Abstract
Identifying cultivars of leguminous crops exhibiting drought resistance has become crucial in addressing water scarcity issues. This investigative study aimed to select soybean and cowpea cultivars with enhanced potential to grow under water restriction during the vegetative stage. Two parallel trials were conducted using seven soybean (AS3810IPRO, M8644IPRO, TMG1180RR, NS 8338IPRO, BMX81I81IPRO, M8808IPRO, and BÔNUS8579IPRO) and cowpea cultivars (Aracê, Novaera, Pajeú, Pitiúba, Tumucumaque, TVU, and Xique-xique) under four water levels (75, 60, 45, and 30% field capacity—FC) over 21 days. Growth, water content, membrane damage, photosynthetic pigments, organic compounds, and proline levels were analyzed. Drought stress significantly impacted the growth of both crops, particularly at 45 and 30% FC for soybean and 60 and 45% FC for cowpea plants. The BÔNUS8579IPRO and TMG1180RR soybean cultivars demonstrated the highest performance under drought, a response attributed to increased amino acids and proline contents, which likely help to mitigate membrane damage. For cowpea, the superior performance of the drought-stressed Xique-xique cultivar was associated with the maintenance of water content and elevated photosynthetic pigments, which contributed to the preservation of the photosynthetic efficiency and carbohydrate levels. Our findings clearly indicate promising leguminous cultivars that grow under water restriction, serving as viable alternatives for cultivating in water-limited environments.
Keywords: Glycine max, Vigna unguiculata L. Walp, drought tolerance, water deficit, leguminous crops
1. Introduction
Water scarcity has emerged as a pressing global concern, and several regions have confronted a critical shortage of water due to the combined effects of population growth, rapid urbanization, and climate change. These problems are directly reflected in detrimental consequences for agriculture, industry, and people’s health. The scarcity of water resources presents not only challenges in fulfilling fundamental human requirements but also puts ecosystems and biodiversity at risk [1,2].
In face of populational growth in recent years, leguminous crops constitute an important solution to ensure food and nutritional security, with a key socioeconomic role [2,3]. Among them, soybean (Glycine max L. Merrill) and cowpea (Vigna unguiculata L. Walp) stand out as a crucial component for humans and ruminant animals. Leguminous crops are also associated with the fixation of biological nitrogen due to their abilities in mutualistic association, thus reducing costs of industrially manufactured chemical fertilizers in supplying nitrogen to plants.
Soybean is among the most explored crops worldwide, and Brazil has become the world’s leading producer of grains, followed by the United States and China [4,5]. Nevertheless, the drought episodes are projected to promote severe decreases from 21.8 to 40% in soybean yields around the world [6,7,8]. On the other hand, the global production of cowpea reached around 9.6 million tons in 2021, with highlights in African countries [9,10]. Moreover, Brazil is among the largest producers with one million hectares of land, despite its production mainly being concentrated in the Northeast region where the drought and soil quality are abiotic factors limiting cowpea’s ability to grow satisfactorily, especially during the pod filling stage, resulting in losses above 30% [4,11,12].
In plants, several processes are disturbed by a water deficit, including morphological, physiological, and biochemical alterations, as well as modulation in gene expression. Under water restriction, plants tend to promote stomatal closure in order to avoid water loss through transpiration, also decreasing CO2 availability for chloroplasts, which impairs the net photosynthesis and carbohydrate biosynthesis [13,14,15]. Consequently, the energy excess in electron transport chain creates oxidative damage to membranes and photosynthetic pigments [16]. In an attempt to defend against drought, plants may activate multiple morphophysiological, biochemical, and enzymatic responses, highlighting osmotic adjustment as the main mechanism for the large majority of plant species [17,18].
Numerous research centers have devoted intensive efforts to searching for methods to improve plant defense to water deficit. Efforts have been made by addressing plant breeding with different genes [19,20], and cross-talk tolerance inducers to increase the photosynthetic performance, antioxidant system, and promote osmotic adjustment [21,22,23]. Nonetheless, despite efforts to develop cultivars that combine high productivity with resistance to biotic and abiotic stresses, challenges remain in the search for cultivars with high ability under water limitation. These cultivars can decisively contribute to maintaining the agribusiness of large grain producers and the productive stability of small-scale farmers located in areas affected by drought episodes. Therefore, studies focusing on the selection of cultivars with elevated performance under low water availability become essential to selecting plants more resistant to drought occurrences.
Our working hypothesis was that soybean and cowpea cultivars display distinct responses to water deficit, which arise from biochemical adjustments to optimize plant performance. To test this hypothesis, seven semiarid-cultivated soybean and cowpea cultivars were exposed to different water availability levels under greenhouse conditions. Growth and some biochemical stress indicators were analyzed in both leguminous crops.
2. Results
2.1. Plant Growth
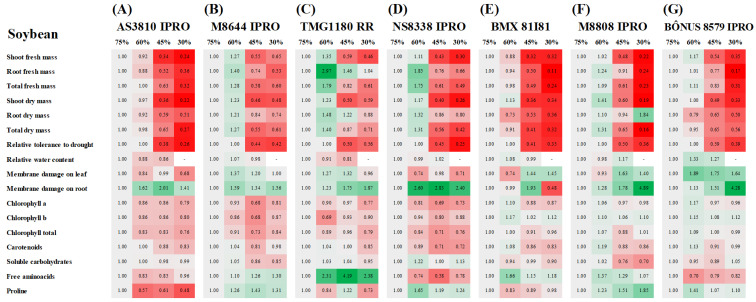
In general, soybean plants grown in soil with 60% field capacity (FC) exhibited values of shoot fresh mass (SFM) and shoot dry mass (SDM), root fresh mass (RFM), root dry mass (RDM), total fresh mass (TFM), and total dry mass (TDM) similar or higher than those of very well-irrigated plants (75% FC treatments) (Figure 1). All growth parameters were dramatically decreased by water limitation in soil, with rare exceptions (45 and 30% FC) (Figure 1 and Table 1). Also, in the majority of soybean cultivars, the fresh and dry mass decrease was intensified by reducing the irrigation water level (from 45 to 30% FC), and the reductions were found to be more prominent in the AS3810 IPRO, NS8338 IPRO BMX 81I81 IPRO, and M8808 IPRO cultivars (Figure 1 and Table 1). On the other hand, the lowest drought-induced reductions in plant growth were registered in BÔNUS8579 IPRO (at 45% FC) and TMG1180 RR (at 30% FC) cultivars, which exhibited the highest values of total dry mass and relative tolerance to drought as compared to other studied soybean cultivars (Figure 1 and Figure 2C,D).
Figure 1.
Clustering analysis of growth and biochemical assays relative to changes due to drought treatments in soybean cultivars: AS3810 IPRO (A), M8644 IPRO (B), TMG1180 RR (C), NS8338 IPRO (D), BMX81I81 IPRO (E), M8808 IPRO (F), and BÔNUS8579 IPRO (G). The trials were carried out in plants 21 days after exposure to four water level treatments (75, 60, 45, and 30% field capacity—FC). Each row characterizes an individual analysis. For all cases, green color specifies an increase, and red denotes a decrease in the analyzed indexes, taking the data of 75% FC plants as reference. Gray represents no change. The number inside the box and different red and green intensities express the extent of the change according to fold increase or decrease related to reference. For relative tolerance to drought, the total dry mass of plants from the 60% treatment was used as the control, and the plants from 45% to 30% FC were considered water deficit. Sufficient material was not obtained to calculate RWC at 30% FC. For absolute values and statistical details, see Table 1.
Table 1.
Absolute values and statistical details for fresh and dry mass, relative tolerance to drought, membrane damage, photosynthetic pigments, soluble carbohydrates, free amino acids, and proline of soybean plants. The assays were carried out on cultivars of AS3810 IPRO, M8644 IPRO, TMG1180 RR, NS8338 IPRO, BMX 81I81 IPRO, M8808 IPRO, and BÔNUS8579 IPRO, 21 days after exposure to four water levels treatments (75, 60, 45, and 30% field capacity—FC).
| Soybean Cultivars | 75% | 60% | 45% | 30% | 75% | 60% | 45% | 30% |
|---|---|---|---|---|---|---|---|---|
| Shoot fresh mass (g/plant) | Shoot dry mass (g/plant) | |||||||
| AS3810 IPRO | 8.26 Aa | 7.57 Aa | 2.80 Ba | 1.94 Ba | 1.71 Aa | 1.66 Aa | 0.61 Ba | 0.37 Ba |
| M8644 IPRO | 4.20 Ac | 5.32 Ab | 2.33 Ba | 2.72 Ba | 1.09 Ab | 1.35 Ab | 0.50 Ba | 0.53 Ba |
| TMG1180 RR | 5.61 Bb | 7.60 Aa | 3.30 Ca | 2.57 Ca | 1.33 Ab | 1.64 Aa | 0.67 Ba | 0.79 Ba |
| NS8338 IPRO | 7.29 Aa | 8.10 Aa | 3.11 Ba | 2.20 Ba | 1.65 Aa | 1.94 Aa | 0.66 Ba | 0.43 Ba |
| BMX81I81 IPRO | 7.20 Aa | 6.36 Ab | 2.31 Ba | 2.27 Ba | 1.29 Ab | 1.45 Ab | 0.46 Ba | 0.44 Ba |
| M8808 IPRO | 5.77 Ab | 5.89 Ab | 2.77 Ba | 1.24 Ca | 0.99 Bb | 1.40 Ab | 0.60 Ca | 0.18 Da |
| BÔNUS8579 IPRO | 7.89 Aa | 9.20 Aa | 4.27 Ba | 2.76 Ca | 1.85 Aa | 1.85 Aa | 0.91 Ba | 0.61 Ba |
| Root fresh mass (g/plant) | Root dry mass (g/plant) | |||||||
| AS3810 IPRO | 4.07 Ab | 3.58 Ab | 2.11 Ba | 1.45 Ba | 0.44 Ac | 0.40 Aa | 0.26 Bb | 0.23 Bb |
| M8644 IPRO | 3.47 Bb | 4.94 Aa | 2.34 Ca | 1.67 Ca | 0.39 Ac | 0.47 Aa | 0.30 Ba | 0.23 Bb |
| TMG1180 RR | 2.03 Cc | 6.03 Aa | 2.96 Ba | 2.10 Ca | 0.34 Bd | 0.46 Aa | 0.37 Bb | 0.27 Cb |
| NS8338 IPRO | 2.94 Bc | 4.92 Aa | 2.01 Ca | 1.74 Ca | 0.31 Bd | 0.41 Aa | 0.27 Bb | 0.25 Bb |
| BMX81I81 IPRO | 5.27 Aa | 4.95Aa | 2.65 Ba | 0.58 Cb | 0.70 Aa | 0.46 Ba | 0.33 Ca | 0.23 Db |
| M8808 IPRO | 2.47 Ac | 3.05 Ab | 2.23 Aa | 0.59 Bb | 0.25 Bd | 0.28 Bb | 0.24 Bb | 0.42 Aa |
| BÔNUS8579 IPRO | 3.97 Ab | 3.99 Ab | 3.05 Ba | 0.67 Cb | 0.54 Ab | 0.47 Aa | 0.35 Ba | 0.28 Bb |
| Total fresh mass (g/plant) | Total dry mass (g/plant) | |||||||
| AS3810 IPRO | 12.33 Aa | 11.15 Ac | 4.90 Bc | 3.40 Ba | 2.15 Aa | 2.27 Aa | 0.87 Ba | 0.60 Bb |
| M8644 IPRO | 7.68 Bc | 10.26 Ac | 4.67 Cc | 4.39 Ca | 1.49 Ab | 1.81 Ab | 0.80 Ba | 0.76 Bb |
| TMG1180 RR | 7.64 Bc | 13.62 Aa | 6.26 Bb | 4.27 Ca | 1.67 Bb | 2.10 Aa | 1.04 Ca | 1.17 Ca |
| NS8338 IPRO | 10.23 Bb | 13.02 Ab | 5.12 Cc | 3.94 Ca | 1.96 Ba | 2.35 Aa | 0.93 Ca | 0.60 Cb |
| BMX81I81 IPRO | 13.59 Aa | 12.14 Ab | 4.96 Bc | 2.85 Cb | 2.28 Aa | 1.91 Bb | 0.79 Ca | 0.67 Cb |
| M8808 IPRO | 7.23 Bc | 8.95 Ad | 5.00 Cc | 1.83 Db | 1.24 Bb | 1.68 Ab | 0.83 Ca | 0.60 Cb |
| BÔNUS8579 IPRO | 11.86 Ba | 14.47 Aa | 7.94 Ca | 3.43 Da | 2.39 Aa | 2.32 Aa | 1.26 Ba | 0.89 Ca |
| Relative tolerance to drought (%) * | Relative water content (%) ** | |||||||
| AS3810 IPRO | 100 Aa | 100 Aa | 38.39 Bc | 26.34 Cc | 52.6 Aa | 46.3 Aa | 45.4 Ab | - |
| M8644 IPRO | 100 Aa | 100 Aa | 44.06 Bc | 41.76 Bb | 44.9 Aa | 48.2 Aa | 44.2 Ab | - |
| TMG1180 RR | 100 Aa | 100 Aa | 49.64 Bb | 55.86 Ca | 49.5 Aa | 45.2 Aa | 40.3 Ab | - |
| NS8338 IPRO | 100 Aa | 100 Aa | 44.64 Bc | 25.43 Cc | 56.9 Aa | 56.1 Aa | 58.3 Aa | - |
| BMX81I81 IPRO | 100 Aa | 100 Aa | 41.41 Bc | 35.07 Cb | 49.3 Aa | 53.5 Aa | 49.0 Ab | - |
| M8808 IPRO | 100 Aa | 100 Aa | 49.65 Bb | 35.80 Cb | 52.0 Aa | 50.7 Aa | 61.1 Aa | - |
| BÔNUS8579 IPRO | 100 Aa | 100 Aa | 58.75 Ba | 38.54 Cb | 42.5 Ba | 56.6 Aa | 53.9 Aa | - |
| Membrane damage in leaves (%) | Membrane damage in roots (%) | |||||||
| AS3810 IPRO | 30.4 Aa | 25.7 Aa | 30.1 Ba | 20.6 Ba | 35.9 Ba | 58.1 Aa | 72.1 Aa | 50.6 Bb |
| M8644 IPRO | 22.8 Bb | 31.1 Aa | 27.4 Aa | 22.8 Ba | 32.2 Aa | 51.1 Aa | 43.2 Aa | 50.3 Ab |
| TMG1180 RR | 23.7 Bb | 30.1 Aa | 31.3Aa | 22.8 Ba | 31.5 Ba | 38.1 Ba | 55.2 Aa | 58.9 Ab |
| NS8338 IPRO | 28.4 Aa | 21.1 Bb | 27.7 Aa | 20.3 Ba | 19.4 Ba | 50.5 Aa | 55.0 Aa | 46.6 Ab |
| BMX81I81 IPRO | 17.6 Bc | 13.1 Bc | 25.4 Aa | 25.5 Aa | 34.7 Ba | 34.3 Ba | 66.9Aa | 16.5 Bc |
| M8808 IPRO | 16.3 Bc | 15.2 Bc | 26.6 Aa | 22.9 Aa | 34.6 Ca | 44.3 Ca | 61.8 Ba | 169.3 Aa |
| BÔNUS8579 IPRO | 16.4 Bc | 31.1 Aa | 28.7 Aa | 26.9 Aa | 36.5 Ba | 41.1 Ba | 54.9 Ba | 156.2 Aa |
| Chl a (μg g−1 DM) | Chl b (μg g−1 DM) | |||||||
| AS3810 IPRO | 4419 Aa | 3801 Bb | 3821 Ba | 3478 Ba | 1376 Aa | 1182 Ba | 1180 Ba | 1095 Ba |
| M8644 IPRO | 3556 Ab | 3289 Ac | 2425 Bb | 2896 Bb | 1108 Ab | 952 Bb | 758 Bb | 959 Bb |
| TMG1180 RR | 3990 Aa | 3603 Ac | 3875 Aa | 3059 Bb | 1263 Aa | 873 Bb | 1169 Aa | 1132 Aa |
| NS8338 IPRO | 4179 Aa | 3399 Bc | 2883 Bb | 3043 Bb | 1106 Ab | 1035 Ab | 886 Ab | 975 Ab |
| BMX81I81 IPRO | 3888 Ab | 4289 Aa | 3436 Ba | 3368 Ba | 1036 Ab | 1215 Aa | 1057 Aa | 1156 Aa |
| M8808 IPRO | 3658 Ab | 3881 Ab | 3557 Aa | 3596 Aa | 1045 Ab | 1145 Aa | 1112 Aa | 1153 Aa |
| BÔNUS8579 IPRO | 3657 Bb | 4279 Aa | 3565 Ba | 3499 Ba | 1013 Ab | 1163 Aa | 1097 Aa | 1134 Aa |
| Chl total (μg g−1 DM) | Carotenoids (μg g−1 DM) | |||||||
| AS3810 IPRO | 6039 Aa | 5016 Ba | 5035 Ba | 4602 Ba | 951 Aa | 950 Aa | 837 Aa | 787 Aa |
| M8644 IPRO | 4695 Ab | 4278 Aa | 3408 Bb | 3934 Ba | 740 Aa | 772 Ab | 603 Ab | 725 Aa |
| TMG1180 RR | 5286 Aa | 4722 Aa | 5082 Aa | 4200 Aa | 836 Aa | 870 Ab | 835 Aa | 712 Aa |
| NS8338 IPRO | 5343 Aa | 4466 Ba | 3795 Bb | 4042 Ba | 942 Aa | 836 Ab | 665 Bb | 676 Ba |
| BMX81I81 IPRO | 4977 Ab | 4960 Aa | 4525 Aa | 4780 Aa | 882 Aa | 955 Aa | 759 Ba | 735 Ba |
| M8808 IPRO | 4744 Ab | 5067 Aa | 4177 Aa | 4778 Aa | 893 Ba | 1060 Aa | 784 Ba | 772 Ba |
| BÔNUS8579 IPRO | 4716 Ab | 5154 Aa | 4695 Aa | 4660 Aa | 853 Ba | 962 Aa | 774 Aa | 845 Aa |
| Soluble carbohydrates (μmol g−1 DM) | Free amino acids (μmol g−1 DM) | |||||||
| AS3810 IPRO | 1287 Aa | 1283 Aa | 1266 Aa | 1275 Aa | 217.8 Ab | 180.1 Ab | 184.1 Ac | 210.1 Aa |
| M8644 IPRO | 1228 Aa | 1287 Aa | 1058 Ba | 1048 Bb | 154.2 Ac | 169.0 Ab | 194.5 Ab | 200.5 Aa |
| TMG1180 RR | 1163 Aa | 1199 Aa | 1206 Aa | 1100 Ab | 77.4 Cb | 178.6 Bb | 324.1 Aa | 183.9 Ba |
| NS8338 IPRO | 863 Ab | 1050 Ab | 863 Ab | 973 Ab | 263.2 Aa | 195.9 Bb | 153.0 Cc | 205.5 Ba |
| BMX81I81 IPRO | 1173 Aa | 1101 Ab | 1157 Aa | 1057 Ab | 196.6 Bb | 326.5 Aa | 225.5 Bb | 232.1 Ba |
| M8808 IPRO | 1106 Aa | 1124 Ab | 845 Bb | 774 Bc | 163.9 Bc | 224.4 Ab | 211.2 Ab | 175.9 Ba |
| BÔNUS8579 IPRO | 1101 Aa | 1042 Ab | 984 Ab | 1152 Aa | 214.8 Ab | 150.5 Bb | 169.4 Bc | 177.1 Ba |
| Proline (μmol g−1 DM) | ||||||||
| AS3810 IPRO | 6.69 Aa | 3.79 Bb | 4.07 Ba | 3.24 Bb | ||||
| M8644 IPRO | 2.11 Ac | 2.67 Ab | 3.02 Ab | 2.76 Ab | ||||
| TMG1180RR | 2.65 Ac | 2.22 Ab | 3.23 Ab | 1.95 Ab | ||||
| NS8338 IPRO | 3.75 Bb | 6.17 Aa | 4.46 Ba | 4.66 Ba | ||||
| BMX81I81 IPRO | 3.37 Ab | 2.82 Ab | 3.00 Ab | 3.30 Ab | ||||
| M8808 IPRO | 2.48 Bc | 3.05 Bb | 3.74 Aa | 4.58 Aa | ||||
| BÔNUS8579 IPRO | 2.36 Ac | 3.33 Ab | 2.53 Ab | 2.61 Ab | ||||
In the same line, different capital letters represent significant differences due to drought stress within the same soybean cultivar. In the same column, different lowercase letters represent significant alterations among soybean cultivars within the same stress level, according to Scott–Knott’s test (p < 0.05). * For relative tolerance to drought, the total dry mass of plants from the 60% treatment was used as the control, and the plants 45% and 30% FC were considered water deficit. ** Sufficient material was not obtained to calculate RWC at 30% FC.
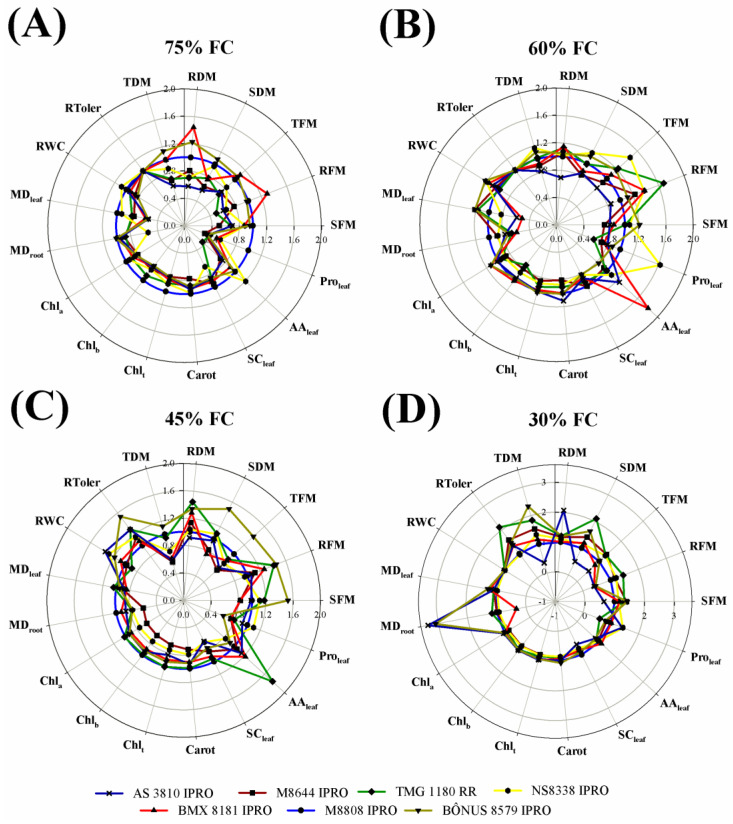
Figure 2.
Overall representation of contrasting responses from Glycine max L. cultivars, AS3810 IPRO (dark blue line), M8644 IPRO (dark red line), TMG1180 RR (green line), NS8338 IPRO (yellow line), BMX81I81 IPRO (red line), M8808 IPRO (blue line), and BÔNUS8579 IPRO (dark yellow line), subject to different water levels treatments: 75 (A), 60 (B), 45 (C), and 30% (D) field capacity (FC). The data refer to relative alterations in the following parameters: shoot (SFM), root (RFM), and total fresh mass (TFM); shoot (SDM), root (RDM), and total dry mass (TDM); relative tolerance to drought (RToler); relative water content (RWC); membrane damage in leaf (MDleaf) and roots (MDroot); contents of chlorophyll a (Chla), b (Chlb), total (Chlt), and carotenoids (Carot); contents of soluble carbohydrates (SCleaf), free amino acids (AAleaf), and proline (Proleaf) in the leaves. The radar plot was designed using the data of the M8808 cultivar (blue line) as a reference.
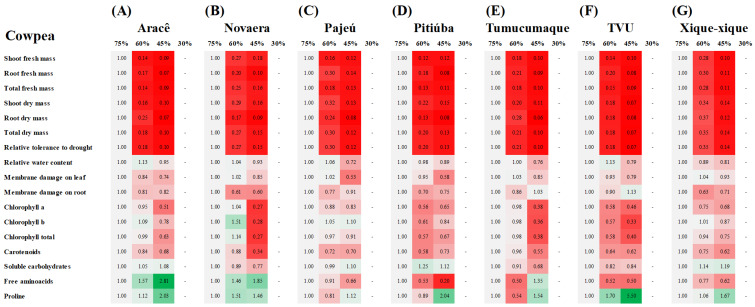
Water deprivation also promoted a strong decrease in the fresh and dry mass of tissues from all cowpea cultivars, with main reductions varying from 63 to 88% and 86 to 94% in plants growing under 60 and 45% FC as compared to the control (75% FC), respectively (Figure 3 and Table 2). At 30% FC, the drought deleterious effects were lethal for cowpea, and the plants exhibited severe symptoms of stress and died from the tenth to the fourteenth day of treatment. In absolute terms, drought-stressed Xique-xique plants displayed the highest values of shoot dry mass at 60% FC, whereas TVU plants showed the lowest ones at 45% FC, in relation to other cowpea cultivars (Figure 4B,C and Table 2). Similarly, the total dry mass under drought stress was higher in Xique-xique, Novaera and Pajeú (only at 60% FC) plants than in other cowpea cultivars at both levels of water limitation (60 and 45% FC). As a consequence of biomass accumulation, the Xique-xique, Novaera, and Pajeú plants exhibited the highest relative tolerance indexes for 60% FC treatments, respectively; whereas Xique-xique and Novaera were found to be the most tolerant under 45% FC (Figure 4B,C and Table 2).
Figure 3.
Clustering analysis of growth and biochemical assays relative to changes due to drought treatments in cowpea cultivars: Aracê (A), Novaera (B), Pajeú (C), Pitiúba (D), Tumucumaque (E), TVU (F), and Xique-xique (G). The trials were carried out in plants 21 days after exposure to four water levels treatments (75, 60, 45, and 30% field capacity—FC). Each row characterizes an individual analysis. For all cases, green color specifies an increase, and red denotes a decrease in the analyzed indexes, taking the data of 75% FC plants as reference. Cowpea plants did not support the 30% FC drought level and died before the harvest. Gray represents no change. Numbers inside the box and different red and green intensities express the extent of the change according to fold increase/decrease related to reference. For absolute values and statistical details, see Table 2.
Table 2.
Absolute values and statistical details for fresh and dry mass, relative tolerance to drought, membrane damage, photosynthetic pigments, soluble carbohydrates, free amino acids, and proline of cowpea plants. The assays were carried out on cultivars of Aracê, Novaera, Pajeú, Pitiúba, Tumucumaque, TVU, and Xique-xique, 21 days after exposure to four water level treatments (75, 60, 45, and 30% field capacity—FC). Cowpea plants did not support the 30% FC drought level and died before the harvest.
| Cowpea Cultivars | 75% | 60% | 45% | 30% | 75% | 60% | 45% | 30% |
|---|---|---|---|---|---|---|---|---|
| Shoot fresh mass (g/plant) | Shoot dry mass (g/plant) | |||||||
| Aracê | 64.83 Aa | 8.83 Ba | 5.96 Ba | - | 7.18 Aa | 1.18 Ba | 0.75 Ba | - |
| Novaera | 38.20 Ab | 10.20 Ba | 7.02 Ba | - | 6.19 Aa | 1.79 Ba | 0.98 Ba | - |
| Pajeú | 39.32 Ab | 6.29 Ba | 4.90 Ba | - | 5.09 Aa | 1.61 Ba | 0.68 Ba | - |
| Pitiúba | 46.48 Ab | 5.69 Ba | 5.55 Ba | - | 5.54 Aa | 1.20 Ba | 0.81 Ba | - |
| Tumucumaque | 43.41 Ab | 7.683 Ba | 4.30 Ba | - | 5.42 Aa | 1.07 Ba | 0.60 Ba | - |
| TVU | 42.02 Ab | 6.04 Ba | 4.07 Ba | - | 6.21 Aa | 1.15 Ba | 0.45 Ba | - |
| Xique-xique | 55.22 Aa | 15.37 Ba | 5.76 Ba | - | 6.56 Aa | 2.24 Ba | 0.93 Ba | - |
| Root fresh mass (g/plant) | Root dry mass (g/plant) | |||||||
| Aracê | 13.91 Aa | 2.41 Ba | 1.02 Ba | - | 1.14 Ab | 0.29 Ba | 0.08 Ba | - |
| Novaera | 12.35 Aa | 2.51 Ba | 1.25 Ba | - | 1.45 Aa | 0.25 Ba | 0.13 Ba | - |
| Pajeú | 7.93 Ab | 2.40 Ba | 1.13 Ba | - | 1.16 Ab | 0.28 Ba | 0.09 Ba | - |
| Pitiúba | 11.26 Ab | 2.08 Ba | 0.95 Ba | - | 1.13 Ab | 0.15 Ba | 0.09 Ba | - |
| Tumucumaque | 14.88 Aa | 3.08 Ba | 1.38 Ba | - | 1.33 Aa | 0.37 Ba | 0.08 Ca | - |
| TVU | 9.86 Ab | 1.94 Ba | 0.82 Ba | - | 1.12 Ab | 0.20 Ba | 0.09 Ba | - |
| Xique-xique | 10.55 Ab | 3.12 Ba | 1.16 Ba | - | 0.96 Ab | 0.36 Ba | 0.12 Ba | - |
| Total fresh mass (g/plant) | Total dry mass (g/plant) | |||||||
| Aracê | 78.75 Aa | 11.23 Ba | 6.97 Ba | - | 8.33 Aa | 1.46 Bb | 0.82 Bb | - |
| Novaera | 50.55 Ac | 12.70 Ba | 8.27 Ba | - | 7.65 Aa | 2.04 Ba | 1.11 Ba | - |
| Pajeú | 47.25 Ac | 8.69 Ba | 6.03 Ba | - | 6.25 Aa | 1.88 Ba | 0.77 Bb | - |
| Pitiúba | 57.74 Ac | 7.766 Ba | 6.50 Ba | - | 6.67 Aa | 1.36 Bb | 0.89 Bb | - |
| Tumucumaque | 58.29 Ac | 10.76 Ba | 5.69 Ba | - | 6.75 Aa | 1.44 Bb | 0.68 Bb | - |
| TVU | 51.88 Ac | 7.97 Ba | 4.89 Ba | - | 7.33 Aa | 1.35 Bb | 0.54 Bb | - |
| Xique-xique | 65.77 Ab | 18.49 Ba | 6.93 Ca | - | 7.53 Aa | 2.60 Ba | 1.05 Ba | - |
| Relative tolerance to drought (%) * | Relative water content (%) | |||||||
| Aracê | 100 Aa | 10.1 Bb | 8.3 Bb | - | 84.5% Ba | 95.2% Aa | 80.2% Ba | - |
| Novaera | 100 Aa | 28.7 Ba | 15.8 Ba | - | 78.5% Aa | 81.5% Ab | 72.7% Aa | - |
| Pajeú | 100 Aa | 32.4 Ba | 13.8 Cb | - | 77.2% Aa | 81.7% Ab | 55.7% Bb | - |
| Pitiúba | 100 Aa | 13.7 Bb | 21.2 Ba | - | 84.2% Aa | 82.5% Ab | 75.5% Aa | - |
| Tumucumaque | 100 Aa | 12.8 Bb | 9.0 Bb | - | 83.0% Aa | 82.7% Ab | 63.0% Bb | - |
| TVU | 100 Aa | 18.9 Bb | 7.6 Bb | - | 82.5% Aa | 92.7% Aa | 65.2% Bb | - |
| Xique-xique | 100 Aa | 36.7 Ba | 15.5 Ca | - | 89.2% Aa | 79.2% Bb | 72.2% Ba | - |
| Membrane damage in leaves (%) | Membrane damage in roots (%) | |||||||
| Aracê | 82.5% Aa | 69.0% Ba | 61.5% Ba | - | 60.5% Aa | 48.7% Ba | 49.5% Ba | - |
| Novaera | 70.0% Ab | 72.0% Aa | 59.5% Ba | - | 68.0% Aa | 42.0% Ba | 41.2% Ba | - |
| Pajeú | 74.2% Aa | 76.2% Aa | 39.7% Bb | - | 47.0% Ab | 36.2% Aa | 43.0% Aa | - |
| Pitiúba | 80.5% Aa | 76.2% Aa | 47.0% Bb | - | 64.5% Aa | 45.0% Ba | 48.0% Ba | - |
| Tumucumaque | 62.0% Ab | 64.2% Aa | 53.0% Ab | - | 51.7% Ab | 44.5% Aa | 53.5% Aa | - |
| TVU | 77.7% Aa | 72.2% Aa | 61.7% Ba | - | 44.0% Ab | 39.7% Aa | 49.2% Aa | - |
| Xique-xique | 70.7% Ab | 73.7% Aa | 66.0% Aa | - | 70.5% Aa | 44.2% Ba | 50.0% Ba | - |
| Chl a (μg g−1 DM) | Chl b (μg g−1 DM) | |||||||
| Aracê | 3353 Ab | 2535 Ab | 1366 Bb | - | 1084 Ab | 1181 Aa | 847 Aa | - |
| Novaera | 2858 Ab | 2959 Aa | 764 Bc | - | 892 Bb | 1351 Aa | 252 Cb | - |
| Pajeú | 2785 Ab | 2461 Ab | 2324 Aa | - | 841 Ab | 883 Ab | 921 Aa | - |
| Pitiúba | 3144 Aa | 1769 Bc | 2030 Ba | - | 1101 Ab | 670 Bb | 925 Aa | - |
| Tumucumaque | 3335 Aa | 3256 Aa | 1273 Bb | - | 1459 Aa | 1427 Aa | 532 Bb | - |
| TVU | 3353 Aa | 1958 Bc | 1559 Bb | - | 1504 Aa | 858 Bb | 501 Cb | - |
| Xique-xique | 2777 Ab | 2095 Bc | 1893 Ba | - | 974 Ab | 984 Ab | 849 Aa | - |
| Chl total (μg g−1 DM) | Carotenoids (μg g−1 DM) | |||||||
| Aracê | 3744 Ab | 3699 Ab | 2375 Ba | - | 492 Aa | 414 Ab | 332 Ba | - |
| Novaera | 3775 Ab | 4292 Aa | 1022 Bc | - | 542 Aa | 479 Aa | 185 Bc | - |
| Pajeú | 3573 Ab | 3464 Ab | 3245 Aa | - | 543 Aa | 393 Bb | 379 Ba | - |
| Pitiúba | 4261 Aa | 2442 Bc | 2845 Ba | - | 541 Aa | 313 Bb | 396 Ba | - |
| Tumucumaque | 4780 Aa | 4669 Aa | 1803 Bb | - | 510 Aa | 489 Aa | 282 Bb | - |
| TVU | 4839 Aa | 2808 Bc | 1922 Bb | - | 587 Aa | 375 Bb | 365 Ba | - |
| Xique-xique | 3632 Ab | 3404 Ab | 2732 Aa | - | 552 Aa | 415 Bb | 344 Ba | - |
| Soluble carbohydrates (μmol g−1 DM) | Free amino acids (μmol g−1 DM) | |||||||
| Aracê | 194 Ab | 204 Ab | 209 Ab | - | 535 Cb | 843 Ba | 1504 Aa | - |
| Novaera | 267 Aa | 238 Aa | 204 Bb | - | 514 Bb | 753 Aa | 949 Ac | - |
| Pajeú | 268 Aa | 264 Aa | 295 Aa | - | 703 Ab | 592 Aa | 466 Bd | - |
| Pitiúba | 224 Ab | 280 Aa | 250 Aa | - | 583 Ab | 310 Bb | 119 Be | - |
| Tumucumaque | 256 Aa | 233 Aa | 175 Bb | - | 861 Ba | 429 Cb | 1160 Ab | - |
| TVU | 229 Ab | 187 Ab | 192 Ab | - | 959 Aa | 496 Bb | 477 Bd | - |
| Xique-xique | 229 Ab | 263 Aa | 273 Aa | - | 767 Aa | 643 Ba | 478 Bd | - |
| Proline (μmol g−1 DM) | ||||||||
| Aracê | 65 Ba | 72 Ba | 131 Ab | - | ||||
| Novaera | 64 Aa | 97 Aa | 93 Ac | - | ||||
| Pajeú | 75 Aa | 61 Aa | 84 Ac | - | ||||
| Pitiúba | 77 Ba | 69 Ba | 158 Ab | - | ||||
| Tumucumaque | 115 Ba | 62 Ca | 177 Ab | - | ||||
| TVU | 62 Ca | 105 Ba | 341 Aa | - | ||||
| Xique-xique | 87 Ba | 92 Ba | 145 Ab | - | ||||
In the same line, different capital letters represent significant differences due to drought stress within the same soybean cultivar. In the same column, different lowercase letters represent significant alterations among soybean cultivars within the same stress level, according to Scott–Knott’s test (p < 0.05). * For relative tolerance to drought, the total dry mass of plants from the 75% treatment was used as the control, and the plants 60% and 45% FC were considered water deficit.
Figure 4.
Overall representation of contrasting responses from Vigna unguiculata L. Walp cultivars, Aracê (yellow line), Novaera (dark blue line), Pajeú (dark red line), Pitiúba (red line), Tumucumaque (dark yellow line), TVU (blue line), and Xique-xique (green line), subject to different water levels treatments: 75 (A), 60 (B), and 45% (C) field capacity (FC). The data refer to relative alterations in the following parameters: shoot (SFM), root (RFM), and total fresh mass (TFM); shoot (SDM), root (RDM), and total dry mass (TDM); relative tolerance to drought (RToler); relative water content (RWC); membrane damage in leaf (MDleaf) and roots (MDroot); contents of chlorophyll a (Chla), b (Chlb), total (Chlt), and carotenoids (Carot); contents of soluble carbohydrates (SCleaf), free amino acids (AAleaf), and proline (Proleaf) in the leaves. The radar plot was designed using the data of TVU cultivar (blue line) as a reference.
2.2. Relative Water Content and Membrane Damage
In soybean, relative water content (RWC) was significantly altered by water limitation only in the BÔNUS8579 IPRO cultivar, where plants growing under 45% FC displayed a 26.8% increase as compared to those from 75% FC treatment (Figure 1F,G and Table 1). Under drought (45% FC), the highest RWC values were registered in M8808 IPRO, NS8338 IPRO, and BÔNUS8579 IPRO in comparison to other studied cultivars (Figure 2C and Table 1).
The membrane damage was significantly increased by water deficit in the leaf and roots of soybean plants as compared to respective controls (75% FC well-irrigated plants), depending on cultivar (Figure 1 and Table 1). In the leaves, the effects were more evident in the BMX 81I81 IPRO, M8808 IPRO, and BÔNUS8579 IPRO plants, while in roots they were observed in the TMG1180 RR, M8808 IPRO, and BÔNUS8579 IPRO ones. However, the drought-induced damages were more conspicuous in the roots from 30% FC treatments, where stressed M8808 IPRO and BÔNUS8579 IPRO plants showed values 389 and 327% higher than those of 75% FC-treated plants, respectively (Figure 1F,G and Figure 2D).
Significant decreases in the RWC of cowpea were observed only in Pajeú, Tumucumaque, and TVU under 45% FC, and Xique-xique plants for both 60 and 45% FC treatments (Figure 3 and Table 2). Under drought, the highest RWC values were registered in Aracê and TVU plants grown at 60% FC, and in Novaera, Pitiúba, and Xique-xique cultivars at 45% FC (Figure 4B,C). Interestingly, the water restriction treatments did not increase the membrane damage to cowpea plants in comparison to well-irrigated plants (75% FC) (Figure 3), and significant alterations among drought-stressed cowpea cultivars were exclusively registered in leaves from plants grown under 45% FC treatments (Figure 4C and Table 2).
2.3. Accumulation of Photosynthetic Pigments
The photosynthetic pigments of soybean were found to be regulated differently in response to water deficit, depending on cultivar and stress level (Figure 1 and Table 1). Except for M8808, all soybean cultivars exhibited a significant decrease in content of chlorophyll (Chl) a under 45% and 30% drought treatments (Figure 1). Conversely, the cultivars AS3810 IPRO and M8644 IPRO under drought stress (45% and 30% FC) showed lower contents of Chl b and Chl total compared to their respective controls (75% FC) (Figure 1A,B and Table 1). Additionally, significant decreases in carotenoid levels were only observed in NS8338 IPRO, BMX 81I81 IPRO, and M8808 IPRO due to water deficit (Figure 1D–F and Table 1). Among the drought treatments, the cultivars M8644 IPRO and NS8338 IPRO exhibited the lowest contents of Chl a, Chl b, Chl total (only at 45% FC), and carotenoids (only at 45% FC) compared to other cultivars (Figure 2C,D and Table 1).
In cowpea, drought stress (60% and 45% FC treatments) promoted a significant decrease in the Chl a content of cultivars Pitiúba, TVU, and Xique-xique. A similar response was only observed at 45% FC for the Aracê, Novaera, and Tumucumaque cultivars, compared to their respective controls (Figure 3 and Table 2). TVU plants showed a strong decrease in Chl b content due to drought treatment, with the response being intensified by the level of water restriction (Figure 3F). Novaera, Pitiúba, and Tumucumaque also exhibited decreases in Chl b content but at specific levels of water restriction (Figure 3A,D,E, and Table 2). Significant decreases in Chl total content were observed in Pitiúba and TVU plants exposed to 60% and 45% FC treatments, while it occurred in Aracê, Novaera, and Tumucumaque plants exposed to 45% FC treatments, compared to the control (Figure 3 and Table 2). In addition, carotenoid contents were found to be significantly decreased by all drought treatments in Pajeú, Pitiúba, TVU, and Xique-xique cultivars, whereas the decrease in Aracê, Novaera, and Tumucumaque was observed only at 45% FC, compared to their respective controls. Under moderate drought (60% FC), the highest contents of Chl a, Chl b, and total Chl were registered in the cultivars Tumucumaque, Novaera, and Aracê, compared to other cowpea cultivars (Figure 4 and Table 2). However, the Novaera cultivar was found to display the lowest contents of photosynthetic pigments under the 45% FC drought treatment, followed by Tumucumaque and TVU plants.
2.4. Accumulation of Organic Compounds
Soluble carbohydrates (SC) in soybean plants were found to be significantly reduced by drought in the M8644 IPRO and M8808 IPRO cultivars, at both 45% and 30% FC levels, when compared to the control (Figure 1B,F and Table 1). Under drought conditions at 45% FC, the highest SC contents were registered in AS3810 IPRO, M8644 IPRO, TMG 1180 RR, and BMX81I81 IPRO (Figure 2C and Table 1). On the other hand, at 30% FC, the highest values were observed in AS3810 IPRO and Bônus8579 IPRO, followed by M8644 IPRO, TMG 1180 RR, NS8338 IPRO, and BMX81I81 IPRO; M8808 IPRO exhibited the lowest SC accumulation (Figure 2D and Table 1).
For cowpea, drought stress caused a significant decrease in leaf SC content only in the Novaera and Tumucumaque cultivars, specifically at the 45% FC level, compared to the control (75% FC) (Figure 3B,E and Table 2). Furthermore, at 45% FC, the Pajeú, Pitiúba, and Xique-xique cultivars exhibited higher SC contents than the Aracê, Novaera, Tumucumaque, and TVU plants (Figure 4C and Table 2).
The free amino acids (AA) were differentially regulated by drought treatments and cultivars in both leguminous crops. In soybean, the TMG 1180 RR cultivar exhibited the most notable alterations, showing a significant increase in leaf AA under drought stress (Figure 1C and Table 1). It also displayed the highest AA content at 45% FC compared to other cultivars studied (Figure 2C and Table 1). Conversely, the BÔNUS8579 IPRO cultivar demonstrated decreased AA content at all drought levels studied, compared to their respective controls. In cowpea, the Aracê, Novaera, and Tumucumaque cultivars showed a significant increase in leaf AA content due to drought treatments, with the most prominent response observed in Aracê plants (Figure 3A,B,E and Table S2). In contrast, Pajeú, Pitiúba, TVU, and Xique-xique plants exhibited a significant decrease in AA content under specific levels of drought stress, compared to the control (Figure 3). Additionally, at the 45% FC level, the highest AA accumulation was observed in Aracê plants, followed by Tumucumaque and Novaera, while the lowest values were found in the Pitiúba cultivar (Figure 4C and Table 2).
The leaf proline content of drought-stressed M8808 soybean plants was higher than that of well-irrigated plants, at both 45% and 30% FC levels (Figure 1F and Table 1). For other soybean cultivars, proline levels remained unchanged or decreased due to water restriction treatments. Under the 45% FC drought treatment, the highest proline levels were observed in the AS3810 IPRO, NS8338 IPRO, and M8808 plants (Figure 2C and Table 1); whereas the biggest proline accumulation at 30% FC level was registered in the NS8338 IPRO and M8808 cultivars (Figure 2D and Table 1).
The cowpea cultivars exhibited a significant increase in leaf proline content when subjected to 45% FC drought treatment, compared to well-irrigated plants at the 75% FC level, except Novaera and Pajeú plants (Figure 3 and Table 2). The accumulation of proline was more particularly evident in drought-stressed TVU plants, which displayed intensification of proline content by increasing water restriction (from 60 to 45% FC) (Figure 3F), resulting in the highest proline levels among the cowpea cultivars at 45% FC (Figure 4C and Table 2).
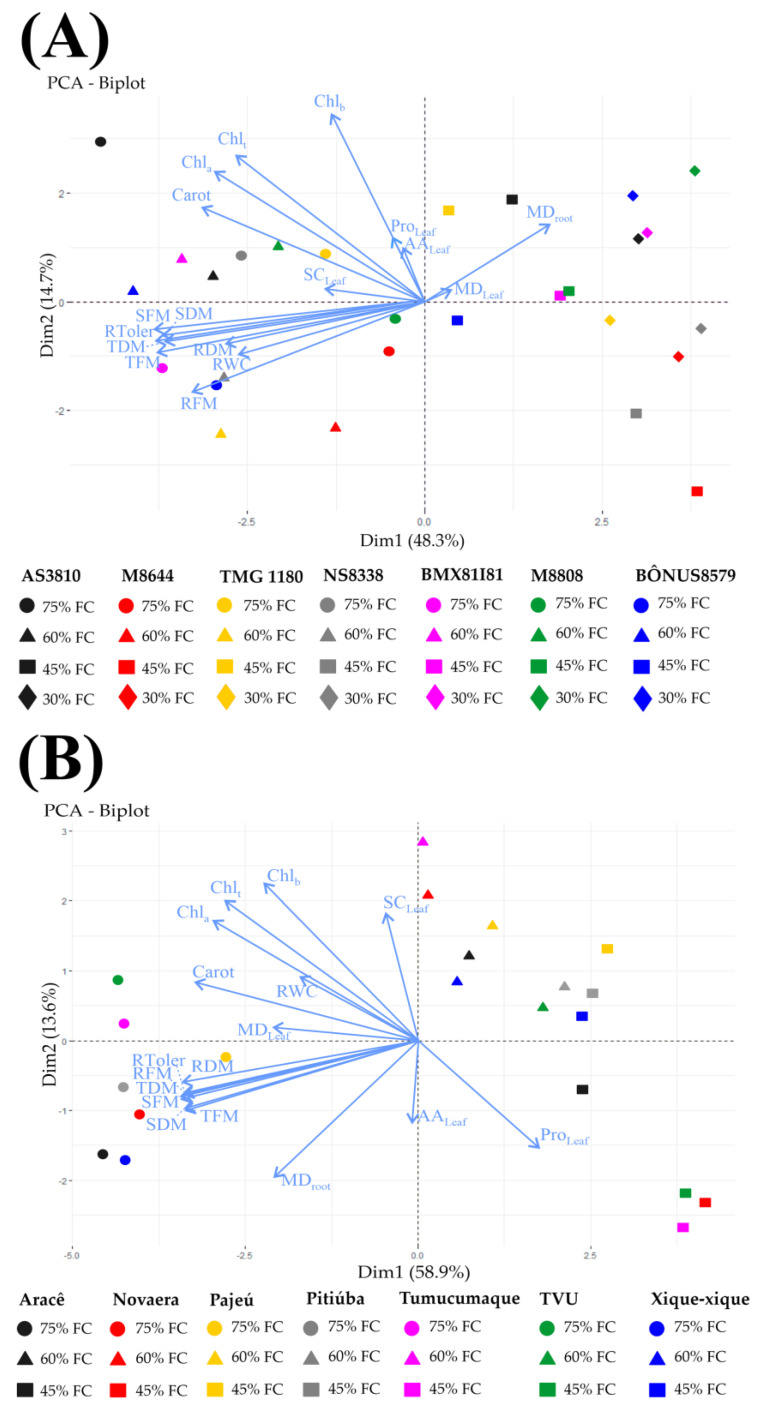
2.5. Principal Component Analysis (PCA)
Principal Component Analysis (PCA) was designed to investigate the correlation within soybean/cowpea crops and the parameters that best separated the cultivars for tolerance to drought (Figure 5). In soybean, the data explained 63.0% of total variation, with 48.3 and 14.7% explaining the first and second components, respectively (Figure 5A). The biplot analysis reveals an overlap between the 75 and 60% FC treatments, indicating similar performance, which distinguished them from the 45 and 30% FC treatments. At 45% FC, the cultivars BÔNUS8579 IPRO and TMG1180 RR exhibited the most remarkable responses, closer to well-irrigated plants. The growth parameters showed significant correlations with RWC, drought tolerance, soluble carbohydrates, and photosynthetic pigments, indicating a positive relationship with well-irrigated plants at 75% and 60% FC. Proline and amino acids were significantly correlated with some 45% FC-stressed plants, while root membrane damage showed a strong correlation with severe water restriction at 30% FC (Figure 5A).
Figure 5.
Principal Component Analysis (PCA). Scatter plots of parameters investigated in soybean (A) and cowpea (B) cultivars under different water availability levels (75, 60, 45, and 30% of field capacity—FC). The X and Y axes indicate the percentage of variance explained by each Principal Component (PC). The loading plot displays the contribution of the following parameters: shoot fresh mass (SFM), root fresh mass (RFM), and total fresh mass (TFM); shoot dry mass (SDM), root dry mass (RDM), and total dry mass (TDM); relative tolerance to drought (RToler); relative water content (RWC); membrane damage in leaves (MDleaf) and roots (MDroot); contents of chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (Chlt), and carotenoids (Carot); contents of soluble carbohydrates (SCleaf), free amino acids (AAleaf), and proline (Proleaf) in the leaves.
For cowpea, the data explained 72.5% of total variation, it being 58.9 and 13.6% for the first and second components, respectively (Figure 5B). The cowpea biplot reveals a good separation between treatments based on water levels, suggesting a performance similar for cowpea plants under drought. Nevertheless, under water restriction, the most expressive responses were observed in drought-stressed Xique-xique plants under 60 and 45% FC which exhibited a closer performance to that of the control plants (Figure 5B). By investigating the Pearson correlation coefficients, growth parameters correlated significantly with photosynthetic pigments, RWC, and drought tolerance, demonstrating a positive correlation with 75% well-irrigated plants (Figure 5B). Soluble carbohydrate was closely related with 60% FC-stressed plants, whereas free amino acids correlated significantly with proline, displaying a high positive correlation with severely stressed plants (45% FC) (Figure 5B).
3. Discussion
3.1. Soybean Crop Has Low Water Requirements for Elevated Growth during the Vegetative Stage and Displays Drought Tolerance Higher Than Cowpea Crop
The data obtained from soybean crop revealed a similar performance between plants grown at 75% and 60% FC treatments. In total, 60% FC-grown plants showed values of fresh and dry biomass comparable (M8808 IPRO, AS3810 IPRO, BMX81I81 IPRO, and BÔNUS8579 IPRO) or higher (M8644 IPRO, TMG1180 RR, and NS8338 IPRO) than those of 75% FC-grown plants (Figure 1, Figure 2 and Figure 5). These results indicate that soybean plants did not experience water stress at 60% FC, and, in fact, this condition constitutes an ideal water availability for cultivation. Clearly, the studied soybean cultivars, cultivated in the semi-arid region, demonstrate a remarkable ability to withstand a significant level of water restriction. Furthermore, our findings suggest innovations for water-saving during the vegetative phase of the crop, a key claim for agricultural production considering future projections of water scarcity [24,25].
Both leguminous crops exhibited distinct patterns of response to water deficit, highlighting that soybean plants tolerate more severe levels of water restriction than cowpea plants. The evidence was that all cowpea cultivars displayed high sensitivity at 30% FC, exhibiting severe stress symptoms, and died between the tenth and fourteenth day of treatment. Therefore, analyzing the data, we defined that 60% FC represents well-irrigated conditions for soybean, 45% FC corresponds to a moderate drought, and 30% FC indicates a severe drought (Figure 1, Figure 2 and Figure 5A). In contrast, the data indicate that 75% FC is an ideal condition for cowpea cultivation, while the 60% and 45% FC treatments represent moderate and severe drought, respectively (Figure 3, Figure 4 and Figure 5B). These findings underscore that the ideal level of water availability depends on the crop and cultivar, and limited soil moisture can significantly impact plant growth and development.
3.2. Cowpea and Soybean Cultivars Display Contrasting Responses to Water Deficit
In the current study, under moderate (45%) and severe (30%) drought for soybean, the highest values of fresh and dry biomass were recorded in the BÔNUS8579 IPRO and TMG1180 RR plants, demonstrating the greatest relative tolerance to water deficit (Figure 2 and Figure 5A). On the contrary, under the same conditions, the M8808 IPRO, BMX81I81 IPRO, M8644 IPRO, and AS3810 IPRO cultivars exhibited the lowest biomass accumulation and were highly sensitive to water deficit (Figure 2 and Figure 5A).
Our data are consistent with previous studies in soybean cultivars, which report that water stress affects growth and carbon partition differently, since some cultivars are less tolerant compared to others [26,27]. Similarly, the highest drought tolerance was found in plants capable of maintaining phenotypic traits in a study investigating 20 soybean cultivars exposed to drought [28]. The authors emphasized the significant correlation between root characteristics and water stress tolerance, underscoring their crucial role in determining agronomic traits during vegetative growth.
The cowpea cultivars also displayed differential responses to the studied treatments, especially under moderate (60% FC) and severe drought (45% FC) (Figure 4 and Figure 5B). In both stress levels, the highest biomass values were recorded in the stressed Xique-xique and Novaera cultivars that demonstrated the highest levels of relative tolerance to drought (Figure 4B,C and Figure 5B). Conversely, the TVU plants showed the lowest biomass accumulation and stood out as the most drought-sensitive cultivar. These data are in concordance with previous studies which demonstrated that water deficit negatively impacts biomass accumulation, revealing a response that varies depending on the cultivar [29,30].
3.3. Leguminous Crops Activate Specific Biochemical Mechanisms for Drought Tolerance
Numerous reports have cited the loss of photosynthetic pigments as a primary signal of responses to water stress [14,24,31]. In agreement, our results revealed a significant decrease in Chl a, Chl b, Chl total, and carotenoids in almost all soybean and cowpea studied cultivars (Figure 1 and Figure 3 and Table 1 and Table 2). The decrease in chlorophyll levels may be a result of both the down regulation of biosynthetic pathways and increased hydrolysis by hydrolytic enzymes, as well as oxidative damage to the chloroplast membrane and chlorophyll degradation [31,32,33]. Herein, the accumulation of photosynthetic pigments in plants exposed to drought suggests the participation of differential mechanisms between leguminous crops. For soybean, a differential response among drought-contrasting stressed cultivars was practically absent, probably due to genetic potential to withstand water deficit impacts (Figure 2C,D and Table 1). On the other hand, for cowpea, a high content of photosynthetic pigments was recorded in the drought-tolerant Xique-xique cultivar and a low content was exhibited in the drought-sensitive TVU cultivar (Figure 4B,C and Figure 5B and Table 2). These data suggest a close relationship between chlorophyll accumulation and growth performance for cowpea plants.
Our findings reinforce the crucial role of biochemical adjustments for soybean performance under drought treatments. The elevated sensitivity to drought observed in the M8808 soybean cultivar was associated with reduced accumulation of soluble carbohydrate, likely due to impaired CO2 assimilation under water limitation (Figure 1F) [33,34]. These implications suggest an energy excess at the PSII level and increased oxidative damage, as supported by the recorded membrane damage in leaves and roots (Figure 1F) [35,36,37]. In contrast, the TMG1180 RR cultivar, which exhibited greater tolerance to drought, showed a smaller decrease in biomass accumulation under drought stress, a response associated with the accumulation of free amino acids. These amino acids likely played a role in plant defense pathways such as osmotic adjustment (Figure 3 and Figure 5A) [38,39,40].
In cowpea, the good performance of the drought-tolerant Xique-xique cultivar was attributed to the maintenance of RWC and photosynthetic pigments, which acted to maintain the photosynthetic efficiency even at low water availability. This argument is corroborated by the unaltered level of soluble carbohydrates in the leaves of stressed plants (Figure 3G). Also, the proline accumulation in severely water-stressed Xique-xique plants might play an active role in the regulation of RWC and membrane damage for growth recovery, as previously reported for maize plants under salt stress (Figure 3G and Figure 5B) [41]. Yet, the Novaera cultivar activated a greater accumulation of free amino acids, probably to act in osmotic adjustment under water limitation (Figure 3B and Figure 5B). These results indicate a likely activation of control mechanisms to maintain water absorption and tissue water content, as well as to prevent harmful damage to cellular components.
Similarly, all stressed cowpea cultivars displayed an excessive accumulation of proline in the leaves, especially under severe drought, with a more pronounced response in drought-sensitive TVU plants (Figure 3 and Figure 5B). Proline is a versatile molecule that can elicit numerous defense responses, like osmotic adjustment, protein and membrane stabilization, scavenging of free radicals, signaling in cellular events, and gene expression [42]. Our results seem to indicate that proline serves as a molecular marker of drought stress, establishing a constitutive defense mechanism in cowpea plants against water deficit.
In general, our findings distinctly highlight pathways to counteract drought-related damages by identifying resilient cultivars and guiding targeted studies for developing effective agricultural advancements. These encompass the exploration of tolerance markers, crossbreeding methods, crosstalk tolerance inducers, and enhanced fertilizer management practices. Taken together, the progress can ensure both food security and environmental sustainability, particularly in regions prone to water scarcity.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
The experiments were carried out in a greenhouse at the Federal University of Piauí with geographic coordinates of 9°05′02.5″ S and 44°19′32.7″ W at approximately 650 m altitude. The analyses were performed at the Laboratories of the Campus Professora Cinobelina Elvas. The plants were grown in 11 dm³ plastic pots filled with soil from experimental site at UFPI (geographic coordinates of 9°04′45.6″ S and 44°19′37.9″ W), which was analyzed chemically and corrected [43]. During the trials, the environmental conditions inside the greenhouse were as follows: maximum and minimum temperatures of 27.4 ± 2.0 °C and 25.9 ± 2.2 °C, respectively; relative air humidity of approximately 68.5 ± 4.0%; and a photoperiod of approximately 12 h.
Two independent experiments were conducted in a randomized complete design, in 7 × 4 factorial schemes composed of seven soybean (Glycine max L.) or cowpea (Vigna unguiculata L. Walp) cultivars and four water level treatments [field capacity (FC) at 75, 60, 45, and 30%]. For soybean trials, the cultivars AS3810 IPRO, M8644 IPRO, TMG1180 RR, NS8338 IPRO, BMX81I81 IPRO, M8808 IPRO, and BÔNUS8579 IPRO were investigated; while the cultivars Aracê, Novaera, Pajeú, Pitiúba, Tumucumaque, TVU, and Xique-xique were studied in the cowpea trials. In all cases, four replications per treatment (one plant per pot) were employed, totaling 112 experimental units per experiment.
The soybean and cowpea cultivars were chosen based on their performance, production stability, and wide adaptability across the agricultural regions of the Brazilian Cerrado and semiarid areas. These regions are characterized by rainfed cultivation and subjected to periods of extended dry spells. The soybean seeds were acquired through Celeiro Sementes farm, a specialized company in soybean seed propagation. Cowpea seeds were obtained from the Active Germplasm Bank (https://av.cenargen.embrapa.br/avconsulta/Passaporte/detalhesBanco.do?idb=772, accessed on 8 March 2023) at the Federal University of Ceará (UFC), in Fortaleza, Ceará, Brazil. Detailed information about the soybean and cowpea cultivars are outlined in Tables S1 and S2, respectively. Before the sowing, the seeds were sterilized with 2% sodium hypochlorite and then sown in soils previously irrigated to 75% FC. At 14 days after sowing, uniform seedlings were selected and the drought treatments were imposed by reducing the water in irrigation to 60, 45, and 30% FC. A group of plants remained under irrigation of 75% FC, constituting the well-irrigated plants. The plants were watered daily to defined water levels using the weighing principle. The harvests were conducted 21 days after beginning the drought treatments, at the end of the vegetative stage.
4.2. Plant Growth
At harvest time, the plants were initially separated into leaves, stem, and roots to measure their fresh mass (FM). Subsequently, the plant material was immediately frozen and lyophilized to measure the dry mass. The index of relative tolerance to drought (RToler) was calculated by comparing the total dry mass of drought-stressed plants to the total dry mass of control plants [44]. Specifically, the total dry mass of plants grown under 60% FC for soybean and 75% FC for cowpea were used as controls for calculating the Rtoler index.
4.3. Relative Water Content and Membrane Damage
To estimate the relative water content (RWC), leaf discs were initially weighed to obtain the fresh weight (FW). Subsequently, the samples were immersed in deionized water for a period of 6 h at room temperature to achieve full turgidity, and the weight was recorded as the turgid weight (TW). Afterward, the samples were subjected to oven drying at 65 °C for 24 h and weighed to determine the dry weight (DW). The RWC was estimated by using the formula RWC (%) = [(FW – DW)/(TW – DW)] × 100.
Electrolyte leakage was employed as a measure of cell membrane damage, utilizing a conductivity meter. Leaf discs and root samples weighing 0.1 g were placed in sealed vials filled with deionized water, and then incubated at room temperature on a rotary shaker for 12 h. Thereafter, the electrical conductivity of the solution (EC1) was measured. Later, the homogenate was subjected to incubation at 100 °C for 15 min, and the conductivity was measured once more (EC2). The percentage of membrane damage (%) was calculated as EC1/C2 × 100.
4.4. Photosynthetic Pigments
The photosynthetic pigments were extracted by incubating leaf discs in dimethyl sulfoxide (DMSO) saturated with CaCO3 at room temperature in the dark for 72 h. The contents of chlorophyll a (Chl a), chlorophyll a (Chl b), chlorophyll total (Chl total), and carotenoids were estimated through spectrophotometry readings at wavelengths of 480, 649, and 665 nm, following the equations defined by Wellburn method [45].
4.5. Soluble Carbohydrates, Free Amino Acids and Proline
Soluble carbohydrates and free amino acids were extracted after homogenizing 10 mg of leaf lyophilized samples in 5 mL of 80% aqueous ethanol at 75 °C for 1.0 h. Then, the homogenate was centrifuged at 3000× g for 10 min at 4 °C, and the resulting supernatant was collected. This extraction process was repeated twice on the remaining precipitate. Subsequently, all the collected supernatants were combined and adjusted to a final volume of 25 mL with 80% ethanol. The content of soluble carbohydrates was estimated by readings at 490 nm, using anhydrous D-glucose as a standard [46]. Yet, the content of free amino acids was measured by spectrophotometric readings at 570 nm, using L-glycine as a standard [47].
The proline content was assessed using the ninhydrin method as outlined by the Bates method [48]. Aqueous extracts were prepared by homogenizing 20 mg of lyophilized leaves in 2.0 mL of deionized water at 75 °C for 1.0 h. Thereafter, the homogenate was centrifuged at 3000× g for 10 min at room temperature. The supernatant was collected and used to measure the proline content by absorbance at 520 nm, with L-proline serving as the standard.
4.6. Statistical Analysis
For each experiment, the data underwent analysis of variance (ANOVA) using the F test at a significance level of 5%. Post hoc comparisons of means were conducted using Scott–Knott’s test (p ≤ 0.05) with the Sisvar software [49]. The clustering analyzes were designed using Excel software, and the radar plot graphs were plotted through Sigma Plot 11.0 software (SPSS Inc., San Jose, California, USA). Additionally, principal component analysis (PCA) was performed on the datasets by using the R software [50].
5. Conclusions
Our findings reveal insights into innovative agricultural practices, underscoring the remarkable efficiency of soybean in managing water requirements, not only in terms of water-saving potential in agricultural production but also in terms of enhanced production strategies. TMG1180 RR and BÔNUS8579 IPRO are the most drought-resistant soybean cultivars, highlighting defense pathways to avoid tissue desiccation. Xique-xique emerged as the most drought-resistant cowpea cultivar, characterized by a high ability to maintain water content, proline, and photosynthetic pigments, which contributed to the preservation of soluble carbohydrates under water restriction. The data might help plant breeders and farmers in mitigating drought-related damages in general, providing suitable information regarding tolerant soybean/cowpea cultivars for advancing plant breeding efforts, and exploring viable alternatives for cultivating leguminous crops in arid and semiarid regions toward sustainable and resilient agriculture.
Acknowledgments
The authors are grateful to Celeiro Sementes Farm for kindly providing the soybean seeds, and to the Active Germplasm Bank (BAG) at the Federal University of Ceará (UFC, Fortaleza, Ceará, Brazil) for kindly provided the cowpea seeds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12173134/s1, Table S1: Soybeans cultivars grown in agricultural areas of the Brazilian Cerrado; Table S2: Cowpea cultivars grown in agricultural areas of the Brazilian semiarid region.
Author Contributions
Conceptualization, R.d.S.M. and E.M.d.S.; methodology, R.R.d.B., A.S.S. and R.H.B.d.S.; software, R.d.S.M., A.S.S. and S.d.O.P.-M.; validation, J.J.P.L., F.d.A.N. and R.d.S.M.; formal analysis, R.H.B.d.S., B.S.F.d.F., D.S.P., J.Y.N.B., W.S.F., M.d.S.L., L.F.N., É.S.d.A. and J.A.F.P.; investigation, R.H.B.d.S., R.R.d.B., J.Y.N.B., D.S.P. and L.F.N.; resources, R.d.S.M. and E.G.-F.; data curation, R.d.S.M., J.Y.N.B. and B.S.F.d.F.; writing—original draft preparation, R.d.S.M.; writing—review and editing, J.H.C., J.J.P.L., C.P.d.S., F.d.A.N. and E.G.-F.; visualization, S.d.O.P.-M. and R.d.S.M.; supervision, R.d.S.M. and E.M.d.S.; project administration, R.d.S.M.; funding acquisition, R.d.S.M. and E.G.-F. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Foundation for Research Support of the State of Piauí (FAPEPI), grant number PPP-Edital FAPEPI/MCT/CNPq/CT-INFRA 007/2018, and the National Council for Scientific and Technological Development (CNPq), grant numbers 427219/2018-3 and 316056/2021-9.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.FAO (Food and Agriculture Organization of the United Nations) The State of the World’s Land and Water Resources for Food and Agriculture: Systems at Breaking Point. FAO; Rome, Italy: 2022. [DOI] [Google Scholar]
- 2.UN (United Nations) The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water. UNESCO; Paris, France: 2023. [(accessed on 21 July 2023)]. Available online: https://www.unesco.org/reports/wwdr/2023/en/download. [Google Scholar]
- 3.Omomowo O.I., Babalola O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Front. Plant Sci. 2021;12:751731. doi: 10.3389/fpls.2021.751731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CONAB (Brazilian Supply Company) Monitoring of Brazilian Grain Harvest 2022/2023. 10th ed. Volume 1. CONAB; Brasília, Brazil: 2023. [(accessed on 15 July 2023)]. Tenth Survey. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos. [Google Scholar]
- 5.USDA (United States Department of Agriculture) World Agricultural Production. Circular Series. [(accessed on 21 July 2023)];2023 Available online: http://www.usda.gov.
- 6.Wang C., Linderholm H.W., Song Y., Wang F., Liu Y., Tian J., Xu J., Song Y., Ren G. Impacts of drought on maize and soybean production in Northeast China during the past five decades. Int. J. Environ. Res. Public Health. 2020;17:2459. doi: 10.3390/ijerph17072459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva E.H.F.M., Antolin L.A.S., Zanon A.L., Andrade-Junior A.S., Souza H.A., Carvalho K.S., Vieira-Junior N.A., Marin F.R. Impact assessment of soybean yield and water productivity in Brazil due to climate change. Eur. J. Agron. 2021;129:126329. doi: 10.1016/j.eja.2021.126329. [DOI] [Google Scholar]
- 8.Nguyen H., Thompson A., Costello C. Impacts of historical droughts on maize and soybean production in the southeastern United States. Agric. Water Manag. 2023;281:108237. doi: 10.1016/j.agwat.2023.108237. [DOI] [Google Scholar]
- 9.FAOSTAT (Food and Agriculture Organization Statistics) Food and Agriculture Data. 2021. [(accessed on 20 July 2023)]. Available online: http://www.fao.org/faostat/en/#data/QC.
- 10.CONAB (Brazilian Supply Company) Monitoring of Brazilian Grain Harvest 2020/2021. 8th ed. Volume 12. CONAB; Brasília, Brazil: 2021. [(accessed on 15 July 2023)]. Twelfth Survey. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos. [Google Scholar]
- 11.Souza P.J.O.P., Farias V.D.S., Pinto J.V.N., Nunes H.G.G.C., Souza E.B., Fraisse C.W. Yield gap in cowpea plants as function of water deficits during reproductive stage. Rev. Bras. Eng. Agr. Amb. 2020;24:372–378. doi: 10.1590/1807-1929/agriambi.v24n6p372-378. [DOI] [Google Scholar]
- 12.Praxedes S.C., Gomes-Filho E., Damatta F.M., Lacerda C.F., Prisco J.T. Salt stress tolerance in cowpea is poorly related to the ability to cope with oxidative stress. Acta Bot Croat. 2014;73:51–62. doi: 10.2478/botcro-2013-0010. [DOI] [Google Scholar]
- 13.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvalho M., Castro I., Moutinho-Pereira J., Correia C., Egea-Cortines M., Matos M., Rosa E., Carnide V., Lino-Neto T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019;241:153001. doi: 10.1016/j.jplph.2019.153001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang A., Liu M., Gu W., Chen Z., Gu Y., Pei L., Tian R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021;21:293. doi: 10.1186/s12870-021-03048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahra N., Hafeez M.B., Kausar A., Zeidi M.A., Asekova S., Siddique K.H.M., Farooq M. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop Sci. 2023;1:1–22. doi: 10.1111/jac.12652. [DOI] [Google Scholar]
- 17.Talbi S., Rojas J.A., Sahrawy M., Rodríguez-Serrano M., Cárdenas K.E., Debouba M., Sandalio L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020;176:104099. doi: 10.1016/j.envexpbot.2020.104099. [DOI] [Google Scholar]
- 18.Mukarram M., Choudhary S., Kurjak D., Petek A., Khan M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021;172:1291–1300. doi: 10.1111/ppl.13423. [DOI] [PubMed] [Google Scholar]
- 19.Fita A., Rodríguez-Burruezo A., Boscaiu M., Prohens J., Vicente O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015;6:1–14. doi: 10.3389/fpls.2015.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazaridi E., Bebeli P.J. Cowpea Constraints and Breeding in Europe. Plants. 2023;12:1339. doi: 10.3390/plants12061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdelaal K.A.A. Effect of salicylic acid and abscisic acid on morpho–physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 2015;6:1771–1788. doi: 10.21608/jpp.2015.52096. [DOI] [Google Scholar]
- 22.Khan M.N., Zhang J., Luo T., Liu J., Rizwan M., Fahad S., Xu Z., Hu L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crop Prod. 2019;140:111597. doi: 10.1016/j.indcrop.2019.111597. [DOI] [Google Scholar]
- 23.Katam R., Shokri S., Murthy N., Singh S.K., Suravajhala P., Khan M.N., Bahmani M., Sakata K., Reddy K.R. Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS ONE. 2022;15:e0233905. doi: 10.1371/journal.pone.0233905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeem M., Li J., Yahya M., Sher A., Ma C., Wang X., Qiu L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019;20:2541. doi: 10.3390/ijms20102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry S., Sidhu G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022;41:1–31. doi: 10.1007/s00299-021-02759-5. [DOI] [PubMed] [Google Scholar]
- 26.Jumrani K., Bhatia V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plant. 2018;24:37–50. doi: 10.1007/s12298-017-0480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesquita R.O., Coutinho F.S., Vital C.E., Nepomuceno A.L., Williams T.C.R., Ramos H.J.O., Loureiro M.E. Physiological approach to decipher the drought tolerance of a soybean genotype from Brazilian savana. Plant Physiol. Bioch. 2020;151:132–143. doi: 10.1016/j.plaphy.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Yan C., Song S., Wang W., Wang C., Li H., Wang F., Li S., Sun X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant. Biol. 2020;20:321. doi: 10.1186/s12870-020-02519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutra W.F., Melo A.S., Suassuna J.F., Dutra A.F., Silva D.C., Maia J.M. Antioxidative responses of cowpea cultivars to water deficit and salicylic acid treatment. Agron. J. 2017;109:895–905. doi: 10.2134/agronj2015.0519. [DOI] [Google Scholar]
- 30.Cui Q., Xiong H., Yufeng Y., Eaton S., Imamura S., Santamaria J., Ravelombola W., Mason R.E., Wood L., Mozzoni L.A., et al. Evaluation of drought tolerance in Arkansas cowpea lines at seedling stage. HortScience. 2020;55:1132–1143. doi: 10.21273/HORTSCI15036-20. [DOI] [Google Scholar]
- 31.Singh J., Thakur J.K. Photosynthesis and abiotic stress in plants. In: Vats S., editor. Biotic and Abiotic Stress Tolerance in Plants. Springer; Singapore: 2018. [DOI] [Google Scholar]
- 32.Ansari W.A., Atri N., Ahmad J., Qureshi M.I., Singh B., Kumar R., Rai V., Pandey S. Drought mediated physiological and molecular changes in muskmelon (Cucumis melo L.) PLoS ONE. 2019;14:e0222647. doi: 10.1371/journal.pone.0222647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goufo P., Moutinho-Pereira J.M., Jorge T.F., Correia C.M., Oliveira M.R., Rosa E.A.S., António C., Trindade H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017;8:586. doi: 10.3389/fpls.2017.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandey K., Kumar R.S., Prasad P., Pande V., Trivedi P.K., Shirke P.A. Coordinated regulation of photosynthesis and sugar metabolism in guar increases tolerance to drought. Environ. Exp Bot. 2022;194:104701. doi: 10.1016/j.envexpbot.2021.104701. [DOI] [Google Scholar]
- 35.Miranda R.S., Mesquita R.O., Freitas N.S., Prisco J.T., Gomes-Filho E. Nitrate: Ammonium nutrition alleviates detrimental effects of salinity by enhancing photosystem II efficiency in sorghum plants. Rev. Bras. Eng. Agr. Amb. 2014;18:8–12. doi: 10.1590/1807-1929/agriambi.v18nsupps8-s12. [DOI] [Google Scholar]
- 36.Araújo G.S., Miranda R.S., Mesquita R.O., Paula S.O., Prisco J.T., Gomes-Filho E. Nitrogen assimilation pathways and ionic homeostasis are crucial for photosynthetic apparatus efficiency in salt-tolerant sunflower genotypes. Plant Growth Regul. 2018;86:375–388. doi: 10.1007/s10725-018-0436-y. [DOI] [Google Scholar]
- 37.Anda A., Simon B., Soós G., Silva J.A.T., Menyhárt L. Water stress modifies canopy light environment and qualitative and quantitative yield components in two soybean varieties. Irrig. Sci. 2021;39:549–566. doi: 10.1007/s00271-021-00728-0. [DOI] [Google Scholar]
- 38.Ebeed H.T., Hassan N.M., Aljarani A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Bioch. 2017;118:438–448. doi: 10.1016/j.plaphy.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Ozturk M., Unal B.T., García-Caparrós P., Khursheed A., Gul A., Hasanuzzaman M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plantarum. 2020;172:1321–1335. doi: 10.1111/ppl.13297. [DOI] [PubMed] [Google Scholar]
- 40.Živanović B., Milić Komić S., Tosti T., Vidović M., Prokić L., Veljović Jovanović S. Leaf soluble sugars and free amino acids as important components of abscisic acid-mediated drought response in tomato. Plants. 2020;9:1147. doi: 10.3390/plants9091147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freitas P.A.F., Carvalho H.H., Costa J.H., Miranda R.S., Saraiva K.D.C., Oliveira F.D.B., Coelho D.G., Prisco J.T., Gomes-Filho E. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019;38:403–416. doi: 10.1007/s00299-019-02382-5. [DOI] [PubMed] [Google Scholar]
- 42.Benitez L.C., Vighi I.L., Auler P.A., Amaral M.N., Moraes G.P., Rodrigues G.S., Maia L.C., Magalhães-Júnior A.M., Braga E.J.B. Correlation of proline content and gene expression involved in the metabolism of this amino acid under abiotic stress. Acta Physiol. Plant. 2016;38:267. doi: 10.1007/s11738-016-2291-7. [DOI] [Google Scholar]
- 43.Souza D.M.G., Lobato E. Cerrado: Correção do Solo e Adubação. 2nd ed. Embrapa Informação Tecnológica; Brasília, Brazil: 2014. 416p [Google Scholar]
- 44.Miranda R.S., Souza F.I.L., Alves A.F., Souza R.R., Mesquita R.O., Ribeiro M.I.D., Santana-Filho J.A., Gomes-Filho E. Salt-acclimation physiological mechanisms at the vegetative stage of cowpea cultivars in soils from a semiarid region. J. Soil. Sci. Plant Nutr. 2021;21:3530–3543. doi: 10.1007/s42729-021-00625-7. [DOI] [Google Scholar]
- 45.Wellburn A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994;144:307–314. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 46.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 47.Yemm E.W., Cocking E.C., Ricketts R.E. The determination of amino acids with ninhydrin. Analyst. 1955;80:209–214. doi: 10.1039/an9558000209. [DOI] [Google Scholar]
- 48.Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 49.Ferreira D.F. Sisvar: A computer statistical analysis system. Cienc. Agrotec. 2011;35:1039–1042. doi: 10.1590/S1413-70542011000600001. [DOI] [Google Scholar]
- 50.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [(accessed on 21 February 2022)]. Available online: http://www.R-project.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.