Abstract
Both enteropathogenic Escherichia coli (EPEC) and an obligate intracellular bacterium, previously referred to as an intracellular Campylobacter-like organism and now designated Lawsonia intracellularis, have been reported as causes of enterocolitis in rabbits. An outbreak of enterocolitis in a group of rabbits, characterized by an unusually high rate of mortality, was found to be associated with dual infection with EPEC and L. intracellularis. The EPEC strain was found to have eaeA gene homology but was negative for afrA homology. The absence of the afrA gene, which encodes the structural subunit for the AF/R1 pilus, indicates that this rabbit EPEC strain is distinct from the prototypic RDEC-1 strain. This finding suggests that rabbit EPEC strains widely reported in Western Europe, which lack AF/R1 pili, are also present in rabbits in the United States. Dual infection with these two pathogens in rabbits has not been previously reported and may have contributed to the unusually high mortality observed in this outbreak.
Enterocolitis is a significant cause of morbidity and mortality in rabbits, and infection with Escherichia coli is perhaps the most important cause of rabbit enterocolitis. The severity of disease presentation in colibacillosis is variable and is dependent on the particular strain of E. coli (19) as well as on the presence of concurrent infectious agents (25). In cases of severe morbidity and mortality, treatment is often not effective and entire rabbit herds must be depopulated and restocked. Therefore, a better understanding of the factors which control disease expression in rabbit colibacillosis is needed. The purpose of this study was to ascertain the etiology of an outbreak of enterocolitis in a group of rabbits, characterized by an unusually high rate of mortality.
The prototypic diarrheagenic E. coli strain of rabbits, designated RDEC-1, was isolated 20 years ago from an outbreak of diarrhea in a rabbit colony (3). This strain is considered an attaching and effacing E. coli (AEEC) strain because it causes attaching and effacing (AE) lesions (24). These lesions are characterized by intimate bacterial adherence to the apical cytoplasmic membrane of intestinal epithelial cells, often with pedestal or cup formation, destruction of the microvillus brush border, and rearrangement of the enterocyte cytoskeleton at the site of bacterial attachment (18). AEEC strains include both enteropathogenic E. coli (EPEC) and certain strains of enterohemorrhagic E. coli (EHEC). EPEC strains do not express the high levels of Shiga toxin which are characteristic of EHEC strains, but all AEEC strains, as well as certain Hafnia alvei strains (1) and Citrobacter rodentium strains (22), appear to have a common 35-kb pathogenicity island located on the chromosome (13), which encodes all of the gene products necessary for production of AE lesions (14). This pathogenicity island is called the locus for enterocyte effacement (LEE). The presence of the LEE-encoded AE genes, and of eaeA in particular, has been used to identify AEEC.
In addition to the AE gene products of the LEE, RDEC-1 expresses plasmid-encoded AF/R1 pili which promote adherence to rabbit intestinal epithelial cells (27). The structural subunit of the AF/R1 pilus is encoded by the afrA gene (28). Although most, if not all, EPEC strains from rabbits have AE genes, including eaeA, few, if any, rabbit EPEC isolates from Western Europe express AF/R1 pili (2, 20). Indeed, a distinct fimbrial adhesin, called AF/R2, has been identified in some of these rabbit EPEC strains (4). The plasmid pRKB35, which is pUC18 containing a 400-bp KpnI fragment from the afrA gene, provides a convenient gene probe for the identification of the RDEC-1 rabbit EPEC strain.
Intracellular Campylobacter-like organisms (ICLOs) have been most frequently recognized in pigs (21) and in hamsters (26), but they have also been reported in other animal species, including rabbits (10, 17, 23) and ferrets (6, 7). These intracellular organisms are gram-negative, curved- to spiral-shaped bacteria which exist free in the apical cytoplasm of intestinal epithelial cells. They cause proliferative ileitis and/or proliferative colitis characterized by epithelial cell hyperplasia and mucosal inflammation. Although the ICLOs have not been cultured on cell-free media, nucleotide sequence determination of the 16S rRNA gene of ICLOs isolated from pigs (15) and from ferrets (5) has shown that the organisms are related to Desulfovibrio desulfuricans but are sufficiently distinct from the Desulfovibrio spp. to warrant placing them in a separate genus, where they comprise a single species called Lawsonia intracellularis (15).
MATERIALS AND METHODS
Animals.
In late 1995, a closed, Pasteurella-free production colony of barn-housed New Zealand White rabbits experienced an outbreak of diarrhea associated with approximately 70% mortality in which over 400 animals died. Clinical signs of disease included acute diarrhea, dehydration, and weight loss in animals from 2 to 4 months of age. Prior to this outbreak, fecal flotation revealed the presence of coccidia in the colony but the rabbits did not have clinical signs of disease and did not suffer significant mortality.
Histologic examination.
Segments of visibly thickened ileum were collected at necropsy from four affected rabbits and were fixed in 10% neutral-buffered formalin. Fixed tissues were processed, paraffin embedded, and sectioned routinely at 5 μm. Sections were stained with hematoxylin and eosin or with a modified Warthin-Starry silver impregnation stain.
Bacterial culture and isolation.
Samples of ileal contents from the four affected rabbits were plated on hektoen enteric agar and on xylose-lysine-deoxycholate agar for enteric pathogens, on cefoxitin-cycloserine-fructose modified agar for Clostridium spp., and on brucella agar supplemented with 5% defibrinated sheep erythrocytes and with cefoperazone (20 mg/liter), vancomycin (10 mg/liter), and amphotericin B (2 mg/liter) (Campy CVA agar; Becton Dickinson Microbiology Systems, Cockeysville, Md.) for Campylobacter spp. and for Helicobacter spp. Enteric media were incubated at 37°C in ambient O2, media for Clostridium spp. were incubated at 37°C in an anaerobic atmosphere (Anaerogen; Oxoid Ltd., Basingstoke, England), and media for Campylobacter spp. and Helicobacter spp. were incubated at 37°C in a microaerobic atmosphere (90% N2, 5% CO2, and 5% H2). E. coli strains were biotyped with a commercial identification system (API 20E; bioMérieux Vitek Inc., St. Louis, Mo.).
DNA isolation.
DNA was isolated from E. coli using Qiagen genomic tips according to the recommendations of the manufacturer (Qiagen Inc., Chatsworth, Calif.).
Southern blot analysis.
Southern blot analysis for E. coli virulence factors was performed with an ECL Direct Kit (Amersham Corp., Arlington Heights, Ill.). Briefly, genomic DNA was digested with HindIII (New England Biolabs, Beverly, Mass.) and separated by electrophoresis. The DNA was then transferred to a nylon membrane by the capillary transfer method and was UV cross-linked. The probe for the eaeA gene was a 1-kb internal restriction fragment from the eaeA gene of a human EPEC strain (12). The probe for the afrA gene, a gift from J. Robert Canty, was a 400-bp internal restriction fragment from the rabbit EPEC strain RDEC-1 (28). These fragments were labeled by using an ECL Direct Kit (Amersham) according to the recommendations of the manufacturer. After hybridization with the probe overnight at 42°C, the membranes were washed twice for 20 min at 42°C in 6 M urea–0.4% sodium dodecyl sulfate–0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). For low-stringency washes, the final concentration of SSC in the primary wash solution was increased from 0.1× to 2×. These washes were followed by two additional 5-min washes at room temperature in 20× SSC. The detection reagents included with the kit were used as directed, and positive signals were detected by exposure to radiographic film (Hyperfilm-ECL; Amersham).
Clostridial toxin assay.
Fifty microliters of fecal filtrate from each intestinal sample was analyzed for Clostridium difficile toxin by using a C. difficile toxin B human foreskin tissue culture assay as outlined by the manufacturer (TechLab, Blacksburg, Va.). The cells were observed for rounding, which indicates a cytotoxic effect, at 24 and at 48 h.
RESULTS AND DISCUSSION
Gross necropsy.
Upon gross examination, the affected animals had thickening of the ileum and the colon as well as mesenteric lymphadenopathy. No other abnormalities were noted.
Histologic examination.
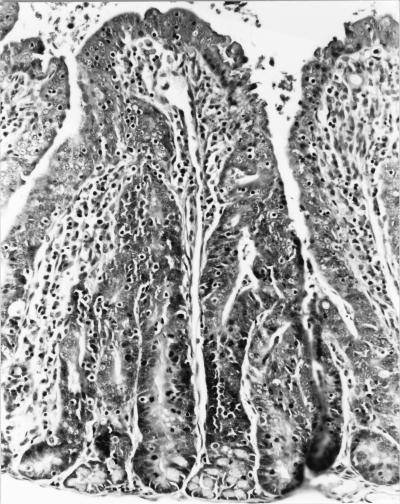
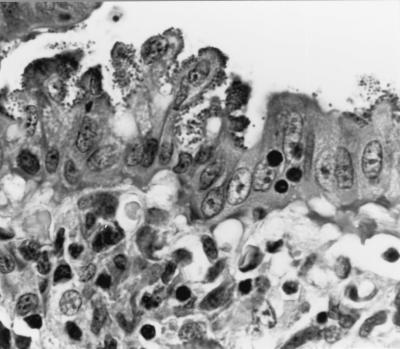
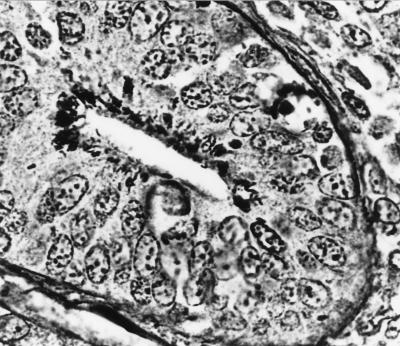
Evaluation of slides stained with hematoxylin and eosin revealed intestinal epithelial cell hyperplasia with blunting of the villi. A moderate inflammatory cell infiltrate was seen in the majority of the sections which exhibited marked epithelial cell hyperplasia. A decrease in the number of goblet cells was also observed (Fig. 1). A histologic diagnosis of proliferative enterocolitis was made. Under high-power magnification, numerous bacterial organisms were found to be closely associated with the surface of differentiated intestinal epithelial cells (Fig. 2). The appearance of these organisms was consistent with that of AEEC. High-power magnification of slides stained with the modified Warthin-Starry silver stain revealed numerous curved- to spiral-shaped intracellular organisms in the apical cytoplasm of intestinal epithelial cells (Fig. 3). The appearance of these organisms was consistent with that of the obligate intracellular bacterium L. intracellularis.
FIG. 1.
Photomicrograph of a section of small intestine from a rabbit with proliferative enterocolitis. The mucosa of the intestine has blunted villi, a moderate inflammatory cell infiltrate, and a decreased number of goblet cells. The crypt epithelial cells are markedly hyperplastic. Hematoxylin and eosin stain was used (magnification, ×40).
FIG. 2.
Photomicrograph of mucosal detail of a section of small intestine from a rabbit with proliferative enterocolitis, demonstrating typical AE lesions on surface intestinal epithelial cells. Hematoxylin and eosin stain was used (magnification, ×1,000).
FIG. 3.
Photomicrograph of a section of small intestine from a rabbit with proliferative enterocolitis, demonstrating numerous curved- to spiral-shaped bacteria in the apical cytoplasm of the intestinal epithelial cells. Modified Warthin-Starry silver stain was used (magnification, ×1,000).
Pathogenic synergism, related to the severity of disease in weanling rabbits, has been reported between EPEC and rotavirus (25). Experimental inoculation of weanling rabbits with >106 CFU of RDEC-1 in conjunction with rotavirus resulted in increased morbidity and mortality compared to inoculation with RDEC-1 alone. There was no histologic evidence of rotavirus infection in these rabbits, but specific assays were not performed. It may be that dual infection with EPEC and L. intracellularis in these rabbits also resulted in pathogenic synergism, leading to increased morbidity and mortality compared to infection with either agent alone.
A similar pathogenic synergism in hamsters was reported by Frisk and Wagner (8). In that study, animals were inoculated with ileal suspensions from naturally infected hamsters suffering from proliferative ileitis, resulting in dual infection with an organism indistinguishable from L. intracellularis and with an E. coli strain. The E. coli organism, which produced histologic changes suggestive of an enteroinvasive E. coli, was also found intracellularly, and in some cases the two bacterial species were found within the same intestinal epithelial cell. In general, the E. coli strain was more frequently observed in areas of nonhyperplastic, acute enteritis, whereas the L. intracellularis organisms were consistently associated with areas of hyperplasia. The authors hypothesized that infection with the E. coli strain preceded, and might facilitate subsequent infection with, the L. intracellularis organism (8). It may be that infection with EPEC also increases the susceptibility of rabbits to infection with L. intracellularis. However, L. intracellularis infection can occur independent of EPEC infection in rabbits (10, 23).
Bacterial culture.
No Clostridium spp. were recovered from anaerobic cultures of the ileal contents from the rabbits, and no clostridial toxins were detected. Aerobic culture of the ileal contents from the rabbits yielded E. coli. The E. coli isolates were clonal as judged by the API 20E biochemistry profile (Table 1). The strain was positive for fermentation of mannitol, sorbitol, rhamnose, melibiose, and arabinose and negative for fermentation of inositol, sucrose, and amygdalin. These isolates are nonmotile biotype 3 (biotype 3−) in the simplified biotyping scheme of Peeters et al. (19). Peeters et al. found that biotype 3− strains were highly correlated with severe disease, accounted for 35.5% of the isolates from weanling rabbits with diarrhea, and were not recovered from healthy rabbits (19).
TABLE 1.
Biochemical reactions of rabbit EPEC strainsa
| Test or characteristicb | Rabbit EPECc | RDEC-1 |
|---|---|---|
| ONPG | + | + |
| Arginine | − | − |
| Lysine | + | + |
| Ornithine | + | + |
| Citrate | − | − |
| H2S | − | − |
| Urease | − | − |
| TDA | − | − |
| Indole | + | + |
| Voges-Proskauer | − | − |
| Gelatin hydrolysis | − | − |
| Acid produced from: | ||
| Glucose | + | + |
| Mannitol | + | + |
| Inositol | − | − |
| Sorbitol | + | + |
| Rhamnose | + | + |
| Sucrose | − | + |
| Melibiose | + | + |
| Amygdalin | − | − |
| Arabinose | + | + |
| Oxidase | − | − |
| Motility | − | − |
+, positive for test or characteristic; −, negative for test or characteristic.
ONPG, o-nitrophenyl-β-d-galactopyranoside; TDA, tryptophan deaminase agar.
Identical results were obtained from three independent E. coli isolates from each of four different rabbits with dual infections with EPEC and L. intracellularis.
Southern blot analysis of E. coli isolates.
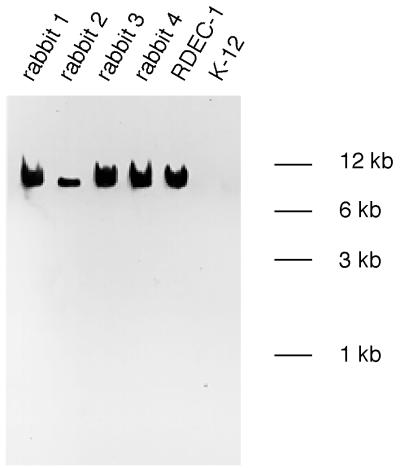
Genomic DNA from the E. coli isolates did possess eaeA gene homology, which was detected under conditions of high stringency (Fig. 4). Even under conditions of low stringency, there was no evidence of afrA gene homology in the DNA from the E. coli isolates (data not shown). DNA from the positive control, RDEC-1, did possess afrA gene homology, which was detected under conditions of both high and low stringency.
FIG. 4.
Southern blot of HindIII-digested genomic DNA from E. coli strains probed with the eaeA gene probe under conditions of high stringency. The lanes contain independent isolates from four rabbits with dual infections with EPEC and L. intracellularis, RDEC-1, and a nonpathogenic E. coli K-12 strain.
Rabbit EPEC strains from Western Europe, even those of the same serogroup as RDEC-1 (O15:H−), do not express AF/R1 pili. Some serogroup O103 rabbit EPEC strains do express an analogous initial adhesin, called AF/R2 (4), but the recovery of these strains from rabbits in North America has not been reported. The absence of the afrA gene in these isolates indicates that rabbit EPEC strains such as those reported in Western Europe are also present in rabbits in the United States. We did not test for the AF/R2 gene, nor did we serotype the isolates in this study. However, the fact that these isolates are biotype 3− suggests that these strains may also belong to serogroup O15:H−. The possible existence of yet another initial adhesin in the rabbit EPEC O15:H− strains other than RDEC-1 remains to be determined.
We have demonstrated that the rabbits in this outbreak had a dual infection with EPEC and L. intracellularis. The rabbit EPEC strain was typical of those biotype 3− isolates from Western Europe which are associated with severe disease and which do not express AF/R1 pili. While these isolates appear to be of the same biotype and may indeed also belong to the same O15:H− serogroup as the prototypic RDEC-1 strain, they are distinct from RDEC-1. To our knowledge, such a rabbit EPEC isolate has not been previously reported in rabbits in the United States. The prevalence of strains such as these in rabbits in the United States remains to be determined. It is also not clear if the high mortality associated with this outbreak was attributable to the virulence of this rabbit EPEC strain, to pathogenic synergism between EPEC and L. intracellularis, or to both of these factors. Additional experiments, including experimental inoculation studies, are being conducted to address these questions.
The source of the EPEC and the L. intracellularis has not been established. No new rabbits had been introduced into this colony prior to the outbreak of disease. It is interesting that at approximately the time that morbidity and mortality developed in the rabbits, a herd of pigs was introduced onto the farm in an adjacent facility. It is possible that the rabbits were exposed to fomites contaminated with feces from the pig facility. Both EPEC (9) and L. intracellularis (21) have been reported to cause disease in pigs, raising the possibility of cross-species transmission of these agents from pigs to rabbits. There are no experimental data to support this hypothesis, but cross-species transmission of L. intracellularis from pigs to hamsters has been reported previously (11, 16). Also, the zoonotic cross-species transmission of EHEC from livestock to humans has a significant impact on public health. We speculate that pigs might indeed be the source of EPEC and/or L. intracellularis infection for the rabbits in this outbreak. Unfortunately, specimens from the affected pigs were not available for analysis. Determination of the host range of these enteric pathogens will require experimental inoculation studies using naive pigs.
ACKNOWLEDGMENTS
We thank J. Robert Cantey for the gift of the pRKB35 plasmid and the RDEC-1 strain.
This work was supported in part by grants CA63112 to D.B.S. and RR07036 to J.G.F. from the National Institutes of Health.
REFERENCES
- 1.Albert M J, Faruque S M, Ansaruzzaman M, Islam M M, Haider K, Alam K, Kabir I, Robins-Browne R. Sharing of virulence-associated properties at the phenotypic and genetic levels between enteropathogenic Escherichia coli and Hafnia alvei. J Med Microbiol. 1992;27:310–314. doi: 10.1099/00222615-37-5-310. [DOI] [PubMed] [Google Scholar]
- 2.Blanco J E, Blanco M, Blanco J, Mora A, Balaguer L, Mourino M, Juarez A, Jansen W H. O serogroups, biotypes, and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J Clin Microbiol. 1996;34:3101–3107. doi: 10.1128/jcm.34.12.3101-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantey J R, Blake R K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977;135:454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- 4.Fiederling F, Boury M, Petit C, Milon A. Adhesive factor/rabbit 2, a new fimbrial adhesin and a virulence factor from Escherichia coli O103, a serogroup enteropathogenic for rabbits. Infect Immun. 1997;65:847–851. doi: 10.1128/iai.65.2.847-851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox J G, Dewhirst F E, Fraser G J, Paster B J, Shames B, Murphy J C. Intracellular Campylobacter-like organism from ferrets and hamsters with proliferative bowel disease is a Desulfovibrio sp. J Clin Microbiol. 1994;32:1229–1237. doi: 10.1128/jcm.32.5.1229-1237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox J G, Lawson G H. Campylobacter-like omega intracellular antigen in proliferative colitis of ferrets. Lab Anim Sci. 1988;38:34–36. [PubMed] [Google Scholar]
- 7.Fox J G, Murphy J C, Ackerman J I, Prostak K S, Gallagher C A, Rambow V J. Proliferative colitis in ferrets. Am J Vet Res. 1982;43:858–864. [PubMed] [Google Scholar]
- 8.Frisk C S, Wagner J E. Experimental hamster enteritis: an electron microscopic study. Am J Vet Res. 1977;38:1861–1868. [PubMed] [Google Scholar]
- 9.Hélie P, Morin M, Jacques M, Fairbrother J M. Experimental infection of newborn pigs with an attaching and effacing Escherichia coli O45:K“E65” strain. Infect Immun. 1991;59:814–821. doi: 10.1128/iai.59.3.814-821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss C E, Shames B, Perkins S E, Fox J G. Proliferative enteropathy of rabbits: the intracellular Campylobacter-like organism is closely related to Lawsonia intracellularis. Lab Anim Sci. 1996;46:623–627. [PubMed] [Google Scholar]
- 11.Jasni S, McOrist S, Lawson G H. Reproduction of proliferative enteritis in hamsters with a pure culture of porcine ileal symbiont intracellularis. Vet Microbiol. 1994;41:1–9. doi: 10.1016/0378-1135(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 12.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 15.McOrist S, Gebhart C J, Boid R, Barns S M. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820–825. doi: 10.1099/00207713-45-4-820. [DOI] [PubMed] [Google Scholar]
- 16.McOrist S, Lawson G H. Possible relationship of proliferative enteritis in pigs and hamsters. Vet Microbiol. 1987;15:293–302. doi: 10.1016/0378-1135(87)90017-4. [DOI] [PubMed] [Google Scholar]
- 17.Moon H W, Cutlip R C, Amtower W C, Matthews P J. Intraepithelial Vibrio associated with acute typhlitis of young rabbit. Vet Pathol. 1974;11:313–326. doi: 10.1177/030098587401100404. [DOI] [PubMed] [Google Scholar]
- 18.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters J E, Geeroms R, Orskov F. Biotype, serotype, and pathogenicity of attaching and effacing enteropathogenic Escherichia coli strains isolated from diarrheic commercial rabbits. Infect Immun. 1988;56:1442–1448. doi: 10.1128/iai.56.6.1442-1448.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohl P H, Peeters J E, Jacquemin E R, Lintermans P F, Mainil J G. Identification of eae sequences in enteropathogenic Escherichia coli strains from rabbits. Infect Immun. 1993;61:2203–2206. doi: 10.1128/iai.61.5.2203-2206.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowland A C, Lawson G H. Intestinal adenomatosis in the pig: immunofluorescent and electron microscopic studies. Res Vet Sci. 1974;17:323–330. [PubMed] [Google Scholar]
- 22.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoeb T R, Fox J G. Enterocecocolitis associated with intraepithelial Campylobacter-like bacteria in rabbits (Oryctolagus cuniculus) Vet Pathol. 1990;27:73–80. doi: 10.1177/030098589002700201. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi A, Inman L R, O’Hanley P D, Cantey J R, Lushbaugh W B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978;19:686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thouless M E, DiGiacomo R F, Deeb B J. The effect of combined rotavirus and Escherichia coli infections in rabbits. Lab Anim Sci. 1996;46:381–385. [PubMed] [Google Scholar]
- 26.Wagner J E, Owens D R, Troutt H F. Proliferative ileitis of hamsters: electron microscopy of bacteria in cells. Am J Vet Res. 1973;34:249–252. [PubMed] [Google Scholar]
- 27.Wolf M K, Andrews G P, Fritz D L, Sjogren R W, Jr, Boedeker E C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988;56:1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf M K, Boedeker E C. Cloning of the genes for AF/R1 pili from rabbit enteroadherent Escherichia coli RDEC-1 and DNA sequence of the major structural subunit. Infect Immun. 1990;58:1124–1128. doi: 10.1128/iai.58.4.1124-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]