Abstract
Background
Lung cancer patients with coronavirus disease 2019 (COVID-19) infection experience high mortality rates. The study aims to determine the risk factors for mortality in lung cancer patients with COVID-19 infection.
Materials and methods
Followed the PRISMA reporting guidelines, PubMed, Embase, and Web of Science were systematically searched to February 20, 2023, for studies of lung cancer patients with COVID-19 infection. The main outcome of interest was the risk factor for mortality. We also compared the mortality rate of those patients among different continents. A pooled risk ratio (RR) with 95% CI was presented as the result of this meta-analysis.
Results
Meta-analysis of 33 studies involving 5018 patients showed that pooled mortality rate of lung cancer in COVID-19 patients was 0.31 (95% CI: 0.25–0.36). Subgroup analysis based on the continents showed significant difference of the mortality rate was observed between Asia and the rest of world (χ2 = 98.96, P < 0.01). Older age (SMD: 0.24, 95% CI: 0.09–0.40, P < 0.01), advanced lung cancer (RR: 1.14, 95% CI: 1.04–1.26, P < 0.01), coexisting comorbidities such as hypertension (RR: 1.17, 95% CI: 1.01–1.35, P = 0.04) and cardiovascular disease (RR: 1.40, 95% CI: 1.03–1.91, P = 0.03) were associated with higher risk of mortality rate in those patients.
Conclusions
Findings of this meta-analysis confirms an increased risk of mortality in lung cancer patients with COVID-19 infection, whose risk factors for these patients appear to be exacerbated by older age, advanced-stage lung cancer, and comorbidities such as hypertension and cardiovascular disease.
Introduction
The coronavirus disease 2019 (COVID-19) outbreak, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted more than 676 million confirmed cases worldwide as of March 10, 2023 [1–4]. This unprecedented crisis continues to challenge global public health systems as the number of new cases continues to rise. Compared to the general population, cancer patients are more susceptible to infections due to immunological impairment, either directly from the cancer itself or from immunosuppressive curative/palliative treatments [5–8].
Lung cancer remains the leading cause of cancer‐related death worldwide [9], and lung cancer patients are thought to be at a higher risk of severe COVID-19 complications [5, 10–12]. Evidence suggests that SARS-CoV-2 primarily infects the respiratory tract through the angiotensin-converting enzyme 2 (ACE2) receptor on alveolar epithelial cells, causing respiratory complications in the lung that may worsen lung cancer patients’ prognosis [13, 14]. Numerous studies have attempted to determine the outcomes of lung cancer patients with COVID-19 infection to aid in risk stratification and clinical management [8, 11, 12, 15–53]. In a recent multicenter cohort study conducted by Mengyuan Dai [8], it was found that patients with hematologic cancer, lung cancer, or metastatic cancer (stage IV) had the highest frequency of severe events. This supports the findings by Garassino [11] that patients with thoracic cancer have a high mortality rate. However, there is a lack of meta-analyses specifically focused on patients with lung cancer and COVID-19. The existing published studies have mainly analyzed whether lung cancer is a poor prognostic factor for COVID-19 [54–56]. Oldani [54] compared the disease severity and mortality rate between lung cancer patients and non-cancer patients, as well as patients with other malignancies. The results showed that the mortality rate of lung cancer patients was significantly higher than that of non-cancer patients or patients with other malignancies. However, it was not statistically significant to consider lung cancer as a risk factor for SARS-CoV-2 infection. In the overall meta-analysis conducted by Haike Lei [55], no significant differences in mortality rates were found between lung cancer and other tumor subgroups. This suggests that there may be other potential risk factors for lung cancer patients with COVID-19, which could be the underlying causes of the increased mortality.
Therefore, the specific risk factors that contribute to the higher mortality rate of lung cancer patients with COVID-19 remain uncertain. Additionally, there is a lack of meta-analysis reports on this topic. Thus, the objective of our study is to identify the risk factors associated with mortality in lung cancer patients who have contracted COVID-19, by analyzing the existing published data. We anticipate that the findings from this meta-analysis will offer significant insights for safeguarding individuals who are vulnerable to COVID-19.
Materials and methods
Study protocol
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines [57], this study protocol has been registered in the International Prospective Registry of Systematic Reviews (PROSPERO) (number: CRD42022306866) and published in PLOS ONE [58].
Search strategy and literature search
We performed repeated searches of PubMed, Embase, and Web of Science for English-language articles published up to February 20, 2023. We identified relevant articles from the references of these articles and removed duplicates. The search strategy included the following terms: ("COVID-19" or " SARS-CoV-2" or "coronavirus disease 2019" or "2019-nCoV") and ("cancer" or "neoplasms" or "carcinoma" or "malignancy") and ("lung" or "pulmonary"). Details of the results of the literature search can be found in S1 Table in the supplementary material.
Study selection
Two authors (M.W. and S.L.) independently screened all titles and abstracts after the initial search, and discrepancies were resolved by consultation with a third author (J.L.).
Inclusion criteria were as follows: (1) any cohort studies, case-control, and cross-sectional studies; (2) lung cancer patients with COVID-19 infection confirmed through Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) or highly suspected COVID-19 infection identified by CT scan or symptoms, to ensure data integrity; and (3) studies reporting clinical characteristics or outcomes.
Exclusion criteria were as follows: (1) publication types such as abstracts, editorials, letters, reviews, commentaries, guidelines, or non-human studies; (2) studies with data that could not be obtained from the corresponding author; and (3) repetitive data published in the literature.
Data extraction
Two authors (M.W. and S.L.) independently performed data extraction and quality assessment, and cross-checked the extracted data to reach consensus. The extraction included: (1) study information: first author, single or multi-center study type (retrospective or prospective), study period, study region, diagnostic methods of COVID-19, and study sample; (2) demographic or clinical characteristics: age, sex, smoking history (former/current smoker vs. non-smoker), lung cancer type (NSCLC vs. other), lung cancer stage (advanced stage or other), laboratory parameters, common COVID-19 symptoms, and comorbidities; and (3) outcomes: death status of lung cancer patients. Advanced cancer included stage III/IV or metastasis, regardless of whether the cancer type was NSCLC or other.
Statistical analysis
We assessed the quality of the included articles using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control and cohort studies [59], and the Prevalence Study Quality for cross-sectional studies [60]. We assessed publication bias between studies by visually inspecting a funnel plot for asymmetry and performing Egger’s test (P < 0.05 was considered to indicate significant publication bias) [61].
The main outcome of interest was the risk factor for mortality in lung cancer patients with COVID-19 infection. Therefore, we compared the collected demographic or clinical characteristics mentioned above between living and dead lung cancer patients with confirmed COVID-19 infection. We also compared the mortality of lung cancer patients with COVID-19 infection between different continents. Qualitative results of this meta-analysis were presented as pooled risk ratios (RRs) with 95% confidence intervals (CIs), and quantitative results were presented as standard mean differences (SMD) with 95% CIs.
Statistical heterogeneity was quantified using the I2 statistic [62]. The common-effects model was used when the heterogeneity was not significant (P ≥ 0.05, I2 ≤ 50%), whereas the random-effects model was performed when heterogeneity between studies was present (P < 0.05, I2 > 50%) [63]. For the confounding variables, we have incorporated six factors to examine the influence of these variables on the study. These factors include the center type, continents, publication year, study design, study type, and diagnosis methods for COVID-19. We have employed meta-regression analysis to ascertain if these confounding factors have a noteworthy impact on the established effect model. Subgroup analysis was then performed to dissect significant heterogeneity for those variables with a P value less than 0.05. Sensitivity analysis was also used to assess the stability of the results by iteratively repeating the analyses, discarding one dataset each time.
Meta-analysis was performed using R software (version 4.1.2). A two-sided P < 0.05 indicated statistical significance.
Results
Literature search results
A total of 7,308 articles were initially retrieved for this study. After removing irrelevant or duplicate articles, 6,040 records remained for title and abstract screening. Of these, we selected 49 articles for full-text screening. To avoid duplication of the population, we retained studies with the largest number of samples for duplicate sample sources. Ultimately, according to the pre-specified inclusion criteria, 33 studies remained for this systematic review and meta-analysis, including 8 studies that reported detailed information between living and dead lung cancer patients with COVID-19 infection. Details of the PRISMA chart for study selection are shown in Fig 1.
Fig 1. Details of the PRISMA chart for study selection.
Global distribution of studies
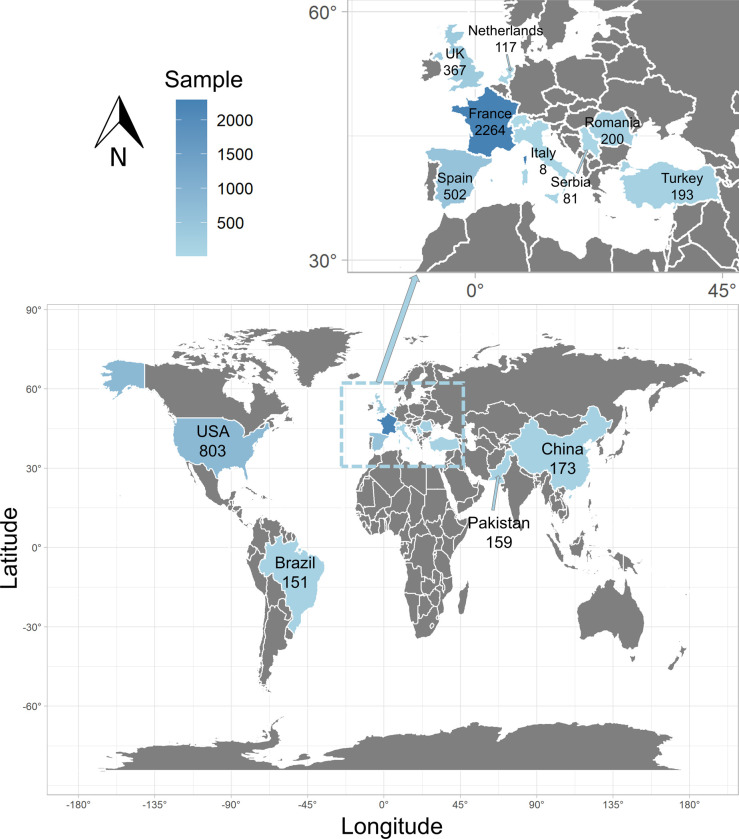
The 33 studies included 5,018 patients with lung cancer and COVID-19 infection from 12 countries on 4 continents. Europe had the highest number of patients recruited (n = 3,539, 70.53%), followed by North America (n = 803, 16.00%), Asia (n = 525, 10.46%), and South America (n = 151, 3.01%). The distribution map of lung cancer patients across the 33 studies is shown in Fig 2, which was generated using the ggplot2 package in the R software program.
Fig 2. The distribution of lung cancer patients across 33 studies.
Characteristics of included studies
Basic information about the 33 included studies is summarized in Table 1. These studies consisted of 25 retrospective, 7 prospective, and 1 retro-prospective study. More than half of the included studies were multi-center studies (n = 21, 63.64%). The diagnosis of patients with COVID-19 infection was mainly confirmed by RT-PCR. The overall quality of the 33 studies was high, with quality scores ranging from 5 to 9 (S2 and S3 Tables) (S1 Fig). The funnel plot is shown in S2 Fig, and Egger’s test (t = - 0.19, P = 0.85) indicated no significant publication bias among the included studies.
Table 1. Characteristics of the included 33 studies.
| Author/Year | Region | Lung Cancer Patients with COVID-19 | Single/Multi-center | Study design | Study type | Diagnosis method for Covid-19 | Quality Score |
|
|---|---|---|---|---|---|---|---|---|
| Total (n = 5018) | Deaths (n = 1624) | |||||||
| Beltramo et al., 2021 [40] | France | 977 | 402 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Bernard et al., 2021 [41] | France | 873 | 359 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Chen et al., 2022 [42] | USA | 676 | 63 | Multi-center | Prospective | Cohort study | RT-PCR | 6 |
| Provencio et al., 2021 [15] | Spain | 447 | 146 | Multi-center | Prospective | Cohort study | RT-PCR | 5 |
| Lièvre et al.,2020 [43] | France | 311 | 113 | Multi-center | Retro-prospective | Cohort study | RT-PCR/CT/Clinical | 7 |
| Várnai et al.,2022 [44] | UK | 265 | 131 | Multi-center | Prospective | Cohort study | RT-PCR | 6 |
| Haineala et al., 2021 [22] | Romania | 200 | 64 | Multi center | Prospective | Cohort study | RT-PCR/CT/Clinical | 7 |
| Farooque et al., 2021 [45] | Pakistan | 159 | 7 | Multi-center | Prospective | Cohort study | RT-PCR | 8 |
| Özdemir et al.,2020 [46] | Turkey | 157 | 18 | Multi-center | Retrospective | Cohort study | RT-PCR | 7 |
| Joode et al., 2022 [53] | Netherlands | 117 | 44 | Multi-center | Retrospective | Cohort study | RT-PCR/Antibodies | 8 |
| Peixoto et al., 2022 [27] | Brazil | 110 | 58 | Single | Retrospective | Cohort study | RT-PCR/CT/Clinical | 7 |
| Luo et al., 2020 [19] | USA | 102 | 25 | Single | Retrospective | Cohort study | RT-PCR | 7 |
| Lee et al.,2020 [47] | UK | 90 | 32 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Benderra et al., 2021 [48] | France | 85 | 37 | Multi-center | Retrospective | Cohort study | RT-PCR | 5 |
| Bursać et al., 2022 [24] | Serbia | 81 | 16 | Single | Retrospective | Case control study | RT-PCR | 7 |
| Song et al.,2021 [49] | China | 61 | 16 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Nie et al., 2021 [16] | China | 45 | 11 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Kahya et al., 2021 [18] | Turkey | 36 | 2 | Single | Retrospective | Case control study | RT-PCR | 8# |
| Calles et al., 2020 [25] | Spain | 23 | 8 | Single | Retrospective | Cross-sectional study | RT-PCR | 6# |
| Dai et at., 2020 [8] | China | 22 | 4 | Multi-center | Retrospective | Case control study | RT-PCR | 8 |
| Zhang et al., 2020 [31] | China | 21 | 5 | Multi-center | Retrospective | Cross-sectional study | RT-PCR | 8# |
| Basse et al., 2021 [36] | France | 18 | 6 | Single | Prospective | Cohort study | RT-PCR/CT | 8 |
| Fernandes et al., 2021 [50] | Brazil | 18 | 6 | Single | Retrospective | Cross-sectional study | RT-PCR | 7# |
| Rogado et al., 2020 [17] | Spain | 17 | 9 | Single | Retrospective | Cohort study | RT-PCR | 7 |
| Ferrari et al., 2021 [51] | Brazil | 16 | 7 | Multi-center | Prospective | Cohort study | RT-PCR | 6 |
| Yarza et at., 2020 [32] | Spain | 15 | 6 | Single | Retrospective | Cohort study | RT-PCR/CT/Clinical | 7 |
| Yang et al.,2020 [33] | China | 24 | 6 | Multi-center | Retrospective | Cohort study | RT-PCR | 7 |
| Khusid et al.,2021 [52] | USA | 14 | 6 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| Mehta et al.,2020 [35] | USA | 11 | 6 | Single | Retrospective | Cohort study | RT-PCR | 7 |
| Stroppa et al.,2020 [37] | Italy | 8 | 2 | Single | Retrospective | Cohort study | RT-PCR | 6 |
| Fraser et al., 2021 [29] | UK | 7 | 2 | Multi-center | Retrospective | Cohort study | RT-PCR | 6 |
| de Melo et al., 2020 [34] | Brazil | 7 | 4 | Single | Retrospective | Cohort study | RT-PCR | 7 |
| Hogan et al., 2020 [38] | UK | 5 | 3 | Multi-center | Retrospective | Cohort study | RT-PCR | 7 |
# Quality of studies was evaluated through Prevalence Study Quality for cross-sectional studies, while others by Newcastle-Ottawa quality assessment scale.
The mortality of lung cancer patients with COVID-19 infection
All included studies reported the mortality of lung cancer patients with COVID-19 infection, resulting in 1,624 deaths among 5,018 patients. The mortality varied among different regions, with the highest in Brazil (75/151, 49.67%) and the lowest in Pakistan (7/159, 4.40%) (S3 Fig). The pooled mortality of lung cancer in COVID-19 patients was 0.31 (95% CI: 0.25–0.36) (S4 Fig). Sensitivity analysis revealed that our results were robust (S5 Fig). However, our analysis showed that the heterogeneity between studies was substantial (I2 = 94%, P < 0.01). The result of the meta-regression analysis showed that there was significant heterogeneity in the continents of the included studies (Z = 4.93, P < 0.01) (S4 Table).
As shown in Fig 3, subgroup analysis by continent revealed that the pooled lung cancer mortality in COVID-19 patients was 0.50 (95% CI: 0.42–0.58) in South America, followed by Europe [0.37 (95% CI: 0.33–0.42)], North America [0.28 (95% CI: 0.11–0.50)], and Asia [0.15 (95% CI: 0.09–0.23)]. A significant difference in mortality was observed between Asia and the rest of the world (χ2 = 98.96, P < 0.01). However, the source of heterogeneity could not be further determined due to limited information in the 33 studies.
Fig 3. Pooled mortality of lung cancer in COVID-19 patients after subgroup analysis based on the continents.

Risk factor for mortality in lung cancer patients with COVID-19 infection
Eight of the 33 studies (24.24%), including 260 deaths among 771 patients, reported detailed information between living and dead lung cancer patients with confirmed COVID-19 infection by RT-PCR. Considering that these studies reported different demographic or clinical characteristics, the corresponding data were scrutinized, and insufficient information reported by less than 3 studies was excluded. Finally, age, sex, smoking history, lung cancer type, lung cancer stage, and comorbidities, including hypertension, cardiovascular disease, respiratory disease, and diabetes mellitus, were selected to assess the risk factors for mortality (Table 2).
Table 2. The detailed information for lung cancer patients with COVID-19 infection.
| Group | Study | Sample | Age | Gender | Smoking status | Cancer type | Cancer stage | Comorbidities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Former/Current | Non-smoker | NSCLC | Others | Advanced-stage | Others | Hypertension | Cardiovascular disease | Respiratory disease | Diabetes mellitus | ||||
| Dead | Provencio et al., 2021 [15] | 146 | 68.00 ± 9.40 | 108 | 38 | 128 | 18 | 120 | 26 | 125 | 21 | 73 | 45 | 42 | 27 |
| Peixoto et al., 2022 [27] | 58 | 67.00 ± 8.00 | 32 | 26 | 46 | 12 | 53 | 5 | 40 | 18 | 28 | NR | 19 | 22 | |
| Luo et al., 2020 [19] | 25 | 71.79 ± 13.36 | 14 | 11 | NR | NR | NR | NR | 15 | 10 | 18 | 6 | 18 | 9 | |
| Nie et al., 2020 [16] | 11 | 72.57 ± 11.03 | 10 | 1 | 3 | 8 | 5 | 6 | 9 | 2 | 7 | 3 | NR | 3 | |
| Dai et at., 2020 [8] | 4 | 67.75 ±13.12 | 2 | 2 | 2 | 2 | 1 | 3 | 2 | 2 | 2 | NR | NR | 1 | |
| Zhang et al., 2020 [31] | 5 | 70.60 ± 8.50 | 4 | 1 | 2 | 3 | NR | NR | 3 | 2 | 3 | 1 | 2 | 2 | |
| Rogado et al., 2020 [17] | 9 | 64.87 ± 11.38 | 6 | 3 | NR | NR | NR | NR | 7 | 2 | 6 | NR | 4 | NR | |
| Fraser et al., 2021 [29] | 2 | 64.35 ± 5.94 | 2 | 0 | 1 | 1 | NR | NR | NR | NR | 2 | 0 | 0 | 1 | |
| Living | Provencio et al., 2021 [15] | 301 | 66.60 ± 9.90 | 224 | 77 | 255 | 46 | 257 | 44 | 229 | 72 | 134 | 69 | 95 | 75 |
| Peixoto et al., 2022 [27] | 52 | 63.00 ± 10.00 | 24 | 28 | 41 | 11 | 48 | 4 | 26 | 26 | 29 | NR | 17 | 19 | |
| Luo et al., 2020 [19] | 77 | 67.30 ± 9.07 | 35 | 42 | NR | NR | NR | NR | 53 | 24 | 39 | 1 | 34 | 18 | |
| Nie et al., 2020 [16] | 34 | 63.49 ± 11.61 | 21 | 13 | 8 | 26 | 10 | 24 | 22 | 12 | 8 | 4 | NR | 3 | |
| Dai et at., 2020 [8] | 18 | 66.78 ± 6.83 | 16 | 2 | 12 | 6 | 9 | 9 | 12 | 6 | 3 | 2 | 1 | 4 | |
| Zhang et al., 2020 [31] | 16 | 67.00 ± 6.11 | 12 | 4 | 3 | 13 | NR | NR | 7 | 9 | 8 | 1 | 2 | 2 | |
| Rogado et al., 2020 [17] | 8 | 69.26 ± 11.85 | 7 | 1 | NR | NR | NR | NR | 4 | 4 | 4 | NR | 5 | NR | |
| Fraser et al., 2021 [29] | 5 | 74.18 ± 7.21 | 3 | 2 | 4 | 1 | NR | NR | NR | NR | 5 | 0 | 1 | 0 | |
Age was presented as mean and standard deviation, and others were the number of patients
NR, Not reported; NSCLC, non-small cell lung cancer; advanced-stage cancer including stage III/IV or metastasis
As shown in Fig 4, eight studies provided data on the age of living and dead lung cancer patients with COVID-19 infection. The result showed that older patients had a higher risk of death (SMD: 0.24, 95% CI: 0.09–0.40, P < 0.01). Seven studies reported the cancer stage information, and the result demonstrated that advanced-stage lung cancer including stage III/IV or metastasis was also associated with a higher risk of death compared with others (RR: 1.14, 95% CI: 1.04–1.26, P < 0.01). In term of comorbidities, there were significant differences in hypertension (RR: 1.17, 95% CI: 1.01–1.35, P = 0.04) between living and dead lung cancer patients with COVID-19 infection. As for cardiovascular disease reported by four studies, an apparent asymmetry was observed when assessing for publication bias (S6 Fig). Thus, a sensitivity analysis was performed using the leave-one-out approach and a significant change in the result was observed, showing that cardiovascular disease was also associated with an increased rate of death (RR: 1.40, 95% CI: 1.03–1.91, P = 0.03). However, the results showed that sex, smoking history, lung cancer type, respiratory disease, and diabetes mellitus were not associated with a higher risk of death (P = 0.39, 0.47, 0.51, 0.64, 0.99, respectively).
Fig 4. Forest plot for risk factors of case mortality of lung cancer patients with COVID-19 infection.
Discussion
A major strength of our study is the detailed investigation of risk factors for mortality in lung cancer patients with COVID-19 infection. Our findings indicate that older age, advanced-stage lung cancer, and comorbidities such as hypertension and cardiovascular disease contribute to increased mortality in this patient population. These observations align with previous research on risk factors for COVID-19-related mortality in the general population [64, 65]. Interestingly, our study did not identify any significant associations between gender, smoking history, lung cancer subtype, respiratory disease, and diabetes mellitus and the risk of death in lung cancer patients with COVID-19 infection.
According to the results of two previous meta-analyses on COVID-19 and cancer, cancer was associated with an increased risk of severe COVID-19 or death compared to patients without cancer [66, 67]. Considering that the lung is a primary target of SARS-CoV-2, and given the inherent vulnerability of lung cancer patients [5–8, 10–12], three previous meta-analyses sought to determine whether lung cancer was a poor prognostic factor for COVID-19 [54–56]. However, these studies varied in size and methodology, produced inconsistent results when assessing the differences in mortality between lung cancer patients and those with other malignancies and COVID-19 infection. Moreover, these meta-analyses were limited by the absence of detailed information comparing lung cancer patients who survived and those who succumbed to COVID-19 infection. Thus, it remains unclear which risk factors would increase mortality in lung cancer patients with COVID-19 infection.
A strength of our study is the comprehensive systematic review of 33 individual studies with a wide geographical distribution to represent the global impact on lung cancer patients with COVID-19 infection. Our results indicate that the pooled mortality in lung cancer patients with COVID-19 infection is estimated to be 0.31 (95% CI: 0.25–0.36), which is higher than that observed in the general population with COVID-19 infection as reported in recently published studies [1, 35, 68, 69]. Furthermore, our continent-based subgroup analysis shows that the pooled lung cancer mortality in COVID-19 patients is 0.15 (95% CI: 0.09–0.23) in Asia, which is significantly lower than the rest of the world (χ2 = 98.96, P < 0.01). This disparity is mainly due to the different approaches to handling the SARS-CoV-2 pandemic in different regions and countries, where health policies and lifestyles may have influenced mortality rates from lung cancer or COVID-19 infection. Nevertheless, the results of our study underscore the need for continued data sharing and international collaboration on a global scale.
Another crucial issue addressed in our study is to the underlying risk factors associated with COVID-19-related mortality in lung cancer patients. In particular, recent studies have identified older age and smoking as the most significant risk factors for lung cancer [64, 65, 70]. Other risk factors include air pollution, previous radiotherapy, respiratory disease, and family history [70, 71]. Our systematic review and meta-analysis support the hypothesis that older age, advanced-stage lung cancer, and comorbidities such as hypertension and cardiovascular disease are associated with a higher risk mortality in lung cancer patients with COVID-19 infection. Previously, older age was reported to be an important risk factor for mortality in SARS-CoV-2 infection. Additionally, age-dependent defects in B-cell and T-cell function could result in prolonged proinflammatory responses and a deficiency in controlling viral replication, potentially leading to poor outcomes [72]. Given that older age is also a risk factor for lung cancer[65], special treatment should be considered for these patients. Patients with advanced-stage lung cancer often have decreased immune function, reduced lung function, and severe infections, which may contribute to worse outcomes in this subpopulation. Other risk factors related to mortality include comorbidities such as hypertension and cardiovascular disease. Crackower et al.’s evidence [73] demonstrated that SARS-CoV-2 could directly impair cardiac function by targeting disruption of ACE2 in mice, resulting in severe cardiac contractility defects, increased angiotensin II levels, and upregulation of hypoxia-induced genes in the heart. Previous studies have also shown that circulating ACE2 levels are higher in patients with hypertension and cardiovascular disease [74, 75]. As a result, lung cancer patients with these comorbidities may have a potentially worse prognosis and an increased risk of death from COVID-19 infection due to high expression of the ACE2 receptor. Based on these findings, we recommend that high-risk lung cancer patients be evaluated and treated in a clinical setting with comprehensive medical resources.
This study has several limitations. Firstly, demographic or clinical characteristics were only reported in 8 of the 33 individual studies (24.24%). Additionally, the lack of more detailed information on anticancer treatment may impact the baseline prognosis of lung cancer patients with COVID-19 infection. Second, the potential mortality of SARS-CoV-2 variants could not be examined, as this information was not available. Thirdly, the source of heterogeneity could not be further determined when evaluating the pooled mortality of the 33 studies due to limited information. However, no significant heterogeneity was found when assessing the risk factor for mortality using the leave-one-out approach of the sensitivity analysis. Finally, due to the nature of retrospective studies and only including articles written in English, it was not possible to completely avoid any potential bias in selecting the articles for this study.
Thus, this systematic review and meta-analysis brought attention to possible directions for future investigation, such as (1) conducting additional prospective and multi-center cohort studies involving diverse regions and languages; (2) gathering detailed data on the systemic therapy employed for cancer treatment; (3) exploring the potential impact of different variants of the SARS-CoV-2 virus on mortality. Additionally, to ascertain the effectiveness of nationwide vaccination campaigns, it is important for future studies to assess COVID-19 mortality rates among lung cancer patients who have been vaccinated. It would also be valuable to analyze the impact of vaccine dosage and type on the antibody response.
Conclusions
Based on our meta-analysis, we have found that there is an increased mortality risk in lung cancer patients with COVID-19. The risk factors for these patients seem to be amplified by older age, advanced lung cancer, and comorbidities such as hypertension and cardiovascular disease. Implementing evidence-based best practices will help mitigate the impact of the COVID-19 pandemic on lung cancer patients and reduce the risk of death from COVID-19 infection.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
The maximal score for Newcastle-Ottawa scale is 9 stars: 4 stars for the selection process, 2 stars for comparability, and 3 stars for outcome.
(DOCX)
The maximal score for Prevalence Study Quality is 11, and only YES means 1 score.
(DOCX)
# variables were set as the reference. *P value < 0.05.
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020; 382(18): 1708–1720. doi: 10.1056/NEJMoa2002032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020; 395(10223): 507–513. Epub 2020/02/03. doi: 10.1016/S0140-6736(20)30211-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University Coronavirus Resource Center [cited 2023 March 10]. Available from: https://coronavirus.jhu.edu/map.html.
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020; 395(10223): 497–506. Epub 2020/01/28. doi: 10.1016/S0140-6736(20)30183-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology. 2020; 21(3): 335–337. Epub 2020/02/19. doi: 10.1016/S1470-2045(20)30096-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong B, Liu T, Luo P, Wei Y, Zhou Y, Liu M, et al. Prominent Hypercoagulability Associated With Inflammatory State Among Cancer Patients With SARS-CoV-2 Infection. Frontiers in oncology. 2020; 10: 1345. Epub 2020/08/09. doi: 10.3389/fonc.2020.01345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addeo A, Friedlaender A. Cancer and COVID-19: Unmasking their ties. Cancer treatment reviews. 2020; 88: 102041. Epub 2020/06/10. doi: 10.1016/j.ctrv.2020.102041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer discovery. 2020; 10(6): 783–791. Epub 2020/04/30. doi: 10.1158/2159-8290.CD-20-0422 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. 2023; 73(1): 17–48. Epub 2023/01/13. doi: 10.3322/caac.21763 . [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA oncology. 2020; 6(7): 1108–1110. Epub 2020/03/27. doi: 10.1001/jamaoncol.2020.0980 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. The Lancet Oncology. 2020; 21(7): 914–922. Epub 2020/06/17. doi: 10.1016/S1470-2045(20)30314-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai MY, Chen Z, Leng Y, Wu M, Liu Y, Zhou F, et al. Patients With Lung Cancer Have High Susceptibility of COVID-19: A Retrospective Study in Wuhan, China. Cancer control: journal of the Moffitt Cancer Center. 2020; 27(1): 1073274820960467. Epub 2020/09/18. doi: 10.1177/1073274820960467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shieh WJ, Hsiao CH, Paddock CD, Guarner J, Goldsmith CS, Tatti K, et al. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Human pathology. 2005; 36(3): 303–309. Epub 2005/03/26. doi: 10.1016/j.humpath.2004.11.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadalin S, Flego V, Pavlić SD, Volarić D, Radojčić Badovinac A, Kapović M, et al. Association between the ACE‑I/D polymorphism and nicotine dependence amongst patients with lung cancer. Biomed Rep. 2020; 13(6): 58. doi: 10.3892/br.2020.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provencio M, Mazarico Gallego JM, Calles A, Antoñanzas M, Pangua C, Mielgo Rubio X, et al. Lung cancer patients with COVID-19 in Spain: GRAVID study. Lung cancer (Amsterdam, Netherlands). 2021; 157: 109–115. Epub 2021/05/22. doi: 10.1016/j.lungcan.2021.05.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie L, Dai K, Wu J, Zhou X, Hu J, Zhang C, et al. Clinical characteristics and risk factors for in-hospital mortality of lung cancer patients with COVID-19: A multicenter, retrospective, cohort study. Thoracic cancer. 2021; 12(1): 57–65. Epub 2020/11/04. doi: 10.1111/1759-7714.13710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Pérez-Pérez M, et al. Covid-19 and lung cancer: A greater fatality rate? Lung cancer (Amsterdam, Netherlands). 2020; 146: 19–22. Epub 2020/06/07. doi: 10.1016/j.lungcan.2020.05.034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahya Y, Çınar G, Konuk Balcı BM, Yüksel C, Memikoğlu KO. Clinical characteristics of lung cancer patients with COVID-19: Retrospective case series. Tuberkuloz ve toraks. 2021; 69(4): 499–509. Epub 2021/12/28. doi: 10.5578/tt.20219608 . [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Rizvi H, Preeshagul IR, Egger JV, Hoyos D, Bandlamudi C, et al. COVID-19 in patients with lung cancer. Annals of oncology: official journal of the European Society for Medical Oncology. 2020; 31(10): 1386–1396. Epub 2020/06/21. doi: 10.1016/j.annonc.2020.06.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokce A, Hatipoglu M, Akboga SA, Sezen AI, Akkas Y, Kocer B. Critical care for lung cancer patients: surgical treatment during COVID-19 pandemic. Bratislavske lekarske listy. 2022; 123(2): 125–128. Epub 2022/01/24. doi: 10.4149/BLL_2022_019 . [DOI] [PubMed] [Google Scholar]
- 21.Hekimoglu B, Beyoglu MA. Early outcomes of lung resections in non-small cell lung cancer after COVID-19 pneumonia. Asian journal of surgery. 2022; 45(8): 1553–1558. Epub 2022/05/10. doi: 10.1016/j.asjsur.2022.04.080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haineala B, Zgura A, Badiu DC, Iliescu L, Anghel RM, Bacinschi XE. Lung Cancer, Covid-19 Infections and Chemotherapy. In vivo (Athens, Greece). 2021; 35(3): 1877–1880. Epub 2021/04/30. doi: 10.21873/invivo.12450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclère JB, Fournel L, Etienne H, Al Zreibi C, Onorati I, Roussel A, et al. Maintaining Surgical Treatment of Non-Small Cell Lung Cancer During the COVID-19 Pandemic in Paris. The Annals of thoracic surgery. 2021; 111(5): 1682–1688. Epub 2020/10/11. doi: 10.1016/j.athoracsur.2020.08.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bursac D, Zaric B, Bokan D, Kovacevic T, Stojsic V, Petkov S, et al. Mortality of COVID-19 pneumonia during anticancer treatment in lung cancer patients. Vojnosanitetski pregled. 2022; 79: 18–18. 10.2298/VSP211120018B. [DOI] [Google Scholar]
- 25.Calles A, Aparicio MI, Alva M, Bringas M, Gutierrez N, Soto J, et al. Outcomes of COVID-19 in Patients With Lung Cancer Treated in a Tertiary Hospital in Madrid. Frontiers in oncology. 2020; 10: 1777. Epub 2020/10/13. doi: 10.3389/fonc.2020.01777 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villena-Vargas J, Lutton EM, Mynard N, Nasar A, Voza F, Chow O, et al. Safety of lung cancer surgery during COVID-19 in a pandemic epicenter. The Journal of thoracic and cardiovascular surgery. 2022; 164(2): 378–385. Epub 2022/04/24. doi: 10.1016/j.jtcvs.2021.11.092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peixoto D, Callia JPB, Bittencourt MS, Generoso G, Anastácio VM, Alves-Jr JL, et al. Clinical presentation and in-hospital prognosis of lung cancer patients presenting with suspected and confirmed COVID-19. Brazilian journal of medical and biological research=Revista brasileira de pesquisas medicas e biologicas. 2022; 55: e12140. Epub 2022/09/15. doi: 10.1590/1414-431X2022e12140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pages PB, Cottenet J, Bonniaud P, Tubert-Bitter P, Piroth L, Cadranel J, et al. Impact of the SARS-CoV-2 Epidemic on Lung Cancer Surgery in France: A Nationwide Study. Cancers. 2021; 13(24). Epub 2021/12/25. doi: 10.3390/cancers13246277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser S, Baranowski R, Patrini D, Nandi J, Al-Sahaf M, Smelt J, et al. Maintaining safe lung cancer surgery during the COVID-19 pandemic in a global city. EClinicalMedicine. 2021; 39: 101085. Epub 2021/08/26. doi: 10.1016/j.eclinm.2021.101085 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assaad S, Avrillon V, Fournier ML, Mastroianni B, Russias B, Swalduz A, et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. European journal of cancer (Oxford, England: 1990). 2020; 135: 251–259. Epub 2020/06/17. doi: 10.1016/j.ejca.2020.05.028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Wang L, Chen Y, Wu Q, Chen G, Shen X, et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. 2020; 126(17): 4023–4031. Epub 2020/06/24. doi: 10.1002/cncr.33042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarza R, Bover M, Paredes D, López-López F, Jara-Casas D, Castelo-Loureiro A, et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. European journal of cancer (Oxford, England: 1990). 2020; 135: 242–250. Epub 2020/06/27. doi: 10.1016/j.ejca.2020.06.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. The Lancet Oncology. 2020; 21(7): 904–913. Epub 2020/06/02. doi: 10.1016/S1470-2045(20)30310-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Melo AC, Thuler LCS, da Silva JL, de Albuquerque LZ, Pecego AC, Rodrigues LOR, et al. Cancer inpatients with COVID-19: A report from the Brazilian National Cancer Institute. PloS one. 2020; 15(10): e0241261. Epub 2020/10/27. doi: 10.1371/journal.pone.0241261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer discovery. 2020; 10(7): 935–941. Epub 2020/05/03. doi: 10.1158/2159-8290.CD-20-0516 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basse C, Diakite S, Servois V, Frelaut M, Noret A, Bellesoeur A, et al. Characteristics and Outcome of SARS-CoV-2 Infection in Cancer Patients. JNCI cancer spectrum. 2021; 5(1): pkaa090. Epub 2021/02/20. doi: 10.1093/jncics/pkaa090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stroppa EM, Toscani I, Citterio C, Anselmi E, Zaffignani E, Codeluppi M, et al. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future oncology (London, England). 2020; 16(20): 1425–1432. Epub 2020/05/15. doi: 10.2217/fon-2020-0369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joharatnam-Hogan N, Hochhauser D, Shiu KK, Rush H, Crolley V, Wilson W, et al. Outcomes of the 2019 novel coronavirus in patients with or without a history of cancer: a multi-centre North London experience. Therapeutic advances in medical oncology. 2020; 12: 1758835920956803. Epub 2020/09/25. doi: 10.1177/1758835920956803 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B, Choi H, Lee SK, Chung SJ, Yeo Y, Shin YM, et al. Risk of Coronavirus Disease 2019 Occurrence, Severe Presentation, and Mortality in Patients with Lung Cancer. Cancer research and treatment. 2021; 53(3): 678–684. Epub 2021/01/11. doi: 10.4143/crt.2020.1242 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beltramo G, Cottenet J, Mariet AS, Georges M, Piroth L, Tubert-Bitter P, et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. The European respiratory journal. 2021; 58(6). Epub 2021/05/22. doi: 10.1183/13993003.04474-2020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard A, Cottenet J, Bonniaud P, Piroth L, Arveux P, Tubert-Bitter P, et al. Comparison of Cancer Patients to Non-Cancer Patients among COVID-19 Inpatients at a National Level. Cancers. 2021; 13(6). Epub 2021/04/04. doi: 10.3390/cancers13061436 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen UI, Xu H, Krause TM, Greenberg R, Dong X, Jiang X. Factors Associated With COVID-19 Death in the United States: Cohort Study. JMIR public health and surveillance. 2022; 8(5): e29343. Epub 2022/04/05. doi: 10.2196/29343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lièvre A, Turpin A, Ray-Coquard I, Le Malicot K, Thariat J, Ahle G, et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). European journal of cancer (Oxford, England: 1990). 2020; 141: 62–81. Epub 2020/11/01. doi: 10.1016/j.ejca.2020.09.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Várnai C, Palles C, Arnold R, Curley HM, Purshouse K, Cheng VWT, et al. Mortality Among Adults With Cancer Undergoing Chemotherapy or Immunotherapy and Infected With COVID-19. JAMA network open. 2022; 5(2): e220130. Epub 2022/02/22. doi: 10.1001/jamanetworkopen.2022.0130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farooque I, Farooque U, Karimi S, Syed MUS, Nadeem Z, Zulfiqar A, et al. Clinical Presentations and Outcomes of Coronavirus Disease 2019 in Patients With Solid Tumors. Cureus. 2021; 13(6): e15452. Epub 2021/07/16. doi: 10.7759/cureus.15452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Özdemir N, Dizdar Ö, Yazıcı O, Aksoy S, Dede DS, Budakoğlu B, et al. Clinical features and outcomes of COVID-19 in patients with solid tumors: Turkish National Registry Data. International journal of cancer. 2020. Epub 2020/12/08. doi: 10.1002/ijc.33426 . [DOI] [PubMed] [Google Scholar]
- 47.Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet (London, England). 2020; 395(10241): 1919–1926. Epub 2020/06/01. doi: 10.1016/S0140-6736(20)31173-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benderra MA, Aparicio A, Leblanc J, Wassermann D, Kempf E, Galula G, et al. Clinical Characteristics, Care Trajectories and Mortality Rate of SARS-CoV-2 Infected Cancer Patients: A Multicenter Cohort Study. Cancers. 2021; 13(19). Epub 2021/10/14. doi: 10.3390/cancers13194749 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song K, Gong H, Xu B, Dong X, Li L, Hu W, et al. Association between recent oncologic treatment and mortality among patients with carcinoma who are hospitalized with COVID-19: A multicenter study. Cancer. 2021; 127(3): 437–448. Epub 2020/11/03. doi: 10.1002/cncr.33240 . [DOI] [PubMed] [Google Scholar]
- 50.Fernandes GA, Feriani D, França ESILA, Mendonça ESDR, Arantes PE, Canteras JDS, et al. Differences in mortality of cancer patients with COVID-19 in a Brazilian cancer center. Seminars in oncology. 2021; 48(2): 171–180. Epub 2021/02/13. doi: 10.1053/j.seminoncol.2021.01.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari BL, Ferreira CG, Menezes M, De Marchi P, Canedo J, Melo AC, et al. Determinants of COVID-19 Mortality in Patients With Cancer From a Community Oncology Practice in Brazil. JCO global oncology. 2021; 7: 46–55. Epub 2021/01/13. doi: 10.1200/GO.20.00444 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khusid JA, Becerra AZ, Gallante B, Sadiq AS, Atallah WM, Badani KK, et al. Cancer, Mortality, and Acute Kidney Injury among Hospitalized Patients with SARS-CoV-2 Infection. Asian Pacific journal of cancer prevention: APJCP. 2021; 22(2): 517–522. Epub 2021/03/01. doi: 10.31557/APJCP.2021.22.2.517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Joode K, Tol J, Hamberg P, Cloos M, Kastelijn EA, Borgers JSW, et al. Life-prolonging treatment restrictions and outcomes in patients with cancer and COVID-19: an update from the Dutch Oncology COVID-19 Consortium. European journal of cancer (Oxford, England: 1990). 2022; 160: 261–272. Epub 2021/11/21. doi: 10.1016/j.ejca.2021.10.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oldani S, Petrelli F, Dognini G, Borgonovo K, Parati MC, Ghilardi M, et al. COVID-19 and Lung Cancer Survival: An Updated Systematic Review and Meta-Analysis. Cancers. 2022; 14(22). Epub 2022/11/27. doi: 10.3390/cancers14225706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei H, Yang Y, Zhou W, Zhang M, Shen Y, Tao D, et al. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung cancer (Amsterdam, Netherlands). 2021; 157: 60–65. Epub 2021/05/15. doi: 10.1016/j.lungcan.2021.05.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peravali M, Joshi I, Ahn J, Kim C. A Systematic Review and Meta-Analysis of Clinical Characteristics and Outcomes in Patients With Lung Cancer with Coronavirus Disease 2019. JTO clinical and research reports. 2021; 2(3): 100141. Epub 2021/01/14. doi: 10.1016/j.jtocrr.2020.100141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. Jama. 2018; 319(4): 388–396. Epub 2018/01/25. doi: 10.1001/jama.2017.19163 . [DOI] [PubMed] [Google Scholar]
- 58.Wu M, Liu S, Yang Y, Lin J, Liu J. Clinical characteristics and outcomes of lung cancer patients with COVID-19: A systematic review and meta-analysis protocol. PloS one. 2022; 17(8): e0273691. Epub 2022/09/01. doi: 10.1371/journal.pone.0273691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010; 25(9): 603–605. Epub 2010/07/24. doi: 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 60.Rostom A DC, Cranney A. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US) 2004 Sep [cited 2023 3/25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK35156/.
- 61.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997; 315(7109): 629–634. Epub 1997/10/06. doi: 10.1136/bmj.315.7109.629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003; 327(7414): 557–560. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research synthesis methods. 2010; 1(2): 97–111. Epub 2010/04/01. doi: 10.1002/jrsm.12 . [DOI] [PubMed] [Google Scholar]
- 64.Aredo JV, Luo SJ, Gardner RM, Sanyal N, Choi E, Hickey TP, et al. Tobacco Smoking and Risk of Second Primary Lung Cancer. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2021; 16(6): 968–979. Epub 2021/03/17. doi: 10.1016/j.jtho.2021.02.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. Jama. 2021; 325(10): 962–970. Epub 2021/03/10. doi: 10.1001/jama.2021.1117 . [DOI] [PubMed] [Google Scholar]
- 66.Khoury E, Nevitt S, Madsen WR, Turtle L, Davies G, Palmieri C. Differences in Outcomes and Factors Associated With Mortality Among Patients With SARS-CoV-2 Infection and Cancer Compared With Those Without Cancer: A Systematic Review and Meta-analysis. JAMA network open. 2022; 5(5): e2210880. Epub 2022/05/10. doi: 10.1001/jamanetworkopen.2022.10880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arayici ME, Kipcak N, Kayacik U, Kelbat C, Keskin D, Kilicarslan ME, et al. Effects of SARS-CoV-2 infections in patients with cancer on mortality, ICU admission and incidence: a systematic review with meta-analysis involving 709,908 participants and 31,732 cancer patients. Journal of cancer research and clinical oncology. 2022: 1–14. Epub 2022/07/14. doi: 10.1007/s00432-022-04191-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020; 584(7821): 430–436. Epub 2020/07/09. doi: 10.1038/s41586-020-2521-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. Jama. 2020; 323(18): 1775–1776. Epub 2020/03/24. doi: 10.1001/jama.2020.4683 . [DOI] [PubMed] [Google Scholar]
- 70.Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Annals of internal medicine. 2018; 169(10): 684–693. Epub 2018/10/12. doi: 10.7326/M18-1250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang D, Liu Y, Bai C, Wang X, Powell CA. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer letters. 2020; 468: 82–87. Epub 2019/10/11. doi: 10.1016/j.canlet.2019.10.009 . [DOI] [PubMed] [Google Scholar]
- 72.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005; 41 Suppl 7: S504–512. Epub 2005/10/21. doi: 10.1086/432007 . [DOI] [PubMed] [Google Scholar]
- 73.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002; 417(6891): 822–828. Epub 2002/06/21. doi: 10.1038/nature00786 . [DOI] [PubMed] [Google Scholar]
- 74.Li R, Qiao S, Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. The Journal of infection. 2020; 80(4): 469–496. Epub 2020/02/25. doi: 10.1016/j.jinf.2020.02.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; 181(2): 271–280 e278. Epub 2020/03/07. doi: 10.1016/j.cell.2020.02.052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
The maximal score for Newcastle-Ottawa scale is 9 stars: 4 stars for the selection process, 2 stars for comparability, and 3 stars for outcome.
(DOCX)
The maximal score for Prevalence Study Quality is 11, and only YES means 1 score.
(DOCX)
# variables were set as the reference. *P value < 0.05.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.