Abstract
This review discusses the driving principles which may underly neurodegeneration in dementia, represented most dominantly by Alzheimer’s disease (AD). While a myriad of different disease risk factors contributes to AD, these ultimately converge to a common disease outcome. Based on decades of research, a picture emerges where upstream risk factors combine in a feedforward pathophysiological cycle, culminating in a rise of cytosolic calcium concentration ([Ca2+]c) that triggers neurodegeneration. In this framework, positive AD risk factors entail conditions, characteristics or lifestyles that initiate or accelerate self-reinforcing cycles of pathophysiology, whereas negative risk factors or therapeutic interventions -particularly those mitigating elevated [Ca2+]c- oppose these effects and therefore have neuroprotective potential.
Keywords: Dementia, Alzheimer’s disease, calcium, neurodegeneration, calcineurin, synaptic function
1. Introduction
Dementia refers to a group of progressive, age-related neurodegenerative disorders including AD, vascular dementia (VaD), Lewy body disease (LBD) and frontotemporal lobe dementia (FTLD). Despite their differences in etiology and pathology, there exists a common set of symptoms of impaired cognitive abilities involving memory, thinking, social interactions and decision making. The underlying forces driving AD dementia result in impaired function, and ultimately atrophy, of the cortex and hippocampus, key brain areas responsible for cognition and behavior. AD is by far the most common form representing about 60–70% of dementia cases and therefore is the focus of this review. Nevertheless, despite the differences in symptoms and pathology across the different forms of dementia, the basic mechanisms underlying neurodegeneration in AD are likely also instructive to non-AD dementia.
To adapt and learn, the central nervous system relies on the ability to restructure and/or rewire neuronal networks in response to external or internal stimuli. For instance, memory formation -or erasure- underlies increasing or decreasing synaptic connectivity in networks controlling cognition. Other examples entail synaptic pruning of underutilized synapses or dedicated mechanisms to drive neuronal death, such as apoptosis or ferroptosis. Hence, intrinsic neurophysiological processes operate to physically alter neural network architecture to secure optimal brain function. From this perspective, it is conceivable that, under pathological conditions such as in dementia, these neurophysiological processes are deregulated, impairing the brain’s ability to adapt, learn, repair, or even survive.
A key regulator of these neurophysiological processes is calcium (Ca2+), a metal ion which serves as a versatile intracellular signal to regulate diverse Ca2+ sensitive effector pathways (BOX 1). In the resting, unstimulated neuron, a steep Ca2+ concentration gradient is maintained between the extracellular space and intracellular organelles versus the cytosol. However, upon stimulation, Ca2+ can be mobilized from both extracellular and intracellular stores to increase cytosolic levels, where it acts as a secondary messenger to regulate Ca2+ sensitive effector pathways. Pathological states impairing optimal Ca2+ homeostasis are consequently expected to inappropriately regulate pathways that support neuronal function and survival.
BOX 1. Principles of calcium (Ca2+) signaling.
Ca2+ is a highly versatile intracellular signal that operates to regulate a broad spectrum of neurophysiological processes required for brain function. For a comprehensive review see 122.
A key attribute to specify the signaling function of Ca2+ entails a very steep Ca2+ concentration gradient from a relatively a low concentration in the cytosol to high concentrations in intracellular organelles and the extracellular environment.
Extracellular stimuli that transiently disrupt the gradient lead to influxes of Ca2+ in the cytosol subsequently impacting Ca2+ sensitive effector pathways to control neurophysiological functions or processes.
The precise physiological outcome of these effector pathways is specified by the subcellular localization and kinetics (concentration and duration) of Ca2+ levels in the cytosol (e.g., synaptic potentiation vs synaptic depression).
Extensive systems are in place to maintain optimal Ca2+ homeostasis to prevent inappropriate Ca2+ signaling and consequential suboptimal neuronal function or even demise.
Ca2+ dyshomeostasis is the physiological state in which the Ca2+ gradient is suboptimal (i.e., the Ca2+ signal is inappropriately specified) for optimal function and survival.
The mitochondria, ER and lysosomes are organelles constituting important elements of the Ca2+ homeostatic system by acting as Ca2+ buffers and/or stores.
Intra-organelle Ca2+ regulates the functions of these organelles such as energy production by mitochondria, protein folding in the ER, acidity in lysosomes.
In states of impaired Ca2+ homeostasis the function of these organelles to compartmentalize Ca2+ in neurons also becomes impaired and so further contributing to inappropriate effector pathways outcomes which are harmful to the cell. For review, see 123.
So far, no interventions are available to effectively treat dementia and preserve cognitive function, but we know an effective treatment should improve, or at least preserve, synaptic function as a means to preserve memory encoding 1,2. Hence, a key question in the field is to understand the mechanisms governing synaptic transmission and neuronal survival, particularly in relation to Ca2+ homeostasis. This review accommodates this need by discussing how dementia risk factors lead to deranged calcium homeostasis enabling neurodegeneration and catalysing the development of pathophysiology. We do not aim to recapitulate and reiterate the multitude of different pathophysiological elements of AD as these have been reviewed extensively by others. Instead, we aim to synthesize these findings into a general principle or concept unifying the most prominent hypotheses of the field to explain the driving principles underlying familial (fAD) and sporadic AD (sAD) dementia. We aim to provide a theoretical framework of how AD risk factors promote onset and progression of neurodegeneration through Ca2+ dyshomeostasis, allowing for predictions of effective therapeutic approaches for AD.
2. Alzheimer’s disease - a manifestation of dementia in a continuum of overlapping pathologies
Pathophysiological hallmarks of AD neurons include dysregulated intracellular Ca2+ homeostasis, impaired proteostasis, dysfunctional mitochondrial bioenergetics with concomitant elevated reactive oxygen species (ROS), and increased neuroinflammatory responses. These are not specific for AD and are illustrative of other dementias as well. The presence of two insoluble protein aggregates, extracellular amyloid β (Aβ) plaques consisting of aggregated Aβ peptide, and intracellular tangles of hyper-phosphorylated tau, are hallmark pathologies used as diagnostic criteria of AD. However, such categorization is certainly not absolute as different forms of dementia have overlapping protein pathologies. For instance, alpha-synuclein pathology, a hallmark of Lewy Body Disease (LBD), is also found in AD, whereas, in LBD, tau pathology is also observed. Such continuum of pathologies across different forms of dementia again suggests similar or at least overlapping underlying mechanisms driving neurodegeneration.
In the early phase of AD, symptoms include short-term memory deficits, but as the disease advances, other symptoms like disorientation and behavioral changes can develop as well. At the cellular level, impaired synaptic structure and function are among the earliest functional changes 1 providing a logical explanation for the cognitive decline early in the disease, whereas subsequent loss of presumably already dysfunctional neurons comes later 2. Notably, of the known features of AD, synaptic loss correlates most strongly with cognitive decline, which makes sense as synaptic plasticity is the cellular correlate of learning and memory 3. This sequence of events indicates that synapses are the most vulnerable neuronal structures; therefore, it seems likely that mechanisms eliciting synaptic dysfunction set the stage for more advanced degeneration of neurons over time 2.
The underlying risk factors of AD responsible for triggering the cascade of events leading to synaptic dysfunction and neuronal loss are diverse and multifactorial. These risk factors could be of genetic (fAD) or non-genetic sporadic (sAD) origin and are positive or negative (protective). The disease etiology of each AD patient is unique and shaped by a constellation of risk factors that define onset and progression of neurodegeneration. However, neuronal degeneration in AD is not a passive process, but instead entails an active series of signaling and biochemical decisions 4,5. In other words, complexity and diversity of the initial risk factors notwithstanding, the pathophysiological processes ultimately converge to a common mechanism executing or enabling neurodegeneration. Elucidating this mechanism is key to understanding AD and to conceptualize effective therapeutic interventions in the heterogeneous AD population.
3. Ca2+ from intracellular stores - the enemy within
Altered calcium release from intracellular stores (Fig. 1) plays a major role in disrupting synaptic structure and plasticity, intracellular signaling cascades, and lysosome and mitochondria function 6,7. The endoplasmic reticulum (ER) maintains a large reservoir of calcium in the micromolar concentration range, orders of magnitude greater than the surrounding cytosol. ER calcium release occurs through two receptor/channel complexes: The inositol 1,4,5-trisphosphate receptor (IP3R) and the ryanodine receptor (RyR). Replenishment of ER calcium stores occurs via activation of Orai-store operated calcium entry (SOCE) channels by the ER-localized Ca2+ sensing proteins stromal interaction molecule (STIM) 1 and 2. Cytosolic calcium is then transported into the ER lumen against a concentration gradient by the sarco-endoplasmic reticulum Ca2+ ATPase pump (SERCA). Recent work shows neuronal SOCE to be linked with calcium/calmodulin-dependent protein kinase II (CaMKII) in the process of dendritic remodeling of the hippocampus to promote learning and memory, such that knockdown of CaMKIIβ downregulates neuronal SOCE in dendritic spines, impacting spine structure 8.
Fig. 1.
Dysregulated calcium storage leads to Ca2+ dyshomeostasis
Elevated [Ca2+]c concentration disrupts the balance between three crucial calcium stores: the mitochondria, lysosome, and endoplasmic reticulum (ER). This dysregulation is both a cause and product of pathological responses seen in these three cellular components, as, once excess calcium has been introduced to the cytosol either from the extracellular environment or intracellular stores, feedforward mechanisms such as CICR further potentiate insults seen as downstream effects in all compartments.
The IP3R, activated by the second messenger, IP3, is localized predominantly in the soma, nuclear envelope, and proximal dendrites, and plays key roles in second messenger signaling, calcium-activated gene transcription, synaptic plasticity and cellular bioenergetics 9. In the brain, the RyR, and predominantly the RyR2 isoform, is localized in hippocampus, cortex, and other brain regions essential for memory encoding 10,11. It is activated by calcium itself, a process termed calcium induced calcium release (CICR), to further amplify Ca2+ signaling 12. As intracellular calcium signaling regulates a wide array of functions and processes, disruptions in calcium levels can trigger a vast array of downstream pathological events 13. In AD, both familial and sporadic, this can take the form of accelerated amyloid and tau pathology by upregulating Ca2+-regulated cleavage enzymes (e.g. beta secretase) and kinase activity (GSK3b and cdk5), respectively 14,15(p5), deficits in synaptic structure and function in brain regions implicated in memory encoding and storage that manifest prior to widespread neuronal death and plaque deposition 16,17, impediments in cellular autophagy and debris clearance 6, and mitochondrial dysfunction, oxidative stress and apoptosis 18,19, among many others 7,20. Several of these mechanisms will be discussed in further detail below.
Early studies identifying ER-calcium dyshomeostasis in AD conducted in fibroblasts from fAD patients demonstrated a marked increase in IP3R-evoked calcium responses relative to their non-AD siblings or age-matched controls 21,22. Subsequent studies in model cells expressing mutant presenilin genes (PSEN), which leads to fAD in an autosomal dominant fashion, resulted in similar excess ER Ca2+ release 23-25. More detailed mechanistic studies conducted in a series of AD mouse models also demonstrated exaggerated IP3R and RyR-evoked calcium responses in neurons which contributed to deficits in synaptic structure and plasticity 17,26,27. Notably, while the IP3R-calcium responses were predominant in the somatic regions, the excess RyR-calcium release was most pronounced in synaptic compartments and distal dendrites and was responsible for loss of dendritic spines and impaired synaptic transmission 28. These so called “leaky” RyR channels are a well-characterized factor in cardiomyopathies, which are now being explored as avenues of dementia and neurodegenerative disease.29,30 Presynaptically, Ca2+ is required for tethering proteins regulating vesicle release, such as SNAP, SNARE, and syntaxin proteins. Interestingly, the link between tethering proteins and calcium also appears to be affected directly by RyR isoforms interacting with SNARE associated SNAPIN domains, potentially resulting in sensitized RyR channels31. In AD models, consequences of dysregulated Ca2+ are seen through increased spontaneous vesicle release, impaired presynaptic plasticity mechanisms, and reduced neurotransmitter vesicles within active zones 17,32. In neurons derived from induced pluripotent stem cells (iPSCs) obtained from familial AD (fAD) patients, increased Aβ42 and phospho-tau was associated with exaggerated ER calcium responses 33. Notably, normalizing the ER calcium responses with RyR allosteric modulators such as dantrolene and Ryanodex not only restored normal intracellular calcium handling, but also reduced phospho-tau, amyloid, and the above described pre- and postsynaptic deficits, thus implicating the RyR as a potential therapeutic target in AD 11,34,35. AD human brain tissue samples also show altered levels of RyR expression 36,37, a phenotype mirrored in AD mouse models 27,36 Although RyR allosteric modulators do not directly affect expression of SNAP, the degree to which they may influence RyR/SNARE/SNAPIN interactions remains unstudied35. These more recently discovered interactions may provide more insight to the success of currently hypothesized therapeutic interventions.
In both fAD and sAD, ER calcium dysregulation is both a result and key contributor to impaired SERCA activity and/or excessive IP3R/RyR activity. PSEN1 mutations also impact channel properties of RyR and IP3R 24,38, effects that can be rescued by dantrolene 26, suggesting a mechanism of enhanced IP3R/RyR activity at least in fAD. While the exact underpinnings remain elusive, mutated PSEN1 may lead to defects in closing or suppressing channel activity, providing a prolonged open state 25. RyR2 and IP3R are also regulated at the transcriptional level 39 and by post-translational protein modifications such as phosphorylation, nitrosylation, and oxidation 30,40. Collectively, the ER is a prominent driver of calcium dyshomeostasis in AD, not only because of mechanisms to maintain the appropriate concentration gradient versus the cytosol are impaired, but also because it amplifies calcium entry through plasma membrane channels such as through glutamatergic N-methyl-D-aspartate receptors (NMDAR) 28 by CICR.
Mechanistically, one way the cell buffers elevated [Ca2+]c is uptake by mitochondria41. This mechanism may work well to counter transient increases of elevated [Ca2+]c, however, under conditions of more chronic calcium dyshomeostasis (such as in AD), it may lead to dysfunctional mitochondria. Ca2+ is essential for optimal cellular respiration 9 and running of the tricarboxylic acid cycle (TCA) and thus an overload of mitochondrial Ca2+ will initially result in an overactive electron transfer chain (ETC) and hyperpolarization of mitochondria with concomitant elevated reactive oxygen species (ROS) production 42. If left unchecked, ROS accumulation will increase opening frequency of the mitochondrial permeability transition pore (mPTP), ultimately leading to mitochondria depolarization and enabling a net release of calcium to the cytosol together with death signals like cytochrome c. Cytosolic cytochrome c eventually generates the apoptosome, resulting in recruitment of caspase-9 and maturation of caspase-3, a pro-apoptotic factor 43. Thus, buffering of [Ca2+]c by the mitochondria can be seen as both necessary for proper functioning for a neuron stressed by calcium dysregulation as a product of AD. A persistent Ca2+ overload, however, leads to depolarized and dysfunctional mitochondria with impaired [Ca2+]c buffering capacity contributing further to elevated levels of calcium in the cytosol. Recent studies indicate that Ca2+ uptake by the mitochondria in pathogenic states is also driven by a spatial and temporal component. These ion and lipid transfer junctions, known as ER-mitochondria contact sites (ERMCs) and mitochondria-associated membranes (MAMs), allow for direct transfer of Ca2+ between the ER and mitochondria, and are formed by tethering proteins such as IP3R and voltage dependent calcium channels 44. Although only recently emphasized in the field of neurodegeneration, these organelle linking spots are crucial for spatial and temporal regulation of mitochondria and Ca2+ whose compartmentalization is important for synaptic plasticity and dendritic spine formation45. Collectively, these data indicate that, in diseased neurons, the intracellular Ca2+ gradient across cytosol and organelles is disturbed, which at the one hand may compromise organellar function including their Ca2+ storage capacity (see also below) and on the other hand result in abnormally elevated levels of Ca2+ in the cytosol. Current and future research will continue to shed light on the healthy physiological functioning of these Ca2+ transport mechanisms along with their pathological states to further support Ca2+ dyshomeostasis at the organelle level as a pathway to neurodegeneration.
4. Aβ and tau– partners in crime inciting excitotoxicity
A small subpopulation of AD (1-2%) constitutes familial Alzheimer’s disease (fAD) which shares the same pathological hallmarks as the vast majority of late-onset or sporadic AD (sAD). fAD patients carry disease-causing mutations in the amyloid precursor protein (APP) or presenilin (PSEN1 or PSEN2) genes. The resulting protein products alter the APP-processing machinery towards production of aggregation-prone Aβ species. These findings led to the proposition that pathological Aβ is at least one element driving neurodegeneration in AD and is a crucial supportive element of the amyloid cascade hypothesis. In this hypothesis, Aβ plaque aggregation is the underlying culprit of AD symptoms. However, a growing body of evidence is calling the primacy of this hypothesis for sporadic AD into question, as there is little correlation between amyloid plaque levels and cognitive function 46,47. Nevertheless, fAD has provided a unique context to unveil mechanisms underlying synaptic toxicity triggered by pathological Aβ species. This has been instructive in understanding how AD risk factors converge to a common pathological mechanism leading to synaptic dysfunction and neuronal cell loss.
Amyloidogenic processing of APP is a highly regulated process entailing the sequential cleavage of APP in endosomal compartments 48. Upon fusion of endosomes with the plasma membrane, Aβ peptides are released in the extracellular space where plaque aggregation occurs. APP is also cleaved in a non-amyloidogenic process by alpha and gamma secretases after synthesis in the ER and post-translational modifications in the Golgi apparatus prior to reaching the plasma membrane. The end products, the so-called P3 and APP intracellular domain fragments 49, do not have the intrinsic property to assemble in neurotoxic aggregates.
The evolutionary conservation of these highly complex processing events suggests it may have physiological functions which remain elusive. However, pathological assemblies of Aβ peptides, such as soluble Aβ oligomers (Aβos) 50 may inappropriately activate glutamatergic transmission through NMDAR 51 with associated elevated Ca2+ influx into the cytosol. As such, this may underlie neuronal hyperactivity phenomenon in AD and may explain seizure activity as co-morbidity. The NMDAR can mediate activity-dependent increase (long term potentiation, LTP) or decrease (long term depression, LTD) of synaptic strength - the molecular correlates of memory formation and erasure 52. Generation of LTP upon a high frequency stimulus requires a large, but transient influx through the NMDAR of Ca2+ triggering Ca2+/calmodulin-dependent protein kinase II (CAMKII) mediated signaling to increase the number of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors at the post-synaptic membrane (Fig. 2). However, the opposite result (LTD) after a low frequency stimulus is also controlled by NMDAR-dependent Ca2+ influx. This involves a moderate and more sustained Ca2+ influx that selectively activates calcineurin, a phosphatase with a much higher affinity for Ca2+ than CAMKII resulting in synaptic depression and subsequent apoptosis of neurons 53.
Fig. 2.
Schematic of the mechanism as to how differences in kinetics of post-synaptic Ca2+ influx have two opposing outcomes (LTP vs LTD).
Apart from NMDAR, pathological assemblies of Aβ also facilitate excessive Ca2+ influx from the extracellular environment through a multitude of Ca2+ gating activities at the plasma membrane 54 as well as from internal stores (Fig. 1). Irrespective of the precise mechanism, an important consequence of the elevated Ca2+ influx into the cytosol entails inappropriate activation of Ca2+ responsive pathways mediating synaptic depression (i.e. memory erasure) and ultimately neuronal apoptosis that characterizes AD 55.
While the Aβ hypothesis of AD has been heavily explored, the intended function of Aβ in healthy, normally aging brains is less understood. Crucially, loss of APP or BACE1, which are substrates essential for Aβ generation, results in impaired LTP and cognitive deficits, as studied in knockout mouse models 56,57. Thus, solely targeting Aβ as a therapeutic strategy may yield more harmful effects than protective. Research has also focused on specific pathogenic intermediaries leading to plaque development, such as Aβos, which exert neurotoxic effects on the CNS including LTP inhibition, downstream calcium dysregulation, and ultimately selective neuronal death 50,58. As a mechanism for downstream calcium dysregulation, Aβos can interact with cellular prion protein (PrPc) to mediate mGluR5 toxicity, resulting in altered NMDAR and fynomer (fyn) kinase activity as well as tau hyperphosphorylation, and inhibition of fyn kinase activity can rescue synaptic deficit in AD mice 59-61.
Tau hyperphosphorylation and aggregation into full-blown tangles is another pathological hallmark of AD. Tau is a protein with diverse functions, best known for its ability to bind and stabilize microtubules, but has other prominent functions particularly in regulating signaling pathways 7. Pathological tau plays a major role in NMDAR mediated excitotoxity in AD and other degenerative conditions, like stroke or chronic stress, involving potentiation of NMDAR activity by phospho-tau dependent recruitment of fyn kinase. Hence, pathological Aβ and tau appear to have similar effects which may not act independently. This is dramatically illustrated by tau lowering approaches such as deletion of the endogenous tau-encoding MAPT gene, which prevents or reduces neurodegeneration in various disease models of neuronal degeneration 62-64. This combined action on Fyn kinase by both tau and Aβos allows for at least a partial explanation of the additive effects of both pathologies. Thus, more synergistic effects are kept in consideration for potential therapeutic development 65. Besides Fyn kinase, tau interacts with a plethora of signal transducers such as phospholipase C 66, implying a modulatory role on the respective signaling cascades that regulate ER calcium release and, and more indirectly activates SOCE channel activity 67, all pointing to a far more extensive impact on Ca2+ signaling besides modulating NMDAR activity.
Between these two classical components of AD, an overall picture emerges in which both pathological Aβ and tau synergistically facilitate an abnormally high Ca2+ influx, thereby inducing calcium-responsive signaling cascades underlying memory erasure and neuronal apoptosis 55. These cascades not only act together to result in the development of AD pathology, but are also intrinsically linked to Ca2+ mishandling as both a driving force and product of their toxic activity (see Fig 3).
Fig. 3.
A schematic depicting how mechanistically [Ca2+]c impacts AD pathophysiology (indicated in yellow boxes) reciprocally.
Aβ/tau pathology - Ca2+ promotes intracellular Aβ aggregation directly 125,126, which activates APP-processing resulting in higher levels of amyloidogenic Aβ peptide 127-129. Calcium signaling steers the expression of APP-processing secretase, β-site APP cleaving enzyme (BACE) 130-132 and of glutaminyl cyclase 133, an enzyme mediating the formation of aggregation-prone pyroglutamylated Aβ. Fusion of lysosomes and autophagosomes is impacted via excessive Ca2+ efflux, causing inappropriate fusion of dysfunctional lysosomes to autophagosomes, ultimately increasing waste build up 134 and an increase of Aβ and p-tau. Aβ peptide clearance is compromised largely via mutated PS1 protein. PS1 results in impaired trafficking of v-ATPase to the lysosomal membrane 72. Improper v-ATPase activity results in a more alkaline lysosomal lumen, which is incapable of activating cathepsin B to break down Aβ. [Ca2+]c acts as a second messenger activating mitogen-activated protein kinase (MAPK) signaling, calpain-cyclin dependent kinase 5 (Cdk5), calcineurin and glycogen synthase kinase 3 (GSK3) pathways mediating phosphorylation and subsequent aggregation of tau 135,136, tau secretion and possibly its spreading by exocytosis 137. In turn, pathological Aβ favors assembly into Ca2+ permeable pores in the plasma membrane 138,139; activates receptors and voltage-operated calcium channels and IP3 mediated release of calcium from internal stores 48,140,141; and impairs Ca2+ buffer capacity of mitochondria 88,90,142. Phosphorylated tau promotes neuronal excitability by potentiating NMDAR activity and 62 lowering Kv4.2 channel activity 143; hyperphosphorylated tau damages mitochondria impairing Ca2+-buffering 144.
Mitochondrial dysfunction - Elevated [Ca2+]c impacts mitochondrial function directly 91 as it leads to an overload of Ca2+ in the mitochondrial matrix. This disturbs oxidative phosphorylation thereby increasing the generation of reactive oxygen species (ROS). Probably transient increases of mitochondrial Ca2+ will be relatively well tolerated but when not resolved the excess of Ca2+ and ROS trigger the opening frequency of mPTP, leading to loss of mitochondrial membrane potential, decreased ATP production and release of pro-apoptotic factors. In turn, dysfunctional mitochondria have impaired buffering capacity of [Ca2+]c 91 and have increased ROS production which impact calcium homeostasis: ROS activate intracellular calcium channels IP3R and RyR increasing calcium influx from the ER 42, whereas oxidative modification of PMCA decrease extrusion of calcium from the cytosol 145. Excessive Ca2+ decreases autophagy/mitophagy promoting AD pathology 146
Inflammation – Calcineurin signaling underlies activation of microglia and neuroinflammation 84. Microglia are activated by Aβ pathology a direct result of elevated calcium (see above) 147. In turn, inflammatory cytokines produced by microglia elevate [Ca2+]c by promoting neuronal excitability 147, increasing ROS production 79 or by increased expression of the IP3R by tumor necrosis factor alpha (TNFα) signaling 148.
Calcium dysregulation – Through calcium-induced calcium release (CICR), an increase in [Ca2+]c activates RyRs, releasing more Ca2+ and consequently increasing [Ca2+]c + 12,29. IP3R and RyR are both regulated by presenilin and are implicated in FAD. Additionally, cytochrome-c, which binds to IP3R on the ER membrane 7, is crucial for blocking the negative feedback function of IP3R in excess Ca2+ 149. Moreover, the Ca2+ depleted ER-stores will activate SOCE which triggers an influx of extracellular Ca2+, hence to a further accumulation of calcium in the cytosol.
5. Mishandling of intracellular Ca2+ impairs proteostasis
Improper aggregation and deposition of misfolded and denatured protein is a key pathological hallmark of AD. This aspect appears to go beyond aggregation of the well-known players Aβ and Tau as plaques and tangles contain hundreds of other co-aggregated proteins. Perhaps the best known being α-synuclein as the “non-amyloid component” of plaques 68. To what extent insoluble aggregates contribute to neurodegeneration remains to be seen, but it signifies that protein folding and/or degradation processes are impaired in diseased neurons and as such contribute to the formation of the hallmark pathological features.
For clearing cellular debris, denatured proteins, and unwanted peptide fragments, post-mitotic cells such as neurons rely on autophagy, as opposed to cells which have division as a method of waste clearance 69. Several steps in autophagic degradation involve Ca2+ regulation, and the excess Ca2+ released from ER stores in AD neurons interferes with lysosome and autophagosome function, and thus contributes to proteinopathy in AD 70. Under physiological normal conditions, the vacuolar [H+] ATPase (v-ATPase) proton pump on the lysosome maintains the acidic luminal pH 71 necessary for proteolytic degradation. With impaired PS1 function, as seen in AD, trafficking of v-ATPase subunits to the lysosomal membrane is disrupted 72, contributing to an alkaline lysosomal pH, inefficient protease activity, 73 and autophagosome accumulation 74. Recent studies have shown that faulty autolysosome acidification leads to the build-up of Aβ in neurons and generation of precursors to senile plaques in AD mouse models 75. While these findings lend support to the overall generation and pathology of Aβ plaques as a result of AD, they also challenge the conventionally accepted sequential nature of the disease, by proposing a precursor neuron to senile plaques, termed “PANTHOS”75.
v-ATPase deficiency in PS1 loss-of-function states causes lysosomal/autophagy deficits and [Ca2+]c elevation by triggering abnormal Ca2+ efflux through the lysosomal Ca2+ channel mucolipin, a transient receptor potential subfamily member (TRPML1). This cascade further disrupts lysosome-autophagosome fusion, a calcium regulated event, and contributes to incomplete degradation of pathogenic protein fragments such as phospho-tau 76,77. The multiple previously defined sources of intracellular calcium dysregulation, such as PSEN1 and RyR2 mutations, link these AD-related pathogenic processes through a common molecular mechanism 72,78. In support of this linking hypothesis, mouse models with RyR2 gating mutations display rescued AD phenotypes, including restoration of LTP, improved clearance of Aβ, and restored lysosome-autophagosome fusion58. Continued study of aberrant Ca2+ signaling and its impacts on autophagy will lead to a broader array of therapeutic opportunities for AD and other proteinopathies.
The folding and trafficking of proteins in the ER is also impaired in AD, such as the aforementioned v-ATPase subunits 72,78. The ER contains a host of calcium binding enzymes and chaperones controlling the post-translational modifications and folding of nascent peptides. Sustained ER-calcium dyshomeostasis as seen in AD impairs protein-folding and the ensuing accumulation of misfolded proteins lead to ER-stress and activation of the unfolded protein response (UPR). ER stress in conjugation with mitochondrial dysfunction (the result of excessive [Ca2+]c) leads to a rise of ROS levels and so further aggravates protein folding impairments in the ER 79(p2). When not resolved, persistent UPR triggers apoptosis and as such contributes to neurodegeneration.
Not to be overlooked, AD hippocampal astrocytes derived from triple transgenic (3xTg) AD mice also display upregulated UPR, elevated ROS, and altered ER-mitochondria interaction80,81. While relatively understudied, it is important to consider the global consequences of Ca2+ dysregulation causing neurodegenerative phenotypes such as ER stress in other cell types to derive a complete picture of CNS dysfunction as driven by aforementioned, feed-forward, cyclical mechanisms.
In conjunction, impaired proteostasis in AD resulting from lysosome deacidification, hampered lysosome-autophagosome fusion, RyR2 gating abnormalities, and persistent UPR can be seen as both the promotors and results of neurodegeneration driven by calcium dyshomeostasis in AD. Due to the feed-forward nature of these varied pathologies, treating only one facet of this heterogeneous onslaught will likely not result in a holistic therapy. Thus, the therapeutic importance of targeting the sources of increased [Ca2+]c to end the cycle is highlighted.
6. [Ca2+]c amplifies AD pathophysiology– a vicious cycle
As outlined above, one of the earliest effects of neurons challenged with AD risk factors entails elevated influx of Ca2+ in the cytosol from both extra and intracellular sources. Not surprisingly, given the diverse roles and functions of [Ca2+]c, inappropriately increasing its second messenger activity has profound effects in neurons, ranging from altered signaling and gene expression to mitochondrial dysfunction and catalysis of Aβ-aggregation (Fig. 3). In turn, these deranged physiological processes such as increased excitability further promote influxes of Ca2+ in the cytosol (Fig. 3). In other words, elevated [Ca2+]c is the cause and consequence of AD pathophysiology, thus constituting a self-reinforcing cycle that, when set in motion by AD risk factors, autonomously drives the development of full-blown pathophysiology. In this scenario, heterogeneous AD risk factors ultimately contribute to the same consequence; namely, a rise in [Ca2+]c and the development of AD pathophysiology. For instance, in fAD subjects bearing mutations in APP, this cycle is initiated by increased formation of pathological Aβ, whereas in sAD it may involve a mix of weakly penetrant risk factors that vary considerably from one individual to the next.
The pathological rise of [Ca2+]c modulates active mechanisms or processes which ultimately lead to neurodegeneration 4,82 (Fig 4). As already outlined above, [Ca2+]c selectively activates calcineurin to enable synaptic depression in an acute fashion 83. However, more chronic elevations of calcium trigger neuronal apoptosis 55 which also involves calcineurin 84, activation of calpains 5,85, 82, necrosome activation 86, sustained extracellular signal-regulated kinase (ERK) signaling 87 and the release of cell death signals from mitochondria 88-91. In this scenario, there appears a time window, during which neurons have dysfunctional synapses but have not yet undergone apoptosis 2, where synaptic function can be restored if the underlying driver of pathology is removed. Below, we describe an example entailing calcineurin inhibitors that capitalizes this concept
Fig. 4.
Ca2+ responsive pathways enable neurodegeneration (see main text for details).
Collectively, development of AD pathophysiology leading to neurodegeneration is not a linear cascade but comprised of a self-reinforcing cycle of events (Fig. 3) ultimately spiraling to a progressive accumulation of [Ca2+]c where it sets-off neurodegeneration (Fig. 4).
7. AD risk factors offset neuronal physiology towards increasing cytosolic calcium
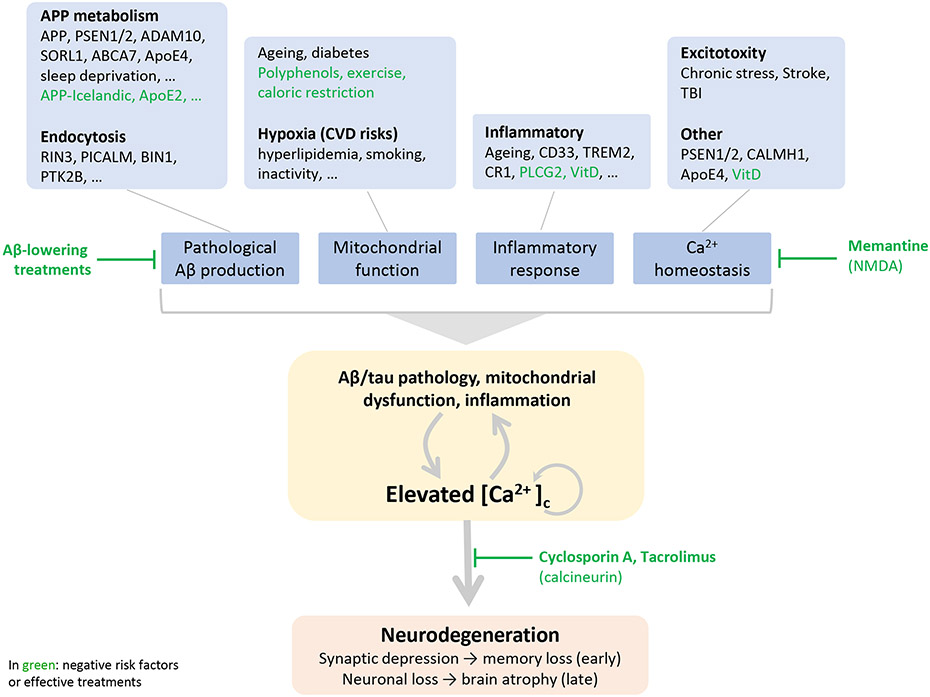
A risk factor entails a characteristic, condition, or behavior that increases the chances of developing the disease. Accordingly, AD risk factors alter one or more processes underlying the pathophysiology leading to neurodegeneration. In AD, many either positive or negative (protective) risk factors have been identified which can be loosely categorized according to their impact on key pathological features in neurons: Ca2+ homeostasis, Aβ formation, inflammation and mitochondrial dysfunction (Fig 5). In fAD, pathology is triggered primarily by altered APP metabolism and subsequent increased formation of amyloidogenic Aβ. sAD risk factors differ from patient to patient, but ageing is the most prominent risk factor shared between all forms of AD. However, not all aged people develop AD, or have signs of advanced aging. Therefore, studying aging as both an unavoidable risk factor and a healthy physiological process with the potential to be altered by genetic and epigenetic markers may leave clues as to the mechanisms of neurodegeneration. At the other end of the spectrum, negative risk factors slow down formation of disease-related pathophysiology. An interesting example is the Icelandic mutation in APP which lower amyloidogenic processing of APP 92 or phospholipase-C-gamma-2 (PLCG2) 93 presumably mitigating inflammation.
Fig. 5.
Towards a unifying hypothesis - an outline of how AD risk factors trigger a self-enforcing cycle of pathophysiology ultimately converging to abnormally elevated [Ca2+]c executing neurodegeneration.
Conditions which favor pathological Aβ formation include mutations in genes regulating APP processing such as in PSEN1/2 or endocytosis which increase the residence time of amyloidogenic processing on endocytic membranes 150. Factors which directly impact Ca2+ homeostasis include apolipoprotein E (ApoE4) 151 and calcium homoeostasis modulator 1 (CALMH1) which facilitates Ca2+ influx in the cytosol 152. Presenilins do not have only a role in APP processing but also increase influx of calcium in the cytosol by facilitating the gating function of IP3R 25. Inflammation, an important risk factor of AD, is the result of diverse risk factors associated with AD such as ageing, activation of microglia by pathological Aβ or clinical mutations. Inflammatory cytokines impact neurons to modulate calcium for instance by elevated ROS production by activated microglia 153 or increased expression of the IP3R by TNFα signaling 148. Causes of excitotoxicity like TBI or stroke involve increased Ca2+ influx in an acute fashion and more chronically an inflammatory response which facilitates the formation of Aβ pathology 154. Subsequent neuronal degeneration requires tau 63 indicating this mechanism recapitulates closely AD pathophysiology. Brain hypoxia is a main AD risk factor and cerebrovascular disease or other conditions which limit the oxygen supply to the brain have a negative impact on mitochondrial function and increase ROS formation 155. A prominent effect of ageing involves “wear-and-tear” of mitochondria (for instance due to accumulation of mutations in the mitochondrial DNA) becoming progressively less functional. Moreover ageing is associated with an increased inflammatory state 156.
Indicated in green are negative risk factors or effective treatments 103,113 which are (presumed) protective and delay disease onset: the Icelandic mutation in APP 92, ApoE2 157, PLCG2 93, exercise to keep the vascular system in better shape 158, antioxidants such resveratrol 159 and vitamin D 160 which through the vitamin D receptor regulates expression of genes controlling Ca2+ homeostasis and ROS production 161.
Microarray and high-throughput gene expression technologies combined with network analysis are additional tools advancing our understanding of how dysregulated gene pathways in disease are being identified. By analyzing publicly available databases and employing novel bioinformatics approaches, recent studies have identified several alternatively expressed gene pathways in sAD patients that may provide key mechanistic insight as well as therapeutic targets for AD 94-97. This level of analysis at the molecular level is a novel way to provide insight as to how gene-level changes and risk factors result in AD and cognitive decline. Gene networks associated with cognitive decline include signaling pathways that regulate Ca2+ handling, kinase activation, and mitochondrial bioenergetics. These include PLD, EGR1, PI3K, CALM3, FOXO3, and ITPBK, among others 95,96,98-100.
Implicating Ca2+ dyshomeostasis as a common process preceding other pathological hallmarks begs the question: how does Ca2+ fluctuate over a lifetime of regular aging that differs from a disease state? At this point, the limitations of the field are put on trial. Current animal models not only struggle to perfectly mirror all human components of the disease, but also healthy human aging. Recent advances to the field include the use of human induced pluripotent stem cells (iPSC) and direct reprogramming of fibroblasts, but future directions include better temporal analysis of calcium dynamics and biomarker detection. We do not have (at least at present) the tools to observe real-time calcium dynamics in the human brain noninvasively in the clinic. However, recent methods have emerged attempting to bridge this gap while technology advances. For example, by studying functional connectivity in key brain regions affected by AD pre-amyloid burden, researchers can compare the early effects of calcium dysregulation in AD rodent astrocytes to what we see in human disease development 101. Not only is this technique promising, but it emphasizes the link between whole brain calcium dysregulation and astrocyte activity, which will likely be studied heavily with the advent of high-throughput sequencing and transcriptomics 102.
Limitations in the field notwithstanding, the findings from previous studies aggregate intoa model in which various AD risk factors come together to result in compromised Ca2+ handling. This consequently sets off a cascade of events leading to neurodegeneration. Fig 5 outlines the basic tenets of such a scenario: 1) risk factors offset one or more processes leading to AD pathophysiology including most prominently increased influx of Ca2+ in the cytosol; 2) elevated [Ca2+]c amplifies AD pathophysiology in a feed-forward fashion; 3) neurodegeneration constitutes an active process, enabled by a global increase of [Ca2+]c. In other words, [Ca2+]c plays a central role in the fate and function of a neuron. Or metaphorically, [Ca2+]c judges the accumulated impact of risk factors either for good or bad, rules on the verdict, and executes the death sentence. Hence, maintenance and regulation of [Ca2+]c is crucial to secure optimal neuronal function and survival.
We believe this hypothesis unifies the broader scientific principles addressing AD mechanisms within the context of calcium dynamics in aging and neurodegeneration. Testing this hypothesis entails experimental approaches which selectively lower the global increase of [Ca2+]c to normal levels, preferably in conceptually different disease models to recapitulate the multifactorial nature of AD. Box 2 discusses the assertions, implications, and predictions of this hypothesis, detailing its ultimate test; intervention studies targeting Ca2+ dyshomeostasis in AD patients.
BOX 2. Towards a unifying hypothesis.
Fig. 5 outlines a comprehensive testable hypothesis providing a simple and plausible explanation of mechanisms underlying neurodegeneration and corresponding symptoms in AD. It unifies the most prominent hypotheses in the field and anticipates Ca2+ dyshomeostasis as a final common pathway to enable neurodegeneration124. This unified hypothesis posits assertions (listed below) addressing key questions and postulates in the field 20 and more importantly provide predictions of experimental outcomes to test its validity.
Assertions
Positive AD risk factors of AD are all conditions, lifestyles, or characteristics which directly or indirectly challenge Ca2+ homeostatic systems to maintain the optimal concentration gradient of [Ca2+]c concentration versus intracellular Ca2+ stores and the extracellular environment, whereas negative risk factors (protective) act in the opposite direction. As the term “gradient” already implies this is not a binary on or off process but rather a shift from an optimal average [Ca2+]c concentration to elevated suboptimal levels which may evolve over time to high, catastrophic levels. These translates in a correspondingly excessive activation of otherwise physiological effector pathways towards initially synaptic dysfunction and depression and ultimately to neurite degeneration or even neuronal demise.
Elevated [Ca2+]c is necessary and sufficient to enable neurodegeneration. In other words, AD risk factors are risk factors because these disrupt Ca2+ homeostasis. This implies that AD risk factors are tolerated as long as Ca2+ homeostatic systems can cope with these to maintain the gradient.
Ageing is a universal risk factor impacting, like any other risk factor, Ca2+ homeostasis contributing to age-related deterioration (biological ageing) of the brain.
AD risk factors accelerate biological ageing. A prominent mechanism of age-related degeneration (biological ageing) entails progressive damaging of mitochondria by ROS. Dysfunctional mitochondria in-turn have lowered the capacity to buffer excessive cytosolic Ca2+, whereas chronically elevated [Ca2+]c impairs mitochondrial function. This asserts that by impacting Ca2+ homeostasis, AD risk factors contribute to mitochondrial dysfunction and hence accelerate biological ageing.
Excessive [Ca2+]c drives the formation of AD pathophysiology and vice versa in a self-enforcing vicious cycle. This bidirectional interaction explains the common AD pathophysiology (such as tau tangles, Aβ aggregates, inflammation) in AD and as to how fAD risk factors (e.g., clinical mutations in APP) drive neurodegeneration.
Prolonged periods of mild Ca2+ dyshomeostasis or acute, large Ca2+ increases in the cytosol lead to equivalent damage increasing the vulnerability of neurons. In particular, a massive Ca2+ influx directly after acute brain injury (a major AD risk factor) may lead to similar overall accumulated ROS-instigated mitochondrial damage as different, less penetrant risk factors do over a longer period. In both cases the accumulated damage in mitochondria (signifying biological ageing) makes neurons less capable to control optimal Ca2+ homeostasis to cope with ongoing or future risk factors.
Disease onset and progression in a given patient is determined by the penetrance of AD risk factors. Highly penetrant ‘early onset” risk factors (such as in fAD) have a stronger impact on Ca2+ homeostasis compared to low penetrant risk factors reflecting the heterogeneity in onset and progression of neurodegeneration among patients.
The hypothesis outlined here asserts the most vulnerable neurons are those which are the least capable of maintaining optimal Ca2+ homeostasis and/or the most sensitive towards excessive Ca2+ signaling. It seems likely that selective neuronal vulnerability is the result of a combination of attributes such as neuronal type, activity, and selective receptiveness towards AD risk factors to off-set Ca2+ homeostasis. As a hypothetical example, neurons receiving intense glutamatergic Ca2+ signaling and expressing glucocorticoid receptors would be relatively sensitive to risk factors such as hypoxia, stress hormones, physical inactivity and ageing which impact mitochondrial function to maintain optimal Ca2+ buffering. Consistent with this notion is the fact that the hippocampus (one of the most vulnerable brain regions in AD) is the most vascularized and therefore most sensitive to conditions leading of decreased oxygen supply.
Predictions
The ultimate test of the proposed hypothesis would entail interventions or lifestyle changes towards normalization of Ca2+ homeostasis in AD patients:
Interventions which fully and selectively lower abnormally elevated [Ca2+]c level to non-pathological levels (without interfering with physiological Ca2+ signaling) would be most effective and safe to delay or halt neurodegeneration and the associated development of AD pathology. Moreover it has the potential to restore synaptic activity of dysfunctional neurons. Such interventions thus would have both symptomatic and “disease-modifying” effects (or perhaps more appropriately phrased sustained symptomatic benefit).
Treatments which partially lower [Ca2+]c level will be partially effective. Thus, targeting just one element of the pathophysiology (i.e. pathological Aβ or tau); or treatments with limited impact on the overall Ca2+ dyshomeostasis (such as memantine) are expected to have limited effect size. However, combination therapies addressing effectively multiple elements of the pathophysiology are expected to have added benefit.
Lifestyle changes which oppose the formation of AD pathophysiology (see Fig 5) are expected to be beneficial, but again, given the multifactorial nature of risk factors in sAD, likely result in limited effect size.
8. Therapeutic concepts and future directions – targeting the weakest link
If the objective of a therapeutic intervention is to generate clinically relevant effects in the heterogeneous sAD population, targeting singular risk factors may not be very effective. In such an approach, specific treatments would only be applicable to the corresponding AD subpopulation in which that targeted risk factor is a significant and direct contributor to the disease state. Rather, a more efficient approach would engage a pathway at which the various risk factors converge. From the model outlined in Fig. 5, this boils down to the question: which therapeutic approach most effectively lowers elevated [Ca2+]c to normal physiological levels? Concepts along those lines are currently being evaluated or considered, such as mitigating pathological Aβ, tau, or interventions aimed to restore mitochondrial function. However, given the complex, self-amplifying, and redundant nature of the different pathophysiological elements, it remains to be seen whether targeting just one element at this level will lead to a strong effect size in the heterogeneous patient population. An interesting recent example underlining this notion entails the Aβ lowering biologic lecanemab 103 which slows cognitive decline in sAD by 27% without acute symptomatic benefit.
Although still in preliminary stages, work has been done developing therapeutics for previously mentioned leaky RyR channels, both in the cardio-myopic and muscular domains as well as from the perspective of neurodegeneration 104. Many advances in developing RyR based therapeutics are dependent on a wholistic approach spanning genomic studies and structural imaging, both of which have seen rapid development since the inception of the calcium hypothesis. Interventions aimed at directly lowering abnormally increased [Ca2+]c to normal levels are expected to exert broader therapeutic outcomes, as this Ca2+elevation is at the core of synaptic dysfunction, inciting pathophysiological cascades, and inducing neuronal apoptosis. Given that Ca2+-signaling is a fundamental component of cellular functions, therapeutic modulation of this system without unacceptable side effects will not be easy but is not unprecedented. Memantine is an example of an effective drug in AD patients that decreases Ca2+ influx through partial antagonist action on the NMDA receptor 105. Although this illustrates conceptually that lowering elevated influx of calcium is clinically validated, the effect size is very limited because the action of memantine on the overall cellular Ca2+ homeostasis is also very limited 106. After all, the elevation of [Ca2+]c in AD neurons comprise mechanisms which go far beyond NMDAR activation alone (as discussed above).
Notably, there is a large body of work demonstrating broad therapeutic effects using negative allosteric modulators of the RyR, such as dantrolene and Ryanodex. As shown in numerous AD mouse models, these compounds serve to normalize RyR Ca2+ release, while reducing amyloid and tau pathology and preserving synaptic structure and function, as well as maintaining cognitive function 17,30,33-35,70,107-109. This profound therapeutic effect demonstrated by targeting a single Ca2+ channel speaks to the critical upstream pathogenic role of dysregulated RyR Ca2+ handling. Importantly, therapeutic potential has been demonstrated in human neurons derived from AD patients, in which not only Ca2+ signaling is normalized, but lysosome, autophagosome and mitochondrial functions as well 33,70. In a similar vein, studies in model cells have implicated related pathways important in ER Ca2+ handling, such as STIM2 and SOCE, in driving upstream AD pathophysiology 8,110.
Another clinical intervention geared towards neutralizing elevated [Ca2+]c is calcineurin (or protein phohphatase 2B) inhibition 111. This phosphatase responds with high affinity to [Ca2+]c and is a major player in many aspects of neurodegeneration including synaptic depression, inflammation, and neuronal apoptosis 53,84,112. Fig. 6 shows the outcome of a retrospective study of elderly patients who had undergone organ transplantation and were treated with tacrolimus or cyclosporine 113. The data indicate that the incidence of dementia was virtually absent compared to the general population suggesting that at least a prophylactic treatment with calcineurin inhibitors is highly effective in preventing dementia and warrants prospective clinical studies. This data back-translates well to non-clinical studies demonstrating improved cognitive function by calcineurin inhibition in different mouse models (transgenic APP and MAPT) of AD 114. In particular, LTP was restored to normality after just 7 days of treatment illustrating the point made above that synaptic function of “diseased” or “silent” neurons can be restored.
Fig. 6.
Prophylactic treatment with calcineurin inhibitors (CNI) lowers the incidence of dementia. Data from 113.
It is hopeful that the development of new model systems that offer more clinically-relevant insight into disease mechanisms and therapeutic potential will invigorate the field. Until fairly recently, transgenic mouse models expressing human familial AD mutations were the primary experimental tool to study pathological mechanisms, biomarkers, and therapeutic strategies for AD. Stem cell biology and cellular reprogramming techniques have advanced to a point where human neurons can be generated directly or indirectly (via iPSCs) from non-neuronal cells, such as fibroblasts or blood cells. This powerful approach allows for clinically-relevant cell types to be generated from AD patients and maintains many of the key genetic and epigenetic features that contribute to AD risk 115,116. Studies utilizing these human-derived neurons show profound abnormalities in intracellular calcium signaling, and markedly increased RyR-Ca2+ release from ER stores 33,70; these signaling abnormalities feed directly into protein handling deficits in the endolysosome-autophagosome pathways and results in accumulation of amyloid and phospho-tau. Thus, this provides evidence for a direct pathway by which altered calcium homeostasis contributes to proteinopathy in human AD neurons. It is also likely that the excess calcium feeds into aberrant mitochondrial bioenergetics and increased free radical production 117. Human organoids also provide a 3-dimensional matrix for generating multiple brain cell types (neurons, astrocytes, microglia) to examine more complex pathological interactions by which altered calcium homeostasis can drive a broad range of AD phenotypes 118,119. Collectively, the development of human neuron-based model systems, particularly when combined with the vast offerings of the ‘omics and bioinformatics explosion, can catapult the field and provide deeper understanding of mechanistic causes of AD and identify and validate effective therapeutic treatments.
Overall, we support the hypothesis that the most effective intervention point in the disease cascade entails restoring elevated [Ca2+]c to within normal physiological levels, and this could be attained through a variety of means. Additional experimental approaches to that end could include enhanced extrusions through activation of plasma membrane calcium-ATPase (PMCA) calcium channel activity, decreasing elevated calcium efflux or leakage from ER stores, increasing Ca2+ buffering/storage capacity of organelles or lowering elevated Ca2+ influx from the extracellular environment. We anticipate therapeutic modulation of targets directly involved in Ca2+ signaling (e.g., ligand gated calcium ion channels) are less attractive, because their modulation may not be well tolerated, and the effects on overall [Ca2+]c in the neuron might be limited. The mixed efficacy data of voltage-gated calcium channel (VGCC) inhibition in patients 120 or in animal models supports this consideration 121. Given that [Ca2+]c influences both synaptic functionality and neuronal survival, we anticipate that drugs capable of effectively normalizing Ca2+ homeostasis will have both symptomatic and disease-modifying actions in AD.
9. Conclusions
Here we propose a model of AD neurodegeneration which features Ca2+ dyshomeostasis as an upstream common process driving the major disease features. Risk factors of AD entail conditions, characteristics or lifestyles which when combined result in altered Ca2+ homeostasis and mishandling of Ca2+ gradients essential for optimal function of neurons. The resulting elevated increase of [Ca2+]c drives AD pathophysiology which may lead to further disruptions of Ca2+ homeostasis. This ultimately spirals [Ca2+]c from suboptimal to catastrophic levels paralleling the evolution from early synaptic dysfunction to neuronal death. Hence, therapeutic strategies aimed to counter the detrimental effects of risk factors on Ca2+ handling have the potential to restore synaptic function, reduce proteinopathy, preserve cellular metabolism and mitigate neuronal degeneration.
Research In Context.
Systematic review: The authors assessed peer-reviewed scientific literature using traditional sources. The objective of this review is to discuss the driving principles in dementia of mechanisms as to how widely different disease risk factors ultimately converge to a common disease outcome.
Interpretation: The author’s findings led to a conceptual outline presented in the manuscript proposing calcium dyshomeostasis as a common final pathway leading to synaptic dysfunction with corresponding symptoms and ultimately neuronal loss in dementia.
Future directions: The manuscript provides testable hypotheses as to the role of calcium dysregulation with respect to neuronal degeneration in function of AD risk factors. Further it provides a framework to conceptualize effective and much needed therapeutic strategies aimed to normalise calcium homeostasis in AD.
Acknowledgements
GES is funded by NIH/NIA 1RF1AG065628
Footnotes
Conflicts of interests
GG is consultant for reMYND. MF is full time employee of reMYND. GG and MF own reMYND warrants and/or stocks. EKW and GES have nothing to disclose.
10. References
- 1.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society. 1991;30(4):572–580. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi T, Duszkiewicz AJ, Morris RG. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1633):20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nature Cell Biology 2003 5:12. 2003;5(12):1041–1043. doi: 10.1038/ncb1203-1041 [DOI] [PubMed] [Google Scholar]
- 5.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature reviews Molecular cell biology. 2003;4(7):552–565. doi: 10.1038/nrm1150 [DOI] [PubMed] [Google Scholar]
- 6.Mustaly-Kalimi S, Littlefield AM, Stutzmann GE. Calcium Signaling Deficits in Glia and Autophagic Pathways Contributing to Neurodegenerative Disease. Antioxidants & Redox Signaling. 2018;29(12):1158–1175. doi: 10.1089/ars.2017.7266 [DOI] [PubMed] [Google Scholar]
- 7.Stutzmann GE. The Pathogenesis of Alzheimers Disease—Is It a Lifelong “Calciumopathy”? Neuroscientist. 2007;13(5):546–559. doi: 10.1177/1073858407299730 [DOI] [PubMed] [Google Scholar]
- 8.Zernov N, Bezprozvanny I, Popugaeva E. CaMKIIβ knockdown decreases store-operated calcium entry in hippocampal dendritic spines. IBRO Neuroscience Reports. 2022;12:90–97. doi: 10.1016/j.ibneur.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cárdenas C, Miller RA, Smith I, et al. Essential Regulation of Cell Bioenergetics by Constitutive InsP3 Receptor Ca2+ Transfer to Mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galeotti N, Quattrone A, Vivoli E, Norcini M, Bartolini A, Ghelardini C. Different involvement of type 1, 2, and 3 ryanodine receptors in memory processes. Learning & memory (Cold Spring Harbor, NY). 2008;15(5):315–323. doi: 10.1101/lm.929008.During [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiess F, Yao J, Song Z, et al. Subcellular localization of hippocampal ryanodine receptor 2 and its role in neuronal excitability and memory. Communications Biology. 2022;5(1). doi: 10.1038/s42003-022-03124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roderick HL, Berridge MJ, Bootman MD. Calcium-induced calcium release. Current Biology. 2003;13(11):R425. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ. Calcium Signalling and Alzheimer’s Disease. Neurochemical Research. 2011;36(7):1149–1156. doi: 10.1007/s11064-010-0371-4 [DOI] [PubMed] [Google Scholar]
- 14.De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910 [DOI] [PubMed] [Google Scholar]
- 15.Jayapalan S, Natarajan J. The role of CDK5 and GSK3B kinases in hyperphosphorylation of microtubule associated protein tau (MAPT) in Alzheimer’s disease. Bioinformation. 2013;9(20):1023–1030. doi: 10.6026/97320630091023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakroborty S, Kim J, Schneider C, Jacobson C, Molgó J, Stutzmann GE. Early Presynaptic and Postsynaptic Calcium Signaling Abnormalities Mask Underlying Synaptic Depression in Presymptomatic Alzheimer’s Disease Mice. J Neurosci. 2012;32(24):8341. doi: 10.1523/JNEUROSCI.0936-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakroborty S, Hill ES, Christian DT, et al. Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):7. doi: 10.1186/s13024-019-0307-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends in Neurosciences. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Q, Sopher BL, Furukawa K, et al. Alzheimer’s Presenilin Mutation Sensitizes Neural Cells to Apoptosis Induced by Trophic Factor Withdrawal and Amyloid β-Peptide: Involvement of Calcium and Oxyradicals. J Neurosci. 1997;17(11):4212. doi: 10.1523/JNEUROSCI.17-11-04212.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzheimer’s Association Calcium Hypothesis Workgroup, Khachaturian ZS. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer’s & Dementia. 2017;13(2):178–182.e17. doi: 10.1016/j.jalz.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 21.Etcheberrigaray R, Hirashima N, Nee L, et al. Calcium Responses in Fibroblasts from Asymptomatic Members of Alzheimer’s Disease Families. Neurobiology of Disease. 1998;5(1):37–45. doi: 10.1006/nbdi.1998.0176 [DOI] [PubMed] [Google Scholar]
- 22.Ito E, Oka K, Etcheberrigaray R, et al. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proceedings of the National Academy of Sciences. 1994;91(2):534–538. doi: 10.1073/pnas.91.2.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leissring MA, Paul BA, Parker I, Cotman CW, LaFerla FM. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem. 1999;72(3):1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x [DOI] [PubMed] [Google Scholar]
- 24.Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275(24):18195–18200. doi: 10.1074/jbc.M000040200 [DOI] [PubMed] [Google Scholar]
- 25.Cheung KH, Mei L, Mak DOD, et al. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer’s disease-linked presenilin mutants in human cells and mouse neurons. Science signaling. 2010;3(114). doi: 10.1126/SCISIGNAL.2000818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stutzmann GE, Smith I, Caccamo A, Oddo S, LaFerla FM, Parker I. Enhanced Ryanodine Receptor Recruitment Contributes to Ca2+ Disruptions in Young, Adult, and Aged Alzheimer’s Disease Mice. J Neurosci. 2006;26(19):5180. doi: 10.1523/JNEUROSCI.0739-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakroborty S, Goussakov I, Miller MB, Stutzmann GE. Deviant Ryanodine Receptor-Mediated Calcium Release Resets Synaptic Homeostasis in Presymptomatic 3xTg-AD Mice. J Neurosci. 2009;29(30):9458. doi: 10.1523/JNEUROSCI.2047-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci. 2010;30(36):12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chami M, Checler F. Alterations of the Endoplasmic Reticulum (ER) Calcium Signaling Molecular Components in Alzheimer’s Disease. Cells. 2020;9(12):2577. doi: 10.3390/cells9122577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacampagne A, Liu X, Reiken S, et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol. 2017;134(5):749–767. doi: 10.1007/s00401-017-1733-7 [DOI] [PubMed] [Google Scholar]
- 31.Zissimopoulos S, West DJ, Williams AJ, Lai FA. Ryanodine receptor interaction with the SNARE-associated protein snapin. Journal of cell science. 2006;119(11):2386–2397. [DOI] [PubMed] [Google Scholar]
- 32.Huang JK, Ma PL, Ji SY, et al. Age-dependent alterations in the presynaptic active zone in a Drosophila model of Alzheimer’s Disease. Neurobiology of Disease. 2013;51:161–167. doi: 10.1016/j.nbd.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Schrank S, McDaid J, Briggs CA, et al. Human-Induced Neurons from Presenilin 1 Mutant Patients Model Aspects of Alzheimer’s Disease Pathology. International Journal of Molecular Sciences. 2020;21(3). doi: 10.3390/ijms21031030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakroborty S, Briggs C, Miller MB, et al. Stabilizing ER Ca2+ Channel Function as an Early Preventative Strategy for Alzheimer’s Disease. PLOS ONE. 2012;7(12):e52056. doi: 10.1371/journal.pone.0052056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oules B, Del Prete D, Greco B, et al. Ryanodine Receptor Blockade Reduces Amyloid- Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. Journal of Neuroscience. 2012;32(34):11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2012;33(5):1001.e1–6. doi: 10.1016/j.neurobiolaging.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelliher M, Fastbom J, Cowburn RF, et al. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92(2):499–513. doi: 10.1016/s0306-4522(99)00042-1 [DOI] [PubMed] [Google Scholar]
- 38.Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein–protein interaction. Cell Calcium. 2008;44(5):507–518. doi: 10.1016/j.ceca.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 39.Lobos Pedro, Córdova Alex, Vega-Vásquez Ignacio, et al. RyR-mediated Ca2+ release elicited by neuronal activity induces nuclear Ca2+ signals, CREB phosphorylation, and Npas4/RyR2 expression. Proceedings of the National Academy of Sciences. 2021;118(33):e2102265118. doi: 10.1073/pnas.2102265118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harbor perspectives in biology. 2010;2(11):a003996–a003996. doi: 10.1101/cshperspect.a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enomoto M, Nishikawa T, Siddiqui N, Chung S, Ikura M, Stathopulos PB. From Stores to Sinks: Structural Mechanisms of Cytosolic Calcium Regulation. Adv Exp Med Biol. 2017;981:215–251. doi: 10.1007/978-3-319-55858-5_10 [DOI] [PubMed] [Google Scholar]
- 42.Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biology. 2015;6:260. doi: 10.1016/J.REDOX.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423–1433. doi: 10.1038/sj.cdd.4401950 [DOI] [PubMed] [Google Scholar]
- 44.Wilson EL, Metzakopian E. ER-mitochondria contact sites in neurodegeneration: genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ. 2021;28(6):1804–1821. doi: 10.1038/s41418-020-00705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN neuro. 2010;2(5):AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 47.Makin S. The amyloid hypothesis on trial. Nature. 2018;559(7715):S4–S4. [DOI] [PubMed] [Google Scholar]
- 48.Shen JJ, Barrios RJ, Jaiswal AKAAK, et al. NIH Public Access. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;5(3):1–12. doi: 10.1016/j.ejmech.2005.12.002 [DOI] [Google Scholar]
- 49.Mary A, Eysert F, Checler F, Chami M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol Psychiatry. 2023;28(1):202–216. doi: 10.1038/s41380-022-01631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cline EN, Bicca MA, Viola KL, Klein WL. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. Journal of Alzheimer’s Disease. 2018;64(s1):S567–S610. doi: 10.3233/JAD-179941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harbor perspectives in medicine. 2012;2(7). doi: 10.1101/CSHPERSPECT.A006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Citri A, Malenka RC. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology Reviews. 2008;33:18–41. doi: 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- 53.Wang HG, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science (New York, NY). 1999;284(5412):339–343. doi: 10.1126/SCIENCE.284.5412.339 [DOI] [PubMed] [Google Scholar]
- 54.Guan PP, Cao LL, Wang P. Elevating the Levels of Calcium Ions Exacerbate Alzheimer’s Disease via Inducing the Production and Aggregation of β-Amyloid Protein and Phosphorylated Tau. International Journal of Molecular Sciences. 2021;22(11). doi: 10.3390/ijms22115900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berridge MJ. Calcium regulation of neural rhythms, memory and Alzheimer’s disease. The Journal of physiology. 2014;592(Pt 2):281–293. doi: 10.1113/jphysiol.2013.257527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawson GR, Seabrook GR, Zheng H, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the β-amyloid precursor protein. Neuroscience. 1999;90(1):1–13. doi: 10.1016/S0306-4522(98)00410-2 [DOI] [PubMed] [Google Scholar]
- 57.Lombardo S, Chiacchiaretta M, Tarr A, et al. BACE1 partial deletion induces synaptic plasticity deficit in adult mice. Sci Rep. 2019;9(1):19877. doi: 10.1038/s41598-019-56329-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Knight C, Chen SRW, Bezprozvanny I. A gating mutation in ryanodine receptor type 2 rescues phenotypes of Alzheimer’s disease mouse models by upregulating neuronal autophagy. J Neurosci. Published online January 10, 2023. doi: 10.1523/JNEUROSCI.1820-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Um JW, Kaufman AC, Kostylev M, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79(5):887–902. doi: 10.1016/j.neuron.2013.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaufman AC, Salazar SV, Haas LT, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Annals of Neurology. 2015;77(6):953–971. doi: 10.1002/ana.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ittner LM, Ke YD, Delerue F, et al. Dendritic Function of Tau Mediates Amyloid-β Toxicity in Alzheimer’s Disease Mouse Models. Cell. 2010;142(3):387–397. doi: 10.1016/J.CELL.2010.06.036 [DOI] [PubMed] [Google Scholar]
- 63.Bi M, Gladbach A, Van Eersel J, et al. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nature communications. 2017;8(1). doi: 10.1038/S41467-017-00618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopes S, Vaz-Silva J, Pinto V, et al. Tau protein is essential for stress-induced brain pathology. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(26):E3755–E3763. doi: 10.1073/PNAS.1600953113/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23(10):1183–1193. doi: 10.1038/s41593-020-0687-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morris M, Maeda S, Vossel K, Mucke L. The Many Faces of Tau. Neuron. 2011;70(3):410–426. doi: 10.1016/J.NEURON.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ye J, Yin Y, Yin Y, et al. Tau-induced upregulation of C/EBPβ-TRPC1-SOCE signaling aggravates tauopathies: A vicious cycle in Alzheimer neurodegeneration. Aging Cell. 2020;19(9):e13209. doi: 10.1111/ACEL.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uéda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proceedings of the National Academy of Sciences. 1993;90(23):11282–11286. doi: 10.1073/pnas.90.23.11282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terman A, Gustafsson B, Brunk U. Autophagy, organelles and ageing. The Journal of Pathology. 2007;211(2):134–143. doi: 10.1002/path.2094 [DOI] [PubMed] [Google Scholar]
- 70.Mustaly-Kalimi S, Gallegos W, Marr RA, et al. Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proceedings of the National Academy of Sciences. 2022;119(49):e2211999119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mindell JA. Lysosomal Acidification Mechanisms. Annu Rev Physiol. 2012;74(1):69–86. doi: 10.1146/annurev-physiol-012110-142317 [DOI] [PubMed] [Google Scholar]
- 72.Lee JH, McBrayer MK, Wolfe DM, et al. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12(9):1430–1444. doi: 10.1016/j.celrep.2015.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Man SM, Kanneganti TD. Regulation of lysosomal dynamics and autophagy by CTSB/cathepsin B. Autophagy. 2016;12(12):2504–2505. doi: 10.1080/15548627.2016.1239679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S, Sato Y, Nixon RA. Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy. 2011;7(12):1562–1563. doi: 10.4161/auto.7.12.17956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JH, Yang DS, Goulbourne CN, et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci. 2022;25(6):688–701. doi: 10.1038/s41593-022-01084-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jun Kisun, Piedras-Rentería Erika S., Smith Stephen M., et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proceedings of the National Academy of Sciences. 1999;96(26):15245–15250. doi: 10.1073/pnas.96.26.15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian X, Gala U, Zhang Y, et al. A Voltage-Gated Calcium Channel Regulates Lysosomal Fusion with Endosomes and Autophagosomes and Is Required for Neuronal Homeostasis. PLOS Biology. 2015;13(3):e1002103. doi: 10.1371/journal.pbio.1002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lie Pearl P. Y., Yoo Lang, Goulbourne Chris N., et al. Axonal transport of late endosomes and amphisomes is selectively modulated by local Ca2+ efflux and disrupted by PSEN1 loss of function. Science Advances. 8(17):eabj5716. doi: 10.1126/sciadv.abj5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.SanMartín CD, Veloso P, Adasme T, et al. RyR2-Mediated Ca2+ Release and Mitochondrial ROS Generation Partake in the Synaptic Dysfunction Caused by Amyloid β Peptide Oligomers. Frontiers in Molecular Neuroscience. 2017;10. https://www.frontiersin.org/article/10.3389/fnmol.2017.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tapella L, Dematteis G, Moro M, et al. Protein synthesis inhibition and loss of homeostatic functions in astrocytes from an Alzheimer’s disease mouse model: a role for ER-mitochondria interaction. Cell Death Dis. 2022;13(10):1–16. doi: 10.1038/s41419-022-05324-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dematteis G, Vydmantaitė G, Ruffinatti FA, et al. Proteomic analysis links alterations of bioenergetics, mitochondria-ER interactions and proteostasis in hippocampal astrocytes from 3xTg-AD mice. Cell Death Dis. 2020;11(8):1–16. doi: 10.1038/s41419-020-02911-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovascular research. 2012;96(1):32–37. doi: 10.1093/CVR/CVS163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reese L C, Taglialatela G A Role for Calcineurin in Alzheimer’s Disease. Current Neuropharmacology. 2011;9(4):685–692. doi: 10.2174/157015911798376316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reese LC, Taglialatela G. Neuroimmunomodulation by Calcineurin in Aging and Alzheimer’s Disease. Aging and Disease. 2010;1(3):245. [PMC free article] [PubMed] [Google Scholar]
- 85.Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. The FEBS journal. 2006;273(15):3437–3443. doi: 10.1111/J.1742-4658.2006.05352.X [DOI] [PubMed] [Google Scholar]
- 86.Arrázola MS, Court FA. Compartmentalized necroptosis activation in excitotoxicity-induced axonal degeneration: a novel mechanism implicated in neurodegenerative disease pathology. Neural Regeneration Research. 2019;14(8). https://journals.lww.com/nrronline/Fulltext/2019/14080/Compartmentalized_necroptosis_activation_in.19.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stanciu M, Wang Y, Kentor R, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. The Journal of biological chemistry. 2000;275(16):12200–12206. doi: 10.1074/JBC.275.16.12200 [DOI] [PubMed] [Google Scholar]
- 88.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010;1802(1):2–10. doi: 10.1016/J.BBADIS.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 89.Perez Ortiz JM, Swerdlow RH. Mitochondrial dysfunction in Alzheimer’s disease: Role in pathogenesis and novel therapeutic opportunities. British journal of pharmacology. 2019;176(18):3489–3507. doi: 10.1111/BPH.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calvo-Rodriguez M, Hou SS, Snyder AC, et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nature Communications 2020 11:1. 2020;11(1):1–17. doi: 10.1038/s41467-020-16074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Müller M, Ahumada-Castro U, Sanhueza M, Gonzalez-Billault C, Court FA, Cárdenas C. Mitochondria and calcium regulation as basis of neurodegeneration associated with aging. Frontiers in Neuroscience. 2018;12(JUL):470. doi: 10.3389/FNINS.2018.00470/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tambini MD, Norris KA, D’Adamio L. Opposite changes in APP processing and human Aβ levels in rats carrying either a protective or a pathogenic APP mutation. eLife. 2020;9. doi: 10.7554/ELIFE.52612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magno L, Lessard CB, Martins M, et al. Alzheimer’s disease phospholipase C-gamma-2 (PLCG2) protective variant is a functional hypermorph. Alzheimer’s Research & Therapy. 2019;11(1). doi: 10.1186/S13195-019-0469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Potashkin JA, Bottero V, Santiago JA, Quinn JP. Computational identification of key genes that may regulate gene expression reprogramming in Alzheimer’s patients. PLoS One. 2019;14(9):e0222921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bottero V, Powers D, Yalamanchi A, Quinn JP, Potashkin JA. Key disease mechanisms linked to Alzheimer’s disease in the entorhinal cortex. International journal of molecular sciences. 2021;22(8):3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santiago JA, Bottero V, Potashkin JA. Transcriptomic and network analysis identifies shared and unique pathways across dementia spectrum disorders. International Journal of Molecular Sciences. 2020;21(6):2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bottero V, Potashkin JA. Meta-analysis of gene expression changes in the blood of patients with mild cognitive impairment and Alzheimer’s disease dementia. International journal of molecular sciences. 2019;20(21):5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Emilsson L, Saetre P, Jazin E. Alzheimer’s disease: mRNA expression profiles of multiple patients show alterations of genes involved with calcium signaling. Neurobiology of disease. 2006;21(3):618–625. [DOI] [PubMed] [Google Scholar]
- 99.Borjabad A, Volsky DJ. Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and Multiple Sclerosis. Journal of Neuroimmune Pharmacology. 2012;7:914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biological psychiatry. 2015;77(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah D, Gsell W, Wahis J, et al. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease. Cell Reports. 2022;40(8):111280. doi: 10.1016/j.celrep.2022.111280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brandebura AN, Paumier A, Onur TS, Allen NJ. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci. 2023;24(1):23–39. doi: 10.1038/s41583-022-00641-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. New England Journal of Medicine. Published online 2022. [Google Scholar]
- 104.Marks AR. Targeting ryanodine receptors to treat human diseases. J Clin Invest. 2023;133(2). doi: 10.1172/JCI162891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rogawski MA, Wenk GL. The Neuropharmacological Basis for the Use of Memantine in the Treatment of Alzheimer’s Disease. CNS Drug Reviews. 2003;9(3):275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsunaga S, Kishi T, Iwata N. Memantine Monotherapy for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. PLOS ONE. 2015;10(4):e0123289. doi: 10.1371/journal.pone.0123289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng J, Liang G, Inan S, et al. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neuroscience Letters. 2012;516(2):274–279. doi: 10.1016/j.neulet.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H. Dantrolene Ameliorates Impaired Neurogenesis and Synaptogenesis in Induced Pluripotent Stem Cell Lines Derived from Patients with Alzheimer’s Disease. Anesthesiology. 2020;132(5):1062–1079. doi: 10.1097/ALN.0000000000003224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium Signaling, Excitability, and Synaptic Plasticity Defects in a Mouse Model of Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2015;45(2):561–580. doi: 10.3233/JAD-142427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Popugaeva E, Bezprozvanny I. STIM proteins as regulators of neuronal store-operated calcium influx. Neurodegenerative Disease Management. 2018;8(1):5–7. doi: 10.2217/nmt-2017-0053 [DOI] [PubMed] [Google Scholar]
- 111.O’Neal MA, Stallings NR, Malter JS. Alzheimer’s Disease, Dendritic Spines, and Calcineurin Inhibitors: A New Approach? ACS Chem Neurosci. 2018;9(6):1233–1234. doi: 10.1021/acschemneuro.8b00213 [DOI] [PubMed] [Google Scholar]
- 112.Ayton S, Lei P. The Aβ-induced NFAT apoptotic pathway is also activated by GSK-3 inhibition: implications for Alzheimer therapeutics. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(28):9454–9456. doi: 10.1523/JNEUROSCI.2143-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taglialatela G, Rastellini C, Cicalese L. Reduced Incidence of Dementia in Solid Organ Transplant Patients Treated with Calcineurin Inhibitors. Journal of Alzheimer’s disease : JAD. 2015;47(2):329–333. doi: 10.3233/JAD-150065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavallucci V, Berretta N, Nobili A, Nisticò R, Mercuri NB, D’Amelio M. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromolecular medicine. 2013;15(3):541–548. doi: 10.1007/S12017-013-8241-2 [DOI] [PubMed] [Google Scholar]
- 115.Lagomarsino VN, Pearse RV, Liu L, et al. Stem cell-derived neurons reflect features of protein networks, neuropathology, and cognitive outcome of their aged human donors. Neuron. 2021;109(21):3402–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu L, Hsieh YC, Pearse RV, et al. Association of AK4 Protein From Stem Cell-Derived Neurons With Cognitive Reserve: An Autopsy Study. Neurology. 2022;99(20):e2264–e2274. doi: 10.1212/WNL.0000000000201120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calvo-Rodriguez M, Bacskai BJ. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends in Neurosciences. 2021;44(2):136–151. doi: 10.1016/j.tins.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 118.Cenini G, Hebisch M, Iefremova V, et al. Dissecting Alzheimer’s disease pathogenesis in human 2D and 3D models. Molecular and Cellular Neuroscience. 2021;110:103568. doi: 10.1016/j.mcn.2020.103568 [DOI] [PubMed] [Google Scholar]
- 119.Yin J, VanDongen AM. Enhanced Neuronal Activity and Asynchronous Calcium Transients Revealed in a 3D Organoid Model of Alzheimer’s Disease. ACS Biomater Sci Eng. 2021;7(1):254–264. doi: 10.1021/acsbiomaterials.0c01583 [DOI] [PubMed] [Google Scholar]
- 120.Birks J, López-Arrieta J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database of Systematic Reviews. 2002;(3). doi: 10.1002/14651858.CD000147 [DOI] [PubMed] [Google Scholar]
- 121.Sadleir KR, Popovic J, Khatri A, Vassar R. Oral nimodipine treatment has no effect on amyloid pathology or neuritic dystrophy in the 5XFAD mouse model of amyloidosis. PLOS ONE. 2022;17(2):e0263332. doi: 10.1371/journal.pone.0263332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology. 2000;1(1):11–21. doi: 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- 123.Mattson MP. Calcium and neurodegeneration. Aging cell. 2007;6(3):337–350. [DOI] [PubMed] [Google Scholar]
- 124.Khachaturian ZS. Calcium hypothesis of Alzheimer’s disease and brain aging. Annals of the New York Academy of Sciences. 1994;747:1–11. doi: 10.1111/J.1749-6632.1994.TB44398.X [DOI] [PubMed] [Google Scholar]
- 125.Itkin A, Dupres V, Dufrene YF, Bechinger B, Ruysschaert JM, Raussens V. Calcium ions promote formation of amyloid beta-peptide (1-40) oligomers causally implicated in neuronal toxicity of Alzheimer’s disease. PloS one. 2011;6(3):e18250. doi: 10.1371/journal.pone.0018250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Isaacs AM, Senn DB, Yuan M, Shine JP, Yankner BA. Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. The Journal of biological chemistry. 2006;281(38):27916–27923. doi: 10.1074/JBC.M602061200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pierrot N, Ghisdal P, Caumont AS, Octave JN. Intraneuronal amyloid-beta1-42 production triggered by sustained increase of cytosolic calcium concentration induces neuronal death. Journal of neurochemistry. 2004;88(5):1140–1150. doi: 10.1046/J.1471-4159.2003.02227.X [DOI] [PubMed] [Google Scholar]
- 128.Buxbaum JD, Ruefli AA, Parker CA, Cypess AM, Greengard P. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(10):4489–4493. doi: 10.1073/PNAS.91.10.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry. 1994;33(15):4550–4561. doi: 10.1021/BI00181A016 [DOI] [PubMed] [Google Scholar]
- 130.Li L, Ke K, Tan X, et al. Up-regulation of NFATc4 Involves in Neuronal Apoptosis Following Intracerebral Hemorrhage. Cellular and Molecular Neurobiology. 2013;33(7):893–905. doi: 10.1007/s10571-013-9955-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mei Z, Yan P, Tan X, Zheng S, Situ B. Transcriptional Regulation of BACE1 by NFAT3 Leads to Enhanced Amyloidogenic Processing. Neurochemical Research. 2015;40(4):829–836. doi: 10.1007/s11064-015-1533-1 [DOI] [PubMed] [Google Scholar]
- 132.Liang B, Duan BY, Zhou XP, Gong JX, Luo ZG. Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. The Journal of biological chemistry. 2010;285(36):27737–27744. doi: 10.1074/JBC.M110.117960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Kimpe L, Bennis A, Zwart R, van Haastert ES, Hoozemans JJM, Scheper W. Disturbed Ca2+ homeostasis increases glutaminyl cyclase expression; connecting two early pathogenic events in Alzheimer’s disease in vitro. PloS one. 2012;7(9). doi: 10.1371/JOURNAL.PONE.0044674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McDaid J, Mustaly-Kalimi S, Stutzmann GE. Ca(2+) Dyshomeostasis Disrupts Neuronal and Synaptic Function in Alzheimer’s Disease. Cells. 2020;9(12). doi: 10.3390/cells9122655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends in Molecular Medicine. 2009;15(3):112–119. doi: 10.1016/J.MOLMED.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 136.O’Day DH, Eshak K, Myre MA. Calmodulin binding proteins and Alzheimer’s disease. Journal of Alzheimer’s Disease. 2015;46(3):553–569. doi: 10.3233/JAD-142772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pooler AM, Phillips EC, Lau DHW, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO reports. 2013;14(4):389–394. doi: 10.1038/embor.2013.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Quist A, Doudevski I, Lin H, et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10427–10432. doi: 10.1073/PNAS.0502066102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(22):10573–10577. doi: 10.1073/PNAS.90.22.10573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sciaccaluga M, Megaro A, Bellomo G, et al. An Unbalanced Synaptic Transmission: Cause or Consequence of the Amyloid Oligomers Neurotoxicity? International Journal of Molecular Sciences. 2021;22(11). doi: 10.3390/IJMS22115991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. Journal of Biological Chemistry. 2010;285(17):12463–12468. doi: 10.1074/jbc.R109.080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Current molecular medicine. 2008;8(2):119–130. doi: 10.2174/156652408783769571 [DOI] [PubMed] [Google Scholar]
- 143.Hall AM, Throesch BT, Buckingham SC, et al. Tau-Dependent Kv4.2 Depletion and Dendritic Hyperexcitability in a Mouse Model of Alzheimer’s Disease. The Journal of Neuroscience. 2015;35(15):6221. doi: 10.1523/JNEUROSCI.2552-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guha S, Johnson GVW, Nehrke K. The Crosstalk Between Pathological Tau Phosphorylation and Mitochondrial Dysfunction as a Key to Understanding and Treating Alzheimer’s Disease. Molecular neurobiology. 2020;57(12):5103–5120. doi: 10.1007/S12035-020-02084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca(2+)-ATPase. Free radical biology & medicine. 1999;27(7-8):810–821. doi: 10.1016/S0891-5849(99)00128-8 [DOI] [PubMed] [Google Scholar]
- 146.Filadi R, Pizzo P. Defective autophagy and Alzheimer’s disease: is calcium the key? Neural regeneration research. 2019;14(12):2081–2082. doi: 10.4103/1673-5374.262584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park KM, Yule DI, Bowers WJ. Tumor necrosis factor-alpha-mediated regulation of the inositol 1,4,5-trisphosphate receptor promoter. The Journal of biological chemistry. 2009;284(40):27557–27566. doi: 10.1074/JBC.M109.034504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bezprozvanny llya, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of lns(l,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351(6329):751–754. doi: 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- 150.Cirrito JR, Kang JE, Lee J, et al. Endocytosis Is Required for Synaptic Activity-Dependent Release of Amyloid-β In Vivo. Neuron. 2008;58(1):42–51. doi: 10.1016/J.NEURON.2008.02.003/ATTACHMENT/499C70EB-C6AB-40C9-96F6-4F790A465F2D/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. Journal of neuroscience research. 2002;67(3):379–387. doi: 10.1002/JNR.10138 [DOI] [PubMed] [Google Scholar]
- 152.Ma Z, Siebert AP, Cheung KH, et al. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):E1963–E1971. doi: 10.1073/PNAS.1204023109/-/DCSUPPLEMENTAL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140(6):918. doi: 10.1016/J.CELL.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goulay R, Mena Romo L, Hol EM, Dijkhuizen RM. From Stroke to Dementia: a Comprehensive Review Exposing Tight Interactions Between Stroke and Amyloid-β Formation. Translational Stroke Research. 2020;11(4):601–601. doi: 10.1007/S12975-019-00755-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang F, Niu L, Li S, Le W. Pathological Impacts of Chronic Hypoxia on Alzheimer’s Disease. ACS chemical neuroscience. 2019;10(2):902–909. doi: 10.1021/ACSCHEMNEURO.8B00442 [DOI] [PubMed] [Google Scholar]
- 156.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature reviews Cardiology. 2018;15(9):505–522. doi: 10.1038/S41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Z, Shue F, Zhao N, Shinohara M, Bu G. APOE2: protective mechanism and therapeutic implications for Alzheimer’s disease. doi: 10.1186/s13024-020-00413-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meng Q, Lin MS, Tzeng IS. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Frontiers in Neuroscience. 2020;14. doi: 10.3389/FNINS.2020.00131/PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Griñán-Ferré C, Bellver-Sanchis A, Izquierdo V, et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing research reviews. 2021;67. doi: 10.1016/J.ARR.2021.101271 [DOI] [PubMed] [Google Scholar]
- 160.Chai B, Gao F, Wu R, et al. Vitamin d deficiency as a risk factor for dementia and alzheimer’s disease: An updated meta-analysis. BMC Neurology. 2019;19(1):1–11. doi: 10.1186/S12883-019-1500-6/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berridge MJ. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016;371(1700). doi: 10.1098/RSTB.2015.0434 [DOI] [PMC free article] [PubMed] [Google Scholar]