Abstract
Lautropia mirabilis, a pleomorphic, motile, gram-negative coccus, has been isolated from the oral cavities of 32 of 60 (53.3%) children infected with human immunodeficiency virus (HIV) and 3 of 25 (12.0%) HIV-uninfected controls; the association of L. mirabilis isolation with HIV infection is significant (P < 0.001). All children in the study, both HIV-infected children and controls, were born to HIV-infected mothers. The presence of this bacterium was not associated with clinical disease in these children. The HIV-infected children with L. mirabilis did not differ from the HIV-infected children without L. mirabilis in immunological status, clinical status, or systemic medications. The role of HIV infection itself or concomitant factors in the establishment of L. mirabilis in the oral cavity remains to be elucidated.
Lautropia mirabilis, a recently characterized motile gram-negative coccus, has been isolated from oral and pulmonary sites (4). Its pathogenicity is unknown, as it has been recovered from both ill and healthy persons. This report describes the isolation of L. mirabilis from the oral cavities of human immunodeficiency virus (HIV)-infected children and its absence in noninfected children. This association suggests a possible link between immunocompromise and oral colonization with L. mirabilis.
L. mirabilis was described in 1994 by P. Gerner-Smidt et al. (4) and subsequently was given species status (6). The organism is gram negative, facultatively anaerobic, and oxidase positive, with cell morphology varying from coccobacilli 1 μm in diameter to spheroblast-like forms more than 10 μm in diameter. The colonies are pleomorphic. This organism appears to be identical to Sarcina mirabilis, described by Orskov in 1930 (7). Both Orskov and Gerner-Smidt et al. isolated the organism from oral or upper respiratory sites. Subsequently, it has been isolated as the predominant microorganism from the sputum of one Australian patient with cystic fibrosis (1).
MATERIALS AND METHODS
Population.
The study population consisted of 60 children, ranging in age from newborn to 12 years, who had been infected with HIV vertically, i.e., who were born to infected mothers. HIV culture and/or PCR determined the infection status of the children. The control population consisted of 25 uninfected children born to HIV-infected mothers. This prospective study was designed to evaluate the scope and possible causes of oral lesions in HIV-infected children, as oral disease is a cause of significant morbidity in HIV-infected children and adults.
Clinical evaluation.
Each child received a complete oral examination by a pediatric dentist at entry into the study and every 6 months subsequently, or at the time of any illness. During the examination, aerobic and anaerobic cultures were initiated.
Immunological categories for children infected with HIV, as defined by the Centers for Disease Control and Prevention (CDC) (3), range from no immune suppression to severe suppression, based on age-specific CD4 T-lymphocyte counts and percentages of total lymphocytes. The children were categorized by immunologic status at entry into the study. HIV-infected children can also be divided into several CDC-defined standard clinical categories based on disease severity (3). Categories range from not symptomatic to severely symptomatic, depending on the presence of associated conditions and specific infections. Clinical status was measured at entry into the study.
Microbiology.
The gingivae or alveolar mucosae of patients and controls were swabbed, and the swabs were transported to the laboratory in an S/P Culturette (Baxter Diagnostics, Deerfield, Ill.). The specimens were inoculated immediately upon receipt at the Texas Children’s Hospital Microbiology Laboratory onto a variety of media chosen to identify common oral organisms and were subsequently processed in a dedicated portion of the laboratory.
Biochemical identification.
Standard biochemical methods were used. The production of urease was determined by using a heavy inoculum on Christensen’s urea agar slant (Becton Dickinson, Cockeysville, Md.), with incubation at 35°C for 24 to 72 h. Urease was also tested for with Christensen’s urea broth (rapid urea broth; Becton Dickinson) and with the API 20E system (bioMérieux Vitek, Hazelwood, Mo.). Catalase was tested for with 10 and 3% hydrogen peroxide.
Electron microscopy.
Colonies of L. mirabilis were obtained from MacConkey’s II agar (Becton Dickinson) and fixed in 3% glutaraldehyde for 24 h. Postfixation staining was carried out for 90 min in 1% osmium tetroxide, followed by 2% uranyl acetate. The colonies were dehydrated and embedded in plastic, and thin sections were produced for ultrastructural examination.
Statistical analysis.
The statistical significance of associations was tested with the chi-square test, or Fisher’s exact test when the expected cell count was <5. Mean values were compared by the t test.
RESULTS
L. mirabilis was initially isolated from MacConkey’s II agar at 35°C in 6% CO2. The organism was visible in 48 to 72 h. On primary isolation on this agar the colony was circular, less than 1 mm in diameter, and dark purplish; it had a rough surface and was extremely pitted in the agar. With further incubation, the colony increased to 1 to 2 mm in diameter and became irregular in shape, lighter purple, and less pitted in the agar (Fig. 1A). The organism was successfully reisolated on Sabouraud dextrose agar (Emmons modification) (Becton Dickinson), chocolate II agar with bacitracin (Becton Dickinson), and TSA II–5% sheep blood agar (Becton Dickinson). CO2 was not required for growth, and the organism grew at room temperature as well as at 35°C. Subsequent work has shown that growth is best on Regan-Lowe charcoal agar (Becton Dickinson). L. mirabilis was not recovered from specimens processed anaerobically.
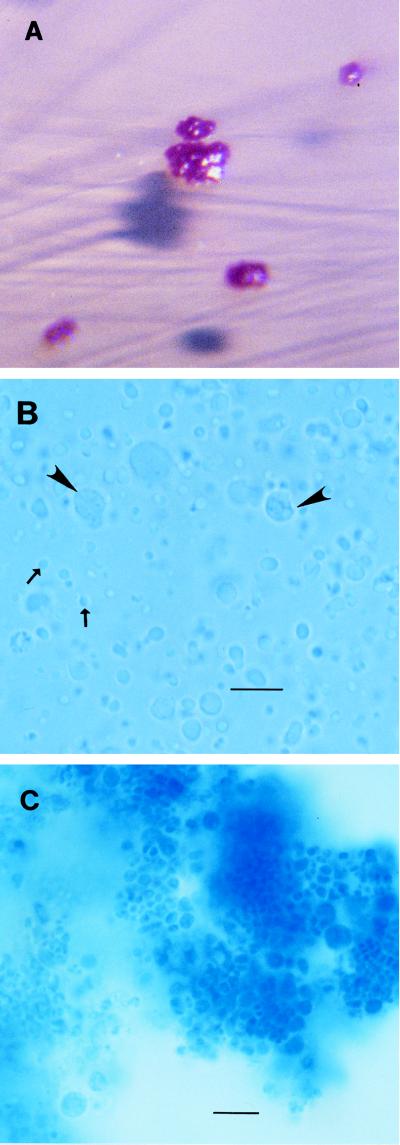
FIG. 1.
(A) L. mirabilis colonies on MacConkey’s agar. (B) Very small forms (1 to 2 μm in diameter; arrows) of L. mirabilis with larger forms (up to 10 μm in diameter; arrowheads). The staining was with lactophenol cotton blue. Magnification, ×100 (bar = 10 μm). (C) Large aggregates of L. mirabilis organisms, with individual organisms at the periphery. The staining was with lactophenol cotton blue. Magnification, ×100 (bar = 10 μm).
Wet mounts of L. mirabilis demonstrated the morphological forms that have previously been described (4). Very small (approximately 1 μm in diameter) round forms frequently showed a rapid, circular motility. Individual cells of larger size, up to 10 μm in diameter, often with a vaguely perceptible internal structure (Fig. 1B), and large aggregates (>10 μm in diameter) of nonmotile cells of various sizes were also seen (Fig. 1C). All forms were gram negative. The cells were also well visualized by lactophenol cotton blue staining; this stain is generally used in mycology and yields results comparable to those with methylene blue.
On several media, L. mirabilis had two colony morphologies: a smooth, mucoid form and a drier, rough form. These morphologies corresponded very approximately to the microscopic morphology, as the small, motile forms were more likely to come from the mucoid colonies. The best long-term viability was noted in Trypticase soy broth with 20% glycerol (Becton Dickinson) at −70°C. The growth of L. mirabilis was sometimes poor, and the organism was resistant to multiple reisolations. Confirmation of the species was provided for three of the isolates by Brita Bruun of the Statens Serum Institut in Copenhagen, Denmark (2).
Biochemical identification.
L. mirabilis was positive for oxidase, urease, catalase, nitrate reduction, esculin, acid formation from glucose, mannitol, sucrose, and maltose. L. mirabilis was negative for o-nitrophenyl-β-d-galactopyranoside, ornithine decarboxylase, citrate utilization, tryptophan deaminase, indole, H2S production, acid formation from inositol, rhamnose, melibiose, amygdalin, arabinose, lactose, inulin, and raffinose. The urease test was positive on Christensen’s urea agar slants or rapid urea broth but was often negative with the API 20E system. The catalase test was weakly positive with 10% hydrogen peroxide, and catalase was undetectable with 3% H2O2. Variable reactions were observed for arginine dihydrolase, lysine decarboxylase, Voges-Proskauer test, gelatin at 35°C, and sorbitol.
Electron microscopy.
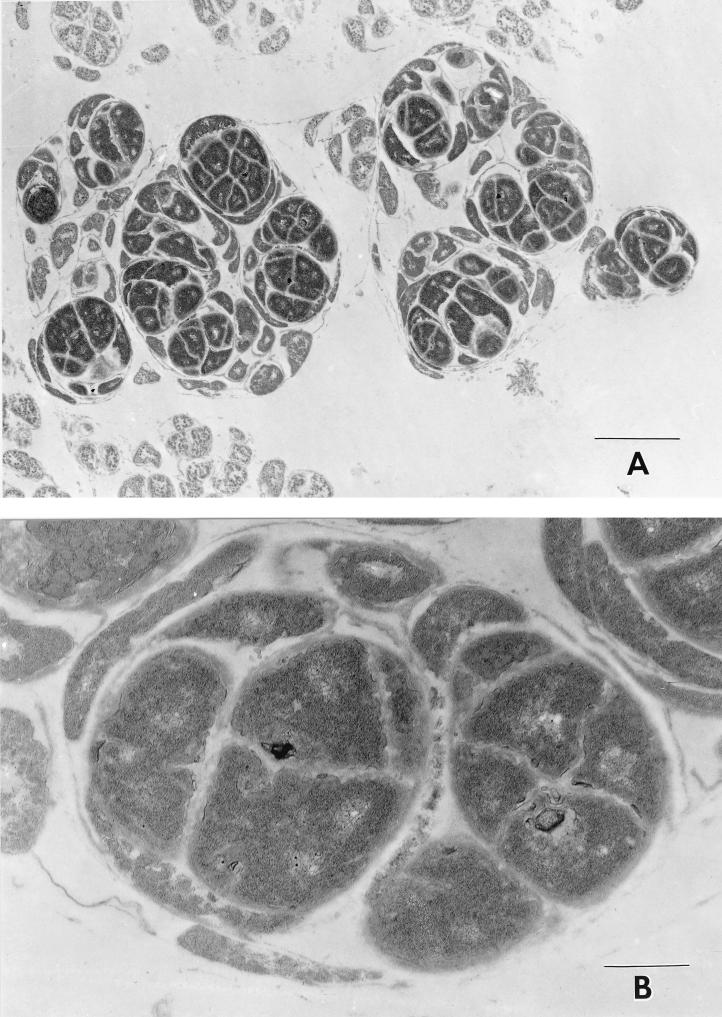
An ultrastructural examination of organisms obtained from the colonies showed large groups of irregular cells surrounded by a thin surface membrane which in many locations was either lost or disrupted (Fig. 2). These large groups of cells measured approximately 2 to 3.5 μm in diameter in aggregate, with individual cells having maximum dimensions varying from 0.5 to 1.2 μm. Septa of intermediate electron density divided the cells. The internal regions of the cells were composed of fine, granular, homogenous, electron-dense material with only occasional myeloid-like figures and fine punctate dense granules. The cells appeared to divide by binary fission. The cell walls were generally characteristic of those described for gram-negative organisms. No flagella or fimbria were identified in the specimens examined, although these have been previously reported (4).
FIG. 2.
Electron microscopic appearance of L. mirabilis colonies at two magnifications. Bars, 2.0 μm (A) and 0.5 μm (B).
Culture.
Of 85 subjects evaluated in this study, 35 (41.4%) harbored cultivable L. mirabilis organisms at some time during the study. L. mirabilis was recovered on two separate occasions from nine of these individuals. No clinical manifestations of this colonization were found. Specifically, those infected showed no increase in the number of oral lesions.
A strong association between HIV infection and the presence of L. mirabilis was found (Table 1). Only 3 of 35 subjects from which L. mirabilis was isolated were not infected with HIV. All three of these children lived with their biological mothers, who were HIV infected. One of these children also had an HIV-infected sibling, who was also infected with L. mirabilis.
TABLE 1.
Prevalence of L. mirabilis in HIV-infected and uninfected childrena
| HIV status | No. (%) of children whose L. mirabilis cultures were:
|
Total | |
|---|---|---|---|
| Negative | Positive | ||
| Infected | 28 (46.7) | 32 (53.3) | 60 (100) |
| Uninfected | 22 (88.0) | 3 (12.0) | 25 (100) |
| Total | 50 | 35 | 85 |
χ2 = 12.447 with 1 df; P < 0.001.
Forty-four of the 85 subjects were male, of whom 21 (48%) had L. mirabilis, 14 of the 41 female subjects (34%) harbored the organism (P = 0.2). Also, differences by race and ethnicity were not statistically significant; most (61 of 85) children in the study were African-American.
When the analysis was limited to the children who were HIV infected, the mean age at the time of culture of those who harbored L. mirabilis (62.6 months) was not significantly different from that of the HIV-infected children who did not harbor L. mirabilis (55.0 months; P = 0.24). The three HIV-negative children who had L. mirabilis were 18.6, 24.0, and 28.1 months old at the time of positive culture.
In terms of the CDC categories for the immunological competence of the HIV-infected children, 6 of 9 of those with no suppression, 14 of 24 of those with moderate suppression, and 12 of 27 of those with severe suppression harbored L. mirabilis (P = 0.5). For HIV-infected children, the mean CD4 percentage, averaged over all observations for each subject, was 21.6% for those from whom L. mirabilis had never been isolated and 21.5% for those from whom L. mirabilis had been isolated at least once (P = 0.97). Similarly, the mean CD8 percentage of those negative for the organism was 40.1%, while those positive had a CD8 percentage of 37.1% (P = 0.38). Grouping the subjects by mean CD4 percentage also did not yield significant differences (data not shown). Finally, a logistic regression analyzing HIV infection status, CD4 percentage, and age yielded only HIV status as a significant variable (P = 0.0001).
In the group of HIV-positive children who were asymptomatic by CDC criteria, seven of nine harbored L. mirabilis. L. mirabilis infection was found in 8 of 11 children in the mildly symptomatic group, in 12 of 25 in the moderately affected group, and in 5 of 15 in the severely affected group. This pattern does not reach statistical significance (P = 0.10).
Neither at the time of entry into the study nor at the time of culture was there an association between the presence of L. mirabilis and the child’s use of systemic medications. Children who were using nystatin oral suspension, commonly prescribed as prophylaxis for oral infections with Candida spp., rarely developed L. mirabilis colonization, but the numbers in this study were not large enough for this observation to reach statistical significance (data not shown).
DISCUSSION
L. mirabilis is an unusual organism in many respects. Both its colonial and microscopic morphologies are striking. Optimal growth conditions remain uncertain; other workers have not successfully isolated this organism on MacConkey’s media (1, 4). The range of biochemical reactions included within strains of this species has also not been fully defined.
Clinically, L. mirabilis has generally been considered a saprophyte (4), although its isolation has occurred in several patients with respiratory disease (1, 4). In our study it has been isolated from a large number of children; this series encompasses the largest number of isolations of L. mirabilis reported to date. No specific disease manifestation has been identified with this unusual bacterium. However, a strong association between the presence of cultivable L. mirabilis and HIV infection in these children is seen.
Recent work on the transmission of oral pathogens has shown a close identity at the molecular level between strains found in children and those found in their parents (5). No direct evidence for the presence of L. mirabilis in the parents of the children in this study is available, as specimens from the parents were not cultured.
In an attempt to elucidate the basis and mechanism of the strong association between HIV and L. mirabilis, several other differences between the infected and uninfected children were considered. Sex did not appear to be an important variable in the acquisition of Lautropia. The ages of the HIV-positive subjects were generally greater than those of the HIV-negative controls; this is a natural effect of the success of current therapy in reducing HIV transmission from HIV-infected pregnant women to their children. Within the HIV-infected group, age was not a significant variable. Data from this study do not suggest that the acquisition of L. mirabilis is simply an age-related phenomenon. However, the effect of age cannot be clearly determined from these limited data.
The lack of correlation between the presence of L. mirabilis and immunological or clinical status could be due to medications, as many children with more advanced disease were receiving antibacterial or antifungal treatment, either for clinical disease or as prophylaxis. Such drugs might affect the usual oral flora, resulting, for example, in increased growth of L. mirabilis by suppression of more numerous and commonly found organisms. An initial examination of the oral flora of these children did not reveal major differences between HIV-infected and uninfected children which might account for the difference in L. mirabilis isolation (8). The HIV-infected children in this study took a wide variety of anti-infective drugs. Of special interest is the possible effect of topical oral agents on the growth of L. mirabilis. The organism has been seen to be sensitive to a wide variety of antimicrobial agents (1, 4). Such an effect would not, of course, account for the lack of L. mirabilis in the noninfected population, as these children would not be receiving this medication. However, this or similar topical agents may play a role in the reduced incidence of L. mirabilis seen in the most severely immunocompromised and most severely clinically affected groups.
The incidence of oral and periodontal disease in HIV-infected individuals is striking, and the possible role of this organism remains to be investigated. Further work is also needed to clarify the role of topical oral agents such as nystatin in the presence of this organism. The association between HIV and L. mirabilis is fascinating, suggesting a possible role for immune function in the acquisition of this little-understood organism.
ACKNOWLEDGMENTS
We are grateful to Lynne Sigler, of the University of Alberta, Edmonton, Canada, for suggesting the identification of this organism. Brita Bruun at the Statens Serum Institut confirmed our identification of three isolates. Peter Gerner-Smidt of the same institute was also helpful. Margaret Price of St. Luke’s Episcopal Hospital, Houston, Tex., performed the macrophotography.
This study was supported by grant NIH-NIDR-1-RO1-DE11363 from the National Institute of Dental Research, National Institutes of Health.
REFERENCES
- 1.Ben Dekhil S M, Peel M M, Lennox V A, Stackebrandt E, Sly L I. Isolation of Lautropia mirabilis from sputa of a cystic fibrosis patient. J Clin Microbiol. 1997;35:1024–1026. doi: 10.1128/jcm.35.4.1024-1026.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruun, B. Personal communication.
- 3.Centers for Disease Control and Prevention. 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 4.Gerner-Smidt P, Keiser-Nielsen H, Dorsch M, Stackebrandt E, Ursing J, Blom J, Christensen A S, Christensen J J, Frederiksen W, Hoffmann S, Holten-Andersen W, Ying Y T. Lautropia mirabilis gen. nov., sp. nov., a Gram-negative motile coccus with unusual morphology isolated from the human mouth. Microbiology. 1994;140:1787–1797. doi: 10.1099/13500872-140-7-1787. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein G, Lamster I. Bacterial transmission in periodontal diseases: a critical review. J Periodontol. 1997;68:421–431. doi: 10.1902/jop.1997.68.5.421. [DOI] [PubMed] [Google Scholar]
- 6.International Journal of Systematic Bacteriology. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. List No. 53. Int J Syst Bacteriol. 1995;45:418–419. [Google Scholar]
- 7.Orskov, J. 1930. Untersuchungen über einen in Mundhöhle und oberen Luftwegen häufig vorkommenden, zur Sarcinagruppe gehörigen Mikroben, der eigentümliche morphologische Verhältnisse aufweist. Acta Pathol. Microbiol. Scand. (Suppl. III):519–541.
- 8.Rossmann S N, Wilson P, Carter B, Hicks J, Flaitz C, Demmler G, Simon C, Cron S, Shearer W, Kline M. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. The effect of age and vertically-acquired HIV on oral flora of children, abstr. V-15; p. 576. [Google Scholar]