Abstract
PURPOSE
Pembrolizumab significantly improves clinical outcomes in advanced/metastatic microsatellite instability high (MSI-H)/deficient mismatch repair (dMMR) solid tumors but is not well studied in the neoadjuvant space.
METHODS
This is a phase II open-label, single-center trial of localized unresectable or high-risk resectable MSI-H/dMMR tumors. Treatment is pembrolizumab 200 mg once every 3 weeks for 6 months followed by surgical resection with an option to continue therapy for 1 year followed by observation. To continue on study, patients are required to have radiographic or clinical benefit. The coprimary end points are safety and pathologic complete response. Key secondary end points are response rate and organ-sparing at one year for patients who declined surgery. Exploratory analyses include interrogation of the tumor immune microenvironment using imaging mass cytometry.
RESULTS
A total of 35 patients were enrolled, including 27 patients with colorectal cancer and eight patients with noncolorectal cancer. Among 33 evaluable patients, best overall response rate was 82%. Among 17 (49%) patients who underwent surgery, the pathologic complete response rate was 65%. Ten patients elected to receive one year of pembrolizumab followed by surveillance without surgical resection (median follow-up of 23 weeks [range, 0-54 weeks]). An additional eight did not undergo surgical resection and received less than 1 year of pembrolizumab. During the study course of the trial and subsequent follow-up, progression events were seen in six patients (four of whom underwent salvage surgery). There were no new safety signals. Spatial immune profiling with imaging mass cytometry noted a significantly closer proximity between granulocytic cells and cytotoxic T cells in patients with progressive events compared with those without progression.
CONCLUSION
Neoadjuvant pembrolizumab in dMMR/MSI-H cancers is safe and resulted in high rates of pathologic, radiographic, and endoscopic response, which has implications for organ-sparing strategies.
INTRODUCTION
Cancers with mismatch repair deficiency (dMMR)/microsatellite instability high (MSI-H) arise across a wide variety of tumor types, most commonly colorectal, endometrial, and gastric cancers.1,2 In addition, dMMR cancers are more commonly seen in early-stage disease.3 Localized dMMR cancers are currently treated as proficient mismatch repair/microsatellite stable tumors, which in general involves surgery, chemotherapy, and/or radiation. However, both preclinical and clinical data have suggested that dMMR cancers demonstrate less benefit from chemotherapy compared with proficient mismatch repair cancers.4-10
CONTEXT
Key Objective
Among patients with a diverse range of localized, solid microsatellite instability high (MSI-H)/deficient mismatch repair (dMMR) tumors, can preoperative pembrolizumab result in pathologic complete response (tumor eradication)?
Knowledge Generated
Seventeen patients (49% of study population) underwent surgical resection and demonstrated a high pathologic complete response rate of 65%. The remaining 18 patients (51% of study population) pursued nonoperative management with durable responses in the majority, suggesting that definitive nonsurgical management with the use of immunotherapy in MSI-H/dMMR tumors is promising and warrants further exploration.
Relevance (E.M. O'Reilly)
This study adds to the growing body of evidence that endorses the role of anti–programmed cell death protein-1 antibody therapy in GI cancers with dMMR/MSI-H, where deep and major pathologic responses are observed in a substantial majority of patients with localized disease.*
*Relevance section written by JCO Associate Editor Eileen M. O'Reilly, MD.
Anti–programmed cell death protein-1 (PD-1) therapy has revolutionized the treatment of metastatic dMMR cancers.11-13 For dMMR colorectal cancer (CRC), the Keynote 177 trial demonstrated the superiority of pembrolizumab over chemotherapy in the frontline treatment of patients with metastatic disease.14 The role of anti–PD-1 therapy for localized dMMR cancers is not fully defined but has demonstrated promising results in CRC15,16 and gastroesophageal cancers.17
Neoadjuvant immunotherapy is appealing since the intact primary tumor provides a source of antigens for immune priming and expansion of activated tumor-specific T cells.18,19 Furthermore, it holds promise not only as a highly active therapy coupled with surgery, but also a therapy that may enable nonoperative management. The study reported here investigates the safety and efficacy of neoadjuvant pembrolizumab in 35 patients with localized dMMR solid cancers.
METHODS
Study Design and Eligibility
This is an investigator-initiated, single-center, open-label, phase II trial conducted at the University of Texas MD Anderson Cancer Center (UTMDACC; ClinicalTrials.gov identifier: NCT04082572). Patients with locally advanced, histologically confirmed, dMMR/MSI-H solid cancers were enrolled and written consent obtained. Locally advanced was defined as nonmetastatic primary cancer with ≥ 20% chance of recurrence with surgical resection alone. The planned treatment course was pembrolizumab 200 mg intravenously once every 3 weeks for eight treatments followed by surgical resection. An option for nonsurgical management was provided in which patients could receive pembrolizumab for 16 treatments followed by observation.
Eligibility included age ≥ 18 years and measurable disease by RECIST version 1.1. Adverse events were categorized using Common Terminology Criteria for Adverse Events (CTCAE; version 4.0) and surgical complications according to the Clavien-Dindo classification. The study was conducted in accordance with the Declaration of Helsinki and the UTMDACC institutional review board. Full details are provided in the Protocol (online only).
Tumor Response Assessment
Following 6 weeks of pembrolizumab, dosed once every 3 weeks, patients were assessed with radiographic imaging. Circulating tumor DNA (ctDNA) was assessed before and after 3 weeks of treatment. Patients not demonstrating clinical benefit were discontinued from the trial.
Following initial 6-week assessment, patients underwent radiographic imaging every three cycles (9 weeks). For luminal tumors, serial endoscopic evaluation was recommended, although not required. Endoscopic response was assessed using the Memorial Sloan Kettering Regression Schema.20
Histopathologic Assessment
Histopathologic examinations of baseline and resection specimens were assessed for regression of tumor with routine hematoxylin and eosin staining. Pathologic complete response (pCR) was defined as absence of any residual viable tumor of the macroscopically identifiable tumor bed or lymph node. All other responses were classified as non-pCR.
Genomic Assessment
ctDNA sampling was performed via a UTMDACC Clinical Laboratory Improvement Amendments–certified 70-gene liquid biopsy panel, which uses digital sequencing of cell-free circulating DNA isolated from plasma.
Baseline formalin-fixed paraffin-embedded tumor samples and germline peripheral blood mononuclear cells were sequenced in a Clinical Laboratory Improvement Amendments environment using a targeted next-generation sequencing platform. Tumor mutation burden was assessed on the basis of the total panel coverage of 237,530 base pairs.
Statistical Analysis
The coprimary end points were safety as determined by CTCAE-assessed toxicity and postsurgical complications as assessed by the Clavien-Dindo classification and the rate of pCR in patients with surgically resected specimens receiving at least three cycles of pembrolizumab (Data Supplement, online only). Secondary end points included best overall RECIST response, rate of organ-sparing at one year for nonsurgical patients, and the rate of pCR for all patients. Exploratory end points included endoscopic response rates and ctDNA kinetics as a surrogate for clinical efficacy. For exploratory end points, multiple testings were not adjusted for the type I error.
A sample size of 35 was chosen to obtain a 95% CI of 0.35 to 0.93 for the primary end point of rate of pCR, assuming 20 patients will undergo surgical resection. Toxicity was monitored continuously using a Bayesian approach.21,22 The protocol dictated stopping new patient enrollment if at any time during the study it was determined that there was more than an 85% chance that the unacceptable toxicity rate exceeded 25%. Unacceptable toxicities were defined as any grade 3 or higher probably or definite treatment-related CTCAE toxicity that occurred during the first 6 months of therapy.
To explore the differences in the tumor immune microenvironment, t-tests comparing differential analyses of cell type abundances (imaging mass cytometry) and cell type distance relationships between progressors and nonprogressors (patients whose disease did or did not progress on study or during follow-up) were performed.
RESULTS
Participants
Thirty-five patients were enrolled between October 31, 2019, and March 25, 2021(Table 1, Fig 1, and Data Supplement). The majority of patients had colorectal adenocarcinoma (77%) and clinical stage III disease (74%). One quarter of all patients had unresectable cancer at presentation.
TABLE 1.
Baseline Patient Characteristics
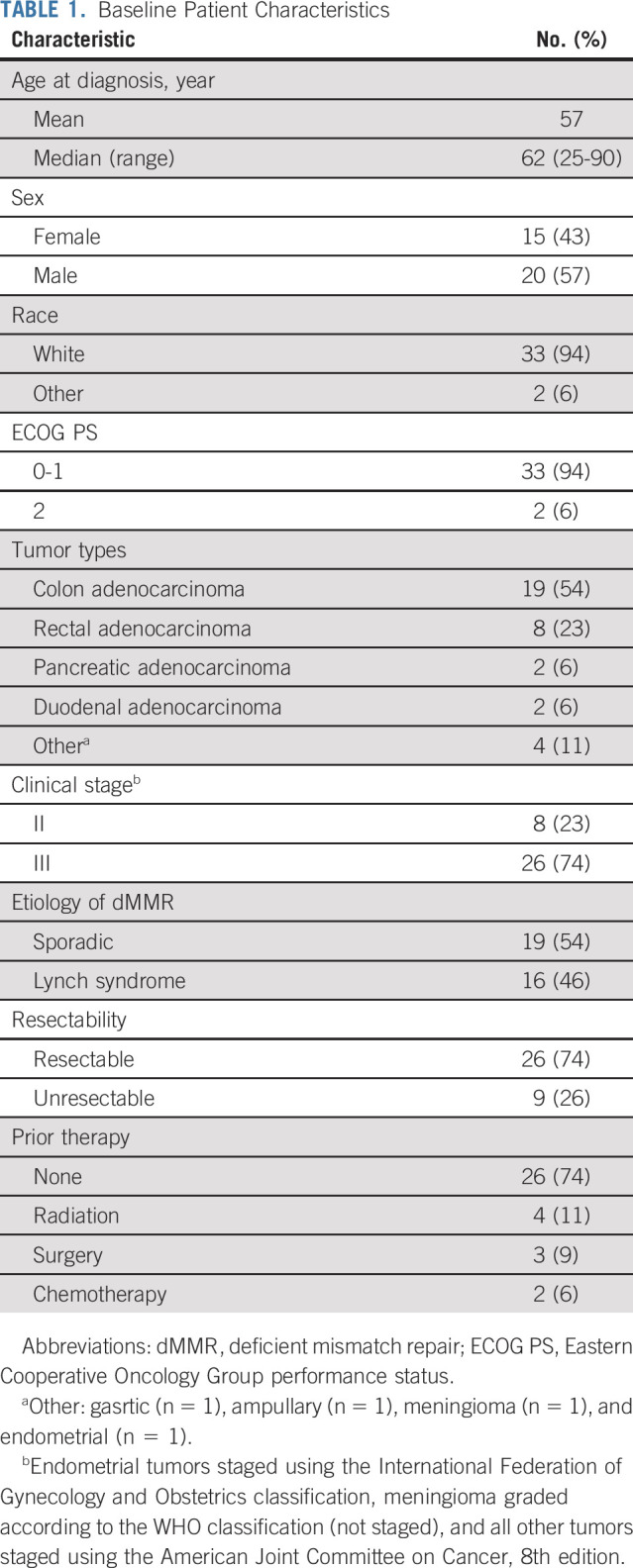
FIG 1.
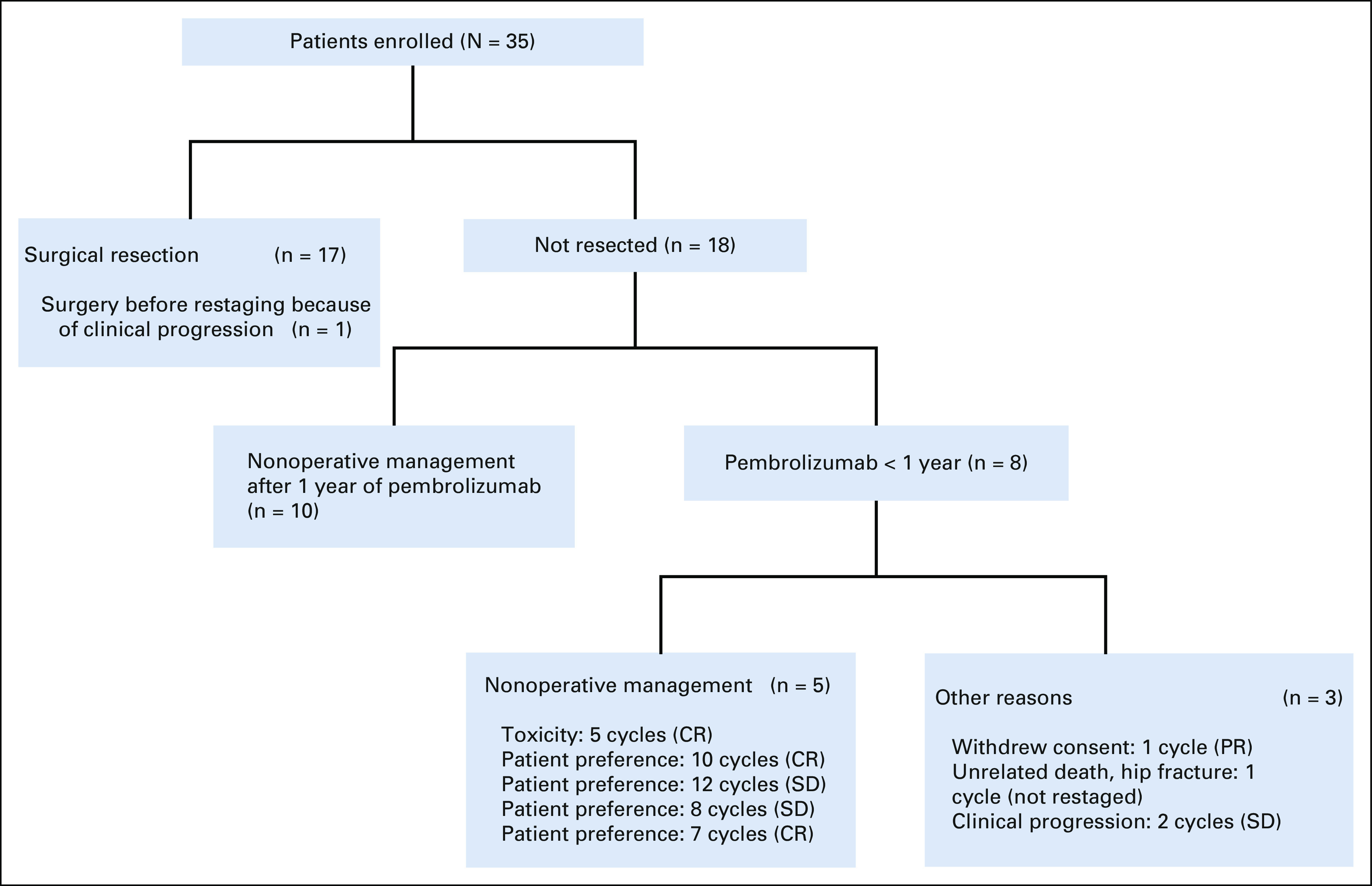
Flow diagram. CR, complete response; PR, partial response; SD, stable disease.
At the time of data cutoff, March 31, 2022, 17 patients (49%) have undergone surgical resection and 18 patients did not undergo surgical resection (Fig 1).
Safety
All-cause adverse events were reported in 35 patients (Data Supplement). Thirteen patients (37%) had grade 1 or 2 treatment-related events. Grade 3 events were reported in two patients (6%), while there were no grade 4 events. At the time of data cutoff, two patients (6%) had expired, both unrelated to treatment.
Three of 17 surgical patients developed postoperative complications (Clavien-Dindo classification): one surgical site abscess (grade 3b), one abdominal wall hematoma (grade 1), and one patient with diarrhea (grade 2).
Pathologic Response
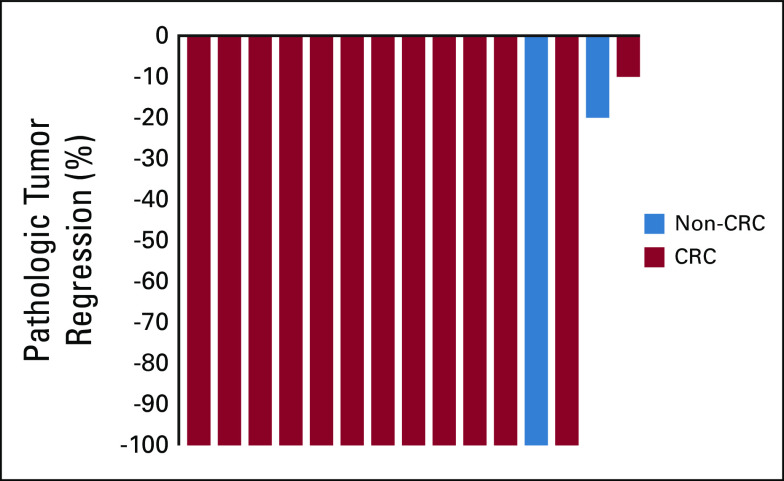
The primary efficacy end point was evaluable in 15 patients, with 10 patients having a pCR of 67% (95% CI, 38 to 88; Data Supplement). In addition to the 15 patients who underwent surgery after three cycles, two additional patients underwent surgical resection following one cycle and two cycles, respectively, with one patient having a pCR. Of the 17 patients who underwent resection, 14 patients had CRC and 11 of these had a pCR (79% in CRC patients). Figure 2 shows the pathologic tumor regression in the primary tumor of all available resected specimens. Six of 17 surgically resected patients did not demonstrate a pCR. Treatment response with pathologic downstaging was seen in five (Data Supplement).
FIG 2.
Waterfall plot of pathologic tumor regression in the primary tumor of resected specimens after neoadjuvant pembrolizumab (n = 15). Patients not depicted above (n = 2): one patient with CRC who underwent surgical resection at an outside facility (ypT4bN0) and one patient with endometrial adenocarcinoma (ypT1aN0) who could not be classified using the tumor regression grade systems used for GI malignancies. CRC, colorectal cancer.
Radiographic Response
Of the 35 patients, 33 were evaluable for radiographic response, with an overall response rate of 82% (n = 27): 10 (30%) complete responses (CRs) and 17 (52%) partial responses (PRs). An additional six patients had stable disease (SD; 18%; Fig 3A). The reasons two patients were not evaluable for response were resection before restaging because of clinical progression, and death following an unrelated hip fracture in a 90-year-old patient before restaging. We did not observe an association between pCR and radiographic CR. Of the 11 patients with pCR, only two had radiographic CR, with the others demonstrating PR. Of the other eight patients with radiographic CR, none underwent surgical resection. Among those patients with partial or complete radiographic response, the median time to response was 6 weeks (range, 4-24 weeks). Figure 3B shows the change in target lesion diameters over time in 33 evaluable patients. Within the limitations of the small sample size, we did not see variations in response according to key subgroups (Data Supplement).
FIG 3.
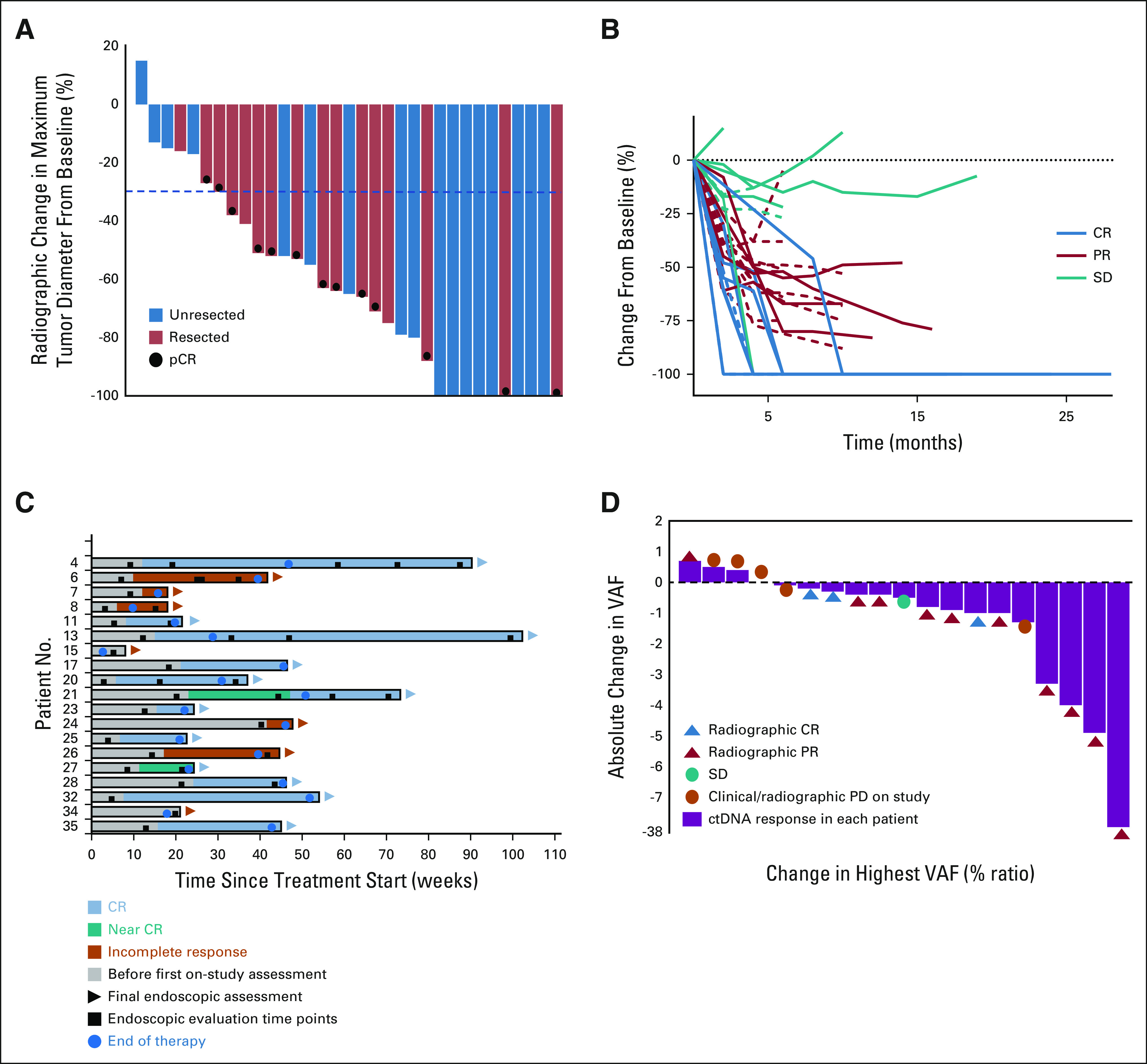
(A) Waterfall plot showing best overall radiographic responses to neoadjuvant pembrolizumab in resected and unresected patients (n = 33). (B) Spider plot of the change in sum of target lesion diameters from baseline over time for all evaluable patients, RECIST version 1.1 (n = 33). Lines are color-coded on the basis of best overall response. Dash lines represent patients who underwent surgical resection. (C) Swimmer plot showing endoscopic responses of evaluable luminal patients (n = 17). (D) Waterfall plot showing absolute change in highest VAF (%) at baseline before pembrolizumab and at 3 weeks after one cycle of pembrolizumab in patients with baseline positive ctDNA values. CR, complete response; ctDNA, circulating tumor DNA; pCR, pathologic complete response; PD, progressive disease; PR, partial response; SD, stable disease; VAF, variant allele frequency.
Endoscopic Response
Eighty-six percent (n = 30) of patients had luminal GI cancers. Nineteen were evaluable for response (six patients did not have on-treatment endoscopy and five responses were not evaluable because of strictures or stents). Endoscopic CR was seen in 12 (63%), while seven (37%) had incomplete responses (Fig 3C). Among those with complete endoscopic response, the median time to response was 17 weeks (range, 6-31 weeks). Of the 12 patients with complete endoscopic response, five underwent surgical resection and all had pCR. Of the seven patients with incomplete endoscopic response, three patients underwent surgical resection: two with pCR and the other with residual disease.
Biomarker Response
Of the 35 patients, 19 had serial samples and detectable ctDNA at baseline. There was a decrease in the absolute value of the highest variant allele frequency (VAF) in 79% (n = 15; Fig 3D). Of the remaining four patients with detectable baseline ctDNA, three had increase in VAF and one had stable VAF. Ultimately, three of four patients with stable or increasing ctDNA VAF experienced progression. Thirteen of 15 patients with ctDNA decrease had no progression on study. Early decrease in ctDNA was a predictor for no future progression events (P = .037).
Nonoperative Cohort
Eighteen patients did not undergo surgical resection. For patients pursuing a nonoperative approach, the protocol recommended the administration of 1 year of pembrolizumab and this was done in 10 patients, none of whom demonstrated disease progression. For the 18 patients who did not undergo surgical resection, the median follow-up was 38 weeks (range, 0-103 weeks) from last pembrolizumab (Data Supplement). Of the eight patients who did not undergo surgical resection and completed < 1 year of pembrolizumab, five patients elected to purse an organ-sparing approach with radiographic response status of CR in 3 and SD in 2. Of these two SD patients, one had an endoscopic CR and the second demonstrated disease progression at 9 months but elected to continue pursuing a nonoperative approach. One patient with a radiographic PR withdrew consent after one cycle. Two patients died without surgical resection: one because of unrelated causes and the second because of disease progression.
Progression Events
During the study course and subsequent follow-up, progression events were seen in six patients. Two patients with pancreatic cancer demonstrated radiographic SD with decreases in cancer antigen 19-9 before radiographic progression occurring at 6 and 4 months, respectively. Both underwent surgical resection. Two colorectal patients had clinical progression at 6 weeks: one received further chemotherapy and subsequently died of disease progression without resection, while the other patient proceeded directly to surgical resection. Two other colorectal patients demonstrated radiographic progression at 6 and 9 months following initial response of PR and SD, respectively. The former proceeded to surgery at progression, while the latter opted to pursue nonoperative management.
Immunologic Correlatives
The results of mass cytometry analyses and associated 43-parameter panel used to characterize the immunologic features of the tumor microenvironment are shown in the Data Supplement. The images, totaling 44.6 mm2 of acquisition area and segmented into a total of 366,953 single cells, are shown in the Data Supplement. The 14 final cell types merged from 50 metaclusters are shown in Figure 4A. The immune microenvironment heavily depended on the tissue of origin, both by multicolored visualization (Data Supplement) and multidimensional scaling (Data Supplement). Since immune composition heavily depends on the tissue context, downstream differential analyses were limited to GI tumors. When the immune profiles between progressors and nonprogressors were compared, progressors demonstrated a trend toward higher presence of granulocytic cell cluster (CD45+HLADR–CD15+; Gran; Fig 4B) and exhausted T cells (CD45+CD3+CD8+TOXhi; TcTOX; Fig 4B). Network visualizations on the basis of average distances between all cell types demonstrated that CD8+ T cells (Tc), which are known to carry out the killing of tumor cells, were particularly in close spatial association with the Gran cluster in progressors compared with nonprogressors (Fig 5A). This was confirmed by direct multicolored visualization of the samples (Fig 5B), illustrating how CD8+ cells seemed to be more surrounded by CD15+ cells in progressors. Quantitatively confirming this relationship, differential analyses of the distances between Tc and other immune cell types demonstrated significantly greater distances between Tc and Gran in nonprogressors (Fig 5C). Also in nonprogressors, Tc cluster was also in closer proximity to CD163–CD206– macrophages (Mac) and B cells (Fig 5C). These findings suggest that the presence of CD15+ granulocytic cell types and their close proximity to CD8+ T cells associate with progression in dMMR cancers.
FIG 4.
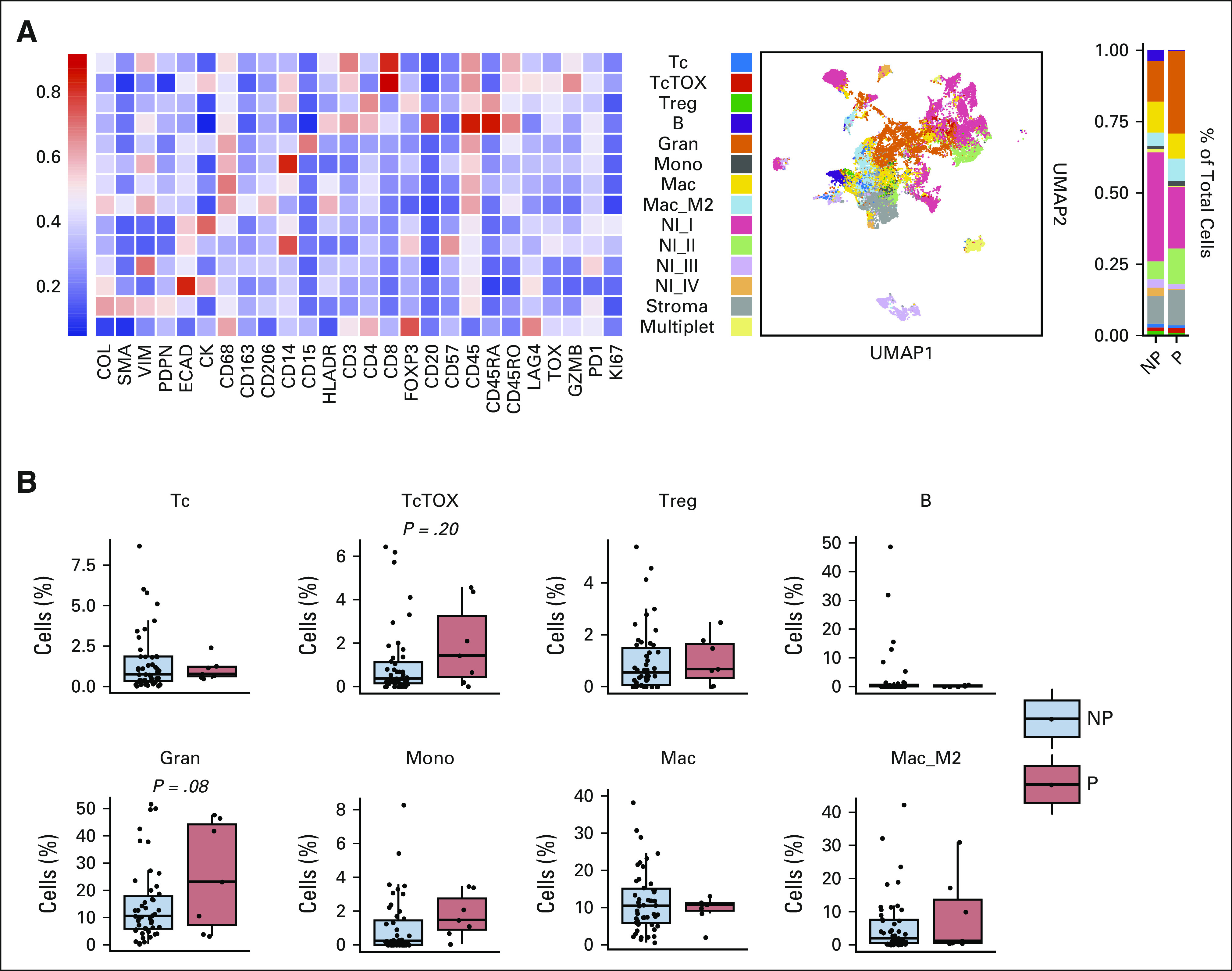
Tumor microenvironment profiling using imaging mass cytometry. (A) Heatmap expression profiles of 14 final annotated clusters (merged from 50 metaclusters) determined by FlowSOM. Accompanying panels show clusters on UMAP and stacked bar plots as a proportion of total cells for NP and P. (B) Boxplots showing differences in abundances of immune cell types as a percentage of total number of cells. Each dot represents one unique region of interest (NP, n = 47 v P, n = 7; t-tests). B, B cells; Gran, granulocytic; Mac, macrophage; Mac_M2, macrophages with CD163+CD206+; Mono, monocytic; NI (I-IV), various nonimmune cells; NP, nonprogressors; P, progressors; Tc, CD8+ T cells; TcTOX, CD8+ T cells with TOXhi; Treg, regulatory T cells.
FIG 5.
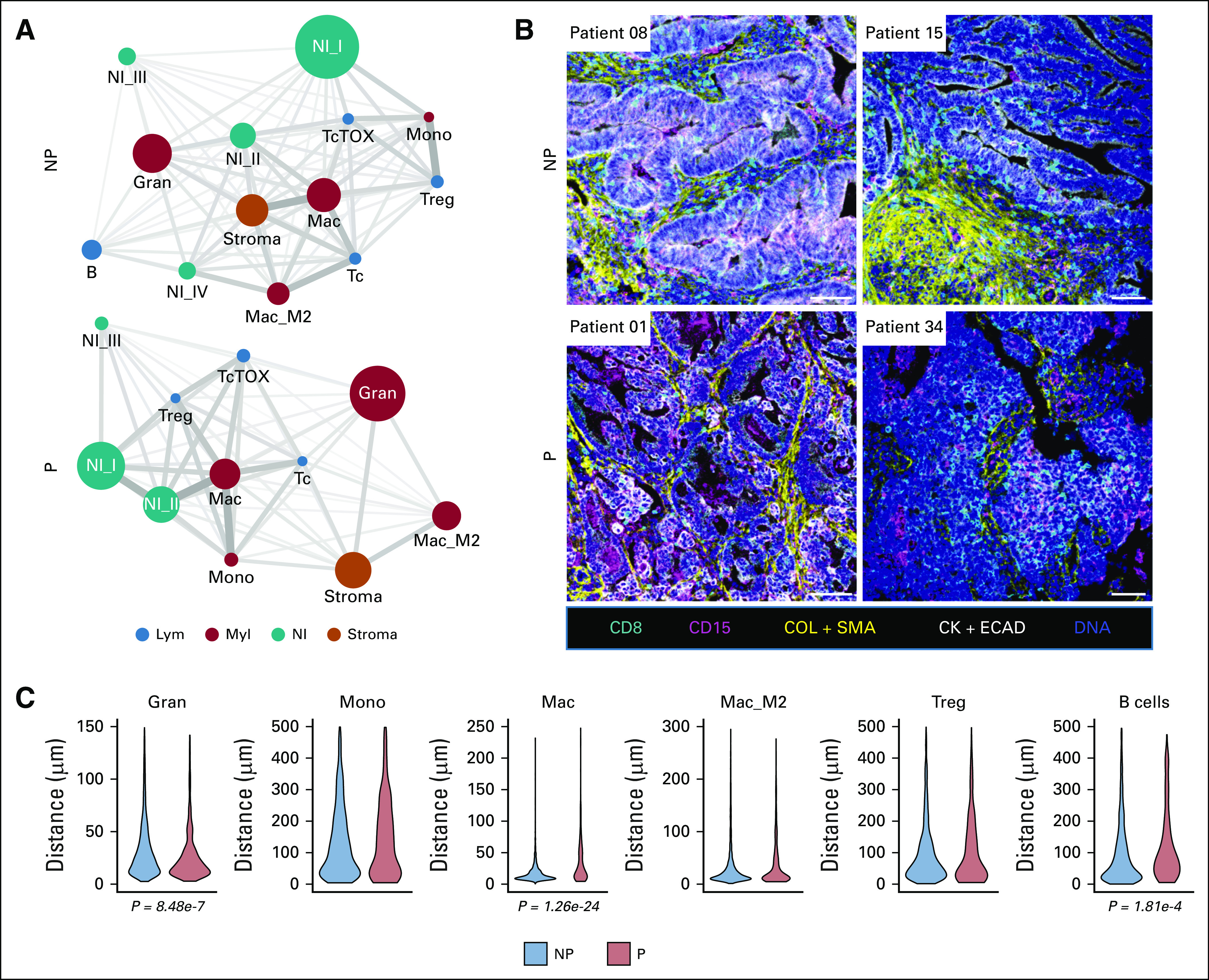
Spatial analysis of immune microenvironment. (A) Network visualization of all spatial relationships among 14 cell types for nonprogressors and progressors. Distance relationships among all cell types were calculated based on the mean of all of the shortest distances between one cell to another. Node sizes represent relative abundance of the cell types. Node colors represent major cell types. Edge width and length both represent the relative similarity of neighbors between the two cell types that the edge connects. (B) Representative images from imaging mass cytometry (scale bar: 100 μm). (C) Violin plots of comparing the distances from Tc to other immune cell types in NP and P (number of cell-cell distances: NP, n = 2,802-3,447 v P, n = 235-508; t-tests). CK + ECAD, pan-cytokeratin + e-cadherin; COL + SMA, collagen + alpha-smooth muscle actin; Gran, granulocytic; Lym, lymphoid; Mac, macrophage; Mac_M2, macrophages with CD163+CD206+; Mono, monocytic; Myl, myeloid; NI, nonimmune; NI (I-IV), various nonimmune cells; NP, nonprogressors; P, progressors; Tc, CD8+ T cells; TcTOX, CD8+ T cells with TOXhi; Treg, regulatory T cells.
DISCUSSION
To our knowledge, this single-center, phase II clinical trial demonstrates for the first time that neoadjuvant pembrolizumab is safe and feasible, and results in a high rate of complete pathologic response in patients with a range of localized dMMR solid tumor cancers. Although the pCR rate of 65% was lower than the study goal of 80%, the majority of resected patients without pCR demonstrated pathologic downstaging and in the CRC only patients, the pCR was 79%.
Although several landmark trials have established the efficacy of checkpoint inhibitors in metastatic dMMR cancers,11,14,23 there is a paucity of data on neoadjuvant checkpoint inhibitors in dMMR cancers. Chalabi and colleagues reported on the clinical activity of nivolumab combined with ipilimumab among 21 patients with dMMR resectable colon cancers demonstrating pCR in 12 (57%) patients,16 while André et al17 demonstrated pCR in 17 (59%) patients with dMMR gastroesophageal resectable cancers. Finally, Cercek et al15 demonstrated complete endoscopic response in 12 (100%) patients with rectal adenocarcinoma receiving 6 months of dostarlimab. A correlation between response, specifically, pCR and survival following neoadjuvant immunotherapy, has been demonstrated in some cancers.24,25
One of the most intriguing findings from this prospective trial is the suggestion that neoadjuvant PD-1–based therapy may represent a definitive approach for dMMR solid tumors enabling organ preservation. With a median follow-up of almost 9.5 months (range, 0-26 months) among 17 patients managed with a nonoperative approach, only two patients demonstrated progression events. This is consistent with data published by Cercek et al15 showing durable responses (range, 6-25 months of follow-up) in patients with dMMR rectal adenocarcinomas treated with dostarlimab precluding the need for surgical resection. In contrast to the study by Cercek et al, we observed more heterogeneity in responses to pembrolizumab among the eight patients with rectal adenocarcinoma. Two patients demonstrated progression events: one with innate resistance with clinical progression following two cycles, and one with adaptive resistance with progression at 9 months. The remaining six patients did not demonstrate progression events with one having undergone surgical resection with pCR. Although organ preservation as a treatment strategy has gained momentum in rectal cancers,26-28 the variety of dMMR solid tumors provides a number of theoretical opportunities for expanding organ preservation to other disease sites. Given the limited sample size of this clinical trial, future studies with larger numbers of patients are needed to validate this approach across the range of dMMR solid tumor types and refine the optimal duration of therapy.
The radiographic response rate of 82% is notably higher than the response rates seen in clinical trials of PD-1–based therapy for metastatic dMMR cancers. In Keynote 177 and Keynote 164, metastatic dMMR CRC and metastatic dMMR non-CRC solid tumors demonstrated response rates of 44% and 34% to pembrolizumab, respectively.14,29 These results raise the hypothesis that PD-1 therapy in earlier-stage dMMR cancers may have greater clinical activity than in stage IV disease, before the development of additional mechanisms of immune evasion that enable metastatic dissemination.3 Clinical benefit was seen across a variety of subgroups; however, one subgroup that appeared to demonstrate less activity was dMMR pancreatic cancer, in which both patients demonstrated adaptive progression and failed to demonstrate a pCR. This is similar to other trials in which activity in pancreatic patients appears less than other patients.30
Because of the tumor-agnostic nature of this trial, endoscopic evaluation for the 30 patients with luminal cancers was recommend but not required. Among patients with luminal cancers, endoscopic evaluation provided a valuable adjunct for complementing radiographic response evaluation. Although limited in numbers, the data suggest a correlation between complete endoscopic response and subsequent pCR. Larger prospective studies are needed to determine the most optimal schedule of endoscopic evaluation, especially considering its potential role in facilitating organ preservation. An additional area of future investigation is defining appropriate endoscopic response criteria for neoadjuvant checkpoint inhibitors. The extent to which the stringent criteria for complete endoscopic response used in rectal adenocarcinoma20 apply to immune-related endoscopic responses is not clear, as both stricturing and papillary mucosal changes were seen in patients in the study who subsequently demonstrated pCR after surgical resection. Although the majority of patients demonstrated radiographic response, complete radiographic response was rare and occurred in only two of the 11 patients with pCR at resection. This differs from the recent report of dostarlimab for dMMR rectal cancer in which all patients had a radiographic CR. The discrepancy between residual radiographic findings and either prolong disease control or pCR following metastectomy has been demonstrated in metastatic dMMR CRC patients treated with PD-1–based therapies.11,31,32 This suggests the need for further guidance in distinguishing residual tumor from noncancer fibrosis, inflammatory change, and/or mucin. An early reduction in ctDNA was predictive of clinical benefit and supports the use of ctDNA as a predictive biomarker for immunotherapy, but, in this localized population, was only detected in 54% of patients.33 These results raise important questions about establishing the best paradigm to assess patients on therapy. These data would suggest potential limitations from radiographic imaging alone and favor the incorporation of endoscopic response assessment when a luminal tumor is present. Innate or adaptive progression events were observed in six (17%) patients. The mechanisms of resistance to checkpoint inhibitors remain unclear. Our exploratory analyses suggest that a higher abundance of CD15+ granulocytic cell types within the tumor immune microenvironment and their proximity to cytotoxic CD8+ T cells may contribute to the lack and/or eventual loss of treatment response. The granulocytic cluster in our data set could represent either tumor-associated neutrophils or granulocytic myeloid-derived suppressor cells. Both of these cell types have been shown to denote poor prognosis in multiple cancer types34 and specifically associate with poor clinical outcomes in the context of PD-1/programmed death ligand-1 inhibition.35,36 Given the limited sample size of the current analysis, further studies to investigate the mechanisms of resistance are warranted among larger cohorts of MSI-H patients.
The study design, in which the patient and/or treating physician chose whether or not to proceed with surgical resection, could have introduced bias into our analysis of the primary and secondary end points of pCR and organ-sparing rate. Furthermore, the study size is small and represents the experience of a single institution. Larger prospective studies are warranted.
In conclusion, in patients with dMMR solid cancers, neoadjuvant pembrolizumab was associated with limited side effects and high clinical activity as reflected by response rate and complete pathologic response in resected patients. We demonstrated that nonoperative management of dMMR/MSI-H localized solid tumors is promising and warrants continued exploration.
Kaysia Ludford
Research Funding: Merck (Inst)
Won Jin Ho
Honoraria: Standard Biotools
Consulting or Advisory Role: Exelixis
Research Funding: Sanofi (Inst), NeoTX (Inst)
Patents, Royalties, Other Intellectual Property: I hold patents and receive royalties from Rodeo Therapeutics
Kanwal P.S. Raghav
Consulting or Advisory Role: AstraZeneca, Bayer, Eisai, Daiichi Sankyo, Seattle Genetics
Speakers' Bureau: Bayer
Research Funding: Bayer (Inst), Roche/Genentech (Inst), Guardant Health (Inst), Daiichi Sankyo/Astra Zeneca (Inst), HiberCell (Inst), Merck Serono (Inst)
Mariela Blum Murphy
Research Funding: Bristol Myers Squibb (Inst), Genentech/Roche (Inst)
Nicole D. Fleming
Consulting or Advisory Role: Tesaro, Pfizer, GlaxoSmithKline, Immunogen
Michael S. Lee
Consulting or Advisory Role: Pfizer, Imvax, G1 Therapeutics, Delcath Systems
Research Funding: Arcus Biosciences (Inst), Erasca Inc (Inst), Repare Therapeutics (Inst), Merck (Inst), TriSalus Life Sciences (Inst), Boehringer Ingelheim (Inst), Xilis (Inst), EpimAb BioTherapeutics (Inst)
Brandon G. Smaglo
Speakers' Bureau: Taiho Pharmaceutical, Sirtex Medical
Travel, Accommodations, Expenses: Marker Therapeutics
Benny Johnson
Consulting or Advisory Role: Gritstone Bio, Incyte, Taiho Oncology, Insmed, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Syntrix Biosystems (Inst), Gateway Foundation (Inst)
Eduardo Vilar
Consulting or Advisory Role: Janssen Research & Development, Recursion Pharmaceuticals, Guardant Health
Research Funding: Janssen Research & Development
Patents, Royalties, Other Intellectual Property: The University of Texas MD Anderson Cancer Center, Vilar E, Wu W, Katayama H, Hanash S, Bommi P. Methods for Prognosing, Diagnosing, and Treating Colorectal Cancer, United States, 63/152,751, 2/23/2021, Filed (Provisional), The University of Texas MD Anderson Cancer Center, Vilar E, Chang K, Wu W, Bowen CM, Sinha K. Methods and Compositions Comprising MHC Class I Peptides, United States, 63/171,137, 4/6/2021, Filed (Provisional)
Travel, Accommodations, Expenses: Janssen Research & Development
Arvind Dasari
Consulting or Advisory Role: Novartis, Voluntis, Personalis, Crinetics Pharmaceuticals
Research Funding: Eisai, Hutchison MediPharma, Merck, Guardant Health
Dipen Maru
Honoraria: GSB Pharma (I)
Speakers' Bureau: Clinical Care Options, Pfizer
Research Funding: Daiichi Sankyo/UCB Japan
Uncompensated Relationships: Bristol Myers Squibb/Medarex
Scott Kopetz
Stock and Other Ownership Interests: Lutris, Iylon, Frontier Medicines, Xilis, Navire
Consulting or Advisory Role: Genentech, EMD Serono, Merck, Holy Stone Healthcare, Novartis, Lilly, Boehringer Ingelheim, AstraZeneca/MedImmune, Bayer Health, Redx Pharma, Ipsen, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, AbbVie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical, Cardiff Oncology, Ono Pharmaceutical, Bristol Myers Squibb/Medarex, Amgen, Tempus, Foundation Medicine, Harbinger Oncology Inc, Takeda, CureTeq, Zentalis, Black Stone Therapeutics, NeoGenomics Laboratories, Accademia Nazionale Di Medicina
Research Funding: Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, EMD Serono, MedImmune, Novartis, Amgen, Lilly, Daiichi Sankyo
Michael J. Overman
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Gritstone Bio, MedImmune, Novartis, Promega, Spectrum Pharmaceuticals, Array BioPharma, Janssen, Pfizer, 3D Medicines, Merck, Eisai
Research Funding: Bristol Myers Squibb, Merck, Roche, MedImmune
No other potential conflicts of interest were reported.
See accompanying editorial on page 2138
PRIOR PRESENTATION
Presented orally at ESMO Congress, Paris Expo Porte de Versailles, France, September 19, 2021. Presented as a poster at ASCO Annual Conference, June 2021.
SUPPORT
Supported by CCSG P30 CA016672, SPORE P50CA221707, Merck (Recipient: M.J.O.), and Kavanagh Family Foundation (Recipient: J.V.T.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Kaysia Ludford, Wei Qiao, Scott Kopetz, Michael J. Overman
Financial support: Michael J. Overman
Administrative support: Michael J. Overman
Provision of study materials or patients: Nicole D. Fleming, Michael S. Lee, Y. Nancy You, Matthew M. Tillman, Benny Johnson, Eduardo Vilar, Arvind Dasari, Xuan Yuan, Wai Chin Foo, Dipen Maru, Scott Kopetz, Michael J. Overman
Collection and assembly of data: Kaysia Ludford, Won Jin Ho, Jane V. Thomas, Mariela Blum Murphy, Nicole D. Fleming, Michael S. Lee, Y. Nancy You, Matthew M. Tillman, Carlos Kamiya-Matsuoka, Selvi Thirumurthi, Benny Johnson, Eduardo Vilar, Arvind Dasari, Sarah Shin, Alexei Hernandez, Xuan Yuan, Hongqui Yang, Wai Chin Foo, Dipen Maru, Scott Kopetz, Michael J. Overman
Data analysis and interpretation: Kaysia Ludford, Won Jin Ho, Jane V. Thomas, Kanwal P.S. Raghav, Nicole D. Fleming, Michael S. Lee, Brandon G. Smaglo, Y. Nancy You, Matthew M. Tillman, Carlos Kamiya-Matsuoka, Craig Messick, Benny Johnson, Arvind Dasari, Sarah Shin, Wai Chin Foo, Dipen Maru, Scott Kopetz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kaysia Ludford
Research Funding: Merck (Inst)
Won Jin Ho
Honoraria: Standard Biotools
Consulting or Advisory Role: Exelixis
Research Funding: Sanofi (Inst), NeoTX (Inst)
Patents, Royalties, Other Intellectual Property: I hold patents and receive royalties from Rodeo Therapeutics
Kanwal P.S. Raghav
Consulting or Advisory Role: AstraZeneca, Bayer, Eisai, Daiichi Sankyo, Seattle Genetics
Speakers' Bureau: Bayer
Research Funding: Bayer (Inst), Roche/Genentech (Inst), Guardant Health (Inst), Daiichi Sankyo/Astra Zeneca (Inst), HiberCell (Inst), Merck Serono (Inst)
Mariela Blum Murphy
Research Funding: Bristol Myers Squibb (Inst), Genentech/Roche (Inst)
Nicole D. Fleming
Consulting or Advisory Role: Tesaro, Pfizer, GlaxoSmithKline, Immunogen
Michael S. Lee
Consulting or Advisory Role: Pfizer, Imvax, G1 Therapeutics, Delcath Systems
Research Funding: Arcus Biosciences (Inst), Erasca Inc (Inst), Repare Therapeutics (Inst), Merck (Inst), TriSalus Life Sciences (Inst), Boehringer Ingelheim (Inst), Xilis (Inst), EpimAb BioTherapeutics (Inst)
Brandon G. Smaglo
Speakers' Bureau: Taiho Pharmaceutical, Sirtex Medical
Travel, Accommodations, Expenses: Marker Therapeutics
Benny Johnson
Consulting or Advisory Role: Gritstone Bio, Incyte, Taiho Oncology, Insmed, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Syntrix Biosystems (Inst), Gateway Foundation (Inst)
Eduardo Vilar
Consulting or Advisory Role: Janssen Research & Development, Recursion Pharmaceuticals, Guardant Health
Research Funding: Janssen Research & Development
Patents, Royalties, Other Intellectual Property: The University of Texas MD Anderson Cancer Center, Vilar E, Wu W, Katayama H, Hanash S, Bommi P. Methods for Prognosing, Diagnosing, and Treating Colorectal Cancer, United States, 63/152,751, 2/23/2021, Filed (Provisional), The University of Texas MD Anderson Cancer Center, Vilar E, Chang K, Wu W, Bowen CM, Sinha K. Methods and Compositions Comprising MHC Class I Peptides, United States, 63/171,137, 4/6/2021, Filed (Provisional)
Travel, Accommodations, Expenses: Janssen Research & Development
Arvind Dasari
Consulting or Advisory Role: Novartis, Voluntis, Personalis, Crinetics Pharmaceuticals
Research Funding: Eisai, Hutchison MediPharma, Merck, Guardant Health
Dipen Maru
Honoraria: GSB Pharma (I)
Speakers' Bureau: Clinical Care Options, Pfizer
Research Funding: Daiichi Sankyo/UCB Japan
Uncompensated Relationships: Bristol Myers Squibb/Medarex
Scott Kopetz
Stock and Other Ownership Interests: Lutris, Iylon, Frontier Medicines, Xilis, Navire
Consulting or Advisory Role: Genentech, EMD Serono, Merck, Holy Stone Healthcare, Novartis, Lilly, Boehringer Ingelheim, AstraZeneca/MedImmune, Bayer Health, Redx Pharma, Ipsen, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, AbbVie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical, Cardiff Oncology, Ono Pharmaceutical, Bristol Myers Squibb/Medarex, Amgen, Tempus, Foundation Medicine, Harbinger Oncology Inc, Takeda, CureTeq, Zentalis, Black Stone Therapeutics, NeoGenomics Laboratories, Accademia Nazionale Di Medicina
Research Funding: Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, EMD Serono, MedImmune, Novartis, Amgen, Lilly, Daiichi Sankyo
Michael J. Overman
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, Gritstone Bio, MedImmune, Novartis, Promega, Spectrum Pharmaceuticals, Array BioPharma, Janssen, Pfizer, 3D Medicines, Merck, Eisai
Research Funding: Bristol Myers Squibb, Merck, Roche, MedImmune
No other potential conflicts of interest were reported.
REFERENCES
- 1.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germano G, Amirouchene-Angelozzi N, Rospo G, et al. : The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov 8:1518-1528, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Battaglin F, Naseem M, Lenz HJ, et al. : Microsatellite instability in colorectal cancer: Overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol 16:735-745, 2018 [PMC free article] [PubMed] [Google Scholar]
- 4.Brueckl WM, Moesch C, Brabletz T, et al. : Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res 23:1773-1777, 2003 [PubMed] [Google Scholar]
- 5.Carethers JM, Chauhan DP, Fink D, et al. : Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology 117:123-131, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carethers JM, Smith EJ, Behling CA, et al. : Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 126:394-401, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cercek A, Dos Santos Fernandes G, Roxburgh CS, et al. : Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res 26:3271-3279, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo WS, Carethers JM: Chemotherapeutic implications in microsatellite unstable colorectal cancer. Cancer Biomark 2:51-60, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers M, Wagner MW, Hwang HS, et al. : Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res 61:5193-5201, 2001 [PubMed] [Google Scholar]
- 10.Ribic CM, Sargent DJ, Moore MJ, et al. : Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247-257, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overman MJ, McDermott R, Leach JL, et al. : Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol 18:1182-1191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andre T, Shiu KK, Kim TW, et al. : Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 383:2207-2218, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Cercek A, Lumish M, Sinopoli J, et al. : PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med 386:2363-2376, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalabi M, Fanchi LF, Dijkstra KK, et al. : Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26:566-576, 2020 [DOI] [PubMed] [Google Scholar]
- 17.André T, Tougeron D, Piessen G, et al. : Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability–high gastric or esophagogastric junction adenocarcinoma: The GERCOR NEONIPIGA phase II study. J Clin Oncol 41:255-265, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germano G, Lamba S, Rospo G, et al. : Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552:116-120, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Kanani A, Veen T, Soreide K: Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg 108:1417-1425, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JJ, Chow OS, Gollub MJ, et al. : Organ preservation in rectal adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 15:767, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thall PF, Simon RM, Estey EH: New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol 14:296-303, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Thall PF, Sung HG: Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 17:1563-1580, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Lenz HJ, Van Cutsem E, Luisa Limon M, et al. : First-Line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J Clin Oncol 40:161-170, 2022 [DOI] [PubMed] [Google Scholar]
- 24.Amaria RN, Reddy SM, Tawbi HA, et al. : Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 24:1649-1654, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Taube JM, Pardoll DM: Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367:eaax0182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald RC: Organ-preserving approaches in oesophageal cancer. Lancet Oncol 19:858-859, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noordman BJ, Spaander MCW, Valkema R, et al. : Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): A prospective multicentre, diagnostic cohort study. Lancet Oncol 19:965-974, 2018 [DOI] [PubMed] [Google Scholar]
- 28.van der Valk MJM, Hilling DE, Bastiaannet E, et al. : Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 391:2537-2545, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Le DT, Kim TW, Van Cutsem E, et al. : Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 38:11-19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macherla S, Laks S, Naqash AR, et al. : Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci 19:3505, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludford K, Cohen R, Svrcek M, et al. : Pathological tumor response following immune checkpoint blockade for deficient mismatch repair advanced colorectal cancer. J Natl Cancer Inst 113:208-211, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andre T, Lonardi S, Wong KYM, et al. : Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann Oncol 33:1052-1060, 2022 [DOI] [PubMed] [Google Scholar]
- 33.Dasari A, Morris VK, Allegra CJ, et al. : ctDNA applications and integration in colorectal cancer: An NCI Colon and Rectal-Anal Task Forces whitepaper. Nat Rev Clin Oncol 17:757-770, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruni D, Angell HK, Galon J: The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 20:662-680, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Germann M, Zangger N, Sauvain MO, et al. : Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol Med 12:e10681, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorría Puga T, Teixidó C, Auclin E, et al. : 184P Association of tumor-associated neutrophils (TAN) with immunotherapy outcomes in patients in advanced non-small cell lung cancer. Ann Oncol 32:S1462, 2021 [Google Scholar]