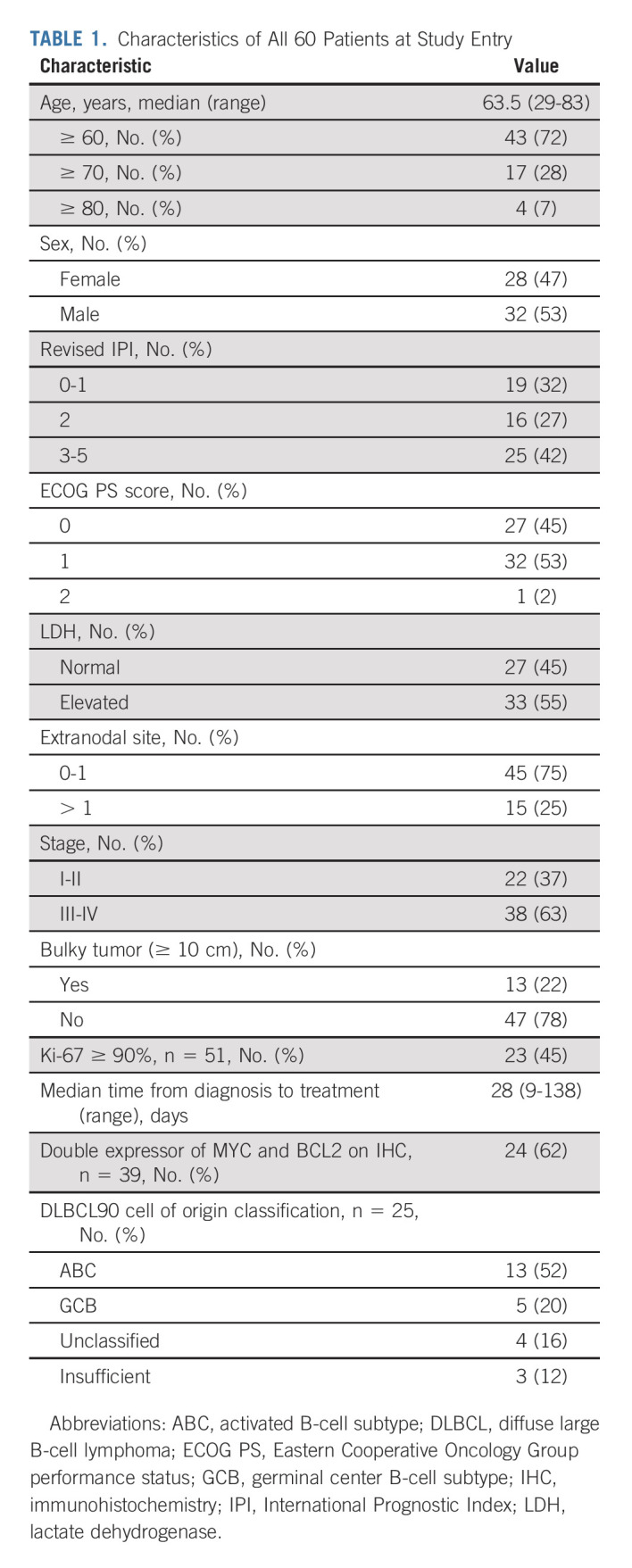
Two patients withdrew consent from the trial before first restaging because of patient preference and were evaluable for safety only (Data Supplement). Two patients received two cycles of RLI therapy only without chemotherapy, one withdrew consent after a CR, and one because of progressive disease and grade 5 CNS aspergillosis infection in the first patient enrolled potentially associated with a steroid prephase therapy administered while awaiting study activation (Data Supplement). Fifty-six patients received RLI combined with chemotherapy, including RLI + EPOCH in 31 patients (one changed to CHOP after three cycles because of physician preference), and RLI + CHOP in 25 patients. One patient had preplanned radiation to the contralateral testicle after completing study therapy with a CR.
Efficacy
Primary end points.
After two cycles of RLI, 58 patients were evaluable; the ORR was 86.2% (95% CI, 74.6 to approximately 93.9, primary end point 1), the CR rate was 36.2% (95% CI, 24.0 to approximately 49.9), and 50% had a partial response (PR, Fig 1). After two cycles of RLI and two cycles of RLI-chemotherapy, 56 patients were evaluable; the ORR was 100% (95% CI, 93.6 to approximately 100%) and the CR rate was 75% (95% CI, 61.6 to approximately 85.6%). At the end of therapy, 55 patients were evaluable; the ORR was 100% (95% CI, 93.5 to approximately 100%) and the CR rate was 94.5% (95% CI, 84.9 to approximately 98.9%, primary end point 2). The response rates were not different between patients treated with EPOCH or CHOP (Data Supplement). Three patients had a PR on end of therapy PET, two of which had a subsequent negative biopsy, and none had subsequent therapy or suffered relapse with > 2 years of follow-up.
FIG 1.
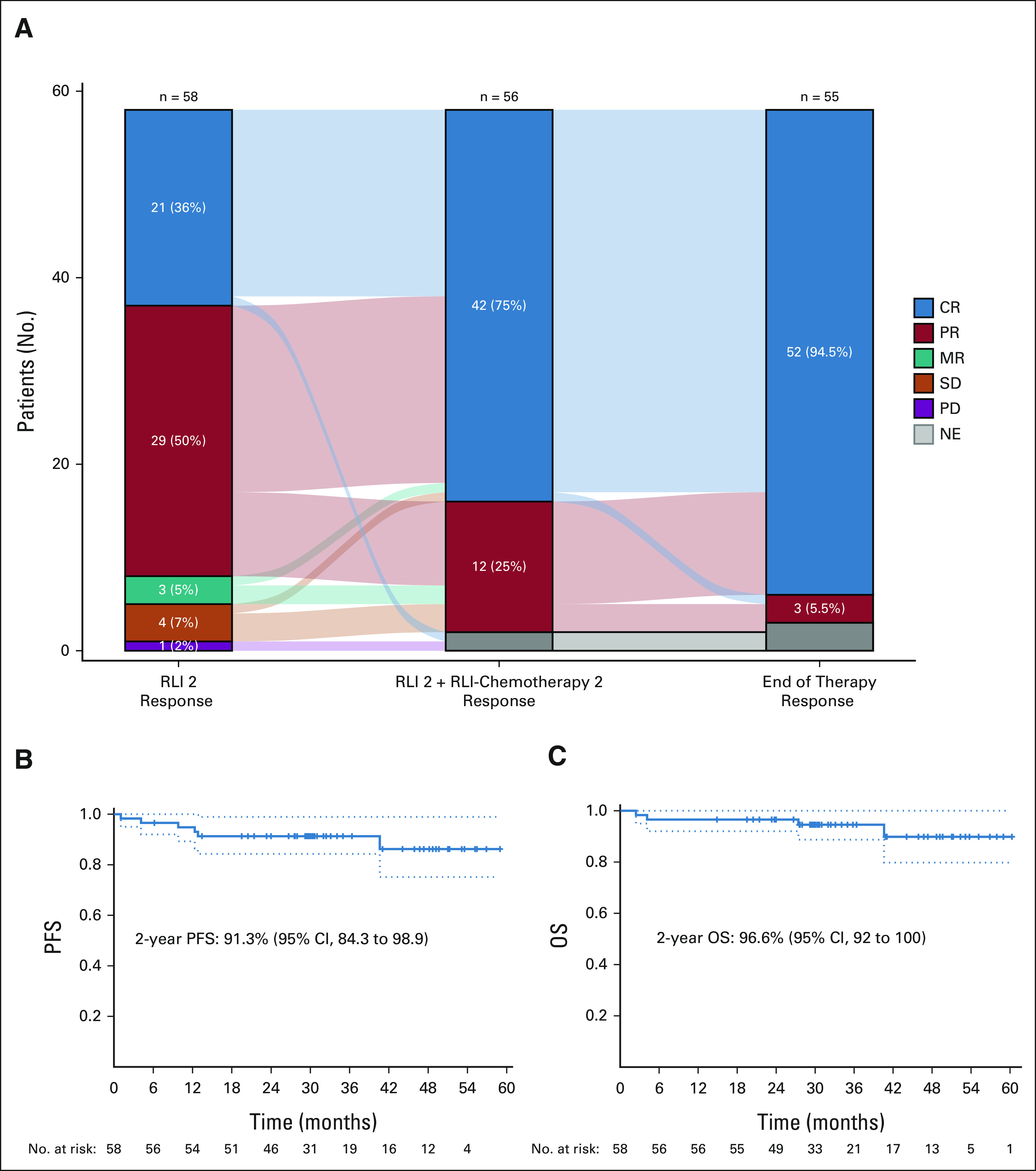
Clinical efficacy. (A) Response to RLI alone and with chemotherapy in a Sankey diagram. After two cycles of RLI, 58 patients were evaluable. Twenty-one (36%) had CR, 29 (50%) had PR, four (7%) had SD, three (5%) had MR, and one (2%) had PD. After two cycles of RLI and two cycles of RLI-chemotherapy, 56 patients were evaluable. Forty-two (75%) had CR and 12 (25%) had PR. At the end of therapy, 55 patients were evaluable. Fifty-two (94.5%) had CR and three (5.5%) had PR. Kaplan-Meier survival curves for (B) PFS and (C) OS. CR, complete response; MR, mixed response; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RLI, rituximab, lenalidomide, and ibrutinib; SD, stable disease.
Secondary end points.
With a median follow-up of 31 months (95% CI, 30 to approximately 40.9), the median PFS was not reached and the PFS rate at 2 years was 91.3% (95% CI, 84.3 to 98.9; Fig 1). The median OS was not reached, and the OS rate at 2 years was 96.6% (95% CI, 92 to 100). Two deaths occurred during therapy (Data Supplement, fungal infection, Clostridium difficile infection), and two additional deaths occurred > 24 months after enrollment (DLBCL progression, unrelated malignancy). The patient who achieved CR after two cycles of RLI and withdrew consent for further therapy is in a confirmed remission ongoing at > 3.9 years.
In patients with sufficient samples for detection of common DLBCL mutations in ctDNA available at baseline and during treatment for clearance and/or emergence of new mutations, 29/36 (81%) were of sufficient quantity (> 10 ng) and quality (low genomic DNA contamination) for library preparation. In two patients with sufficient ctDNA available, high-confidence mutations were not detected at baseline, and thus, they were excluded from additional analysis. Two patients had both EZH2 and TNFRSF14 mutations at baseline, typically synonymous with the GCB subtype of DLBCL (Fig 2).37 There were no baseline mutations that were associated with ORR after cycle 2 of RLI or CR at the end of therapy. In four patients who did not achieve response after cycle two of RLI and had ctDNA data available, no pattern of mutation was associated with lack of response and all subsequently achieved a CR with RLI-chemotherapy. The patient who achieved durable remission after only two cycles of RLI had mutations of both CD79B and PIM1, which were also present in eight and nine other patients, respectively, including in one and two nonresponders to RLI alone. After one cycle of RLI, early molecular responses of ≥ 2-log reduction in ctDNA from baseline were identified in 54.5% (12/22, Fig 2A), and two additional patients were within 1% of achieving this threshold.38
FIG 2.
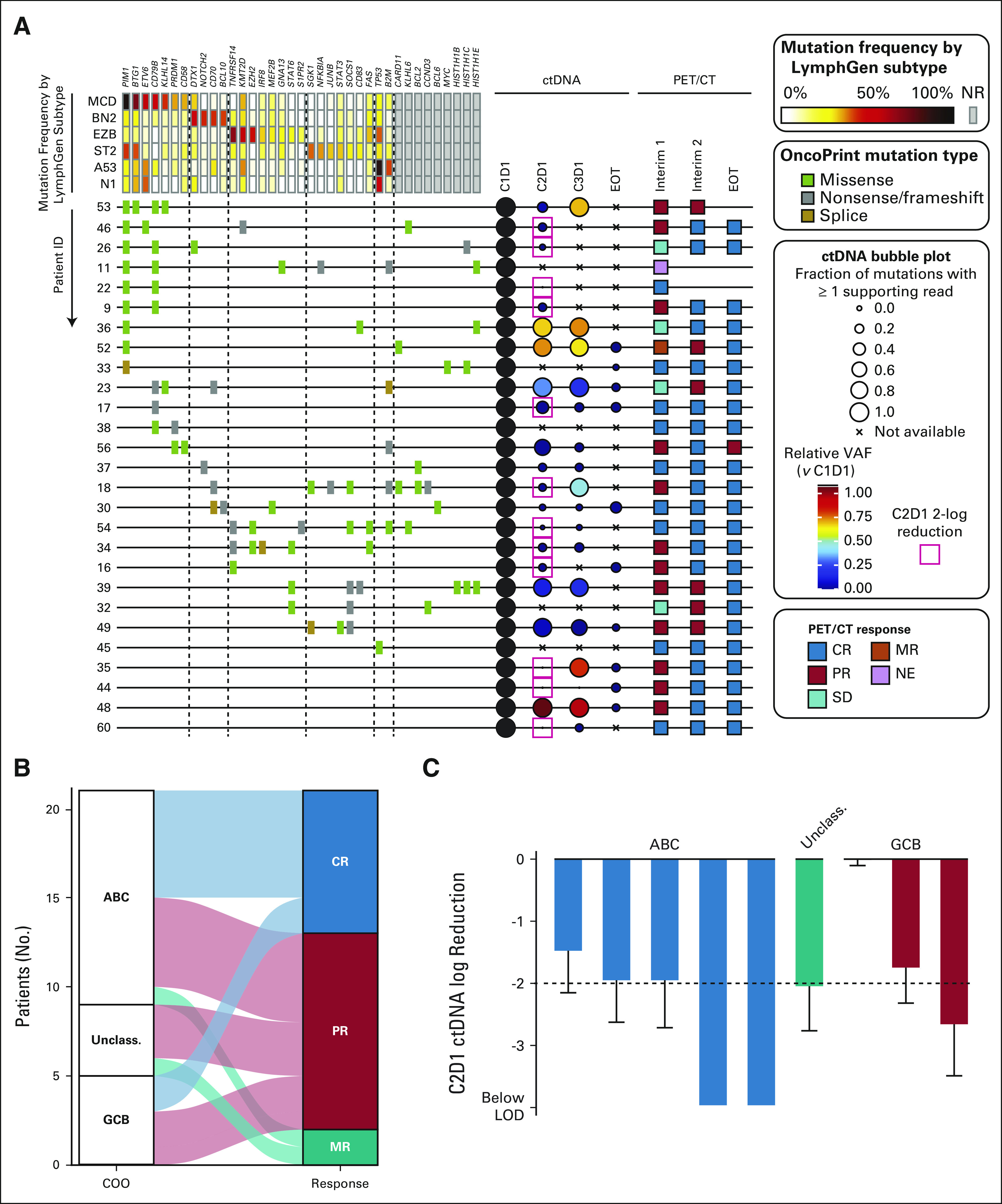
Molecular analysis. (A) Analysis of ctDNA in 27 patients with available samples and detectable pretreatment variants. Samples were available from 36 patients, of which 29 were of sufficient quantity (> 10 ng) and quality (low genomic DNA contamination) from baseline samples for library preparation, two patients had sufficient ctDNA available but did not have detectible high-confidence mutations at baseline, and thus they were excluded from additional analysis. Pathogenic coding variants are shown, arranged according to their reported frequency in LymphGen genetic subtypes. A bubble plot shows the percentage of variants detectable at each time point (size) and the relative variant allele fraction (color). PET-CT assessments for the patients are colored according to response. (B) PET-CT response after two cycles of rituximab, lenalidomide, and ibrutinib is shown for patients with COO subtype by the DLBCL90 assay. (C) Molecular response at C2D1 is shown for patients with both COO subtype by DLBCL90 assay and ctDNA assessment available. ABC, activated B-cell-like; COO, cell of origin; CR, complete response; ctDNA, circulating tumor DNA; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell-like; LOD, limit of detection; MR, mixed response; NE, not evaluable; NR, no response; PET-CT, positron emission tomography-computed tomography; PR, partial response; SD, stable disease; Unclass., unclassified; VAF, variant allele frequency.
DLBCL90 was used to profile the baseline biopsy in 25 patients with sufficient biopsy material. Thirteen were classified ABC, five GCB, four unclassified, and quality criteria were not met in 3. None were classified as double-hit signature–positive by the DLBCL90. Of the two patients with TNFRSF14 and EZH2 mutations by ctDNA, one was also tested with DLBCL90 and confirmed GCB, resulting in six total patients with presumed GCB DLBCL. Although patients classified as ABC were more likely to achieve a CR with RLI, two of the six patients classified as GCB had a CR, and the remaining four had a PR before chemotherapy (Figs 2B and 2C).
Safety
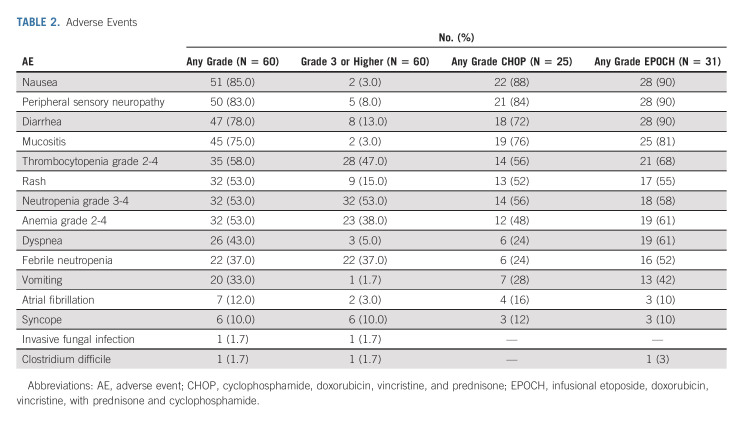
The most common adverse events (AEs, Table 2) were nausea, peripheral sensory neuropathy, diarrhea, and mucositis. Other AEs of interest included rash (grade 3: 16%, no patients discontinued therapy related to rash), febrile neutropenia in 38% of patients (24% of CHOP- and 52% of EPOCH-treated patients, respectively), and atrial fibrillation in 12% of patients.
TABLE 2.
Adverse Events
The mean (± standard deviation) relative dose intensities were 91 ± 17% for lenalidomide and 89 ± 18% for ibrutinib, with eight patients receiving a maximum starting dose of ibrutinib of 420 mg because of a protocol amendment. Overall, 21 and 11 patients had an adjustment to the lenalidomide and ibrutinib dose, respectively. All evaluable patients received two cycles of RLI; however, 16 patients (28.5%) received < 6 RLI-chemotherapy cycles (five cycles: 9, four cycles: 5, zero cycles: 2) because of a combination of physician and/or patient preference after early treatment response and/or toxicity. Treatment delay because of unresolved hematologic toxicity was rare, affecting 1.5% of RLI-chemotherapy cycles.
Features Associated With Treatment Response
Baseline clinical parameters were evaluated for association with CR after cycle 2 of RLI alone: lower LDH levels (median: 427 v 615 U/L, normal < 225, P = .038, Data Supplement), longer time from diagnosis to treatment (median: 32 v 27 days, P = .0499), and lack of bulky disease (P = .04) were significantly associated with CR on univariate analysis. No baseline clinical parameters were associated with end of treatment CR.
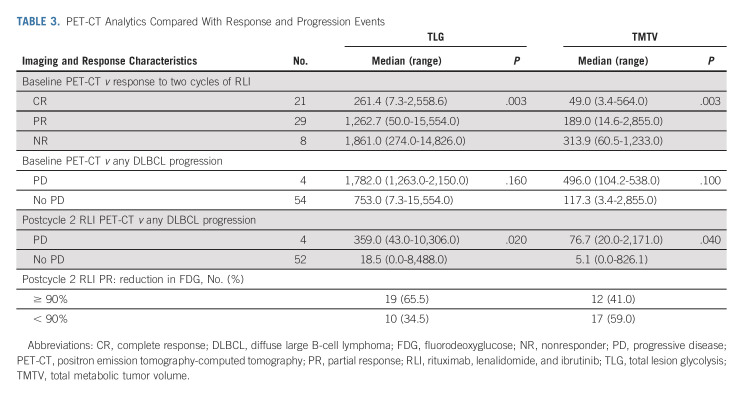
The baseline PET-CT TMTV and TLG correlated with response after cycle 2 of RLI, but not with the end of treatment response or with progression of DLBCL (Table 3). Both TMTV and TLG after cycle 2 of RLI correlated with progression of DLBCL. Of the 50% of patients with PR before chemotherapy, nearly half had a ≥ 90% reduction in tumor burden quantified by fluorodeoxyglucose avidity compared with baseline.
TABLE 3.
PET-CT Analytics Compared With Response and Progression Events
DISCUSSION
The Smart Start trial establishes the safety and efficacy of a targeted therapy combination in patients with newly diagnosed DLBCL before the confounding and/or immunosuppressive effects of chemotherapy, allowing for potential synergy of the targeted agents. The combination of RLI for two cycles had impressive clinical activity in patients with newly diagnosed DLBCL, with 86% achieving an objective response, including 36% having a CR before any chemotherapy, and only one patient having progressive disease. Direct comparisons of the CR rate after two cycles of RLI with historical results from R-CHOP are not possible; however, a large meta-analysis identified the CR rate after one and two cycles of R-CHOP to be 37.7% and 58.6%, respectively.39 The Smart Start trial was designed for all patients to receive consolidation with RLI-chemotherapy, regardless of initial response, because of the known curative potential of chemoimmunotherapy in newly diagnosed DLBCL. After one cycle of RLI, more than half of patients with data available achieved an early molecular response defined by > 2-log reduction of ctDNA, which was previously reported to be an excellent surrogate of early clinical response and to be associated with a longer survival after chemoimmunotherapy for DLBCL.38 Further speaking to the depth of response with RLI, 69% of patients had ≥ 90% reduction in tumor burden defined by TLG before chemotherapy (36.2% with CR and 32.8% with PR). It is unclear whether additional cycles of RLI alone would have further increased the CR rate, and whether CR after RLI may have been durable without chemotherapy consolidation. The patient who elected to stop therapy after only two cycles of RLI resulting in a remission ongoing at > 3.9 years had CD79B and PIM1 mutations, which are most frequent in the MCD and C5 subtypes, predicted to be sensitive to BTK inhibition because of chronic active B-cell receptor signaling and constitutive nuclear factor κB activity.40,41 The 28.5% of patients who received < 8 cycles of therapy, which may have been influenced by high rates of interim clinical responses, had no difference in clinical outcomes, suggesting that targeted therapy combinations could allow for a further reduction in or replacement of chemotherapy, which could decrease toxicity with comparable efficacy. The Smart Start concept establishes a new precedent for the feasibility of window trial designs to evaluate targeted therapy combinations in patients with untreated DLBCL subsets, such as those defined by the LymphGen or Cluster classifications.41,42 Our favorable results, potentially influenced by a small number of patients and single-center accrual at a tertiary referral center, warrant further validation; however, our approach has been preliminarily replicated by other investigators,43,44 and future similar trials are planned. The potential for targeted therapy combinations to reduce or remove the need for chemotherapy is further being explored in an ongoing clinical trial that uses response to targeted therapy to modify the amount of chemotherapy delivered (Smart Stop, ClinicalTrials.gov identifier: NCT04978584), with the planned next steps if successful of conducting a phase III trial comparing this approach with standard chemotherapy. This design concept could allow for unique targeted therapy combinations to be used in different DLBCL subsets with a single control arm.45,46
When RLI was combined with chemotherapy, 100% of evaluable patients responded, including 94.5% with a CR. All patients with PR were without relapse for > 2 years without additional therapy, implying a false-positive PET-CT. Although direct cross-trial comparisons are not feasible, it is informative to consider results from phase III trials that included chemotherapy with either ibrutinib or lenalidomide in patients with newly diagnosed ABC DLBCL. R-CHOP with and without ibrutinib, and with and without lenalidomide resulted in CR rates of 68%, 67.3%, 69%, and 65%, respectively.5,6 With a mature median follow-up of 31 months, Smart Start resulted in PFS and OS rates at 2 years of 91.3% and 96.6%, respectively. R-CHOP with and without ibrutinib resulted in a 3-year PFS rates of 70.8% and 68.1%, respectively, and R-CHOP with and without lenalidomide resulted in a 2-year PFS rates of 67% and 64%, respectively.5,6 Although the efficacy was promising, toxicity was generally comparable with R-CHOP-like trials,5,6,24 including two patients (3.3%) who died from therapy-related infectious AEs. One death was due to an invasive CNS aspergillosis in the only patient who progressed during RLI alone after a steroid prephase treatment was administered while waiting for study activation, which has subsequently been described as occurring in other patients treated with ibrutinib and corticosteroids.47,48 No additional fungal infections were observed when steroid prephase therapy was subsequently prohibited. It is possible the toxicity of ibrutinib and lenalidomide were not additive to chemotherapy because of a reduction in the tumor burden and/or immune-related effects of the initial cycles of RLI alone.
We enrolled non-GCB DLBCL patients, defined by IHC testing, on the assumption RLI would be of marginal benefit in GCB patients. Interestingly, our ctDNA and GEP analyses identified six patients (10%) with presumed GCB DLBCL, discordantly identified as non-GCB via IHC, all of whom responded to RLI alone (CR: 2 and PR: 4), in contrast with the historical 30% ORR with RLI in patients with relapsed GCB DLBCL.22 The ctDNA-classified GCB patients had both EZH2 and TNFRSF14 mutations, which are seed features for the EZB classification and prevalent in the analogous C3 subtype.40,41 The EZB/C3 subtype has genetic similarities to follicular lymphoma, in which rituximab and lenalidomide are highly active in part through repair of dysfunctional immune synapse formation with T cells.49,50 Although BTK inhibition may play a limited role in GCB DLBCL, ibrutinib has also been shown to have immune-potentiating activity.20 The response to RLI in these GCB patients may therefore be influenced more by immunomodulatory effects, potentially amplified by our design of RLI before immunosuppressive chemotherapy, than by direct lymphoma cytotoxicity.19
In conclusion, the combination of RLI alone and with chemotherapy resulted in high response rates and promising survival outcomes in patients with newly diagnosed DLBCL. Our novel trial design of a targeted therapy combination before chemotherapy demonstrates the feasibility of this approach and establishes a new precedent for biologically guided innovative first-line trials for patients with DLBCL.
ACKNOWLEDGMENT
The authors wish to acknowledge the important contributions of their treasured colleague Dr Francesco Turturro, including enrolling on and helping design the trial, and providing excellent care to his patients. The authors also wish to acknowledge the support of the Conquer Cancer Foundation and the Schweitzer Family Foundation who supported the translational research of this trial.
Jason Westin
Consulting or Advisory Role: Novartis, Kite/Gilead, Janssen Scientific Affairs, Amgen, MorphoSys, Curis, Forty Seven, ADC Therapeutics, Karyopharm Therapeutics, Iksuda Therapeutics, IMV, Bristol Myers Squibb/Celgene/Juno, AstraZeneca, Umoja Biopharma, Genentech/Roche, AbbVie, Merck, Monte Rosa Therapeutics
Research Funding: Celgene, Janssen, Genentech, Novartis, Kite/Gilead, Bristol Myers Squibb, Curis, MorphoSys, Forty Seven, AstraZeneca
R. Eric Davis
Research Funding: Karus Therapeutics (Inst)
Fredrick Hagemeister
Consulting or Advisory Role: Genentech
Raphael Steiner
Research Funding: Seattle Genetics, BMSi, Rafael Pharmaceuticals, GlaxoSmithKline
Travel, Accommodations, Expenses: Lymphoma Research Foundation
Hun Ju Lee
Honoraria: Aptitude Health, Cancer Experts Now, Curio Science, Century Therapeutics
Consulting or Advisory Role: BMS, Guidepoint Global
Research Funding: Seattle Genetics, BMS, Takeda, Oncternal Therapeutics, Celgene
Luis Fayad
Consulting or Advisory Role: EUSA Pharma, Roche, Medicamenta Ecuatoriana
Loretta Nastoupil
Honoraria: Gilead Sciences, Novartis, Bayer, Janssen Oncology, TG Therapeutics, Bristol Myers Squibb, ADC Therapeutics, Morphosys, Epizyme, Genmab, Takeda, Genentech/Roche
Research Funding: Janssen Biotech, Celgene, Genentech/Roche, Epizyme, Novartis, IgM Biosciences, Caribou Biosciences, Gilead Sciences, Allogene Therapeutics, Takeda
Sairah Ahmed
Honoraria: Seattle Genetics, Novartis
Consulting or Advisory Role: Tessa Therapeutics, Sanofi, Myeloid Therapeutics
Research Funding: Seattle Genetics (Inst), Merck (Inst), Tessa Therapeutics (Inst), Xencor (Inst), Chimagen (Inst)
Michelle Fanale
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Felipe Samaniego
Uncompensated Relationships: TG THer
Swaminathan P. Iyer
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, Legend Biotech, Yingli Pharma, Secura Bio
Speakers' Bureau: Targeted Oncology
Research Funding: Rhizen Pharmaceuticals (Inst), Seattle Genetics (Inst), Crispr Therapeutics (Inst), Spectrum Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Affimed Therapeutics (Inst), Innate Pharma, Legend Biotech, Merck
Travel, Accommodations, Expenses: Takeda, Gilead Sciences
Ranjit Nair
Honoraria: Incyte, ScienciaCME
Yasuhiro Oki
Employment: Jazz Pharmaceuticals (I), Genentech
Stock and Other Ownership Interests: Jazz Pharmaceuticals (I), Genentech/Roche
Nathan Fowler
Employment: BostonGene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Bayer, Novartis, Bristol Myers Squibb/Pfizer
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie, BeiGene
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead Company, Miltenyi Biomedicine, Moffit Cancer Center, Physicans' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, Bioinvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead Company, Innocare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, Pepromene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead Company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead Company, Physician Education Resources (PER)
Francisco Vega
Honoraria: i3 Health
Research Funding: Crispr Therapeutics, Geron
Chelsea Pinnix
Employment: MD Anderson Cancer Center
Stock and Other Ownership Interests: Neumentum (I)
Honoraria: International Journal of Radiation Oncology Biology Physics
Research Funding: Merck
Travel, Accommodations, Expenses: Merck
Yang Lu
Employment: ORIC Pharmaceuticals (I)
Stock and Other Ownership Interests: Regeneron (I)
Consulting or Advisory Role: GE Healthcare
Sanjit Tewari
Stock and Other Ownership Interests: CoapTech, STARTON Therapeutics, Clovis Oncology (I), Intrommune Therapeutics, Aurie, DocPanel, UE Life Sciences, Lutroo Imaging
Consulting or Advisory Role: CoapTech
Ryan Sun
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Sanofi
David W. Scott
Consulting or Advisory Role: Janssen, AbbVie, AstraZeneca, Incyte
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma; as a member of the LLMPP, I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell of origin in diffuse large B-cell lymphoma; I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma; named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL
Christopher R. Flowers
Stock and Other Ownership Interests: Foresight Diagnostics
Consulting or Advisory Role: Bayer, Gilead Sciences, Spectrum Pharmaceuticals, AbbVie, Celgene, Denovo Biopharma, BeiGene, Karyopharm Therapeutics, Pharmacyclics/Janssen, Genentech/Roche, Epizyme, Genmab, Seattle Genetics, Foresight Diagnostics, Bristol Myers Squibb/Celgene, Curio Science, AstraZeneca, MorphoSys
Research Funding: Acerta Pharma (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), AbbVie (Inst), Millennium (Inst), Alimera Sciences (Inst), Xencor (Inst), 4D Pharma (Inst), Adaptimmune (Inst), Amgen (Inst), Bayer (Inst), Cellectis (Inst), EMD Serono (Inst), Guardant Health (Inst), Iovance Biotherapeutics (Inst), Kite/Gilead (Inst), MorphoSys (Inst), Nektar (Inst), Novartis (Inst), Pfizer (Inst), Sanofi (Inst), Takeda (Inst), ZIOPHARM Oncology (Inst)
Sattva Neelapu
Stock and Other Ownership Interests: Longbow Immunotherapy Inc
Honoraria: Bio Ascend, Medscape, Aptitude Health, MJH Life Sciences
Consulting or Advisory Role: Merck Sharp & Dohme, Kite, a Gilead Company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, Bristol Myers Squibb, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, Bluebird Bio, Sana Biotechnology
Research Funding: Bristol Myers Squibb, Kite, a Gilead Company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patents related to cellular therapy, royalty income from Takeda Pharmaceuticals
Michael R. Green
Stock and Other Ownership Interests: KDAc Therapeutics
Honoraria: Daiichi Sankyo, Tessa Therapeutics, Monte Rosa Therapeutics
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Allogene Therapeutics, Kite, a Gilead Company, Sanofi, AbbVie
Travel, Accommodations, Expenses: AbbVie
No other potential conflicts of interest were reported.
See accompanying editorial on page 724
PRIOR PRESENTATION
Presented at ASCO 2019 annual meeting, Chicago, IL, May 31-June 4, 2019; ICML 2019 meeting, Lugano, Switzerland, June 18-22, 2019; ASH 2019 annual meeting, Orlando, FL, December 7-10, 2019.
SUPPORT
Supported by Conquer Cancer, Schweitzer Family Foundation, Celgene, and Janssen. The trial was in part supported by the NIH through MD Anderson's Cancer Center Support Grant CA016672.
CLINICAL TRIAL INFORMATION
NCT02636322 (Smart Start) and NCT04978584
DATA SHARING STATEMENT
Deidentified patient data used in this publication may be shared for reasonable requests to the corresponding author for up to 2 years after publication.
AUTHOR CONTRIBUTIONS
Conception and design: Jason Westin, R. Eric Davis, Sattva Neelapu
Administrative support: Donna Griffith
Provision of study materials or patients: Fredrick Hagemeister, Loretta Nastoupil, Sairah Ahmed, Alma Rodriguez, Michelle Fanale, Felipe Samaniego, Swaminathan P. Iyer, Ranjit Nair, Yasuhiro Oki, Michael Wang, Donna Griffith, Sattva Neelapu
Collection and assembly of data: Jason Westin, Fredrick Hagemeister, Raphael Steiner, Hun Ju Lee, Loretta Nastoupil, Alma Rodriguez, Swaminathan P. Iyer, Yasuhiro Oki, Nathan Fowler, Donna Griffith, Yang Lu, Sanjit Tewari, David W. Scott, Sattva Neelapu, Michael R. Green
Data analysis and interpretation: Jason Westin, R. Eric Davis, Lei Feng, Fredrick Hagemeister, Raphael Steiner, Luis Fayad, Loretta Nastoupil, Sairah Ahmed, Michelle Fanale, Felipe Samaniego, Ranjit Nair, Nathan Fowler, Michael Wang, Man Chun John Ma, Francisco Vega, Timothy McDonnell, Chelsea Pinnix, Yang Lu, Sanjit Tewari, Ryan Sun, David W. Scott, Christopher R. Flowers, Sattva Neelapu, Michael R. Green
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Smart Start: Rituximab, Lenalidomide, and Ibrutinib in Patients With Newly Diagnosed Large B-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jason Westin
Consulting or Advisory Role: Novartis, Kite/Gilead, Janssen Scientific Affairs, Amgen, MorphoSys, Curis, Forty Seven, ADC Therapeutics, Karyopharm Therapeutics, Iksuda Therapeutics, IMV, Bristol Myers Squibb/Celgene/Juno, AstraZeneca, Umoja Biopharma, Genentech/Roche, AbbVie, Merck, Monte Rosa Therapeutics
Research Funding: Celgene, Janssen, Genentech, Novartis, Kite/Gilead, Bristol Myers Squibb, Curis, MorphoSys, Forty Seven, AstraZeneca
R. Eric Davis
Research Funding: Karus Therapeutics (Inst)
Fredrick Hagemeister
Consulting or Advisory Role: Genentech
Raphael Steiner
Research Funding: Seattle Genetics, BMSi, Rafael Pharmaceuticals, GlaxoSmithKline
Travel, Accommodations, Expenses: Lymphoma Research Foundation
Hun Ju Lee
Honoraria: Aptitude Health, Cancer Experts Now, Curio Science, Century Therapeutics
Consulting or Advisory Role: BMS, Guidepoint Global
Research Funding: Seattle Genetics, BMS, Takeda, Oncternal Therapeutics, Celgene
Luis Fayad
Consulting or Advisory Role: EUSA Pharma, Roche, Medicamenta Ecuatoriana
Loretta Nastoupil
Honoraria: Gilead Sciences, Novartis, Bayer, Janssen Oncology, TG Therapeutics, Bristol Myers Squibb, ADC Therapeutics, Morphosys, Epizyme, Genmab, Takeda, Genentech/Roche
Research Funding: Janssen Biotech, Celgene, Genentech/Roche, Epizyme, Novartis, IgM Biosciences, Caribou Biosciences, Gilead Sciences, Allogene Therapeutics, Takeda
Sairah Ahmed
Honoraria: Seattle Genetics, Novartis
Consulting or Advisory Role: Tessa Therapeutics, Sanofi, Myeloid Therapeutics
Research Funding: Seattle Genetics (Inst), Merck (Inst), Tessa Therapeutics (Inst), Xencor (Inst), Chimagen (Inst)
Michelle Fanale
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Felipe Samaniego
Uncompensated Relationships: TG THer
Swaminathan P. Iyer
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, Legend Biotech, Yingli Pharma, Secura Bio
Speakers' Bureau: Targeted Oncology
Research Funding: Rhizen Pharmaceuticals (Inst), Seattle Genetics (Inst), Crispr Therapeutics (Inst), Spectrum Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Affimed Therapeutics (Inst), Innate Pharma, Legend Biotech, Merck
Travel, Accommodations, Expenses: Takeda, Gilead Sciences
Ranjit Nair
Honoraria: Incyte, ScienciaCME
Yasuhiro Oki
Employment: Jazz Pharmaceuticals (I), Genentech
Stock and Other Ownership Interests: Jazz Pharmaceuticals (I), Genentech/Roche
Nathan Fowler
Employment: BostonGene
Consulting or Advisory Role: Roche/Genentech, TG Therapeutics, Bayer, Novartis, Bristol Myers Squibb/Pfizer
Research Funding: Roche, Celgene, Gilead Sciences, TG Therapeutics, Novartis, AbbVie, BeiGene
Michael Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead Company, Miltenyi Biomedicine, Moffit Cancer Center, Physicans' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, Bioinvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead Company, Innocare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Lilly, Pepromene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead Company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, Innocare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead Company, Physician Education Resources (PER)
Francisco Vega
Honoraria: i3 Health
Research Funding: Crispr Therapeutics, Geron
Chelsea Pinnix
Employment: MD Anderson Cancer Center
Stock and Other Ownership Interests: Neumentum (I)
Honoraria: International Journal of Radiation Oncology Biology Physics
Research Funding: Merck
Travel, Accommodations, Expenses: Merck
Yang Lu
Employment: ORIC Pharmaceuticals (I)
Stock and Other Ownership Interests: Regeneron (I)
Consulting or Advisory Role: GE Healthcare
Sanjit Tewari
Stock and Other Ownership Interests: CoapTech, STARTON Therapeutics, Clovis Oncology (I), Intrommune Therapeutics, Aurie, DocPanel, UE Life Sciences, Lutroo Imaging
Consulting or Advisory Role: CoapTech
Ryan Sun
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Sanofi
David W. Scott
Consulting or Advisory Role: Janssen, AbbVie, AstraZeneca, Incyte
Research Funding: Janssen, Roche/Genentech, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Named inventor on a pending patent describing gene expression profiling in prognostication in classical Hodgkin lymphoma; as a member of the LLMPP, I am potentially a named inventor on a pending patent on the use of gene expression profiling to assign cell of origin in diffuse large B-cell lymphoma; I am a named inventor on a pending patent on the use of gene expression profiling to determine the proliferation signature in mantle cell lymphoma; named inventor on a pending patent describing using gene expression profiling to identify molecular subtypes of GCB-DLBCL
Christopher R. Flowers
Stock and Other Ownership Interests: Foresight Diagnostics
Consulting or Advisory Role: Bayer, Gilead Sciences, Spectrum Pharmaceuticals, AbbVie, Celgene, Denovo Biopharma, BeiGene, Karyopharm Therapeutics, Pharmacyclics/Janssen, Genentech/Roche, Epizyme, Genmab, Seattle Genetics, Foresight Diagnostics, Bristol Myers Squibb/Celgene, Curio Science, AstraZeneca, MorphoSys
Research Funding: Acerta Pharma (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), AbbVie (Inst), Millennium (Inst), Alimera Sciences (Inst), Xencor (Inst), 4D Pharma (Inst), Adaptimmune (Inst), Amgen (Inst), Bayer (Inst), Cellectis (Inst), EMD Serono (Inst), Guardant Health (Inst), Iovance Biotherapeutics (Inst), Kite/Gilead (Inst), MorphoSys (Inst), Nektar (Inst), Novartis (Inst), Pfizer (Inst), Sanofi (Inst), Takeda (Inst), ZIOPHARM Oncology (Inst)
Sattva Neelapu
Stock and Other Ownership Interests: Longbow Immunotherapy Inc
Honoraria: Bio Ascend, Medscape, Aptitude Health, MJH Life Sciences
Consulting or Advisory Role: Merck Sharp & Dohme, Kite, a Gilead Company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, Bristol Myers Squibb, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, Bluebird Bio, Sana Biotechnology
Research Funding: Bristol Myers Squibb, Kite, a Gilead Company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patents related to cellular therapy, royalty income from Takeda Pharmaceuticals
Michael R. Green
Stock and Other Ownership Interests: KDAc Therapeutics
Honoraria: Daiichi Sankyo, Tessa Therapeutics, Monte Rosa Therapeutics
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Allogene Therapeutics, Kite, a Gilead Company, Sanofi, AbbVie
Travel, Accommodations, Expenses: AbbVie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Wild CP, Weiderpass E, Stewart BW (eds): World Cancer Report: Cancer Research for Cancer Prevention. Lyon, France, International Agency for Research on Cancer, 2020 [Google Scholar]
- 2.McKelvey EM, Gottlieb JA, Wilson HE, et al. : Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer 38:1484-1493, 1976 [DOI] [PubMed] [Google Scholar]
- 3. National Comprehensive Cancer Network (NCCN): NCCN Clinical Practice Guidelines in Oncology. B-cell Lymphomas Version 5.2022. National Comprehensive Cancer Network, 2021. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf. [DOI] [PubMed]
- 4.Roschewski M, Staudt LM, Wilson WH: Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol 11:12-23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Sehn LH, Johnson P, et al. : A global, randomized, placebo-controlled, phase 3 study of ibrutinib plus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) in patients with previously untreated non-germinal center B-cell-like (GCB) diffuse large B-cell lymphoma (DLBCL). Blood 132:784-784, 2018 [Google Scholar]
- 6.Nowakowski GS, Chiappella A, Gascoyne RD, et al. : ROBUST: A phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol 39:1317-1328, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehn LH, Salles G: Diffuse large B-cell lymphoma. N Engl J Med 384:842-858, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilly H, Morschhauser F, Sehn LH, et al. : Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med 386:351-363, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherng HJ, Westin J: Why R-CHOP + X is not enough: Lessons learned and next steps in the mission to improve frontline therapy for diffuse large B-cell lymphoma. Leuk Lymphoma 62:1302-1312, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westin J: Ibrutinib and lenalidomide: When 1+1 = >2. Blood 134:996-998, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Hans CP, Weisenburger DD, Greiner TC, et al. : Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275-282, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Rosenwald A, Wright G, Chan WC, et al. : The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937-1947, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Davis RE, Ngo VN, Lenz G, et al. : Chronic active B cell receptor signaling in diffuse large B cell lymphoma. Nature 463:88-92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RE, Brown KD, Siebenlist U, et al. : Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 194:1861-1874, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. : Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 117:5058-5066, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Fowler N, Wagner-Bartak N, et al. : Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: A phase II clinical trial. Leukemia 27:1902-1909, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Shaffer AL III, Emre NC, et al. : Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 21:723-737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson WH, Young RM, Schmitz R, et al. : Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med 21:922-926, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gribben JG, Fowler N, Morschhauser F: Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol 33:2803-2811, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, et al. : Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA 112:E966-E972, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubovsky JA, Beckwith KA, Natarajan G, et al. : Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 122:2539-2549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goy A, Ramchandren R, Ghosh N, et al. : Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non–germinal center B-cell–like DLBCL. Blood 134:1024-1036, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson WH, Phillips T, Popplewell L, et al. : Phase 1b/2 study of ibrutinib and lenalidomide with dose-adjusted EPOCH-R in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma 62:2094-2106, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartlett NL, Wilson WH, Jung SH, et al. : Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: Clinical outcomes of the phase III intergroup trial Alliance/CALGB 50303. J Clin Oncol 37:1790-1799, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. : Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048-3058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasanelli M, Meignan M, Haioun C, et al. : Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 41:2017-2022, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Vercellino L, Cottereau AS, Casasnovas O, et al. : High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood 135:1396-1405, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim TM, Paeng JC, Chun IK, et al. : Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer 119:1195-1202, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Deng Q, Han G, Puebla-Osorio N, et al. : Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med 26:1878-1887, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ennishi D, Jiang A, Boyle M, et al. : Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 37:190-201, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Washington, DC, Department of Health and Human Services, 2010. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Google Scholar]
- 33.Thall PF, Sung HG: Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 17:1563-1580, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Sehn LH, Berry B, Chhanabhai M, et al. : The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857-1861, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Riedell PA, Smith SM: Double hit and double expressors in lymphoma: Definition and treatment. Cancer 124:4622-4632, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Maurer MJ, Ghesquieres H, Link BK, et al. : Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol 36:1603-1610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morin RD, Johnson NA, Severson TM, et al. : Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42:181-185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurtz DM, Scherer F, Jin MC, et al. : Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma. J Clin Oncol 36:2845-2853, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eertink JJ, Burggraaff CN, Heymans MW, et al. : Optimal timing and criteria of interim PET in DLBCL: A comparative study of 1692 patients. Blood Adv 5:2375-2384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz R, Wright GW, Huang DW, et al. : Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378:1396-1407, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapuy B, Stewart C, Dunford AJ, et al. : Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679-690, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright GW, Huang DW, Phelan JD, et al. : A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 37:551-568.e14, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkes EA, Manos K, Smith C, et al. : AvR-CHOP: Feasibility study of induction and maintenance avelumab plus R-CHOP in patients with diffuse large B-cell lymphoma (DLBCL). Blood 134:5332-5332, 2019 [Google Scholar]
- 44.Roschewski M, Phelan JD, Pittaluga S, et al. : Phase 2 study of acalabrutinib window prior to frontline therapy in untreated aggressive B-cell lymphoma: Preliminary results and correlatives of response to acalabrutinib. Blood 138, 2021. (abstr 524) [Google Scholar]
- 45.Zhang M, Xu P, Wang L, et al. : Genetic subtype guided rituximab-based immunochemotherapy improves outcome in newly diagnosed diffuse large B-cell lymphoma: First report of a randomized phase 2 study. Hematologic Oncol 39, 2021 [Google Scholar]
- 46.Liefaard MC, Lips EH, Wesseling J, et al. : The way of the future: Personalizing treatment plans through technology. Am Soc Clin Oncol Ed Book 41:1-12, 2021 [DOI] [PubMed] [Google Scholar]
- 47.Lionakis MS, Dunleavy K, Roschewski M, et al. : Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 31:833-843.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghez D, Calleja A, Protin C, et al. : Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 131:1955-1959, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Morschhauser F, Fowler NH, Feugier P, et al. : Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med 379:934-947, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramsay AG, Clear AJ, Kelly G, et al. : Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implications for the tumor microenvironment and immunotherapy. Blood 114:4713-4720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified patient data used in this publication may be shared for reasonable requests to the corresponding author for up to 2 years after publication.