Abstract
A renal biopsy from a 36-year-old man with AIDS showed a severe tubulointerstitial nephritis with intranuclear inclusions in epithelial cells. Electron microscopy revealed the characteristic findings of a polyomavirus (PyV) infection, and immunofluorescence indicated the presence of BK virus (BKV) antigen. Inoculation of rhesus monkey kidney cell cultures both with urine and with buffy coat blood cells resulted in a cytopathic response which was subsequently confirmed to be due to BKV. Further characterization of the viral DNA from the kidney by PCR amplification and Southern blot analysis with PyV and strain-specific primers and probes indicated that the virus was closely related to the BK(Dun) strain but different in its apparent sequence arrangement. Subsequent cycle sequencing showed a dinucleotide mutation of TG→AA which substitutes hydrophilic Gln for hydrophobic Leu in a sequence homologous to an origin DNA-binding domain of simian virus 40 T antigen. It is suggested that the mutation and a coding region rearrangement of this strain of BKV designated BKV(Cin) has the potential to alter viral DNA replication and enhance pathogenicity.
The human polyomaviruses BK virus (BKV) and JC virus (JCV) are small DNA viruses that infect the majority of the world population (1, 2, 16). Primary infection with each virus usually occurs in childhood (19, 29), possibly by the respiratory route (15, 24, 28), followed by latent infection in the urinary tract (4, 6). Genomic sequences of both viruses have been detected in normal cadaver kidney (10). JCV has been established as an opportunistic human pathogen causing progressive multifocal leukoencephalopathy (PML) with increased frequency in AIDS patients (21). Reactivation of BKV occurs in pregnant women resulting in viruria (14) and in immunodeficient individuals, with frequent viruria and, rarely, hemorrhagic cystitis, particularly in bone marrow transplant recipients (5). BKV has also been repeatedly associated with the development of ureteral stenosis in renal transplants with characteristic intranuclear inclusions limited to the uroepithelial cells (6) and a recently described self-limited viral interstitial nephritis in renal transplant patients (22). However, there have been few previously reported cases of fatal renal tubular injury attributed to BKV. Two of these were in children with immunodeficiency (12, 27), one was in an adult with AIDS (29), and two were in renal allograft recipients (26). In this report we present a case of severe tubulointerstitial nephritis resulting in renal failure in a patient with AIDS. Genomic characterization of the virus revealed point mutations that resulted in a single amino acid substitution, as well as a rearrangement in the regulatory region. Together, these unusual features may be responsible for the increased virulence of this polyomavirus strain designated BKV(Cin). This report illustrates the potential for the emergence of a new and more virulent pathogen from a widely distributed, largely nonpathogenic virus.
CASE REPORT
A 36-year-old white homosexual man who was human immunodeficiency virus positive, was referred for evaluation of renal failure and hypertension. His human immunodeficiency virus status was initially established three years earlier when he presented with oral hairy leukoplakia and recurrent vesicular lesions diagnosed as herpes zoster. At that time his CD4 lymphocyte count was less than 50. He was maintained on daily zidovudine, fluconazole, and monthly pentamidine inhalation. Nine months earlier, a diastolic blood pressure of 117 mm of Hg was noted for the first time. Physical examination revealed a blood pressure of 152/114 mm of Hg. The optic fundi were unremarkable, as was the cardiac examination. No edema was present. The urine analysis revealed an absence of casts, red blood cells, and protein. Blood chemistries showed 145 mM sodium, 4.9 mM potassium, 111 mM chloride, 28 mM bicarbonate, 21 mg of urea nitrogen/dl, and 3.7 mg of creatinine/dl, and 24-h urine showed 379 mg of protein in a total volume of 2,370 ml. The uncorrected creatinine clearance was 32 ml/min.
Two weeks later, a percutaneous renal biopsy was performed, at which time his serum creatinine had risen to 9.7 mg/dl. Attempted virus isolation from the spinal fluid and blood was unsuccessful, but a urine specimen obtained on the day after renal biopsy showed a characteristic cytopathic change in rhesus monkey kidney (RMK) cells. A subsequent culture of buffy coat cells resulted in the isolation of cytomegalovirus (CMV) from inoculated human fibroblasts (MRHF) and a second virus from RMK cells showing the same cytopathic effect as the urine.
Following a subsequent hospitalization for institution of peritoneal dialysis, he abruptly left the hospital without any medication or follow-up. Attempts to contact him and his family were unsuccessful. Subsequently, it was learned that he developed seizures and died 4 weeks after the renal biopsy. Permission for an autopsy was denied.
MATERIALS AND METHODS
Renal biopsy and identification of the virus in renal tissue.
A percutaneous renal biopsy was processed in the usual way for light microscopy, immunofluorescence, and electron microscopy. Following the ultrastructural identification of virus, additional indirect immunofluorescent microscopy was carried out with polyclonal rabbit antibodies to BKV, JCV, and simian virus 40 (SV40) with fluorescein isothiocyanate-labeled anti-rabbit immunoglobulin G. Polyomavirus antisera were kindly provided by Duard L. Walker, University of Wisconsin, Madison.
Virus isolation and characterization.
Urine obtained soon after the biopsy was submitted to the diagnostic virology laboratory and inoculated into four cell lines: RMK, Vero monkey kidney, MRHF, and human epithelial cells (HEp-2). Cultures were incubated on roller drums at 35°C and examined three times a week for evidence of cytopathic change. All cultures were held for 2 weeks, except for the human fibroblasts that were held for 4 weeks. For further characterization of the virus from the kidney, 5-μm-thick frozen sections of the biopsy obtained in Cincinnati, Ohio, were sent frozen to the National Institutes of Health in Bethesda, Md. The sections were scraped from glass slides and used for DNA extraction and PCR amplification with primers specific for BKV, JCV, or SV40. DNA was obtained by digesting the tissue with proteinase K for 1 h at 55°C, followed by boiling for 10 min. PCR with 5 μl of extract or positive control (plasmid DNA) and negative control (extraction buffer) was carried out for 30 cycles with AmpliTaq DNA polymerase (Perkin-Elmer Cetus) and the universal BJU primers (Table 1) with denaturation at 94°C, annealing at 55°C, and extension at 72°C for 1 min each. Following resolution of the products in a 2% agarose gel containing ethidium bromide, they were transferred to a nylon membrane for hybridization with 32P-end-labeled oligonucleotide probes. Probes were labeled by using T4 polynucleoside kinase and hybridized at 107 dpm per 5 ml of solution for 1 h at 55°C. After one wash with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) for 20 min at 55°C, and two washes with 1× SSC–0.1% SDS for 20 min at 55°C, blots were dried and exposed to X-ray film overnight. For direct cycle sequencing of the BKV- and JCV-specific products the amplified bands generated by the BES-3 and BES-6 primers were purified with a GeneClean kit (Bio 101) and sequenced with an AmpliTaq sequencing kit (Perkin-Elmer Cetus) and 32P-end-labeled BES-3 and BES-6 as primers. After 20 cycles between 94 and 63°C, the products were resolved on a denaturing acrylamide gel by autoradiography. Frozen samples of buffy coat cells from the patients’ blood and centrifuged urine sediment were also examined. DNA was extracted for PCR, followed by Western blot and sequence analysis in a manner similar to that described here for the renal biopsy sample.
TABLE 1.
Primers and probes
| Primer or probe | Positiona | Sequence (5′-3′) | Virus and/or fragment size (bp) |
|---|---|---|---|
| Universal primersd | |||
| BJU-1b | (2518–2539) | GGGCAGCCTATGTATGG(T,C)cATGG | JCV, 354 |
| BJU-2 | (2868–2889) | TCTGGACATGGATCAAGCACTG | BKV(Dun), 372 |
| BKV(AS), 360 | |||
| Virus-specific probes | |||
| BDN-1.1 | (2798–2819) | TCTGACCTTTGGGAATCTTCAG | BKV(Dun) specific |
| BAS-1.1 | (2534–2553) | GCTGTGAGGGTTTTCTGAAT | BKV(AS) specific |
| JMG-1.1 | (2650–2671) | TACAGATGTGAAAAGTGCAGTT | JCV specific, Mad-1 and Cin |
| BK virus-specific primers and probes | |||
| BES-3 | (4222–4243) | AATATTATGCCCAGCACACATG | 151 |
| BES-6 | (4393–4372) | CTTTCCCTCTGATCTACACCAG | |
| BES-3.1 | (4287–4304) | TATACAGAATTTGAGCTT | |
| BES-3.3 | (4287–4304) | TATACAGAATTAAAGCTT | |
| BLS-3 | (1818–1837) | GCTTCCCTGTTACAGCACAG | 95 |
| BLS-4 | (1912–1893) | GTACAGTTACAGCCTCCCAC | |
| BLS-3.1 | (1843–1867) | ATTCCCCTCCCCAATTTAAATGAGG | |
| BTP-1 | (3049–3070) | TACAGGCCTAAACCAAATTAGC | 168 |
| BTP-2 | (3216–3195) | GAGTATCCTGTCCCTAAAACCC | |
| BTP-1.1 | (3121–3138) | CTCTGAGTTTTGTAAGGA | |
| BRR-1 | (5018–5047) | CTTTCTCGAGGTGAAATTCCTTACACTTCC | 513 |
| BRR-2 | (377–348) | CAGTACTAGTGGGGACAAGGCCAAGATTCC | |
| JC virus-specific primers and probes | |||
| JES-3 | (4085–4106) | AATATTATGACCCCCAAAACCA | 151 |
| JES-6 | (4256–4235) | CTTTCCTGTAGATCTGCATGCA | |
| JES-3.1 | (4178–4197) | GGTATACACAGCAAAAGAAG | |
| JTP-1 | (2990–3011) | GCAGCTTAGTGATTTTCTCAGG | 141 |
| JTP-2 | (3109–3130) | CACCAAAACAAAAGAACACAGG | |
| JTP-1.1 | (3053–3077) | CTGTAAAGTTCTAGGCACTGAATAT | |
| JRR 5 | (4979–5009) | TCCATGGATCCCTCCCCTATTCAGCACTTTGT | 473 |
| JRR 6 | (315–285) | TTTCACTGCAGCCTTACGTGACAGCTGGCGA |
Each virus numbered after BKV(Dun), JCV(Mad-1), and SV40 (10).
Primers are represented with whole numbers, probes are represented with decimal numbers.
T and C combined at this position in order to match both BKV and JCV sequences.
For amplification of both BKV and JCV.
The regulatory region of BKV was amplified with primers BRR-1 and BRR-2, and the products were cloned and sequenced as previously described (9). The regulatory region of JCV from the kidney was amplified and sequenced similarly with primers JRR-6 and JRR-7.
RESULTS
Renal biopsy.
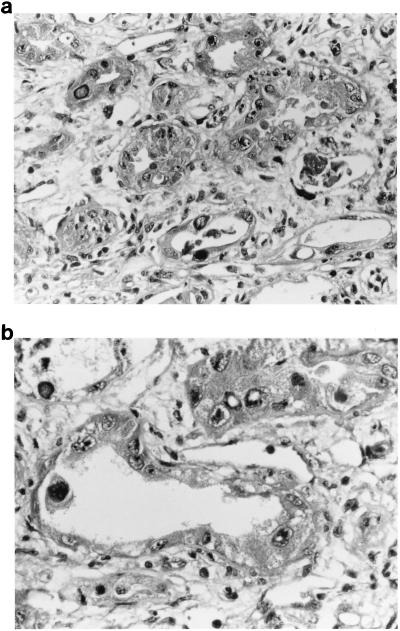
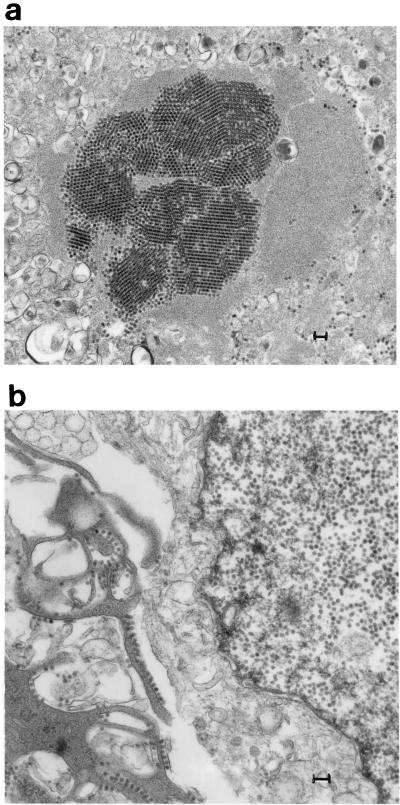
Sections of the renal biopsy revealed an acute, severe interstitial nephritis with a mixed inflammatory infiltrate of polymorphonuclear leukocytes and lymphocytes and necrosis of tubules (Fig. 1A). Individual tubular epithelial cells, many of which had sloughed into the tubular lumen, contained large, dense basophilic intranuclear inclusions (Fig. 1B). Glomeruli showed no specific changes except for occasional intranuclear inclusions in the epithelium lining Bowman’s capsule. Immunofluorescence for immunoglobulins and C3 was negative. Electron microscopy revealed 45-nm-diameter particles in the nuclei of scattered tubular epithelial cells, occasionally forming dense crystalline arrays (Fig. 2A). In the cytoplasm nonenveloped virions were noted to line up along plasma membranes (Fig. 2B). Immunofluorescence with polyvalent antibody to polyomaviruses showed bright staining of scattered tubular epithelial nuclei corresponding to the inclusions seen by light microscopy. An identical pattern of staining was obtained with the antibody for BKV, but there was no labeling with the antibodies specific for JCV or SV40.
FIG. 1.
(a and b) Sections of the renal biopsy stained with hematoxylin and eosin show tubular necrosis with sloughed epithelial cells and interstitial inflammation. Individual epithelial cells contain dense basophilic inclusions. Original magnification, ×250.
FIG. 2.
(a) Transmission electron micrograph of a renal biopsy showing 45-nm particles in the nucleus of a tubular epithelial cell, forming a crystalline array. Bar, 0.1 μm. (b) Transmission electron microscopy showing 45-nm nuclear particles and cytoplasmic nonenveloped virions lined up along plasma membranes. Bar, 0.1 μm.
Tissue culture.
A urine specimen collected on the day following the renal biopsy showed cytopathic effects (CPE) in the RMK cells on day 9, consisting of rounding up and eventual clumping of the involved cells. This was subcultured into RMK, producing CPE in 5 days, and into the other cell lines, without producing any CPE. The original urine specimen had been frozen at −70°C and was reinoculated into RMK, producing CPE in 6 days. The isolate was tested by PCR and identified as BK virus. In addition to the BK virus, CPE were seen in MRHF cells at 6 days, consistent with CMV, and this was confirmed by fluorescent antibody staining with antibody to CMV. A blood buffy coat sample collected approximately 6 weeks after the renal biopsy was inoculated into MRHF and RMK cells. CMV was recovered in the MRHF, and a second virus was recovered in RMK on day 14. This isolate showed the same type of CPE as the urine culture. It was passaged in RMK cells and confirmed as BK virus by PCR.
Characterization of the virus.
The results of PCR amplification of DNA extracted from the kidney biopsy and from urine and buffy coat cells are summarized in Table 2. Probing of the product amplified by primers BJU-1 and BJU-2 with type-specific probes showed the fragment sequence to be like BKV(Dun) rather than BKV(AS). No amplification of JCV sequences with this universal primer pair, which amplifies both BKV and JCV, was detected. However, other primer pairs specific for JCV showed that JCV DNA was, in fact, present in the kidney extracts and was of the type 1 genotype (2, 7). JCV DNA was not detectable in products amplified from urine or buffy coat cells. Three pairs of primers specific for SV40 were negative for all samples. Primer pairs that were specific for BKV DNA (designated BES, BLS, and BTP) amplified bands of appropriate sizes on agarose gels. However, on Southern blots these products did not bind to the appropriate 32P-labeled probe synthesized according to the BKV(Dun) sequence (Table 1). This result suggested that a new variant of wild-type BKV(Dun) was present in extracts of kidney, urine, and buffy coat cells.
TABLE 2.
PCR amplification and hybridization of DNA sequences
| Primers or probe | Test resulta with:
|
|||
|---|---|---|---|---|
| Kidney | Urine | Buffy coat | BKV(Dun) | |
| Primers BJU-1 and 2 | Pos. | Pos. | Pos. | Pos. |
| Probe BDN-1.1 | Pos. | Pos. | Pos. | Pos. |
| Probe BAS-1.1 | Neg. | Neg. | Neg. | Neg. |
| Probe JMG-1.1 | Neg. | Neg. | ||
| Primers BES-3 and 6 | Pos.b | Pos.b | Pos. | Pos. |
| Probe BES-3.1 | Neg. | Neg. | Neg. | Pos. |
| Probe BES-3.3 | Pos. | Pos. | Pos. | Neg. |
| Primers BTP-1 and 2 | Pos. | Pos. | Pos. | Pos. |
| Probe BTP-1.1 | Neg. | Neg. | Neg. | Pos. |
| Primers BLS-3 and 4 | Pos. | Pos. | Pos. | Pos. |
| Probe BLS-3.1 | Neg. | Neg. | Neg. | Pos. |
| Primers BRR-1,2 | Pos.b | Pos.b | Neg. | Pos. |
| Primers JTP-1 and 2 | Pos. | Neg. | Neg. | Neg. |
| Probe JTP-1.1 | Pos. | Neg. | Neg. | |
| Primers JES-3 and 6 | Pos. | Neg. | ||
| Probe JES-3.1 | Pos. | Neg. | ||
| Primers JRR-6 and 7 | Pos. | Neg. | ||
Pos. or Neg. on the Primers line indicates presence or absence of a gel band of the expected length. Pos. or Neg. on the Probe line indicates reactivity of the band following Southern blotting and hybridization with the 32P-labeled probe.
Gel band purified and sequenced directly by cycle sequencing.
The BES-3 and BES-6 primers amplify a fragment in the DNA-binding region of the large-T-antigen coding sequence that, as noted above, did not bind the specific probe, BES-3.1. Direct cycle sequencing of the fragment revealed a mutation in the amplified fragment in which the dinucleotide TG was changed to AA at positions 4298 and 4299 (Fig. 3). On the coding strand the CAA codon for glutamine was changed to TTA, encoding leucine. Resynthesis of the BES-3.1 probe to conform to the mutated sequence (Table 1) produced a probe which bound to the BES-3 and BES-6 products of BKV(Cin) from kidney, urine, and buffy coat cells (Table 2). Otherwise, the sequence of the BES-3 BES-6 fragments from the kidney followed the BKV(Dun) sequence rather than the BKV(AS) sequence (Fig. 3). At nucleotide position 4339, the amplified fragment from buffy coat virus [BKV(Cin/W)] could be distinguished from that of the kidney virus [BKV(Cin/K)] by a G→A substitution. This mutation does not change the amino acid (Thr) predicted by the affected codon.
FIG. 3.
DNA sequence of the BES-3 and BES-6 fragments. There is a mutation in the amplified fragment of the BKV from the kidney, BK(Cin/K), and the buffy coat cells, BK(Cin/W), at positions 4298 to 4299 as compared to the BK(Dun) and BK(AS) strains. At position 4339, the buffy coat virus, BK(Cin/W), differed from the kidney virus by a G→A substitution.
Following amplification of the regulatory region with primers BRR-1 and BRR-2, the products were cloned and sequenced. Of four clones obtained from the kidney, three contained a 48-bp deletion and a 41-bp duplication, while a fourth contained the same deletion but lacked the duplication. No archetypal BKV regulatory region was identified. In contrast, the JCV(Cin) regulatory region from the kidney was of the unrearranged archetypal structure ordinarily found in urine.
DISCUSSION
This is the first case of tubulointerstitial nephritis due to BKV in which an amino acid mutation and regulatory region rearrangement with the potential to alter viral DNA replication have been demonstrated. The rearranged regulatory region was found in viral DNA extracted directly from the kidney biopsy without passage in tissue culture and, therefore, could not have been a tissue culture artifact. Together, these alterations may have contributed to the unusually aggressive BKV infection of the renal tubules, resulting in renal failure. While BKV often infects the kidney persistently, and may occasionally result in ureteral stenosis following renal transplant, permissive and fatal infection of the tubules is rare. Two cases have been reported for children with immunodeficiency (12, 27), and three others have been documented for an adult with AIDS (30) and two others with renal allografts (26). In the AIDS patient, the virus had spread to lung and brain, causing a fatal meningitis (30). In our case, the patient died with seizures after release from the hospital, but an autopsy to confirm central nervous system (CNS) involvement was not performed.
This is also the first case in which BKV and JCV were PCR amplified from the same kidney, although both viruses are sometimes shed together in the urine (1). While the kidney was coinfected with JCV, the JCV regulatory region showed the archetypal (unrearranged) form rather than the PML-type rearranged regulatory region (8, 31). Therefore, it is likely that the JCV renal infection remained latent, in contrast to the productive BKV infection. The JCV DNA was present in much lower amounts than was BKV DNA, since it was not coamplified by the primer pair (BJU-1 and BJU-2) which was designed to amplify simultaneously both BKV and JCV DNAs. JCV DNA was amplified only with JCV-specific primers, and it seems unlikely that JCV contributed to the renal pathology.
The relationship of this BKV strain (Cin) to previously described serotypes and genotypes (17, 18) is not yet clear. It is more closely related in the BJU and BES fragments to the Dun strain than to BKV(AS). However, it is clearly different from BKV(Dun) in the BTP and BLS fragments, so that BKV(Cin) may represent a new group of BKV sequences. Further DNA sequence characterization will be required to answer this question.
The BKV(Cin) dinucleotide mutation (TG→AA) at positions 4298 and 4299 which changes Gln to Leu may be a recent event within this patient or may represent a strain which has been circulating in the population for some time. Since the mutation in the kidney virus [BKV(Cin/K)] was also found in the buffy coat virus [BKV(Cin/W)] (Fig. 3), it would appear that this mutation was present in many, if not all, viral clones from this patient. This would suggest either that the mutation arose very early in the infection, or, more likely, that it was already present in the infecting viral inoculum.
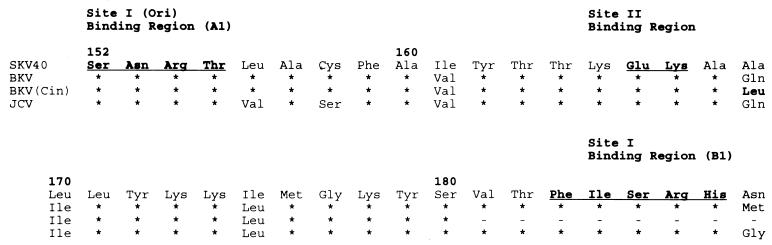
The substitution of hydrophilic Gln by hydrophobic Leu at position 169 occurs in a sequence homologous to an origin DNA-binding domain of SV40 T antigen (28). Residue 169 is located between two ori binding regions in SV40 T antigen defined by mutational analysis (Fig. 4). This residue is not a part of the protein surface implicated in origin-specific recognition, but is located in an α helix (αB) which flanks a central five-stranded antiparallel β-sheet (20). Interestingly, Leu, along with Glu, Met, and Ala, is a strong helix former (12). Thus, we suggest that replacement of Gln with a stronger helix-former (Leu) promotes the correct folding and/or stabilizes the active protein conformation rather than altering its structure and function. The Gln→Leu mutation in αB would extend the hydrophobic surface of the helix and may enhance the stability of its interaction with the hydrophobic face of the central β sheet of this domain. It is also possible that the mutation in T antigen was a factor in the in vivo rearrangement of the BKV regulatory region, although the mechanism by which these rearrangements occur in JCV and BKV remains entirely unknown.
FIG. 4.
Predicted T-antigen amino acid sequence of BKV strain Cin in DNA binding regions A1 and B1. Only changes for the amino acid sequence of SV40 are indicated. Note the change of Ala/Gln at position 169 to Leu. Asterisks denote portions not sequenced. For localization of DNA binding regions, see reference 28.
This case demonstrates that, like the human polyomavirus JC, which causes the fatal demyelinating disease known as PML (25), BKV is also capable of regulatory region rearrangement and progressive infection, particularly in AIDS patients. PML in AIDS was first reported in 1982 (23), and since then there have been many thousands of AIDS patients who have died with PML. In contrast, tubulointerstitial nephritis with renal failure was first reported for an immunodeficient child in 1983 (27), but subsequent reports of similar cases have been very few, although self-limited interstitial nephritis has recently been reported to occur in renal transplant recipients (22). The reason for this difference is not clear, but it may in part be due to the frequency with which the regulatory region rearranges or to a requirement for BKV coding region mutants of increased pathogenicity. It is noteworthy that both regulatory region rearrangements and coding region changes also appear to be associated with increased pathogenicity of JCV in PML (3, 4). Further studies to determine the frequency of the T-antigen mutation both in the general population and in patients with progressive kidney disease are indicated.
REFERENCES
- 1.Agostini H T, Brubaker G R, Shao J, et al. BK virus and a new type of JC virus excreted by HIV-1 positive patients in rural Tanzania. Arch Virol. 1995;140:1919–1934. doi: 10.1007/BF01322682. [DOI] [PubMed] [Google Scholar]
- 2.Agostini H T, Ryschkewitsch C F, Stoner G L. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–164. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostini H T, Ryschkewitsch C F, Mory R, Singer E J, Stoner G L. JC virus (JCV) genotypes in brain tissue from patients with progressive multifocal leukoencephalopathy (PML) and in urine from controls without PML: increased frequency of JCV type 2 in PML. J Infect Dis. 1997;176:1–8. doi: 10.1086/514010. [DOI] [PubMed] [Google Scholar]
- 4.Agostini H T, Ryschkewitsch C F, Singer E J, Stoner G L. JC virus regulatory region rearrangements and genotypes in progressive multifocal leukoencephalopathy: two independent aspects of virus variation. J Gen Virol. 1997;78:659–664. doi: 10.1099/0022-1317-78-3-659. [DOI] [PubMed] [Google Scholar]
- 5.Arthur R R, Shah K V, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 6.Arthur R R, Shah K V. Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. [PubMed] [Google Scholar]
- 7.Ault G S, Stoner G L. Two major types of JC virus defined in progressive multifocal leukoencephalopathy brain by early and late coding region DNA sequences. J Gen Virol. 1992;73:2669–2678. doi: 10.1099/0022-1317-73-10-2669. [DOI] [PubMed] [Google Scholar]
- 8.Ault G S, Stoner G L. Human polyomavirus JC promoter/enhancer rearrangement patterns from progressive multifocal leukoencephalopathy brain are unique derivatives of a single archetypal structure. J Gen Virol. 1993;74:1499–1507. doi: 10.1099/0022-1317-74-8-1499. [DOI] [PubMed] [Google Scholar]
- 9.Ault G S, Stoner G L. Brain and kidney of progressive multifocal leukoencephalopathy patients contain identical rearrangements of the JC virus promoter/enhancer. J Med Virol. 1994;44:298–304. doi: 10.1002/jmv.1890440315. [DOI] [PubMed] [Google Scholar]
- 10.Buckler C E, Salzman N P. Annotated nucleotide sequences and restriction site lists for selected papovavirus strains. In: Salzman N P, editor. The polyomaviruses. New York, N.Y: Plenum; 1986. pp. 382–440. [Google Scholar]
- 11.Chesters P M, Heritage J, McCance D J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 12.De Silva L M, Bale P, De Courcy J, Brown D, Knowles W. Renal failure due to BK virus infection in an immunodeficient child. J Med Virol. 1995;45:192–196. doi: 10.1002/jmv.1890450214. [DOI] [PubMed] [Google Scholar]
- 13.Fasman G D. Prediction of protein structure and the principles of protein conformation. New York, N.Y: Plenum Press; 1989. [Google Scholar]
- 14.Gardner S D, Knowles W A. Human polyomaviruses. In: Zuckerman A G, Banatvala J E, Pattison J R, editors. Principles and practice of clinical virology. Chichester, United Kingdom: Wiley; 1994. pp. 635–651. [Google Scholar]
- 15.Goudsmit J, Wertheim-van Dillen P, Van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10:91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Kitamura T, Ebihara H, et al. Geographical distribution of the human polyomavirus JC virus type A and B and isolation of a new type from Ghana. J Gen Virol. 1996;77:919–927. doi: 10.1099/0022-1317-77-5-919. [DOI] [PubMed] [Google Scholar]
- 17.Jin L, Gibson P E, Booth J C, Clewley J P. Genomic typing of BK virus in clinical specimens by direct sequencing of polymerase chain reaction products. J Med Virol. 1993;41:11–17. doi: 10.1002/jmv.1890410104. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Gibson P E, Knowles W A, Clewley J P. BK virus antigenic variants: sequence analysis within the capsid VP1 epitope. J Med Virol. 1993;39:50–56. doi: 10.1002/jmv.1890390110. [DOI] [PubMed] [Google Scholar]
- 19.Kunitake T, Kitamura T, Guo J, Taguchi F, Kawabe K, Yogo Y. Parent-to-child transmission is relatively common in the spread of the human polyomavirus JC virus. J Clin Microbiol. 1995;33:1448–1451. doi: 10.1128/jcm.33.6.1448-1451.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X L, Sanford D G, Bullock P A, Bachovchin W W. Solution structure of the origin DNA-binding domain of SV40 T-antigen. Nat Struct Biol. 1996;3:1034–1039. doi: 10.1038/nsb1296-1034. [DOI] [PubMed] [Google Scholar]
- 21.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathurm U S, Olson J L, Darragh T M, Yen T J B. Polyomavirus induced interstitial nephritis in two renal transplant recipients: case reports and review of the literature. Am J Kidney Dis. 1997;29:754–758. [PubMed] [Google Scholar]
- 23.Miller J R, Barrett R E, Britton C B, et al. Progressive multifocal leukoencephalopathy in a male homosexual with T-cell immune deficiency. N Engl J Med. 1982;307:1436–1438. doi: 10.1056/NEJM198212023072307. [DOI] [PubMed] [Google Scholar]
- 24.Monaco M C G, Atwood W J, Gravell M, Tornatore C S, Major E O. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padgett B L, Walker D L, ZuRhein G M, Eckroade R J, Dessel B H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 26.Pappo O, Demetris A J, Raikow R B, Randhawa P S. Human polyoma virus infection of renal allografts: histopathologic diagnosis, clinical significance, and literature review. Mol Pathol. 1996;9:105–109. [PubMed] [Google Scholar]
- 27.Rosen S, Harmon W, Krensky A M, et al. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N Engl J Med. 1983;308:1119–1196. doi: 10.1056/NEJM198305193082004. [DOI] [PubMed] [Google Scholar]
- 28.Simmons D T, Loeber G, Tegtmeyer P. Four major sequence elements of simian virus 40 large T antigen coordinate its specific and nonspecific DNA binding. J Virol. 1990;64:1973–1983. doi: 10.1128/jvi.64.5.1973-1983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundsfjord A, Spein A R, Lucht E, Flaegstad T, Morten Seternes O, Traavik T. Detection of BK virus DNA in nasopharyngeal aspirates from children with respiratory infections but not in saliva from immunodeficient and immunocompetent adult patients. J Clin Microbiol. 1994;32:1390–1394. doi: 10.1128/jcm.32.5.1390-1394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallbracht A, Löhler J, Gossmann J, et al. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am J Pathol. 1993;143:29–39. [PMC free article] [PubMed] [Google Scholar]
- 31.Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]