Abstract
Twenty well-characterized isolates of methicillin-resistant Staphylococcus aureus were used to study the optimal resolution and interlaboratory reproducibility of pulsed-field gel electrophoresis (PFGE) of DNA macrorestriction fragments. Five identical isolates (one PFGE type), 5 isolates that produced related PFGE subtypes, and 10 isolates with unique PFGE patterns were analyzed blindly in 12 different laboratories by in-house protocols. In several laboratories a standardized PFGE protocol with a commercial kit was applied successfully as well. Eight of the centers correctly identified the genetic homogeneity of the identical isolates by both the in-house and standard protocols. Four of 12 laboratories failed to produce interpretable data by the standardized protocol, due to technical problems (primarily plug preparation). With the five related isolates, five of eight participants identified the same subtype interrelationships with both in-house and standard protocols. However, two participants identified multiple strain types in this group or classified some of the isolates as unrelated isolates rather than as subtypes. The remaining laboratory failed to distinguish differences between some of the related isolates by utilizing both the in-house and standardized protocols. There were large differences in the relative genome lengths of the isolates as calculated on the basis of the gel pictures. By visual inspection, the numbers of restriction fragments and overall banding pattern similarity in the three groups of isolates showed interlaboratory concordance, but centralized computer analysis of data from four laboratories yielded percent similarity values of only 85% for the group of identical isolates. The differences between the data sets obtained with in-house and standardized protocols could be the experimental parameters which differed with respect to the brand of equipment used, imaging software, running time (20 to 48 h), and pulsing conditions. In conclusion, it appears that the standardization of PFGE depends on controlling a variety of experimental intricacies, as is the case with other bacterial typing procedures.
The use of electric field pulsing techniques in conjunction with agarose gel electrophoresis for discrimination of large DNA molecules was introduced by Schwarz and Cantor in 1984 (9). During the past decade the methodology has been adapted and improved by various research groups to the point that pulsed-field gel electrophoresis (PFGE) for bacterial strain typing is now utilized with relative ease in a variety of laboratories (1). The combination of contour-clamped homogeneous field electrophoresis and PFGE for the molecular analysis of Staphylococcus aureus has been reported since the late 1980s (7, 19). At present, PFGE is considered to have both the reproducibility and resolving power of a standard technique for the epidemiological typing of bacterial isolates (10, 15).
Molecular typing systems can identify different strains within a species, generating data useful for taxonomic or epidemiologic purposes (10, 14). A frequently observed shortcoming of typing systems in general is their lack of reproducibility: most typing systems do not provide a definitive strain identification, which is usually due to the variability of the technique and the lack of large databases containing fragment patterns from a wide variety of organisms to which unknowns can be compared. These problems were recently described in detail for two molecular typing systems. A multicenter study on random amplification of polymorphic DNA for discrimination of S. aureus strains revealed a lack of interlaboratory reproducibility among the banding patterns generated by the participating centers, although the epidemiological interpretation of the data was similar for all the centers involved (16). For PFGE, a similar lack of interlaboratory reproducibility of patterns was observed, although the interpretation of the experimental data also differed per participating center (2). The latter study analyzed 12 different methicillin-resistant S. aureus (MRSA) strains with different techniques optimized in each center and different sources and types of equipment. Since interlaboratory discrepancies with respect to classification of the strains were observed, the study concluded that there is a clear need for standardization of the technique, including the construction of a panel of reference strains to assist the individual researcher in the optimization of the PFGE protocol.
The aim of the present study was to compare the fragment patterns of a well-defined collection of MRSA isolates in 12 laboratories using in-house and a standard set of PFGE parameters to determine whether standardization of experimental parameters (DNA preparation and switching protocols) would improve intercenter reproducibility of PFGE analysis.
MATERIALS AND METHODS
Study design.
Twenty isolates of MRSA were selected for analysis by Wolfgang Witte (Wernigerode, Germany). The collection was composed of 10 genetically unrelated isolates, 5 isolates exhibiting similar but not identical PFGE fingerprints, and 5 isolates with indistinguishable PFGE patterns. The original S. aureus NCTC 8325 was provided by Richard Goering (Omaha, Neb.). This strain served as a source for molecular size standards together with concatameric lambda DNA molecules. Isolates were stored in the coordinating center (EMCR, MM&ID, Rotterdam, The Netherlands), and cultures were coded and distributed in agar stabs to the participating centers. Prior to PFGE analysis, isolates were cultured on blood agar plates at least once, and single colonies were used for further testing.
PFGE with the restriction enzyme SmaI was performed in duplicate on DNA from all isolates in all centers with the equipment available in the individual laboratories. The lambda concatamers were to be run in every sixth lane (i.e., five isolates included between the two sets of markers), although not all laboratories complied with this aspect of the protocol. DNA preparation was performed according to the in-house protocols of each laboratory. In addition, all participants analyzed the isolates by using a recently developed, commercially available PFGE kit (Genepath; Bio-Rad, Veenendaal, The Netherlands) (GP), which contained all ingredients for both plug and gel preparation. Each laboratory performed PFGE by using their in-house switching protocol and PFGE equipment as summarized in Table 1. PFGE of DNA prepared by the commercial kit was performed as follows: initial switching time, 5.3 s; final switching time, 34.9 s; run time, 20 h; 6 V/cm; 120° angle; 14°C. All gels were stained with ethidium bromide and photographed with a Polaroid or charge-coupled device camera. The digital images were set to a resolution of >500 pixels from well to bottom of the gel. For the purpose of additional comparison, bacteriophage-typing and antimicrobial susceptibility testing were performed for all isolates of the test panel. The isolates were typed by arbitrary primed PCR (AP-PCR), binary typing, and target 916–Shine-Dalgarno PCR (tar 916-shida PCR) as described previously (3, 16, 18).
TABLE 1.
Evaluation of the different experimental parameters as applied in the different participating laboratories
| Center identification | Electrophoresis supply | Gels per year | Imaging software | Run time (h) | Voltage (V/cm) | Pulsing protocol | Temperature (°C) | Gel percentage |
|---|---|---|---|---|---|---|---|---|
| 1 | CHEF DR-II | 200 | Bio Image Advanced Q. 2.01 | 20 | 6 | 20 h: 5.3–34.9" | 14 | 1.0 |
| 2 | CHEF DR-II | 40–50 | GelCompar 3.1 | 25 | 6 | 10 h: 5.0–15.0"; 15 h: 15.0–45.0" | 14 | 1.0 |
| 3 | CHEF DR-III | 35 | In-house software | 26 | 6 | 7 h: 5.0–15.0"; 19 h: 15–60" | 14 | 1.0 |
| 4 | CHEF DR-II | 500 | GelCompar 4.0 | 48 | 6 | 48 h: 1.0–80.0" | 12 | 1.2 |
| 5 | CHEF DR-II | 1000 | Taxotron | 20 | 6 | 10 h: 10.0"; 10 h: 25.0" | 10 | 0.8 |
| 6 | Pulsaphor TM | 15 | In-house software | 27 | 235c | 4 h: 5.0"; 6 h: 15.0"; 8 h: 25.0"; 9 h: 35.0" | 12 | 1.2 |
| 7 | Gene Navigator | 100 | Taxotron | 24 | 7 | 24 h: 20–80" | 10 | 1.0 |
| 8 | CHEF Mapper | 40 | GelCompar 4.0 | 20 | 6 | 10 h: 5.0–15.0"; 10 h: 15.0–45.0" | 14 | 1.0 |
| 9 | CHEF DR-II | 150–200 | Molecular Analyst | 20 | 6 | 20 h: 1.0–40.0" | 12 | 1.0 |
| 10 | CHEF DR-II | 100 | Molecular Analyst | 22 | 6 | 22 h: 1.0–34.0" | 14 | 0.8 |
| 11 | CHEF DR-III | 200 | Biogene 6.32 | 21 | 6 | 2 h: 0.5–5.5"; 18 h: 7.5 to 27.5"; 1 h: 50–65" | 14 | 1.0 |
| 12 | CHEF DR-II | 150 | Molecular Analyst | 20 | 6 | 3 h: 1–10"; 17 h: 5–40" | 13 | 1.4 |
| 13a | Optional | NAb | Optional | 20 | 6 | 20 h: 5.3–34.9" | 14 | 1 |
The parameters for center 13 show the settings for the standardized protocol. Exact size of the gel not reported.
NA, not applicable.
The exact size of the gel was not reported, so the result is given as overall voltage.
Local data analysis.
All Polaroid pictures or digital images were interpreted by the individual researchers. Interpretation was performed on the basis of guidelines for interpreting banding pattern differences (10, 15). Specific types were identified by capital letters, with subtypes identified by additional numbers. Each center analyzed both gels by using its own software package to calculate Dice coefficients and to generate a dendrogram by UPGMA (unweighted pair group method using arithmetic averages) clustering. The in-house data and the data generated by using the GP kit were interpreted separately, and the agreement between the two methods was assessed in each center (number of fragments per strain and classification into types and subtypes based on the overall number of band differences). For all isolates, the genome size was calculated based on the cumulative sizes of the restriction fragments observed.
Centralized data analysis.
All pictures and accompanying interpretations were sent to a second center for interlaboratory comparison. The tiff images were imported into Bio Image Advanced Quantifier 1-D Match (AQ) version 2.5 and normalized by using the lambda concatameric standards on each gel. To evaluate center-to-center reproducibility, lanes from each normalized image were compared by using Dice coefficients and a UPGMA-derived dendrogram. For intergel Advanced Quantifier analysis, the same lambda standards were used. One of the gels from the five laboratories that sent data for centralized analysis did not include lambda standards and could not be matched to the other gels. Another laboratory sent only the in-house image for analysis. Therefore, only four in-house gels and three gels prepared with the commercial kits were analyzed with computer-aided technology.
RESULTS
General remarks.
Twelve laboratories in nine countries participated in this study. Although the goal was to compare a standardized PFGE protocol to in-house PFGE protocols, complete data sets on the 20 test isolates were not achieved by all laboratories. Most of the centers found it necessary to modify the commercially standardized GP protocol. In one instance, the restriction enzyme was inactive and had to be replaced by that of another manufacturer. However, most of the problems centered around the deterioration of the agarose plugs during overnight proteinase K treatment at 56°C. The in-house protocols, which proved to be more effective for typing the isolates in most centers, are presented in Table 1. Finally, there was a set order of the strains which did help the reproducibility of data interpretation.
Local analysis.
Table 2 shows the visual interpretation of isolate interrelationships based on the PFGE gels generated by both the standardized and in-house protocols. Four centers (4, 5, 6, and 12) were not able to generate typing data by using the standardized protocol. Of the eight laboratories that did report standardized data, five (1, 2, 8, 9, and 11) correctly categorized all 20 isolates into the indistinguishable, related, and unrelated groupings. Laboratories 3, 7, and 10 correctly classified the indistinguishable and unrelated isolates but misclassified some of the related subtypes.
TABLE 2.
Evaluation of the experimental data obtained by PFGE of MRSA: survey of visual data interpretationa
| MRSA strain | Center 1
|
Center 2
|
Center 3
|
Center 4
|
Center 5
|
Center 6
|
Center 7
|
Center 8
|
Center 9
|
Center 10
|
Center 11
|
Center 12
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | IH | GP | |
| 1 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||||
| 2 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||||
| 3 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||||
| 4 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||||
| 5 | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | A | ||||
| 6 | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | B | ||||
| 7 | B1 | B1 | B1 | B1 | B1 | C | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | B1 | ||||
| 8 | B2 | B2 | B2 | B2 | B2 | D | B2 | B2 | B2 | B2 | B2 | B2 | B2 | B2 | B2 | C | C | B2 | B2 | B2 | ||||
| 9 | B3 | B3 | B3 | B3 | B3 | C1 | B3 | B3 | B3 | B3 | B3 | B3 | B3 | B3 | B3 | D | D | B3 | B2 | B3 | ||||
| 10 | B4 | B4 | B4 | B4 | B4 | C2 | B3 | B4 | B4 | B3 | B3 | B4 | B4 | B4 | B4 | D1 | D1 | B4 | B3 | B4 | ||||
| 11 | C | C | C | C | C | E | C | C | C | C | C | C | C | C | C | E | E | C | C | C | ||||
| 12 | D | D | D | D | D | F | D | D | D | D | D | D | D | D | D | F | F | D | D | |||||
| 13 | E | E | E | E | E | G | E | E | E | E | E | E | E | E | E | G | G | E | E | B3 | ||||
| 14 | F | F | F | F | F | H | F | F | F | F | F | F | F | F | F | H | H | F | F | D | ||||
| 15 | G | G | G | G | G | I | G | G | G | G | G | G | G | G | G | I | I | G | G | E | ||||
| 16 | H | H | H | H | H | J | H | H | H | H | H | H | H | H | H | J | J | H | H | B | ||||
| 17 | I | I | I | I | I | K | I | I | I | I | I | I | I | I | I | K | K | I | I | F | ||||
| 18 | J | J | J | J | J | L | J | J | J | J | J | J | J | J | J | L | L | J | J | G | ||||
| 19 | K | K | K | K | K | M | K | K | K | K | K | K | K | K | K | M | M | K | K | G | ||||
| 20 | L | L | L | L | L | N | L | L | L | L | L | L | L | L | L | N | N | L | L | |||||
Observations differing from the experimental gold standard are highlighted in bold lettering. IH, in-house protocol. The following centers modified the commercial protocol as follows: center 1, other source for SmaI; center 4, use of own plugs and prolonged electrophoresis time; center 8, use of homemade plugs. For centers 4, 5, 6, and 12, no useful GP data could be generated.
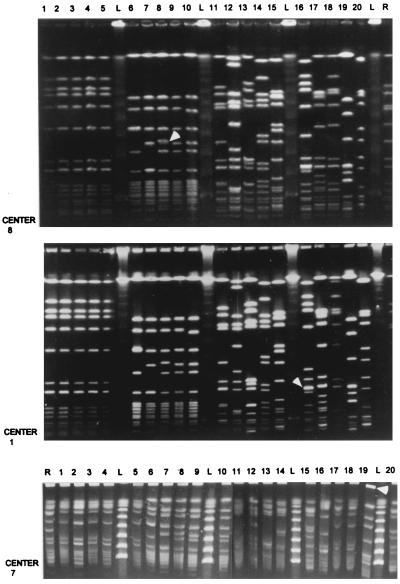
Despite widely differing in-house PFGE protocols and variable quality of the gel pictures (Fig. 1), all of the laboratories were capable of correctly discriminating the five identical isolates (Fig. 1, lanes 1 to 5) from the unrelated isolates (Fig. 1, lanes 11 to 20) (Table 2). Two of 12 laboratories (centers 4 and 7) were unable to discriminate two of the related isolates (MRSA 9 and 10), which were identified as indistinguishable in these laboratories but were considered related subtypes in the other 10 laboratories. This may have been the result of using a pulsing time of up to 80 s or, more likely, tolerance settings applied during the analysis of the data. One laboratory (center 10) identified three types and two subtypes among the related isolates (lanes 5 to 10) by using both the in-house and the standard protocol.
FIG. 1.
Comparative analysis of gel pictures obtained after PFGE of DNA macrorestriction fragments derived from the panel of 20 MRSA isolates. From top to bottom the experimental outputs of participating centers 8, 1, and 7 are shown. White arrowheads in the two top panels highlight potential fragment doublets. Note that these two pictures clearly overlap with respect to resolution and number of DNA fragments. The arrowhead in the lower panel identifies a floating plug. Although the lower panel shows a lesser degree of band resolution, it has to be emphasized that pattern identification obtained from this gel picture was as expected except for patterns belonging to isolates 9 and 10. However, the quality of the PFGE profiles shown in the lower panel is markedly inferior to those in the upper two panels. Numbering above the lanes corresponds with strain numbers, L identifies the lambda concatamers and N indicates the macrorestriction pattern generated for the S. aureus NCTC 8325 reference strain.
Table 3 shows the molecular size values as deduced from the experimental data with concatameric (48.5 kbp)n lambda DNA and/or S. aureus NCTC 8325 DNA macrorestriction fragments as size standards. In general, the spread in the molecular sizes was quite large among centers, and in some instances, the values for the identical isolates (1 to 5) differed even within centers for both the in-house and standard protocols. The average values as calculated and shown in Table 3 demonstrate that the GP procedure indicates smaller genome sizes than those calculated on the basis of results obtained with the in-house procedures. Note that the genome size of these isolates of MRSA seems to vary between 2,153 and 2,768 (in house) or 2,035 and 2,816 (GP) kbp.
TABLE 3.
Evaluation of the experimental data obtained by PFGE of MRSA: survey of molecular size valuesa
| MRSA strain | Center 1
|
Center 2
|
Center 3
|
Center 4
|
Center 5
|
Center 6
|
Center 7
|
Center 8
|
Center 10
|
Center 11
|
Center 12
|
Avg value
|
Avg value
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IH | GP | IH | GP | IH | GP | IH | IH | IH | IH | GP | IH | GP | IH | GP | GP | IH | IH (SD) | GP (SD) | |
| 1 | 2,813 | 2,679 | 2,846 | 2,829 | 2,763 | 2,788 | 2,848 | 2,416 | 2,813 | 2,924 | 2,400 | 2,792 | 2,932 | 2,750 | 2,550 | 2,702 | 2,710 | 2,768 (130) | 2,697 (165) |
| 2 | 2,813 | 2,679 | 2,828 | 2,812 | 2,779 | 2,787 | 2,856 | 2,416 | 2,813 | 2,787 | 2,387 | 2,792 | 2,932 | 2,700 | 2,580 | 2,725 | 2,716 | 2,750 (120) | 2,700 (164) |
| 3 | 2,813 | 2,679 | 2,785 | 2,812 | 2,785 | 2,783 | 2,874 | 2,416 | 2,813 | 2,819 | 2,468 | 2,792 | 2,832 | 2,700 | 2,570 | 2,665 | 2,704 | 2,750 (122) | 2,687 (124) |
| 4 | 2,813 | 2,679 | 2,818 | 2,881 | 2,786 | 2,784 | 2,854 | 2,416 | 2,813 | 2,763 | 2,467 | 2,792 | 2,932 | 2,700 | 2,550 | 2,673 | 2,719 | 2,747 (119) | 2,709 (156) |
| 5 | 2,813 | 2,679 | 2,796 | 2,818 | 2,770 | 2,783 | 2,881 | 2,416 | 2,813 | 2,932 | 2,494 | 2,792 | 2,932 | 2,700 | 2,610 | 2,644 | 2,720 | 2,763 (133) | 2,709 (135) |
| 6 | 2,227 | 2,068 | 2,153 | 2,378 | 2,452 | 2,173 | 2,481 | 1,943 | 2,402 | 2,299 | 2,076 | 2,286 | 2,265 | 2,010 | 2,020 | 2,222 | 2,133 | 2,239 (172) | 2,171 (118) |
| 7 | 2,247 | 2,078 | 2,090 | 2,301 | 2,460 | 2,180 | 2,540 | 1,768 | 2,243 | 2,277 | 2,045 | 2,207 | 2,179 | 2,010 | 2,050 | 2,077 | 2,158 | 2,200 (207) | 2,130 (87) |
| 8 | 2,412 | 2,238 | 2,129 | 2,317 | 2,594 | 2,347 | 2,673 | 1,960 | 2,378 | 2,461 | 2,226 | 2,406 | 2,460 | 2,280 | 2,220 | 2,194 | 2,300 | 2,359 (198) | 2,286 (88) |
| 9 | 2,400 | 2,228 | 2,111 | 2,322 | 2,611 | 2,343 | 2,681 | 2,093 | 2,383 | 2,465 | 2,189 | 2,406 | 2,449 | 2,310 | 2,220 | 2,749 | 2,283 | 2,374 (179) | 2,357 (180) |
| 10 | 2,370 | 2,230 | 2,104 | 2,362 | 2,616 | 2,331 | 2,656 | 1,905 | 2,383 | 2,483 | 2,206 | 2,342 | 2,435 | 2,280 | 2,230 | 2,155 | 2,256 | 2,340 (213) | 2,278 (92) |
| 11 | 2,593 | 2,673 | 2,811 | 2,721 | 2,675 | 2,627 | 2,567 | 2,603 | 2,690 | 3,431 | 2,456 | 2,513 | 2,677 | 2,770 | 2,680 | 2,447 | 2,476 | 2,713 (260) | 2,612 (104) |
| 12 | 2,912 | 2,705 | 2,701 | 2,759 | 2,787 | 2,768 | 2,854 | 1,860 | 2,931 | 2,657 | 2,358 | 2,950 | 3,299 | 2,770 | 2,830 | 2,780 | 2,714 (317) | 2,786 (255) | |
| 13 | 2,586 | 2,483 | 2,670 | 2,701 | 2,547 | 2,541 | 2,431 | 2,125 | 2,716 | 3,023 | 2,499 | 2,403 | 2,571 | 3,080 | 2,580 | 2,464 | 2,278 | 2,586 (287) | 2,548 (74) |
| 14 | 2,892 | 2,752 | 2,954 | 3,052 | 2,731 | 2,753 | 2,776 | 2,371 | 2,867 | 2,360 | 2,969 | 2,674 | 2,841 | 2,900 | 2,650 | 2,694 | 2,706 | 2,705 (193) | 2,816 (136) |
| 15 | 2,672 | 2,470 | 2,725 | 2,790 | 2,516 | 2,491 | 2,542 | 2,080 | 2,536 | 2,540 | 2,574 | 2,464 | 2,596 | 2,400 | 2,680 | 2,502 | 2,539 | 2,501 (166) | 2,586 (107) |
| 16 | 2,735 | 2,544 | 2,807 | 2,726 | 2,141 | 2,587 | 2,708 | 2,531 | 2,929 | 2,821 | 2,327 | 2,631 | 2,709 | 2,810 | 2,650 | 2,805 | 2,708 | 2,682 (208) | 2,628 (154) |
| 17 | 2,514 | 2,307 | 2,788 | 2,833 | 2,434 | 2,396 | 2,787 | 1,940 | 2,621 | 2,770 | 2,133 | 2,296 | 2,733 | 2,330 | 2,410 | 2,282 | 2,104 | 2,458 (279) | 2,442 (233) |
| 18 | 3,929 | 2,794 | 2,766 | 2,817 | 2,407 | 2,709 | 2,471 | 2,192 | 2,799 | 2,645 | 2,432 | 2,572 | 2,722 | 2,710 | 2,830 | 3,107 | 2,613 | 2,710 (441) | 2,773 (185) |
| 19 | 1,909 | 1,737 | 2,479 | 2,593 | 2,527 | 2,441 | 2,612 | 1,402 | 1,878 | 2,726 | 1,497 | 1,656 | 1,846 | 1,800 | 1,870 | 2,263 | 2,542 | 2,153 (448) | 2,035 (372) |
| 20 | 2,404 | 1,925 | 2,633 | 2,133 | 2,770 | 2,785 | 2,820 | 3,077 | 2,671 | 2,966 | 1,947 | 2,577 | 2,349 | 2,210 | 2,860 | 2,117 | 2,681 (253) | 2,302 (354) | |
The molecular length values are given in kilobase pairs. Centers 9 and 11 experienced some difficulties with efficient normalization and band recognition by the available software, hence, the absence of numerical data and the discrepancy of chromosome sizes for the identical isolates 1 to 5. For centers 4, 5, 6, and 11, the GP protocol did not give rise to interpretable results. Center 4 did not perform the GP analysis. SD, standard deviation. The average values are given per strain. When groups of isolates are considered, the following general averages were calculated: isolates 1 to 5, 2,756 (IH) and 2,700 (GP); isolates 6 to 10, 2,302 (IH) and 2,244 (GP); isolates 11 to 20, 2,590 (IH) and 2,552 (GP); IH, in-house protocol.
Table 4 shows the similarity of the isolates in the three different clusters as determined in each center by using Dice coefficients obtained by using commercial or in-house software. The number of bands that the participants detected per group of isolates is also indicated. Overall, the clustering of the identical and related isolates is well documented in all centers as is the unrelated nature of isolates 11 to 20. The in-house procedures produce similar fragment numbers for all of the identical isolates with a single exception (Table 4, center 7), while the standard protocol was associated with a wider range of values (12 to 16). Average clustering values for the indistinguishable isolates were higher for the in-house than the standard protocol (99.8 versus 97.3, respectively). Also, the average clustering values for the related isolates were higher with the in-house than with the GP protocol (87.2 versus 85.9, respectively).
TABLE 4.
Evaluation of the experimental data obtained by PFGE of MRSA: pattern similarity (mean values) and range of the total number of bands per strain by epidemiological group
| Center identification | In-house procedure
|
Standardized procedure
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identical isolates (1 to 5)
|
Clustered isolates (6–10)
|
Independent isolates (11 to 20)
|
Identical isolates (1 to 5)
|
Clustered isolates (6 to 10)
|
Independent isolates (11 to 20)
|
|||||||
| % | na | % | n | % | n | % | n | % | n | % | n | |
| 1 | 100 | 15 | 96 | 15–16 | 64 | 13–16 | 100 | 15 | 92 | 14–16 | 53 | 12–15 |
| 2 | 100 | 16 | 87 | 15–16 | 26 | 13–16 | 98 | 15 | 89 | 14–15 | 31 | 11–16 |
| 3 | 100 | 15 | 93 | 15–16 | 67 | 12–16 | 96 | 13 | 92 | 13–14 | 59 | 11–15 |
| 4 | 100 | 16 | 88 | 16–17 | 27 | 13–18 | —b | — | — | — | — | — |
| 5 | 100 | 15 | 93 | 14–15 | 38 | 10–15 | — | — | — | — | — | — |
| 6 | 100 | 16 | 89 | 16–17 | 22 | 14–18 | — | — | — | — | — | — |
| 7 | 100 | 10–12 | 94 | 9–10 | 30 | 8–12 | 98 | 12 | 87 | 11–12 | 55 | 10–14 |
| 8 | 100 | 16 | 87 | 16–17 | 34 | 12–17 | 92 | 16 | 62 | 17 | 31 | 14–18 |
| 9 | 98 | 16 | 79 | 13–14 | 52 | 13–16 | 94 | 14 | 94 | 13 | 67 | 12–14 |
| 10 | 100 | 16 | 68 | 14–16 | 30 | 11–18 | 100 | 15 | 85 | 14 | 44 | 10–15 |
| 11 | 100 | 15 | — | 14–16 | — | 12–18 | 100 | 15 | — | 15–17 | — | 11–18 |
| 12 | 100 | 17 | 85 | 16–17 | 49 | 16–18 | — | — | — | — | — | — |
| Overall range (no. of fragment) | 10–17 | 9–17 | 8–18 | 12–16 | 11–17 | 10–18 | ||||||
| Avg | 99.8 | 87.2 | 39.9 | 97.3 | 85.9 | 48.6 | ||||||
| SD | 0.6 | 7.6 | 15.0 | 2.8 | 10.2 | 12.8 | ||||||
n: number of bands.
—, no data or nonevaluable data.
Centralized analysis.
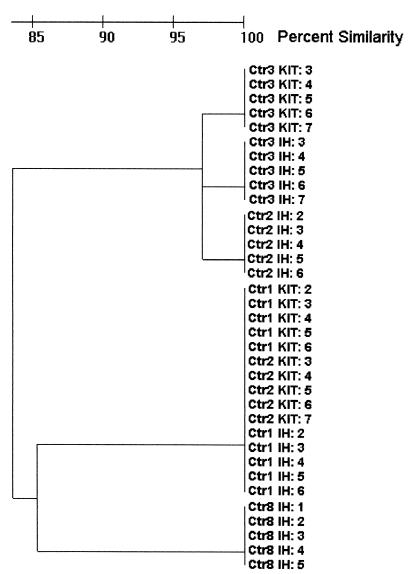
Gel images from seven data sets, four in-house gels and three standardized gels, were available for analysis. The other gel images either did not contain the appropriate lambda standards or were of insufficient quality for analysis. The gels could not be analyzed by GelCompar as a single file because the lambda standards were not sufficient to normalize the gels. Gelcompar was used to assess improvements in data homology upon copying of PFGE conditions. When the primary data obtained in centers 4 and 8 were compared, relatively low homology values were calculated. When center 8 adopted the experimental parameters proposed by center 4, however, the homology between primary data obtained in center 8 and the novel data generated in center 4 increased significantly (results not shown). This emphasizes the importance of exact experimental standardization. The gels could be analyzed in more detail using the Bio Image software. Among the gels analyzed, the number of lambda fragments, which were used for normalization of the gels, varied from 9 to 15 per lane, which hampered the analysis. We focused the analysis on the first five lanes of the gel images containing the five indistinguishable isolates. Using the Bio Image software and a 3.5% band tolerance, which was previously determined at Centers for Disease Control and Prevention to be optimal for analysis of S. aureus isolates (data not shown), the five indistinguishable isolates from seven data sets matched at a level of 84% similarity by using UPGMA clustering (Fig. 2). Both in-house and GP kit data from center 1 and the in-house data from center 2 clustered at 100% similarity, while the GP kit and in-house data from center 3 and the in-house data from center 2 were 97% similar. These two clusters were linked at 84% similarity. Data from center 3 for the kit was not available for analysis.
FIG. 2.
Dendrogram of results of seven data sets, four in-house (IH) and 3 standardized GP kits (KIT) from four centers (Ctr). The numbers after the colons are lane numbers. The percent similarity scale is based on UPGMA clustering of Dice coefficients generated by Bio Image software.
Strain characteristics.
The data were obtained by conventional and genetic analysis of the isolates used for the present study (results not shown). The binary type (18), the tar 916–shida fingerprints (3), and the AP-PCR characteristics (16) for isolates 1 through 5 were indistinguishable. The PFGE subtypes for isolates 6 to 10 appear homogeneous by AP-PCR and tar 916–shida PCR, confirming their relatedness. Binary typing shows some heterogeneity among these isolates, but this is limited to differences detected by a single probe. AP-PCR discriminates each of the isolates in the final group (11 to 20), although tar 916–shida PCR fails to discriminate isolates 11 and 18 from the cluster of indistinguishable isolates (1 to 5, which were PFGE identical). For this panel of 20 isolates, both bacteriophage typing and the antibiogram data are in good agreement with the genotypic data. Since the study isolates were selected on the basis of PFGE differences detected in a single laboratory, the additional typing data were necessary to confirm the interstrain relationships.
DISCUSSION
Standardization of molecular typing methods is an issue of debate in the field of medical microbiology. For several microorganisms, such as Pseudomonas aeruginosa, an array of typing methods has been compared in multiple laboratory studies, leading to suggestions for the appropriate use of molecular data (4, 8, 12). To date, however, only typing based on IS6110 sequences of Mycobacterium tuberculosis has been standardized to any great extent (5, 17). This has resulted in a method and database capable of importing and analyzing new information generated by different laboratories around the world. However, this required several years and the concerted efforts of many individuals and institutions to achieve. This situation is a rare exception in microbiology, and efforts in establishing similar systems for other bacterial species are urgently required. Emerging multiresistant microorganisms are an important group of species in this context; in this study, we have attempted to achieve similar results with MRSA.
Several comparative typing studies have been performed for S. aureus in the recent past (e.g., 2, 6, 11, 13, 16, 18). Some of these studies focused on the detailed analysis of single techniques, performed in single centers (6, 11, 13, 18), whereas others assessed interlaboratory reproducibility (2, 16). The study by Cookson et al. (2) was the first attempt to compare PFGE data from different laboratories and merge the data into a single file for analysis. The present study continues this initiative, by utilizing a set of reference MRSA isolates to investigate interlaboratory reproducibility of PFGE typing, including the use of standardized DNA preparation and electrophoretic switching protocols.
In the present study, experimental problems were encountered during generation of DNA plugs when using the standard protocol. Apparently, the use of agarose of sufficient heat tolerance is critical for resolving band differences. The separation of bands could be improved by altering the pulsing protocol, a parameter which was not optimal in the current version of the procedure, in which large portions of the gel were not used. Some of the participating centers modified the commercial protocol in order to achieve better band separation (see legend to Table 2). The GP protocol needs to be reexamined critically and its major deficiencies need to be corrected before it could be recommended for widespread used.
The in-house procedures performed quite well in all of the participating laboratories. Despite differences in DNA preparation methodology, electrophoretic equipment, and electric current switching times, none of the centers had difficulty in recognizing the identical and unrelated groups of isolates (1 to 5 and 11 to 20, respectively). Differences between centers were noted, however, with interpretation of data concerning the related isolates (numbers 6 to 10). In some instances this was apparently due to electric current switching protocols which did not clearly differentiate closely sized (but nonidentical) restriction fragments in different isolates. However, other differences were not associated with questions of in-house versus standardized PFGE methodologies but, instead, were specifically related to the algorithm that individual investigators employed for pattern interpretation. Investigators were instructed to employ specific guidelines for assessing isolate interrelationships (11, 15). A key aspect of this approach, for the purpose of hospital epidemiology, involves the choice of a predominant (epidemic) type (occurring more than once in the group) to which all other patterns are compared (15). Isolates that differ by three or fewer restriction fragment positions (i.e., up to six band differences when comparing lanes) are considered subtypes of this common strain pattern, while organisms differing in four or more positions are identified as different strain types. With groups of isolates that are identical or clearly different (i.e., a multitude of differently positioned fragments), application of the algorithm is straightforward, leading to reproducible interlaboratory interpretation as demonstrated here. However, an interesting aspect of the study design was the inclusion of only one isolate for each of the related isolates, 6 to 10. It was thus left to the investigator to determine which isolate from within this group would represent the standard type to which the other four would be compared. In addition, two of the isolates (numbers 6 and 7) exhibited an increased staining intensity of specific but different restriction fragments which could be interpreted as a difference between the isolates in comigrating fragment doublets (Fig. 1). These two features were the reason for the different relationships as defined in differing centers, even if the PFGE patterns generated in different laboratories were indistinguishable. As can be deduced from the results in Fig. 1 (reading left to right), most centers chose isolate 6 as the standard and each arrived at the same interpretation for the isolates. Alternative interpretations noted in some instances (Table 2) may reflect differences in the choice of a standard as well as perceived differences in restriction fragment positions. These results underscore the importance of the choice of a predominant PFGE pattern as an issue separate from that of DNA preparation or the reproducibility of electrophoretic separation for purposes of epidemiological interpretation.
An unexpected finding was the fact that data sets generated in different centers did not lend themselves to numerical analysis by GelCompar when combined into a single digitized image. This situation improved when two different in-house protocols were replicated at the coordinating center, but a 100% homology score was never reached despite the fact that the visual data appeared highly similar to that generated by the respective participating center. Different modes of data processing did not result in the improvement of the correlation among data sets. This observation may relate to issues regarding the use of computerized analysis in the comparative normalization of restriction fragment positions between different gels. However, data analysis was somewhat more successful with Bio Image software. When the data sets representing images from four in-house gels and three standard kit gels were merged, the result was two clusters of highly related isolates (>97% similarity) linked to each other at the 84% similarity level. Given the disparity in the positions of standards and the number of identifiable bands in each lambda standard lane, this level of similarity suggests that interlaboratory comparisons are clearly possible, although every effort should be made to standardize the positioning of standards on the gels to facilitate comparisons.
At present, it appears that the computerized analysis of PFGE patterns may be more useful in identifying closely related or identical strains for further testing or analysis than for reliably establishing differentiating nonrelated strains (Table 4). The differences in the number of DNA fragments that were successfully identified in the different laboratories were most probably related to differences in the quality of the gel images, rather than the artifactual absence or presence of specific DNA fragments, since a number of laboratories produced apparently identical gel images with identical numbers of DNA fragments. An important point to make here is that visual inspection is still an essential complementary procedure to so-called automated analysis, which subjects objective computerized analysis to subjective review.
The basis of a scientific method is reproducibility. Different laboratories performing the same procedure in the same way are expected to generate the same results. Based on this premise, one can argue that the present study indicates that standardization of PFGE typing for MRSA has not yet been achieved. In terms of numerical output of computerized PFGE analysis this is clearly the case. Any method involving manipulations performed by hand and data inspections with an element of human visualization possesses an inherent potential for at least some degree of variability and bias. For this reason, absolute numerical standardization of PFGE may never be achieved. But epidemiological questions commonly involve answers which are not an absolute “yes” or “no” but, instead, often involve an assessment of qualitative degrees of interrelationship. In this context the overall qualitative similarity of PFGE results observed here, despite a variety of different in-house DNA preparation procedures, PFGE equipment, and switching protocols, indicates that continuing efforts to minimize the variability of these parameters will lead to acceptable numerical as well as methodological standardization of PFGE procedure and analysis. As a step in this direction, the difficulties with the standardized GP protocol noted in this study should be corrected and reevaluated in a future effort.
ACKNOWLEDGMENTS
The GP kits used for the standardized part of the present study were provided free of charge by the Dutch Bio-Rad agency, Veenendaal, The Netherlands, for which John Kuijpers is gratefully acknowledged. We thank Loretta Carson at CDC (Atlanta, Ga.) for technical assistance. The coordinating center was based in the Department of Medical Microbiology & Infectious Diseases, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands. This study was an initiative of the European Study Group on Epidemiological Markers (ESGEM), which is an official working party of the European Society of Clinical Microbiology and Infectious Diseases.
REFERENCES
- 1.Carle G F. Field inversion gel electrophoresis. In: Burmeister M, Ulanowsky L, editors. Methods in molecular biology: pulsed field gel electrophoresis. Totowa, N.J: The Humana Press Inc.; 1992. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- 2.Cookson B D, Aparicio P, Deplano A, Struelens M, Goering R, Marples R. Inter-center comparison of pulsed field gel electrophoresis for the typing of methicillin resistant Staphylococcus aureus. J Med Microbiol. 1996;44:179–184. doi: 10.1099/00222615-44-3-179. [DOI] [PubMed] [Google Scholar]
- 3.Cuny C, Witte W. Typing of Staphylococcus aureus by PCR for DNA sequences flanked by transposon Tn916 target region and ribosomal binding site. J Clin Microbiol. 1996;34:1502–1505. doi: 10.1128/jcm.34.6.1502-1505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grothues D, Koopman U, von der Hardt H, Tummler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988;26:1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermans P W M, Massadi F, Guebrexhaber H, van Soolingen D, de Haas P E W, Heersma H, de Neeling H, Ayoub A, Portaels F, Frommel D, Zribi M, van Embden J D A. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia and the Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J Infect Dis. 1995;171:1504–1513. doi: 10.1093/infdis/171.6.1504. [DOI] [PubMed] [Google Scholar]
- 6.Morvan A, Aubert S, Godard C, El Solh N. Contribution of a typing method based on IS256 probing of SmaI-digested cellular DNA to discrimination of European phage type 77 methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1997;35:1415–1423. doi: 10.1128/jcm.35.6.1415-1423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A H, Foster T J, Pattee P A. Physical and genetic mapping of the protein A gene in the chromosome of Staphylococcus aureus 8325–4. J Gen Microbiol. 1989;135:1799–1807. doi: 10.1099/00221287-135-7-1799. [DOI] [PubMed] [Google Scholar]
- 8.Renders N, Römling U, Verbrugh H, van Belkum A. Comparative typing of Pseudomonas aeruginosa by random amplification of polymorphic DNA or pulsed-field gel electrophoresis of DNA macrorestriction fragments. J Clin Microbiol. 1996;34:3190–3195. doi: 10.1128/jcm.34.12.3190-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz D C, Cantor C R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984;37:67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- 10.Struelens M J, Bauernfeind A, van Belkum A, Blanc D, Cookson B D, Dijkshoorn L, El Solh N, Etienne J, Garaizar J, Gerner-Smidt P, Legakis N, de Lencastre H, Nicolas M H, Pitt T L, Römling U, Rosdahl V, Witte W. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 11.Struelens M J, Deplano A, Godard C, Maes N, Serruys E. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2599–2605. doi: 10.1128/jcm.30.10.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Struelens M J, Schwam V, Deplano A, Baran D. Genome macrorestriction analysis of diversity and variability of Pseudomonas aeruginosa strains infecting cystic fibrosis patients. J Clin Microbiol. 1993;31:2320–2326. doi: 10.1128/jcm.31.9.2320-2326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenover F C, Arbeit R D, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Hebert G A, Hill B, Hollis R, Jarvis W R, Kreiswirth B, Eisner W, Maslow J, McDougal L K, Miller J M, Mulligan M, Pfaller M A. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover F C, Arbeit R D, Goering R V the Molecular Typing Working Group of the Society for Healthcare Epidemiology of America. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18:426–439. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 15.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Embden J D A, Cave M D, Crawford J D, Dale J W, Eisenach K D, Gicquel B, Hermans P W M, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Leeuwen W, Sijmons M, Sluijs J, Verbrugh H, van Belkum A. On the nature and use of randomly amplified DNA from Staphylococcus aureus. J Clin Microbiol. 1996;34:2770–2777. doi: 10.1128/jcm.34.11.2770-2777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada A, Katayama Y, Hiramatsu K, Yokota T. Southern hybridization analysis of the mecA deletion from methicillin resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1991;176:1319–1325. doi: 10.1016/0006-291x(91)90430-f. [DOI] [PubMed] [Google Scholar]