Abstract
PURPOSE
It remains unknown whether or not short-term androgen deprivation (STAD) improves survival among men with intermediate-risk prostate cancer (IRPC) treated with dose-escalated radiotherapy (RT).
METHODS
The NRG Oncology/Radiation Therapy Oncology Group 0815 study randomly assigned 1,492 patients with stage T2b-T2c, Gleason score 7, or prostate-specific antigen (PSA) value >10 and ≤20 ng/mL to dose-escalated RT alone (arm 1) or with STAD (arm 2). STAD was 6 months of luteinizing hormone–releasing hormone agonist/antagonist therapy plus antiandrogen. RT modalities were external-beam RT alone to 79.2 Gy or external beam (45 Gy) with brachytherapy boost. The primary end point was overall survival (OS). Secondary end points included prostate cancer–specific mortality (PCSM), non-PCSM, distant metastases (DMs), PSA failure, and rates of salvage therapy.
RESULTS
Median follow-up was 6.3 years. Two hundred nineteen deaths occurred, 119 in arm 1 and 100 in arm 2. Five-year OS estimates were 90% versus 91%, respectively (hazard ratio [HR], 0.85; 95% CI, 0.65 to 1.11]; P = .22). STAD resulted in reduced PSA failure (HR, 0.52; P <.001), DM (HR, 0.25; P <.001), PCSM (HR, 0.10; P = .007), and salvage therapy use (HR, 0.62; P = .025). Other-cause deaths were not significantly different (P = .56). Acute grade ≥3 adverse events (AEs) occurred in 2% of patients in arm 1 and in 12% for arm 2 (P <.001). Cumulative incidence of late grade ≥3 AEs was 14% in arm 1 and 15% in arm 2 (P = .29).
CONCLUSION
STAD did not improve OS rates for men with IRPC treated with dose-escalated RT. Improvements in metastases rates, prostate cancer deaths, and PSA failures should be weighed against the risk of adverse events and the impact of STAD on quality of life.
INTRODUCTION
Prostate cancer was diagnosed over 1.4 million times and accounted for 375,000 deaths worldwide in 2020.1 In the United States, nearly 40% of diagnosed have National Comprehensive Cancer Network–classified intermediate-risk prostate cancer (IRPC), comprising the largest proportion of men undergoing definitive therapy and underscoring the need for treatment optimization in these patients.2
CONTEXT
Key Objective
To test in a prospective, randomized trial the hypothesis that the addition of 6 months of androgen deprivation will provide a survival advantage for men with intermediate-risk prostate cancer (IRPC) when added to dose-escalated radiation therapy.
Knowledge Generated
Although 6 months of androgen deprivation did not produce an overall survival (OS) advantage, it was associated with improvements in rates of biochemical failure, distant metastases (DMs), and prostate cancer–specific mortality. Although no patient subgroups showed an OS advantage, nearly all had reduced rates of DMs and prostate-specific antigen failure with androgen deprivation added. Adverse event rates were significantly higher in patients receiving androgen deprivation and must be weighed against its anticipated clinical benefits.
Relevance (M.A. Carducci)
This long-awaited report sheds light on the potential clinical benefits of short-term androgen-deprivation therapy for IRPC. Along with the accompanying report on patient-reported outcomes, these results allow the clinician and patient to determine the risk/benefits of STAD in this patient population.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
Previous studies investigating a combination of short-term androgen deprivation (STAD) with radiotherapy (RT) led to a greater understanding of how to optimally combine these modalities in patients with IRPC.3,4 However, large sample sizes and prolonged follow-up durations are required to demonstrate impacts on critical outcomes such as distant metastases (DMs) and overall survival (OS). These protracted durations can be long enough to affect the direct clinical applicability of a studied intervention.
Such was the case for multiple studies activated in the 1990s demonstrating both disease control and survival benefits for patients receiving STAD with RT compared with RT alone.3,5-8 RT doses used on these studies were in the range of 66-70 Gy. Simultaneously, multiple randomized trials were being conducted showing improved disease control using escalated RT doses, generally in the range of 78-80 Gy.9-11 As such, patients were shown to benefit from two independently tested interventions, the addition of STAD to lower RT doses and escalation of that RT dose to levels currently considered standard. Hence, once the results of the STAD trials matured, they were reported in the context of RT doses since shown to be suboptimal. Despite no demonstrable survival advantage, improvements in prostate-specific antigen (PSA) and clinical progression associated with RT dose escalation led to its acceptance, before this study's design, as a practice standard and a contention that the need for STAD could potentially be obviated by delivering higher RT doses.12-14
The NRG Oncology/Radiation Therapy Oncology Group 0815 study was designed to test the hypothesis that the addition of STAD would continue to provide an OS benefit to patients with IRPC despite intensified local treatment in the form of dose-escalated RT.
METHODS
Patients
Eligible patients had IRPC diagnosed within 6 months of enrollment defined by ≥1 of the following features: stage T2b-T2c (American Joint Committee on Cancer Staging Manual, sixth Edition); Gleason score 7; or pretreatment PSA >10 and ≤20 ng/mL. Patients harboring all three risk factors and ≥50% of biopsy cores positive were ineligible. Pelvic CT or MRI and 99Tc bone scan were mandatory for patients with multiple risk factors. Patients with equivocal bone scan findings were eligible if radiographic evidence supported the absence of metastatic disease. Patients with nodal or distant metastatic disease, Gleason score ≥8, PSA >20 ng/mL, or clinical stage ≥T3 were ineligible. Additional exclusion criteria included prior local therapy (prostatectomy, cryosurgery, or high-intensity focused ultrasound) or systemic therapy (hormonal manipulation or chemotherapy) for prostate cancer. Patients had baseline Zubrod performance status of 0-1 and no other active invasive or hematologic malignancy (other than nonmelanoma skin cancer) within 5 years of enrollment.
Patients underwent baseline assessment using the validated Adult Comorbidity Evaluation 27 (ACE-27) instrument.15 Degrees of decompensation across cardiovascular, respiratory, gastrointestinal, renal, endocrine, neurologic, psychiatric, rheumatologic, immunologic, oncologic, substance abuse, and body weight domains are graded 1-3: mild, moderate, or severe due to conditions in each category, respectively. The final score reflects the maximum grade in any category. Patients with grade 2 scores in multiple domains receive a score of 3.
Participating sites received approval from their respective institutional review boards. Informed consent was obtained from all study participants at enrollment. This clinical trial was supported by grants from the National Cancer Institute with no commercial support provided.
Study Design
Patients were stratified by number of intermediate-risk features (1 v 2-3), pre-enrollment ACE-27 score (<2 v ≥2), and type of RT planned: external-beam RT (EBRT) alone or together with low-dose-rate (LDR) or high-dose-rate (HDR) brachytherapy. Patients were randomly assigned to receive either dose-escalated RT alone or in combination with 6 months of STAD. Trial coordination, data collection, statistical support, and manuscript preparation were conducted centrally by NRG Oncology, an NCI-funded cooperative oncology group.
Protocol Treatment
Treatment initiated within 4 weeks of random assignment. EBRT alone consisted of 79.2 Gy (1.8 Gy/d) delivered to the prostate and proximal 1 cm of seminal vesicle.16 Patients with prostate volume <60 cc on transrectal ultrasound, American Urologic Association symptom score ≤15, and no history of transurethral resection of the prostate were eligible, per physician/patient preference, to receive brachytherapy in combination with EBRT. EBRT for brachytherapy patients was delivered as above to 45 Gy (1.8 Gy/d). LDR brachytherapy was completed within 4 weeks after completion of EBRT. Permitted isotopes were iodine-125 and palladium-103. Boost doses for respective isotopes were 110 Gy and 100 Gy. HDR brachytherapy boost dose was 21 Gy in two fractions of 10.5 Gy (separated by a minimum 6-hour interval), using iridium-192, in single or multiple implant procedures completed during or within one week of the initiation or completion of EBRT.
STAD patients received luteinizing hormone–releasing hormone (LHRH) agonist/antagonist plus antiandrogen for 6 months beginning 8 weeks before RT. Leuprolide, goserelin, buserelin, triptorelin, and degarelix were permitted and administered per manufacturers' instructions. Antiandrogen consisted of bicalutamide (50 mg once daily) or flutamide (250 mg three times daily) beginning within 10 days of patients' first LHRH agonist/antagonist injection.
Patient Assessments
Patients underwent history and physical examination with performance status assessment, serum PSA and testosterone levels, and required imaging within 60 days of registration. Patients completed pre-enrollment AST, ALT, and alkaline phosphatase. CBC was assessed during weeks 1 and 5 of RT delivery, and patients were seen weekly with adverse event (AE) assessment throughout RT. Follow-up consisting of history and physical (including digital rectal examination), performance status assessment, and PSA testing was completed at 3, 6, 9, and 12 months after RT, every 6 months for years 2-5, and annually thereafter. Acute and late AEs were scored using Common Terminology Criteria for Adverse Events version 4.0. Acute and late were defined as the first occurrence of worst severity occurring ≤30 or >30 days, respectively, after RT completion, measured from the date of initiation of protocol therapy.
End Points
End points were measured from the date of random assignment. The primary end point was OS. Secondary end points included PSA failure, local recurrence (LR), DMs, clinical or biochemical failure, prostate cancer–specific mortality (PCSM), non-PCSM (nPCSM), and initiation of salvage androgen-deprivation therapy (ADT). PSA failure was defined as 2 ng/mL above post-treatment nadir value.17 LR was defined as biopsy-proven failure within the prostate or seminal vesicles; DM as clinical and/or radiographic evidence of disease beyond LR; and PCSM/nPCSM as death in the presence or absence of clinically uncontrolled prostate cancer, respectively. Cause of death assignment was completed by study cochairs blinded to treatment arm with complex cases discussed among evaluating individuals.
Statistical Analysis
Prior studies led to a 5-year OS estimate of 90% for patients receiving RT alone. It was hypothesized that adding 6 months of STAD would result in absolute improvement of 3.3% (93.3%) at that time point, corresponding to a 34% relative reduction in annual death rate (hazard ratio of 0.66). Under a one-sided significance level of 0.025 and 85% power, a sample size of 1,520 patients with minimum 218 deaths was required to achieve the stated power and trigger primary end point analysis. Three planned interim analyses were performed following prespecified numbers of deaths in the two treatment arms; these occurred at 55, 109, and 163 events with corresponding P values of 0.0001, 0.0018, and 0.015, respectively, that would trigger premature study closure for efficacy. Futility analyses were also conducted. Stopping criteria were never met.
OS was estimated using the Kaplan-Meier approach,18 with the log-rank test used to compare survival rates between randomization arms.19 The Cox regression model20 was used to estimate hazard ratios, unadjusted and adjusted for covariates, along with 95% CIs for further assessment of treatment differences. Prespecified stratification variables along with age and race were used as covariates for the adjusted analysis. Secondary end points of PSA failure, LR, DM, PCSM, nPCSM, and initiation of salvage ADT were estimated using cumulative incidence curves and compared using cause-specific hazard ratios and the Fine-Gray method21 to account for death as a competing risk. For AEs, chi-square or Fisher's exact test was used to compare frequencies; cumulative incidence curves were generated and multiple logistic regression models fit to compare rates of acute and late events adjusted for covariates. Planned subgroup analyses for OS by RT type were performed, as well as post hoc subgroup analyses for OS, PSA failure, and DM. All P values reported are two-sided, including that for OS.
RESULTS
Patient Characteristics
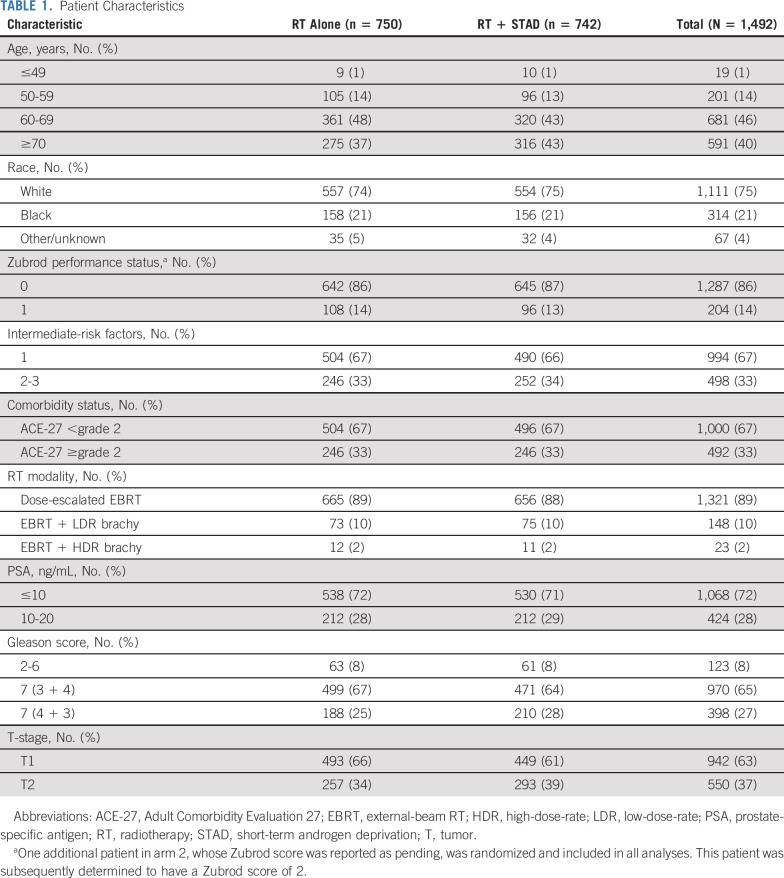
One thousand five hundred thirty-eight patients from 223 centers were randomly assigned to dose-escalated RT alone (arm 1) or with STAD (arm 2) between September 2009 and March 2016 (Fig 1). Forty-six patients found to be ineligible after random assignment were excluded, leaving 1,492 analyzed. The most common reason for exclusion was imaging/laboratory workup outside the protocol-stipulated time frame, and this was equally balanced between randomization arms. Baseline demographics and disease characteristics were well balanced (Table 1), with no significant differences between patients in arm 1 versus arm two except for T-stage (P = .039).
FIG 1.
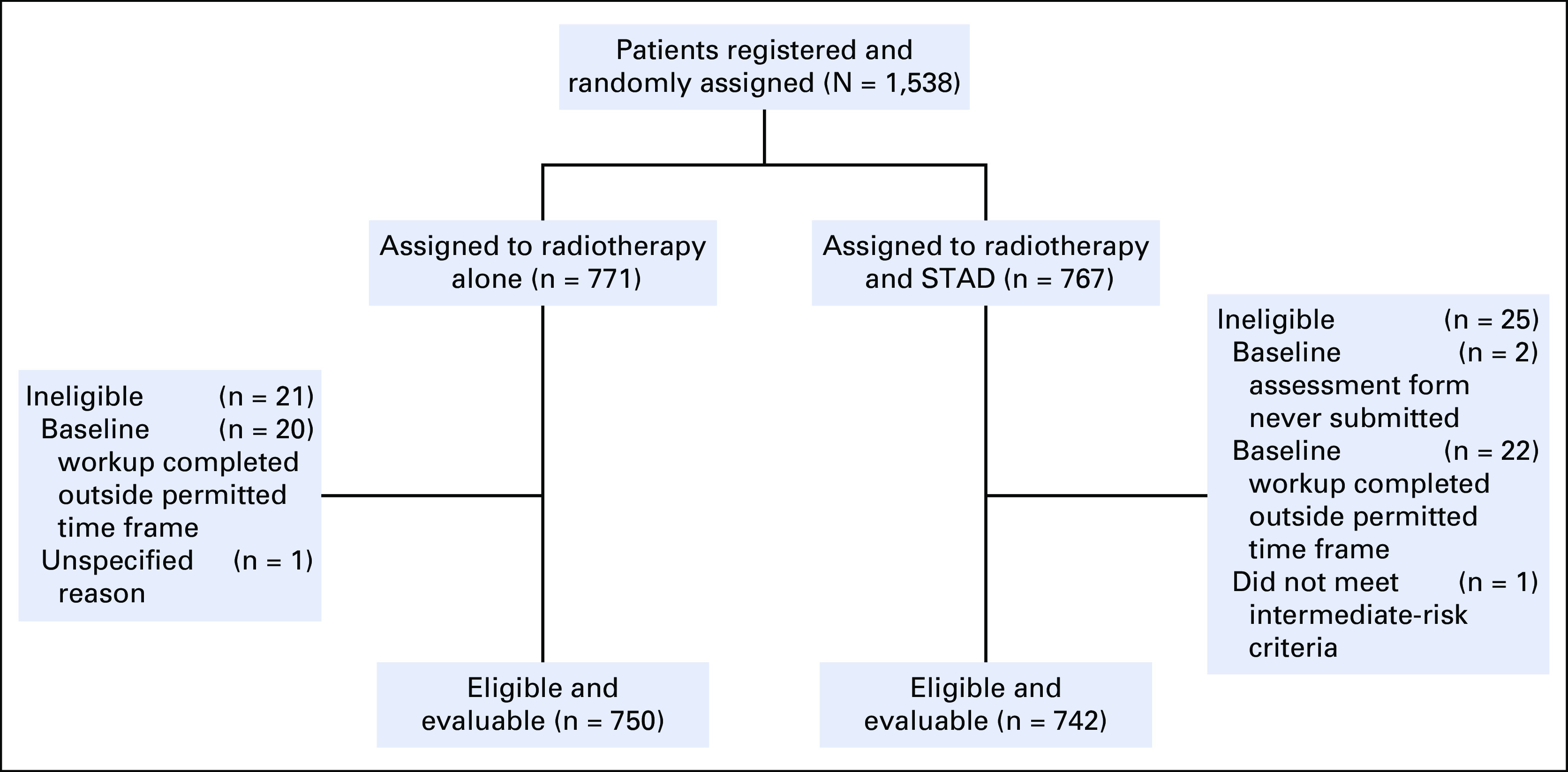
CONSORT diagram. STAD, short-term androgen deprivation.
TABLE 1.
Patient Characteristics
Protocol Compliance
More than 98% of RT plans underwent central review by principal investigators assessing anatomic target/critical structure contours and ensuring dose plans were within study parameters. More than 90% of plans were scored per protocol or variation acceptable with regard to tumor volume contours (91%), critical structure contours (91%), target dose delivery (91%), and critical structure dose constraints (93%), with no significant differences between treatment arms. A 54% random sample of patients (n = 399) in arm 2 underwent STAD therapy review. Ninety-eight percent of these patients received bicalutamide, with 79% receiving 80%-120% of protocol-specified dosing. Ninety-one percent of patients received LHRH agonist therapy per protocol (80%-120% of target dose), with 4% underdosed and 2% overdosed.
Time-to-Event Outcomes
Median follow-up for surviving patients was 6.3 years for arm 1 (range, 0.0-10.3) and 6.4 years for arm 2 (range, 0.01-10.2). Two hundred nineteen deaths occurred, just exceeding the prespecified number triggering this analysis. OS and cumulative incidence rates for PCSM, DM, PSA progression, initiation of salvage ADT, and nPCSM are shown in Figure 2. Five- and 8-year OS rates were 90% and 79% for arm 1 versus 91% and 84% for arm 2, respectively (hazard ratio [HR], 0.85; 95% CI, 0.65 to 1.11; log-rank P = .22; adjusted HR, 0.84; 95% CI, 0.65 to 1.10). Ten patients died of prostate cancer in arm 1 versus 1 patient in arm 2 (cause-specific HR, 0.10; 95% CI, 0.01 to 0.80; Fine-Gray P = .007). Five- and 8-year cumulative incidence rates of DM were 3.1% and 4.3% for arm 1 versus 0.6% and 1.0% for arm 2, respectively (cause-specific HR, 0.25; 95% CI, 0.11 to 0.57; Fine-Gray P <.001). Five- and 8-year PSA failure rates were 14% and 21% for arm 1 versus 8% and 10% for arm 2, respectively (cause-specific HR, 0.52; 95% CI, 0.39 to 0.70; Fine-Gray P <.001). Patients receiving STAD were less likely to receive salvage ADT, with 5- and 8-year rates of 6.1% and 9.8% for arm 1 and 4.2% and 5.8% for arm 2, respectively (cause-specific HR, 0.62; 95% CI, 0.41 to 0.95; Fine-Gray P = .025). There was no significant difference in nPCSM between randomization arms (HR, 0.92; 95% CI, 0.70 to 1.21; Fine-Gray P = .56). LR rates at 5 and 8 years were 2.6% and 3.9% for arm 1 versus 0.6% and 2.0% for arm 2, respectively (cause-specific HR, 0.44; 95% CI, 0.22 to 0.90, Fine-Gray P = .021). Combined rates of clinical or biochemical failure at 5 and 8 years were 14.8% and 22.5% for arm 1 versus 7.9% and 11.4% for arm 2, respectively (cause-specific HR, 0.52; 95% CI, 0.39 to 0.70; Fine-Gray P <.001).
FIG 2.
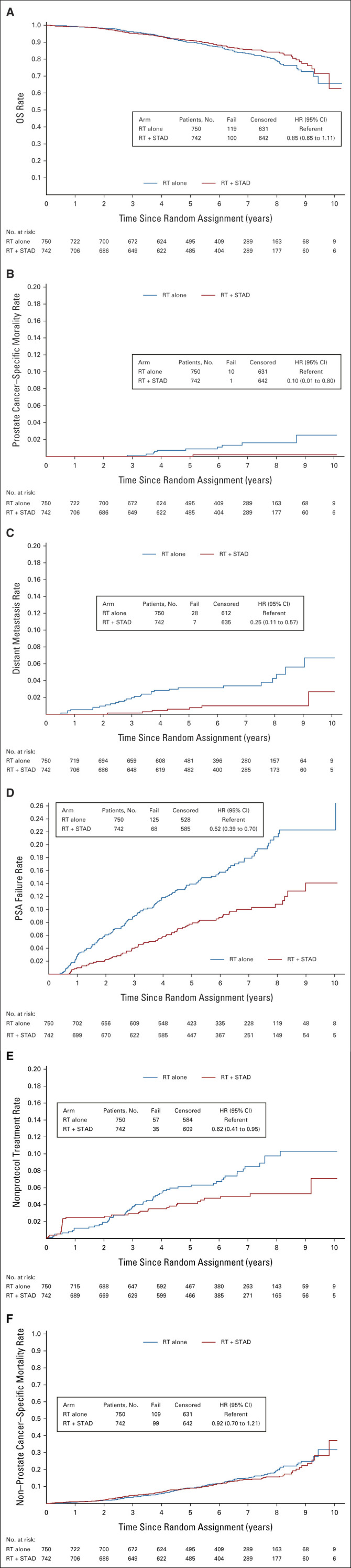
Disease control outcomes: (A) OS, (B) prostate cancer–specific mortality, (C) distant metastasis, (D) PSA progression, (E) rates of salvage ADT, and (F) non–prostate cancer–specific mortality. ADT, androgen-deprivation therapy; HR, hazard ratio; OS, overall survival; PSA, prostate-specific antigen; RT, radiotherapy; STAD, short-term androgen deprivation.
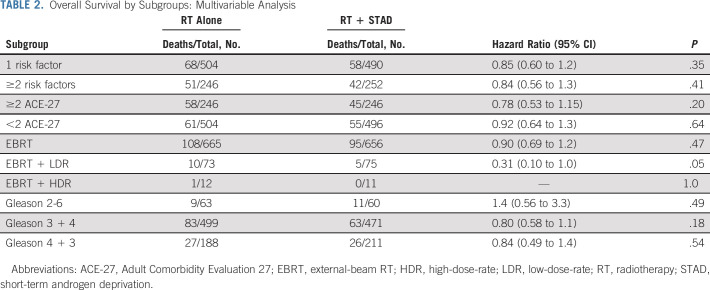
Multivariable analysis for subgroup comparisons of OS is shown in Table 2. STAD did not result in an OS advantage for patients with a single intermediate-risk factor, multiple intermediate-risk factors, ACE-27 score <2 or ≥2, or predominant Gleason pattern (Gleason score 2-6 v 7 [3 + 4] v 7 [4 + 3]). A trend toward improved OS was identified in LDR brachytherapy boost patients. With the exception of patients with Gleason score 2-6, a benefit for adding STAD was found in all subgroups for both PSA failure and DM (Fig 3). For context, 10-year PSA failure-free rates for the overall study population were 75.7% and 84.4% in RT alone and RT + STAD arms, respectively, while 10-year DM-free rates were 92.2% and 96.7%, respectively.
TABLE 2.
Overall Survival by Subgroups: Multivariable Analysis
FIG 3.
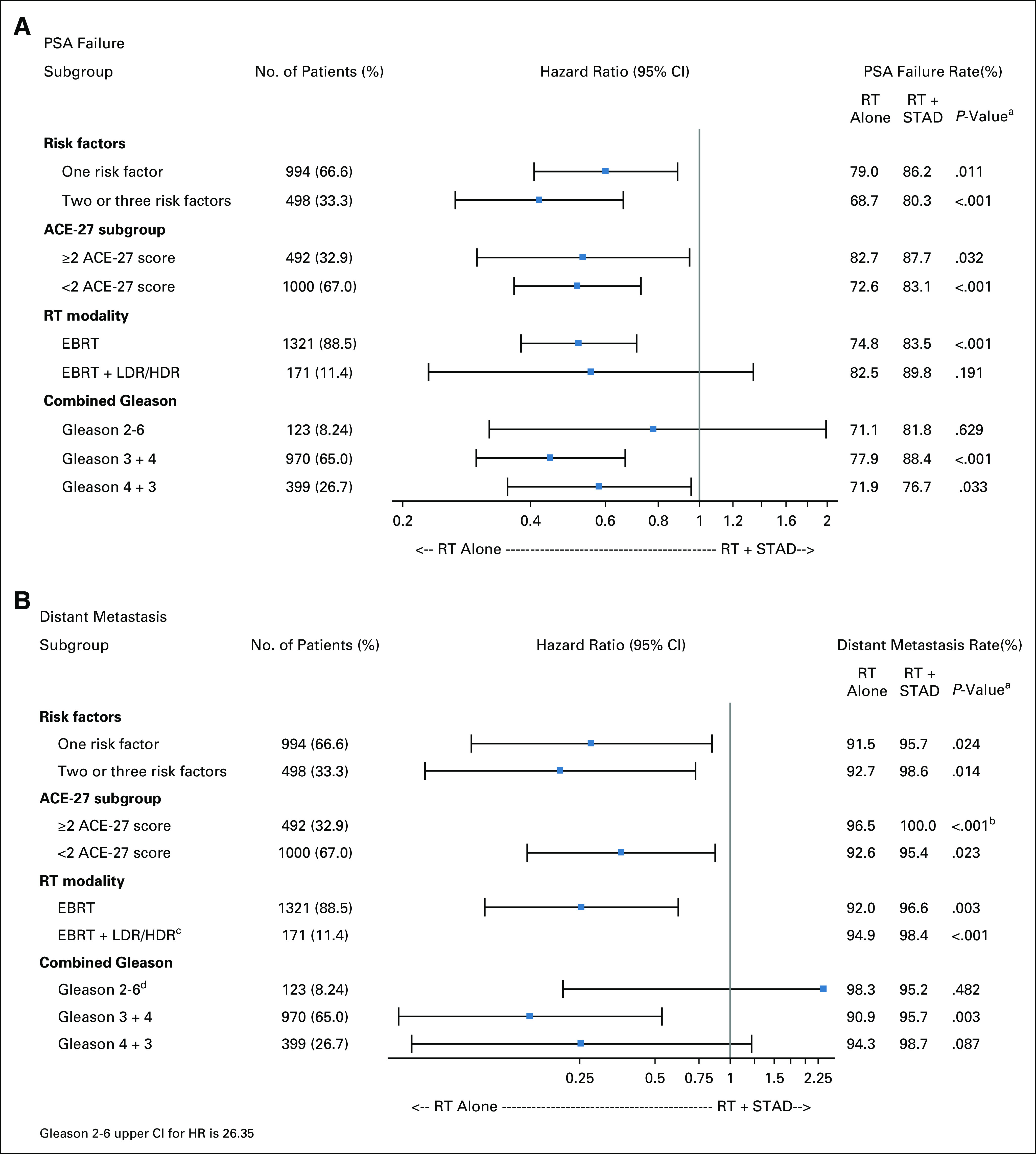
Patient subset analyses for (A) PSA failure and (B) distant metastasis. HR, hazard ratio; PSA, prostate-specific antigen; RT, radiotherapy; STAD, short-term androgen deprivation. aP value obtained from Cox regression analysis unless otherwise indicated. bP value from logrank test. cConfidence interval omitted because of too few events. dGleason 2-6 upper confidence limit for HR is 26.4.
AEs
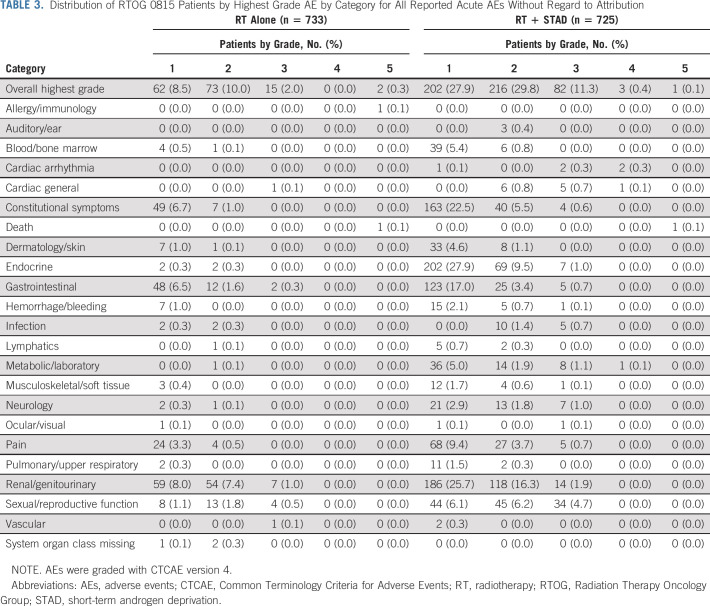
Eleven patients, nine in arm 1 and two in arm 2, never started treatment. In 23 patients, an AE form was never submitted, so these analyses are based on n = 1,458 patients. Acute AE frequencies by system organ class are shown in Table 3. The AE rate (any grade) was higher for patients receiving RT plus STAD (69%) versus those receiving dose-escalated RT alone (21%; odds ratio [OR], 8.70; 95% CI, 6.85 to 11.1; P <.001). Multivariable analysis revealed no association of patient age, number of intermediate-risk factors, RT modality, comorbidity status, or race with increased likelihood of experiencing an acute AE. Significant differences in acute AE rates were detected in domains of endocrine symptoms (38% v 0.5%), sexual/reproductive function (17% v 3.4%), constitutional symptoms (29% v 7.6%), gastrointestinal toxicity (21% v 8.5%), renal/genitourinary toxicities (44% v 16%), and metabolic/laboratory findings (8.1% v 0.1%) with all P values < 0.001. Rates of grade ≥3 acute AEs were 12% for patients receiving RT plus STAD versus 2% for those treated with dose-escalated RT alone (OR, 5.67; 95% CI, 3.30 to 10.28; P < .001). Six patients (0.8%) experienced an acute grade ≥3 general cardiac event on arm 2 compared with one (0.1%) on arm 1 (Fisher's exact P = .068).
TABLE 3.
Distribution of RTOG 0815 Patients by Highest Grade AE by Category for All Reported Acute AEs Without Regard to Attribution
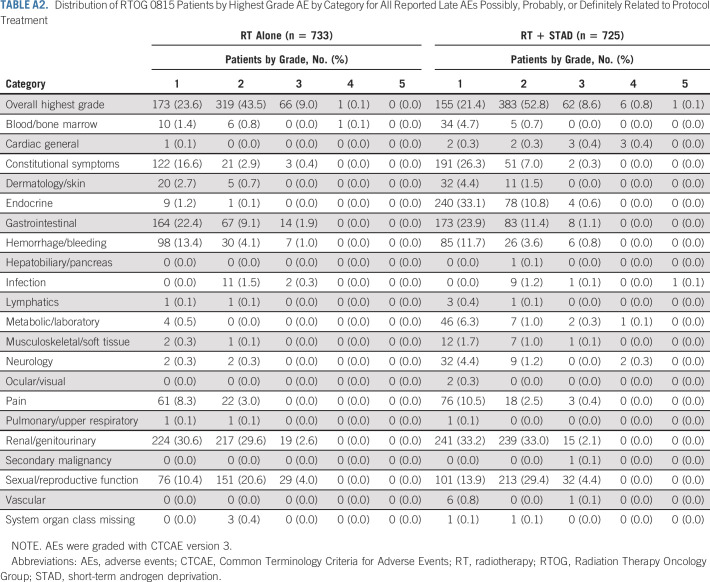
Late AEs (>30 days after completion of RT) by system organ class are tabulated in Table 4. Overall late AE rate was significantly higher in arm 2 (84% in arm 2 v 79% in arm 1; OR, 1.42; 95% CI, 1.07 to 1.87; P = .011). Arm 2 experienced significantly increased rates of late endocrine events (45% v 3.3%; P <.001, 98% grade 1 or 2) and neurologic toxicity (12% v 5.3%; P <.001, 89% grade 1 or 2). In arm 2, 103 (14%), 225 (31%), and 36 patients (5.0%) had grade 1, grade 2, or grade 3 sexual/reproductive symptoms, respectively, compared with 79 (11%), 164 (22%), and 35 (4.8%) in arm 1 (P <.001). Overall late grade 3 or higher AE rate was not affected by randomization arm (15% in arm 2 v 14% in arm 1; OR, 1.17; 95% CI, 0.87 to 1.57; P = .29). Twenty patients (2.8%) experienced late grade ≥3 general cardiac AEs on arm 2 compared with 9 (1.2%) on arm 1 (P = .036). There were no significant differences in late gastrointestinal or genitourinary AEs between treatment arms. Acute and late AEs deemed at least possibly related to study treatment are tabulated in Appendix Tables A1 and A2, online only, respectively.
TABLE 4.
Distribution of RTOG 0815 Patients by Highest Grade AE by Category for All Reported Late AEs Without Regard to Attribution
DISCUSSION
To our knowledge, this study represents the first multi-institutional, randomized clinical trial to directly examine the survival impact of adding STAD to dose-escalated radiation therapy for patients with IRPC. The risk stratification for patient eligibility remains consistent with current IRPC classification. The RT used, from a treatment intensification standpoint, remains consistent with current standard radiation oncology practice. Although shorter EBRT regimens in the range of 4-6 weeks are now used, clinical trials have demonstrated no difference in disease control rates between the two approaches.22,23 Dose escalation using brachytherapy was permitted on the study, per physician discretion, and its impact on treatment outcomes was considered as a stratification variable; however, no conclusions could be drawn regarding STAD impact on the basis of RT modality.
This protocol failed to demonstrate a significant impact on OS for the addition of STAD to RT for patients with IRPC. This analysis was conducted following a prespecified number of mortality events detailed in the statistical design of the study, and it remains unclear whether or not with additional follow-up a survival advantage will become appreciable. This was possibly the result of an overestimation of the survival impact of STAD in the study design and is potentially a significant limitation. Significant reductions in PSA progression, DM, and PCSM were demonstrated for patients randomly assigned to receive STAD, translating into reduced need for salvage ADT. And despite a slight increase in the cardiac AE rate, there was no difference in nPCSM in patients receiving STAD.
Increased acute AE rates in patients receiving STAD was driven largely by grade 1-2 events (83% of all acute AEs in this cohort) and was predominantly associated with anticipated side effects of testosterone suppression. Despite similar rates of late severe AEs, differences in the domains of endocrine, neurologic, and sexual function did persist, as well as a small increased risk of cardiac events. Many of these late AEs, especially in the endocrine domain, were likely driven by their protocol-specified definition of occurrence just 30 days after RT completion. Further details of long-term impacts of STAD are addressed in a separate report of prospectively collected, patient-reported quality-of-life outcomes.
Historical trials demonstrating clinical benefits of STAD in conjunction with RT have predominantly used doses in the range of 66-70 Gy (approximately 12%-17% less than those used in the current protocol) and have shown reduced mortality for patients getting STAD.3,5-8 Additionally, multiple randomized studies have shown clear benefits with escalation of RT doses to 78-80 Gy, consistent biologically with those used in the current study.9-11 RT dose escalation has been shown to result in reduction of both clinical and PSA progression of disease, as well as in reduced need for salvage therapies, but has not been associated with OS improvements. Herein, STAD combined with dose-escalated RT has not resulted in OS improvement as it did in studies using lower RT doses.
Although there remains no evidence that STAD produces an OS benefit when combined with dose-escalated RT, it could be argued that risks associated with STAD remain justified to achieve reduced rates of DM and salvage therapy use. However, the relatively small absolute benefits in these areas could certainly be used as counterpoints. The data derived from this protocol, along with its companion patient-reported quality-of-life analysis by Movsas et al30 will allow clinicians to far more accurately counsel patients with IRPC on the risks/benefits of adding STAD to RT. Patients will be able to make more informed choices on the basis of quantified clinical advantages weighed against prospectively collected, patient-reported quality-of-life impacts.
No prespecified patient subgroup benefitted from STAD in terms of OS. Conversely, with the exception of the small subset of patients in this study with a Gleason score <7, we failed to detect any category of patients who did not benefit from adding STAD in terms of PSA failure or DM rates. Hazard ratios for these events were remarkably similar regardless of number of intermediate-risk features, ACE-27 score, or RT modality. Unplanned subset analysis compared outcomes of Gleason score 7 patients with 3 + 4 versus 4 + 3 growth patterns to better fit a characterization of unfavorable versus favorable IRPC that has been applied more recently,24,25 and patients benefitted similarly from STAD regardless of the predominant histologic growth pattern being 3 or 4.
Ongoing research is looking to identify subsets of patients with IRPC most likely to have their metastatic disease risk reduced by adding STAD and which may be spared the associated toxicity. Genomic biomarker profiles have been shown to prognostically outperform classic clinicopathologic risk factors, albeit retrospectively,26,27 and are being investigated in the next generation of RT clinical trials to better select patients for adjuvant systemic therapies. Additionally, imaging modalities such as multiparametric MRI, which can optimize the accurate staging of primary disease,28 and PET imaging with prostate-specific tracers such as prostate-specific membrane antigen (PSMA)29 hold tremendous potential to more optimally select patients with IRPC likely to benefit from adjuvant STAD. These diagnostic tools were not routinely used at the time this study was initiated and may limit the applicability of these data to patients staged with these modalities.
In conclusion, STAD added to dose-escalated RT did not improve rates of OS for men with IRPC compared with patients treated with dose-escalated RT alone. Reductions in PSA failure and DMs should be weighed against the toxicity added by STAD and its overall impact on patients' quality of life.
APPENDIX
TABLE A1.
Distribution of RTOG 0815 Patients by Highest Grade AE by Category for All Reported Acute AEs Possibly, Probably, or Definitely Related to Protocol Treatment
TABLE A2.
Distribution of RTOG 0815 Patients by Highest Grade AE by Category for All Reported Late AEs Possibly, Probably, or Definitely Related to Protocol Treatment
Gerard Morton
Employment: Odette Cancer Centre—Sunnybrook Hospital
Honoraria: Elekta
Deborah Watkins Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmont Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
Benjamin Movsas
Research Funding: Varian Medical Systems (Inst), Philips Healthcare (Inst), ViewRay (Inst)
Patents, Royalties, Other Intellectual Property: Lung phantom for image guidance, MR-CT imaging-related patent for radiation oncology
Travel, Accommodations, Expenses: Varian Medical Systems, viewray, Alpha Tau
Deborah Citrin
Employment: Mid-Atlantic Permanente Medical Group
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Jason Alexander Efstathiou
Consulting or Advisory Role: Blue Earth Diagnostics, AstraZeneca, Boston Scientific, Merck, Janssen, Genentech, Bayer, Progenics, Pfizer, Gilead Sciences, Myovant Sciences, Lantheus Medical Imaging, Blue Earth Diagnostics
Vivek S. Kavadi
Employment: US Oncology Network
Stock and Other Ownership Interests: McKesson/US Oncology Network
Fabio L. Cury
Consulting or Advisory Role: Knight Pharmaceuticals, Sanofi/Aventis
Speakers' Bureau: Varian Medical Systems
Research Funding: Boston Scientific (Inst), Tolmar (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Michael Lock
Stock and Other Ownership Interests: Myovant Sciences
Consulting or Advisory Role: Sanofi, Tersera
Speakers' Bureau: Ferring, AbbVie, Eisai
Adam Raben
Honoraria: Bristol Myers Squibb
Speakers' Bureau: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at ASTRO 2021, Chicago, IL, October 24-27, 2021.
SUPPORT
This trial was supported by the following grants from the National Cancer Institute: UG1CA189867 to the NCI Community Oncology Research Program; U10CA180868 to NRG Oncology operations; U24CA180803 to the Imaging and Radiation Oncology Core (IROC); and U10CA180822 to the Statistical and Data Management Center.
CLINICAL TRIAL INFORMATION
NCT00936390 (RTOG 0815)
DATA SHARING STATEMENT
According to National Cancer Institute (NCI) requirements, the data from this article will be submitted to the NCI National Clinical Trials Network (NCTN), NCI Community Oncology Research Program (NCORP) data archive (https://nctn-data-archive.nci.nih.gov) no later than 6 months after publication. After the required NCI reviews are completed, it will be released and available in the data archive for data-sharing proposals. The study protocol is available on the ClinicalTrials.gov website.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel J. Krauss, Alvaro A. Martinez, Gerard Morton, Di Yan, Deborah Watkins Bruner, Mohamed Elshaikh, Deborah Citrin, Jeff M. Michalski, Howard M. Sandler
Administrative support: Jeff M. Michalski
Provision of study materials or patients: Gerard Morton, Di Yan, Bruce Hershatter, Jeff M. Michalski, Jason Alexander Efstathiou, Vivek S. Kavadi, Fabio L. Cury, Michael Lock, Adam Raben, Samantha Andrews Seaward, Ali El-Gayed
Collection and assembly of data: Daniel J. Krauss, Gerard Morton, Di Yan, Deborah Watkins Bruner, Deborah Citrin, Bruce Hershatter, Adam Currey, Fabio L. Cury, Michael Lock, Adam Raben, Samantha Andrews Seaward, Ali El-Gayed, Joseph P. Rodgers
Data analysis and interpretation: Daniel J. Krauss, Theodore Karrison, Alvaro A. Martinez, Gerard Morton, Benjamin Movsas, Deborah Citrin, Jeff M. Michalski, Jason Alexander Efstathiou, Adam Currey, Vivek S. Kavadi, Fabio L. Cury, Michael Lock, Adam Raben, Joseph P. Rodgers
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Gerard Morton
Employment: Odette Cancer Centre—Sunnybrook Hospital
Honoraria: Elekta
Deborah Watkins Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmont Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
Benjamin Movsas
Research Funding: Varian Medical Systems (Inst), Philips Healthcare (Inst), ViewRay (Inst)
Patents, Royalties, Other Intellectual Property: Lung phantom for image guidance, MR-CT imaging-related patent for radiation oncology
Travel, Accommodations, Expenses: Varian Medical Systems, viewray, Alpha Tau
Deborah Citrin
Employment: Mid-Atlantic Permanente Medical Group
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Jason Alexander Efstathiou
Consulting or Advisory Role: Blue Earth Diagnostics, AstraZeneca, Boston Scientific, Merck, Janssen, Genentech, Bayer, Progenics, Pfizer, Gilead Sciences, Myovant Sciences, Lantheus Medical Imaging, Blue Earth Diagnostics
Vivek S. Kavadi
Employment: US Oncology Network
Stock and Other Ownership Interests: McKesson/US Oncology Network
Fabio L. Cury
Consulting or Advisory Role: Knight Pharmaceuticals, Sanofi/Aventis
Speakers' Bureau: Varian Medical Systems
Research Funding: Boston Scientific (Inst), Tolmar (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Michael Lock
Stock and Other Ownership Interests: Myovant Sciences
Consulting or Advisory Role: Sanofi, Tersera
Speakers' Bureau: Ferring, AbbVie, Eisai
Adam Raben
Honoraria: Bristol Myers Squibb
Speakers' Bureau: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gandaglia G, Leni R, Bray F, et al. : Epidemiology and prevention of prostate cancer. Eur Urol Oncol 4:877-892, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Burt LM, Shrieve DC, Tward JD: Factors influencing prostate cancer patterns of care: An analysis of treatment variation using the SEER database. Adv Radiat Oncol 3:170-180, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CU, Hunt D, McGowan DG, et al. : Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365:107-118, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Pisansky TM, Hunt D, Gomella LG, et al. : Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation Therapy Oncology Group randomized clinical trial 9910. J Clin Oncol 33:332-339, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilepich MV, Winter K, John MJ, et al. : Phase III Radiation Therapy Oncology Group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 50:1243-1252, 2001 [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Manola J, Loffredo M, et al. : 6-month androgen suppression plus radiation therapy vs. radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 292:821-827, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bolla M, Maingon P, Carrie C, et al. : Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol 34:1748-1756, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Denham JW, Steigler A, Lamb DS, et al. : Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451-459, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Michalski JM, Moughan J, Purdy J, et al. : Effect of standard vs. dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: The NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol 4:e180039, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasalic D, Kuban DA, Allen PK, et al. : Dose escalation for prostate adenocarcinoma: A long-term update on the outcomes of a phase 3, single institution randomized clinical trial. Int J Radiat Oncol Biol Phys 104:790-797, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zietman AL, DeSilvio ML, Slater JD, et al. : Comparison of conventional-dose vs. high-dose conformal radiation therapy in clinically localized carcinoma of the prostate: A randomized controlled trial. JAMA 294:1233-1239, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Krauss D, Kestin L, Ye H, et al. : Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys 80:1064-1071, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Merrick GS, Butler WM, Wallner KE, et al. : Androgen deprivation therapy does not impact cause-specific or overall survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 65:669-677, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Beyer DC, McKeough T, Thomas T: Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 61:1299-1305, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Piccirillo JF, Tierney RM, Costas I, et al. : Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 291:2441-2447, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kestin LL, Goldstein NS, Vicini FA, et al. : Treatment of prostate cancer with radiotherapy: Should the entire seminal vesicles be included in the clinical target volume? Int J Radiat Oncol Biol Phys 54:686-697, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Roach M, Hanks G, Thames H, et al. : Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65:965-974, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 19.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 20.Cox DR: Regression models and life tables (with discussion). J R Stat Soc Series B Stat Methodol 34:187-220, 1972 [Google Scholar]
- 21.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 22.Lee WR, Dignam JJ, Amin MB, et al. : Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol 34:2325-2332, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dearnaley D, Syndikus I, Mossop H, et al. : Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 17:1047-1060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumsteg ZS, Spratt DE, Daskivich TJ, et al. : Effect of androgen deprivation on long-term outcomes of intermediate-risk prostate cancer stratified as favorable or unfavorable. JAMA Netw Open 3:e2015083, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumsteg ZS, Spratt DE, Pei I, et al. : A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external beam radiation therapy. Eur Urol 64:895-902, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Feng FY, Huang HC, Spratt DE, et al. : Validation of a 22-gene genomic classifier in patients with recurrent prostate cancer: An ancillary study of the NRG/RTOG 9601 randomized clinical trial. JAMA Oncol 7:544-552, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen PL, Haddad Z, Ross AE, et al. : Ability of a genomic classifier to predict metastasis and prostate cancer-specific mortality after radiation or surgery based on needle biopsy specimens. Eur Urol 72:845-852, 2017 [DOI] [PubMed] [Google Scholar]
- 28.O’Connor LP, Lebastchi AH, Horuz R, et al. : Role of multiparametric prostate MRI in the management of prostate cancer. World J Urol 39:651-659, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Hofman MS, Lawrentschuk N, Francis RJ, et al. : Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 395:1208-1216, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Movsas B, Rodgers JP, Elshaikh MA, et al. : Dose-escalated radiation alone or in combination with short-term total androgen suppression for intermediate-risk prostate cancer: Patient-reported outcomes from NRG/Radiation Therapy Oncology Group 0815 randomized trial. J Clin Oncol 41:3217-3224, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
According to National Cancer Institute (NCI) requirements, the data from this article will be submitted to the NCI National Clinical Trials Network (NCTN), NCI Community Oncology Research Program (NCORP) data archive (https://nctn-data-archive.nci.nih.gov) no later than 6 months after publication. After the required NCI reviews are completed, it will be released and available in the data archive for data-sharing proposals. The study protocol is available on the ClinicalTrials.gov website.