Abstract
Placenta accreta spectrum is one of the most dangerous conditions in pregnancy and is increasing in frequency. The risk of life-threatening bleeding is present throughout pregnancy but is particularly high at the time of delivery. Although the exact etiology is unknown, the result is clear: severe placenta accreta spectrum distorts the uterus and surrounding anatomy and transforms the pelvis into an extremely high-flow vascular state. Screening for risk factors and assessing placental location by antenatal ultrasound are essential for timely diagnosis. Further evaluation and confirmation of placental accreta spectrum is best performed in referral centers with expertise in antenatal imaging and surgical management of placenta accreta spectrum. In the United States, cesarean hysterectomy with the placenta left in-site after delivery of the fetus is the most common treatment for placental accreta spectrum, but even in experienced referral centers this treatment is often morbid, resulting in prolonged surgery, intraoperative injury to the urinary tract, blood transfusion, and admission to the intensive care unit. Postsurgical complications include high rates of post-traumatic stress disorder, pelvic pain, decreased quality of life, and depression. Team-based, patient-centered, evidence-based care from diagnosis to full recovery is needed to optimally manage this potentially deadly disorder. In a field that has relied mainly on expert opinion, more research is needed to explore alternative treatments and adjunctive surgical approaches to reduce blood loss and postoperative complications.
Precis
Updated guidance for clinicians who encounter placenta accreta spectrum, a disorder for which our understanding of the etiology, diagnosis, classification, and management is rapidly evolving.
INTRODUCTION
Placenta accreta spectrum (PAS) is a morbid pregnancy condition that is characterized by failure of placental detachment at the time of delivery and may result in life-threatening bleeding. By one statewide evaluation, PAS puts pregnant patients at much higher risk of severe maternal morbidity than health conditions considered relative or absolute contraindications to pregnancy including chronic renal disease, preexisting maternal cardiac disease, and pulmonary hypertension.1 Transfusion at delivery occurs in about 50% of patients;2 intensive care unit (ICU) admission after delivery is common; and operative injury to the urinary tract occurs in 5–30% of cases.3 4 Cesarean hysterectomy, and consequent loss of fertility, has long been considered standard treatment in the United States.5 In some instances of PAS, delivery before fetal viability is needed to preserve maternal life, resulting in both termination of pregnancy and often loss of future fertility. Furthermore, morbidity continues beyond the immediate postpartum period. Patients with PAS experience higher rates of repeat hospitalization, need for additional surgeries, long-lasting psychosocial and emotional problems, and decreased quality of life.6–8
Incidence
The incidence of PAS may be increasing. By pooled estimates, PAS now occurs in 0.17% (1 in 588) of pregnancies (95% CI 0.01% to 1.1%),2 compared to a much lower incidence in the 1970s and 1980s at 0.02% (1 in 4027) and 0.04% (1 in 2510), respectively.9 10 Accurate population-based statistics are hard to come by since PAS coding did not exist until the most recent ICD coding nomenclautre11 and since disease definitions are evolving.12–14 The increasing rate of placenta accreta is likely attributable to a change in risk factors, most notably the increased rate of cesarean delivery.15–18
Etiology and Risk Factors
The exact etiology of PAS is unclear. Historically, it was proposed that PAS resulted from abnormal trophoblast invasion much like dysregulated proliferation of cancer cells19 20; however, newer models of PAS focus more on an inciting event of trophoblast attachment to an abnormal uterine wall and subsequent placental growth within a progressively remodeled and dehisced uterine scar.21–23
The most significant risk factor for PAS is placenta previa diagnosed in a patient with a history of cesarean delivery.15 24 In these patients, PAS likely develops because of embryo implantation in or near the area of the uterine scar. In PAS, trophoblasts implant into the myometrium directly with no intervening decidua. Abnormal angiogenic and growth factor signaling, including local abundance of VEGF, results in proliferative transformation of the uterine arterioles and pelvic vessels early in pregnancy.25 26 PAS may also occur in other areas of uterine insult such as after myomectomy or in association with disorders of endometrial scarring or infertility. Very rarely, PAS occurs in patients with no known uterine instrumentation or other known risk factors.
Other risk factors include other uterine surgeries, Asherman’s syndrome, prior endometrial ablation, multifetal pregnancy, and in vitro fertilization, with relative risks in the literature ranging on the order of 2–7 compared to baseline and persisting when adjusted for factors such as maternal age and prior cesarean delivery.27–29
Classification
Classification of PAS is evolving to reflect a newer understanding of the disease. According to historical schemas based on histopathologic assessment alone, abnormal trophoblast location in the myometrium was diagnosed as accreta, increta, or percreta based on increasing depth of perceived invasion within the myometrium. 20 30–32 The usefulness of the historical classification has recently been challenged since its focus on progressive trophoblastic invasion seems contrary to observations from antenatal imaging, intraoperative findings, and targeted pathologic evaluation.22 33 In 2019, the International Federation of Gynecology and Obstetrics (FIGO) proposed a scheme of classification for the clinical diagnosis of PAS disorders.13 In 2020, a panel of experts convened and published a document on classification and reporting of pathological diagnosis of PAS disorders that roughly parallels the FIGO guidelines.14 These contemporary schemas, which are presented in Table 1, identify abnormal trophoblast attachment as the defining and inciting histopathologic characteristic of milder PAS (FIGO 1, PAS Grade 1), but redefine progression of PAS to more severe forms (FIGO 2–3, PAS Grade 2–3) based on the visible appearance and location of the placenta within a remodeled uterus and surrounding maternal structures 13 14 Regardless of underlying pathophysiology, the end result is clear: severe PAS is characterized by significant distortion of the uterine and pelvic anatomy and impressive transformation of the placenta and pelvis into a potential source of massive hemorrhage.
Table 1:
Comparison of Current and Historical Classification Systems for Placenta Accreta Spectrum Based on Contemporary Expert Consensus Guidelines13,14
| Pathology Classification (PAS)14 | Description | Clinica Classification (FIGO)13 | Description | Historical Classification | ||
|---|---|---|---|---|---|---|
| Normal | Layer of decidualized endometrium separates placenta from myometrium | Normal | Placenta able to be separated (spontaneously or manually) after delivery | |||
| Grade 1 | Areas of absent decidua between villi and myometrium; uniform myometrial thickness without thinning | Grade 1 | Adherent placenta, unable to manually develop a clean plane of separation between placenta and myometrium; often requires curettage | Placenta adherent Placenta creta Accreta |
||
| Grade 2 | Irregular placental-myometrial interface, without involvement of outer myometrium (<75% of myometrial thickness); intact uterine serosa | Grade 2 | Abnormal macroscopic findings over placental bed: placental “bulge;” hypervascularity; “Dimple sign,” with uterus pulling in on gentle cord traction | Increta | ||
| Grade 3 | Grade 3 | |||||
| 3A | Irregular placental-myometrial interface, placental involvement of outer myometrium (>75% thickness); intact uterine serosa | 3a | Macroscopic findings over placental bed and placental tissue seen through surface of uterus without extension to other organ Appearance of uterine “window” with base of placenta visible through extremely thin myometrium or single layer of serosa |
Increta or percreta (for histological PAS 3A) and percreta (for Clinical FIGO 3a) | ||
| 3D | Deep myometrial invasion with disruption of serosal surface | 3b | Involvement of urinary bladder – clear surgical plane cannot be identified between uterus and bladder | Percreta | ||
| 3E | Extrauterine extension with placental invasion into, or fibroadipose tissue extension to, extrauterine structures | 3c | Involvement/invasion of other pelvic tissues or organs (may also include bladder) | Percreta | ||
Requires hysterectomy or partial hysterectomy specimen; findings from delivered placentas and curettings are considered separately from PAS and designated basal plate myometrial fibers (BPMF)
Does not require pathological specimen, as grades are assigned based on intraoperative findings
DIAGNOSIS
The tools for diagnosis of PAS are imperfect, and many patients are not diagnosed until the time of delivery. When diagnosis is not made until delivery, management may occur in a hospital setting lacking necessary resources which can result in worse outcomes.34 35 Investigation of promising biomarkers for PAS is underway36 37, but broadly available and clinically useful blood or urine tests to predict PAS are not currently available. Importantly, a patients’ a priori risk should not be dismissed based on a reassuring ultrasound study. Consequently, risk factor assessment and ultrasound should be the foundation of PAS screening and diagnosis. Magnetic resonance imaging (MRI) may be preferred for diagnosis in some institutions, but its routine use is limited by higher cost and limited availability of expertise in MRI interpretation for PAS.
Assessment of Risk
For the clinician, diagnosis starts with understanding risk and developing an appropriate index of suspicion for PAS. Clinicians and ultrasound units should screen for history of cesarean delivery and inquire about any other uterine surgeries. Unlike imaging, this step does not require advanced expertise; and yet, it is probably the most important aspect of screening for PAS since it influences imaging interpretation considerably. For instance, the presence of placental lacunae or subplacental vascularity is significantly more concerning in a patient with four prior cesarean deliveries compared to the identical sonographic findings in a patient with no uterine surgery history. In the former, these findings could confirm the diagnosis; in the latter, the findings would likely be considered normal variations. Other risk factors mentioned above are associated with PAS, albeit less strongly, and should prompt close evaluation for PAS signs.
Patients with risk factors for PAS should undergo systematic evaluation of the placenta at multiple times starting early in pregnancy. Evidence of PAS or cesarean scar ectopic pregnancy (CSEP), particularly abnormal vascularity and low placental implantation, can be detected as early as the viability ultrasound at 6–10 weeks.38–40 In the second trimester, those with any number of prior cesareans should have the placental location defined in relation to the cervix and the area of low-transverse cesarean scar(s). The scarred area is typically located just above or at the level of the cervix in the second and third trimester for patients with prior term low-transverse cesarean(s), although scar location may be higher in cases of prior classical cesarean delivery. If the placenta is located well-above and away from the level of the prior scar(s), the risk of PAS in the lower uterine segment is likely exceedingly low. Conversely, if the placenta overlies the anterior lower uterine segment (i.e. forms a previa or is low-lying and extends into the area of prior hysterotomy) a thorough evaluation for sonographic signs of PAS is recommended.
Ultrasound
In patients at risk, ultrasound is the primary screening and diagnostic modality. The most important sign for PAS is placenta previa, as this modifies the risk for disease and morbidity most strikingly.41 When previa is present in patients with prior cesarean, the risk of PAS is considerable and increases with the number of prior cesarean deliveries (3%, 11%, 40%, 61%, and 67%, for the first, second, third, fourth, and fifth or more cesarean, respectively).15 24 SMFM convened a task force to define the most important ultrasound signs related to PAS.42 Descriptions of these markers are found in Appendix 1, available online at http://links.lww.com/xxx. The most common signs are a loss the normal hypoechoic zone between the placenta and myometrium or “clear zone”, myometrial thinning (less than 1 mm), placental lacunae, subplacental hypervascularity, and bridging vessels, although these and other “signs of PAS” are surprisingly common in unaffected pregnancies.43 Additional signs include placental bulging, bladder wall interruption, exophytic mass, and uterovesical hypervascularity. Investigations continue in the search for new, potentially more helpful signs.44 45 Transvaginal imaging with sweeps of the lower uterine segment can be invaluable to evaluate the lower uterine segment in higher resolution.46 No individual sign is highly predictive of PAS, so a combination of findings and a patient’s a priori risk helps to determine the overall suspicion.
Models to predict the risk of PAS based on the number and character of sonographic signs have been proposed, but these are not yet adequately validated in external cohorts. Predictive models to date suffer from imprecision and unsatisfactory rates of false-negative results (missed diagnoses).47 Importantly, the absence of ultrasound findings for PAS does not rule out the diagnosis in patients with previa and clinical risk factors.
Magnetic Resonance Imaging
The role of magnetic resonance imaging (MRI) in PAS diagnosis is less well defined. MRI may provide helpful information in select cases and in institutions with special expertise in interpreting placental MRI.5 In high-risk cohorts, MRI may be equivalent to ultrasound in diagnostic accuracy.48 However, caution is warranted in interpreting these data since MRI research for PAS is typically performed in busy referral centers and only in patients who have very high suspicion for PAS based on ultrasound.
The most common MRI features associated with PAS are abnormal uterine bulging, dark intraplacental bands on T2-weighted imaging, disruption of the uteroplacental zone, disorganized placental vasculature, and heterogeneous placental signal intensity.49 On MRI, placenta previa and placental bulging are the most reproducible signs 50, although these are often present in non-accreta cases.
Use of MRI for diagnosis may be preferred in some institutions and its use is under active investigation in PAS research centers, but clinicians should not presume that MRI will be a helpful adjunct to ultrasound in every setting.51 Expertise in the diagnosis of PAS varies by institution and, similar to ultrasound, diagnostic accuracy of MRI is likely worse outside of experienced referral centers. Given its cost and uncertain utility, we believe that MRI should be used only when it is likely to offer important clinical information about the disease character or location beyond what ultrasound can provide.
Antenatal Staging and Morbidity Prediction: The Future of PAS Diagnosis?
Detecting PAS is an important first step of diagnosis, but it is not the whole story. PAS investigators and surgeons are further interested in questions of location and extent (or topography) of PAS as well. This is important work, as the surgical teams preparing for management of PAS need better predictive models for morbidity and better staging paradigms for PAS. Antenatal staging paradigms exist, but few have been validated outside of the facilities from which they originate.52
On a fundamental level, members of the PAS diagnostics team should be able to provide answers to key questions that will be helpful to the surgical team. How certain are we that this patient has PAS? Where is the placenta located in the uterus and pelvis? Where can surgical difficulty be anticipated? Answering these questions helps to formulate the right team, the right time to deliver, and the right approach to treatment. An ideal staging paradigm, therefore, would incorporate the history with easily identifiable imaging signs into a model to accurately predict PAS morbidity and surgical expectations rather than simply the presence of PAS.
In summary, even at highly specialized referral centers, PAS diagnosis is difficult and imperfect. When approaching the challenge of screening and diagnosis, clinicians should maintain a high index of suspicion and a low threshold for referral to specialty centers with PAS expertise.
SYSTEMS PREPAREDNESS
System preparedness may be more important than an individual clinician’s experience or skill in ensuring safe and effective care for patients with known or suspected PAS. Preparing a health system, hospital, and clinical team for PAS care is challenging due to the relatively low incidence, high level of expertise required, and extraordinary number of hospital-based resources needed. Leading international organizations have provided guidelines for management of PAS including lists of resources and services necessary to plan and manage cases of PAS.5 53 54. Further, the American College of Obstetricians & Gynecologists (ACOG) with the Society for Maternal-Fetal Medicine (SMFM) endorse a practice of referral to specialized centers and regionalization of care for PAS5 55, but health care professional surveys and state-level data suggest that referral to higher levels of care has not yet become universal practice.56–58
Unfortunately, the rate of missed diagnosis of PAS remains high (up to 50%).59 60 All facilities offering obstetric care should therefore prepare an action plan for unexpected cases, and routinely engage in comprehensive self-assessment to determine if routine PAS care should be performed locally (see section Assessment of Resources for PAS) or referred to more experienced centers. To improve readiness, preparation, and response to cases of PAS, the SMFM developed checklists (found at https://www.smfm.org/checklists-and-safety-bundles), which can be used for self-assessment. These checklists also specifically address unexpected cases of PAS and can assist institutions that may be under-resourced to manage unexpected cases of PAS.
Assessment of Resources for PAS
A first step for hospitals providing obstetric care is to determine whether they have the resources and expertise to manage planned cases of PAS. At minimum, ACOG and SMFM state that hospitals planning to manage patients with “suspected accreta or placenta previa with prior uterine surgery” should have the available resources of a Maternal Level of Care III (subspecialty) or higher facility.55 Specifically, hospitals should have immediate 24-hour access to the following: (1) blood bank services with ability for massive transfusion, (2) neonatal and adult intensive care facilities, and (3) obstetric and surgical expertise in managing complex maternal and obstetric complications like PAS. Admittedly, not enough is known about quality metrics and case volumes to make recommendations about what constitutes a minimum level of expertise required for PAS care. However, if the relationship between case volume and quality metrics in PAS mirrors that in other surgeries, then low-volume surgeons and low-volume facilities are likely to experience worse outcomes compared to high-volume surgeons and centers.61 62 Determining what constitutes ‘adequate expertise’ for PAS management is therefore a critical knowledge gap in the field. This and other high-priority items are noted in Table 1.
Optimizing care and preparing for PAS
Hospitals and teams serving as PAS referral centers should work systematically to ensure optimization of PAS care. PAS referral centers should have a designated interdisciplinary team committed to case review, iterative team learning, and a culture of continuous quality improvement. An effective interdisciplinary PAS team is characterized by the following: (1) coordination by a program champion or champions, (2) membership from maternal fetal medicine, obstetrics, gynecologic surgery, anesthesia, critical care, interventional radiology, and neonatology, (3) ability to quickly mobilize an experienced surgical team at all times, (4) interdisciplinary treatment planning meetings or formalized communication, and (5) standardized evidence-based approaches to PAS diagnosis, staging, and management.
Conversely, obstetric hospitals and teams with lesser resources or experience should establish pathways and partnerships for referral to higher levels of care. Referral should occur as early as possible - at the time of diagnosis or when there is uncertainty about the diagnosis. This allows for early confirmation or reevaluation of the diagnosis, ideally prior to 26 – 32 weeks gestation, and care coordination and case planning in the facility that is the most appropriate for the level of concern. Referral at the time of delivery in unexpected cases is also possible and preferable in some circumstances (Figure 1).
Figure 1:
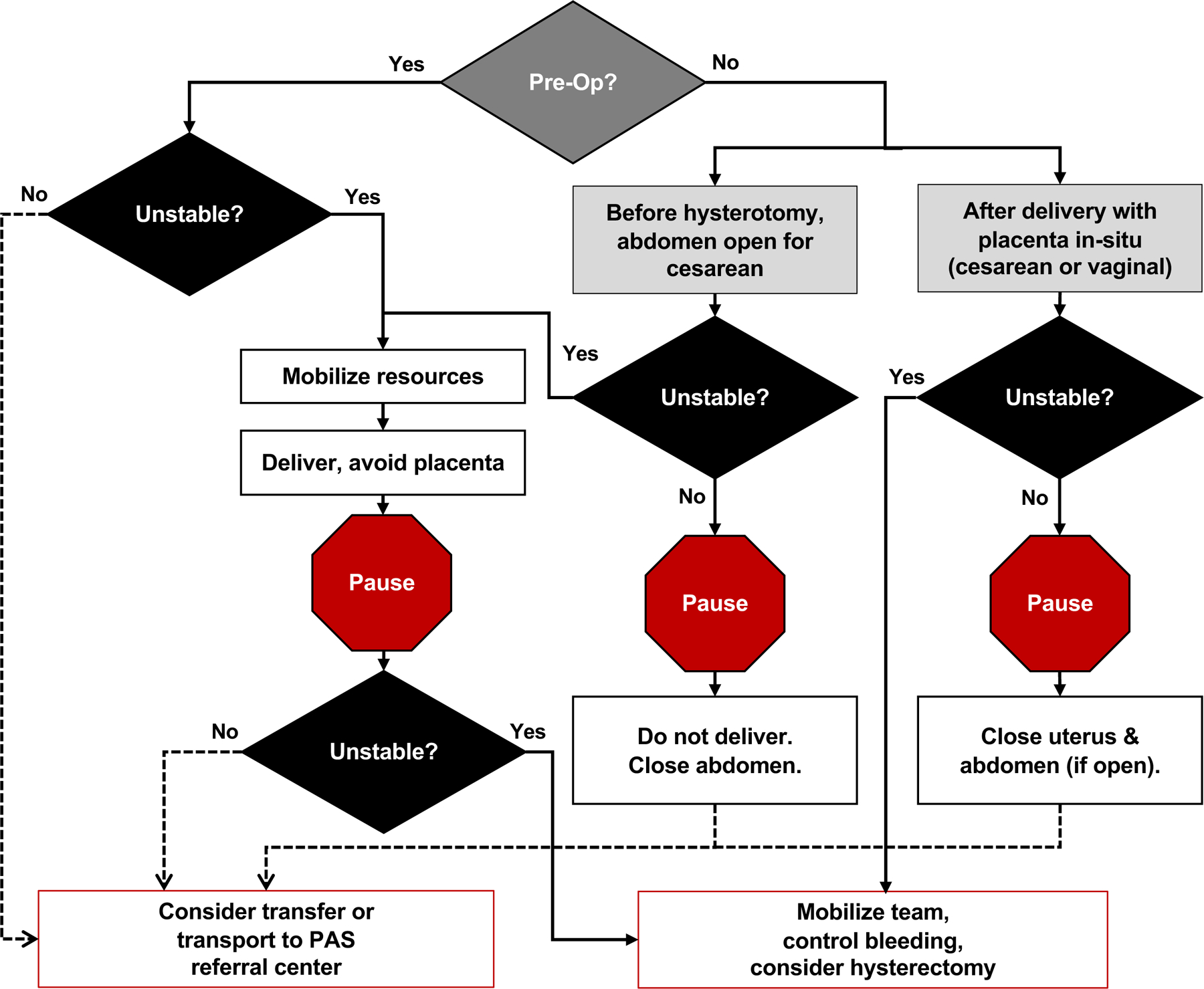
Managing unexpected and intraoperative placenta accreta spectrum (PAS) cases outside of PAS specialty centers. Real-time discovery of previously undiagnosed PAS is possible at any center that provides obstetric care, presenting an urgent need for complex clinical decision-making. Critical stability assessment of the pregnant patient and fetus is required throughout resource mobilization and consideration for potential transfer. Opportunities for transfer to a PAS referral center may be present before delivery of the infant, after delivery of the infant but prior to placental delivery (particularly if the placenta was not disrupted at hysterotomy), and following attempted removal of the placenta but prior to initiation of hysterectomy.
COORDINATED ANTENATAL MANAGEMENT
Antenatal Care
Comprehensive antenatal care for patients with suspected PAS begins with detailed risk stratification for tailored counseling and goals of care discussion. Identification of any PAS risk by either obstetric history or routine imaging warrants additional dedicated discussion of uterine procedural history and plan for timely obstetric imaging focused on PAS severity assessment as described in the diagnosis section. Personalized counseling for PAS needs to be both practical and emotionally supportive, taking into account the best estimate of a priori risk from thorough risk factor assessment, predicted severity of disease incorporating current clinical factors and imaging findings, and potential for morbidity including possible threat to future fertility. Although risk stratification may evolve over serial imaging studies in the pregnancy, the conversation that first introduces the possibility of PAS needs to emphasize where a patient can turn for reliable information. Availability of information is as easy as accessing the internet; however, the range and intensity of information relating to PAS can be overwhelming. For the vast majority of pregnant patients, the possibility of mortality is unexpected, so discussing the concept of PAS, its associated threat to health, and medical management options during pregnancy is important as soon as the a priori risk is determined to be high, or the diagnosis is suspected.
From a practical standpoint, once clinically significant PAS is suspected, the essential components of antenatal care include proactive mitigation of hemorrhage risk, arranging the timing and location of delivery, and coordination of the multidisciplinary team. If adequate resources and expertise are not available locally, referral to a center with PAS expertise is strongly advised. The predominant risk with a PAS diagnosis is obstetric hemorrhage, making timely assessment of hematologic parameters and iron stores a necessity. Laboratory screening for anemia at 24–28 weeks and treatment for hemoglobin levels under 10.5 g/dL and/or serum ferritin levels under 30 ng/dL, which is recommended in all pregnancies, is critical in this population.63 Optimizing hemoglobin prior to delivery may require high concentration iron replacement using IV iron formulations. Treatment thresholds specific to PAS for hemoglobin or serum ferritin have not been established, but given safety and efficacy data, late second or early third trimester administration of IV iron rather than oral iron is reasonable for any degree of anemia or iron deficiency.64 For patients with unique circumstances (e.g., refusal of blood products, complex antibodies), additional considerations may be warranted including perioperative erythropoietin, intraoperative cell salvage, or acute normovolemic hemodilution.65
Anticipation of blood loss and plans for intraoperative management should be discussed in coordination with anesthesiologists familiar with obstetric hemorrhage and resuscitation. Early consultations with anesthesiologists and surgical specialists who can provide expert review and knowledgeable predictions of what the patient and family may anticipate around the time of delivery is fundamental to prepare both the patient and the care team. Neonatal expectations must be discussed as well, given that most PAS deliveries will occur preterm (i.e., prior to 37 weeks gestation). Antenatal corticosteroid administration may be indicated when a preterm delivery is planned.
In addition to planning intended delivery scenarios, the team needs to establish contingency plans for how and where the pregnant patient can seek appropriate care in the case of complications such as antenatal hemorrhage or early labor signs. Consideration of inpatient admission is individualized, balancing both clinical factors and availability of resources in close proximity to the patient’s home. Throughout the pregnancy, effective and frequent communication between the pregnant patient and the multidisciplinary team should be prioritized.
Delivery Timing
Delivery timing recommendations are driven by expert opinion and are strongly influenced by data from pregnant patients with placenta previa. International professional organizations including ACOG and SMFM advocate for delivery of patients with suspected or confirmed PAS at 34 0/7 to 35 6/7 weeks gestation in the absence of bleeding or labor.5 Later delivery near term may be safe in selected cases, including those without placenta previa, but it is unclear what combination of other reassuring factors (e.g. history of term birth, long cervical length, or less severe disease) might contribute to robust risk stratification for later delivery.66 A systematic inquiry of high-volume centers reported wide variation in scheduled delivery timing for PAS, without directly associated alterations in outcomes for the pregnant patients.67 Additional studies are needed to validate proposed expert recommendation for delivery between 34 0/7 to 35 6/7 weeks of gestation.
Preoperative Preparation
There are several additional aspects of immediate preoperative preparation to consider. Preoperative checklists can assist in preparation for these complex surgical cases in the days leading up to delivery. If not already obtained, consultations with the appropriate services are needed. A blood type and antibody screen should be obtained, and if complex antibodies are present special preparations and consultation with the blood bank should be performed well in advance of surgery.
On the day of surgery, preparing for the possibility of significant resuscitative efforts and massive transfusion is warranted.65 At minimum, this includes obtaining adequate intravenous access for fluid and blood resuscitation with multiple large bore intravenous catheters and use of direct invasive arterial monitoring. Additionally, PAS teams should consider the use of cell salvage and rapid infusion devices to expedite transfusion of blood products.68 Since risk of rapid bleeding is difficult to predict, we bring a cooler to the operating room with multiple units of packed red cells, fresh frozen plasma, and platelets to be ready for rapid transfusion when needed.69
Coordination with an anesthesiologist accustomed to PAS care or managing massive obstetric hemorrhage, rapid large volume transfusion, and invasive hemodynamic monitoring is crucially important.68 Often, this is a person or team with subspecialty training in obstetric anesthesia. The optimal approach to anesthesia for PAS surgery is unknown and differs based on the intention of initial surgical treatment. Although general endotracheal anesthesia is often chosen, many referral centers use neuraxial analgesia for either all of the case or part of the case up until the time of delivery.70
The optimal operating theater for PAS surgery in each hospital varies depending on local resources and preferences, proximity to blood bank and neonatal intensive care unit, the goals of treatment (see section on Treatment below), and the urgency of the case. Many referral centers perform PAS surgery in the main operating room, where access to key equipment and surgical consultants is most readily available. In other centers, the hybrid operating room71 or labor and delivery are appropriate for PAS surgery.
Patient positioning in the operating room depends on the surgical approach. In general, positioning should allow for adequate surgical exposure for a large laparotomy; sufficient space for rapid intubation and multiple intravenous and arterial lines; leftward tilt until the fetus is delivered; and access to the perineum to periodically assess blood loss from below, allow for vaginal procedures or assistance, and perform cystoscopy and stent placement if indicated.72 Depending on the surgical plan, some centers routinely make use of preoperative or intraoperative cystoscopy to delineate the position of the bladder and ureters, and prophylactic ureteral stent placement has been associated with reduced incidence of genitourinary injury in cases of PAS undergoing hysterectomy.73
DELIVERY AND TREATMENT
High-quality comparative data is lacking to determine best treatments for patients with PAS at the time of delivery, and this lack of evidence contributes to wide variation in both clinical practice and surgical outcomes. In the absence of data, surgeons rely on collective local experience to determine treatment approach. The optimal surgical approach for an individual with PAS depends on multiple factors that vary substantially between patients and between centers. These include, but are not limited to, individual disease characteristics, gestational age at time of delivery, surgical team experience, and institutional resources. In addition, the preferences and values of the patient should inform plans. Dozens of approaches have been described in the literature, but none is clearly superior. These strategies can be condensed into four groups:
Cesarean hysterectomy following delivery of the newborn with the placenta left in situ.
Conservative (or expectant) in situ management of the placenta.
Planned delayed hysterectomy weeks after in situ management.
Partial myometrial resection and uterine reconstruction.
The treatment options for PAS discovered very early in gestation or cesarean scar ectopic pregnancy (CSEP) – which is now recognized as a precursor to PAS – are not discussed here in detail, although these are conditions for which PAS diagnostic and surgical teams are increasingly asked to contribute opinions on treatment.39 74 If diagnosis is made before viability, gravid hysterectomy with placenta and fetus left in-situ is a rare but reasonable options for patients with PAS.
Cesarean Hysterectomy
Cesarean hysterectomy – removal of the uterus with the placenta left in-situ following delivery of the fetus – is the most common approach practiced in the United States and hysterectomy is typically the most appropriate approach for PAS with active hemorrhage and hemodynamic instability.5 Hysterectomy is also the default backup plan or salvage approach when other treatment strategies fail. In the absence of very severe disease (FIGO 3B or 3C or PAS Grade 3E, see Box 1), and in the hands of an experienced surgical team, some experts believe this approach reduces the risk of the worst outcomes compared to alternative approaches.
Box 1: Suggested innovations, knowledge gaps, and research priorities for the future of PAS care.
Contemporary Innovations
-
Characteristics of PAS referral centers:
Coordination by a program champion or champions
Membership from all involved specialties
Capability for 24/7 mobilization of PAS surgical team
Interdisciplinary treatment planning meetings or formalized communication
Standardized evidence-based approaches to PAS diagnosis, staging, and management.
Standardized intraoperative staging and pathologic classification based on visible appearance of the placenta, remodeled uterus, and surrounding maternal structures
Peer Support Networks and Care Navigators
Urgent Clinical Needs
Externally validated antenatal staging schemes correlated to clinical outcomes
Integrative risk assessment combining a priori risk, primarily from prior uterine surgery, with imaging
Identification of best treatments for PAS and CSEP prior to viability (gravid hysterectomy, D&E, resection)
Techniques for improving uterine scar integrity to prevent PAS, CSEP, and other scar morbidities
Approaches and infrastructure to mitigate antenatal anxiety and postnatal trauma
Structured systems for education of new clinicians who provide PAS care (diagnosis, management, and surgery)
Key Research Gaps
Further advances in the field of PAS will require prospective systematic assessment in the following areas:
Systems
Objective core outcome sets & quality metrics
Defining case volume and competency for PAS expertise
Regionalization of PAS care
Diagnosis
Staging that predicts morbidity (not just disease presence)
Biomarker development
First trimester identification
Antenatal
Individualized delivery timing
Define patient-centered outcomes
Delivery/Treatment
Comparative, randomized studies of alternative treatments
-
Evaluation of surgical adjuncts:
Ureteral stents
Uterotonics
Anesthesia approaches (general, neuraxial, combined)
Adjuncts to Surgery
Randomized trials comparing endovascular intervention modalities, focusing on both efficacy and harm
Post-Delivery
Interventions to improve psychological and patient-centered outcomes
PAS, placenta accreta spectrum; CSEP, cesarean scar ectopic pregnancy
Cesarean hysterectomy performed in the setting of PAS is technically complex and hazardous, and it should ideally be performed by a surgical team with PAS experience. Patients with PAS are particularly vulnerable to bleeding for several reasons related to their underlying pathophysiology. First, the architecture designed to generate a high-flow low-resistance circuit to support the growing fetus creates a precarious surgical field, with rapid blood loss ensuing from even minor disruption of placental tissue, uterine tissue, or uterine vasculature. Second, the third trimester gravid uterus is 30- to 40-fold in size compared with a non-gravid uterus, with significant decrease in collagen concentration and increase in water concentration.75 76 Third, a 10-fold increase in blood flow, as high as 17% of cardiac output or 700 mL per minute, makes any surgical misstep an inciting event for life-threatening hemorrhage. The result is uterine tissue that is soft, edematous, and vascular, requiring dramatic adaptation of tissue handling as compared to a non-pregnant hysterectomy. PAS surgeons must be comfortable operating in the retroperitoneum near the pelvic side wall since most cases involve altered anatomy including a widened and distorted lower uterine segment, often with aberrant locations of the uterine arteries and ureters and a distorted bladder. Further complexity results from the presence of abdominopelvic adhesive disease related to prior uterine surgery or abdominal surgery.
Although there is tremendous practice variability regarding surgical approaches, several techniques have been proposed by experts at high-volume centers to reduce intraoperative morbidity.77 78 A vertical midline incision for abdominal entry is generally recommended to allow for adequate exposure of the uterus and for pelvic dissection in the setting of the gravid uterus. Infant delivery should be through a hysterotomy well away from the placental site to avoid placental disruption. This is best accomplished by first mapping the placental location with a sterile intraoperative ultrasound directly on the uterine surface and performing the uterine incision away from the placental site. Some centers routinely enter the uterus using serial clamping to devascularize an area for entry or hysterotomy extension with a stapler device.79 During hysterectomy, stepwise devascularization of the uterus beginning at the utero-ovarian pedicles allows for progressive mobility of the post-gravid uterus and reduction in collateral blood supply and reserves the most difficult dissection for last. The difficult areas of dissection are also those with highest vascularity, including bladder and lateral isolation of vascular pedicles, so approaching them last allows for expedited specimen amputation if bleeding is encountered.
Electrodissection is employed throughout the case, and many centers use vessel sealing devices.78 Of note, each of the currently marketed vessel sealing devices report a maximum vessel diameter for efficacy at 7mm which may be exceeded by the size of vascular pedicles in PAS. Thus, a combination of sealing device and traditional suture ligation techniques is typically used. Placement of an EEA sizer device or Breisky retractor within the vagina may assist in identification of the top of the vagina, given that tactile determination of the transition from cervix to vagina is much more challenging with a pregnancy-remodeled cervix particularly if also altered by placenta previa.80
Conservative in situ Management
Conservative in situ management (or expectant management) is practiced commonly in some parts of the world, both as a potential way of preserving the uterus for future pregnancy and as a potential way to reduce surgical morbidity.81 In this technique, the newborn is delivered via cesarean away from the placenta. The cord is trimmed short and tied off near the cord insertion. The non-adherent portions of the placenta may be trimmed away, or the entire placenta left in situ undisturbed. The uterine incision is then closed. If no immediate bleeding complications arise intraoperatively during the initial period of observation, the abdomen is closed, and expectant management continues until there is complete resorption of the placenta or until an indication to abandon conservative management occurs.82
Conservative management may reduce risk of major surgical morbidity and bleeding, although the absolute number of cases described in the literature remains quite low. Non-randomized and observational studies of conservative management, including the recent PACCRETA study from France83, have found reductions of up to 70% in the odds of transfusion, blood loss, and operative injury to the urinary tract or colon compared to immediate hysterectomy. Conservative management was also successful in avoiding hysterectomy in most cases (78–94%).83
There are several concerns with conservative management including the risk of emergent reoperation due to a complication of retained placenta and the need for prolonged and complex follow-up. In one study, endometritis occurred in 11% for those undergoing conservative management and the chance of hospital readmission was much higher (29% vs 3.4%).83 In some cases, endometritis can be successfully managed with antibiotic treatment without surgery. Additionally, in many individuals who require repeat operation or hysterectomy, the surgery is not emergent and can be scheduled when an indication arises such as ongoing severe pain or persistent bleeding. However, some patients undergoing conservative management will require prompt or emergent treatment for life-threatening bleeding or severe uterine infection with sepsis.
In the United States, unique barriers to conservative management exist. Patients living far from the hospital face logistical and cost barriers.84 Local clinicians may be unwilling to manage the perceived risks of retained PAS far from access to a referral center. Insurance coverage may end prior to the completion of placental resorption or fail to adequately cover the cost of additional care. Also, not all pregnant patients with PAS are eligible, as up to one-third develop pre-delivery or intra-operative bleeding. Other patients may not be willing to accept the burden of prolonged follow-up and uncertainty of conservative management.
Additionally, there remains a healthy skepticism among U.S. clinicians about whether the promising outcomes of conservative management studies can be reproduced. Selection bias almost certainly exists in uncontrolled studies of conservative management, likely resulting in more favorable outcomes. Patients undergoing conservative management had fewer antenatal complications, were more likely to have a non-emergent delivery on their planned surgical date, and were less likely to be hemorrhaging during or before delivery. Additionally, an inherent problem in prior studies of conservative management is the lack of histopathologic confirmation following delivery. Use of standardized definitions of disease staging, and statistical methods controlling for differences in baseline characteristics may reduce the risk of selection bias, but these methods can not eliminate bias.
Planned Delayed Hysterectomy
One proposed approach to minimize the risks associated with both immediate hysterectomy and conservative management is performing an interval hysterectomy weeks after delivery. This allows for definitive treatment of PAS while potentially mitigating risks of immediate hysterectomy, particularly in cases of severe PAS. Before the delivery of the newborn via cesarean, a systematic evaluation of the abdomen and pelvis is performed by the surgical team to document the extent of disease. This assessment includes evaluation of the remodeled uterus, bladder, parametria, adhesive disease, and other pelvic organ involvement. If the surgical team agrees that immediate hysterectomy can be carried out in a reasonably safe manner, this is performed instead of delayed hysterectomy. If the surgical team elects for delayed hysterectomy, the newborn is delivered and another systematic evaluation of the abdomen and pelvis is performed to ensure integrity of hysterotomy and absence of clinically significant vaginal bleeding, which would be a contraindication to delayed management. The value of adjunctive procedures for delayed hysterectomy, including femoral access, ureteral catheter placement, resuscitative endovascular balloon occlusion of the aorta, pelvic artery embolization, and postoperative antibiotics remains open for investigation.54
Delayed hysterectomy remains an investigational approach, although data from small studies shows lower estimated blood loss (750–850 mL vs. 2000–3000 mL), lower transfusion volumes, and fewer transfusions > 4 units (14% versus 55%) compared to immediate hysterectomy.85 86 Since this strategy requires two planned surgeries and possibly multiple hospitalizations, it is associated with longer total surgical times and longer postoperative lengths of stay.86 Rates of surgical and post-operative complications are similar to those undergoing immediate hysterectomy.85 The most common complications reported across studies of delayed hysterectomy were infection of the urinary tract and surgical site.85–87
Partial Myometrial Resection and Uterine Reconstruction
Single-surgery partial myometrial resection and reconstruction of the uterus is a treatment approach in some areas of the world.88–90 This strategy involves en bloc resection of the placenta and attached myometrium in well-selected cases followed by repair and reconstruction of the remaining uterus and cervix.91 This strategy is typically performed during the same surgery as cesarean delivery. Important to the success of this approach is experience in determining at the time of surgery whether to attempt resection or proceed directly to hysterectomy based on individual patient characteristics. These factors include sufficient viable myometrial tissue surrounding the affected area, degree of hypervascularity, and location and severity of pelvic adhesive disease. Knowing when to use this approach is a nuanced skill that comes only with experience. Some investigators couple resection and repair with pelvic devascularization procedures, compression suturing, incorporating the cervix into the closure, or tourniquet placement to reduce blood loss during uterine repair and reconstruction.92 93
This approach has not been commonly used (or at least has not been widely reported) in the U.S. SMFM and ACOG state that this approach should be “rare and considered individually.”5 Patients considering this approach should understand that resection may not be possible or may result in significant bleeding that would require hysterectomy. There are questions about generalizability of the data describing resection and reconstruction. Reports of success most often come from uncontrolled case series at single centers, sometimes with unclear selection criteria. Resection and reconstruction may be useful in select individuals.89 91 94 Before this technique is used more broadly, prospective research is needed to define the best candidates and compare outcomes to other approaches.
There are major unresolved questions regarding future pregnancies after uterine preservation. Patients undergoing uterine reconstruction after severe PAS are left with a considerably deformed and scarred uterus that may, in future pregnancies, place them at considerable risk of adverse outcomes including recurrent PAS, uterine rupture, and preterm birth. The few case series addressing these questions are not definitive to rule out significant harm.95
Intrapartum Diagnosis of PAS
Intrapartum identification of PAS presents a unique situation where teams may face an urgent need for complex clinical decision-making. For patients undergoing cesarean delivery, evaluation of the uterus prior to hysterotomy may show evidence of PAS such as bulging and an abnormally vascular lower uterine segment, or extrusion of placenta through a serosal defect [Figure 2]. Recognizing signs of PAS prior to delivery allows for careful assessment of resources as well as meticulous planning of hysterotomy well away from the placental location. The period following delivery, either vaginally and by cesarean, is another critical timepoint for assessment of expected placental separation. Failure of spontaneous separation, or an absent plane between the placenta and uterine wall on manual examination, are important signs or PAS and should prompt assessment and mobilization of resources for management of PAS.
Figure 2:
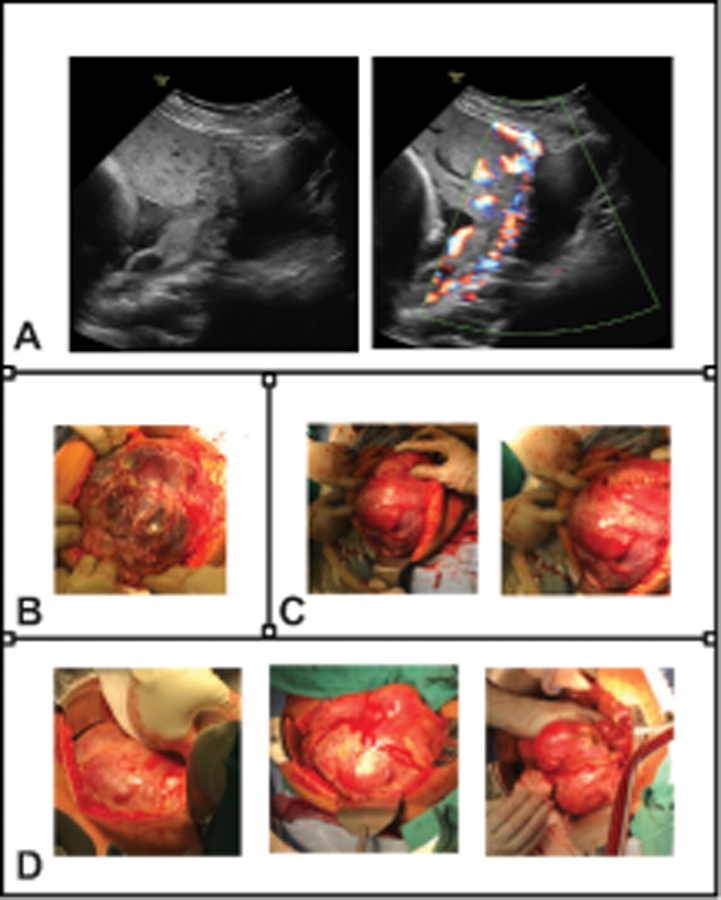
Appearance of severe placenta accreta spectrum (PAS). Visual correlation with patient outcomes at each stage of diagnosis and management is important for honing skills of PAS recognition, developing classification schemes predictive of clinical morbidity, and iterative learning for the multidisciplinary PAS care team. All images and photographs in this figure are from the same patient case. A. Representative antenatal ultrasound images (29 weeks of gestation) show complete placenta previa with placental bulging toward the bladder, loss of retroplacental clear zone, myometrial thinning, and extreme uterovesical hypervascularity with bridging vessels visible by color Doppler (right panel). B. Taken at the time of abdominal entry for scheduled delivery at 35 weeks of gestation, this photograph demonstrates obvious massive dehiscence of the previous cesarean scar with a large portion of the placental base visible through a thin translucent layer of uterine serosa. C. The placental edge was mapped with intraoperative ultrasonography to create a hysterotomy well away from the placental edge. The infant was delivered, avoiding placental disruption, and the hysterotomy was closed. Reduction in uterine volume with infant delivery allowed for full pelvic anatomy assessment, and a clear surgical plane could not be identified between the bladder and uterus. The patient was deemed a candidate for delayed interval hysterectomy. D. These photographs, taken at the time of completion laparotomy 40 days later, demonstrate the degree of tissue involution and reduction in vasculature that is the primary impetus for the delayed hysterectomy approach.
When an obstetric team encounters undiagnosed PAS, optimal management depends on the specific situation [Figure 1]. In cases of fetal or maternal instability, rapid delivery may be necessary; however, definitive management of PAS with hysterectomy or alternative strategy is not absolutely or immediately required once stabilization has occurred. In fact, a finding of PAS at laparotomy in an otherwise stable patient is an opportunity to pause and assess whether proceeding with delivery is in the patient’s best interest. Often, when a center lacks resources or expertise, transport prior to delivery is the best course of action. Another timepoint for assessment is after delivery. If PAS is encountered following delivery of the fetus, closing the uterus and abdomen with the placenta in situ may be safer than attempting hysterectomy in low-resource settings. The same is true for PAS suspected after vaginal delivery, where failure of placental separation necessitates surgical planning. Depending on a facility’s resources and expertise, this is a time point to decide whether transport to a higher level of care is preferable and feasible to proceeding with delivery and or hysterectomy. This decision is ideally based on prior and ongoing assessment of system resources, expertise, and an established relationship and action plan for transitioning care to a designated referral center. Throughout, continued assessment of a patient’s hemodynamic stability and provision of life-saving and resuscitative care such as volume repletion, blood product transfusion, antifibrinolytic agent is paramount.
ENDOVASCULAR APPROACHES TO REDUCE BLOOD LOSS
Even with advanced surgical expertise and optimal preoperative planning in experienced referral centers, rapid life-threating hemorrhage during delivery and treatment for PAS can occur. The distinct challenge for PAS management comes from the proliferative neovasculature, which can arise from essentially anywhere in the pelvis, including internal iliac, external iliac and possibly aortic or ovarian vessels. These vessels are hypertrophied and have a very high flow rate.26 96 Anticipating the potential for a large intraoperative blood loss, some centers collaborate with interventional radiologists to pursue endovascular approaches to reduce blood loss, including balloon occlusion and embolization. There is tremendous heterogeneity in reported studies, resulting in severely limited generalizability and limited opportunity to assess comparative efficacy.
Endovascular approaches may be beneficial in some scenarios. For immediate hysterectomy, endovascular adjunctive procedures may simplify dissection by “drying” the surgical field.97 Similarly, diminishing blood supply to the uterus and placenta may hasten involution for either immediate or delayed hysterectomy or conservative management.86
Balloon Occlusion
Balloon occlusion can be applied at multiple levels, from distal to proximal including placement in uterine arteries (UA), internal iliac arteries (IIA), common iliac arteries (CIA), or the distal abdominal aorta (AA) [Figure 3]. Small case reports, case series, and retrospective cohort studies yield mixed results for balloon occlusion.98 99 A randomized controlled trial of IIA occlusion balloons reported no improvement in blood loss volume or need for hysterectomy.100 A meta-analysis suggests that endovascular intervention may result in less blood loss compared to no endovascular intervention, particularly if occlusion is done at the level of the abdominal aorta101, but data are largely from uncontrolled studies with highly varied inclusion criteria, indications for use, and techniques for balloon inflation.102 Ultimately, more prospective research is needed to confirm and define benefits of balloon occlusion.
Figure 3:
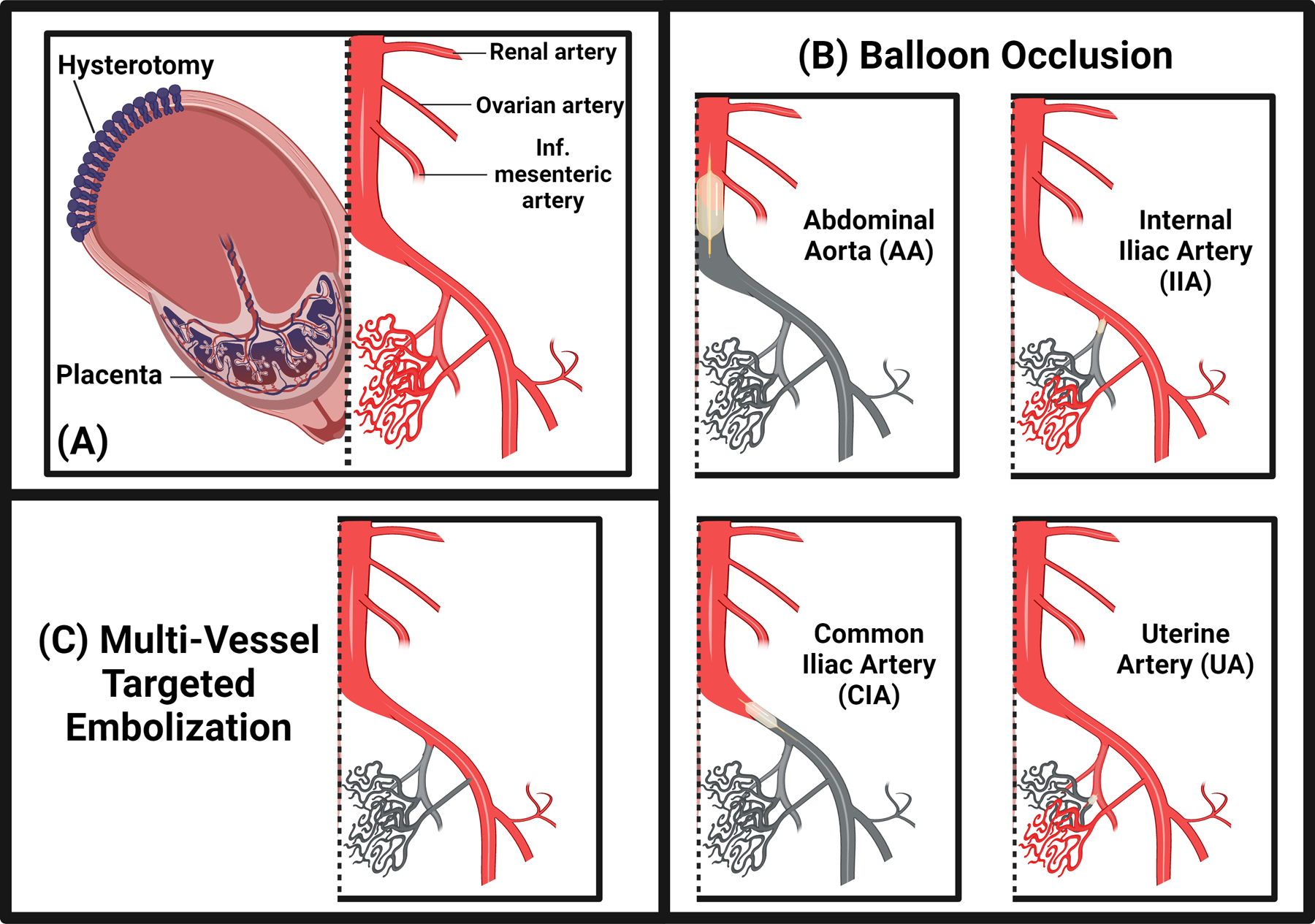
Endovascular approaches to reducing blood loss in placenta accreta spectrum (PAS). Endovascular adjunctive approaches, most commonly performed by interventional radiologists, can be used as salvage therapy in the case of active bleeding, or in a prophylactic manner to simplify dissection by “drying” the surgical field. In cases where the uterus is maintained in situ after delivery, these techniques are thought to hasten involution of the uterus, placenta, and pelvic vasculature. A. Diagrammatic representation of uteroplacental anatomy (left of dashed line) and major abdominopelvic vasculature (right of dashed line) in PAS after infant delivery. A hallmark of PAS disease, and the predominant source of its morbidity, is pervasive hypertrophied pelvic neovasculature, which can arise from any major artery in the lower abdomen and pelvis. B. Balloon occlusion approaches range from distal to proximal, with placement in uterine arteries (UA), internal iliac arteries (IIA), common iliac arteries (CIA), or the distal abdominal aorta (AA). More proximal placement can be faster and result in greater reduction in pelvic blood flow, but also carries greater risk for nontarget ischemic sequelae. C. Multivessel targeted embolization may require more specialized interventional radiology experience for optimization, but early data has demonstrated comparable blood loss reduction to abdominal aortic occlusion with dramatic reduction in nontarget vascular sequelae.
Serious complications of balloon occlusion are primarily vascular and result from balloon migration, balloon rupture, vessel intimal injury or pseudoaneurysm, claudication from downstream ischemia, and arterial thrombosis.98 103 Arterial thrombosis occurs in 9–15% of those undergoing IIA or AA occlusion.104–106 Extended inflation time may result in complications including limb ischemia necrosis or multi organ dysfunction syndrome.107 Radiation exposure is another significant concern prior to fetal delivery due to fluoroscopy exposure at balloon placement, which is generally higher for more distal placement.103
Prophylactic multivessel embolization
Given concerns with the complication rates and uncertain efficacy of balloon occlusion, some centers alternatively opt for prophylactic embolization. A promising approach includes targeted multivessel embolization after delivery of the newborn to reduce average blood loss while drastically reducing the potential for non-target ischemia that can result from aortic or internal iliac balloon occlusion.86 108 Similar to most balloon occlusion data, prophylactic embolization data are retrospective, but the reported reduction in blood loss and transfusion rates spark interest for a prospective study.
POSTPARTUM CARE
Short term postpartum care
After initial treatment for PAS, patients require attention to specialized needs, often including critical care, in addition to routine postpartum care. Anticipation of critical care needs depends on clear communication between the obstetric team and critical care team(s). This process can start early with antenatal consults at PAS diagnosis and should allow for coordinated multidisciplinary daily care including the obstetrician even in critical care units that traditionally place care under the full responsibility of the intensivist. The PAS patient needs the intensivists’ expertise in standard measures to reduce the effects of post-intensive care syndrome (PICS) 109 110, as well as the obstetricians’ expertise in achieving attainable elements of the normal postpartum experience.
The simple act of normalization can go a long way toward healing from a highly atypical birth experience inherent in PAS disease. Routine postpartum elements can be brought to the patient in any unit, such as early initiation of lactation support and creative attempts to bring the birthing parent and newborn together. This may come in the form of video connections between maternal and neonatal hospital locations, methods of keeping the recovering birthing parent involved in the care progress of the (often premature) neonate, or nursing-supervised visits to the neonatal unit once the birthing parent is stabilized.
Patients with PAS have unique needs beyond postpartum care. Those needs inevitably bridge multiple medical disciplines, making them unfamiliar to health care professionals at the bedside. It is the responsibility of the core PAS team to clearly communicate expectations to nurses, house staff, consultants, physical therapists, case managers, patients, and their support persons. Postoperative milestones such as pain levels, urinary function, foley catheter removal, epidural discontinuation, expectations for bleeding, understanding of intraoperative events, and return of appetite are just some examples of care elements that may fall outside the “standard” postpartum and postoperative course and must be preemptively addressed through education and counseling. Small reminders and consistent communication to the full care team can avoid missteps such as routine nursing assessments for fundal height or postpartum evaluations that may interpret excessive post-hysterectomy vaginal bleeding as normal lochia.
Long-term post-PAS care
A common thread of the PAS experience is the extreme and unexpected nature of the experience, particularly when compared with pregnancy expectations prior to diagnosis. As such, it is misguided to consider the work and care provision of the PAS team to be complete once the birthing parent and their neonate are safely discharged from the hospital after delivery. Regardless of innate resilience, this circumstance sets up patients and families for emotional distress, fear, feelings of loss, and varying degrees of trauma. Furthermore, the cognitive, psychiatric, and physical effects of PICS can persist for weeks or months after hospital discharge.
Formal development of an integrated care pathway has been proposed to address these serious unmet needs. Patients and families affected by PAS may find helpful the provision of educational materials, standardized planning and recovery protocols, and ready access to PAS specialist review in all stages of the experience from diagnosis through post-delivery recovery and what has been termed “living beyond PAS.”111 The engagement of a Nurse Navigator or peer support networks, or a core team serving as primary contacts for the patient and family, can also address the long-term processing required for this major physical and emotional transition. Consistent presence can bring peace of mind at a broad level and can expedite connecting the patient to the right member of a large care team at any point in their PAS experience.
Long-awaited research is emerging to explore the unique challenges to recovery for the growing population of PAS patients and families. Several recent qualitative research studies have extracted themes from the lived experience from those affected by PAS.6–8 111–113 These first-hand accounts are invaluable for framing future goals to better support patients with PAS in their short-term and long-term recovery. Importantly, the existing medical literature represents only a fraction of the demographic affected by PAS disease, leaving much work to be done to address varying cultural implications, economic effects, and support needs where large gaps remain in PAS care provision [Box 1].
CONCLUSION
Placenta accreta spectrum is a condition that places patients at high risk of hemorrhage, surgical complications, and even death. As such, their care is best accomplished at centers with ample experience and resources to manage the breadth of both physical and psychosocial needs attendant to a PAS diagnosis. Clinical risk assessment, mainly a history of prior uterine surgery, in addition to a detailed ultrasound assessment of the placenta in at risk patients, is the first line diagnostic approach. In prenatally diagnosed cases, cesarean hysterectomy is the most common and definitive approach. Considering conservative or alternative management approaches depends on an institution’s expertise and resources, PAS severity, and the patient’s preferences. Any institution that offers obstetric care must anticipate the potential for encountering undiagnosed PAS and have a pathway for escalation to a higher level of care.
An intrapartum diagnosis of PAS is often associated with increased bleeding, and in cases where stabilization can occur, transfer to an experienced PAS center may be the best option.
Although understanding of pathophysiology, ideal diagnostic tools, and optimal treatment approach is growing, much remains to be discovered. Future directions for PAS investigation are vast and include the following: how to designate and/or build a PAS referral center; optimal surgical approach and techniques; person-centered outcomes related to experience, psychosocial effects, and strategies to mitigate trauma; and improved diagnosis through discovery of biomarkers for PAS.
Supplementary Material
Acknowledgments
Brett D. Einerson was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, K23HD106009) during the completion of this work to study conservative management as an alternative to hysterectomy for placenta accreta spectrum.
Footnotes
Financial Disclosure
Lisa C. Zuckerwise reports money was paid to her institution from Laborie Medical Technologies Corp for an ongoing clinical study unrelated to placenta accreta spectrum. She received payment from Gershon, Willoughby & Getz, LLC, and Huff, Powell & Bailey, LLC for legal expert consulting not related to placenta accreta spectrum. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Contributor Information
Brett D. Einerson, University of Utah Health, Department of Obstetrics & Gynecology.
Jennifer B. Gilner, Duke University, 2608 Erwin Rd. Suite 200, DUMC 3967, Durham, NC 27710.
Lisa C. Zuckerwise, Vanderbilt University Medical Center, 1161 Medical Center Avenue South, C1120, Nashville, TN 37273..
REFERENCES
- 1.Leonard SA, Kennedy CJ, Carmichael SL, et al. An Expanded Obstetric Comorbidity Scoring System for Predicting Severe Maternal Morbidity. Obstet Gynecol 2020;136(3):440–49. doi: 10.1097/aog.0000000000004022 [published Online First: 2020/August/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jauniaux E, Bunce C, Grønbeck L, et al. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol 2019;221(3):208–18. doi: 10.1016/j.ajog.2019.01.233 [published Online First: 2019/February/05] [DOI] [PubMed] [Google Scholar]
- 3.Eller AG, Porter TF, Soisson P, et al. Optimal management strategies for placenta accreta. BJOG 2009;116(5):648–54. doi: 10.1111/j.1471-0528.2008.02037.x [published Online First: 2009/February/05] [DOI] [PubMed] [Google Scholar]
- 4.Morlando M, Schwickert A, Stefanovic V, et al. Maternal and neonatal outcomes in planned versus emergency cesarean delivery for placenta accreta spectrum: A multinational database study. Acta Obstet Gynecol Scand 2021;100 Suppl 1:41–49. doi: 10.1111/aogs.14120 [published Online First: 2021/March/14] [DOI] [PubMed] [Google Scholar]
- 5.Placenta accreta spectrum. Obstetric Care Consensus No. 7. American College of Obstetricians and Gynecologists. Obstetrics and Gynecology 2018;132:e259–75. [DOI] [PubMed] [Google Scholar]
- 6.Grover B, Einerson BD, Keenan KD, et al. Patient-Reported Health Outcomes and Quality of Life after Peripartum Hysterectomy for Placenta Accreta Spectrum. Am J Perinatol 2020 doi: 10.1055/s-0040-1715465 [published Online First: 2020/August/21] [DOI] [PubMed] [Google Scholar]
- 7.Tol ID, Yousif M, Collins SL. Post traumatic stress disorder (PTSD): The psychological sequelae of abnormally invasive placenta (AIP). Placenta 2019;81:42–45. doi: 10.1016/j.placenta.2019.04.004 [published Online First: 2019/May/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels HC, Terlizzi K, Cooney N, et al. Quality of life and sexual function after a pregnancy complicated by placenta accreta spectrum. Aust N Z J Obstet Gynaecol 2021;61(5):708–14. doi: 10.1111/ajo.13338 [published Online First: 2021/March/26] [DOI] [PubMed] [Google Scholar]
- 9.Read JA, Cotton DB, Miller FC. Placenta accreta: changing clinical aspects and outcome. Obstet Gynecol 1980;56(1):31–4. [published Online First: 1980/July/01] [PubMed] [Google Scholar]
- 10.Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol 1997;177(1):210–4. doi: 10.1016/s0002-9378(97)70463-0 [published Online First: 1997/July/01] [DOI] [PubMed] [Google Scholar]
- 11.Butwick AJ, Walsh EM, Kuzniewicz M, et al. Accuracy of international classification of diseases, ninth revision, codes for postpartum hemorrhage among women undergoing cesarean delivery. Transfusion 2018;58(4):998–1005. doi: 10.1111/trf.14498 [published Online First: 2018/January/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jauniaux E, Chantraine F, Silver RM, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int J Gynaecol Obstet 2018;140(3):265–73. doi: 10.1002/ijgo.12407 [published Online First: 2018/February/07] [DOI] [PubMed] [Google Scholar]
- 13.Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet 2019;146(1):20–24. doi: 10.1002/ijgo.12761 [published Online First: 2019/June/08] [DOI] [PubMed] [Google Scholar]
- 14.Hecht JL, Baergen R, Ernst LM, et al. Classification and reporting guidelines for the pathology diagnosis of placenta accreta spectrum (PAS) disorders: recommendations from an expert panel. Mod Pathol 2020 doi: 10.1038/s41379-020-0569-1 [published Online First: 2020/May/18] [DOI] [PubMed] [Google Scholar]
- 15.Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol 1985;66(1):89–92. [published Online First: 1985/July/01] [PubMed] [Google Scholar]
- 16.Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol 2005;192(5):1458–61. doi: 10.1016/j.ajog.2004.12.074 [published Online First: 2005/May/20] [DOI] [PubMed] [Google Scholar]
- 17.Betran AP, Ye J, Moller AB, et al. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health 2021;6(6) doi: 10.1136/bmjgh-2021-005671 [published Online First: 2021/June/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Givens M, Einerson BD, Allshouse AA, et al. Trends in Unplanned Peripartum Hysterectomy in the United States, 2009–2020. Obstet Gynecol 2022;139(3):449–51. doi: 10.1097/aog.0000000000004673 [published Online First: 2022/February/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irving C, Hertig A. A study of placenta accreta. Surg Gynecol Obstet 1937;64:178–200. [Google Scholar]
- 20.Luke RK, Sharpe JW, Greene RR. Placenta accreta: the adherent or invasive placenta. Am J Obstet Gynecol 1966;95(5):660–8. doi: 10.1016/s0002-9378(16)34741-x [published Online First: 1966/July/01] [DOI] [PubMed] [Google Scholar]
- 21.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008;29(7):639–45. doi: 10.1016/j.placenta.2008.04.008 [published Online First: 2008/June/03] [DOI] [PubMed] [Google Scholar]
- 22.Einerson BD, Comstock J, Silver RM, et al. Placenta Accreta Spectrum Disorder: Uterine Dehiscence, Not Placental Invasion. Obstet Gynecol 2020;135(5):1104–11. doi: 10.1097/AOG.0000000000003793 [published Online First: 2020/April/14] [DOI] [PubMed] [Google Scholar]
- 23.Jauniaux E, Hussein AM, Elbarmelgy RM, et al. Failure of placental detachment in accreta placentation is associated with excessive fibrinoid deposition at the utero-placental interface. Am J Obstet Gynecol 2021 doi: 10.1016/j.ajog.2021.08.026 [published Online First: 2021/August/31] [DOI] [PubMed] [Google Scholar]
- 24.Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol 2006;107(6):1226–32. doi: 10.1097/01.AOG.0000219750.79480.84 [published Online First: 2006/June/02] [DOI] [PubMed] [Google Scholar]
- 25.Duzyj CM, Buhimschi IA, Laky CA, et al. Extravillous trophoblast invasion in placenta accreta is associated with differential local expression of angiogenic and growth factors: a cross-sectional study. Bjog 2018;125(11):1441–48. doi: 10.1111/1471-0528.15176 [published Online First: 2018/February/23] [DOI] [PubMed] [Google Scholar]
- 26.Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol 2018;218(1):75–87. doi: 10.1016/j.ajog.2017.05.067 [published Online First: 2017/June/11] [DOI] [PubMed] [Google Scholar]
- 27.Kohn JR, Shamshirsaz AA, Popek E, et al. Pregnancy after endometrial ablation: a systematic review. BJOG 2018;125(1):43–53. doi: 10.1111/1471-0528.14854 [published Online First: 2017/September/28] [DOI] [PubMed] [Google Scholar]
- 28.Modest AM, Toth TL, Johnson KM, et al. Placenta Accreta Spectrum: In Vitro Fertilization and Non-In Vitro Fertilization and Placenta Accreta Spectrum in a Massachusetts Cohort. Am J Perinatol 2020 doi: 10.1055/s-0040-1713887 [published Online First: 2020/July/06] [DOI] [PubMed] [Google Scholar]
- 29.Miller HE, Leonard SA, Fox KA, et al. Placenta Accreta Spectrum Among Women With Twin Gestations. Obstet Gynecol 2021;137(1):132–38. doi: 10.1097/aog.0000000000004204 [published Online First: 2020/December/06] [DOI] [PubMed] [Google Scholar]
- 30.Benirschke K, Burton GJ, Baergen R. Pathology of the human placenta (6th ed.). 6 ed. New York: Springer; 2012. [Google Scholar]
- 31.Cramer SF, Heller DS. Placenta Accreta and Placenta Increta: An Approach to Pathogenesis Based on the Trophoblastic Differentiation Pathway. Pediatr Dev Pathol 2016;19(4):320–33. doi: 10.2350/15-05-1641-oa.1 [published Online First: 2015/October/23] [DOI] [PubMed] [Google Scholar]
- 32.da Cunha Castro EC, Popek E. Abnormalities of placenta implantation. Apmis 2018;126(7):613–20. doi: 10.1111/apm.12831 [published Online First: 2018/August/22] [DOI] [PubMed] [Google Scholar]
- 33.Jauniaux E, Hecht JL, Elbarmelgy RA, et al. Searching for placenta percreta: a prospective cohort and systematic review of case reports. Am J Obstet Gynecol 2022;226(6):837.e1–37.e13. doi: 10.1016/j.ajog.2021.12.030 [published Online First: 2022/January/02] [DOI] [PubMed] [Google Scholar]
- 34.Warshak CR, Ramos GA, Eskander R, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol 2010;115(1):65–9. doi: 10.1097/AOG.0b013e3181c4f12a [published Online First: 2009/December/23] [DOI] [PubMed] [Google Scholar]
- 35.Eller AG, Bennett MA, Sharshiner M, et al. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol 2011;117(2 Pt 1):331–7. doi: 10.1097/aog.0b013e3182051db2 [published Online First: 2011/February/12] [DOI] [PubMed] [Google Scholar]
- 36.Shainker SA, Silver RM, Modest AM, et al. Placenta accreta spectrum: biomarker discovery using plasma proteomics. Am J Obstet Gynecol 2020 doi: 10.1016/j.ajog.2020.03.019 [published Online First: 2020/March/23] [DOI] [PubMed] [Google Scholar]
- 37.Afshar Y, Dong J, Zhao P, et al. Circulating trophoblast cell clusters for early detection of placenta accreta spectrum disorders. Nat Commun 2021;12(1):4408. doi: 10.1038/s41467-021-24627-2 [published Online First: 2021/August/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. Am J Obstet Gynecol 2012;207(1):14–29. doi: 10.1016/j.ajog.2012.03.007 [published Online First: 2012/April/21] [DOI] [PubMed] [Google Scholar]
- 39.Miller R, Gyamfi-Bannerman C. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy. Am J Obstet Gynecol 2022;227(3):B9–b20. doi: 10.1016/j.ajog.2022.06.024 [published Online First: 2022/July/20] [DOI] [PubMed] [Google Scholar]
- 40.Jauniaux E, Zosmer N, De Braud LV, et al. Development of the utero-placental circulation in cesarean scar pregnancies: a case-control study. Am J Obstet Gynecol 2022;226(3):399.e1–99.e10. doi: 10.1016/j.ajog.2021.08.056 [published Online First: 2021/September/08] [DOI] [PubMed] [Google Scholar]
- 41.Hessami K, Salmanian B, Einerson BD, et al. Clinical Correlates of Placenta Accreta Spectrum Disorder Depending on the Presence or Absence of Placenta Previa: A Systematic Review and Meta-analysis. Obstet Gynecol 2022;140(4):599–606. doi: 10.1097/aog.0000000000004923 [published Online First: 2022/September/09] [DOI] [PubMed] [Google Scholar]
- 42.Shainker SA, Coleman B, Timor-Tritsch IE, et al. Special Report of the Society for Maternal-Fetal Medicine Placenta Accreta Spectrum Ultrasound Marker Task Force: Consensus on definition of markers and approach to the ultrasound examination in pregnancies at risk for placenta accreta spectrum. Am J Obstet Gynecol 2021;224(1):B2–b14. doi: 10.1016/j.ajog.2020.09.001 [published Online First: 2021/January/03] [DOI] [PubMed] [Google Scholar]
- 43.Philips J, Gurganus M, DeShields S, et al. Prevalence of Sonographic Markers of Placenta Accreta Spectrum in Low-Risk Pregnancies. Am J Perinatol 2019;36(8):733–80. doi: 10.1055/s-0038-1676488 [published Online First: 2018/December/24] [DOI] [PubMed] [Google Scholar]
- 44.Shih JC, Kang J, Tsai SJ, et al. The “rail sign”: an ultrasound finding in placenta accreta spectrum indicating deep villous invasion and adverse outcomes. Am J Obstet Gynecol 2021;225(3):292.e1–92.e17. doi: 10.1016/j.ajog.2021.03.018 [published Online First: 2021/March/22] [DOI] [PubMed] [Google Scholar]
- 45.Allwood RX, Self A, Collins SL. Separation sign: novel ultrasound sign for ruling out diagnosis of placenta accreta spectrum. Ultrasound Obstet Gynecol 2022;60(3):390–95. doi: 10.1002/uog.26021 [published Online First: 2022/July/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jauniaux E, D’Antonio F, Bhide A, et al. Modified Delphi study of ultrasound signs associated with placenta accreta spectrum. Ultrasound Obstet Gynecol 2023 doi: 10.1002/uog.26155 [published Online First: 2023/January/08] [DOI] [PubMed] [Google Scholar]
- 47.Happe SK, Yule CS, Spong CY, et al. Predicting Placenta Accreta Spectrum: Validation of the Placenta Accreta Index. J Ultrasound Med 2020 doi: 10.1002/jum.15530 [published Online First: 2020/October/16] [DOI] [PubMed] [Google Scholar]
- 48.Carniello MO, Oliveira Brito LG, Sarian LOZ, et al. Diagnosis of placenta accreta spectrum in high-risk women using ultrasonography or magnetic resonance imaging: systematic review to compare accuracy of tests. Ultrasound Obstet Gynecol 2022 doi: 10.1002/uog.24861 [published Online First: 2022/January/19] [DOI] [PubMed] [Google Scholar]
- 49.Jha P, Pōder L, Bourgioti C, et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol 2020;30(5):2604–15. doi: 10.1007/s00330-019-06617-7 [published Online First: 2020/February/11] [DOI] [PubMed] [Google Scholar]
- 50.Einerson BD, Rodriguez CE, Silver RM, et al. Accuracy and Interobserver Reliability of Magnetic Resonance Imaging for Placenta Accreta Spectrum Disorders. Am J Perinatol 2020 doi: 10.1055/s-0040-1701196 [published Online First: 2020/January/28] [DOI] [PubMed] [Google Scholar]
- 51.Einerson BD, Rodriguez CE, Kennedy AM, et al. Magnetic resonance imaging is often misleading when used as an adjunct to ultrasound in the management of placenta accreta spectrum disorders. Am J Obstet Gynecol 2018;218(6):618 e1–18 e7. doi: 10.1016/j.ajog.2018.03.013 [published Online First: 2018/March/25] [DOI] [PubMed] [Google Scholar]
- 52.Cali G, Forlani F, Lees C, et al. Prenatal ultrasound staging system for placenta accreta spectrum disorders. Ultrasound Obstet Gynecol 2019;53(6):752–60. doi: 10.1002/uog.20246 [published Online First: 2019/March/06] [DOI] [PubMed] [Google Scholar]
- 53.Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol 2015;212(5):561–8. doi: 10.1016/j.ajog.2014.11.018 [published Online First: 2014/December/03] [DOI] [PubMed] [Google Scholar]
- 54.Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol 2019;220(6):511–26. doi: 10.1016/j.ajog.2019.02.054 [published Online First: 2019/March/09] [DOI] [PubMed] [Google Scholar]
- 55.Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol 2019(134):e41–55. [DOI] [PubMed] [Google Scholar]
- 56.Jolley JA, Nageotte MP, Wing DA, et al. Management of placenta accreta: a survey of Maternal-Fetal Medicine practitioners. J Matern Fetal Neonatal Med 2012;25(6):756–60. doi: 10.3109/14767058.2011.594467 [published Online First: 2011/August/11] [DOI] [PubMed] [Google Scholar]
- 57.Wright JD, Silver RM, Bonanno C, et al. Practice patterns and knowledge of obstetricians and gynecologists regarding placenta accreta. J Matern Fetal Neonatal Med 2013;26(16):1602–9. doi: 10.3109/14767058.2013.793662 [published Online First: 2013/April/10] [DOI] [PubMed] [Google Scholar]
- 58.Couret M, Huang Y, Khoury-Collado F, et al. Patterns of care for women with placenta accreta spectrum. J Matern Fetal Neonatal Med 2019:1–7. doi: 10.1080/14767058.2019.1684471 [published Online First: 2019/November/21] [DOI] [PubMed] [Google Scholar]
- 59.Fitzpatrick KE, Sellers S, Spark P, et al. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS One 2012;7(12):e52893. doi: 10.1371/journal.pone.0052893 [published Online First: 2013/January/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailit JL, Grobman WA, Rice MM, et al. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol 2015;125(3):683–9. doi: 10.1097/AOG.0000000000000680 [published Online First: 2015/March/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright JD, Herzog TJ, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol 2010;115(6):1194–200. doi: 10.1097/AOG.0b013e3181df94e8 [published Online First: 2010/May/27] [DOI] [PubMed] [Google Scholar]
- 62.Matsuo K, Youssefzadeh AC, Mandelbaum RS, et al. Hospital surgical volume-outcome relationship in caesarean hysterectomy for placenta accreta spectrum. BJOG 2021 doi: 10.1111/1471-0528.16993 [published Online First: 2021/November/08] [DOI] [PubMed] [Google Scholar]
- 63.James AH. Iron Deficiency Anemia in Pregnancy. Obstet Gynecol 2021;138(4):663–74. doi: 10.1097/aog.0000000000004559 [published Online First: 2021/October/09] [DOI] [PubMed] [Google Scholar]
- 64.Gatta LA, Lockhart EL, James AH. Blood Products in the Management of Abnormal Placentation. Clin Obstet Gynecol 2018;61(4):828–40. doi: 10.1097/grf.0000000000000400 [published Online First: 2018/October/05] [DOI] [PubMed] [Google Scholar]
- 65.Butwick A, Lyell D, Goodnough L. How do I manage severe postpartum hemorrhage? Transfusion 2020;60(5):897–907. doi: 10.1111/trf.15794 [published Online First: 2020/April/23] [DOI] [PubMed] [Google Scholar]
- 66.Perlman NC, Little SE, Thomas A, et al. Patient selection for later delivery timing with suspected previa-accreta. Acta Obstet Gynecol Scand 2017;96(8):1021–28. doi: 10.1111/aogs.13140 [published Online First: 2017/April/05] [DOI] [PubMed] [Google Scholar]
- 67.Salmanian B, Einerson BD, Carusi DA, et al. Timing of delivery for placenta accreta spectrum: the Pan-American Society for the Placenta Accreta Spectrum experience. Am J Obstet Gynecol MFM 2022;4(6):100718. doi: 10.1016/j.ajogmf.2022.100718 [published Online First: 2022/August/18] [DOI] [PubMed] [Google Scholar]
- 68.Warrick CM, Rollins MD. Peripartum Anesthesia Considerations for Placenta Accreta. Clin Obstet Gynecol 2018;61(4):808–27. doi: 10.1097/grf.0000000000000403 [published Online First: 2018/October/13] [DOI] [PubMed] [Google Scholar]
- 69.Shaylor R, Weiniger CF, Austin N, et al. National and International Guidelines for Patient Blood Management in Obstetrics: A Qualitative Review. Anesth Analg 2017;124(1):216–32. doi: 10.1213/ane.0000000000001473 [published Online First: 2016/August/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markley JC, Farber MK, Perlman NC, et al. Neuraxial Anesthesia During Cesarean Delivery for Placenta Previa With Suspected Morbidly Adherent Placenta: A Retrospective Analysis. Anesth Analg 2018;127(4):930–38. doi: 10.1213/ane.0000000000003314 [published Online First: 2018/February/27] [DOI] [PubMed] [Google Scholar]
- 71.Clark A, Farber MK, Sviggum H, et al. Cesarean delivery in the hybrid operating suite: a promising new location for high-risk obstetric procedures. Anesth Analg 2013;117(5):1187–9. doi: 10.1213/ANE.0b013e3182a00aff [published Online First: 2013/September/14] [DOI] [PubMed] [Google Scholar]
- 72.Einerson BD, Weiniger CF. Placenta accreta spectrum disorder: updates on anesthetic and surgical management strategies. Int J Obstet Anesth 2021;46:102975. doi: 10.1016/j.ijoa.2021.102975 [published Online First: 2021/March/31] [DOI] [PubMed] [Google Scholar]
- 73.Scaglione MA, Allshouse AA, Canfield DR, et al. Prophylactic Ureteral Stent Placement and Urinary Injury During Hysterectomy for Placenta Accreta Spectrum. Obstet Gynecol 2022;140(5):806–11. doi: 10.1097/aog.0000000000004957 [published Online First: 2022/October/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol 2014;44(3):346–53. doi: 10.1002/uog.13426 [published Online First: 2014/June/04] [DOI] [PubMed] [Google Scholar]
- 75.Uldbjerg N, Ekman G, Malmström A, et al. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol 1983;147(6):662–6. doi: 10.1016/0002-9378(83)90446-5 [published Online First: 1983/November/15] [DOI] [PubMed] [Google Scholar]
- 76.Rehman KS, Yin S, Mayhew BA, et al. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod 2003;9(11):681–700. doi: 10.1093/molehr/gag078 [published Online First: 2003/October/17] [DOI] [PubMed] [Google Scholar]
- 77.Einerson BD, Branch DW. Surgical Management of Placenta Accreta Spectrum. Clin Obstet Gynecol 2018;61(4):774–82. doi: 10.1097/GRF.0000000000000406 [published Online First: 2018/October/10] [DOI] [PubMed] [Google Scholar]
- 78.Kingdom JC, Hobson SR, Murji A, et al. Minimizing surgical blood loss at cesarean hysterectomy for placenta previa with evidence of placenta increta or placenta percreta: the state of play in 2020. Am J Obstet Gynecol 2020;223(3):322–29. doi: 10.1016/j.ajog.2020.01.044 [published Online First: 2020/February/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belfort MA, Shamshiraz AA, Fox K. Minimizing blood loss at cesarean-hysterectomy for placenta previa percreta. Am J Obstet Gynecol 2017;216(1):78.e1–78.e2. doi: 10.1016/j.ajog.2016.10.030 [published Online First: 2016/December/17] [DOI] [PubMed] [Google Scholar]
- 80.Belfort MA, Shamshirsaz AA, Fox KA. A technique to positively identify the vaginal fornices during complicated postpartum hysterectomy. Am J Obstet Gynecol 2017;217(2):222.e1–22.e3. doi: 10.1016/j.ajog.2017.05.001 [published Online First: 2017/May/11] [DOI] [PubMed] [Google Scholar]
- 81.Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol 2010;115(3):526–34. doi: 10.1097/AOG.0b013e3181d066d4 [published Online First: 2010/February/24] [DOI] [PubMed] [Google Scholar]
- 82.Fox KA, Shamshirsaz AA, Carusi D, et al. Conservative management of morbidly adherent placenta: expert review. Am J Obstet Gynecol 2015;213(6):755–60. doi: 10.1016/j.ajog.2015.04.034 [published Online First: 2015/May/04] [DOI] [PubMed] [Google Scholar]
- 83.Sentilhes L, Seco A, Azria E, et al. Conservative management or cesarean hysterectomy for placenta accreta spectrum: the PACCRETA prospective study. Am J Obstet Gynecol 2022;226(6):839.e1–39.e24. doi: 10.1016/j.ajog.2021.12.013 [published Online First: 2021/December/17] [DOI] [PubMed] [Google Scholar]
- 84.Pineles BL, Sibai BM, Sentilhes L. Is conservative management of placenta accreta spectrum disorders practical in the United States? Am J Obstet Gynecol MFM 2022:100749. doi: 10.1016/j.ajogmf.2022.100749 [published Online First: 2022/September/17] [DOI] [PubMed] [Google Scholar]
- 85.Zuckerwise LC, Craig AM, Newton JM, et al. Outcomes following a clinical algorithm allowing for delayed hysterectomy in the management of severe placenta accreta spectrum. Am J Obstet Gynecol 2020;222(2):179.e1–79.e9. doi: 10.1016/j.ajog.2019.08.035 [published Online First: 2019/August/31] [DOI] [PubMed] [Google Scholar]
- 86.Gatta LA, Weber JM, Gilner JB, et al. Transfusion Requirements with Hybrid Management of Placenta Accreta Spectrum Incorporating Targeted Embolization and a Selective Use of Delayed Hysterectomy. Am J Perinatol 2022;29(14):1503–13. doi: 10.1055/s-0042-1754321 [published Online First: 2022/August/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee PS, Kempner S, Miller M, et al. Multidisciplinary approach to manage antenatally suspected placenta percreta: updated algorithm and patient outcomes. Gynecol Oncol Res Pract 2017;4:11. doi: 10.1186/s40661-017-0049-6 [published Online First: 2017/August/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandraharan E, Rao S, Belli AM, et al. The Triple-P procedure as a conservative surgical alternative to peripartum hysterectomy for placenta percreta. Int J Gynaecol Obstet 2012;117(2):191–4. doi: 10.1016/j.ijgo.2011.12.005 [published Online First: 2012/February/14] [DOI] [PubMed] [Google Scholar]
- 89.Aryananda RA, Aditiawarman A, Gumilar KE, et al. Uterine conservative-resective surgery for selected placenta accreta spectrum cases: Surgical-vascular control methods. Acta Obstet Gynecol Scand 2022;101(6):639–48. doi: 10.1111/aogs.14348 [published Online First: 2022/March/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palacios-Jaraquemada JM, Fiorillo A, Hamer J, et al. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective-reconstructive technique. J Matern Fetal Neonatal Med 2022;35(2):275–82. doi: 10.1080/14767058.2020.1716715 [published Online First: 2020/January/28] [DOI] [PubMed] [Google Scholar]
- 91.Nieto-Calvache AJ, Palacios-Jaraquemada JM, Aryananda R, et al. How to perform the one-step conservative surgery for placenta accreta spectrum move by move. Am J Obstet Gynecol MFM 2022;5(2):100802. doi: 10.1016/j.ajogmf.2022.100802 [published Online First: 2022/November/14] [DOI] [PubMed] [Google Scholar]
- 92.Dawood AS, Elgergawy AE, Elhalwagy AE. Evaluation of three-step procedure (Shehata’s technique) as a conservative management for placenta accreta at a tertiary care hospital in Egypt. J Gynecol Obstet Hum Reprod 2019;48(3):201–05. doi: 10.1016/j.jogoh.2018.10.007 [published Online First: 2018/October/15] [DOI] [PubMed] [Google Scholar]
- 93.Abo-Elroose AA, Ahmed MR, Shaaban MM, et al. Triple P with T-shaped lower segment suture; an effective novel alternative to hysterectomy in morbidly adherent anterior placenta previa. J Matern Fetal Neonatal Med 2021;34(19):3187–91. doi: 10.1080/14767058.2019.1678145 [published Online First: 2019/October/17] [DOI] [PubMed] [Google Scholar]
- 94.Ghaleb MM, Safwat S, Purohit R, et al. Conservative stepwise surgical approach for management of placenta previa accreta: A prospective case series study. Int J Gynaecol Obstet 2022;157(2):383–90. doi: 10.1002/ijgo.13887 [published Online First: 2021/September/23] [DOI] [PubMed] [Google Scholar]
- 95.Palacios-Jaraquemada JM, Basanta N, Labrousse C, et al. Pregnancy outcome in women with prior placenta accreta spectrum disorders treated with conservative-reconstructive surgery: analysis of 202 cases. J Matern Fetal Neonatal Med 2021:1–5. doi: 10.1080/14767058.2021.1910671 [published Online First: 2021/April/13] [DOI] [PubMed] [Google Scholar]
- 96.Khong TY, Robertson WB. Placenta creta and placenta praevia creta. Placenta 1987;8(4):399–409. doi: 10.1016/0143-4004(87)90067-1 [published Online First: 1987/July/01] [DOI] [PubMed] [Google Scholar]
- 97.Lee AY, Ballah D, Moreno I, et al. Outcomes of balloon occlusion in the University of California Morbidly Adherent Placenta Registry. Am J Obstet Gynecol MFM 2020;2(1):100065. doi: 10.1016/j.ajogmf.2019.100065 [published Online First: 2020/December/22] [DOI] [PubMed] [Google Scholar]
- 98.Shrivastava V, Nageotte M, Major C, et al. Case-control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol 2007;197(4):402.e1–5. doi: 10.1016/j.ajog.2007.08.001 [published Online First: 2007/October/02] [DOI] [PubMed] [Google Scholar]
- 99.Ballas J, Hull AD, Saenz C, et al. Preoperative intravascular balloon catheters and surgical outcomes in pregnancies complicated by placenta accreta: a management paradox. Am J Obstet Gynecol 2012;207(3):216.e1–5. doi: 10.1016/j.ajog.2012.06.007 [published Online First: 2012/July/27] [DOI] [PubMed] [Google Scholar]
- 100.Salim R, Chulski A, Romano S, et al. Precesarean Prophylactic Balloon Catheters for Suspected Placenta Accreta: A Randomized Controlled Trial. Obstet Gynecol 2015;126(5):1022–28. doi: 10.1097/aog.0000000000001113 [published Online First: 2015/October/08] [DOI] [PubMed] [Google Scholar]
- 101.Dai M, Zhang F, Li K, et al. The effect of prophylactic balloon occlusion in patients with placenta accreta spectrum: a Bayesian network meta-analysis. Eur Radiol 2022;32(5):3297–308. doi: 10.1007/s00330-021-08423-6 [published Online First: 2021/December/01] [DOI] [PubMed] [Google Scholar]
- 102.Liu C, Yang DD, Qu HB, et al. Efficacy and safety of prophylactic abdominal aortic balloon occlusion versus internal iliac arterial balloon occlusion for placenta accreta spectrum disorder: A systematic review and meta-analysis. Clin Imaging 2021;78:250–55. doi: 10.1016/j.clinimag.2021.06.020 [published Online First: 2021/June/26] [DOI] [PubMed] [Google Scholar]
- 103.Hawthorn BR, Ratnam LA. Role of interventional radiology in placenta accreta spectrum (PAS) disorders. Best Pract Res Clin Obstet Gynaecol 2021;72:25–37. doi: 10.1016/j.bpobgyn.2021.01.007 [published Online First: 2021/March/01] [DOI] [PubMed] [Google Scholar]
- 104.Picel AC, Wolford B, Cochran RL, et al. Prophylactic Internal Iliac Artery Occlusion Balloon Placement to Reduce Operative Blood Loss in Patients with Invasive Placenta. J Vasc Interv Radiol 2018;29(2):219–24. doi: 10.1016/j.jvir.2017.08.015 [published Online First: 2017/November/13] [DOI] [PubMed] [Google Scholar]
- 105.Shahin Y, Pang CL. Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. Eur Radiol 2018;28(7):2713–26. doi: 10.1007/s00330-017-5222-0 [published Online First: 2018/February/07] [DOI] [PubMed] [Google Scholar]
- 106.Ioffe YJM, Burruss S, Yao R, et al. When the balloon goes up, blood transfusion goes down: a pilot study of REBOA in placenta accreta spectrum disorders. Trauma Surg Acute Care Open 2021;6(1):e000750. doi: 10.1136/tsaco-2021-000750 [published Online First: 2021/September/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li K, Zou Y, Sun J, et al. Prophylactic balloon occlusion of internal iliac arteries, common iliac arteries and infrarenal abdominal aorta in pregnancies complicated by placenta accreta: a retrospective cohort study. Eur Radiol 2018;28(12):4959–67. doi: 10.1007/s00330-018-5527-7 [published Online First: 2018/June/07] [DOI] [PubMed] [Google Scholar]
- 108.Melber DJ, Berman ZT, Jacobs MB, et al. Placenta Accreta Spectrum Treatment With Intraoperative Multivessel Embolization: the PASTIME protocol. Am J Obstet Gynecol 2021;225(4):442.e1–42.e10. doi: 10.1016/j.ajog.2021.07.001 [published Online First: 2021/July/11] [DOI] [PubMed] [Google Scholar]
- 109.Mikkelsen ME, Still M, Anderson BJ, et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit Care Med 2020;48(11):1670–79. doi: 10.1097/ccm.0000000000004586 [published Online First: 2020/September/19] [DOI] [PubMed] [Google Scholar]
- 110.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care 2011;17(1):43–9. doi: 10.1097/MCC.0b013e3283427243 [published Online First: 2010/December/21] [DOI] [PubMed] [Google Scholar]
- 111.Bartels HC, Horsch A, Cooney N, et al. Living beyond placenta accreta spectrum: parent’s experience of the postnatal journey and recommendations for an integrated care pathway. BMC Pregnancy Childbirth 2022;22(1):397. doi: 10.1186/s12884-022-04726-8 [published Online First: 2022/May/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bartels HC, Mulligan KM, Lalor JG, et al. A life changing experience: An interpretative phenomenological analysis of women’s experiences of placenta accreta spectrum. Eur J Obstet Gynecol Reprod Biol 2020;254:102–08. doi: 10.1016/j.ejogrb.2020.09.014 [published Online First: 2020/September/21] [DOI] [PubMed] [Google Scholar]
- 113.Einerson BD, Watt MH, Sartori B, et al. Lived experiences of patients with placenta accreta spectrum in Utah: a qualitative study of semi-structured interviews. BMJ Open 2021;11(11):e052766. doi: 10.1136/bmjopen-2021-052766 [published Online First: 2021/November/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


