Abstract
Salmonella typhi was isolated from 369 and Salmonella paratyphi A was isolated from 6 of 515 Vietnamese patients with suspected enteric fever. Compared with conventional broth culture of blood, direct plating of the buffy coat had a diagnostic sensitivity of 99.5% (95% confidence interval [CI], 97.1 to 100%). Blood bacterial counts were estimated by the pour plate method. The median S. typhi count in blood was 1 CFU/ml (range, <0.3 to 387 CFU/ml), of which a mean of 63% (95% CI, 58 to 67%) were intracellular. The mean number of bacteria per infected leukocyte was 1.3 (interquartile range [IQR], 0.7 to 2.4) CFU/cell (n = 81). Children (<15 years old; n = 115) had higher median blood bacterial counts than adults (n = 262): 1.5 (range, <0.3 to 387) versus 0.6 (range, <0.3 to 17.7) CFU/ml (P = 0.008), and patients who excreted S. typhi in feces had higher bacteremias than those who did not: a median of 3 (range, <0.3 to 32) versus 1 (range, <0.3 to 68) CFU/ml (P = 0.02). Blood bacterial counts declined with increasing duration of illness (P = 0.002) and were higher in infections caused by multidrug-resistant S. typhi (1.3 [range, <0.3 to 387] CFU/ml; n = 313) than in infections caused by antibiotic-sensitive S. typhi (0.5 [range, <0.3 to 32] CFU/ml; n = 62) (P = 0.006). In a multivariate analysis this proved to be an independent association, suggesting a relationship between antibiotic resistance and virulence in S. typhi.
Typhoid fever is a prolonged illness characterized by bacteremia with Salmonella typhi, a highly evolved gram-negative bacterial parasite that infects only humans. Despite the bacteriological similarities between S. typhi and other enterobacteriaceae, the clinical picture of typhoid is usually distinctive and differs in many respects from that of other septicemias caused by gram-negative organisms. In general, typhoid patients are less severely ill, and severity in typhoid usually reflects localization of the infection to the Peyer’s patches, and consequent intestinal ulceration, rather than fulminant septicemia. There is little information on the numbers of bacteria in the blood and their relationship with disease state (17). With standard broth culture, salmonellae have been found in the blood of 30 to 90% of patients with clinically suspected cases of enteric fever, and the proportion of typhoid fever patients with positive blood cultures decreases with increasing duration of illness (3, 5, 20, 21). The volume of blood taken and the laboratory methods used for isolation are also important factors determining the yields from blood culture (3–6, 10, 13, 17, 18, 20, 24). S. typhi is able to survive and reproduce inside monocytic phagocytes, and in typhoid fever S. typhi is reported to be confined to the monocyte-platelet fraction of the blood (5, 17, 18, 24). In order to investigate the relationship between bacterial counts in blood and clinical and laboratory features of typhoid, we have performed quantitative bacteriological cultures for a large series of patients with uncomplicated enteric fever.
MATERIALS AND METHODS
Patients.
Adults and children admitted with suspected uncomplicated typhoid fever were studied over a 3-year period at two separate sites in Vietnam: the Centre for Tropical Diseases, Ho Chi Minh City, an infectious disease referral hospital, and the Friendship Hospital, Cao Lanh City, a large provincial hospital in Dong Thap Province in the Mekong Delta. These patients were recruited for prospective treatment studies of short-course fluoroquinolone therapy on the basis of either clinical suspicion or a positive blood culture. The criteria for the clinical diagnosis of typhoid fever were usually fever for ≥6 days with no obvious focus of infection, a negative malaria blood smear, and abdominal discomfort with change of bowel habits. In some cases the patients’ withdrawn or apathetic behavior in the presence of fever was also a diagnostic pointer. Patients with severe or complicated typhoid (except those with a history of melena only) and those who had already received effective antibiotic treatment (i.e., antibiotics active against S. typhi) were not included in these studies. Before treatment was started, blood was taken for routine laboratory tests and cultures as described below, and when it was possible without delaying the start of treatment, a stool sample was cultured for Salmonella. Fully informed consent to blood sampling was obtained from all patients. This study was approved by the Scientific and Ethical Committees of the participating institutions.
Laboratory methods.
The same laboratory methods were used at both centers. Five to ten milliliters of venous blood was drawn aseptically from each patient and inoculated into 50 ml of brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) containing 0.05% sodium polyanetholesulfonate (Sigma, Poole, United Kingdom). A minimum blood-to-broth ratio of 1 to 10 was maintained. Blood culture broths were incubated for 7 days, and subcultures were performed at 24 h and after 7 days. All bottles were also examined daily, and if a bottle showed visible signs of growth, subculture onto sheep blood agar was performed. Stool cultures were performed after enrichment of a 1-g sample in 10 ml of selenite broth for 24 h. Five microliters from the top of the broth was then plated onto XLD agar (Oxoid). Bacterial isolates were identified by standard biochemical tests (glucose, lactose fermentation and production of gas, indole and H2S production, citrate utilization, motility, and ability to split urea) and agglutination with Salmonella 09- and Vi-specific antisera (Wellcome Diagnostics, Dartford, United Kingdom). Antibiotic disc sensitivities were performed by using a modification of the method of Bauer et al. (1). Organisms resistant to chloramphenicol, ampicillin, trimethoprim, and sulfamethoxazole but sensitive to ofloxacin and ceftriaxone were described as multidrug resistant.
Quantitative cultures.
Venous blood for quantitative culture and for broth culture was taken before administration of antimicrobials for typhoid fever. The blood (3 to 9 ml from adults and 1.5 to 6 ml from children) was collected in a sterile heparinized tube and transported immediately to the laboratory. Quantitative whole-blood cultures (QBC) were carried out by a pour plate method. In brief, three measured aliquots of blood (usually 1 ml; 0.5 ml for small children) were mixed with 19 ml of molten (50°C) Columbia agar (Unipath, Basingstoke, United Kingdom) in a sterile petri dish, allowed to set, and then incubated at 37°C. After 2 to 4 days colonies were counted and recorded as CFU per milliliter. Up to five colonies were picked from the surface of the agar for identification. After reincubation for 24 h standard biochemical tests and agglutination with the specific antisera were performed. Plates were discarded as negative if no colonies were visible after 4 days of incubation.
To determine the number of intraleukocytic CFU in blood samples, quantitative cultures were performed on peripheral blood buffy coats. This study was performed on a randomly chosen subgroup of patients. Heparinized whole blood (2.5 or 5 ml) taken at the same time as QBC was centrifuged at 2,700 × g for 10 min. The plasma was removed carefully with a sterile plastic pipette, and 0.1 ml including the buffy coat layer was aspirated with a sterile 1-ml syringe. Quantitative cultures were performed on this 0.1-ml sample by mixing it with 19 ml of molten Columbia agar, pouring the mixture as an agar plate, and incubating it for up to 4 days. Peripheral blood phagocytes containing more than one bacterium will produce a single colony in solid culture medium. Thus, for whole-blood and buffy coat samples, results were expressed as CFU per milliliter. To quantitate precisely the number of intracellular bacteria per infected phagocyte, a second 0.1-ml buffy coat sample was taken and the leukocytes were lysed by incubation with 0.1 ml of 0.1% digitonin (Sigma) for 10 min at 37°C. This should have released the intracellular bacteria. Quantitative cultures were then performed on this sample in the same way as for the first sample, i.e., by mixing with 19 ml of molten Columbia agar, pouring the mixture as an agar plate, and incubating it at 37°C for up to 4 days. In order to exclude the possibility that the localization of bacteria in the buffy coat was an artifact of the centrifugation step, 10 ml of blood from a healthy volunteer was seeded with 104 CFU of S. typhi and centrifuged immediately at 2,700 × g for 10 min. A 0.1-ml volume of buffy coat and 0.1 ml of the erythrocyte layer were collected with sterile 1-ml syringes, mixed with 19 ml of molten Columbia agar, and allowed to set as described above. Bacterial colonies were counted after 4 days of incubation at 37°C.
Calculation of bacterial counts.
The number of S. typhi bacteria per milliliter of blood (QBC) was estimated from the number of CFU on each pour plate. The minimum volume of whole blood used for quantitative methods from any one patient was 1.5 ml for small children and 3 ml for adults and larger children. This gives a lower limit of detection of 0.7 or 0.3 CFU per ml, respectively. When reporting the number of bacteria in buffy coat and lysed buffy coat samples the denominator was the volume of whole blood from which the buffy coat was collected. In patients who were broth culture positive but QBC negative, the quantitative value was estimated from the total volume of blood cultured: 0.7 to 1.0 CFU/ml from 1.5 ml of blood and 0.3 to 1.0 CFU/ml from 3 ml of blood. The viable bacteria within the 0.1-ml buffy coat sample were considered to be intraleukocytic, and the organisms in the remainder of the blood were assumed to be in the plasma. Extracellular bacterial counts were therefore calculated by the following formula: (whole-blood CFU/ml) − (buffy coat CFU/ml). The average number of viable bacteria per infected leukocyte was calculated by the following formula: lysed buffy coat counts (in bacteria per milliliter)/unlysed buffy coat counts (in infected cells per milliliter).
Statistical analysis.
SPSS for Windows (SPSS Benelux Inc., Gorinchem, The Netherlands) release 6 was used for all statistical analyses. Partial correlation coefficients were calculated for associations between the continuous variables and bacterial counts. Differences in bacterial counts between groups defined by dichotomous variables were tested by the Mann-Whitney U tests. Potentially confounding variables (site and/or age) were controlled for, and all non-normally distributed data were ranked before correlation tests on the ranked data were performed. Interactions were assessed by multivariate analysis.
RESULTS
Broth blood culture was performed on 515 adults and children with suspected enteric fever between March 1993 and March 1996 (Table 1). All patients received treatment with oral ofloxacin (total dose, 60 to 135 mg/kg of body weight given over 2 to 5 days) as reported elsewhere (4, 22), and all made a good recovery. Enteric fever pathogens were isolated from 375 (72.8%) patients: S. typhi from 369 patients and Salmonella paratyphi A from 6 patients. For two patients the pour plate QBC was positive but the broth blood cultures were negative. Salmonella derby was isolated from the blood of one patient, and this patient was considered negative for enteric fever pathogens and excluded from the analysis. The majority of S. typhi isolates (313; 80%) were multidrug resistant, i.e., resistant to chloramphenicol, trimethoprim-sulfamethoxazole, and ampicillin. Both whole-blood and buffy coat bacterial counts were performed for 187 patients. These patients had admission clinical parameters similar to those of the 188 patients with enteric fever for whom only whole-blood QBC were performed. Simultaneous whole-blood, buffy coat, and lysed buffy coat samples were taken from a subgroup of 81 patients. These patients had a slightly shorter duration of illness before admission, i.e., a median of 7 (range, 2 to 33) days compared with a median of 9 (range, 2 to 33) days overall, but in all other respects their clinical presentations were similar.
TABLE 1.
Clinical and laboratory variables for patients with suspected enteric fever
| Variable | Value for:
|
|
|---|---|---|
| All patients enrolled (n = 514) | Patients with positive blood cultures (n = 375) | |
| Age (yr)a | 12.9 (9) | 14 (9.5) |
| Preceding fever duration (days)b | 9 (7 to 12; 2 to 33) | 10 (7 to 13; 2 to 33) |
| Temp (°C)a | 39.3 (1.0) | 39.1 (1.0) |
| Hepatomegalyc | 337 of 511 (66) | 237 of 375 (64) |
| Melenac | 22 of 512 (4) | 14 of 374 (4) |
| Vomitingc | 91 of 508 (18) | 69 of 378 (18) |
| Abdominal painc | 161 of 509 (32) | 106 of 378 (62) |
| Diarrheac | 325 of 506 (64) | 234 of 378 (62) |
| Jaundicea | 5 of 512 (1) | 4 of 378 (1.1) |
| Peak temp (°C)a | 39.7 (0.9) | 39.7 (0.9) |
| Rose spotsc | 18 of 482 (4) | 17 of 378 (5) |
| Leukocyte count/μla | 7,300 (3,402) | 7,629 (3,121) |
| Hematocrit (%)a | 33.2 (4.9) | 33.6 (5.1) |
| Platelet count/μla | 180,000 (89,000) | 196,615 (84,795) |
Shown as mean (standard deviation).
Non-normally distributed continuous data shown as median (IQR; range).
Shown as number of patients positive (percentage).
Direct plating of buffy coat.
Direct plating of buffy coat was performed for 192 patients with cases of broth culture-positive enteric fever: 190 of 191 S. typhi and 1 of 1 S. paratyphi A cultures were positive, a sensitivity of 99.5% (95% confidence interval [CI], 97.1 to 100%). Although the precise time for colony formation from the buffy coat cultures was not recorded, counts were usually possible after overnight incubation. Significant contamination did not occur.
Quantitative culture results.
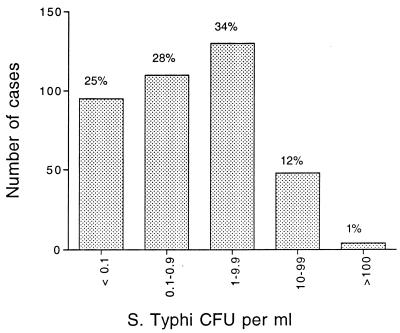
The median S. typhi count in whole blood from patients with uncomplicated enteric fever (n = 375) was 1 CFU/ml (interquartile range [IQR], <0.3 to 5; range, <0.3 to 387) (Fig. 1). A mean of 37.5% (95% CI, 32.9 to 42.1%) of the CFU were from organisms distributed in the erythrocyte and plasma layers (n = 187), and the remaining 62.5% of viable bacteria were concentrated in the buffy coat layer. The mean number of bacteria per infected leukocyte was 1.3 (IQR, 0.7 to 2.4) CFU/cell (n = 81). Children less than 15 years old (n = 115) had higher whole-blood counts than did adults (n = 262): a median of 1.5 (IQR, <0.3 to 6.0; range, <0.3 to 387) versus a median of 0.6 (IQR, <0.3 to 2.8; range, <1.3 to 17.7) CFU/ml (P = 0.008). Extracellular bacterial counts were also higher in children less than 15 years of age than in adults: a median of 1.5 (range, 0 to 93) versus a median of 0.6 (range, 0 to 17.7) (P = 0.005). When blood from a healthy volunteer was seeded with S. typhi, the concentration of organisms cultured from the erythrocyte layer was the same as that in the buffy coat layer. No bacteria were cultured from the plasma layer. Thus, S. typhi cells were not concentrated in the buffy coat as a result of centrifugation.
FIG. 1.
Distribution of blood bacterial counts in acute typhoid fever.
Antibiotic resistance and blood bacterial counts.
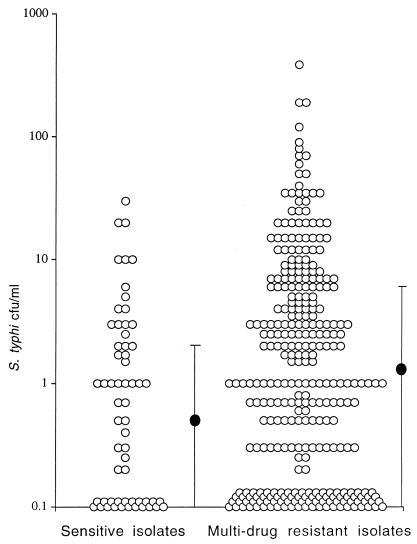
The QBC counts were higher in patients infected by multidrug-resistant S. typhi (n = 313) than in those infected by more sensitive isolates (n = 62): a median of 1.3 (range, 0 to 387) versus a median of 0.5 (range, 0 to 32) CFU/ml (P = 0.006) (Fig. 2). Patients infected by multidrug-resistant strains tended to be younger than patients infected by more sensitive strains: a median of 10 (IQR, 6 to 15) versus a median of 13 (IQR, 7 to 24) years (P = 0.02). The duration of the preceding illness was also slightly shorter in infections by multidrug-resistant strains: a median of 9 (IQR, 6 to 12) versus a median of 10 (IQR, 6 to 15) days (P = 0.07). In a multivariate analysis, duration of preceding illness, age of the patient, and multidrug resistance in the isolated S. typhi proved to be associated independently with the blood bacterial count (P = 0.04, P = 0.003, and P = 0.02, respectively). The association between blood bacterial counts and multidrug resistance was found to be robust and could not be explained by confounding factors.
FIG. 2.
Blood bacterial counts in patients infected by antibiotic-sensitive and multidrug-resistant S. typhi. •, median; the bars indicate the IQR. Each circle represents one patient.
Duration of illness.
There was a decline in whole-blood bacterial counts with increasing duration of illness. The median whole-blood counts were 1.7 (range, <0.2 to 387) CFU/ml in the first week of illness, 1 (range, 0.2 to 68) CFU/ml in the second week, 1 (range, <0.2 to 23) CFU/ml in the third week, and 0.3 (range, <0.2 to 19) CFU/ml in the fourth week of illness (P < 0.001). This remained statistically significant when age and site were both controlled for in the partial correlation analysis (P = 0.002). Similar trends were observed for intracellular (P = 0.06) and extracellular (P = 0.03) counts. The proportion of organisms that was extracellular also declined with duration of illness, but in a multiple regression analysis, controlling for age and multidrug resistance, this trend did not reach statistical significance (P = 0.058).
Stool cultures.
S. typhi was isolated from 24 of 167 patients’ stool samples. The median blood bacterial count in patients with positive stool cultures was significantly higher than that in those with negative cultures: 3 (IQR, 0.7 to 7.3) CFU/ml compared to 1 (IQR, <0.3 to 3.0) CFU/ml (P = 0.02). The relative risk for stool carriage in patients with infections by multidrug-resistant S. typhi compared to that for patients with infections by fully sensitive S. typhi was 3.0 (95% CI, 0.75 to 12.3) (P = 0.15).
DISCUSSION
Enteric fever is a characteristic clinical syndrome which differs clinically and pathologically from other septicemias caused by gram-negative bacteria. The illness has a subacute onset and chronic course when untreated, but it rarely causes endotoxic shock and carries a significantly lower mortality than other septicemias caused by gram-negative bacteria (6, 12, 15, 16, 23). Despite these differences the levels of bacteremia in patients with uncomplicated typhoid (median count, 1 CFU/ml; mortality, <1%) (27) are similar to those reported in bacteremias caused by other enterobacteriaceae (mortality 10 to 50%) (6, 16, 27). Approximately 50% of patients with typhoid fever whose blood cultures are positive have less than 1 CFU/ml of blood, and 10% have greater than 50 CFU/ml. Thus, the pathological differences between septicemia caused by enteric fever pathogens and that caused by other enterobacteriaceae cannot be explained by differences in the numbers of organisms circulating in the blood. In a previous study of 25 patients with typhoid fever reported by Butler et al., blood bacterial counts were higher: five patients had counts of more than 100 CFU/ml (3). In that series a small volume (0.1 ml) of blood was plated directly onto bile-containing agar, which may have released intracellular organisms. The patients were also more severely ill (three had intestinal hemorrhages, and two died). However, intestinal perforation and hemorrhage are usually signs of a late stage of typhoid and are associated with lower diagnostic yields from blood culture (5, 17). In the present study severely ill patients, who comprise less than 5% of all cases in this area, were not included (although 14 of 374 patients did have evidence of upper gastrointestinal bleeding). The range of bacterial counts in blood in this large study of typhoid fever is similar to those in the much-smaller studies reported previously by Rubin et al., using first a semiquantitative DNA hybridization method (19) and, second, direct cultures (18).
In terms of intrinsic toxicity there is little difference between enterobacteriaceae. The purified endotoxin of S. typhi is similar in cytotoxicity and ability to induce proinflammatory cytokine release to that of Escherichia coli (26). However, septicemias caused by gram-negative bacteria are often sudden in onset, whereas the subacute progression of typhoid may allow some endotoxin tolerance to develop (11, 14). The salmonellae, including S. typhi, have evolved mechanisms for downregulating the inflammatory response, presumably to facilitate persistence and transmission (7). As in other severe bacterial infections, higher bacteremias were found more commonly in children with typhoid fever and were associated with higher peak temperatures. This may reflect lower background immunity. The principal difference between systemic infection with salmonellae and that with other enterobacteriaceae is that two-thirds of the bacteria in the circulatory system are located within phagocytic cells, where they remain viable. Thus, the numbers of extracellular organisms in the bloodstream in patients with typhoid tends to be lower than in those with other systemic infections.
The concentration of typhoid bacilli in the buffy coat (presumably in monocytes and polymorphonuclear leukocytes) means that direct plating of this cell layer provides an alternative and more rapid method of diagnosis than whole-blood broth culture. Colonies were usually evident after an overnight incubation. In this study the average number of organisms per phagocytic cell was 1.3, a figure identical to that reported by Rubin et al. (18). Lysing of the phagocytic cells increased absolute numbers of CFU identified but not the sensitivity of the procedure. Overall, buffy coat culture was as sensitive as whole-blood broth culture and allowed earlier organism identification and antimicrobial sensitivity testing. Contamination was rare in this study. Buffy coat culture might be particularly useful for young children, who tend to have higher bacteremias, as it allows a smaller total volume of blood to be taken. Plasma from the same sample can be used for both biochemical and serological tests.
In this study bacterial counts in blood declined with increasing duration of illness. The declining diagnostic sensitivity of blood culture with increasing duration of illness in typhoid has been well known throughout this century. In 1907 a review of the literature reported that 89% of blood cultures were positive in the first week of typhoid fever, 73% were positive in the second, 60% were positive in the third, and only 26% were positive in the fourth and subsequent weeks (5). Modern blood culture media contain sodium polyanetholesulfonate (Liquoid) and may be better at growing lower numbers of bacteria than those used previously (8, 9), but the trend in this large study was the same. The declining numbers of bacteria in blood coincide with an increasing risk of small bowel ulceration and bleeding as the typhoid bacilli concentrate in the Peyer’s patches and areas of necrosis form in the gut wall.
In other studies in which multidrug resistance has been linked to severity of typhoid, the association has been ascribed to incorrect initial treatment and thus further progression of the disease and later presentation to hospital (2, 25). In this study patients with typhoid caused by multidrug-resistant strains had higher numbers of S. typhi bacteria in the blood than patients infected by antibiotic-sensitive organisms. They also had a slightly shorter preceding duration of illness, so previous treatment with ineffective antimicrobials is unlikely to explain the higher bacteremias in this group. Although ineffective previous treatment cannot be definitely excluded, as many patients are unsure of the medications they have received, fluoroquinolones were the antibiotics most likely to have been prescribed outside the hospital in the study sites (22a), and these should have been equally effective against drug-resistant and sensitive bacteria. Furthermore, fluoroquinolones usually sterilize the blood within 2 days in patients with typhoid, so it is very unlikely that significant antibiotic treatment courses had been given. Patients with infections by multidrug-resistant bacteria tended to be younger, but in a multivariate analysis drug resistance was associated independently with higher bacteremias. This suggests that the multidrug-resistant phenotype may be associated with virulence in S. typhi. Multidrug resistance in S. typhi is encoded by genes carried in large transferable plasmids. Whether these plasmids carry other virulence genes or whether the resistance mechanisms themselves somehow enhance bacterial survival and multiplication in vivo is not known.
Single-admission stool cultures were positive for S. typhi in 14.3% of 167 patients. Carriage of S. typhi in the stool was associated with significantly higher bacterial counts in the blood. As multidrug resistance was also positively associated with bacterial counts in the blood, patients infected by multidrug-resistant bacteria were more likely to excrete the organisms in the stool, although this difference did not reach statistical significance. If studies of larger series of patients confirm this observation, then the association of drug resistance with increased bacteremias and also increased transmission potential would provide a plausible explanation for the recent very rapid spread of multidrug-resistant typhoid bacilli in the southern part of Vietnam.
ACKNOWLEDGMENTS
We are very grateful to the directors and staffs of the Dong Thap Provincial Hospital and the Centre for Tropical Diseases, Ho Chi Minh City. We are particularly grateful to the Microbiology Departments in both institutions for their help, and to Julie Simpson for statistical advice.
This study was supported by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Bauer A W, Kirby W M, Sherris J C, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1978;45:493–496. [PubMed] [Google Scholar]
- 2.Bhutta Z A, Nagvi S H, Razzaq R A, Farooqui B J. Multi-drug resistant typhoid fever in children: presentation and clinical features. Rev Infect Dis. 1991;13:832–836. doi: 10.1093/clinids/13.5.832. [DOI] [PubMed] [Google Scholar]
- 3.Butler T, Bell W R, Levin J, Linh N N, Arnold K. Typhoid fever; studies of blood coagulation, bacteremia, and endotoxemia. Arch Intern Med. 1978;138:407–410. doi: 10.1001/archinte.138.3.407. [DOI] [PubMed] [Google Scholar]
- 4.Chinh N T, Solomon T, Thong M X, Ly N T, Hoa N T T, Wain J, Diep T S, Smith M D, Day N P J, Phi L T, Parry C M, White N J. Short courses of ofloxacin for the treatment of enteric fever. Trans R Soc Trop Med Hyg. 1997;91:347–349. doi: 10.1016/s0035-9203(97)90102-4. [DOI] [PubMed] [Google Scholar]
- 5.Coleman W, Buxton B H. The bacteriology of the blood in typhoid fever: an analysis of 1602 cases. Am J Med Sci. 1907;133:896–903. [Google Scholar]
- 6.DuPont H L, Spink W W. Infections due to Gram negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota medical center, 1958–1966. Medicine. 1969;48:307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Eisenstein T K, Al-Ramadi B K, Huang D. Immunity and immunosuppression induced by attenuated Salmonella: the role of nitric oxide. In: Cabello F, editor. Biology of salmonella. New York, N.Y: Plenum Press; 1993. pp. 265–276. [Google Scholar]
- 8.Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119–123. doi: 10.1128/jcm.1.2.119-123.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escamilla J, Santiago L T, Sangalang R P, Ranoa C P, Cross J H. Comparative study of three blood culture systems for the isolation of enteric fever Salmonella. Southeast Asian J Trop Med Public Health. 1984;15:161–166. [PubMed] [Google Scholar]
- 10.Foster W D. Laboratory diagnosis of typhoid fever. Lancet. 1975;ii:80. doi: 10.1016/s0140-6736(75)90527-9. [DOI] [PubMed] [Google Scholar]
- 11.Greisman S E, Woodward C L. In vivo studies on the role of the liver in endotoxin fever and tolerance. J Clin Invest. 1970;49:37a–38a. [Google Scholar]
- 12.Hall W H, Gold D. Shock associated with bacteremia. Arch Intern Med. 1955;96:403–412. doi: 10.1001/archinte.1955.00250140125014. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman S L, Punjabi N H, Rockhill R C, Sutomo A, Rivai A R, Pulungsih S P. Duodenal string-capsule culture compared with bone-marrow, blood, and rectal-swab cultures for diagnosing typhoid and paratyphoid fever. J Infect Dis. 1984;149:157–161. doi: 10.1093/infdis/149.2.157. [DOI] [PubMed] [Google Scholar]
- 14.Hornick R B, Greisman S E, Woodward T E, DuPont H L, Dawkins A T, Snyder M J. Typhoid fever: pathogenesis and immunological control. N Engl J Med. 1970;283:686–691. doi: 10.1056/NEJM197009242831306. and 739–746. [DOI] [PubMed] [Google Scholar]
- 15.Kluge R M, DuPont H L. Factors affecting mortality of patients with bacteremia. Surg Gynecol Obstet. 1973;137:137–267. [PubMed] [Google Scholar]
- 16.Kreger B E, Craven D E. Gram negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980;68:332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- 17.Parker M T. Salmonella. In: Wilson G, Miles A, Parker M T, editors. Topley and Wilson’s principles of bacteriology, virology and immunity. 7th ed. London, England: Edward Arnold; 1983. pp. 332–355. [Google Scholar]
- 18.Rubin F A, McWhirter P D, Burr D, Punjabi N H, Lane E, Kumala S, Sudarmono P, Pulungsih S P, Lesmana M, Tjaniadi P, Sukri N, Hoffman S L. Rapid diagnosis of typhoid fever through identification of Salmonella typhi within 18 hours of specimen acquisition by culture of the mononuclear cell-platelet fraction of blood. J Clin Microbiol. 1990;28:825–827. doi: 10.1128/jcm.28.4.825-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin F A, McWhirter P D, Punjabi N H, Lane E, Sudarmono P, Pulungsih S P, Lesmana M, Kumala S, Kopecko D J, Hoffman S L. Use of a DNA probe to detect Salmonella typhi in the blood of patients with typhoid fever. J Clin Microbiol. 1989;27:1112–1114. doi: 10.1128/jcm.27.5.1112-1114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw A B, Mackay H A F. Factors influencing the effects of blood cultures in enteric fever. J Hyg. 1951;49:315–323. doi: 10.1017/s0022172400044181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuart M D, Pullen R L. Typhoid: clinical analysis of three hundred and sixty cases. Arch Intern Med. 1946;78:629–661. doi: 10.1001/archinte.1946.00220060002001. [DOI] [PubMed] [Google Scholar]
- 22.Vinh H, Wain J, Hanh V T N, Nga C N, Chinh M T, Bethell D, Hoa N T T, Diep T S, Dung N M, White N J. Two or three days of ofloxacin treatment for uncomplicated multidrug-resistant typhoid fever in children. Antimicrob Agents Chemother. 1996;40:958–961. doi: 10.1128/aac.40.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Wain, J., and C. M. Parry. Unpublished observation.
- 23.Walsh A L, Smith M D, Wuthiekanun V, Suputtamongkol Y, Chaowagul W, Dance D A B, Angus B, White N J. Prognostic significance of quantitative bacteremia in septicemic melioidosis. Clin Infect Dis. 1995;21:1498–1500. doi: 10.1093/clinids/21.6.1498. [DOI] [PubMed] [Google Scholar]
- 24.Watson K. Isolation of Salmonella typhi from the blood stream. J Lab Clin Med. 1955;46:128–134. [PubMed] [Google Scholar]
- 25.White N J, Parry C M. The treatment of typhoid. Curr Opin Infect Dis. 1996;9:298–302. [Google Scholar]
- 26.Wilson B M G, Severn A, Rapson N T, Chana J, Hopkins P. A convenient whole blood system for studying the regulation of tumour necrosis factor release by bacterial lipopolysaccharide. J Immunol Methods. 1991;139:233–240. doi: 10.1016/0022-1759(91)90193-j. [DOI] [PubMed] [Google Scholar]
- 27.Yagupsky P, Nolte F S. Quantitative aspects of septicemia. Clin Microbiol Rev. 1990;3:269–279. doi: 10.1128/cmr.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]