Mantle cell lymphoma (MCL) is a rare and heterogeneous subgroup of B-cell lymphomas. The clinical course of MCL varies from an indolent form to an extremely aggressive phenotype. MCL is still an incurable disease. However, intensified chemoimmunotherapy regimens and targeted therapies have increased the overall survival (OS) of patients <65 years old from 5.6 years to 8.9 years.1 Today, the standard treatment for fit patients <65–70 years is high-dose cytarabine-containing immunochemotherapy regimens followed by autologous stem cell transplantation (ASCT).2 The MCL2 protocol developed by the Nordic Lymphoma Group is one of these approaches.3,4 The MCL2 protocol has shown a median OS of 12.7 years at the 15-year follow-up and a median progression-free survival (PFS) of 8.5 years.4 Similar results have been obtained in other high-dose regimen trials.5–7 This study aimed to evaluate the efficacy of the MCL2 protocol in a population-based retrospective analysis and to compare treatment results in patients fulfilling the trial eligibility criteria with those who would have been trial ineligible.
We collected data on patients diagnosed with MCL from 7 hospitals in Finland and 1 hospital in Spain from 2000 to 2020. This study was approved by the Finnish Social and Health Data Permit Authority. In total, 520 patients were included in this study. Of these, 198 patients (38%) received treatment according to the MCL2 protocol (the MCL2 group). The MCL2 protocol started with the induction treatment of rituximab–maxi-CHOP, alternating with rituximab–high-dose-cytarabine. ASCT, preceded by BEAM therapy (carmustine–etoposide–cytarabine–melphalan), was used as a consolidation therapy after 6 immunochemotherapy cycles.3 Rituximab maintenance therapy has been used as an option after ASCT in recent years. The remaining 322 patients (the other group) received rituximab combined with CHOP/CHOP-like regimens, bendamustine, or high-dose cytarabine-based regimens, MCL-FI (a Finnish protocol for elderly patients),8 single-agent rituximab, or irradiation. To evaluate the impact of the patient selection criteria on survival, we divided the patients treated with the MCL2 protocol into trial-eligible and trial-ineligible subgroups, following the MCL2 trial inclusion criteria, which were an Eastern Cooperative Oncology Group (ECOG) performance test status of 0–2, stage II–IV disease, and age <66 years.3 To obtain comparable groups, we excluded the patients with missing trial inclusion parameters in all categories. The patient-specific characteristics of the other group, the MCL2 group, and the subgroups of trial-eligible and trial-ineligible patients, along with the actual MCL2 trial,3 are summarized in Table 1. The median age was higher in the trial-ineligible group (67 years) than in the trial-eligible group (59 years), and Mantle Cell Lymphoma International Prognostic Index (MIPI) high-risk disease was more common in the trial-ineligible group (37.7% versus 20.9%).
Table 1.
Patient-specific Characteristics of the Other Group, all MCL2-treated, Trial-eligible (Stage II–IV, Age <66 Years, ECOG 0–2), and Trial-ineligible Patients
| Other | MCL2 | Trial Eligible | Trial Ineligible | Clinical MCL2 Trial |
|
|---|---|---|---|---|---|
| Patient number | 322 | 198 | 129 | 69 | 160 |
| Male | 229 (69.0%) | 153 (77.3%) | 104 (80.6%) | 49 (71.0%) | 113 (70.6%) |
| Median age, y | 75 (43–91) | 62 (27–79) | 59 (32–65) | 67 (27–79) | 56 (32–65) |
| <66 years | 39 (12.1%) | 145 (73.2%) | 129 (100%) | 16 (23.2%) | 160 (100%) |
| Stage | |||||
| I | 27 (8.4%) | <5 (2.5%) | 0 | <5 (7.1%) | 0 |
| II | 26 (8.1%) | 17 (8.5%) | 9 (7.0%) | 8 (11.4%) | 24 (15%) |
| III | 63 (19.6%) | 23 (11.6%) | 15 (11.6%) | 8 (11.4%) | |
| IV | 206 (63.9%) | 154 (77.4%) | 105 (81.4%) | 49 (68.1%) | 136 (85%) |
| ECOGa | |||||
| 0 | 80 (26.5%) | 92 (47.4%) | 65 (50.4%) | 27 (41.5%) | 148 (93%) |
| 1 | 137 (45.4%) | 77 (39.7%) | 54 (41.8%) | 23 (35.4%) | |
| 2 | 50 (16.6%) | 15a (7.7%) | 10 (7.8%) | <5 (7.7%) | 12 (7%) |
| 3 | 33 (10.9%) | 5 (2.6%) | 0 | 5 (7.7%) | 0 |
| 4 | <5 (1.6%) | <5 (2.6%) | 0 | <5 (7.7%) | 0 |
| MIPI risk groups | (15.9% missing) | (14.6% missing) | (17.0% missing) | (10.1% missing) | (1.9% missing) |
| Low | 12 (3.7%) | 45 (22.7%) | 38 (29.5%) | 7 (10.1%) | 79 (50%) |
| Intermediate | 78 (24.2%) | 71 (35.9%) | 42 (32.6%) | 29 (42.1%) | 41 (26%) |
| High | 181 (56.2%) | 53 (26.8%) | 27 (20.9%) | 26 (37.7%) | 37 (24%) |
aIn one of the centers, ECOG 0–2 patients were assigned to the same group (performance status at least ECOG 2).
ECOG = Eastern Cooperative Oncology Group; MCL = mantle cell lymphoma; MIPI = Mantle Cell Lymphoma International Prognostic Index.
The MCL2 trial patient characteristics, as referenced in the last column, have been adapted from Geisler et al.3
The MCL subtype was not reported in most pathology reports; therefore, the subgroup unclassified was used in addition to indolent, conventional, blastoid, and pleomorphic histologies. The MCL subtypes were as follows: the trial-eligible group, 77 unclassified, 21 conventional, 2 pleomorphic, 23 blastoid, and 6 missing; the trial-ineligible group, 29 unclassified, 1 indolent, 8 conventional, 2 pleomorphic, 11 blastoid, and 18 missing; and the other group, 162 unclassified, 7 indolent, 46 conventional, 12 pleomorphic, 32 blastoid, and 63 missing.
The OS (the time from diagnosis to death by any cause or last follow-up date), age- and gender-standardized net survival (NS), and PFS (the time from diagnosis to progression or death by any cause) were calculated according to the Kaplan–Meier method. Analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) version 27.0 (IBM Corp, Armonk, NY) and R software version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). A P-value of <0.05 was considered statistically significant. The MIPI risk classification was calculated according to Hoster et al.9
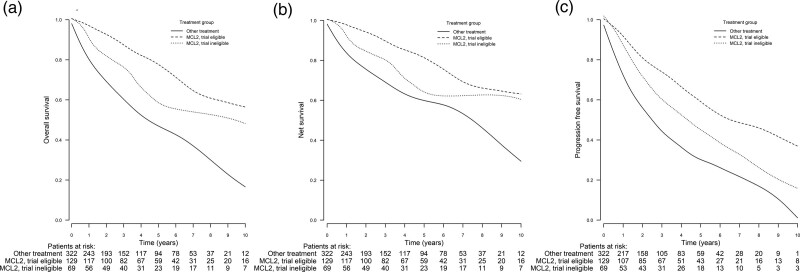
The estimated median OS for the entire MCL2 group was 12.8 years (95% confidence interval [CI], 7.9-17.6). The median OS of the other group was 4.3 years (95% CI, 3.3-5.4). The OS was significantly better in the MCL2 group than in the other group (P < 0.001). Of the 198 patients treated with the MCL2 protocol, 129 fulfilled all MCL2 trial inclusion criteria (Table 1). Age was the major discriminator that made many of the MCL2-treated patients ineligible. The 5-year OS rate of the trial-eligible group was 79% (95% CI, 71-87) compared with 59% (95% CI, 46-74) of the trial-ineligible group. For the other group, the 5-year OS rate was 47% (95% CI, 41-53) (Figure 1A; also, 2- and 10-year OS rates are included for additional information). The median OS of the trial-eligible patients was 14.3 years (95% CI, 7.20-21.3), and the median OS of the trial-ineligible patients was 9.8 years (95% CI, 6.30-13.3). The OS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001). In addition, the median OS in the trial-eligible group was improved compared with the trial-ineligible group (P < 0.001).
Figure 1.
Comparison of 2-, 5-, and 10-year OS, NS, and PFS of the MCL2-treated trial-eligible and trial-ineligible groups and the other group, who did not receive MCL2 treatment. (A) OS at 2, 5, and 10 years. The trial-eligible group: 93% (95% CI, 89-98), 79% (95% CI, 71-87), and 57% (95% CI, 45-72). The trial-ineligible group: 84% (95% CI, 75-94), 59% (95% CI, 46-74), and 49% (95% CI, 34-70). The other group: 70% (95% CI, 65-75), 47% (95% CI, 41-53), and 17% (95% CI, 11-25). The OS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001 in both comparisons). The median OS in the trial-eligible group was also improved compared with the trial-ineligible group (P < 0.001). (B) NS at 2, 5, and 10 years. The trial-eligible group: 95% (95% CI, 90-99), 82% (95% CI, 74-91), and 64% (95% CI, 51-80). The trial-ineligible group: 86% (95% CI, 77-96), 64% (95% CI, 51-81), and 61% (95% CI, 43-87). The other group: 76% (95% CI, 71-82), 60% (95% CI, 53-69), and 29% (95% CI, 20-44). NS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001 in both comparisons). There was no statistically significant difference between the trial-eligible and trial-ineligible groups (P = 0.368). (C) PFS at 2, 5, and 10 years. The trial-eligible group: 81% (95% CI, 74-89), 62% (95% CI, 53-73), and 40% (95% CI, 29-56). The trial-ineligible group: 72% (95% CI, 62-85), 46% (95% CI, 34-62), and 23% (95% CI, 11-45). The other group: 57% (95% CI, 51-63), 30% (95% CI, 25-37), and 2% (95% CI, 0-13). PFS was significantly improved both in the trial-eligible group (P < 0.001) and trial-ineligible (P = 0.003) group compared with the other group. The median PFS in the trial-eligible group was also improved compared with the trial-ineligible group (P = 0.030). CI = confidence interval; MCL = mantle cell lymphoma; NS = net survival; OS = overall survival; PFS = progression-free survival.
To determine whether the observed expansion of trial inclusion criteria in clinical practice leads to increased mortality and differences in frontline disease control, NS and PFS rates were calculated (Figure 1B, C). The 5-year NS rate of the trial-eligible group was 82% (95% CI, 74-91) compared with 64% (95% CI, 51-81) of the trial-ineligible group. For the other group, the 5-year NS rate was 60% (95% CI, 53-69) (Figure 1B; 2- and 10-year NS rates are also included for additional information). The NS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001). There was no significant difference between the trial-eligible and trial-ineligible groups (P = 0.368). The 5-year PFS rate was 46% (95% CI, 34-62) in the trial-ineligible group and 62% (95% CI, 53-73) in the trial-eligible group. The PFS rate in the other group was 30% (95% CI, 25-37) at 5 years (Figure 1C; 2- and 10-year PFS rates are also included for additional information). The estimated median PFS was 6.4 years (95% CI, 3.2-9.6) in the trial-eligible group, 4.4 years (95% CI, 2.6-6.8) in the trial-ineligible group, and 2.7 years (95% CI, 2.2-3.1) in the other group. The PFS rate was significantly improved both in the trial-eligible (P < 0.001) and trial-ineligible (P < 0.003) groups compared with the other group. There was also a significant PFS improvement in the trial-eligible group compared with the trial-ineligible group (P = 0.030). There was shorter survival in the trial-ineligible group after the first relapse, with a median OS of 4.2 years, compared with 1.8 years among trial-eligible patients (data not shown). After the first relapse, 73% of the trial-ineligible patients and 88% of the trial-eligible patients received salvage chemotherapy.
Rituximab maintenance every 2–3 months for 2 years is offered as a treatment option after ASCT, and in other treatment modalities for patients with MCL. In our study population, 33 patients (26%) in the trial-eligible group and 27 (39%) in the trial-ineligible group received rituximab maintenance. In the other group, 81 (25%) patients received rituximab maintenance. Only 10 patients discontinued rituximab treatment.
We found that patients selected to receive the MCL2 protocol were older (median age of 62 years, compared with the trial setting, where the median age was 56 years; Table 1), but younger than the other group, whose median age was 75 years. In the MCL2 trial3 and in our study, the patients selected to receive MCL2 treatment were fit and had stage II–IV disease. Many patients had a high-risk MIPI class in the other group (56.2%) compared with the MCL2 group (26.8%) and the MCL2 trial (24%; Table 1). A previous retrospective study comparing the efficacy of R-bendamustine, R-CHOP, and MCL2 as frontline therapies showed similar age, ECOG, and MIPI class distribution in MCL2-treated patients.10 We observed an increase in MIPI high-risk patients in the trial-ineligible group (37.7%), and the inclusion of ECOG 3–4 patients, showing that aggressive disease presentation is an important factor in selecting treatment strategies in clinical practice.
A previous phase 3 trial showed that an intensified treatment that includes high-dose cytarabine postpones the time-to-treatment failure in patients <65 years of age compared with anthracycline-based induction.7 Also, the Nordic MCL2 phase 2 study demonstrated a promising median OS of 12.7 years and a PFS of 8.5 years. Since the MCL2 and MCL3 trials3,11 were conducted, the upper age limit for this therapy has gradually increased to 75 years at some centers, and the therapy has been offered to patients with ECOG > 2 and more comorbidities, as also shown in this study. To date, no data on whether the results of the MCL2 and MCL3 trials can be extrapolated to real-world populations exist. We demonstrated an even higher OS of 14.3 years in a real-world setting among trial-eligible patients. Among trial-ineligible patients, the median OS was also remarkable (9.8 years). Albertsson-Linblad and colleagues10 reported a median OS of 4.9 years in patients receiving any systemic therapy in the frontline and also showed that both MCL2 and RB are superior over R-CHOP. We observed that the PFS improvement was greater in trial-eligible patients than in trial-ineligible patients (6.4 versus 4.4 years). The trial-ineligible patients were older and probably frailer with more comorbidities; therefore, the trial-eligible patients may also have better frontline disease control. Notably, we found no significant difference in the NS, suggesting that the MCL2 approach outside clinical trial inclusion does not substantively increase mortality. However, there are several limitations in our study. This was a retrospective study and the MCL subtype was not reported in most pathology reports. Also the patients with missing trial inclusion criteria data were excluded, although the subgroups were balanced for the analyses. To conclude, this study confirmed that the MCL2 protocol is an effective treatment approach for real-world patients with MCL. Importantly, the data show the importance of the expansion of trial inclusion criteria in real-world practice, as patients over 65 years of age may also benefit from the MCL2 strategy.
AUTHOR CONTRIBUTIONS
MH and TK did research design, data collection, analysis, and writing. OK and ARö did research design, funding, analysis, and writing. MS did data collection, analysis, and writing. TS did data analysis and figure preparation. MK, RP, EK, EA, ARa, KS, AJ, EJ, SK, JMS, and HK did data collection. All authors have read and approved the article.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was funded by North Ostrobothnia Health Care District.
Footnotes
MH, TK, ARö and OK have contributed equally to this work.
REFERENCES
- 1.Wu H, Wang J, Zhang X, et al. Survival trends in patients under age 65 years with mantle cell lymphoma, 1995-2016: a SEER-based analysis. Front Oncol. 2020;10:588314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddocks K. Update on mantle cell lymphoma. Blood. 2018;132:1647–1656. [DOI] [PubMed] [Google Scholar]
- 3.Geisler C, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175:410–418. [DOI] [PubMed] [Google Scholar]
- 5.Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53. [DOI] [PubMed] [Google Scholar]
- 6.Chihara D, Cheah CY, Westin JR, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol. 2016;172:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–575. [DOI] [PubMed] [Google Scholar]
- 8.Räty R, Honkanen T, Jantunen E, et al. Prolonged immunochemotherapy with rituximab, cytarabine and fludarabine added to cyclophosphamide, doxorubicin, vincristine and prednisolone and followed by rituximab maintenance in untreated elderly patients with mantle cell lymphoma: a prospective study by the Finnish Lymphoma Group. Leuk Lymphoma. 2012;53:1920–1928. [DOI] [PubMed] [Google Scholar]
- 9.Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol. 2014;32:1338–1346. [DOI] [PubMed] [Google Scholar]
- 10.Albertsson-Lindblad A, Palsdottir T, Smedby KE, et al. Survival in mantle cell lymphoma after frontline treatment with R-bendamustine, R-CHOP and the Nordic MCL2 regimen - a real-world study on patients diagnosed in Sweden 2007-2017. Haematologica. 2022;107:740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskelund CW, Dimopoulos K, Kolstad A, et al. Detailed long-term follow-up of patients who relapsed after the Nordic Mantle Cell Lymphoma Trials: MCL2 and MCL3. HemaSphere. 2020;5:e510. [DOI] [PMC free article] [PubMed] [Google Scholar]