Abstract
We investigated the utility of PCR to detect Burkholderia cepacia directly in sputum samples at two cystic fibrosis (CF) centers serving children and adults. Following liquefaction of the sputa by using N-acetyl-l-cysteine, DNA was isolated and analyzed by PCRs with three different primer pairs directed toward bacterial rRNA loci. Two primer pairs were putatively specific for B. cepacia. The other pair, which universally amplifies a band from all bacteria, served as a control. Sputum samples were obtained from 219 patients and analyzed independently by culture and by PCR to detect B. cepacia. The analyses were performed blinded with respect to each other. The results of the PCR with sputa demonstrated that the primers directed to the 16S loci demonstrated approximately 95% concordance with culture results and were more specific than those amplifying the 16S to 23S spacer region. In addition, the 16S primer pair putatively identified B. cepacia in seven patients whose sputa were culture negative at this time. Of these culture-negative patients, five had sputum samples that were culture positive for B. cepacia either prior or subsequent to this study. The results of this study indicate the utility of PCR as a diagnostic method for the rapid identification of B. cepacia in sputum samples of CF patients. We anticipate that improvements in our taxonomic understanding may allow the design of more specific primers for detection of each species of the B. cepacia complex in sputum samples.
Burkholderia cepacia is an important opportunistic pathogen which causes pulmonary infections among individuals with cystic fibrosis (CF) and chronic granulomatous disease (10, 12, 15, 20, 24). Isolates from patients display multidrug resistance and are refractory to treatment despite extensive drug regimens. Colonization with B. cepacia is associated with poor clinical prognosis, and as many as 20% of colonized individuals will succumb to B. cepacia syndrome, a necrotizing pneumonia associated with fever which culminates in a rapid and fatal clinical deterioration (12). Molecular and classic epidemiological investigations have confirmed person-to-person and nosocomial transmission (14, 17, 18, 21, 25). As a result, segregation policies have been adopted at most CF centers in attempts to limit the spread of B. cepacia to noncolonized individuals. Such drastic policies cause problems for the management of CF centers and may also lead to devastating social disruptions for the CF patient (1, 9). These factors have led to great interest in the prevention of acquisition of B. cepacia. However, efforts are hindered by the inability to accurately culture and identify B. cepacia in clinical samples. Selective media for B. cepacia are available. However, they may require up to 72 h for growth to become visible and they also support the growth of other gram-negative nonfermenting bacilli such as Comamonas acidovorans and Stenotrophomonas maltophila (basonym Xanthomonas maltophila) (2, 8, 10). Thus, presumptive isolates require further characterization by commercial multitest systems. For example, more complete biochemical testing of isolates previously identified as B. cepacia by commercial multitest systems has indicated that these isolates may be the closely related species B. gladioli (4). Accurate identification is further complicated by recent reports detailing the genetic diversity of B. cepacia. Although once thought to be a single species, B. cepacia comprises a complex of five different genomovars which may represent five new species (10, 27). B. cepacia isolates representative of each genomovar have been isolated from CF patients (28). These observations cast further doubt on the ability of microbiology laboratories to distinguish among the members of the B. cepacia complex.
A method to accurately detect all members of the B. cepacia complex directly in sputum, at low concentration, would offer opportunities to clarify some of these issues. Previously, we demonstrated the efficacy of the PCR in the epidemiologic analysis of B. cepacia in a number of outbreaks and the ability of PCR with B. cepacia-specific primers to identify the organism in sputum samples. In this study, we examined the ability of two sets of putatively B. cepacia-specific PCR primers to detect the B. cepacia complex directly in clinical samples and we compared the detection capability of PCR with that of the standard culture method.
MATERIALS AND METHODS
Study population.
Sputum samples were collected from patients attending the adult CF clinic at the Wellesley Hospital, Toronto, Ontario, Canada, during a 2-month period in 1995. This group contained 96 adults aged 17 through 56 years with a mean age of 30.1 years. Females represented 40.6% of this group. At the time of the study, the adult CF clinic at the Wellesley Hospital served 250 patients with a prevalence of B. cepacia, determined by culture, of 45%. An additional 110 sputum samples were collected from patients attending the CF center at the Hospital for Sick Children, Toronto, Ontario, Canada, over a 5-month period in 1995. This group contained 88 children aged 5 through 17 years with a mean age of 11.7 years and 5 adults aged 27.9 through 39.5 years with a mean age of 34.3 years. Females represented 54.5% of the subjects from the children’s clinic.
Collection of sputum samples.
Expectorated sputum was collected, and a portion of each sample was analyzed for B. cepacia and other bacteria by culture while the remainder was frozen and stored at −70°C prior to the PCR analysis. Sputum samples were collected from patients who were too young to expectorate by nasopharyngeal aspiration (23).
Culture and identification.
Sputum samples were analyzed by selectively plating for B. cepacia on MacConkey agar containing polymyxin B, and the plates were incubated for up to 5 days. Gram-negative bacilli isolated from the selective media were confirmed as B. cepacia if they were also aminoglycoside resistant and oxidase positive and displayed β-galactosidase activity. As an alternative to the substrate o-nitrophenyl-β-d-galactopyranoside (ONPG), the activity of β-galactosidase was determined by using the more sensitive fluorescent substrate 4-methylumbelliferyl-β-d-galactoside (MUG) (6). Isolates not readily identified as B. cepacia were forwarded to the Ontario Provincial Laboratories for a more detailed analysis.
Liquefaction of sputum samples.
Sputum samples were liquefied by the method of Reischl et al. (22). Approximately 1 ml of sputum was mixed with an equal volume of 1% N-acetyl-l-cysteine–2% NaOH solution and centrifuged at 10,000 × g for 10 min. The resulting pellet was suspended in 100 μl of extraction buffer (1% Triton X-100, 0.5% Tween 20, 10 mM Tris-HCl, 1 mM EDTA) and subjected to five cycles of freezing in liquid nitrogen for 3 min and heating for 1 min in boiling water to lyse the bacteria. Following a second centrifugation step at 10,000 × g for 5 min, the supernatant, containing total DNA, was extracted for use in the PCR.
PCR analysis.
Supernatants derived from liquefied sputum were analyzed by using three primer pairs. Two of these primer pairs were putatively specific for B. cepacia and amplified regions of the rRNA locus. Primer pairs PSL1-PSR1 and PSL-PSR have been previously described (3). Each pair targets a region of the 16S rRNA gene and amplifies a 209-bp gene fragment from B. cepacia and a 310-bp fragment from all bacteria, respectively (3). The other primer pair, G1 (5′-TCGGAATCCTGCTGAGAGGC-3′)-G2 (5′-GCCATGGATACTCCAAAAGGA-3′), targets the 16S to 23S spacer region, amplifying a 1,300-bp DNA, and is putatively specific for B. cepacia. PCRs with each primer pair were optimized by using genomic DNA isolated from in vitro cultures of B. cepacia. Reactions were performed with the Rapid Cycler thermocycle machine (Idaho Technologies, Idaho Falls). All reaction mixtures had an initial denaturation at 95°C for 5 min with a subsequent 30 cycles of amplification and contained 100 μM each primer, 10 μl of liquefied sputum supernatant, 200 μM each deoxynucleoside triphosphate, and 2 U of Taq DNA polymerase (U.S. Biochemicals, Cleveland, Ohio) in a 2 mM MgCl2 PCR buffer (Idaho Technologies), in a total volume of 50 μl. For primer pairs PSL-PSR and PSL1-PSR1, the cycle parameters consisted of annealing at 58°C for 5 s, extension at 72°C for 30 s, and denaturation at 95°C for 5 s. For the primer pair G1-G2, the reaction parameters consisted of annealing at 55°C for 20 s, extension at 72°C for 100 s, and denaturation at 95°C for 20 s. Following amplification, 25 μl of each reaction mixture was subjected to electrophoresis in an 0.8% agarose gel in 0.5× Tris-borate-EDTA buffer (pH 8.0) next to a 100-bp molecular ladder. The PCR products were visualized and photographed after ethidium bromide staining. Positive results were assessed by the amplification of a band of the correct size for the primer pair used (Fig. 1). Samples that failed to amplify a product with these two primer pairs were further analyzed with the PSL-PSR primer pair (3) to determine if the reaction was inhibited by components within the sputum sample (5).
FIG. 1.
Analysis of PCR using the PSL1-PSR1 (lanes A to D) and the G1-G2 (lanes E to H) primer pairs. Lanes: A and E, B. cepacia genomic DNA; B and F, control free of B. cepacia genomic DNA; C and G, PCR-positive clinical sample; D and H, PCR-negative clinical sample. Lane M contains a 100-bp molecular ladder; the most intense band corresponds to 600 bp.
Specificity and sensitivity.
The specificity and sensitivity of the PCRs were calculated from the experimental results by using the criteria of true-positive responders and true-negative responders determined from the culture status of the sputum sample.
RESULTS
Culture analysis of sputum samples.
Culture of the sputum samples indicated that the samples contained, variously, B. cepacia, Pseudomonas aeruginosa, Candida albicans, Haemophilus influenzae, Staphylococcus aureus, group C streptococci, Aspergillus spp., and yeast (non-Candida) species (Table 1).
TABLE 1.
Comparison of detection of B. cepacia by culture and PCR
| Sample no.a | Detectionb of B. cepacia by:
|
Other organisms detectedc | ||
|---|---|---|---|---|
| Culture | PSL1-PSR1 PCR | G1-G2 PCR | ||
| A6 | X | X | X | |
| A19 | X | X | X | |
| A20 | X | X | X | P. aeruginosa |
| A21 | X | X | X | P. aeruginosa |
| A22 | X | X | X | P. aeruginosa |
| A24 | X | X | ||
| A31 | X | X | X | C. albicans |
| A32 | X | X | X | P. aeruginosa |
| A33 | X | X | X | |
| A34 | X | X | X | P. aeruginosa |
| A35 | X | X | X | P. aeruginosa, H. influenzae |
| A36 | X | X | X | |
| A37 | X | X | X | |
| A38 | X | X | X | |
| A47 | X | X | ||
| A48 | X | X | P. aeruginosa | |
| A49 | X | X | S. aureus | |
| A50 | X | X | X | |
| A52 | X | X | X | P. aeruginosa |
| A53 | X | X | X | |
| A59 | X | X | X | |
| A61 | X | X | P. aeruginosa, H. influenzae | |
| A62 | X | X | X | |
| A63 | X | X | P. aeruginosa, C. albicans | |
| A64 | X | X | X | |
| A67 | X | X | X | P. aeruginosa |
| A68 | X | X | X | |
| A69 | X | P. aeruginosa, S. aureus | ||
| A71 | X | X | X | P. aeruginosa |
| A72 | X | X | X | |
| A73 | X | X | X | P. aeruginosa, GCS |
| A74 | X | X | P. aeruginosa | |
| A75 | X | X | X | S. aureus, Aspergillus spp. |
| A76 | X | X | S. aureus | |
| A77 | X | X | P. aeruginosa, YNC | |
| A78 | X | X | X | P. aeruginosa |
| A80 | X | X | X | |
| A81 | X | X | X | |
| A82 | X | X | X | |
| A84 | X | X | ||
| A89 | X | |||
| A90 | X | X | X | |
| A91 | X | X | X | P. aeruginosa |
| A92 | X | X | P. aeruginosa | |
| A93 | X | X | X | |
| A94 | X | Aspergillus spp. | ||
| A95 | X | X | X | P. aeruginosa |
| A96 | X | P. aeruginosa | ||
| A97 | X | X | ||
| A102 | X | X | X | YS |
| A103 | X | X | X | |
| A104 | X | X | X | H. influenzae |
| A105 | X | X | X | |
| A107 | X | X | ||
| A108 | X | X | X | P. aeruginosa |
| A115 | X | X | X | |
| A116 | X | X | X | |
| A117 | X | X | GNB, Aspergillus spp. | |
| A118 | X | X | P. aeruginosa | |
| A122 | X | X | X | P. aeruginosa |
| C22 | X | X | ||
| C23 | X | X | X | |
| C24 | X | X | X | |
| C30 | X | X | ||
| C33 | X | X | X | |
| C38 | X | X | ||
| C93 | X | X | X | |
| C94 | X | X | X | |
| C95 | X | X | ||
| C98 | X | |||
| Total | 63 | 66 | 52 | |
Arbitrarily assigned sample number. Samples prefixed with “A” denote samples from the adult clinic; samples prefixed with “C” indicate samples from the children’s clinic.
X, positive result.
GCS, group C Streptococcus; YNC, yeast (non-Candida) species; GNB, gram-negative bacteria; YS, yeast species.
Development of a PCR analysis for B. cepacia in sputum samples.
To assess the potential sensitivity of the B. cepacia primers, sputa inoculated with graded concentrations of B. cepacia were examined. A B. cepacia isolate whose genomic DNA was amplified with the primers (data not shown) was grown in liquid culture and analyzed to determine the CFU per milliliter. Following determination of the cell count, B. cepacia-free sputum was mixed with the thawed bacteria over the range of 1 to 105 bacteria per ml. The suspension was processed to liquefy the sputum, lyse the bacteria, and separate the genomic DNA as described in Materials and Methods. Ten microliters of the extracted DNA was used in the PCRs. Sputum containing 102 CFU of B. cepacia per ml of sputum reliably yielded a PCR product.
PCR analysis of clinical samples.
A portion of sputum was removed for culture analysis, and the remainder was frozen at −70°C and shipped to the Children’s Hospital, Oklahoma City, Okla. Upon receipt, the samples were thawed, the sputum was liquefied, and the DNA containing fraction was isolated. Ten microliters of the DNA containing supernatant was used in separate PCRs containing primer pairs PSL1-PSR1 and G1-G2. A control PCR, lacking template, was performed with each primer pair. Samples that failed to give an amplification product with primer pairs PSL1-PSR1 and G1-G2 were further analyzed with primer pair PSL-PSR (3) to ensure that the reaction was not inhibited by components within the sputum (5).
Comparison of culture and PCR methods to detect B. cepacia.
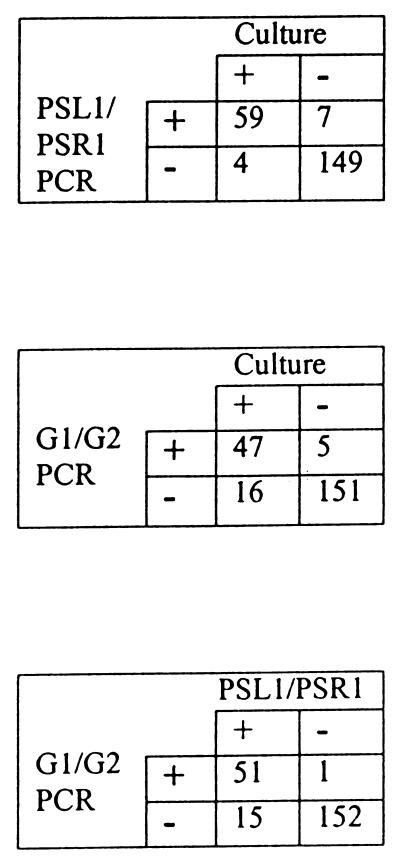
Both researchers performing the culture and those doing the PCR analyses were blinded with respect to the results of the other study. Upon completion of the two analyses, the two groups exchanged data and compared the results. The results from the analysis of the sputum samples are shown in Table 1. Culture analysis of the 109 sputum samples from the adult clinic indicated that 55 contained B. cepacia. PCR analysis, using primer pair PSL1-PSR1, identified 57 samples as B. cepacia positive, while primer pair G1-G2 identified 45 samples. Primer pair PSL1-PSR1 correctly identified 52 of the 55 culture-positive samples (95%), while primer pair G1-G2 correctly identified 42 samples (74%) (Fig. 2). Of the three culture-positive samples that PSL1-PSR1 failed to identify, two of these were also negative with the G1 and G2 primers. The PSL1 and PSR1 primers identified B. cepacia in five culture-negative samples. Of these, three were also PCR positive with primer pair G1-G2. Primer pair G1-G2 also identified one sample that was culture positive but not PCR positive with the PSL1 and PSR1 primers. Culture analysis of the 110 samples from the children’s clinic indicated that 8 samples contained B. cepacia. Of these culture-positive samples, primer pair PSL1-PSR1 identified seven positive samples (87%) while primer pair G1-G2 identified five positive samples (62%). Both PSL1-PSR1 and G1-G2 primer pairs identified B. cepacia in two culture-negative samples.
FIG. 2.
Comparison of the B. cepacia detection methods.
All samples that were B. cepacia negative with the two putatively B. cepacia-specific primers amplified a band when the universal primers PSL and PSR were used, indicating that failure to amplify a DNA fragment with the PSL1-PSR1 and G1-G2 primer pairs was not due to the presence of inhibitors of the PCR in the specimen.
DISCUSSION
Identification of microorganisms by using species-specific PCR protocols is a rapidly expanding field. Such diverse species as Yersinia enterocolytica and Listeria monocytogenes in human feces and Mycobacterium tuberculosis, Pneumocystis carinii, Chlamydia pneumoniae, Chlamydia psittaci, and Pseudomonas aeruginosa in sputum samples have been identified (7, 11, 13, 16, 19, 26). Campbell et al. reported species-specific primers to allow the identification of B. cepacia isolates (3). In the present study, we analyzed the efficacy of these primers and another putatively species-specific primer pair to identify B. cepacia in sputum samples and compared the results with those of the current culture detection protocol. Comparison of the results of analysis of sputa from the adult clinic indicated that the primer pair PSL1-PSR1 more accurately matched the culture results than did primer pair G1-G2. Of the 55 culture-positive samples identified during this study, primer pair PSL1-PSR1 identified 52 of them while primer pair G1-G2 identified 42. Three culture-positive sputum samples failed to give an amplification product with primer pair PSL1-PSR1. Two of these samples (A84 and A89) were collected from the same patient, 4 days apart, while the other sample (A94) was from a patient whose sputum was intermittently culture positive. Interestingly, the analysis of the two samples from the same patient displayed different results when primer pair G1-G2 was used: sample A89 was negative, and sample A84 was positive. This was the only instance in which the G1 and G2 primers yielded a positive result where the PSL1 and PSR1 primers yielded a negative one. Primer pair PSL1-PSR1 identified five samples that were culture negative, three of which primer pair G1-G2 also identified as positive. Of these five samples, sample A69 was from a patient who had been culture positive for B. cepacia twice, once during 1985 and again in 1986, at two different CF centers, but who had been culture negative since. Samples A74, A117, and A118 were from patients who were intermittently culture positive. Patient A117 had yielded 16 culture-positive sputum samples since 1985, and in each case the B. cepacia growth was very slight. Patient A118 has yielded 15 positive samples since 1989 and a single positive sample in 1995. Sample A96 was from a patient who has not had a culture-positive B. cepacia sample at any time prior to this study.
By using the same methodology, DNA derived from sputa collected at the children’s clinic was analyzed by PCR. Comparison of the results of the samples from the children’s clinic indicated three discrepant results between the culture and PCR analysis methods. Sample C30 was derived from a child who never produced a B. cepacia-positive culture. This patient died subsequent to providing the sample. Patient C38 was known to be B. cepacia positive by culture since 1988 and still expectorates B. cepacia-positive sputum. This sample was one of three samples provided by this patient that was culture negative over a 9-year period. Sample C98 was culture positive and PCR negative. This sample was the only culture-positive one submitted by this patient and was thought by the microbiology laboratory, at the time of culture, to represent a contaminated sample. Analysis of the data after inclusion of samples from patients with previously positive results (samples A69, A74, A117, A118, and C38) and exclusion of the “contaminated” sample from the children’s clinic (C98) with those identified as culture positive in this study gives a sensitivity and specificity for primer pair PSL1-PSR1 of 95.5 and 98.7%, respectively, and for primer pair G1-G2 of 76.1 and 99.3%, respectively. Overall, these analyses show that the results of PCR with primer pair PSL1-PSR1 have high concordance with the results obtained by culture. In addition, these results indicate that the PCR approach may be more accurate than culture, since it was able to detect B. cepacia in patients displaying intermittent colonization when the culture was negative. The samples that were culture positive but PCR negative may be explained by the recent report that the species B. cepacia comprises five distinct species, or genomovars (10, 27), and indicate the need for continued refinement of the detection methods. Standard microbiology laboratory identification is unable to discern genomovars. The primers used in this study target the 16S and 23S rRNA genes. These genes have regions of heterogeneity at the genus, species, and strain levels. Thus, it may be possible that the primers are targeting the species-specific region and are not capable of recognizing one or more of the genomovars. From the results, it would appear that PSL1-PSR1 detects more genomovars than G1-G2. PCR examination of prototypical strains representing the five B. cepacia genomovars with primer pair PSL1-PSR1 gave a positive result for each, while primer pair G1-G2 recognized only three strains (data not shown). This raises the interesting possibility of designing PCR primers that are genomovar specific for B. cepacia.
In summary, we have reported the efficacy of PCR to rapidly and accurately identify B. cepacia in sputum samples from patients with CF. Future studies will be performed with a broader study population, while new sets of primers will be investigated to identify each species of the B. cepacia complex directly in sputum samples.
ACKNOWLEDGMENTS
We thank Mary G. Corey and Karen B. Carter for their help in the production and presentation of data in this manuscript.
This work was supported by Cystic Fibrosis Foundation grant G904 to T.L.S.
REFERENCES
- 1.Anonymous. Pseudomonas cepacia—more than a harmless commensal? Lancet. 1992;339:1385–1386. [PubMed] [Google Scholar]
- 2.Butler S L, Doherty C J, Hughes J E, Nelson J W, Govan J R. Burkholderia cepacia and cystic fibrosis: do natural environments present a potential hazard? J Clin Microbiol. 1995;33:1001–1004. doi: 10.1128/jcm.33.4.1001-1004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell P W, III, Phillips III J A, Heidecker G J, Krishnamani M R S, Zahorchak R, Stull T L. Detection of Pseudomonas (Burkholderia) cepacia using PCR. Pediatr Pulmonol. 1995;20:44–49. doi: 10.1002/ppul.1950200109. [DOI] [PubMed] [Google Scholar]
- 4.Christenson J C, Welch D F, Mukwaya G, Muszynski M J, Weaver R E, Brenner D J. Recovery of Pseudomonas gladioli from respiratory tract specimens of patients with cystic fibrosis. J Clin Microbiol. 1989;27:270–273. doi: 10.1128/jcm.27.2.270-273.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deneer H G, Knight I. Inhibition of the polymerase chain reaction by mucolytic agents. Clin Chem. 1994;40:171–172. [PubMed] [Google Scholar]
- 6.Dick H L N, Borezyk A, Kiani G, et al. Abstracts of the Ninth Annual North American CF Conference 1995. 1995. Rapid identification of Burkholderia cepacia from cystic fibrosis patients. [Google Scholar]
- 7.Eisenach K D, Sifford M D, Cave M D, Bates J H, Crawford J T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991;144:1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- 8.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govan J R, Brown P H, Maddison J, Doherty C J, Nelson J W, Dodd M, Greening A P, Webb A K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 10.Govan J R, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim A, Liesack W, Stackebrandt E. Polymerase chain reaction-gene probe detection system specific for pathogenic strains of Yersinia enterocolitica. J Clin Microbiol. 1992;30:1942–1947. doi: 10.1128/jcm.30.8.1942-1947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 13.Jaton J, Sahli R, Bille J. Development of polymerase chain reaction assays for detection of Listeria monocytogenes in clinical cerebrospinal fluid samples. J Clin Microbiol. 1992;30:1931–1936. doi: 10.1128/jcm.30.8.1931-1936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostman J R, Edlind T D, LiPuma J J, Stull T L. Molecular epidemiology of Pseudomonas cepacia determined by polymerase chain reaction ribotyping. J Clin Microbiol. 1992;30:2084–2087. doi: 10.1128/jcm.30.8.2084-2087.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy D E, Spencer D A, Goldstein A, Weller P H, Darbyshire P. Chronic granulomatous disease presenting in childhood with Pseudomonas cepacia septicaemia. J Hosp Infect. 1993;21:199–204. doi: 10.1016/0163-4453(93)92271-w. [DOI] [PubMed] [Google Scholar]
- 16.Lipschik G Y, Gill V J, Lundgren J D, Andrawis V A, Nelson N A, Nielsen J O, Ognibene F P, Kovacs J A. Improved diagnosis of Pneumocystis carinii infection by polymerase chain reaction on induced sputum and blood. Lancet. 1992;340:203–206. doi: 10.1016/0140-6736(92)90469-j. [DOI] [PubMed] [Google Scholar]
- 17.LiPuma J J, Dasen S E, Nielson D W, Stern R C, Stull T L. Person-to-person transmission of Pseudomonas cepacia between patients with cystic fibrosis. Lancet. 1990;336:1094–1096. doi: 10.1016/0140-6736(90)92571-x. [DOI] [PubMed] [Google Scholar]
- 18.LiPuma J J, Marks-Austin K A, Holsclaw D S, Jr, Winnie G B, Gilligan P H, Stull T L. Inapparent transmission of Pseudomonas (Burkholderia) cepacia among patients with cystic fibrosis. Pediatr Infect Dis J. 1994;13:716–719. doi: 10.1097/00006454-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh I, Govan J R, Brock D J. Detection of Pseudomonas aeruginosa in sputum from cystic fibrosis patients by the polymerase chain reaction. Mol Cell Probes. 1992;6:299–304. doi: 10.1016/0890-8508(92)90005-i. [DOI] [PubMed] [Google Scholar]
- 20.O’Neill K M, Herman J H, Modlin J F, Moxon E R, Winkelstein J A. Pseudomonas cepacia: an emerging pathogen in chronic granulomatous disease. J Pediatr. 1986;108:940–942. doi: 10.1016/s0022-3476(86)80934-9. [DOI] [PubMed] [Google Scholar]
- 21.Pegues C F, Pegues D A, Ford D S, Hibberd P L, Carson L A, Raine C M, Hooper D C. Burkholderia cepacia respiratory tract acquisition: epidemiology and molecular characterization of a large nosocomial outbreak. Epidemiol Infect. 1996;116:309–317. doi: 10.1017/s0950268800052626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reischl U, Pulz M, Ehret W, Wolf H. PCR-based detection of mycobacteria in sputum samples using a simple and reliable DNA extraction protocol. BioTechniques. 1994;17:844–845. [PubMed] [Google Scholar]
- 23.Sadeghi E, Matlow A, Maclusky I, Karmali M A. Utility of gram stain in evaluation of sputa from patients with cystic fibrosis. J Clin Microbiol. 1994;32:54–58. doi: 10.1128/jcm.32.1.54-58.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tablan O C, Chorba T L, Schidlow D V, White J W, Hardy K A, Gilligan P H, Morgan W M, Carson L A, Martone W J, Jason J M, et al. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 25.Taylor R F H, Dalla Costa L, Kaufmann M E, Pitt T L, Hodson M E. Pseudomonas cepacia pulmonary infection in adults with cystic fibrosis: is nosocomial acquisition occurring? J Hosp Infect. 1992;21:199–204. doi: 10.1016/0195-6701(92)90076-x. [DOI] [PubMed] [Google Scholar]
- 26.Tong C Y, Sillis M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J Clin Pathol. 1993;46:313–317. doi: 10.1136/jcp.46.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandamme P. 20th European Cystic Fibrosis Conference. 1995. Emerging new “Pseudomonas” species in cystic fibrosis. [Google Scholar]
- 28.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R W. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and the proposal of Burkholderia multivorans sp. nov. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]