Abstract
PURPOSE
Cisplatin is widely used and highly ototoxic, but patient-reported functional impairment because of cisplatin-related hearing loss (HL) and tinnitus has not been comprehensively evaluated.
PATIENTS AND METHODS
Testicular cancer survivors (TCS) given first-line cisplatin-based chemotherapy completed validated questionnaires, including the Hearing Handicap Inventory for Adults (HHIA) and Tinnitus Primary Function Questionnaire (TPFQ), each of which quantifies toxicity-specific functional impairment. Spearman correlations evaluated associations between HL and tinnitus severity and level of functional handicap quantified with the HHIA and TPFQ, respectively. Associations between HL or tinnitus and five prespecified adverse health outcomes (cognitive dysfunction, fatigue, depression, anxiety, and overall health) were evaluated.
RESULTS
HL and tinnitus affected 137 (56.4%) and 147 (60.5%) of 243 TCS, respectively. Hearing aids were used by 10% TCS (14/137). Of TCS with HL, 35.8% reported clinically significant functional impairment. Severe HHIA-assessed functional impairment was associated with cognitive dysfunction (odds ratio [OR], 10.62; P < .001), fatigue (OR, 5.48; P = .003), and worse overall health (OR, 0.19; P = .012). Significant relationships existed between HL severity and HHIA score, and tinnitus severity and TPFQ score (P < .0001 each). TCS with either greater hearing difficulty or more severe tinnitus were more likely to report cognitive dysfunction (OR, 5.52; P = .002; and OR, 2.56; P = .05), fatigue (OR, 6.18; P < .001; and OR, 4.04; P < .001), depression (OR, 3.93; P < .01; and OR, 3.83; P < .01), and lower overall health (OR, 0.39; P = .03; and OR, 0.46; P = .02, respectively).
CONCLUSION
One in three TCS with HL report clinically significant functional impairment. Follow-up of cisplatin-treated survivors should include routine assessment for HL and tinnitus. Use of the HHIA and TPFQ permit risk stratification and referral to audiologists as needed, since HL adversely affects functional status and is the single largest modifiable risk factor for cognitive decline and dementia in the general population.
INTRODUCTION
Cisplatin is one of the most ototoxic drugs in clinical use,1,2 causing permanent, bilateral sensorineural hearing loss (HL) in up to 80% of cancer survivors,3-6 with many experiencing tinnitus.7,8 Despite recognition of cisplatin's ototoxicity over 40 years ago9 and its retention in the cochlea indefinitely,10 to our knowledge, no study has quantified its impact on the functional status of cancer survivors using validated, otologic-specific patient-reported outcome measures. In-depth investigations of common treatment toxicities are increasingly recognized as important in survivorship follow-up care.11
CONTEXT
Key Objective
Cisplatin is widely used and highly ototoxic, but patient-reported functional impairment because of cisplatin-related hearing loss (HL) and tinnitus has not been comprehensively evaluated. We quantified the impact of ototoxicity using validated, otologic-specific patient-reported outcomes: the Hearing Handicap Inventory for Adults, and Tinnitus Primary Function Questionnaire. These provide quantitative clinically actionable scores that can be used for risk stratification.
Knowledge Generated
Clinically significant functional impairments attributed to cisplatin-related HL and tinnitus were reported by 36% and 44% testicular cancer survivors, respectively. Significant relationships existed between HL severity and Hearing Handicap Inventory for Adults score, and tinnitus severity and Tinnitus Primary Function Questionnaire score.
Relevance (M.A. Carducci)
Attention to survivorship issues such as HL after platinum treatment for germ cell cancers is essential and includes follow-up with audiology, patient education, and thorough survivorship plans highlighting impact of HL.*
*Relevance section written by Michael A. Carducci, MD, FACP, FASCO.
In the general population, HL begins in midlife,12,13 with two thirds of individuals age ≥ 70 years having bilateral HL,13 but for cancer survivors where treatment occurs earlier in life, cisplatin-related ototoxicity can exacerbate age-related HL.3 Testicular cancer (TC) is one example, with a median diagnosis age of only 30 years,14 and with TC the leading malignancy among men age 20-39 years.15 Because of the effectiveness of cisplatin-based chemotherapy (CBCT), overall 10-year relative survival rates now exceed 95%.14 Thus, TC survivors (TCS) are at risk for both short- and long-term adverse CBCT-related effects,16 including HL and tinnitus, with no preventive or protective measures available. The sudden development of HL can be devastating,17,18 and often more consequential than the slow progression of age-related HL. HL, even with a late age at onset, is significantly related to increased risks of cognitive decline and dementia,19-23 decreased health-related quality of life,24-27 poor mental and physical functioning,28-32 and increased social isolation.33-36 Tinnitus can lead to further social isolation, increased stress, anxiety, and, in extreme cases, mental health sequalae such as suicide.37,38
Nonetheless, to our knowledge, no investigation to date has comprehensively evaluated and quantified the effect of CBCT-associated ototoxicity on functional status in adult-onset cancer survivors.16 To address this important gap, we quantified severity and administered HL- and tinnitus-specific handicap patient-reported measures, along with other validated questionnaires,39-41 to a subset of TCS in a large multicenter investigation (The Platinum Study).3,42,43 These HL- and tinnitus-specific questionnaires are unique in asking patients to partition functional deficits into those directly attributable to each toxicity and quantify them.44,45 Resultant scores can then be used clinically to accurately risk-stratify patients for audiologic and other interventions.
PATIENTS AND METHODS
Patients
The Platinum Study enrolled cisplatin-treated TCS at eight cancer centers (2012-2018).3,42,43 At enrollment, participants completed questionnaires and underwent physical examinations and extensive audiologic testing.3,42,43,46,47 Administration of a subsequent survey to TCS enrolled at Indiana University, University of Pennsylvania, University of Rochester, Dana-Farber Cancer Institute, and Memorial Sloan Kettering Cancer Center was approved by the appropriate institutional review boards. This report includes 243 TCS with complete surveys through February 27, 2022. Standardized questionnaires collected demographic and clinical data, including information on medical history, lifestyle, and comorbidities. Validated instruments collected outcome data,39-41,48-58 with questions and scoring criteria in Appendix 1 (online only).
Identifying Patients With HL and Tinnitus
HL was ascertained as a binary variable using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy-20 Scale40; Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN)39; and questions regarding hearing aid use, and difficulty hearing in crowds (Appendix 1). HL severity was quantified with, “During the past 4 weeks, did you have difficulty hearing?” with responses of not at all, a little, quite a bit, and very much.40 Tinnitus was ascertained as a binary variable by patients describing ringing or buzzing or with questions in the SCIN,39 which also quantified severity, “Have you suffered in the last 4 weeks from ringing or buzzing in your ears (ie, tinnitus)?” with responses of not at all, a little, quite a bit, and very much.39
Effect of Severity of HL or Tinnitus on Patient-Reported Functional Status
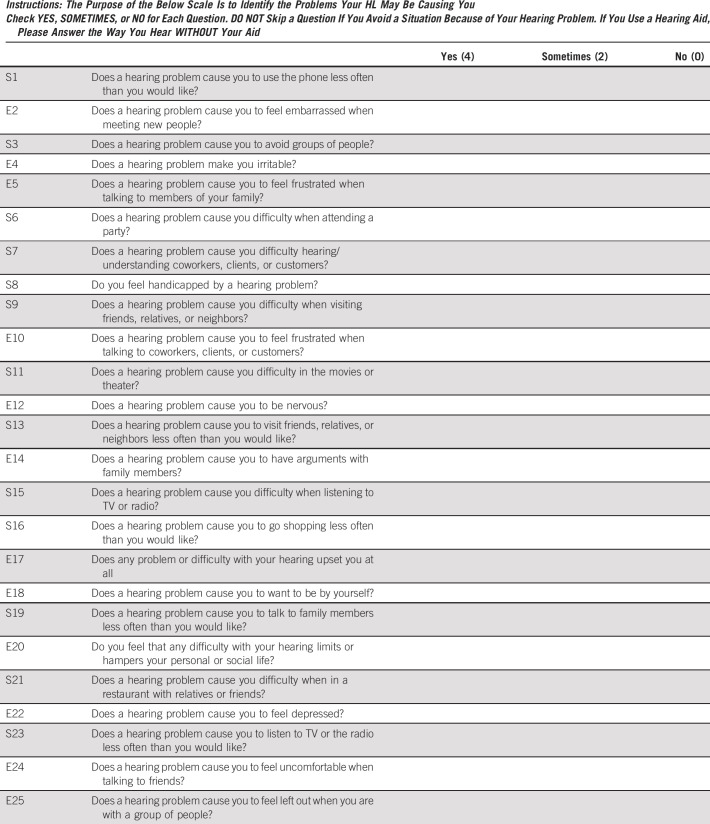
TCS with HL were administered the Hearing Handicap Inventory for Adults (HHIA),45,59-62 a 25-item self-assessment quantifying the impact of HL, with 13 questions assessing emotional effects and 12 questions assessing social effects (see Appendix 2 [online only] for instrument validity, questions/scoring). Overall HHIA scores range from 0% to 100% (higher scores indicate greater handicap attributable to HL) and were grouped using standard clinical categories: none/minimal, 0%-16%; mild/moderate, 17%-42%; and severe, 43%-100%.45,62 Patients with mild/moderate or greater handicap are typically referred for audiologic evaluation/treatment.
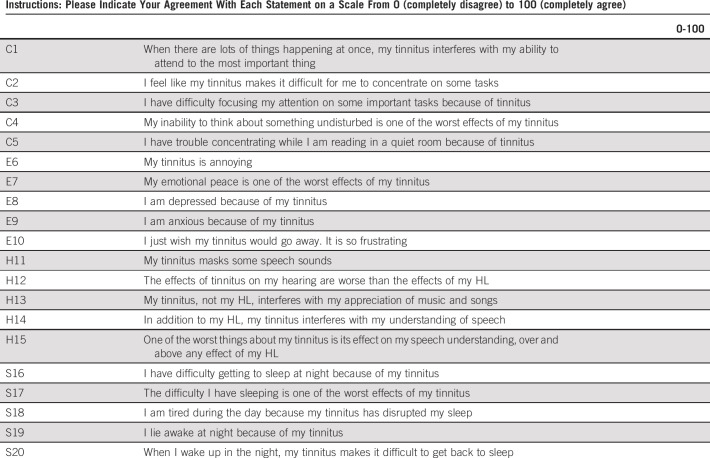
TCS with tinnitus were administered the Tinnitus Primary Function Questionnaire (TPFQ),44 a 20-item self-assessment quantifying tinnitus' impact on four functional subdomains: concentration, emotion, hearing, and sleep (see Appendix 3 [online only] for instrument validity, questions/scoring). Subdomain and overall scores range from 0% to 100% (higher scores indicate greater handicap attributable to tinnitus). Standard clinical categories were used to group scores: none/minimal, 0%-16%; mild/moderate, 17%-42%; and severe, 43%-100%.63,64 Ratings of mild/moderate or greater handicap are considered clinically actionable, with patients referred for available interventions.
Effect of Severity of HL or Tinnitus on Patient-Reported Adverse Health Outcomes
We identified five patient-reported adverse health outcomes (AHOs), a priori, for which to evaluate relationships with HL and tinnitus: cognitive dysfunction, fatigue, anxiety, depression, and overall health (see Appendix 1 for definitions/scoring). For all TCS, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale40 and SCIN39 severity grading methods were used: not at all, a little, or quite a bit/very much. Quite a bit and very much were combined for modeling to increase precision and because they exhibited similar effect sizes. For TCS with HL and tinnitus, we also evaluated the HHIA and TPFQ clinical ratings (none/minimal, mild/moderate, and severe) and patient-reported AHOs.
Statistical Analyses
Descriptive statistics are provided as frequencies (proportions) for categorical variables or medians (interquartile range) for continuous variables. Logistic (binary or multinomial) or linear regression, as appropriate, were applied to bivariate comparisons. We conducted Spearman correlations between (1) HL severity and audiometrically measured HL, tinnitus severity, and HHIA handicap scores (total, subdomains); and (2) tinnitus severity and TPFQ handicap scores (total, subdomains). To evaluate associations of HL severity, tinnitus severity, HHIA, and TPFQ scores with prespecified AHOs, we performed multivariable regression analyses adjusted for age, body mass index, cumulative cisplatin dose, years since chemotherapy, tobacco use (never/ever), and hypertension.3,43,65,66 Specifically, binary logistic regression was used for presence/absence of cognitive dysfunction, fatigue, anxiety, and depression, whereas partial proportional odds logistic regression was used for overall health (dependent variables). In partial proportional odds, ordered logit functions are used, except for covariates violating the proportion odds assumption for which multinomial functions are used. For covariates under the ordered logit relationship, a single odds ratio (OR) describes the assumed constant odds across the overall health categories. When the proportional odds assumption was violated for TPFQ score (a primary independent variable), overall health was dichotomized and binary logistic regression used.
RESULTS
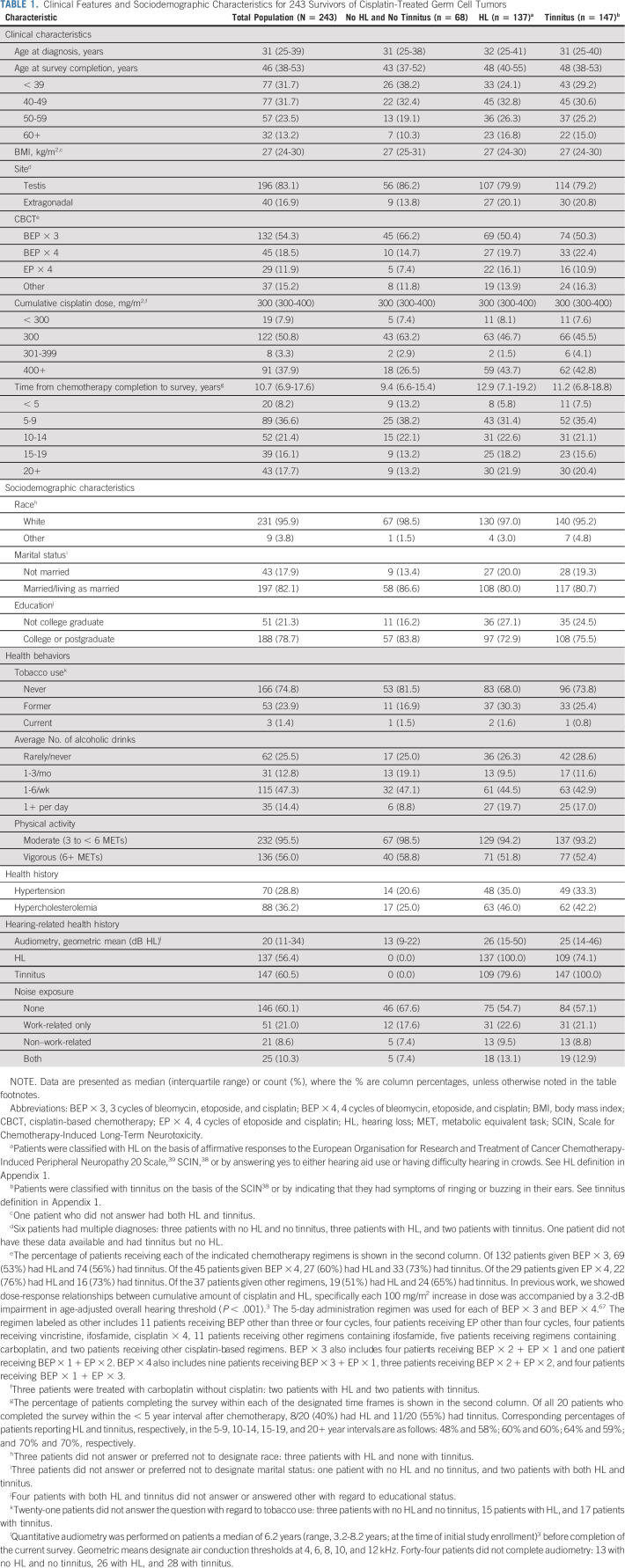
Table 1 presents clinical and sociodemographic characteristics for all TCS and subgroups with HL or tinnitus. Among 243 TCS (median age at evaluation, 46 years [interquartile range, 38-53 years]), HL and tinnitus were reported by 137 (56.4%) and 147 (60.5%), respectively. Only 68 (28.0%) of TCS reported neither HL or tinnitus, and 109 (44.9%) reported both.
TABLE 1.
Clinical Features and Sociodemographic Characteristics for 243 Survivors of Cisplatin-Treated Germ Cell Tumors
HL Severity
TCS with self-reported HL as a binary variable (n = 137) rated the severity of HL in the past 4 weeks as not at all (12.4%), a little (62.1%), quite a bit (12.4%), or very much (13.1%; Table 2). HL severity was significantly associated with audiometrically defined HL (P < .001), documenting participants' ability to accurately self-assess HL. For TCS with very much self-reported HL, audiometric results were consistent with moderately severe HL,47 and 50% (9/18) used hearing aids.
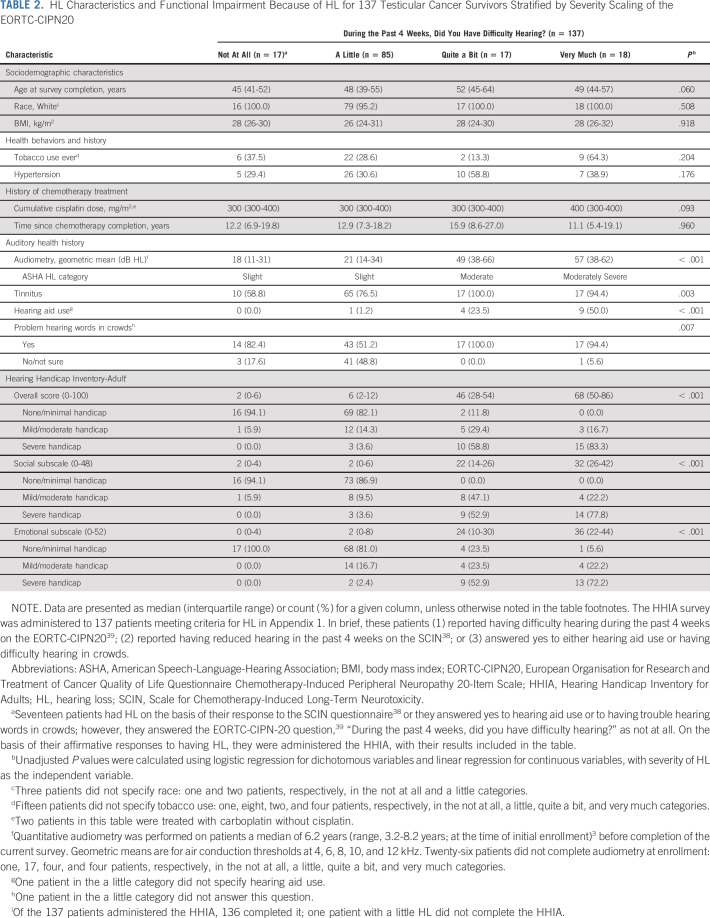
TABLE 2.
HL Characteristics and Functional Impairment Because of HL for 137 Testicular Cancer Survivors Stratified by Severity Scaling of the EORTC-CIPN20
All 137 TCS with HL were administered the HHIA, with a 99% completion rate (n = 136), resulting in 21 reporting mild/moderate overall handicap and 28 reporting severe overall handicap attributed to HL. Highly significant correlations (P < .0001 each) existed between greater HL severity and higher scores on the HHIA social and emotional subdomains (Spearman's rho = 0.68 and 0.66, respectively). Among TCS with either quite a bit or very much difficulty hearing, the overall degree of impairment ascribed to HL was noted as severe by 58.8% and 83.3%, respectively. A highly significant positive correlation existed between greater HL severity and worse overall handicap (Spearman's rho = 0.68; P < .0001; Fig 1A).
FIG 1.
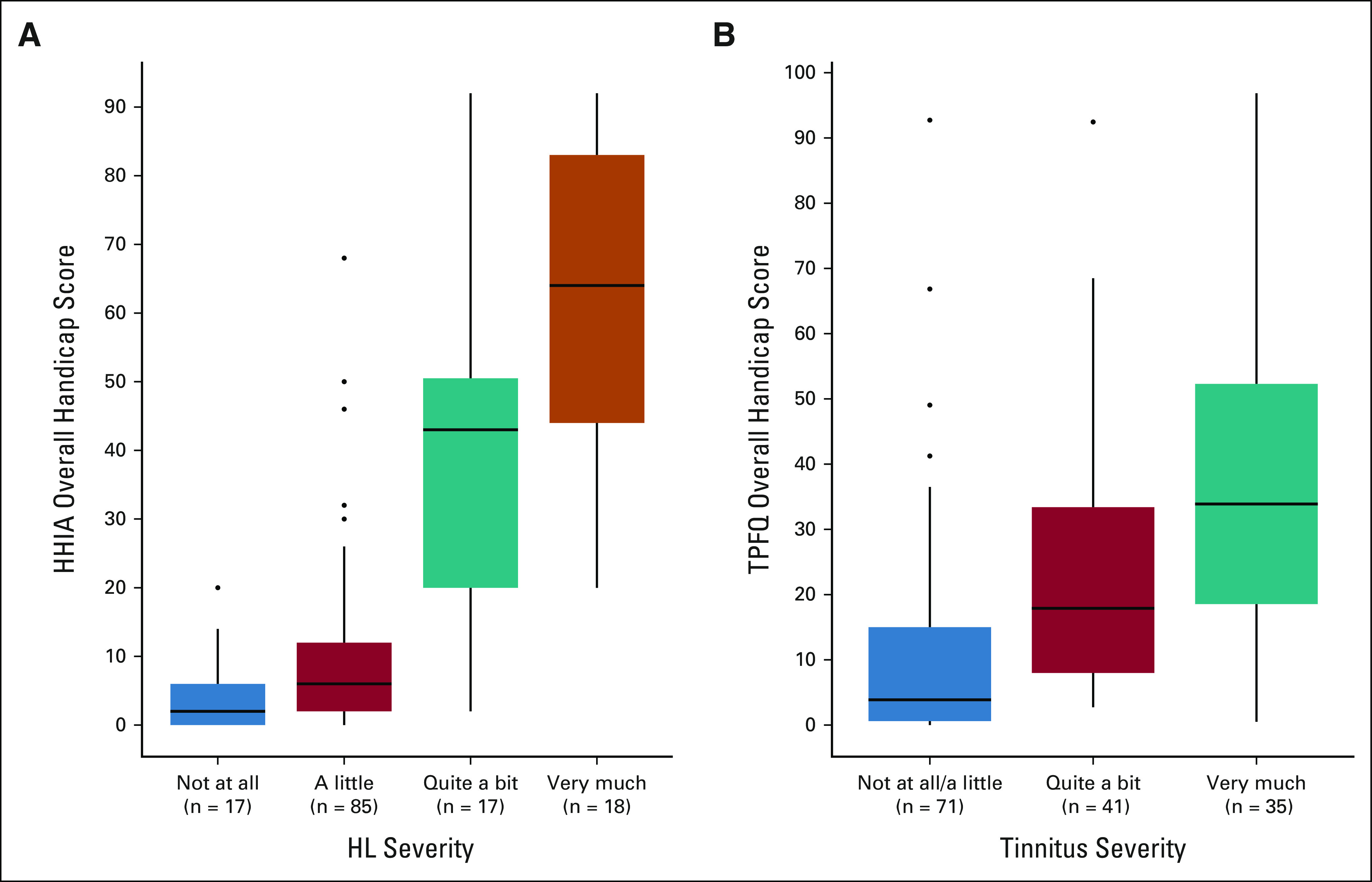
Correlation between various degrees of hearing loss and tinnitus and patient-reported functional impairment using the overall handicap scores in the Hearing Handicap Inventory for Adults (HHIA; A) and the Tinnitus Primary Frequency Questionnaire (TPFQ; B), respectively. Data are presented as box and whisker plot diagrams and correlation analysis using Spearman's rank correlation coefficient. The Spearman's correlation between (A) self-reported hearing loss severity and overall HHIA handicap is ρ = 0.68; P < .001 and between (B) self-reported tinnitus severity and overall TPFQ handicap is ρ = 0.55; P < .001. HHIA, Hearing Handicap Inventory for Adults; HL, hearing loss; TPFQ, Tinnitus Primary Function Questionnaire.
Tinnitus Severity
TCS with tinnitus described its severity in the past 4 weeks as a little (45.6%), quite a bit (27.9%), or very much (23.8%). Given sparse numbers (n = 4) in not at all, this category was combined with a little (Table 3). Worse tinnitus severity was significantly associated with worse HL severity (Spearman's rho = 0.48, P < .0001). TCS with tinnitus described as quite a bit or very much were older at survey completion (P = .004), had greater audiometrically defined HL (P < .001), had longer time since chemotherapy (P = .04), and had greater hearing aid use (P = .001).
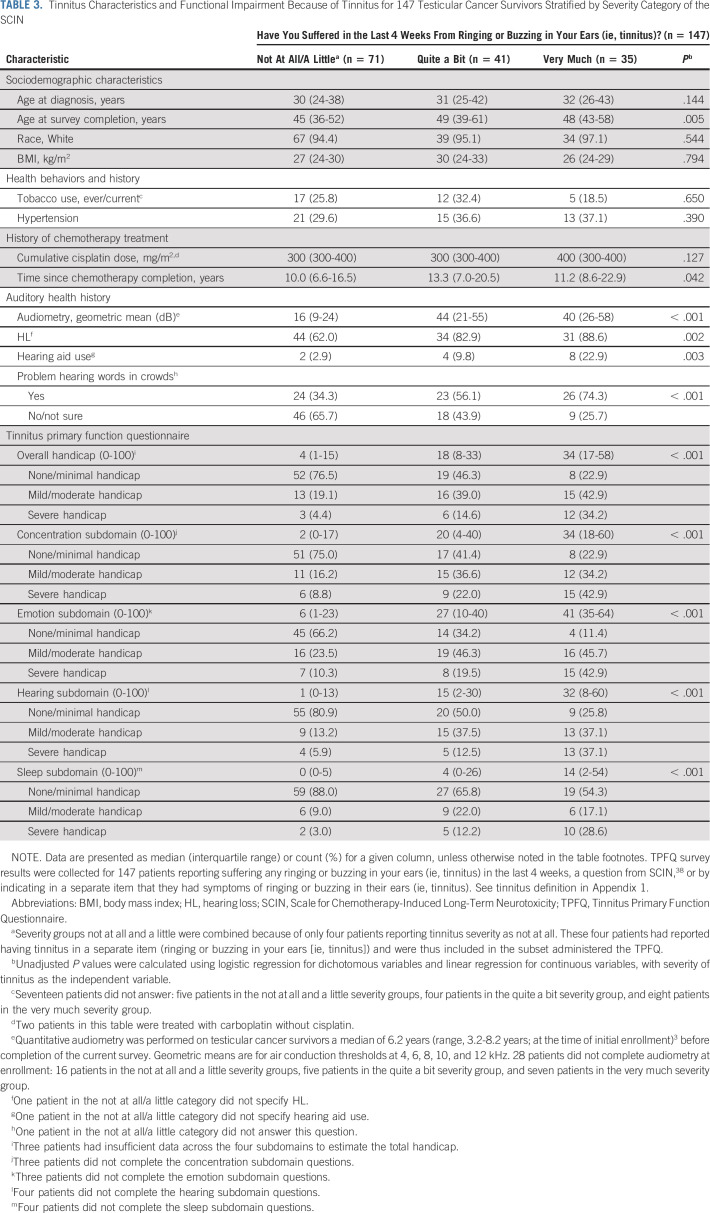
TABLE 3.
Tinnitus Characteristics and Functional Impairment Because of Tinnitus for 147 Testicular Cancer Survivors Stratified by Severity Category of the SCIN
All 147 TCS with tinnitus were administered the TPFQ, with 65 (44.2%) reporting a clinically actionable overall handicap of mild/moderate or severe. Worse tinnitus severity was significantly correlated (all P < .0001) with worse handicap in each TPFQ subdomain: concentration (Spearman's rho = 0.47), emotion (Spearman's rho = 0.57), hearing (Spearman's rho = 0.49), and sleep (Spearman's rho = 0.43), and overall (Spearman's rho = 0.55; Fig 1B). The TPFQ overall handicap attributed to tinnitus was rated as severe by 14.6%, and 34.2% of TCS with tinnitus described as quite a bit or very much
Effect of Severity of HL or Tinnitus on Prespecified AHOs
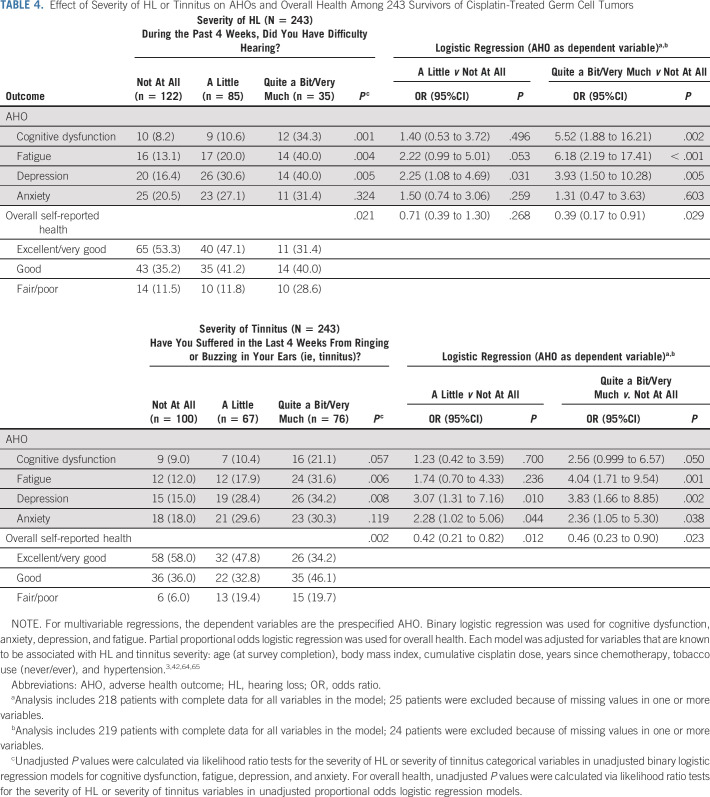
Table 4 shows relationships between AHOs and the degree of hearing and tinnitus difficulty for all 243 TCS. Hearing difficulty was significantly related to cognitive dysfunction, fatigue, and depression. After covariate adjustment, increasing hearing difficulty, in particular quite a bit/very much versus not at all, remained significantly associated with cognitive dysfunction (OR, 5.52; P = .002), fatigue (OR, 6.18; P < .001), and depression (OR, 3.93; P = .005), as well as with worse overall health (proportional odds logistic OR, 0.39; P = .029), indicating greater HL was associated with lower odds of better overall health.
TABLE 4.
Effect of Severity of HL or Tinnitus on AHOs and Overall Health Among 243 Survivors of Cisplatin-Treated Germ Cell Tumors
Tinnitus severity was significantly related to cognitive dysfunction, fatigue, and depression. After covariate adjustment, increasing tinnitus severity, in particular quite a bit/very much versus not at all, was significantly associated with each AHO: cognitive dysfunction (OR, 2.56; P = .050), fatigue (OR, 4.04, P = .001), depression (OR, 3.83, P = .002), and anxiety (OR, 2.36; P = .038). Greater tinnitus severity was also significantly associated with worse overall health (proportional odds logistic OR, 0.46; P = .023).
Effect of Clinically Scaled HHIA and TPFQ Results on Prespecified AHOs
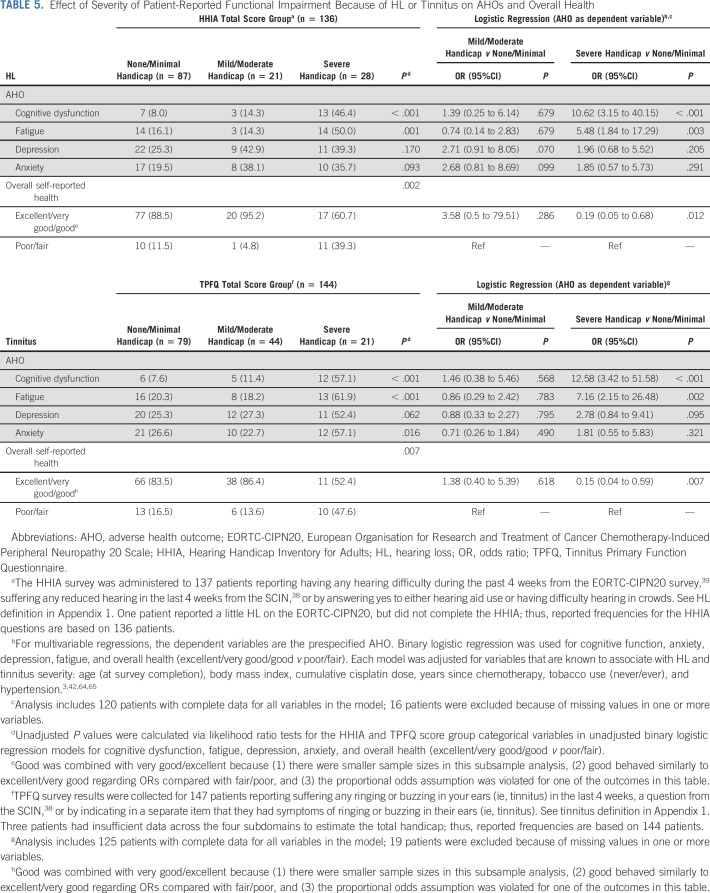
Among TCS with HL, functional impairment because of HL quantified with the HHIA was significantly related to cognitive dysfunction and fatigue (Table 5). After covariate adjustment, severe hearing handicap (v none/minimal) remained significantly associated with both outcomes (OR, 10.62; P < .001 and OR, 5.48; P = .003, respectively), and with worse overall health (binary logistic OR, 0.19; P = .012).
TABLE 5.
Effect of Severity of Patient-Reported Functional Impairment Because of HL or Tinnitus on AHOs and Overall Health
Tinnitus handicap quantified with the TPFQ was significantly related to cognitive dysfunction, fatigue, anxiety, and overall health. After covariate adjustment, severe tinnitus handicap (v none/minimal) remained significantly associated with cognitive dysfunction (OR, 12.58; P < .001), fatigue (OR, 7.16; P = .003), and worse overall health (binary logistic OR, 0.15; P = .007).
DISCUSSION
To our knowledge, this is the first study to examine the toxicity-specific functional effect of cisplatin-associated HL and tinnitus, and the severity of these symptoms, in adult-onset cancer survivors. We provide evidence of the deleterious impact on social and emotional functioning directly related to HL and tinnitus in a well-characterized population. After CBCT, 56% of TCS report HL, and overall, one in five survivors with HL indicates a severe handicap in social and emotional functioning attributable to HL. The proportion of severe functional impairment increases to 83% among TCS who describe very much hearing difficulty. Tinnitus occurred in 60% of TCS and, similar to HL, a significant positive correlation existed between greater severity and worse overall handicap attributed to tinnitus. The overall degree of functional impairment because of tinnitus was described as severe by one in three TCS with very much tinnitus. Cognitive dysfunction, fatigue, depression, and lower overall health were significantly associated with both HL and tinnitus severity. These and other new findings are discussed below.
HL is the third most prevalent disability worldwide,68,69 and associated with cognitive decline, fatigue, social isolation, depression, and other AHOs.20,31,33,35,36,70,71 An estimated $750 billion US dollars is spent annually on 466 million people with disabling HL, with many cases developing gradually and age-related.13 After CBCT, however, HL develops rapidly because of inner ear damage including the inner and outer sensory hair cells, spiral-ganglion neurons, stria vascularis, and injury to central auditory pathways.1,72 The HL is permanent, becoming a chronic health condition.
Previously, we reported detailed audiometric findings in this well-characterized cohort,3,46 including dose-response relationships with CBCT.3 We now show the deleterious impact of ototoxicity on patient-reported functional status. Over 33% of TCS with HL indicated more than a mild handicap in social and emotional functioning attributable to HL. More than a mild handicap suggests significant hearing problems warranting diagnostic evaluation and clinical intervention.73 In the general population, HL is associated with depression74,75 and fatigue,76,77 and our study demonstrates similar relationships in adult-onset cancer survivors. Nearly 40% of TCS with severe HL handicap quantified with the HHIA reported depression and 50% noted fatigue. HL-related listening fatigue is thought to be due to the increased cognitive load and extra effort needed to process speech, thereby depleting cognitive resources and resulting in fatigue.78 Listening fatigue and increased cognitive load are hypothesized as one underlying mechanism explaining robust independent associations observed between HL and cognitive decline.79 TCS with more severe HL handicap were also 10-fold more likely to indicate cognitive dysfunction than TCS with no/minimal handicap.
Despite recommendations for clinical intervention and the known AHOs related to untreated HL,73,80 only 10% of TCS with HL used hearing aids, indicating that most TCS are not receiving treatment options. This low prevalence is alarming, considering robust evidence that hearing health care including hearing aids can reduce listening fatigue76 and depression,74,75 and improve communication,81 cognition,19,74 and health-related quality of life.82-84 In a 2017 Cochrane Review of five randomized controlled trials involving 825 older adults using hearing aids, Ferguson et al84 reported large improvements in listening ability,85,86 and a large beneficial effect on hearing-specific quality of life using the Hearing Handicap Inventory for the Elderly60,87 compared with unaided/placebo conditions. HL is a modifiable chronic condition, and interventions must include evidence-based approaches with provision of hearing assistive technologies along with self-management support.88 US adults face structural barriers to accessing hearing health care, including high costs and difficulty navigating the hearing health care system,89 which may in part explain the low hearing aid use here. This low uptake is troubling, given the increasing amount of evidence that relates untreated HL in the general population to AHOs as well as to increased risks of dementia and cognitive decline.22,23 In fact, untreated HL has been identified as the single largest modifiable risk factor for dementia,22 and may be especially important as TCS become older and susceptible to additional age-related HL. Cognitive decline in older adults also results in negative relational, socioeconomic, and public health implications.22,23
Tinnitus affects 10%-15% of adults worldwide.90 Severity is key to consider, as increasing tinnitus severity is strongly associated with AHOs.91,92 Suffering from tinnitus is not the same as tinnitus perception,91 and only 3%-5% of the general population actually suffers with tinnitus.64,93 Neuroimaging has revealed that individuals reporting severe tinnitus have different brain activity and connectivity patterns.94-96 We found a strong relationship between more severe tinnitus and cognitive dysfunction, fatigue, anxiety, depression, and overall health. The severity scaling of tinnitus on these relationships is noteworthy, as suffering related to tinnitus can result in social isolation, increased stress, and, in extreme cases, suicide.37,38 We previously reported a significantly elevated two- to three-fold risk of prescription medications for anxiety and depression in TCS with tinnitus.97
Tinnitus severity was also significantly associated with greater TPFQ-quantified functional impairment. Among TCS with tinnitus, 45% indicated more than a mild/moderate degree of overall handicap attributable to tinnitus. There are no medicinal treatments for tinnitus; however, rehabilitative approaches may be beneficial and are often offered through a structured hierarchical manner to provide an individual patient-centered approach. If a patient presents with both HL and tinnitus, the provision of hearing aids can ameliorate not only HL, but often reduce tinnitus.98,99 Effective psychologic treatments for tinnitus include cognitive-behavioral approaches.100,101
We provide evidence of the deleterious impact on social and emotional functioning related to HL and tinnitus in a well-characterized clinical TCS cohort. Strengths of our study include the homogenous CBCT, quantitative hearing assessments, and use of validated clinical instruments to quantify functional impairment attributable to HL and tinnitus. Given their efficacy, the HHIA and TPFQ are used worldwide and have been translated into multiple languages, including Spanish, Arabic, and Chinese.102-106 Furthermore, the existence and validation of these surveys across multiple languages facilitates clinical implementation globally. With these reliable instruments, we quantified the negative impact of cisplatin-related ototoxicity in TCS and risk-stratified patients for available interventions. Although the HHIA and TFPQ assess toxicity-specific impact, it should be recognized that cisplatin-treated TCS may have comorbidities.42,43 The extent to which comorbidities might influence the perception of HL- or tinnitus-specific effects is unknown; however, in the general population, both HL and tinnitus typically affect older individuals with multiple comorbidities, and the HHIA and TPFQ have been extensively validated in these populations.44,45,62,107,108 Moreover, each question is phrased to specifically query and isolate the impact of either HL or tinnitus per se on functional status. Although we found strong associations between worse HL or tinnitus and greater cognitive dysfunction using rigorous definitions (Appendix 1), we relied on self-report; thus, this finding requires confirmation in studies with additional objective measures. Some investigations with objective neurocognitive testing show that TCS have impaired cognitive function,109-111 but others do not,112 as recently reviewed.43 Although response bias is a potential concern in any questionnaire study, we found no differences in survey completion rates on the basis of whether or not the patient had ototoxicity.
Ten-year TC survival rates now exceed 95%, given the effectiveness of cisplatin-based treatments14; thus, the high benefit-versus-risk ratio in using cisplatin to treat and cure TC, especially in young patients, is noteworthy. Accordingly, cisplatin should not be avoided, but attention must be turned to survivorship, including an awareness of the functional impact of ototoxicity. Routine follow-up of adult-onset cisplatin-treated ototoxicity in cancer survivors should begin with prechemotherapy baseline measurements, resume shortly after treatment, and include annual query for HL/tinnitus status and severity, especially as patients age, so that they are presented with available treatment strategies. For patients with HL or tinnitus, administration of the HHIA or TPFQ should be considered to accurately risk-stratify survivors for available interventions, as discussed above, with referral to audiologists and other specialists for treatments. Among TCS with HL here, approximately 1/3 reported mild/moderate or greater handicap on the HHIA overall score and would have been referred for audiologic evaluation and intervention in a general practice. Moreover, TCS with more than a mild clinical rating on the HHIA and TPFQ could be at higher risk for AHO, including cognitive dysfunction, fatigue, and poorer self-reported health. The potentially severe, negative impact of cisplatin-related ototoxicity on functional status warrants clinical intervention, survivorship support, and education.
APPENDIX
APPENDIX 1. Definitions Used for Adverse Health Outcomes and Other Variables
Hearing loss: Answered yes to any of the following questions: (1) a little, quite a bit, or very much for difficulty hearing40; (2) a little, quite a bit, or very much for reduced hearing39; (3) problems hearing words, sounds, or language in crowds; and (4) required a hearing aid.
Tinnitus: Answered a little, quite a bit, or very much for for ringing or buzzing your ears39 or yes to ringing or buzzing in your ears.
Fatigue: Answered yes to “In the past 7 days, have you experienced any type of fatigue?” and had a T-score ≥ 55 on the Patient-Reported Outcomes Measurement Information System (PROMIS) Fatigue Short Form 6a.41,50,53,54 Only participants who replied yes were administered the latter form.
Cognitive dysfunction: Answered yes to “Have you had any problems in your ability to think, concentrate, or remember items (ie, cognitive function) in the past 7 days?” and had a T-score ≤ 45 on the PROMIS Cognitive Function Abilities Short Form 4a.48,55 Only participants who replied yes were administered the latter form.
Depression: Score ≥ 3 on the Two-Question Screening Survey for Depression52 or reported the use of prescription medications for depression.
Anxiety: Reported the use of prescription medications for anxiety, or answered yes to “In the past 7 days, have you had any persistent fearfulness or worry (ie, anxiety)?” and had a T-score ≥ 55 on the PROMIS Anxiety Short Form 4.41,49 Only participants who replied yes were administered the latter form.
Hypertension: Answered yes to “Have you ever been told by a doctor or other health care provider that you had one of the following conditions: (a) hypertension” or reported the use of prescription medications for hypertension.
Hypercholesterolemia: Answered yes to “Have you ever been told by a doctor or other health care provider that you had one of the following conditions: (b) high cholesterol” or reported the use of prescription medications for cholesterol.
Overall self-reported health: Possible responses of excellent, very good, good, fair, or poor to “In general, would you say your health is?” from the PROMIS 10-item Global Health v1.2.51
Noise exposure: Categorized as none, work-related only, non–work-related only, or both from the following questions: (1) “Have you ever had a job where you were exposed to loud noise for 5 or more hours a week? (Loud noise means noise so loud that you had to speak in a raised voice to be heard)”; (2) “Outside of a job, have you ever been exposed to steady loud noise or music for 5 or more hours a week? (This is noise so loud that you have to raise your voice to be heard. Examples are noise from power tools, lawn mowers, farm machinery, cars, trucks, motorcycles, or loud music).”
Physical activity: Exercise was assessed with a validated questionnaire57,58 that asked participants to report their average time per week (over the past year) spent in each of nine recreational activities: walking or hiking (including walking to work); jogging (> 10 min/mile); running (≤ 10 min/mile); bicycling (including stationary bike); aerobic exercise/dance or exercise machines; lower-intensity exercise, yoga, stretching, or toning; tennis, squash, or racquetball; lap swimming; weight lifting or strength training; and other: please specify activity. Each physical activity was assigned a metabolic equivalent task (MET) value, which is a commonly used metric for describing the relative energy expenditure of a specific type of physical activity (1 MET = 1 kcal/kg/h or the energy cost of sitting quietly).57,58 The physical activities were then grouped on the basis of the MET values into categories of vigorous (≥ 6 METs) and moderate (3 to < 6 METs) physical activities.56
Race: Categorized as White if responded only as White to “Do you identify yourself as being (check all that apply)”; categorized as other if responded as Black or African American, Asian, American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, other, please specify, or in any combination with or without White.
Education: Categorized as not college graduate if responded 1-8 years (grade school), 9-12 years (high school), but did not graduate, completed high school/General Educational Development, training after high school, other than college/university, or some college/university to “What is the highest grade or level of schooling that you have completed?”; categorized as college or postgraduate if responded college/university graduate or postgraduate level.
Marital Status: Categorized as married/living as married if responded married or living as married to “Which of these possibilities best describes your current marital status?”; categorized as not married if responded single or never married, divorced, widowed, or separated or no longer living as married.
APPENDIX 2. Hearing Handicap Inventory for Adults
The Hearing Handicap Inventory for Adults (HHIA) is a widely accepted measure in the field of audiology and otolaryngology. The questionnaire is highly reliable (test-retest; r = 0.97) with high internal consistency (Cronbach's α = .93).61 In addition, Cronbach's α was calculated in our study, with scores of .95 and .94 for the emotional and social domains, respectively, and .97 overall. The HHIA has three response categories that are differently weighted (4, 2, 0), which are summed to a 0-100 total score. The questionnaire has items related to emotional (E) and social (S) domains. Patients were asked to complete the HHIA if they self-reported hearing loss (HL) as described in Appendix 1. Consistent with previous reports,45,62 three degrees of severity ratings were applied: none/minimal, 0%-16%; mild/moderate, 17%-42%; and severe, 43%-100%.
Emotional (E) and social (S) domain scores are sums of the respective 13 and 12 items. Each item is scored either 4 (yes), 2 (sometimes), or 0 (no). The emotional domain has a possible score of 0-52. The social domain has a possible score of 0-48. If at least seven emotional items are answered, missing emotional items are mean-imputed. If at least six social items are answered, missing social items are mean-imputed. If < seven emotional items or six social items are answered, then the emotional domain score or social domain score is set to missing, respectively. Domain scores are rounded to the nearest whole number. Domain percent scores are percentages of the total possible domain score. The total score is the sum of the two domain scores. The total score is set to missing when either the emotional or social domain scores are missing.
APPENDIX 3. Tinnitus Primary Function Questionnaire
The Tinnitus Primary Function Questionnaire44 is a 20-item self-assessment quantifying the impact of tinnitus per se on four functional domains: concentration (C), emotion (E), hearing (H), and sleep (S). The questionnaire is highly reliable with high construct validity (r = 0.77) and high internal consistency (Cronbach's α = .92).44 In addition, Cronbach's α was calculated in our study for all four subscales: concentration (.880), emotion (.84), hearing (.81), and sleep (.94). Patients were asked to complete the Tinnitus Primary Function Questionnaire if they self-reported ‘tinnitus’ through two questions as described in Appendix 1. Similar to prior reports and uses,63,64 and for consistency with the Hearing Handicap Inventory for Adults categorization, three categories of severity are used: none/minimal, 0%-16%; mild/moderate, 17%-42%; and severe, 43%-100%.
Concentration (C), emotion (E), hearing (H), and sleep (S) domain scores are the means of the respective five domain items. Each item has a possible score of 0-100. If at least three items are answered from a respective domain, the missing domain items are mean-imputed. If less than three items are answered from a respective domain, then the respective domain score is set to missing. The total score is the mean of the nonmissing domain scores. The total score is set to missing when < 10 total items are answered.
APPENDIX 4. Supplemental Methods
The Supplemental Methods provide additional detail to the Patients and Methods section in the manuscript.
Study Population
The Platinum Study enrolled testicular cancer survivors (TCS) from eight cancer centers in the United States, Canada, and Great Britain (2012-2018), as described in detail elsewhere.3,42,43 Eligible participants were age at least 18 years at consent and ≤ 60 years at diagnosis, had a serologically or histologically confirmed germ cell tumor, and were treated with cisplatin-based chemotherapy. Patients were well characterized in terms of diagnostic and treatment information abstracted from medical records, including cumulative cisplatin dose. We included all patients with complete surveys as of the cutoff date for this report (February 24, 2022): this comprised 243 TCS (66%) of 371 TCS who consented to participate. To evaluate whether patients with hearing loss (HL) or tinnitus were more likely to fill out the questionnaire (eg, response bias), we determined how many of the 371 patients had reported HL/tinnitus at prior audiometric examination. Of the 371 patients, 175 (47%) had reported HL and 164 (69%) reported tinnitus; of these patients, 122/175 (69%) with HL and 113/164 (69%) with tinnitus completed the survey. Of the 371 patients, 196 (53%) had not reported HL at the time of prior audiometric examination, with 121/196 (62%) completing the survey; and 207 did not report tinnitus at the time of prior audiometric examination, with 130/207 (63%) completing the survey. There were no statistically significant differences in questionnaire completion between those with and without HL (P = .11) or those with and without tinnitus (P = .21). See Appendix 1 for additional details on questions and scoring criteria.
Audiometric Testing
At initial enrollment, participants also underwent extensive audiologic testing.3,42,43,46,47 Air-conduction audiometric thresholds for left and right ears were measured at each of 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, and 12k Hz.3,46 The geometric mean of the air-conduction audiometric thresholds of left and right ears at the five upper frequencies that were evaluated (4, 6, 8, 10, and 12k Hz) was used to define an aggregate measure.3,46 For each patient, the extent of HL using this aggregate measure was determined by applying criteria of the American Speech-Language- Hearing Association (ASHA),47 which defines hearing in decibels referenced to hearing level (dB-HL; Frank T: American Journal of Audiology 6:29-32, 1997) as normal (< 15), slight (16-25), mild (26-40), moderate (41-55), moderately severe (56-70), severe (71-90), or profound HL (> 90).
Identifying Patients With HL and Tinnitus: Effect of Severity of HL or Tinnitus on Patient-Reported Functional Status
The 137 patients who met criteria for HL as defined in Appendix 1 were also asked to complete a questionnaire (the Hearing Handicap Inventory for Adults [HHIA])45,59-62 that was designed for patients with HL and validated in patients with HL. The HHIA asks patients with HL 25 questions about the handicap imposed by HL in two functional domains: social and emotional (Appendix 2).45 If participants reported using hearing aids (a small minority in the present investigation), they were instructed to answer questions with respect to their functionality without hearing aids. The characteristics and functional impairment measured with the HHIA stratified by the severity of HL according to the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale are shown in Table 2.
The 147 patients who met criteria for tinnitus as defined in Appendix 1 were also asked to complete a questionnaire (the Tinnitus Primary Function Questionnaire [TPFQ]) designed for patients with tinnitus and validated in patients with tinnitus. The TPFQ asks patients with tinnitus 20 questions about the handicap imposed by their tinnitus in four functional domains: concentration, emotion, hearing, and sleep (Appendix 3). Of the 147 patients identified with tinnitus, three patients had insufficient data across the four subdomains to estimate the total handicap; thus, reported frequencies are based on 144 patients. The characteristics and functional impairment measured with the TPFQ stratified by the severity of tinnitus according to the Scale for Chemotherapy-Induced Long-Term Neurotoxicity are shown in Table 3.
Measurement of Adverse Health Outcomes
For all 243 TCS in the study, data with regard to patient-reported adverse health outcomes (AHOs) that were defined a priori (ie, cognitive dysfunction, anxiety, depression, fatigue, and overall health) were collected with validated instruments including those from the National Institutes of Health–derived Patient-Reported Outcomes Measurement Information System (Andersen BL et al: J Clin Oncol 32:1605-1619, 2014).41,48-52 Each question and its scoring criteria are shown in Appendix 1. The results for the 243 TCS are shown in Table 4 stratified by the patient's response to the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale with regard to HL and by his response to the Scale for Chemotherapy-Induced Long-Term Neurotoxicity with regard to tinnitus.
For the subgroup of 137 TCS who met criteria for HL and were thus administered the HHIA, we also evaluated the effect of HHIA category (ie, none/minimal, mild/moderate, and severe handicap) on the five prespecified patient-reported AHOs. The results for the 136 (99%) patients who completed the HHIA are shown in the upper half of Table 5.
For the subgroup of 147 TCS who met criteria for tinnitus and were thus administered the TPFQ, we also evaluated the effect of TPFQ category (ie, none/minimal, mild/moderate, and severe handicap) on these five patient-reported AHOs. The results for the 144 (98%) TCS with tinnitus who completed the entire TPFQ are shown in the bottom half of Table 5.
Statistical Analyses
As noted above, questions and scoring of all study end points are detailed in Appendices 1, 2, and 3. We also calculated the internal reliability of the HHIA and TPFQ with Cronbach alphas for this study, with the results shown in Appendices 2 and 3.
Victoria A. Sanchez
Consulting or Advisory Role: Autifony Therapeutics
Speakers' Bureau: Sonova
Research Funding: Otonomy Inc (Inst), Frequency Therapeutics (Inst)
Howard D. Sesso
Honoraria: BASF
Research Funding: Pfizer (Inst), Mars Symbioscience (Inst), Pure Encapsulations (Inst)
Darren R. Feldman
Consulting or Advisory Role: Cigna
Research Funding: Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma
Other Relationship: UpToDate
Chunkit Fung
Consulting or Advisory Role: Novartis, Exelixis
Research Funding: Astellas Pharma (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/450635
Robert D. Frisina
Patents, Royalties, Other Intellectual Property: Patent awarded for new neural stimulation technique involving laser light and gold nanoparticles (Inst), Patent awarded for a new drug combination (hormone, anti-inflammatory) to treat age-related hearing loss (Inst), Patent awarded on self-inflating flotation devices to prevent drowning in children and adults (Inst), I was awarded a US patent on Peristaltic Micropumps and Fluid Delivery Devices that Incorporate Them (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2022 annual meeting of the American Society of Clinical Oncology.
SUPPORT
M.M.S., R.D.F., M.E.D., H.D.S., D.R.F., P.O.M., and L.B.T. were supported by 2 R01 CA157823 funded by the National Cancer Institute. V.A.S. was supported by R01DC019408-01 funded by the National Institute of Deafness and Other Communication Disorders.
V.A.S. and M.M.S. contributed equally as first authors to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Victoria A. Sanchez, Megan M. Shuey, Paul C. Dinh, M. Eileen Dolan, Lawrence H. Einhorn, Chunkit Fung, Robert D. Frisina, Lois B. Travis
Financial support: Lois B. Travis
Administrative support: Victoria A. Sanchez, Megan M. Shuey, Paul C. Dinh, Lois B. Travis
Provision of study materials or patients: Lawrence H. Einhorn, David J. Vaughn, Darren R. Feldman, Lois B. Travis
Collection and assembly of data: Victoria A. Sanchez, Megan M. Shuey, Paul C. Dinh, Lawrence H. Einhorn, David J. Vaughn, Darren R. Feldman, Lois B. Travis
Data analysis and interpretation: Victoria A. Sanchez, Megan M. Shuey, Paul C. Dinh, Patrick O. Monahan, Sophie D. Fosså, Howard D. Sesso, Lawrence H. Einhorn, Neil E. Martin, Darren R. Feldman, Kurt Kroenke, Robert D. Frisina, Lois B. Travis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Functional Impairment Due to Hearing Loss and Tinnitus After Cisplatin-Based Chemotherapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Victoria A. Sanchez
Consulting or Advisory Role: Autifony Therapeutics
Speakers' Bureau: Sonova
Research Funding: Otonomy Inc (Inst), Frequency Therapeutics (Inst)
Howard D. Sesso
Honoraria: BASF
Research Funding: Pfizer (Inst), Mars Symbioscience (Inst), Pure Encapsulations (Inst)
Darren R. Feldman
Consulting or Advisory Role: Cigna
Research Funding: Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma
Other Relationship: UpToDate
Chunkit Fung
Consulting or Advisory Role: Novartis, Exelixis
Research Funding: Astellas Pharma (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/450635
Robert D. Frisina
Patents, Royalties, Other Intellectual Property: Patent awarded for new neural stimulation technique involving laser light and gold nanoparticles (Inst), Patent awarded for a new drug combination (hormone, anti-inflammatory) to treat age-related hearing loss (Inst), Patent awarded on self-inflating flotation devices to prevent drowning in children and adults (Inst), I was awarded a US patent on Peristaltic Micropumps and Fluid Delivery Devices that Incorporate Them (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Santos NAGD, Ferreira RS, Santos ACD: Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem Toxicol 136:111079, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Paken J, Govender CD, Pillay M, et al. : Cisplatin-associated ototoxicity: A review for the health professional. J Toxicol 2016:1809394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frisina RD, Wheeler HE, Fossa SD, et al. : Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 34:2712-2720, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ardeshirrouhanifard S, Fossa SD, Huddart R, et al. : Ototoxicity after cisplatin-based chemotherapy: Factors associated with discrepancies between patient-reported outcomes and audiometric assessments. Ear Hear 43:794-807, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight K, Kraemer D, Winter C, et al. : Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol 25:1190-1195, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Landier W, Knight K, Wong FL, et al. : Ototoxicity in children with high-risk neuroblastoma: Prevalence, risk factors, and concordance of grading scales—A report from the Children's Oncology Group. J Clin Oncol 32:527-534, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Charif O, Mapes B, Trendowski MR, et al. : Clinical and genome-wide analysis of cisplatin-induced tinnitus implicates novel ototoxic mechanisms. Clin Cancer Res 25:4104-4116, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cianfrone G, Pentangelo D, Cianfrone F, et al. : Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur Rev Med Pharmacol Sci 15:601-636, 2011 [PubMed] [Google Scholar]
- 9.Schacht J, Talaska AE, Rybak LP: Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat Rec (Hoboken) 295:1837-1850, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breglio AM, Rusheen AE, Shide ED, et al. : Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun 8:1654, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nekhlyudov L, Ganz PA, Arora NK, et al. : Going beyond being lost in transition: A decade of progress in cancer survivorship. J Clin Oncol 35:1978-1981, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal Y: Prevalence of hearing loss and differences by demographic characteristics among US adults: Data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med 168:1522-1530, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Goman AM, Lin FR: Prevalence of hearing loss by severity in the United States. Am J Public Health 106:1820-1822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEER*Explorer: An Interactive Website for SEER Cancer Statistics. Surveillance Research Program, National Cancer Institute. https://seer.cancer.gov/explorer/ [Google Scholar]
- 15.Hayes-Lattin B, Nichols CR: Testicular cancer: A prototypic tumor of young adults. Semin Oncol 36:432-438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercieca-Bebber R, Naher SK, Rincones O, et al. : Patient-reported outcomes associated with treatments for testicular cancer: A systematic review. Patient Relat Outcome Meas 12:129-171, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn M, Heman-Ackah SE, Shaikh JA, et al. : Sudden sensorineural hearing loss. Trends Amplif 15:91-105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuser J, Knoop T: Sudden idiopathic hearing loss: Psychopathology and antecedent stressful life-events. Br J Med Psychol 59:245-251, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Amieva H, Ouvard C, Giulioli C, et al. : Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: A 25-year study. J Am Geriatr Soc 63:2099-2104, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Lin FR: Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci 66A:1131-1136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loughrey DG, Kelly ME, Brennan S, et al. : Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 144:115-126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livingston G, Huntley J, Sommerlad A, et al. : Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396:413-446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingston G, Sommerlad A, Orgeta V, et al. : Dementia prevention, intervention, and care. Lancet 390:2673-2734, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Chia EM, Wang JJ, Rochtchina E, et al. : Hearing impairment and health-related quality of life: The Blue Mountains Hearing Study. Ear Hear 28:187-195, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Dalton DS, Cruickshanks KJ, Klein BE, et al. : The impact of hearing loss on quality of life in older adults. Gerontologist 43:661-668, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Carabellese C, Appollonio I, Rozzini R, et al. : Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc 41:401-407, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Mulrow CD, Aguilar C, Endicott JE, et al. : Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc 38:45-50, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Viljanen A, Kaprio J, Pyykko I, et al. : Hearing acuity as a predictor of walking difficulties in older women. J Am Geriatr Soc 57:2282-2286, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Li L, Simonsick EM, Ferrucci L, et al. : Hearing loss and gait speed among older adults in the United States. Gait Posture 38:25-29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gispen FE, Chen DS, Genther DJ, et al. : Association between hearing impairment and lower levels of physical activity in older adults. J Am Geriatr Soc 62:1427-1433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deal JA, Reed NS, Kravetz AD, et al. : Incident hearing loss and comorbidity. JAMA Otolaryngol Head Neck Surg 145:36-43, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C-M, Zhang X, Hoffman HJ, et al. : Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005-2010. JAMA Otolaryngol Head Neck Surg 140:293-302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strawbridge WJ, Wallhagen MI, Shema SJ, et al. : Negative consequences of hearing impairment in old age: A longitudinal analysis. Gerontologist 40:320-326, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Weinstein BE, Ventry IM: Hearing impairment and social isolation in the elderly. J Speech Hear Res 25:593-599, 1982 [DOI] [PubMed] [Google Scholar]
- 35.Sung YK, Li L, Blake C, et al. : Association of hearing loss and loneliness in older adults. J Aging Health 28:979-994, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Pronk M, Deeg DJ, Smits C, et al. : Hearing loss in older persons: Does the rate of decline affect psychosocial health? J Aging Health 26:703-723, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Cunningham LL, Tucci DL: Hearing loss in adults. N Engl J Med 377:2465-2473, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugo A, Trpchevska N, Liu X, et al. : Sex-specific association of tinnitus with suicide attempts. JAMA Otolaryngol Head Neck Surg 145:685-687, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldenburg J, Fosså SD, Dahl AA: Scale for chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res 15:791-800, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Postma TJ, Aaronson NK, Heimans JJ, et al. : The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer 41:1135-1139, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Cella D, Choi SW, Condon DM, et al. : PROMIS((R)) adult health profiles: Efficient short-form measures of seven health domains. Value Health 22:537-544, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kerns SL, Fung C, Monahan PO, et al. : Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: A multi-institutional study. J Clin Oncol 36:1505-1512, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fung C, Sesso HD, Williams AM, et al. : Multi-institutional assessment of adverse health outcomes among North American testicular cancer survivors after modern cisplatin-based chemotherapy. J Clin Oncol 35:1211-1222, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyler R, Ji H, Perreau A, et al. : Development and validation of the tinnitus primary function questionnaire. Am J Audiol 23:260-272, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Newman CW, Weinstein BE, Jacobson GP, et al. : The hearing handicap inventory for adults: Psychometric adequacy and audiometric correlates. Ear Hear 11:430-433, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Trendowski MR, Wilkinson E, et al. : Pharmacogenomics of cisplatin‐induced neurotoxicities: Hearing loss, tinnitus, and peripheral sensory neuropathy. Cancer Med 11:2801-2816, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark J: Uses and abuses of hearing loss classification. ASHA 23:493-500, 1981 [PubMed] [Google Scholar]
- 48.PROMIS—Cognitive Function Abilities Short Form 4a. http://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20SF%20v2.0-Cognitive%20Abilities%20Subset%204a%201-2-2020.pdf [Google Scholar]
- 49.PROMIS—Anxiety Short Form 4a. http://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20SF%20v1.0%20-%20ED-Anxiety%204a%206-2-2016.pdf [Google Scholar]
- 50.PROMIS—Fatigue Short Form 6a. http://www.healthmeasures.net/administrator/components/com_instruments/uploads/PROMIS%20SF%20v1.0%20-%20Fatigue%206a%206-2-2016.pdf [Google Scholar]
- 51.Hays RD, Bjorner JB, Revicki DA, et al. : Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res 18:873-880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisch MJ, Loehrer PJ, Kristeller J, et al. : Fluoxetine versus placebo in advanced cancer outpatients: A double-blinded trial of the Hoosier Oncology Group. J Clin Oncol 21:1937-1943, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Cella D, Lai JS, Jensen SE, et al. : PROMIS fatigue item bank had clinical validity across diverse chronic conditions. J Clin Epidemiol 73:128-134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook KF, Jensen SE, Schalet BD, et al. : PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol 73:89-102, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saffer BY, Lanting SC, Koehle MS, et al. : Assessing cognitive impairment using PROMIS((R)) applied cognition-abilities scales in a medical outpatient sample. Psychiatry Res 226:169-172, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Ainsworth BE, Haskell WL, Herrmann SD, et al. : Compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc 43:1575-1581, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. : Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 7:81-86, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Taylor HL, Jacobs DR Jr, Schucker B, et al. : A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31:741-755, 1978 [DOI] [PubMed] [Google Scholar]
- 59.Lichtenstein MJ, Bess FH, Logan SA: Diagnostic performance of the Hearing Handicap Inventory for the elderly (screening version) against differing definitions of hearing loss. Ear Hear 9:208-211, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Ventry IM, Weinstein BE: Idenification of elderly people with hearing problems. ASHA 25:37-42, 1983 [PubMed] [Google Scholar]
- 61.Newman CW, Weinstein BE: The Hearing Handicap Inventory for the Elderly as a measure of hearing aid benefit. Ear Hear 9:81-85, 1988 [DOI] [PubMed] [Google Scholar]
- 62.Newman CW, Weinstein BE, Jacobson GP, et al. : Test-retest reliability of the hearing handicap inventory for adults. Ear Hear 12:355-357, 1991 [DOI] [PubMed] [Google Scholar]
- 63.Skarżyński PH, Rajchel JJ, Gos E, et al. : A revised grading system for the Tinnitus Handicap Inventory based on a large clinical population. Int J Audiol 59:61-67, 2020 [DOI] [PubMed] [Google Scholar]
- 64.Zhou F, Zhang T, Jin Y, et al. : Worldwide tinnitus research: A bibliometric analysis of the published literature between 2001 and 2020. Front Neurol 13:828299, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar A, Gulati R, Singhal S, et al. : The effect of smoking on the hearing status—A hospital based study. J Clin Diagn Res 7:210-214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomson RS, Auduong P, Miller AT, et al. : Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Invest Otolaryngol 2:69-79, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Wit R, Roberts JT, Wilkinson PM, et al. : Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: A randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 19:1629-1640, 2001 [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization : World Report on Hearing. Geneva, Switzerland, World Health Organization, 2021 [Google Scholar]
- 69.Vos T, Allen C, Arora M, et al. : Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545-1602, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mick P, Kawachi I, Lin FR: The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 150:378-384, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Choi JS, Betz J, Li L, et al. : Association of using hearing aids or cochlear implants with changes in depressive symptoms in older adults. JAMA Otolaryngol Head Neck Surg 142:652-657, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Tang Q, Wang X, Jin H, et al. : Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm 163:60-71, 2021 [DOI] [PubMed] [Google Scholar]
- 73.American Speech-Language-Hearing Association : Guidelines for Audiologic Screening, Rockville, MD, American Speech-Language-Hearing Association, 1997 [Google Scholar]
- 74.Dawes P, Emsley R, Cruickshanks KJ, et al. : Hearing loss and cognition: The Role of hearing aids, social isolation and depression. PLOS ONE 10:e0119616, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma RK, Chern A, Golub JS: Age-related hearing loss and the development of cognitive impairment and late-life depression: A scoping overview. Semin Hear 42:10-25, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holman JA, Drummond A, Naylor G: The effect of hearing loss and hearing device fitting on fatigue in adults: A systematic review. Ear Hear 42:1-11, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alhanbali S, Dawes P, Lloyd S, et al. : Hearing handicap and speech recognition correlate with self-reported listening effort and fatigue. Ear Hear 39:470-474, 2018 [DOI] [PubMed] [Google Scholar]
- 78.Pichora-Fuller MK, Kramer SE, Eckert MA, et al. : Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear Hear 37:5S-27S, 2016 [DOI] [PubMed] [Google Scholar]
- 79.Powell DS, Oh ES, Lin FR, et al. : Hearing impairment and cognition in an aging world. J Assoc Res Otolaryngol 22:387-403, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.American Speech-Language-Hearing Association : Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines]. 1994. Available from www.asha.org/policy [Google Scholar]
- 81.Stark P, Hickson L: Outcomes of hearing aid fitting for older people with hearing impairment and their significant others. Int J Audiol 43:390-398, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Kitterick PT, Ferguson MA: Hearing aids and health-related quality of life in adults with hearing loss. JAMA 319:2225-2226, 2018 [DOI] [PubMed] [Google Scholar]
- 83.Chisolm TH, Johnson CE, Danhauer JL, et al. : A systematic review of health-related quality of life and hearing aids: Final report of the American Academy of Audiology Task Force on the health-related quality of life benefits of amplification in adults. J Am Acad Audiol 18:151-183, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Ferguson M, Kitterick PT, Chong L, et al. : Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev 9:CD012023, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cox RM, Gilmore C: Development of the profile of hearing aid performance (PHAP). J Speech Hear Res 33:343-357, 1990 [DOI] [PubMed] [Google Scholar]
- 86.Cox RM, Alexander GC: The abbreviated profile of hearing aid benefit. Ear Hear 16:176-186, 1995 [DOI] [PubMed] [Google Scholar]
- 87.Ventry IM, Weinstein BE: The Hearing Handicap Inventory for the elderly: A new tool. Ear Hear 3:128-134, 1982 [DOI] [PubMed] [Google Scholar]
- 88.Barnett M, Hixon B, Okwiri N, et al. : Factors involved in access and utilization of adult hearing healthcare: A systematic review. Laryngoscope 127:1187-1194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arnold ML, Hyer K, Chisolm T: Medicaid hearing aid coverage for older adult beneficiaries: A state-by-state comparison. Health Aff (Millwood) 36:1476-1484, 2017 [DOI] [PubMed] [Google Scholar]
- 90.Bhatt JM, Lin HW, Bhattacharyya N: Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg 142:959-965, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Ridder D, Schlee W, Vanneste S, et al. : Chapter 1—Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal), in Schlee W, Langguth B, Kleinjung T, et al. (eds): Progress in Brain Research. Elsevier, 2021, pp 1-25 [DOI] [PubMed] [Google Scholar]
- 92.Coles RRA: Epidemiology of tinnitus: (1) prevalence. J Laryngol Otology 98:7-15, 1984 [DOI] [PubMed] [Google Scholar]
- 93.Axelsson A, Ringdahl A: Tinnitus—A study of its prevalence and characteristics. Br J Audiol 23:53-62, 1989 [DOI] [PubMed] [Google Scholar]
- 94.Hullfish J, Abenes I, Kovacs S, et al. : Functional connectivity analysis of fMRI data collected from human subjects with chronic tinnitus and varying levels of tinnitus-related distress. Data Brief 21:779-789, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maudoux A, Lefebvre P, Cabay J-E, et al. : Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res 1485:10-21, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Mohan A, De Ridder D, Idiculla R, et al. : Distress‐dependent temporal variability of regions encoding domain‐specific and domain‐general behavioral manifestations of phantom percepts. Eur J Neurosci 48:1743-1764, 2018 [DOI] [PubMed] [Google Scholar]
- 97.Ardeshirrouhanifard S, Dinh PC, Monahan PO, et al. : Use of medications for treating anxiety or depression among testicular cancer survivors: A multi-institutional study. Cancer Epidemiol Biomarkers Prev 30:1129-1138, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henry JA, Thielman EJ, Zaugg TL, et al. : Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with progressive tinnitus management. J Speech Lang Hear Res 60:1378-1397, 2017 [DOI] [PubMed] [Google Scholar]
- 99.Shekhawat GS, Searchfield GD, Stinear CM: Role of hearing aids in tinnitus intervention: A scoping review. J Am Acad Audiol 24:747-762, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Andersson G, Lyttkens L: A meta-analytic review of psychological treatments for tinnitus. Br J Audiol 33:201-210, 1999 [DOI] [PubMed] [Google Scholar]
- 101.Martinez‐Devesa P, Waddell A, Perera R, et al. : Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev:CD005233, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Carrillo A, del Mar Medina M, Polo R, et al. : Validation of the Hearing Handicap Inventory for Adults Scale for Spanish-speaking patients. Otol Neurotol 40:e947-e954, 2019 [DOI] [PubMed] [Google Scholar]
- 103.Shin J, Heo S, Lee H-K, et al. : Reliability and validity of a Korean version of the tinnitus primary function questionnaire. Am J Audiol 28:362-368, 2019 [DOI] [PubMed] [Google Scholar]
- 104.Talaat HS, Ali RH, Zein El Abedein AM: Trans-adaptation and standardization of Arabic version of tinnitus primary function questionnaire. Egypt J Ear Nose Throat Allied Sci 21:51-55, 2020 [Google Scholar]
- 105.Xin Y, Tyler R, Yao ZM, et al. : Tinnitus Assessment: Chinese Version of Tinnitus Primary Function Questionnaire. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lu T, Liu J-H, Li G, et al. : Reliability and validity of the mandarin version of the tinnitus primary function questionnaire: A preliminary observational study. Medicine 98:e16104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cassarly C, Matthews LJ, Simpson AN, et al. : The revised Hearing Handicap Inventory and screening tool based on psychometric reevaluation of the hearing handicap inventories for the elderly and adults. Ear Hear 41:95-105, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Theodoroff SM: Tinnitus Questionnaires for Research and Clinical Use, in Searchfield GD, Zhang J (eds): The Behavioral Neuroscience of Tinnitus. Current Topics in Behavioral Neurosciences, vol 51. Cham, Springer, 2020, pp 403-418 [DOI] [PubMed] [Google Scholar]
- 109.Chovanec M, Vasilkova L, Setteyova L: Long-term cognitive functioning in testicular germ-cell tumor survivors. Oncologist 23:617-623, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wefel J, Vidrine D, Marani S, et al. : A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psychooncology 23:626-633, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skoogh J, Steineck G, Stierner U: Testicular-cancer survivors experience compromised language following chemotherapy: Findings in a Swedish population-based study 3-26 years after treatment. Acta Oncol 51:185-197, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Schagen SB, Boogerd W, Muller MJ, et al. : Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol 47:63-70, 2008 [DOI] [PubMed] [Google Scholar]