It is well-established that human papillomavirus (HPV) is etiologically responsible for a distinct subset of oropharyngeal squamous cell carcinomas (OPSCCs) and is an independent biomarker of improved prognosis. In light of this association, the National Comprehensive Cancer Network (NCCN) guidelines,1 College of American Pathologists,2 and ASCO3 recommend assessment of HPV tumor status at diagnosis using either direct methods of HPV testing (eg, in situ hybridization or polymerase chain reaction) or a surrogate marker of HPV (ie, p16 immunohistochemistry) from primary tumor or nodal metastasis.
HPV-related OPSCC generally has a good prognosis with a 5-year overall survival rate of 80%-91% and a recurrence-free survival rate of 78%-90%.4-9 The majority of recurrences, approximately 66%-80%, occur within the first two years after treatment and are locoregional (54%).10-13 Surgical salvage of recurrent disease is associated with better overall survival.12 Furthermore, overall survival is significantly improved for patients with locoregional compared with distant recurrence.11-13 Critical to optimal survival after recurrence is early identification when salvage is possible. Current NCCN guidelines for surveillance after treatment recommend history and clinical examination including mirror or fiberoptic examination every 1-3 months in the first year and every 3-6 months in the second year.1 There are no imaging recommendations beyond obtaining post-treatment imaging only once to assess the response to radiation-based therapy or to establish baseline after primary surgical resection. The NCCN recommends against routine imaging surveillance; instead, the guidelines and data support obtaining imaging only for new symptoms or physical exam findings.1
The relationship between HPV and OPSCC is analogous to another virally mediated head and neck cancer—Epstein-Barr virus (EBV)–related nasopharyngeal cancer (NPC). In NPC, EBV is etiologically responsible for a subset of malignancies and is an independent biomarker of prognosis.1,14 Determination of EBV tumor status at diagnosis is recommended by the NCCN.1 For patients with EBV-related NPC and detectable circulating tumor DNA (ctEBV DNA) before treatment, ctEBV DNA is a dynamic biomarker of disease state and burden of disease.14 Thus, ctEBV DNA levels are used to assess treatment response and are the basis for clinical treatment decision making. For example, the presence of ctEBV DNA post-treatment is associated with worse prognosis and is being evaluated as an indication for consolidation chemotherapy.14,15
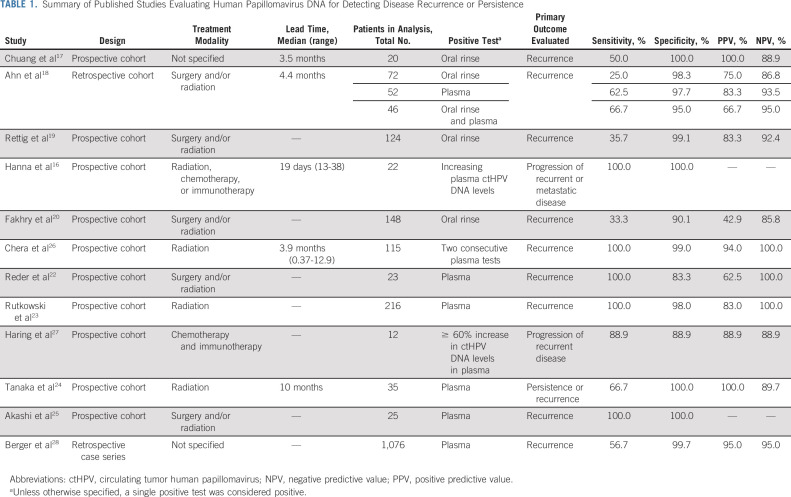
Similar to EBV-related NPC, it appears that the detection of circulating tumor HPV DNA (ctHPV DNA) after treatment of HPV-related OPSCC is associated with worse prognosis and is predictive of clinical recurrence. Several observational cohort studies have shown that HPV DNA in oral rinse or plasma precedes clinical detection of disease recurrence (Table 1)16-28; however, this has not been investigated in clinical trials with uniform study design which limits translation to clinical practice. In the past year, a plasma ctHPV DNA assay has become commercially available (and others are in development), which advertises for determination of genotype at diagnosis, assessment of clinical response, and disease surveillance.29 This assay uses droplet digital polymerase chain reaction (PCR) which, compared with conventional PCR, is able to identify DNA of interest with improved sensitivity, reproducibility, and precision.30 The assay detects E6 and E7 genes encoded by HPV 16 and E7 gene for HPV 18, 31, 33, and 35.26
TABLE 1.
Summary of Published Studies Evaluating Human Papillomavirus DNA for Detecting Disease Recurrence or Persistence
While there is great enthusiasm that ctHPV DNA can be used in surveillance for earlier detection of recurrence in HPV-OPSCC, there are many unanswered questions within the head and neck cancer community. The literature that served as the basis for integration of ctEBV DNA in clinical decision making in both treatment and surveillance for EBV-related NPC may serve as a template for HPV-related OPSCC. In large population-based screening studies, detection of ctEBV DNA led to earlier diagnosis of NPC31 as well as earlier detection of recurrence in the post-treatment setting.14 After the positive ctEBV DNA test, magnetic resonance imaging was associated with increased odds of clinical findings relative to fiberoptic nasopharyngoscopy.32,33
In the published literature to date on HPV-related OPSCC, the lead time between positive plasma ctHPV DNA and clinical evidence of disease ranged from 19 days to 10 months, with the largest study identifying a median lead time of 3.9 months (range, 0.4-12.9 months).26 Unfortunately, these analyses were not derived from prospective clinical studies with sample collection, clinical examination, and imaging at regular prescribed intervals, but rather from prospective studies with sampling at the time of clinical visits. In a retrospective case series analyzing 1,076 patients across the country, the time interval between the first two ctHPV DNA tests ranged between 9 and 367 days.28 This introduces interval censoring, which suggests that the true lead time could not be observed as it lies between currently observed time points of clinical follow-up and imaging. Whether the exact lead time is shorter or longer than the current data suggest is unknown. To address this uncertainty and define a robust estimate of lead time, larger prospective studies comparing study participants with a designated schedule and order of sample collection, clinical examination, and imaging to participants undergoing NCCN recommended surveillance are needed.
Such rigorous studies are needed to inform whether the addition of ctHPV DNA to the present NCCN endorsed clinical surveillance alters the disease course in a clinically meaningful manner. Data are needed to determine whether differences in timing, type and extent of recurrence diagnosed, morbidity of salvage therapy, quality of life, and cost-effectiveness exist. If ctHPV DNA is observed to have clinical utility, such prospective studies will also inform frequency for ctHPV DNA use and its utility as an adjunct or alternative to clinical examination. A recent study suggests that testing for ctHPV DNA every 3 months is more cost-effective for post-treatment surveillance compared with currently used strategies, particularly for equivocal results that are resulting in repeated imaging studies.34
Critical to elucidating the reliability of an assay and its clinical role is understanding its performance characteristics and reproducibility. Generally, performance characteristics appear to vary based on the type of sample collection (oral v plasma), the DNA detection method (real-time PCR, droplet digital PCR, and NGS), and type of recurrence (local, regional, and/or distant). Pooled sensitivity for oral HPV DNA detection has been shown to be moderate (72%; 95% CI, 45 to 89) ranging from 25% to 100%, while specificity is higher with a pooled estimate of 92% (95% CI, 82 to 97) and a range of 88%-100%. The positive predictive value of oral HPV DNA to detect recurrence has ranged from 42.9% to 100% while the negative predictive value (NPV) has ranged from 85.8% to 100%.35 Performance properties in plasma ctHPV DNA appear to be improved with ranges of sensitivity 63%-100%, specificity 83%-100%, positive predictive value 63%-100%, and NPV 89%-100% (Table 1). These studies consistently identify a high NPV. With a low complement to the NPV or false omission rate, it is expected that few patients with recurrent or persistent disease have undetectable ctHPV DNA. If the NPV of ctHPV DNA proves to be reliably high, it may be possible to investigate whether the frequency of clinical surveillance can be reduced for survivors with negative tests or if ctHPV DNA could supplant clinical examination. ctHPV DNA may also offer the opportunity to extend the window of surveillance beyond the currently accepted 3-5 years to identify and understand late recurrences. As with other surveillance tools, a positive ctHPV DNA test may assist with identifying patients who require further evaluation with diagnostic tests; however, prospective studies are needed to elucidate what the clinical and/or radiographic evaluation should entail after a positive ctHPV DNA test.
At present, ctHPV DNA detection without concurrent clinical or radiographic correlates represents an outcome without actionable implications outside of clinical trials. The magnitude of a positive ctHPV DNA test appears to be associated with disease burden21,36; however, there are no established cutoffs to guide a diagnostic evaluation to a locoregional or distant site, and the clinical significance of ctHPV DNA variation as a continuous variable is unknown. Moreover, the definition of an abnormal test has varied between studies—while one study defined two consecutive abnormal ctHPV DNA tests as criteria for positive,26 others considered one abnormal test to be positive.17-25 Notably, studies to date and commercially available assays have used heterogeneous HPV detection assays; validation is needed, especially of commercially available tests, as methods are expected to influence thresholds of positivity and performance characteristics. Establishing clear definitions of clinically meaningful positivity will be important for physicians and survivors.
Another important consideration in the absence of prospective data is the potential harmful psychological impact of ctHPV DNA on survivors between a positive test and clinical recurrence, and the impact of false positive tests and lead time bias. With better understanding of the kinetics, dynamics, and prognostic value of ctHPV DNA, we will be able to counsel patients on the meaning and significance of their test results when it is used as a method of surveillance.
Future studies should be designed with the goal of refining our understanding of lead time, clinical course following positive tests, and quality of life implications. In addition, robust prospective studies will allow us to also determine whether ctHPV DNA levels vary by race or gender. While HPV-OPSCC incidence is highest among White men, the prevalence of HPV-positive tumors is increasing significantly across all race and gender groups.37-39 If ctHPV DNA is included in clinical workflows, the acceptance of p16 as a surrogate for HPV status at diagnosis may need to be revisited, as determining the tumor type infection has been shown to be of relevance in the interpretation of ctHPV DNA levels.19,20 Finally, the ctHPV DNA commercial assay presently only applies to plasma; however, published data support a role for evaluating HPV DNA in saliva,21 oral rinses,17-20,40 or pharyngeal brushings.41 The performance characteristics for these, in addition to reproducibility and validation, will need to be determined, as the ease of saliva and oral rinse collection relative to venipuncture may be appealing to survivors and health care teams if the performance characteristics are similar.
In sum, while there is great enthusiasm for the emerging role of ctHPV DNA in the surveillance of HPV-OPSCC, it is incumbent upon us to recognize that physicians and survivors are in uncharted territory. There are significant knowledge gaps at this time, which introduce uncertainty as a commercially available assay is routinely used.
Carmen Kut
Stock and Other Ownership Interests: Pfizer (I), Moderna Therapeutics (I)
Research Funding: Regeneron
Patents, Royalties, Other Intellectual Property: Quantitative tissue property mapping for real time tumor detection and interventional guidance. Patent EP3122238A1/US20170086675A1
Harry Quon
Employment: Johns Hopkins Hospital
Leadership: Pistevo Health
Stock and Other Ownership Interests: Oncospace, Pistevo Health
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Pinnacle Biologics, Tactile Medical
Research Funding: Toshiba, Vibrent Health
Patents, Royalties, Other Intellectual Property: Filed under Hopkins, we have several patents related to mobile informatics solutions
Other Relationship: EPIC (I)
Tanguy Y. Seiwert
Honoraria: Merck, Nanobiotix, Coherus Biosciences
Consulting or Advisory Role: Merck, Bayer, Nanobiotix, Innate Pharma, Sanofi, CUE Biopharma, VIR Biotechnology, BioNTech SE, IO Biotech, Nektar, BostonGene
Research Funding: Merck (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Nanobiotix (Inst), AstraZeneca (Inst), Cue Biopharma (Inst), Dracen (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Nanobiotix, BostonGene, Coherus Biosciences
Carole Fakhry
Consulting or Advisory Role: Merck
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Carole Fakhry
Financial support: Carole Fakhry
Administrative support: Carole Fakhry
Provision of study materials or patients: Harry Quon, Tanguy Y. Seiwert, Carole Fakhry
Collection and assembly of data: Deborah X. Xie
Data analysis and interpretation: Deborah X. Xie, Carmen Kut, Carole Fakhry
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Uncertainties of Circulating Tumor DNA in Human Papillomavirus–Related Oropharyngeal Squamous Cell Carcinoma in the Absence of National Comprehensive Cancer Network Guidelines
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carmen Kut
Stock and Other Ownership Interests: Pfizer (I), Moderna Therapeutics (I)
Research Funding: Regeneron
Patents, Royalties, Other Intellectual Property: Quantitative tissue property mapping for real time tumor detection and interventional guidance. Patent EP3122238A1/US20170086675A1
Harry Quon
Employment: Johns Hopkins Hospital
Leadership: Pistevo Health
Stock and Other Ownership Interests: Oncospace, Pistevo Health
Honoraria: Sanofi/Regeneron
Consulting or Advisory Role: Pinnacle Biologics, Tactile Medical
Research Funding: Toshiba, Vibrent Health
Patents, Royalties, Other Intellectual Property: Filed under Hopkins, we have several patents related to mobile informatics solutions
Other Relationship: EPIC (I)
Tanguy Y. Seiwert
Honoraria: Merck, Nanobiotix, Coherus Biosciences
Consulting or Advisory Role: Merck, Bayer, Nanobiotix, Innate Pharma, Sanofi, CUE Biopharma, VIR Biotechnology, BioNTech SE, IO Biotech, Nektar, BostonGene
Research Funding: Merck (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Nanobiotix (Inst), AstraZeneca (Inst), Cue Biopharma (Inst), Dracen (Inst), Regeneron (Inst)
Travel, Accommodations, Expenses: Nanobiotix, BostonGene, Coherus Biosciences
Carole Fakhry
Consulting or Advisory Role: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pfister DG, Spencer S, Adelstein D, et al. : Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18:873-898, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Lewis JS Jr, Beadle B, Bishop JA, et al. : Human papillomavirus testing in head and neck carcinomas: Guideline from the College of American Pathologists. Arch Pathol Lab Med 142:559-597, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Fakhry C, Lacchetti C, Rooper LM, et al. : Human papillomavirus testing in head and neck carcinomas: ASCO clinical practice guideline endorsement of the College of American Pathologists guideline. J Clin Oncol 36:3152-3161, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Gillison ML, Trotti AM, Harris J, et al. : Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 393:40-50, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin BM, Wang H, D'Souza G, et al. : Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer 119:3462-3471, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yver CM, Shimunov D, Weinstein GS, et al. : Oncologic and survival outcomes for resectable locally-advanced HPV-related oropharyngeal cancer treated with transoral robotic surgery. Oral Oncol 118:105307, 2021 [DOI] [PubMed] [Google Scholar]
- 7.Fakhry C, Zhang Q, Gillison ML, et al. : Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer 125:2027-2038, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, et al. : Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24-35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeman JE, Li JG, Pei X, et al. : Patterns of treatment failure and postrecurrence outcomes among patients with locally advanced head and neck squamous cell carcinoma after chemoradiotherapy using modern radiation techniques. JAMA Oncol 3:1487-1494, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SH, Perez-Ordonez B, Weinreb I, et al. : Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol 49:79-85, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. : Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 32:3365-3373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T, Qualliotine JR, Ha PK, et al. : Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 121:1977-1984, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraji F, Eisele DW, Fakhry C: Emerging insights into recurrent and metastatic human papillomavirus-related oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol 2:10-18, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevisiol C, Gion M, Vaona A, et al. : The appropriate use of circulating EBV-DNA in nasopharyngeal carcinoma: Comprehensive clinical practice guidelines evaluation. Oral Oncol 114:105128, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Chen YP, Chan ATC, Le QT, et al. : Nasopharyngeal carcinoma. Lancet 394:64-80, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Hanna GJ, Supplee JG, Kuang Y, et al. : Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann Oncol 29:1980-1986, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Chuang AY, Chuang TC, Chang S, et al. : Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol 44:915-919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn SM, Chan JYK, Zhang Z, et al. : Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg 140:846-854, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rettig EM, Wentz A, Posner MR, et al. : Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol 1:907-915, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhry C, Blackford AL, Neuner G, et al. : Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol 5:985-992, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna GJ, Lau CJ, Mahmood U, et al. : Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol 95:120-126, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Reder H, Taferner VF, Wittekindt C, et al. : Plasma cell-free human papillomavirus oncogene E6 and E7 DNA predicts outcome in oropharyngeal squamous cell carcinoma. J Mol Diagn 22:1333-1343, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Rutkowski TW, Mazurek AM, Śnietura M, et al. : Circulating HPV16 DNA may complement imaging assessment of early treatment efficacy in patients with HPV-positive oropharyngeal cancer. J Transl Med 18:167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka H, Takemoto N, Horie M, et al. : Circulating tumor HPV DNA complements PET-CT in guiding management after radiotherapy in HPV-related squamous cell carcinoma of the head and neck. Int J Cancer 148:995-1005, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Akashi K, Sakai T, Fukuoka O, et al. : Usefulness of circulating tumor DNA by targeting human papilloma virus-derived sequences as a biomarker in p16-positive oropharyngeal cancer. Sci Rep 12:5722022, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chera BS, Kumar S, Shen C, et al. : Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol 38:1050-1058, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haring CT, Bhambhani C, Brummel C, et al. : Human papilloma virus circulating tumor DNA assay predicts treatment response in recurrent/metastatic head and neck squamous cell carcinoma. Oncotarget 12:1214-1229, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger BM, Hanna GJ, Posner MR, et al. : Detection of occult recurrence using circulating tumor tissue modified viral HPV DNA among patients treated for HPV-driven oropharyngeal carcinoma. Clin Cancer Res 28:4292-4301, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naveris . https://naveris.com/
- 30.Postel M, Roosen A, Laurent-Puig P, et al. : Droplet-based digital PCR and next generation sequencing for monitoring circulating tumor DNA: A cancer diagnostic perspective. Expert Rev Mol Diagn 18:7-17, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Chan KA, Woo JKS, King A, et al. : Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 377:513-522, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Li H, Yu KJ, et al. : Comparison of new magnetic resonance imaging grading system with conventional endoscopy for the early detection of nasopharyngeal carcinoma. Cancer 127:3403-3412, 2021 [DOI] [PubMed] [Google Scholar]
- 33.King AD, Woo JKS, Ai QY, et al. : Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol 30:977-982, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Kowalchuk RO, Kamdem Talom BC, Van Abel KM, et al. : Estimated cost of circulating tumor DNA for posttreatment surveillance of human papillomavirus-associated oropharyngeal cancer. JAMA Netw Open 5:e2144783, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gipson BJ, Robbins HA, Fakhry C, et al. : Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol 77:52-56, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chera BS, Kumar S, Beaty BT, et al. : Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res 25:4682-4690, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahal BA, Catalano PJ, Haddad RI, et al. : Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev 28:1660-1667, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Gillison ML, Chaturvedi AK, Anderson WF, et al. : Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 33:3235-3242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Souza G, Westra WH, Wang SJ, et al. : Differences in the prevalence of human papillomavirus (HPV) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and HPV detection method. JAMA Oncol 3:169-177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerndt SP, Ramirez RJ, Wahle BM, et al. : Evaluating a clinically validated circulating tumor HPV DNA assay in saliva as a proximal biomarker in HPV+ oropharyngeal squamous cell carcinoma. J Clin Oncol 39, 2021(15 suppl; abstr 6063) [Google Scholar]
- 41.Kofler B, Borena W, Dudas J, et al. : Post-treatment HPV surface brushings and risk of relapse in oropharyngeal carcinoma. Cancers (Basel) 12:1069, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]