Abstract
COI1-mediated perception of jasmonate is critical for plant development and responses to environmental stresses. Monocots such as rice have two groups of COI genes due to gene duplication: OsCOI1a and OsCOI1b that are functionally equivalent to the dicotyledons COI1 and OsCOI2 whose function remains unclear. In order to assess the function of OsCOI2 and its functional redundancy with COI1 genes, we developed a series of rice mutants in the 3 genes OsCOI1a, OsCOI1b and OsCOI2 by CRISPR Cas9-mediated editing and characterized their phenotype and responses to jasmonate. Characterization of OsCOI2 uncovered its important roles in root, leaf and flower development. In particular, we show that crown root growth inhibition by jasmonate relies on OsCOI2 and not on OsCOI1a nor on OsCOI1b, revealing a major function for the non-canonical OsCOI2 in jasmonate-dependent control of rice root growth. Collectively, these results point to a specialized function of OsCOI2 in the regulation of plant development in rice and indicate that sub-functionalisation of jasmonate receptors has occurred in the monocot phylum.
Introduction
Jasmonoyl-isoleucine (JA-Ile), the bioactive form of jasmonic acid (JA), regulates many physiological and developmental processes in plants such as flower and root development [1, 2]. Plants from monocot and eudicot lineages accumulate JA-Ile in response to stresses, such as injury or insect attack [3, 4]. Jasmonate perception and transcriptional regulation of JA-responsive genes relies on a conserved core protein complex, the main components of which are the F-box protein CORONATINE INSENSITIVE1 (COI1) protein, the JASMONATE ZIM DOMAIN (JAZ) transcriptional repressors and transcription factors (TFs) [5–9]. In the absence of JA-Ile, target gene expression is repressed due to TF and JAZ protein interaction within the TOPLESS (TPL)-NOVEL INTERACTORS OF JAZ (NINJA) repressor complex [10]. Upon JA-Ile accumulation, JAZ proteins are recruited by COI1, ubiquitinated and rapidly degraded in a proteasome-dependent manner, thus freeing TFs and leading to the de-repression of JA-responsive genes and subsequent physiological responses.
In eudicot species such as Arabidopsis and tomato, JA-Ile is perceived by a single receptor encoded by COI1, that interacts with members of the JAZ family [5–7]. In contrast, monocots such as rice, maize and wheat have two groups of COI genes due to gene duplication [4, 11, 12]. In rice, there are three COI genes, named OsCOI1a, OsCOI1b, and OsCOI2. OsCOI1a and OsCOI1b are orthologous to AtCOI1 as shown by complementation of the loss-of-function coi1-1 mutant in Arabidopsis [13]. Several studies in rice demonstrated the involvement of OsCOI1a and OsCOI1b in plant defence. Transgenic rice plants with a reduced expression of both OsCOI1a and OsCOI1b genes display a phenotype similar to gibberellic acid over-accumulating plants, including elongated internodes which suggests that jasmonate signaling promotes defence response over growth [14]. In a second study, silencing of OsCOI1a and OsCOIb gene expression led to an increased susceptibility to the rice leaf-folder insect [15] and to the rice stripe virus, the resistance to which is known to be conferred by JA signalling elements [16].
In contrast, very little is known about OsCOI2 function in rice. OsCOI2 fails to rescue the fertility and defence response defect of coi1-1 Arabidopsis plants, nor does it interact with any AtJAZ co-receptor [13], suggesting that OsCOI2 may have as-yet unidentified function(s) distinct from that of OsCOI1. Similarly, in maize (Zea mays), three closely related ZmCOI1 genes could complement the coi1-1 mutation in Arabidopsis, but ZmCOI2 did not [11]. Interestingly, a substitution in OsCOI2 of one amino acid predicted to be involved in JA-Ile interaction allowed it to interact with AtJAZs and to rescue coi1-1 deficiency [13]. Monocot and dicot lineages split about 140 to 150 million years ago [17]. Phylogenetic analysis revealed that OsCOI2 shares higher similarity to COI2 proteins from other monocots than to COI1 proteins from monocots and eudicots, suggesting that the monocot-specific COI1-COI2 duplication and divergence occurred early in the monocot phylum [11]. This high similarity in the sequence of COI2 proteins from monocot species, and the failure of OsCOI2 and ZmCOI2 to complement the Arabidopsis coi1-1 mutant raised fundamental questions about the conservation of function of COI2 and whether it was involved in jasmonate signalling or not. Here genetic analysis allowed us to address in parallel the functions of OsCOI1a/b and OsCOI2, and to uncover the unique roles of OsCOI2 in jasmonate-dependent developmental processes.
Materials and methods
Edited rice lines
To generate the JA perception mutants oscoi1a/b and oscoi2, the CRISPR-Cas9 system was used on the background cultivar Kitaake (Oryza sativa L. ssp. japonica) as described in [18]. Briefly, the CRISPR Guides tool on Benchling platform (https://benchling.com/crispr) was used to design the gRNAs. One gRNA was designed for each gene, OsCOI1a and OsCOI1b, to obtain the oscoi1a/b double mutants and two different pairs of gRNAs were designed to obtain the oscoi2 mutants. The sequences of the gRNAs were first inserted into the pUC57-sgRNA vector, then transferred by LR recombination into the binary vector pOS-Cas9 [19]. The pOS-Cas9-gRNAs vectors were introduced in the Agrobacterium tumefaciens strain EHA105 by electroporation. Rice embryogenic calli were transformed via Agrobacterium-mediated transformation protocols. The regenerated plants were checked for transformation, by PCR using T-DNA specific primers (HPT and Cas9, S1 Table in S1 File), and for mutation, by PCR using primers flanking the targeted sequences (S1 Table in S1 File) and sequencing (Eurofins). Edited plants were transferred to a greenhouse for multiplication. Edited lines were counter-selected from T1 generation to obtain T3 homozygous lines without T-DNA insertion, except for oscoi2-1 for which the limited number of seeds prevented such selection.
Plant phenotyping
Seeds were sterilized in 70% ethanol for 1 minute, then in 40% commercial bleach for 30 minutes, and were rinsed 6 times in sterilized water. Disinfected seeds were then germinated on half-strength Murashige & Skoog (½MS) medium including Gamborg B5 vitamins (Duchefa Biochemie BV, Haarlem, the Netherlands) supplied with 0.7% plant agar (Duchefa) for 6 days. Plantlets of uniform size were transferred into soil and grown in a greenhouse under a 16h-day/8h-night photoperiod, at 28°C/24°C with a 75% relative humidity. Tiller and internode lengths were measured at the mature stage on the 3 highest tillers from 18 T3 homozygous lines and wild type (WT) plants. Fertility rate was measured on the same tillers as the ratio between the number of fertile spikelets and the total number of spikelets. Leaf necrotic lesions and adventitious roots were observed under an Axiozoom microscope (Zeiss). Spikelet and anther morphology were observed under a stereo microscope (Nikon SMZ1500).
Hormone treatments
Seeds were sterilized and sown as described above. Two days after germination, seedlings with both coleoptile and primary root were transferred into glass tubes containing 20 ml of ½MS including Gamborg B5 vitamins supplied with 0.2% phytagel (Sigma) and grown in a growth chamber at 26°C under a 12h-day/12h-night photoperiod with a 70% relative humidity. For phenotyping, 5 μM JA (Sigma), 0.5 μM COR (Sigma) both dissolved in DMSO, or DMSO only (control plants) were added to the media just before pouring. Eight days after transplanting, length of the crown roots was measured for each plant. For each line, 20–24 plants were used except for the oscoi2-1 line for which very few seeds were available. Percentages of root growth inhibition were determined by randomly comparing the length values of the treated plants to those of control plants.
For gene expression analyses, plants were transferred 4 days after transplantation from the tubes into liquid media containing either 5 μM JA or DMSO for control plants. Crown root tips (around 1 cm) from 20–24 plants were collected 6 hours after and immediately frozen in liquid nitrogen. Five independent biological samples were collected and analysed.
Gene expression analyses
Total RNAs were extracted from rice root samples using the RNeasy Plant mini kit (Qiagen, France), with addition of an on-column DNase I digestion. First-strand cDNAs were synthesized from 1 μg of total RNA in 20 μl final volume using an oligo-dT(18)-MN primer (Eurogentec, France) and the Omniscript RT kit (Qiagen). Specific primers were designed from O. sativa Nipponbare sequences using Primer3Plus (https://primer3plus.com/) (S1 Table in S1 File) and checked for specificity on O. sativa Kitaake sequences. Quantitative-PCR assays were performed on cDNAs samples (diluted 1/50e) in an Mx30005P thermal cycler (Stratagene, USA) using the Brilliant III Ultra-fast SYBR® Green QPCR Master mix with low ROX (Agilent, Santa Clara, CA, USA). Amplifications were performed in duplicate from the five biological samples. The EXP gene (LOC_Os06g11070) was used as a reference gene to normalize data. Relative gene expression levels were calculated using the 2-ΔΔCt method.
Statistical analyses
Experimental data were analysed using GraphPad Prism (version 9.3.0). Values were considered statistically significant when p ≤ 0.05.
Results
OsCOI2 regulates plant development and its function is not redundant with that of OsCOI1a and OsCOI1b
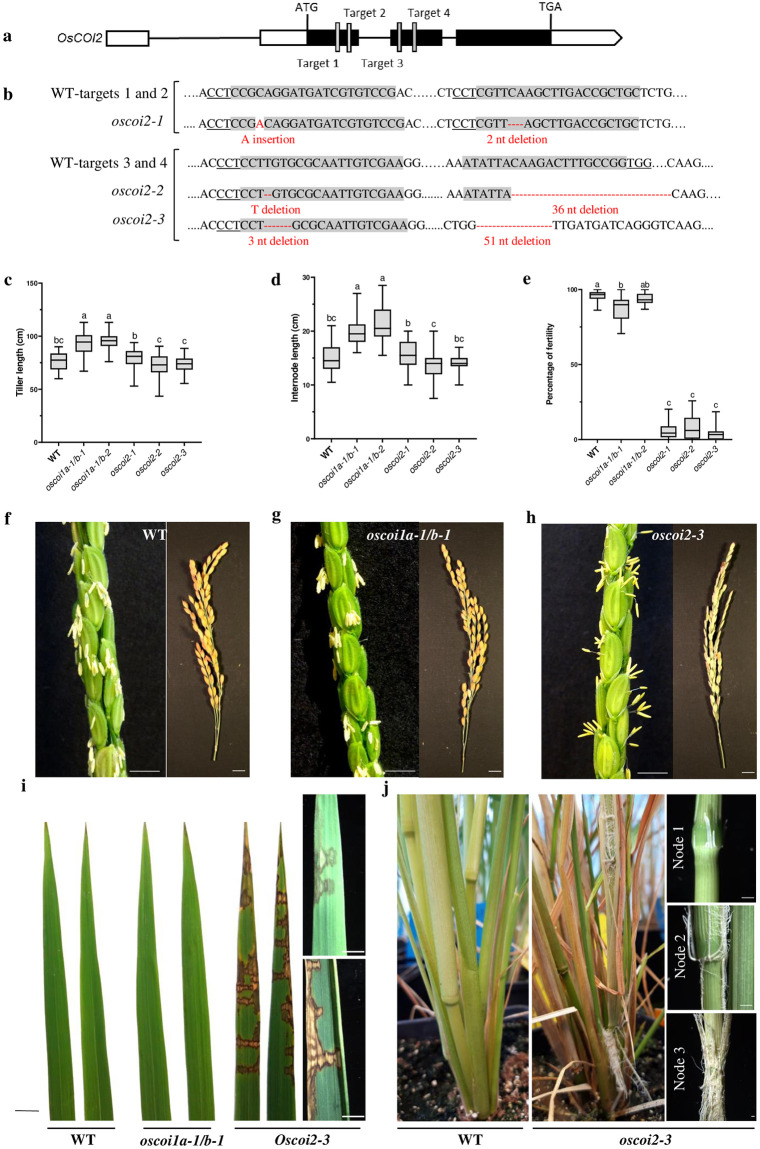
To investigate COI2 functions in monocots, CRISPR-Cas9 technology was used to generate oscoi2 mutant alleles in rice. Two CRISPR-Cas9 constructs targeting the first (target #1 and #2), and the second exon (target #3 and #4) of OsCOI2, respectively, were used to generate edited lines in the rice cultivar Kitaake (Fig 1a). Three homozygous mutant lines, named oscoi2-1, oscoi2-2 and oscoi2-3, were isolated and further characterized. The alleles oscoi2-1 and oscoi2-2 include short nucleotide insertions and deletions in the first and second exon leading to a translational frame shift and premature stop codon truncating 467 amino acids and 372 amino acids in the predicted proteins, respectively. The third allele oscoi2-3 displays fragments deleted in exon 2 eliminating 18 amino acids in the LRR domain, which can affect the protein stability (Fig 1b) [20]. In order to compare their phenotype with that of oscoi2 mutants and assess the putative functional redundancy between OsCOI2 and OsCOI1, we also generated two double oscoi1a oscoi1b mutant lines (thereafter named oscoi1a-1/b-1 and oscoi1a-1/b-2) by CRISPR-Cas9 (S1 Fig in S1 File).
Fig 1. OsCOI2 regulates vegetative and reproductive developmental programmes in rice distinct from OsCOI1a/b.
a, OsCOI2 gene structure and CRISPR-Cas9 target sites. b, insertion and deletion sites of three allelic mutations (oscoi2-1, oscoi2-2 and oscoi2-3) generated by two different pairs of gRNA. c, quantification of tiller length and d, quantification of internode length. Letters indicate significant differences between lines (45<n<54, One way-ANOVA with Tuckey’s multiple comparisons test, p<0.05). e, Percentage of fertile spikelet. Letters indicate significant differences between lines (43<n<53, Kruskall-Wallis multiple comparisons test, p<0.05). In the boxplots, whiskers denote minimum/maximum values, the box defines the interquartile range and the centre line represents the median. (c, d, e). f-h images of WT (f), oscoi1a-1/b-1(g) and oscoi2-3 (h) panicles. i, flag leaves. j, nodes. (f-j) Scale bar = 1 cm.
Phenotypically, the oscoi1a-1/b-1 and oscoi1a-1/b-2 double mutant lines exhibited increased plant height in comparison with oscoi2 single mutant and WT plants (Fig 1c, S2 and S3 Figs in S1 File). In addition, internode length was increased in the oscoi1a-1/b-1 and oscoi1a-1/b-2 compared to WT and oscoi2 mutant lines (Fig 1d, S2 Fig in S1 File). These oscoi1a/b phenotypes are similar to those reported for the rice coi1-RNAi lines in which both OsCOI1a and OsCOI1b expression is reduced [14]. Thus, we used these new oscoi1a-1/b-1 and oscoi2 knockout lines to compare OsCOI1a/b and OsCOI2-mediated regulation of plant development and JA signalling in rice.
The most prominent phenotype observed in Arabidopsis coi1-1 and tomato jai1-1 JA receptor mutants is sterility [5, 21], but earlier studies reported only a weak reduction in fertility in oscoi1-RNAi and oscoi1b-T-DNA rice mutant lines [14, 22]. Consistent with these reports, seed-setting in our oscoi1a/b double mutant lines was close to WT level (Fig 1e–1g). In contrast, all three of our independent oscoi2 mutant alleles conferred reduced seed setting (Fig 1e and 1h). None of the oscoi1a/b, nor oscoi2 mutant lines displayed any abnormality in floret architecture (S4 Fig in S1 File); however, most of the oscoi2 anthers did not dehisce (Fig 1f–1h and S4 Fig in S1 File). Hence, OsCOI2, rather than OsCOI1a or OsCOI1b, plays an important role during anther dehiscence stage in rice reproductive development. Importantly, even though OsCOI2 does not restore fertility in the Arabidopsis coi1-1 mutant background [13], our results indicated that OsCOI2 is an essential factor for rice plant fertility, probably via jasmonate-dependent regulation of flower or seed development.
During the vegetative to reproductive transition, oscoi2 mutant lines exhibited three distinct phenotypes not observed in WT or in oscoi1a/b plants. First, all three oscoi2 mutant alleles showed spontaneous lesions on flag leaves and the last leaf mimicking disease symptoms, despite plants not being infected (Fig 1i). Second, when the main oscoi2 panicle started to flower, above-ground adventitious roots emerged from node 1 to node 3, which resembles adventitious rooting induced by flooding in deep-water rice [23]. Third, 25 to 35% of the plants showed leaf rolling followed by tiller senescence and eventually plant death without imposing any water stress (S5 Fig in S1 File). Collectively, these data reveal that OsCOI2 regulates plant development and that its function is not redundant with that of OsCOI1a and OsCOI1b jasmonate receptors.
OsCOI2 is required for JA perception in rice crown roots
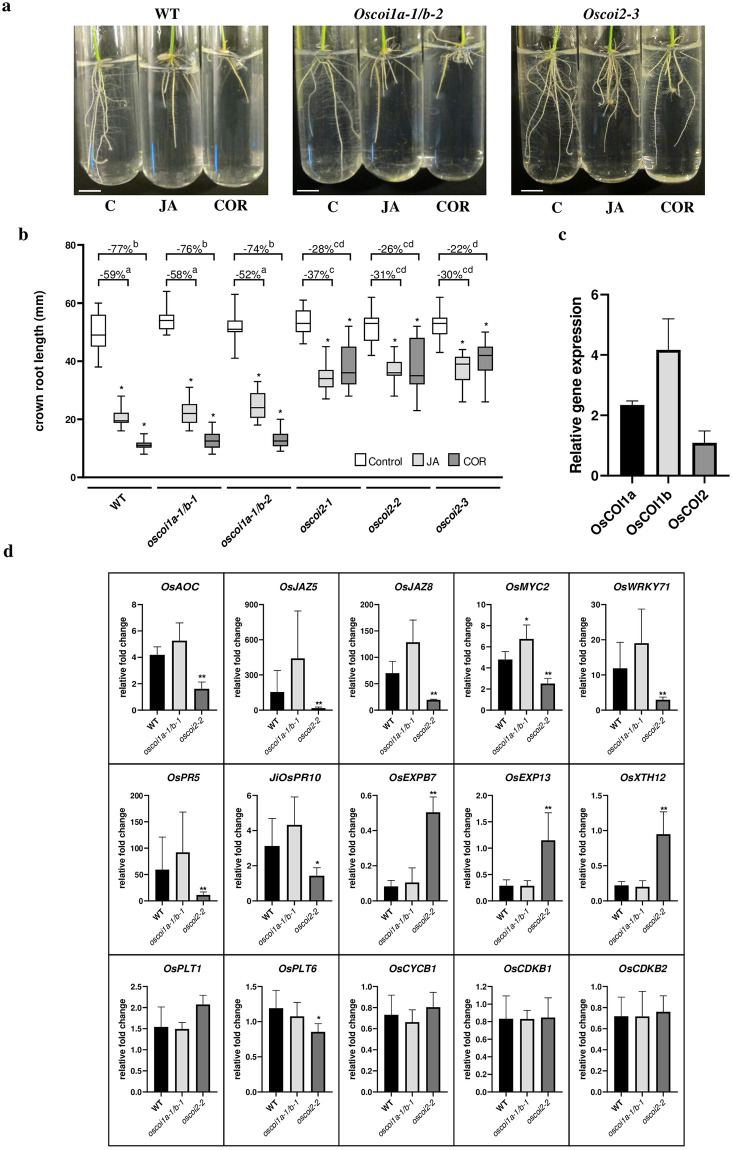
Next we tested the hypothesis that OsCOI2 participates in JA signalling in rice. Inhibition of seedling root growth by jasmonate or jasmonate analogue treatments has been extensively used as a bioassay to identify jasmonate response mutants in plants [5, 21, 24]. Under our growth conditions, JA treatment at 5 μM inhibited significantly WT crown root growth (S6 Fig in S1 File). Interestingly, at this concentration, JA also inhibited root growth of oscoi1a/b double mutant plants to the same extent as in the WT (Fig 2a and 2b). In contrast, crown root growth was significantly less sensitive to exogenous JA treatment in oscoi2-1, oscoi2-2, and oscoi2-3 genetic backgrounds (Fig 2a and 2b and S7 Fig in S1 File). Similarly, coronatine (COR), a bacterial mimic of JA-Ile, strongly inhibited root growth of WT and oscoi1a/b lines but had a significantly weaker effect on root growth of oscoi2 mutant alleles (Fig 2a and 2b).
Fig 2. OsCOI2 regulates jasmonate response in crown roots.
a, Phenotype of the (WT) and two allelic mutations (oscoi1a-1/b-2 and oscoi2-3) of roots in response to jasmonic acid (JA, 5 μM), coronatine (COR, 0.5 μM) and mock control (C, DMSO). Scale bars = 1 cm. b, effect of JA and COR compared to the mock treatment (DMSO) on crown root length in WT oscoi1a/b (oscoi1a-1/b-1 and oscoi1a-1/b-2) and OsCOI2 (oscoi2-1, oscoi2-2 and oscoi2-3) mutant lines. In the boxplots, whiskers denote minimum/maximum values, the box defines the interquartile range and the centre line represents the median. Asterisks indicate significant differences between treated and control plants (20<n<24 except for oscoi2-1 where 7<n<21, One way-ANOVA with Bonferroni’s multiple comparisons test, p<0.05). The percentages of crown root growth inhibition by JA and COR treatments are indicated above the respective plots from the WT, oscoi1a-1/b-1, oscoi1a-1/b-2, oscoi2-1, oscoi2-2 and oscoi2-3 mutants. Letters indicate significant differences between lines and treatments (21<n<24, One way-ANOVA with Tuckey’s multiple comparisons test, p<0.05). c. expression of OsCOI1a, OsCOI1b and OsCOI2 genes in crown root tips from wild-type plants. Data are presented as the means +/- SD from five biological replicates. d, regulatory effects of JA on the transcription of jasmonate responsive genes, cell elongation and division and defence genes. Data are presented as means +/- SD from five biological replicates. Asterisks indicate significant differences between the WT and the double oscoi1a-1/b-1 mutant or the oscoi2-2 mutant (Mann-Whitney tests, * p <0.05, **p<0.01).
To explore the molecular mechanisms associated with inhibition of crown root growth by jasmonate, we focused on crown root tips, where cell proliferation and elongation occur. All three JA receptor genes OsCOI1a, OsCOI1b and OsCOI2 were expressed in crown root tips (Fig 2c). Expression of the OsCOIa and OsCOI1b was similar in WT and oscoi2 mutant crown root tips thus indicating that no genetic compensation occurred (S8 Fig in S1 File). Similarly, OsCOI2 expression was not different in WT and oscoi1a-1/b-1 plants.
Induction of jasmonate responsive genes related to JA biosynthesis (OsAOC) and signalling (OsJAZ5, OsJAZ8 and OsMYC2) by exogenous JA application was compromised in oscoi2-2 crown roots relative to WT, but not in the oscoi1a-1/b-1 mutant background (Fig 2d, top panels). Consistent with the crown root growth phenotype, expression of the cell elongation-related genes OsEXPB7, OsEXP13 and OsXTH12 were significantly less repressed by JA treatment in oscoi2-2 crown roots than in WT and in oscoi1a-1/b-1 mutant background (Fig 2d). Conversely, expression of cell cycle or meristem marker genes (OsCYCB1, OsCDKB1, OsCDKB2, OsPLT1 and OsPLT6) was not affected by JA treatment [25]. Furthermore, genes associated with defence responses (OsPR5, OsPR10 and OsWRKY71) were significantly less induced by JA treatment in oscoi2-2 than in WT and oscoi1a-1/b-1. Hence, expression profiling revealed that OsCOI2, and not OsCOI1a/b, is required for JA-dependent signalling in rice crown root.
Discussion
Here, we report that a monocot-specific member of the COI family OsCOI2 mediates the regulation of a range of jasmonate-dependent vegetative and reproductive developmental processes. Significantly, the loss of OsCOI2 function (and not that of OsCOI1a and OsCOI1b genes) was shown to strongly alter male fertility in rice, a phenotype that is AtCOI1-dependent in Arabidopsis. In rice, jasmonate is not only necessary for stamen fertility, but also required for floret development [26, 27]. Loss-of-function mutations or misexpression in key genes in the jasmonate biosynthetic or signalling pathways cause floral morphological alterations such as longer sterile lemmas and extra glume-like organs [24, 26, 27]. We found that oscoi2 anthers did not dehisce properly, a phenotype also observed in the rice osjar1 mutant impaired in JA-Ile biosynthesis [28]. Furthermore, previous reports showed that OsCOI2 does not restore fertility and JA signal transduction in the Arabidopsis coi1 mutant, unless the OsCOI2 His-391 was substituted with Tyr-391 [13]. Recently, two reports also described that oscoi2 mutant anthers do not dehisce properly [29, 30]. In addition, Wang et al., 2023 showed that the pollen germination rate of oscoi2 mutants was significantly lower than that of WT plants. Collectively, these data point to a specialized function of COI2 in the regulation of plant reproductive development in the Monocot phylum.
COI1 was shown to be the receptor of the jasmonate signal in Arabidopsis and in rice. We investigated the possible role of OsCOI2 in jasmonate signalling using the JA-mediated repression of crown root growth. Surprisingly, our data suggested that inhibition of crown root growth by JA relies primarily on OsCOI2. Although the roots of oscoi2 mutant plants showed better resistant to JA than that of oscoi1a and oscoi1b double mutant, its growth is still inhibited to some extent suggesting the minor contribution of OsCOI1a and OsCOI1b. Our observation is also in line with the report in Inagaki et al., (2023) and Wang et al., (2023) [29, 30], although the sensitivity to JA treatment varies to some extent between studies probably due to the differences in genetic background, alleles chosen, type of roots, and/or treatments. Consistently, expression profiling revealed that OsCOI2 is specifically required for JA-dependent signalling in crown roots. This suggests that sub-functionalisation of JA receptors has occurred in the monocot lineage.
Besides its role in crown root growth inhibition, jasmonate is a potential regulator of adventitious root initiation. For example, JA treatment inhibited adventitious root formation in Arabidopsis hypocotyl [31]. Accordingly, atcoi1-1 mutant produced more adventitious root compared to the WT indicating a negative role of JA in this developmental process [31]. Here, our oscoi2 mutants also produced adventitious root on the above-ground nodes, a specific phenotype that has not been reported by Inagaki et al., (2023) and Wang et al., (2023) [29, 30]. In rice, adventitious root emergence is inducible by flooding and is tightly regulated by various hormones such as ethylene and auxin [32, 33]. Constitutive formation of adventitious roots from oscoi2 nodes suggests that this non-canonical jasmonate receptor could be a central regulator of shoot-borne root development in rice.
Specific functions of OsCOI2 could derive from (i) its spatial expression pattern being different from OsCOI1a/b, and/or (ii) distinct protein-protein interactions involving a subset of OsJAZs, and/or (iii) the perception of distinct jasmonate forms. For instance, the liverwort Marchantia polymorpha MpCOI1 receptor was shown to interact with distinct hormone forms that the eudicot Arabidopsis COI1 receptor did not bind [34]. Specifically, instead of perceiving JA-Ile, MpCOI1 binds the JA precursor dn-OPDA as an ancestral ligand to initiate jasmonate signalling [34]. In rice, despite of JA-Ile functions being established, OPDA- and distinct jasmonic acid- amino acid conjugates have been shown to be involved in defence response and could be additional ligands of OsCOI proteins [35, 36]. OsCOI2 was shown to bind with different jasmonate derivatives and to interact with OsJAZ proteins with some selectivity compared to OsCOI1a and OsCOI1b JA receptors [29, 30]. This could explain the specificity of OsCOIs function.
In summary, we report that COI jasmonate receptor sub-functionalisation has occurred in rice, consistent with the hypothesis that different bioactive forms of jasmonate could modulate distinct responses to developmental cues and environmental stresses via OsCOI1a/b and OsCOI2 perception. Our collection of oscoi mutant alleles offers a unique genetic resource for future dissection of the function of jasmonate receptor proteins in monocots.
Supporting information
(PDF)
Acknowledgments
We thank B.K. Pandey and M. Bennett for advice while drafting the manuscript. We thank E. Guillon and P. Serin for technical assistance with the IRD glasshouse work.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by the Consultative Group for International Agricultural Research Program on rice-agrifood systems (CRP-RICE, 2017–2022). HTN is funded by a PhD fellowship from the French Embassy in Vietnam. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yuan Z, Zhang D. Roles of jasmonate signalling in plant inflorescence and flower development. Curr Opin Plant Biol. 2015; 27: 44–51. doi: 10.1016/j.pbi.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 2.Lakehal A, Ranjan A, Bellini C. Multiple roles of jasmonates in shaping rhizotaxis: emerging integrators. Methods Mol Biol. 2020; 2085: 3–22. doi: 10.1007/978-1-0716-0142-6_1 [DOI] [PubMed] [Google Scholar]
- 3.Lyons R, Manners JM, Kazan K. Jasmonate biosynthesis and signaling in monocots: a comparative overview. Plant Cell Rep. 2013; 32: 815–827. doi: 10.1007/s00299-013-1400-y [DOI] [PubMed] [Google Scholar]
- 4.Schluttenhofer C. Origin and evolution of jasmonate signaling. Plant Sci. 2020; 298: 110542. doi: 10.1016/j.plantsci.2020.110542 [DOI] [PubMed] [Google Scholar]
- 5.Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998; 280: 1091–1094. [DOI] [PubMed] [Google Scholar]
- 6.Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007; 448: 666–671. doi: 10.1038/nature06006 [DOI] [PubMed] [Google Scholar]
- 7.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007; 448: 661–665. doi: 10.1038/nature05960 [DOI] [PubMed] [Google Scholar]
- 8.Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007; 19: 2470–2483. doi: 10.1105/tpc.107.050708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010; 468: 400–405. doi: 10.1038/nature09430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010; 464: 788–791. doi: 10.1038/nature08854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An L, Ahmad RM, Ren H, Qin J, Yan Y. Jasmonate signal receptor gene family ZmCOIs restore male fertility and defense response of Arabidopsis mutant coi1-1. J Plant Growth Regul. 2019; 38: 479–493. [Google Scholar]
- 12.Qi X, Guo S, Wang D, Zhong Y, Chen M, Chen C, et al. ZmCOI2a and ZmCOI2b redundantly regulate anther dehiscence and gametophytic male fertility in maize. Plant J. 2022. 10: 849–862. [DOI] [PubMed] [Google Scholar]
- 13.Lee HY, Seo JS, Cho JH, Jung H, Kim JK, Lee JS, et al. Oryza sativa COI homologues restore jasmonate signal transduction in Arabidopsis coi1-1 mutants. PLoS One. 2013; 8: e52802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci USA. 2012; 109: 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye M, Song Y, Long J, Wang R, Baerson SR, Pan Z, et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc Natl Acad Sci USA. 2013; 110: 3631–3639. doi: 10.1073/pnas.1305848110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Huang Y, Yang J, Yao S, Zhao K, Wang D, et al. Jasmonate signaling enhances RNA silencing and antiviral defense in rice. Cell Host Microbe. 2020; 28: 89–103. doi: 10.1016/j.chom.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Chaw SM, Chang CC, Chen HL, Li WH. Dating the monocot-dicot divergence and the origin of core eudicots using whole chloroplast genomes. J Mol Evol. 2004; 58: 424–441. doi: 10.1007/s00239-003-2564-9 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TH, Mai HTT, Moukouanga D, Lebrun M, Bellafiore S, Champion A. CRISPR/Cas9-Mediated Gene Editing of the Jasmonate Biosynthesis OsAOC Gene in Rice. Methods Mol Biol. 2020; 2085: 199–209. [DOI] [PubMed] [Google Scholar]
- 19.Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, et al. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013; 23: 1233–1236. doi: 10.1038/cr.2013.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009; 21: 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004; 16: 126–143. doi: 10.1105/tpc.017954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Sakuraba Y, Lee T, Kim KW, An G, Lee HY, et al. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J Integr Plant Biol. 2015; 57: 562–576. [DOI] [PubMed] [Google Scholar]
- 23.Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999; 119: 21–30. doi: 10.1104/pp.119.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao L, Tian J, Liu Y, Chen X, Li S, Persson S, et al. Ectopic expression of OsJAZ6, which interacts with OsJAZ1, alters JA signaling and spikelet development in rice. Plant J. 2021; 108: 1083–1096. [DOI] [PubMed] [Google Scholar]
- 25.Zou X, Liu L, Hu Z, Wang X, Zhu Y, Zhang J, et al. Salt-induced inhibition of rice seminal root growth is mediated by ethylene-jasmonate interaction. J Exp Bot. 2021; 72: 5656–5672. doi: 10.1093/jxb/erab206 [DOI] [PubMed] [Google Scholar]
- 26.Cai Q, Yuan Z, Chen M, Yin C, Luo Z, Zhao X, et al. Jasmonic acid regulates spikelet development in rice. Nat Commun. 2014; 5: 3476–3489. doi: 10.1038/ncomms4476 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TH, Thi Mai To H, Lebrun M, Bellafiore S, Champion A. Jasmonates-the master regulator of rice development, adaptation and defense. Plants. 2019; 8: 339. doi: 10.3390/plants8090339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Y, Chen Y, Charnikhova T, Mulder PP, Heijmans J, Hoogenboom A, et al. OsJAR1 is required for JA-regulated floret opening and anther dehiscence in rice. Plant Mol Biol. 2014; 86: 19–33. [DOI] [PubMed] [Google Scholar]
- 29.Inagaki H, Hayashi K, Takaoka Y, Ito H, Fukumoto Y, Yajima-Nakagawa A, et al. Genome Editing Reveals Both the Crucial Role of OsCOI2 in Jasmonate Signaling and the Functional Diversity of COI1 Homologs in Rice. Plant Cell Physiol. 2023; 64: 405–421. doi: 10.1093/pcp/pcac166 [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Chen Y, Liu S, Fu W, Zhuang Y, Xu J, et al. Functional dissection of rice jasmonate receptors involved in development and defense. New Phytol. 2023; 238: 2144–2158. doi: 10.1111/nph.18860 [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez L, Mongelard G, Floková K, Pacurar DI, Novák O, Staswick P, et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012; 24:2515–2527. doi: 10.1105/tpc.112.099119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mergemann H, Sauter M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 2000; 124: 609–614. doi: 10.1104/pp.124.2.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin C, Sauter M. Polar auxin transport determines adventitious root emergence and growth in rice. Front Plant Sci. 2019; 10:444. doi: 10.3389/fpls.2019.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monte I, Ishida S, Zamarreño AM, Hamberg M, Franco-Zorrilla JM, García-Casado G, et al. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nature Chem Biol. 2018; 14: 480–488. doi: 10.1038/s41589-018-0033-4 [DOI] [PubMed] [Google Scholar]
- 35.Shinya T, Miyamoto K, Uchida K, Hojo Y, Yumoto E, Okada K, et al. Chitooligosaccharide elicitor and oxylipins synergistically elevate phytoalexin production in rice. Plant Mol Biol. 2021; 109: 595–609. doi: 10.1007/s11103-021-01217-w [DOI] [PubMed] [Google Scholar]
- 36.Fu W, Jin G, Jiménez-Alemán GH, Wang X, Song J, Li S, et al. The jasmonic acid-amino acid conjugates JA-Val and JA-Leu are involved in rice resistance to herbivores. Plant Cell Environ. 2022; 45: 262–272. doi: 10.1111/pce.14202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.