Abstract
Randomly amplified polymorphic DNA (RAPD) genotyping was applied to one representative strain of each of the 84 electrophoretic types (ETs) of Neisseria meningitidis serogroup A previously defined by multilocus enzyme electrophoresis (MEE) (J.-F. Wang et al., Infect. Immun. 60:5267–5282, 1992). Twenty-seven additional isolates comprising six ETs were also tested. MEE and RAPD genotyping yielded similar dendrograms at the subgroup level. Similar results were obtained by both methods for 18 serogroup A meningococci isolated in The Netherlands between 1989 and 1993. Ten of these isolates defined a new subgroup, designated subgroup IX. One isolate belonged to the ET-5 complex, normally associated with serogroup B strains (D. A. Caugant et al., Proc. Natl. Acad. Sci. USA 83:4927–4931, 1986). By RAPD genotyping, meningococci can be linked to previously characterized genotypes by using a computerized database, and dendrograms based on cluster analyses can easily be generated. RAPD analysis offers advantages over MEE since intermediate numbers of isolates of serogroup A meningococci can quickly be assigned to known subgroups and new subgroups can be defined.
Neisseria meningitidis is an encapsulated gram-negative bacterium that causes meningitis and septicemia worldwide. Classification according to capsule polysaccharide type revealed 11 capsule types. Serogroup A, B, and C isolates are the causes of about 90% of the cases of meningitis (13). Serogroup A strains are the leading cause of epidemics, whereas serogroup B and C strains generally cause endemic cases of infection and small outbreaks. Since World War II, large epidemics caused by serogroup A have not occurred in Europe or the United States, but such epidemics still prevail in the People’s Republic of China and the Sahel zone of Africa (the so-called meningitis belt) (1). In addition to the classification into serogroups, meningococci are divided serologically into serotypes and serosubtypes on the basis of differences in the class 2/3 outer membrane protein (OMP) and the class 1 OMP (P1), respectively (7). However, differences in serotype or serosubtype do not necessarily reflect genotypic differences, because OMP variation is caused by horizontal gene transfer (8).
Multilocus enzyme electrophoresis (MEE) has been used for the genotyping of meningococci, to identify specific clones, and to study the genetic diversity of the organism (3, 11). Currently, this method is considered the reference “gold standard” for the typing of meningococci (3). Although the correlation between the electrophoretic migration of individual enzymes and the genotype may be disrupted by horizontal gene transfer (6), the use of multiple enzymes makes MEE a fairly robust typing method. After completion of this work, a new technique, multilocus sequence typing (MLST) of six gene fragments, was applied to a representative collection of meningococci and was shown to recognize the same clonal lineages recognized by MEE (9).
PCR of randomly amplified polymorphic DNA (RAPD) (17) has potential advantages for the typing of meningococcal isolates. It requires only modest effort, its cost is relatively low, and no prior sequence information is necessary. In RAPD analyses short (10-bp) arbitrary primers that can each anneal at various sites on the chromosome are used. Several DNA fragments of different lengths which can be analyzed by conventional agarose gel electrophoresis are generated. Strains differing in the presence of annealing sites and the distances between them yield different sets of DNA fragments and are considered to have different genotypes. RAPD analysis is reproducible, provided that uniform template DNA (4) and MgCl2 (12) concentrations are used.
Previously, Woods et al. (18) used RAPD analysis to type N. meningitidis serogroup B, C, and Y strains isolated during an outbreak at a university. They noted the potential of RAPD analysis for determining the global epidemiology of meningococcal disease and the population structure of N. meningitidis if it were validated by direct comparison to MEE. Here we compare the characterizations of a large collection of serogroup A meningococci (16) obtained by MEE and RAPD analysis. Furthermore, recent isolates from The Netherlands were genotyped by both RAPD analysis and MEE, leading to the identification of new genotypes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Eighty-four strains representing the individual electrophoretic types (ETs) of serogroup A meningococci described by Wang et al. (16) were used to compare the results of RAPD analysis with those of MEE. A selection of 27 strains of ETs 19 (subgroup I; 3 additional strains), 41 (subgroup II; 1 strain), 48 (subgroup III; 7 strains), 50 (subgroup III; 2 strains), 67 (subgroup IV-1; 7 strains), and 28 (subgroup I; 7 strains) from diverse sources (16) was also included. In addition, 18 formerly serotyped and serosubtyped but undescribed strains from the collection of The Netherlands Reference Laboratory for Bacterial Meningitis (Rijksinstituut voor Volkgezondheid en Milieuhygiëne/Academic Medical Center, Bilthoven/Amsterdam, The Netherlands) were assessed by RAPD analysis and, with one exception, by MEE (Table 1). These strains represent the four serotype-serosubtype combinations found for serogroup A strains isolated during the period from 1989 to 1993. The marker DNA used to generate a standard pattern on all gels was isolated from strain 770377, a serogroup B meningococcus from The Netherlands Reference Laboratory for Bacterial Meningitis. Meningococci were grown on heated blood (chocolate) agar plates at 37°C in a humidified atmosphere of 5% CO2.
TABLE 1.
Properties of 18 Dutch serogroup A strains
| Strain | Yr of isolation | Serotype | Serosubtype | Genotype |
|---|---|---|---|---|
| 890461 | 1989 | NTa | P1.16 | Subgroup IX |
| 890592 | 1989 | NT | P1.16 | Subgroup IX |
| 890867 | 1989 | NT | P1.16 | Subgroup IX |
| 891780 | 1989 | 4 | P1.9 | NDb |
| 892411 | 1989 | 4 | P1.10 | ET-33 |
| 892665 | 1989 | 15 | NSTc | ET-5 complex |
| 900973 | 1990 | 4 | P1.9 | ET-48 |
| 901335 | 1990 | NT | P1.16 | Subgroup IX |
| 902488 | 1990 | 4 | P1.10 | ET-33 |
| 911652 | 1991 | NT | P1.16 | Subgroup IX |
| 911960 | 1991 | NT | P1.16 | Subgroup IX |
| 920054 | 1992 | 4 | P1.9 | ET-48 |
| 920521 | 1992 | NT | P1.16 | Subgroup IX |
| 921051 | 1992 | 4 | P1.10 | New |
| 921268 | 1992 | NT | P1.16 | Subgroup IX |
| 921710 | 1992 | 4 | P1.9 | ET-48 |
| 931114 | 1993 | NT | P1.16 | Subgroup IX |
| 931192 | 1993 | NT | P1.16 | Subgroup IX |
NT, not typeable.
ND, not determined.
NST, not subtypeable.
Chromosomal DNA preparation.
Chromosomal DNA was isolated as described by Akopyanz et al. (2). The concentration of the chromosomal DNA in the samples was assessed by measuring the optical density at 260 nm with an Ultraspec 2000 spectrophotometer (Pharmacia).
PCR of RAPD.
The primers used for PCR of RAPD were primer 1254 (5′-CCG CAG CCA A-3′), primer 1281 (5′-AAC GCG CAA C-3′) (2), primer NM03 (5′-CCG CTG CCT T-3′), and primer NM04 (5′-GCA CGG ATC A-3′); all primers were synthesized by Perkin-Elmer Nederland B.V., Gouda, The Netherlands. The mixture used for PCR of RAPD contained 20 ng of template DNA, 3.2 μM (primers 1254 and 1281) or 4.8 μM (primers NM03 and NM04) primer, each nucleotide (dATP, dCTP, dGTP, dTTP) at 250 μM, 0.01% (wt/vol) bovine serum albumin, 3.0 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl (pH 8.8), and 1.25 U of Taq polymerase (Perkin-Elmer Cetus) in a final volume of 25 μl. Reactions were performed in a Trio Thermoblock (Biometra). The PCR program consisted of 4 cycles (5 min at 94°C, 5 min at 36°C, and 5 min at 72°C), followed by 30 cycles (1 min at 94°C, 1 min at 36°C, and 2 min at 72°C). A final 10-min extension was performed at 72°C.
Agarose gel electrophoresis.
Electrophoretic separation of the amplified products was performed for 4 h at 4 V/cm on 1% agarose gels in 1× Tris-borate-EDTA buffer. Both gel and buffer contained 1 mg of ethidium bromide per liter.
Consistency marker.
The RAPD pattern generated with primer 1254 from strain 770377 contains evenly spread bands and was used as a marker (consistency marker). This marker was prepared fresh each time that a set of strains was tested and was used as a quality control for the reagents used for PCR of RAPD.
Computer-assisted analysis of RAPD patterns.
The RAPD patterns were visualized by UV illumination, and an image was captured with a video camera. Analysis of the images was performed with the Windows version of the Gelcompar software (version 3.1; Applied Maths, Kortrijk, Belgium). The patterns were normalized with the bands of the consistency marker and bands that were uniformly present in all patterns. The patterns generated with each of the four primers were combined for each strain. The resulting combined band patterns were compared by the unweighted pair group cluster method with arithmetic averages, and the Dice coefficient (5) was applied. Computer-assisted analysis and the methods and algorithms used in this study were carried out according to the instructions of the manufacturer. A tolerance in the band positions of 0.2% was applied during the comparison of the RAPD patterns.
MEE.
MEE of the 18 serogroup A meningococcal isolates from The Netherlands was performed with 14 enzymes as described previously (3): malic enzyme (EC 1.1.1.40), glucose-6-phosphate dehydrogenase (EC 1.1.1.49), a peptidase (EC 3.4), isocitrate dehydrogenase (EC 1.1.1.42), aconitase (EC 4.2.1.3), NAD-linked glutamate dehydrogenase (EC 1.4.1.2), NADP-linked glutamate dehydrogenase (EC 1.4.1.4), alcohol dehydrogenase (EC 1.1.1.1), fumarase (EC 4.2.1.2), alkaline phosphatase (EC 3.1.3.1), two indophenol oxidases (EC 1.9.3.1), adenylate kinase (EC 2.7.4.3), and an unidentified dehydrogenase (UDH).
Sequence analysis of porA.
The primers used for sequence analysis of porA were AB01 (5′-TGT AAA ACG ACG GCC AGT GTT TGC CCG ATG TTT TTA GGT T-3′), AB02 (5′-CAG GAA ACA GCT ATG ACC CGG CGT ATA GGC GGA CTT GCT G-3′), AB03 (5′-TGT AAA ACG ACG GCC AGT CAG CGG CAG CGT CCA ATT CGT T-3′), and AB04 (5′-CAG GAA ACA GCT ATG ACC CGT ATC CGC TTC ACC GCC CCG A-3′). The underlined sequences are identical to the −21 M13 primer or M13 reverse primer sequences. Overlapping parts of the porA gene were amplified by PCR with primers AB01-AB02 and AB03-AB04. The products were used as templates for sequencing with fluorescent dye-labelled universal M13 primers. Analysis was performed on an automatic sequencer (model 373), according to the instructions supplied by Applied Biosystems Incorporated (Foster City, Calif.).
Nucleotide sequence accession number.
The porA nucleotide sequence data will appear in the EMBL/Genbank/DDBJ nucleotide sequence databases under accession no. AF026890.
RESULTS
Reproducibility.
To assess the reproducibility of RAPD analysis, 10 different chromosomal DNA preparations were prepared from one strain of N. meningitidis and used as templates in PCRs with primers 1254 and 1281. The resulting patterns were identical. DNA from 14 isolates from blood and their paired isolates from cerebrospinal fluid resulted in identical RAPD patterns for each pair of isolates with primer 1254 (data not shown).
Comparison of RAPD analysis and MEE.
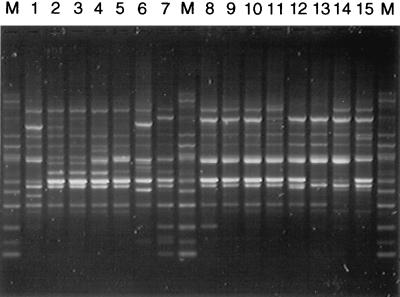
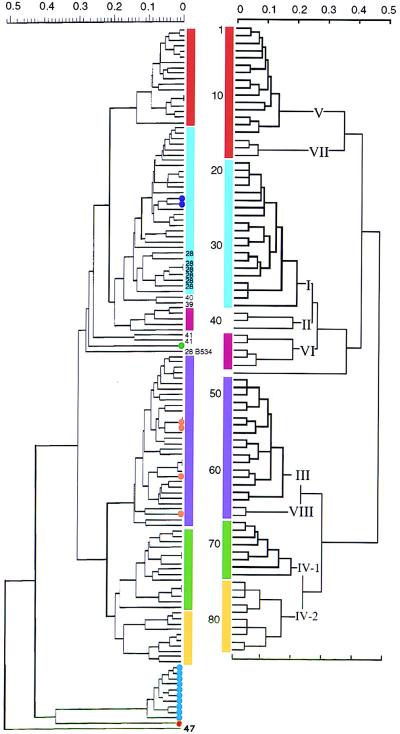
We tested one representative strain of each of the 84 ETs previously identified by Wang et al. (16) by RAPD analysis with four primers. In addition, 27 strains from six epidemic-related ETs from MEE subgroups I, II, III, and IV-1 were also tested. Representative RAPD patterns obtained for 15 strains are shown in Fig. 1. The results obtained with all four primers (10 to 20 specific bands per strain) were combined for cluster analysis. The resulting dendrogram resembled the subgroup distribution obtained by MEE (Fig. 2).
FIG. 1.
Representative RAPD patterns obtained with primer 1254 for representative serogroup A isolates. Lanes: M, marker; 1, ET-11 strain; 2, ET-38 strain; 3, ET-33 strain; 4, ET-39 strain; 5, ET-44 strain; 6, ET-28 strain; 7, ET-41 strain; 8, ET-55 strain; 9, ET-48 strain; 10, ET-75 strain; 11, ET-78 strain; 12, ET-79 strain; 13, ET-83 strain; 14, ET-70 strain; 15, ET-72 strain.
FIG. 2.
Comparison of the genetic relationships inferred by RAPD analysis (left) and MEE (right) (data for MEE are adapted from reference 14). The dendrograms resulting from cluster analysis show the genetic distance at which the clusters divided according to the unweighted pair group average clustering algorithm. In the dendrogram obtained by MEE, subgroups are indicated by Roman numerals and individual ETs are numbered 1 to 84 (top to bottom). Subgroups containing the same ETs in the dendrograms obtained by MEE and RAPD analysis are indicated by colored bars. Subgroup II is split into ET-39 and ET-40 isolates versus ET-41 isolates, as indicated by the numbers at the end of the branches. ET-28 isolate B534 is indicated by its strain number. Other ET-28 strains are labelled 28 at the ends of the branches. The recent Dutch isolates that have been tested are marked with dots: purple dots, ET-33 (strains 892411 and 902488); orange dots, ET-48 (strains 900973, 920054, and 921710) and strain 891780; green dot, new genotype within the serogroup A strains (strain 921051); blue dots, subtype P1.16 strains (strains 890461, 890592, 890867, 901335, 911652, 911960, 920521, 921268, 931114, and 931192); red dot, serotype 15 strain (strain 892665) related to the serogroup B ET-5 complex (see text).
Only a few differences between the dendrogram obtained by RAPD analysis and the one obtained by MEE were noted. Subgroups V and VII and subgroups III and VIII obtained by MEE were not distinguished by RAPD analysis. The two available strains of ET-41 (subgroup II) were distinct from all other strains tested, although the other subgroup II strains (ETs 39 and 40) were closely related to subgroup I strains by RAPD analysis (Fig. 2), as expected. The ET-28 strain, B534, did not fall in the expected subgroup, subgroup I. It was unrelated to all other serogroup A strains by RAPD analysis, even when another frozen subculture of the same strain was tested. Six additional ET-28 isolates did fall within the subgroup I branch by RAPD analysis, as expected. The other 21 strains tested also fell in the same subgroup as the representative strains of those ETs.
Characterization of 18 Dutch serogroup A meningococci.
The data obtained for the 84 representative serogroup A strains by RAPD analysis were stored in a computerized database. This database allows rapid identification of unknown serogroup A isolates. Eighteen serotyped and serosubtyped serogroup A meningococci isolated in The Netherlands from 1989 to 1993 (Table 1) were characterized by RAPD analysis, and their RAPD patterns were compared to those in the database of RAPD patterns (dots in Fig. 2). These 18 Dutch isolates were also typed by MEE, with one exception.
Two of the A:4:P1.10 isolates (purple dots in Fig. 2) were assigned to subgroup I by RAPD analysis and to ET-33 of subgroup I by MEE. Four A:4:P1.9 isolates (orange dots) were assigned to the combined subgroups III and VIII by RAPD analysis; the three isolates that were typed by MEE were assigned to ET-48 of subgroup III. One A:4:P1.10 isolate (green dot) was different from all other serogroup A strains by both RAPD analysis and MEE. The A:15:NST (where NST is not typeable) strain (red dot) was also different from all other serogroup A strains by RAPD analysis. MEE showed that this strain was closely related to the ET-5 complex, which is normally associated with serogroup B meningococci (3).
The 10 A:NT:P1.16 isolates (blue dots in Fig. 2) formed a distinct cluster by both methods, and the isolates in this cluster were unrelated to other known serogroup A strains. To our knowledge, the P1.16 subtype was found only once previously among serogroup A strains (14). Therefore, we sequenced the porA gene of Dutch P1.16 strain 931192 in order to determine whether this gene corresponded to a known porA allele. The sequence (accession no. AF026890) encodes the P1.21, 16a epitopes and differs from known P1.21,16a porA alleles outside the epitope-encoding regions.
DISCUSSION
Both RAPD analysis and the MEE method subdivided clonal serogroup A meningococci into similar subgroups, with only a few exceptions.
RAPD analysis did not distinguish between subgroups III and VIII obtained by MEE. Of 15 cytoplasmic enzymes and four OMPs tested by MEE, only 1 enzyme and one OMP differed between these subgroups (16). Similarly, no differences were found by MLST (9).
MEE subgroups V and VII also clustered together in the dendrogram obtained by RAPD analysis. The strains from these subgroups possess related porA alleles (14), identical iga alleles (10), and serologically indistinguishable pilin classes (16). They were indistinguishable by MLST (9) and consistently differed at only 2 of 15 housekeeping enzymes and two of four OMPs by MEE (16).
Subgroup II obtained by MEE was split into two clusters by RAPD analysis, a cluster of ETs 39 and 40 that was closely related to subgroup I and a cluster of ET-41. Strains from the two clusters were isolated from different geographical locations over an interval of more than 20 years. ET-41 strains were isolated in the United States prior to World War II, whereas the ET-39 and ET-40 strains were isolated in Djibouti in the 1960s. These strains have not yet been tested by MLST. Finally, the supposed ET-28 strain B534 was found to be distinct from ET-28 and other subgroup I strains. Subsequent to this analysis, MLST confirmed that B534 is distinct from all known serogroup A meningococci.
Both RAPD analysis and MEE differentiated subgroups III, IV-1, and IV-2. Almost identical iga and opa alleles as well as a 5-kb DNA stretch flanking one of the opa alleles of older strains from these subgroups was taken as evidence (10) that strains in these three subgroups had descended from a common ancestor since 1800. More recent strains, isolated over the past two to three decades, showed microevolution due to mutations, translocations, and import of DNA from unrelated neisseria. The results obtained by both RAPD analysis and MEE are consistent with the accumulation of differences between these subgroups since their descent from a common ancestor. Furthermore, RAPD analysis and MEE are more sensitive than MLST, which did not differentiate between subgroups IV-1 and IV-2 (9).
Six of the Dutch serogroup A strains isolated in The Netherlands between 1989 and 1993 clustered within the known subgroups I and III. Subgroup I strains had been isolated from Holland in the 1970s (11), and pilgrims returning from the epidemic of 1987 at Mecca brought subgroup III strains to Europe (10). These results indicate that new isolates can be linked to previously identified genotypes with a computerized database of RAPD patterns.
Interestingly, 10 endemic serogroup A Dutch strains were assigned to a novel subgroup which had not been formerly detected among strains causing epidemic disease. We propose the designation subgroup IX for this subgroup. It is unclear whether subgroup IX strains have epidemic potential and will be isolated from other countries in the future or whether they will remain restricted to the population in The Netherlands. Strains from some other subgroups have also been isolated only from single regions (subgroups V, VII, and VIII from the People’s Republic of China, subgroup VI from the former East Germany and Scandinavian countries, and MEE ET-47 from Scotland). Strains belonging to subgroup V have caused large epidemics in the People’s Republic of China, although they have never been isolated elsewhere (16). These results indicate that the current collection of serogroup A meningococcal strains is incomplete and that additional subgroups will be recognized as strains from other geographical areas are tested.
One strain of serotype 15 was closely related to serogroup B strains of the ET-5 complex (3) by MEE. Other inconsistencies between serogroup and clonal structure have been described. Subgroup VI contains strains of serogroups A, B, and C, and a serogroup B strain belonging to subgroup III has been described (16). Sequence data have indicated that serogroup B and C meningococci can exchange genes encoding the capsular polysaccharide serogroup, probably by natural transformation (15). Similar mechanisms are probably responsible for the discrepancy between serogroup and clonal structure described here, namely, that the genes encoding serogroup B or C capsular polysaccharide had been replaced by a gene cassette encoding the A polysaccharide within an ancestor of the serotype 15 strain.
In conclusion, RAPD analysis is reproducible, yields results that are similar to those of MEE and MLST, is more easily implemented in many laboratories than MEE, and is considerably cheaper than MLST. RAPD analysis is a relatively simple method for the rapid identification of strains from endemic and epidemic situations, especially when its use is combined with the use of a computerized database that enables one to make correlations between strains analyzed at different times. Like MEE and MLST, RAPD analysis is based on multiple loci scattered around the chromosome and is less sensitive to the effects of horizontal genetic exchange than methods based on single loci.
ACKNOWLEDGMENTS
We thank Wim van Est and Eelco Roos for expert artwork.
ADDENDUM IN PROOF
The RAPD data for subgroup II and subgroup IX are supported by MLST analysis of strains from these subgroups. The ET-39 strain and the ET-40 strain were indistinguishable from subgroup I by MLST analysis, whereas the ET41 strains differ by one allele out of six alleles tested from the subgroup I strains. MLST analysis of five subgroup IX isolates showed that these differ in four (four isolates) or three (one isolate) of six alleles tested from all other strains tested by MLST. Subgroup IX isolates are therefore unrelated to known strains.
REFERENCES
- 1.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons, Ltd.; 1995. pp. 159–175. [Google Scholar]
- 2.Akopyanz N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caugant D A, Frøholm L O, Bøvre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davin-Regli A, Abed Y, Charrel R N, Bollet C, de Micco P. Variations in DNA concentrations significantly affect the reproducibility of RAPD fingerprint patterns. Res Microbiol. 1995;146:561–568. doi: 10.1016/0923-2508(96)80562-6. [DOI] [PubMed] [Google Scholar]
- 5.Dice L R. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 6.Feil E, Carpenter G, Spratt B G. Electrophoretic variation in adenylate kinase of Neisseria meningitidis is due to inter- and intraspecies recombination. Proc Natl Acad Sci USA. 1995;92:10535–10539. doi: 10.1073/pnas.92.23.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frasch C E, Zollinger W D, Poolman J T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985;7:504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- 8.Maiden M C J, Malorny B, Achtman M. A global gene pool in the neisseriae. Mol Microbiol. 1996;21:1297–1298. doi: 10.1046/j.1365-2958.1996.981457.x. [DOI] [PubMed] [Google Scholar]
- 9.Maiden M C J, Bygraves J A, Feil E, Morelli G, Russell J E, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant D A, Feavers I M, Achtman M, Spratt B G. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998;95:3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morelli G, Malorny B, Müller K, Seiler A, Wang J-F, del Valle J, Achtman M. Clonal descent and microevolution of Neisseria meningitidis during 30 years of epidemic spread. Mol Microbiol. 1997;25:1047–1064. doi: 10.1046/j.1365-2958.1997.5211882.x. [DOI] [PubMed] [Google Scholar]
- 11.Olyhoek T, Crowe B A, Achtman M. Clonal population structure of Neisseria meningitidis serogroup A isolated from epidemics and pandemics between 1915 and 1983. Rev Infect Dis. 1987;9:665–692. doi: 10.1093/clinids/9.4.665. [DOI] [PubMed] [Google Scholar]
- 12.Park Y-H, Kohel R J. Effect of concentration of MgCl2 on random-amplified DNA polymorphism. BioTechniques. 1994;16:652–655. [PubMed] [Google Scholar]
- 13.Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983;5:71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Suker J, Feavers I M, Achtman M, Morelli G, Wang J-F, Maiden M C J. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol. 1994;12:253–265. doi: 10.1111/j.1365-2958.1994.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 15.Swartley J S, Marfin A A, Edupuganti S, Liu L J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J-F, Caugant D A, Li X, Hu X, Poolman J T, Crowe B A, Achtman M. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People’s Republic of China. Infect Immun. 1992;60:5267–5282. doi: 10.1128/iai.60.12.5267-5282.1992. . (Erratum, 62:5706, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams J G K, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods J P, Kersulyte D, Tolan R W, Jr, Berg C M, Berg D E. Use of arbitrarily primed polymerase chain reaction analysis to type disease and carrier strains of Neisseria meningitidis isolated during a university outbreak. J Infect Dis. 1994;169:1384–1389. doi: 10.1093/infdis/169.6.1384. [DOI] [PubMed] [Google Scholar]