Abstract
Select life stressors having enduring physiological and behavioral consequences, in part by eliciting dramatic signaling shifts in monoamine neurotransmitters. High monoamine levels can overwhelm selective transporters like the serotonin transporter. This is when polyspecific transporters like plasma membrane monoamine transporter (PMAT, Slc29a4) are hypothesized to contribute most to monoaminergic signaling regulation. Here, we employed two distinct counterbalanced stressors – fear conditioning, and swim stress – to systematically determine how reductions in PMAT function affect heterotypic stressor responsivity. We hypothesized male heterozygotes would exhibit augmented stressor responses relative to female heterozygotes. Decreased PMAT function enhanced context fear expression, an effect unexpectedly obscured by a sham stress condition. Impairments in cued fear extinction retention and enhanced context fear expression in males were conversely unmasked by sham swim condition. Abrogated corticosterone levels in male heterozygotes that underwent swim stress after context fear conditioning did not map on to any measured behaviors. In sum, male heterozygous fear behaviors proved malleable in response to preceding stressor or sham stress exposure. Combined, these data indicate reduced male PMAT function elicits a form of stress-responsive plasticity. Future studies should assess how PMAT is differentially affected in the sexes, and identify downstream consequences of the stress-shifted corticosterone dynamics.
Introduction
Under stressful environmental conditions, signaling patterns of monoamine neurotransmitters like dopamine and serotonin change dramatically [1-4]. Duration and magnitude of monoamine neurotransmitter signaling is regulated, in part, by transporter-mediated uptake. Monoamine neurotransmitter transporters are broadly categorized into two groups: uptake 1 and uptake 2 (see reviews [5-7]). Uptake 2 have greater capacity for substrate transport than uptake 1, but are less selective about the substrates they transport than uptake 1. One uptake 2 is plasma membrane monoamine transporter (PMAT, Slc29a4). PMAT preferentially transports dopamine and serotonin over other monoamine neurotransmitters like norepinephrine or histamine [8] (see review [9]). Thus, PMAT function likely impacts dopamine and serotonin signaling, particularly during high signaling periods like stressful environmental conditions.
Previous studies in mice show constitutive reductions in, or loss of, PMAT function affect behavioral responses to stressful environmental conditions, such as a swim stress [10] or tail suspension test [11]. Moreover, these behavioral responses were sex-specific. Mice constitutively lacking organic cation transporter 2 (OCT2, Slc22a2) – another uptake 2 – exhibit augmented behavioral responses to both acute (both sexes used, but sex differences not analyzed; [12]) and chronic (males only; [13]) stressors. In contrast, male mice constitutively deficient in, or lacking, OCT3 (Slc22a3) exhibited no changes in the resident-intruder test nor in Morris water maze performance [14]. Findings with the latter test suggest that the uptake 2 OCT3 does not affect spatial learning or memory processes, whereas the outcomes with both tests indicate that OCT3 isn’t involved in behavioral responses to stressors (aggressive encounters or water immersion).
Surprisingly few evaluations have examined how uptake 2 contribute to learning and memory processes. Moreover, no studies have assessed fear conditioning either in uptake 2 knockout mice or after administration of the broad uptake 2 inhibitor, decynium-22. Beyond the Morris water maze study mentioned earlier, a couple groups have assessed conditioned place preference (CPP) - a form of classical conditioning - in uptake 2 knockout mice. Gautron’s group observed no influence of OCT3 knockout on amphetamine CPP (sex(es) not stated; [15]), whereas Daws’ group reported that males (but not females) lacking OCT3 had attenuated amphetamine CPP [16]. The latter group used a dose half that of the former group, which may explain some of the discrepancy between findings. In contrast, Daws’ group found that females (but not males) deficient in PMAT exhibited enhanced amphetamine CPP. Thus, some evidence exists that sex-specific influences of uptake 2 could influence learning and memory processes. Still, because amphetamine affects monoamine signaling, disentangling the effects of uptake 2 deficiency upon responses to amphetamine from those upon learning and memory is difficulty to do.
Here, we evaluated how PMAT deficiency influences classical conditioning in the absence of any drug exposure. We accomplished this using both contextual and cued fear conditioning paradigms in conjunction with exposure to a second, different form of stressor – swim stress. Contextual and cued fear conditioning preferentially engage activity within the dorsal hippocampus and amygdala [17-19] (see reviews [20-22]), whereas swim stress predominantly increases hypothalamus and amygdala activity [23-25]. Here, we assessed directional influences of these two different stressor formats – context/cued fear conditioning before/after swim stress – by evaluating stress-responsive behaviors specific to each paradigm (freezing; swimming, climbing, immobility; respectively). Because we’ve previously observed sex-specific stressor responses in PMAT-deficient mice [10,11], these studies were likewise performed in mice of both biological sexes. Finally, we intentionally used only wildtype (+/+) and heterozygous (+/−) PMAT mice, given potential translational relevance to humans with functional reductions in PMAT resulting from common polymorphisms [26-29].
We hypothesized that attenuated PMAT function in heterozygous mice would enhance behavioral responses to both initial and secondary stressors due to reduced clearance of elevated dopamine and serotonin. Further, we hypothesized that male heterozygotes would exhibit augmented stressor responses relative to females, given previous indications of such in male PMAT-deficient mice [11], plus literature evidence suggesting overall sex differences in stressor responsivity in mice [30,31].
Methods
Animals
Adult (≥90 days old) male and female PMAT-deficient mice maintained on a C57BL/6J background and bred in-house were used for all experiments. This line of mice was developed by Dr. Joanne Wang’s lab at the University of Washington [32]. Our PMAT-deficient colony is maintained in accordance with a material transfer agreement between the University of Washington and Kent State University. Males and females were run through all experiments separately; if both sexes were run on the same day, all males were always run before any females. All mice were group housed (2-5 per cage) within the same sex on 7090 Teklad Sani-chip bedding (Envigo, East Millstone, NJ). Mice had ad libitum access to LabDiet 5001 rodent laboratory chow (LabDiet, Brentwood, MO) and drinking water. The vivarium was maintained at 22 ± 1°C, on a 12:12 light:dark cycle, with lights on at 07:00. All procedures adhered to the National Research Council’s Guide for the Care and Use of Laboratory Animals, 8th Ed. [33], and were approved by the Kent State University Institutional Animal Care and Use Committee.
Genotyping
At postnatal day 21 (P21), mice were weaned, and 2 mm ear punches were collected for DNA extraction. Extensive details regarding buffer compositions, and procedures for DNA extraction, PCR (including primer sequences), and agarose gel electrophoresis are published [10,32], including in an open access journal [11].
Fear conditioning
Mice underwent either contextual fear training or cued fear training (Fig. 1). Regardless of the type of training, all mice were trained in ‘Context A’ in chambers made by Coulbourn Instruments (7 in D × 7 in W × 12 in H; Allentown, PA). These chambers consisted of two opposite clear acrylic walls and two opposite aluminum panel walls. In ‘Context A’, the chamber contained a metal shock grid floor, had a blue dotted pattern hung behind one of the clear acrylic walls, was illuminated with visible light, and was cleaned with 70% ethanol as a scent cue. Sound-attenuating enclosures surrounded each separate chamber, and every chamber had a camera mounted at the top to record behavior. FreezeFrame (v. 5.201, Coulbourn Instruments) software was used to quantify freezing behavior in real time. Freezing behavior is defined as the absence of all movement except that required for breathing. Testing commenced 48 h after training for both contextual and cued fear conditioning paradigms. Mice were brought directly from the vivarium to the fear behavior room on every day of testing and training in a designated individual transport cage. Differences between context and cued fear conditioning paradigms are described below.
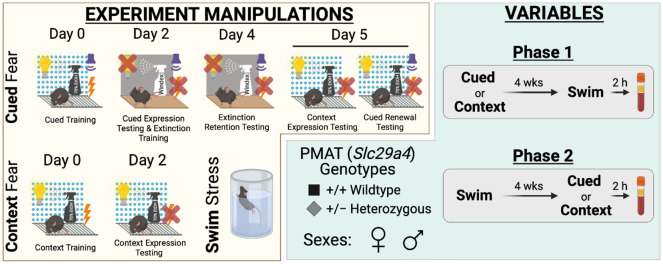
Figure 1. Experimental manipulations and variables for study.
Experimental manipulations involved cued fear conditioning, context fear conditioning, and swim stress (yellow compartment, left side). Cued fear conditioning (top, yellow compartment) involved cued fear training in Context A (visible light, grid floor, patterned background, ethanol scent) on Day 0, followed by cued fear expression testing and cued extinction training on Day 2 in Context B (infrared light, smooth floor, no background, Windex scent). On Day 4, testing of extinction retention occurred in Context B, then Day 5 involved testing mice in Context A for context expression testing followed immediately by cued fear renewal testing. Context fear conditioning (bottom left, yellow compartment) involved context fear training in Context A on Day 0, with testing occurring in Context A on Day 2. Swim stress involved a six min inescapable immersion in room temperature water (bottom right, yellow compartment). Variables involved plasma membrane monoamine transporter (PMAT, Slc29a4) genotype, sex, and the timeline of stressor exposure (Phase 1 or 2) (green compartment, right side). Wildtype (+/+) or heterozygous (+/−) mice of both sexes were used (bottom left, green compartment). Phase 1 (top right, green compartment) involved exposing mice to either cued or context fear conditioning, followed 4 wks later by swim stress; 2 h after swim stress, blood was collected for serum corticosterone analyses. Phase 2 (bottom right, green compartment) had mice undergo swim stress, followed 4 wks later by either cued or context fear conditioning; 2 h after the last test (Day 5, cued; Day 2, context), blood was collected for serum corticosterone analyses.
Cued fear conditioning
Following a 2 min baseline, training for cued fear involved five tone-shock pairings, with each 4 kHz, 30 s tone co-terminating with a 1 s, 0.8 mA scrambled mild foot shock. Inter-tone-intervals (ITIs) of 90 s were used, and the entire training duration including baseline lasted 11 min. Percent freezing was measured for each 30 s period when a tone was played; this was graphed as cued fear training. Testing for cued fear began 48 h after training (Fig. 1), and involved three stages. The first stage was for cued fear expression and cued fear extinction training; this included a 2 min baseline followed by fifteen 30 s, 4 kHz tone presentations separated by 30 s ITIs [34]. The second stage of testing began 48 h after the first testing stage. This had the exact same structure as the first stage of testing, but the purpose was to evaluate cued fear extinction retention, plus further cued fear extinction training. The first and second stages of testing occurred in ‘Context B’. Context B had a smooth acrylic floor, no pattern, was illuminated only with infrared light, and was cleaned with Windex® (SC Johnson, Racine, WI) as the scent cue. The third, final stage of testing occurred 24 h after the second stage. This third stage of testing occurred in Context A, and contained two portions. First, behavior was observed in Context A for 10 min in the absence of any tones, to evaluate contextual fear expression and extinction. Then, the second portion began immediately at the 10 min point by presentation of five 30 s, 4 KHz tones separated by 30 s ITIs to assess cued fear renewal [35-37].
Context fear conditioning
Following a 2 min baseline, training for context fear involved pseudorandom delivery of five, 1 s, 0.8 mA scrambled mild foot shocks delivered at 137, 186, 229, 285, and 324 s. The entire training duration including baseline lasted 6 min (Fig. 1). Percent freezing was measured for each 30 s period – averaged across six 5 s bins - that followed each foot shock, starting with the first 5 s bin that did not include the foot shock. This was graphed as context fear training. Testing for context fear occurred 48 h after training, in Context A. Testing lasted for 10 min; freezing from min 2 through 6 was averaged to assess contextual fear expression [38,39]. The full time course of the testing period was evaluated to determine contextual fear extinction. No shocks were administered during testing.
Swim stress
Mice were moved to a holding room approximately 30 ft away from the swim stress testing room a minimum of 1 h prior to test commencement to acclimate. Control (“no swim”) mice were included with every cohort. These mice experienced a sham stressor, involving moving them to the holding room, acclimating, then being moved to individual transport cages during the ‘test’ period and put half-on a heating pad. Mice that did undergo a swim stress were, after the acclimation period, brought in an individual transport cage directly to the swim stress testing room and immediately (and gently) placed in a tank of water (26 cm radius × 36.8 cm high) that was between 21.5-24 °C. This swim stress lasted for 6 min, and the entirety was recorded with a digital video camera for offline hand scoring of behaviors. An experimenter, remaining silent and still, watched each entire swim in real time to ensure no mouse was ever at risk of becoming submerged below the water surface. At test end, mice were immediately (and gently) removed from the water, hand-dried with clean paper towels, and then placed in an individual transport cage half-on a heating pad. Mice remained half-on heating pads in their individual transport cages for at least 15 min, or until their fur was completely dry, whichever came second.
Study phases
Two phases were conducted for this study, each with separate mice (Fig. 1). Phase 1 involved mice first undergoing context or cued fear conditioning, followed 4 wks after the last fear test by swim stress. Phase 2 was the reverse, with mice first undergoing a swim stress, then 4 wks later commencing either cued or context fear conditioning. No swim mice were used as controls in both Phase 1 and Phase 2. All mice underwent fear conditioning, because we have previously published on swim stress behavior in the absence of fear conditioning or any other stressor [10]. This approach, combined with our within-subjects design for each Phase, was to minimize the number of mice used in accordance with the three Rs [40].
Tissue collection
Tissue was collected 2 h after swim stress (or placement half-on heating pad, for no swim controls) for Phase 1, and 2 h after the final fear test for Phase 2 (Fig. 1). Previously, we observed no differences in serum corticosterone levels 30 min after swim stress (see Supplemental Figure S1), the time point at which corticosterone peaks following an acute stressor. Given this information, plus our experimental design of heterotypic stressors spaced 4 wks apart, we intentionally evaluated corticosterone levels 2 h after the last behavioral test for each Phase. This allowed us to determine if the descending limb of the corticosterone curve was impacted by PMAT deficiency, biological sex, stressor history, or any interaction thereof. Just prior to tissue collection, mice were briefly anesthetized with isoflurane, then rapidly decapitated to obtain trunk blood. Ears were also collected at this time for re-verification of genotype. Blood was allowed to clot at room temperature (20 ± 2°C) for 30 min, then was spun in a tabletop centrifuge at 3,500 rpm and 4°C for 30 min. Serum supernate was collected and placed in a clean tube, then serum and ears were frozen and stored at −80°C until analyses. Serum corticosterone levels were quantified using corticosterone ELISA kits (ADI-900-097, Enzo Life Sciences, Inc., Farmingdale, NY). Log-transformation of serum corticosterone levels was performed prior to analyses to correct for typical skewness of these data [39,41-43].
Data graphing & statistical analyses
Data were graphed using GraphPad Prism (v 10.0.2 (171); GraphPad Software, San Diego, CA), showing the mean ± 95% confidence interval (CI), plus individual data points when not showing repeated measures data. Data were analyzed with GraphPad Prism and IBM SPSS Statistics (v 29.0.1.0 (171), IBM, Armonk, NY). Significance thresholds were set a priori at p<0.05, and non-significant trends (p<0.10) were only examined if their corresponding partial η2>0.060. Analyses were performed within each Phase and each form of fear conditioning (e.g., Phase 1 cued, Phase 2 context, etc.). Repeated measures data were analyzed within each training/testing stage and within each sex, using 3-way repeated measures ANOVAs (time × PMAT genotype × swim condition) and pairwise comparisons with Bonferroni correction, or two-way repeated measures ANOVAs (PMAT genotype × sex) and Holm-Šídák post-hoc testing. Greenhouse- Geisser corrections were employed for within-subjects analyses. Average contextual fear expression (minutes 2 through 6) were analyzed in Phase 1 by a 2-way ANOVA (PMAT genotype × sex; because no effects of swim detected, so data were collapsed across swim condition), and in Phase 2 by a 3-way ANOVA (PMAT genotype × sex × swim condition), all with Holm-Šídák post-hocs. Measurements of serum corticosterone were analyzed within each Phase and form of fear conditioning (cued or context) by a 3-way ANOVA (PMAT genotype × sex × swim condition) and Holm-Šídák post-hocs. Some data loss occurred for the following reasons: software malfunctions (e.g., file did not save); equipment malfunction (e.g., camera was not displaying real-time images); operator error (e.g., chamber door left open by experimenter, and mouse departed chamber). Additionally, some mouse behavior indicated impairments in fear learning or excessive unconditioned fear. Exclusion criteria were as follows: 1) freezing >75% in any 5 (context) or 30 (cued) s bin prior to the first mild foot shock being administered; or 2) freezing <25% for first five tones (first stage of cued fear testing in Context B), for every 30 s bin of testing (context fear testing), or for all five tones of cued fear testing in Context A (i.e., cued fear renewal). Specific details of all instances are in the Supplemental Material. The criterion to exclude outliers was a priori assigned as >5 standard deviations ± mean.
Results
Fear Behavior
Phase 1
Because this is the first report of fear conditioning in PMAT-deficient mice, we began with Phase 1 experiments, which involved first performing fear conditioning followed four weeks thereafter by swim stress. This allowed for initial identification of any influences of PMAT deficiency upon fear processing, independent of prior swim stress exposure. As expected, there were no significant interactions with swim stress condition, nor any main effects of swim, for Phase 1 cued (Tables 1,2) and Phase 1 context (Tables 3,4) experiments across sexes. Consequently, graphed data were collapsed across swim condition to focus upon effects of time and genotype, and the interactions thereof as applicable.
Table 1.
Repeated measures ANOVAs of Phase 1 Cued Female data.
| Phase 1 Cued Females | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=0.531 | 0.471 | 0.015 |
| Swim | F(1,35)=0.003 | 0.957 | 0.000 |
| Genotype × Swim | F(1,35)=0.881 | 0.354 | 0.025 |
| Time | F(3.122,109.266)=183.4 | <0.001 | 0.840 |
| Time × Genotype | F(3.122,109.266)=0.676 | 0.574 | 0.019 |
| Time × Swim | F(3.122,109.266)=0.585 | 0.633 | 0.016 |
| Time × Genotype × Swim | F(3.122,109.266)=0.964 | 0.415 | 0.027 |
| Expression Testing & Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=0.471 | 0.497 | 0.013 |
| Swim | F(1,35)=0.455 | 0.504 | 0.013 |
| Genotype × Swim | F(1,35)=0.751 | 0.392 | 0.021 |
| Time | F(9.728,340.487)=8.714 | <0.001 | 0.199 |
| Time × Genotype | F(9.728,340.487)=3.865 | <0.001 | 0.099 |
| Time × Swim | F(9.728,340.487)=0.802 | 0.624 | 0.022 |
| Time × Genotype × Swim | F(9.728,340.487)=1.138 | 0.334 | 0.031 |
| Extinction Retention Testing & More Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=1.000 | 0.324 | 0.028 |
| Swim | F(1,35)=0.477 | 0.494 | 0.013 |
| Genotype × Swim | F(1,35)=0.508 | 0.481 | 0.014 |
| Time | F(9.774,342.097)=8.024 | <0.001 | 0.186 |
| Time × Genotype | F(9.774,342.097)=1.034 | 0.414 | 0.029 |
| Time × Swim | F(9.774,342.097)=1.523 | 0.131 | 0.042 |
| Time × Genotype × Swim | F(9.774,342.097)=1.191 | 0.296 | 0.033 |
| Context Fear Expression - Timecourse | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=0.001 | 0.976 | 0.000 |
| Swim | F(1,35)=0.110 | 0.742 | 0.003 |
| Genotype × Swim | F(1,35)=0.027 | 0.871 | 0.001 |
| Time | F(6.926,242.403)=5.297 | <0.001 | 0.131 |
| Time × Genotype | F(6.926,242.403)=0.641 | 0.720 | 0.018 |
| Time × Swim | F(6.926,242.403)=0.586 | 0.765 | 0.016 |
| Time × Genotype × Swim | F(6.926,242.403)=0.531 | 0.809 | 0.015 |
| Cued Fear Renewal | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=0.009 | 0.926 | 0.000 |
| Swim | F(1,35)=0.075 | 0.786 | 0.002 |
| Genotype × Swim | F(1,35)=0.421 | 0.521 | 0.012 |
| Time | F(3.712,129.923)=12.40 | <0.001 | 0.262 |
| Time × Genotype | F(3.712,129.923)=0.574 | 0.670 | 0.016 |
| Time × Swim | F(3.712,129.923)=0.745 | 0.554 | 0.021 |
| Time × Genotype × Swim | F(3.712,129.923)=0.184 | 0.937 | 0.005 |
Table 2.
Repeated measures ANOVAs of Phase 1 Cued Male data.
| Phase 1 Cued Males | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,54)=3.148 | 0.082 | 0.055 |
| Swim | F(1,54)=1.825 | 0.182 | 0.033 |
| Genotype × Swim | F(1,54)=0.166 | 0.685 | 0.003 |
| Time | F(3.156,170.433)=205.7 | <0.001 | 0.792 |
| Time × Genotype | F(3.156,170.433)=1.554 | 0.200 | 0.028 |
| Time × Swim | F(3.156,170.433)=0.475 | 0.710 | 0.009 |
| Time × Genotype × Swim | F(3.156,170.433)=0.477 | 0.708 | 0.009 |
| Expression Testing & Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,54)=1.051 | 0.310 | 0.019 |
| Swim | F(1,54)=0.074 | 0.787 | 0.001 |
| Genotype × Swim | F(1,54)=2.257 | 0.139 | 0.040 |
| Time | F(10.594,572.057)=9.786 | <0.001 | 0.153 |
| Time × Genotype | F(10.594,572.057)=0.538 | 0.872 | 0.010 |
| Time × Swim | F(10.594,572.057)=0.554 | 0.860 | 0.010 |
| Time × Genotype × Swim | F(10.594,572.057)=0.718 | 0.716 | 0.013 |
| Extinction Retention Testing & More Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,53)=0.173 | 0.679 | 0.003 |
| Swim | F(1,53)=0.035 | 0.852 | 0.001 |
| Genotype × Swim | F(1,53)=1.543 | 0.220 | 0.028 |
| Time | F(10.125,536.64)=11.09 | <0.001 | 0.173 |
| Time × Genotype | F(10.125,536.64)=1.004 | 0.439 | 0.019 |
| Time × Swim | F(10.125,536.64)=1.339 | 0.205 | 0.025 |
| Time × Genotype × Swim | F(10.125,536.64)=0.633 | 0.788 | 0.012 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,49)=0.250 | 0.619 | 0.005 |
| Swim | F(1,49)=0.684 | 0.412 | 0.014 |
| Genotype × Swim | F(1,49)=1.147 | 0.289 | 0.023 |
| Time | F(10.889,533.558)=11.58 | <0.001 | 0.191 |
| Time × Genotype | F(10.889,533.558)=0.631 | 0.801 | 0.013 |
| Time × Swim | F(10.889,533.558)=1.197 | 0.286 | 0.024 |
| Time × Genotype × Swim | F(10.889,533.558)=1.319 | 0.210 | 0.026 |
| Cued Fear Renewal | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,49)=0.033 | 0.856 | 0.001 |
| Swim | F(1,49)=0.874 | 0.354 | 0.018 |
| Genotype × Swim | F(1,49)=0.081 | 0.777 | 0.002 |
| Time | F(3.82,187.198)=11.17 | <0.001 | 0.186 |
| Time × Genotype | F(3.82,187.198)=0.190 | 0.938 | 0.004 |
| Time × Swim | F(3.82,187.198)=0.182 | 0.942 | 0.004 |
| Time × Genotype × Swim | F(3.82,187.198)=0.999 | 0.407 | 0.020 |
Table 3.
Repeated measures ANOVAs of Phase 1 Context Female data.
| Phase 1 Context Females | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.904 | 0.348 | 0.026 |
| Swim | F(1,34)=0.014 | 0.907 | 0.000 |
| Genotype × Swim | F(1,34)=0.061 | 0.806 | 0.002 |
| Time | F(3.626,123.285)=125.3 | <0.001 | 0.787 |
| Time × Genotype | F(3.626,123.285)=0.252 | 0.893 | 0.007 |
| Time × Swim | F(3.626,123.285)=0.818 | 0.506 | 0.023 |
| Time × Genotype × Swim | F(3.626,123.285)=0.412 | 0.782 | 0.012 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=2.936 | 0.096 | 0.079 |
| Swim | F(1,34)=0.540 | 0.468 | 0.016 |
| Genotype × Swim | F(1,34)=0.555 | 0.462 | 0.016 |
| Time | F(9.138,310.686)=8.874 | <0.001 | 0.207 |
| Time × Genotype | F(9.138,310.686)=1.369 | 0.200 | 0.039 |
| Time × Swim | F(9.138,310.686)=0.494 | 0.881 | 0.014 |
| Time × Genotype × Swim | F(9.138,310.686)=0.984 | 0.454 | 0.028 |
Table 4.
Repeated measures ANOVAs of Phase 1 Context Male data.
| Phase 1 Context Males | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.553 | 0.462 | 0.014 |
| Swim | F(1,39)=2.221 | 0.144 | 0.054 |
| Genotype × Swim | F(1,39)=0.081 | 0.777 | 0.002 |
| Time | F(3.226,125.803)=12.92 | <0.001 | 0.249 |
| Time × Genotype | F(3.226,125.803)=1.424 | 0.237 | 0.035 |
| Time × Swim | F(3.226,125.803)=0.441 | 0.738 | 0.011 |
| Time × Genotype × Swim | F(3.226,125.803)=0.158 | 0.934 | 0.004 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=4.555 | 0.039 | 0.105 |
| Swim | F(1,39)=0.027 | 0.871 | 0.001 |
| Genotype × Swim | F(1,39)=1.280 | 0.265 | 0.032 |
| Time | F(9.546,372.282)=13.94 | <0.001 | 0.263 |
| Time × Genotype | F(9.546,372.282)=1.142 | 0.331 | 0.028 |
| Time × Swim | F(9.546,372.282)=1.319 | 0.221 | 0.033 |
| Time × Genotype × Swim | F(9.546,372.282)=0.840 | 0.586 | 0.021 |
Phase 1 Cued
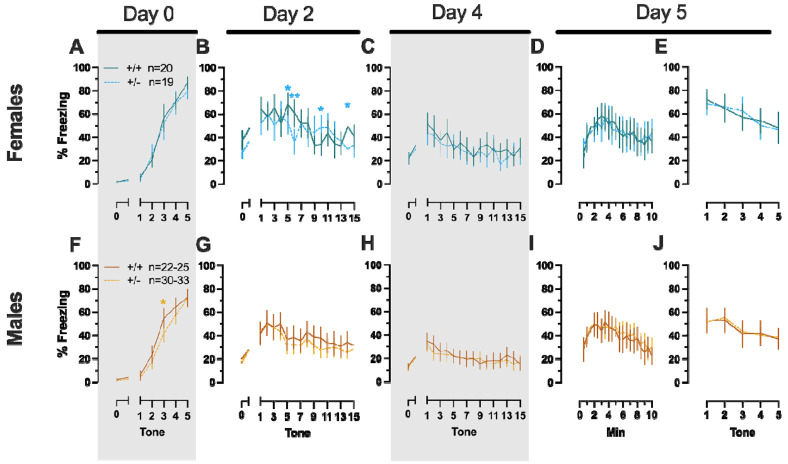
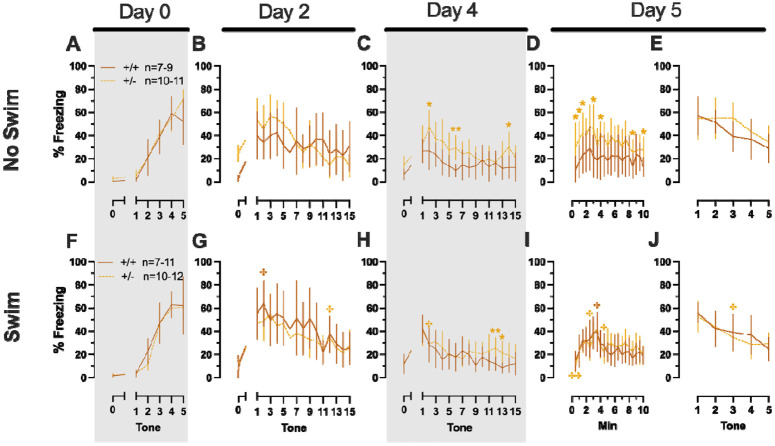
In Phase 1 cued females, there was an expected main effect of time for training, extinction retention testing, context fear expression, and cued fear renewal (Table 1; Fig. 1A,C-E). While there were no interactions with, nor main effects of, genotype for any of these either, there was a significant time × genotype interaction in Phase 1 cued females for cued expression testing and extinction training (Table 1; Fig. 1B). Post-hoc testing reflects that female heterozygous mice exhibited a temporally distinct pattern of cued fear expression and cued fear extinction from wildtype females at multiple timepoints (Fig. 1B). Unlike females that underwent Phase 1 cued procedures, Phase 1 cued males had no interactions with or main effects of genotype at any stage (Table 2; Fig. 1F-J). The anticipated main effect of time was present for all stages in Phase 1 cued males (Table 2; Fig. 1F-J). Combined, these data indicate that PMAT deficiency was largely without consequence on cued fear processing in males, whereas it had a modest impact upon cued extinction learning in females.
Phase 1 Context
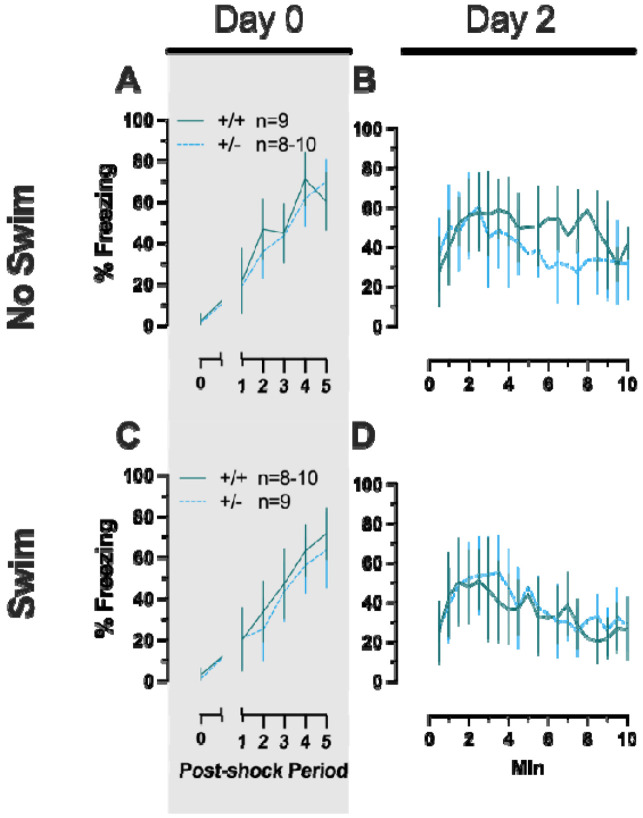
Females assigned to the Phase 1 context condition exhibited no interactions of time × genotype, and no main effect of genotype, but the expected main effect of time during context fear training (Table 3; Fig. 2A). Similarly, testing of context fear expression in these females revealed a significant main effect of time (Table 3; Fig. 2B). A non-significant trend of genotype was noted for females during context fear testing, but pairwise comparisons did not indicate any select timepoints that differed significantly across genotype. Males that underwent context fear conditioning were similar to females in that only a main effect of time was detected for training (Table 4; Fig. 2C). Unlike females, however, context fear testing in males showed main effects of both genotype and time (Table 4; Fig. 2D). Heterozygous males exhibited increased context fear expression compared to wildtype males (Table 4; Fig. 3D; Supplemental Table S2; Supplemental Fig. S2), suggesting typical PMAT function could sex-selectively suppress expression of context fear.
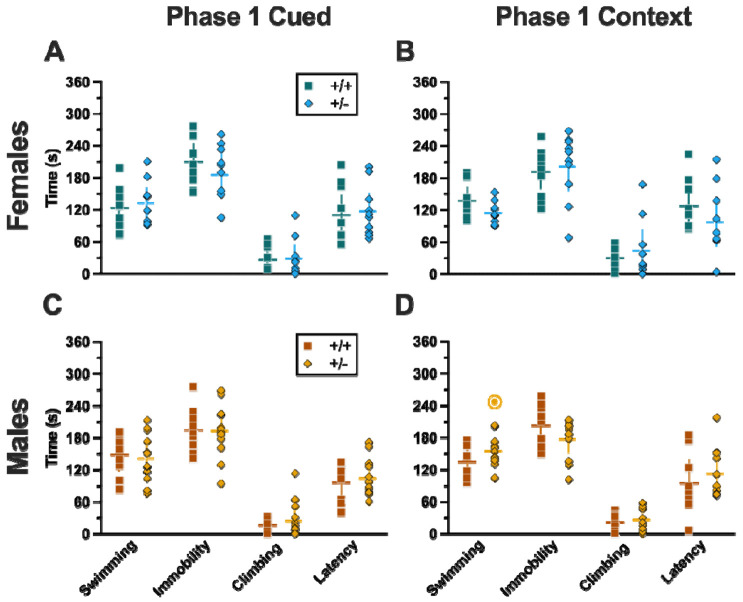
Figure 2. Phase 1 cued fear conditioning in female and male mice, collapsed across swim condition.
Female (A-E) wildtypes are represented by teal solid lines, and female heterozygotes are represented by blue dashed lines. Male (F-J) wildtypes are represented by orange solid lines, and male heterozygotes are represented by yellow dashed lines. Fear conditioning commenced 4 weeks prior to swim stress exposure for Phase 1. Mice were trained on Day 0 in Context A by pairing five 4 kHz, 30 s tones with a 1 s, 0.8 mA foot shock that co-terminated with the tone (A,F), each pairing separated by a 90 s inter-tone interval (ITIs). Two days later (Day 2), mice were placed in Context B, where they were presented with fifteen 30 s tones with 30 s ITIs, starting after a 2 min baseline (B,G). This served as cued fear expression testing as well as cued fear extinction training; two days thereafter, mice underwent the identical procedure for the purposes of testing extinction retention (Day 4; C,H) One day later, mice were placed in Context A, and in the absence of any tones for 10 min, were tested for context fear expression (D,I). Immediately after these 10 min, five 4 kHz tones were played with 30 s ITIs in the absence of any foot shock to evaluate cued fear renewal (E,J). Data are percent time spent freezing for each 30 s period indicated. Female wildtypes, n=20; female heterozygotes, n=19; male wildtype training, n=25; male heterozygous training, n=33; male wildtype cued expression testing and extinction training, n=25; male heterozygous cued expression testing and extinction training, n=33; male wildtype extinction retention testing, n=25; male heterozygous extinction retention testing, n=32; male wildtype context fear expression, n=23; male heterozygous context fear expression, n=30; male wildtype cued fear renewal, n=22; male heterozygous cued fear renewal, n=31. Data are graphed as mean ± 95% confidence interval. *p=0.027, *p=0.042, *p=0.016 (left to right, panel B); *p=0.021 (panel F); **p=0.002 (panel B); indicate difference between heterozygous and wildtype within same sex at the indicated timepoint.
Figure 3. Phase 1 context fear conditioning in female and male mice, collapsed across swim condition.
Female (A-B) wildtypes are represented by teal solid lines, and female heterozygotes are represented by blue dashed lines. Male (C-D) wildtypes are represented by orange solid lines, and male heterozygotes are represented by yellow dashed lines. Fear conditioning commenced 4 weeks prior to swim stress exposure for Phase 1. Mice were trained on Day 0 in Context A by administering five pseudo-randomized 1 s, 0.8 mA foot shocks following a two minute baseline (A,C). Two days later (Day 2), mice were placed back in Context A to test for context fear expression (B,D). Data are percent time spent freezing for each 30 s period following the foot shock (A,C), or every 30 s of testing (B,D). Female wildtypes, n=20; female heterozygotes, n=18; male wildtypes, n=20; male heterozygotes, n=23. Data are graphed as mean ± 95% confidence interval. *p=0.039 indicates difference between heterozygous and wildtype within same sex at the indicated timepoint.
Phase 2
After determining how reductions in PMAT function impact cued and context fear processing in the absence of any preceding stressors, we next evaluated how heterotypic stressor exposure interacted with PMAT function and sex. To do this, mice underwent cued or context fear conditioning procedures identical to those in Phase 1, except these procedures occurred four weeks after a swim stressor.
Phase 2 Cued
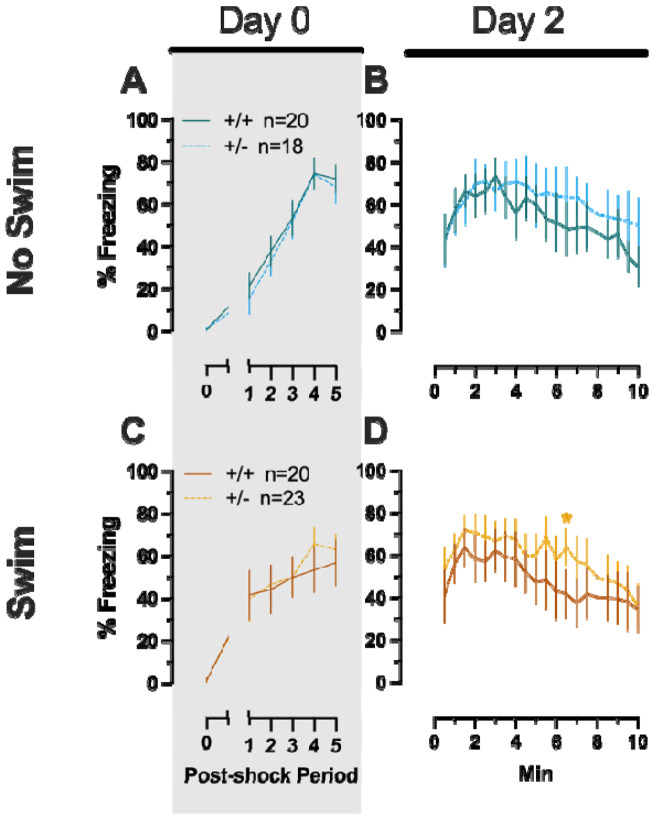
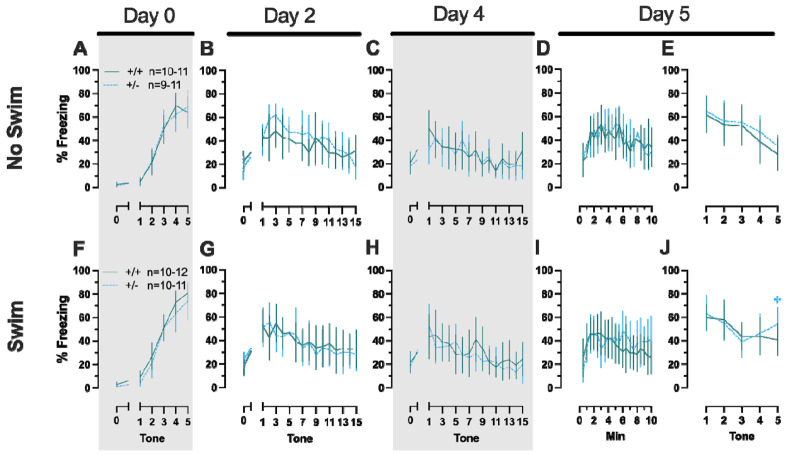
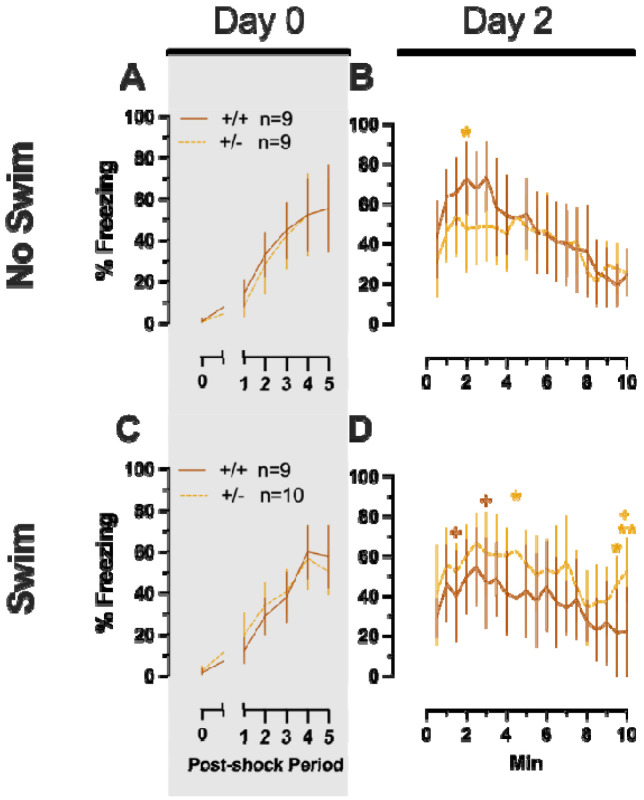
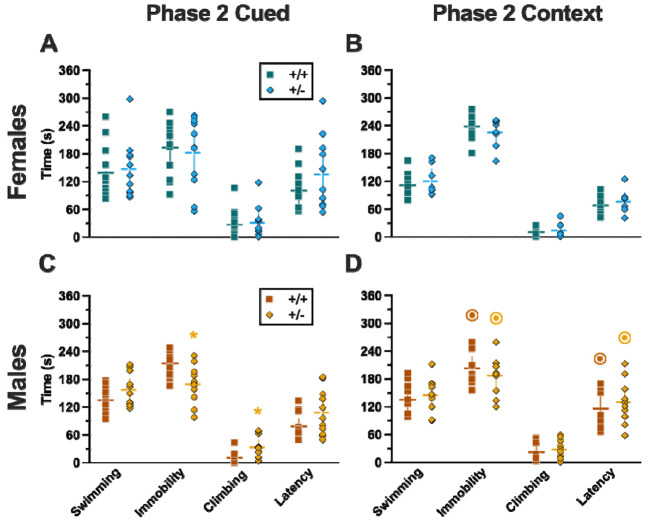
Repeated measures ANOVAs of females in the Phase 2 cued condition indicated that there were no three-way time × genotype × swim interactions in any of the five stages: training, cued expression testing and extinction training, extinction retention testing, context fear expression, or cued fear renewal (Table 5; Fig. 4). There were also no time × genotype interactions across the five stages, nor were any main effects of genotype or swim detected (Table 5; Fig. 4). The first four stages all had the expected main effect of time (Table 5; Fig. 4A-D,F-I). Cued fear renewal was the only stage with a significant time × swim interaction (Table 5; Fig. 4E,J). With pairwise comparisons, we determined that this appeared to be driven by reduced extinction of cued fear renewal over time in mice that previously underwent a swim stressor (Fig. 4J). This was most prominent in heterozygous females, reaching significance for cued fear in response to the final tone between heterozygous females that had a swim stressor compared to heterozygous females that did not undergo swim stress (Fig. 4J). Phase 2 cued males likewise showed no three-way time × genotype × swim interactions, nor any two-way interactions across all five stages (Table 6; Fig. 5). No main effect of swim was detected at any stage, whereas significant main effects of time occurred for all stages (Table 6; Fig. 5). Significant main effects of genotype were found for Phase 2 cued males for both extinction retention testing (Fig. 5C,H) and context fear expression (Fig. 5D,I), the latter reflecting what was found for Phase 1 context males (Fig. 3D), but not context testing in Phase 1 cued males (Fig. 2I). Pairwise comparisons highlights that no swim heterozygous males in Phase 2 cued extinction retention testing exhibit impaired retention of extinction training relative to wildtypes (Fig. 5C). Further, pairwise comparisons suggest that the genotype effect in context fear expression appears to be mostly driven by males in the no swim condition (Fig. 5D), despite the absence of any significant swim effects or interactions. These Phase 2 findings provide further support for a sex-dependent role of intact PMAT function attenuating expression of context fear, and additionally suggest that PMAT might typically function to facilitate retention of cued extinction in males.
Table 5.
Repeated measures ANOVAs of Phase 2 Cued Female data.
| Phase 2 Cued Females | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,41)=0.676 | 0.416 | 0.016 |
| Swim | F(1,41)=1.273 | 0.266 | 0.030 |
| Genotype × Swim | F(1,41)=0.922 | 0.343 | 0.022 |
| Time | F(3.605,147.811)=159.1 | <0.001 | 0.795 |
| Time × Genotype | F(3.605,147.811)=0.679 | 0.593 | 0.016 |
| Time × Swim | F(3.605,147.811)=0.970 | 0.420 | 0.023 |
| Time × Genotype × Swim | F(3.605,147.811)=0.169 | 0.942 | 0.004 |
| Expression Testing & Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,41)=0.299 | 0.587 | 0.007 |
| Swim | F(1,41)=0.036 | 0.850 | 0.001 |
| Genotype × Swim | F(1,41)=0.951 | 0.335 | 0.023 |
| Time | F(8.741,358.393)=6.623 | <0.001 | 0.139 |
| Time × Genotype | F(8.741,358.393)=0.852 | 0.566 | 0.020 |
| Time × Swim | F(8.741,358.393)=0.726 | 0.681 | 0.017 |
| Time × Genotype × Swim | F(8.741,358.393)=0.437 | 0.911 | 0.011 |
| Extinction Retention Testing & More Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,35)=0.400 | 0.531 | 0.011 |
| Swim | F(1,35)=0.093 | 0.763 | 0.003 |
| Genotype × Swim | F(1,35)=0.009 | 0.924 | 0.000 |
| Time | F(8.864,310.252)=11.15 | <0.001 | 0.242 |
| Time × Genotype | F(8.864,310.252)=0.590 | 0.803 | 0.017 |
| Time × Swim | F(8.864,310.252)=0.822 | 0.594 | 0.023 |
| Time × Genotype × Swim | F(8.864,310.252)=1.133 | 0.339 | 0.031 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,41)=0.256 | 0.615 | 0.006 |
| Swim | F(1,41)=0.498 | 0.484 | 0.012 |
| Genotype × Swim | F(1,41)=0.060 | 0.807 | 0.001 |
| Time | F(10.762,441.257)=5.923 | <0.001 | 0.126 |
| Time × Genotype | F(10.762,441.257)=1.001 | 0.444 | 0.024 |
| Time × Swim | F(10.762,441.257)=1.15 | 0.321 | 0.027 |
| Time × Genotype × Swim | F(10.762,441.257)=1.064 | 0.389 | 0.025 |
| Cued Fear Renewal | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.637 | 0.430 | 0.016 |
| Swim | F(1,39)=0.050 | 0.825 | 0.001 |
| Genotype × Swim | F(1,39)=0.066 | 0.799 | 0.002 |
| Time | F(3.57,139.219)=11.74 | <0.001 | 0.231 |
| Time × Genotype | F(3.57,139.219)=0.690 | 0.584 | 0.017 |
| Time × Swim | F(3.57,139.219)=3.592 | 0.011 | 0.084 |
| Time × Genotype × Swim | F(3.57,139.219)=0.282 | 0.870 | 0.007 |
Figure 4. Phase 2 cued fear conditioning in female mice, separated by swim condition.
Female wildtypes are represented by teal solid lines, and female heterozygotes are represented by blue dashed lines. Fear conditioning occurred 4 weeks after swim stress exposure for Phase 2. Mice were trained on Day 0 in Context A by pairing five 4 kHz, 30 s tones with a 1 s, 0.8 mA foot shock that co-terminated with the tone (A,F), each pairing separated by a 90 s ITIs. Two days later (Day 2), mice were placed in Context B, where they were presented with fifteen 30 s tones with 30 s ITIs, starting after a 2 min baseline (B,G). This served as cued fear expression testing as well as cued fear extinction training; two days thereafter, mice underwent the identical procedure for the purposes of testing extinction retention (Day 4; C,H) One day later, mice were placed in Context A, and in the absence of any tones for 10 min, were tested for context fear expression (D,I). Immediately after these 10 min, five 4 kHz tones were played with 30 s ITIs in the absence of any foot shock to evaluate cued fear renewal (E,J). Data are percent time spent freezing for each 30 s period indicated. No Swim: Female wildtype training, n=11; female heterozygous training, n=11; female wildtype cued expression testing and extinction training, n=11; female heterozygous cued expression testing and extinction training, n=11; female wildtype extinction retention testing, n=10; female heterozygous extinction retention testing, n=9; female wildtype context fear expression, n=11; female heterozygous context fear expression, n=11; female wildtype cued fear renewal, n=10; female heterozygous cued fear renewal, n=11. Swim: Female wildtype training, n=12; female heterozygous training, n=11; female wildtype cued expression testing and extinction training, n=12; female heterozygous cued expression testing and extinction training, n=11; female wildtype extinction retention testing, n=10; female heterozygous extinction retention testing, n=10; female wildtype context fear expression, n=12; female heterozygous context fear expression, n=11; female wildtype cued fear renewal, n=11; female heterozygous cued fear renewal, n=11. Data are graphed as mean ± 95% confidence interval. Indicates p=0.021 difference between no swim and swim conditions within same sex and genotype at the indicated timepoint.
Table 6.
Repeated measures ANOVAs of Phase 2 Cued Male data.
| Phase 2 Cued Males | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,36)=0.033 | 0.858 | 0.001 |
| Swim | F(1,36)=0.140 | 0.710 | 0.004 |
| Genotype × Swim | F(1,36)=1.226 | 0.276 | 0.033 |
| Time | F(2.7,97.197)=123.1 | <0.001 | 0.774 |
| Time × Genotype | F(2.7,97.197)=1.581 | 0.203 | 0.042 |
| Time × Swim | F(2.7,97.197)=0.872 | 0.449 | 0.024 |
| Time × Genotype × Swim | F(2.7,97.197)=0.978 | 0.400 | 0.026 |
| Expression Testing & Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,30)=0.196 | 0.661 | 0.006 |
| Swim | F(1,30)=1.649 | 0.209 | 0.052 |
| Genotype × Swim | F(1,30)=0.725 | 0.401 | 0.024 |
| Time | F(8.933,267.996)=10.55 | <0.001 | 0.260 |
| Time × Genotype | F(8.933,267.996)=1.029 | 0.417 | 0.033 |
| Time × Swim | F(8.933,267.996)=0.987 | 0.451 | 0.032 |
| Time × Genotype × Swim | F(8.933,267.996)=1.631 | 0.107 | 0.052 |
| Extinction Retention Testing & More Extinction Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,38)=6.914 | 0.012 | 0.154 |
| Swim | F(1,38)=0.052 | 0.820 | 0.001 |
| Genotype × Swim | F(1,38)=1.033 | 0.316 | 0.026 |
| Time | F(7.79,296.005)=8.583 | <0.001 | 0.184 |
| Time × Genotype | F(7.79,296.005)=0.955 | 0.470 | 0.025 |
| Time × Swim | F(7.79,296.005)=1.211 | 0.293 | 0.031 |
| Time × Genotype × Swim | F(7.79,296.005)=1.249 | 0.272 | 0.032 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,37)=4.175 | 0.048 | 0.101 |
| Swim | F(1,37)=0.273 | 0.604 | 0.007 |
| Genotype × Swim | F(1,37)=2.728 | 0.107 | 0.069 |
| Time | F(9.633,356.405)=5.218 | <0.001 | 0.124 |
| Time × Genotype | F(9.633,356.405)=0.390 | 0.947 | 0.010 |
| Time × Swim | F(9.633,356.405)=1.470 | 0.152 | 0.038 |
| Time × Genotype × Swim | F(9.633,356.405)=1.183 | 0.302 | 0.031 |
| Cued Fear Renewal | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.164 | 0.688 | 0.005 |
| Swim | F(1,34)=1.950 | 0.172 | 0.054 |
| Genotype × Swim | F(1,34)=0.625 | 0.435 | 0.018 |
| Time | F(3.835,130.383)=24.30 | <0.001 | 0.417 |
| Time × Genotype | F(3.835,130.383)=0.851 | 0.492 | 0.024 |
| Time × Swim | F(3.835,130.383)=0.898 | 0.464 | 0.026 |
| Time × Genotype × Swim | F(3.835,130.383)=1.335 | 0.262 | 0.038 |
Figure 5. Phase 2 cued fear conditioning in male mice, separated by swim condition.
Male wildtypes are represented by orange solid lines, and male heterozygotes are represented by yellow dashed lines. Fear conditioning occurred 4 weeks after swim stress exposure for Phase 2. Mice were trained on Day 0 in Context A by pairing five 4 kHz, 30 s tones with a 1 s, 0.8 mA foot shock that co-terminated with the tone (A,F), each pairing separated by a 90 s ITIs. Two days later (Day 2), mice were placed in Context B, where they were presented with fifteen 30 s tones with 30 s ITIs, starting after a 2 min baseline (B,G). This served as cued fear expression testing as well as cued fear extinction training; two days thereafter, mice underwent the identical procedure for the purposes of testing extinction retention (Day 4; C,H) One day later, mice were placed in Context A, and in the absence of any tones for 10 min, were tested for context fear expression (D,I). Immediately after these 10 min, five 4 kHz tones were played with 30 s ITIs in the absence of any foot shock to evaluate cued fear renewal (E,J). Data are percent time spent freezing for each 30 s period indicated. No Swim: Male wildtype training, n=9; male heterozygous training, n=11; male wildtype cued expression testing and extinction training, n=7; male heterozygous cued expression testing and extinction training, n=10; male wildtype extinction retention testing, n=9; male heterozygous extinction retention testing, n=11; male wildtype context fear expression, n=9; male heterozygous context fear expression, n=11; male wildtype cued fear renewal, n=7; male heterozygous cued fear renewal, n=10. Swim: Male wildtype training, n=9; male heterozygous training, n=11; male wildtype cued expression testing and extinction training, n=7; male heterozygous cued expression testing and extinction training, n=10; male wildtype extinction retention testing, n=10; male heterozygous extinction retention testing, n=12; male wildtype context fear expression, n=10; male heterozygous context fear expression, n=12; male wildtype cued fear renewal, n=10; male heterozygous cued fear renewal, n=11. . Data are graphed as mean ± 95% confidence interval. *p=0.023, *p=0.015 (left to right, panel C); *p=0.021, *p=0.029,*p=0.026, *p=0.023, *p=0.043, *p=0.046, *p=0.015 (left to right, panel D); *p=0.039 (panel H); **p=0.002 (panel C); **p=0.008 (panel H) indicate difference between heterozygous and wildtype within same sex at the indicated timepoint. *p=0.025, *p=0.019 (left to right, panel G); *p=0.031 (panel H); *p=0.019, *p=0.035, *p=0.013 (left to right, panel I); **p=0.009 (panel I); *p=0.015 (panel J); indicates difference between no swim and swim conditions within same sex and genotype (indicated by color) at the indicated timepoint.
Phase 2 Context
No three- or two-way interactions were found for females in the Phase 2 context condition during either training or testing, and the only main effects were those of time (Table 7, Fig. 6). Males in Phase 2 context similarly had no significant three-way interactions for training or testing. No two-way interactions were found for training, and neither time × genotype nor time × swim interactions were significant for testing (Table 8; Fig. 7). A non-significant trend was observed for swim × genotype during context fear testing in males (Table 8; Fig. 7B,D). Pairwise comparisons suggest context fear expression was lowered by previous swim stress exposure in wildtypes, while swim stress in heterozygous males increased their context fear expression (Fig. 7B,D). These changes in context fear expression across genotype as a result of an earlier stressor in males appear to be the inverse of what is observed for context fear expression in males that underwent cued fear conditioning (Fig. 5). Expected main effects of time were observed for testing and training, but no other main effects were found (Table 8; Fig. 7). Though complex in directionality and circumstance, overall the fear behavior data indicate that reductions in PMAT function result in much more prominent behavioral effects in males versus females. Further, the present findings suggest that the form of fear conditioning and stressor history can sex-specifically enhance or mask the influence of diminished PMAT function.
Table 7.
Repeated measures ANOVAs of Phase 2 Context Female data.
| Phase 2 Context Females | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.713 | 0.404 | 0.021 |
| Swim | F(1,34)=0.346 | 0.560 | 0.010 |
| Genotype × Swim | F(1,34)=0.065 | 0.800 | 0.002 |
| Time | F(3.029,102.984)=76.52 | <0.001 | 0.692 |
| Time × Genotype | F(3.029,102.984)=1.116 | 0.346 | 0.032 |
| Time × Swim | F(3.029,102.984)=1.963 | 0.124 | 0.055 |
| Time × Genotype × Swim | F(3.029,102.984)=0.978 | 0.407 | 0.028 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,30)=0.362 | 0.552 | 0.012 |
| Swim | F(1,30)=2.066 | 0.161 | 0.064 |
| Genotype × Swim | F(1,30)=1.549 | 0.223 | 0.049 |
| Time | F(9.209,276.265)=8.773 | <0.001 | 0.226 |
| Time × Genotype | F(9.209,276.265)=1.094 | 0.367 | 0.035 |
| Time × Swim | F(9.209,276.265)=0.985 | 0.454 | 0.032 |
| Time × Genotype × Swim | F(9.209,276.265)=1.251 | 0.263 | 0.040 |
Figure 6. Phase 2 context fear conditioning in female mice, separated by swim condition.
Female wildtypes are represented by teal solid lines, and female heterozygotes are represented by blue dashed lines. Fear conditioning occurred 4 weeks after swim stress exposure for Phase 2. Mice were trained on Day 0 in Context A by administering five pseudo-randomized 1 s, 0.8 mA foot shocks following a two minute baseline (A,C). Two days later (Day 2), mice were placed back in Context A to test for context fear expression (B,D). Data are percent time spent freezing for each 30 s period following the foot shock (A,C), or every 30 s of testing (B,D). Female wildtype no swim training and testing, n=9; female heterozygous no swim training, n=10; female heterozygous no swim testing, n=8; female wildtype swim training, n=10; female heterozygous swim training and testing, n=9; female wildtype swim testing, n=8. Data are graphed as mean ± 95% confidence interval.
Table 8.
Repeated measures ANOVAs of Phase 2 Context Male data.
| Phase 2 Context Males | |||
|---|---|---|---|
| Training | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,33)=0.042 | 0.839 | 0.001 |
| Swim | F(1,33)=0.075 | 0.786 | 0.002 |
| Genotype × Swim | F(1,33)=0.180 | 0.674 | 0.005 |
| Time | F(3.284,108.38)=66.40 | <0.001 | 0.668 |
| Time × Genotype | F(3.284,108.38)=0.190 | 0.917 | 0.006 |
| Time × Swim | F(3.284,108.38)=0.979 | 0.411 | 0.029 |
| Time × Genotype × Swim | F(3.284,108.38)=1.062 | 0.372 | 0.031 |
| Context Fear Expression | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,33)=0.524 | 0.474 | 0.016 |
| Swim | F(1,33)=0.024 | 0.877 | 0.001 |
| Genotype × Swim | F(1,33)=3.400 | 0.074 | 0.093 |
| Time | F(8.625,284.634)=14.87 | <0.001 | 0.311 |
| Time × Genotype | F(8.625,284.634)=1.447 | 0.171 | 0.042 |
| Time × Swim | F(8.625,284.634)=1.160 | 0.322 | 0.034 |
| Time × Genotype × Swim | F(8.625,284.634)=0.763 | 0.646 | 0.023 |
Figure 7. Phase 2 context fear conditioning in male mice, separated by swim condition.
Male wildtypes are represented by orange solid lines, and male heterozygotes are represented by yellow dashed lines. Fear conditioning occurred 4 weeks after swim stress exposure for Phase 2. Mice were trained on Day 0 in Context A by administering five pseudo-randomized 1 s, 0.8 mA foot shocks following a two minute baseline (A,C). Two days later (Day 2), mice were placed back in Context A to test for context fear expression (B,D). Data are percent time spent freezing for each 30 s period following the foot shock (A,C), or every 30 s of testing (B,D). Male wildtype no swim, n=9; male heterozygous no swim, n=9; male wildtype swim, n=9; male heterozygous swim, n=10. Data are graphed as mean ± 95% confidence interval. *p=0.034 (panel B); *p=0.048, p=0.013 (left to right, panel D); **p=0.004 (panel D); indicate difference between heterozygous and wildtype within same sex at the indicated timepoint. *p=0.030, *p=0.035, *p=0.011 (left to right, panel D); indicate difference between no swim and swim conditions within same sex and genotype (indicated by color) at the indicated timepoint.
Serum corticosterone
Blood was collected from all mice 2 h after their last test to measure serum corticosterone levels. For Phase 1 mice, this was 2 h after swim stress; for Phase 2 mice, this was 2 h after the context testing and cued fear renewal test. This 2 h timepoint was to capture the descending limb of the corticosterone curve to assess how elevated corticosterone levels remained after the established 30 min peak post-stressor [44-47]. This 2 h timepoint was the focus here because no sex nor genotype differences were detected in PMAT mice when serum corticosterone was measured 30 min following a single acute swim stressor (Supplemental Table S1; Supplemental Fig.S1). All serum corticosterone levels were log-transformed (hereafter referred to as “cort”) to ensure normal distribution, as previously reported [39,41-43].
Phase 1
Phase 1 Cued
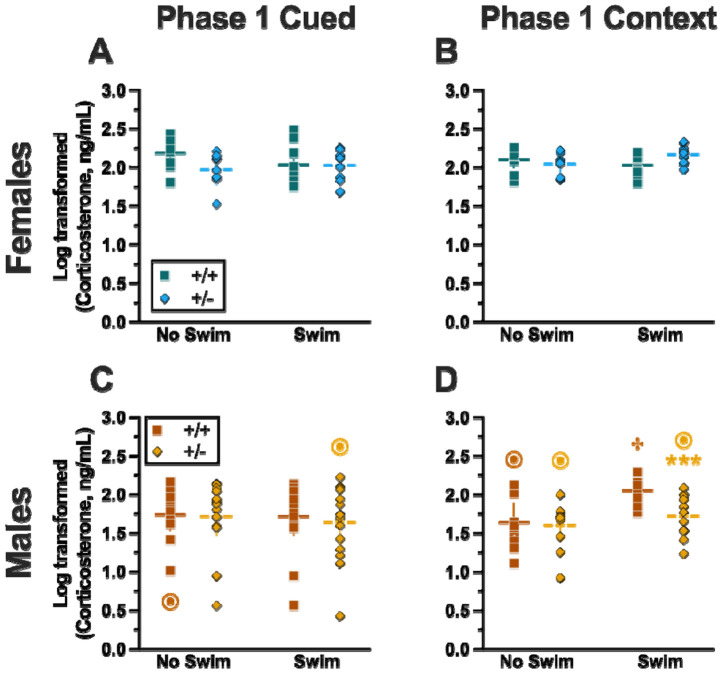
Analyzing Phase 1 cued cort levels via three-way ANOVA indicated no significant three- or two-way interactions (Table 9; Fig. 8). The only significant main effect was one of sex (Table 9). Post-hoc testing indicated that male wildtype mice in the no swim condition exhibited significantly lower cort levels than female wildtype no swim mice (Fig. 8A,C). Conversely, in the swim condition, male heterozygous mice had lower cort levels than female heterozygotes (Fig. 8A,C). Such sex differences in descending cort levels have been reported previously [48-52] (see review [53]).
Table 9.
Three-way ANOVAs of log-transformed corticosterone levels taken 2 h following final testing (Phase 1 – swim stress; Phase 2 – context testing & cued fear renewal).
| Phase 1 Cued | |||
|---|---|---|---|
| F statistic | p | partial η2 | |
| Genotype | F(1,82)=0.962 | 0.330 | 0.012 |
| Sex | F(1,82)=17.77 | <0.001 | 0.178 |
| Swim | F(1,82)=0.301 | 0.584 | 0.004 |
| Genotype × Sex | F(1,82)=0.119 | 0.731 | 0.001 |
| Genotype × Swim | F(1,82)=0.170 | 0.681 | 0.002 |
| Sex × Swim | F(1,82)=0.000 | 0.992 | 0.000 |
| Genotype × Sex × Swim | F(1,82)=0.639 | 0.426 | 0.008 |
| Phase 1 Context | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,72)=2.007 | 0.161 | 0.027 |
| Sex | F(1,72)=45.11 | <0.001 | 0.385 |
| Swim | F(1,72)=8.382 | 0.005 | 0.104 |
| Genotype × Sex | F(1,72)=4.839 | 0.031 | 0.063 |
| Genotype × Swim | F(1,72)=0.239 | 0.626 | 0.003 |
| Sex × Swim | F(1,72)=5.938 | 0.017 | 0.076 |
| Genotype × Sex × Swim | F(1,72)=6.029 | 0.016 | 0.077 |
| Phase 2 Cued | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,79)=2.285 | 0.135 | 0.028 |
| Sex | F(1,79)=17.40 | <0.001 | 0.180 |
| Swim | F(1,79)=0.967 | 0.328 | 0.012 |
| Genotype × Sex | F(1,79)=1.157 | 0.285 | 0.014 |
| Genotype × Swim | F(1,79)=1.748 | 0.190 | 0.022 |
| Sex × Swim | F(1,79)=0.390 | 0.534 | 0.005 |
| Genotype × Sex × Swim | F(1,79)=1.235 | 0.270 | 0.015 |
| Phase 2 Context | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,63)=0.001 | 0.979 | 0.000 |
| Sex | F(1,63)=1.437 | 0.235 | 0.022 |
| Swim | F(1,63)=0.670 | 0.416 | 0.011 |
| Genotype × Sex | F(1,63)=0.186 | 0.668 | 0.003 |
| Genotype × Swim | F(1,63)=4.377 | 0.040 | 0.065 |
| Sex × Swim | F(1,63)=0.100 | 0.753 | 0.002 |
| Genotype × Sex × Swim | F(1,63)=0.282 | 0.597 | 0.004 |
Figure 8. Log-transformed corticosterone levels in mice from Phase 1.
Mice in Phase 1 had blood collected 2 h following swim stress to measure serum corticosterone levels. Female (A-B) wildtypes are represented by teal squares, and female heterozygotes are represented by blue diamonds. Male (C-D) wildtypes are represented by orange squares, and male heterozygotes are represented by yellow diamonds. Phase 1 Cued data (A,C) and Phase 1 Context data (B,D) are graphed in columns separated by sex. Data are log-transformed serum corticosterone levels, showing individual data points. Phase 1 Cued No Swim: Female wildtypes, n=10; female heterozygotes, n=8; male wildtypes, n=12; male heterozygotes, n=15. Phase 1 Cued Swim: Female wildtypes, n=10; female heterozygotes, n=10; male wildtypes, n=15; male heterozygotes, n=16. Phase 1 Context No Swim: Female wildtypes, n=10; female heterozygotes, n=8; male wildtypes, n=10; male heterozygotes, n=11. Phase 1 Context Swim: Female wildtypes, n=10; female heterozygotes, n=10; male wildtypes, n=10; male heterozygotes, n=11. Horizontal lines are shown as the mean, with vertical lines as ± 95% confidence interval. ***p=0.001 (panel D) indicates difference between heterozygous and wildtype within same sex and same swim condition. ***p<0.001 indicates difference between no swim and swim conditions within same sex and genotype. ⊙p=0.008, ⊙p=0.014 (left to right, panel C); ⊙p<0.001, ⊙p<0.001, ⊙p<0.001 (left to right, panel D) indicates difference between sexes within same genotype and swim condition.
Phase 1 Context
Unlike Phase 1 Cued cort levels, there was a significant three-way interaction of genotype × sex × swim (Table 9, Fig. 8). Pairwise comparisons showed that male heterozygotes in the swim condition had significantly lower cort levels than male wildtypes in the same condition (Fig. 8D). Male wildtypes that were swam also had significantly higher cort levels than male wildtypes not exposed to swim stress (Fig. 8D). Several sex differences were also detected; specifically, cort levels for males were lower than females for all swim-genotype combinations except swam wildtypes (Fig. 8B,D). These data could indicate that prior context fear conditioning augments cort elevations to subsequent acute stressors in wildtype males, but that reduced PMAT function dampens this response.
Phase 2
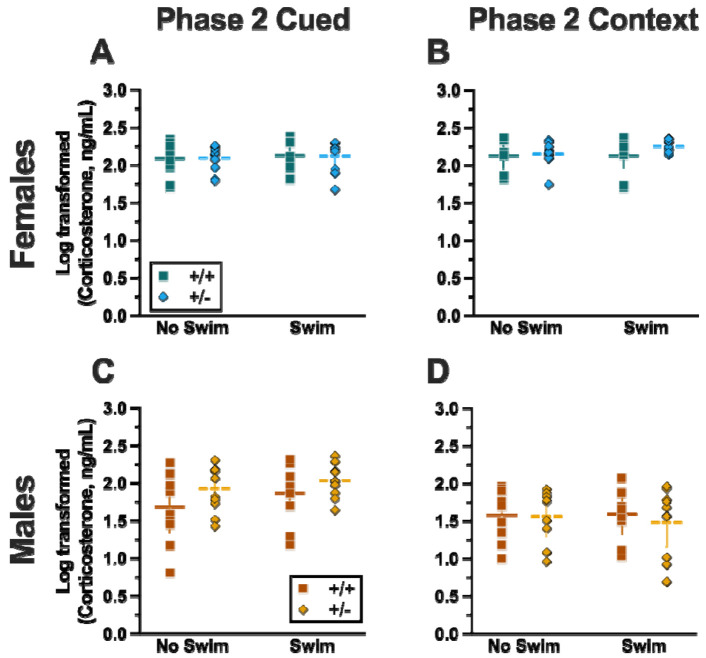
Phase 2 Cued
Similar to Phase 1 Cued, no significant three- nor two-way interactions were detected in cort levels for Phase 2 Cued (Table 9). As with Phase 1 Cued, the only significant main effect in Phase 2 Cued was of sex (Table 9; Fig. 9). Unlike Phase 1 cort levels though, pairwise comparisons indicated no select differences between individual pairs of groups (Fig. 9A,C). The absence of any swim effect for Phase 2 cued mice indicates that an acute swim stress 4 wks prior to undergoing cued fear conditioning was not impactful enough to alter cort responses long-term.
Figure 9. Log-transformed corticosterone levels in mice from Phase 2.
Mice in Phase 2 had blood collected 2 h following context fear testing and cued fear renewal to measure serum corticosterone levels. Female (A-B) wildtypes are represented by teal squares, and female heterozygotes are represented by blue diamonds. Male (C-D) wildtypes are represented by orange squares, and male heterozygotes are represented by yellow diamonds. Phase 2 Cued data (A,C) and Phase 2 Context data (B,D) are graphed in columns separated by sex. Data are log-transformed serum corticosterone levels, showing individual data points. Phase 2 Cued No Swim: Female wildtypes, n=11; female heterozygotes, n=11; male wildtypes, n=10; male heterozygotes, n=11. Phase 2 Cued Swim: Female wildtypes, n=12; female heterozygotes, n=11; male wildtypes, n=12; male heterozygotes, n=12. Phase 2 Context No Swim: Female wildtypes, n=9; female heterozygotes, n=10; male wildtypes, n=9; male heterozygotes, n=9. Phase 2 Context Swim: Female wildtypes, n=10; female heterozygotes, n=9; male wildtypes, n=9; male heterozygotes, n=10. Horizontal lines are shown as the mean, with vertical lines as ± 95% confidence interval.
Phase 2 Context
No significant three-way interaction of genotype × sex × swim (Table 9) was detected. Though genotype × sex and sex × swim interactions were not significant, a genotype × swim interaction was significant for Phase 2 Context (Table 9). As with Phase 2 Cued though, post-hoc testing indicated no select differences between any two groups (Fig. 9B,D). Considering these data along with the cort levels of Phase 2 Cued mice, it is possible that the prolonged testing for cued fear conditioning obscured any lasting cort regulatory changes. In contrast, context fear conditioning’s more concise timeline could have facilitated a glimpse into the impact of PMAT genotype upon cort levels in Phase 1 following an earlier acute stressor.
Swim stress
For both Phases 1 and 2, only those mice assigned to the swim stress condition underwent an acute 6 min swim stress. Mice assigned to the no swim condition were transported to the same room and treated the same as mice that underwent swim (i.e., placed in clean cages half-on heating pads), but were not swam. All swim stresses were video recorded, then later hand-scored offline by two blinded observers to quantify swimming, climbing, and immobility behaviors for subsequent analyses. Additionally, latency to the first bout of immobility (i.e., latency) and the number of fecal boli (Supplemental Table S3, Supplemental Fig. S4) were analyzed.
Phase 1
Phase 1 Cued
Mice that underwent cued fear conditioning 4 wks prior to swim stress exhibited no significant interactions between genotype × sex, nor any main effects of either genotype nor sex (Table 10). Accordingly, no significant post-hoc tests were observed either (Fig. 10A,C). This aligns with the absence of any cort level differences detected in Phase 1 Cued mice (Fig. 8A,C).
Table 10.
Two-way ANOVAs for behaviors during swim stress in Phase 1 Cued mice.
| Phase 1 Cued | |||
|---|---|---|---|
| Swimming | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.010 | 0.922 | 0.000 |
| Sex | F(1,39)=1.825 | 0.185 | 0.045 |
| Genotype × Sex | F(1,39)=0.419 | 0.521 | 0.011 |
| Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.801 | 0.376 | 0.020 |
| Sex | F(1,39)=0.065 | 0.801 | 0.002 |
| Genotype × Sex | F(1,39)=0.570 | 0.455 | 0.014 |
| Climbing | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.403 | 0.529 | 0.010 |
| Sex | F(1,39)=0.626 | 0.434 | 0.016 |
| Genotype × Sex | F(1,39)=0.134 | 0.716 | 0.003 |
| Latency to 1st Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,39)=0.356 | 0.554 | 0.009 |
| Sex | F(1,39)=1.051 | 0.312 | 0.026 |
| Genotype × Sex | F(1,39)=0.003 | 0.957 | 0.000 |
Figure 10. Behaviors during swim stressor in Phase 1 mice.
Mice in Phase 1 assigned to swim stress experienced an acute 6 min swim stressor 4 wks after undergoing Cued (A,C) or Context (B,D) fear conditioning. Female (A-B) wildtypes are represented by teal squares, and female heterozygotes are represented by blue diamonds. Male (C-D) wildtypes are represented by orange squares, and male heterozygotes are represented by yellow diamonds. Data are the amount of time in seconds spent swimming, immobile, or climbing; or the amount of time until the first bout of immobility (i.e., latency). Phase 1 Cued: Female wildtype, n=10; female heterozygous, n=10; male wildtype, n=9; male heterozygous, n=14. Phase 1 Context: Female wildtype, n=10; female heterozygous, n=10; male wildtype, n=9; male heterozygous, n=11. Horizontal lines are shown as the mean, with vertical lines as ± 95% confidence interval. ⊙p=0.003 (panel D) indicates difference between sexes within same genotype and swim behavior.
Phase 1 Context
Mice that were swam 4 wks after undergoing context fear conditioning had a significant genotype × sex interaction (Table 11). Post-hoc testing indicated that male heterozygotes displayed significantly more swimming behavior than female heterozygotes (Fig. 10D). Despite the absence of any genotype-specific behavioral changes observed in swim behaviors, cort measurements indicate that heterotypic stressor exposure across the 4 week experimental timeframe did indeed have a physiological impact in males that appears to be moderated by PMAT deficiency (Fig. 8B,D).
Table 11.
Two-way ANOVAs for behaviors during swim stress in Phase 1 Context mice.
| Phase 1 Context | |||
|---|---|---|---|
| Swimming | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,36)=0.013 | 0.911 | 0.000 |
| Sex | F(1,36)=4.250 | 0.047 | 0.106 |
| Genotype × Sex | F(1,36)=5.572 | 0.024 | 0.134 |
| Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,36)=0.283 | 0.598 | 0.008 |
| Sex | F(1,36)=0.181 | 0.673 | 0.005 |
| Genotype × Sex | F(1,36)=1.360 | 0.251 | 0.036 |
| Climbing | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,36)=0.765 | 0.388 | 0.021 |
| Sex | F(1,36)=1.538 | 0.223 | 0.041 |
| Genotype × Sex | F(1,36)=0.189 | 0.667 | 0.005 |
| Latency to 1st Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,36)=0.151 | 0.700 | 0.004 |
| Sex | F(1,36)=0.259 | 0.614 | 0.007 |
| Genotype × Sex | F(1,36)=2.022 | 0.164 | 0.053 |
Phase 2
Phase 2 Cued
In Phase 2, mice underwent a swim stress 4 wks before fear conditioning. Mice that went through cued fear conditioning after swim stress had no significant differences in swimming (Table 12). Though no significant interactions or main effects were detected for either immobility or climbing, both had non-significant trends for genotype (Table 12). Latency to first immobility, while not having a significant genotype × sex interaction, did have a significant main effect of genotype (Table 12; Fig. 11A,C). Post-hoc testing demonstrated that male heterozygotes that went through swim stress 4 wks before cued fear conditioning displayed less immobility, and more climbing behavior, than male wildtypes that went through the same procedures (Fig. 11C). The male-specific influences of reduced PMAT function on swim stress behavior reflect the largely consistent trend observed here where male behavior and physiology was more affected than females.
Table 12.
Two-way ANOVAs for behaviors during swim stress in Phase 2 Cued mice.
| Phase 2 Cued | |||
|---|---|---|---|
| Swimming | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,42)=1.062 | 0.309 | 0.025 |
| Sex | F(1,42)=0.048 | 0.828 | 0.001 |
| Genotype × Sex | F(1,42)=0.271 | 0.605 | 0.006 |
| Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,42)=3.221 | 0.080 | 0.071 |
| Sex | F(1,42)=0.065 | 0.800 | 0.002 |
| Genotype × Sex | F(1,42)=1.161 | 0.287 | 0.027 |
| Climbing | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,42)=3.024 | 0.089 | 0.067 |
| Sex | F(1,42)=0.867 | 0.357 | 0.020 |
| Genotype × Sex | F(1,42)=1.483 | 0.230 | 0.034 |
| Latency to 1st Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,42)=4.679 | 0.036 | 0.100 |
| Sex | F(1,42)=2.722 | 0.106 | 0.061 |
| Genotype × Sex | F(1,42)=0.030 | 0.863 | 0.001 |
Figure 11. Behaviors during swim stressor in Phase 2 mice.
Mice in Phase 2 assigned to swim stress experienced an acute 6 min swim stressor 4 wks before undergoing Cued (A,C) or Context (B,D) fear conditioning. Female (A-B) wildtypes are represented by teal squares, and female heterozygotes are represented by blue diamonds. Male (C-D) wildtypes are represented by orange squares, and male heterozygotes are represented by yellow diamonds. Data are the amount of time in seconds spent swimming, immobile, or climbing; or the amount of time until the first bout of immobility (i.e., latency). Phase 2 Cued: Female wildtype, n=12; female heterozygous, n=11; male wildtype, n=12; male heterozygous, n=11. Phase 2 Context: Female wildtype, n=10; female heterozygous, n=8; male wildtype, n=10; male heterozygous, n=10. *p=0.049, *p=0.43 (left to right, panel C) indicate difference between heterozygous and wildtype within same sex for the same swim behavior. ⊙p=0.032, ⊙p=0.030, ⊙p=0.006, ⊙p=0.003 (left to right, panel D) indicates difference between sexes within same genotype and swim behavior.
Phase 2 Context
Four weeks before going through Phase 2 context fear conditioning, mice were subjected to swim stress. Across all four measures of behavior, there was no significant genotype × sex interaction and no main effect of genotype, but there was a significant main effect of sex (Table 13; Fig. 11 B,D). Post-hoc tests emphasized significantly less time spent immobile, and an accompanying increase in latency to first immobility bout, in males of both genotypes compared to females of the same genotype (Fig. 11 B,D). Why these sex differences were not also present in Phase 2 Cued mice (Fig. 11 A,C) is not clear, though could be attributable to the somewhat greater variability observed in Phase 2 Cued females versus Phase 2 context females.
Table 13.
Two-way ANOVAs for behaviors during swim stress in Phase 2 Context mice.
| Phase 2 Context | |||
|---|---|---|---|
| Swimming | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.694 | 0.411 | 0.020 |
| Sex | F(1,34)=4.996 | 0.032 | 0.128 |
| Genotype × Sex | F(1,34)=0.003 | 0.954 | 0.000 |
| Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=1.512 | 0.227 | 0.043 |
| Sex | F(1,34)=10.09 | 0.003 | 0.229 |
| Genotype × Sex | F(1,34)=0.013 | 0.909 | 0.000 |
| Climbing | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.930 | 0.342 | 0.027 |
| Sex | F(1,34)=5.611 | 0.024 | 0.142 |
| Genotype × Sex | F(1,34)=0.020 | 0.890 | 0.001 |
| Latency to 1st Immobility | |||
| F statistic | p | partial η2 | |
| Genotype | F(1,34)=0.845 | 0.364 | 0.024 |
| Sex | F(1,34)=18.76 | <0.001 | 0.356 |
| Genotype × Sex | F(1,34)=0.087 | 0.770 | 0.003 |
Discussion
In addition to being the first assessment of how reduced PMAT function impacts classical conditioning to an aversive stimulus, the present findings additionally are an inaugural foray into systematically examining interactions between PMAT function and heterotypic stressor exposure. Previously, we noticed that sequential brief stressors in male PMAT-deficient mice altered behavior [11]. Consequently, we sought to explore this phenomenon in more depth, while simultaneously assessing how functional PMAT reductions affect fear processing measures. Here, we observed that diminished PMAT expression shifts the time course of cued fear expression and cued extinction training in females, while augmenting expression of context fear in males. Notably, PMAT function appeared to be without substantive impact upon acquisition of cued or context fear conditioning. This allows conclusions about how function of PMAT moderates expression of fear to be made independent of any concerns regarding confounds of acquisition. Certainly though, a contribution of PMAT to consolidation of aversive memories remains to be clearly defined.
Previously, we and others have found sex-specific effects of PMAT deficiency on behavior [10,11,16] (see review [5]). Similar outcomes were found here. With the exception of cued fear expression and extinction training in females, the broad theme in the present findings was that females were largely unaffected by PMAT reductions nor heterotypic stress exposure in their fear behavior, swim stress behavior, and serum corticosterone levels. In contrast, decreased PMAT function in males enhanced context fear expression prior to any preceding stress exposures (i.e., in Phase 1). This remained true for Phase 2 PMAT-deficient males that encountered a brief swim stressor 4 weeks before being trained in context fear conditioning. Also counter to our hypothesis, Phase 2 cued PMAT-deficient males exhibited behavior that mostly mirrored that of behavior by pre-swim males in Phase 1 cued. Paradoxically, male Phase 2 context PMAT-deficient mice that experienced a sham procedure (no swim) instead of a swim stress exhibited reduced context fear expression relatively to their wildtype counterparts. Adding to the confusion were Phase 2 cued male heterozygous no swim mice that exhibited enhanced context fear (like Phase 1 context males) preceding by impaired cued extinction retention.
While initially perplexing, we think these data could indicate a narrow window of plasticity that typical PMAT function might typically obscure. Modestly arousing experiences – such as those of no swim mice being temporarily relocated for a sham swim procedure – are eliciting shifts in later heterozygous behavior during multi-day conditioning procedures. Given PMAT can transport not only dopamine and serotonin, but also epinephrine and norepinephrine [8] – the latter of which is important for arousal [54-56] – it could be that reduced PMAT function enhances arousal and promotes plasticity. In contrast, prior exposure to an overt, albeit brief, stressor appears to obscure this plasticity window in heterozygotes, resulting in behavioral responses to conditioning procedures that are nearly indistinguishable from those of heterozygous mice with no prior acute stressor encounters. Moreover, these behavioral changes in fear expression and retention were selective to males, suggesting a sex hormone component [57-59]. Indeed, recent studies are beginning to parse apart the molecular underpinnings of PMAT’s sex-specific functions [60,61]. Until further experiments can be performed, of course, this interpretation of plasticity remains speculative.
Evaluating cort levels 2 h following the last test helped determine if PMAT deficiency interacted with prior stressor exposure to influence return of cort levels to baseline. This was the focus here given previous evidence that reduced or ablated PMAT function had no impact on cort levels 30 min after an acute swim stressor (Supplemental Fig. S1); a time point accepted to be the peak of cort response to an acute stress [44-47]. Expected sex differences [49,62,63] were repeatedly, albeit not uniformly, observed in Phase 1 mice. Only for Phase 2 Context were sex differences not observed, and instead a genotype × swim interaction predominated. Because testing of fear expression, in the absence of any unconditioned stimulus, is by nature less discrete of a stressor, the absence of selective sex-, genotype- and swim-specific differences is not necessarily surprising for Phase 2 cort levels. This might also help explain why Phase 1 Cued cort levels didn’t display a particular pattern – because the cued fear conditioning procedure spans 5 days, rather than the 2 days for context fear conditioning. Indeed, Phase 1 Context males displayed cort levels that suggested an enduring interaction between heterotypic stress exposure and PMAT deficiency. Males in Phase 1 Context that didn’t undergo a swim stress had comparable cort levels across genotypes, and male wildtypes that were swam displayed higher cort levels than male wildtypes that weren’t. However, swam male heterozygotes had significantly lower cort levels than swam male wildtypes. Thus, reduced PMAT function seems to result in less sustainment of cort when an acute stressor is experienced weeks after previous stressor of a different form. While intriguing, these cort level differences in Phase 1 Context males don’t appear to map on to either fear or swim behavior, indicating that these physiological changes are likely having other influences that were not captured by the present study. Future evaluations of behaviors like risk assessment [64], depressive-like behavior [65], or social interaction [41] could uncover what corticosterone regulation changes are affecting.
The swim behaviors exhibited by mice after or before fear conditioning were modestly different across Phases. A swimming-specific genotype × sex interaction was found for Phase 1 Context mice, where male heterozygotes displayed higher swimming behavior than female heterozygotes. Otherwise, prior exposure to either cued or context fear conditioning did not drastically alter swim behaviors. When Phase 2 swim behaviors were evaluated, 4 wks before those mice would ever experience any form of fear conditioning, there was an unanticipated disparity in the overall patterns observed. Genotype effects were more prominent in Phase 2 Cued mice, whereas sex effects dominated in Phase 2 Context mice. Previously, we also observed sex × genotype interactions during swim stress [10]. Because of the timing of the swim stressors in the current study though, differences between Phase 2 Cued and Phase 2 Context should not exist, particularly considering the same two blinded observers scored all swim behaviors after all behavior testing had concluded. Thus, the findings noted for these should be interpreted with caution. In line with data for fear behavior and cort levels, effects were specific to males, with Phase 2 Cued heterozygotes showing less immobility and a corresponding increase in climbing behaviors. Across genotypes, males had less immobility and greater latencies to first immobility than females. As with fear behavior, swim behaviors in both Phases did not map on to cort levels, suggesting PMAT function influences other physiological processes that drive swim behaviors, likely serotonin signaling [3,66,67] among others.
The present study supports a contribution of PMAT function to behavioral and physiological heterotypic stress responses. Limitations of the study include the aforementioned differences in swim behavior prior to any conditioning exposure, a consequence of quantifying swim behaviors later than would have been optimal. Additionally, the discordance between cort changes and behavioral shifts indicate that alternative physiologic/behavior measures might have instead been better suited to detect concordance [41,64,65]. Absence of a complete time course of cort levels would have been helpful, but would have either resulted in a potentially confounding stress source, or use of more mice than we could ethically justify for the purpose of this investigation. In hindsight, focusing specifically on context fear conditioning and swim stress, and incorporating instead a behavioral measure of appetitive learning (e.g., lever pressing for a food reinforcer), might have provided better insight into the behavioral consequences of PMAT function upon heterotypic stress responsivity.
Nonetheless, the present findings provide important information upon which future experiments can be based to better focus efforts into understanding PMAT’s roles. Such studies should take a deeper look at learning and memory processes, and explore both behavioral and molecular changes occurring from PMAT reductions and stressor encounters. Investigations employing orchidectomies could also provide insight into the organizational and/or activational interactions of sex hormones with PMAT function. This is only the second study to date to use classical conditioning in PMAT mice [16], and the first to employ fear conditioning, so more remains to be learned in this domain, including long-term memory, cue discrimination, and generalization among other parameters. Moreover, expanding studies into evaluations of PMAT’s role in operant conditioning procedures could be informative. And as always with PMAT, development of drugs that selectively inhibit this transporter would be a tremendous boon to understanding the functional influences of this protein.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the assistance of undergraduate research assistants Aliyah Ross, Anna Anello, and Kaden Ruffin. This work was supported by a 2017 NARSAD Young Investigator Grant (26249) from the Brain & Behavior Research Foundation, and Vital Projects Fund, Inc., to TLG; a National Institute of Mental Health grant R15 MH118705 to TLG; and support from Kent State University, including its Brain Health Research Institute and University Research Council. Biorender.com (Toronto, ON) was used to create Figure 1.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Puglisi-Allegra S.; Kempf E.; Schleef C.; Cabib S. Repeated Stressful Experiences Differently Affect Brain Dopamine Receptor Subtypes. Life Sci. 1991, 48, 1263–1268, doi: 10.1016/0024-3205(91)90521-c. [DOI] [PubMed] [Google Scholar]
- 2.Finlay J.M.; Zigmond M.J.; Abercrombie E.D. Increased Dopamine and Norepinephrine Release in Medial Prefrontal Cortex Induced by Acute and Chronic Stress: Effects of Diazepam. Neuroscience 1995, 64, 619–628, doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 3.Fujino K.; Yoshitake T.; Inoue O.; Ibii N.; Kehr J.; Ishida J.; Nohta H.; Yamaguchi M. Increased Serotonin Release in Mice Frontal Cortex and Hippocampus Induced by Acute Physiological Stressors. Neurosci. Lett. 2002, 320, 91–95, doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- 4.Matuszewich L.; Filon M.E.; Finn D.A.; Yamamoto B.K. Altered Forebrain Neurotransmitter Responses to Immobilization Stress Following 3,4-Methylenedioxymethamphetamine. Neuroscience 2002, 110, 41–48, doi: 10.1016/s0306-4522(01)00539-5. [DOI] [PubMed] [Google Scholar]
- 5.Weber B.L.; Beaver J.N.; Gilman T.L. Summarizing Studies Using Constitutive Genetic Deficiency to Investigate Behavioural Influences of Uptake 2 Monoamine Transporters. Basic Clin. Pharmacol. Toxicol. 2022, doi: 10.1111/bcpt.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daws L.C. Organic Cation Transporters in the Central Nervous System. Handb Exp Pharmacol 2021, 266, 215–239, doi: 10.1007/164_2021_473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koepsell H. Organic Cation Transporters in the Central Nervous System. Handb Exp Pharmacol 2021, 266, 1–39, doi: 10.1007/164_2021_449. [DOI] [PubMed] [Google Scholar]
- 8.Duan H.; Wang J. Selective Transport of Monoamine Neurotransmitters by Human Plasma Membrane Monoamine Transporter and Organic Cation Transporter 3. J Pharmacol Exp Ther 2010, 335, 743–753, doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bönisch H. Organic Cation Transporters in the Central Nervous System. Handb Exp Pharmacol 2021, 266, 119–167, doi: 10.1007/164_2021_516. [DOI] [PubMed] [Google Scholar]
- 10.Gilman T.L.; George C.M.; Vitela M.; Herrera Rosales M.; Basiouny M.S.; Koek W.; Daws L.C. Constitutive Plasma Membrane Monoamine Transporter (PMAT, Slc29a4) Deficiency Subtly Affects Anxiety like and Coping Behaviours. Eur J Neurosci 2018, 48, 1706–1716, doi: 10.1111/ejn.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaver J.N.; Weber B.L.; Ford M.T.; Anello A.E.; Kassis S.K.; Gilman T.L. Uncovering Functional Contributions of PMAT (Slc29a4) to Monoamine Clearance Using Pharmacobehavioral Tools. Cells 2022, 11, 1874, doi: 10.3390/cells11121874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacq A.; Balasse L.; Biala G.; Guiard B.; Gardier A.M.; Schinkel A.; Louis F.; Vialou V.; Martres M.-P.; Chevarin C.; et al. Organic Cation Transporter 2 Controls Brain Norepinephrine and Serotonin Clearance and Antidepressant Response. Mol Psychiatr 2012, 17, 926–939, doi: 10.1038/mp.2011.87. [DOI] [PubMed] [Google Scholar]
- 13.Couroussé T.; Bacq A.; Belzung C.; Guiard B.; Balasse L.; Louis F.; Guisquet A.-M.L.; Gardier A.M.; Schinkel A.H.; Giros B.; et al. Brain Organic Cation Transporter 2 Controls Response and Vulnerability to Stress and GSK3β Signaling. Mol Psychiatr 2015, 20, 889–900, doi: 10.1038/mp.2014.86. [DOI] [PubMed] [Google Scholar]
- 14.Wultsch T.; Grimberg G.; Schmitt A.; Painsipp E.; Wetzstein H.; Breitenkamp A.F.S.; Gründemann D.; Schömig E.; Lesch K.-P.; Gerlach M.; et al. Decreased Anxiety in Mice Lacking the Organic Cation Transporter 3. J Neural Transm 2009, 116, 689–697, doi: 10.1007/s00702-009-0205-1. [DOI] [PubMed] [Google Scholar]
- 15.Vialou V.; Balasse L.; Callebert J.; Launay J.; Giros B.; Gautron S. Altered Aminergic Neurotransmission in the Brain of Organic Cation Transporter 3 deficient Mice. J Neurochem 2008, 106, 1471–1482, doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- 16.Clauss N.J.; Koek W.; Daws L.C. Role of Organic Cation Transporter 3 and Plasma Membrane Monoamine Transporter in the Rewarding Properties and Locomotor Sensitizing Effects of Amphetamine in Male andFemale Mice. Int J Mol Sci 2021, 22, 13420, doi: 10.3390/ijms222413420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logue S.F.; Paylor R.; Wehner J.M. Hippocampal Lesions Cause Learning Deficits in Inbred Mice in the Morris Water Maze and Conditioned-Fear Task. Behav Neurosci 1997, 111, 104–113, doi: 10.1037/0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 18.Huff N.C.; Rudy J.W. The Amygdala Modulates Hippocampus-Dependent Context Memory Formation and Stores Cue-Shock Associations. Behav. Neurosci. 2004, 118, 53–62, doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- 19.Zelikowsky M.; Hersman S.; Chawla M.K.; Barnes C.A.; Fanselow M.S. Neuronal Ensembles in Amygdala, Hippocampus, and Prefrontal Cortex Track Differential Components of Contextual Fear. J. Neurosci. 2014, 34, 8462–8466, doi: 10.1523/jneurosci.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maren S. Pavlovian Fear Conditioning as a Behavioral Assay for Hippocampus and Amygdala Function: Cautions and Caveats. Eur. J. Neurosci. 2008, 28, 1661–1666, doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 21.Farrell M.R.; Sengelaub D.R.; Wellman C.L. Sex Differences and Chronic Stress Effects on the Neural Circuitry Underlying Fear Conditioning and Extinction. Physiol. Behav. 2013, 122, 208–215, doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaaya N.; Battle A.R.; Johnson L.R. An Update on Contextual Fear Memory Mechanisms: Transition between Amygdala and Hippocampus. Neurosci. Biobehav. Rev. 2018, 92, 43–54, doi: 10.1016/j.neubiorev.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Yanagida S.; Motomura K.; Ohashi A.; Hiraoka K.; Miura T.; Kanba S. Effect of Acute Imipramine Administration on the Pattern of Forced Swim-Induced c-Fos Expression in the Mouse Brain. Neurosci. Lett. 2016, 629, 119–124, doi: 10.1016/j.neulet.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 24.Duncan G.E.; Inada K.; Farrington J.S.; Koller B.H.; Moy S.S. Neural Activation Deficits in a Mouse Genetic Model of NMDA Receptor Hypofunction in Tests of Social Aggression and Swim Stress. Brain Res. 2009, 1265, 186–195, doi: 10.1016/j.brainres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y.F.; Bertram K.; Perides G.; McEwen B.S.; Wang D. Stress Induces Activation of Stress activated Kinases in the Mouse Brain. J. Neurochem. 2004, 89, 1034–1043, doi: 10.1111/j.1471-4159.2004.02391.x. [DOI] [PubMed] [Google Scholar]
- 26.Dawed A.Y.; Zhou K.; van Leeuwen N.; Mahajan A.; Robertson N.; Koivula R.; Elders P.J.M.; Rauh S.P.; Jones A.G.; Holl R.W.; et al. Variation in the Plasma Membrane Monoamine Transporter (PMAT, Encoded in SLC29A4) and Organic Cation Transporter 1 (OCT1, Encoded in SLC22A1) and Gastrointestinal Intolerance to Metformin in Type 2 Diabetes: An IMI DIRECT Study. Diabetes Care 2019, 42, dc182182, doi: 10.2337/dc18-2182. [DOI] [PubMed] [Google Scholar]
- 27.Moeez S.; Khalid S.; Shaeen S.; Khalid M.; Zia A.; Gul A.; Niazi R.; Khalid Z. Clinically Significant Findings of High-Risk Mutations in Human SLC29A4 Gene Associated with Diabetes Mellitus Type 2 in Pakistani Population. J Biomol Struct Dyn 2021, 1–14, doi: 10.1080/07391102.2021.1975561. [DOI] [PubMed] [Google Scholar]
- 28.Christensen M.M.H.; Brasch-Andersen C.; Green H.; Nielsen F.; Damkier P.; Beck-Nielsen H.; Brosen K. The Pharmacogenetics of Metformin and Its Impact on Plasma Metformin Steady-State Levels and Glycosylated Hemoglobin A1c. Pharmacogenet Genom 2011, 21, 837–850, doi: 10.1097/fpc.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Gómez N.; Fernández-Ortega M.D.; Elizari-Roncal M.; Santos-Mazo E.; Maza-Pereg L. de la; Calvo S.; Alcaraz R.; Sanz-Solas A.; Vinuesa R.; Saiz-Rodríguez M. Identification of Clinical and Pharmacogenetic Factors Influencing Metformin Response in Type 2 Diabetes Mellitus. Pharmacogenomics 2023, 0, doi: 10.2217/pgs-2023-0109. [DOI] [PubMed] [Google Scholar]
- 30.Yohn N.L.; Blendy J.A. Adolescent Chronic Unpredictable Stress Exposure Is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice. Neuropsychopharmacology 2017, 42, 1670–1678, doi: 10.1038/npp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sillivan S.E.; Joseph N.F.; Jamieson S.; King M.L.; Chévere-Torres I.; Fuentes I.; Shumyatsky G.P.; Brantley A.F.; Rumbaugh G.; Miller C.A. Susceptibility and Resilience to Posttraumatic Stress Disorder–like Behaviors in Inbred Mice. Biol. Psychiatry 2017, 82, 924–933, doi: 10.1016/j.biopsych.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan H.; Wang J. Impaired Monoamine and Organic Cation Uptake in Choroid Plexus in Mice with Targeted Disruption of the Plasma Membrane Monoamine Transporter (Slc29a4) Gene. J Biol Chem 2013, 288, 3535–3544, doi: 10.1074/jbc.m112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Animals C. for the U. of the G. for the C. and U. of L.; Research, I. for L.A.; Studies, D. on E. and L.; Council, N.R. Guide for the Care and Use of Laboratory Animals. 2011, doi: 10.17226/25801. [DOI] [Google Scholar]
- 34.Gilman T.L.; DaMert J.P.; Meduri J.D.; Jasnow A.M. Grin1 Deletion in CRF Neurons Sex-Dependently Enhances Fear, Sociability, and Social Stress Responsivity. Psychoneuroendocrino 2015, 58, 33–45, doi: 10.1016/j.psyneuen.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Radford K.D.; Berman R.Y.; Jaiswal S.; Kim S.Y.; Zhang M.; Spencer H.F.; Choi K.H. Enhanced Fear Memories and Altered Brain Glucose Metabolism (18F-FDG-PET) Following Subanesthetic Intravenous Ketamine Infusion in Female Sprague–Dawley Rats. Int. J. Mol. Sci. 2022, 23, 1922, doi: 10.3390/ijms23031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C.-M.; Zhang Y.-F.; Lin Z.-Q.; Cai Y.-F.; Fu X.-Y.; Lin Z.-H. Pre-Extinction Activation of Hippocampal AMPK Prevents Fear Renewal in Mice. Pharmacol. Res. 2020, 161, 105099, doi: 10.1016/j.phrs.2020.105099. [DOI] [PubMed] [Google Scholar]
- 37.Jasnow A.M.; Ehrlich D.E.; Choi D.C.; Dabrowska J.; Bowers M.E.; McCullough K.M.; Rainnie D.G.; Ressler K.J. Thy1-Expressing Neurons in the Basolateral Amygdala May Mediate Fear Inhibition. J Neurosci 2013, 33, 10396–10404, doi: 10.1523/jneurosci.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch J.F.; Winiecki P.; Gilman T.L.; Adkins J.M.; Jasnow A.M. Hippocampal GABAB(1a) Receptors Constrain Generalized Contextual Fear. Neuropsychopharmacol 2017, 42, 914–924, doi: 10.1038/npp.2016.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaver J.N.; Weber B.L.; Ford M.T.; Anello A.E.; Ruffin K.M.; Kassis S.K.; Gilman T.L. Generalization of Contextual Fear Is Sex-Specifically Affected by High Salt Intake. PLOS ONE 2023, 18, e0286221, doi: 10.1371/journal.pone.0286221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell W.; Burch R. The Principles of Humane Experimental Technique. Nature 1959, 184, 1675–1676, doi: 10.1038/1841675b0. [DOI] [Google Scholar]
- 41.Gilman T.L.; George C.M.; Andrade M.A.; Mitchell N.C.; Toney G.M.; Daws L.C. High Salt Intake Lowers Behavioral Inhibition. Front Behav Neurosci 2019, 13, 271, doi: 10.3389/fnbeh.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uarquin D.G.; Meyer J.S.; Cardenas F.P.; Rojas M.J. Effect of Overcrowding on Hair Corticosterone Concentrations in Juvenile Male Wistar Rats. J Am Assoc Laboratory Animal Sci Jaalas 2016, 55, 749–755. [PMC free article] [PubMed] [Google Scholar]
- 43.Teilmann A.C.; Kalliokoski O.; Sørensen D.B.; Hau J.; Abelson K.S.P. Manual versus Automated Blood Sampling: Impact of Repeated Blood Sampling on Stress Parameters and Behavior in Male NMRI Mice. Lab Anim 2014, 48, 278–291, doi: 10.1177/0023677214541438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romeo R.D.; Bellani R.; Karatsoreos I.N.; Chhua N.; Vernov M.; Conrad C.D.; McEwen B.S. Stress History and Pubertal Development Interact to Shape Hypothalamic-Pituitary-Adrenal Axis Plasticity. Endocrinology 2006, 147, 1664–1674, doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- 45.Romeo R.D.; Karatsoreos I.N.; McEwen B.S. Pubertal Maturation and Time of Day Differentially Affect Behavioral and Neuroendocrine Responses Following an Acute Stressor. Horm. Behav. 2006, 50, 463–468, doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Hare B.D.; Beierle J.A.; Toufexis D.J.; Hammack S.E.; Falls W.A. Exercise-Associated Changes in the Corticosterone Response to Acute Restraint Stress: Evidence for Increased Adrenal Sensitivity and Reduced Corticosterone Response Duration. Neuropsychopharmacology 2014, 39, 1262–1269, doi: 10.1038/npp.2013.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClennen S.J.; Cortright D.N.; Seasholtz A.F. Regulation of Pituitary Corticotropin-Releasing Hormone-Binding Protein Messenger Ribonucleic Acid Levels by Restraint Stress and Adrenalectomy. Endocrinology 1998, 139, 4435–4441, doi: 10.1210/endo.139.11.6311. [DOI] [PubMed] [Google Scholar]
- 48.Alele P.E.; Devaud L.L. Sex Differences in Steroid Modulation of Ethanol Withdrawal in Male and Female Rats. J. Pharmacol. Exp. Ther. 2007, 320, 427–436, doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- 49.Albrechet-Souza L.; Schratz C.L.; Gilpin N.W. Sex Differences in Traumatic Stress Reactivity in Rats with and without a History of Alcohol Drinking. Biol. Sex Differ. 2020, 11, 27, doi: 10.1186/s13293-020-00303-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palumbo M.C.; Dominguez S.; Dong H. Sex Differences in Hypothalamic–Pituitary–Adrenal Axis Regulation after Chronic Unpredictable Stress. Brain Behav. 2020, 10, e01586, doi: 10.1002/brb3.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shors T.J.; Chua C.; Falduto J. Sex Differences and Opposite Effects of Stress on Dendritic Spine Density in the Male Versus Female Hippocampus. J. Neurosci. 2001, 21, 6292–6297, doi: 10.1523/jneurosci.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aoki M.; Shimozuru M.; Kikusui T.; Takeuchi Y.; Mori Y. Sex Differences in Behavioral and Corticosterone Responses to Mild Stressors in ICR Mice Are Altered by Ovariectomy in Peripubertal Period. Zool. Sci. 2010, 27, 783–789, doi: 10.2108/zsj.27.783. [DOI] [PubMed] [Google Scholar]
- 53.Goel N.; Workman J.L.; Lee T.T.; Innala L.; Viau V. Comprehensive Physiology. Compr. Physiol. 2021, 4, 1121–1155, doi: 10.1002/cphy.c130054. [DOI] [PubMed] [Google Scholar]
- 54.Reitman M.E.; Tse V.; Mi X.; Willoughby D.D.; Peinado A.; Aivazidis A.; Myagmar B.-E.; Simpson P.C.; Bayraktar O.A.; Yu G.; et al. Norepinephrine Links Astrocytic Activity to Regulation of Cortical State. Nat. Neurosci. 2023, 26, 579–593, doi: 10.1038/s41593-023-01284-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.España R.A.; Schmeichel B.E.; Berridge C.W. Norepinephrine at the Nexus of Arousal, Motivation and Relapse. Brain Res. 2016, 1641, 207–216, doi: 10.1016/j.brainres.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunsley M.S.; Palmiter R.D. Altered Sleep Latency and Arousal Regulation in Mice Lacking Norepinephrine. Pharmacol. Biochem. Behav. 2004, 78, 765–773, doi: 10.1016/j.pbb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 57.Aikey J.L.; Nyby J.G.; Anmuth D.M.; James P.J. Testosterone Rapidly Reduces Anxiety in Male House Mice (Mus Musculus). Horm. Behav. 2002, 42, 448–460, doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 58.Honk J. van; Peper J.S.; Schutter D.J.L.G. Testosterone Reduces Unconscious Fear but Not Consciously Experienced Anxiety: Implications for the Disorders of Fear and Anxiety. Biol. Psychiatry 2005, 58, 218–225, doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Chen L.-S.; Tzeng W.-Y.; Chuang J.-Y.; Cherng C.G.; Gean P.-W.; Yu L. Roles of Testosterone and Amygdaloid LTP Induction in Determining Sex Differences in Fear Memory Magnitude. Horm. Behav. 2014, 66, 498–508, doi: 10.1016/j.yhbeh.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Wei R.; Gust S.L.; Tandio D.; Maheux A.; Nguyen K.H.; Wang J.; Bourque S.; Plane F.; Hammond J.R. Deletion of Murine Slc29a4 Modifies Vascular Responses to Adenosine and 5 hydroxytryptamine in a Sexually Dimorphic Manner. Physiological Reports 2020, 8, e14395, doi: 10.14814/phy2.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Y.; Zhang N.; Zhu S.; Lu S.; Jiang H.; Zhou H. Estradiol Reduced 5-HT Reuptake by Downregulating the Gene Expression of Plasma Membrane Monoamine Transporter (PMAT, Slc29a4) through Estrogen Receptor β and the MAPK/ERK Signaling Pathway. Eur J Pharmacol 2022, 924, 174939, doi: 10.1016/j.ejphar.2022.174939. [DOI] [PubMed] [Google Scholar]
- 62.Daviu N.; Andero R.; Armario A.; Nadal R. Sex Differences in the Behavioural and Hypothalamic–Pituitary–Adrenal Response to Contextual Fear Conditioning in Rats. Horm Behav 2014, 66, 713–723, doi: 10.1016/j.yhbeh.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Xia J.; Wang H.; Zhang C.; Liu B.; Li Y.; Li K.; Li P.; Song C. The Comparison of Sex Differences in Depression-like Behaviors and Neuroinflammatory Changes in a Rat Model of Depression Induced by Chronic Stress. Front. Behav. Neurosci. 2023, 16, 1059594, doi: 10.3389/fnbeh.2022.1059594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodgers R.J.; Haller J.; Holmes A.; Halasz J.; Walton T.J.; Brain P.F. Corticosterone Response to the Plus-Maze High Correlation with Risk Assessment in Rats and Mice. Physiol. Behav. 1999, 68, 47–53, doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 65.Kokras N.; Krokida S.; Varoudaki T.Z.; Dalla C. Do Corticosterone Levels Predict Female Depressive like Behavior in Rodents? J. Neurosci. Res. 2021, 99, 324–331, doi: 10.1002/jnr.24686. [DOI] [PubMed] [Google Scholar]
- 66.Porsolt R.D.; Bertin A.; Blavet N.; Deniel M.; Jalfre M. Immobility Induced by Forced Swimming in Rats: Effects of Agents Which Modify Central Catecholamine and Serotonin Activity. Eur. J. Pharmacol. 1979, 57, 201–210, doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- 67.Ehlinger D.G.; Commons K.G. Cav1.2 L-Type Calcium Channels Regulate Stress Coping Behavior via Serotonin Neurons. Neuropharmacology 2019, 144, 282–290, doi: 10.1016/j.neuropharm.2018.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.