Abstract
Two series of pyrazolo[3,4-b]pyridine derivatives, 9a–h and 14a–h, are synthesized and evaluated for their anti-cancer potency towards Hela, MCF7, and HCT-116 cancer cell lines. Compound 9a showed the highest anticancer activity with IC50 = 2.59 µM against Hela when compared with doxorubicin (IC50 = 2.35 µM). Compound 14g revealed cytotoxicity IC50 = 4.66 and 1.98 µM towards MCF7 and HCT-116 compared to doxorubicin with IC50 = 4.57 and 2.11 µM, respectively. Compound 9a exhibited cell cycle arrest at the S phase for Hela, whereas 14g revealed an arresting cell cycle for MCF7 at G2/M phase and an arresting cell cycle at S phase in HCT-116. In addition, 9a induced a significant level of early and late apoptosis in Hela when compared with the control cells, whereas 14g induced an apoptosis in MCF7 and HCT-116, respectively. Compounds 9a (IC50 = 26.44 ± 3.23 µM) and 14g (IC50 = 21.81 ± 2.96 µM) showed good safety profiles on normal cell line WI-38. Compounds 9a and 14g showed good inhibition activity towards CDK2, with IC50 = 1.630 ± 0.009 and 0.460 ± 0.024 µM, respectively, when compared with ribociclib (IC50 = 0.068 ± 0.004). Furthermore, 9a and 14g showed inhibitory activity towards CDK9 with IC50 = 0.262 ± 0.013 and 0.801 ± 0.041 µM, respectively, related to IC50 of ribociclib = 0.050 ± 0.003. Docking study for 9a and 14g exhibited good fitting in the CDK2 and CDK9 active sites.
Keywords: pyrazolo[3,4-b]pyridines; anti-cancer; cell cycle; apoptosis; CDK2; CDK9
1. Introduction
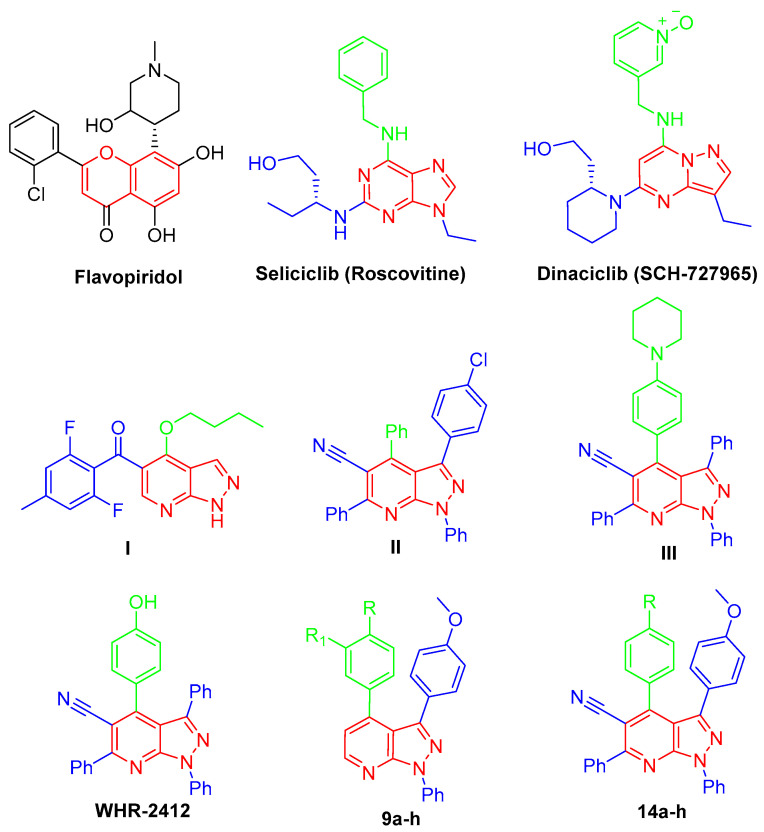
Biomedical research has focused on how living cells grow and divide since the theory of spontaneous generation was disproved in the late seventeenth century, and the interest in the subject only increased as it became clear that sustained cellular proliferation was central to the initiation and progression of cancer, and today, sustained proliferative capacity is considered a hallmark of cancer [1]. Regulation of proliferation is defined by the work of Hartwell, Nurse, and Hunt, and they also defined the role of cyclin-dependent kinases (CDKs); hence, the 2001 Nobel Prize in Physiology and Medicine was awarded to them [2]. The cyclin-dependent protein kinases (CDKs) are protein-serine/threonine kinases that belong to the CMGC family (CDKs, mitogen-activated protein kinases, glycogen synthase kinases, and CDK-like kinases) [3]. The human genome encodes 21 CDKs, although only 7 (CDK 1, 2, 3, 4, 6, 10, 11) have a direct crucial role in the progression of the cell cycle, while other CDKs play an indirect role via activation of other CDKs (CDK 3), regulation of transcription (CDK7, 8, 9) or neuronal function (CDK 5). Different families of cyclins, the regulatory subunits required for CDK activity, have been identified, and their expression fluctuates significantly throughout the phases of the cell cycle [4]. CDK inhibitors may be used to treat cancer, diabetes, kidney disease, neurological illness, and infectious disease [5,6]. A variety of chemical classes, typically planar heteroaromatic structures, such as flavonoid, purine, indenopyrazole, arylcarbazole, indolinone, oxindole, pyrimidine, thiazole, and indirubin, have been described as CDK inhibitors [7]. CDKs have been considered promising targets for the treatment of cancers and other diseases due to their crucial roles in the regulation of cell cycle and transcription. Since the 1990s, the first-generation CDK inhibitors have been explored [8]. These molecules include benzopyran derivative flavopiridol [9,10], imidazolo [4,5-d]pyrimidine roscovitine [11,12], and pyrazolo [1,5-a]pyrimidine dinaciclib (SCH-727965) [13,14] (Figure 1).
Figure 1.
Chemical structures of flavopiridol, roscovitine, dinaciclib, pyrazolo[3,4-b]pyridines I–III, WHR-2412, and targeted compounds 9a–h and 14a–h.
In the last few decades, anticancer drugs have been developed from chemically synthesized compounds including pyrazole derivatives where their anticancer activity is due to the inhibition of various targets such as CDKs [15]. Important pyrazole-containing drugs were available in the market, such as Celecoxib, which showed very promising anticancer activity against prostate cancer cells [16,17]. On the other hand, pyridine derivatives serve as promising anticancer agents in the field; they acquired huge attention in current medicinal research due to their impact in curing numerous vicious ailments, such as breast cancer, myeloid leukemia, and idiopathic respiratory fibrosis [18,19]. Hybridization of pyrazole and pyridine scaffold is of great importance in cancer treatment, such as via sorafenib, regorafenib, vismodegib, and crizotinib. Pyrazolo[3,4-b]pyridine scaffold is an important central moiety of several anticancer agents, such as in the case of compounds I–III [20,21,22,23], which showed an interesting anti-cancer activity through different mechanisms, in addition to WHR-2412, which can exert its antitumor impact through CDK2 inhibition [24,25] (Figure 1).
There are three binding sites that have been reported in the CDK monomer: the ATP-competitive binding site (Site I) in addition to two non-competitive binding sites, II and III. Site I, the main target site of CDK2 inhibitors, has the impressive capacity to accommodate various ligands, including flat heterocyclic rings. On the other side, site III accommodates short peptide ligands, whereas the binding mechanism of Site II remains ambiguous. Furthermore, when CDK is subjected to the cyclin binding process, the resulting conformational changes give rise to a variation of the ATP binding site and then generate an allosteric binding site, i.e., Site IV, which requires the binding inhibitors to possess extraordinarily high inhibitory affinities. In precis, for designing potent CDK2 and CDK9 inhibitors, we can arrange the following principles: (1) developing inhibitors with flat heterocyclic rings for site I; (2) designing short peptide ligands for Site III; and (3) employing binding molecules which have extraordinarily high inhibitory affinities for Site IV [26,27].
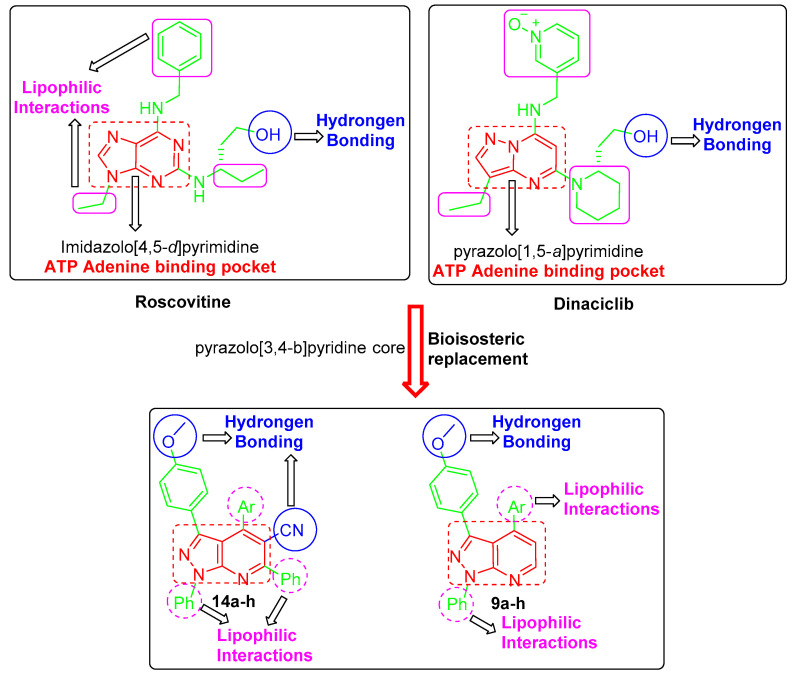
In the light of the mentioned data, several drug design approaches were exploited in order to develop the target pyrazolo[3,4-b]pyridine derivatives, 9a–h and 14a–h, (Figure 2). First, the bioisosteric replacement strategy was utilized to replace the core heterocycle pyrazolo [1,5-a]pyrimidine in dinaciclib and imidazolo [4,5-d]pyrimidine core in roscovitine by the pyrazolo[3,4-b]pyridine core in the target structures. These fused heterocyclic are expected to be fitted into the ATP adenine binding pocket. Moreover, both roscovitine and dinaciclib are tethered with the hydroxyl functionality that is responsible for the hydrogen bonding. As a result, the methoxy functionality was grafted into the structure of our target compounds to ensure the requisite hydrogen bonding.
Figure 2.
Design of the targeted pyrazolo[3,4-b]pyridine derivatives 9a–h and 14a–h.
Furthermore, the cyano functional group was incorporated in target compounds 14a–h to enhance the hydrogen bonding interactions (Figure 2). Moreover, the target structures were decorated with diverse lipophilic motifs to establish the required hydrophobic interactions within the CDK2 binding site. The variation of substitutions in 9a–h and 14a–h was adopted to provide different lipophilic environments (Figure 2). Interestingly, a pilot molecular modeling study showed a plausible binding mode and different interactions of the target molecules within their proposed target CDK2. As expected, a preliminary molecular modeling
study showed that the designed pyrazolo[3,4-b]pyridine derivatives 9a–h and 14a–h succeed in achieving the required hydrogen bonding and hydrophobic interactions.
2. Results and Discussion
2.1. Chemistry
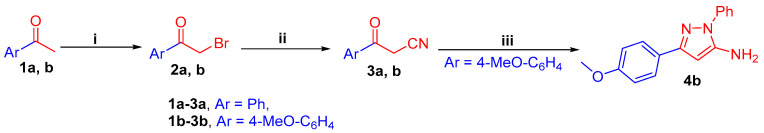
3-(Phenyl/4-methoxyphenyl)-3-oxopropanenitrile 3a, b, are prepared via the bromination of acetophenones 1a, b to afford 2-bromo-1-(phenyl/4-methoxyphenyl)ethan-1-ones 2a, b, followed by cyanation reaction (Scheme 1). Then, the key intermediate 3-(4-methoxyphenyl)-pyrazol-5-amine derivative 4b is synthesized through the reaction of oxopropanenitrile 3b with phenylhydrazine in refluxing ethanol (Scheme 1).
Scheme 1.
(i) Br2, AcOH, stirring, 1 h, rt; (ii) KCN, EtOH/H2O, stirring, 2 h, rt; (iii) PhNHNH2, EtOH, reflux, 6 h.
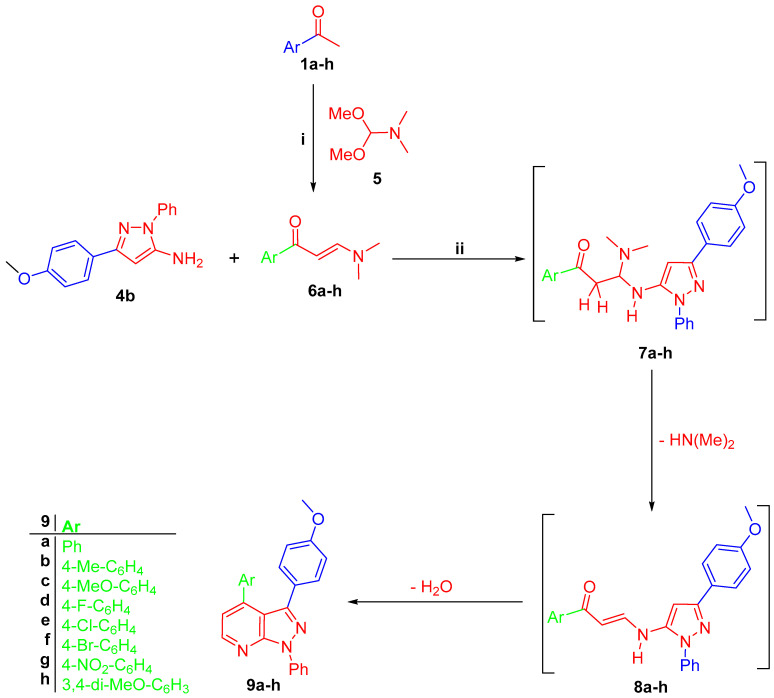
Amino pyrazole derivative 4b reacted with prop-2-en-1-ones 6a–h, which was prepared by the reaction of acetophenones 1a, b with dimethylformamide-dimethylacetal (DMF-DMA) 5 in anhydrous xylene, to give 3-(4-methoxyphenyl)-pyrazolo[3,4-b]pyridines 9a–h, respectively (Scheme 2). The latter reaction is proposed to proceed through michael addition of exocyclic NH2 of amino pyrazole 4b into the double bond of enaminones 6a–h to form intermediate 7a–h, which lost a dimethylamine molecule to give intermediate 7a–h, which gave the final targeted compounds 9a–h through an intramolecular cyclo-condensation reaction (Scheme 2).
Scheme 2.
(i) DMF-DMA, anhydrous xylene, reflux 8 h; (ii) glacial AcOH, refluxed for 4–6 h.
The 1HNMR of 9a–h revealed the doublet signals of pyridine H2 and H3 around δ 8.60 and 8.00 ppm, respectively, with coupling constant (J) around 6.5 Hz. 1HNMR of 9a–h showed the singlet signal of the methoxy group of the pyrazole 4-methoxyphenyl group around δ 3.87 ppm, whereas compounds 9b and 9c revealed an additional signal of methyl and methoxy groups at δ 2.41 and 3.855 ppm, respectively. Moreover, the signals of two methoxy groups of 9h appeared at δ 3.86 and 3.93 ppm, respectively. 13CNMR of 9a–h showed the signals of pyrazole aryl –CH3O-C6H4 carbon at δ 55.75 ppm, in addition to the signals of SP3 carbons of pyridine aryl for –CH3-C6H4 in 9b, –CH3O-C6H4 in 9c and para and meta–CH3O-C6H3 in 9h at δ 21.38, 55.79, 55.90, and 56.07 ppm, respectively.
On the other side, one-step three components reaction of pyrazol-5-amine 4b, 3-oxo-3-phenylpropanenitrile (3a) and the aromatic aldehydes 10a–h give pyrazolo[3,4-b]pyridine-5-carbonitriles 14a–h, respectively (Scheme 3). The IR of 14a–h appeared as the characteristic sharp band of C≡N at 2218–2226 cm−1. 1HNMR of 14a–h exhibited the singlet signal of the methoxy group of the pyrazole 4-methoxyphenyl group around δ 3.72–3.76 ppm. The 1HNMR of 14g showed the D2O exchangeable signal of -OH as a broad singlet at δ 9.90 ppm, whereas the 1HNMR of 14f showed the signal of two methyl groups of -N(Me) as one singlet with integration equal to 6 protons at δ 2.93 ppm. The latter reaction proceeded via the condensation reaction between 3-oxo-3-phenylpropanenitrile (3b) and aldehydes 10a–h, then the addition of H4 of pyrazole to give intermediate 11a–h. The intramolecular cyclocondensation of 11a–h gives the target pyrazolo[3,4-b]pyridine derivatives 14a–h, respectively (Scheme 3).
Scheme 3.
(i) EtOH, TEA (catalytic), reflux 6–10 h.
2.2. Biological Evaluation
2.2.1. Anti-Cancer Activity of 9a–h and 14a–h against Hela, HCT-116, and MCF7 Cancer Cell Lines
The cytotoxicity of compounds 9a–h and 14a–h against three cell lines, namely, cervical cancer Hela, breast cancer MCF7, and colon HCT-116, compared with doxorubicin as a reference compound was performed using MTT assay. The cytotoxic concentration (IC50 in µM) is illustrated in Table 1. Among the screened compounds, 9a and 14g showed significant anticancer activity. Compound 9a showed the highest anticancer activity, with IC50 = 2.59 µM against Hela cell lines when compared with doxorubicin (IC50 = 2.35 µM) (Table 1). Compounds 14g revealed cytotoxicity IC50 = 4.66 and 1.98 µM towards MCF7 and HCT-116 cell lines compared to doxorubicin with IC50 = 4.57 and 2.11 µM, respectively (Table 1).
Table 1.
Anticancer activity of 9a–h and 14a–h against MCF7, HCT-116, and Hela cancer cell lines.
| Compound | IC50% µM | ||
|---|---|---|---|
| Hela | MCF7 | HCT116 | |
| 9a | 2.59 ± 0.16 | 6.39 ± 0.46 | 15.26 ± 1.46 |
| 9b | 13.96 ± 1.25 | 22.35 ± 3.18 | 18.01 ± 1.16 |
| 9c | 26.63 ± 3.37 | 59.04 ± 5.52 | 43.17 ± 3.26 |
| 9d | 19.59 ± 1.68 | 13.01 ± 1.33 | 17.46 ± 0.98 |
| 9e | 48.45 ± 4.20 | 20.31 ± 3.21 | 16.58 ± 1.34 |
| 9f | 20.44 ± 1.74 | 35.56 ± 4.64 | 16.78 ± 0.76 |
| 9g | 28.26 ± 4.23 | 26.28 ± 2.85 | 40.02 ± 3.19 |
| 9h | 7.92 ± 1.11 | 9.70 ± 1.00 | 9.69 ± 0.56 |
| 14a | 23.05 ± 1.63 | 20.72 ± 1.73 | 25.82 ± 2.58 |
| 14b | 20.98 ± 2.18 | 21.39 ± 2.05 | 36.29 ± 1.83 |
| 14c | 41.27 ± 2.79 | 39.34 ± 3.74 | 36.73 ± 2.02 |
| 14d | 21.07 ± 2.03 | 37.21 ± 2.48 | 31.14 ± 3.54 |
| 14e | 37.33 ± 3.32 | 34.36 ± 2.59 | 37.77 ± 2.63 |
| 14f | 46.14 ± 3.70 | 19.56 ± 1.26 | 39.96 ± 4.32 |
| 14g | 12.65 ± 1.53 | 4.66 ± 0.38 | 1.98 ± 0.17 |
| 14h | 31.69 ± 2.48 | 38.12 ± 2.76 | 3.69 ± 0.11 |
| Doxorubicin | 2.35 ± 0.08 | 4.57 ± 0.28 | 2.11 ± 0.20 |
Data are presented as the mean of the IC50 values ± SD from three different experiments.
2.2.2. SAR Studies
The potent anticancer activity of compounds 9a and 14g exhibited the impact of the electronic characteristics of substitution phenyl in position 4 of the pyridine ring with respect to its substituents in the para position. In compounds 9a–h, these substitutions in 9b–h gave low anticancer activity when compared with para-unsubstituted phenyl in 9a with respect to Hela cell line. The 3,4-dimethoxyphenyl in 9h improves the anticancer activity against Hela cell line when compared with 9b–f, but it is still less than that of 9a. Compound 14g, with para-hydroxy substitution in the same phenyl ring, showed the highest anticancer activity against Hela among series 14a–h but still less than the standard reference drug. Compound 14g, with OH group in the phenyl group of position 4 in pyridine moiety, in addition to the Ph group in position 2 and the CN function in position 3 of the pyridine moiety, enhanced its anticancer activity towards MCF7 and HCT-116 cell lines. In the light of previous data, it can be noticed that the pyrazolo[3,4-b]pyridine system in compounds 9a–h and 14a–h, in addition to the substitutions around it, are necessary for their anticancer activity.
2.2.3. Cell Cycle and Apoptosis
Cell Cycle
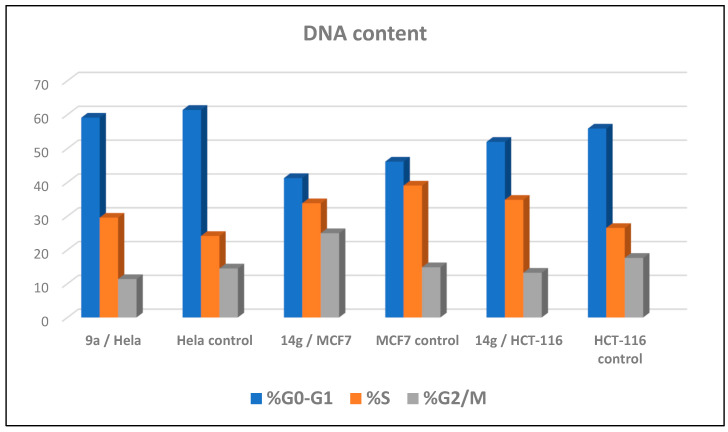
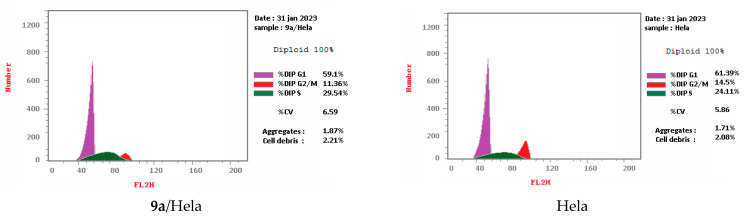
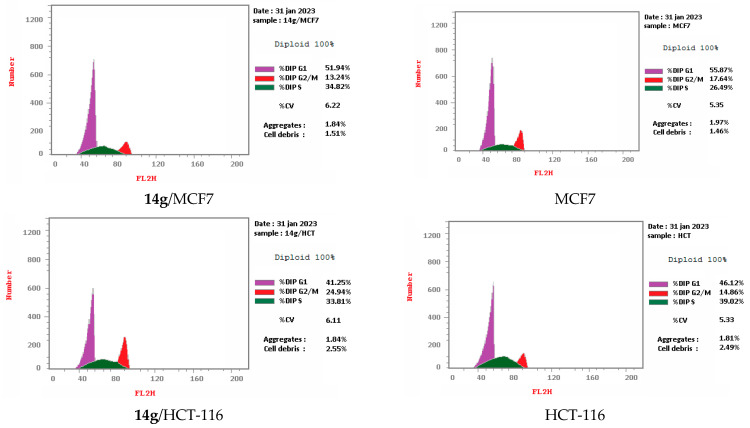
Cell cycle arrest of cervical cancer cells Hela, breast cancer cells MCF7, and colon cancer cells HCT-116 were assessed for compounds 9a and 14g at their IC50. Compound 9a exhibited high cell accumulation (29.54%, 1.23 fold) for the Hela cell line at the S phase (Control; 24.11%) that signifies cell cycle arrest at the S phase (Table 2, Figure 3 and Figure 4). Compound 14g exhibited cell cycle arrest for breast cell line MCF7 at G2/M—the phase with DNA accumulation, 24.94%, (1.68 fold), compared with the control, 14.86% (Table 2, Figure 2 and Figure 3). In addition, the compound 14g arresting cell cycle at S phase in HCT-116 cell lines with cell accumulation = 34.82% (1.97 fold) when compared with the control cells (17.64%) (Table 2, Figure 3 and Figure 4).
Table 2.
Cell cycle analyses for compounds 9a and 14g against MCF7, HCT-116, and Hela cancer cell lines.
| Compound/Cell Line | DNA Content | Comment | ||
|---|---|---|---|---|
| %G0-G1 | %S | %G2/M | ||
| 9a/Hela | 59.1 | 29.54 | 11.36 | cell growth arrest@ S |
| Hela control | 61.39 | 24.11 | 14.5 | --- |
| 14g/MCF7 | 41.25 | 33.81 | 24.94 | cell growth arrest@ G2/M |
| MCF7 control | 46.12 | 39.02 | 14.86 | --- |
| 14g/HCT-116 | 51.94 | 34.82 | 13.24 | cell growth arrest@ S |
| HCT-116 control | 55.87 | 26.49 | 17.64 | --- |
Figure 3.
Representative diagram for cell cycle analyses for 9a against Hela and 14g against MCF7 and HCT-116, respectively.
Figure 4.
Cell cycle analyses for 9a against Hela and 14g against MCF7 and HCT-116, respectively.
Apoptosis Assay
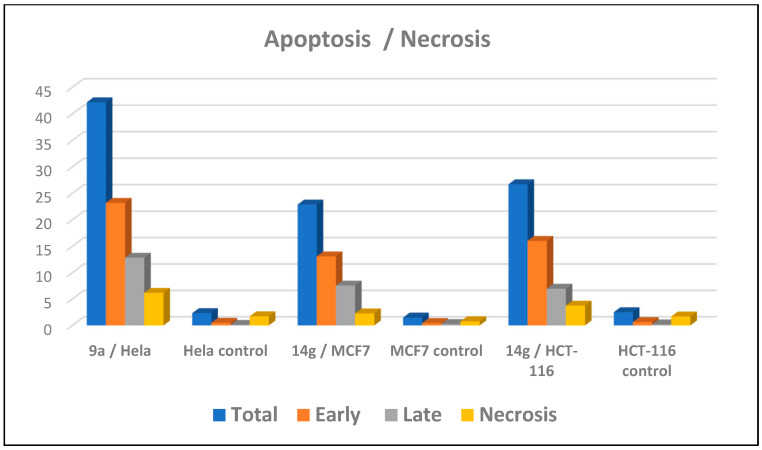
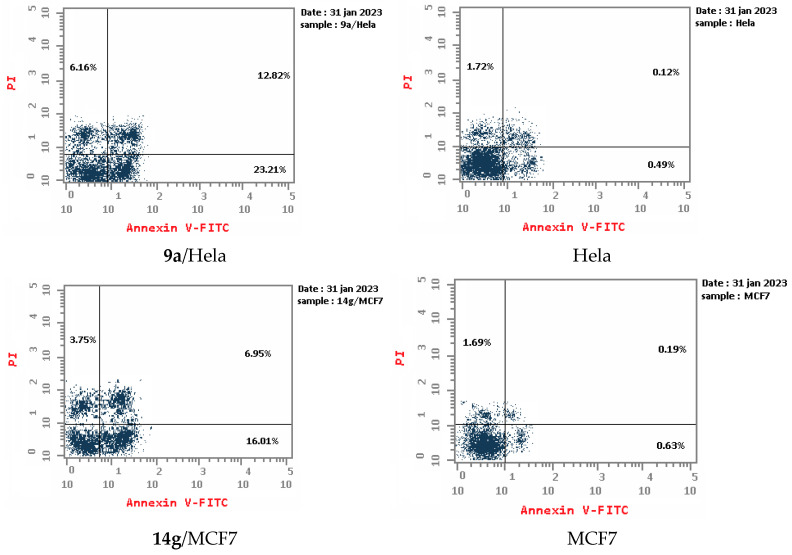
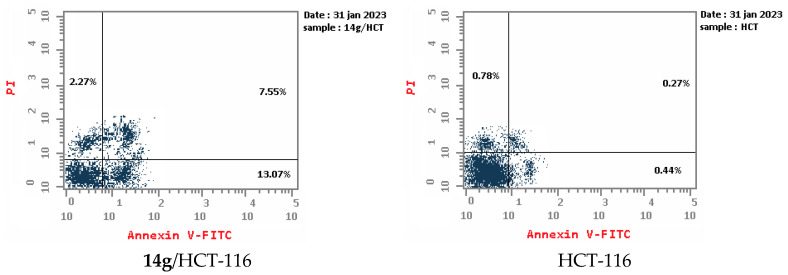
An apoptotic assay using Annexin V/PI analysis for compounds 9a and 14g is evaluated. Compound 9a induced a significant level of early and late apoptosis (total = 42.19) in the Hela cell line when compared with the control cells (Table 3, Figure 5 and Figure 6). Compound 14g revealed total apoptosis = 22.89 and 26.71 in MCF7 and HCT-116 cell lines, respectively, compared with the control cells (Table 3, Figure 5 and Figure 6).
Table 3.
Apoptosis analysis for 9a and 14g against MCF7, HCT-116, and Hela cancer cell lines.
| Compound | Apoptosis | Necrosis | ||
|---|---|---|---|---|
| Total | Early | Late | ||
| 9a/Hela | 42.19 | 23.21 | 12.82 | 6.16 |
| Hela control | 2.33 | 0.49 | 0.12 | 1.72 |
| 14g/MCF7 | 22.89 | 13.07 | 7.55 | 2.27 |
| MCF7 control | 1.49 | 0.44 | 0.27 | 0.78 |
| 14g/HCT-116 | 26.71 | 16.01 | 6.95 | 3.75 |
| HCT-116 control | 2.51 | 0.63 | 0.19 | 1.69 |
Figure 5.
Representative diagram for apoptosis analyses for 9a against Hela and 14g against MCF7 and HCT-116, respectively.
Figure 6.
Apoptosis analyses for 9a against Hela and 14g against MCF7 and HCT-116, respectively.
2.2.4. Cytotoxicity of 9a and 14g on Normal Cell Line
The cytotoxicity of the most effective compounds 9a and 14g towards human normal cell line WI-38 was evaluated comparing with doxorubicin as a reference drug using MTT assay. Compounds 9a (IC50 = 26.44 ± 3.23 µM) and 14g (IC50 = 21.81 ± 2.96 µM) showed good safety profiles on normal cell line WI-38 when compared with doxorubicin (IC50 = 15.60 ± 0.37 µM) (Table 4).
Table 4.
In vitro anticancer activities of 9a and 14g against human normal WI-38 cells.
| Compound | In Vitro Cytotoxicity on WI-38 (IC50, µM) a |
|---|---|
| 9a | 26.44 ± 3.23 |
| 14g | 21.81 ± 2.96 |
| Doxorubicin | 15.60 ± 0.37 |
a Data are presented as the mean of the IC50 values ± SD from three different experiments.
2.2.5. CDK2 and CDK9 Enzyme Assay Inhibition of 9a and 14g
The in vitro CDK2 and CDK9 inhibitory activity of 9a and 14g was assayed using ribociclib as a standard CDK inhibitor, and their IC50 (µM) are illustrated in Table 5. Compounds 9a and 14g showed good inhibition activity towards CDK2 with IC50 = 1.630 ± 0.009 and 0.460 ± 0.024 µM, respectively, when compared with ribociclib (IC50 0.068 ± 0.004). Furthermore, compounds 9a and 14g showed inhibitory activity towards CDK9 with IC50 = 0.262 ± 0.013 and 0.801 ± 0.041 µM, respectively, related to IC50 of ribociclib = 0.050 ± 0.003.
Table 5.
CDK2 and CDK9 inhibitory activity (IC50, µM) for 9a, 14g and ribociclib.
| Compound | IC50 (µM) | |
|---|---|---|
| CDK2 | CDK9 | |
| 9a | 1.630 ± 0.009 | 0.262 ± 0.013 |
| 14g | 0.460 ± 0.024 | 0.801 ± 0.041 |
| Ribociclib | 0.068 ± 0.004 | 0.050 ± 0.003 |
2.3. Molecular Modeling
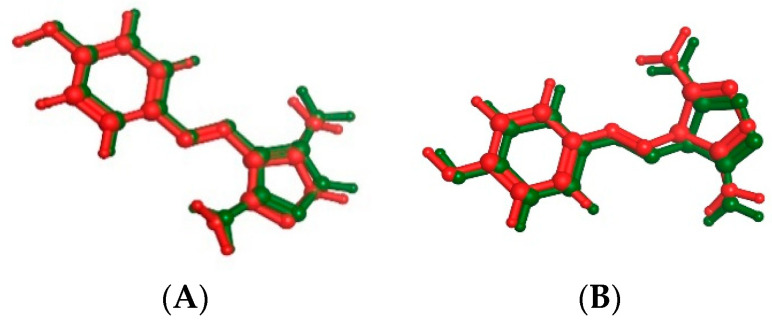
A molecular docking study on compounds 9a and 14g in the active site of CDK2 and CDK9 enzyme to correlate the in vitro enzyme activity with their binding mode with enzymes’ active sites using the molecular operating environment (MOE) 2019.02 and 3D coordinates of CDK2 and CDK9 (PDB IDs 3tnw and 3tn8 with resolution 2 and 2.95 Å, respectively). Valid docking protocol is established through the complexing of CDK2 and CDK9 with CAN508 as a potent CDK inhibitor with 0.265 and 0.4337 Å (Figure 7). The interaction modes of the ligand with the active sites should not be only determined as the highest energy scored protein–ligand complex used during docking but also bears in mind the conformers of each compound which are mostly associated with bioactive conformations. Based on the binding free energies and their correlation with the inhibitory activities, we can give a more quantitative explanation of the structure–activity relationship of the inhibitory mechanism for these compounds [28,29,30]. The output of the docking simulation is the scoring function which reflects the binding free energy dG in Kcal/mol. This step showed an energy score of −11.7195 and −10.045 kcal/mol for CAN508 in CDK2 and CDK9, respectively.
Figure 7.
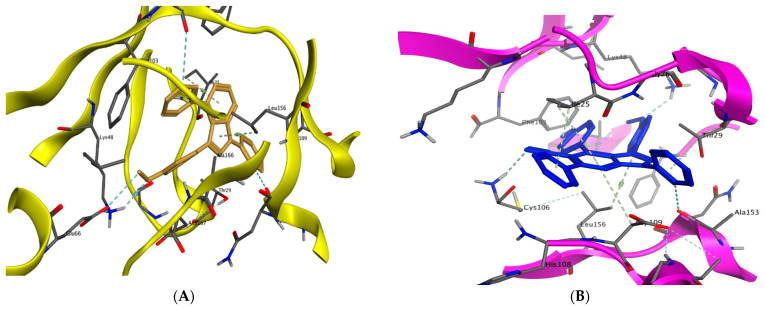
The superimposition of co-crystallized (red) and docking pose (green) of CAN508 in CDK2 (A) and CAN508 in CDK9 (B) active sites.
2.3.1. Docking of 9a and 14g in CDK2 Binding Site
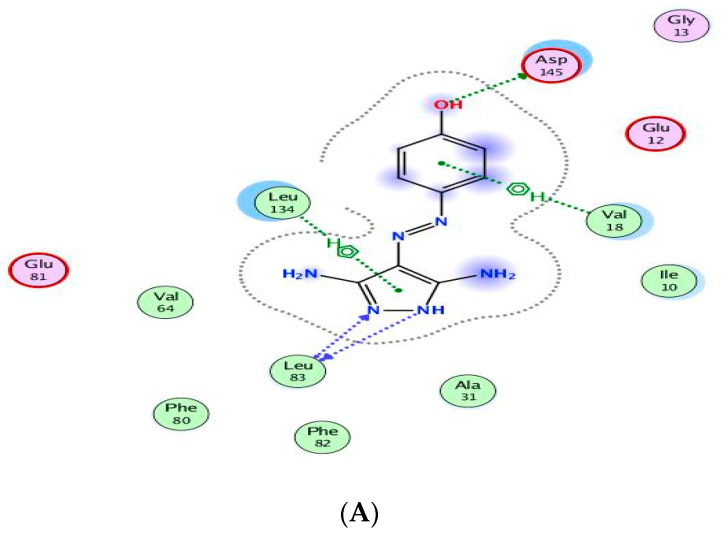
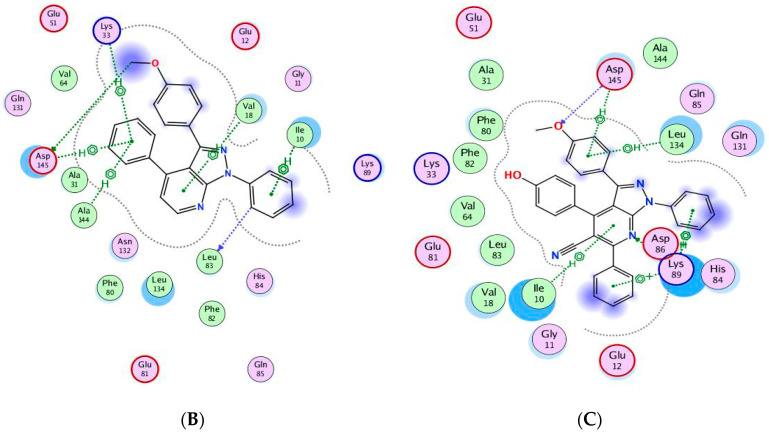
The binding of CAN508 with CDK2 active site showed the formation of two H-bonds with Asp145 and Leu83 residues, and two pi–H bonds with Val18 and Leu134 (Figure 8A). Docking simulations for 9a and 14g exhibited a good fit in the CDK2 active site with docking scores −13.0738 and −14.924 kcal/mol, respectively, when compared with that of CAN508 (−11.7195 kcal/mol). The higher binding free energy of 9a and 14g, compared with CAN508, explained their good affinity to CDK2 active site. The binding patterns of 9a (Figure 8B) and 14g (Figure 8C) are consistent with the crystallographic binding of CAN508 in the CDK2 active site.
Figure 8.
Two-dimensional binding interactions of CAN508 (A), 9a (B), and 14g (C) in the CDK2 binding site in which green arrows represent H-bond interaction in the sidechain, blue arrows represent H-bond interaction in the backbone, the benzene/H structure on the green bond represents pi–hydrogen interactions, and the benzene/+ structure on the green bond represents pi–cation interactions.
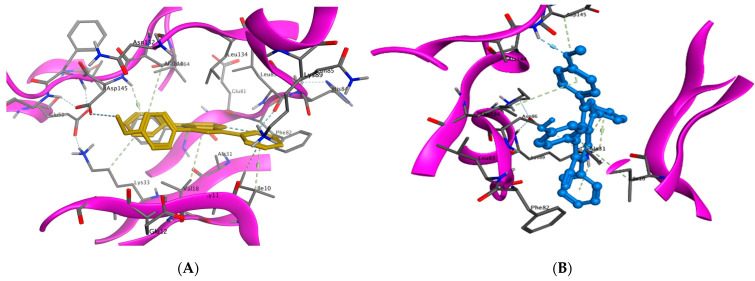
For example, the methoxy group in the phenylmethoxy ring of both compounds 9a and 14g was H-bonded with the key residue Asp145. The latter H-bond highlighted the important role of the methoxy group of the phenyl ring in these compounds in CDK9 inhibition. Moreover, binding with Asp145 by pi–hydrogen bond through the phenyl ring of pyridine in 9a and the phenylmethoxy ring in 14g are noticed. Both compounds bind with Ile10 by pi–hydrogen bond through a phenyl ring attached to pyrazole in compound 9a and the pyridine ring in compound 14g. Compound 9a binds the key residue Leu83 by a hydrogen bond through its phenyl ring attached to pyrazole. Further pi–hydrogen interactions are observed between compound 9a and Val18, Lys33 and Ala144 (Figure 9A). Compound 14g is anchored by the key residue Lys89 by hydrogen, pi–cation, and pi–hydrogen bonds. Further pi–hydrogen interaction between compound 14g and Leu134 is observed (Figure 9B).
Figure 9.
Binding interactions (3D diagram) for 9a (A) and 14g (B) in the CDK2 binding site.
2.3.2. Docking of 9a and 14g in CDK9 Binding Site
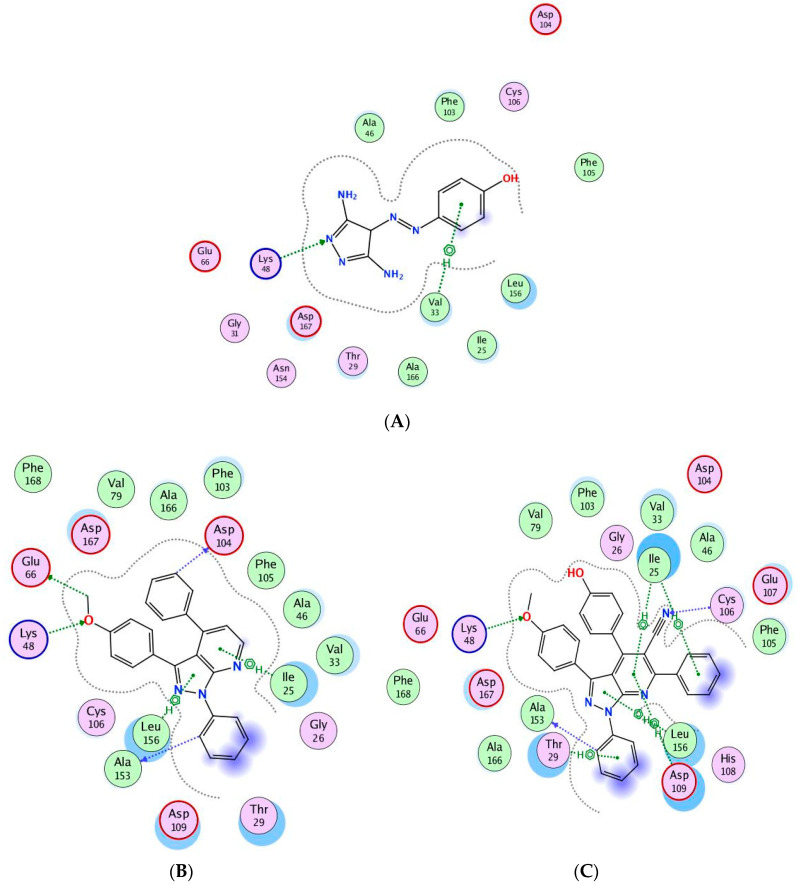
Compounds 9a and 14g showed good docking scores with high free binding energy (−10.6742 and −10.1599 kcal/mol) compared to CAN508 (−10.045 kcal/mol). Figure 10A shows that CAN508 formed an H-bond with the Lys48 residue and a pi–hydrogen interaction with Val33 in the CDK9 active site. Interestingly, 9a and 14g revealed essential interactions in CDK9 active sites (Figure 10B,C). For instance, both compounds formed an H-bond with essential key residue Lys48 via the oxygen atom of the phenylmethoxy ring. This illustrates the importance of additional methoxy group in our compounds. The phenyl ring attached to pyrazole forms a hydrogen bond with the key residue Ala153 in both compounds. Both compounds form pi–hydrogen interactions leu156 and Ile25 via pyrazole and pyridine rings, respectively, noting that there is an additional interaction in compound 14g via the phenyl group of the pyridine ring and this explains the stronger binding and score in this compound.
Figure 10.
Binding interactions (2D diagram) for CAN508 (A), 9a (B), and 14g (C) in the CDK9 binding site in which green arrows represent H-bond interaction in the sidechain, blue arrows represent H-bond interaction in the backbone, and the benzene/H structure on the green bond represents pi–hydrogen interactions.
Compound 9a forms two hydrogen bonds with Glu66 and Asp104 via the methoxy group and phenyl ring attached to pyridine, respectively. Compound 14g forms a hydrogen bond with the key residue Cys106 via the nitrogen atom in the nitrile group in addition to two pi–hydrogen interactions with Asp104 and Leu156 (Figure 11A,B).
Figure 11.
Binding interactions (3D diagram) of 9a (A) and 14g (B) in the CDK9 binding site.
3. In Silico ADME Study
A small molecule’s therapeutic activity is determined by its ability to reach the aimed target at a sufficient concentration, which can be determined by its ADME properties. Lipinski’s rule-of-five states if a small molecule has the potential to be a medication. SwissADME provides rapid but reliable prediction data about a small molecule’s physicochemical parameters (such as molecular weight, partition coefficient, solubility, topological surface area, and so on), as well as pharmacokinetics and drug likeness [31,32]. This method may forecast lead molecules with desired drug-like features and can be used to change or improve a molecule such that it has all desirable lead-like or drug-like properties. As a result, we employed the SwissADME model to forecast the properties of our newly created molecules 9a–h and 14a–h. According to the data gathered from this web tool, all compounds obey the Veber rule (Table 6) with zero violations indicating their drug-likeliness.
Table 6.
SwissADME prediction of physicochemical properties and bioavailability of 9a–9h and 14a–h.
| Compound | Fraction Csp3 | Rotatable Bonds | H-bond Acceptors | H-bond Donors | MR | TPSA | Consensus Log P | ESOL Class | GI Absorption | BBB Permeant | Pgp Substrate | Lipinski Violations | Veber Violations | Bioavailability Score | PAINS Alerts | Brenk Alerts |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9a | 0.04 | 4 | 3 | 0 | 116.23 | 39.94 | 4.97 | Poorly soluble | High | Yes | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9b | 0.08 | 4 | 3 | 0 | 121.19 | 39.94 | 5.29 | Poorly soluble | High | No | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9c | 0.08 | 5 | 4 | 0 | 122.72 | 49.17 | 4.91 | Poorly soluble | High | Yes | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9d | 0.04 | 4 | 4 | 0 | 116.19 | 39.94 | 5.27 | Poorly soluble | High | No | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9e | 0.04 | 4 | 3 | 0 | 121.24 | 39.94 | 5.49 | Poorly soluble | High | No | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9f | 0.04 | 4 | 3 | 0 | 123.93 | 39.94 | 5.57 | Poorly soluble | High | No | Yes | 1 | 0 | 0.55 | 0 | 0 |
| 9g | 0.04 | 5 | 5 | 0 | 125.05 | 85.76 | 4.16 | Poorly soluble | High | No | No | 0 | 0 | 0.55 | 0 | 2 |
| 9h | 0.11 | 6 | 5 | 0 | 129.21 | 58.4 | 4.92 | Poorly soluble | High | No | Yes | 0 | 0 | 0.55 | 0 | 0 |
| 14a | 0.03 | 5 | 4 | 0 | 146.38 | 63.73 | 6.03 | Poorly soluble | Low | No | No | 1 | 0 | 0.55 | 0 | 0 |
| 14b | 0.06 | 5 | 4 | 0 | 151.35 | 63.73 | 6.38 | Poorly soluble | Low | No | No | 1 | 0 | 0.55 | 0 | 0 |
| 14c | 0.06 | 6 | 5 | 0 | 152.87 | 72.96 | 6 | Poorly soluble | Low | No | No | 2 | 0 | 0.17 | 0 | 0 |
| 14d | 0.03 | 5 | 4 | 0 | 151.39 | 63.73 | 6.55 | Poorly soluble | Low | No | No | 2 | 0 | 0.17 | 0 | 0 |
| 14e | 0.03 | 5 | 4 | 0 | 154.08 | 63.73 | 6.63 | Poorly soluble | Low | No | No | 2 | 0 | 0.17 | 0 | 0 |
| 14f | 0.09 | 6 | 4 | 0 | 160.59 | 66.97 | 5.99 | Poorly soluble | Low | No | No | 2 | 0 | 0.17 | 0 | 0 |
| 14g | 0.03 | 5 | 5 | 1 | 148.4 | 83.96 | 5.64 | Poorly soluble | Low | No | No | 1 | 0 | 0.55 | 0 | 0 |
| 14h | 0.09 | 7 | 6 | 0 | 159.36 | 82.19 | 5.94 | Poorly soluble | Low | No | No | 1 | 0 | 0.55 | 0 | 0 |
Compounds 9g and 9h have zero violations of Lipinski’s rule of five, whereas the rest have one violation, with the exception of 14c–f, which has two violations. All compounds had acceptable clogP values in the range of 4.00–6.00. Topological polar surface area (TPSA) is the surface sum of all the polar atoms in a molecule, and the acceptable range is 20–130 Å, with all of our compounds falling inside this range. Except for 9a and 9c, none of the compounds penetrated the blood–brain barrier (BBB). Pgp is an efflux transporter that pushes xenobiotics out of cells, resulting in clearance [31,32]. Except for 9a–f and 9h, all compounds were anticipated to be non-substrates for P-gp. Except for 14c–f compounds, all compounds had a bioavailability score of 0.55. Compounds 9a–h have good GI absorption, whereas compounds 14a–h have poor GI absorption. Except for 9g, which has two brenk alerts, all compounds have zero pain and brenk alerts.
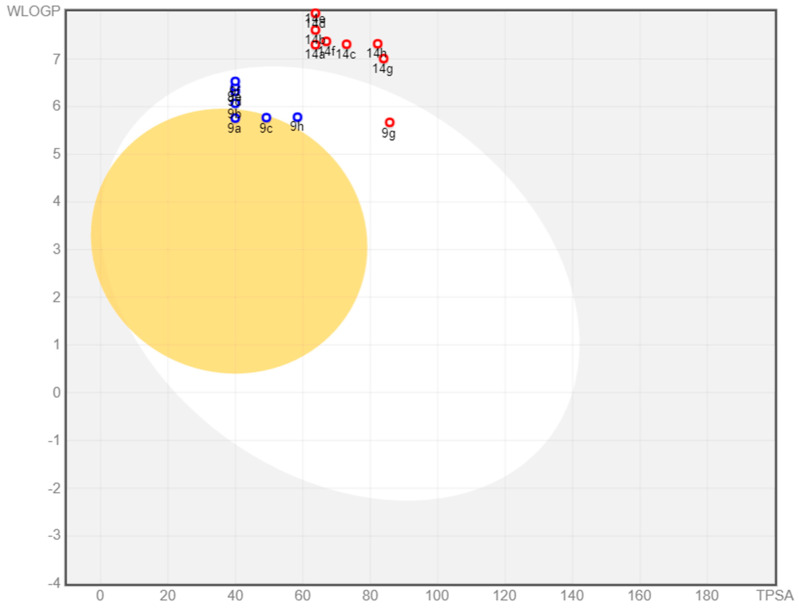
SwissADME provides a BOILED-Egg intrinsic model for predicting BBB entry and passive gastrointestinal absorption (HIA). It is a fantastic strategy that is based on two descriptors: WLOGP (lipophilicity) and TPSA (apparent polarity). The white region of the BOILED-Egg indicates a high likelihood of passive absorption through the gastrointestinal tract, but the yellow part indicates a high likelihood of reaching the brain. P-gp is a multidrug resistance efflux pump that is in charge of drug clearance. The presence or absence of PGP in a drug candidate is shown by blue or red spots in the BOILED-Egg plot.
Figure 12 illustrates the distribution of 9a–h and 14a–h on BOILED-Egg. Only 9a and 9c compounds were found in the yellow zone, indicating that they are BBB penetrant, whereas the remaining compounds are not. The presence of 9a–h compounds in the white region indicated proper absorption, whereas 14a–h compounds were identified outside the white region, indicating inadequate GI absorption. Among all compounds, seven were anticipated to be P-gp substrates (PGP+) (blue dot), while the remaining compounds were not subjected to the active efflux P-gp pump (PGP-) (red dot).
Figure 12.
Spread of 9a–h and 14a–h compounds on the BOILED-Egg plot.
4. Conclusions
Novel two series pyrazolo[3,4-b]pyridine derivatives 9a–h and 14a–h were prepared and assessed for their anti-cancer activity against Hela, MCF7, and HCT-116 cancer cell lines. Compounds 9a and 14g showed the highest anticancer activity, the highest cell cycle arrest, and a significant level of early and late apoptosis. They showed good safety profiles on normal cell line WI-38. They also showed good inhibition activity towards CDK2 and CDK9. Compounds 9a and 14g showed essential interactions in their docking in CDK2 and CDK9 active sites. In the light of these results, the situation definitely calls for additional research to understand the effect of substitutions around pyrazolo[3,4-b]pyridine central moiety and the activity of a such class of compounds.
5. Experimental Section
5.1. Chemistry
2-Bromo-1-(phenyl/4-methoxyphenyl)ethan-1-ones 2a, b [33], 3-(phenyl/4-methoxyphenyl)-3-oxopropanenitriles 3a, b [34], aminopyrazole 4b [35], and enamenones 5a–g [36] were prepared following the reported method. Instruments and thermal analysis results are listed in Supporting Data.
5.1.1. Synthesis of Pyrazolo[3,4-b]pyridine Derivatives 9a–h
A mixture of 3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-5-amine (4b) (0.265 g, 1 mmol) and enamenones 6a–h (1 mmol) in glacial acetic acid (30 mL) was refluxed for 4–6 h. After cooling, the formed precipitate was filtered, washed with EtOH, and crystallized from EtOH/DMF to yield the corresponding pyrazolo[3,4-b]pyridine derivatives 9a–h, respectively.
3-(4-Methoxyphenyl)-1,4-diphenyl-1H-pyrazolo[3,4-b]pyridine (9a)
White powder, 72% yield; mp 161–163 °C; IR νmax/cm−1 1593 (C=N), 1501 (C=C), 1250 (C–O); 1H-NMR δ 3.87 (s, 3H, OMe), 7.16 (d, J = 8.8 Hz, 2H, ArHs), 7.39 (t, J = 7.4 Hz, 1H, ArH), 7.51–7.67 (m, 5H, ArHs), 8.01 (d, J = 8.5 Hz, 1H, H3 of pyridine), 8.09 (d, J = 8.7 Hz, 2H, ArHs), 8.27 (d, J = 7.3 Hz, 2H, ArHs), 8.46 (d, J = 7.2 Hz, 2H, ArHs), 8.70 (d, J = 8.5 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.77 (C of OMe), 113.95, 115.03 (2C), 115.99, 121.05 (2C), 125.01, 126.23, 127.80 (2C), 128.85 (2C), 129.48 (2C), 129.70 (2C), 130.33, 132.65, 138.62, 139.68, 143.92, 151.39, 156.42, 160.38; MS m/z (%) 377.08 (M+,21.80). For C25H19N3O (377.45): Calc.: C, 79.55; H, 5.07; N, 11.13. Found: C, 79.31; H, 5.20; N, 11.40.
3-(4-Methoxyphenyl)-1-phenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine (9b)
White powder, 75% yield; mp 186–188 °C; IR νmax/cm−1 1593 (C=N), 1501 (C=C), 1250 (C–O); 1H-NMR δ 2.41 (s, 3H, Me), 3.87 (s, 3H, OMe), 7.16 (d, J = 8.8 Hz, 2H, ArHs), 7.39 (d, J = 8.0 Hz, 3H, ArHs), 7.64 (t, J = 7.8 Hz, 2H, ArHs), 7.99 (d, J = 8.6 Hz, 1H, H3 of pyridine), 8.09 (d, J = 8.9 Hz, 2H, ArHs), 8.17 (d, J = 8.4 Hz, 2H, ArHs), 8.46 (d, J = 8.6 Hz, 2H, ArHs), 8.69 (d, J = 8.5 Hz, 1H, H2 of pyridine); 13C-NMR δ 21.38 (C of Me), 55.75 (C of OMe), 113.73, 115.01 (2C), 115.70, 120.99 (2C), 125.05, 126.17, 127.68 (2C), 128.82 (2C), 129.68 (2C), 130.07 (2C), 132.50, 135.84, 139.72, 140.07, 143.88, 151.40, 156.42, 160.35; MS m/z (%) 391.47 (M+, 52.80). For C26H21N3O (391.47): Calc.: C, 79.77; H, 5.41; N, 10.73. Found: C, 79.54; H, 5.68; N, 10.98.
3,4-Bis(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9c)
White powder, 66% yield; mp 198–200 °C; IR νmax/cm−1 1600 (C=N), 1501(C=C), 1254 (C–O); 1H-NMR δ 3.855 (s, 3H, OMe), 3.865 (s, 3H, OMe), 7.09–7.18 (m, 4H, ArHs), 7.37 (t, J = 7.4 Hz, 1H, ArH), 7.63 (t, J = 7.8 Hz, 2H, ArHs), 7.94 (d, J = 8.6 Hz, 1H, H3 of pyridine), 8.07 (d, J = 8.6 Hz, 2H, ArHs), 8.23 (d, J = 8.7 Hz, 2H, ArHs), 8.45 (d, J = 8.0 Hz, 2H, ArHs), 8.63 (d, J = 8.5 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.75 (C of OMe), 55.79 (C of OMe), 113.38, 114.86 (2C), 115.01 (2C), 115.34, 120.96 (2C), 125.09, 126.13, 128.81 (2C), 129.24 (2C), 129.67 (2C), 130.98, 132.42, 139.75, 143.88, 151.44, 156.20, 160.33, 161.29; MS m/z (%) 407.33 (M+, 14.18). For C26H21N3O2 (407.47): Calc.: C, 76.64; H, 5.19; N, 10.31. Found: C, 76.86; H, 5.32; N, 10.47.
4-(4-Fluorophenyl)-3-(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9d)
White powder, 74% yield; mp 171–173 °C; IR νmax/cm−1 1597 (C=N), 1505 (C=C), 1250 (C–O), 1227 (C-F); 1H-NMR δ 3.87 (s, 3H, OMe), 7.15 (d, J = 8.7 Hz, 2H, ArHs), 7.40 (q, J = 8.6 Hz, 3H, ArHs), 7.63 (t, J = 7.9 Hz, 2H, ArHs), 8.00 (d, J = 8.5 Hz, 1H, H3 of pyridine), 8.08 (d, J = 8.7 Hz, 2H, ArHs), 8.33 (dd, J = 8.7, 5.7 Hz, 2H, ArHs), 8.43 (d, J = 8.6 Hz, 2H, ArHs), 8.70 (d, J = 8.5 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.77 (C of OMe), 113.86, 115.03 (2C), 115.82, 116.27, 116.49, 121.11 (2C), 124.96, 126.28, 128.86 (2C), 129.71 (2C), 130.02, 130.11, 132.77, 134.76, 139.62, 143.93, 151.28, 155.36, 160.39, 165.03; MS m/z (%) 395.64 (M+, 26.35). For C25H18FN3O (395.44): Calc.: C, 75.93; H, 4.59; N, 10.63. Found: C, 76.09; H, 4.72; N, 10.91.
4-(4-Chlorophenyl)-3-(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9e)
White powder, 72% yield; mp 210–212 °C; IR νmax/cm−1 1599 (C=N), 1501 (C=C), 1254 (C–O); 1H-NMR δ 3.87 (s, 3H, OMe), 7.15 (d, J = 8.4 Hz, 2H, ArHs), 7.39 (t, J = 7.4 Hz, 1H, ArH), 7.63 (dd, J = 8.3, 6.3 Hz,, 4H, ArHs), 8.03 (d, J = 8.5 Hz, 1H, H3 of pyridine), 8.09 (d, J = 8.6 Hz, 2H, ArHs), 8.29 (d, J = 8.3 Hz, 2H, ArHs), 8.42 (d, J = 8.0 Hz, 2H, ArHs), 8.72 (d, J = 8.6 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.78 (C of OMe), 110.10, 115.08, 115.96, 121.18 (2C), 122.66, 124.92, 126.63, 128.89, 129.56 (2C), 129.75 (2C), 135.61, 137.43, 139.56, 144.00, 149.39, 151.27, 152.74, 153.76, 155.17, 160.42, 161.98; MS m/z (%) 412.40 (M++1, 36.04), 411.71 (M+, 53.37). For C25H18ClN3O (411.89): Calc.: C, 72.90; H, 4.41; N, 10.20. Found: C, 73.14; H, 4.60; N, 10.43.
4-(4-Bromophenyl)-3-(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9f)
Pale yellow powder, 65% yield; mp 217–219 °C; IR νmax/cm−1 1596 (C=N), 1501 (C=C), 1254 (C–O), 1227 (C–F); 1H-NMR δ 3.86 (s, 3H, OMe), 7.14 (d, J = 8.2 Hz, 2H, ArHs), 7.38 (t, J = 7.4 Hz, 1H, ArHs), 7.62 (t, J = 7.8 Hz, 2H, ArHs), 7.76 (d, J = 8.1 Hz, 2H, ArHs), 7.99 (d, J = 8.5 Hz, 1H, H3 of pyridine), 8.07 (d, J = 8.2 Hz, 2H, ArHs), 8.19 (d, J = 8.1 Hz, 2H, ArHs), 8.41 (d, J = 8.0 Hz, 2H, ArHs), 8.69 (d, J = 8.5 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.76 (C of OMe), 107.26, 114.15, 115.23 (2C), 115.92, 121.15 (2C), 126.47, 128.85, 129.65, 129.76 (2C), 131.76 (2C), 132.13, 143.93, 139.75, 145.47, 151.24, 154.43, 154.97, 157.59, 162.42, 166.54; MS m/z (%) 456.20 (M+, 23.09). For C25H18BrN3O (456.34): Calc.: C, 65.80; H, 3.98; N, 9.21. Found: C, 65.97; H, 4.06; N, 9.42.
3-(4-Methoxyphenyl)-4-(4-nitrophenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9g)
Orange powder, 68% yield; mp > 300 °C; IR νmax/cm−1 1597 (C=N), 1520 (C-NO2), 1497 (C=C), 1343 (C–NO2), 1250 (C–O); 1H-NMR δ 3.88 (s, 3H, OMe), 7.18 (d, J = 8.3 Hz, 2H, ArHs), 7.42 (s, 1H, ArH), 7.67 (d, J = 8.2 Hz, 3H, ArHs), 8.16–8.43 (m, 7H, 6 ArHs + H3 of pyridine), (d, J = 8.1 Hz, 4H, ArHs), 8.56 (d, J = 8.4 Hz, 2H, H2 of pyridine); MS m/z (%) 422.27 (M+, 43.75). For C25H18NO3 (422.44): Calc.: C, 71.08; H, 4.30; N, 13.26. Found: C, 70.89; H, 4.57; N, 13.47.
4-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-1-phenyl-1H-pyrazolo[3,4-b]pyridine (9h)
White powder, 76% yield; mp 165–167 °C; IR νmax/cm−1 1593 (C=N), 1520, 1505 (C=C), 1250 (C–O–C); 1H-NMR δ 3.86 (s, 3H, OMe), 3.87 (s, 3H, OMe), 3.93 (s, 3H, OMe), 7.14 (dd, J = 8.5, 6.0 Hz, 3H, ArHs), 7.37 (t, J = 7.4 Hz, 1H, ArHs), 7.59–7.67 (m, 2H, ArHs), 7.82–7.91 (m, 2H, ArHs), 8.00 (d, J = 8.6 Hz, 1H, H3 of pyridine), 8.08 (d, J = 8.8 Hz, 2H, ArHs), 8.48 (d, J = 7.4 Hz, 2H, ArHs), 8.63 (d, J = 8.6 Hz, 1H, H2 of pyridine); 13C-NMR δ 55.74 (C of OMe), 55.90 (C of OMe), 56.07 (C of OMe), 110.77, 112.28, 115.01, 115.44 (2C), 120.72, 120.90 (2C), 125.07, 126.14, 128.80 (2C), 129.63 (2C), 131.06, 132.30, 139.69, 143.89, 149.43, 151.02, 151.32, 156.12, 160.32; MS m/z (%) 437.66 (M+, 21.67). For C27H23N3O3 (437.50): Calc.: C, 74.13; H, 5.30; N, 9.60. Found: C, 74.40; H, 5.42; N, 9.78.
5.1.2. Synthesis of Pyrazolo[3,4-b]pyridine Derivatives 14a–g
A mixture of aminopyrazole 4b (0.265 g, 1 mmol), propanenitrile 3a (0.145 g, 1 mmol), and the appropriate aromatic aldehydes 10a–g (1 mmol) in absolute EtOH (30 mL) was refluxed for 6–10 h in the presence of TEA (catalytic). The formed precipitated was filtered, washed with EtOH, and crystallized from EtOH/DMF to yield pyrazolo[3,4-b]pyridine derivatives 14a–h, respectively.
3-(4-Methoxyphenyl)-1,4,6-triphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14a)
White powder, 74% yield; mp 229–231 °C; IR νmax/cm−1 2218 (C≡N), 1610 (C=N), 1559, 1497 (C=C), 1250 (C–O); 1H-NMR δ 3.72 (s, 3H, OMe), 6.65 (d, J = 8.7 Hz, 2H, ArHs), 7.03 (d, J = 8.6 Hz, 2H), 7.32 (t, J = 7.5 Hz, 2H, ArHs), 7.39–7.47 (m, 4H, ArHs), 7.60–7.66 (m, 5H, ArHs), 8.00–8.02 (m, 2H, ArHs), 8.29 (d, J = 7.4 Hz, 2H); 13C-NMR δ 55.61 (C of OMe), 102.37, 112.26, 113.54 (2C), 118.02, 122.04 (2C), 123.87, 127.35, 128.50 (2C), 128.99 (2C), 129.78 (2C), 129.85 (2C), 129.94 (2C), 130.07, 130.54 (2C), 130.70, 133.93, 138.10, 138.66, 147.06, 150.28, 153.02, 159.66, 160.76; MS m/z (%) 478.58 (M+, 15.55). For C32H22N4O (478.56): Calc.: C, 80.32; H, 4.63; N, 11.71. Found: C, 80.09; H, 4.74; N, 11.94.
3-(4-Methoxyphenyl)-1,6-diphenyl-4-(p-tolyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14b)
White powder, 68% yield; mp 203–205 °C; IR νmax/cm−1 2218 (C≡N), 1609, (C=N), 1559, 1509 (C=C), 1250 (C–O); 1H-NMR δ 2.34 (s, 3H, Me), 3.72 (s, 3H, OMe), 6.64 (d, J = 8.7 Hz, 2H, ArHs), 7.00 (d, J = 8.7 Hz, 2H, ArHs), 7.10 (d, J = 7.9 Hz, 2H, ArHs), 7.25 (d, J = 8.1 Hz, 2H, ArHs), 7.42 (t, J = 7.5 Hz, 1H, ArHs), 7.57–7.65 (m, 5H, ArHs), 7.99–8.01 (m, 2H, ArHs), 8.28 (d, J = 8.5 Hz, 2H, ArHs); 13C-NMR δ 21.32 (C of Me), 55.64 (C of OMe), 102.28, 112.39, 113.46 (2C), 118.11, 122.05 (2C), 123.91, 127.34, 128.97 (2C), 129.79 (2C), 129.92 (2C), 130.58 (2C), 130.67, 131.04, 138.13, 138.67, 139.85, 147.10, 150.27, 153.19, 159.70, 160.79; MS m/z (%) 492.77 (M+, 58.66). For C33H24N4O (492.58): Calc.: C, 80.47; H, 4.91; N, 11.37. Found: C, 80.68; H, 4.85; N, 11.53.
3,4-Bis(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14c)
White powder, 65% yield; mp 207–209 °C; IR νmax/cm−1 2218 (C≡N), 1609 (C=N), 1551, 1509 (C=C), 1250 (C–O); 1H-NMR δ 3.74 (s, 3H, OMe), 3.78 (s, 3H, OMe), 6.69 (d, J = 8.7 Hz, 2H, ArHs), 6.84 (d, J = 8.7 Hz, 2H, ArHs), 7.04 (d, J = 8.7 Hz, 2H, ArHs), 7.31 (d, J = 8.6 Hz, 2H, ArHs), 7.43 (t, J = 7.4 Hz, 1H, ArHs), 7.58–7.68 (m, 5H, ArHs), 7.99 - 8.02 (m, 2H, ArHs), 8.29 (d, J = 8.5 Hz, 2H, ArHs); 13C-NMR δ 55.66 (C of OMe), 55.83 (C of OMe), 102.36, 112.51, 113.58 (2C), 113.97 (2C), 118.26, 122.09 (2C), 124.06, 125.96, 127.35, 128.98 (2C), 129.82 (2C), 129.95 (2C), 130.66 (3C), 131.56 (2C), 138.23, 138.73, 147.22, 150.33, 152.99, 159.70, 160.87, 160.94; MS m/z (%) 508.37 (M+, 24.90). For C33H24N4O2 (508.58): Calc.: C, 77.94; H, 4.76; N, 11.02. Found: C, 78.12; H, 4.92; N, 11.24.
4-(4-Chlorophenyl)-3-(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14d)
White powder, 70% yield; mp 258–260 °C; IR νmax/cm−1 2226 (C≡N), 1610 (C=N), 1493 (C=C), 1250 (C–O); 1H-NMR δ 3.74 (s, 3H, OMe), 6.69 (d, J = 6.5 Hz, 2H, ArHs), 7.02 (d, J = 6.4 Hz, 2H, ArHs), 7.45–7.32 (m, 5H, ArHs) 7.59–7.63 (m, 5H, ArHs), 8.01 (d, J = 6.7, Hz, 2H, ArHs), 8.28 (d, J = 8.2 Hz, 2H, ArHs); 13C-NMR δ 55.71 (C of OMe), 112.77, 113.61 (2C), 115.61, 117.88, 122.07 (2C), 123.73, 127.44, 128.48 (2C), 129.04 (3C), 129.85 (3C), 129.93 (2C), 130.72 (2C), 130.77 (2C), 131.75 (2C), 132.69, 135.16, 138.04, 138.65, 150.23, 159.87; MS m/z (%) 514.87, (M++1, 19.95), 513.02 (M+, 46.40). For C32H21ClN4O (513.00): Calc.: C, 74.92; H, 4.13; N, 10.92. Found: C, 74.81; H, 4.29; N, 11.15.
4-(4-Bromophenyl)-3-(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14e)
White powder, 82% yield; mp 221–223 °C; IR νmax/cm−1 2226 (C≡N), 1609 (C=N), 1524, 1489 (C=C), 1250 (C-O); 1H-NMR δ 3.76 (s, 3H, OMe), 6.70 (d, J = 8.7 Hz, 2H, ArHs), 7.02 (d, J = 8.7 Hz, 2H, ArHs), 7.32 (d, J = 8.4 Hz, 2H, ArHs), 7.42 (t, J = 7.4 Hz, 1H, ArHs), 7.50 (d, J = 8.5 Hz, 2H, ArHs), 7.58–7.69 (m, 5H, ArHs), 8.00–8.02 (m, 2H, ArHs), 8.29 (d, J = 7.3 Hz, 2H, ArHs); 13C-NMR δ 55.68 (C of OMe), 102.07, 112.40, 113.55 (2C), 117.87, 121.93 (2C), 123.63, 123.85, 127.37, 129.03 (2C), 129.80 (2C), 129.90 (2C), 130.67 (2C), 130.78, 131.38 (2C), 131.90 (2C), 132.97, 137.96, 138.60, 146.98, 150.18, 151.70, 159.81, 160.70; MS m/z (%) 559.62 (M++2, 7.68), 557.96 (M+, 25.99). For C32H21BrN4O (557.45): Calc.: C, 68.95; H, 3.80; N, 10.05. Found: C, 69.07; H, 3.94; N, 10.21.
4-(4-(Dimethylamino)phenyl)-3-(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14f)
Pale yellow powder, 63% yield; mp 230–232 °C; IR νmax/cm−1 2218 (C≡N), 1613 (C=N), 1524, 1489 (C=C), 1250 (C–O); 1H-NMR δ 2.93 (s, 6H, 2NMe), 3.74 (s, 3H, OMe), 6.55 (d, J = 8.9 Hz, 2H, ArHs), 6.68 (d, J = 8.8 Hz, 2H, ArHs), 7.05 (d, J = 8.7 Hz, 2H, ArHs), 7.17 (d, J = 8.8 Hz, 2H, ArHs), 7.42 (t, J = 7.5 Hz, 1H, ArHs), 7.57–7.66 (m, 5H, ArHs), 7.98–8.01 (m, 2H, ArHs), 8.29 (d, J = 7.3 Hz, 2H); 13C-NMR δ 40.31 (2C of 2Me), 55.60 (C of OMe), 101.81, 111.59 (2C), 112.28, 113.57 (2C), 118.69, 120.52, 122.08, 124.36, 127.26, 128.92 (2C), 129.78 (2C), 129.95 (2C), 130.54, 130.67 (2C), 131.22 (2C), 138.42, 138.81, 147.39, 150.45, 151.75, 153.68, 159.56, 161.08; MS m/z (%) 521.28 (M+, 19.10). For C34H27N5O (521.62): Calc.: C, 78.29; H, 5.22; N, 13.43. Found: C, 78.43; H, 5.46; N, 13.67.
4-(4-Hydroxyphenyl)-3-(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14g)
White powder, 77% yield; mp 295–297 °C; IR νmax/cm−1 2222 (C≡N), 1613 (C=N), 1555, 1512 (C=C), 1250 (C–O); 1H-NMR δ 3.74 (s, 3H, OMe), 6.68 (dd, J = 13.4, 8.7 Hz, 4H, ArHs), 7.06 (d, J = 8.7 Hz, 2H, ArHs), 7.18 (d, J = 8.6 Hz, 2H, ArHs), 7.41 (t, J = 7.4 Hz, 1H, ArH), 7.63–7.57 (m, 5H, ArHs), 8.02–7.97 (m, 2H, ArHs), 8.28 (dd, J = 8.7, 1.2 Hz, 2H, ArHs), 9.90 (s, D2O exchangeable, 1H, OH); 13C-NMR δ 55.68 (C of OMe), 102.30, 112.32, 113.57 (2C), 115.25 (2C), 118.38, 122.05, 124.19, 124.34, 127.28, 128.94 (2C), 129.77 (2C), 129.94 (2C), 130.60, 130.71 (2C), 131.65 (2C), 138.28, 138.75, 147.23, 150.39, 153.39, 159.44, 159.70, 160.92; MS m/z (%) 494.63 (M+, 13.48). For C32H22N4O2 (494.55): Calc.: C, 77.72; H, 4.48; N, 11.33. Found: C, 77.50; H, 4.52; N, 11.50.
4-(3,4-Dimethoxyphenyl)-3-(4-methoxyphenyl)-1,6-diphenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (14h)
White powder, 69% yield; mp 212–214 °C; IR νmax/cm−1 2222 (C≡N), 1609 (C=N), 1555, 1505 (C=C), 1258 (C–O); 1H-NMR δ 3.37 (s, 3H, OMe), 3.73 (s, 3H, OMe), 3.82 (s, 3H, OMe), 6.70 (d, J = 8.4 Hz, 2H, ArHs), 6.77 (d, J = 2.1 Hz, 1H, ArHs), 7.03 (dd, J = 14.1, 8.3 Hz, 3H, ArHs), 7.15 (dd, J = 8.2, 2.0 Hz, 1H, ArHs), 7.42 (t, J = 7.4 Hz, 1H, ArHs), 7.58–7.66 (m, 5H, ArHs), 7.99–8.02 (m, 2H, ArHs), 8.28 (d, J = 8.0 Hz, 2H, ArHs); 13C-NMR δ 55.60 (C of OMe), 55.73 (C of OMe), 56.22 (C of OMe), 102.24, 111.90, 112.33, 113.52 (2C), 114.48, 118.36, 122.13 (2C), 122.83, 124.36, 126.01, 127.36, 128.97 (2C), 129.81 (2C), 129.95 (2C), 130.64, 130.68 (2C), 138.25, 138.72, 147.18, 148.68, 150.35, 150.68, 152.93, 159.78, 160.94; MS m/z (%) 538.16 (M+, 27.45). For C34H26N4O3 (538.61): Calc.: C, 75.82; H, 4.87; N, 10.40. Found: C, 75.96; H, 4.98; N, 10.68.
5.2. Biological Screening
5.2.1. MTT Assay for Cytotoxicity
Standard MTT colorimetric assay was applied to test the IC50 of compounds 9a–h and 14a–h against three cell lines, namely, cervical cancer Hela, breast cancer MCF7, and colon HCT-116, compared with doxorubicin as a reference compound with the typical reported methodology of MTT colorimetric assay [37].
5.2.2. Cell Cycle Analysis and Apoptotic Assay
Cell cycle analysis and apoptosis study of the 2 most active compounds, 9a and 14g, was accomplished using propidium iodide (PI) flow cytomertric analysis according to the reported procedure [38,39]. An apoptotic study using the Annexin V-FITC Apoptosis Detection Kit (K101–25, BioVision®, 980 Linda Vista Avenue, Mountain View, CA, USA) at their IC50 concentration values was used on the specified cell after 24 h in three successive steps according to the manufacturer’s instructions.
5.2.3. In Vitro CDK Enzyme Assay
The CDK in vitro inhibitory activity of 9a–h and 14a–h toward CDK2 and CDK9 was performed using CDK Assay Kit (BPS Bioscience, USA) at different dilutions of 30, 10, 3, 1, 0.3, 0.1, 0.03, and 0.01 µM, measuring the chemiluminescence using BioTek™ Synergy2 Microplate Reader (BioTek, Santa Clara, CA, USA) [40].
5.3. Molecular Docking
Docking studies of compounds 9a and 14g were performed using Molecular Operating Environment (MOE 2019.02) software [41,42,43], and the crystal structures of CDK2 and CDK9 in complex with CAN508 were downloaded from the protein databank PDB IDs 3tnw and 3tn8, respectively.
5.4. In-Silico SwissADME Predictions
SwissADME is a reliable, free online utility [44] for calculating the physicochemical properties of compounds. By uploading the structures to the website http://www.swissadme.ch/, accessed on 10 March 2023, the bioavailability and pharmacokinetic parameters of any synthetic compound can be determined. The smiles of 9a–9h and 14a–14h structures were imported into the SwissADME environment. Different physiological and pharmacokinetic parameter measurements were exported as a CSV file, from which data were extracted and analyzed.
Acknowledgments
The authors acknowledge the support from National Institute for Health Research under project No. SNIH-RO-HRT01-2302-KSU-36598045, Riyadh, Saudi Arabia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176428/s1, Figure S1−S64: NMR spectra; Figure S65−S80: IR spectra; Figure S81−S94: Mass spectra.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, F.A.B., A.A.-M.A.-A. and S.T.A.-R.; investigation, B.S.A.; resources, data curation, writing—original draft preparation, writing—review and editing, visualization, B.S.A., F.A.B., A.A.-M.A.-A. and S.T.A.-R.; supervision, project administration, F.A.B., A.A.-M.A.-A. and S.T.A.-R.; funding acquisition, S.T.A.-R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This research was funded by National Institute for Health Research, grant number SNIH-RO-HRT01-2302-KSU-36598045, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Balter M., Vogel G. Cycling toward Stockholm. Science. 2001;294:502–503. doi: 10.1126/science.294.5542.502. [DOI] [PubMed] [Google Scholar]
- 3.Roskoski R., Jr. Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol. Res. 2016;107:249–275. doi: 10.1016/j.phrs.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Malumbres M., Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Yin T., Lallena M.J., Kreklau E.L., Fales K.R., Carballares S., Torrres R., Wishart G.N., Ajamie R.T., Cronier D.M., Iversen P.W. A novel CDK9 inhibitor shows potent antitumor efficacy in preclinical hematologic tumor models. Mol. Cancer Ther. 2014;13:1442–1456. doi: 10.1158/1535-7163.MCT-13-0849. [DOI] [PubMed] [Google Scholar]
- 6.Jessen B.A., Lee L., Koudriakova T., Haines M., Lundgren K., Price S., Nonomiya J., Lewis C., Stevens G.J. Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. J. Appl. Toxicol. Int. J. 2007;27:133–142. doi: 10.1002/jat.1177. [DOI] [PubMed] [Google Scholar]
- 7.Abate A.A., Pentimalli F., Esposito L., Giordano A. ATP-noncompetitive CDK inhibitors for cancer therapy: An overview. Exp. Opin. Investig. Drugs. 2013;22:895–906. doi: 10.1517/13543784.2013.798641. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Martinez C., Gelbert L.M., Lallena M.J., de Dios A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg. Med. Chem. Lett. 2015;25:3420–3435. doi: 10.1016/j.bmcl.2015.05.100. [DOI] [PubMed] [Google Scholar]
- 9.Awan F.T., Jones J.A., Maddocks K., Poi M., Grever M.R., Johnson A., Byrd J.C., Andritsos L.A. A phase 1 clinical trial of flavopiridol consolidation in chronic lymphocytic leukemia patients following chemoimmunotherapy. Ann. Hematol. 2016;95:1137–1143. doi: 10.1007/s00277-016-2683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidner J.F., Foster M.C., Blackford A.L., Litzow M.R., Morris L.E., Strickland S.A., Lancet J.E., Bose P., Levy M.Y., Tibes R. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100:1172. doi: 10.3324/haematol.2015.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicenas J., Kalyan K., Sorokinas A., Stankunas E., Levy J., Meskinyte I., Stankevicius V., Kaupinis A., Valius M. Roscovitine in cancer and other diseases. Ann. Trans. Med. 2015;3:135. doi: 10.3978/j.issn.2305-5839.2015.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meijer L., Borgne A., Mulner O., Chong J.P., Blow J.J., Inagaki N., Inagaki M., Delcros J.G., Moulinoux J.P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 13.Saqub H., Proetsch-Gugerbauer H., Bezrookove V., Nosrati M., Vaquero E.M., de Semir D., Ice R.J., McAllister S., Soroceanu L., Kashani-Sabet M., et al. Dinaciclib, a cyclin-dependent kinase inhibitor, suppresses cholangiocarcinoma growth by targeting CDK2/5/9. Sci. Rep. 2020;10:18489. doi: 10.1038/s41598-020-75578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paruch K., Dwyer M.P., Alvarez C., Brown C., Chan T.Y., Doll R.J., Keertikar K., Knutson C., McKittrick B., Rivera J., et al. Discovery of Dinaciclib (SCH 727965): A Potent and Selective Inhibitor of Cyclin-Dependent Kinases. ACS Med. Chem. Lett. 2010;1:204–208. doi: 10.1021/ml100051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennani F.E., Doudach L., Cherrah Y., Ramli Y., Karrouchi K., Ansar M., Faouzi M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020;97:103470. doi: 10.1016/j.bioorg.2019.103470. [DOI] [PubMed] [Google Scholar]
- 16.Palomer A., Cabré F., Pascual J., Campos J., Trujillo M.A., Entrena A., Gallo M.A., García L., Mauleón D., Espinosa A. Identification of novel cyclooxygenase-2 selective inhibitors using pharmacophore models. J. Med. Chem. 2002;45:1402–1411. doi: 10.1021/jm010458r. [DOI] [PubMed] [Google Scholar]
- 17.Kulp S.K., Yang Y.-T., Hung C.-C., Chen K.-F., Lai J.-P., Tseng P.-H., Fowble J.W., Ward P.J., Chen C.-S. 3-phosphoinositide-dependent protein kinase-1/Akt signaling represents a major cyclooxygenase-2-independent target for celecoxib in prostate cancer cells. Cancer Res. 2004;64:1444–1451. doi: 10.1158/0008-5472.CAN-03-2396. [DOI] [PubMed] [Google Scholar]
- 18.Staben S.T., Heffron T.P., Sutherlin D.P., Bhat S.R., Castanedo G.M., Chuckowree I.S., Dotson J., Folkes A.J., Friedman L.S., Lee L. Structure-based optimization of pyrazolo-pyrimidine and-pyridine inhibitors of PI3-kinase. Bioorg. Med. Chem. Lett. 2010;20:6048–6051. doi: 10.1016/j.bmcl.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 19.Chiacchio M.A., Iannazzo D., Romeo R., Giofrè S.V., Legnani L. Pyridine and pyrimidine derivatives as privileged scaffolds in biologically active agents. Curr. Med. Chem. 2019;26:7166–7195. doi: 10.2174/0929867325666180904125400. [DOI] [PubMed] [Google Scholar]
- 20.Tadesse S., Caldon E.C., Tilley W., Wang S. Cyclin-Dependent Kinase 2 Inhibitors in Cancer Therapy: An Update. J. Med. Chem. 2019;62:4233–4251. doi: 10.1021/acs.jmedchem.8b01469. [DOI] [PubMed] [Google Scholar]
- 21.Donaire-Arias A., Montagut A.M., de la Bellacasa R.P., Estrada-Tejedor R., Teixidó J., Borrell J.I. 1H-Pyrazolo[3,4-b]pyridines: Synthesis and Biomedical Applications. Molecules. 2022;27:2237. doi: 10.3390/molecules27072237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farahat A.A., Samir E.M., Zaki M.Y., Serya R.A., Abdel-Aziz H.A. Synthesis and in vitro antiproliferative activity of certain novel pyrazolo[3,4-b]pyridines with poten- tial p38α MAPK-inhibitory activity. Archiv der Pharmazie. 2022;355:2100302. doi: 10.1002/ardp.202100302. [DOI] [PubMed] [Google Scholar]
- 23.Hassan G.S., Georgey H.H., Mohammed E.Z., George R.F., Mahmoud W.R., Omar F.A. Mechanistic selectivity investigation and 2D-QSAR study of some new antipro- liferative pyrazoles and pyrazolopyridines as potential CDK2 inhibitors. Eur. J. Med. Chem. 2021;218:113389. doi: 10.1016/j.ejmech.2021.113389. [DOI] [PubMed] [Google Scholar]
- 24.Elewa M.A.F., Eldehna W.M., Hamdan A.M.E., Abd El-kawi S.H., El-Kalaawy A.M., Majrashi T.A., Barghash R.F., Abdel-Aziz H.A., Hashem K.S., Al-Gayyar M.M.H. WRH-2412 alleviates the progression of hepatocellular carcinoma through regulation of TGF-β/β-catenin/α-SMA pathway. J. Enz. Inhib. Med. Chem. 2023;38:2185761. doi: 10.1080/14756366.2023.2185761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barghash R.F., Eldehna W.M., Kovalová M., Vojáčková V., Kryštof V., Abdel- Aziz H.A. One-pot three-component synthesis of novel pyrazolo[3,4-b]pyridines as po- tent antileukemic agents. Eur. J. Med. Chem. 2022;227:113952. doi: 10.1016/j.ejmech.2021.113952. [DOI] [PubMed] [Google Scholar]
- 26.Li Y., Zhang J., Gao W., Zhang L., Pan Y., Zhang S., Wang Y. Insights on structural characteristics and ligand binding mechanisms of CDK2. Int. J. Mol. Sci. 2015;16:9314–9340. doi: 10.3390/ijms16059314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Łukasik P., Baranowska-Bosiacka I., Kulczycka K., Gutowska I. Inhibitors of cyclin-dependent kinases: Types and their mechanism of action. Int. J. Mol. Sci. 2021;22:2806. doi: 10.3390/ijms22062806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramalho T.C., Rocha M.V., da Cunha E.F., Oliveira L.C., Carvalho K.T. Understanding the molecular behavior of organotin compounds to design their effective use as agrochemicals: Exploration via quantum chemistry and experiments. J. Biomol. Struct. Dyn. 2010;28:227–238. doi: 10.1080/07391102.2010.10507355. [DOI] [PubMed] [Google Scholar]
- 29.Hammoud M.M., Khattab M., Abdel-Motaal M., Van der Eycken J., Alnajjar R., Abulkhair H.S., Al-Karmalawy A.A. Synthesis, structural characterization, DFT calculations, molecular docking, and molecular dynamics simulations of a novel ferrocene derivative to unravel its potential antitumor activity. J. Biomol. Struct. Dyn. 2023;41:5199–5216. doi: 10.1080/07391102.2022.2082533. [DOI] [PubMed] [Google Scholar]
- 30.de Lima W.E.A., Pereira A.F., de Castro A.A., da Cunha E.F.F., Ramalho T.C. Flexibility in the molecular design of acetylcholinesterase reactivators: Probing representative conformations by chemometric techniques and docking/QM calculations. Lett. Drug Des. Discov. 2016;13:360–371. doi: 10.2174/1570180812666150918191550. [DOI] [Google Scholar]
- 31.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daina A., Zoete V. A boiled-egg to predict gastrointestinal absorption and brain penetration of smallmolecules. Chem. MedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuriakose D., Thumpakara R.K., Jesna A., Jacob J.P. Substituent effects in the formation of a few acenaphthenone-2-ylidene ketones and their molecular docking studies and in silico ADME profile. J. Mol. Struct. 2021;1224:129209. doi: 10.1016/j.molstruc.2020.129209. [DOI] [Google Scholar]
- 34.Yoon S.H., Kim S.J., Kim I. One-Pot Four-Component Coupling Approach to Polyheterocycles: 6H-Furo[3,2-f]pyrrolo[1,2-d][1,4]diazepine. J. Org. Chem. 2020;85:15082–15091. doi: 10.1021/acs.joc.0c01971. [DOI] [PubMed] [Google Scholar]
- 35.Zeng L.Y., Liu T., Yang J., Yang Y., Cai C., Liu S. “On-Water” Facile Synthesis of Novel Pyrazolo[3,4-b]pyridinones Possessing Anti-influenza Virus Activity. ACS Comb. Sci. 2017;19:437–446. doi: 10.1021/acscombsci.7b00016. [DOI] [PubMed] [Google Scholar]
- 36.Kantevari S., Chary M.V., Vuppalapati S.V.N. A highly efficient regioselective one-pot synthesis of 2,3,6-trisubstituted pyridines and 2,7,7-trisubstituted tetrahydroquinolin-5-ones using K5CoW12O40·3H2O as a heterogeneous recyclable catalyst. Tetrahedron. 2007;63:13024–13031. doi: 10.1016/j.tet.2007.10.014. [DOI] [Google Scholar]
- 37.Edmondson J.M., Armstrong L.S., Martinez A.O. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J. Tissue Cult. Methods. 1988;11:15–17. doi: 10.1007/BF01404408. [DOI] [Google Scholar]
- 38.Pozarowski P., Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 2004;281:301. doi: 10.1385/1-59259-811-0:301. [DOI] [PubMed] [Google Scholar]
- 39.Crowley L.C., Scott A.P., Marfell B.J., Boughaba J.A., Chojnowski G., Waterhouse N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016;2016:647. doi: 10.1101/pdb.prot087163. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Gan Y., Li H., Yin J., He X., Lin L., Xu S., Fang Z., Kim B.W., Gao L., et al. Inhibition of the CDK2 and Cyclin A complex leads to autophagic degradation of CDK2 in cancer cells. Nat. Commun. 2022;13:2835. doi: 10.1038/s41467-022-30264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilar S., Cozza G., Moro S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008;8:1555–1572. doi: 10.2174/156802608786786624. [DOI] [PubMed] [Google Scholar]
- 42.Scholz C., Knorr S., Hamacher K., Schmidt B. DOCKTITE, A Highly Versatile Step-by-Step Workflow for Covalent Docking and Virtual Screening in the Molecular Operating Environment. J. Chem. Inf. Model. 2015;55:398–406. doi: 10.1021/ci500681r. [DOI] [PubMed] [Google Scholar]
- 43.Abdelsalam E.A., Abd El-Hafeez A.A., Eldehna W.M., El Hassab M.A., Marzouk H.M.M., Elaasser M.M., Abou Taleb N.A., Amin K.M., Abdel-Aziz H.A., Ghosh P., et al. Discovery of novel thiazolyl-pyrazolines as dual EGFR and VEGFR-2 inhibitors endowed with in vitro antitumor activity towards non-small lung cancer. J. Enzym. Inhib. Med. Chem. 2022;37:2265–2282. doi: 10.1080/14756366.2022.2104841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SwissADME. [(accessed on 10 March 2023)]. Available online: http://www.swissadme.ch/index.php#.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.