Abstract
The endoplasmic reticulum (ER) extends throughout neurons and regulates many neuronal functions, including neurite outgrowth, neurotransmission, and synaptic plasticity. Mutations in proteins that control ER shape are linked to the neurodegenerative disorder Hereditary Spastic Paraplegia (HSP), yet the ultrastructure and dynamics of neuronal ER remain largely unexplored, especially at presynaptic terminals. Using super-resolution and live imaging in D. melanogaster larval motor neurons, we investigated ER structure at presynaptic terminals of wild-type animals and null mutants of the ER shaping protein and HSP-linked gene, Atlastin. Previous studies using an ER luminal marker reported diffuse localization at Atlastin mutant presynaptic terminals, which was attributed to ER fragmentation. However, using an ER membrane marker, we discovered that Atlastin mutant ER forms robust networks with only mild defects in structural dynamics, indicating the primary defect is functional rather than architectural. We demonstrate that Atlastin mutants progressively displace overexpressed luminal ER proteins to the cytosol during larval development, specifically at synapses, while these proteins remain correctly localized in cell bodies, axons, and muscles. This synaptic-specific displacement phenotype, previously unreported in non-neuronal cells, emphasizes the importance of studying neurons to understand HSP pathogenesis.
Introduction:
The endoplasmic reticulum (ER) is a continuous organelle that extends to the periphery of neurons and regulates many neuronal functions, including neurite outgrowth, neurotransmission, and synaptic plasticity (de Juan-Sanz et al., 2017; Liu et al., 2019; Oliva et al., 2020; Perez-Alvarez et al., 2020; Raffaello et al., 2016; Raiborg et al., 2015). Structurally, this essential organelle consists of two domains: ER sheets (enriched in cell bodies) and ER tubules (abundant in dendrites and axons) (Öztürk et al., 2020; Wu et al., 2017). Recent studies have revealed even finer levels of organization, with increasing evidence to suggest further structural and functional differences in neuronal ER tubules. For instance, different regions of the neuron show distinct features, including varying densities of ER-organelle contacts (Wu et al., 2017), specialized ER-endosome contacts that regulate lysosome size in cell bodies (Özkan et al., 2021), and unique ER ladder structures in developing axons and dendrites (Benedetti et al., 2024; Zamponi et al., 2022). These regional specializations underscore the importance of examining ER structure and function across all neuronal regions. However, the cell biology and ultrastructure of the neuronal ER, particularly at presynaptic terminals, have been under-investigated. Conventional microscopy, used in most studies of neuronal ER structure, lacks the resolution needed to visualize individual ER tubules in small structures like presynaptic terminals (Chouhan et al., 2010; Oliva et al., 2020; Orso et al., 2009; Pérez-Moreno et al., 2023; Summerville et al., 2016; Yalçın et al., 2017). Other techniques such as electron microscopy (EM) have typically been limited to thin 2D sampling and cannot be used in live samples. These limitations make it difficult to distinguish the ER from synaptic vesicles at synapses and prevent the examination of live dynamics (Orso et al., 2009; Wu et al., 2017; Zhu et al., 2022).
We must overcome the technical challenges of studying neuronal ER as mutations in ER-shaping genes cause neurodegenerative disorders such as Hereditary Spastic Paraplegia (HSP) and Charcot Marie Tooth Disease, indicating that ER structure is uniquely essential for neuronal physiology (Boutry et al., 2019; Fowler et al., 2019; Öztürk et al., 2020). One essential ER tubule-shaping protein is Atlastin, a GTPase that regulates the homotypic fusion and tethering of ER tubules to form 3-way junctions (Lü et al., 2020). In humans, mutations in Atlastin underlie one of the most common forms of autosomal dominant HSP, highlighting the importance of examining its neuron-specific functions in regulating ER structure (Finsterer et al., 2012). In various experimental systems, Atlastin mutants exhibit numerous neuronal phenotypes, including defects in synaptic vesicle cycling and trafficking of neuronal cargoes (Gregorio et al., 2017), synapse development (Gregorio et al., 2017; Lee et al., 2009; Zhu et al., 2006), and axon regeneration (Rao et al., 2016). However, significant gaps remain in our understanding of how Atlastin-dependent changes in the structure and dynamics of neuronal ER lead to these functional defects. For example, using electron microscopy others found Drosophila Atlastin mutants exhibited shorter ER tubules in their motor neuron cell bodies(Orso et al., 2009) and diffuse localization of an ER lumen marker in their presynaptic terminals, attributed to extensive ER fragmentation (Summerville et al., 2016). However, this fragmentation has not been validated by EM or by fluorescence using ER membrane markers. Further, in C. elegans, Atlastin mutant ER networks in neuronal dendrites were not fragmented but were prone to retraction, leading to disrupted microtubule structures and mitochondrial fission (Liu et al., 2019). Finally, a ladder-like ER expansion was observed in spinal axons in a mouse model of Atlastin-dependent HSP (Zhu et al., 2022). In contrast to these neuronal findings, knockdown of Atlastin in Hela or Cos-7 cells leads to long and unbranched ER tubules (Hu et al., 2009, 2015; Niu et al., 2019). Thus, loss of Atlastin causes distinct ER phenotypes depending on several factors, including cell type, which region of the neuron was examined, and the reporter used to visualize the ER. To resolve the functions of this vital protein, comprehensive imaging studies of ER organization in the different regions of the neuron are needed, using complementary labeling strategies.
Another critical gap is our limited understanding of neuronal ER dynamics. High-resolution imaging in non-neuronal cells has revealed transitions from ER tubules to ER sheets (Lu et al., 2009; Sun et al., 2011), ER fragmentation (Espadas et al., 2019), tubule extension and retraction (Friedman et al., 2011; Hoyer et al., 2018; Rowland et al., 2014), and alterations in the number of 3-way junctions (Spits and Neefjes, 2021). These dynamics have been linked to ER functions such as endosome or mitochondrial fission (Friedman et al., 2011; Hoyer et al., 2018; Rowland et al., 2014) and calcium signaling (Crapart et al., 2023; Sun et al., 2011). In neurons, ER rearrangements have been suggested to alter calcium compartmentalization (Subramanian and Meyer, 1997), local protein synthesis (Deng et al., 2021; Schwarz and Blower, 2016), organelle contacts (Lu et al., 2020) and transport within the ER (Scott et al., 2021). However, the types and functions of neuronal ER dynamics, particularly in presynaptic terminals, remain largely unknown due to the challenges of imaging ER networks over time in small compartments. New insights into the mechanisms that regulate ER structure and ER dynamics in neurons are crucial for understanding why and how mutations in ER shaping proteins, such as Atlastin, predominantly affect the nervous system. Here, we addressed this gap by examining the structure and dynamics of the ER at the Drosophila neuromuscular junction (NMJ) of wild-type and Atlastin mutants using in vivo imaging and super-resolution Airyscan microscopy.
Results
High resolution imaging of the ER in Drosophila motor neuron cell bodies, axons, and presynaptic terminals.
At presynaptic terminals of the Drosophila larval NMJ, the ER is highly sensitive to fixation, limiting imaging experiments to live samples, often acquired on upright microscopes using water dipping objectives (Chouhan et al., 2010; Oliva et al., 2020; O’Sullivan et al., 2012; Pérez-Moreno et al., 2023; Summerville et al., 2016; Yalçın et al., 2017). However, the axial resolution limit of typical water dipping objectives is >300 nm, which cannot distinguish the densely packed ER tubules at presynaptic terminals (Espadas et al., 2019; Wu et al., 2017). To overcome this limitation, we created a reusable microscopy imaging slide to capture live super-resolution images or movies of neuronal ER in Drosophila larval fillets through glass coverslips, utilizing high numerical aperture oil objectives and Airyscan microscopy (Figure 1A). Our system provides an axial resolution of ~170 nm after image processing (Del Signore et al., 2022). We imaged larval fillets using this imaging slide on both inverted and upright microscopes within 40–50 min from dissection, a time frame in which this preparation maintains muscle potential and the capacity for evoked neurotransmitter release, even once the axon is severed from the cell body (Feng et al., 2004).
Figure 1. In vivo imaging of ER networks in Drosophila motor neurons.
(A) The schematic setup for live imaging experiments in this study consists of a glass microscope slide with a mounted silicone isolator filled with Sylgard. Small metal pins were used to stretch the larvae (small round dots). Finally, a coverslip was placed on top of the larval fillet for imaging with an oil objective on upright or inverted microscopes. (B) A representative single slice of an Airyscan microscopy Z-stack of the center (top) or periphery (bottom) of the cell body of a motor neuron expressing the luminal ER marker BiP:sfGFP:HDEL. Pink dotted boxes indicate ER tubules and sheets. (C) Maximum projections of Z-stacks acquired with an Airyscan microscope of two different motor neuron axons expressing the luminal ER marker BiP:sfGFP:HDEL. (D) Representative maximum projection of a Z-stack acquired with an Airyscan microscope of a motor neuron presynaptic terminal expressing the luminal ER marker BiP:sfGFP:HDEL. The magenta outline highlights the neuronal boundary. Arrows indicate boutons containing ER baskets or ladder-like ER structures.
To study the live dynamics of the ER, we used Vglut-Gal4 to express either the luminal ER marker BiP:sfGFP:HDEL (Figure 1B–D, 2C) or the transmembrane ER marker tdTomato:Sec61β (Figure 2B) in Drosophila 3rd instar larval motor neurons. These markers were previously shown to co-localize at this synapse, indicating they are comparable reporters for visualizing synaptic ER (Summerville et al., 2016). Our imaging approach allowed more precise visualization of ER tubules and networks labeled by these reporters in cell bodies, axons, and presynaptic terminals compared to previously reported results (Figure 1B–D, 2). As previously observed using lower-resolution imaging (Summerville et al., 2016), we detected ER tubule structures in all neuronal regions examined, including ER tubules and ER sheets in cell bodies, and ER tubules in axons and presynaptic terminals. In axons, ER tubules were long, and we sometimes observed a ladder-like structure consisting of pairs of interconnected ER tubules joined by rungs or cross-bridges, similar to structures previously described in developing axons of mammalian cultured neurons and Drosophila dendrites (Benedetti et al., 2024; Zamponi et al., 2022) (Figure 1C). At presynaptic terminals, ER tubules in some boutons appeared in a highly interconnected basket-like network, while others were transversed by several parallel ER tubules, similar to the ladder-like structures observed in axons (Figure 1D). Using this improved imaging approach, we achieved high-resolution visualization of individual ER tubules within networks of Drosophila presynaptic terminals, enabling detailed analysis of their structure and dynamics over time.
Figure 2. Dynamics of luminal and membrane ER markers at presynaptic terminals.
(A) Diagram of a presynaptic terminal of Drosophila motor neurons highlighting en passant and terminal boutons with the ER in grey. (B, top) Maximum intensity projection of Airyscan Z-stacks from the beginning (t=0) and the end (t=39 sec) of a timelapse movie (Movie S1) of a single wild-type bouton expressing the ER membrane marker tdTomato:Sec61β. (B, bottom) Magnification of the region in the dotted red box of dynamics observed from 0 sec to 1.4 sec of the movie. (C, top) Maximum intensity projection of Airyscan Z-stacks from the beginning (t=0) and the end (t=72 sec) of a timelapse movie (Movie S4) of a single wild-type bouton expressing the luminal ER marker BiP:sfGFP:HDEL. (C, bottom) Magnification of the region in the dotted red box of dynamics observed from 0 sec to 1.4 sec of the movie. (D) Presynaptic terminals exhibiting ER budding, focus dynamics and various types of ER tubule dynamics (ER tubule displacement, tubule extension, and tubule retraction). (E) Qualitative categorization of the percentage of en passant and terminal boutons that are dynamic or static expressing the ER membrane marker tdTomato: Sec61β. (F) Qualitative categorization of the percentage of dynamic terminal boutons expressing the ER membrane marker tdTomato:Sec61β that exhibit ER budding, foci dynamics or ER tubule dynamics. (G) Qualitative categorization of the percentage of dynamic terminal boutons expressing the ER membrane marker tdTomato:Sec61β that exhibit tubule displacement, tubule extension, tubule retraction and both tubule extension and retraction. The same control dataset used in E-G was used in Figure 5, and Figure 5_Supplement.
We next examined ER dynamics in en passant and terminal boutons at the Drosophila NMJ (Figure 2A). Terminal boutons have distinct cytoskeletal organization and signaling that could regulate the dynamics of the ER differently (Deshpande et al., 2016; Fernandes et al., 2023; Roos et al., 2000). To examine the dynamics of the ER at presynaptic terminals, we collected Z stacks of single boutons expressing the ER membrane marker for 40 sec at 0.92 sec/stack (Movie S1–S3) or the luminal ER marker for 90 sec at 0.73 sec/stack (Movie S4–S6). Both markers were distributed unevenly across the ER network (Figure 2B–2C). In particular, the luminal ER marker exhibited numerous discrete round foci that moved within ER tubules with fast dynamics (<1 sec) (Figure 2C). These foci were less frequently observed with the ER membrane marker (Figure 2B, bottom), consistent with the model that proteins in the ER lumen and ER membrane may differentially cluster and move within the ER (Holcman et al., 2018; Obara et al., 2024). Conversely, static regions of high intensity (which we saw for both markers) may reflect stacked ER tubules (Costantini et al., 2012).
We focused on the ER membrane marker to define dynamic events since its labeling pattern had fewer confounding foci. Analysis revealed three distinct types of ER dynamics: (1) ER budding events, in which a fragment of ER dissociates from the network; (2) Focus dynamics, in which round foci move within the ER network; and (3) ER tubule dynamics, including overall displacement of the tubule in the XY plane, as well as lengthening (extension) and shortening (retraction) of the tubule (Figure 2D). We qualitatively categorized boutons as “static” if we observed no change in ER network structure or “dynamic” if we observed at least one change. On many occasions, we observed multiple types of ER dynamics within the same bouton. We found that terminal boutons are highly dynamic (89%) while en passant boutons were moderately dynamic (38%) (Figure 2E). This difference in dynamics suggests that the ER is regulated differently in different regions of presynaptic terminals and could have specialized functions.
Next, we characterized the frequency of the three main types of ER network dynamics at terminal boutons, where we observed the most remodeling. Among terminal boutons, 93% exhibited tubule movements, making this the most common type of ER remodeling (Figure 2F). Less frequently, we observed that 28% of boutons had ER budding and 12% had moving foci. We further classified tubule movements into four categories: displacement, extension, retraction, and extension followed by retraction. The most common category was extension followed by retraction, observed in 81% of terminal boutons (Figure 2G). Simple displacement occurred in 44% of boutons, 24% showed extension alone, and 18% showed retraction alone. These results demonstrate our ability to capture diverse ER behaviors at high resolution in live Drosophila motor neurons.
Presynaptic terminals of Atlastin mutants form robust ER networks.
To investigate the relationship between ER structure and function at synapses, we examined mutants of Atlastin, a GTPase that regulates ER tubule fusion. Drosophila has a single homolog while mammals have three Atlastin homologs, with Atlastin-1 enriched in the brain (Rismanchi et al., 2008). The diverse neuronal phenotypes in Atlastin mutants, including defects in synaptic growth, vesicle cycling, cargo trafficking, and axon regeneration, make it an excellent model for understanding how ER architecture impacts cellular function (Audhya et al., 2007; Gregorio et al., 2017; Lee et al., 2009; Rao et al., 2016; Zhu et al., 2006). We analyzed transheterozygotes of an Atlastin null allele (atl2) and a deficiency removing the Atlastin locus to avoid second-site effects (Lee et al., 2009). These Atlastin null mutants (hereafter referred to as Atlastin mutants) were mainly pharate lethal and showed increased satellite boutons at larval NMJs (Figure 3A–B), indicating defects in synaptic development as previously reported (Gregorio et al., 2017; Lee et al., 2009).
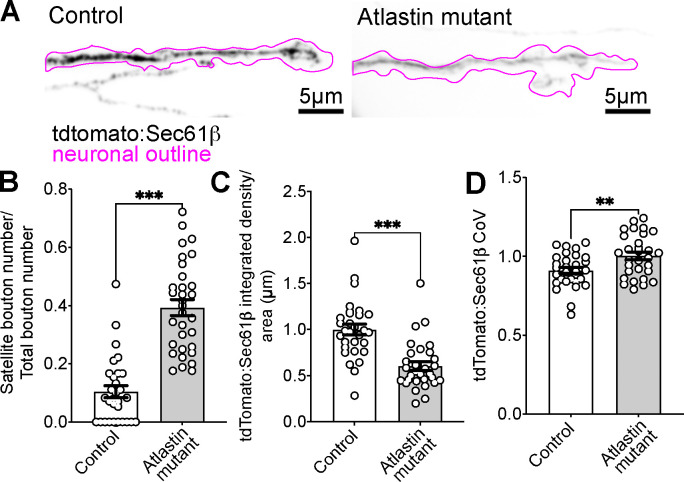
Figure 3. Atlastin mutants have lower levels of the ER membrane tdTomato: Sec61β at presynaptic terminals.
(A) Representative maximum intensity projection of a spinning disk confocal Z-stack from fixed larvae expressing the ER membrane marker tdTomato:Sec61β (black) in control (left) and Atlastin mutants (right). The neuronal outline (derived from the α-HRP mask) is shown in magenta. (B) Quantification of satellite boutons normalized to total bouton number in control and Atlastin mutants. (C) Quantification of the intensity of the ER membrane marker tdTomato:Sec61β, normalized to wild-type control. (D) Coefficient of variation (CoV) of the ER membrane marker tdTomato:Sec61β. (B-D) Graphs show mean +/− s.e.m. from 30 control and 30 Atlastin mutant NMJs.
Using confocal microscopy, Summerville et al. (2016) observed that the ER lumen marker BiP:sfGFP:HDEL was diffusely distributed in Atlastin mutant presynaptic terminals and concluded that the ER was extensively fragmented. To ask whether an ER membrane marker would show similar fragmentation, we used spinning disk and Airyscan microscopy to examine ER network structures at Atlastin mutant presynaptic terminals expressing the ER membrane marker tdTomato:Sec61β. We found that tdTomato:Sec61β labeled intact ER networks in Atlastin mutant synapses (Figure 3A, Figure 3_Supplement). Our results suggest that ER networks exist in Atlastin mutant presynaptic terminals, and the previously observed defects in synaptic function are likely due to more subtle ER structural or dynamic properties rather than fragmentation. However, we found that the marker tdTomato:Sec61β exhibited lower fluorescence levels in Atlastin mutants, as quantified by its mean intensity within the entire presynaptic terminal in fixed preparations (Figure 3C). This reduction in ER membrane marker intensity has also been observed in other HSP mutants, suggesting this is a common feature of ER shaping mutants and could indicate changes in ER membrane composition, integrity, or tubule thickness (Pérez-Moreno et al., 2023). We also noted that the coefficient of variation (CoV) of tdTomato:Sec61β signal was significantly higher in Atlastin mutants (Figure 3D), suggesting that it is more concentrated in subsynaptic structures compared to wild-type synapses (notably the opposite of what would be expected for fragmentation).
Figure 3 – Figure Supplement. Atlastin mutants expressing the ER membrane tdTomato:Sec61β exhibit robust ER networks at presynaptic terminals.
(A, B) Representative maximum projection of a Z-stack acquired live by Airyscan microscopy of control and Atlastin mutant NMJs expressing the ER membrane marker tdTomato: Sec61β at presynaptic terminals. These images are not contrast matched because Atlastin mutants exhibit lower levels of the tdTomato:Sec61β (See Figures 3C).
Next, we measured the levels of tdTomato:Sec61β in various motor neuron regions to determine if its reduced levels are specific to presynaptic terminals or a cell-wide phenotype (Figure 4). Using Vglut-Gal4 to label motor neurons, we measured clusters of cell bodies in the ventral ganglion and axon bundles extending towards body-wall muscles (Figure 4A–B). Atlastin mutants have significantly increased tdTomato:Sec61β intensity levels in axon bundles (Figure 4C) and unchanged levels in cell body clusters (Figure 4F). We also found that the volume occupied by tdTomato:Sec61β-labeled structures was significantly increased in axon bundles and decreased in cell body clusters of Atlastin mutants. Finally, we observed a significant decrease in the CoV of the tdTomato:Sec61β signal in both axon bundles and cell body clusters, suggesting less concentrated ER structures (the opposite of what we observed at presynaptic terminals). Our results suggest that the changes observed in cell body clusters, axon bundles, and presynaptic terminals are local, indicating that the ER has neuronal region-specific phenotypes regulated by Atlastin. These changes in tdTomato:Sec61β distribution could reflect alterations in ER structure or ER membrane protein density.
Figure 4. Atlastin mutants expressing the ER membrane tdTomato:Sec61β in axon bundles and soma clusters have distinct phenotypes.
(A) Representative middle slice of axon bundles in control (top) and Atlastin mutants (bottom) expressing tdTomato:Sec61β. (B) Representative top or middle slices of somas in control (top) and Atlastin mutants (bottom) expressing tdTomato:Sec61β. (C,F) Quantification of the intensity of the ER membrane marker tdTomato:Sec61β. (D,G) Quantification of the area of the ER membrane marker tdTomato:Sec61β. (E,H) Coefficient of variation (CoV) of the ER membrane marker tdTomato:Sec61β. For axon bundles, graphs show mean +/− s.e.m. from 29 control and 32 Atlastin mutant NMJs. For soma clusters, graphs show mean +/− s.e.m. from 26 control and 33 Atlastin mutant NMJs.
Atlastin mutants show mild defects in synaptic ER dynamics
We hypothesized that Atlastin could regulate synaptic function by controlling the dynamics of the ER network. We captured rapid time-lapse images of the ER labeled with tdTomato:Sec61β in en passant and terminal boutons at 0.92 sec/stack (Figure 2D–G, Figure 5A, Movies 7–14). We blinded movies from controls and Atlastin mutants to qualitatively categorize boutons as “dynamic” or “static”, as described above (Figure 2E). Compared to the 89% of terminal boutons in control animals that exhibited dynamics (previously shown in Figure 2B, E), we found a small but significant reduction in dynamic boutons in Atlastin mutants (76%), with a concomitant increase in the fraction of non-dynamic boutons (24%) (Figure 5B). In en passant boutons, we observed similar ER dynamics in controls (62% static/38% dynamic) and Atlastin mutants (69% static/31% dynamic). These findings indicate that Atlastin mutants exhibit mild defects in ER dynamics, specifically at terminal boutons, with no significant changes in en passant boutons. This suggests that Atlastin’s ER tubule tethering and fusion roles are critical in highly dynamic ER networks like those found in terminal boutons.
Figure 5. Atlastin mutants have mild ER dynamics phenotypes.
(A) Representative images of terminal (left) or en passant (right) boutons in controls (top) and Atlastin mutants (bottom) (Movies S7–14). (B) Percentage of dynamic versus static boutons in controls and Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β, analyzed separately for terminal and en passant boutons. Terminal bouton analysis included 75 control (65 dynamic, 10 static) and 83 Atlastin mutant boutons (63 dynamic, # static). En passant bouton analysis included 29 control (11 dynamic, 18 static) and 36 Atlastin mutant boutons (11 dynamic, 25 static). (C) Distribution of ER dynamic types (budding, foci dynamics, or ER tubule dynamics) in dynamic terminal boutons expressing tdTomato:Sec61β. (D) Distribution of ER dynamics types in dynamic en passant boutons expressing tdTomato:Sec61β. Dynamic boutons were further analyzed in Figure 5_Supplement. Data from control animals were also used in Figure 2 and Figure 5_Supplement.
We performed a further comprehensive analysis of ER dynamics in Atlastin mutants, examining multiple structural rearrangements, including tubule dynamics, ER budding, and the movement of concentrated protein foci. Surprisingly, despite Atlastin being the sole homolog in Drosophila and a key player in ER membrane fusion, we found only modest changes in ER structure and dynamics. While we observed slight shifts in the relative frequencies of different dynamic events (Figure 5C–D, Figure 5A-Supplement), none of these changes reached statistical significance, and the overall dynamic capacity of the ER network remained intact (Figure 5B-Supplement). The subtlety of these phenotypes suggests either substantial functional redundancy with other ER-shaping proteins or the existence of compensatory mechanisms that maintain ER organization and dynamics in neurons upon loss of Atlastin. Moreover, given the numerous synaptic defects previously reported in Atlastin mutants, our findings suggest that these phenotypes may arise from defects in ER function rather than gross changes in ER structure or dynamics.
Figure 5 – Supplement.
(A) A qualitative categorization of the percentage of dynamic terminal boutons in controls and Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β that exhibit tubule displacement, tubule extension, tubule retraction, and both tubule extension and retraction. (B) A qualitative categorization of the number of dynamic categories per terminal bouton in control and Atlastin mutants. The same control and Atlastin mutant dataset were used in Figure 2, and Figure 5.
Atlastin mutant presynaptic terminals exhibit distinct BiP:sfGFP:HDEL and tdTomato:Sec61β distributions.
Our results showing intact ER networks in Atlastin mutants using an ER membrane marker sharply contrast with those from a prior study examining Atlastin mutants using the luminal marker BiP:sfGFP:HDEL, where its diffuse distribution was interpreted as ER fragmentation (Summerville et al., 2016). To further explore this discrepancy, we used Airyscan microscopy to re-examine BiP:sfGFP:HDEL in Atlastin mutants. Unlike its strict localization to the ER network in control synapses, we reproduced the observation that a significant fraction of BiP:sfGFP:HDEL was diffusely localized in Atlastin mutant synapses, even at the resolution of Airyscan microscopy. Further, the marker filled the interiors of boutons and extended to their periphery, suggesting that it may be mislocalized to the cytosol instead of within ER fragments (Figure 6A–B).
Figure 6. Luminal ER proteins are displaced to the cytosol in Atlastin mutants.
Maximum projection of a Z-stack acquired live with an Airyscan microscope of control (A) and Atlastin mutant (B) NMJs expressing the ER lumen marker BiP:sfGFP:HDEL. (B) Atlastin mutants exhibit two distinct phenotypes at presynaptic terminals that were categorized as partial loss (B, top) or complete loss (B, bottom). Control and Atlastin mutant images are contrast matched. (C) A qualitative categorization of presynaptic terminals in control and Atlastin mutants exhibiting “partial loss”, “complete loss” or “no phenotype”. (D) Histogram of normalized mean intensities of the ER lumen marker BiP:sfGFP:HDEL. Displacement does not strongly correlate with steady-state levels of BiP:sfGFP:HDEL. (E) Quantification of the intensity of the luminal ER marker BiP:sfGFP:HDEL. (F) Coefficient of variation (CoV) of the luminal ER marker BiP:sfGFP:HDEL in neurons of control and Atlastin mutant. (E, F) Graphs show mean +/− s.e.m. from 8 control and 15 Atlastin mutant NMJs.
We identified two distinct ER network phenotypes in Atlastin mutants: NMJs with “Partial loss” had both diffuse BiP:sfGFP:HDEL and an identifiable ER network structure, while NMJs with “Complete loss” had a more severe phenotype with no visible ER network structures. We blinded our images and categorized control and Atlastin mutant datasets as “Partial loss,” “Complete loss,” or “No phenotype” (Figure 6C). In Atlastin mutants, we found that 45% of NMJs exhibited partial loss, 55% exhibited complete loss, and 10% had no phenotype. We observed partial loss and complete loss phenotypes in both anterior (segment A2) and posterior (segment A5) muscle segments, in Is (phasic) and Ib (tonic) motor neuron subtypes, and in neurons innervating multiple different body wall muscles, suggesting that it reflects a widespread phenomenon in NMJs of Atlastin mutants (Figure 6–Figure 6-Supplement). Presynaptic terminals in Atlastin mutants show similar mean intensities of the luminal ER marker compared to controls, suggesting that the marker’s redistribution in mutant neurons does not affect its overall levels (Figure 6D–E). However, we observed a significant change in the CoV in Atlastin mutants due to the redistribution of the luminal marker (Figure 6F). Along with our earlier finding that the ER membrane marker, tdTomato:Sec61β, does not exhibit fragmentation, these results suggest the possibility that the luminal ER marker is displaced to the cytosol.
Figure 6 – Supplement. Is and Ib motor neurons in various muscles and abdominal segments of 3rd instar Atlastin mutant larvae exhibit similar cytosolic displacement of BiP:sfGFP:HDEL.
Maximum projection of a live Airyscan Z-stack from larvae expressing the ER lumen marker BiP:sfGFP:HDEL at muscles 12–13 (A, left), muscles 6–7 (A, right), muscle 4 in segment A2 (B, left) and muscle 4 in segment A4 (B, right).
Atlastin mutants may be particularly sensitized to overexpression of the BiP:sfGFP:HDEL marker because we could not assess the co-localization of BiP:sfGFP:HDEL and tdTomato:Sec61β, as very few larvae co-expressing these markers survived. The few that did had grossly defective NMJ morphologies and large aggregates of both markers, even in wild-type flies (data not shown). Further, control adult flies expressing BiP:sfGFP:HDEL were unhealthy and could not be maintained as a stock, suggesting that this marker is toxic.
Next, we asked whether the luminal ER marker displacement phenotype is specific to all neuronal regions or specific to presynaptic terminals. First, we examined the ER in body wall muscles of Atlastin mutants by overexpressing the BiP:sfGFP:HDEL with the muscle driver BG57-Gal4. We did not find the luminal ER marker displaced to the cytosol (Figure 7, right). However, as noted in earlier studies (Lee et al., 2009), we observed a decrease in network complexity. Next, we examined the cell body and axons of Atlastin mutants to determine if the displacement of the luminal ER marker was a global or local phenotype within neurons. As previously reported (Summerville et al., 2016), we did not observe a diffuse signal for the luminal ER marker in cell bodies or distal axons (within ~30μm of the synapse) (Fig 7, middle + left). However, we did observe ER fragmentation in some axons (Fig 7, left). These results indicate that the displacement of BiP:sfGFP:HDEL in Atlastin mutants is a synapse-specific phenotype.
Figure 7. Luminal ER proteins are not displaced in other neuronal compartments or cell types in Drosophila 3rd instar larvae.
All images were acquired by live Airyscan microscopy from larvae expressing the ER lumen marker BiP:sfGFP:HDEL with Vglut-GAL4 (motor neurons) or BG57-GAL4 (body wall muscles). (Left panel) Maximum projection of a Z-stack of motor neuron axons. (Middle panel) A single slice of motor neuron cell bodies. (Right panel) A single slice of body wall muscles.
The luminal ER phenotype in Atlastin mutants is progressive
Atlastin dysfunction is linked to the neurodegenerative disorder HSP, in which patients progressively lose neuronal function over time (Blackstone, 2018, 2012; Blackstone et al., 2011). Drosophila Atlastin mutants recapitulate progressive motor dysfunction as observed in HSP patients (Gregorio et al., 2017). We examined 1st instar larvae to test the hypothesis that the partial loss phenotype may similarly progress to the complete loss phenotype through larval development. We categorized control and Atlastin mutant datasets as described above (Figure 6). We anticipated that a progressive phenotype would decrease the proportion of NMJs showing BiP:sfGFP:HDEL displacement and an increase in presynaptic terminals displaying no phenotype. Indeed, we observed that the percentage of NMJs with complete loss increased from 12.5% in 1st instar to 55% in 3rd instar, while NMJs with no phenotype decreased from 38% to 10% across these developmental stages (compare Figure 8A–C to Figure 6). These results suggest that displacement of the luminal ER marker progresses over time and that this phenotype is initiated early in neuronal development.
Figure 8. The luminal displacement phenotype in Atlastin mutants is progressive.
(A, B) Maximum intensity projections of a live Airyscan Z-stack acquired of 1st instar larvae expressing the ER lumen marker BiP:sfGFP:HDEL (black) with a neuronal outline (magenta, derived from the α-HRP mask) in control (A) and Atlastin mutants (B). (C) A qualitative categorization of presynaptic terminals in control and Atlastin mutant 1st instar larvae expressing the ER lumen marker BiP:sfGFP:HDEL that exhibit “partial loss”, “complete loss” or “no phenotype”. Data are shown from 16 control NMJs and 16 Atlastin mutant NMJs.
Atlastin mutants have disrupted luminal ER protein dynamics
To examine ER network continuity and the dynamic properties of luminal ER proteins in Atlastin mutants, we used fluorescence recovery after photobleaching (FRAP) to track BiP:sfGFP:HDEL mobility at presynaptic terminals (Figure 9A). In this analysis, the rate and extent of fluorescence recovery provide key insights into protein behavior: continuous ER networks show robust recovery due to the movement of unbleached proteins across the connected network, while fragmented ER networks fail to recover due to physical barriers between compartments (Dayel et al., 1999; Lippincott-Schwartz et al., 2018). Further, if the BiP:sfGFP:HDEL protein had leaked into the cytosol, we would expect rapid recovery due to the higher diffusion rates of cytosolic proteins compared to ER-localized proteins (Dayel et al., 1999). Our FRAP analysis examined single synaptic boutons co-expressing BiP:sfGFP:HDEL and RFP:mCD8, which labels the neuronal plasma membrane. We photobleached en passant boutons in the middle of a synaptic branch to ensure BiP:sfGFP:HDEL could recover from ER networks surrounding the FRAP region. We recorded movies of a single NMJ slice, simultaneously imaging both markers. After photobleaching with a 405nm laser, we captured recovery dynamics by imaging every 10 seconds for 2 minutes (Figure 9, Movies 15–19).
Figure 9. FRAP analysis of BiP:sfGFP:HDEL recovery in Atlastin mutants.
(A) Diagram of the recovery of BiP:sfGFP:HDEL in controls and Atlastin mutants with partial loss or complete loss. (B) Representative slices from movies of controls and Atlastin mutants with partial loss or complete loss co-expressing mCD8:RFP and BiP:sfGFP:HDEL pre-bleach, after FRAP and 2 min post-FRAP (Movies S15–19). (C) Mean recovery of mCD8:RFP in control (black squares) and Atlastin mutants with partial loss (empty lime green triangles) and complete loss (green triangles). (D) Mean recovery of BiP:sfGFP:HDEL in control (black squares) and Atlastin mutants with partial loss (empty lime green triangles) and complete loss (green triangles). The table contains the results from the non-linear fit one-phase association of the data for each dataset. The s.e.m. are represented by solid lines and shading: controls (n=10) have black lines with grey shading, Atlastin mutants with partial loss (n=6) have lime green lines and lime green shading, and Atlastin mutants with complete loss (n= 8) have green lines with light green shading.
We analyzed the recovery after photobleaching of RFP:mCD8 and BiP:sfGFP:HDEL to characterize protein dynamics in Atlastin mutant presynaptic terminals. RFP:mCD8 served as a control because it showed similar plasma membrane distribution patterns at synaptic boutons in both control and Atlastin mutants, suggesting that plasma membrane organization was unaffected and would exhibit comparable dynamics across all genotypes. While the recovery of RFP:mCD8 could not be fitted to a non-linear equation due to the absence of a plateau, its similar recovery across genotypes indicates no significant changes in its diffusion at the plasma membrane (Figure 9B–C). We next analyzed BiP:sfGFP:HDEL recovery using a one-association non-linear model, focusing first on the plateau to estimate the mobile fraction of the luminal ER marker in the FRAP region (Figure 9B, D). We found that Atlastin mutants with partial loss of ER networks had the least recovery, with a mobile fraction of 50.61%. By contrast, controls and Atlastin mutants with complete loss had a higher mobile fraction of 65.73% and 66.34%, respectively. We also measured how quickly BiP:sfGFP:HDEL recovered by calculating the time to reach 50% of maximum recovery. Atlastin mutants with partial loss exhibited the slowest BiP:sfGFP:HDEL recovery, reflected by a longer half-life (38.26 sec) compared to controls (26.91 sec), while those with complete loss had the fastest recovery (14.62 sec). The reduced mobile fraction in partial loss Atlastin mutants likely reflects discontinuous ER networks (Figure 9B, blue arrows). In contrast, the rapid recovery and absence of visible ER structures in complete loss Atlastin mutants suggests that BiP:sfGFP:HDEL is largely displaced into the cytosol. Our analyses indicate that Atlastin dysfunction impairs luminal ER protein diffusion and subcellular localization. Surprisingly, these defects occur while maintaining relatively normal synaptic ER network structure (Figure 3–4) and dynamics (Figure 5). These findings reveal a novel pathogenic mechanism where Atlastin dysfunction primarily impacts ER protein distribution rather than ER network morphology, potentially disrupting synaptic function through the mislocalization of essential ER proteins.
Luminal proteins are displaced to the cytosol at presynaptic terminals of Atlastin mutants
A fundamental question emerged from our observations: does the altered BiP:sfGFP:HDEL distribution in Atlastin mutants arise from ER fragmentation or genuine protein displacement to the cytosol? Previous research shows that ER network morphology relies on a delicate balance between ER shaping proteins; disrupting this balance may result in ER fragmentation (Wang et al., 2016). In contrast, the displacement of luminal proteins to the cytosol suggests changes in the ER integrity and potential activation of ER stress responses (Merighi and Lossi, 2022). To definitively distinguish between these models, we conducted two independent experiments to track BiP:sfGFP:HDEL sub-cellular localization at presynaptic terminals. First, we examined whether BiP:sfGFP:HDEL in Atlastin mutants is sensitive to the mild detergent digitonin, which gently permeabilizes the plasma membrane and extracts proteins from the cytosol but not the ER (Le Gall et al., 2004). Second, we examined the direct interaction between BiP:sfGFP:HDEL and a plasma membrane-tethered nanobody, which would only occur if the protein was displaced to the cytosol.
We hypothesized that digitonin treatment would lead to loss of fluorescence in Atlastin mutants, proportional to the fraction of the luminal ER marker protein in the cytosol (Figure 10B-Supplement). We treated presynaptic terminals for 5 min with digitonin (Figure 10A-Supplement) and imaged the same presynaptic terminal (expressing either cytosolic GFP or BiP:sfGFP:HDEL) before and after digitonin treatment. We also labeled our live samples with α-HRP antibodies to identify presynaptic terminals (Snow et al., 1987). We measured the fraction change in mean intensity to determine the effects of digitonin treatment on cytosolic and ER markers (Figure 10C-Supplement). We observed a significant loss in mean fluorescence of cytosolic GFP following digitonin treatment. Control presynaptic terminals expressing the luminal ER marker BiP:sfGFP:HDEL became highly fragmented, possibly due to osmolarity changes in the cytosol, which are known to promote ER fragmentation (King et al., 2020). Despite this fragmentation, most ER remained within the synaptic terminal, suggesting limited extraction or permeabilization by this treatment. In half the control samples, the marker redistributed to bright puncta and showed an unexpected increase in mean intensity. One interpretation is that digitonin treatment allows the exchange of small molecules into the ER (Le Gall et al., 2004), potentially altering the fluorescent properties of sfGFP. The other half of the samples had slightly reduced mean intensity following digitonin treatment, possibly due to moderate loss of ER fragments. We next analyzed Atlastin mutants categorized as “Partial loss” or “Complete loss”. Digitonin treatment yielded no significant differences between controls and Atlastin mutants with partial loss. We also observed that Atlastin mutant synapses exhibiting partial loss had puncta following digitonin treatment that were similar to the control. This is likely because the partial loss presynaptic terminals have some ER network visible with the luminal marker. In contrast, we observe a significant decrease in fluorescence intensity following digitonin treatment in Atlastin mutant presynaptic terminals with complete loss, which do not exhibit visible ER networks, as indicated by the luminal marker. These results suggest that luminal ER molecules in Atlastin mutants are likely displaced to the cytosol.
Figure 10 – Supplement. Luminal ER proteins displaced to the cytosol are sensitive to digitonin treatment.
(A) Maximum projections of Z-stacks acquired live with a spinning disk confocal of boutons expressing with Vglut-Gal4 GFP (control 1), BiP:sfGFP:HDEL (control 2), BiP:sfGFP:HDEL in Atlastin mutants exhibiting partial loss and BiP:sfGFP:HDEL in Atlastin mutants exhibiting complete loss. (B) Diagram of the expected distribution patterns before and after digitonin treatment of GFP and BiP:sfGFP:HDEL. Note that digitonin treatment causes partial ER fragmentation. (C) Fractional change of fluorescence intensity at presynaptic terminals expressing GFP (cytosolic protein) or BiP:sfGFP:HDEL in control and Atlastin mutants following digitonin treatment. Graphs show mean +/− s.e.m. from 18 control and 20 Atlastin mutant NMJs. Statistical significance was only calculated between BiP:sfGFP:HDEL samples since the baseline fluorescence and distribution of this marker was sensitive to digitonin treatment in a manner distinct from cytosolic GFP.
From the digitonin experiments, we cannot rule out the possibility that BiP:sfGFP:HDEL resides in small ER membrane fragments that easily diffuse out of permeabilized plasma membranes. We hypothesized that if BiP:sfGFP:HDEL is displaced to the cytosol in Atlastin mutants, it would interact with plasma membrane proteins topologically oriented toward the cytosol. However, if BiP:sfGFP:HDEL were within a small membrane-bound ER compartment, it could not interact with a cytosolically oriented ligand. To test this, we co-expressed BiP:sfGFP:HDEL with a plasma membrane-tethered GFP nanobody fused to RFP (referred to as morphotrapInt,) (Harmansa et al., 2017)). The GFP nanobody in morphotrapInt faces the cytosol, even when tethered to membranes, allowing it to interact only with cytosolic BiP:sfGFP:HDEL. Given that the morphotrapInt is localized to the plasma membrane, it should cause the relocation of cytosolic BiP:sfGFP:HDEL, but not luminal BiP:sfGFP:HDEL. Second, since a membrane-tethered nanobody is likely to have slower diffusion than the putatively cytosolic BiP:sfGFP:HDEL, morphotrapInt co-expression would be predicted to reduce cytosolic BiP:sfGFP:HDEL dynamics, but not luminal BiP:sfGFP:HDEL dynamics (Figure 10A).
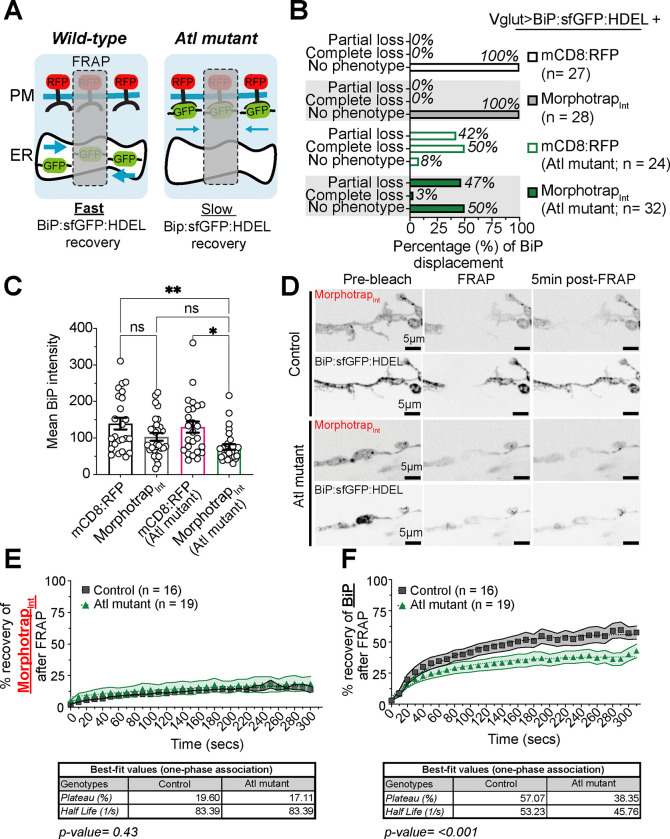
Figure 10. BiP:sfGFP:HDEL in the cytosol binds morphotrapInt in Atlastin mutants.
(A) Diagram of the recovery of BiP:sfGFP:HDEL in controls and Atlastin mutants co-expressing morphotrapInt. (B) Distribution of BiP:sfGFP:HDEL phenotypes (partial loss, complete loss, or no phenotype) in control and Atlastin mutant presynaptic terminals co-expressing either mCD8:RFP or morphotrapInt. (C) Intensity of BiP:sfGFP:HDEL in control and Atlastin mutant terminals co-expressing either mCD8:RFP or morphotrapInt. (D) Representative maximum projections of controls (Movies S20–22) and Atlastin mutants (Movies S23–24) co-expressing morphotrapInt and BiP:sfGFP:HDEL pre-bleach, after FRAP and 5 min post-FRAP. (E) Mean recovery of mCD8:RFP in control (black squares) and Atlastin mutants (green triangles). (F) Mean recovery of BiP:sfGFP:HDEL in control (black squares) and Atlastin mutants (green triangles). The tables contain the results from the non-linear fit one-phase association equation of all the datasets. The s.e.m. are represented by solid lines and shading: controls have black lines with grey shading, Atlastin mutants have green lines with light green shading. For 10A-B, there were 27 controls co-expressing BiP:sfGFP:HDEL and mCD8:RFP, 28 controls co-expressing BiP:sfGFP:HDEL and morphotrapInt, 24 Atlastin mutants co-expressing BiP:sfGFP:HDEL and mCD8:RFP, and 24 Atlastin mutants co-expressing BiP:sfGFP:HDEL and morphotrapInt. For 10 E-F, there were 16 controls co-expressing BiP:sfGFP:HDEL and morphotrapInt and 19 Atlastin mutants co-expressing BiP:sfGFP:HDEL and morphotrapInt.
First, we blinded our samples and categorized control and Atlastin mutants as “No phenotype”, “Partial loss” and “Complete loss”, as we had done previously (Figure 6C). We found that Atlastin mutants co-expressing morphotrapInt and BiP:sfGFP:HDEL had 47% of NMJs with partial loss, 3% with complete loss, and 50% with no phenotype (Figure 10B). This redistribution is consistent with morphotrapInt binding and relocalizing cytosolic BiP:sfGFP:HDEL. Interestingly, rather than accumulating at the plasma membrane, the overall levels of BiP:sfGFP:HDEL were reduced, suggesting that the displaced protein is targeted for degradation (Figure 10C). Further, the reduction in partial and complete loss phenotypes compared to BiP:sfGFP:HDEL expression alone (Figure 6C vs 10B) indicates that sequestering BiP:sfGFP:HDEL at the plasma membrane uncovers residual ER networks masked by the diffuse cytoplasmic signal. To test the specificity of this effect, we co-expressed BiP:sfGFP:HDEL with RFP:mCD8, which has the same protein domain composition as morphotrapInt but lacks the GFP nanobody. BiP:sfGFP:HDEL levels were unaffected in those co-expressing RFP:mCD8 in Atlastin mutants and controls. We also found that 42% of NMJs had partial loss, 50% had complete loss, and 8% had no phenotype (Figure 10B), similar to what we observed when only BiP:sfGFP:HDEL was overexpressed in Atlastin mutants (Figure 6C). By contrast, in wild-type controls, co-expression of BiP:sfGFP:HDEL with either morphotrapInt or mCD8:RFP did not result in any relocalization of BiP:sfGFP:HDEL. Our results suggest that BiP:sfGFP:HDEL protein at presynaptic terminals is displaced to the cytosol and binds to morphotrapInt in Atlastin mutants, leading to its altered distribution and removal.
Next, we performed a FRAP experiment to test the dynamics of BiP:sfGFP:HDEL in the presence of morphotrapInt. First, we examined the dynamics of morphotrapInt itself following photobleaching. We found that morphotrapInt had very slow recovery and plateaued at 20% in wild-type and 17% in Atlastin mutants (Figure 10D–E). We hypothesized that the slow diffusion of morphotrapInt would restrict the movement of any cytosolic BiP:sfGFP:HDEL in Atlastin mutants. Indeed, we found that wild-type expressing morphotrapInt and BiP:sfGFP:HDEL had a mobile fraction of 57%, while Atlastin mutants had a mobile fraction of 38% (Figure 10F). By contrast, in Atlastin mutants co-expressing BiP:sfGFP:HDEL and non-membrane-targeted RFP:mCD8, NMJs with partial loss had a mobile fraction of 51%, and those with complete loss had a plateau of 66% (Figure 9). We hypothesize that the reduction in the mobile fraction in the presence of morphotrapInt results from the binding of BiP:sfGFP:HDEL in the cytosol to morphotrapInt in Atlastin mutants. There was a minor decrease to 57% in wild-type larvae co-expressing BiP:sfGFP:HDEL and morphotrapInt, while those co-expressing BiP:sfGFP:HDEL with RFP:mCD8 plateaued at 66%. These results suggest the presence of a small cytosolic population of BiP:sfGFP:HDEL in controls. Finally, we examined the recovery kinetics of BiP:sfGFP:HDEL in larvae co-expressing morphotrapInt. We found the recovery in controls is slower, with a half-life of 53.23 s, while Atlastin mutants had a faster recovery with a half-life of 45.76 s. The faster half-life in Atlastin mutants could reflect that a small pool of BiP:sfGFP:HDEL remains mobile, perhaps contained within a discontinuous section of the ER. Our findings provide multiple lines of evidence that BiP:sfGFP:HDEL is displaced from the ER lumen to the cytosol in Atlastin mutants, potentially reflecting disruption to ER integrity and function at presynaptic terminals.
Discussion
This study revealed the architecture and dynamic behaviors of Drosophila neuronal ER networks through a super-resolution in vivo imaging approach that can be adapted to visualize fluorescently labeled proteins at high resolution in any larval tissue. Using this approach, we provided the first characterization of presynaptic ER network dynamics in vivo, establishing a foundation for investigating the functional significance of structural rearrangements of the ER. Our analysis of Atlastin mutants uncovered an unexpected phenotype: while these mutants maintain largely intact ER networks with only minor defects in dynamics, they exhibit displacement of luminal ER proteins to the cytosol of presynaptic terminals but not in other neuronal regions. These findings reveal that Atlastin serves distinct, synapse-specific functions in maintaining both ER structure and function, providing mechanistic insight into the synaptic deficits previously observed in Atlastin mutants.
ER network dynamics at presynaptic terminals
We observed three categories of ER dynamics at presynaptic terminals: (1) budding and fusion of vesicular structures, (2) ER tubule dynamics, and (3) dynamics of foci that are retained within the ER network. Disruptions in these dynamics, as seen in Atlastin mutants, could impair synaptic signaling and contribute to neurodegenerative diseases linked to ER dysfunction. ER network dynamics may depend on Atlastin-mediated ER tubule tethering and fusion to distribute lipids and ER membrane proteins throughout the network to ensure proper distribution and prevent fragmentation. Future work is needed to examine the specific functions of these dynamics at presynaptic terminals, but we speculate below on some potential roles:
First, ER-derived vesicles could act as localized calcium signaling hubs (Burgoyne et al., 2015) or serve as sites for compartmentalized local translation if they carry ribosomes (Carter et al., 2020; Deng et al., 2021). The synaptic ER vesicle-like structures we observed are unlikely to be involved in the secretory pathway or ER-phagy (Chino and Mizushima, 2020) because we observe these vesicles re-fusing with the ER. We speculate the ER-derived vesicles observed may be REEP1–4 positive, which regulates ER vesicle formation in mammalian cells (Shibata et al., 2024). Notably, while overexpression of Atlastin can regulate REEP1–4 vesicle fusion, it is not essential for vesicle formation. Second, ER tubule dynamics may play multiple roles, such as maintaining the tension of the ER network (Espadas et al., 2019; Niu et al., 2019) and forming ER-organelle contacts (Friedman et al., 2011; Hoyer et al., 2018; Rowland et al., 2014). ER contacts occur at the tips and edges of ER tubules, and they have diverse functions, such as promoting mitochondrial and endosome fission (Friedman et al., 2011; Hoyer et al., 2018; Rowland et al., 2014). To understand how these functions are regulated at presynaptic terminals, future studies could examine ER tubule interactions with other organelles using co-localization experiments or ER-organelle sensors. Finally, we identified foci dynamics within ER networks. We hypothesize that foci emerge due to changes in the dynamics of protein clusters at the ER membrane or within the lumen. Foci dynamics occur at higher levels with the luminal ER marker in contrast to the ER membrane marker. This differential distribution suggests distinct organizational principles for luminal versus membrane proteins in presynaptic ER.
Distinct Membrane and Luminal ER Phenotypes in Atlastin Mutants Suggest Local Stress Responses
Using the membrane marker tdTomato:Sec61β, we discovered that Atlastin mutants have robust ER networks at presynaptic terminals. The relatively mild structural phenotypes stand in sharp contrast to the diffuse distribution we and others observe for the luminal marker BiP:sfGFP:HDEL, and argue against excessive ER fragmentation in Atlastin mutants. Despite being the sole Atlastin homolog in flies, we found that loss of Atlastin causes surprisingly subtle changes in ER structure and dynamics. However, we did observe specific defects with our FRAP experiments, which revealed Atlastin mutants have discontinuous ER networks. The subtle nature of these defects also suggests that other proteins may partially compensate for Atlastin’s function in maintaining ER structure and dynamics at presynaptic terminals.
Considering the distinct phenotypes displayed by luminal and membrane ER markers, which one best represents the Atlastin mutant phenotype in the absence of a marker? We favor the hypothesis that the true Atlastin ER phenotype is more likely to be similar to the tdTomato:Sec61β distribution, for the following reasons: (1) tdTomato:Sec61β-expressing animals still show Atlastin-associated functional defects such as defective synaptic growth, indicating that Atlastin-dependent ER functions are disrupted without dramatic defects in ER structure; (2) The BiP:sfGFP:HDEL phenotype becomes progressively more severe throughout larval development, suggesting this marker’s displacement is a secondary effect rather than a direct reflection of ER architecture. Future studies in other ER-shaping mutants should examine both ER membrane and ER lumen markers, as specific defects could affect one and not the other, as we observed in Atlastin mutants.
Why, then, might Atlastin mutants displace luminal proteins to the cytosol? A key observation that constrains possible mechanisms is that this displacement occurs specifically at presynaptic terminals - the luminal ER marker remains properly localized in cell bodies, axons, and other cell types such as muscles. This spatial specificity argues against several potential global mechanisms: First, overall disruption of ER integrity seems unlikely to explain the phenotype; while Atlastin mutants show reduced levels of the ER membrane marker Sec61β and altered network structure at presynaptic terminals, catastrophic loss of ER membrane integrity would likely release calcium and other ER components into the cytosol, which would be incompatible with cell survival (Wang et al., 2011). Second, general defects in protein translocation are unlikely, since these would affect all neuronal compartments, especially the cell body where most protein translation occurs (Glock et al., 2021; McKibbin et al., 2012). Third, we found no evidence for apoptosis-induced ER permeability, as Atlastin mutant neurons lack the characteristic debris observed after neuronal death at the NMJ (Aponte-Santiago et al., 2020; Graf et al., 2011).
Instead, the presynaptic specificity suggests more localized mechanisms. One possibility is compartment-specific activation of ER stress responses like UPR (Untranslated Protein Response), ERAD (ER-associated degradation) or ERCYS (ER-to-cytosol signaling) (Christianson et al., 2023; Igbaria et al., 2019; Lajoie and Snapp, 2020; Read and Schröder, 2021; Sicari et al., 2021; Wu and Rapoport, 2018). These pathways can actively transport luminal proteins to the cytosol through distinct mechanisms: in UPR and ERAD, proteins are targeted for proteasomal degradation, while in ERCYS, they can acquire novel functions in the cytosol. Previous work has shown that Atlastin mutants and other HSP-linked genes have activated UPR (Lai et al., 2014; O’Sullivan et al., 2012; Zhao et al., 2016). In the case of our overexpressed BiP:sfGFP:HDEL marker, either pathway could explain its accumulation in the cytosol. If UPR or ERAD is activated, BiP:sfGFP:HDEL might be continuously displaced from the ER but accumulate when the proteasome cannot keep up with its degradation. Alternatively, if ERCYS is activated, BiP:sfGFP:HDEL might accumulate in the cytosol as part of a regulated signaling response. We cannot distinguish between these possibilities, but multiple lines of evidence support the general activation of ER stress pathways in Atlastin mutants. First, the phenotype progresses from 1st to 3rd instar larvae, consistent with gradually activated stress responses. Second, synaptic terminals show a specific reduction in Sec61β levels, suggesting local defects in protein homeostasis. Given these observations, what might trigger an ER stress response in Atlastin mutants? The underlying mechanism remains to be determined, but the progressive nature of the phenotype and the eventual failure of luminal ER protein retention suggest a gradual deterioration of ER homeostasis at synaptic terminals. Future work will be needed to identify the specific cellular changes that initiate this stress response.
ER network structure and function are compartmentalized across neuronal regions
Our findings reveal distinct requirements for Atlastin across neuronal regions. At presynaptic terminals, loss of Atlastin leads to the displacement of luminal proteins and reduced levels of Sec61β. This reduction in Sec61β suggests other ER membrane proteins, such as ER tethers, could also be affected, potentially disrupting ER-dependent processes like endosome trafficking that rely on proper ER-organelle contacts. This reduction in Sec61β mirrors findings in Drosophila null mutants of Reticulon-1, another ER shaping and HSP-linked gene, in which levels of STIM and Stukorf are also decreased on ER tubules (Pérez-Moreno et al., 2023). Yet, a key distinction emerges as Reticulon-1 null mutants maintain normal luminal ER protein localization, suggesting unique features of Atlastin mutant ER networks. These region-specific phenotypes highlight fundamental differences in ER organization and function across neuronal regions. Further evidence for neuronal ER compartmentalization comes from studies of mammalian spinal axons, where Atlastin-fusion incompetent mutants showed ladder-like ER expansion (Zhu et al., 2022). We observed similar ladder-like structures with three-way junctions in both control and Atlastin mutant axons labeled with tdTomato:Sec61β, indicating that Atlastin is not solely responsible for ER tubule fusion in axons. This neuronal-specific organization contrasts with the long, unbranched ER tubules found in Atlastin-deficient Hela or Cos-7 cells (Hu et al., 2009; Niu et al., 2019), suggesting neurons possess unique compensatory tubule-fusion machinery. Future work should comprehensively examine the common and unique defects associated with HSP gene dysfunction across neuronal compartments.
Methods
Drosophila stocks
Drosophila melanogaster was cultured on a standard medium at 22–25°C. Detailed genotypes and sexes used for experiments are described in Table S1. Stocks used in this study include: Vglut-Gal4 (Bloomington Drosophila Stock Center (BDSC_24635), BG57-Gal4 (gift from Vivian Budnik), atl2 (gift from James McNew), third chromosome deficiency (BDSC_7948), UAS-BiP:sfGFP:HDEL (BDSC_64748), 20XUAS-tdTomato:Sec61β (BDSC_64746), UAS-CD8:RFP (BDSC_27391) and UAS-morphotrapInt (gift from Chi-Kuang Yao).
Live imaging of luminal and membrane ER markers
1st and wandering 3rd instar larvae were dissected in Ca2+-free HL3.1 (Feng et al., 2004) and axons were severed from the central nervous system. Dissections were performed in the live imaging slide described below. Larvae were imaged at room temperature with a 63X (n.a. 1.4) oil immersion objective, using an Airyscan LSM 880 microscope in super-resolution mode and Zen Black software. Raw image stacks were processed in Zen Blue or Zen Black using 3D Airyscan processing with automatic settings.
For 3rd instar larvae, Z-stacks of soma clusters, axons or presynaptic type Ib terminals at muscle 4 in segment A5 (unless otherwise noted) were acquired to measure the levels of luminal ER markers, to categorize ER network phenotypes, or for morphological measurements. For 1st instar larvae, live larval fillets were stained with 1:250 dilution of 1.5 mg/ml HRP Alexa Fluor 647 (Jackson ImmunoResearch) in HL3.1 for 5 min followed by a 1 min wash. Next, Z-stacks of 1st instar presynaptic terminals at muscle 4 in segment A3-A5 were acquired and used to categorize ER network phenotypes.
To record the dynamics of the luminal ER marker BiP:sfGFP:HDEL, 16-bit Z-stacks of a single bouton (8 slices with 0.5 micron spacing) were collected for 100 frames at a frame rate of 0.73 seconds/stack. To record ER dynamics with the membrane ER marker tdTomato:Sec6β, 8-bit Z-stacks of a single bouton (20 slices with 0.1850 micron spacing) were collected for 60 frames at a frame rate of 0.92 seconds/stack.
Construction of in vivo imaging slide for Drosophila larvae
To make the reusable imaging slide, Press-To-Seal Silicone Isolators (Grace Bio-labs; CQS-13R-2.0) were mounted onto rectangular glass slides, which then were filled from their centers to their tops with Krayden Dow Sylgard 184 Silicone (Thermo Fisher Scientific). Each slide with a Press-To-Seal Silicone Isolator was leveled to ensure the silicone cured evenly at room temperature. Minutien pins (Fine Science Tools) were cut with nail clippers and used to stretch the larvae and fix it in place. Pins were pressed into the cured Sylgard 184 Silicone and a few drops of hemolymph-like solution HL3.1 were added. Next, a coverslip was placed on top of the dissected larvae, each corner was gently pressed down, and excess HL3.1 was removed with a Kimwipe, thus sealing the coverslip to the silicone isolator.
Fixed tissue staining
Wandering 3rd instar larvae were dissected in Ca2+-free HL3.1 and fixed for 17 min in Ca2+-free HL3.1 containing 4% PFA. Larvae were blocked and permeabilized overnight in PBS containing 0.25% Saponin, 2.5% normal goat serum (NGS), 2.5% bovine serum albumin (BSA), and 0.1% sodium azide. Fixed larvae (Figure 3) were stained with 1:500 FluoTag®-X4 anti-RFP (#N0404, Nanotag Biotechnologies) at 4°C for 24 hrs and with 1:100 HRP Alexa Fluor 647 at room temperature for 2hrs. Stained larvae were mounted in ProLong Diamond Antifade Mountant (#P36970; Thermo-Fisher Scientific, Waltham, MA, USA). Z-stacks were collected of Drosophila larval NMJs in muscle 4 of segment A5 at room temperature on a Nikon Ni-E upright microscope equipped with 100x (n.a. 1.45) oil immersion objective, a Yokogawa CSU-W1 spinning-disk head, and an Andor iXon 897U EMCCD camera. Images were collected using Nikon Elements AR software.
FRAP experiment
Imaging was conducted using a Nikon super-resolution spinning disk SoRA system in W1-spinning disk mode, equipped with an Apo 60x Oil DIC N2 objective and set to a 12-bit depth. We photobleached boutons in the middle of a synaptic branch to ensure BiP:sfGFP:HDEL could recover from both the top and bottom of the photobleached region. To capture the rapid recovery of BiP:sfGFP:HDEL, we imaged a single slice of NMJs at muscle 4 and sequentially acquired 488 and 561 channels with an exposure time of 100ms. For all NMJs, we photobleached single boutons within a 6.8×7.8 microns region in the middle of neuronal branch. Each movie acquired used an ND sequence acquisition consisting of three phases: (1) we acquired five frames at maximum speed (100Mhz) before photobleaching; (2) we photobleached BiP:sfGFP:HDEL and RFP:mCD8 or morphotrapInt using a 405 nm depletion laser with a dwell time of 100μs for a duration of 2s; and (3) we imaged the recovery of both BiP:sfGFP:HDEL and RFP:mCD8 or morphotrapInt with a 10s interval for 2 minutes for experiments in Figure 9 and 5 minutes for experiments in Figure 10.
Digitonin experiments
Digitonin experiments were conducted in live fillets from larvae expressing the luminal ER marker, UAS-BiP:sfGFP:HDEL, under the control of the neuronal driver Vglut-GAL4. Fillets were stained with 1:500 1.5 mg/ml HRP Alexa Fluor 594 (Jackson ImmunoResearch) or 1:250 HRP Alexa Fluor 647 in HL3.1 for 5 min, followed by a 1 min HL3.1 wash. Next, pre-digitonin Z-stacks were acquired from muscle 4 NMJs from segments A3 to A5 (both hemisegments) using the spinning disk confocal microscope described above. Fillets were subsequently incubated with 0.05% digitonin in HL3.1 for 5 min, then rinsed with HL3.1 and re-incubated with HRP Alexa Fluor 594 or HRP Alexa Fluor 647 for 5 min. Preps were then washed for 1 min with HL3.1. Finally, post-digitonin Z-stacks were acquired of the same NMJs.
Image analysis
Debris, axons, and large fluorescent aggregates (sometimes observed for tdTomato:Sec61β) were manually removed from all images before analyses of maximum projections or Z-stacks. Neuronal masks were created by first conducting a background subtraction of the image (rolling ball, 50 pixel radius), and then introducing a gaussian blur before thresholding using Otsu or Li algorithms. For live image analysis, maximum intensity projections of each timepoint were corrected for (1) photobleaching using histogram matching and (2) sample drift or muscle contraction using the StackReg plugin (Thevenaz et al., 1998).
Presynaptic levels of ER membrane marker tdTomato:Sec61β (Figure 3):
A custom FIJI macro consisting of the following steps was employed on maximum intensity projections,: (1) An NMJ mask was created using the α-HRP neuronal marker; (2) The integrated density of the ER membrane marker tdTomato:Sec61β was measured within the α-HRP mask; (3) The mean intensity of the ER membrane marker at presynaptic terminals was calculated by dividing the tdTomato:Sec61β integrated density by the area of the presynaptic terminal.
Analysis of NMJ morphology:
Satellite and total bouton numbers were manually counted from blinded maximum intensity projections of fixed and α-HRP labeled larvae. Satellite boutons were defined as any string of 5 or fewer boutons extending from the main axis of the NMJ.
Categorization of ER dynamics:
For dynamics analysis, we used the same dataset as the control in Figure 2, Figure 5, and Figure 5_Supplement. Maximum intensity projection timelapse movies were blinded and replayed 3–4 times before categorization. We identified three distinct categories of ER dynamics in presynaptic terminals of Drosophila motor neurons (note that a single bouton can exhibit multiple types of dynamics): (1) ER budding – small vesicle-like structures emerging from the ER network and re-fusing in a different region; (2) ER tubule dynamics, encompassing four subcategories as follows: (a) Tubule displacement, involving tubules that changed their localization within boutons without detaching from the ER network; (b) Tubule extension, where an ER tubule emerged and extended from the ER network; (c) Tubule retraction, characterized by the retraction and disappearance of an ER tubule from the ER network; and (d) Tubule extension and retraction, indicating an ER tubule both extending and retracting from the ER network within the timelapse. (3) Foci dynamics – round, bright puncta moving within the ER network and (4) static – boutons that lacked observable dynamics.
Presynaptic levels of luminal ER proteins at Drosophila motor neurons:
A custom FIJI macro consisting of the following steps was employed on Z-stacks: (1) We created an NMJ mask using the luminal ER protein marker. (2) Using this mask, we measured the neuron volume and the integrated density of the marker. (3) We calculated the mean intensity of the marker by dividing the integrated density by the volume of the presynaptic terminal.
Categorization of luminal ER marker distribution:
Images were blinded before analysis to ensure unbiased qualitative analysis of luminal ER marker BiP:sfGFP:HDEL in controls and Atlastin mutants. Maximum intensity projections were then sorted into three phenotypic categories: (1) “Partial loss” - luminal ER marker localizes to ER networks but is partially displaced to the cytosol, (2) “Complete loss” - luminal ER marker mostly displaced to the cytosol and ER networks are not detectable, and (3) “No phenotype” - luminal ER marker completely localizes to ER networks.
Presynaptic levels of cytosolic or luminal ER proteins pre- and post-digitonin treatment:
Images were blinded and maximum projections were created for further analysis. Neuronal outlines were obtained by manually tracing the α-HRP-positive region in FIJI. Next, the integrated density of UAS-GFP or UAS-BiP:sfGFP:HDEL was quantified within the manually traced neuronal outlines. Mean intensity was calculated by dividing the integrated density by the area of the presynaptic terminal.
BiP:sfGFP:HDEL levels before and after photobleaching:
Images were blinded for analysis. The 20 frames corresponding to the photobleaching event were removed since this prevented the Stackreg plugin from working correctly. Movies with uncorrectable drift were excluded. For the analysis, we drew 3 ROIs corresponding to the region that was photobleached, a region away from the bleach site and the background. A Fiji macro was then used to measure the mean intensities at these 3 regions per movie. The mean intensities at the site of photobleaching of BiP:sfGFP:HDEL, RFP:mCD8 or morphotrapInt were background subtracted and normalized to the mean intensity of the non-bleached region. We also used the same approach to measure the levels of BiP:sfGFP:HDEL before FRAP (Figure 10C).
Statistics
GraphPad Prism software was used to perform statistical analyses and generate graphs. Statistical analyses were conducted using parametric tests (e.g. one-way ANOVA, Student’s t-test) for normally distributed data, and non-parametric tests (e.g. Kruskal-Wallis, Mann-Whitney tests) if the data was not normally distributed. Chi-squared tests were used for categorical data. p-values were set to *<0.05, **<0.01, ***<0.001.
Supplementary Material
Acknowledgements
We thank the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN, NIH P40OD018537) and James McNew for fly lines, Steve Del Signore for help with image analysis, and Bruce Goode for use of the SoRA microscope. We also thank Chi-Kuang Yao for sharing reagents and stimulating discussion. This work was supported by NINDS grants R01 NS103967 to A.A.R., and T32 007292 and K99 NS136720-02 to M.C.Q.F.
Footnotes
Movies S1–3. Movies acquired at a frame rate of 0.65 frames per second of presynaptic terminals expressing the ER membrane marker tdTomato:Sec61β. These movies are played at a frame rate of 10 frames per second and the scale bar is 1 μm.
Movies S4–6: Movies acquired at a frame rate of 0.73 frames per second of presynaptic terminals expressing the ER membrane marker tdTomato:Sec61β. These movies are played at a frame rate of 10 frames per second and the scale bar is 1 μm.
Movie S7: Example movie of a static proximal bouton of controls expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S8: Example movie of a dynamic proximal bouton of controls expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S9: Example movie of a static terminal bouton of controls expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S10: Example movie of a dynamic terminal bouton of controls expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S11: Example movie of a static proximal bouton of Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S12: Example movie of a dynamic proximal bouton of Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S13: Example movie of a static terminal bouton of Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S14: Example movie of a dynamic terminal bouton of Atlastin mutants expressing the ER membrane marker tdTomato:Sec61β acquired at a frame rate of 0.92 seconds/stack. The movie is played at a frame rate of 5 frames per second and the scale bar is 2 μm.
Movie S15: Representative movie of a control NMJ expressing mCD8:RFP pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S16: Representative movie of a control NMJ expressing BiP:sfGFP:HDEL pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S17: Representative movie of an Atlastin mutant NMJ with partial loss expressing mCD8:RFP pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S18: Representative movie of an Atlastin mutant NMJ with partial loss expressing BiP:sfGFP:HDEL pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S19: Representative movie of an Atlastin mutant NMJ with complete loss expressing mCD8:RFP pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S20: Representative movie of an Atlastin mutant NMJ with complete loss expressing BiP:sfGFP:HDEL pre-bleach and after FRAP every 10 secs for 2 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S21: Representative movie of a control NMJ expressing morphotrapint pre-bleach and after FRAP every 10 secs for 5 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S22: Representative movie of a control NMJ expressing BiP:sfGFP:HDEL pre-bleach and after FRAP every 10 secs for 5 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S23: Representative movie of a Atlastin mutant NMJ expressing morphotrapint pre-bleach and after FRAP every 10 secs for 5 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
Movie S24: Representative movie of a Atlastin mutant NMJ expressing BiP:sfGFP:HDEL pre-bleach and after FRAP every 10 secs for 5 min. The movie is played at a frame rate of 5 frames per second, and the scale bar is 5 μm.
References:
- Aponte-Santiago NA, Ormerod KG, Akbergenova Y, Littleton JT. 2020. Synaptic Plasticity Induced by Differential Manipulation of Tonic and Phasic Motoneurons in Drosophila. J Neurosci 40:6270–6288. doi: 10.1523/JNEUROSCI.0925-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Desai A, Oegema K. 2007. A Role for Rab5 in Structuring the Endoplasmic Reticulum. The Journal of Cell Biology 178:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti L, Fan R, Weigel AV, Moore AS, Houlihan PR, Kittisopikul M, Park G, Petruncio A, Hubbard PM, Pang S, Xu CS, Hess HF, Saalfeld S, Rangaraju V, Clapham DE, Camilli PD, Ryan TA, Lippincott-Schwartz J. 2024. Periodic ER-plasma membrane junctions support long-range Ca2+ signal integration in dendrites. doi: 10.1101/2024.05.27.596121 [DOI] [PubMed] [Google Scholar]
- Blackstone C. 2018. Hereditary spastic paraplegia. Handbook of Clinical Neurology, Vol. 148:633–651. doi: 10.1016/B978-0-444-64076-5.00041-7 [DOI] [PubMed] [Google Scholar]
- Blackstone C. 2012. Cellular Pathways of Hereditary Spastic Paraplegia. Annu Rev Neurosci 35:25–47. doi: 10.1146/annurev-neuro-062111-150400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C, O’Kane CJ, Reid E. 2011. Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat Rev Neurosci 12:31–42. doi: 10.1038/nrn2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M, Morais S, Stevanin G. 2019. Update on the Genetics of Spastic Paraplegias. Curr Neurol Neurosci Rep 19:18. doi: 10.1007/s11910-019-0930-2 [DOI] [PubMed] [Google Scholar]
- Burgoyne T, Patel S, Eden ER. 2015. Calcium signaling at ER membrane contact sites. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 13th European Symposium on Calcium 1853:2012–2017. doi: 10.1016/j.bbamcr.2015.01.022 [DOI] [PubMed] [Google Scholar]
- Carter SD, Hampton CM, Langlois R, Melero R, Farino ZJ, Calderon MJ, Li W, Wallace CT, Tran NH, Grassucci RA, Siegmund SE, Pemberton J, Morgenstern TJ, Eisenman L, Aguilar JI, Greenberg NL, Levy ES, Yi E, Mitchell WG, Rice WJ, Wigge C, Pilli J, George EW, Aslanoglou D, Courel M, Freyberg RJ, Javitch JA, Wills ZP, Area-Gomez E, Shiva S, Bartolini F, Volchuk A, Murray SA, Aridor M, Fish KN, Walter P, Balla T, Fass D, Wolf SG, Watkins SC, Carazo JM, Jensen GJ, Frank J, Freyberg Z. 2020. Ribosome-associated vesicles: A dynamic subcompartment of the endoplasmic reticulum in secretory cells. Sci Adv 6:eaay9572. doi: 10.1126/sciadv.aay9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino H, Mizushima N. 2020. ER-Phagy: Quality Control and Turnover of Endoplasmic Reticulum. Trends in Cell Biology 30:384–398. doi: 10.1016/j.tcb.2020.02.001 [DOI] [PubMed] [Google Scholar]
- Chouhan AK, Zhang J, Zinsmaier KE, Macleod GT. 2010. Presynaptic Mitochondria in Functionally Different Motor Neurons Exhibit Similar Affinities for Ca2+ But Exert Little Influence as Ca2+ Buffers at Nerve Firing Rates In Situ. J Neurosci 30:1869–1881. doi: 10.1523/JNEUROSCI.4701-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JC, Jarosch E, Sommer T. 2023. Mechanisms of substrate processing during ER-associated protein degradation. Nat Rev Mol Cell Biol 1–20. doi: 10.1038/s41580-023-00633-8 [DOI] [PubMed] [Google Scholar]
- Costantini LM, Fossati M, Francolini M, Snapp EL. 2012. Assessing the Tendency of Fluorescent Proteins to Oligomerize under Physiologic Conditions. Traffic 13:643–649. doi: 10.1111/j.1600-0854.2012.01336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapart C, Scott ZC, Konno T, Sharma A, Bailey DM, Avezov E, Koslover EF. 2023. Luminal transport rates through intact endoplasmic reticulum limit the magnitude of localized Ca2+ signals. doi: 10.1101/2023.06.23.546357 [DOI] [PMC free article] [PubMed]
- Dayel MJ, Hom EFY, Verkman AS. 1999. Diffusion of Green Fluorescent Protein in the Aqueous-Phase Lumen of Endoplasmic Reticulum. Biophysical Journal 76:2843–2851. doi: 10.1016/S0006-3495(99)77438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Juan-Sanz J, Holt GT, Schreiter ER, de Juan F, Kim DS, Ryan TA. 2017. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron 93:867–881.e6. doi: 10.1016/j.neuron.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Signore SJ, Mitzner MG, Silveira AM, Fai TG, Rodal AA. 2022. An approach for quantitative mapping of synaptic periactive zone architecture and organization. Mol Biol Cell mbcE22080372. doi: 10.1091/mbc.E22-08-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Moradi M, Reinhard S, Ji C, Jablonka S, Hennlein L, Lüningschrör P, Doose S, Sauer M, Sendtner M. 2021. Dynamic remodeling of ribosomes and endoplasmic reticulum in axon terminals of motoneurons. Journal of Cell Science 134:jcs258785. doi: 10.1242/jcs.258785 [DOI] [PubMed] [Google Scholar]
- Deshpande M, Feiger Z, Shilton AK, Luo CC, Silverman E, Rodal AA. 2016. Role of BMP receptor traffic in synaptic growth defects in an ALS model. MBoC 27:2898–2910. doi: 10.1091/mbc.E16-07-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadas J, Pendin D, Bocanegra R, Escalada A, Misticoni G, Trevisan T, Velasco del Olmo A, Montagna A, Bova S, Ibarra B, Kuzmin PI, Bashkirov PV, Shnyrova AV, Frolov VA, Daga A. 2019. Dynamic constriction and fission of endoplasmic reticulum membranes by reticulon. Nat Commun 10:5327. doi: 10.1038/s41467-019-13327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu C-F. 2004. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet 18:377–402. doi: 10.1080/01677060490894522 [DOI] [PubMed] [Google Scholar]
- Fernandes AR, Martins JP, Gomes ER, Mendes CS, Teodoro RO. 2023. Drosophila motor neuron boutons remodel through membrane blebbing coupled with muscle contraction. Nat Commun 14:3352. doi: 10.1038/s41467-023-38421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Löscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. 2012. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. Journal of the Neurological Sciences 318:1–18. doi: 10.1016/j.jns.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Fowler PC, Garcia-Pardo ME, Simpson JC, O’Sullivan NC. 2019. NeurodegenERation: The Central Role for ER Contacts in Neuronal Function and Axonopathy, Lessons From Hereditary Spastic Paraplegias and Related Diseases. Front Neurosci 13. doi: 10.3389/fnins.2019.01051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. 2011. ER tubules mark sites of mitochondrial division. Science 334:358–362. doi: 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glock C, Biever A, Tushev G, Nassim-Assir B, Kao A, Bartnik I, tom Dieck S, Schuman EM. 2021. The translatome of neuronal cell bodies, dendrites, and axons. Proceedings of the National Academy of Sciences 118:e2113929118. doi: 10.1073/pnas.2113929118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Heerssen HM, Wright CM, Davis GW, DiAntonio A. 2011. Stathmin is Required for Stability of the Drosophila Neuromuscular Junction. J Neurosci 31:15026–15034. doi: 10.1523/JNEUROSCI.2024-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio CD, Delgado R, Ibacache A, Sierralta J, Couve A. 2017. Drosophila Atlastin in motor neurons is required for locomotion and presynaptic function. J Cell Sci 130:3507–3516. doi: 10.1242/jcs.201657 [DOI] [PubMed] [Google Scholar]
- Harmansa S, Alborelli I, Bieli D, Caussinus E, Affolter M. 2017. A nanobody-based toolset to investigate the role of protein localization and dispersal in Drosophila. eLife 6:e22549. doi: 10.7554/eLife.22549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcman D, Parutto P, Chambers JE, Fantham M, Young LJ, Marciniak SJ, Kaminski CF, Ron D, Avezov E. 2018. Single particle trajectories reveal active endoplasmic reticulum luminal flow. Nat Cell Biol 20:1118–1125. doi: 10.1038/s41556-018-0192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer MJ, Chitwood PJ, Ebmeier CC, Striepen JF, Qi RZ, Old WM, Voeltz GK. 2018. A Novel Class of ER Membrane Proteins Regulates ER-Associated Endosome Fission. Cell 175:254–265.e14. doi: 10.1016/j.cell.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Zhu P-P, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. 2009. A Class of Dynamin-like GTPases Involved in the Generation of the Tubular ER Network. Cell 138:549–561. doi: 10.1016/j.cell.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Wu F, Sun S, Yu W, Hu J. 2015. Human atlastin GTPases mediate differentiated fusion of endoplasmic reticulum membranes. Protein Cell 6:307–311. doi: 10.1007/s13238-015-0139-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbaria A, Merksamer PI, Trusina A, Tilahun F, Johnson JR, Brandman O, Krogan NJ, Weissman JS, Papa FR. 2019. Chaperone-mediated reflux of secretory proteins to the cytosol during endoplasmic reticulum stress. Proc Natl Acad Sci U S A 116:11291–11298. doi: 10.1073/pnas.1904516116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Sengupta P, Seo AY, Lippincott-Schwartz J. 2020. ER membranes exhibit phase behavior at sites of organelle contact. Proceedings of the National Academy of Sciences 117:7225–7235. doi: 10.1073/pnas.1910854117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y-S, Stefano G, Brandizzi F. 2014. ER stress signaling requires RHD3, a functionally conserved ER-shaping GTPase. Journal of Cell Science 127:3227–3232. doi: 10.1242/jcs.147447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Snapp EL. 2020. Size-dependent secretory protein reflux into the cytosol in association with acute endoplasmic reticulum stress. Traffic 21:419–429. doi: 10.1111/tra.12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Neuhof A, Rapoport T. 2004. The Endoplasmic Reticulum Membrane Is Permeable to Small Molecules. MBoC 15:447–455. doi: 10.1091/mbc.e03-05-0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Paik SK, Lee M-J, Kim Y-J, Kim S, Nahm M, Oh S-J, Kim H-M, Yim J, Lee CJ, Bae YC, Lee S. 2009. Drosophila Atlastin regulates the stability of muscle microtubules and is required for synapse development. Developmental Biology 330:250–262. doi: 10.1016/j.ydbio.2009.03.019 [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Snapp EL, Phair RD. 2018. The Development and Enhancement of FRAP as a Key Tool for Investigating Protein Dynamics. Biophys J 115:1146–1155. doi: 10.1016/j.bpj.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Guo X, Niu L, Li X, Sun F, Hu J, Wang X, Shen K. 2019. Atlastin-1 regulates morphology and function of endoplasmic reticulum in dendrites. Nat Commun 10:1–15. doi: 10.1038/s41467-019-08478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Ladinsky MS, Kirchhausen T. 2009. Cisternal Organization of the Endoplasmic Reticulum during Mitosis. MBoC 20:3471–3480. doi: 10.1091/mbc.e09-04-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü L, Niu L, Hu J. 2020. “At last in” the physiological roles of the tubular ER network. Biophys Rep 6:105–114. doi: 10.1007/s41048-020-00113-y [DOI] [Google Scholar]
- Lu M, Tartwijk FW van, Lin JQ, Nijenhuis W, Parutto P, Fantham M, Christensen CN, Avezov E, Holt CE, Tunnacliffe A, Holcman D, Kapitein L, Schierle GSK, Kaminski CF. 2020. The structure and global distribution of the endoplasmic reticulum network are actively regulated by lysosomes. Science Advances 6:eabc7209. doi: 10.1126/sciadv.abc7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin C, Mares A, Piacenti M, Williams H, Roboti P, Puumalainen M, Callan AC, Lesiak-Mieczkowska K, Linder S, Harant H, High S, Flitsch SL, Whitehead RC, Swanton E. 2012. Inhibition of protein translocation at the endoplasmic reticulum promotes activation of the unfolded protein response. Biochemical Journal 442:639–648. doi: 10.1042/BJ20111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Lossi L. 2022. Endoplasmic Reticulum Stress Signaling and Neuronal Cell Death. International Journal of Molecular Sciences 23:15186. doi: 10.3390/ijms232315186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu L, Ma T, Yang F, Yan B, Tang X, Yin H, Wu Q, Huang Y, Yao Z-P, Wang J, Guo Y, Hu J. 2019. Atlastin-mediated membrane tethering is critical for cargo mobility and exit from the endoplasmic reticulum. PNAS 116:14029–14038. doi: 10.1073/pnas.1908409116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara CJ, Nixon-Abell J, Moore AS, Riccio F, Hoffman DP, Shtengel G, Xu CS, Schaefer K, Pasolli HA, Masson J-B, Hess HF, Calderon CP, Blackstone C, Lippincott-Schwartz J. 2024. Motion of VAPB molecules reveals ER-mitochondria contact site subdomains. Nature 626:169–176. doi: 10.1038/s41586-023-06956-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva MK, Pérez-Moreno JJ, O’Shaughnessy J, Wardill TJ, O’Kane CJ. 2020. Endoplasmic Reticulum Lumenal Indicators in Drosophila Reveal Effects of HSP-Related Mutations on Endoplasmic Reticulum Calcium Dynamics. Front Neurosci 14. doi: 10.3389/fnins.2020.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A. 2009. Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature 460:978–983. doi: 10.1038/nature08280 [DOI] [PubMed] [Google Scholar]
- O’Sullivan NC, Jahn TR, Reid E, O’Kane CJ. 2012. Reticulon-like-1, the Drosophila orthologue of the Hereditary Spastic Paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum Mol Genet 21:3356–3365. doi: 10.1093/hmg/dds167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan N, Koppers M, van Soest I, van Harten A, Jurriens D, Liv N, Klumperman J, Kapitein LC, Hoogenraad CC, Farías GG. 2021. ER - lysosome contacts at a pre-axonal region regulate axonal lysosome availability. Nat Commun 12:4493. doi: 10.1038/s41467-021-24713-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk Z, O’Kane CJ, Pérez-Moreno JJ. 2020. Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration. Frontiers in Neuroscience 14:1–33. doi: 10.3389/fnins.2020.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alvarez A, Yin S, Schulze C, Hammer JA, Wagner W, Oertner TG. 2020. Endoplasmic reticulum visits highly active spines and prevents runaway potentiation of synapses. Nature Communications 11:5083. doi: 10.1038/s41467-020-18889-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Moreno JJ, Smith RC, Oliva MK, Gallo F, Ojha S, Müller KH, O’Kane CJ. 2023. Drosophila SPG12 ortholog, reticulon-like 1, governs presynaptic ER organization and Ca2+ dynamics. J Cell Biol 222:e202112101. doi: 10.1083/jcb.202112101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. 2016. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends in Biochemical Sciences 41:1035–1049. doi: 10.1016/j.tibs.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, Johansen T, Stenmark H. 2015. Repeated ER–endosome contacts promote endosome translocation and neurite outgrowth. Nature 520:234–238. doi: 10.1038/nature14359 [DOI] [PubMed] [Google Scholar]
- Rao K, Stone MC, Weiner AT, Gheres KW, Zhou C, Deitcher DL, Levitan ES, Rolls MM. 2016. Spastin, atlastin, and ER relocalization are involved in axon but not dendrite regeneration. MBoC 27:3245–3256. doi: 10.1091/mbc.E16-05-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read A, Schröder M. 2021. The Unfolded Protein Response: An Overview. Biology (Basel) 10:384. doi: 10.3390/biology10050384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rismanchi N, Soderblom C, Stadler J, Zhu P-P, Blackstone C. 2008. Atlastin GTPases are required for Golgi apparatus and ER morphogenesis. Hum Mol Genet 17:1591–1604. doi: 10.1093/hmg/ddn046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klämbt C, Davis GW. 2000. Drosophila Futsch Regulates Synaptic Microtubule Organization and Is Necessary for Synaptic Growth. Neuron 26:371–382. doi: 10.1016/S0896-6273(00)81170-8 [DOI] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. 2014. ER Contact Sites Define the Position and Timing of Endosome Fission. Cell 159:1027–1041. doi: 10.1016/j.cell.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz DS, Blower MD. 2016. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73:79–94. doi: 10.1007/s00018-015-2052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ZC, Brown AI, Mogre SS, Westrate LM, Koslover EF. 2021. Diffusive search and trajectories on tubular networks: a propagator approach. Eur Phys J E 44:80. doi: 10.1140/epje/s10189-021-00083-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Mazur EE, Pan B, Paulo JA, Gygi SP, Chavan S, Valerio LSA, Zhang J, Rapoport TA. 2024. The membrane curvature-inducing REEP1–4 proteins generate an ER-derived vesicular compartment. Nat Commun 15:8655. doi: 10.1038/s41467-024-52901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari D, Centonze FG, Pineau R, Le Reste P- J, Negroni L, Chat S, Mohtar MA, Thomas D, Gillet R, Hupp T, Chevet E, Igbaria A. 2021. Reflux of Endoplasmic Reticulum proteins to the cytosol inactivates tumor suppressors. EMBO Rep 22:e51412. doi: 10.15252/embr.202051412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow PM, Patel NH, Harrelson AL, Goodman CS. 1987. Neural-specific carbohydrate moiety shared by many surface glycoproteins in Drosophila and grasshopper embryos. J Neurosci 7:4137–4144. doi: 10.1523/JNEUROSCI.07-12-04137.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits M, Neefjes J. 2021. Mobile late endosomes modulate peripheral endoplasmic reticulum network architecture. EMBO reports 22:e50815. doi: 10.15252/embr.202050815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian K, Meyer T. 1997. Calcium-Induced Restructuring of Nuclear Envelope and Endoplasmic Reticulum Calcium Stores. Cell 89:963–971. doi: 10.1016/S0092-8674(00)80281-0 [DOI] [PubMed] [Google Scholar]
- Summerville JB, Faust JF, Fan E, Pendin D, Daga A, Formella J, Stern M, McNew JA. 2016. The effects of ER morphology on synaptic structure and function in Drosophila melanogaster. J Cell Sci 129:1635–1648. doi: 10.1242/jcs.184929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yu F, Ullah A, Hubrack S, Daalis A, Jung P, Machaca K. 2011. Endoplasmic Reticulum Remodeling Tunes IP3-Dependent Ca2+ Release Sensitivity. PLOS ONE 6:e27928. doi: 10.1371/journal.pone.0027928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. 1998. A pyramid approach to subpixel registration based on intensity. IEEE Trans on Image Process 7:27–41. doi: 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- Wang S, Tukachinsky H, Romano FB, Rapoport TA. 2016. Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. eLife 5:e18605. doi: 10.7554/eLife.18605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Olberding KE, White C, Li C. 2011. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ 18:38–47. doi: 10.1038/cdd.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Rapoport TA. 2018. Mechanistic insights into ER-associated protein degradation. Curr Opin Cell Biol 53:22–28. doi: 10.1016/j.ceb.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF, Camilli PD. 2017. Contacts between the endoplasmic reticulum and other membranes in neurons. PNAS 114:E4859–E4867. doi: 10.1073/pnas.1701078114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalçın B, Zhao L, Stofanko M, O’Sullivan NC, Kang ZH, Roost A, Thomas MR, Zaessinger S, Blard O, Patto AL, Sohail A, Baena V, Terasaki M, O’Kane CJ. 2017. Modeling of axonal endoplasmic reticulum network by spastic paraplegia proteins. eLife 6:1–27. doi: 10.7554/eLife.23882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi E, Meehl JB, Voeltz GK. 2022. The ER ladder is a unique morphological feature of developing mammalian axons. Dev Cell 57:1369–1382.e6. doi: 10.1016/j.devcel.2022.05.002 [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhu P-P, Renvoisé B, Maldonado-Báez L, Park SH, Blackstone C. 2016. Mammalian knock out cells reveal prominent roles for atlastin GTPases in ER network morphology. Experimental Cell Research 349:32–44. doi: 10.1016/j.yexcr.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Zhu P-P, Hung H-F, Batchenkova N, Nixon-Abell J, Henderson J, Zheng P, Renvoisé B, Pang S, Xu CS, Saalfeld S, Funke J, Xie Y, Svara F, Hess HF, Blackstone C. 2022. Transverse endoplasmic reticulum expansion in hereditary spastic paraplegia corticospinal axons. Hum Mol Genet 31:2779–2795. doi: 10.1093/hmg/ddac072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P-P, Soderblom C, Tao-Cheng J-H, Stadler J, Blackstone C. 2006. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum Mol Genet 15:1343–1353. doi: 10.1093/hmg/ddl054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.