Abstract
PURPOSE
Preclinical cancer models harboring CCNE1 amplification were more sensitive to adavosertib treatment, a WEE1 kinase inhibitor, than models without amplification. Thus, we conducted this phase II study to assess the antitumor activity of adavosertib in patients with CCNE1-amplified, advanced refractory solid tumors.
PATIENTS AND METHODS
Patients aged ≥ 18 years with measurable disease and refractory solid tumors harboring CCNE1 amplification, an Eastern Cooperative Oncology Group performance status of 0-1, and adequate organ function were studied. Patients received 300 mg of adavosertib once daily on days 1 through 5 and 8 through 12 of a 21-day cycle. The trial followed Bayesian optimal phase II design. The primary end point was objective response rate (ORR).
RESULTS
Thirty patients were enrolled. The median follow-up duration was 9.9 months. Eight patients had partial responses (PRs), and three had stable disease (SD) ≥ 6 months, with an ORR of 27% (95% CI, 12 to 46), a SD ≥ 6 months/PR rate of 37% (95% CI, 20 to 56), a median progression-free survival duration of 4.1 months (95% CI, 1.8 to 6.4), and a median overall survival duration of 9.9 months (95% CI, 4.8 to 15). Fourteen patients with epithelial ovarian cancer showed an ORR of 36% (95% CI, 13 to 65) and SD ≥ 6 months/PR of 57% (95% CI, 29 to 82), a median progression-free survival duration of 6.3 months (95% CI, 2.4 to 10.2), and a median overall survival duration of 14.9 months (95% CI, 8.9 to 20.9). Common treatment-related toxicities were GI, hematologic toxicities, and fatigue.
CONCLUSION
Adavosertib monotherapy demonstrates a manageable toxicity profile and promising clinical activity in refractory solid tumors harboring CCNE1 amplification, especially in epithelial ovarian cancer. Further study of adavosertib, alone or in combination with other therapeutic agents, in CCNE1-amplified epithelial ovarian cancer is warranted.
INTRODUCTION
Cell cycle progression is orchestrated by the orderly expression of cyclins (regulatory subunits), which sequentially activate the cyclin-dependent kinases (CDKs) that govern the cell division machinery.1,2 Cyclin E (E1 and E2) is most abundant between G1 phase and S phase. Cyclin E binds to Cdk2 to form a unique configuration that is required for the transition from G1 to S phase of the cell cycle and determines the initiation of DNA duplication.3,4 Overexpression of cyclin E has been shown to promote genomic instability by causing DNA replication stress and deregulating the G1 to S transition.4-10
CONTEXT
Key Objective
CCNE1 amplification is frequently identified in many types of malignancies, associated with resistance to chemotherapy and short survivals duration. In this phase II trial, we investigated the clinical activity of the WEE1 kinase inhibitor adavosertib in advanced malignancies harboring CCNE1 amplification.
Knowledge Generated
Adavosertib demonstrated promising preliminary antitumor activity, with a response rate of 27% in 30 patients with metastatic malignancies harboring CCNE1 amplification. A response rate of 36% was observed in 14 patients with metastatic epithelial ovarian cancer, associated with a median duration of response of 6.3 months.
Relevance (G.F. Fleming)
-
No WEE1 kinase inhibitors are currently US Food and Drug Administration approved, but WEE1 inhibition holds promise as a therapy for patients whose tumors exhibit CCNE1 amplification, particularly women with ovarian cancer.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
WEE1 kinase, which arrests cells in G2/M through inhibition of CDK1/2 to block premature mitotic entry, is essential to prevent massive DNA damage and cell death in cyclin E-overexpressed cells,11-13 which can be reversed by WEE1 kinase inhibition with adavosertib, resulting in mitotic catastrophe and apoptosis induced by unrepaired DNA damage.13-16 Laboratory studies showed that cyclin E overexpression was predictive of antitumor response to adavosertib,17 which provided preclinical proof-of-mechanistic evidence to support the use of cyclin E as a potential biomarker of response to adavosertib.18
CCNE1 is frequently amplified in many human tumors, such as in uterine, ovarian, stomach, esophageal, lung, and pancreatic cancer and sarcoma, with a frequency of 2%-40%.19,20 We hypothesized that CCNE1 amplification in cancer cells leads to an overexpression of cyclin E1 protein, which sensitizes cancer cells to adavosertib. Therefore, we conducted a multicenter open-label, phase II clinical trial (ClinicalTrials.gov identifier: NCT03253679, NCI 10136) to evaluate the antitumor activity of adavosertib in patients with metastatic solid tumors harboring CCNE1 amplification.
PATIENTS AND METHODS
Patient Selection
This was a single-arm phase II study (NCI 10136) conducted at six different cancer centers in the United States through the National Cancer Institute (NCI) Experimental Therapeutics Clinical Trials Network. We identified all patients, aged 18 years or older, who had histologically confirmed metastatic solid tumors harboring CCNE1 amplification, for which there was no effective standard-of-care therapy available. CCNE1 amplification was preidentified by a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory, such as Foundation One, UW-OncoPlex—Cancer Gene Panel, MSK-IMPACT, or Solid Tumor Genomic Assay by Life Technologies. There was no limitation on the number of prior treatment lines, but WEE1 kinase inhibitors were not allowed. Other eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease by RECIST 1.1,21 and adequate organ function (absolute neutrophil count of ≥ 1,500/µL, hemoglobin ≥ 9 g/dL, platelets ≥ 100,000/µL, serum creatinine < 1.5 × upper limit of normal [ULN] or creatinine clearance ≥ 45 mL/min, total bilirubin concentration ≤ ULN or ≤ 1.5 × ULN in patients with known hepatic metastases, ALT and AST concentrations ≤ 3 × ULN or ≤ 5 × ULN in patients with known hepatic metastases), recovery to ≤ grade 1 toxicity or baseline from prior cancer therapy, the ability to swallow and absorb oral medications, and willingness and ability to comply with study treatment and follow-up procedures.
This study was reviewed and approved by the NCI Central Institutional Review Board, the six individual site Institutional Review Boards, and the US Food and Drug Administration; it was conducted in accordance with ethical principles per the Declaration of Helsinki. The trial was registered on ClinicalTrials.gov (identifier: NCT03253679).
Treatment Plan
After patients had provided written informed consent, they received oral adavosertib at a starting dose of 300 mg once daily on days 1 through 5 and 8 through 12 of a 21-day cycle. All patients received a 5-hydroxytryptamine-3 antagonist and dexamethasone (unless contraindicated or not well-tolerated) before each dose of adavosertib. Treatment continued until disease progression, unacceptable toxicity, intercurrent illness that prevented further treatment, or patient withdrawal from this study. Dose delay and reduction by 50 mg were allowed, depending on the grade of the adverse event.
Efficacy and Safety Assessments
All patients underwent periodic safety assessments through in-person office visits or telemedicine. Laboratory tests, including CBC and comprehensive metabolic panel, were assessed weekly during cycle one and then once every subsequent cycle, or more frequently, as clinically indicated. Safety and adverse events were assessed and graded according to the NCI Common Terminology Criteria for Adverse Events version 5.0.
Tumor response and progression were evaluated using the revised RECIST guidelines (version 1.1).21 The same imaging technique used during the screening, or more sophisticated studies, was performed once every 9 weeks or sooner as clinically indicated. Tumor markers, if applicable, were tested once with each imaging study or more frequently as indicated.
Correlative Biomarker Assays
In all patients, CCNE1 amplification (ie, CCNE1 amplification > 7 on the basis of the targeted custom AmpliSeq panel on the Ion Torrent Personal Genome Machine or CCNE1 amplification on alternate CLIA platforms such as Foundation One, Solid Tumor Genomic Assay by Life Technologies) had been detected by a CLIA-certified Molecular Diagnostic Laboratory, with genomic testing performed as part of their routine care. After informed consent had been obtained, the results of these genomic tests were obtained before therapy began.
Statistical Analyses
The primary objective of this phase II study was to evaluate the objective response rate (ORR) to adavosertib in patients with advanced refractory cancers harboring CCNE1 amplification. Subgroup analysis was not designed in this study. The response was defined as a complete response (CR) or partial response (PR) within 6 months of the start of therapy, per RECIST version 1.1. The duration of response was measured from the date of the initial CR or PR to the first date that recurrent or progressive disease was objectively documented. The trial used a two-stage Bayesian optimal phase II (BOP2) design22 with the null hypothesis of ORR = 5% and the alternative hypothesis of ORR = 20%. In the first stage, 10 patients were enrolled. If one or more responses were observed, this study continued to the second stage of enrolling additional 19 patients. If four or more responses were observed among the total of 29 patients, the null hypothesis would be rejected, concluding that adavosertib was promising. For this trial, the BOP2 design was identical to Simon's two-stage design.23 The design yielded 80% power, while controlling one-sided type 1 error rate at the level of 5%. There was a 49% chance of stopping after the first stage under the null hypothesis.
Descriptive summary statistics were used to characterize demographics, safety, and antitumor activity. Categorical data were summarized using frequencies, percentages, and 95% exact CIs. Differences in categorical variables were assessed by Fisher's exact tests. Continuous data were summarized by medians with 95% CIs and ranges. Progression-free survival (PFS) and overall survival (OS) durations were estimated using the Kaplan-Meier method in all patients who received at least one dose of the study agent. PFS duration was defined as the time from enrollment to death or disease progression (whichever was first). Patients without evidence of progression or death were censored at the date of the last radiographic assessment of disease progression. OS duration was defined as the time from enrollment to death or September 28, 2021, at which time the patients' data were censored. Log-rank tests were used to compare PFS and OS duration distributions between patients with epithelial ovarian cancer and those with other cancers. Statistical inferences were based on two-sided tests at a significance level of P < .05. Statistical analyses were carried out using SPSS Statistics version 24 (IBM, Armonk, NY).
RESULTS
Patient Characteristics
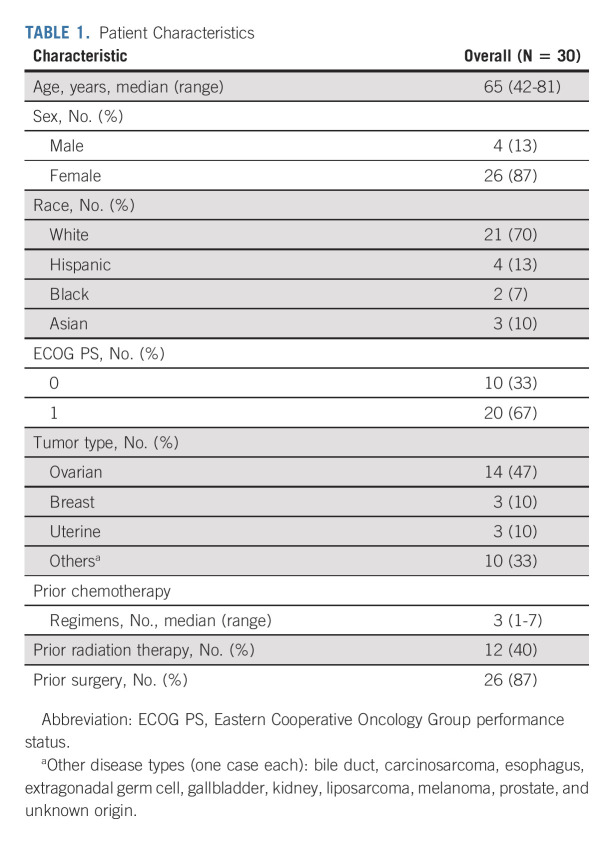
Between January 10, 2019, and May 5, 2020, 31 patients had signed informed consent for study enrollment at six individual NCI Experimental Therapeutics Clinical Trials Network cancer centers. One patient (No. 25) did not receive any study agent. Thus, 30 patients were considered evaluable; the baseline characteristics are presented in Table 1. Most (87%) were female, and 14 had epithelial ovarian cancer. The patients' median age was 65 years, and the median number of prior systemic therapies was three (range, 1-7).
TABLE 1.
Patient Characteristics
Antitumor Activity
Antitumor activity was assessed in all patients. At the data cutoff date on September 28, 2021, the median follow-up period was 9.9 months. One patient was undergoing active therapy (updated on May 24, 2022), while the remaining 29 were removed from the study for the following reasons: disease progression (n = 22), treatment intolerance (n = 2), death on study due to tumor progression (n = 3), alternative therapy (n = 1), and patient withdrawal 1 week after beginning protocol therapy (n = 1). Twenty-seven patients had sufficient tumor measurement data for tumor response evaluation (Fig 1). Three patients could not be evaluated since they had experienced rapid clinical deterioration before a restaging imaging study could be performed. Figure 2 shows changes in tumor measurements over time. We observed no CR, eight PRs, and three stable diseases (SDs) of ≥ 6 months, leading to an ORR of 27% (n = 8; 95% CI, 12 to 46), a SD ≥ 6 months/PR rate of 37% (n = 11; 95% CI, 20 to 56), a median PFS duration of 4.1 months (95% CI, 1.8 to 6.4), and a median OS duration of 9.9 months (95% CI, 4.8 to 15), as shown in Figure 3. Therefore, the null hypothesis of ORR = 5% was rejected because the number of responses ≥ 4, and the treatment was regarded as promising. Among patients who experienced a PR, the median duration of response was 2.1 months (95% CI, 0.2 to 4), ranging from 1.9 months to 10.3 months.
FIG 1.
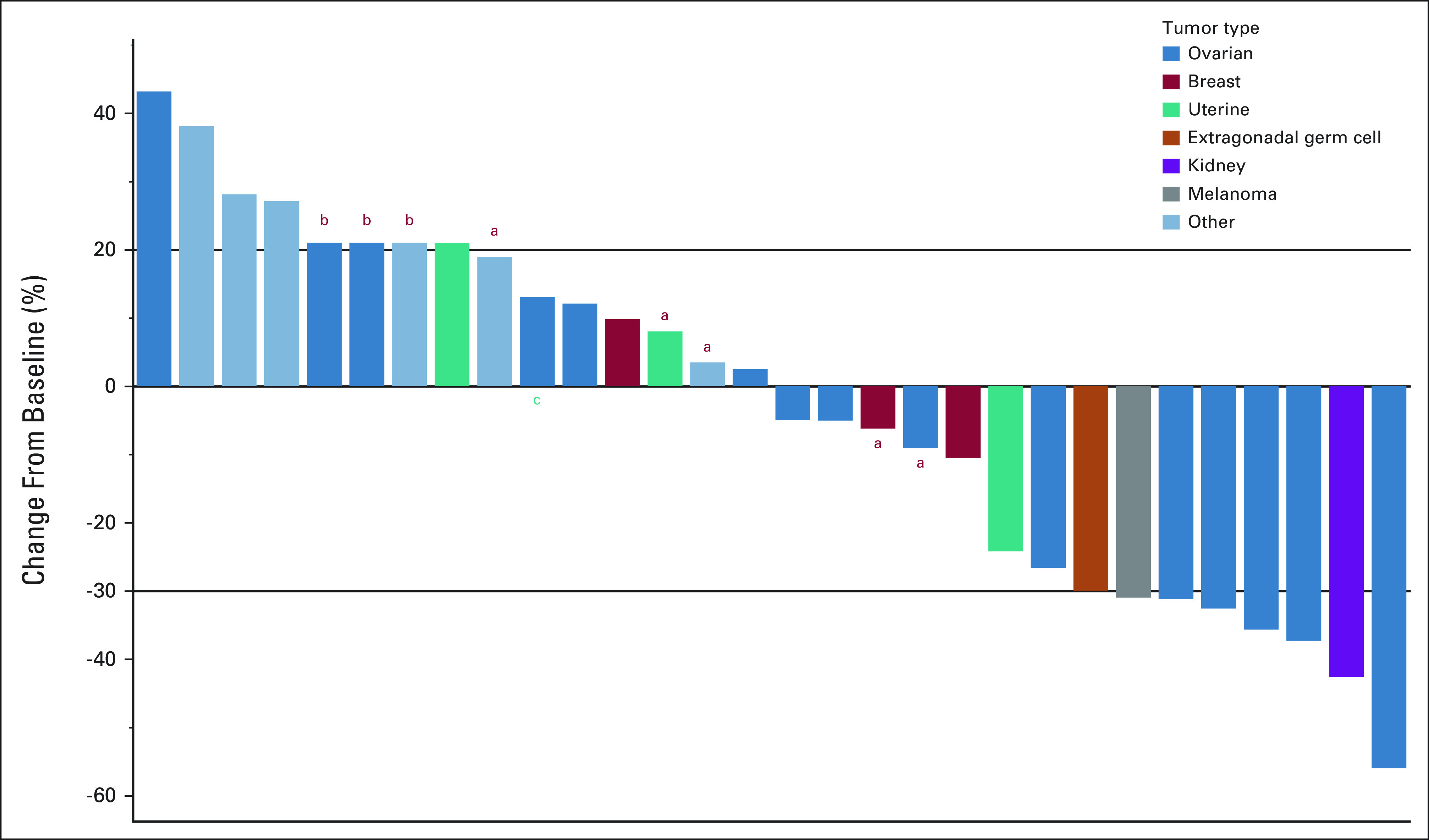
A waterfall plot shows best tumor responses in all 30 patients. aPatients had overall progressive disease with < 20% increase on target lesions but had new nontarget lesions. bPatients had rapid clinical deterioration before a restaging imaging study could be performed, who are documented a 21% tumor increase. cOne patient was still on treatment at the last updated date on May 24, 2022.
FIG 2.
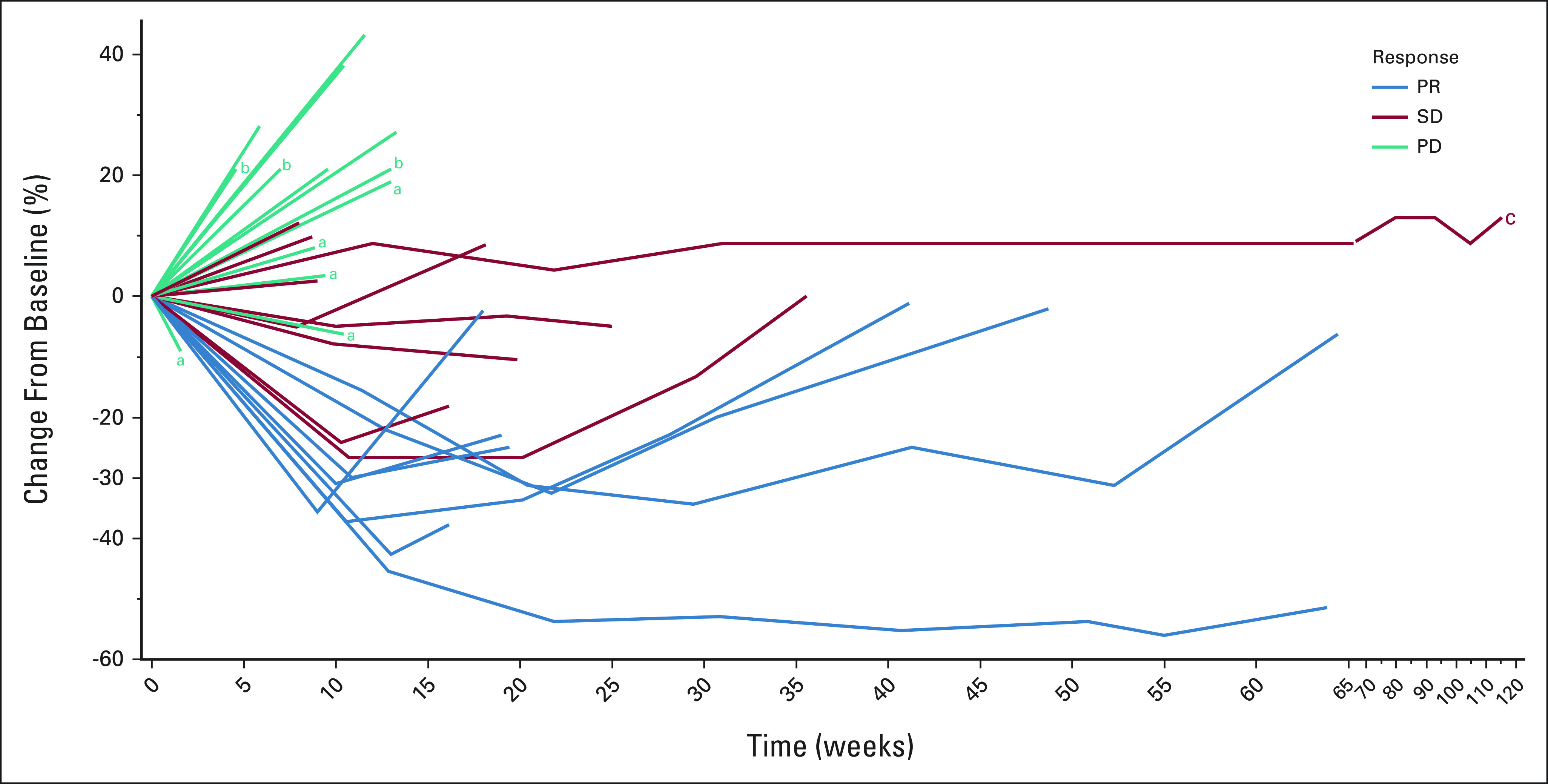
A spider plot of tumor measurement changes over time in all 30 patients. aPatients had overall PD with < 20% increase on target lesions but had new nontarget lesions. bPatients had rapid clinical deterioration before a restaging imaging study could be performed, who are documented a 21% tumor increase. cOne patient was still on treatment at the last updated date on May 24, 2022. PD, progressive disease; PR, partial response; SD, stable disease.
FIG 3.
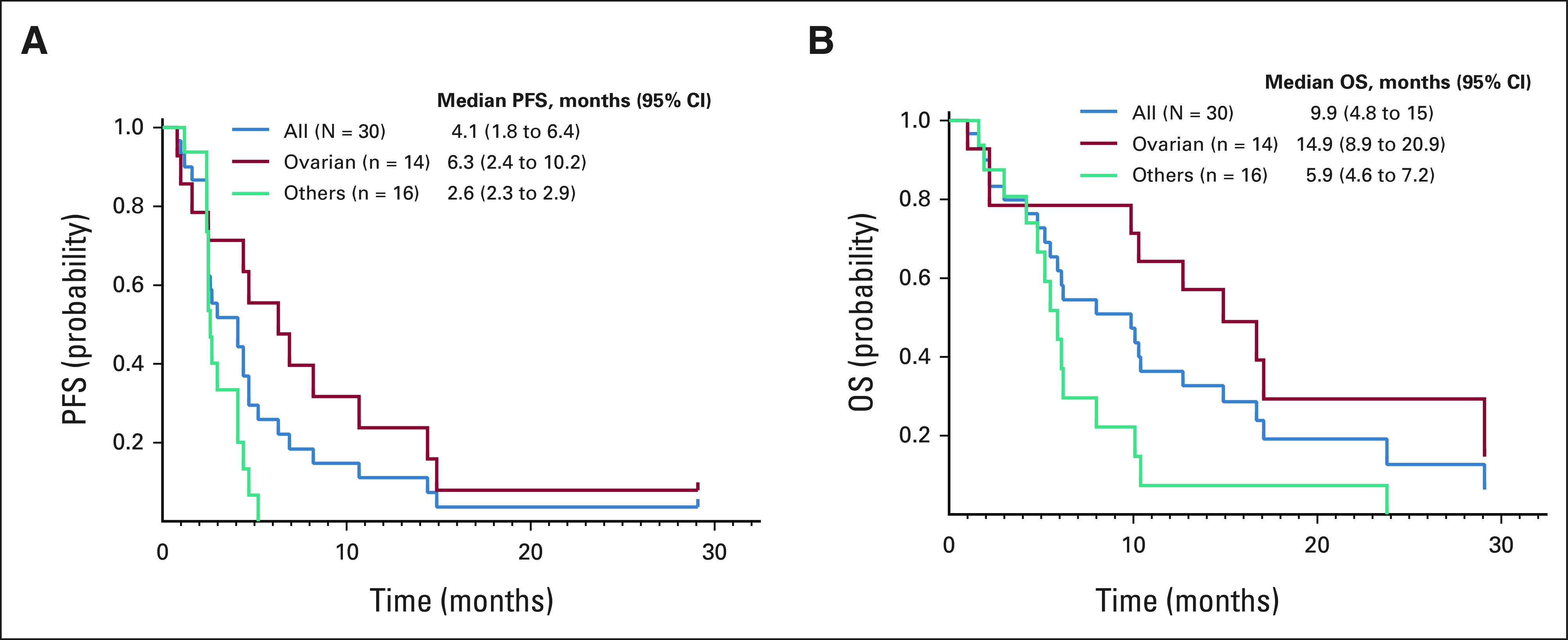
Kaplan-Meier plots show the probabilities of (A) PFS and (B) OS in all 30 patients (blue curve), in 14 patients with epithelial ovarian cancer (red curve), and 16 patients with metastatic malignancies other than epithelial ovarian cancer (teal curve). 95% CI are indicated in parentheses. OS, overall survival; PFS, progression-free survival.
Among 14 patients with epithelial ovarian cancer, five experienced PRs and three had SD ≥ 6 months, leading to an ORR of 36% (n = 5; 95% CI, 13 to 65), a SD ≥ 6 months/PR rate of 57% (n = 8; 95%, 29 to 82), a median PFS duration of 6.3 months (95% CI, 2.4 to 10.2), a median OS duration of 14.9 months (95% CI, 8.9 to 20.9), and a median duration of response of 6.3 months (95% CI, 1.6 to 11). Patients who did not have epithelial ovarian cancer had a median PFS duration, OS duration, and duration of response of 2.6 months (95% CI, 2.3 to 2.9), 5.9 months (95% CI, 4.6 to 7.2), and 2 months (95% CI, 1.9 to 2.1), respectively.
Safety
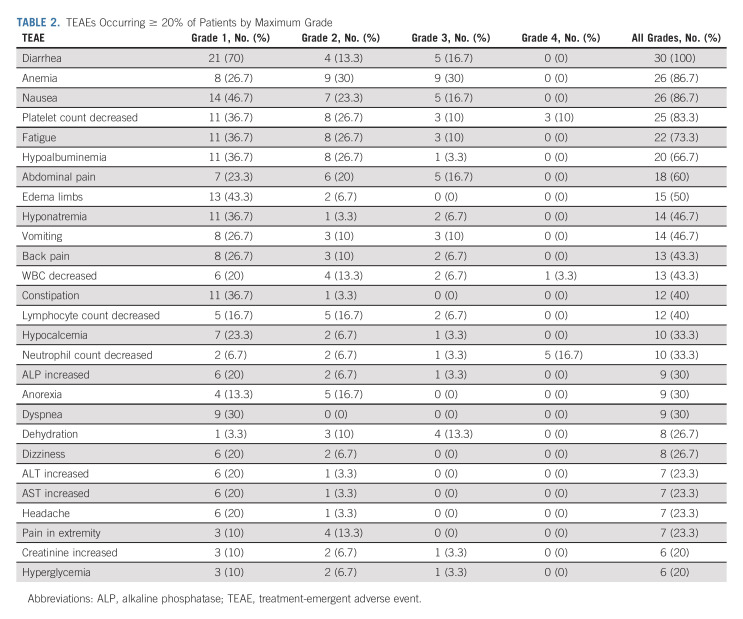
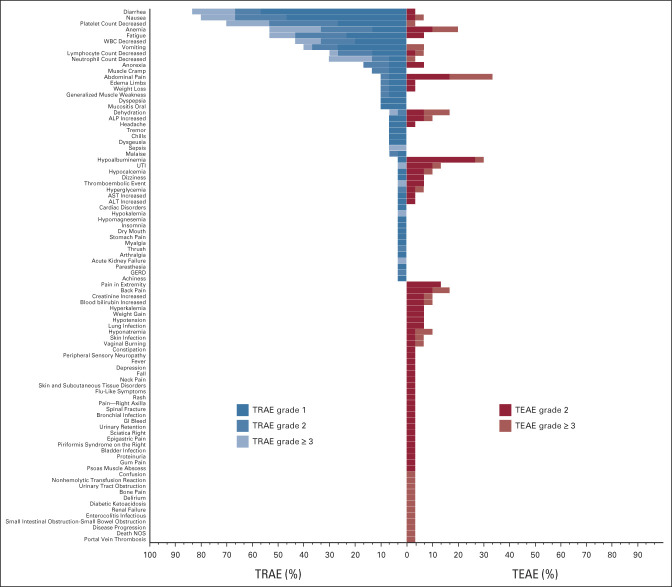
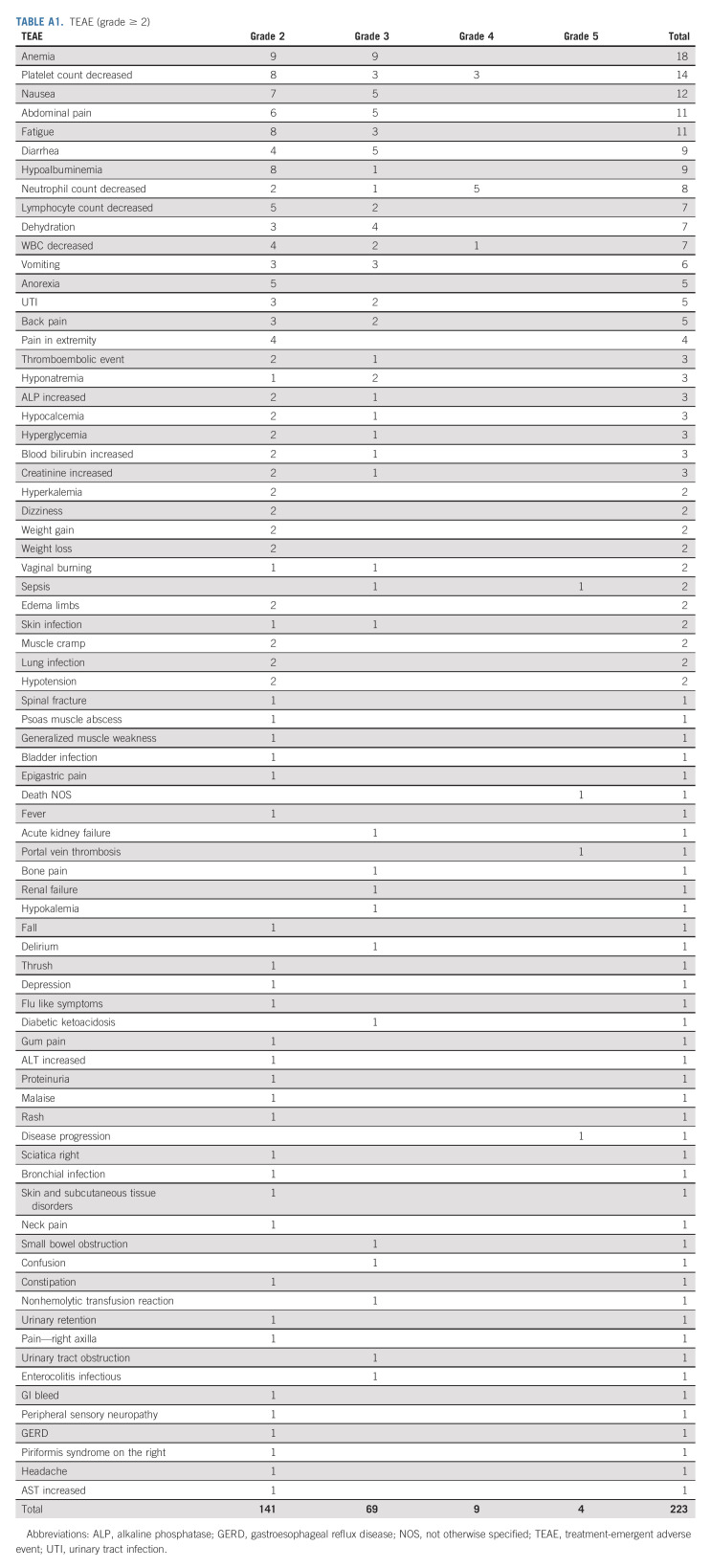
All 30 patients who had received at least one dose of adavosertib were evaluable for toxicity. Treatment-emergent (TEAE) and treatment-related adverse events are presented in Appendix Figure A1 (online only) and Appendix Tables A1 and A2 (online only). Three patients died during the study due to disease progression. There was no treatment-related death. Table 2 presents common TEAEs. Twenty-four patients experienced ≥ grade 3 TEAEs (80%). Eighteen patients experienced ≥ grade 3 treatment-related adverse events (60%), including anemia (20%), decreased neutrophil count (17%), diarrhea (17%), decreased platelet count (13%), nausea (13%), fatigue (10%), decreased WBC (10%), sepsis (7%), decreased lymphocyte count (3%), vomiting (3%), Urinary tract infection (3%), hypokalemia (3%), acute kidney failure (3%), dehydration (3%), and thromboembolic events (3%). Two patients were removed from the study for treatment intolerance. Dose reduction of adavosertib to 250 mg occurred in 17 patients (57%) and then further to 200 mg in nine patients (30%). Six patients had a dose reduction after cycle 1, five after cycle 2, two after cycle 3, one after cycle 4, and three after cycle 6 and beyond. One patient had a second dose reduction after cycle 1, three after cycle 2, four after cycle 3, and one after cycle 6 and beyond.
TABLE 2.
TEAEs Occurring ≥ 20% of Patients by Maximum Grade
Potential Biomarker Exploration
Molecular profiles, per FoundationOne CDx (n = 14), MDACC Solid Tumor Genomic Assay 2018 (n = 10), Guardant360 CDx (n = 2), Columbia Combined Cancer Panel (n = 1), GEM ExTra (n = 1), Invitae Breast and Gyn Cancer Panel (n = 1), and TEMPUS x T597 Gene Panel (n = 1), were obtained before trial therapy, as presented in Appendix Figure A2 (online only). Baseline molecular profiles of the 30 patients with CCNE1 amplification showed concurrent TP53 gene aberration (90%), AKT2 amplification (23%), MYC amplification (17%), CCND2 amplification (10%), and NOTCH1 mutations (10%). Selected genes are arranged according to their tumor responses, as shown in Figure 4. One patient was found to have a BRCA1 variant of unknown significance. No deleterious BRCA1 and BRCA2 mutation was identified in this cohort of patients. Among 17 patients tested, no microsatellite instability-high was detected. One patient with melanoma had a high tumor mutation burden of 28.4 mutations per megabase, per the Columbia Combined Cancer Panel, and experienced a PR. Another patient with epithelial ovarian cancer was identified to have homologous recombination deficiency that was associated with a loss of heterozygosity score of ≥ 16%, per FoundationOne CDx, and died of tumor progression 54 days after starting the study. Besides concurrent TP53 aberrations in responders, we identified one patient with concurrent RAD51 deletion, PIM1 amplification, and HRAS mutation; one with deletion of NF1 and mutations of BRIP1, DICER1, FAT1, and MAX; and one with amplification of KRAS, RAD51C, and RNF43.
FIG 4.
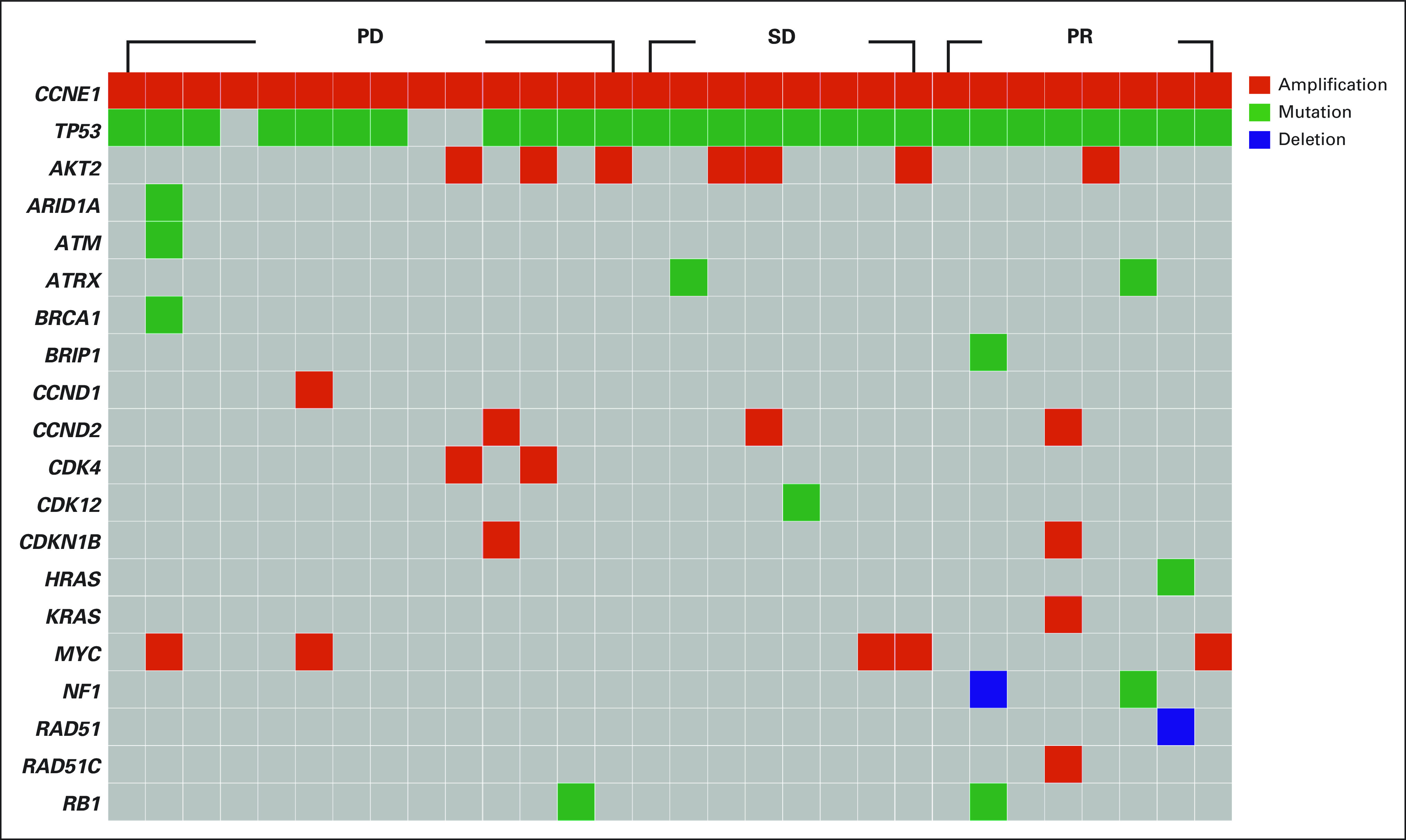
Baseline molecular profiling was performed per next-generation sequencing in a Clinical Laboratory Improvement Amendments–certified molecular diagnostic laboratory; selected genes are arranged according to their tumor responses, as shown in a heatmap. PD, progressive disease; PR, partial response; SD, stable disease.
DISCUSSION
The results of this multicenter, open-label, single-arm phase II trial showed that adavosertib displayed promising preliminary antitumor activity in advanced malignancies harboring CCNE1 amplification, especially in 14 patients with CCNE1-amplified epithelial ovarian cancer: ORR = 36%; SD ≥ 6 months/PR = 57%, a median PFS duration of 6.3 months, and a median OS duration of 14.9 months. All patients who experienced a PR or SD for at least 6 months had a concurrent TP53 mutation.
More than 50 clinical trials of the WEE1 kinase inhibitor adavosertib, alone or in combination, have been conducted to determine its roles in sensitization to chemotherapy and radiation therapy.24-31 Antitumor activities were observed with adavosertib, as well as other WEE1 kinase inhibitors such as ZN-c3,32-36 supporting the use of a WEE1 kinase inhibitor for cancer therapy.
To the best of our knowledge, our reported findings represent the results of the first biomarker-driven study to demonstrate that adavosertib has substantial antitumor activity as a single agent in CCNE1-amplified malignancies, especially in epithelial ovarian carcinoma harboring CCNE1 amplification. Our results support the hypothesis that WEE1 kinase inhibition might be most active against cells that lose G1/S cell-cycle checkpoint control; these results are supported by the results of a randomized phase II study comparing adavosertib and gemcitabine with placebo and gemcitabine in patients with recurrent platinum-resistant or platinum-refractory ovarian cancer27; a phase I trial of adavosertib in advanced solid tumors that demonstrated baseline cyclin E1 overexpression in two responding patients and in none of the three nonresponding patients31; and a recently reported phase II study of adavosertib that showed 6% CR (n = 2) and 44% PR (n = 14) in the initial 32 patients with cyclin E1 overexpression and nonamplified CCNE1 patients with platinum-resistant high-grade serous ovarian cancer overexpressing cyclin E1 protein detected by immunohistochemistry (H-score > 50) without ≥ eight copies of CCNE1 by fluorescent in situ hybridization.37
In view of our observations, one important question is whether adavosertib treatment would be an effective mechanism-driven therapeutic strategy in a biomarker-driven patient selection trial. In a biomarker-driven study in recurrent small-cell lung cancer patients with MYC amplification or CDKN2A and TP53 coalterations, no response to adavosertib was observed.38 TP53 biomarker-driven clinical studies showed modest clinical benefit with adavosertib alone or in combination in patients with TP53-mutant platinum-resistant or platinum-refractory ovarian cancer,39,40 TP53-mutant platinum sensitive ovarian cancer,28 and TP53- and RAS-mutant metastatic colorectal cancer who were stable or responding after 16 weeks of chemotherapy.41
In this report, we did not identify concurrent deleterious BRCA1/2 mutations or microsatellite instability-high in this cohort of patients. Approximately 90% of patients with CCNE1 amplification had concurrent TP53 mutations. Objective responses were observed in eight of 27 patients with concurrent TP53 mutations but not in the three patients without. These data support the hypothesis that WEE1 kinase inhibition might be most active against cells that lose G1/S cell-cycle checkpoint control.
Proportionally high numbers of new lesions or rapid clinical deterioration were observed in eight patients treated with adavosertib, which might be due to mechanism-driven heterogeneity in response to WEE1 kinase inhibition. When several cell checkpoints simultaneously lose control as a result of genetic defects, such as CCNE1 amplification and TP53 aberration, and pharmacologic intervention through WEE1 kinase inhibition, cells are forced to go through cell cycling. Cells with low levels of accumulated DNA damage will continue to undergo cell cycling, resulting in tumor growth that may present clinically as new lesions, until those tumor cells accumulate high levels of DNA damage, leading to lethal genomic instability, mitotic catastrophe, and cell death and resulting in tumor shrinkage that may present clinically as a tumor response. If this theory is true, this may pose a clinical challenge for the further development of WEE1 kinase inhibition in the treatment of advanced malignancies using this synthetic lethality strategy.
When considering the clinical findings of this study, several caveats should be borne in mind. First, patient selection bias that is associated with the eligibility criteria may limit the generalizability of our findings, as it does in many clinical trials. Second, the small sample sizes in the subgroup analyses may limit the validity of the statistical assessments of individual pathological diagnoses. Third, this study might not test an optimal phase II dose for cancer therapy, in part because patients required excessive antiemetic support with steroid (10 days every 21 days), which might limit its potential systemic antitumor activity for its immunosuppressive effect.42,43 Fourth, this study allowed several different CLIA-certified molecular diagnostic assays to preidentify eligible patients, which might result in enrolling patients with different copy numbers of CCNE1 gene since the cutoffs for these assays may not be same. The ongoing correlative biomarker exploration including cyclin E1 expression levels by immunohistochemistry and RNA-seq, as well as copy numbers of CCNE1 gene by targeted genomic next-generation sequencing on tumor specimens obtained from this study, may facilitate learning and thinking of the definition of CCNE1 amplification in different assays.
In conclusion, 300 mg once daily of adavosertib, given by mouth for 5 days a week for 2 weeks, with 1 week off, was tolerated by patients with refractory solid tumors that harbored CCNE1 amplification, although a dose reduction may be eventually needed over time for better tolerance. The traditional strategy of including unselected patients with cancer may not meet the challenges of the current landscape of early drug development. Further evaluation of adavosertib or other WEE1-directed therapy, alone or in combination with other therapeutic agents targeting concurrent gene aberrations,44 is warranted in patients with advanced malignancies harboring CCNE1 amplification and TP53 aberration.
ACKNOWLEDGMENT
The authors thank the patients for their participation in this phase II clinical trial, and Ann M Sutton from Editing Services, Research Medical Library, The University of Texas MD Anderson Cancer Center, for a critical review of the manuscript.
APPENDIX
FIG A1.
Toxicity assessment board for frequencies and grades of (right) TEAEs and (left) TRAEs. ALP, alkaline phosphatase; GERD, gastroesophageal reflux disease; NOS, not otherwise specified; TEAE, treatment-emergent adverse event; TRAE, treatment-related adverse event; UTI, urinary tract infection.
FIG A2.
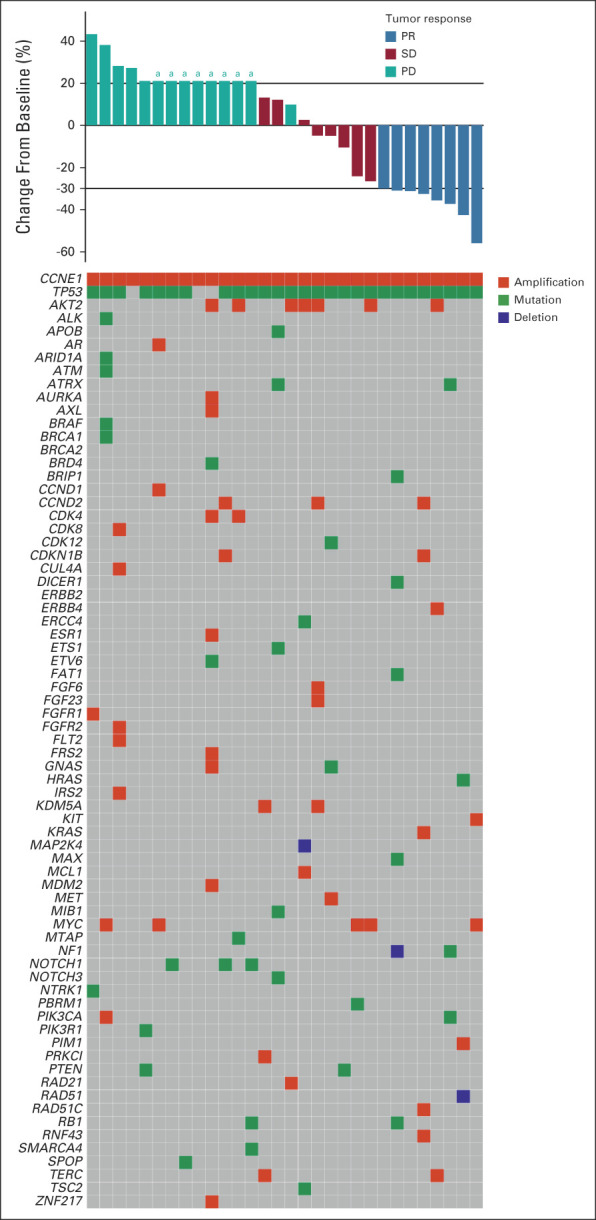
Baseline molecular profiling was performed per next-generation sequencing in a Clinical Laboratory Improvement Amendments–certified molecular diagnostic laboratory; the results are arranged according to their best tumor responses, as shown in the waterfall plot. aPatients had overall PD with < 20% increase on target lesions but had new nontarget lesions, or patients had rapid clinical deterioration. PD, progressive disease; PR, partial response; SD, stable disease.
TABLE A1.
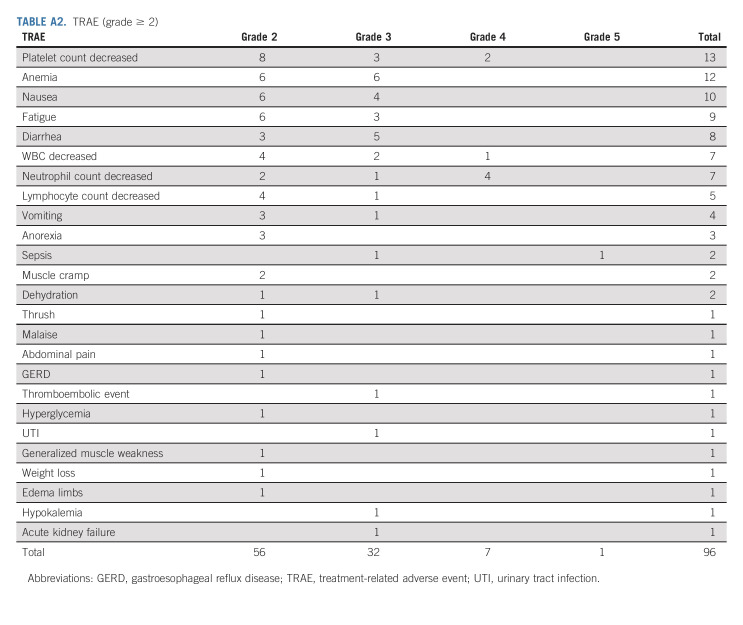
TEAE (grade ≥ 2)
TABLE A2.
TRAE (grade ≥ 2)
Yuan Yuan
Consulting or Advisory Role: Novartis, Pfizer, Eisai, Genentech, Immunomedics, Diiachi, BCI Pharma, Guardant Health
Speakers' Bureau: Eisai, Genentech, Daiichi Sankyo/Lilly, AstraZeneca, Gilead Sciences, Merck, Pfizer
Research Funding: Pfizer, Merck, Novartis, Genentech, Imugene
Expert Testimony: Novartis
Rebecca A. Previs
Employment: Labcorp
Consulting or Advisory Role: Myriad Genetics, Natera
Anthony D. Elias
Stock and Other Ownership Interests: AbbVie, Merck, Gilead Sciences, Allergan, Pfizer, Abbott Laboratories, Amgen, Bristol Myers Squibb, United Health Group, Align Oncology, Illumina, Exact Sciences, Lilly, Agilent, Cigna, Alexion Pharmaceuticals, Biogenerix
Research Funding: Astellas Pharma (Inst), Genentech (Inst), Deciphera (Inst), xencor (Inst), Infinity Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), TopAlliance BioSciences Inc (Inst), Orinove (Inst), BioAtla (Inst)
Uncompensated Relationships: Seiyax
Richard D. Carvajal
Consulting or Advisory Role: Merck, Aura Biosciences, Castle Biosciences, Immunocore, PureTech, Sorrento Therapeutics, Chimeron Bio, Rgenix, InxMed, Pierre Fabre, TriSalus Life Sciences, Iovance Biotherapeutics, Oncosec, Regeneron, Genzyme, Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb/Medarex (Inst), Corvus Pharmaceuticals (Inst), IDEAYA Biosciences (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Plexxikon (Inst), Roche/Genentech (Inst), Alkermes, Bristol Myers Squibb/Celgene, Delcath Systems, Eisai, Hengrui Pharmaceutical, Novartis
Speakers' Bureau: Bristol Myers Squibb/Medarex
Research Funding: Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), Bellicum Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals (Inst), Lilly (Inst), Immunocore (Inst), Incyte (Inst), Macrogenics (Inst), Merck (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Plexxikon (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), IDEAYA Biosciences (Inst), Regeneron (Inst)
Thomas J. George
Consulting or Advisory Role: Tempus, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Lilly (Inst), Bayer (Inst), Incyte (Inst), Ipsen (Inst), Seattle Genetics (Inst), Genentech (Inst), Astellas Pharma (Inst), BioMed Valley Discoveries (Inst), GlaxoSmithKline (Inst), Amgen (Inst), OncoC4 (Inst)
Open Payments Link: https://https://openpaymentsdata.cms.gov/physician/321938
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Ecaterina E. Dumbrava
Consulting or Advisory Role: Bolt Biotherapeutics
Research Funding: Bayer (Inst), Immunocore (Inst), Amgen (Inst), Aileron Therapeutics (Inst), Compugen (Inst), TRACON Pharma (Inst), Unum Therapeutics (Inst), Bolt Biotherapeutics (Inst), Aprea Therapeutics (Inst), Bellicum Pharmaceuticals (Inst), PMV Pharma (Inst), Triumvira Immunologics, Inc (Inst), Seattle Genetics (Inst), Mereo BioPharma 5 (Inst), Sanofi (Inst), Astex Pharmaceuticals (Inst), Immunomedics/Gilead (Inst), Rain Therapeutics (Inst), Gateway Foundation (Inst)
Daniel D. Karp
Research Funding: Phosplatin Therapeutics (Inst)
Travel, Accommodations, Expenses: Phosplatin Therapeutics
Sarina A. Piha-Paul
Consulting or Advisory Role: CRC Oncology
Research Funding: AbbVie (Inst), Aminex (Inst), Biomarin (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squib (Inst), Cerulean Pharma (Inst), Chugai Pharma (Inst), Curis (Inst), Five Prime Therapeutics (Inst), Genmab (Inst), GlaxoSmithKline (Inst), Helix BioPharma (Inst), Incyte (Inst), Jacobio (Inst), MedImmune (Inst), Medivation (Inst), Merck Sharp and Dohme Corp (Inst), Novartis (Inst), Pieris Pharmaceuticals (Inst), Pfizer (Inst), Principa Biopharma (Inst), Puma Biotechnology (Inst), RAPT Therapeutics (Inst), Seattle Genetics (Inst), Taiho Oncology (Inst), Tesaro (Inst), TransThera Biosciences (Inst), Amphivena Therapetics, Inc (Inst), Alkermes (Inst), Daichi Sanko (Inst), Lilly (Inst), ABM (Inst), Acepodia (Inst), ENB Therapeutics (Inst), Gene Quantum (Inst), Silverback Therapeutics (Inst), NIH/NCI (Inst), Cyclacel (Inst), F-Star Beta Limited (Inst), F-Star Therapeutics, Ltd (Inst), HiberCell (Inst), Immunomedics (Inst), Lytix Biopharma (Inst), Synologic Therapeutics (Inst), Gilead Sciences (Inst), Phanes Therapeutics (Inst), Purinomia Biotech, Inc (Inst), ZielBio, Inc (Inst), Hengrui Pharmaceuticals, Co, Ltd (Inst), Replimune (Inst)
Apostolia M. Tsimberidou
Consulting or Advisory Role: Vincerx Pharma, Diaccurate
Research Funding: IMMATICS (Inst), Karus Therapeutics (Inst), OBI Pharma (Inst), Tempus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Agenus (Inst), Novocure (Inst), Tvardi Therapeutics (Inst)
Jordi Rodon Ahnert
Consulting or Advisory Role: Peptomyc, Kelun Pharmaceuticals/Klus Pharma, Ellipses Pharma, Molecular Partners, iOnctura
Research Funding: Blueprint Medicines (Inst), Black Diamond Therapeutics (Inst), Merck Sharp & Dohme (Inst), Hummingbird (Inst), Yingli Pharma (Inst), Vall d'Hebron Institute of Oncology/Cancer Core Europe (Inst), Novartis (Inst), Spectrum Pharmaceuticals (Inst), Symphogen (Inst), BioAtla (Inst), Pfizer (Inst), Genmab (Inst), CytomX Therapeutics (Inst), Kelun (Inst), Takeda/Millennium (Inst), GlaxoSmithKline (Inst), Taiho Pharmaceutical (Inst), Roche (Inst), Bicycle Therapeutics (Inst), Merus (Inst), Curis (Inst), Bayer (Inst), AADi (Inst), Nuvation Bio (Inst), Fore Biotherapeutics (Inst), BioMed Valley Discoveries (Inst), Loxo (Inst), Hutchison MediPharma (Inst), Cellestia Biotech (Inst), Deciphera (Inst), IDEAYA Biosciences (Inst), Amgen (Inst), Tango Therapeutics (Inst), Mirati Therapeutics (Inst), Linnaeus Therapeutics (Inst)
Travel, Accommodations, Expenses: ESMO
Other Relationship: Vall d'Hebron Institute of Oncology/Ministerio De Empleo Y Seguridad Social, Chinese University of Hong Kong, Boxer Capital, Tang Advisors
Khandan Keyomarsi
Stock and Other Ownership Interests: pfizer
Research Funding: Repare Therapeutics (Inst), Apeiron Biologics (Inst), Blueprint Medicines (Inst), Schrodinger, Inc (Inst), NIH, National Cancer Institute R01 grants CA255960 and CA223772 and CPRIT multi-investigator grant RP180712
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Xencor, Debiopharm Group, Roche, PACT Pharma, eFFECTOR Therapeutics, Kolon Life Sciences, Tyra Biosciences, Zymeworks, Zentalis, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seattle Genetics, Loxo, Silverback Therapeutics
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seattle Genetics (Inst), Klus Pharma (Inst), Takeda (Inst)
No other potential conflicts of interest were reported.
See accompanying article on page 1770
SUPPORT
Supported by Cancer Therapy Evaluation Program National Cancer Institution (NCI CTEP). This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA016672.
CLINICAL TRIAL INFORMATION
NCI Experimental Therapeutics Clinical Trials Network (ETCTN) trial: NCI 10136
DATA SHARING STATEMENT
Adavosertib is an investigational agent; hence, the data collected for this study will not be made available to others.
AUTHOR CONTRIBUTIONS
Conception and design: Siqing Fu, Ying Yuan, Naoko Takebe, Khandan Keyomarsi, Funda Meric-Bernstam
Financial support: Siqing Fu, Funda Meric-Bernstam
Administrative support: Siqing Fu, Naoko Takebe, Funda Meric-Bernstam
Provision of study materials or patients: Siqing Fu, Yuan Yuan, Rebecca A. Previs, Anthony D. Elias, Richard D. Carvajal, Thomas J. George, Ying Yuan, Shannon N. Westin, Yan Xing, Ecaterina E. Dumbrava, Daniel D. Karp, Sarina A. Piha-Paul, Apostolia M. Tsimberidou, Naoko Takebe, Karen Lu, Funda Meric-Bernstam
Collection and assembly of data: Yuan Yuan, Anthony D. Elias, Richard D. Carvajal, Ying Yuan, Lihou Yu, Yan Xing, Daniel D. Karp, Sarina A. Piha-Paul, Apostolia M. Tsimberidou, Jordi Rodon Ahnert, Karen Lu, Funda Meric-Bernstam
Data analysis and interpretation: Siqing Fu, Shuyang Yao, Yuan Yuan, Rebecca A. Previs, Anthony D. Elias, Thomas J. George, Richard D. Carvajal, Ying Yuan, Lihou Yu, Shannon N. Westin, Yan Xing, Ecaterina E. Dumbrava, Daniel D. Karp, Sarina A. Piha-Paul, Apostolia M. Tsimberidou, Jordi Rodon Ahnert, Naoko Takebe, Karen Lu, Khandan Keyomarsi, Funda Meric-Bernstam
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Multicenter Phase II Trial of the WEE1 Inhibitor Adavosertib in Refractory Solid Tumors Harboring CCNE1 Amplification
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Siqing Fu
Research Funding: Novartis (Inst), NeuPharma, Inc (Inst), BeiGene (Inst), MacroGenics (Inst), BioAtla (Inst), Parexel International, LLC (Inst), Boehringer Ingelheim (Inst), Abbisko (Inst), Lilly (Inst), Hookipa Pharma (Inst), IMV (Inst), Innovent Biologics (Inst), Lyvgen Biopharma (Inst), Millennium (Inst), Nerviano Medical Sciences (Inst), NIH/NCI (Inst), Sellas Life Sciences (Inst), Soricimed (Inst), NovoCure (Inst), Turnstone Bio (Inst), Taiho Oncology (Inst), NCCN (Inst), Exelis (Inst), K-Group Beta (Inst), NextCure (Inst), Ningbo NewBay Medical Technology (Inst), PureTech (Inst), SQZ Biotech (Inst), Sumitomo Dainippon Pharma Oncology (Inst), Treadwell Therapeutics (Inst), Tyligand Bioscience (Inst), Vaccibody (Inst), Greenfire Bio (Inst)
Yuan Yuan
Consulting or Advisory Role: Novartis, Pfizer, Eisai, Genentech, Immunomedics, Diiachi, BCI Pharma, Guardant Health
Speakers' Bureau: Eisai, Genentech, Daiichi Sankyo/Lilly, AstraZeneca, Gilead Sciences, Merck, Pfizer
Research Funding: Pfizer, Merck, Novartis, Genentech, Imugene
Expert Testimony: Novartis
Rebecca A. Previs
Employment: Labcorp
Consulting or Advisory Role: Myriad Genetics, Natera
Anthony D. Elias
Stock and Other Ownership Interests: AbbVie, Merck, Gilead Sciences, Allergan, Pfizer, Abbott Laboratories, Amgen, Bristol Myers Squibb, United Health Group, Align Oncology, Illumina, Exact Sciences, Lilly, Agilent, Cigna, Alexion Pharmaceuticals, Biogenerix
Research Funding: Astellas Pharma (Inst), Genentech (Inst), Deciphera (Inst), xencor (Inst), Infinity Pharmaceuticals (Inst), Karyopharm Therapeutics (Inst), TopAlliance BioSciences Inc (Inst), Orinove (Inst), BioAtla (Inst)
Uncompensated Relationships: Seiyax
Richard D. Carvajal
Consulting or Advisory Role: Merck, Aura Biosciences, Castle Biosciences, Immunocore, PureTech, Sorrento Therapeutics, Chimeron Bio, Rgenix, InxMed, Pierre Fabre, TriSalus Life Sciences, Iovance Biotherapeutics, Oncosec, Regeneron, Genzyme, Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bristol Myers Squibb/Medarex (Inst), Corvus Pharmaceuticals (Inst), IDEAYA Biosciences (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Plexxikon (Inst), Roche/Genentech (Inst), Alkermes, Bristol Myers Squibb/Celgene, Delcath Systems, Eisai, Hengrui Pharmaceutical, Novartis
Speakers' Bureau: Bristol Myers Squibb/Medarex
Research Funding: Amgen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), Bellicum Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Corvus Pharmaceuticals (Inst), Lilly (Inst), Immunocore (Inst), Incyte (Inst), Macrogenics (Inst), Merck (Inst), Mirati Therapeutics (Inst), Novartis (Inst), Pfizer (Inst), Plexxikon (Inst), Roche/Genentech (Inst), Array BioPharma (Inst), IDEAYA Biosciences (Inst), Regeneron (Inst)
Thomas J. George
Consulting or Advisory Role: Tempus, Pfizer
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Lilly (Inst), Bayer (Inst), Incyte (Inst), Ipsen (Inst), Seattle Genetics (Inst), Genentech (Inst), Astellas Pharma (Inst), BioMed Valley Discoveries (Inst), GlaxoSmithKline (Inst), Amgen (Inst), OncoC4 (Inst)
Open Payments Link: https://https://openpaymentsdata.cms.gov/physician/321938
Shannon N. Westin
Consulting or Advisory Role: Roche, AstraZeneca, Genentech, Medscape, Clovis Oncology, Gerson Lehrman Group, Vaniam Group, Merck, BioAscent, OncLive, Targeted Oncology, Curio Science, GlaxoSmithKline, Eisai, Zentalis, Agenus, EQRX, Lilly, Vincerx Pharma, Mereo BioPharma, Immunogen, Mersana
Research Funding: AstraZeneca (Inst), Novartis (Inst), Bayer (Inst), Cotinga Pharmaceuticals (Inst), Clovis Oncology (Inst), Roche/Genentech (Inst), GOG Foundation (Inst), Mereo BioPharma (Inst), Bio-Path Holdings, Inc (Inst), GlaxoSmithKline (Inst), OncXerna Therapeutics (Inst), Zentalis (Inst)
Ecaterina E. Dumbrava
Consulting or Advisory Role: Bolt Biotherapeutics
Research Funding: Bayer (Inst), Immunocore (Inst), Amgen (Inst), Aileron Therapeutics (Inst), Compugen (Inst), TRACON Pharma (Inst), Unum Therapeutics (Inst), Bolt Biotherapeutics (Inst), Aprea Therapeutics (Inst), Bellicum Pharmaceuticals (Inst), PMV Pharma (Inst), Triumvira Immunologics, Inc (Inst), Seattle Genetics (Inst), Mereo BioPharma 5 (Inst), Sanofi (Inst), Astex Pharmaceuticals (Inst), Immunomedics/Gilead (Inst), Rain Therapeutics (Inst), Gateway Foundation (Inst)
Daniel D. Karp
Research Funding: Phosplatin Therapeutics (Inst)
Travel, Accommodations, Expenses: Phosplatin Therapeutics
Sarina A. Piha-Paul
Consulting or Advisory Role: CRC Oncology
Research Funding: AbbVie (Inst), Aminex (Inst), Biomarin (Inst), Boehringer Ingelheim (Inst), Bristol Myers Squib (Inst), Cerulean Pharma (Inst), Chugai Pharma (Inst), Curis (Inst), Five Prime Therapeutics (Inst), Genmab (Inst), GlaxoSmithKline (Inst), Helix BioPharma (Inst), Incyte (Inst), Jacobio (Inst), MedImmune (Inst), Medivation (Inst), Merck Sharp and Dohme Corp (Inst), Novartis (Inst), Pieris Pharmaceuticals (Inst), Pfizer (Inst), Principa Biopharma (Inst), Puma Biotechnology (Inst), RAPT Therapeutics (Inst), Seattle Genetics (Inst), Taiho Oncology (Inst), Tesaro (Inst), TransThera Biosciences (Inst), Amphivena Therapetics, Inc (Inst), Alkermes (Inst), Daichi Sanko (Inst), Lilly (Inst), ABM (Inst), Acepodia (Inst), ENB Therapeutics (Inst), Gene Quantum (Inst), Silverback Therapeutics (Inst), NIH/NCI (Inst), Cyclacel (Inst), F-Star Beta Limited (Inst), F-Star Therapeutics, Ltd (Inst), HiberCell (Inst), Immunomedics (Inst), Lytix Biopharma (Inst), Synologic Therapeutics (Inst), Gilead Sciences (Inst), Phanes Therapeutics (Inst), Purinomia Biotech, Inc (Inst), ZielBio, Inc (Inst), Hengrui Pharmaceuticals, Co, Ltd (Inst), Replimune (Inst)
Apostolia M. Tsimberidou
Consulting or Advisory Role: Vincerx Pharma, Diaccurate
Research Funding: IMMATICS (Inst), Karus Therapeutics (Inst), OBI Pharma (Inst), Tempus (Inst), Parker Institute for Cancer Immunotherapy (Inst), Agenus (Inst), Novocure (Inst), Tvardi Therapeutics (Inst)
Jordi Rodon Ahnert
Consulting or Advisory Role: Peptomyc, Kelun Pharmaceuticals/Klus Pharma, Ellipses Pharma, Molecular Partners, iOnctura
Research Funding: Blueprint Medicines (Inst), Black Diamond Therapeutics (Inst), Merck Sharp & Dohme (Inst), Hummingbird (Inst), Yingli Pharma (Inst), Vall d'Hebron Institute of Oncology/Cancer Core Europe (Inst), Novartis (Inst), Spectrum Pharmaceuticals (Inst), Symphogen (Inst), BioAtla (Inst), Pfizer (Inst), Genmab (Inst), CytomX Therapeutics (Inst), Kelun (Inst), Takeda/Millennium (Inst), GlaxoSmithKline (Inst), Taiho Pharmaceutical (Inst), Roche (Inst), Bicycle Therapeutics (Inst), Merus (Inst), Curis (Inst), Bayer (Inst), AADi (Inst), Nuvation Bio (Inst), Fore Biotherapeutics (Inst), BioMed Valley Discoveries (Inst), Loxo (Inst), Hutchison MediPharma (Inst), Cellestia Biotech (Inst), Deciphera (Inst), IDEAYA Biosciences (Inst), Amgen (Inst), Tango Therapeutics (Inst), Mirati Therapeutics (Inst), Linnaeus Therapeutics (Inst)
Travel, Accommodations, Expenses: ESMO
Other Relationship: Vall d'Hebron Institute of Oncology/Ministerio De Empleo Y Seguridad Social, Chinese University of Hong Kong, Boxer Capital, Tang Advisors
Khandan Keyomarsi
Stock and Other Ownership Interests: pfizer
Research Funding: Repare Therapeutics (Inst), Apeiron Biologics (Inst), Blueprint Medicines (Inst), Schrodinger, Inc (Inst), NIH, National Cancer Institute R01 grants CA255960 and CA223772 and CPRIT multi-investigator grant RP180712
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: Xencor, Debiopharm Group, Roche, PACT Pharma, eFFECTOR Therapeutics, Kolon Life Sciences, Tyra Biosciences, Zymeworks, Zentalis, Infinity Pharmaceuticals, AbbVie, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seattle Genetics, Loxo, Silverback Therapeutics
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seattle Genetics (Inst), Klus Pharma (Inst), Takeda (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Malumbres M, Barbacid M: Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer 9:153-166, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Otto T, Sicinski P: Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17:93-115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lew DJ, Dulic V, Reed SI: Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66:1197-1206, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Pils D, Bachmayr-Heyda A, Auer K, et al. : Cyclin E1 (CCNE1) as independent positive prognostic factor in advanced stage serous ovarian cancer patients - a study of the OVCAD consortium. Eur J Cancer 50:99-110, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Keyomarsi K, Tucker SL, Buchholz TA, et al. : Cyclin E and survival in patients with breast cancer. N Engl J Med 347:1566-1575, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Santala S, Talvensaari-Mattila A, Soini Y, et al. : Cyclin E expression correlates with cancer-specific survival in endometrial endometrioid adenocarcinoma. Anticancer Res 35:3393-3397, 2015 [PubMed] [Google Scholar]
- 7.Cassia R, Moreno-Bueno G, Rodriguez-Perales S, et al. : Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J Pathol 201:589-595, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Huang LN, Wang DS, Chen YQ, et al. : Meta-analysis for cyclin E in lung cancer survival. Clin Chim Acta 413:663-668, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Bandla S, Ye J, et al. : Cyclin E involved in early stage carcinogenesis of esophageal adenocarcinoma by SNP DNA microarray and immunohistochemical studies. BMC Gastroenterol 14:78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsina M, Landolfi S, Aura C, et al. : Cyclin E amplification/overexpression is associated with poor prognosis in gastric cancer. Ann Oncol 26:438-439, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Beck H, Nahse-Kumpf V, Larsen MS, et al. : Cyclin-dependent kinase suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption. Mol Cell Biol 32:4226-4236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan K, Mahajan NP: WEE1 tyrosine kinase, a novel epigenetic modifier. Trends Genet 29:394-402, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aarts M, Sharpe R, Garcia-Murillas I, et al. : Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov 2:524-539, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Kogiso T, Nagahara H, Hashimoto E, et al. : Efficient induction of apoptosis by wee1 kinase inhibition in hepatocellular carcinoma cells. PLoS One 9:e100495, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mir SE, De Witt Hamer PC, Krawczyk PM, et al. : In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 18:244-257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PS, Venkataraman S, Alimova I, et al. : Integrated genomic analysis identifies the mitotic checkpoint kinase WEE1 as a novel therapeutic target in medulloblastoma. Mol Cancer 13:72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Low KH, Alexander A, et al. : Cyclin E overexpression sensitizes triple-negative breast cancer to Wee1 kinase inhibition. Clin Cancer Res 24:6594-6610, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer TM, Jones SF, Greenlees C, et al. : A phase 1b, open-label, multi-center study to assess the safety, tolerability, pharmacokinetics and anti-tumor activity of AZD1775 monotherapy in patients with advanced solid tumors: Expansion cohorts. J Clin Oncol 34, 2016. (suppl 15; abstr TPS2608) [Google Scholar]
- 19.Loden M, Stighall M, Nielsen NH, et al. : The cyclin D1 high and cyclin E high subgroups of breast cancer: Separate pathways in tumorogenesis based on pattern of genetic aberrations and inactivation of the pRb node. Oncogene 21:4680-4690, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Gorski JW, Ueland FR, Kolesar JM: CCNE1 amplification as a predictive biomarker of chemotherapy resistance in epithelial ovarian cancer. Diagnostics (Basel) 10:279, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Lee JJ, Yuan Y: BOP2: Bayesian optimal design for phase II clinical trials with simple and complex endpoints. Stat Med 36:3302-3314, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Simon R: Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1-10, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Fu S, Wang Y, Keyomarsi K, et al. : Strategic development of AZD1775, a Wee1 kinase inhibitor, for cancer therapy. Expert Opin Investig Drugs 27:741-751, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Kong A, Mehanna H: WEE1 inhibitor: Clinical development. Curr Oncol Rep 23:107, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JF, Xiong N, Campos SM, et al. : Phase II study of the WEE1 inhibitor adavosertib in recurrent uterine serous carcinoma. J Clin Oncol 39:1531-1539, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Lheureux S, Cristea MC, Bruce JP, et al. : Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397:281-292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oza AM, Estevez-Diz M, Grischke EM, et al. : A biomarker-enriched, randomized phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer. Clin Cancer Res 26:4767-4776, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Keenan TE, Li T, Vallius T, et al. : Clinical efficacy and molecular response correlates of the WEE1 inhibitor adavosertib combined with cisplatin in patients with metastatic triple-negative breast cancer. Clin Cancer Res 27:983-991, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuneo KC, Morgan MA, Sahai V, et al. : Dose escalation trial of the Wee1 inhibitor adavosertib (AZD1775) in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer. J Clin Oncol 37:2643-2650, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takebe N, Naqash AR, O'Sullivan Coyne G, et al. : Safety, antitumor activity, and biomarker analysis in a phase I trial of the once-daily Wee1 inhibitor adavosertib (AZD1775) in patients with advanced solid tumors. Clin Cancer Res 27:3834-3844, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang PQ, Boren BC, Hegde SG, et al. : Discovery of ZN-c3, a highly potent and selective Wee1 inhibitor undergoing evaluation in clinical trials for the treatment of cancer. J Med Chem 64:13004-13024, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Tolcher A, Mamdani H, Chalasani P, et al. : Clinical activity of single-agent ZN-c3, an oral WEE1 inhibitor, in a phase 1 dose-escalation trial in patients with advanced solid tumors. Cancer Res 81, 2021. (suppl 13; abstr CT016) [Google Scholar]
- 34.Meric-Bernstam F, Chalsani P, Mamdani H, et al. : Safety and clinical activity of single-agent ZN-c3, an oral WEE1 inhibitor, in a phase 1 trial in subjects with recurrent or advanced uterine serous carcinoma (USC). Cancer Res 82, 2022. (suppl 12; abstr CT029) [Google Scholar]
- 35.Fu S, Pasic A, Richardson G, et al. : 562TiP A phase Ib dose-escalation study of ZN-c3, a WEE1 inhibitor, in combination with chemotherapy in patients with platinum-resistant or -refractory ovarian, peritoneal, or fallopian tube cancer. Ann Oncol 32:S618, 2021 [Google Scholar]
- 36.Pasic A, Richardson G, Vranjes Z, et al. : A phase 1b dose-escalation study of ZN-c3, a WEE1 inhibitor, in combination with chemotherapy (CT) in subjects with platinum-resistant or refractory ovarian, peritoneal, or fallopian tube cancer. Cancer Res 82, 2022. (abstr CT148) [Google Scholar]
- 37.Au-Yeung G, Bressel M, Prall O, et al. : IGNITE: A phase II signal-seeking trial of adavosertib targeting recurrent high-grade, serous ovarian cancer with cyclin E1 overexpression with and without gene amplification. J Clin Oncol 40, 2022. (suppl 16; abstr 5515) [Google Scholar]
- 38.Park S, Shim J, Mortimer PGS, et al. : Biomarker-driven phase 2 umbrella trial study for patients with recurrent small cell lung cancer failing platinum-based chemotherapy. Cancer 126:4002-4012, 2020 [DOI] [PubMed] [Google Scholar]
- 39.Leijen S, van Geel RM, Sonke GS, et al. : Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol 34:4354-4361, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Embaby A, Geenen JJ, Kutzera J, et al. : Adavosertib in combination with carboplatin in advanced TP53-mutated platinum-resistant ovarian cancer. J Clin Oncol 40, 2022. (suppl 16; abstr 5516) [DOI] [PubMed] [Google Scholar]
- 41.Seligmann JF, Fisher DJ, Brown LC, et al. : Inhibition of WEE1 is effective in TP53- and RAS-mutant metastatic colorectal cancer: A randomized trial (FOCUS4-C) comparing adavosertib (AZD1775) with active monitoring. J Clin Oncol 39:3705-3715, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olejniczak I, Oster H, Ray DW: Glucocorticoid circadian rhythms in immune function. Semin Immunopathol 44:153-163, 2022 [DOI] [PubMed] [Google Scholar]
- 43.Taves MD, Ashwell JD: Glucocorticoids in T cell development, differentiation and function. Nat Rev Immunol 21:233-243, 2021 [DOI] [PubMed] [Google Scholar]
- 44.Yao S, Meric-Bernstam F, Hong D, et al. : Clinical characteristics and outcomes of phase I cancer patients with CCNE1 amplification: MD Anderson experiences. Sci Rep 12:8701, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Adavosertib is an investigational agent; hence, the data collected for this study will not be made available to others.