Abstract
Background:
Available literature has reported the association of Helicobacter pylori (H pylori) infection with inflammatory bowel disease (IBD) in adults. However, only a few studies have addressed the disease in children.
Aim:
To ascertain the correlation of H pylori infection with IBD among children.
Methods:
The aim of this systematic review and meta-analysis is to assess the association between H pylori infection and IBD in children. We searched databases including Cochrane, EMBASE, Google Scholar, PubMed, Medline, and Web of Science to select relevant studies. Ultimately, based on predetermined inclusion criteria, we included 6 studies that met the requirements. Review Manager and Stata software were used to extract and analyze the data from the relevant studies. In the methods, we employed both qualitative and quantitative approaches for comprehensive analysis. Qualitative analysis involved describing study designs, sample characteristics, and results, while quantitative analysis involved statistical tests such as calculating pooled risk ratios and 95% confidence intervals to evaluate the association between H pylori infection and IBD in children. Lastly, by combining the results of the individual studies, our objective is to provide a comprehensive understanding of the relationship between H pylori infection and IBD in children.
Results:
In totality, we involved 2236 participants that were recruited in 6 studies. We detected no significant difference in H pylori prevalence (9.8% vs 12.7%, P = .12) by comparing the children IBD group to controls. Among the IBD children, we estimated odds ratio (OR) of H pylori infection to 0.62 [(95% confidence interval (CI) of 0.34–1.12)]. In children suffering from ulcerative colitis (UC) and Crohn disease (CD), the H pylori infection rates were higher than in those with IBD-unclassified (IBDU).When analyzed stratified by disease of study design, In CD group [OR = 1.42, 95% CI: 0.72–2.80)] (I2 = 0%, P = .64). but no significant difference in CD group.
Conclusions:
No correlation was found between H pylori infection and the occurrence of IBD in children.
Keywords: Children, Crohn disease, Helicobacter pylori infection, Inflammatory disease of the bowel, ulcerative colitis
1. Introduction
About 3.5 million people worldwide suffer from inflammatory bowel disease (IBD), which is characterized as a chronic recurrent idiopathic gastrointestinal tract disease with 2 main types, namely ulcerative colitis (UC) and Crohn disease (CD).[1] Reduced quality of life and increased colorectal cancer risk has been associated with IBD.[2] Globally, existing literature has indicated marked increase in IBD incidence particularly in children, wherein this rise has been ascribed to parallels of global industrialization,[3–5] amid placing a huge burden on public health.[6]
However, the etiology of IBD is still unknown.[7] Immunological differences exist between pediatric patients and adults with IBD[8]; however, no significant difference has been detected in genotype,[9] thus suggesting that pediatric phenotype might be related to environmental factors. Although microbial system has been considered critical to the pathogenesis of IBD,[10,11]the underlying microorganism has not yet been identified.[12,13]
Previous studies have indicated a certain degree of association between Helicobacter pylori (H pylori) infection and IBD in both adults and children.[14] Through a systematic review and meta-analysis, it was found that there might be a potential link between H pylori infection and IBD in adults and children.[14] Some studies have proposed H pylori infection as a potential factor in the development of IBD in children. The results of the meta-analysis suggest that H pylori infection may be associated with an increased risk of developing IBD in adults and children. Additionally, certain studies have demonstrated a correlation between H pylori infection and the severity of IBD in both children and adults. However, further research is needed to better understand the underlying mechanisms of the association between H pylori infection and IBD.
As a gut bacterium, H pylori has been linked to stomach cancer and peptic ulcers.[15] Conflicting evidence from human and animal studies supports Helicobacter as an agent causing IBD.[16–22] Pediatric data on this aspect are limited and based on a small sample. Some studies have suggested negative association of H pylori infection in children with IBD,[23] while others disagreed.[24] Currently, there is no pediatric comprehensive, large-scale, systematic review on the aforesaid controversy. This work sought to assess the correlation of H pylori infection with IBD in children.
There is currently significant controversy regarding the association between H pylori infection and IBD in children. Some studies suggest a possible negative correlation, while others hold different opinions. There is a lack of large-scale, systematic reviews in the pediatric field to address this issue. Therefore, the question of whether H pylori infection is associated with IBD in children has not been definitively resolved.
2. Materials and methods
2.1. Strategy for literature search and selection
We carried out this meta-analysis on the account of preferred report item statement for systematic reviews and meta analysis (PRISMA).[25] The following databases were searched: Cochrane Central-Register of Controlled Trials, EMBASE, Google Scholar, PubMed, MEDLINE, and Web of Science. Search duration was from inception to December 2022. Search sensitivity was improved by search strategy through usage of MeSH terms and free text words. Search terms that were employed included: “Bowel Diseases, Inflammatorylcer,” “Bowel Diseases, Inflammatory Disease,” “Campylobacter pylori,” “C. pylori subsp.” “C. pyloridis,” “CD,” “Colitis Gravis,” “CD,” “Crohns Disease,” “CD”, Crohn Enteritis,” “Colitis, Granulomatous”, Enteritis, Granulomatous,” “Granulomatous Colitis,” “Enteritis, Regional,” “Granulomatous Enteritis,” “H pylori,” “H. nemestrinae”, Pylori,” “H pylori,” “IBD,” “IBD-unclassified (IBDU),” “Idiopathic Proctocolitis,” “Ileocolitis,” “Ileitis, Regional,” “Ileitis, Terminal,” “IBD,” “Inflammatory Bowel Disease 1,” “Inflammatory Bowel Disease, Ulcerative Colitis Type,” “Ulcerative Colitis,” “Regional Enteritis,” “Regional Ileitides,” “Regional Ileitis,” and “Terminal Ileitis.” Later, the title and summary of the search results were checked before their inclusion in the study. In case of discrepancy, the corresponding author could be contacted.
2.2. Article selection and eligibility criteria
Systematic review study usually must meet eligibility criteria, namely: Inclusively, reported the association between IBD and H pylori infection, diagnosed as H pylori infection by rapid urea test (RUT), serology, stool antigen, urea breath test (UBT) or histology, <18 years of age, all case-control studies with non-IBD as the control group, human studies, and calculation of H pylori infection rate in IBD and control groups is based on sufficient reported data. The following exclusion criteria were used: case reports, conference abstracts, letters or reviews, duplicate or overlapping data, adults, cell or animal studies, and lack of accurate data.
2.3. Extraction of data and assessment of quality
Extraction of data was independently done by 2 reviewers (Guiping Kong and Yan Lu) via selected studies using standardized forms for extracting data to purposively minimize biased reporting and data collection errors. Creation of tables by research team was based on the following items: author, title, publication year, study design, criteria of inclusion and exclusion, methods of diagnosing H pylori infection, methods of diagnosing IBD, number of CD, UC and IBDU patients, including negative and positive H pylori, and number of H pylori positive and negative patients in control group. Three researchers (Yan Lu, Mei Li, and Hongmei Guo) used the Newcastle–Ottawa Scale[26] for cohort and case-control reports, wherein for cross sectional surveys, the Agency for Healthcare Research and Quality (AHRQ-11),[27] was utilized to accurately assess the quality of the methodology incorporated into the study. Assessment of quality of studies was carried out with the aforementioned criteria: design of study, diagnostic methods for H pylori, diagnostic methods for IBD, inclusion method (continuous vs selected), and whether rate of H pylori infection was main or minor result of the report. High quality was denoted by scale scores greater or equal to 7 and moderate quality was represented by scores equal to 4 to 7, while poor quality was depicted by scores <4.
The study complied with the Declaration of Helsinki and was approved by the local research ethics committee. Written informed consent was obtained from all patients.
2.4. Statistical analysis
Pooled odds ratio (OR) of H pylori infection with IBD compared to controls was considered the primary outcome of this analysis. Ratio of probability of H pylori infection in patients with IBD to control was used to describe OR. Review Manager 5.3 software was used for data processing. The dichotomous variable OR was used as the efficacy analysis statistical tool, while weighted mean difference was used as the efficacy analysis parameter. All effect sizes were expressed with 95% confidence interval (CI). Chi square test was employed to analyze the heterogeneity among the studies. For a high degree of statistical heterogeneity among the studies, we used random effects model; IBD patients comprised of case group, while the non-IBD subjects composed of control group. The OR and corresponding 95% CI were calculated for both case and control groups. All data were synthesized and analyzed with Stata software (version 15, StataCorp, College Station, TX).
3. Results
3.1. Search for literature search and selection of studies
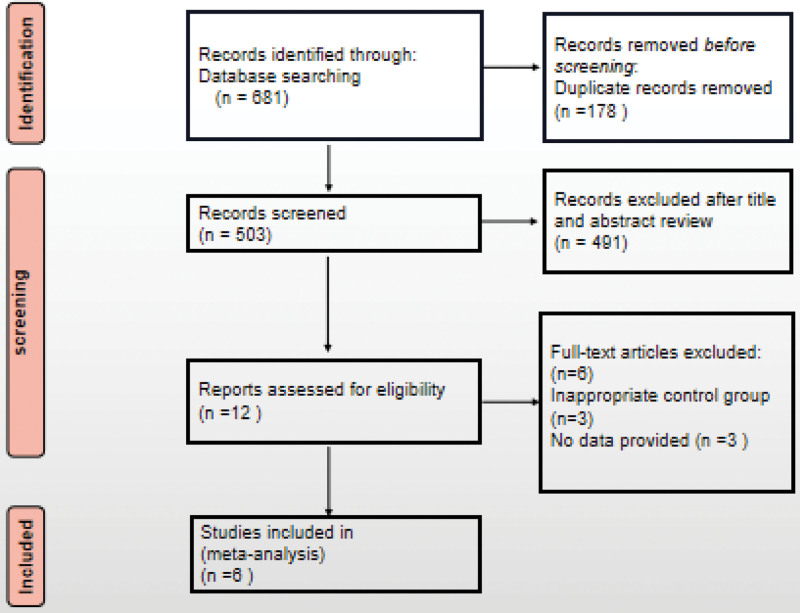
In totality, 681 articles were screened out by searching keywords, wherein 503 were retained after removal of duplicates. Afterwards, we excluded 491 articles by reviewing abstracts and titles. Among the remaining 12 records, 6 were removed for various reasons. Finally, 6 eligible case-control studies were included (Fig. 1).[13,28–30]
Figure 1.
Study selection flow chart.
3.2. Included studies features
The baseline features of included studies (6) are listed in Table 1, wherein it involved 356 IBD patients and 1880 non-IBD controls. The diagnostic methods and results of H pylori infection and IBD were recorded. Among these, 4 studies employed a case-control study design, and 2 were cohort studies. All studies were published between 2010 and 2014, with a wide geographic representation across 5 countries. The H pylori was detected with PCR, serology and histology from different sampling sites, including the stomach (4 studies), colon (3 studies), and liver (1 study). IBD was mainly diagnosed according to endoscopy, histology, radiology and clinical manifestations. Table 2 shows the proportion of H pylori-positive subtypes of IBD, namely CD, UC, and IBDU.
Table 1.
Basic characteristics of included research literature.
| Report | Country | Design | H pylori methods (sampling sites) | IBD methods | Result | Age (yr) | Participant (CD/UC/IBDU) | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| Kaakoush, 2010 | Australia | Case-control | PCR/RUT/histology (colon) | Endoscopy Histology Radiology |
main | 11.4 ± 3.9 vs 10.2 ± 3.9* vs 9.1 ± 4.2† | 77/102 (77/0/0) | 8 9 |
| Casswall, 2010 | Sweden | Case-control | PCR/serum/histology (liver and stomach) | Endoscopy Histology Clinical |
main | NA | 26/51 (4/21/1) | 8 9 |
| Hansen, 2011 | United Kingdom | Cohort | PCR (colon) | Endoscopy Histology Clinical |
main | NA | 24/26 (12/8/4) | 7 9 |
| Sonnenberg, 2011 | United States | Case-control | Histology (stomach) | Endoscopy Histology Clinical |
minor | NA | 26/216 (8/15/3) | 9 9 |
| Hansen, 2013 | United Kingdom | Cohort | PCR/histology/serum (colon, stomach) | Endoscopy Histology Radiology |
minor | 11.9 ± 2.9 vs 10.6 ± 3.5 | 44/42 (29/13/2) | 7 9 |
| Roka, 2014 | Greece | Case-control | Histology/UBT (stomach) | Endoscopy Histology Clinical |
main | 9.3 ± 4.2 vs 7.3 ± 4.4 | 159/1443 (66/34/59) | 8 9 |
CD = Crohn disease, H pylori = Helicobacter pylori, IBD = inflammatory bowel disease, IBDU = inflammatory bowel disease-unclassified, NA = not available, RUT = rapid urea test, UBT = urea breath test, UC = ulcerative colitis.
Non-IBD with pathology.
Non-IBD with no pathology.
Table 2.
Research results of different inflammatory bowel disease subtypes.
| Included study | H pylori positive | ||||
|---|---|---|---|---|---|
| % IBD | % CD | % UC | % IBDU | % controls | |
| Kaakoush, 2010 | 14 (18.2%) | 14 (18.2%) | 0 | 0 | 23 (22.5%) |
| Casswall, 2010 | 6 (23.1%) | 0 | 6 (28.6%) | 0 | 10 (19.6%) |
| Hansen, 2011 | 3 (12.5%) | NA | NA | NA | 2 (7.7%) |
| Sonnenber, 2011 | 0 | 0 | 0 | 0 | 3 (1.4%) |
| Hansen, 2013 | 6 (13.6%) | 4 (13.8%) | 2 (15.4%) | 0 | 11 (26.2%) |
| Roka, 2014 | 6 (3.8%) | 3 (4.5%) | 2 (5.8%) | 1 (1.7%) | 190 (13.2%) |
CD = Crohn disease, H pylori = Helicobacter pylori, IBD = inflammatory bowel disease, IBDU = inflammatory bowel disease-unclassified, NA = not available, UC = ulcerative colitis.
3.3. Data analysis
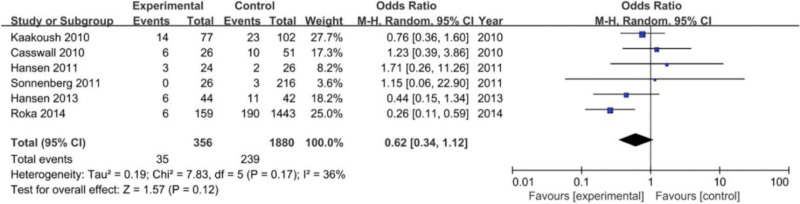
A total of 35 out of 356 (9.8%) cases were H pylori-positive in the pediatric IBD group, while 239 out of 1880 (12.7%) in the control group. Moreover, 4 studies provided data on the negative correlation between H pylori (stomach) and IBD, which is the primary outcome in this meta-analysis. I² = 36 < 50%, P = .17 > 0.05 revealed that there was no substantial heterogeneity, due to which the meta-analysis was conducted using a fixed effects model.
Through statistical analysis, it was established that H pylori infection did not show any direct correlation with IBD in children (OR = 0.62, 95% CI: 0.34–1.12, P = .12) (Fig. 2).
Figure 2.
Forest plotting of correlation of Helicobacter pylori (H pylori) infection with inflammatory bowel disease (IBD) in children.
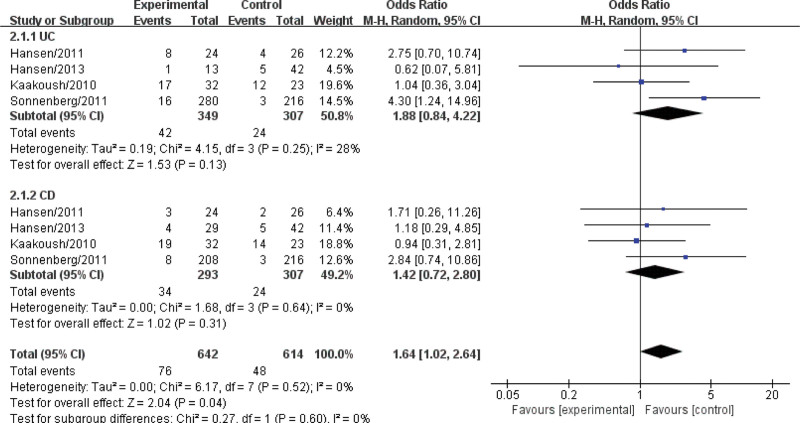
When analyzed stratified by disease of study design, in CD group [OR = 1.42, 95% CI: 0.72–2.80)] (I2 = 0%, P = .64), but no significant difference in CD group (Fig. 3).
Figure 3.
Forest plotting for subgroup analysis of Helicobacter pylori (H pylori) infection with inflammatory bowel disease (IBD) in children.
4. Discussion
Epidemiologically, IBD occurs in adults and children. Several studies have been carried out on the risk factors of IBD, however factors that can reduce or prevent IBD are still unclear. Pathogenesis of IBD is driven by the immune response of genetically susceptible hosts to intestinal dysbacteriosis.[1,31] Reportedly, H pylori protects against some immune-mediated diseases, including IBD, by regulating the host immune defense and other mechanisms.[32,33] H pylori infection could be acquired in childhood, while the infected individual without treatment could serve as a carrier.[14] Available report has suggested that H pylori infection was detected in adults influenced by IBD.[34] However, the involvement of H pylori in pathological process of IBD is still controversial, especially among children.[11,28,35–37]
In this work, meta-analysis was employed to delve into association of H pylori with IBD in children. In this regard, we analyzed in total 6 reports comprising 2236 cases in the current meta-analysis. Most importantly, compared to the control group, our data demonstrated that H pylori did not significantly associate with IBD in children, thus indicating insufficient evidence to prove that H pylori is involved in occurrence of pediatric IBD. According to included studies,[13,24,28,29] H pylori usually settles in the external gastric tissue, including colon and liver. A negative correlation was established between gastric H pylori and IBD, while other sites, such as colon and liver, did not show similar results. H pylori sampling via gastric biopsy alone is random and could be missed.[38,39] Therefore, regarding the correlation between H pylori and IBD, we should consider the stomach and also collect samples of H pylori from various sites for a comprehensive analysis.
To analyze accurately and correctly, the choice of detection method is important. The results of 4 studies[13,24,28,29] that used multiple methods including PCR to diagnose H pylori were inconsistent with others[23,30] that only utilized histology or UBT. The former reported significant infection rates of H pylori in IBD patients[28,29] or no significant difference between groups.[13,24] The latter showed a lower infection of H pylori in IBD patients,[23,30] thereby implying that several tests might be needed to acquire accurate data to diagnose H pylori. Typically, the diagnosis of IBD in children (excluding differences in race, gender, systemic antibiotics, steroids and immunosuppressants) has been confirmed by endoscopy, histology, radiology and clinical manifestations. The stomach, colon (sectioned materials) and liver were examined using UBT, RUT, histology and PCR, respectively. Other confounding factors including baseline characteristics (living environment and matching of control information) and pathological differences of IBD (subtype, disease location and stage). Reportedly, the protective effect of H pylori on autoimmune diseases might originate from cagA + strain,[40,41] and hence, the type of H pylori strains should be identified according to the positive results.
Despite the differences, a low rate of H pylori infection in patients with IBD has been reported by all other works. Three studies excluded previous H pylori infections when subjects were recruited.[13,28,30] Thus, we speculated that the low rate of H pylori infection may be as a result of microbiological system adjustment due to the development of IBD disease rather than the cause. In addition, we observed a statistical difference in the prevalence of H pylori among CD, UC and IBDU. The positive rate of H pylori in individuals with BDU was significantly lower comparable to CD and UC patients, which was similar to previous report.[30]The UC exposure was slightly higher than that of CD patients, albeit insignificance, wherein it was consistent with other report.[42]
In terms of main strengths, this meta-analysis is the first report to investigate the correlation of H pylori with IBD in children, amid the sample size being large. To improve data reliability, we included several factors in the current study, namely multisite sampling (stomach, colon and liver) and multiple tests (serum, UBT, RUT, histology and PCR).
Nevertheless, the systematic review with meta-analysis had some limitations. Firstly, there was a lack of prolonged follow up of IBD individuals with H pylori infection, and hence, the impact of H pylori eradication on IBD prognosis is unknown. Secondly, most of the studies were retrospective, thus lacking long-term follow up data on the non-IBD control group, wherein it was unable to determine the H pylori infection status when IBD occurs in future. Thirdly, lack of data on previous antibiotic use might affect H pylori outcomes, especially for IBD patients. Lastly, due to racial differences in the risk factors for IBD,[43] additional studies encompassing several ethnicities are needed to confirm this conclusion.
In conclusion, the current study did not find any explicit correlation of H pylori infection with occurrence of IBD among children. The overall infection rate of H pylori in IBD group was low. The H pylori infection rate in IBDU was higher comparable to UC and CD groups. In the future, a larger, better-designed, prospective study is needed to further confirm our findings.
Author contributions
Conceptualization: Guiping Kong.
Data curation: Guiping Kong.
Formal analysis: Guiping Kong.
Investigation: Yan Lu, Mei Li.
Software: Yan Lu, Mei Li.
Supervision: Zhifeng Liu, Hongmei Guo.
Validation: Zhifeng Liu, Yan Lu, Mei Li, Hongmei Guo.
Writing – original draft: Guiping Kong.
Writing – review & editing: Zhifeng Liu.
Abbreviations:
- CD
- Crohn disease
- CI
- confidence interval
- H pylori
- Helicobacter pylori
- IBD
- inflammatory bowel disease
- IBDU
- IBD-unclassified
- OR
- odds ratio
- RR
- relative risk
- RUT
- rapid urea test
- UBT
- urea breath test
- UC
- ulcerative colitis
Preparation of the manuscript by the authors was based on the Checklist of PRISMA 2020, after they have thoroughly read the Checklist.
This project was supported by the National Natural Science Foundation of China under grant [No. 62001241].
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Kong G, Liu Z, Lu Y, Li M, Guo H. The association between Helicobacter pylori infection and inflammatory bowel disease in children: A systematic review with meta-analysis. Medicine 2023;102:36(e34882).
Contributor Information
Guiping Kong, Email: kgping@163.com.
Yan Lu, Email: luyan_cpu@163.com.
Mei Li, Email: Limei6389@aliyun.com.
References
- [1].Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henderson P, Hansen R, Cameron FL, et al. Rising incidence of pediatric inflammatory bowel disease in Scotland. Inflamm Bowel Dis. 2012;18:999–1005. [DOI] [PubMed] [Google Scholar]
- [4].Hope B, Shahdadpuri R, Dunne C, et al. Rapid rise in incidence of Irish paediatric inflammatory bowel disease. Arch Dis Child. 2012;97:590–4. [DOI] [PubMed] [Google Scholar]
- [5].Martín-de-Carpi J, Rodríguez A, Ramos E, et al. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996-2009): the SPIRIT registry. Inflamm Bowel Dis. 2013;19:73–80. [DOI] [PubMed] [Google Scholar]
- [6].Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- [7].Binder V. Epidemiology of IBD during the twentieth century: an integrated view. Best Pract Res Clin Gastroenterol. 2004;18:463–79. [DOI] [PubMed] [Google Scholar]
- [8].Kugathasan S, Saubermann LJ, Smith L, et al. Mucosal T-cell immunoregulation varies in early and late inflammatory bowel disease. Gut. 2007;56:1696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Imielinski M, Baldassano RN, Griffiths A, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arnold IC, Hitzler I, Müller A. The immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol. 2012;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hansen R, Thomson JM, Fox JG, et al. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol. 2011;61:1–14. [DOI] [PubMed] [Google Scholar]
- [12].Sonnenberg A. Protective role of Helicobacter pylori against inflammatory bowel disease: a hypothesis. Pract Gastroenterol. 2009;33:23–33. [Google Scholar]
- [13].Hansen R, Berry SH, Mukhopadhya I, et al. The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: the BISCUIT study. PLoS One. 2013;8:e58825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luther J, Dave M, Higgins PD, et al. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lahat A, Kopylov U, Neuman S, et al. Helicobacter pylori prevalence and clinical significance in patients with quiescent Crohn’s disease. BMC Gastroenterol. 2017;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gravina AG, Prevete N, Tuccillo C, et al. Peptide Hp(2-20) accelerates healing of TNBS-induced colitis in the rat. United European Gastroenterol J. 2018;6:1428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bell SJ, Chisholm SA, Owen RJ, et al. Evaluation of Helicobacter species in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:481–6. [DOI] [PubMed] [Google Scholar]
- [19].Grehan M, Danon S, Lee A, et al. Absence of mucosa-associated colonic Helicobacters in an Australian urban population. J Clin Microbiol. 2004;42:874–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bohr UR, Glasbrenner B, Primus A, et al. Identification of enterohepatic Helicobacter species in patients suffering from inflammatory bowel disease. J Clin Microbiol. 2004;42:2766–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L, Day A, McKenzie G, et al. Nongastric Helicobacter species detected in the intestinal tract of children. J Clin Microbiol. 2006;44:2276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laharie D, Asencio C, Asselineau J, et al. Association between entero-hepatic Helicobacter species and Crohn’s disease: a prospective cross-sectional study. Aliment Pharmacol Ther. 2009;30:283–93. [DOI] [PubMed] [Google Scholar]
- [23].Sonnenberg A, Melton SD, Genta RM. Frequent occurrence of gastritis and duodenitis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:39–44. [DOI] [PubMed] [Google Scholar]
- [24].Hansen R, Mukhopadhya I, Russell RK, et al. The role of microaerophilic colonic mucosal bacteria in de novo paediatric inflammatory bowel disease. Gut. 2011;60(Suppl 1):A147–A147. [Google Scholar]
- [25].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3:e123–130. [PMC free article] [PubMed] [Google Scholar]
- [26].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [27].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [28].Kaakoush NO, Holmes J, Octavia S, et al. Detection of Helicobacteraceae in intestinal biopsies of children with Crohn’s disease. Helicobacter. 2010;15:549–57. [DOI] [PubMed] [Google Scholar]
- [29].Casswall TH, Németh A, Nilsson I, et al. Helicobacter species DNA in liver and gastric tissues in children and adolescents with chronic liver disease. Scand J Gastroenterol. 2010;45:160–7. [DOI] [PubMed] [Google Scholar]
- [30].Roka K, Roubani A, Stefanaki K, et al. The prevalence of Helicobacter pylori gastritis in newly diagnosed children with inflammatory bowel disease. Helicobacter. 2014;19:400–5. [DOI] [PubMed] [Google Scholar]
- [31].Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shah SC, Tepler A, Peek RM, Jr., et al. Association between Helicobacter pylori exposure and decreased odds of eosinophilic esophagitis-a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:2185–2198.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Castaño-Rodríguez N, Kaakoush NO, Lee WS, et al. Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut. 2017;66:235–49. [DOI] [PubMed] [Google Scholar]
- [34].Fallone CA, Bitton A. Is IBD caused by a Helicobacter pylori infection? Inflamm Bowel Dis. 2008;14(Suppl 2):S37–8. [DOI] [PubMed] [Google Scholar]
- [35].Man SM, Zhang L, Day AS, et al. Detection of enterohepatic and gastric helicobacter species in fecal specimens of children with Crohn’s disease. Helicobacter. 2008;13:234–8. [DOI] [PubMed] [Google Scholar]
- [36].Keenan JI, Beaugie CR, Jasmann B, et al. Helicobacter species in the human colon. Colorectal Dis. 2010;12:48–53. [DOI] [PubMed] [Google Scholar]
- [37].Thomson JM, Hansen R, Berry SH, et al. Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS One. 2011;6:e17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Genta RM, Gürer IE, Graham DY, et al. Adherence of Helicobacter pylori to areas of incomplete intestinal metaplasia in the gastric mucosa. Gastroenterology. 1996;111:1206–11. [DOI] [PubMed] [Google Scholar]
- [39].Sonnenberg A. Environmental influence in ulcerative colitis starts in early childhood. J Epidemiol Community Health. 2008;62:992–4. [DOI] [PubMed] [Google Scholar]
- [40].Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One. 2008;3:e4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang S, Zhong B, Chao K, et al. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J Clin Microbiol. 2011;49:1987–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].O’Brien CL, Bringer MA, Holt KE, et al. Comparative genomics of Crohn’s disease-associated adherent-invasive Escherichia coli. Gut. 2017;66:1382–9. [DOI] [PubMed] [Google Scholar]