Abstract
The transmissible spongiform encephalopathies are a heterogeneous group of fatal neurodegenerative disorders occurring in humans, mink, cats, and ruminant herbivores. The occurrence of novel transmissible spongiform encephalopathies in cattle in the United Kingdom and Europe and in mule deer and elk in parts of the United States has emphasized the need for reliable diagnostic tests with standardized reagents. Postmortem diagnosis is performed by histologic examination of brain sections from affected animals. The histopathological criteria for transmissible spongiform encephalopathies include gliosis, astrocytosis, neuronal degeneration, and spongiform change. These lesions vary in intensity and anatomic location depending on the host species and genetics, stage of disease, and infectious agent source. Diagnosis by histopathology alone may be ambiguous in hosts with early cases of disease and impossible if the tissue is autolyzed. Deposition of the prion protein (an abnormal isoform of a native cellular sialoglycoprotein) in the central nervous system is a reliable marker for infection, and immunohistochemical detection of this marker is a useful adjunct to histopathology. In the present paper we describe monoclonal antibody (MAb) F89/160.1.5, which reacts with prion protein in tissues from sheep, cattle, mule deer, and elk with naturally occurring transmissible spongiform encephalopathies. This MAb recognizes a conserved epitope on the prion protein in formalin-fixed, paraffin-embedded sections after hydrated autoclaving. MAb F89/160.1.5 will be useful in diagnostic and pathogenesis studies of the transmissible spongiform encephalopathies in these ruminant species.
The transmissible spongiform encephalopathies (TSEs) are a heterogeneous group of fatal neurodegenerative disorders characterized by deposition of an abnormal isoform (prion protein Sc [PrP-Sc]) of a normal cellular glycoprotein (PrP-C) in neural tissue. PrP-Sc, either alone or in association with another protein, may represent a novel transmissible agent, the prion (28), which propagates by catalyzing the conversion of PrP-C to PrP-Sc through a nucleation or polymerization event (9, 14). Data in support of this “protein-only” hypothesis are based largely on rodent models of the ovine TSE, scrapie, in which PrP-Sc is the major component of infectious tissue extracts (3). PrP-C and PrP-Sc are derived from the same single-copy host gene (25) but differ in their physicochemical qualities including solubility in detergent and relative resistance to digestion by proteinase K (PK) (22). PK hydrolysis removes only the 60 to 70 residues at the amino terminus of the protein, leaving two or three fragments representing the unglycosylated peptide and one or more differentially glycosylated forms migrating between 19 and 28 kDa. Expression of PrP-C by host genes, by transgenes, or in engrafted tissue is required for the development of clinical disease, PrP-Sc propagation, and brain lesions (2, 4, 5, 7). Conversion of PrP-C to PrP-Sc has been demonstrated in a cell-free system (19) and by direct contact of recombinant PrP-C with PrP-Sc in frozen brain slices (1). The mechanisms of neurotoxicity in the TSEs have not yet been delineated. Morphologic and functional changes have been reported in neurons, microglia, and astrocytes in vivo and in vitro in response to infection or exposure to neurotoxic peptide fragments of PrP (6, 11, 29, 30).
TSEs occur naturally in humans, mink, cats, and ruminant herbivores. Sheep scrapie is endemic in many parts of the world, and control efforts have been hampered by the long incubation time and a lack of tools for early diagnosis. Bovine spongiform encephalopathy (BSE), a novel TSE of cattle and exotic ruminants (34), poses a more serious threat because of its proposed causative relationship with a new variant of human Creutzfeldt-Jakob disease (8). Chronic wasting disease (CWD) is a relatively rare disorder reported in mule deer, white tail deer, and elk originating from a small area of the western United States (39). Diagnosis of ruminant TSEs is based on the appearance of neuronal vacuolation, spongiform changes, gliosis, and astrocytosis (15, 35, 37, 39) in neural tissue collected postmortem. The histological lesion profiles vary in intensity and anatomic location among species and individuals (13, 15); diagnosis by histopathology alone may be equivocal for hosts with early cases of disease or autolyzed tissue (24). Detection of PrP-Sc by immunoassay of fixed tissue is a useful confirmatory assay (10, 12, 23, 24, 33). With one exception (20), monoclonal antibodies (MAbs) and polyclonal antibodies recognize PrP-C as well as PrP-Sc and immunodetection protocols must include a process for the selective elimination of the reactivity of PrP-C. PrP-C, which is sensitive to formalin fixation and routine tissue processing procedures (21), is usually not detectable in formalin-fixed tissue; epitopes on the PrP-Sc in these samples are unmasked by heat, acid, or enzyme pretreatment (12, 16, 18). The efficacies of the fixation and pretreatment protocols in which PrP-C reactivity is eliminated and PrP-Sc staining is enhanced are monitored by staining tissues from TSE-affected and healthy animals in parallel. Under these conditions, immunohistochemical analysis with validated reagents will provide useful diagnostic tests. Rabbit antisera reactive with ruminant PrP-Sc cannot be standardized for widespread use due to limitations in its quantity and specificity. In this paper, we report the use of MAb F89/160.1.5 in the immunohistochemical analysis of formalin-fixed central nervous system samples from cattle, sheep, mule deer, and elk with naturally occurring TSEs.
MATERIALS AND METHODS
Antigen preparation and MAb production.
A synthetic peptide, representing residues 146 to 159 of the bovine prion protein (17) (NH2-SRPLIHFGSDYEDR-COOH), was coupled to maleimide-activated keyhole limpet hemocyanin (Pierce Chemical Company). Five 6-week-old BALB/c mice were each inoculated subcutaneously at two sites with a total of 10 μg of conjugated peptide emulsified in 200 μl of Freund’s complete adjuvant. Two booster inoculations of 10 μg of conjugated peptide in 200 μl of Freund’s incomplete adjuvant were administered at 14-day intervals. Three days before cell fusion, the mice were immunized intravenously with 10 μg of conjugated peptide in phosphate-buffered saline without adjuvant. Cell fusion and cloning by limiting dilution were performed by following standard protocols (41). Supernatants from primary and cloned hybridomas were screened by a recombinant ovine PrP-C enzyme-linked immunosorbent assay (ELISA). Clone 1.5 from cell line F89/160 was selected and was transferred to an artificial capillary cell culture system (CellMax; CellCo Inc.) for the in vitro production of an MAb supernatant. The heavy-chain isotype was identified by ELISA, and the MAb concentration was determined by immunodiffusion.
Recombinant sheep PrP-C ELISA.
Supernatants from primary and cloned hybridomas were screened by ELISA with recombinant sheep PrP-C as the antigen.
(i) Production of recombinant sheep PrP-C in Escherichia coli.
Genomic DNA was isolated from the peripheral blood mononuclear cells of a Suffolk sheep. The PrP open reading frame was amplified with flanking primers (38) modified to incorporate EcoRI restriction sites (forward primer, 5′-ATCGAATTCAAGAAGCGACCAAAAC-3′; reverse primer, 5′-ATCGAATTCAGACACCACCACT-3′). The 786-bp PCR product was digested with EcoRI, purified on agarose gels, and ligated into the vector pMal-cRI. Transformation of E. coli DH5 was performed by conventional techniques. Transformants were screened by PCR of colony minipreps with the cloning primers. One positive clone (pMal-1) was selected for large-scale fusion protein expression. The fusion product ShPrP–maltose-binding protein (MBP) was isolated from bacterial lysates by affinity chromatography on amylose resin columns and was eluted with 10 mM maltose. Fractions were screened by Western immunoblotting with a rabbit antiserum to PrP peptide NH2GQGGGTHNQWNKPSK (R2843) (26).
(ii) Recombinant ShPrP ELISA.
Each well of Immulon 2 plates (Dynatech, Chantilly, Va.) was coated with 6.25 μg of the recombinant ShPrP-MBP fusion protein in 50 μl of 0.05 M carbonate buffer (pH 9.6), and the plates were incubated overnight at 4°C. The plates were blocked with a 1:15 dilution of commercially available milk-based blocker (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 1 h. Fifty microliters of antiserum or hybridoma supernatant was incubated in each well for 30 min at room temperature. The plates were developed with goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRPO) and 2,2′-azino-di[3-ethyl-benzthiazoline sulfonate (6)] (ABTS; Kirkegaard & Perry Laboratories). The optical density was read at 405 nm. Negative controls included supernatants from isotype-matched MAbs of irrelevant specificity or tissue culture medium adjusted to contain 15% fetal calf serum. Positive control wells were incubated with rabbit anti-PrP peptide antiserum (R2843) and were developed with goat anti-rabbit IgG-HRPO and ABTS. Positive wells had optical densities at 405 nm higher than 2 standard deviations above the mean for four negative control wells.
Source of brain tissue from ruminant herbivores with naturally occurring TSEs and from control herbivores.
Brain tissues from 34 sheep with histopathological lesions of scrapie were tested for reactivity with MAb F89/160.1.5 by immunohistochemical analysis. PrP-Sc had been detected immunohistochemically with a rabbit anti-mouse PrP polyclonal antiserum in 20 of these samples and by Western immunoblotting in 6 of the 20 samples (23). Tissues from 3 sheep with no histological lesions of scrapie and no PrP-Sc detectable by Western blot analysis were used as negative controls, as were tissues from an additional 12 sheep with no clinical signs of scrapie and no histological lesions. These tissues were provided by pathologists in veterinary medical colleges and state diagnostic laboratories or by personnel from the Animal and Plant Health Inspection Service, U.S. Department of Agriculture, Ames, Iowa.
Unstained brain sections from 19 cattle with BSE and 5 BSE-negative cattle were provided by the Pathobiology Laboratory, National Veterinary Services Laboratories, Animal and Plant Health Inspection Service, U.S. Department of Agriculture. The source of paraffin blocks for these sections was Gerald Wells, Ministry of Agriculture, Fisheries and Food, Central Veterinary Laboratory, New Haw, Surrey, United Kingdom. In addition, brain sections from 15 U.S.-born cattle raised at the National Animal Disease Center, Ames, Iowa, were examined as negative control tissues.
Brain samples from 10 mule deer (Odocoileus hemionus hemionus) and 4 elk (Cervus elaphus nelsoni) with naturally occurring CWD and from 15 mule deer and 12 elk with no clinical or histological evidence of CWD were provided by the Colorado State Diagnostic Laboratory and the Colorado Division of Wildlife.
All TSE-affected animals had neuropil spongiosis, intraneuronal vacuoles, and gliosis within selected brain stem and midbrain nuclei, lesions diagnostic of TSE (15, 36, 40). The myelencephalon (brain stem) at the level of the obex of all TSE-affected and normal control animals was examined by immunohistochemical analysis. Other areas examined in some TSE-affected and healthy control animals included the telencephalon (cerebral cortex), diencephalon (rostral midbrain) at the level of the thalamus, mesencephalon (caudal midbrain) at the level of the rostral colliculus, and metencephalon (rostral brainstem) at the level of the middle cerebellar peduncle.
Western immunoblot analysis.
PrP-Sc was isolated from the brains of sheep by differential centrifugation from a high-salt Sarkosyl buffer (31). Precipitated proteins were digested with 10 μg of PK per ml for 1 h at 37°C and were analyzed in aliquots equivalent to 125 mg of starting material on 15% polyacrylamide minigels (Bio-Rad), followed by transfer to polyvinylidene difluoride membranes (Schleicher & Schuell). The filters were developed with 3 μg of MAb F89/160.1.5 per ml or a control antibody of the same isotype, goat anti-mouse IgG–HRPO, and a chemiluminescent substrate (Amersham). Filters were exposed to film (Amersham HyperFilm) for 8 to 20 min with no increase in background chemiluminescence.
Immunohistochemistry.
Brains were fixed in 10% buffered formalin by immersion and were embedded in paraffin. One section from each block was stained with hematoxylin and eosin for routine histopathology. Additional tissue sections were mounted on positively charged glass slides (Probe-On Plus; Fisher Scientific) for immunohistochemical analysis. Sections for immunohistochemical analysis were deparaffinized and hydrated and then autoclaved in distilled water at 121°C for 30 min (16) and allowed to cool. The slides were immunostained, using capillary flow technology in an automated immunostainer (Code-On Slide Stainer; Fisher Scientific) as described previously (17, 18), with a biotinylated second antibody, streptavidin-alkaline phosphate complex (Biomeda Corp), and an alkaline phosphatase substrate-chromagen (Vector Red; Vector Laboratories). Additional sections of selected ovine samples were immunostained as described above except that bound primary antibody was detected with biotinylated horse anti-mouse IgG second antibody, avidin-biotin-HRPO complex (ABC-peroxidase; Vector Laboratories), and a peroxidase substrate-chromagen (AEC; Dako Corp). All slides were counterstained with Mayer’s hematoxylin. Negative control procedures consisted of (i) substitution of MAb F89/160.1.5 with a similar concentration of an irrelevant control MAb of the same isotype and (ii) incubation of MAb F89/160.1.5 with brain tissue from scrapie-free sheep, cattle, or mule deer with no evidence of TSE as indicated by histopathology of samples from all three species and Western immunoblot analysis of ovine tissues.
Epitope mapping and PrP gene sequences.
An overlapping set of octamer peptides spanning SRPLIHFGSDYEDR was synthesized on a membrane support with commercial reagents and by following the instructions of the manufacturer (SPOTs Test; Genosys Biotechnologies, The Woodlands, Tex.). The ability of MAb F89/160.1.5 to bind to individual octamer peptides was determined visually following incubation with β-galactosidase-conjugated secondary antibody and substrate.
Conservation of the amino acid sequence bound by MAb F89/160.1.5 was demonstrated by direct DNA sequencing of PCR-amplified genomic DNA from some of the TSE-affected ruminants examined by immunohistochemical analysis (12 scrapie-affected sheep and 10 mule deer and 2 elk with CWD). The Colorado Division of Wildlife provided additional samples from healthy mule deer and elk. The open reading frame of the PrP gene from sheep was amplified by PCR as described above, and both strands of the polymorphic region from codons 112 to 240 were sequenced by automated fluorescent dye-labelled dideoxy strand termination (27). Mule deer and elk genomic DNAs were amplified with the cervid-specific primer pair 5′-CTGCAAGAAGCGACCAAAACC (forward primer) and 5′-CACAGGAGGGGAGGAGAAGAGGAT (reverse primer) under standard conditions, except that the Mg2+ concentration was increased to 2.5 mM. Both strands of the PCR products were sequenced with forward primer 5′-GGCTATCCACCTCAGGGAG and reverse primer 5′-TCACACTTGCCCCCTCTTTGGT, which typically yielded sequence information on codons 106 to 224.
Mule deer and elk PrP gene sequences.
Three alleles of the mule deer PrP sequence were identified. Alleles 138S2 and 138N1 encode Ser and Asn at codon 138, respectively. Allele 138S1 differs from allele 138S2 by a silent mutation. Two alleles of the elk PrP gene were found and encode an M→L substitution at codon 132.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers of alleles 138S2, 138N1, and 138S1 and the two alleles of the elk PrP gene are AF009180, U97331, AF009181, AF016227, and AF016228, respectively.
RESULTS
Production of MAbs.
Five mice were immunized with a keyhole limpet hemocyanin-conjugated synthetic peptide previously demonstrated to generate polyclonal antisera reactive with bovine and ovine PrP-C proteins in Western immunoblots (17). Antisera and hybridoma supernatants were screened by ELISA with a recombinant sheep PrP fusion protein as the antigen. Cell line 160 produced antibodies reactive in the ELISA and was selected for two rounds of cloning by limiting dilution and propagated in an in vitro artificial capillary cell culture production system. Pooled supernatants from cell line F89/160.1.5 (heavy-chain isotype immunoglobulin G1) had a concentration of 3.64 mg/ml. The MAb from this pool was further characterized by epitope mapping, Western immunoblot analysis, and immunohistochemical analysis.
Epitope mapping and sequence determination.
The epitope recognized by MAb F89/160.1.5 was mapped with a panel of overlapping peptides (Table 1) immobilized on a derivatized cellulose membrane. Sequential deletion of amino-terminal residues S, R, P, and L (peptides 2, 3, 4, and 5, respectively) did not eliminate antibody binding. Peptide 6, lacking the I residue, and peptides 7 and 8, lacking IH and IHF, respectively, failed to bind to MAb F89/160.1.5. Therefore, only the sequence IHFG is common to all peptides bound by the MAb. This sequence is conserved in the deduced amino acid sequences reported to date for cattle, sheep, mule deer, and elk PrP in the samples from the present study for which frozen tissue was available (tissue from 12 sheep, 10 mule deer, and 2 elk) and for a larger sample of CWD-affected mule deer (n = 26). Samples with amino acid polymorphisms outside the antibody binding site (ovine codon 112, A to V; mule deer codon 138, N to S) showed no difference in the intensity or distribution of immunostaining with MAb F89/160.1.5.
TABLE 1.
Reactivity of MAb F89/160.1.5 with overlapping peptide octamers of the immunogen SRPLIHFGSDYEDR
| Peptide no. | Octameric peptide sequence | Reactivity with MAb F89/160.1.5 |
|---|---|---|
| 1 | SRPLIHFG | + |
| 2 | RPLIHFGS | + |
| 3 | PLIHFGSD | + |
| 4 | LIHFGSDY | + |
| 5 | IHFGSDYE | + |
| 6 | HFGSDYED | − |
| 7 | FGSDYEDR | − |
Western immunoblotting reactivity with PrP-Sc from TSE-affected sheep.
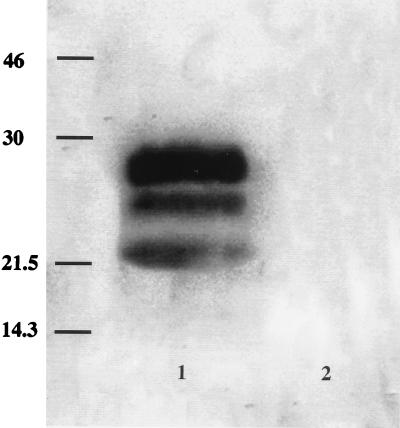
MAb F89/160.1.5 was generated by inoculation into mice with a synthetic peptide and was selected by screening the MAb against a recombinant form of PrP-C. To determine whether MAb F89/160.1.5 binds to the disease-specific, protease-resistant fragments (PrP-Sc), brain extracts from sheep with natural scrapie and from healthy sheep were treated with PK, which hydrolyzes PrP-C and which leaves the hallmark multiple peptide bands of PK-resistant PrP-Sc (22). PK-treated extracts from scrapie-affected sheep typically showed two or three peptide bands with apparent molecular weights of between 19,000 and 28,000 (Fig. 1, lane 1). No bands were detected in extracts from healthy sheep brain (Fig. 1, lane 2) or when an isotype-matched control MAb was used to probe the Western immunoblots (data not shown).
FIG. 1.
Western immunoblot, stained with MAb F89/160.1.5, of PK-treated preparation from brain of a sheep with scrapie, showing PK-resistant glycopeptides characteristic of PrP-Sc (lane 1). No PrP-Sc was demonstrated in brain collected from a sheep with no known exposure to scrapie (lane 2) processed under identical conditions. Bars in the left lane correspond to molecular size markers (in kilodaltons).
Immunohistochemical analysis of tissues from healthy and TSE-affected ruminants.
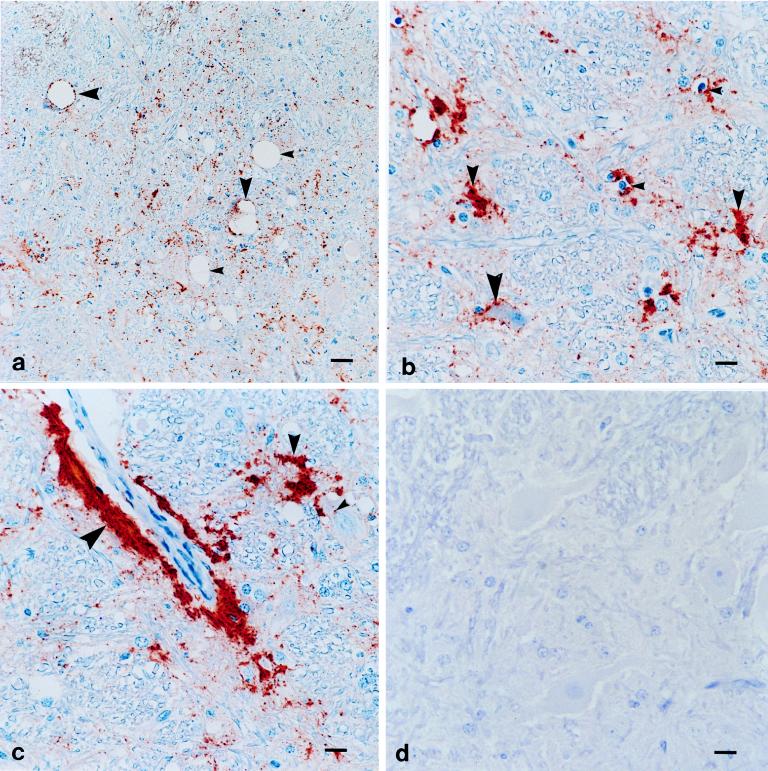
Positive immunostaining by MAb F89/160.1.5 immunohistochemical analysis was detected for the brains of all TSE-affected animals examined (36 sheep, 19 cattle, 10 mule deer, and 4 elk); no immunostaining was detected for the brains of any healthy control animals (15 sheep, 15 mule deer, 12 elk, and 20 cattle). Examples of positive and negative immunostaining are shown in Fig. 2a to d. The pattern of immunoreactivity for TSE-affected animals was basically similar for all animals. PrP-Sc immunoreactivity was present in the brain stem and midbrain from the level of the hypothalamus rostrally to the obex caudally. Immunostaining was concentrated within specific neurologic nuclei. At low magnification, most immunostaining consisted of random dense granules, globules, and plaques within the gray matter neuropil admixed with spongiform lesions (Fig. 2a). The great majority of PrP-Sc immunoreactivity aggregated adjacent to or surrounding glial cell nuclei (Fig. 2b) and sometimes accumulated in a branching pattern around glial cells identified histologically as microglia (small, oval to angular hyperchromatic nuclei without a recognizable cytoplasm) (Fig. 2b) (32). There also was rim-like perivascular and subependymal immunostaining reminiscent of astroglial foot processes (Fig. 2c). The PrP-Sc immunoreactivities of neurons consisted of punctate immunostaining within neuronal perikarya (Fig. 2c) or distinct rimming around the periphery of neuronal perikarya (Fig. 2b) or around the peripheral membranes of intraneuronal vacuoles (Fig. 2a). Both neurons with and without intraneuronal vacuoles had PrP-Sc immunoreactivity.
FIG. 2.
MAb F89/160.1.5 immunohistochemical analysis assay of the brain stem of a scrapie-affected sheep (a to c) and negative control scrapie-free sheep (d). (a) PrP-Sc antigen accumulation (red) within a brain stem nucleus with spongiform lesions consisting of neuropil spongiosis (small arrowheads) and intraneuronal vacuoles (large arrowheads). Immunoreactivity comprising granular and globular foci randomly within the neuropil or around the periphery of intraneuronal vacuoles (large arrowheads) is shown. ABC immunoperoxidase counterstained with Mayer’s hematoxylin was used. Bar, 60 μm. (b) PrP-Sc antigen accumulation (red) within a brain stem nucleus. Immunoreactivity comprising linear rimming around neurons (large arrowhead), plaques in the neuropil (medium arrowheads), and aggregations around glial cells with small hyperchromatic nuclei consistent with microglia (small arrowheads) is shown. ABC immunoperoxidase counterstained with Mayer’s hematoxylin was used. Bar, 15 μm. (c) PrP-Sc antigen accumulation (red) within a brain stem nucleus. Immunoreactivity comprising linear rimming around blood vessels (large arrowheads), plaques in the neuropil (medium arrowhead), and punctate granules within soma of neurons without intraneuronal vacuoles (small arrowhead) is shown. ABC immunoperoxidase counterstained with Mayer’s hematoxylin was used. Bar, 15 μm. (d) No PrP-Sc antigen accumulation within an anatomically matched brain stem nucleus of a scrapie-free sheep (negative control tissue). Similar results were obtained with brain tissue from a scrapie-affected sheep immunostained with irrelevant isotype-matched MAb. ABC immunoperoxidase counterstained with Mayer’s hematoxylin was used. Bar, 15 μm.
Sections from comparable regions of TSE-affected animals and healthy control animals were prepared and immunostained in parallel. Negative control tissues consisted of brain tissue from animals with no histologic evidence of TSE. These negative samples showed no reactivity when they were immunostained with MAb F89/160.1.5 (Fig. 2d). In addition, brain sections from 34 scrapie-affected sheep and 19 BSE-positive cattle incubated with an isotype control MAb rather than MAb F89/160.1.5 failed to show immunostaining. No antibody binding was observed in formalin-fixed tissues from scrapie-affected sheep immunostained with MAb F89/160.1.5 without pretreatment by hydrated autoclaving.
DISCUSSION
PrP-Sc is a marker protein for TSEs, and detection of PrP-Sc by immunohistochemical analysis is a useful adjunct to histopathology for the diagnosis of these diseases in ruminant animals. MAbs reactive with conserved epitopes on ovine, bovine, and cervid PrP-Sc proteins will be useful reagents for standardized diagnostic testing and comparative pathology studies. We immunized mice with a peptide representing an immunogenic region of the bovine PrP protein and screened the resulting MAbs for their reactivities to recombinant sheep PrP-C proteins to select cross-reacting antibodies. One of these MAbs, MAb F89/160.1.5, was shown to react with PrP-Sc by Western immunoblotting of PK-digested preparations from the brains of sheep with natural scrapie. The MAb was also shown to be reactive with PrP-Sc in formalin-fixed tissues from sheep, cattle, mule deer, and elk under tissue fixation and pretreatment conditions which eliminated the reactivity of PrP-C. The immunohistochemical staining pattern of MAb F89/160.1.5 was similar to the patterns described for the brains of scrapie-affected sheep and obtained with polyclonal rabbit antisera to ovine or mouse PrP (23, 24). The present data also demonstrate the utility of MAb F89/160.1.5 in the diagnosis of TSE from brain tissues of cattle and wild ruminants. We are characterizing the accumulation of PrP-Sc in extraneural tissues using MAb F89/160.1.5 and other MAbs to ovine PrP to determine the utility of antigen-based assays for antemortem and preclinical diagnosis of scrapie, CWD, and BSE. MAb reagents to conserved epitopes on PrP-Sc or cocktails of MAbs to multiple variable epitopes provide specific, reliable, and flexible tools for the accurate diagnosis of TSE in mammals.
ACKNOWLEDGMENTS
This work was supported by the Agricultural Research Service, U.S. Department of Agriculture (grant CWU 5348-32000-011-00D).
We acknowledge the technical assistance of L. Mickelsen, W. Harwood, L. Kappmeyer, P. Dilbeck, T. McReynolds, D. Bradley, D. Orcutt, and J. Bulgin. E. Williams, M. Miller, and M. Wild provided blood and tissue samples from healthy and CWD-affected deer and elk. We thank A. Jenny, National Veterinary Services Laboratory, Animal and Plant Health Inspection Service, U.S. Department of Agriculture, for identifying and providing tissues from scrapie-affected sheep.
REFERENCES
- 1.Bessen R A, Raymond G J, Caughey B. In situ formation of protease-resistant prion protein in transmissible spongiform encephalopathy-infected brain slices. J Biol Chem. 1997;272:15227–15231. doi: 10.1074/jbc.272.24.15227. [DOI] [PubMed] [Google Scholar]
- 2.Blattler T, Brandner S, Raeber A J, Klein M A, Voigtlander T, Weissmann C, Aguzzi A. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 3.Bolton D C, McKinley M P, Prusiner S B. Identification of a protein that purifies with the scrapie protein. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 4.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissman C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 5.Brandner S, Raeber A, Sailer A, Blättler T, Fischer M, Weissmann C, Aguzzi A. Normal host prion protein (PrPc) is required for scrapie spread within the central nervous system. Proc Natl Acad Sci USA. 1996;93:13148–13151. doi: 10.1073/pnas.93.23.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D R, Schmidt B, Kretzschmar H A. A neurotoxic prion protein fragment enhances proliferation of microglia but not astrocytes in culture. Glia. 1996;18:59–67. doi: 10.1002/(SICI)1098-1136(199609)18:1<59::AID-GLIA6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Bueler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M, Weissman C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 8.Collinge J, Rossor M. A new variant of prion disease. Lancet. 1996;347:916–917. doi: 10.1016/s0140-6736(96)91407-5. [DOI] [PubMed] [Google Scholar]
- 9.Come J H, Fraser P E, Lansbury P T. A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci USA. 1993;90:5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farquhar C F, Somerville R A, Ritchie L A. Post-mortem immunodiagnosis of scrapie and bovine spongiform encephalopathy. J Virol Methods. 1989;24:215–222. doi: 10.1016/0166-0934(89)90023-2. [DOI] [PubMed] [Google Scholar]
- 11.Forloni G, Del Bo R, Angeretti N, Chiesa R, Smiroldo S, Doni R, Ghibaudi E, Salmona M, Porro M, Verga L, et al. A neurotoxic prion protein fragment induces rat astroglial proliferation and hypertrophy. Eur J Neurosci. 1994;6:1415–1422. doi: 10.1111/j.1460-9568.1994.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 12.Foster J D, Wilson M, Hunter N. Immunolocalisation of the prion protein (PrP) in the brains of sheep with scrapie. Vet Rec. 1996;139:512–515. doi: 10.1136/vr.139.21.512. [DOI] [PubMed] [Google Scholar]
- 13.Fraser H. Diversity in the neuropathology of scrapie-like diseases in animals. Br Med Bull. 1993;49:792–809. doi: 10.1093/oxfordjournals.bmb.a072647. [DOI] [PubMed] [Google Scholar]
- 14.Gajdusek D C. Genetic control of nucleation and polymerization of host precursors to infectious amyloids in the transmissible amyloidoses of brain. Br Med Bull. 1993;49:913–931. doi: 10.1093/oxfordjournals.bmb.a072653. [DOI] [PubMed] [Google Scholar]
- 15.Hadlow W J. Differing neurohistologic images of scrapie, transmissible mink encephalopathy, and chronic wasting disease of mule deer and elk. In: Gibbs C J Jr, editor. Bovine spongiform encephalopathy. The BSE dilemma. New York, N.Y: Springer; 1996. pp. 122–137. [Google Scholar]
- 16.Haritani M, Spencer Y I, Wells G A H. Hydrated autoclave pretreatment enhancement of prion protein immunoreactivity in formalin-fixed bovine spongiform encephalopathy-affected brain. Acta Neuropathol. 1992;87:86–90. doi: 10.1007/BF00386258. [DOI] [PubMed] [Google Scholar]
- 17.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of the prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol. 1995;76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 18.Kitamoto T, Ogomori K, Tateishi J, Prusiner S B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987;57:230–236. [PubMed] [Google Scholar]
- 19.Kocisko D A, Come J H, Priola S A, Chesebro B, Raymond G J, Lansbury P T, Caughey B. Cell-free formation of protease-resistant prion protein. Nature. 1994;370:471–474. doi: 10.1038/370471a0. [DOI] [PubMed] [Google Scholar]
- 20.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrP-Sc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 21.McBride P A, Eickelenboom P, Kraal G, Fraser H, Bruce M E. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J Pathol. 1992;168:413–418. doi: 10.1002/path.1711680412. [DOI] [PubMed] [Google Scholar]
- 22.McKinley M P, Bolton D C, Prusiner S B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- 23.Miller J M, Jenny A L, Taylor W D, Marsh R F, Rubenstein R, Race R E. Immunohistochemical detection of prion protein in sheep with scrapie. J Vet Diagn Invest. 1993;5:309–316. doi: 10.1177/104063879300500301. [DOI] [PubMed] [Google Scholar]
- 24.Miller J M, Jenny A L, Taylor W D, Race R E, Ernst D R, Katz J B, Rubenstein R. Detection of prion protein in formalin-fixed brain by hydrated autoclaving immunohistochemistry for the diagnosis of scrapie in sheep. J Vet Diagn Invest. 1994;6:366–368. doi: 10.1177/104063879400600315. [DOI] [PubMed] [Google Scholar]
- 25.Oesch B, Westaway D, Walchi M, McKinley M P, Kent S B H, Aebersold R, Barry R A, Tempst P, Teplow D B, Hood L E, Prusiner S B, Weissmann C. A cellular gene encodes scrapie PrP27-30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 26.O’Rourke K I, Huff T P, Leathers C W, Robinson M M, Gorham J R. SCID mouse spleen does not support scrapie agent replication. J Gen Virol. 1994;75:1511–1514. doi: 10.1099/0022-1317-75-6-1511. [DOI] [PubMed] [Google Scholar]
- 27.O’Rourke K I, Melco R P, Mickelson J R. Allelic frequencies of an ovine scrapie susceptibility gene. Anim Biotechnol. 1996;7:155–162. [Google Scholar]
- 28.Prusiner S B. Human prion diseases and neurodegeneration. Curr Top Microbiol Immunol. 1996;207:1–17. doi: 10.1007/978-3-642-60983-1_1. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein R, Hui D, Race R, Ju W, Scalici C, Papini M, Kascsak R, Carp R. Replication of scrapie strains in vitro and their influence on neuronal functions. Ann N Y Acad Sci. 1994;724:331–337. doi: 10.1111/j.1749-6632.1994.tb38924.x. [DOI] [PubMed] [Google Scholar]
- 30.Selvaggini C, De Gioia L, Cantù L, Ghibaudi E, Diomede L, Passerini F, Forloni G, Bugiani O, Tagliavini F, Salmona M. Molecular characteristics of a protease-resistant, amyloidogenic and neurotoxic peptide homologous to residues 106–126 of the prion protein. Biochem Biophys Res Commun. 1993;194:1380–1386. doi: 10.1006/bbrc.1993.1977. [DOI] [PubMed] [Google Scholar]
- 31.Stack M J, Keyes P, Scott A C. The diagnosis of bovine spongiform encephalopathy and scrapie by the detection of fibrils and the abnormal protein isoform. In: Baker H F, Ridley R M, editors. Prion diseases. Totowa, N.J: Humana Press; 1996. pp. 85–103. [Google Scholar]
- 32.Summers B A, Cummings J F, de Lahunta A. Principles of neuropathology. In: Summers B A, Cummings J F, de Lahunta A, editors. In Veterinary neuropathology. St. Louis, Mo: Mosby-Year Book Inc.; 1995. [Google Scholar]
- 33.van Keulen L J M, Schreuder B E C, Meloen R H, Poelen-van den Berg M, Mooij-Harkes G, Vromans M E W, Langveld J P M. Immunohistochemical detection and localization of prion protein in brain tissue of sheep with natural scrapie. Vet Pathol. 1995;32:299–308. doi: 10.1177/030098589503200312. [DOI] [PubMed] [Google Scholar]
- 34.Wells G A H, Scott A C, Johnson C T, Gunning R D, Hancock R D, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987;121:419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- 35.Wells G A H, McGill I S. Recently described scrapie-like encephalopathies of animals: case definitions. Res Vet Sci. 1992;53:1–10. doi: 10.1016/0034-5288(92)90076-e. [DOI] [PubMed] [Google Scholar]
- 36.Wells G A H, Spencer Y I, Haritani M. Configurations and topographic distribution of PrP in the central nervous system in bovine spongiform encephalopathy: an immunohistochemical study. Ann N Y Acad Sci. 1994;724:350–352. doi: 10.1111/j.1749-6632.1994.tb38928.x. [DOI] [PubMed] [Google Scholar]
- 37.Wells G A H, Simmons M M. The essential lesion profile of bovine spongiform encephalopathy (BSE) in cattle is unaffected by breed or route of infection. Neuropathol Appl Neurobiol. 1996;22:453. [Google Scholar]
- 38.Westaway D, Zuliani V, Cooper C M, Da Costa M, Neuman S, Jenny A L, Detwiler L, Prusiner S B. Homozygosity for prion protein alleles encoding glutamine-171 renders sheep susceptible to natural scrapie. Genes Dev. 1994;8:959–969. doi: 10.1101/gad.8.8.959. [DOI] [PubMed] [Google Scholar]
- 39.Williams E S, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 40.Wood J L N, McGill I S, Done S H, Bradley R. Neuropathology of scrapie: a study of the distribution patterns of brain lesions in 222 cases of natural scrapie in sheep, 1982–1991. Vet Rec. 1997;140:167–174. doi: 10.1136/vr.140.7.167. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama W M. Production of monoclonal antibodies. In: Coligan J E, editor. Current protocols in immunology. New York, N.Y: Wiley Intersciences; 1994. pp. 2.2.1–2.5.17. [Google Scholar]