Abstract
Purpose:
To determine agreement between diurnal variation testing (DVT) of intraocular pressure (IOP) with Goldmann applanation tonometer (GAT) and iCare HOME (IH) by an optometrist (OP) and home monitoring by participants (PT).
Methods:
Patients (18–80 years) with glaucoma and suspects were enrolled. IH IOP and GAT were taken by an OP at 2 h intervals from 8 AM to 4 PM on Day 1 and PT between 6 AM and 9 PM, for the next 2 days. IOP, date, and time were viewed via iCare LINK software.
Results:
In total, 72.9% (51/70) PT trained were able to take reliable readings. One hundred two eyes (51 patients, age 53 ± 16 yrs) were analyzed. Correlation between optometrist (OP) and participants (PT) was strong and positive {IH OP-IH PT- r = 0.90, p-0.0001;IH PT-GAT- r = 0.79, p-0.0001}. Agreement by Bland Altman plots was limited {IH OP-IH PT mean 0.1 mmHg (95% LOA -5.3 to 5.5), IH PT-GAT 2.2 mmHg (-5.7 to 10.1)}. Intraclass correlation coefficient for IH OP-IH PT was 1.18 (95% CI 1.37-1.09). Intradevice {0.95 (95% CI 0.94-0.97)} and interrater repeatability {0.91 (0.79–0.96)} were good. 37% of eyes had a synchronous peak on GAT and IH during the day DVT.
Conclusion:
Home tonometry by iCare HOME is easy, feasible, but due to limited agreement cannot substitute GAT DVT.
Keywords: Home tonometry, iCare HOME, glaucoma
Twenty-four hour IOP variation can have implications for the pathogenesis and management of glaucoma.[1,2] Studies have shown that intraocular pressure (IOP) peaks and fluctuations are better predictors of progression in patients with apparently well-controlled glaucoma.[1-5] Office day diurnal variation testing (DVT) is a more practical and cost-effective substitute for 24-h IOP phasing. Moodie et al.[6] demonstrated no significant change in management as a result of DVT between daytime and 24 h. However, office day DVT is also time and resource consuming, for both the clinician and the patient. Home IOP monitoring allows for wider phasing times, IOP checks during routine activities in an ambient environment, and reduced hospital waiting time.
Goldmann applanation tonometry (GAT), the current reference standard for IOP measurement, can be performed only in the clinic. iCare HOME (IH, TA022, iCare Finland Oy, Vantaa, Finland) is a USFDA-approved rebound tonometer. It is portable, does not require the use of topical anesthetic, and has eye recognition technology to distinguish right and left eyes. It has forehead, and cheek rests customizable to varied facial anatomy, red-green colored light indicators to guide patients with respect to device alignment – making it a patient-friendly device for self-monitoring. It needs some training by a healthcare professional.
Previous studies have shown that nearly 75% of patients were able to use the IH for self-tonometry.[7,8] IOP measurements by the two instruments have been shown to have a strong correlation and an interdevice agreement within 5 mmhg.[7-10] Very few studies exist where the IH was used by the patient in their habitual environment or to measure diurnal fluctuations. The ones which exist have involved young healthy individuals.[10] Limited data exists regarding home tonometry in the Indian population.
In such a setting, it would be interesting to note the feasibility and clinical utility of home tonometry for diurnal IOP monitoring, in relatively older glaucoma patients, seen routinely in the clinic, and whether it can merit consideration as a substitute for day DVT. Our objectives were to (i) to determine the correlation and agreement between IOP measurements made using GAT and IH by a trained OP and IH by PT after three days of hands-on experience. (ii) To determine whether out of office IOP measurement is providing additional beneficial information on patient’s IOP profile and (iii) To determine PT’s ease in using the device via a questionnaire.
Methods
This study received approval from the Institutional ethics committee and was in accordance with the tenets of Declaration of Helsinki. It was a prospective observational study conducted between January 2021 and March 2022. Patients (aged 18–80 years) visiting the glaucoma clinic underwent visual acuity assessment by Snellen’s chart, IOP measurement with GAT, gonioscopy with four mirror gonioscope, anterior segment examination by slit-lamp biomicroscopy, central corneal thickness (CCT) by ultrasound pachymeter, keratometry reading by automated keratometer, and fundus evaluation.
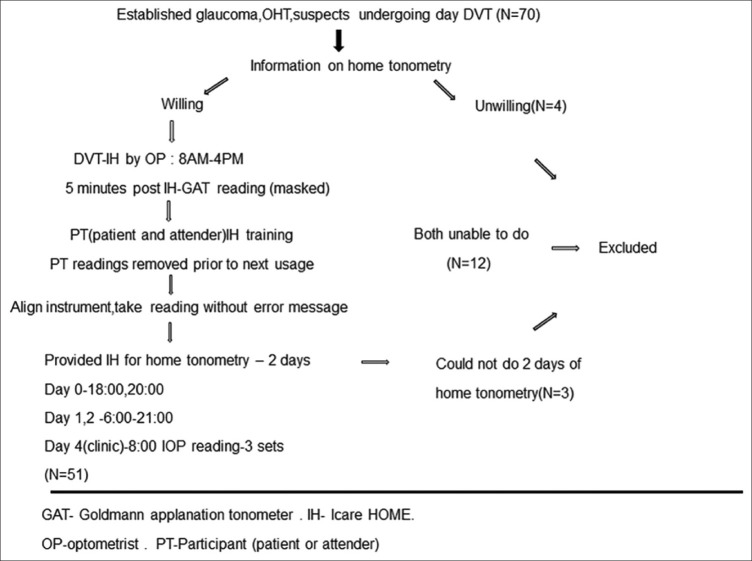
Consecutive, eligible patients with established glaucoma, ocular hypertensives, and glaucoma suspects undergoing day DVT as deemed necessary by their treating clinician were given information regarding home tonometry. [Methodology illustrated in Fig. 1]. A patient with glaucoma was included if he/she had glaucomatous optic disc changes with corresponding typical visual field defects that were reliable. Ocular hypertensives had IOP >21 mm Hg, open angles, and no disc or visual field changes. Patients with cup disc ratio >0.7, rim width <0.1-disc diameter, presence of any retinal nerve fiber layer defect, disc hemorrhage with normal visual field or primary angle closure suspects were grouped as glaucoma suspects for the study. IH IOP measurements were taken by a trained OP at two-hour intervals starting from 8 AM to 4 PM. Five minutes post IH readings, the same OP masked to the IH readings took the IOP on GAT. The IH readings were then transferred to a personal computer by iCare LINK software. The patient received training on using IH,in the presence of his/her attender. If the patient was unable to handle the device, the attender was given training. The hands-on training were spaced and repeated, every time the patient came for a DVT reading. All participants (PT) were trained to use the right hand for the right eye and the left hand for left eye measurements. Measurements made by the PT were removed from the device prior to next usage. Only the 8 AM IOP reading was measured thrice. An information sheet and explanatory video (https://www.youtube.com/watch?v=bXgEoQV0orM) on how to use the device were provided to the patient during his/her waiting period in the hospital. After the 4 PM IOP reading by the OP, the PT had to demonstrate the use of IH without assistance from the OP. If the PT was able to align the instrument correctly, and take readings without getting error messages, then the patient was provided the instrument for home tonometry, being explained clearly that it is meant only for that individual’s use. The PT then had to take two readings the same day at 18:00 and between 20:00-21:00. Multiple IOP measurements were made next two days. Patients were asked to take one IOP reading between 6:00 and 7:00, then at 8:00, 12:00, 16:00, 18:00, and one reading between 19 and 21:00. All IOP measurements were made in the upright sitting position. It could be done in front of a mirror for ease. Patients were asked to maintain a log of sleep, wake time, activity just prior to IOP measurement, time of antiglaucoma medication application. All IOP readings were taken just prior to eyedrop application. On the fourth day, the patient returned the instrument to the clinic. Three readings at 8 AM were taken by the PT who has done the home tonometry and also by the OP. Since the readings are not immediately displayed on the instrument, the OP masked to these readings took the IOP on GAT as well. IOP measurements, time, date, and quality of each measurement were viewed via the iCare LINK software and uploaded into the patient’s electronic medical record. PT also gave feedback on difficulties/ease of use of device, what additional support they would have preferred during their training session, and whether they would want to use home-tonometry again.
Figure 1.
Flowchart illustrating study methodology
Inclusion Criteria: a) Patients with ocular hypertension, established glaucoma, and glaucoma suspects undergoing a day DVT b) Willing to consent and ability to use IH tonometer.
Exclusion Criteria: a) Patients unwilling to give consent or reliably use IH tonometer, b) age less than 18 and more than 80 years, C) patients with eso/exotropia, nystagmus, poor/eccentric fixation, D) visual acuity <3/60, e) physical disability that hinders proper use of instrument, f) corneal scarring, edema, g) corneal astigmatism >3 D and keratoconus, h) contact lens use, i) active ocular surface disease, and j) patient and or caregiver unable to perform the IH IOP measurements correctly, in front of the health care professional, following training session.
Analysis
The statistical analysis was performed using SPSS version 23.0 for Windows (IBM). Bland Altman plots of the agreement were calculated using MedCalc Statistical Software version 20.118 (MedCalc Software Ltd, Ostend, Belgium). Mean GAT IOP was the mean of day DVT performed on day one by the optometrist (OP) and IH Mean was mean of the diurnal readings taken by the OP using IH on day one. Home mean IOP was the mean of the diurnal IOP measurements made using IH by the participant (PT) at home during the next two consecutive days. Pearson correlation coefficient and Bland Altman plots for the agreement were drawn for IOP taken by the OP using GAT and IH and that by the PT following home tonometry on the fourth day at the clinic. Intradevice repeatability was evaluated according to intraclass correlation coefficient (ICC) using three consecutive IOP readings at 8 AM. Since both eyes of the study participants were included for analysis, generalized estimating equations (GEE) were used to study factors affecting the difference in IOP measured by the two tonometers.
Results
Seventy patients undergoing day DVT had their IOP measurements also done by IH and were offered information regarding home tonometry. Of these, four patients were unwilling and twelve patients (and attenders) were unable to take readings reliably after hands-on session. Three patients did not complete two days of home tonometry and all of them were excluded. Data from 51 patients (102 eyes) were analyzed. Table 1 summarizes the clinical and demographic data of patients.
Table 1.
Clinical and demographic data of patients who did home tonometry (n=51)
| Measurement | Mean (SD) |
|---|---|
| Age (years) | 53.41±16.66 |
| Gender (Male: Female) | 32:19 |
| Visual acuity (log mar) | 0.11±0.33 |
| Diagnosis (number of eyes) | |
| Glaucoma suspects | 38 |
| Primary glaucoma | 49 |
| Ocular hypertension | 12 |
| Secondary glaucoma | 3 |
| Prior trabeculectomy | 2 |
| Number of IOP lowering agents | 0.53±1.14 |
| CCT (microns) | 528.17±39.37 |
| Number of eyes: CCT<500 | 22 |
| 500–600 | 77 |
| >600 | 3 |
| Vertical palpebral fissure height (mm) | 11.36±0.70 |
| GAT IOP (mmHg) | 16.84±5.50 |
| IH IOP (mmHg) | 15.28±4.0 |
| Self-tonometry | 19 |
| Severity of established glaucoma | |
| MD≤6 | 20 |
| 6–12 | 17 |
| ≥12 | 15 |
CCT: Central corneal thickness; GAT: Goldmann applanation tonometer; IH: iCare HOME IOP: Intraocular pressure
In total, 39.2% (40/102 eyes) had IH mean IOP (measured by OP) within 2 mmHg of GAT IOP, 56.86% (58/102 eyes) had within 3 mmHg, and 77.84% (79/102 eyes) had within 5 mmHg of GAT IOP. It was found that 37% (38/102) of eyes had a synchronous peak on GAT and IH during the day DVT.
Correlation and agreement between GAT IOP and iCare HOME IOP
Correlation and agreement between readings made by OP on GAT and IH device and by the PT following training and two days of home tonometry, while returning the device during the second clinic visit was made. Due to peak COVID pandemic times, this comparison data was available for 21/51 patients who did two days of home tonometry. The correlation for the IOP measurements was found to be strong and positive {IH OP-GAT (R = 0.798, P < 0.0001), IH OP-IH PT (R = 0.905, P < 0.0001), GAT-IH PT (R = 0.791, P < 0.0001)}.
Bland Altman plots drawn for agreement showed limited agreement as enumerated in Table 2 and Fig. 2. The ICC for agreement between IH IOP measurement between OP and PT was 1.18 (95% CI 1.37-1.09), ICC for IH by OP, and GAT IOP was 0.69 (95% CI 0.38-0.84), ICC for IH IOP by PT and GAT IOP was 0.83 (95% CI 0.55-0.92).
Table 2.
Bland Altman plots for agreement between various settings in the study and relevant bias
| Mean difference IH Vs GAT (mmHg) | 95% Limits of agreement (lower and upper) | Bias | |
|---|---|---|---|
| IH OP Vs GAT | 2.3 | −5.2 to 9.74 | systematic bias (Higher the IOP, higher the IH value) |
| IH OP Vs IH PT | 0.1 | −5.3 to 5.5 | systematic bias |
| IH PT Vs GAT | 2.2 | −5.7 to 10.1 | random bias |
GAT – Goldmann applanation tonometer ; IH OP – iCare HOME IOP by optometrist ; IH PT – iCare HOME IOP by participant
Figure 2.
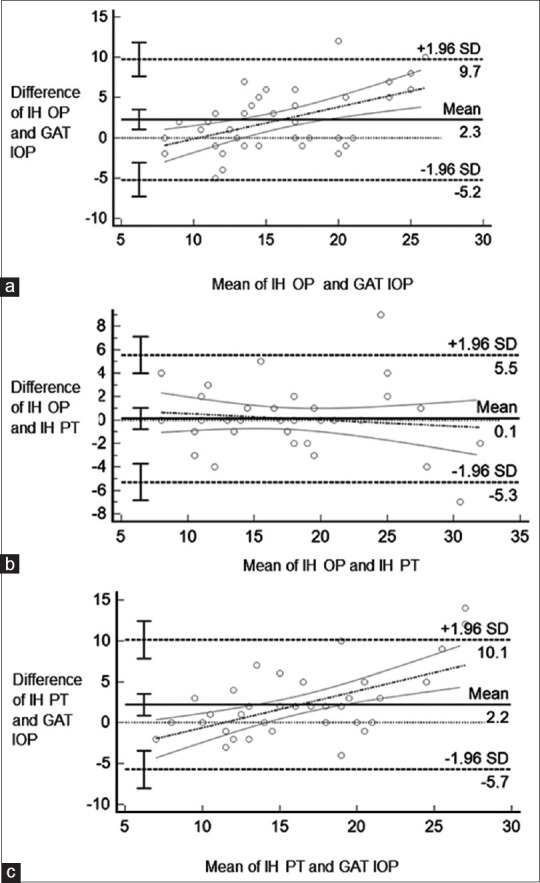
Bland Altman plots of agreement for IOP measurements made by a) Optometrist on IH and GAT, b) IH by optometrist and patient, c) IH by patient and GAT by optometrist
The factors affecting IOP differences between tonometers were studied using GEE. The mean GAT IOP and mean IH IOP measured by the OP during first clinic visit was used. CCT and GAT IOP were found to be significantly affecting the IOP difference between IH and GAT {CCT:-0.029(-0.4 to-0.016),p=-0.0001}{mean GAT:-0.274 (−0.40 to − 0.14), P = −0.0001}.
When CCT was stratified into three groups for analysis, it was found that with increasing CCT, the difference between the two tonometers increased significantly with IH overestimating IOP compared to GAT. [Fig. 3]. For CCT <500 μm, mean IOP difference was −1.13 (-2.46 to 0.18); for CCT 500–600 μm, it was −3.05 (−3.76 to − 2.35), for CCT >600 μm, mean −7.4 (−10.98 to −3.81).
Figure 3.
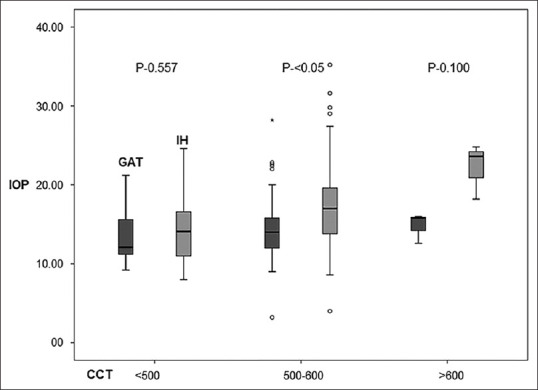
IOP difference between the two tonometers according to CCT variation
Effect of IH on peak IOP and management
Of the 102 eyes of 51 patients who did two days of home tonometry, 25 eyes (24.5%) had their peak IOP out-of-office hours. 5/25 eyes (20%) had CCT <500 μm and 1/25 (4%) had CCT > 600 μm in this group (beyond the manufacturer’s recommendation for IH use). Peak IOP difference of at least 3 mmHg detected beyond office hours measurement was seen in 10/102 eyes (9.8%), 4 mmHg difference in 7/102 eyes (6.86%), and greater than 8 mm Hg difference was seen in 1/102 eyes (0.98%). A treatment change post DVT was instituted in 36 eyes (35 in terms of medication, 1 selective laser trabeculoplasty). However, these management changes were not specifically related to out of office IOP measurements by IH alone.
Repeatability
The intradevice and interrater repeatability for the IH device was very good. The ICC value for GAT was 0.975 (95% CI 0.966–0.983) and for IH was 0.957 (95% CI 0.940–0.969). The interrater repeatability between two trained optometrists was also calculated using IOP measurements made on 20 patients. The ICC for GAT was 0.947 (95% CI 0.907–0.970) and for IH was 0.916 (95% CI 0.787–0.960).
Home tonometry experience and patient feedback
Nineteen patients did home self-tonometry (mean age: 46.5 ± 18.9 years).Thirty-two patients (mean age: 57 ± 2.45) had their attenders (mean attender age: 43.9 ± 13.4 years) take the readings. Cumulative time to train a participant (PT) to take IH readings was 30–60 minutes. All study PT reported right-hand dominance. Good adherence to timings mentioned in the logbook was observed (100% were within ± 10 minutes of prescribed timings). PT also rated the overall experience with the device from 1 to 5 with 1 being difficult (3/51, 5.9%), 2 - somewhat difficult (6/51,12%), 3 - okay (10/51, 20%), 4 - somewhat easy (16/51,31%), and 5 - very easy and comfortable (16/51, 31.%) Among the difficulties expressed by the PTs, alignment issue was the most common (22/51, 43%). Others reasons mentioned were anxiety about high IOP reading, about their ability to get a correct reading, and avoiding blink reflex. Among those who were able to do, acceptance level was high and when asked if they would like to do home tonometry again, 55% (28/51) replied in the affirmative. The OP observed that long eyelashes (which comes in contact with the probe tip during excursion resulting in “repeat reading” error), deep set eyes, and poor dexterity due to which device stabilization and alignment becomes difficult were the most common reasons why some patients who received the training could not qualify to do home self-tonometry.
Discussion
IOP is the only known modifiable risk factor for glaucoma currently. It has been shown that large diurnal IOP fluctuations can lead to glaucoma progression.[1,3,4] Studies have demonstrated that nearly 50% of glaucoma may not be adequately treated due to single office IOP measurement.[11] Continuous IOP monitoring is also ridden with practical difficulties.[12,13] Home monitoring provides a more realistic snapshot of a patient’s IOP profile.
Varied populations have been included in different studies, demonstrating that the feasibility of IH use is not affected by population demographics.[14] Studies have noted that gender, handedness, education level, refractive error, and vertical palpebral aperture do not appear to affect usage of device. Physical disability and systemic conditions like Parkinson’s disease and arthritis causing tremors and affecting dexterity may hinder self-tonometry.[8,14] We attempted training attendants in these cases or excluded them, if both patient and caregiver were elderly and not able.
IH readings seem to agree better with GAT in the mid-range level between 10 and 20 mmHg. This is similar to findings reported by Cvenkel[15] and Mudie et al.[7] wherein IH overestimated GAT in groups with higher IOPs and underestimated in those with lower IOP. Nearly 79 eyes (77.45%) had IH mean IOP (taken by optometrist) within ± 5 mmHg of GAT IOP. Mudie et al.[7] in their study found 91% and Pronin et al.[16] had 56% IH measurements within 5 mmHg of GAT. Chen et al.[17] showed that 68% of IH measurements made by patients and 71% of IH measurements made by staff were within ± 3 mmHg of GAT measurements.
In our study, the mean difference between IH IOP done by the patient after two days of home tonometry and GAT was 2.3 mmHg (95% limits of agreement −5.2 to 9.7 mmHg). Noguchi et al.[10] in his study with young healthy individuals doing self-tonometry reported the mean difference to be 1.03 mmHg (−3.91 to 5.98 mmHg). In most studies, the mean difference in IOP (GAT-IH) ranged from -0.7 mmHg to 2.66 mmHg.[14] We also observed that with increase in GAT IOP, the difference between the two tonometers increased. The manufacturer has validated IH usage only when the CCT is between 500 and 600 μm. Similar to other studies we found that the difference between the two tonometers increased when CCT increased, with IH overestimating IOP.[7,8,16,18,19]
IOP peaks outside office hours may occur in 50–70% of patients.[2,20,21] Prospective studies have shown that self-tonometry with IH is feasible and is able to identify higher IOP peak and fluctuation at home than in the clinic.[15,22,23] In our study, we found only 7 of 102 eyes (6.86%) showing an IOP peak >4 mmhg beyond office hours measurement in comparison to Chen et al.[17] who found 16%. This could possibly be because of the fact that our sample group had many glaucoma suspects, patients on treatment and a smaller number of progressors. Further this study was not directed to detect out of office hours peak as the primary objective. We found the intradevice and interrater repeatability to be good, similar to other studies.[7,10,18]
Studies have shown that with training more than 75% of patients can obtain accurate IOP measurements on iCare HOME in comparison to GAT.[7,9,16] Time needed for training was an average of 15–20 minutes.[8,15,16] In our study, we found a lesser percentage of participants capable of taking reliable readings (72%) and self-tonometry (27%). The cumulative time taken to train them was also more (30–60 minutes). Possible reasons for this discrepancy seem manifold. In our study, patients were given the instrument for home tonometry if they were able to align the device correctly and take measurements without getting error messages. IH manufacturers have prescribed criteria for training which include i) correct alignment ii) first of three iCare readings taken by the patient and GAT by optometrist should differ by 5 mm Hg or less iii) range of three readings by the patient is 7 mm Hg or less. Noguchi and Termuhlen had healthy participants and do not mention their training/certification process.[10,18] Takagi et al.[9] had patients with visual acuity 20/25 or better, and Noguchi et al.[10] had a younger age group (mean age 28.3 ± 4.7 mmHg). Some studies have had only a single IOP measurement by the patient in the office[18] and in some the investigator was unmasked to both readings.[18] Hence, these numbers need to be interpreted with caution and cannot be directly co-related with our findings. Participants reported that ease of use of the device improved from day 1 to day 4 of hands-on experience, but since we removed the readings taken by them on day one of training session, we could not substantiate this statement quantitatively. Ogle et al.[24] assessed the reliability of patient IOP measurements over a seven-day period and found no significant trend in test–retest variability across the seven days of use.
The strength of our study is its prospective nature, inclusion of older age group, patients with advanced field loss, masking of the optometrist to IH values while taking GAT, and the fact that the participants got to use the device at home, in the absence of optometrist supervision, wherein training, recall and confidence in handling device independently is put to test. Agreement data between OP and PT IOP could be collected for only 21/51 patients doing home tonometry, because in peak COVID times, it was impractical to have a second clinic visit. Although we tried to look at out-of-office hours IOP, we restricted IOP measurements until bedtime and did not have the patient wake up to take nocturnal measurements. We also did not have many patients who had glaucoma progression or showing wide IOP fluctuations in our study sample. Despite the manufacturer advocating the instrument for self-tonometry, in our setting, it was more pragmatic to allow the attender also to take the measurements. These are some of our study limitations.
Conclusion
The COVID-19 pandemic has changed many aspects of glaucoma practice worldwide and teleconsultations are becoming more popular. In such a scenario, home tonometers like the IH can possibly be an important addition to our diagnostic armamentarium allowing IOP measurements, electronic storage, and data transfer to the clinician. Home tonometry by IH is easy (82%) and feasible (72.8%), with some training from a healthcare professional and assistance from the attendant. But due to its limited agreement, it cannot substitute GAT DVT. Structured training programs for healthcare professionals in order to train more patients, troubleshoot errors while handling the device, design modification to suit more varied facial anatomy, and potential future applications need to be explored.
Financial support and sponsorship
Icare Home units for the study were under an unconditional loan from the manufacturer.They had no role in study results and manuscript preparation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Vishwanathan Natarajan, Department of Biostatistics, Medical Research Foundation.
References
- 1.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124:793–7. doi: 10.1001/archopht.124.6.793. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.De Moraes CG, Juthani VJ, Liebmann JM, Teng CC, Tello C, Susanna R, Jr, et al. Risk factors for visual field progression in treated glaucoma. Arch Ophthalmol. 2011;129:562–8. doi: 10.1001/archophthalmol.2011.72. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner SK, Johnson CA, Demirel S. Factors predicting the rate of functional progression in early and suspected glaucoma. Invest Ophthalmol Vis Sci. 2012;53:3598–604. doi: 10.1167/iovs.11-9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moodie J, Wilde C, Rotchford AP, Vernon SA, King AJ. 24-Hour versus daytime intraocular pressure phasing in the management of patients with treated glaucoma. Br J Ophthalmol. 2010;94:999–1002. doi: 10.1136/bjo.2009.160267. [DOI] [PubMed] [Google Scholar]
- 7.Mudie LI, LaBarre S, Varadaraj V, Karakus S, Onnela J, Munoz B, et al. The iCare HOME (TA022) Study: Performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123:1675–84. doi: 10.1016/j.ophtha.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Dabasia PL, Lawrenson JG, Murdoch IE. Evaluation of a new rebound tonometer for self-measurement of intraocular pressure. Br J Ophthalmol. 2016;100:1139–43. doi: 10.1136/bjophthalmol-2015-307674. [DOI] [PubMed] [Google Scholar]
- 9.Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, iCare HOME: Comparison with Goldmann applanation tonometer. J Glaucoma. 2017;26:613–8. doi: 10.1097/IJG.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi A, Nakakura S, Fujio Y, Fukuma Y, Mori E, Tabuchi H, et al. A pilot evaluation assessing the ease of use and accuracy of the new self/home-tonometer iCareHOME in healthy young subjects. J Glaucoma. 2016;25:835–41. doi: 10.1097/IJG.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 11.Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: A systematic review. Surv Ophthalmol. 2009;54:643–62. doi: 10.1016/j.survophthal.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Sit AJ. Continuous monitoring of intraocular pressure: Rationale and progress toward a clinical device. J Glaucoma. 2009;18:272–9. doi: 10.1097/IJG.0b013e3181862490. [DOI] [PubMed] [Google Scholar]
- 13.Kakaday T, Hewitt AW, Voelcker NH, Li JS, Craig JE. Advances in telemetric continuous intraocular pressure assessment. Br J Ophthalmol. 2009;93:992–6. doi: 10.1136/bjo.2008.144261. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, De Francesco T, Schlenker M, Ahmed II. iCare home tonometer: A review of characteristics and clinical utility. Clin Ophthalmol. 2020;14:4031–45. doi: 10.2147/OPTH.S284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cvenkel B, Velkovska MA, Jordanova VD. Self-measurement with iCare HOME tonometer, patients'feasibility and acceptability. Eur J Ophthalmol. 2020;30:258–63. doi: 10.1177/1120672118823124. [DOI] [PubMed] [Google Scholar]
- 16.Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol. 2017;135:1030–6. doi: 10.1001/jamaophthalmol.2017.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quérat L, Chen E. Monitoring daily intraocular pressure fluctuations with self-tonometry in healthy subjects. Acta Ophthalmol. 2017;95:525–9. doi: 10.1111/aos.13389. [DOI] [PubMed] [Google Scholar]
- 18.Termühlen J, Mihailovic N, Alnawaiseh M, Dietlein TS, Rosentreter A. Accuracy of measurements with the iCare HOME rebound tonometer. J Glaucoma. 2016;25:533–8. doi: 10.1097/IJG.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 19.Brown L, Foulsham W, Pronin S, Tatham AJ. The influence of corneal biomechanical properties on intraocular pressure measurements using a rebound self-tonometer. J Glaucoma. 2018;27:511–8. doi: 10.1097/IJG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 20.Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: A retrospective review. J Glaucoma. 2003;12:232–6. doi: 10.1097/00061198-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Nakakura S, Nomura Y, Ataka S, Shiraki K. Relation between office intraocular pressure and 24-hour intraocular pressure in patients with primary open-angle glaucoma treated with a combination of topical antiglaucoma eye drops. J Glaucoma. 2007;16:201–4. doi: 10.1097/IJG.0b013e31802ff85f. [DOI] [PubMed] [Google Scholar]
- 22.McGarva E, Farr J, Dabasia P, Lawrenson JG, Murdoch IE. Initial experience in self-monitoring of intraocular pressure. Eur J Ophthalmol. 2021;31:1326–32. doi: 10.1177/1120672120920217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatham AJ, Young SL, Chew E, Brown L. A Comparison of short-term intraocular pressure fluctuation with office-based and home tonometry. Ophthalmol Glaucoma. 2021;4:113–4. doi: 10.1016/j.ogla.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Ogle JJ, Soo Hoo WC, Chua CH, Yip LWL. Accuracy and Reliability of Self-measured Intraocular Pressure in Glaucoma Patients Using the iCare HOME Tonometer. J Glaucoma. 2021;30:1027–32. doi: 10.1097/IJG.0000000000001945. [DOI] [PubMed] [Google Scholar]