SUMMARY
Increased PD-L1 expression in cancer cells is known to enhance immunosuppression, but the mechanism underlying PD-L1 upregulation is incompletely characterized. We show that PD-L1 expression is upregulated through internal ribosomal entry site (IRES)-mediated translation upon mTORC1 inhibition. We identify an IRES element in the PD-L1 5′-UTR that permits cap-independent translation and promotes continuous production of PD-L1 protein despite effective inhibition of mTORC1. eIF4A is found to be a key PD-L1 IRES-binding protein that enhances PD-L1 IRES activity and protein production in tumor cells treated with mTOR kinase inhibitors (mTORkis). Notably, treatment with mTORkis in vivo elevates PD-L1 levels and reduces the number of tumor-infiltrating lymphocytes in immunogenic tumors, but anti-PD-L1 immunotherapy restores antitumor immunity and enhances the therapeutic efficacy of mTORkis. These findings report a molecular mechanism for regulating PD-L1 expression through bypassing mTORC1-mediated cap-dependent translation and provide a rationale for targeting PD-L1 immune checkpoint to improve mTOR-targeted therapy.
Graphical Abstract

In brief
Cao et al. identify IRES-mediated translation as a crucial bypass process that upregulates PD-L1 expression, resulting in tumor immune evasion and resistance to mTOR kinase inhibitors. The co-targeted inhibition of PD-L1 and mTOR presents a promising therapeutic strategy to enhance tumor suppression and overcome therapeutic resistance.
INTRODUCTION
The mammalian target of rapamycin (mTOR) is a central regulator of cell proliferation, growth, survival, and metabolism by integrating both intracellular and extracellular signals.1 It forms two protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The mRNA translation for protein synthesis is by far the best-characterized process controlled by mTORC1. mTORC1 directly phosphorylates eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), which results in release of the mRNA cap-binding protein eIF4E and enables it to recruit the scaffolding protein eIF4G and the RNA helicase eIF4A to assemble the eIF4F complex for initiation of cap-dependent translation.2 The mTORC2 predominantly controls cell survival and cytoskeleton organization by phosphorylating AKT and paxillin, among other proteins. Dysregulation of mTOR signaling is a common event in the majority of cancers,1 making it an attractive therapeutic target. However, first-generation allosteric mTOR inhibitors, rapamycin, and related rapalogs, exhibit limited efficacy attributed to their insufficient inhibition of 4E-BP1 phosphorylation and feedback activation of AKT.1,3-8 Although second-generation ATP-competitive dual mTORC1/2 kinase inhibitors (mTORkis) surpass rapamycin in their potent antitumor effect by effectively inhibiting 4E-BP1 phosphorylation and AKT activity,9-11 they are unable to fully overcome the inherent resistance mechanisms that target upstream signaling molecules.
Programmed death ligand 1 (PD-L1), an immune-checkpoint molecule, binds to PD-1 on T cells, enabling immune evasion and T cell exhaustion.12,13 Increased PD-L1 expression in cancer cells contributes to immunosuppression and immune escape, leading to tumor progression and therapeutic resistance.14-16 PD-L1 expression can be regulated by various mechanisms.17 However, it remains unclear if PD-L1 expression is regulated by mTORC1-promoted cap-dependent translation, and how such translational regulation affects the tumor immune microenvironment and therapeutic response to mTOR inhibitors.
Here, we used pharmacological and genetic inhibition of mTORC1/4E-BP1-mediated cap-dependent translation activity to investigate these questions. We demonstrate that effective inhibition of mTORC1/4E-BP1 signaling fails to reduce PD-L1 protein levels and instead enhances its protein production through an internal ribosomal entry site (IRES)-mediated translation. Inhibition of mTOR kinase increases PD-L1 levels in tumors and leads to exhaustion of the tumor infiltrating lymphocytes (TILs). However, targeting PD-L1 restores antitumor immunity and improves the efficacy of mTORkis in immunocompetent mouse models of colorectal cancer (CRC). These findings highlight a new aspect of translational regulation of PD-L1 expression in modulating antitumor immunity and response to mTOR kinase inhibition.
RESULTS
PD-L1 translation and expression are specifically enhanced rather than inhibited by mTORkis
mTORC1 is well known to promote cap-dependent mRNA translation by phosphorylation of the translational repressor 4E-BP1.1 Rapamycin and related rapalogs are modest inhibitors of mTORC1 activity. In contrast to rapalogs, mTORkis totally block mTORC1 activity by effectively inhibiting 4E-BP1 phosphorylation, thereby leading to strong repression of eIF4E-initiated cap-dependent translation and protein synthesis.18 To explore the possible translational regulation of PD-L1 expression by mTORC1/4E-BP1 signaling, we first examined the effect of the clinical grade mTOR kinase inhibitor INK128 (also known as MLN0128, TAK-228, and sapanisertib)19,20 on PD-L1 expression in two CRC cell lines, HCT116 and DLD-1. Consistent with our previous observations,21 INK128 at a concentration of 100 nM effectively inhibited 4E-BP1 phosphorylation at any of its four phosphorylation sites, confirmed with an altered migration of 4E-BP1, and resulted in the dephosphorylated 4E-BP1 bound to the eIF4E-mRNA cap complex; these events repressed cap-dependent translation in HCT116 and DLD-1 cells (Figures 1A, S1A, and S1B). Unexpectedly, INK128 increased rather than decreased PD-L1 protein levels for up to 24 h after drug exposure in these cells, whereas the elevated PD-L1 protein expression did not correspond to any change in mRNA expression (Figures 1A-1C). These findings suggest that INK128 is unlikely to alter the transcription level of PD-L1. Similarly, exposure to another mTOR kinase inhibitor, AZD8055,9 led to a robust increase in PD-L1 levels associated with inhibition of 4E-BP1 phosphorylation and cap-dependent translation activity in both HCT116 and DLD-1 cells (Figures S1A-S1C). These findings were also observed in four additional CRC cell lines (HCT15, pt93, CT26, and MC38) and two breast cancer cell lines (MDA-MB-231 and MCF7) treated with both INK128 and AZD8055 (Figure S1D). A recent study also showed that mTOR kinase inhibition by either INK128 or AZD8055 could elevate PD-L1 protein levels in a panel of lung cancer cell lines.22
Figure 1. Inhibition of mTORC1/4E-BP1 signaling enhances PD-L1 expression at the level of translation.
(A–C) HCT116 or DLD-1 cells were treated with the indicated concentrations of INK128 for 24 h (A) and (C) or with 100 nM INK128 for the indicated times (B), followed by western blot (A and B) or qPCR analysis (C).
(D and E) HCT116 cells were treated with 100 nM INK128 or DMSO as control for 12 h, followed by polysome analysis. The vertical blue line separates the polysomal (P) and monosomal (M) fractions (D). The P/M ratio (E) was calculated by comparing areas under the polysome and monosome peaks using NIH ImageJ. The results are expressed as a percentage of the P/M ratio relative to values from the DMSO-treated controls.
(F) Quantitative PCR analysis for PD-L1 mRNA levels in monosomal and polysomal fractions as indicated in (D).
(G–I) HCT116 cells with stable expression of two different sets of raptor (G), rictor (H) short hairpin RNAs (shRNAs), control shRNA (ShCtrl), or stable expression of HA-tagged 4E-BP1 wild-type (WT), 4E-BP1-4A or vector control (I) were analyzed by western blot.
(J) HCT116 cells stably expressing 4E-BP1 shRNA or control shRNA were treated with the indicated concentration of INK128 for 24 h, followed by western blot analysis. The relative expression levels of PD-L1 were obtained by normalizing to β-actin (A, B, and G–J). All graphic data are presented as mean ± SEM (n = 3 replicates) from one representative of three (C) or two (E) and (F) independent experiments. Statistical analysis by one-way ANOVA (C) and Student’s t test (E and F), *p < 0.05, **p < 0.02, ***p < 0.01; ns, not significant. See also Figures S1 and S7.
As mRNA is actively translated in polysomes, we further assessed the effect of mTORkis on global mRNA translation and PD-L1-specific mRNA translation. Consistent with the overall inhibition of cap-dependent translation by INK128, analysis of polysome profiles demonstrated a profound decrease of global translation by INK128, as indicated with a 24% reduction in the ratio of polysomal (P) to monosomal (M) fractions (Figures 1D and 1E), an index of translational efficiency.21,23 Remarkably, we found that PD-L1 mRNA translation was instead enhanced by INK128, as revealed by a shift in PD-L1 mRNA association toward actively translating polysomal fractions (Figure 1F). Given that INK128 inhibits both mTORC1 and mTORC2, we evaluated the specific activities of mTORC1 and mTORC2 in regulation of PD-L1 expression by silencing their key components, raptor and rictor, respectively, in HCT116 cells. Knockdown of raptor expression markedly elevated PD-L1 levels, whereas this effect was not observed with knockdown of rictor expression (Figures 1G and 1H). Similar to increased levels of dephosphorylated 4E-BP1 induced by mTORkis (Figure 1A), ectopic expression of a non-phosphorylatable 4E-BP1 mutant (4E-BP1-4A) that binds constitutively to eIF4E and inhibits eIF4E-initiated cap-dependent translation23 upregulated PD-L1 protein expression as compared with the expression of wild-type 4E-BP1 or vector control in HCT116 cells (Figure 1I). Notably, PD-L1 expression was also markedly elevated in AZD8055- or INK128-resistant HCT116 clones accompanied by pronounced inhibition of 4E-BP1 phosphorylation, but minimally altered in rapamycin-resistant clones in which 4E-BP1 phosphorylation was unchanged or only slightly inhibited (Figure S1E). In contrast, the increased PD-L1 levels induced by INK128 were not observed when 4E-BP1 was knocked down in HCT116 cells (Figure 1J). These data indicated that PD-L1 translation and protein expression are specifically increased in cancer cells upon marked inhibition of mTORC1-mediated phosphorylation of 4E-BP1, perhaps through a cap-independent translation mechanism.
PD-L1 upregulation by mTORkis is mediated by IRES-dependent translation
Initiation of mRNA translation can occur by two distinct mechanisms: cap-dependent scanning and internal ribosome entry. The latter mechanism requires an IRES element located in the 50 untranslated region (UTR) of the mRNA.24 Because PD-L1 mRNA was translated efficiently even when the overall rate of cap-dependent translation was inhibited by mTORkis, we suspected that the human PD-L1 mRNA might contain an IRES element responsible for the bypass translation. To assess this, we first analyzed the 5′-UTR of the PD-L1 mRNA using an online version of UTRscan program. After entering the 108 nucleotides (nt) of 5′-UTR along with an additional 52 nt of the open reading frame, a putative IRES element from nt 79 to 160 was identified (Figure 2A). The sequence was derived from GenBank NM_014143. To verify the IRES activity of the PD-L1 5′-UTR, we next inserted the 5′-UTR and its derivatives with a series of deleted fragments of the 1 to 160 sequence into the pRL-FL dicistronic vector that contains the Renilla luciferase (RL) reporter gene and Firefly luciferase (FL) reporter gene (Figure 2B). Expression of RL is cap-dependent, whereas expression of FL is dependent on the presence of the IRES element between these two reporter genes.25 IRES activity was determined by the ratio of FL to RL. Each of these vectors, including the pRL-FL control vector that did not contain any IRES elements, was transfected into HCT116 cells. Upon assessment of luciferase activities, we discovered that the sequence between nt 79 and 108 in the PD-L1 5′-UTR serves as the core IRES region and is crucial for PD-L1 IRES activity (Figure 2C). To ensure that the FL was translated by internal ribosome entry rather than enhanced ribosomal readthrough or re-initiation, an inverted repeat sequence that forms a very stable RNA hairpin structure was inserted into the upstream RL cistron (Figure 2D). As shown in Figure 2E, this inserted hairpin strongly inhibited the cap-dependent translation of RL, while the presence of the 79 to 108 fragment of PD-L1 5′-UTR in the vector allowed initiation of FL translation. The FL activity stimulated by the 79 to 108 fragment with the hairpin added (phpRL-FL) was comparable to that of the transfected vector with pRL-FL containing the fragment without the hairpin. These results confirmed that the FL activity induced in the dicistronic vector was indeed stimulated by the 79 to 108 fragment, which can act as IRES does and was not a product of enhanced ribosomal readthrough or re-initiation. Furthermore, reverse transcription PCR analysis revealed no alteration in FL mRNA expression by insertion of the 79 to 108 fragment in cells transfected with the dicistronic reporter plasmids (Figure 2F), suggesting that the fragment-stimulated FL activity occurs solely at the translational level.
Figure 2. PD-L1 translation is upregulated upon mTOR kinase inhibition in an IRES-mediated manner.
(A) The sequence of the human PD-L1 5′-UTR (1–108 nt) along with an additional 52 nt of the open reading frame (ORF). The putative IRES element in PD-L1 5′-UTR is highlighted in red.
(B) Schematic illustration of the dicistronic reporter vectors containing a series of deleted fragments of the sequence from 1 to 160 nt in (A).
(C) IRES activity was determined by calculating the ratio of FL/RL activities in HCT116 cells transfected with the indicated dicistronic vectors for 36 h. The results are presented as a fold increase over the IRES activity found in the pRL-FL vector control cells.
(D) Schematic illustration of the dicistronic vectors used for the reporter assays.
(E and F) HCT116 cells were transfected with the indicated dicistronic vectors for 36 h, followed by measurement of FL and RL activities (E) or RT-PCR analyses of FL and RL mRNA levels (F).
(G) HCT116 cells were co-transfected with the pGL3-based FL reporter containing 5′-UTR 79–108 or PD-L1 promoter or the pGL3-basic reporter control along with an internal control RL vector for 36 h, and then cultured in the absence or presence of 10 ng/mL IFNγ for 24 h. Promoter activity was determined by normalizing FL activity to RL activity. The results are presented as a fold increase over the activity found in the PGL3-basic vector control cells without IFNγ stimulation.
(H and I) HCT116 cells were transfected with the indicated dicistronic vectors and then treated with 100 nM INK128 or DMSO for 24 h. After treatment, cells were assessed for measurement of IRES activity (H) as described in (C), and western blot or RT-PCR analysis of FL and RL protein (I, top) or mRNA levels (I, bottom). The relative expression levels of FL and RL were obtained by normalizing to β-actin. All graphic data are presented as mean ± SEM (n = 3 replicates) from one representative of three independent experiments. Statistical analysis by Student’s t test, *p < 0.01, **p < 0.001, ***p < 0.001; ns, not significant. See also Figures S2, S7, and S8.
Previous reports suggested that some so-called IRES activities are actually the result of a cryptic promoter element in the 5′-UTR of the given mRNAs.24,26 To differentiate between the observed IRES activity and cryptic promoter activity of the 30 base sequence (79–108) of the PD-L1 5′-UTR, we constructed a monocistronic vector by inserting the 30 base fragment into a promoterless pGL3-basic vector.21 We also used the PGL3-based PD-L1 promoter reporter vector as a positive control. By similar transfection and reporter assays in HCT116 cells, we found that the 30 base sequence did not stimulate FL activity in the PGL3-basic vector, even in the presence of interferon (IFN)γ, a cytokine known to stimulate PD-L1 expression (Figure 2G). In contrast, the control PD-L1 promoter reporter showed a notable increase in the FL activity, which was further enhanced by stimulation with IFNγ. Taken together, these findings clearly demonstrate that only the 30 base sequence between 79 and 108 of PD-L1 5′-UTR is an IRES and responsible for the regulation of PD-L1 translation, and that the sequence is not a promoter mediating PD-L1 transcription.
We next performed gene transfection and reporter assays to determine whether the mTORkis INK128 enhances PD-L1 IRES activity. As shown in Figures 2H, 2I, and S2, INK128 treatment in HCT116 cells could further significantly enhance the 30 base PD-L1 IRES sequence-mediated FL translation activity and its protein expression but did not alter the FL mRNA expression. In contrast, INK128 inhibited cap-dependent translation of RL and its protein production. Collectively, these data substantiated that the elevated PD-L1 levels induced by mTORkis in cancer cells are mediated by its 5′-UTR containing IRES-dependent translation activity.
eIF4A is required for IRES-mediated PD-L1 translation and upregulation upon mTOR kinase inhibition
Several lines of evidence have demonstrated that IRES-containing mRNAs may also utilize the canonical initiation factors such as eIF4A for translation initiation.27,28 Given that dephosphorylated 4E-BP1 induced by mTORkis competed with eIF4G for binding to eIF4E,2 leading to the release of eIF4A from the eIF4E-mRNA cap complex (Figure S1A), we hypothesized that the released eIF4A induced by mTORkis may function as a switch to enhance IRES-mediated translation of PD-L1. To explore this possibility, we used the MS2 in vivo biotin tagged RNA affinity purification (MS2-BioTRAP) approach as described29 to identify whether eIF4A is a regulatory factor of PD-L1 IRES activity. MS2 is a bacteriophage RNA binding protein that specifically binds to an RNA stem-loop target sequence30 (Figure S3A), which enables MS2 to capture target RNAs in vitro from cell extract systems.29 We constructed a PD-L1 IRES expression plasmid (tagged-PD-L1 IRES) in which the identified IRES sequence from the 79 to 108 fragment of PD-L1 5′-UTR (Figure 2A) was cloned upstream of the FL coding sequence (Figure S3B) and four MS2-targeted stem-loops were cloned at the 3′ end of the FL open reading frame.29 A stem-loop tagged-Cap construct (tagged-Cap)29 lacking the IRES element (Figure S3C) was used as control to analyze canonical cap-dependent translation and identify the specific IRES interacting proteins. To effectively isolate MS2-associated RNA-protein complexes, we next generated an HCT116 cell line with stable expression of biotinylated MS2 (MS2-HB, Figure S3A). As shown in Figure S3D, expression of MS2-HB was not detrimental to FL production by either the IRES or Cap transcripts. The MS2-HB was effectively captured by streptavidin beads from whole cell extracts (Figure S3E). Furthermore, the full-length tagged-PD-L1 IRES-FL mRNA (~1.65 kb) was specifically co-purified with MS2-HB, whereas the mRNA was not isolated in the absence of MS2-HB (Figure S3F). Additionally, both tagged-PD-L1 IRES and tagged-Cap mRNA showed a similar level of their binding to MS2-HB (Figure S3G). These results indicated that MS2-HB is effective for the isolation of functional stem-loop tagged RNAs.
Using the MS2-BioTRAP approach, we found that treatment with INK128 resulted in a decrease in the level of eIF4A bound to tagged-Cap mRNA but increased the level of eIF4A bound to tagged-PD-L1 IRES mRNA associated with marked inhibition of 4E-BP1 phosphorylation in HCT116 cells (Figure 3A). The increased level of eIF4A bound to tagged-PD-L1 IRES mRNA was also observed in INK128- and AZD8055-resistant HCT116 cells (Figure S4). To determine whether eIF4A interacts physically with the PD-L1 IRES, we performed RNA electrophoretic mobility-shift assay (EMSA) using a biotin-labeled PD-L1 IRES probe. EMSA analysis revealed the mobility of the PD-L1 IRES probe shifted with the addition of eIF4A using HCT116 cell extracts with and without immuno-depletion of eIF4A (Figures 3B and 3C). The specific binding was further ascertained by the fact that preincubation with unlabeled PD-L1 IRES RNA abolished the mobility shift. Conversely, unlabeled irrelevant RNA, such as iron-responsive element (IRE) RNA, was not effective in competing with PD-L1 IRES RNA for binding to eIF4A in these assays (Figures 3B and 3C). Notably, pharmacological inhibition of eIF4A activity with silvestrol18 profoundly repressed PD-L1 IRES activity and its protein expression, but had no effect on its mRNA expression (Figures 3D-3F). Furthermore, the elevated PD-L1 expression upon INK128 treatment was abrogated by silvestrol or by deletion of both eIF4A1 and eIF4A2, whereas ablation of either eIF4A1 or eIF4A2 was insufficient to eliminate INK128-induced PD-L1 elevation (Figures 3G and 3H). Taken together, these results highlighted that both eIF4A1 and eIF4A2 are key binding proteins of PD-L1 IRES to enhance PD-L1 translation and expression upon mTOR kinase inhibition.
Figure 3. eIF4A binds to PD-L1 IRES to enhance bypass of PD-L1 translation upon mTOR kinase inhibition.
(A) MS2-HB-expressing HCT116 cells were transfected with Tagged-Cap, Tagged-PD-L1 IRES, or vector control and then treated with or without 100 nM INK128 for 24 h. The cell lysates were incubated with streptavidin (SA) beads to pull down biotinylated MS2, followed by western blot analysis for the co-precipitated proteins or whole cell lysates (WCLs).
(B) HCT116 cell extracts with or without immunodepletion (ID) of eIF4A were incubated in the presence or absence of biotin-labeled or unlabeled PD-L1 IRES RNA or IRE control RNA probe for competitive reaction, followed by RNA electrophoretic mobility shift assay for detection of eIF4A-PD-L1 IRES RNA complexes.
(C) The membrane in (B) was re-probed by western blot for eIF4A protein.
(D–F) HCT116 cells were treated with 25 nM silvestrol for 24 h, followed by measurement of PD-L1 IRES activity (D) as described in Figure 2C, western blot analysis (E), or qPCR analysis (F). Data are presented as mean ± SEM (n = 3 replicates) from one representative of three independent experiments. Statistical analysis by Student’s t test, *p < 0.01; ns, not significant.
(G) HCT116 cells were treated with 100 nM AZD8055, 100 nM INK128, and 25 nM silvestrol alone or in combination for 24 h, followed by western blot analysis.
(H) HCT116 cells with eIF4A1/2 wild-type (WT), eIF4A1 knockout (KO), eIF4A2 KO, or eIF4A2 KO plus siRNA-mediated eIF4A1 knockdown were treated with or without 100 nM INK128 for 24 h, followed by western blot analysis. The relative expression levels of PD-L1 were obtained by normalizing to β-actin (E), (G), and (H). See also Figures S3, S4, S7, and S8.
PD-L1 blockade enhances mTOR kinase-targeted therapy by restoring activated TILs
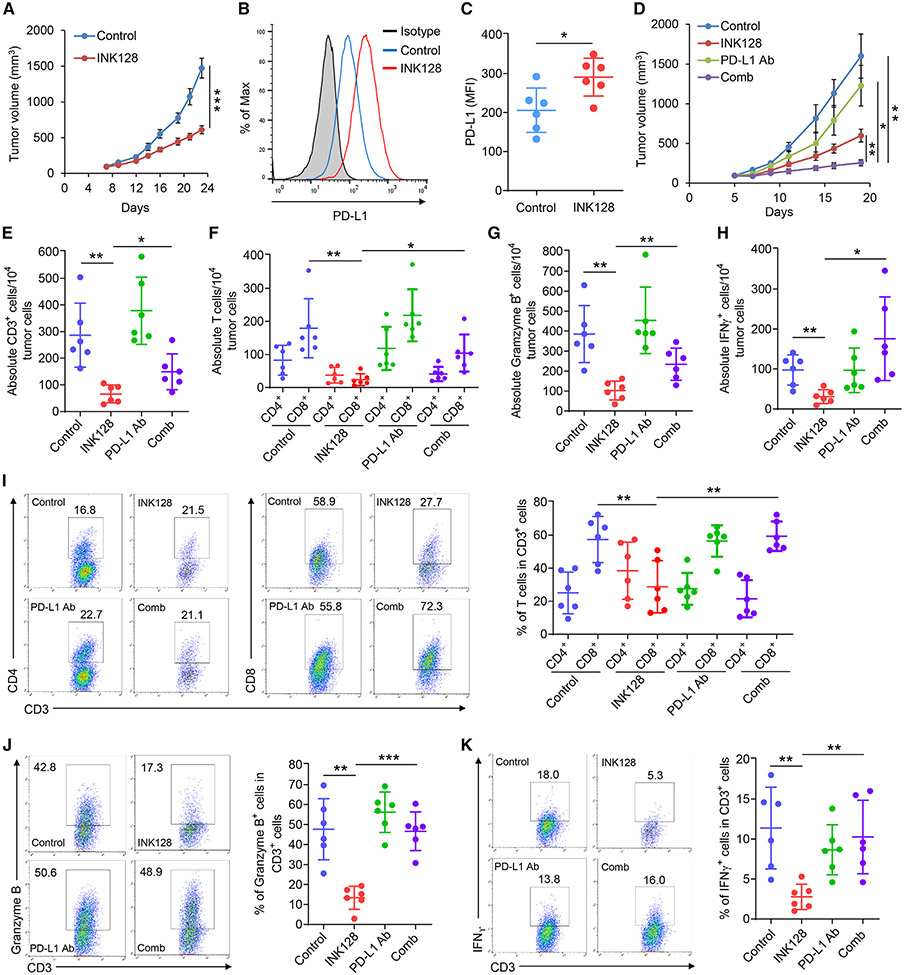
To determine whether treatment with mTORkis can induce PD-L1 elevation in tumor cells in vivo, thereby affecting the function of tumor infiltrating lymphocytes (TILs), we investigated the treatment effect of INK128 in the absence or presence of anti-PD-L1 immune-checkpoint inhibition in immunocompetent mice bearing syngeneic CT26 colon tumors. Our initial study21 showed that chronic treatment with INK128 at 1.5 mg/kg for 16 days effectively inhibited 4E-BP1 phosphorylation and slowed CT26 tumor growth; however, the tumors still grew significantly (Figures 4A and S5A). Flow cytometry analysis of excised tumor cell suspension revealed a significant increase in PD-L1 levels on CD45 negative tumor cells (Figures 4B and 4C) associated with a decrease in the absolute number of TILs, including CD3+, CD8+, Granzyme B+, and IFNγ+ cells, in response to treatment with INK128 (Figures 4E-4H). Importantly, co-administration of anti-PD-L1 antibody with INK128 treatment restored TILs to essentially normal levels and greatly enhanced the efficacy of INK128 in suppression of tumor growth with only marginal weight loss (e.g., <7%) in mice (Figures 4D, 4I-4K, and S5B-S5D). Similar antitumor effects were observed when combining INK128 and PD-L1 blockade in a second immunogenic colon tumor model, MC38 (Figure S6A). To further determine the relative contribution of PD-L1 elevation in tumors upon treatment with INK128, we generated PD-L1 knockout (KO) CT26 isogenic cell lines (Figures S7B and S7C). PD-L1-KO CT26 cells showed a comparable growth rate to wild-type (WT) cells in vitro (Figure S7D). However, PD-L1 depletion resulted in noticeable suppression of CT26 tumor growth when the PD-L1-KO cells were injected subcutaneously into immunocompetent mice (Figures S7E and S7F). Similar to the treatment with anti-PD-L1 antibody, PD-L1 depletion in combination with INK128 markedly suppressed CT26 tumor growth compared with INK128 treatment or PD-L1 depletion alone (Figures S7E and S7F). In marked contrast, PD-L1 depletion or anti-PD-L1 antibody did not affect CT26 tumor growth or enhance the antitumor effect of INK128 when tumor cells were inoculated into athymic nude mice lacking functional T cells (Figure S7G), indicating that PD-L1-mediated suppression of T cell immunity accounts for the limited antitumor response to mTOR kinase inhibition. Altogether, these findings revealed that treatment with mTORkis could lead to tumor PD-L1 elevation to a level sufficient to impair T cell immunity, ultimately reducing the overall antitumor effect of mTORkis.
Figure 4. PD-L1 blockade restores antitumor immunity and enhances the therapeutic efficacy of INK128.
(A) BALB/c mice bearing CT26 tumors were treated daily with INK128 or vehicle control.
(B and C) Tumors from mice euthanized 6 h after the final treatment with INK128 or vehicle control as in (A) were analyzed by flow cytometry for PD-L1 levels in CD45− tumor cells.
(D) BALB/c mice bearing CT26 tumors were treated with INK128, anti-PD-L1 Ab, the combination (comb) of INK128 and anti-PD-L1 Ab, or vehicle and immunoglobulin control.
(E–K) Tumors from mice euthanized 6 h after the final treatments as indicated agents in (D) were analyzed by flow cytometry for the absolute number of CD3+, CD4+, CD8+, Granzyme B+, and IFNγ+ TILs cells (E–H) or the percentage of CD4+, CD8+, Granzyme B+, and IFNγ+ in CD3+ TILs cells (I–K). All results are presented as mean ± SEM (n = 6 mice/group). Statistical analysis by Student’s t test, *p < 0.03, **p < 0.01, ***p < 0.001. See also Figures S5 and S6.
DISCUSSION
mTOR regulation of PD-L1 expression in tumors has been reported in several studies.22,31,32 Our findings are consistent with two recent studies showing a substantial increase in cell surface PD-L1 levels following mTORC1 inhibition in different types of human and murine cancer cells.22,31 However, an earlier study reported opposite results—that inhibition of PI3K/mTORC1 signaling could reduce PD-L1 levels in human non-small cell lung cancer cell lines.32 The discordant mTORC1 effect on PD-L1 expression is purportedly due to non-specific and failed anti-PD-L1 antibody used in that report.22,32,33 In this study, we uncovered an unexpected mechanism of translational control that leads to elevated PD-L1 levels. We identified an IRES element within the PD-L1 5′-UTR that initiates cap-independent translation, increasing PD-L1 protein levels by recruiting the translation initiation factor eIF4A. This effect occurs when mTORkis strongly repress global cap-dependent translation via dephosphorylated 4E-BP1. Interestingly, mTORkis did not elevate PD-L1 levels in 4E-BP1-depleted cells (Figure 1J), likely because 4E-BP1 depletion prevented eIF4A release from the eIF4F complex, hindering further increase in the level of eIF4A bound to PD-L1 IRES to enhance IRES-mediated PD-L1 translation (Figure S7). A recent study revealed an additional mechanism of PD-L1 upregulation by inhibition of mTORC1/p70S6K signaling through targeting β-TrCP for degradation to enhance PD-L1 stability.22 Together with our current cap-independent translational control mechanism, we propose that both mechanisms may cooperate to modulate PD-L1 levels upon mTORC1 inhibition. It is also important to note that translation of the PD-L1 mRNA can be regulated by the upstream open reading frames (uORFs) in its 5′-UTR.34-36 Recent studies demonstrated that cellular stress enables bypass of the uORF-mediated translational repression, leading to enhanced PD-L1 translation and expression34,35 by increasing phosphorylation of eIF2α, a key component of the eIF2 ternary complex.37,38 By analyzing eIF2α phosphorylation levels in all cell lines used in this study, we found that neither AZD8055 nor INK128 had any (or only marginal) effect on eIF2α phosphorylation (Figure S8). These data suggest that eIF2α phosphorylation did not make a major contribution to mTORkis-induced bypass of PD-L1 translation and upregulation. Thus, our work establishes IRES-mediated translation initiation as an additional important bypass process for the translational regulation of PD-L1, leading to increased PD-L1 abundance following mTOR kinase inhibition.
The increased level of PD-L1 induced by INK128, along with its suppression of antitumor immunity and limited efficacy, suggests that PD-L1 expression could determine the therapeutic response to mTORkis. Several mechanisms of resistance to mTOR inhibitors, including incomplete inhibition of downstream effector signaling, release of feedback inhibition of upstream receptor tyrosine kinases, and activation of alternative proliferative and survival signaling pathways, have been identified, emphasizing the need for combination therapies involving mTOR inhibitors.39 However, these mechanisms have mainly been studied in immunodeficient mouse tumor models lacking mature T cells. Evidence is emerging that mTOR inhibitors can modulate immune responses, supporting the potential of combining mTOR inhibitors with immune-checkpoint inhibitors for improved anticancer therapy.40 While mTOR inhibitors are traditionally considered immunosuppressive,41 recent studies have shown immunostimulatory effects, particularly in promoting memory CD8+ T cell generation.42,43 The effects of mTOR inhibitors on immune response appear to be context-dependent, influenced by dosing and treatment schedules, and may vary among different inhibitors.40,44 A recent study demonstrated that vistusertib, another mTOR kinase inhibitor, potentiated antitumor immunity when combined with different immune-checkpoint blockades, including anti-CTLA-4, anti-PD-1, or anti-PD-L1, by promoting proinflammatory cytokine production in resident tumor antigen-presenting cells and by enhancing CD8+ T-effector cell survival using intermediate doses; these events significantly suppressed CT26 or MC38 tumor growth as compared with monotherapies.45 Notably, distinct from the treatment with INK128 at 1.5 mg/kg as shown in this study, treatment with vistusertib at 15 mg/kg did not significantly suppress CT26 tumor growth.45 While parallel evaluation of these two mTORkis regarding drug-dosing regimens in the modulation of PD-L1 expression, immune response, and their therapeutic efficacies is needed in future investigation, their differential effects are also likely related to distinct structures and properties of these two mTORkis. Together with our findings that mTOR kinase inhibition paradoxically promotes IRES-mediated PD-L1 translation and protein production leading to reduction of antitumor immunity, these studies provide a rationale for the complementary molecular combination of mTORkis with anti-PD-1/PD-L1 immunotherapy to enhance tumor suppression. Given that several mTOR inhibitors are being actively tested in advanced cancers, our findings also suggest that incorporating the analysis of PD-L1 expression in response to mTOR inhibition in tumors may help to prospectively identify resistance to mTOR inhibitors in clinical studies.
Limitations of the study
This study employed qPCR-based polysomal profiling analysis to determine the polysome distribution of the PD-L1 mRNAs in cancer cells treated with mTORkis. Although this analysis has been widely used for identifying a specific mRNA that is translationally regulated, it lacks genome-wide analysis of translatomes. We could have missed other translationally upregulated genes including those containing IRES sequences that can bypass inhibition of mTORC1/4E-BP1-mediated cap-dependent translation to cause cancer cell resistance to mTOR inhibition. Furthermore, additional evidence by measuring the newly synthesized PD-L1 protein level will be important to confirm the mTORkis-induced upregulation of the IRES-mediated PD-L1 translation. Finally, we used eIF4A-immunodepleted cell extracts to prove that eIF4A is involved in the binding to PD-L1 IRES in vitro, but further validation could be significant such as by using pure recombinant eIF4A protein to clarify if it directly interacts with PD-L1 IRES.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Qing-Bai She (qing-bai.she@uky.edu).
Materials availability
All materials generated in this study are available from the lead contact upon request.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell lines
Human colorectal (HCT116, DLD-1, HCT15) and breast (MDA-MB-231, MCF7) cancer cell lines, and mouse CT26 colon cancer cell line were obtained from American Type Culture Collection (ATCC) and cultured in the appropriate medium with supplements as recommended by ATCC. The patient-derived Pt93 primary CRC cells as established previously46 were cultured in DMEM supplemented with 10% FBS. The mouse MC38 colon cancer cell line was obtained from Kerafast and cultured in DMEM supplemented with 10% FBS, 2 mmol/L glutamine, 0.1 mmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, and 10 mmol/L HEPES. HCT116 cells with stable expression of HA-4E-BP1, HA-4E-BP1-4A and shRNAs against raptor, rictor or 4E-BP1, or their vector or shRNA controls were generated in our previous studies.21,23,49,50 All cell lines were subjected to regular mycoplasma testing via PCR using e-Myco Plus kit (iNtRON Biotechnology) and underwent short tandem repeat (STR) profiling (Genetica). The rapamycin, INK128 or AZD8055 resistant cells were generated by continuous exposure of parental HCT116 cells to 1 μM of drug until the emergence of resistant colonies.
Animal studies
Animal experiments were performed under a protocol approved by the University of Kentucky Institutional Animal Care and Use Committee. CT26 or MC38 xenograft tumors were established by subcutaneously injecting CT26 or MC38 cells (1 × 106 per mouse) in a 1:1 mixture of media and Matrigel (Corning) into the right flank of 8-week-old BALB/c (for CT26), athymic nude (for CT26) or C57BL/6 (for MC38) female and male mice (Jackson Laboratory). Mice were randomized among control and treated groups (n = 6 per group) when tumors were well-established (~100 mm3). INK128 was formulated in 5% N-methyl-2-pyrrolidone plus 15% polyvinylpyrrolidone as described,21 and delivered orally at 1.5 mg/kg once per day. PD-L1 mAb (Bio X Cell) was administered intraperitoneally at 200 μg/mouse in 200 μL PBS every three days. For combination treatment, both drugs were given concurrently. Control mice received vehicle and isotype IgG control. Tumor dimensions were measured using a caliper and tumor volumes were calculated as mm3 = π/6 x larger diameter x (smaller diameter).2 Tumors were isolated and processed as single cell suspensions for flow cytometry analysis of TILs as indicated below.
METHOD DETAILS
Plasmid construction
The human PD-L1 promoter (position −831/+1)51 was amplified by PCR from HCT116 genomic DNAs, and then cloned into the pGL3 basic reporter vector that contains firefly luciferase (Promega). The pRL-FL dicistronic reporter vector was generated from pcDNA3-RL-Polio IRES-FL vector21 by deletion of Polio IRES. The full length and various deleted fragments of the PD-L1 5′-UTR were obtained by PCR using a HCT116 cDNA library, and then each was inserted between RL and FL of the pRL-FL vector. The phpRL-FL vector was generated by insertion of an inverted repeat sequence that forms a very stable RNA hairpin structure derived from phpBCL2-FL (Addgene) into the upstream RL of the pRL-FL vector. The Tagged-Cap and Tagged-LEF1 IRES plasmids were obtained from Addgene. The Tagged-PD-L1 IRES plasmid was constructed by replacing the LEF1 IRES with PD-L1 IRES from the Tagged-LEF1 IRES. The pLenti6.3-MS2-HB was generated by PCR using MS2-HB (Addgene) as a template to amplify MS2-HB, which was then cloned into the pLenti6.3 vector.21 All sequences were verified by automated DNA sequencing.
Generation of cell lines expressing MS2-HB
Lentiviruses were produced by co-transfection of the pLenti6.3-MS2-HB vector with packaging (psPAX2) and envelope (pMD2.G) plasmids into HEK293T cells using Lipofectamine 3000 (ThermoFisher Scientific) as described.21 Medium was changed the next day; virus-containing supernatants were collected at 48 h after transfection and passed through a 0.45 μM filter. HCT116 cells were infected with the filtered viral supernatants three times within a 36 h period in the presence of 8 μg/mL, and cells were given fresh complete medium. After 24 h, virus-infected HCT116 cells were treated with puromycin (2 μg/mL) for selection of cells with stable expression of MS2-HB.
Generation of gene knockout cell lines
Human eIF4A1 and eIF4A2 and mouse PD-L1 depleted cells were generated by CRISPR/Cas9-mediated knockout system using the PX459 vector (Addgene) as described.52 Short guide RNA (sgRNA) sequences were designed using Benchling’s CRISPR tool and cloned into the PX459 vector. Cells were transiently transfected with the PX459 vector containing the indicated sgRNA using Lipofectamine 3000 reagent according to the manufacturer’s protocol. After 48 h transfection, single cell colonies were selected in puromycin-containing medium before proceeding to experiments. The eIF4A1, eIF4A2 or PD-L1-deficient clones were confirmed by Western blot and/or flow cytometry analysis.
Gene silencing by siRNA
Human eIF4A1 siRNA and negative control siRNA were obtained from Sigma. Cells were transfected with 100 nM siRNA using Lipofectamine RNAiMAX reagent (ThermoFisher Scientific) according to the manufacturer’s protocol. After 36 h transfection, cells were subjected to assays as indicated.
Luciferase reporter assay
For the cap-dependent translation assay, cells were transfected using Lipofectamine 3000 reagent with the dicistronic luciferase reporter plasmid, pcDNA3-RL-Polio IRES-FL, which directs cap-dependent translation of the RL gene and cap-independent Polio IRES-mediated translation of the FL gene.21 After 24 h transfection, cells were treated with AZD8055 or INK128 for 24 h. Cell lysates were prepared and assayed for RL and FL activities using a Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocol. Cap-dependent RL activity was normalized against cap-independent FL activity as the internal control. The ratio of RL/FL activity was calculated for cap-dependent translational activity.21 To identify the presence of IRES activity in the PD-L1 5′-UTR, cells were transfected with dicistronic PD-L1 5′-UTR reporter plasmids. For PD-L1 promoter assay, cells were co-transfected with the FL reporter containing the human PD-L1 promoter along with an RL vector pHRL-TK (for normalization). Thirty-six hours post-transfection, cell lysates were prepared and analyzed similarly using the Dual-Luciferase Reporter Assay Kit (Promega). IRES- or promoter-dependent FL activity was normalized to RL activity.
Real-time quantitative reverse-transcription PCR
Total RNA was isolated using the RNeasy Plus Mini Kit (Qiagen). Equal amounts of RNA were used as templates for all reactions. cDNA was synthesized with a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific). Real-time PCR was performed on a StepOne Real-Time PCR system (Applied Biosystems) in triplicate with Maxima SYBR Green/ROX qPCR Master Mix (ThermoFisher Scientific). GAPDH or β-actin was used as an internal control for normalization, and the relative expression level was calculated by the comparative CT (ΔΔCT) method.
Polysome-associated mRNA analysis
Sucrose density gradient centrifugation was employed to separate the ribosome fractions as described previously.21 Briefly, 15 min before cell collection, cycloheximide (100 μg/mL) was added to the culture medium. Cells were washed in ice-cold PBS containing 100 μg/mL cycloheximide and harvested in polysome lysis buffer (5 mM Tris-HCl, pH7.5, 2.5 mM MgCl2, 1.5 μM KCl, 2 mM DTT, 0.5% Triton X-100, 0.5% sodium deoxycholate, 100 μg/mL cycloheximide, 200 U/ml RNAsin, 0.2 mg/mL heparin and protease inhibitors). Cells were incubated on ice for 15 min and then centrifuged at 10,000 × g for 10 min at 4°C. The supernatant (4 mg protein) was layered on a pre-chilled 10–50% linear sucrose gradient preparing in the gradient buffer (5 mM Tris-HCl, pH7.5, 2.5 mM MgCl2, 1.5 mM KCl, 2 mM DTT, 100 μg/mL cycloheximide, 40 U/ml RNAsin, 0.1 mg/mL heparin and protease inhibitors), and then centrifuged in a Beckman SW40Ti rotor at 250,000 × g for 2.5 h at 4°C. Gradients were fractionated while monitoring absorbance at A254 with a Density Gradient Fractionation System (Brandel). RNA was isolated from gradient fractions using TRIzol Reagent (ThermoFisher Scientific) and LiCl precipitation for RNA purification (ThermoFisher Scientific) according to the manufacturer’s protocol. Purified RNA with equal volumes of each fraction was reverse transcribed to cDNA using a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific) followed by real-time PCR analysis as described above. The percent distribution of the mRNAs across the fractions was calculated using the cycle threshold (CT) values.
Western blot analysis and immunoprecipitation
Cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 10% glycerol, protease and phosphatase inhibitor cocktail). Protein concentrations were measured using the BCA protein assay reagent (ThermoFisher Scientific). Equal amounts of protein were resolved by SDS-PAGE and transferred to PVDF membranes. After transfer, membranes were blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 and incubated with the primary antibody at 4°C overnight, followed by incubation with the secondary antibody conjugated with horseradish peroxidase. The bands were visualized with chemiluminescence substrate (ThermoFisher Scientific). For immunoprecipitation, the cell lysates (250 μg protein) were immunoprecipitated with 1 μg of eIF4E antibody (Santa Cruz Biotechnology) overnight followed by incubation with a 50% slurry of protein G Sepharose beads (Cytiva) for 3 h at 4°C. The beads were washed three times with the lysis buffer, and the precipitated protein complexes were resuspended in 2× Laemmli sample buffer followed by Western blot analysis of the indicated proteins. Antibodies for PD-L1 (1:1,000), p-4E-BP1 (Thr37/46) (1:1,000), p-4E-BP1 (Ser65) (1:1,000), p-4E-BP1 (Thr70) (1:1,000), 4E-BP1 (1:1,000), p-eIF2α (Ser51) (1:1,000), eIF4E (1:1,000), eIF4A1 (1,000), eIF2α (1:1,000), raptor (1,000), rictor (1:1,000) and HA-tag (1:1,000) were from Cell Signaling Technology. Antibodies for eIF4AI/II (1,000), eIF4AII (1:1,000), eIF4G (1:1,000) and firefly luciferase (1:1,000) were from Santa Cruz Biotechnology. Antibodies for renilla luciferase (1:1,000), MS2 (1:10,000) and β-actin (1:10,000) were from Sigma-Aldrich. Mouse PD-L1 antibody (2 μg/mL) was from R&D Systems, and biotin antibody (1:10,000) was from Bethyl Laboratories.
RNA affinity purification
HCT116 cells with stable expression of MS2-HB were transfected with Tagged-PD-L1 IRES and Tagged-Cap using Lipofectamine 3000 reagent (ThermoFisher Scientific). After 24 h transfection, cells were treated with INK128 for 24 h. Cells were then lysed in buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM EDTA, 5 mM MgCl2, 0.5% NP-40, 10% glycerol, 40 U/ml RNAsin, protease and phosphatase inhibitor cocktail. The cell lysates (1 mg protein) were incubated with streptavidin beads (ThermoFisher Scientific) and rotated at 4°C overnight to pull down RNA-protein complexes. After rotation, the beads were washed three times with the lysis buffer, and the beads with bound and unbound proteins and RNAs were subjected to Western blot and PCR analyses, respectively.
RNA electrophoretic mobility shift assay (EMSA)
EMSA experiments were conducted using a Light Shift Chemiluminescent RNA EMSA kit (ThermoFisher Scientific) according to the manufacturer’s protocol. Cell were lysed in 20 mM HEPES, pH 7.3, 40 mM KCl, 1 mM DTT, 40 U/ml RNAsin, 1% Triton X-100, and protease and phosphatase inhibitor cocktail by incubation on ice for 15 min, followed by centrifugation at 16,000 × g for 10 min at 4°C. To deplete eIF4A from the cell lysates, a portion of the supernatant was immunoprecipitated with eIF4AI/II antibody (Santa Cruz Biotechnology) as described above. Biotin-labeled or unlabeled PD-L1 IRES RNA probe was synthesized and obtained from Sigma-Aldrich. The unlabeled IRE control RNA probe was provided by the EMSA kit (ThermoFisher Scientific). The cell lysates with or without immunodepletion of eIF4A were incubated in the presence or absence of unlabeled PD-L1 IRES RNA or IRE control RNA probe for competitive reaction at room temperature for 15 min in reaction buffer (10 mM HEPES, pH 7.3, 20 mM KCl, 1 mM MgCl2, 1 mM DTT, 5% glycerol). Next, the biotin-labeled PD-L1 IRES RNA probe was added into the reaction system and incubated for 30 min at room temperature. Samples were separated with 6% polyacrylamide gel in 0.5 × TBE (Tris-borate-EDTA) buffer. After transfer to a nylon membrane (ThermoFisher Scientific), labeled probes were cross-linked by UV, probed with HRP-conjugated streptavidin, and incubated with substrates for detection of biotin signals.
Cell viability assay
Cell viability was determined by trypan blue incorporation as previously described.21 PD-L1-WT or PD-L1-KO CT26 cells (1 × 105/well) were seeded in 6-well plates in triplicate and assessed by incubation at 37°C for cell growth over 4 days. The number of viable cells was counted using the Vi-CELL XR 2.06 (Beckman Coulter).
Flow cytometry analysis of TILs
Tumors were minced and digested with 5 mL of 2 mg/mL collagenase D (Sigma-Aldrich) and 0.2 mg/mL DNase I (Sigma-Aldrich) in DMEM with 5% FBS for 1 h at 37°C to generate single cell suspensions. Cells were then filtered through a 40 μM strainer and collected by centrifugation. Cell pellets were suspended and lysed in red blood cell lysis buffer (BioLegend) for 5 min, followed by incubation with TruStain fcX antibody (BioLegend) to block nonspecific staining of antibodies. A portion of cells was used to co-stain with PE-conjugated PD-L1 antibody (BioLegend), APC-conjugated CD45 antibody (BioLegend) and 7-AAD (BioLegend). The remaining cells were fixed and permeabilized using the Fixation buffer (BioLegend) and Intracellular Staining Permeabilization Wash Buffer (BioLegend), respectively, according to the manufacturer’s protocol. The cells were then co-stained with antibodies against CD3 (BioLegend, APC conjugated), granzyme B (BioLegend, FITC-conjugated) and IFNγ (BioLegend, PE-conjugated), or co-stained with antibodies against CD3 (BioLegend, APC conjugated), CD4 (BioLegend, FITC-conjugated) and CD8 (BioLegend, PE-conjugated). The corresponding isotype IgG controls (BioLegend) were used. The cells were incubated with corresponding antibodies for 30 min in Cell Staining Buffer (BioLegend). Cells were washed with the Cell Staining Buffer and analyzed by flow cytometry.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis for each experiment was performed as described in the corresponding figure legends. Data between groups were compared using a two-tailed unpaired Student’s t test and one-way ANOVA test as appropriate. All quantitative data are presented as mean ± SEM, and p < 0.05 is considered statistically significant. GraphPad Prism 9 software was used for these analyses.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-PD-L1 | Cell Signaling Technology | Cat# 13684; RRID:AB_2687655 |
| Mouse anti-PD-L1 | R&D Systems | Cat# MAB90781 |
| Mouse monoclonal anti-eIF4E | Santa Cruz Biotechnology | Cat# sc-271480; RID:AB_10649368 |
| Rabbit monoclonal anti-eIF4E | Cell Signaling Technology | Cat# 2067; RRID:AB_2097675 |
| Rabbit monoclonal anti-phospho-4E-BP1 (Thr37/46) | Cell Signaling Technology | Cat# 2855; RRID:AB_560835 |
| Rabbit monoclonal anti-phospho-4E-BP1 (Ser65) | Cell Signaling Technology | Cat# 13443; RRID:AB_2728761 |
| Rabbit monoclonal anti-phospho-4E-BP1 (Thr70) | Cell Signaling Technology | Cat# 13396 |
| Rabbit monoclonal anti-4E-BP1 | Cell Signaling Technology | Cat# 9644; RRID:AB_2097841 |
| Rabbit monoclonal anti-HA-tag | Cell Signaling Technology | Cat# 3724; RRID:AB_1549585 |
| Rabbit monoclonal anti-phospho-eIF2α (Ser51) | Cell Signaling Technology | Cat# 3398; RRID:AB_2096481 |
| Rabbit monoclonal anti-eIF2α | Cell Signaling Technology | Cat# 5324; RRID:AB_10692650 |
| Rabbit monoclonal anti-raptor | Cell Signaling Technology | Cat# 2280; RRID:AB_561245 |
| Rabbit monoclonal anti-rictor | Cell Signaling Technology | Cat# 2114; RRID:AB_2179963 |
| Rabbit monoclonal anti-eIF4A1 | Cell Signaling Technology | Cat# 2490; RRID:AB_823487 |
| Mouse monoclonal anti-eIF4AI/II | Santa Cruz Biotechnology | Cat# sc-377315; RRID:AB_2868449 |
| Mouse monoclonal anti-eIF4AII | Santa Cruz Biotechnology | Cat# sc-137148; RRID:AB_2097384 |
| Mouse monoclonal anti-eIF4G | Santa Cruz Biotechnology | Cat# sc-133155; RRID:AB_2095748 |
| Mouse monoclonal anti-firefly luciferase | Santa Cruz Biotechnology | Cat# sc-74548; RRID:AB_1125118 |
| Rabbit polyclonal anti-Biotin | Bethyl Laboratories | Cat# A150-109A; RRID:AB_67327 |
| Mouse monoclonal anti-β-actin | Sigma-Aldrich | Cat# A5441; RRID:AB_476744 |
| Mouse monoclonal anti-renilla luciferase | Sigma-Aldrich | Cat# MAB4400; RRID:AB_95116 |
| Rabbit polyclonal anti-MS2 | Sigma-Aldrich | Cat# ABE76-I; RRID:AB_2827507 |
| InVivoPlus anti-mouse PD-L1 | Bio X Cell | Cat# BE0101; RRID:AB_10949073 |
| 7-AAD | BioLegend | Cat# 420403; RRID:AB_2869266 |
| TruStain FcX(TM) (anti-mouse CD16/32) | BioLegend | Cat# 101319; RRID:AB_1574973 |
| PE-conjugated anti-mouse PD-L1 | BioLegend | Cat# 124308; RRID:AB_2073556 |
| APC-conjugated CD45 antibody | BioLegend | Cat# 103112; RRID:AB_312977 |
| APC-conjugated anti-mouse CD3 | BioLegend | Cat# 100236; RRID:AB_2561456 |
| FITC-conjugated anti-human/mouse Granzyme B | BioLegend | Cat# 515403; RRID:AB_2114575 |
| PE-conjugated anti-mouse IFNγ | BioLegend | Cat# 505808; RRID:AB_315402 |
| FITC-conjugated anti-mouse CD4 | BioLegend | Cat# 100510; RRID:AB_312713 |
| PE-conjugated anti-mouse CD8a | BioLegend | Cat# 100708; RRID:AB_312747 |
| Chemicals, peptides, and recombinant proteins | ||
| INK128 | MedChemExpress | Cat# HY-13328; CAS: 1224844-38-5 |
| AZD8055 | MedChemExpress | Cat# HY-10422; CAS: 1009298-09-2 |
| Silvestrol | MedChemExpress | Cat# HY-13251; CAS: 697235-38-4 |
| Rapamycin | Selleck Chemicals | S1039; CAS: 53123-88-9 |
| Puromycin Dihydrochloride | Research Product International | Cat# P33020; CAS: 58-58-2 |
| Cycloheximide | Research Product International | Cat# C81040; CAS: 66-81-9 |
| Polybrene | Sigma-Aldrich | Cat# TR-1003-G |
| Heparin | Sigma-Aldrich | H4784; CAS. 9041-08-1 |
| Collagenase D | Sigma-Aldrich | Cat# 11088866001 |
| DNase I | Sigma-Aldrich | Cat# 10104159001 |
| Fetal Bovine Serum | Sigma-Aldrich | Cat# F0926 |
| Protease Inhibitor Cocktail | ThermoFisher Scientific | Cat# 78439 |
| Phosphatase Inhibitor Cocktail | ThermoFisher Scientific | Cat# 78420 |
| RNasin Plus RNase Inhibitor | Promega | Cat# N2615 |
| Recombinant Human IFN-γ | PeproTech | Cat# AF-300-02 |
| Matrigel | Corning | Cat# 356237 |
| Lipofectamine 3000 Transfection Reagent | ThermoFisher Scientific | Cat# L3000015 |
| Lipofectamine RNAiMAX Transfection Reagent | ThermoFisher Scientific | Cat# 13778150 |
| TRIzol Reagent | ThermoFisher Scientific | Cat# 15596026 |
| Streptavidin Agarose Resin | ThermoFisher Scientific | Cat# 3419 |
| Protein G Sepharose beads | Cytiva | Cat# 20353 |
| Red Blood Cell (RBC) Lysis Buffer | BioLegend | Cat# 420301 |
| Fixation Buffer | BioLegend | Cat# 420801 |
| Intracellular Staining Permeabilization Wash Buffer | BioLegend | Cat# 421002 |
| Cell Staining Buffer | BioLegend | Cat# 420201 |
| Critical commercial assays | ||
| e-Myco Plus Mycoplasma PCR Detection Kit | iNtRON Biotechnology | Cat# 25237 |
| Dual-Luciferase® Reporter Assay System | Promega | Cat# E1910 |
| RNeasy Plus Mini Kit | Qiagen | Cat# 74134 |
| BCA Protein Assay Kit | ThermoFisher Scientific | Cat# 23227 |
| High-Capacity cDNA Reverse Transcription Kit | ThermoFisher Scientific | Cat# 4368814 |
| Maxima SYBR Green/ROX qPCR Master Mix (2X) | ThermoFisher Scientific | Cat# K0223 |
| LightShift Chemiluminescent RNA EMSA Kit | ThermoFisher Scientific | Cat# 20158 |
| Experimental models: Cell lines | ||
| Human: HCT116 cells | ATCC | Cat# CCL-247; RRID:CVCL_0291 |
| Human: DLD-1 cells | ATCC | Cat# CCL-221; RRID:CVCL_0248 |
| Human: HCT15 cells | ATCC | Cat# CCL-225; RRID:CVCL_0292 |
| Human: MDA-MB-231 cells | ATCC | Cat# HTB-26; RRID:CVCL_0062 |
| Human: MCF7 cells | ATCC | Cat# HTB-22; RRID:CVCL_0031 |
| Human: HEK293T cells | ATCC | Cat# CRL-3216; RRID:CVCL_0063 |
| Human: Pt93 Primary CRC cells | Huang et al., 201946 | N/A |
| Mouse: CT26 cells | ATCC | CRL-2638; RRID:CVCL_7256 |
| Mouse: MC38 cells | Kerafast | ENH204-FP; RRID:CVCL_B288 |
| Experimental models: Organisms/strains | ||
| BALB/c mice | Jackson Laboratory | Strain# 000651; RRID:IMSR_JAX:000651 |
| C57BL/6 mice | Jackson Laboratory | Strain #:000664; RRID:IMSR_JAX:000664 |
| Nu/J mice | Jackson Laboratory | Strain #:002019; RRID:IMSR_JAX:002019 |
| Oligonucleotides | ||
| MS2-HB_fwd: ATGGCTTCTAACTTTACTCAG | Sigma-Aldrich | N/A |
| MS2-HB_rev: TTAATGATGGTGGTGATGATG | Sigma-Aldrich | N/A |
| PD-L1 promoter_fwd: GGTCAAGGAGTTCGAGAAAAG | Sigma-Aldrich | N/A |
| PD-L1 promoter_rev: GCCAGGCCCGGAGGCGGGG | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–160_fwd: GGCGCAACGCTGAGCAGC | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–160_rev: CTTTCTGGAATGCCCTGCAG | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–108_fwd: GGCGCAACGCTGAGCAGC | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–108_rev: CTTTCTGGAATGCCCTGCAG | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–78_fwd: GGCGCAACGCTGAGCAGC | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 1–78_rev: GCGCGGCTGGTGCGGAGCC | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 79–160_fwd: TTCTGTCCGCCTGCAGGGC | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 79–160_rev: CTTTCTGGAATGCCCTGCAG | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 79–108_fwd: TTCTGTCCGCCTGCAGGGCA TTCCAGAAAG | Sigma-Aldrich | N/A |
| PD-L1 5′-UTR 79–108_rev: CTTTCTGGAATGCCCTGCAG GCGGACAGAA | Sigma-Aldrich | N/A |
| Human eIF4A1 sgRNA: GCCCCCGATACAGGCGTGAC | Sigma-Aldrich | N/A |
| Human eIF4A2 sgRNA: TTTTCTCAATACGAGGCGCA | Sigma-Aldrich | N/A |
| Mouse PD-L1 sgRNA: TGCTGCATAATCAGCTACGG | Sigma-Aldrich | N/A |
| PD-L1 qPCR_fwd: TGTCAGTGCTACACCAAGGC | Sigma-Aldrich | N/A |
| PD-L1 qPCR_rev: ACAGCTGAATTGGTCATCCC | Sigma-Aldrich | N/A |
| β-actin qPCR_fwd: CATGTACGTTGCTATCCAGGC | Sigma-Aldrich | N/A |
| β-actin qPCR_rev: CTCCTTAATGTCACGCACGAT | Sigma-Aldrich | N/A |
| GAPDH qPCR_fwd: ACAACTTTGGTATCGTGGAAGG | Sigma-Aldrich | N/A |
| GAPDH qPCR_rev: GCCATCACGCCACAGTTTC | Sigma-Aldrich | N/A |
| FL semi qPCR_fwd: ACATCTCATCTACCTCCCGG | Sigma-Aldrich | N/A |
| FL semi qPCR_rev: TCCGGAATGATTTGATTGCC | Sigma-Aldrich | N/A |
| RL semi qPCR_fwd: TGATCCAGAACAAAGGAAACGG | Sigma-Aldrich | N/A |
| RL semi qPCR_rev: AATGCCAAACAAGCACCCCA | Sigma-Aldrich | N/A |
| eIF4A1 siRNA | Sigma-Aldrich | SASI_Hs02_00331809 |
| Negative control siRNA | Sigma-Aldrich | Cat# SIC001 |
| PD-L1 IRES RNA probe: UUCUGUCCGCCUGCAGGGC AUUCCAGAAAG | Sigma-Aldrich | N/A |
| IRE control RNA probe: UCCUGCUUCAACAGUGCUUG GACGGAAC | ThermoFisher Scientific | Cat# 20158 |
| Recombinant DNA | ||
| pcDNA3-RL-Polio IRES-FL | She et al., 201023 | N/A |
| pBABE-puro | She et al., 201023 | N/A |
| pBABE-HA-4E-BP1 | She et al., 201023 | N/A |
| pBABE-HA-4E-BP1-4A | She et al., 201023 | N/A |
| pLKO.1 control shRNA | Sigma-Aldrich | Cat# SHC002 |
| pLKO raptor shRNA_1 | Addgene Sarbassov et al., 200547 | # 1857 |
| pLKO raptor shRNA_2 | Addgene Sarbassov et al., 200547 | # 1858 |
| pLKO rictor shRNA_1 | Addgene Sarbassov et al., 200547 | # 1853 |
| pLKO rictor shRNA_2 | Addgene Sarbassov et al., 200547 | # 1854 |
| pLKO 4E-BP1 shRNA | Sigma-Aldrich | TRCN0000040203 |
| pcDNA3-RL-FL | This paper | N/A |
| pcDNA3-RL-PD-L1 5′UTR (1–160)-FL | This paper | N/A |
| pcDNA3-RL-PD-L1 5′UTR (1–108)-FL | This paper | N/A |
| pcDNA3-RL-PD-L1 5′UTR (1–78)-FL | This paper | N/A |
| pcDNA3-RL-PD-L1 5′UTR (79–160)-FL | This paper | N/A |
| pcDNA3-RL-PD-L1 5′UTR (79–108)-FL | This paper | N/A |
| phpBCL2-FL | Addgene Sherrill et al., 200425 | # 42593 |
| pCDNA3-phpRL-FL | This paper | N/A |
| pCDNA3-phpRL-PD-L1 5′UTR (79–108)-FL | This paper | N/A |
| pGL3 basic luciferase reporter vector | Promega | Cat# E1751 |
| pGL3-basic PD-L1 5′UTR (79–108) | This paper | N/A |
| pGL3-basic PD-L1 promoter | This paper | N/A |
| plenti6.3 vector | Wang et al., 201721 | N/A |
| MS2-HB | Addgene Tsai et al., 201129 | # 35573 |
| plenti6.3-MS2-HB | This paper | N/A |
| psPAX2 | Addgene A gift from Didier Trono | #12260 |
| pMD2.G | Addgene A gift from Didier Trono | #12259 |
| Tagged-Cap | Addgene Tsai et al., 201129 | # 35572 |
| Tagged-LEF1 IRES | Addgene Tsai et al., 201129 | # 35570 |
| Tagged-PD-L1 IRES | This paper | N/A |
| PX459 vector | Addgene Ran et al., 201348 | # 62988 |
| PX459-eIF4A1 KO | This paper | N/A |
| PX459-eIF4A2 KO | This paper | N/A |
| PX459-PD-L1 KO | This paper | N/A |
| Software and algorithms | ||
| Prism 9 (v9.5.1) | GraphPad | https://www.graphpad.com/; RRID:SCR_002798 |
| ImageJ | NIH | https://ImageJ.nih.gov/ij/; RRID:SCR_003070 |
| FlowJo (v10.8.0) | FlowJo | https://www.flowjo.com/; RRID:SCR_008520 |
| UTRscan | Institute for Biomedical Technologies | http://bioinformatics.ba.itb.cnr.it/ ?Software___UTRscan |
| Density Gradient Fractionation System (Peak Chart) | Brandel | http://www.brandel.com/fractgradient.html |
| CRISPR Design Tools | Benchling | https://www.benchling.com/ |
Highlights.
mTORC1 inhibition enhances PD-L1 expression via IRES-mediated translation bypass
eIF4A acts as a switch to initiate and enhance the IRES-dependent PD-L1 translation
Upregulated PD-L1 by mTORkis leads to immunosuppression and resistance to mTORkis
Anti-PD-L1 restores antitumor immunity and improves the efficacy of mTORkis
ACKNOWLEDGMENTS
We thank the Markey Cancer Center’s Research Communication Office for assistance with manuscript preparation. This work was supported, in part, by NIH grants R01CA175105 (Q.-B.S.), R01CA203257 (Q.-B.S.), R21ES031712 (Q.-B.S.), T32CA165990 (M.M.), and the pilot grant (Q.-B.S.) from the University of Kentucky Center for Cancer and Metabolism (NIH P20M121327). This work was also supported in part by the Flow Cytometry and Immune Monitoring Shared Resource Facility of the University of Kentucky Markey Cancer Center (NIH P30CA177558).
INCLUSION AND DIVERSITY
We support inclusive, diverse, and equitable conduct of research.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112764.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Laplante M, and Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149, 274–293. 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonenberg N, and Hinnebusch AG (2009). Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745. 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin D, Colombi M, Moroni C, and Hall MN (2011). Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov 10, 868–880. 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 4.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, et al. (2011). The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–1322. 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choo AY, and Blenis J (2009). Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle 8, 567–572. 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 6.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, and Khuri FR (2005). Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 65, 7052–7058. 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. (2006). mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508. 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mi W, Ye Q, Liu S, and She QB (2015). AKT inhibition overcomes rapamycin resistance by enhancing the repressive function of PRAS40 on mTORC1/4E-BP1 axis. Oncotarget 6, 13962–13977. 10.18632/oncotarget.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, et al. (2010). AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 70, 288–298. 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 10.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, and Shokat KM (2009). Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38. 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-García C, Ibrahim YH, Serra V, Calvo MT, Guzmán M, Grueso J, Aura C, Pérez J, Jessen K, Liu Y, et al. (2012). Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin. Cancer Res 18, 2603–2612. 10.1158/1078-0432.CCR-11-2750. [DOI] [PubMed] [Google Scholar]
- 12.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, and Freeman GJ (2007). Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122. 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou W, and Chen L (2008). Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol 8, 467–477. 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 14.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, and Minato N (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 99, 12293–12297. 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567. 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleki Vareki S, Garrigós C, and Duran I (2017). Biomarkers of response to PD-1/PD-L1 inhibition. Crit. Rev. Oncol. Hematol 116, 116–124. 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Cha JH, Chan LC, Li CW, Hsu JL, and Hung MC (2019). Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 76, 359–370. 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, and Topisirovic I (2015). Targeting the translation machinery in cancer. Nat. Rev. Drug Discov 14, 261–278. 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 19.Janes MR, Vu C, Mallya S, Shieh MP, Limon JJ, Li LS, Jessen KA, Martin MB, Ren P, Lilly MB, et al. (2013). Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia 27, 586–594. 10.1038/leu.2012.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghobrial IM, Siegel DS, Vij R, Berdeja JG, Richardson PG, Neuwirth R, Patel CG, Zohren F, and Wolf JL (2016). TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: A phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenstrom’s macroglobulinemia. Am. J. Hematol 91, 400–405. 10.1002/ajh.24300. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Ye Q, Cao Y, Guo Y, Huang X, Mi W, Liu S, Wang C, Yang HS, Zhou BP, et al. (2017). Snail determines the therapeutic response to mTOR kinase inhibitors by transcriptional repression of 4E-BP1. Nat. Commun 8, 2207. 10.1038/s41467-017-02243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, Qian G, Zhang S, Zheng H, Fan S, Lesinski GB, Owonikoko TK, Ramalingam SS, and Sun SY (2019). Inhibition of mTOR complex 1/p70 S6 kinase signaling elevates PD-L1 levels in human cancer cells through enhancing protein stabilization accompanied with enhanced beta-TrCP degradation. Oncogene 38, 6270–6282. 10.1038/s41388-019-0877-4. [DOI] [PubMed] [Google Scholar]
- 23.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, Solit DB, and Rosen N (2010). 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 18, 39–51. 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shatsky IN, Dmitriev SE, Terenin IM, and Andreev DE (2010). Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol. Cells 30, 285–293. 10.1007/s10059-010-0149-1. [DOI] [PubMed] [Google Scholar]
- 25.Sherrill KW, Byrd MP, Van Eden ME, and Lloyd RE (2004). BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem 279, 29066–29074. 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- 26.Han B, and Zhang JT (2002). Regulation of gene expression by internal ribosome entry sites or cryptic promoters: the eIF4G story. Mol. Cell Biol 22, 7372–7384. 10.1128/MCB.22.21.7372-7384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komar AA, and Hatzoglou M (2011). Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 10, 229–240. 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spriggs KA, Cobbold LC, Jopling CL, Cooper RE, Wilson LA, Stoneley M, Coldwell MJ, Poncet D, Shen YC, Morley SJ, et al. (2009). Canonical initiation factor requirements of the Myc family of internal ribosome entry segments. Mol. Cell Biol 29, 1565–1574. 10.1128/MCB.01283-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai BP, Wang X, Huang L, and Waterman ML (2011). Quantitative profiling of in vivo-assembled RNA-protein complexes using a novel integrated proteomic approach. Mol. Cell. Proteomics 10, M110.007385. 10.1074/mcp.M110.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keryer-Bibens C, Barreau C, and Osborne HB (2008). Tethering of proteins to RNAs by bacteriophage proteins. Biol. Cell (Paris) 100, 125–138. 10.1042/BC20070067. [DOI] [PubMed] [Google Scholar]
- 31.Hirayama Y, Gi M, Yamano S, Tachibana H, Okuno T, Tamada S, Nakatani T, and Wanibuchi H (2016). Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma. Cancer Sci. 107, 1736–1744. 10.1111/cas.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, et al. (2016). Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 76, 227–238. 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 33.Sun SY (2020). Searching for the real function of mTOR signaling in the regulation of PD-L1 expression. Transl. Oncol 13, 100847. 10.1016/j.tranon.2020.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Poggio M, Jin HY, Shi Z, Forester CM, Wang Y, Stumpf CR, Xue L, Devericks E, So L, et al. (2019). Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med 25, 301–311. 10.1038/s41591-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suresh S, Chen B, Zhu J, Golden RJ, Lu C, Evers BM, Novaresi N, Smith B, Zhan X, Schmid V, et al. (2020). eIF5B drives integrated stress response-dependent translation of PD-L1 in lung cancer. Nat. Can. (Que.) 1, 533–545. 10.1038/s43018-020-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Xie J, Jin X, Lenchine RV, Wang X, Fang DM, Nassar ZD, Butler LM, Li J, and Proud CG (2020). eEF2K enhances expression of PD-L1 by promoting the translation of its mRNA. Biochem. J 477, 4367–4381. 10.1042/BCJ20200697. [DOI] [PubMed] [Google Scholar]
- 37.Palam LR, Baird TD, and Wek RC (2011). Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem 286, 10939–10949. 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starck SR, Tsai JC, Chen K, Shodiya M, Wang L, Yahiro K, Martins-Green M, Shastri N, and Walter P (2016). Translation from the 5’ untranslated region shapes the integrated stress response. Science 351, aad3867. 10.1126/science.aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Formisano L, Napolitano F, Rosa R, D’Amato V, Servetto A, Marciano R, De Placido P, Bianco C, and Bianco R (2020). Mechanisms of resistance to mTOR inhibitors. Crit. Rev. Oncol. Hematol 147, 102886. 10.1016/j.critrevonc.2020.102886. [DOI] [PubMed] [Google Scholar]
- 40.El Hage A, and Dormond O (2021). Combining mTOR Inhibitors and T Cell-Based Immunotherapies in Cancer Treatment. Cancers 13, 1359. 10.3390/cancers13061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halloran PF (2004). Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med 351, 2715–2729. 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 42.Powell JD, Pollizzi KN, Heikamp EB, and Horton MR (2012). Regulation of immune responses by mTOR. Annu. Rev. Immunol 30, 39–68. 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, and Ahmed R (2009). mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112. 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Long L, Zhou P, Chapman NM, and Chi H (2020). mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol. Rev 295, 15–38. 10.1111/imr.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langdon S, Hughes A, Taylor MA, Kuczynski EA, Mele DA, Delpuech O, Jarvis L, Staniszewska A, Cosulich S, Carnevalli LS, and Sinclair C (2018). Combination of dual mTORC1/2 inhibition and immune-checkpoint blockade potentiates anti-tumour immunity. OncoImmunology 7, e1458810. 10.1080/2162402X.2018.1458810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Ye Q, Chen M, Li A, Mi W, Fang Y, Zaytseva YY, O’Connor KL, Vander Kooi CW, Liu S, and She QB (2019). N-glycosylation-defective splice variants of neuropilin-1 promote metastasis by activating endosomal signals. Nat. Commun 10, 3708. 10.1038/s41467-019-11580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarbassov DD, Guertin DA, Ali SM, and Sabatini DM (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101. 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 48.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc 8, 2281–2308. 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye Q, Cai W, Zheng Y, Evers BM, and She QB (2014). ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene 33, 1828–1839. 10.1038/onc.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai W, Ye Q, and She QB (2014). Loss of 4E-BP1 function induces EMT and promotes cancer cell migration and invasion via cap-dependent translational activation of snail. Oncotarget 5, 6015–6027. 10.18632/oncotarget.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang G, Wen Q, Zhao Y, Gao Q, and Bai Y (2013). NF-kappaB plays a key role in inducing CD274 expression in human monocytes after lipopolysaccharide treatment. PLoS One 8, e61602. 10.1371/journal.pone.0061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Y, Ye Q, Deng P, Cao Y, He D, Zhou Z, Wang C, Zaytseva YY, Schwartz CE, Lee EY, et al. (2020). Spermine synthase and MYC cooperate to maintain colorectal cancer cell survival by repressing Bim expression. Nat. Commun 11, 3243. 10.1038/s41467-020-17067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.