Abstract
Resting energy expenditure (REE) may be useful for individualizing energy intake (EI) and physical activity (PA) goals, and in turn, regulating gestational weight gain (GWG). Limited research, however, has examined the association between REE and GWG. This study examined (1) change in REE from 14 to 28 gestation, (2) time-varying associations between REE and GWG, and (3) EI and PA patterns during the weeks when REE and GWG were significantly associated. Pregnant women with overweight/obesity (N = 27) participating in the Healthy Mom Zone study completed weekly point estimates of EI (back-calculation), PA (wrist-worn activity monitor), REE (mobile metabolism device), and weight (Wi-Fi scale) from 14 to 28 weeks gestation. Analyses included descriptives and time-varying effect modeling. REE fluctuated, increasing on average from 14 to 28 weeks gestation, but decreased at gestational weeks 17, 20, 21, 23, 26, and 28. Most women increased in REE; however there was large between-person variability in the amount of change. Associations between REE and GWG were small but time-varying; low REE was associated with high GWG between gestational weeks 25 to 28 when there was observably larger fluctuation in REE. Moreover, over half of the women were categorized as having excessive EI and most as low active during this time. EI needs may be overestimated and PA needs may be underestimated when REE is fluctuating, which may increase the risk for high second trimester GWG. Researchers should consider the role of REE to inform EI and PA goals to regulate GWG.
Keywords: Resting energy expenditure, Gestational weight gain, Energy intake, Physical activity
Introduction
Pregnant women with overweight/obesity (PW-OW/OB) are at high risk for excessive gestational weight gain (GWG; i.e., > 11.5 kg total or > 0.33 kg/week for women with overweight, > 9 kg total or > 0.27 kg/week for women with obesity) in part due to their unique challenges with regulating weight (e.g., overestimating GWG recommendations, underestimating their body mass index [BMI]) [1-6]. Entering pregnancy with overweight/obesity and/or having excessive GWG increases the risk for adverse perinatal and neonatal outcomes such as gestational diabetes, hypertensive disorders, macrosomia, and childhood obesity [1, 2]. Past research has primarily focused on how energy intake (EI) and physical activity (PA) behaviors can be used to regulate GWG [7-14]. Unfortunately, most PW-OW/OB have difficulty adhering to prenatal EI goals (e.g., 2600 kcals/day) [7, 15] and PA recommendations (i.e., 150 min of moderate PA/week) [16]. Thus, there is a need to identify appropriate strategies to promote individualized intake and activity goals in this population to effectively regulate GWG.
Resting energy expenditure (REE) accounts for 60–70% of total energy expenditure and is often used to inform EI and PA goals to achieve weight loss in non-pregnant adults [17-21]. Given that weight loss is not typically recommended for pregnant women, this approach has not been widely adopted among pregnant women. For example, in an effort to create EI goals for PW-OW/OB to regulate GWG, Symons Downs and colleagues [22] applied the recommendations of Vesco and colleagues [23] and mathematically reduced the established recommendations for pregnant women with normal weight by 20–30% within the Health Mom Zone study. The Healthy Mom Zone study evaluated GWG each week and made decisions to adapt the intensity of the intervention dosage based on whether a woman was meeting or exceeding GWG guidelines. However, given the limited research regarding the relationship between REE and GWG at the time the intervention was implemented, women’s caloric goals were not adapted over the course of the intervention based on the influence of their REE. In order to design interventions to use REE to inform EI and PA goals to regulate GWG in PW-OW/OB, it is first necessary to examine associations between REE and GWG and to identify the extent to which these associations are time-varying at the weekly level.
There is some evidence that low prenatal REE and/or fluctuations in REE may be a “warning sign” of high GWG [19, 20]. For example, findings from Leonard and Symons Downs (under review) showed that 52% of the variance in second trimester GWG was explained by EI, PA, and REE, with low REE emerging as the strongest determinant of GWG followed by EI (PA was not a significant determinant) in PW-OW/OB. Also, Berggren and colleagues [19] found that in a sample of 51 pregnant women, while 75% increased in REE from pre-pregnancy to 34–36 weeks gestation and the remaining 25% decreased, an increase in REE was associated with an increase in fat free mass and a decrease in fat mass. However, this study only included “pre-post” (i.e., 3 months preconception to 34-36 weeks gestation) change in REE and GWG and did not examine time-varying associations between REE and GWG to better understand how variation in REE over the course of pregnancy may influence GWG. Further, little is known about how REE is related to GWG during critical periods of fetal growth such as the second trimester because the middle of pregnancy is often overlooked within “pre-post” study designs. Specifically, the second trimester is often marked by rapid increase in GWG, especially for PW-OW/OB, which is attributed to the nearly 50% increase in blood volume and amniotic fluid that is needed to accommo-date the growing fetus [1, 24]. Also, it has been shown that large fluctuations in REE may be more apparent during the second trimester [25].
Vander Wyst and colleagues [20] partly addressed these limitations and examined change in REE in pregnant women every two weeks from ~14 to 28 weeks gestation using a mobile indirect calorimetry (Breezing™) device to assess REE. They observed between-person variation in REE at each study visit such that 53–63% of women increased in REE, whereas 38-50% decreased in REE. They also found that although changes in REE from 21 to 28 weeks gestation (mean = 128 kcal increase) were nearly twice as much as the changes in REE from 14 to 21 weeks gestation (mean = 72 kcal increase), there were no significant associations between changes in REE and GWG. The researchers suggested that the lack of significant associations may have been due to the large variation in the amount of increase and decrease in REE across individuals. The researchers hypothesized that wide fluctuations (e.g., increase by 350 kcal/day or decrease by 620 kcal/day compared to 10 kcal/day) in REE from 14 to 28 weeks gestation, rather than the mean level of REE, may increase their risk of excessive GWG. However, this study was still based on limited assessments of REE during the second trimester. Examining the associations between REE and GWG at the weekly level may provide unique insight about how REE can be used to help PW-OW/OB regulate their EI and PA behaviors to meet weekly GWG ranges. This is particularly important because the GWG guidelines suggest a small amount of weekly weight gain for PW-OW (i.e., 0.05–0.07) and PW-OB (i.e., 0.04–0.06) to achieve their overall GWG goals [1].
The first purpose of this secondary analysis from the Healthy Mom Zone study was to describe patterns in mean change in REE from 14 to 28 weeks gestation and the proportion of women who increased and decreased in REE. It was hypothesized that REE would increase overall from 14 to 28 weeks gestation [19, 20, 25]. However, it was hypothesized that not all women would increase such that 60–75% of women would increase and 25–40% would decrease [19, 20]. Due to the limited research, an a priori hypothesis was not established about the magnitude of change in REE for the weekly point estimates. The second purpose was to examine associations between weekly point estimates of REE and weekly point estimates of GWG and the extent to which these associations changed over time from 14 to 28 weeks gestation. It was hypothesized that low REE would be associated with high weekly GWG during each week from 14 to 28 weeks gestation [19, Leonard & Symons Downs, under review]. The third purpose was to descriptively examine EI and PA patterns during the weeks when REE and GWG were significantly associated. It was hypothesized that during the weeks when low REE was associated with high GWG, the majority of women would be categorized as exceeding EI recommendations and low active [Leonard & Symons Downs, under review].
Methods
Participants
Women (N = 31) were PW-OW/OB participating in the Healthy Mom Zone feasibility study, a theoretically-based behavioral intervention that adapted the intervention dosage and intensity over time in PW-OW/OB to regulate GWG [22]. PW-OW/OB were recruited using on-site clinic, community-based, and web-based strategies. Potentially eligible women were then screened by a trained staff member who explained the study, obtained verbal assent to ask questions, and determined eligibility. Eligible women were randomized to an intervention or usual care control group from ~8–36 weeks gestation. Women were eligible to participate in the randomized control trial if they were 18–40 years old and (1) had overweight/obesity (BMI range 25–45 kg/m2; > 40 kg/m2 with physician consultation), (2) had a singleton pregnancy ≥8 weeks gestation, (3) obtained physician consent to participate, and (4) were English-speaking, residing in or near Central Pennsylvania. Exclusion criteria were (1) multiple gestation, (2) diabetes at study entry, (3) not having overweight/obesity, (4) severe allergies or dietary restrictions, (5) contraindications to prenatal PA [16] and, (6) not residing in area for duration of study. A more detailed explanation of the Healthy Mom Zone study can be found elsewhere [22]. Of the N = 31 women, n = 3 had miscarriages prior to starting the intervention and n = 1 woman withdrew, resulting in a total sample of N = 27, who were all eligible to be included in this secondary analysis.
Procedures
The Healthy Mom Zone study was approved by the Pennsylvania State University Institutional Review Board (IRB Study# 00000122). Women completed a 30-min baseline session at ~ 8 weeks gestation at the University’s Clinical Research Center. Study procedures were explained to the participants, and written informed consent to participate and publish was obtained for each woman. Women received an Aria Fitbit Wi-Fi scale to weigh themselves daily from 8 to 36 weeks gestation. Weights were transmitted via Bluetooth to research investigators each day. Women also wore a wrist-worn activity monitor daily throughout the study period to assess PA. Women were instructed to wear an ActiGraph GT3x+ intermittently as a supplement to the wrist-worn activity monitor given the ActiGraph is a widely utilized gold standard approach. Next, a study staff person explained how to download the Breezing™ application onto each woman’s smartphone. The Breezing™ device is an indirect calorimetry analyzer of REE and is compatible with both iOS and Android software platforms. All women were trained on how to use the Breezing™ device and sent home with detailed instructions. They were instructed to use the Breezing™ device one time per week (on the same day of the week) at the same time each morning from 8 to 36 weeks gestation. Specifically, women were instructed to obtain a Breezing™ measurement after 8 h of sleep, immediately after waking up while lying down and in a fasting state (e.g., no food for the past 8-12 hours). First, they opened the Breezing™ application on their phone and followed a checklist of instructions to obtain a measurement. Second, they scanned the QR code on the package of provided sensor cartridges. Sensor cartridges were used to determine the rate of oxygen consumption and carbon dioxide production. The scanned QR code carries calibration parameters for the sensor cartridge. Third, they placed a nose clip onto their nose and practiced breathing in the mouthpiece alone until breathing was consistent. Finally, they were prompted to insert one sensor cartridge into the tracking device and preform an actual measurement. Women picked up the tracker and blew into the mouthpiece until the application said the test was completed (1–2 min). The device did not produce results if the woman had “irregular breathing” (i.e., too slow or fast and/or irregular rate, as detected by the device). Women were instructed to complete another measurement until a successful assessment was obtained. On average, 5% of the sample had to repeat at least one measurement due to irregular breathing. All data was transmitted via Bluetooth to the women’s smartphone and sent to the research team. At ~ 36 weeks gestation, women completed their last assessment and returned the device.
Measures
Energy Intake (EI)
EI, matched on the day REE was obtained, was estimated using a validated back-calculation method [22, 26-28]:
The variables are as follows: k = 1, 2, …, N corresponding to day 1-day N. W represents maternal weight in kg, while T represents sampling time which in this case was T = 1 day. PA represents physical activity in kcals. REE represents resting energy expenditure in kcals calculated using Breezing™ or the estimated equation depending on which REE method was used for that data point.
Physical Activity (PA)
Active kcal (calories burned during activity) was obtained from the Jawbone UP3 wrist-worn activity monitor for each day REE was measured. Symons Downs et al. [29] found that the Jawbone UP3 and the ActiGraph activity monitor had only a minor discrepancy of approximately 79 kcals. Thus, ActiGraph data was used when wrist-worn activity monitor data was missing. Based on Evenson and Wen [30] and Mâsse and colleagues [31] suggestions, if there was no data from the Jawbone or the ActiGraph on the day an REE assessment was obtained, active kcal was mean replaced by the weekly average if there was ≥ 4 days of data in the week. There were no significant differences in the active kcals between week-days and weekends on weeks that were mean replaced. If there was < 4 days of data in the week, mean replacement was not completed and the value was considered missing.
Resting Energy Expenditure (REE)
REE was assessed using the Breezing™ device. The algorithm within the Breezing™ device then calculated REE according to the Weir equation, a well-known equation used with indirect calorimetry [32, 33]:
represents the volume of oxygen consumed and represents the volume of carbon dioxide produced. If there was a missing Breezing™ assessment, estimated REE (eREE) was calculated by function of maternal weight for each woman by using an estimated quadratic regression equation that was proposed by Thomas and colleagues [34] based on data collected on pregnant women by Butte and colleagues [35, 36]:
where W represents maternal weight in kilograms.
Weekly Gestational Weight Gain (GWG)
Weekly GWG was calculated as weekly point estimate of weight from the Aria Wi-Fi weight scale at the week after the current week minus the weekly point estimate of weight at the current week (e.g., GWG for week 14 = weight at 15 weeks minus weight at 14 weeks). The Aria Wi-Fi weight scale has been shown to be a valid and reliable tool to estimate weight in the general population [37].
Demographics and Personal Characteristics
At baseline, women self-reported personal demographics (i.e., age, race/ethnicity, income, marital status, education, employment) and current gestational age. Pre-pregnancy BMI was calculated from self-reported pre-pregnancy weight and height.
Data Analyses
Data were analyzed using SPSS v25 (IBM, Armonk, NY) and SAS v9 (SAS Institute, Cary, NC). Evidence from [Leonard & Symons Downs, under review] showed agreement between REE assessed with the estimated equation and Breezing™ at gestational weeks 7-10, 12-22, 24-31, 34, and 36. However, there was a lack of agreement between the two methods at gestational weeks 11, 23, 32, 33, and 35 (noting, however, that there was an outlier at week 35 and once removed, there was subsequent agreement at this time point). Given these mixed findings, the analyses in the current study were run with (1) REE from Breezing™ only and (2) REE from Breezing™ and eREE based on the estimated equation (i.e., with eREE values imputed for missing Breezing™ REE assessments) to test whether the discrepancy between the two methods influenced the study analyses.
Means, standard deviations, and percentages were used to examine demographic variables and descriptives of second trimester REE. Estimates that were ±3 standard deviations away from each woman’s mean second trimester REE were considered as outliers [38]. Time-varying effect modeling (TVEM) [39] was used to examine the associations between REE and weekly GWG across 14–28 weeks gestation. Specifically, REE was included as the time-varying independent variable to model its effect on the dependent variable of weekly GWG over time. The model produced beta coefficients of REE on weekly GWG. Coefficients above 0 signify positive associations and coefficients below 0 signify negative associations. The 95% confidence intervals of the coefficients across time with TVEM were also calculated. Confidence intervals overlapping with 0 indicate non-significant associations at α = .05. The TVEM analysis also included study group assignment (randomized to intervention vs. control) and pre-pregnancy BMI (overweight vs. obese) as time-invariant covariates to control for their effect on REE and weekly GWG. TVEM returned standardized beta coefficients for effect sizes, the magnitude of which can be considered small, medium, and large for effect size values of 0.20, 0.50, and 0.80, respectively [40]. It is important to note that TVEM analyses require adequate coverage of data across time points, which criterion was met in the study [39].
To descriptively examine EI patterns, women were categorized as meeting versus exceeding EI guidelines for each week using back-calculated EI /day. Several guidelines recommend that women should intake an additional 340 kcals/day in the second trimester [1, 15, 41, 42]. Therefore, EI/day for each week was subtracted from average EI/day during the first trimester to obtain change in average EI/day in the second trimesters. If the change was ≤ 340 kcals women were categorized as meeting EI guidelines. If the change was > 340 kcals women were categorized as exceeding the guidelines. Using activity kcal/day, women were categorized as low active vs. active. Researchers have shown that on average, PW-OB are considered low active and expend 569 active kcals/day during pregnancy [43]. Given there are no clinical recommendations for the number of active kcals to expend per day, a threshold of 569 active kcals/day was used. Women who had an active kcal/day < 569 were categorized as low active, and women who had an active kcal/day ≥ 569 were categorized as active.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. Mean age of study participants was 30.6 (SD = 3.0) years, mean gestational week at study entry was 10.2 (SD = 1.7), and mean pre-pregnancy BMI was 31.6 kg/m2 (SD = 7.0; overweight = 59%, obese = 41%). The sample was Non-Hispanic, White (96%), married (92%), employed full-time (89%), reported a family income ≥ $40,000 (77%), and almost half of the sample had a graduate/professional degree (48%).
Table 1.
Participant characteristics (N = 27)
| Mean | SD | N (%) | |
|---|---|---|---|
| Age | 30.6 | 3.0 | |
| Gestational week at study entry | 10.2 | 1.7 | |
| Pre-pregnancy BMI | 31.6 | 7.0 | |
| OW | 16 (59) | ||
| OB | 11 (41) | ||
| Race | |||
| White | 26 (96) | ||
| Asian | 1 (4) | ||
| Employment | |||
| Full time | 24 (89) | ||
| Other | 3 (11) | ||
| Education | |||
| High school | 1 (4) | ||
| College | 13 (48) | ||
| Graduate/professional | 13 (48) | ||
| Family income | |||
| $10–20,000 | 1 (4) | ||
| $20–40,000 | 5 (19) | ||
| $40–100,000 | 12 (44) | ||
| > $100,000 | 9 (33) | ||
| Marital status | |||
| Married | 25 (92) | ||
| Single | 1 (4) | ||
| Divorced | 1 (4) | ||
| Parity | |||
| Nulliparous | 20 (74) | ||
| Primiparous | 7 (26) |
SD standard deviation, BMI body mass index
REE Missing Data and Outliers
From 14 to 28 weeks gestation across all participants, there were 166 instances (41%) when Breezing™ data were missing; there were only 3 women who had no Breezing™ missing data at any time point. Of the 166 instances, 159 (96%) were replaced with the estimated equation resulting in a total of 398 total REE data points across 14 to 28 weeks gestation (7 missing points). Each week ranged from 9 to 15 substitutions of the estimated equation. Analyses revealed there were no outliers in the data. The primary analyses did not significantly differ when the eREE equation was used in place of missing Breezing™ REE data, thus, the analyses are presented below using the estimated equation when Breezing™ was missing across all time points.
REE Descriptives
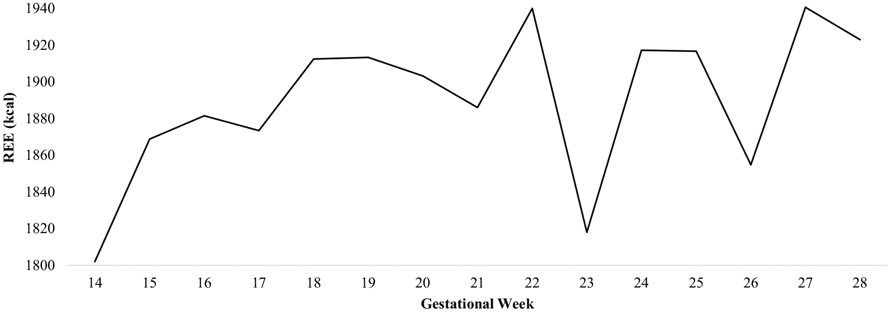
Mean second trimester REE values across the total sample are depicted in Fig. 1. While average REE/day increased by 113.99 kcals (SD = 225.36) from 14 to 28 weeks gestation on average across the total sample, 78% (n = 21) of women increased (range = 2.97–670.00 kcals) in REE, 19% (n = 5) of women decreased (range = −80.00 to −302.41 kcals), and 3% (n = 1) had no change. Further there was evidence of fluctuation within this time period. Specifically, REE increased from gestational weeks 14–16, 17–19, 21–22, 23–24, and 26–27 on average for the total sample and specifically for 26, 30, 63, 63, and 59% of women, respectively. REE decreased from gestational weeks 16–17, 19–21, 22–23, 24–26, and 27–28 on average for the total sample and specifically for 48, 15, 59, 41,, and 37% of women, respectively.
Fig. 1.
Time course of mean resting energy expenditure across the second trimester. REE resting energy expenditure, GW gestational week
Time-Varying Effect
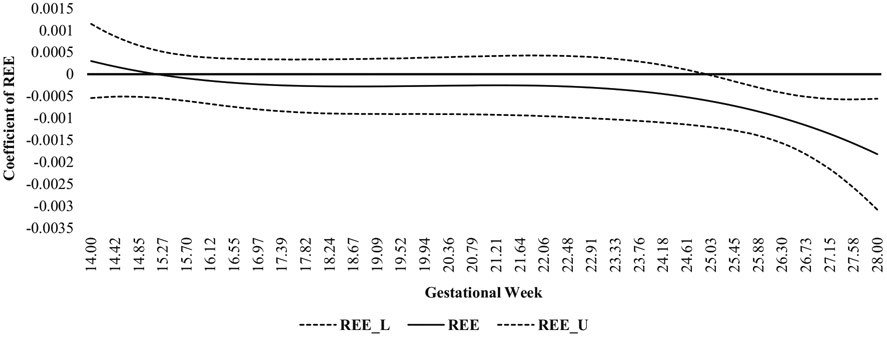
There were no significant associations between REE and weekly GWG from gestational weeks 14 to 24 (Fig. 2). The effect sizes from gestational weeks 14 to 24 were small and ranged from 0.0003 to 0.0006. However, there were significant associations between REE and weekly GWG from gestational weeks 25 to 28 such that low REE was associated with high weekly GWG; the strength of these associations were nevertheless small, but they slightly increased during this time (range = 0.0006–0.002).
Fig. 2.
Time-varying effect model of the associations between resting energy expenditure and gestational weight gain across the second trimester. REE resting energy expenditure, L lower bound 95% confidence interval, U upper bound 95% confidence interval
Energy Intake
Slightly over half of the women (54%) exceeded EI recommendations at gestational week 25, 50% exceeded recommendations at gestational week 26, 58% exceeded recommendations at gestational week 27, and 35% exceeded recommendations at gestational week 28 (Table 2). In contrast, 46% of women met EI recommendations at gestational week 25, 50% met EI recommendations at gestational week 26, 42% met EI recommendations at gestational week 27, and 65% met EI recommendations at gestational week 28. During gestational weeks 25 to 28, women who exceeded EI recommendations (M = 3220.61, range = 3051.78–3505.32) consumed an average of 616.46 (range = 371.78–1092.74) kcals higher each day than women who met EI recommendations (M = 2604.15, range = 2412.58–2750.22). Also, REE was 62.5 kcals/day higher and GWG was 0.01 kg/week higher for women who exceeded EI recommendations (REE: M = 1955.50, range = 1845.00–2156.00; GWG: M = 0.22, range = −0.26 to 0.49) compared to women who met EI recommendations (REE: M = 1893.00, range = 1799.00–2002.00; GWG: M = 0.21, range = −0.25 to 1.07).
Table 2.
Descriptives of energy intake (kcals/day) by energy intake recommendations and physical activity (kcals/day) by physical activity categorization
| Gestational week | Energy intake | Physical activity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meeting | Exceeding | Low active | Active | |||||||||
| % | Mean | SD | % | Mean | SD | % | Mean | SD | % | Mean | SD | |
| 25 | 54 | 2680.00 | 441.11 | 46 | 3051.78 | 795.73 | 77 | 299.31 | 145.56 | 23 | 689.65 | 120.76 |
| 26 | 50 | 2573.83 | 683.32 | 50 | 3074.98 | 680.02 | 76 | 369.99 | 141.93 | 24 | 964.25 | 254.39 |
| 27 | 58 | 2750.22 | 742.61 | 42 | 3250.37 | 813.86 | 80 | 321.09 | 121.73 | 20 | 932.63 | 389.23 |
| 28 | 35 | 2412.58 | 479.96 | 65 | 3505.32 | 934.74 | 76 | 306.80 | 116.24 | 24 | 987.94 | 232.82 |
SD standard deviation
Physical Activity
The majority (77%) of women were categorized as low active at gestational week 25, 76% were categorized as low active at gestational week 26, 80% were categorized as low active at gestational week 27, and 76% were categorized as low active at gestational week 28. In contrast, 23% of women were categorized as active at gestational week 25, 24% were categorized as active at gestational week 26, 20% were categorized as active at gestational week 27, and 24% were categorized as active at gestational week 28. During gestational weeks 25–28, women who were categorized as low active (M = 324.30, range = 299.31 to 369.99) expended fewer kcals (M = 569.32, range = 371.78 to 1092.74) each day than women categorized as active (M = 893.62, range = 689.65 to 987.94). REE was 525 kcals/day higher and GWG was 0.05 kg/week lower for women categorized as active (REE: M = 2237.50, range = 2210.00–2293.00; GWG: M = 0.03, range = −0.24 to 0.32) compared with women categorized as low active (REE: M = 1712.50, range = 1634.00–1764.00; GWG: M = 0.09, range = −0.68 to 0.78).
Discussion
The overall objective of this secondary analysis study was to describe weekly point estimates of REE in relation to weekly GWG over the second trimester in PW-OW/OB in an effort to understand whether future interventions can use REE as a strategy to inform EI and PA goals to effectively regulate GWG. In summary, REE fluctuated during the second trimester with the majority of women increasing in REE overall. Also, there were small but significant time-varying associations between REE and GWG such that low REE was associated with high GWG during times of observable fluctuation in REE. Moreover, during these times of fluctuation, slightly over half of the women were categorized as having excessive EI whereas the majority of women were categorized as low active. These findings suggest there is wide variability in REE during the second trimester and that significant associations during the weeks of REE fluctuation may be attributed to engaging in poor EI and PA behaviors. These findings are described in more detail below.
In support of the first hypothesis, REE increased on average by 113.99 kcals from 14 to 28 weeks gestation. A similar pattern was found by Vander Wyst and colleagues [20] who found that women increased in REE by 200 kcals from 14 to 28 weeks gestation. This current study also revealed that not all women increased in REE. Specifically, 78% of women increased, 19% of women decreased, and 3% had no change. Further, there was a wide range in the amount of increase and decrease. Upon further exploration, descriptives revealed that personal characteristics (i.e., BMI, fat-free mass, age) between women that increased versus decreased or had no change in REE were similar except for maternal age. Specifically, women were more likely to increase in REE from 14 to 28 weeks gestation if they were <35 years old compared with if they were ≥ 35 years old. This is consistent with past research showing that increases in REE are more apparent in younger individuals than older individuals [17]. Moreover, the current study findings revealed that on average, there was fluctuation in REE from 14 to 28 weeks gestation. The greatest amount of fluctuation was observed from gestational weeks 22 to 28. It is important to note that there was a large decrease in REE observed from week 22 to 23, followed by a large increase in REE from week 23 to 24. Analyses revealed that there were no outliers and that this large decrease in REE was not due to missing data and/or due to substituting in the estimated equation for missing Breezing™ REE. There was also no apparent influence of missing data on the pattern of change in REE at any other time point. This suggests that the fluctuations, particularly during the large peaks (i.e., weeks 22–24) were not a result of measurement error but potentially influenced by other factors and behaviors attributed to pregnancy (e.g., EI). For example, exploratory analyses revealed that at week 22, the majority of women were categorized as exceeding weekly EI recommendations, whereas at weeks 23 and 24, the majority of women were categorized as meeting weekly EI recommendations. Given that high EI is associated with high REE [17], it is likely that the large decrease in REE from gestational weeks 22 to 23 reflects a decrease in EI, although reasons for this decrease during this week are unknown. One possible explanation may be that women typically have their anatomical ultrasound somewhere between 21 and 25 weeks of pregnancy which can often generate feelings of anxiety and stress in anticipation of seeing their baby (often for the first time) and hoping there are no abnormalities (e.g., fetal growth or placental issues, markers for Down Syndrome). Significant events such as this can often impact women’s dietary intake patterns as they eat or restrict eating in response to psychosocial stressors [44, 45]. Nevertheless, the fluctuations in REE are consistent with past researchers’ conclusions that have shown some prenatal REE patterns that do not follow a linear trajectory [25], and highlight the importance of examining week-to-week variations in REE over the course of pregnancy to better understand how these fluctuations at key points in gestation may impact maternal and infant outcomes.
In partial support of the second hypothesis and past research [19], Leonard and Symons Downs, under review], there were time-varying associations between REE and weekly GWG. Low REE was associated with high weekly GWG from 25 to 28 weeks gestation, but it was not associated with weekly GWG from 14 to 24 weeks gestation. Although the strength of the associations increased over time, it is important to note that all non-significant and significant associations were very small (range 0.00007–0.002). Similar to the patterns of change in REE noted above, the strength of the associations were not influenced by outliers and/or missing data. Instead, when the associations between REE and GWG were stronger and significant (i.e., weeks 25 to 28), there was an observable fluctuation in REE such that there were large increase and decreases during this time. In contrast, during gestational weeks 14–24, REE did not fluctuate as much, but rather increased. In contrast to Vander Wyst and colleagues [20] who suggested that a drastic fluctuation in REE may be indicative of excessive GWG, these current findings suggest that it may not be the fluctuation itself that is a warning sign of high GWG, but rather low REE during times of fluctuation.
In partial support of the third hypothesis, slightly over half of the women were categorized as exceeding EI recommendations at most of the time points and the majority were categorized as low active from gestational week 25 to 28. This pattern was also observed from gestational weeks 14–24. These patterns of high EI and low PA may yield useful insight regarding how REE can be used to inform EI and PA recommendations to regulate GWG, which are non-existent for PW-OW/OB. For example, the traditional assumption is that there is a linear increase in prenatal REE [17, 19, 21, 35, 36, 46], which has partly informed the need for extra energy requirements during pregnancy [1, 15, 41, 42]. Within the context of GWG interventions, it may be that intervention scientists are not accounting for changes and/or fluctuations in REE, and thus, when REE is low, may be overestimating EI needs and underestimating PA needs for PW-OW/OB which increases the risk of high GWG [47]. For example, rather than reducing EI goals that were developed for women with normal weight by a set amount (e.g., 20–30%) and creating a blanket goal, intervention scientists or clinicians (e.g., registered dieticians) may want to individualize goals for each PW-OW/OB particularly during the second trimester and during critical period of fluctuation in REE based on her actual REE and total energy expenditure in an effort to regulate GWG. If an initial EI goal is set for a PW-OW/OB at 2600 kcal/day and her PA goal is set for 350 kcal/day, but her REE is 1900 kcal, researchers/clinicians may want to adjust her EI and/or PA goals. That is, her EI goal should be decreased to 2300 kcal/day to account for a total energy expenditure of approximately 2250 kcal (i.e., 1900 kcal from REE + 350 kcal from PA). On the other hand, if the goal is to increase a woman’s total energy expenditure to 2350 kcal, researchers/clinicians would use the REE value of 1900 kcal to appropriately inform her PA goal. That is, her PA goal should be increased to 450 kcal to result in a total energy expenditure of 2350 kcal. As the current study illustrated, REE may vary from week to week and thus measuring and understanding REE on a weekly basis may be important for individualizing EI and PA goals from week to week. In other words, EI and PA goals do not need to be “set in stone” and can be adjusted weekly based on each woman’s unique energy needs to personalize GWG regulation. Successfully using REE to inform EI and PA goals can remove the guesswork of how to best moderate EI and increase PA to effectively regulate GWG. However, because few, if any, studies have used REE to adjust prenatal EI and PA goals to regulate GWG, there is a need for research to better understand the impact of these adjustments over time on total GWG.
The current study findings are novel as they are among the first to document that although the associations between REE and GWG in PW-OW/OB during the second trimester are small, they are time-varying. Given the small associations, moderating EI and increasing PA remain important targets for effective GWG regulation in PW-OW/OB. There are several strengths to this study. First, REE and GWG were assessed frequently over the course of the second trimester compared with pre-post assessments. Second, obtaining REE assessments following a true fasting state (i.e., 8–12 h) avoided the possibility of not being in a true rested state due to eating and digestion, which strengthened the quality of the REE data. Third, the focus during the second trimester is an additional strength given the second trimester is a time of rapid GWG, particularly for PW-OW/OB. However, future research is needed to examine the time-varying associations between REE and GWG over the entire pregnancy to understand how transitions between the first and second trimesters and second and third trimesters may influence overall GWG. Fourth, TVEM is an innovative statistical approach to examine associations over time and allows researchers to understand whether there are certain gestational periods when associations may be stronger or non-apparent. This increases the ability for future intervention scientists to identify opportune windows for personalization of GWG regulation interventions during specific gestational periods. Some noteworthy limitations include the homogenous (i.e., White, highly educated, married) group of PW-OW/OB residing in Central Pennsylvania, limiting the generalizability of study findings. Future research with a more diverse sample is needed to confirm and expand the current study findings. Also, future research is needed to understand if the same pattern of fluctuation in REE and its influence on GWG holds in pregnant women with normal weight. In addition, while the use of device-based measurements strengthened the quality of the data, some limitations may include rates of lost data, technical issues, and adherence. In an effort to improve upon these limitations, participants were trained and given weekly resources and instructions to address any issues they may have faced with respect to any of the device-based measurements. Finally, although this study expands upon the current literature to include weekly point estimates of REE, future research should consider including more frequent REE assessments to better reflect the true average REE pattern at the weekly level.
In summary, this study found that REE in PW-OW/OB fluctuated during the second trimester, and low REE was associated with high GWG particularly during times of great fluctuation in REE (i.e., 25 to 28 weeks gestation). During weeks 25–28, slightly over half of the women were categorized as exceeding EI recommendations and most as low active. These findings highlight that EI needs may be overestimated, and PA needs may be underestimated during this critical period when REE is fluctuating, which in turn, may increase the risk for high second trimester GWG. This study highlights the importance of future research to better understand prenatal REE in addition to moderating EI and promoting PA to identifying points of fluctuation in REE over the entire pregnancy. Educating women about REE may be useful for developing personalized EI and PA goals to more effectively regulate GWG, and in turn, improve short- and long-term maternal and infant well-being.
Acknowledgments
We would like to acknowledge Dr. Erica Forzani and David Jackemeyer for supplying some of the Breezing™ devices and materials, Drs. Michael Blair Evans and Joshua M. Smyth for providing comments on the manuscript, and Drs. Jennifer S. Savage, Daniel E. Rivera, Abigail Pauley, Emily Hohman, and Penghong Guo and Katherine McNitt for their contributions to the Healthy Mom Zone Study (grant R01HL119245-01). Permission from all individuals has been obtained.
Funding
Support of this work has been provided by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health through grant R01HL119245-01 (PI Downs), the National Center for Advancing Translational Sciences (NCATS), NIH through grant UL1 TR000127 and TR002014, and the United States Department of Agriculture National Institute of Food and Agriculture (#2011-67001-30117, A2121 Childhood Obesity Prevention Training Program of The Pennsylvania State University).
Footnotes
Availability of Data and Material Upon request to the corresponding author.
Code Availability Upon request to the corresponding author.
Conflict of Interest All authors have no conflicts of interest to disclose.
Ethics Approval The Healthy Mom Zone study was approved by the Pennsylvania State University Institutional Review Board (IRB Study# 00000122). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to Participate Study procedures were explained to the participants and written informed consent was obtained for each woman at their baseline session.
Consent for Publication The following statement was included in the informed consent form – “In the event of any publication or presentation resulting from the research, no personally identifiable information will be shared.
References
- 1.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: Reexamining the guidelines. Yaktine AL, Rasmussen KM (eds). Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 2.McDowell M, Cain MA, Brumley J. Excessive gestational weight gain. J Midwifery Womens Health. 2019;64:46–54. 10.1111/jmwh.12927. [DOI] [PubMed] [Google Scholar]
- 3.Daly N, Mitchell C, Farren M, Kennelly MM, Hussey J, Turner MJ. Maternal obesity and physical activity and exercise levels as pregnancy advances: An observational study. Ir J Med Sci. 2016;185:357–70. 10.1007/s11845-015-1340-3. [DOI] [PubMed] [Google Scholar]
- 4.de Jersey SJ, Nicholson JM, Callaway LK, Daniels LA. An observational study of nutrition and physical activity behaviours, knowledge, and advice in pregnancy. BMC Pregnancy Childbirth. 2013;13:115. 10.1186/1471-2393-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagourney EM, Goodman D, Lam Y, Hurley KM, Henderson J, Surkan PJ. Obese women’s perceptions of weight gain during pregnancy: A theory-based analysis. Public Health Nutr. 2019;22:2228–36. 10.1017/S1368980019000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Symons Downs D, Savage JS, Rauff EL. Falling short of guidelines? Nutrition and weight gain knowledge in pregnancy. J Womens Health Care. 2014;3:1000184. 10.4172/2167-0420.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno R, Petrella E, Bertarini V, Pedrielli G, Neri I, Facchinetti F. Adherence to a lifestyle programme in overweight/obese pregnant women and effect on gestational diabetes mellitus: A randomized controlled trial. Matern Child Nutr. 2017;13:e12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: A systematic review of randomized trials. BJOG. 2010;117:1316–26. [DOI] [PubMed] [Google Scholar]
- 9.Dodd JM, Turnbull D, Phee AJ, et al. Antenatal lifestyle advice for women who are overweight of obese: LIMIT randomized trial. BMJ. 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: A randomized controlled trial. Am J Clin Nutr. 2010;91:373–80. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins M, Hosker M, Marcus BH, Rosal MC, Braun B, Stanek EJ III, et al. A pregnancy lifestyle intervention to prevent gestational diabetes risk factors in overweight Hispanic women: A feasibility randomized controlled trial. Diabet Med. 2015;32:108–15. [DOI] [PubMed] [Google Scholar]
- 12.Szmeja MA, Cramp C, Grivell RM, Deussen AR, Yelland LN, Dodd JM. Use of a DVD to provide dietary and lifestyle information to pregnant women who are overweight or obese: A nested randomized trial. BMC Pregnancy Childbirth. 2014;14:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, Wing RR. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: The Fit for Delivery Study. Am J Clin Nutr. 2011;93:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redman LM, Gilmore LA, Breaux J, Thomas DM, Elkind-Hirsch K, Stewart T, et al. Effectiveness of SmartMoms, a novel eHealth intervention for management of gestational weight gain: Randomized controlled pilot trial. JMIR Mhealth Uhealth. 2017;5:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Obstetrics and Gynecologists. Nutrition during pregnancy. In Your pregnancy and childbirth: Month to month (6th ed., pp. 313–327). Washington, D.C.: ACOG; 2016 [Google Scholar]
- 16.American College of Obstetrics and Gynecologists. Physical activity and exercise during pregnancy and the postpartum period. Committee Opinion No. 650. Obstet Gynecol 2015; 126: e135–e142. [DOI] [PubMed] [Google Scholar]
- 17.Taousani E, Savvaki D, Tsirou E, et al. Regulation of basal metabolic rate in uncomplicated pregnancy and in gestational diabetes mellitus. Hormones (Athens). 2017; 16: 235–50. doi: 10.14310/horm.2002.1743. [DOI] [PubMed] [Google Scholar]
- 18.McClave SA, Snider HL. Use of indirect calorimetry in clinical nutrition. Nutr Clin Pract. 1992;7:207–21. [DOI] [PubMed] [Google Scholar]
- 19.Berggren EK, O’Tierney-Ginn P, Lewis S, Presley L, Hauguel De-Mouzon S, Catalano PM. Variations in resting energy expenditure: impact on gestational weight gain. Am J Obstet Gynecol. 2017;217:445.e1–445.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vander Wyst KB, Buman MP, Shaibi GQ, Petrov ME, Reifsnider E, Whisner CM. Resting energy expenditure relationship with macronutrients and gestational weight gain: a pilot study. Nutrients. 2020;12:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano PM. Pregnancy and lactation in relation to range of acceptable carbohydrate and fat intake. Eur J Clin Nutr. 1999;53:S124–35. [DOI] [PubMed] [Google Scholar]
- 22.Symons Downs D, Savage JS, Rivera DE, Smyth JM, Rolls BJ, Hohman EE, et al. Individually tailored, adaptive intervention to manage gestational weight gain: protocol for a randomized controlled trial in women with overweight and obesity. JMIR Res Protoc. 2018;7:e150. 10.2196/resprot.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vesco KK, Karanja N, King JC, Gillman MW, Perrin N, McEvoy C, et al. Healthy Moms, a randomized trial to promote and evaluate weight maintenance among obese pregnant women: Study design and rationale. Contemp Clinc Trials. 2012;33:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overcash RT, Hull AD, Moore TR, LaCoursiere DY. Early second trimester weight gain in obese women predicts excessive gestational weight gain in pregnancy. Matern Child Health J. 2015;19:2412–8. [DOI] [PubMed] [Google Scholar]
- 25.Jackemeyer D, Forzani E, Whisner C. Study of resting energy expenditure and weight changes during pregnancy. Glob J Obes Diabetes Metab Syndr. 2017;4:016–23. [Google Scholar]
- 26.Guo P, Rivera DE, Symons Downs D, et al. Semi-physical identification and state estimation of energy intake for interventions to manage gestational weight gain. 2016 American Control Conference; July 6-8, 2016; Boston, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo P, Rivera DE, Pauley AM, Leonard KS, Savage JS, Downs DS. A “Model-on-Demand” methodology for energy intake estimation to improve gestational weight control interventions. Proc IFAC World Congress. 2018;51:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo P, Rivera DE, Savage JS, Hohman EE, Pauley AM, Leonard KS, et al. System identification approaches for energy intake estimation: Enhancing interventions for managing gestational weight gain. IEEE Trans Control Syst Technol. 2020;28:63–78. 10.1109/TCST.2018.2871871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Symons Downs D, Savage JS, Rivera DE, et al. Using mHealth and eHealth methods to promote weight control among women. Ann Behav Med. 2017;51:1587–8. [Google Scholar]
- 30.Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med. 2011;53:39–43. [DOI] [PubMed] [Google Scholar]
- 31.Mâsse LC, Fuemmeler BF, Anderson CB, et al. Accelerometer data reduction: A comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–54. [DOI] [PubMed] [Google Scholar]
- 32.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xian X, Quach A, Bridgeman D, Tsow F, Forzani E, Tao N. Personalized indirect calorimeter for energy expenditure (EE) measurement. Glob J Obes Diabetes Metab Syndr. 2015;2:004–8. [Google Scholar]
- 34.Thomas DM, Navarro-Barrientos JE, Rivera DE, Heymsfield SB, Bredlau C, Redman LM, et al. Dynamic energy-balance model predicting gestational weight gain. Am J Clin Nutr. 2012;95:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–32. [DOI] [PubMed] [Google Scholar]
- 36.Butte NF, Wong WW, Treuth MS, Ellis KJ, O’Brian SE. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79:1078–87. [DOI] [PubMed] [Google Scholar]
- 37.Hood KM, Marr C, Kirk-Sorrow J, Farmer J IV, Lee M, Kern M. Validity and reliability of a Wi-Fi smart scale to estimated body composition. Heal Technol. 2019;9:839–56. [Google Scholar]
- 38.Ruan D, Chen G, Kerre EE. Intelligent data mining: techniques and applications (Vol. 5). Springer Science & Business Media; 2005. [Google Scholar]
- 39.Tan X, Shiyko MP, Li R, Li Y, Dierker L. (2012). A time-varying effect model for intensive longitudinal data. Psychol Methods. 2012;17:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd Edition). Lawrence Earlbaum Associates: Hillsdale, NJ; 1988. [Google Scholar]
- 41.Kaiser LL, Campbell CG, Academy Positions Committee Workgroup. Practice paper of the Academy of Nutrition and Dietetics: Nutrition and lifestyle for a healthy pregnancy outcome. J Acad Nutr Diet. 2014;114:1447. [DOI] [PubMed] [Google Scholar]
- 42.Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Med Clin N Am. 2016;100:1199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Most J, Vallo PM, Gilmore LA, St. Amant M, Hsia DS, Altazan AD, et al. Energy expenditure in pregnant women with obesity does not support energy intake recommendations. Obesity (Silver Spring). 2018;26:992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blau LE, Lipsky LM, Dempster KW, Eisenberg Colman MH, Siega-Riz AM, Faith MS, et al. Women’s experience and understanding of food cravings in pregnancy: A qualitative study in women receiving prenatal care at the university of North Carolina-Chapel Hill. J Acad Nutr Diet. 2020;120:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savage JS, Hohman EE, McNitt KM, et al. Uncontrolled eating during pregnancy predicts fetal growth: The healthy mom zone trial. Nutrients. 2019;11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Most J, Dervis S, Harman F, Adamo KB, Redman LM. Energy intake requirements in pregnancy. Nutrients. 2019;11:1812. 10.3390/nu11081812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melzer K, Schutz Y, Soehnchen N, Othenin Girard V, Martinez de Tejada B, Pichard C, et al. Prepregnancy body mass index and resting energy expenditure during pregnancy. Ann Nutr Metab. 2010;57:221–7. 10.1159/000322369. [DOI] [PubMed] [Google Scholar]