Abstract
PURPOSE
Brexucabtagene autoleucel (brexu-cel) is an autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy approved for relapsed/refractory mantle cell lymphoma (MCL). This therapy was approved on the basis of the single-arm phase II ZUMA-2 trial, which showed best overall and complete response rates of 91% and 68%, respectively. We report clinical outcomes with brexu-cel in the standard-of-care setting for the approved indication.
PATIENTS AND METHODS
Patients who underwent leukapheresis between August 1, 2020 and December 31, 2021, at 16 US institutions, with an intent to manufacture commercial brexu-cel for relapsed/refractory MCL, were included. Patient data were collected for analyses of responses, outcomes, and toxicities as per standard guidelines.
RESULTS
Of 189 patients who underwent leukapheresis, 168 (89%) received brexu-cel infusion. Of leukapheresed patients, 79% would not have met ZUMA-2 eligibility criteria. Best overall and complete response rates were 90% and 82%, respectively. At a median follow-up of 14.3 months after infusion, the estimates for 6- and 12-month progression-free survival (PFS) were 69% (95% CI, 61 to 75) and 59% (95% CI, 51 to 66), respectively. The nonrelapse mortality was 9.1% at 1 year, primarily because of infections. Grade 3 or higher cytokine release syndrome and neurotoxicity occurred in 8% and 32%, respectively. In univariable analysis, high-risk simplified MCL international prognostic index, high Ki-67, TP53 aberration, complex karyotype, and blastoid/pleomorphic variant were associated with shorter PFS after brexu-cel infusion. Patients with recent bendamustine exposure (within 24 months before leukapheresis) had shorter PFS and overall survival after leukapheresis in intention-to-treat univariable analysis.
CONCLUSION
In the standard-of-care setting, the efficacy and toxicity of brexu-cel were consistent with those reported in the ZUMA-2 trial. Tumor-intrinsic features of MCL, and possibly recent bendamustine exposure, may be associated with inferior efficacy outcomes.
INTRODUCTION
Mantle cell lymphoma (MCL) is a mature B-cell lymphoma with heterogenous clinical behavior ranging from indolent to aggressive.1 Traditional high-risk features2 include high MCL international prognostic index (MIPI),3,4 high Ki-67 proliferation index,5 blastoid or pleomorphic variant,6 TP53 aberration,7-9 complex karyotype,10,11 and progression of disease within 24 months of first-line therapy (POD24).12,13 Treatment of relapsed or refractory (R/R) MCL is challenging. Bruton's tyrosine kinase (BTK) inhibitors (BTKi) are efficacious but not curative,14-19 and the prognosis of R/R MCL after BTKi failure is poor.20-22
CONTEXT
Key Objective
Sixteen US centers sought to delineate the characteristics and outcomes of patients with relapsed or refractory mantle cell lymphoma treated with brexucabtagene autoleucel (brexu-cel), an autologous anti-CD19 chimeric antigen receptor T-cell product, in standard-of-care practice.
Knowledge Generated
Compared with ZUMA-2, more patients with high-risk features and/or comorbidities were treated with brexu-cel in standard-of-care practice. Sixty-five percent of patients would have been ineligible for ZUMA-2 because of disease status or comorbidities. Despite this, safety and efficacy outcomes were comparable with ZUMA-2. Tumor-intrinsic high-risk features were associated with inferior progression-free survival.
Relevance (J.W. Friedberg)
-
These results further inform the use of brexu-cel for patients with relapsed mantle cell lymphoma, emphasize a higher risk of infectious deaths than previously observed in pivotal trials, and suggest that recent bendamustine exposure may contribute to inferior outcomes in this setting.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Brexucabtagene autoleucel (brexu-cel) is the first US Food and Drug Administration (FDA)–approved autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy23 for R/R MCL. In the pivotal ZUMA-2 study, the objective response rate (ORR) and the complete response (CR) rate were 91% and 68%, respectively.23,24 Three-year follow-up of this study demonstrated durable responses, with a median duration of response (DOR) of 28.2 months, a median progression-free survival (PFS) of 25.8 months, and a median overall survival (OS) of 46.6 months in all 68 treated patients.24
Patients treated in clinical trials often differ from those treated in standard-of-care practice. The ZUMA-2 study had stringent eligibility criteria requiring prior BTKi exposure, adequate organ function, and limited comorbidities and only allowed BTKi and/or corticosteroids for bridging therapy after leukapheresis but before conditioning chemotherapy. By contrast, the standard-of-care indication on the basis of the US FDA label for brexu-cel includes all adult patients with R/R MCL regardless of comorbidities and prior treatment.
We investigated the safety and efficacy of brexu-cel in R/R MCL in standard-of-care practice among US Lymphoma CAR T Consortium25 centers. Subset analyses were explored, including outcomes in BTKi-naïve patients and potential impact of bridging therapy and prior bendamustine exposure.
PATIENTS AND METHODS
Study Design and Participants
Sixteen centers participated in this retrospective study (Appendix Fig A1, online only), and each center obtained independent institutional review board approval. All patients who underwent leukapheresis between August 1, 2020 and December 31, 2021, with an intent to manufacture commercial brexu-cel, were included. Baseline clinical and pathologic characteristics at leukapheresis were abstracted retrospectively, and eligibility for ZUMA-2 was retrospectively determined.
Treatment and Clinical Assessment
Bridging therapy was at the discretion of treating physicians. Conditioning chemotherapy with cyclophosphamide and fludarabine was administered in the same dose and schedule as in ZUMA-2.23 Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded according to American Society for Transplantation and Cellular Therapy criteria.26 Lymphoma response to therapy was assessed by treating physicians according to 2014 Lugano criteria.27
Statistical Methods
PFS was defined as the time from brexu-cel infusion (or leukapheresis in intention-to-treat [ITT] analysis) to disease progression or death. OS was defined as the time from brexu-cel infusion (or leukapheresis in ITT analysis) to death. DOR was defined as the time from initial response to disease progression or death. The Kaplan-Meier method was used to estimate DOR, PFS, and OS rates. Cox proportional hazards models were used to evaluate the association of clinical and pathologic variables with PFS or OS. Cumulative incidences of nonrelapse mortality and disease progression/relapse were analyzed in a competing risk model. Chi-square or Fisher's exact test was used to evaluate the association between categorical variables. The Wilcoxon rank-sum test or Kruskal-Wallis test was used to evaluate the difference in a continuous variable between patient groups. 95% CIs were used for point estimates, and all P values reported are unadjusted for multiple comparisons. Statistical analyses were performed using the IBM SPSS Statistics software (v25, Armonk, NY).
RESULTS
Patient Characteristics
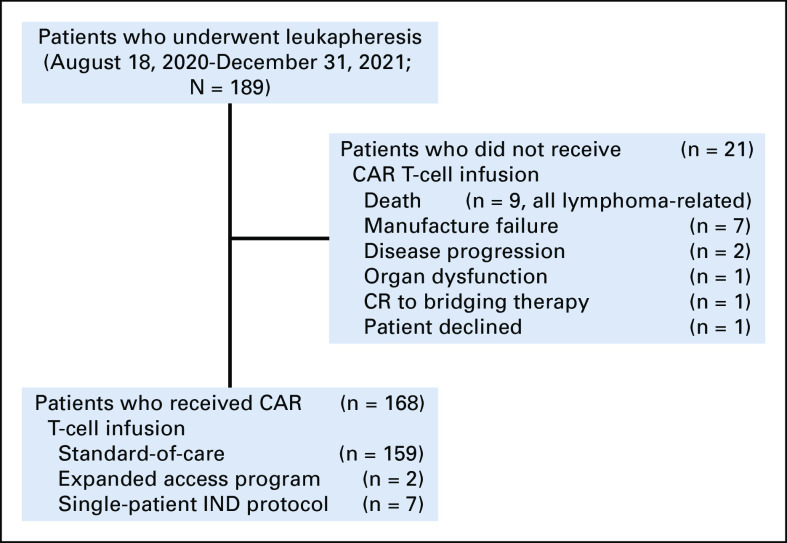
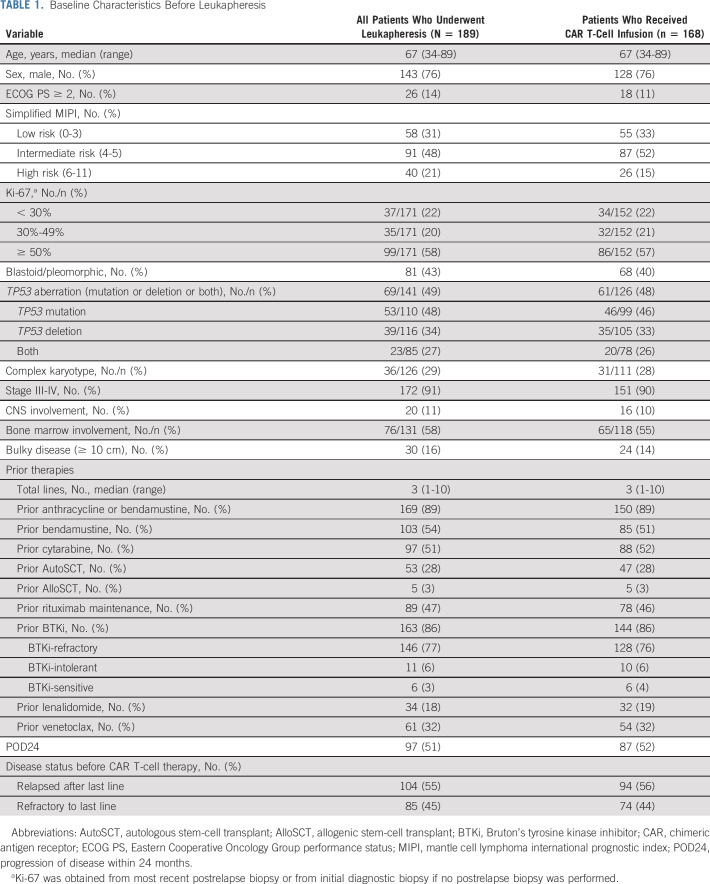
As of December 31, 2021, 189 patients completed leukapheresis (Fig 1). Baseline characteristics of all patients are summarized in Table 1. The median age was 67 (range, 34-89) years, and 76% were male. High-risk prognostic features included high-risk simplified MIPI in 21%, Ki-67 ≥ 50% in 58%, blastoid/pleomorphic variant in 43%, TP53 aberration (mutation, deletion, or both) in 49%, complex karyotype in 29%, and POD24 in 51%. The median number of prior lines of therapy was three (range, 1-10), and 77% had disease progression on a BTKi.
FIG 1.
Patient flow diagram. CAR, chimeric antigen receptor; CR, complete response; IND, investigational new drug.
TABLE 1.
Baseline Characteristics Before Leukapheresis
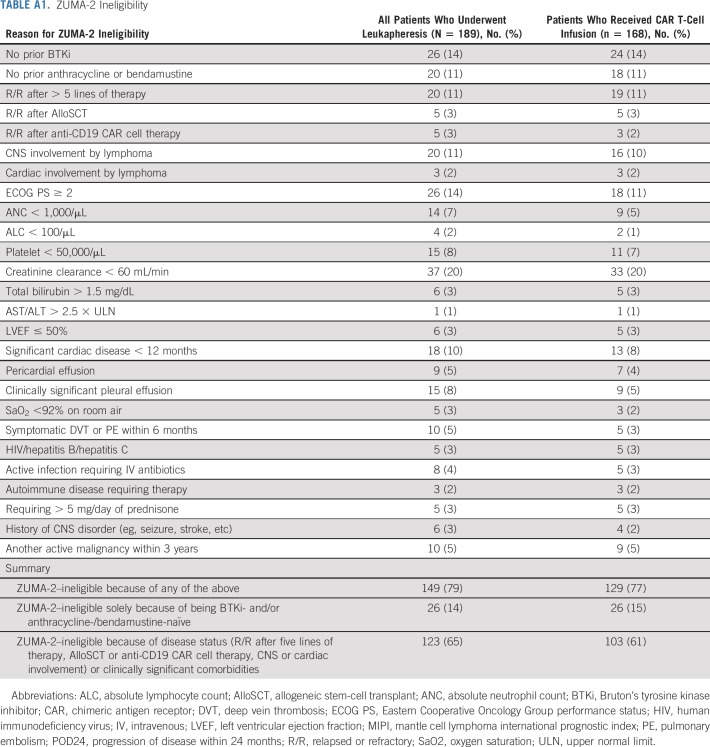
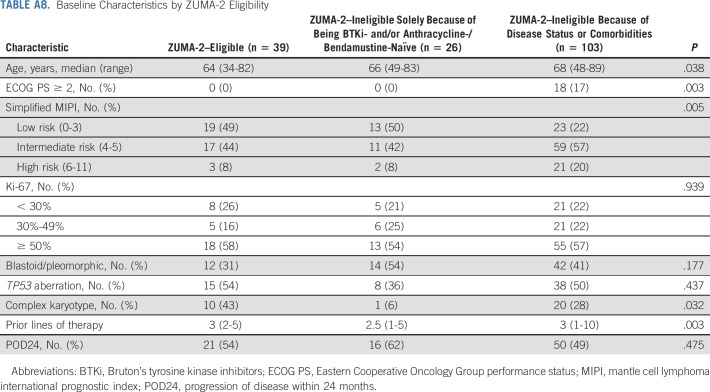
Of all leukapheresed patients, 149 (79%) patients would not have met ZUMA-2 eligibility criteria, and the most common reasons included prior therapies (eg, BTKi-naïve 14%, anthracycline-/bendamustine-naïve 11%, and > 5 lines of prior therapy 11%), disease status (eg, CNS involvement 11%), and comorbidities (eg, creatinine clearance < 60 mL/min 20%, Eastern Cooperative Oncology Group performance status [ECOG PS] ≥ 2 14%, cardiac disease 10%, pleural effusion 8%, platelet < 50,000/µL 8%, and absolute neutrophil count < 1,000/µL 7%; Appendix Table A1, online only). In total, 14% of leukapheresed patients would have been ineligible for ZUMA-2 on the sole basis of being BTKi- and/or anthracycline-/bendamustine-naïve and 65% of patients would have been ineligible for ZUMA-2 on the basis of disease status or clinically significant comorbidities.
Bridging Therapy and Infusion
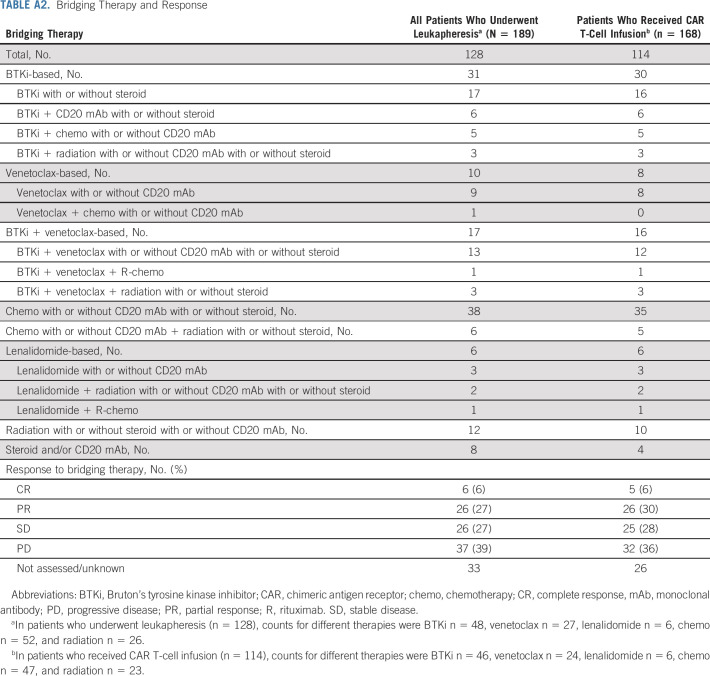
Bridging therapy was used in 128 (68%) patients and included BTKi-based (n = 31), venetoclax-based (n = 10), BTKi and venetoclax combination–based (n = 17), chemotherapy-based (n = 44), lenalidomide-based (n = 6), radiation-based (n = 12), and anti-CD20 antibodies and/or corticosteroids (n = 8; Appendix Table A2, online only). Response to bridging was assessed in 95 (74%) of 128 patients. In assessed patients, the ORR to bridging therapies was 33% (6% CR and 27% partial response [PR]).
Twenty-one (11%) patients did not receive brexu-cel infusion, because of the following reasons: death before infusion (n = 9), manufacturing failure (n = 7), disease progression (n = 2), organ dysfunction (n = 1), CR to bridging therapy (n = 1), or patient declined to proceed (n = 1). Of the 168 patients who received CAR T-cell infusion, 159 received commercial brexu-cel and nine patients received an out-of-specification product through the Expanded Access Program (n = 2) or on single-patient Investigational New Drug protocols (n = 7; Fig 1).
Baseline characteristics of the 168 patients who received brexu-cel infusion are summarized in Table 1, and ZUMA-2 ineligibility and bridging therapy characteristics are summarized in Appendix Tables A1 and A2, respectively. The median time from leukapheresis to conditioning chemotherapy was 28 days (range, 17-140), and the median time from conditioning chemotherapy to brexu-cel infusion was 5 days (range, 5-15).
Safety
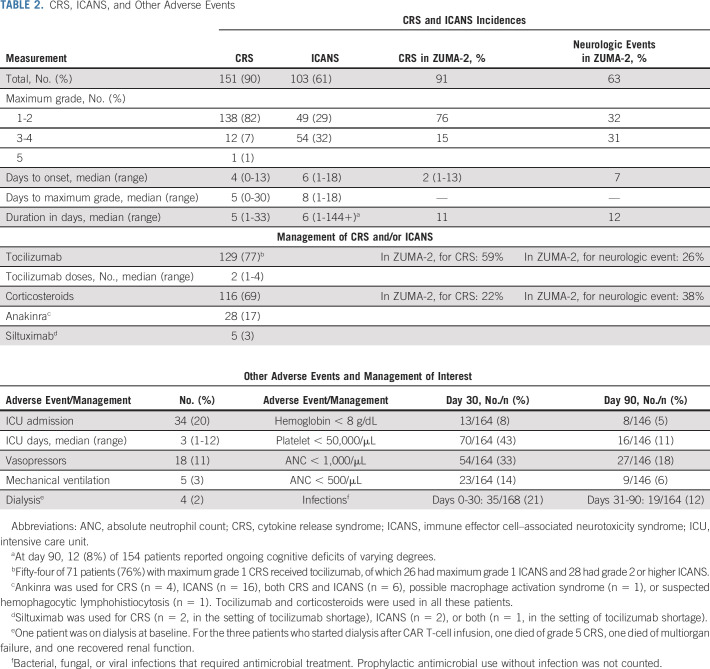
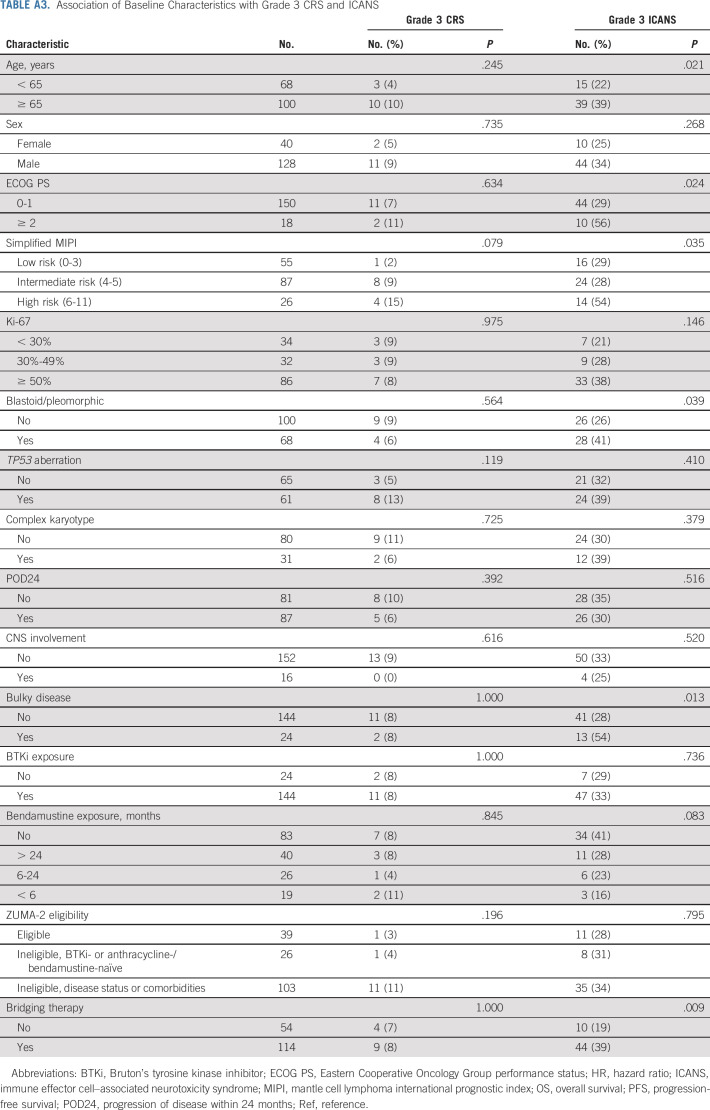
In patients who received brexu-cel infusion, the incidence rate of CRS was 90% (8% grade ≥ 3) and the incidence rate of ICANS was 61% (32% grade ≥ 3), similar to ZUMA-2 data (Table 2). One patient had grade 5 CRS. The median time to CRS onset was 4 days (range, 0-13), and the median duration of CRS was 5 (range, 1-33) days. The median time to ICANS onset was 6 (range, 1-18) days, and the median duration of ICANS was 6 days (range, 1-144+). Age ≥ 65 years, ECOG PS ≥ 2, high-risk simplified MIPI, blastoid/pleomorphic variant, bulky disease, and bridging therapy were associated with higher rates of grade ≥ 3 ICANS, whereas CNS involvement was not (Appendix Table A3, online only).
TABLE 2.
CRS, ICANS, and Other Adverse Events
Medications used to manage CRS and/or ICANS included tocilizumab (77%; median number of doses 2 [range, 1-4]), corticosteroids (69%), anakinra (17%), and siltuximab (3%). Twenty percent of patients required intensive care unit admission, with a median stay of 3 days (range, 1-12); 11% required vasopressors, 3% required mechanical ventilation, and 2% required dialysis. Prolonged significant anemia, thrombocytopenia, and neutropenia at day 90 occurred in 5%, 11%, and 18%, respectively, and infections requiring antimicrobial treatment occurred in 21% before day 30 and 12% between day 31 and day 90 (Table 2).
Response to brexu-cel Therapy
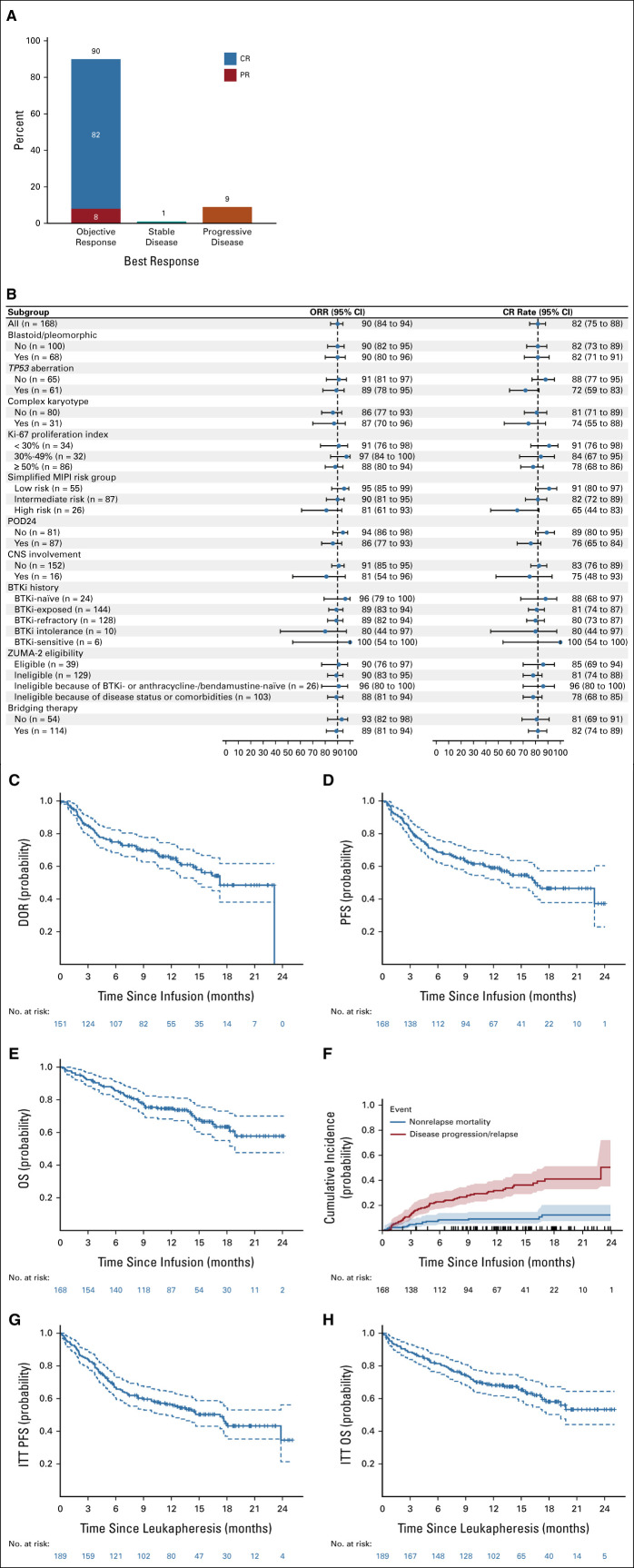
The median follow-up time after infusion was 14.3 months (95% CI, 12.7 to 15.9). Among all patients who received brexu-cel infusion, the best ORR was 90%, with 82% CR and 8% PR (Fig 2A and Appendix Table A4, online only). In responding patients, the median time to best response was 30 days (range, 16-193). ORR and CR rate for subgroups are shown in Figure 2B. TP53 aberration (72% v 88%, P = .029), high-risk simplified MIPI (65% v 82%–91%, P = .019), and POD24 (76% v 89%, P = .028) were associated with lower CR rates.
FIG 2.
Efficacy of brexu-cel. (A) Best response rate (n = 168). (B) Forest plot of ORR and CR rates in subgroups. (C) Duration of response in patients who achieved an objective response. (D) PFS in patients who received brexu-cel infusion. (E) OS in patients who received brexu-cel infusion. (F) Cumulative incidence of nonrelapse mortality. Tick marks above the x-axis indicate censoring; shading around the curves indicates 95% CI. (G) ITT analysis of PFS in patients who underwent leukapheresis. (H) ITT analysis of OS in patients who underwent leukapheresis. brexu-cel, brexucabtagene autoleucel; BTKi, Bruton's tyrosine kinase inhibitor; CR, complete response; ITT, intention-to-treat; MIPI, mantle cell lymphoma international prognostic index; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; POD24, progression of disease within 24 months; PR, partial response.
Time-to-Event Outcomes
The median duration of response was 17.2 months (95% CI, 14.4 to not estimable [NE]). The rate of continuous response at 6 and 12 months was 75% (95% CI, 68 to 82) and 65% (95% CI, 56 to 72), respectively (Fig 2C). The median PFS after brexu-cel infusion was 16.4 months (95% CI, 12.7 to NE), and the 6- and 12-month PFS rate was 69% (95% CI, 61 to 75) and 59% (95% CI, 51 to 66), respectively (Fig 2D). The median OS after brexu-cel infusion was not reached (95% CI, 18.7 to NE), and the 6- and 12-month OS rate was 86% (95% CI, 79 to 90) and 75% (95% CI, 67 to 81), respectively (Fig 2E). The nonrelapse mortality rates were 2.4% (95% CI, 0.8 to 5.6) at day 30, 4.8% at day 90 (95% CI, 2.2 to 8.8), and 9.1% at 1 year (95% CI, 5.3 to 14.1; Fig 2F and Appendix Table A5, online only). In all patients who underwent leukapheresis, the median PFS after leukapheresis was 17.3 months (95% CI, 10.7 to NE; Fig 2G) and the median OS after leukapheresis was not reached (95% CI, 17.7 to NE; Fig 2H).
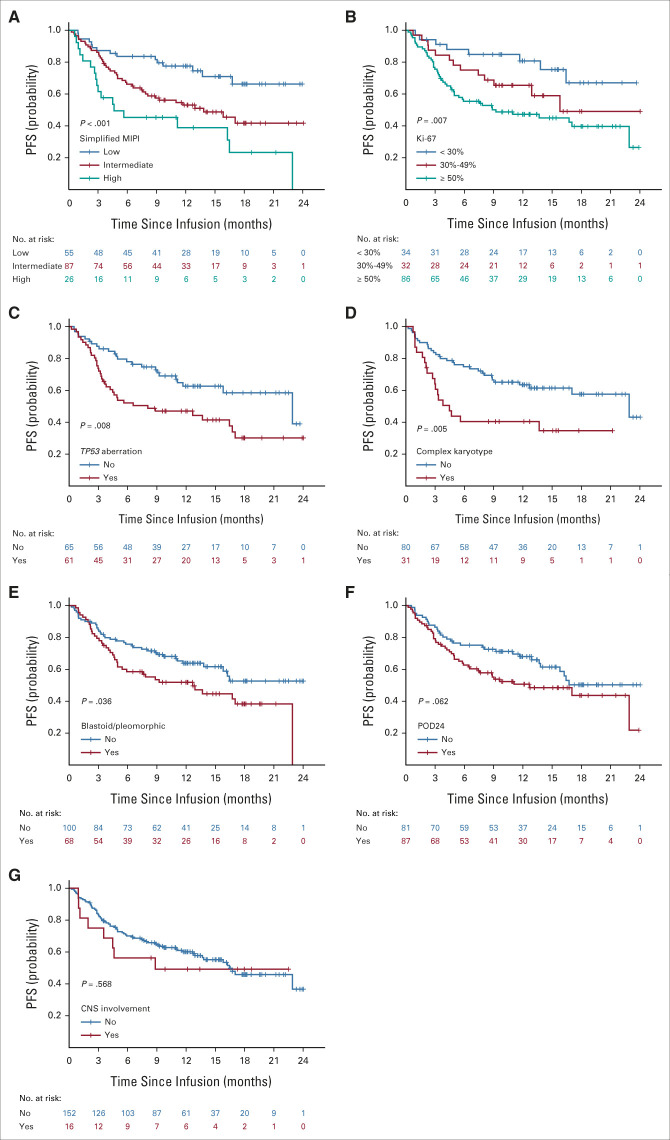
Outcomes according to Prognostic Subgroups
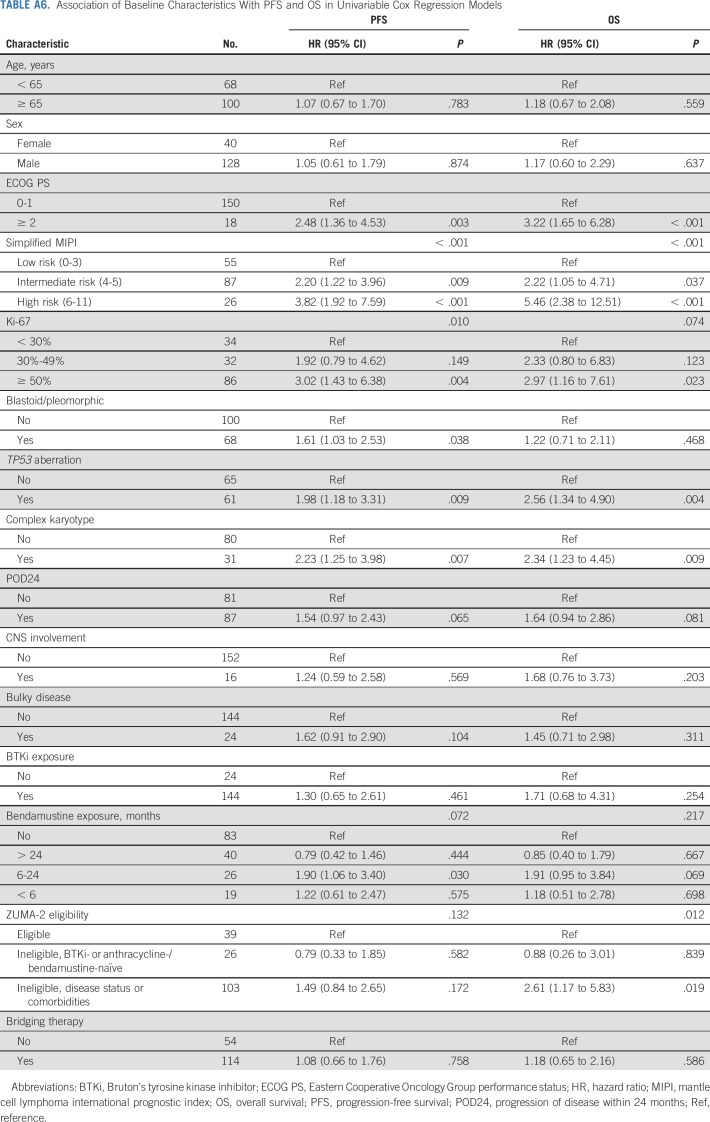
PFS was inferior in patients with high-risk simplified MIPI (hazard ratio [HR], 3.82; 95% CI, 1.92 to 7.59; log-rank P < .001, Fig 3A), Ki-67% ≥ 50% (HR, 3.02; 95% CI, 1.43 to 6.38; log-rank P = .007, Fig 3B), TP53 aberration (HR, 1.98; 95% CI, 1.18 to 3.31; log-rank P = .008, Fig 3C), complex karyotype (HR, 2.23; 95% CI, 1.25 to 3.98; log-rank P = .005, Fig 3D), or blastoid/pleomorphic variant (HR, 1.61; 95% CI, 1.03 to 2.53; log-rank P = .036, Fig 3E) and was also numerically shorter for patients with POD24 (HR, 1.54; 95% CI, 0.97 to 2.43; log-rank P = .062, Fig 3F) although the difference was not statistically significant. CNS involvement was not associated with PFS (Fig 3G). High-risk simplified MIPI, TP53 aberration, and complex karyotype were associated with inferior OS (Appendix Fig A2, online only). The associations of additional variables with PFS and OS by univariable Cox regression analyses are shown in Appendix Table A6 (online only).
FIG 3.
PFS according to prognostic subgroups. PFS by (A) simplified MIPI, (B) Ki-67, (C) TP53, (D) complex karyotype, (E) morphology, (F) POD24, and (G) CNS involvement. MIPI, mantle cell lymphoma international prognostic index; PFS, progression-free survival; POD24, progression of disease within 24 months.
Five patients had prior allogeneic stem-cell transplant (AlloSCT), of whom three achieved CR after brexu-cel (ongoing at 8.0, 14.3, and 17.0 months, respectively) and two had early disease progression (0.9 and 1.6 months after infusion, respectively). One patient had graft-versus-host disease before CAR T-cell therapy, which flared after brexu-cel infusion requiring reinitiation of ruxolitinib that had previously been tapered before infusion. The other four patients did not have graft-versus-host disease before or after infusion. Three patients had prior experimental CD19 CAR T-cell therapy, of whom one did not respond to brexu-cel and two had short-lived response (1 CR lasting for 2.1 months and 1 PR lasting for 0.7 months).
Outcomes According to BTKi Exposure, ZUMA-2 Eligibility, and Bridging Therapy
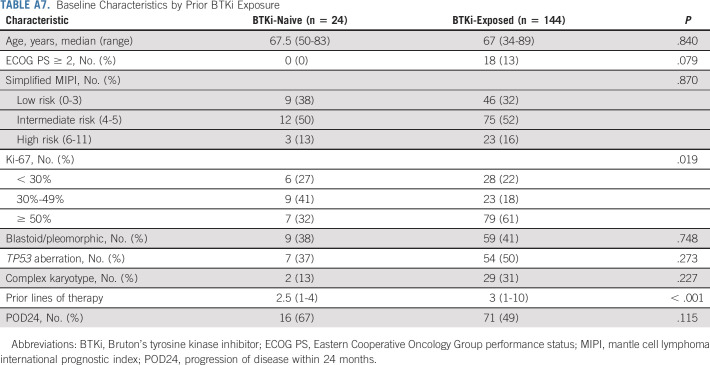
BTKi-naïve and BTKi-exposed patients had similar baseline characteristics except for more frequent Ki-67 ≥ 50% and more prior lines of therapy in BTKi-exposed patients (Appendix Table A7, online only). No statistically significant difference was found in PFS (Fig 4A) or OS (Appendix Fig A3A, online only) between the two groups.
FIG 4.
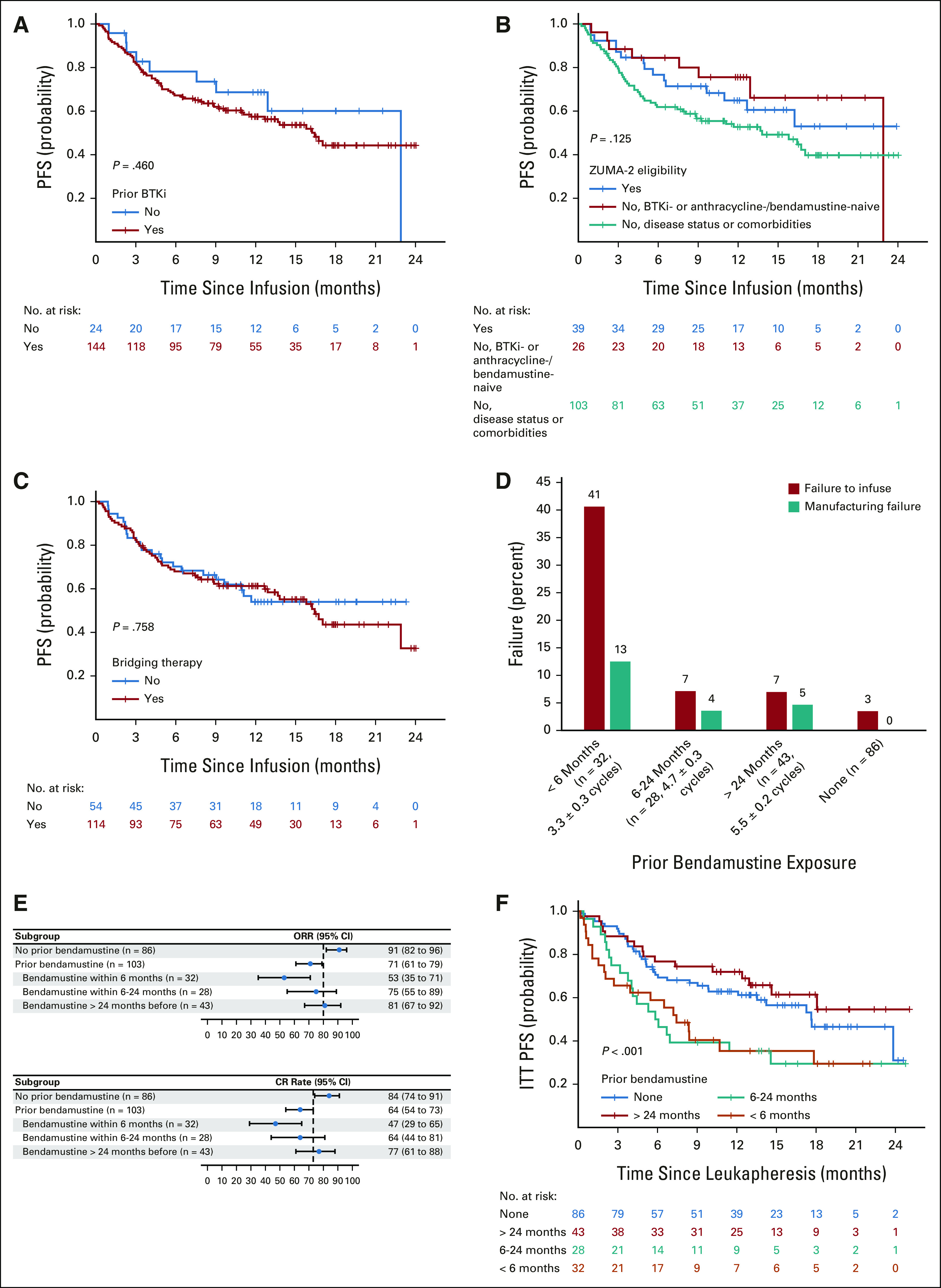
Efficacy by BTKi exposure, ZUMA-2 eligibility, bridging therapy, and bendamustine exposure. PFS by (A) prior BTKi exposure, (B) ZUMA-2 eligibility, and (C) bridging therapy. (D) Rates of failure to infuse and manufacturing failure by prior bendamustine exposure. Cycles were denoted in mean ± standard deviation. (E) ITT analysis of best response rate by prior bendamustine exposure. (F) ITT analysis of PFS from leukapheresis by prior bendamustine exposure. BTKi, Bruton's tyrosine kinase inhibitor; CR, complete response; ITT, intention-to-treat; ORR, objective response rate; PFS, progression-free survival.
Patients ineligible for ZUMA-2 because of disease status or comorbidities were older and had poorer ECOG PS, higher Ki-67, and more prior lines of therapy compared with patients eligible for ZUMA-2 or ineligible for ZUMA-2 solely because of being BTKi- or anthracycline-/bendamustine-naïve (Appendix Table A8, online only). No statistically significant difference was found in PFS among the three groups (Fig 4B), but OS was worse in patients ineligible because of disease status or comorbidities (Appendix Fig A3B and Appendix Table A6).
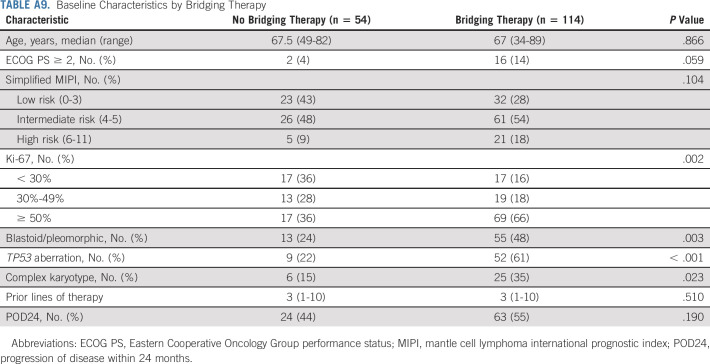
Patients who received bridging therapy had higher Ki-67 and more frequent blastoid/pleomorphic variant, TP53 aberration, and complex karyotype, compared with those who did not (Appendix Table A9, online only). However, no statistically significant difference was found in PFS (Fig 4C) or OS (Appendix Fig A3C) between the two groups. Response to bridging therapy was not associated with PFS or OS (Appendix Fig A3D-A3E).
Association Between Bendamustine Exposure and Outcomes after Leukapheresis
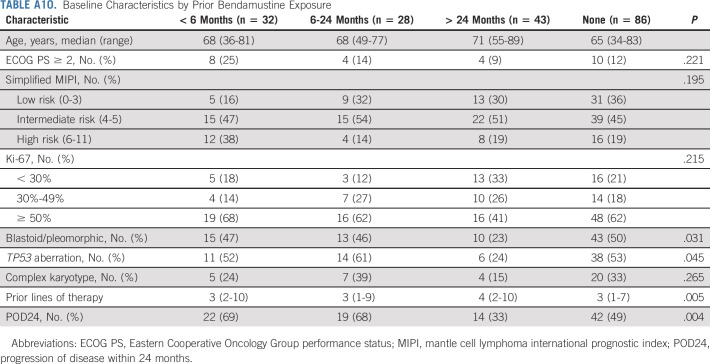
Baseline characteristics of patients with different exposure to bendamustine before leukapheresis are shown in Appendix Table A10 (online only). A higher proportion of patients who had bendamustine exposure within 6 months before leukapheresis did not receive brexu-cel infusion (41% v 3%-7%, P < .001) because of manufacturing failure (13% v 0%-5%) or other reasons (Fig 4D). These patients also had lower ORR (53% v 71%-91%, P < .001) and CR rate (47% v 64%-84%, P < .001; Fig 4E). In ITT analysis, patients who had bendamustine exposure within 6 months or 6-24 months before leukapheresis had inferior PFS (< 6 months v no exposure: HR, 1.90, 95% CI, 1.11 to 3.28; 6-24 months v no exposure: HR, 1.90, 95% CI, 1.10 to 3.30; log-rank P < .001, Fig 4F) and OS (log-rank P = .009, Appendix Fig A3F) compared with those with no bendamustine exposure before leukapheresis. However, after adjusting for simplified MIPI (continuous) and Ki-67 (continuous), the association of prior bendamustine exposure with PFS (< 6 months v no exposure: HR, 1.30, 95% CI, 0.72 to 2.32; 6-24 months v no exposure: HR, 1.30, 95% CI, 0.73 to 2.32) and OS was no longer statistically significant.
DISCUSSION
This study provides important standard-of-care data on feasibility, safety, and efficacy of brexu-cel in R/R MCL. Our data and other real-world studies28-31 further confirm the significant impact of brexu-cel in patients with R/R MCL in routine practice.
Compared with ZUMA-2, our study had a higher proportion of patients with intermediate- or high-risk simplified MIPI (69% v 59%), blastoid/pleomorphic variant (43% v 31%), and TP53 mutation (48% v 17%). In addition, patients were more heavily pretreated and had more comorbidities; 65% of the patients would not have met ZUMA-2 eligibility criteria because of disease status (eg, R/R ≥ 5 lines, prior AlloSCT or CD19 CAR cell therapy, CNS or cardiac involvement) or clinically significant comorbidities. In the standard-of-care setting, brexu-cel was successfully manufactured in 96% and infused to 89% of the patients who underwent leukapheresis, comparable with the rates in ZUMA-2 (96% and 92%, respectively). The rates of CRS (90% v 91%) and neurotoxicity (61% v 63%) were comparable with those in ZUMA-2, but the rate of grade ≥ 3 CRS was lower (8% v 15%) in our study, which may be related to earlier and higher usage of tocilizumab and corticosteroids compared with ZUMA-2. Nearly one third of the patients developed grade ≥ 3 neurotoxicity, similar to ZUMA-2 data24 and comparable with axicabtagene ciloleucel for R/R large B-cell lymphoma.25,32 The nonrelapse mortality was 9.1% at 1 year, primarily because of infections. This is higher than that reported with axicabtagene ciloleucel in large B-cell lymphoma,25,33,34 and further study is needed to understand disease-specific risks and potential mitigation strategies.
The ORR in our study was comparable with that in ZUMA-2 (90% v 91%), but CR rate appeared to be higher. Bridging therapy might have accounted for this difference. ZUMA-2 only allowed BTKi or corticosteroids for bridging therapy, which was used in 37% of the patients and was ineffective in this BTKi-exposed population, with increased median tumor burden in the majority of patients despite bridging therapy.23 By contrast, the choice of the bridging therapy was less restricted in our study, with diverse use of BTKi, venetoclax, lenalidomide, chemotherapy, radiation, or combinations. In some of our patients, holding therapy was started before leukapheresis and often continued after leukapheresis as bridging therapy, ie, extended therapy in the evaluation/planning to infusion brain to vein window, instead of just the leukapheresis to infusion vein to vein window. A better disease control before brexu-cel infusion possibly contributed to a better response with a higher CR rate. In addition, patients who received bridging therapy had more high-risk features, yet the PFS and OS were similar compared with those who did not receive bridging therapy, again highlighting the potential benefit of bridging therapy with effective modalities.
The 12-month estimates for DOR and PFS rate appeared to be comparable with those in ZUMA-2,23,24 which is encouraging considering that there were more patients with high-risk features and comorbidities in our study. The traditional prognostic factors such as simplified MIPI, Ki-67%, blastoid/pleomorphic variant, TP53 aberration, complex karyotype, or POD24 had a varying impact on outcomes after brexu-cel therapy. Strategies to improve on CAR T-cell therapy for high-risk patients are needed. Interestingly, TP53 aberration and complex genomic features have also been reported to associate with poorer outcome after CAR T-cell therapy in large B-cell lymphoma,35,36 and strategies are needed to overcome tumor-intrinsic resistance to CAR T-cell therapy. Patients with CNS involvement did not have higher incidence of grade ≥ 3 ICANS and had a CR rate of 75% and a 12-month PFS rate of 60%, suggesting the safety and efficacy of brexu-cel in this difficult-to-treat population.
Although the FDA approval of brexu-cel was for all R/R MCL regardless of prior BTKi exposure, National Comprehensive Cancer Network guidelines recommend that brexu-cel should only be used after a BTKi,37 likely because BTKi-naïve patients were not included in ZUMA-2. In this setting, our study provides a critical first set of data of brexu-cel efficacy in BTKi-naïve patients. We observed high ORR (96%) and CR (88%) rates and 12-month PFS and OS rates of 69% and 87%, respectively, suggesting high efficacy of brexu-cel in this population. Prospective trials are needed, particularly in high-risk groups, to determine the optimal sequencing of therapy options in MCL.
Bendamustine use may attenuate T-cell fitness and therefore possibly affect CAR T-cell manufacturing and function.38 Indeed, in longer follow-up of ZUMA-2, a poorer pharmacokinetic profile and reduced product doubling time were observed in patients who had bendamustine exposure within 6 months before leukapheresis.24 In our study, we observed higher rates of manufacturing failure and failure to infuse in patients with bendamustine exposure within 6 months. In addition, patients with bendamustine use within 6 months or 6-24 months had inferior PFS and OS than those with remote or no bendamustine exposure. These findings appear to be consistent with prior observations regarding the negative impact on T cells of bendamustine. Larger studies are needed to investigate whether recent bendamustine exposure is independently associated with CAR T-cell therapy outcomes. In our cohort, the group with recent bendamustine exposure was enriched for patients with high-risk disease features, confounding the interpretation. Nevertheless, it may not be unreasonable to consider avoiding bendamustine just before leukapheresis when CAR T-cell therapy is planned and to consider deferring leukapheresis and CAR T-cell manufacture, if alternatives are available, in patients who relapse within 6 months of bendamustine-based therapy.
The strength of our study includes a large observational cohort, consecutive patient inclusion, detailed data collection, and inclusion of BTKi-naïve patients. The limitations include heterogenous peri-infusion managements including bridging therapy and CRS and ICANS management, lack of central response assessment, and lack of serial biomarker and pharmacokinetic studies of CAR T cells. In addition, this study involved only 16 academic centers, and generalization of outcomes to all, including community, cell therapy centers cannot be assumed.
In conclusion, this study demonstrated encouraging safety and efficacy results of brexu-cel in standard-of-care practice that were comparable with those in ZUMA-2, supporting continuous and expanded use of brexu-cel for R/R MCL in routine practice.
ACKNOWLEDGMENT
Dr Y. Wang would like to acknowledge the ASCO Conquer Cancer Career Development Award. Dr M.T. Jacobs would like to acknowledge K12CA167540 (Paul Calabresi K12 Career Development Award for Clinical Oncology), ASCO Conquer Cancer Young Investigator Award, and Dean's Scholars Award from the Washington University Division of Physician-Scientists (funded by the Burroughs Wellcome Fund Physician-Scientist Institution Award).
APPENDIX
FIG A1.
Case numbers contributed by each center.
FIG A2.
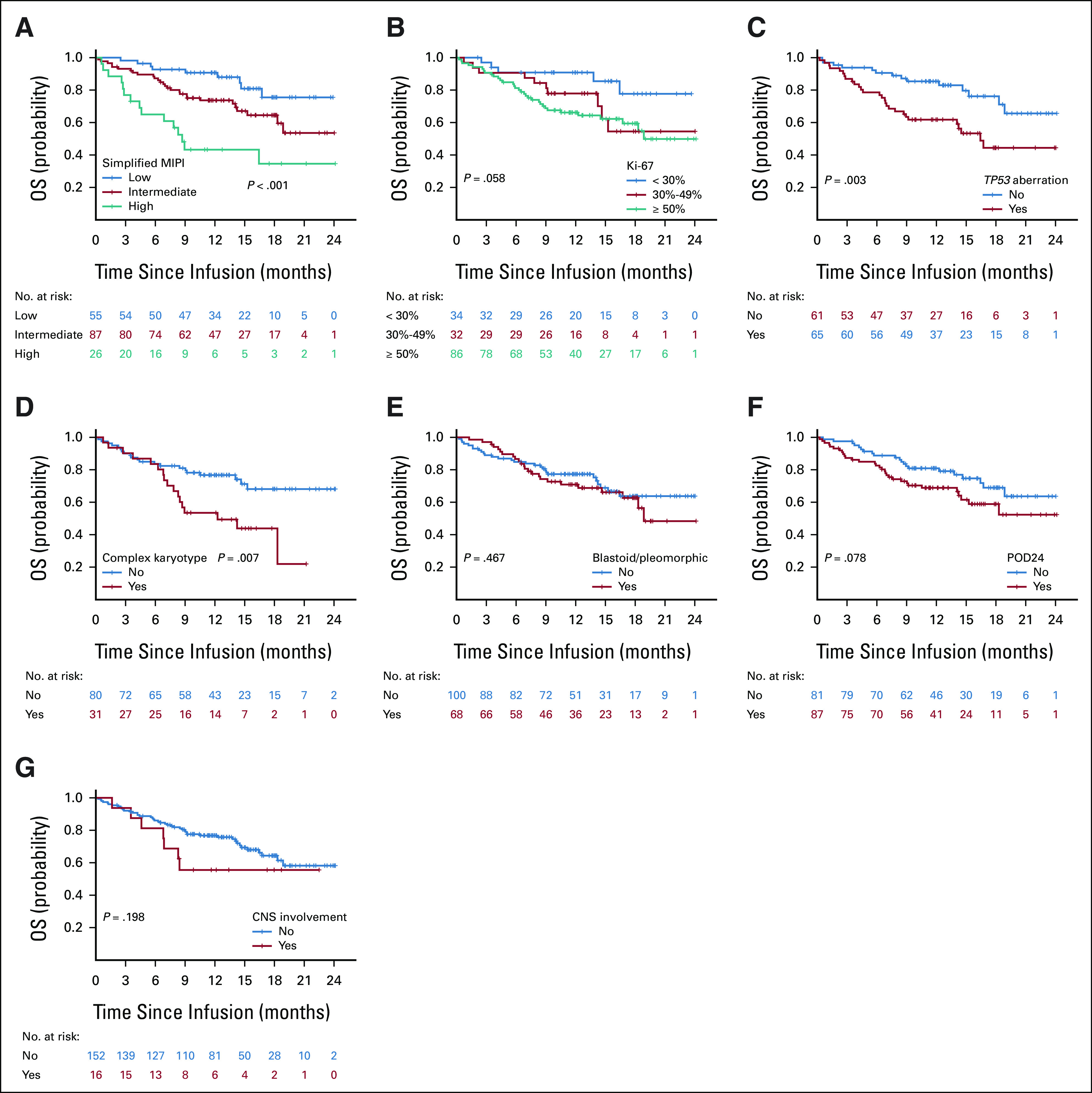
OS according to prognostic subgroups. OS by (A) simplified MIPI, (B) Ki-67, (C) TP53, (D) complex karyotype, (E) morphology, (F) POD24, and (G) CNS involvement. MIPI, mantle cell lymphoma international prognostic index; OS, overall survival; POD24, progression of disease within 24 months.
FIG A3.
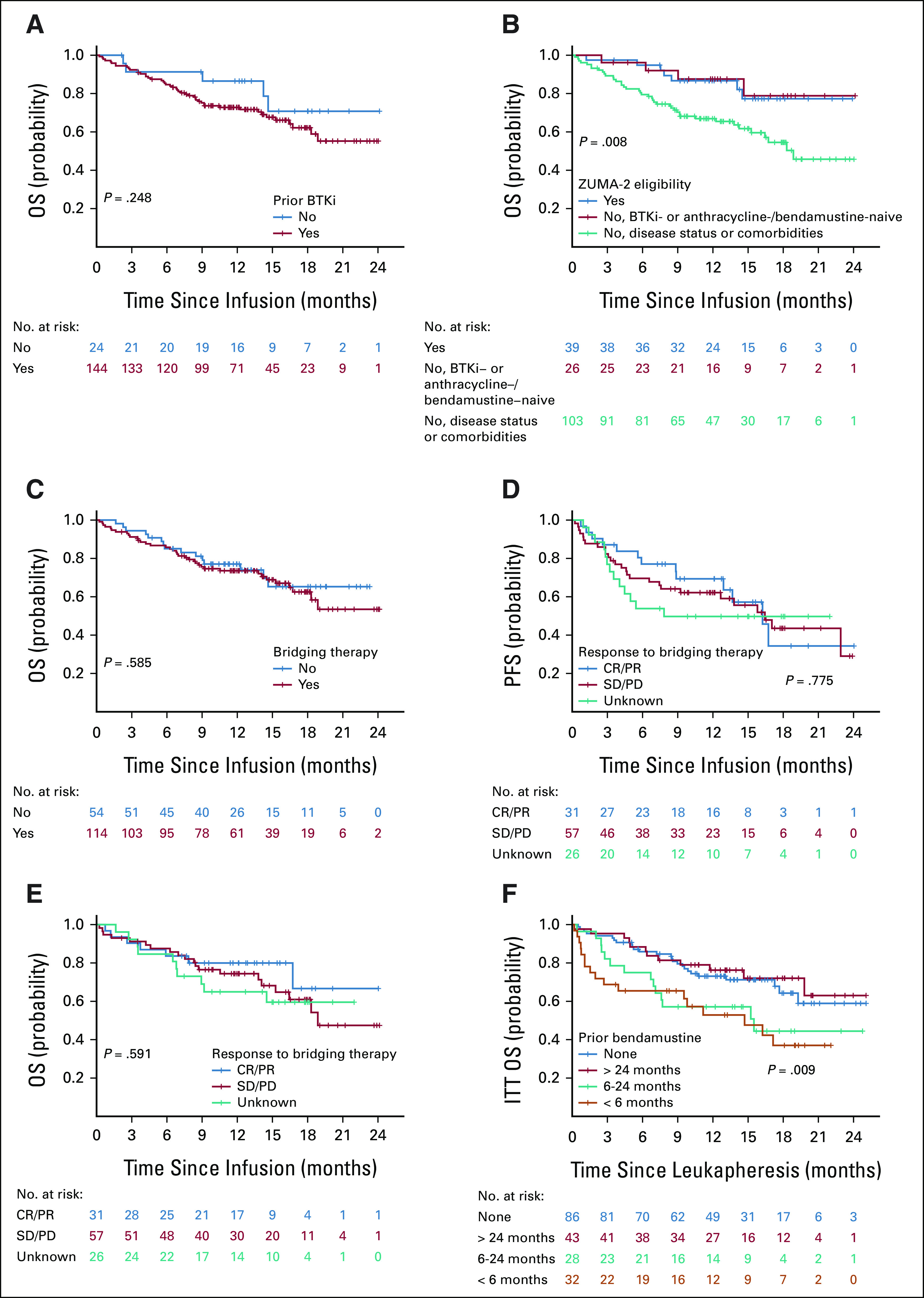
OS by (A) prior BTKi exposure, (B) ZUMA-2 eligibility, and (C) bridging therapy. (D) PFS by response to bridging therapy. (E) OS by response to bridging therapy. (F) ITT analysis of OS from leukapheresis by prior bendamustine exposure. BTKi, Bruton's tyrosine kinase inhibitor; CR, complete response; ITT, intention-to-treat; OS, overall survival; PD, progression of disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
TABLE A1.
ZUMA-2 Ineligibility
TABLE A2.
Bridging Therapy and Response
TABLE A3.
Association of Baseline Characteristics with Grade 3 CRS and ICANS
TABLE A4.
Responses at Day 30 and Best Responses
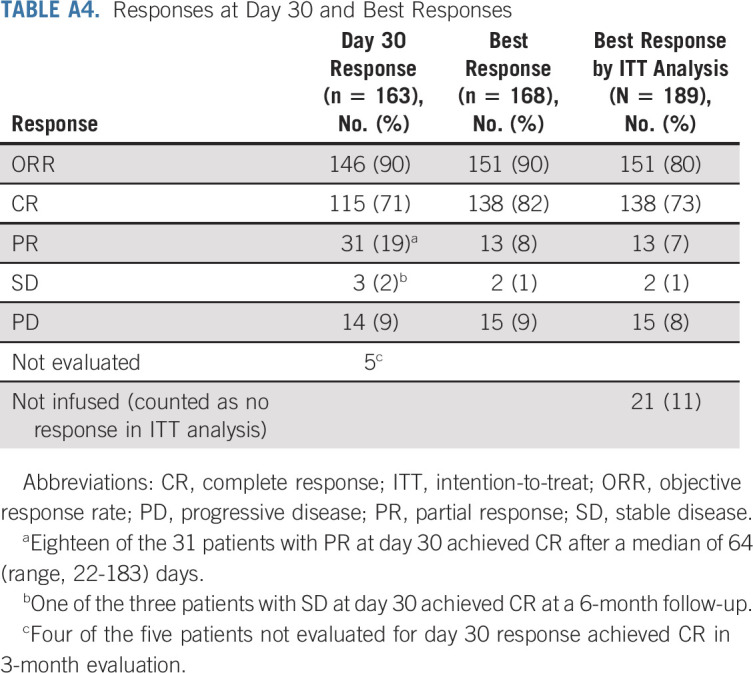
TABLE A5.
Causes of Death in Cases with Nonrelapse Mortality
TABLE A6.
Association of Baseline Characteristics With PFS and OS in Univariable Cox Regression Models
TABLE A7.
Baseline Characteristics by Prior BTKi Exposure
TABLE A8.
Baseline Characteristics by ZUMA-2 Eligibility
TABLE A9.
Baseline Characteristics by Bridging Therapy
TABLE A10.
Baseline Characteristics by Prior Bendamustine Exposure
Yucai Wang
Employment: Merck (I)
Stock and Other Ownership Interests: Merck (I)
Honoraria: Kite, a Gilead company (Inst)
Consulting or Advisory Role: Loxo (Inst), Incyte (Inst), InnoCare (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), Lilly (Inst)
Research Funding: InnoCare (Inst), Incyte (Inst), Novartis (Inst), Genentech (Inst), Loxo (Inst), MorphoSys (Inst), Genmab (Inst)
Preetesh Jain
Honoraria: Lilly, Kite, a Gilead company
Consulting or Advisory Role: Lilly
Speakers' Bureau: Loxo/Lilly
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, Umoja Biopharma
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells With Enhanced Metabolic Fitness. Serial No.: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial No.: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T Cell Therapy. Serial No.: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, A2 Biotherapeutics
Matthew J. Maurer
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Consulting or Advisory Role: Genmab, Adaptive Biotechnologies
Research Funding: MorphoSys (Inst), Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Genmab (Inst)
Matthew J. Frank
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Honoraria: Kite/Gilead, Adaptive Biotechnologies
Consulting or Advisory Role: Kite, a Gilead company, Cargo, INc
Research Funding: Kite, a Gilead company (Inst), Allogene Therapeutics, Adaptive Biotechnologies
Javier L. Munoz
Honoraria: Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, Physicians' Education Resource
Consulting or Advisory Role: Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, Bristol Myers Squibb, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys, Aurobindo, Lilly, Secura Bio
Speakers' Bureau: Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, Verastem, Celgene, AbbVie/Genentech
Research Funding: Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, Millennium
Saurabh Dahiya
Consulting or Advisory Role: Kite/Gilead
Amer M. Beitinjaneh
Honoraria: Kite, a Gilead company
Consulting or Advisory Role: Kite, a Gilead company
Joseph P. Mcguirk
Honoraria: Kite, a Gilead company, AlloVir, Magenta Therapeutics, Nektar, Sana Biotechnology
Consulting or Advisory Role: Kite, a Gilead company, Juno Therapeutics, AlloVir, Magenta Therapeutics, EcoR1 Capital, CRISPR Therapeutics
Speakers' Bureau: Kite/Gilead
Research Funding: Novartis (Inst), Fresenius Biotech (Inst), Astellas Pharma (Inst), Bellicum Pharmaceuticals (Inst), Novartis (Inst), Gamida Cell (Inst), Pluristem Therapeutics (Inst), Kite, a Gilead company (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, syncopation, SITC—ACCC
Julie M. Vose
Honoraria: Acerta Pharma/AstraZeneca, MorphoSys, Johnson and Johnson, MEI Pharma, Lilly, AbbVie, Merck
Research Funding: Celgene (Inst), Incyte (Inst), Acerta Pharma (Inst), Kite, a Gilead company (Inst), Seattle Genetics (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Loxo, Epizyme
Andre Goy
Employment: Regional Cancer Care Associates, OM Pharmaceutical Industries
Leadership: COTA, Genomic Testing Cooperative, Resilience Care
Stock and Other Ownership Interests: COTA, Genomic Testing Cooperative, Resilience Care, Alloplex Biotherapeutics Inc
Honoraria: Alloplex Biotherapeutics Inc, Clinical Advances in Hematology & Oncology, Kite, a Gilead company, Vincerx board meetings, Janssen Biotech
Consulting or Advisory Role: Kite, a Gilead company (Inst), Physicians' Education Resource, Janssen, Vincerx Pharma/Vincerx Pharma, Clinical Advances in Hematology & Oncology
Speakers' Bureau: Bristol Myers Squibb/Celgene
Research Funding: Kite/Gilead (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Genentech/Roche (Inst), Infinity Pharmaceuticals (Inst), Infinity/Verastem (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Pharmacyclics (Inst), Bristol Meyers, MorphoSys, Seattle Genetics, Verastem, Constellation Pharmaceuticals
Travel, Accommodations, Expenses: Physicians' Education Resource
Other Relationship: AstraZeneca
Charalambos Andreadis
Consulting or Advisory Role: Gilead Sciences, Kite, a Gilead company, Karyopharm Therapeutics, Atara Biotherapeutics, Incyte, TG therapeutics, Epizyme
Research Funding: Novartis, Merck, BMS, Genmab
Brian T. Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, Novartis, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
Kathleen A. Dorritie
Honoraria: Onc Live, DAVA Pharmaceuticals
Research Funding: Kite, a Gilead company, Juno Therapeutics, Genentech/Roche, Genmab/Seattle Genetics, Janssen Research & Development
Olalekan O. Oluwole
Consulting or Advisory Role: Kite/Gilead, Legend Biotech, Curio Science, Novartis, ADC Therapeutics, Syncopation Life Sciences, Nektar, Gilead Sciences, Epizyme
Research Funding: Kite, a Gilead company (Inst)
Abhinav Deol
Consulting or Advisory Role: Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, Adicet Bio
Jonas Paludo
Research Funding: Karyopharm Therapeutics (Inst), Biofourmis (Inst)
Bijal Shah
Honoraria: Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences
Consulting or Advisory Role: Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, Novartis, Pfizer, Pfizer, Amgen, Precision Biosciences, Kite, a Gilead company, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus, Lilly, Pepromene
Research Funding: Incyte, Jazz Pharmaceuticals (Inst), Kite/Gilead (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite, a Gilead company
Open Payments Link: https://openpaymentsdata.cms.gov/physician/204581
Trent Wang
Consulting or Advisory Role: Sanofi
Rahul Banerjee
Honoraria: Curio Science, Clinical Care Options, i3 Health
Consulting or Advisory Role: SparkCures, Sanofi Pasteur, Guidepoint Global, Clearview Healthcare Partners, Genentech/Roche, Janssen Oncology, Bristol Myers Squibb/Celgene
Research Funding: Pack Health (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1768167
David B. Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, Janssen
Research Funding: Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics supporting ibrutinib for cGVHD (no royalty claim)
Armin Ghobadi
Consulting or Advisory Role: Kite/Gilead, Amgen, Atara Biotherapeutics, EUSA Pharma, WUGEN, Inc, Celgene, CRISPR therapeutics, CovACE Nanotechnology
Research Funding: Kite, a Gilead company, Amgen
Sattva S. Neelapu
Stock and Other Ownership Interests: Longbow Immunotherapy, Inc
Honoraria: Bio Ascend, Medscape, Aptitude Health, MJH Life Sciences
Consulting or Advisory Role: Merck Sharp & Dohme, Kite, a Gilead company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, Bristol Myers Squibb, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, Bluebird Bio, Sana Biotechnology
Research Funding: Bristol Myers Squibb, Kite, a Gilead company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patents related to cellular therapy, Royalty income from Takeda Pharmaceuticals
Yi Lin
Consulting or Advisory Role: Kite/Gilead (Inst), Novartis (Inst), Bluebird Bio (Inst), Celgene (Inst), Juno Therapeutics (Inst), Bristol Myers Squibb (Inst), Gamida Cell (Inst), Legend Biotech (Inst), Sorrento Therapeutics (Inst), Vineti (Inst), Janssen Oncology (Inst), Pfizer (Inst)
Research Funding: Janssen Oncology (Inst), Janssen Oncology (Inst), Merck (Inst), Takeda (Inst), Boston Scientific (Inst), Kite/Gilead (Inst), Bristol Myers Squibb (Inst), Bluebird Bio (Inst)
Michael L. Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead company, Miltenyi Biomedicine, Moffit Cancer Center, Physicians' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, BioInvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Juno Therapeutics, Lilly, Pepromene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, InnoCare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead company, Physician Education Resources (PER)
Michael D. Jain
Consulting or Advisory Role: Kite/Gilead, Novartis, Bristol Myers Squibb, MyeloidTx
Research Funding: Kite/Gilead, Incyte
Travel, Accommodations, Expenses: Kite/Gilead
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 63rd American Society of Hematology Annual Meeting, Atlanta, GA, December 13, 2021, and at the European Hematology Association 2022 Congress, Vienna, Austria, June 10, 2022.
SUPPORT
NCI cancer support grant to the Moffitt Cancer Center (P30 CA076292).
Y.W., P.J. and F.L.L. are cofirst authors; Y.L., M.L.W., and M.D.J. are cosenior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Yucai Wang, Preetesh Jain, Frederick L. Locke, Matthew J. Frank, Javier L. Munoz, Saurabh Dahiya, Joseph P. Mcguirk, Olalekan O. Oluwole, Abhinav Deol, David B. Miklos, Aaron P. Rapoport, Armin Ghobadi, Sattva S. Neelapu, Yi Lin, Michael L. Wang, Michael D. Jain
Financial support: Frederick L. Locke, Michael L. Wang
Administrative support: Yucai Wang, Preetesh Jain, Frederick L. Locke, Olalekan O. Oluwole, Lazaros Lekakis, Sattva S. Neelapu, Michael L. Wang, Michael D. Jain
Provision of study materials or patients: Yucai Wang, Preetesh Jain, Frederick L. Locke, Javier L. Munoz, Miriam T. Jacobs, Joseph P. Mcguirk, Julie M. Vose, Charalambos Andreadis, Bijal Shah, David B. Miklos, Lazaros Lekakis, Sattva S. Neelapu, Yi Lin, Michael L. Wang, Michael D. Jain
Collection and assembly of data: Yucai Wang, Preetesh Jain, Frederick L. Locke, Matthew J. Frank, Javier L. Munoz, Saurabh Dahiya, Amer M. Beitinjaneh, Miriam T. Jacobs, Joseph P. Mcguirk, Julie M. Vose, Charalambos Andreadis, Brian T. Hill, Olalekan O. Oluwole, Abhinav Deol, Trent Wang, Rahul Banerjee, David B. Miklos, Aaron P. Rapoport, Lazaros Lekakis, Sattva S. Neelapu, Yi Lin, Michael L. Wang, Michael D. Jain
Data analysis and interpretation: Yucai Wang, Preetesh Jain, Frederick L. Locke, Matthew J. Maurer, Matthew J. Frank, Javier L. Munoz, Saurabh Dahiya, Amer M. Beitinjaneh, Joseph P. Mcguirk, Julie M. Vose, Andre Goy, Charalambos Andreadis, Brian T. Hill, Kathleen A. Dorritie, Olalekan O. Oluwole, Abhinav Deol, Jonas Paludo, Bijal Shah, Trent Wang, David B. Miklos, Aaron P. Rapoport, Lazaros Lekakis, Armin Ghobadi, Sattva S. Neelapu, Yi Lin, Michael L. Wang, Michael D. Jain
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma in Standard-of-Care Practice: Results from the US Lymphoma CAR T Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yucai Wang
Employment: Merck (I)
Stock and Other Ownership Interests: Merck (I)
Honoraria: Kite, a Gilead company (Inst)
Consulting or Advisory Role: Loxo (Inst), Incyte (Inst), InnoCare (Inst), TG Therapeutics (Inst), Kite, a Gilead company (Inst), Lilly (Inst)
Research Funding: InnoCare (Inst), Incyte (Inst), Novartis (Inst), Genentech (Inst), Loxo (Inst), MorphoSys (Inst), Genmab (Inst)
Preetesh Jain
Honoraria: Lilly, Kite, a Gilead company
Consulting or Advisory Role: Lilly
Speakers' Bureau: Loxo/Lilly
Frederick L. Locke
Consulting or Advisory Role: Novartis, Celgene, Calibr, Alimera Sciences, Gerson Lehrman Group, EcoR1 Capital, Amgen, Bluebird Bio, Bristol Myers Squibb, Iovance Biotherapeutics, Legend Biotech, Cowen, Kite, a Gilead company, Umoja Biopharma, Takeda, Sana Biotechnology, Daiichi Sankyo/UCB Japan, Bristol Myers Squibb/Celgene, Janssen, A2 Biotherapeutics, Miltenyi Biotec, Caribou Biosciences, Takeda, Umoja Biopharma
Research Funding: Kite, a Gilead company (Inst), Alimera Sciences (Inst), Novartis (Inst), Bluebird Bio (Inst), Bristol Myers Squibb/Celgene (Inst)
Patents, Royalties, Other Intellectual Property: Double Mutant Survivin Vaccine. US010414810B2 (Inst), CAR T Cells With Enhanced Metabolic Fitness. Serial No.: 62/939,727 (Inst), Methods of Enhancing CAR T Cell Therapies. Serial No.: 62/892,292 (Inst), Evolutionary Dynamics of Non-Hodgkin Lymphoma CAR-T Cell Therapy. Serial No.: 62/879,534 (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, A2 Biotherapeutics
Matthew J. Maurer
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Consulting or Advisory Role: Genmab, Adaptive Biotechnologies
Research Funding: MorphoSys (Inst), Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Genmab (Inst)
Matthew J. Frank
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Honoraria: Kite/Gilead, Adaptive Biotechnologies
Consulting or Advisory Role: Kite, a Gilead company, Cargo, INc
Research Funding: Kite, a Gilead company (Inst), Allogene Therapeutics, Adaptive Biotechnologies
Javier L. Munoz
Honoraria: Kyowa Hakko Kirin, Seattle Genetics, Targeted Oncology, Onc view, Curio Science, Physicians' Education Resource
Consulting or Advisory Role: Kite, a Gilead company, Pfizer, Pharmacyclics, Bayer, Alexion Pharmaceuticals, Bristol Myers Squibb, Janssen, Seattle Genetics, Gilead Sciences, Kyowa Hakko Kirin, Juno Therapeutics, Genentech, Celgene, BeiGene, Fosun Kite, Innovent Biologics, Debiopharm Group, Karyopharm Therapeutics, Genmab, ADC Therapeutics, Epizyme, Servier, Novartis, MorphoSys, Aurobindo, Lilly, Secura Bio
Speakers' Bureau: Kite, a Gilead company, Bayer, Pharmacyclics/Janssen, AstraZeneca, Gilead Sciences, Seattle Genetics, Kyowa Hakko Kirin, Acrotech Biopharma, BeiGene, Verastem, Celgene, AbbVie/Genentech
Research Funding: Kite, a Gilead company, Celgene, Portola Pharmaceuticals, Incyte, Genentech/AbbVie, Pharmacyclics/Janssen, Seattle Genetics, Millennium
Saurabh Dahiya
Consulting or Advisory Role: Kite/Gilead
Amer M. Beitinjaneh
Honoraria: Kite, a Gilead company
Consulting or Advisory Role: Kite, a Gilead company
Joseph P. Mcguirk
Honoraria: Kite, a Gilead company, AlloVir, Magenta Therapeutics, Nektar, Sana Biotechnology
Consulting or Advisory Role: Kite, a Gilead company, Juno Therapeutics, AlloVir, Magenta Therapeutics, EcoR1 Capital, CRISPR Therapeutics
Speakers' Bureau: Kite/Gilead
Research Funding: Novartis (Inst), Fresenius Biotech (Inst), Astellas Pharma (Inst), Bellicum Pharmaceuticals (Inst), Novartis (Inst), Gamida Cell (Inst), Pluristem Therapeutics (Inst), Kite, a Gilead company (Inst), AlloVir (Inst)
Travel, Accommodations, Expenses: Kite, a Gilead company, syncopation, SITC—ACCC
Julie M. Vose
Honoraria: Acerta Pharma/AstraZeneca, MorphoSys, Johnson and Johnson, MEI Pharma, Lilly, AbbVie, Merck
Research Funding: Celgene (Inst), Incyte (Inst), Acerta Pharma (Inst), Kite, a Gilead company (Inst), Seattle Genetics (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Loxo, Epizyme
Andre Goy
Employment: Regional Cancer Care Associates, OM Pharmaceutical Industries
Leadership: COTA, Genomic Testing Cooperative, Resilience Care
Stock and Other Ownership Interests: COTA, Genomic Testing Cooperative, Resilience Care, Alloplex Biotherapeutics Inc
Honoraria: Alloplex Biotherapeutics Inc, Clinical Advances in Hematology & Oncology, Kite, a Gilead company, Vincerx board meetings, Janssen Biotech
Consulting or Advisory Role: Kite, a Gilead company (Inst), Physicians' Education Resource, Janssen, Vincerx Pharma/Vincerx Pharma, Clinical Advances in Hematology & Oncology
Speakers' Bureau: Bristol Myers Squibb/Celgene
Research Funding: Kite/Gilead (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), Celgene (Inst), Genentech/Roche (Inst), Infinity Pharmaceuticals (Inst), Infinity/Verastem (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Pharmacyclics (Inst), Bristol Meyers, MorphoSys, Seattle Genetics, Verastem, Constellation Pharmaceuticals
Travel, Accommodations, Expenses: Physicians' Education Resource
Other Relationship: AstraZeneca
Charalambos Andreadis
Consulting or Advisory Role: Gilead Sciences, Kite, a Gilead company, Karyopharm Therapeutics, Atara Biotherapeutics, Incyte, TG therapeutics, Epizyme
Research Funding: Novartis, Merck, BMS, Genmab
Brian T. Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Bayer, AstraZeneca, Novartis, Pfizer, Celgene, Karyopharm Therapeutics, Epizyme, BeiGene, Novartis, MorphoSys
Consulting or Advisory Role: Novartis, Genentech, AbbVie, Gilead Sciences, Karyopharm Therapeutics, AstraZeneca, Epizyme, MorphoSys, BeiGene
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), Celgene (Inst), Takeda (Inst), Amgen (Inst), Genentech (Inst), Kite/Gilead (Inst), TG Therapeutics (Inst)
Kathleen A. Dorritie
Honoraria: Onc Live, DAVA Pharmaceuticals
Research Funding: Kite, a Gilead company, Juno Therapeutics, Genentech/Roche, Genmab/Seattle Genetics, Janssen Research & Development
Olalekan O. Oluwole
Consulting or Advisory Role: Kite/Gilead, Legend Biotech, Curio Science, Novartis, ADC Therapeutics, Syncopation Life Sciences, Nektar, Gilead Sciences, Epizyme
Research Funding: Kite, a Gilead company (Inst)
Abhinav Deol
Consulting or Advisory Role: Novartis, Kite/Gilead, Agios, Juno/Celgene, Janssen, Adicet Bio
Jonas Paludo
Research Funding: Karyopharm Therapeutics (Inst), Biofourmis (Inst)
Bijal Shah
Honoraria: Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences
Consulting or Advisory Role: Adaptive Biotechnologies, Bristol Myers Squibb/Celgene, Novartis, Pfizer, Pfizer, Amgen, Precision Biosciences, Kite, a Gilead company, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus, Lilly, Pepromene
Research Funding: Incyte, Jazz Pharmaceuticals (Inst), Kite/Gilead (Inst), Servier (Inst)
Travel, Accommodations, Expenses: Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite, a Gilead company
Open Payments Link: https://openpaymentsdata.cms.gov/physician/204581
Trent Wang
Consulting or Advisory Role: Sanofi
Rahul Banerjee
Honoraria: Curio Science, Clinical Care Options, i3 Health
Consulting or Advisory Role: SparkCures, Sanofi Pasteur, Guidepoint Global, Clearview Healthcare Partners, Genentech/Roche, Janssen Oncology, Bristol Myers Squibb/Celgene
Research Funding: Pack Health (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1768167
David B. Miklos
Honoraria: Janssen, Fosun Kite Biotechnology
Consulting or Advisory Role: Adaptive Biotechnologies, Juno/Celgene, Pharmacyclics, Janssen
Research Funding: Pharmacyclics, Novartis, Roche/Genentech, Kite, a Gilead company, Adaptive Biotechnologies, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patent held with Pharmacyclics supporting ibrutinib for cGVHD (no royalty claim)
Armin Ghobadi
Consulting or Advisory Role: Kite/Gilead, Amgen, Atara Biotherapeutics, EUSA Pharma, WUGEN, Inc, Celgene, CRISPR therapeutics, CovACE Nanotechnology
Research Funding: Kite, a Gilead company, Amgen
Sattva S. Neelapu
Stock and Other Ownership Interests: Longbow Immunotherapy, Inc
Honoraria: Bio Ascend, Medscape, Aptitude Health, MJH Life Sciences
Consulting or Advisory Role: Merck Sharp & Dohme, Kite, a Gilead company, Novartis, Incyte, Gilead Sciences, Alimera Sciences, Bristol Myers Squibb, Adicet Bio, Calibr, Athenex, Sellas Life Sciences, Bluebird Bio, Sana Biotechnology
Research Funding: Bristol Myers Squibb, Kite, a Gilead company, Cellectis, Poseida Therapeutics, Unum Therapeutics, Gilead Sciences, Alimera Sciences, Precision Biosciences, Adicet Bio
Patents, Royalties, Other Intellectual Property: Patents related to cellular therapy, Royalty income from Takeda Pharmaceuticals
Yi Lin
Consulting or Advisory Role: Kite/Gilead (Inst), Novartis (Inst), Bluebird Bio (Inst), Celgene (Inst), Juno Therapeutics (Inst), Bristol Myers Squibb (Inst), Gamida Cell (Inst), Legend Biotech (Inst), Sorrento Therapeutics (Inst), Vineti (Inst), Janssen Oncology (Inst), Pfizer (Inst)
Research Funding: Janssen Oncology (Inst), Janssen Oncology (Inst), Merck (Inst), Takeda (Inst), Boston Scientific (Inst), Kite/Gilead (Inst), Bristol Myers Squibb (Inst), Bluebird Bio (Inst)
Michael L. Wang
Honoraria: Janssen Research & Development, Dava Oncology, OM Pharmaceutical Industries, AstraZeneca, CAHON, Hebei Cancer Prevention Federation, Mumbai Hematology Group, Acerta Pharma, Chinese Anti-Cancer Association, BeiGene, Clinical Care Options, Epizyme, Imedex, Kite, a Gilead company, Miltenyi Biomedicine, Moffit Cancer Center, Physicians' Education Resource, Breast-Gynecological International Cancer Society, Pharmacyclics/Janssen, Eastern Virginia Medical School, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, OncLive/MJH Life Sciences, Practice Point Communications, First Hospital Zhejiang University, BioInvent
Consulting or Advisory Role: AstraZeneca, Janssen Research & Development, Juno Therapeutics, Bioinvent, Pharmacyclics/Janssen, Loxo, Kite, a Gilead company, InnoCare, Oncternal Therapeutics, CStone Pharmaceuticals, Genentech, BeiGene, DTRM, Epizyme, Miltenyi Biomedicine, VelosBio, Deciphera, Juno Therapeutics, Lilly, Pepromene
Research Funding: AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite, a Gilead company, Juno Therapeutics, BeiGene, Acerta Pharma, Oncternal Therapeutics, Bioinvent, Loxo, VelosBio, Celgene, Molecular Templates, Lilly, InnoCare, Genmab, Genentech, Vincerx Pharma
Travel, Accommodations, Expenses: Janssen Research & Development, AstraZeneca, Celgene, Dava Oncology, OM Pharmaceutical Industries, Kite, a Gilead company, Physician Education Resources (PER)
Michael D. Jain
Consulting or Advisory Role: Kite/Gilead, Novartis, Bristol Myers Squibb, MyeloidTx
Research Funding: Kite/Gilead, Incyte
Travel, Accommodations, Expenses: Kite/Gilead
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jain P, Wang ML: Mantle cell lymphoma in 2022-A comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol 97:638-656, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Jain P, Dreyling M, Seymour JF, et al. : High-risk mantle cell lymphoma: Definition, current challenges, and management. J Clin Oncol 38:4302-4316, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Hoster E, Dreyling M, Klapper W, et al. : A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111:558-565, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Hoster E, Klapper W, Hermine O, et al. : Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 32:1338-1346, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Hoster E, Rosenwald A, Berger F, et al. : Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 34:1386-1394, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Dreyling M, Klapper W, Rule S: Blastoid and pleomorphic mantle cell lymphoma: Still a diagnostic and therapeutic challenge. Blood 132:2722-2729, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Halldorsdottir AM, Lundin A, Murray F, et al. : Impact of TP53 mutation and 17p deletion in mantle cell lymphoma. Leukemia 25:1904-1908, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Eskelund CW, Dahl C, Hansen JW, et al. : TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 130:1903-1910, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Aukema SM, Hoster E, Rosenwald A, et al. : Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 131:417-420, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Sarkozy C, Terre C, Jardin F, et al. : Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosomes Cancer 53:106-116, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Greenwell IB, Staton AD, Lee MJ, et al. : Complex karyotype in patients with mantle cell lymphoma predicts inferior survival and poor response to intensive induction therapy. Cancer 124:2306-2315, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visco C, Tisi MC, Evangelista A, et al. : Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br J Haematol 185:940-944, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Bond DA, Switchenko JM, Villa D, et al. : Early relapse identifies MCL patients with inferior survival after intensive or less intensive frontline therapy. Blood Adv 5:5179-5189, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ML, Rule S, Martin P, et al. : Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 369:507-516, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ML, Blum KA, Martin P, et al. : Long-term follow-up of MCL patients treated with single-agent ibrutinib: Updated safety and efficacy results. Blood 126:739-745, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Rule S, Zinzani PL, et al. : Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. The Lancet 391:659-667, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Rule S, Zinzani PL, et al. : Durable response with single-agent acalabrutinib in patients with relapsed or refractory mantle cell lymphoma. Leukemia 33:2762-2766, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Zhou K, Zou D, et al. : Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res 26:4216-4224, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Zhou K, Zou D, et al. : Zanubrutinib in relapsed/refractory mantle cell lymphoma: Long-term efficacy and safety results from a phase 2 study. Blood 139:3148-3158, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin P, Maddocks K, Leonard JP, et al. : Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 127:1559-1563, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 183:578-587, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Outcomes of relapsed mantle cell lymphoma patients after discontinuing acalabrutinib. Am J Hematol 96:E137-E140, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Munoz J, Goy A, et al. : KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331-1342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Munoz J, Goy A, et al. : Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol 41:555-567, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nastoupil LJ, Jain MD, Feng L, et al. : Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: Results from the US Lymphoma CAR T Consortium. J Clin Oncol 38:3119-3128, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32:3059-3067, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heini AD, Bacher U, Kronig MN, et al. : Chimeric antigen receptor T-cell therapy for relapsed mantle cell lymphoma: Real-world experience from a single tertiary care center. Bone Marrow Transplant 57:1010-1012, 2022 [DOI] [PubMed] [Google Scholar]
- 29.Iacoboni G, Rejeski K, Villacampa G, et al. : Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv 6:3606-3610, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbaux C, Bret C, Di Blasi R, et al. : Kte-X19 in relapsed or refractory mantle-cell lymphoma, a "Real-Life" study from the Descar-T Registry and Lysa Group. Blood 138, 2021. (suppl 1; abstr 743) [Google Scholar]
- 31.Romancik JT, Goyal S, Gerson JN, et al. : Analysis of outcomes and predictors of response in patients with relapsed mantle cell lymphoma treated with brexucabtagene autoleucel. Blood 138, 2021. (suppl 1; abstr 1756) [Google Scholar]
- 32.Neelapu SS, Locke FL, Bartlett NL, et al. : Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson CA, Locke FL, Ma L, et al. : Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transplant Cell Ther 28:581.e1-e581.e8, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bethge WA, Martus P, Schmitt M, et al. : GLA/DRST real-world outcome analysis of CAR T-cell therapies for large B-cell lymphoma in Germany. Blood 140:349-358, 2022 [DOI] [PubMed] [Google Scholar]
- 35.Shouval R, Alarcon Tomas A, Fein JA, et al. : Impact of TP53 genomic alterations in large B-cell lymphoma treated with CD19-chimeric antigen receptor T-cell therapy. J Clin Oncol 40:369-381, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain MD, Ziccheddu B, Coughlin CA, et al. : Whole-genome sequencing reveals complex genomic features underlying anti-CD19 CAR T-cell treatment failures in lymphoma. Blood 140:491-503, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network : B-Cell Lymphomas (version 5.2022). https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
- 38.Duell J, Lukic DS, Karg M, et al. : Functionally defective T cells after chemotherapy of B-cell malignancies can Be activated by the tetravalent bispecific CD19/CD3 antibody AFM11. J Immunother 42:180-188, 2019 [DOI] [PubMed] [Google Scholar]